Abstract
Polystyrene (PS) is an important model polymer for the investigation of effects of microplastic (MP) and nanoplastic (NP) particles on living systems. Aqueous dispersions of PS MP or NP contain residual monomers of styrene. In consequence, it is not clear if the effects observed in standard (cyto)toxicity studies are evoked by the polymer (MP/NP) particle or by residual monomers. We addressed that question by comparing standard PS model particle dispersions with in-house synthesized PS particle dispersions. We proposed a rapid purification method of PS particle dispersions by dialysis against mixed solvents and developed a simple method of UV–vis spectrometry to detect residual styrene in the dispersions. We found that standard PS model particle dispersions, which contain residual monomers, exerted a low but significant cytotoxicity on mammalian cells, while the in-house synthesized PS, after rigorous purification to reduce the styrene content, did not. However, the PS particles per se but not the residual styrene in both PS particle dispersions resulted in immobilization of Daphnia. Only by using freshly monomer-depleted particles, will it be possible in the future to assess the (cyto)toxicities of PS particles, avoiding an otherwise not controllable bias effect of the monomer.
Keywords: microplastics, polystyrene model particles, residual monomer styrene, simple detection method, rapid purification, cytotoxicity, immobilization test, biased conclusions
Short abstract
We discovered that toxic effects ascribed to polystyrene particles stem from residual styrene monomers, which could result in ambiguous interpretations of polystyrene micro- and nanoplastic impact on cells, tissues, and organisms. In microplastic toxic studies such as MTT assays of cell viability or acute immobilization test of Daphnia, model plastic particles are often used. Spherical PS particles are one of the most popular candidates for that purpose. Nevertheless, we questioned the reliability of model particles such as PS particles used in such studies owing to the possibility of monomeric contamination that is highly possible due to incomplete purification of the monomer. Therefore, residual styrene content analysis is of importance. A simple yet sensitive method of UV−vis to analyze residual styrene and PS concentration was developed because in PS particle aqueous dispersion, the reported methods of detecting styrene are still tedious. This graphic not only shows our concept but also reveals a corner of our method.
1. Introduction
Commodity plastics are manufactured in hundreds of million tons per year worldwide.1 Plastics are mostly used in personal care products such as cosmetics and toiletries2,3 as well as in coatings such as paints1,4 that are sources of primary microplastics (MPs, plastic particles <5 mm) and in packaging and engineering that serve as sources of secondary MPs. Although the use of primary MPs in cosmetics is on the decline,2,5 the plastic particles in paints are still not widely recognized as MPs.4 However, they should strictly be considered as MP since polymer particles from paints also end up in the environment.6−10 The types of polymers that paints and plastics share are based mainly on alkyd, epoxy, polyacrylate, polyurethane, and polystyrene.4,10 MPs and NPs (nanoplastic particles, 1–1000 nm)11 derived from paints or other plastic applications have raised concerns, given their putative impact on environmental and human health.3−5,12−15 It has already been shown that they could lead to adverse effects on subcellular and organismic levels16 in the community17 and eventually on the ecological level.18
To investigate the effects of various MPs/NPs on cells19−21 and organisms,22,23 model plastic particles, most of them being spherical polystyrene (PS) particles, are commonly used because of their commercial availability.24−27 Nevertheless, PS particle dispersion used as standard model particles might contain a non-negligible amount of residual monomers. The residual monomers are toxic28,29 and difficult to remove completely from the PS particles. Furthermore, they are rarely considered when assessing the toxicity of the corresponding particles. For example, some research studies, without revealing the residual monomer content, found that standard PS model particles (∼10 μm) can greatly decrease the cell viability of L929 cells.30
Thus, it is important to thoroughly investigate the residual monomer content of the model particle dispersion to test whether the observed adverse effects originate from the particles per se or the residual monomer, thereby judging whether the standard model particle dispersions are qualified. To answer this, we used commercially available PS particle dispersions, referred to as standard particle dispersions, and in-house synthesized PS particle dispersions (∼500 nm) with known monomer contents. We compared the toxicity of both types of particles on a cellular and organismic level, i.e., murine fibroblasts (L929) and Daphnia magna. The size of 500 nm was chosen because this size of PS particles per se does not result in decrease in cell viability of L929 cells, thereby ruling out the effect of PS particles if PS particle dispersion is qualified.31 The L929 cell lines are continuous cell lines and routinely used for biological tests. They are recommended for in vitro biological reactivity tests in contact with polymeric materials by the United States Pharmacopeia Convention (USP 43–NF 38) and are also recommended by the International Organization for Standardization (ISO10993-5: 2009) because of their reproducibility and biological responses. We used Daphnia magna to test the toxicity in organismic level since Daphnia, with high sensitivity to toxicants, is among the most common model organisms for biological studies such as ecotoxicity of MP.26,32,33Daphnia is a keystone species in freshwater ecosystems since it serves as link between primary production and higher trophic levels. As an unselective filter feeder, it is likely exposed to particulate contaminants such as MP.
2. Materials and Methods
2.1. Materials
Styrene (Sigma-Aldrich, ≥99%), potassium persulfate (Aldrich, >99%), potassium carbonate (Fisher Scientific, ≥99.5%), aluminum oxide (Sigma-Aldrich, activated), methanol (VWR chemicals, 99.9%), CDCl3 (Deutero Gmbh, 99.8%), methanol-d4 (Deutero Gmbh, 99.8%), Milli-Q water (18 MΩ·cm), THF (GPC grade), dimethylsulfoxide (DMSO, Sigma, sterile-filtered, Bioperformance certified, meets EP, USP testing specifications), and standard PS model particle dispersion (Polysciences, Cat # 07307-15, 2.7%, 500 nm) were used. Greiner Bio-One was used as the supplier for cell culture materials. Minimum essential medium without phenol red was from Gibco. Eagle’s minimal essential medium, Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+, trypsin/EDTA solution, l-glutamine solution, and penicillin/streptomycin solution were from Lonza. Fetal calf serum (FCS) and isopropanol (analytical grade) were from Sigma Aldrich. Trypan blue solution was from VWR International (0.4%). 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT, 98%) was from Alfa Aesar, M4 medium.34
2.2. Emulsifier-Free Emulsion Polymerization of Styrene
Styrene went through a column with activated aluminum oxide and potassium carbonate to remove the inhibitor, 4-tert-butylcatechol. The inhibitor was adsorbed in the aluminum oxide gel, thereby resulting in destabilized styrene, which was collected with a Schlenk flask under argon. The collected styrene was degassed under argon for 30 min before addition to the reactor.
To 150 mL of Milli-Q water in a 500 mL three-necked flask, styrene (260 mM), NaCl (24.9 mM), and potassium persulfate (2.7 mM) were added. The reaction mixture was maintained at 80 °C for 24 h, followed by cooling down to room temperature. Then, 130 mL of Milli-Q water was added to the flask and degassed for 30 min. To the reactor, 4.6 mL of degassed and destabilized styrene was added, and the mixture was degassed for an additional 10 min. 225 mg of NaCl was dissolved in 10 mL of Milli-Q water. To tune the particle size, the NaCl solution was degassed for 20 min and then added to the reactor. The emulsion was then heated to 80 °C under stirring at 200 rpm. In a 50 mL flask, 112.5 mg of potassium persulfate was dissolved in 10 mL of Milli-Q water and then degassed under argon for 20 min. Then, 10 mL of solution of potassium persulfate was added to the styrene emulsion in one step. After the reaction proceeded at 80 °C under argon with stirring at 200 rpm for 24 h, the reactor was cooled to room temperature in an ice bath.
The reaction conversion (86.7%) was calculated as follows:
The PS concentration (19.36 mg/mL) was determined by freeze-drying after PS was purified by dialysis against Milli-Q water for 40 days.
2.3. Purification of Synthesized PS Dispersion by Dialysis against Water
A portion of the reaction mixture was charged into a dialysis membrane with a molecular weight cutoff of ca. 3500 Da. Air bubbles were removed out of the membrane, and the membrane tube was sealed by clamps with metal bars. The dialysis tube was immersed into Milli-Q water in a 4 L beaker. The ratio of dialysis medium Milli-Q water and the PS dispersion was 40. The dialysis was performed under constant stirring. Fresh Milli-Q water was changed twice per day for the first week, and afterward, once per day. The dialysis was allowed to proceed for 40 days, resulting in dispersion named SIP (“S” refers to in-house synthesized PS particle dispersion; “IP” refers to intermediate purity). Its PS concentration was determined by freeze-drying.
2.4. Rapid Purification of PS Dispersion by Dialysis against Mixed Solvents
The standard PS model particle dispersion was named MIP (“M” for standard PS model particle dispersion, “IP” for intermediate purity). To eliminate the residual styrene further, we dialyzed SIP and MIP with a rapid process, boosted by mixed solvents of distilled methanol/Milli-Q water (50/50 volumetric ratio). The rapid dialysis was against mixed solvents for 8 days then against Milli-Q water for 12 days, resulting in SHP and MHP (“HP” means high purity). In detail, 7 mL of PS dispersion was transferred into a dialysis tube (MWCO ca. 3500 Da), air bubbles were removed out of the membrane, and the membrane tube was sealed by clamps equipped with a metal bar. The prepared tube was placed into a 250 Erlenmeyer flask with a wide neck. 200 mL of mixed solvents of distilled methanol/Milli-Q water (100/100 volumetric) was added. The dialysis medium was stirred at 100 rpm at room temperature. The mixed solvents were refreshed twice a day for the first 8 days. Afterward, the dialysis medium was changed as Milli-Q water, and the dialysis was allowed to proceed for another 12 days at the same stirring rate with water refreshed once a day. Before the MTT assays, the PS particle dispersions were autoclaved (15 min, 121 °C) in a standard laboratory autoclave. Water in the PS particle dispersions can prevent the PS particles from over-heating at such autoclaving conditions. The “A” in “SIPA”, “SHPA”, “MIPA”, and “MHPA” means that they were sterilized by an autoclave. For all PS particle dispersions, PS concentrations and residual styrene contents were determined by a UV–vis spectrometer—at a wavelength 280 and 246 nm,35 respectively. With the styrene determined, the purity of PS in dispersions was calculated as below. We calculated the purity of the PS dispersions based on the residual styrene content due to the synthesis method of emulsifier-free emulsion polymerization, where styrene was believed to be the main impurity. Although the standard PS model particle dispersion contains unknown proprietary surfactants, we used the same equation to obtain purity for these preparations, assuming again residual styrene to constitute the main impurity.
2.5. Quantification of Styrene and PS by a UV–vis Spectrometer: UV Method
Styrene detection was traditionally measured by gas chromatography–mass spectrometry (GC–MS),36−38 high-performance liquid chromatography with a UV detector (HP-LC),39,40 or an electrochemical method.41 Back in 1951, UV–vis was used for styrene detection in solid PS samples.42 The ISO (ISO 2561:2015; 1974) issued the standard method of residual styrene determination in PS products by GC. Few studies used GC,43 GC–MS37 and HP-LC39,40 to detect styrene in aqueous solutions. However, these techniques were either tedious or expensive because the columns are easily contaminated. Hence, we developed a simple yet sensitive method to measure styrene in aqueous solutions. Compared with the GC and LC methods, the limit of detection of our UV method (limit of detection, 0.1 μg/mL) is in the same range as for the GC method37 while higher than for the LC method.39,40
The calibration curve of styrene was built. A mixed solvent system of distilled methanol/water (always 90/10 volumetric ratio unless stated otherwise) was used to conduct the calibration for the UV method with a UV–vis spectrometer (UV–vis). The mixed solvents served as the blank for UV–vis measurement. Sample preparation and measurements were performed at room temperature. Specifically, 82.60 mg of styrene (0.091 mL) was weighed under a fine balance and then dissolved in methanol/water (5.565 mL taken by pipetting). It had a concentration of 14.60 mg/mL. This solution was diluted 20 × 20 × 7.3 times. A 5 μg/mL of stock solution of styrene resulted. From this, 4, 3, 2, 1, and 0.5 μg/mL styrene solutions were prepared. They were measured by UV–vis (500–200 nm, 400 nm/min, data interval: 1 nm). The absorbance value A246 at peak 246 nm35 for styrene was chosen to build its calibration curve. The A246 of around 0.07 for 0.5 μg/mL styrene solution was recorded to make sure the calibration curve can cover low styrene concentrations for the highly purified PS particle dispersions. The whole procedure of calibration was repeated twice.
The quantification of styrene in the PS particle dispersion, for instance, SHPA, was conducted by UV–vis. Specifically, 0.3 mL of the PS particle dispersion was mixed with 2.7 mL of methanol, which was then shaken at 1000 rpm for 10 min to molecularly dissolve the styrene in the mixed solvent. It was centrifuged at 21,000 g for 8 min to rule out interference by remaining PS beads. The supernatant was centrifuged again in the same condition to remove any remaining PS beads. Then, its UV absorbance was measured.
The calibration curve of spherical PS particles (using SIP) was built. PS particle dispersions of known concentrations within 5–15 μg/mL were prepared by diluting the stock dispersion (19.36 mg/mL) with Milli-Q water and measuring the A280 absorbance. The absorbance values for diluted samples were between 0.65 and 0.25. The calibration procedure was also repeated twice.
The average UV absorbance value and the standard deviation were plotted and linearly fitted in Origin software (Figure S4C, S4D).
Other methods are presented in Supporting Information.
3. Results and Discussion
3.1. Synthesis and Characterization of PS Particle Dispersions
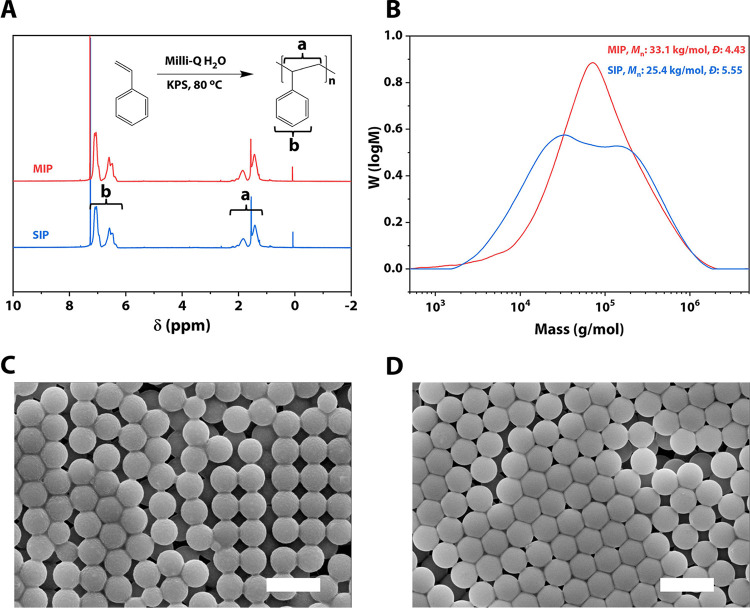
The in-house synthesized spherical PS particle dispersions [number average molar mass Mn = 2.54 × 104, dispersity Đ = 5.55, diameter 515 ± 17 nm and hydrodynamic diameter, 578 ± 173 nm] (Figures 1, S2 and S3) by emulsifier-free emulsion polymerization and standard PS particle dispersion [number average molar mass Mn = 3.31 × 104, dispersity Đ = 4.43, diameter 507 ± 7 nm, and hydrodynamic diameter 555 ± 157 nm] were tested. In the nomenclature of our study “MIPA” and “MHPA”, “SIPA” and “SHPA”, “M” is for samples serving as standard model particle dispersion, “S” is for in-house synthesized samples, “IP” is for intermediate purity, “HP” is for high purity, and “A” is for sterilization by an autoclave. “MIP”, “MHP”, “SIP”, and “SHP” mean that these samples were not autoclaved. We used monodispersed PS particles in our study. The size of the PS particles in both M and S samples is similar (Figures 1 and S2). However, the hydrodynamic diameters of the S samples are slightly higher but still fall within the range of the M samples (Figure S3).
Figure 1.
Preparation of spherical PS particles sized ∼500 nm by emulsifier-free emulsion polymerization and its molecular weight and size comparison with the standard PS model particles sized ∼500 nm. (A) 1H NMR spectra of standard PS as received (MIP) in red and in-house synthesized PS dialysis against Milli-Q water for 40 days (SIP) in blue. (B) Molecular weight measured by GPC of standard PS as received (MIP) in red, Mn = 3.31 × 104, dispersity Đ = 4.43 and of in-house synthesized PS dialyzed against Milli-Q water for 40 days (SIP) in blue, Mn = 2.54 × 104, dispersity Đ = 5.55. (C) Size determined by SEM for SIP, 515 ± 17 nm. (D) Size determined by SEM for MIP, 507 ± 7 nm. Scale in (C, D): 1 μm.
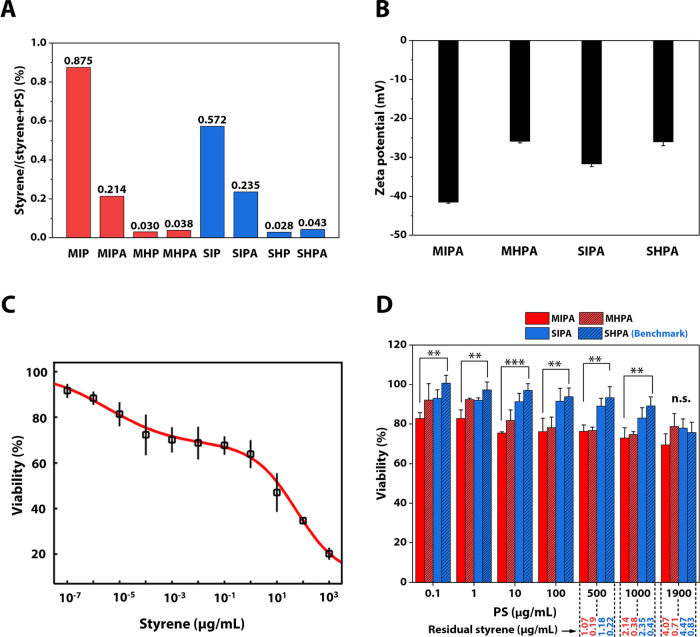
The in-house synthesized PS particle dispersion was first purified by dialysis against Milli-Q water (referred to as “intermediate purity”) and this process takes up to 40 days. Therefore, to speed up this process, the PS particle dispersion and the standard model particle dispersion were further dialyzed against mixed solvents of methanol/water (50/50 volumetric ratio) (referred to as “high purity”). The mixed solvents for dialysis enhanced the diffusion of residual styrene to the dialysis medium, thereby accelerating the purification process. The purity of the PS particle dispersions is defined as the percentage of the PS mass accounting for the total mass of PS and styrene in the dispersions. Residual styrene concentrations (Figure 2A, Table 1) were therefore detected according to the calibration curve (Figure S4C). The standard PS MIP (used as received) contained nearly 0.9% of styrene, higher than in-house synthesized SIP at 0.6% (Figure 2A). Its autoclaved counterpart MIPA contained nearly same styrene at 0.2% for SIPA. Further rapid purification efficiently reduced residual styrene contents in MHP, SHP, MHAP, and SHAP to about 0.03%. Autoclaving was conducted to sterilize the PS dispersions and glassware for the mammalian cell-based studies to avoid other unknown factors other than PS particles or styrene evoking cytotoxicity. Nevertheless, styrene amounts in MHPA and SHPA stored for 13 months are even more than double than those in MIPA and SIPA (Figure S6A). The styrene in MHP stored for 14 months, however, increased to nearly the amount of MIP that maintained a stable styrene content of around 0.8%, while that in SHP in 14 months nearly doubled, contrast to SIP (Figure S6B). The PS concentration in dispersions (Table 1) was determined by the UV method (Figure S4D). Thus, we can calculate the standard PS MIP and MIPA (autoclaved) at 1 mg/mL of the PS concentration concomitantly maintained at 8.8 and 2.1 μg/mL of residual styrene (Table 1), respectively. Likewise, SIP and SIPA with intermediate purity bore the residual styrene over 2 μg/mL at the same PS concentration. In contrast, MHPA and SHPA after rapid dialysis only have styrene monomer below 1 μg/mL even at the highest particle concentration investigated (1.9 mg/mL). Being stored over 1 to 2 years, all PS dispersions investigated have residual styrene contents of at least 1.5 μg/mL at PS particles of 0.3 mg/mL (Table S1). In addition to the styrene content, the zeta potential of the PS particle dispersions was also measured. An increase in the zeta potential was witnessed from −41 to −26 mV for the standard PS model particle dispersion, while it was from −31 to −26 mV for the case of in-house synthesized PS particle dispersion that did not use surfactants for polymerization (Figure 2B).
Figure 2.
MTT assays for styrene and PS particle dispersions, residual styrene, and zeta potential of the PS particle dispersions. (A) Residual styrene determined by UV–vis. (B) Zeta potential. (C) L929 cell viability for styrene; LC50 (50% lethal concentration: 1.5 μg/mL) evaluation was performed by Dr-Fit software fitting;47 this figure was generated by Dr-Fit software. (D) L929 cell viability for in-house synthesized PS particle (∼500 nm) dispersion and standard PS model particle (∼500 nm) dispersion. One-way ANOVA with a Tukey post hoc test was performed to show significant differences between treatments; *P < 0.05, **P < 0.01, ***P < 0.001, n.s. means no significant difference. Significant differences were only between MIPA and SHPA; SIPA and MHPA on the other hand did not have any significant differences even at P < 0.05.
Table 1. Concentrations of PS Particle Dispersions and Residual Styrene Concentration at Varying PS Contents in Cell Culturea.
| nomenclature | PS (μg/mL) | styrene (%) | PS (%) | residual
styrene at varying PS (μg/mL) |
|||
|---|---|---|---|---|---|---|---|
| 300 | 500 | 1000 | 1900 | ||||
| MIP | 27,400 | 0.875 | 99.13 | 2.65 | 4.41 | 8.82 | 16.77 |
| MIPA | 30,500 | 0.214 | 99.79b | 0.64 | 1.07 | 2.14 | 4.07 |
| MHP | 17,300 | 0.030 | 99.97 | 0.09 | 0.15 | 0.30 | 0.56 |
| MHPA | 21,030 | 0.038 | 99.96b | 0.11 | 0.19 | 0.38 | 0.71 |
| S0 | 86.7 | ||||||
| SIP | 19,360 | 0.572 | 99.43 | 1.73 | 2.88 | 5.75 | 10.93 |
| SIPA | 20,780 | 0.235 | 99.77b | 0.71 | 1.18 | 2.35 | 4.47 |
| SHP | 15,510 | 0.028 | 99.97 | 0.08 | 0.14 | 0.28 | 0.53 |
| SHPA | 17,260 | 0.043 | 99.96b | 0.13 | 0.22 | 0.43 | 0.83 |
Calculated final styrene concentration for PS particle dispersions at PS concentrations of 300, 500, 1000, and 1900 μg/mL.
The purity of the autoclaved dispersions changed. This can be ascribed to the overall outcome of the evaporation and condensation of water and styrene (boiling point 145 °C, density 0.91 g/mL) in the glass vial during the autoclaving process (121 °C). Purity refers to the percentage of the PS mass accounting for the total mass of PS and styrene in the dispersions. MIP, standard PS model particle dispersion as received; MIPA, autoclaved MIP; MHP, rapidly dialyzed MIP against methanol/water (50/50) mixed solvents; MHPA, autoclaved MHP; S0, in-house synthesized PS particle dispersion without purification; SIP, S0 dialyzed against water for 40 days; SIPA, autoclaved SIP; SHP, rapidly dialyzed SIP against methanol/water (50/50) mixed solvents; SHPA, autoclaved SHP.
3.2. Diffusion of Styrene in PS Dispersions
In storage, diffusion of styrene between PS particles and water medium happened. It comprises two processes of the leaching of styrene and the absorbing of styrene (Figure S7). Although the amounts of styrene in MIPA and MHPA at the beginning were of dramatical difference, yet they increased and reached a similar level around 0.5% after being stored for 13 months (Figure S6). This was a process of styrene leaching from Z1 to Z2 and to Z3 (LPS-H2O), resulting in more “free” styrene (Table S1). As for MIP and MHP, the former had 29 times more styrene than the latter. The styrene in the former slowly dropped by 10% within 1.5 years to a level of 0.78% that the styrene in the latter caught up within 14 months. The process happening in MHP was still an LPS-H2O, while that happening in MIP is the opposite path of an AH2O-PS (styrene absorbing from H2O to PS), leading to a decrease in “free” styrene. This tells that for standard PS model dispersions, styrene at 0.78% might already be the stable level in the equilibrium state. Deviating from this level, either LPS-H2O or AH2O-PS took place.
Whereas for the in-house synthesized PS, styrene in SHPA reached around 0.5% same as that of MIPA stored for the same time, that in SIPA surpassed that value by over 0.1%. For SIP and SHP, styrene in the latter stored for 14 months reached 1%, exceeding the stable level of MIP and MHP. Styrene in SIP, however, climbed even higher to 1.4% on being stored for a prolonged time to 22 months. However, the leaching process was slow; within about 2 years, styrene still did not reach its saturated concentration in water44 (Table S1). This indicated that the standard model PS with surfactants was a more stable system than the in-house synthesized PS without any.45 Surfactants, toxic,46 are able to lower styrene contents in the equilibrium state and therefore shortened the duration of returning to that state.
3.3. MTT Assays for Styrene and PS Particle Dispersions
Some investigations including ours on PS particle cytotoxicity19,20 used maximal PS concentration at 1 mg/mL in cytotoxicity assays that lead to detectable toxicity of the PS particle dispersions in murine macrophages and intestinal epithelial cell lines.19 To scrutinize if styrene monomers played a role in such studies, we analyzed the threshold toxic concentration of styrene at the cellular level (murine fibroblast L929 cells) using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Figure 2C). The detected 50% lethal concentration (LC50) value for styrene was 1.5 μg/mL, indicating that styrene is highly toxic to the cells. At some tested PS concentrations, the residual styrene concentrations in the particle dispersions measured by the UV method (Table 1) are above the LC50 value and hence expected to be partly responsible for the cytotoxicity of the PS dispersions.
The LC50 value of cellular studies of styrene is a good reference, yet it is worth distinguishing whether all styrene molecules participated in reducing the cell viability. In the cell culture (i.e., containing 10% FCS) of the cell-based assay, residual styrene could be present in various states (Figure S7), the “free” state of molecularly dissolved styrene-water complexes since styrene solubility in water is 300 μg/mL at 25 °C,44 the “trapped” state of PS-styrene complexes due to their chemical similarity,48 and protein-styrene complexes because of molecular interaction with serum proteins. The “free” styrene will be more toxic than the “trapped” styrene because the former is directly diffusible to cells, thus inducing cytotoxicity. However, styrene in the “trapped” state might be released particularly when PS-styrene and proteins-styrene come near the plasma membrane that consists of the phospholipid bilayer.
To monitor whether “free” styrene is still sufficient to interfere with the results of cell study, we performed MTT assays of PS particle dispersions (Figure 2D). Only between MIPA and SHPA is there a statistical significance for the whole range of tested PS concentrations except for the highest one. SHPA led to the highest cell survival among the tested samples for PS ≤ 1 mg/mL. This indicates that the PS particles themselves are not toxic and that the concentration of contaminating styrene (“free” styrene <0.5 μg/mL at PS 1 mg/mL) is too low to negatively influence the cell viability. Styrene is genotoxic and carcinogenic. Its cytotoxicity is related to the metabolism in cells. It can be metabolized to styrene oxide that indicates styrene’s reproductive toxicity.29,49 While PS NPs (70 nm) can be internalized by cells, it can further result in cytotoxicity and disturb the gene transcript and protein expression. Such internalization of NPs is size-dependent.50 Internalized larger PS NPs (200 nm) lead to slight cytotoxicity to L929 cells.51 The highly purified SHPA here, however, is not cytotoxic to L929 cells, which is consistent with the literature.31 Therefore, we treated SHPA as the current benchmark.
In detail, SHPA introduced less residual styrene to the cells, and therefore triggered lower cell death rate than SIPA (PS ≤ 1 mg/mL). This is consistent with the results observed for the standard MIP-based preparations MHPA and MIPA (PS < 500 μg/mL). SHPA’s low zeta potential of −26 mV (Figure 2B), however, might lead to higher cytotoxicity because of weaker electrostatic repulsion between particles and the negatively charged cell surfaces.52,53 Besides, SHPA contains no potentially cytotoxic surfactant46 thanks to the preparation technique of emulsifier-free emulsion polymerization. According to our supplier, some residual proprietary surfactant is present in the standard PS model particle dispersions. The dramatic zeta potential change between the M samples and the much small change between the surfactant-free S samples can support this. Thus, SIPA performed better than MIPA, so did SHPA than MHPA. The surfactant and other possible proprietary chemicals in standard PS model particle dispersion might explain why MHPA and MIPA tend to have the same cell survival rate at PS ≥ 500 μg/mL. Apart from the chemical reasons, the effects of particle sizes on cytotoxicity are well known.20,27,31,53,54 Though the S and M samples possess similar particle size, the S samples have slightly higher hydrodynamic diameters than the M samples. However, such a slight difference in hydrodynamic diameters is unlikely to cause observable difference in cytotoxicity.20,27,31 Hence, it cannot be excluded that the cytotoxicity of standard PS model particle dispersions could be due to the free styrene monomers.
While SHPA, the benchmark along with other tested samples at PS of 1.9 mg/mL held survival rates greater than 70% and approximately in the same range (Figure 2D). SIPA and MIPA at the highest tested concentration contain more than twice the amount of residual styrene than the LC50 of styrene, yet they did not cause a significant decrease in the cell viability. The reason behind this, apart from a decrease in “free” styrene, could be that PS particles at a high concentration will cover the cells, building a “layer” blocking the “free” styrene and interfering with gas diffusion, nutrients, and metabolite supply. Thus, for a given concentration, cell viability will be similar for all samples for these combined reasons.
3.4. Acute Immobilization Test for Styrene and PS Particle Dispersions
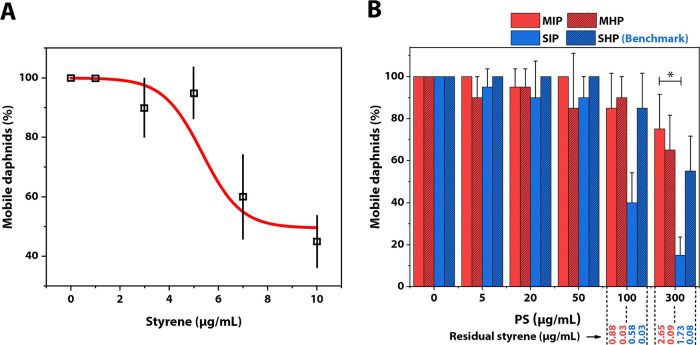
Besides the cytotoxicity of styrene at the cellular level, we investigated the influence of styrene and PS at the organismic level using the OECD Daphnia sp. acute immobilization test.55Daphnia magna was exposed to pure styrene monomers (0–10 μg/mL) for 48 h or to PS particle dispersions (0–300 μg/mL) for 96 h. After the given experimental time, we counted the number of immobilized daphnids and plotted the percentage of mobile daphnids exposed to styrene or PS at a range of concentrations. The half-maximal effective concentration (EC50) for styrene in this case was 9 μg/mL, being slightly higher than the reported value,56 which could be due to differences in the experimental setup (static vs flow through). Hence, we found a higher tolerance of daphnids to styrene compared to the cells (Figure 3A). The EC50 for styrene indicates that at the maximum tested PS concentration (300 μg/mL), the residual styrene (2.65 μg/mL for MIP, Table 1) should have only little effect on the mobility of daphnids. Subsequently, an immobilization test was conducted with the nonautoclaved PS dispersions, MIP, MHP, SIP, and SHP. To observe the effect of PS particles per se, the incubation time for the daphnids was extended to 96 h.57 Except for SIP, we found a similar trend for all PS particle dispersions, that is, only at the highest concentrations, the ratio of mobile daphnids dropped by 20 to 50% (Figure 3B). The mobility of daphnids exposed to SIP already dropped at 100 μg/mL to around 40% and at 300 μg/mL to around 20%. Consequently, only the EC50 of SIP (106.8 μg/mL) was within the tested range of up to 300 μg/mL. Predictions of EC50 for the other batches were 497.4 μg/mL (MIP), 485.7 μg/mL (MHP), and 320 μg/mL (SHP). Differences within in-house synthesized or standard model beads (SIP vs SHP/MIP vs MHP) may be related to the amount of residual styrene as here, the only difference was the amount of residual styrene. Differences between the standard model and in-house synthesized beads (M vs S) may be related to other factors than residual styrene like the remaining surfactants or the surface charge.
Figure 3.
Acute test of daphnids in the presence of styrene and PS particle dispersions. (A) Mobility of daphnids incubated in the presence of styrene after 48 h. (B) Mobility of daphnids incubated in the presence of PS particle dispersions after 96 h. * indicates P ≤ 0.05 (Dunn’s test); Error bars show mean + SE.
Overall, the PS beads dominated the observed effects but not the styrene. By ruling out the effects of styrene, the in-house synthesized PS SHP constitutes a more suitable candidate for acute tests.
SHPA and SHP featuring high purity and contamination-free surfactants serve as the benchmark in biological studies in cellular and organismic level. Nevertheless, they should be freshly purified and used quickly due to the diffusion of styrene between PS particles and water medium. The aged one is not suitable for cell studies anymore. At PS of 300 μg/mL, residual styrene in all dispersions stored over 1 year was at least 1.5 μg/mL (Table S1), the LC50 value of styrene to L929 cells.
We proposed a facile and highly sensitive method to measure styrene concentrations in aqueous dispersions of spherical PS using a UV method that has not been reported before. We also provided synthesis and purification procedures allowing the production of benchmark PS particle dispersion with reduced amounts of residual monomeric styrene. The benchmark PS particle dispersion, however, has to be not only styrene depleted but also freshly purified since leaching of styrene in a benchmark dispersion dramatically increases during storage even in a proper condition. Our finding revealed that the residual monomer in the standard PS model particle dispersions other than the PS bead of the given size and spherical shape itself induces adverse effects at the cellular level, while PS bead per se other than the residual monomer leads to negative effects at the organismic level. Here, our study underlines the problem of residual monomer contamination in the present standard model particles and highlights the necessity of thorough removal of residual chemicals of the model particle dispersions, especially the residual monomers, to avoid interference or biased conclusion in assays involving these particles. Our study might also promote the method of standardization in the studies of MPs and NPs. This will help to address the threat of plastics without bias by unexpected contaminations.
3.5. Implications
We proposed a set of simple methods to provide qualified PS particle dispersions that served as the benchmark here. The standard PS model particle dispersions are not qualified in cell viability assays due to the presence of sufficient residual styrene monomer and therefore need to be thoroughly characterized and purified before being used in relevant investigations. Although the toxicity of styrene alone was well documented,29,58 its role in standard PS model particle dispersion was still not well recognized. The toxicity of NP and MP is in part due to their additives; plastic, a commercial organic material, comprises a polymer or polymer blends as the major part and additives as the minor part for better functionality. Any part of plastics can cause environmental problems. Polymers contain oligomers and unreacted monomers. Additives are important and mainly composed of antioxidants, flame retardants, plasticizers, lubricants, colorants, fillers, and impact modifiers. Most of the additives are organic molecules.15,59 Plastics weathered in the environment are gradually fragmented into MPs and further NPs and even degraded into small organic molecules. MPs and NPs can adsorb and release toxic chemicals to the environment3,4,14 and cause problems in cellular, organismic, and exosystemic levels.17,26,28,31−33
Thus, to cope with the pollution problems of plastics, one aspect is to broadly realize the toxic small chemicals such as monomers, oligomers, degraded byproducts, and additive leachates. More efficient methods and higher degree of postpurification of polymers, development of new materials such as biodegradable polymers, and usage of environmentally friendly additives can be a useful path to a pollution-reduced environment.
Acknowledgments
We thank R. Schneider for GPC measurements. We acknowledge the use of the SEM machine in the KeyLab Electron and Optical Microscopy and the use of zeta-sizer at Physical Chemistry II at the University of Bayreuth. Funding: This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB 1357 – 391977956. Y.H.Z. thanks the scholarship support from the China Scholarship Council (CSC).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c01134.
Validation of accuracy and precision of the UV-Method; diffusion of styrene in PS dispersions stored over time; 1H NMR spectra of monomer styrene in the PS particle dispersion; GPC measurements; SEM images of PS particles; hydrodynamic diameter determined by DLS; MTT assays; acute immobilization test of daphnids; statistical analysis; calibration curves of styrene and PS particles by UV-vis spectroscopy; representative UV-vis spectra of residual styrene and PS particle content detection by UV-vis spectroscopy; residual styrene concentrations in PS particle dispersions being stored in a 4 °C fridge over time; states of styrene in aqueous dispersions of PS particles and a proposed mechanism of styrene leaching; and residual styrene contents at given PS concentrations of PS particle dispersions being stored in a 4 °C fridge over time (PDF)
Author Contributions
A.G., Y.H.Z., V.J., R.F., and C.L. conceived and supervised the project. Y.H.Z. synthesized and characterized the PS beads with DLS, zeta-sizer, and SEM, purified PS particle dispersions, developed UV method of styrene detection in PS aqueous dispersions, and determined residual styrene contents. T.P. performed MTT assays with the technical assistance of M. Völkl and determined the LC50 of styrene to cells. J.B. performed acute test to daphnia and determined EC50 of styrene to daphnids. Y.H.Z. drafted the original manuscript led by A.G., which was reviewed by Y.H.Z., J.B., C.L., V.J., R.F., and A.G. and edited by Y.H.Z. All authors analyzed and discussed the data and approved the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Geyer R.; Jambeck J. R.; Law K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C. F.; Lin W. H.; Voulvoulis N. Evaluating alternatives to plastic microbeads in cosmetics. Nat. Sustainability 2021, 4, 366–372. 10.1038/s41893-020-00651-w. [DOI] [Google Scholar]
- Koelmans A. A.; Bakir A.; Burton G. A.; Janssen C. R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. Paint particles in the marine environment: An overlooked component of microplastics. Water Res.: X 2021, 12, 100110 10.1016/j.wroa.2021.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerranti C.; Martellini T.; Perra G.; Scopetani C.; Cincinelli A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. 10.1016/j.etap.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Verschoor, A.; de Poorter, L.; Dröge, R.; Kuenen, J.; de Valk, E. Emission of microplastics and potential mitigation measures: Abrasive cleaning agents, paints and tyre wear (2016–0026, Rijksinstituut voor Volksgezondheid en Milieu, 2016).
- Galafassi S.; Nizzetto L.; Volta P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019, 693, 133499. 10.1016/j.scitotenv.2019.07.305. [DOI] [PubMed] [Google Scholar]
- Frias J.; Nash R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Imhof H. K.; Laforsch C.; Wiesheu A. C.; Schmid J.; Anger P. M.; Niessner R.; Ivleva N. P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2016, 98, 64–74. 10.1016/j.watres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Gaylarde C. C.; Neto J. A. B.; Da Fonseca E. M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021, 162, 111847 10.1016/j.marpolbul.2020.111847. [DOI] [PubMed] [Google Scholar]
- Gigault J.; Ter Halle A.; Baudrimont M.; Pascal P. Y.; Gauffre F.; Phi T. L.; Hadri H. E.; Grassl B.; Reynaud S. Current opinion: What is a nanoplastic?. Environ. Pollut. 2018, 235, 1030–1034. 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- MacLeod M.; Arp H. P. H.; Tekman M. B.; Jahnke A. The global threat from plastic pollution. Science 2021, 373, 61–65. 10.1126/science.abg5433. [DOI] [PubMed] [Google Scholar]
- Santos R. G.; Machovsky-Capuska G. E.; Andrades R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 2021, 373, 56–60. 10.1126/science.abh0945. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Foekema E. M. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014, 187, 49–54. 10.1016/j.envpol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Hermabessiere L.; Dehaut A.; Paul-Pont I.; Lacroix C.; Jezequel R.; Soudant P.; Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere 2017, 182, 781–793. 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- Trotter B.; Wilde M. V.; Brehm J.; Dafni E.; Aliu A.; Arnold G. J.; Thomas F.; Laforsch C. Long-term exposure of Daphnia magna to polystyrene microplastic (PS-MP) leads to alterations of the proteome, morphology and life-history. Sci. Total Environ. 2021, 795, 148822 10.1016/j.scitotenv.2021.148822. [DOI] [PubMed] [Google Scholar]
- Redondo-Hasselerharm P. E.; Gort G.; Peeters E. T. H. M.; Koelmans A. A. Nano- and microplastics affect the composition of freshwater benthic communities in the long term. Sci. Adv. 2020, 6, eaay4054 10.1126/sciadv.aay4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T. S.; Cole M.; Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Rudolph J.; Völkl M.; Jérôme V.; Scheibel T.; Freitag R. Noxic effects of polystyrene microparticles on murine macrophages and epithelial cells. Sci. Rep. 2021, 11, 15702. 10.1038/s41598-021-95073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.; Choi D.; Han S.; Jung S. Y.; Choi J.; Hong J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. 10.1038/s41598-020-64464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsperger A. F. R. M.; Narayana V. K. B.; Gross W.; Mohanraj J.; Thelakkat M.; Greiner A.; Schmalz H.; Kress H.; Laforsch C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211 10.1126/sciadv.abd1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof H. K.; Rusek J.; Thiel M.; Wolinska J.; Laforsch C. Do microplastic particles affect Daphnia magna at the morphological, life history and molecular level?. PLoS One 2017, 12, e0187590 10.1371/journal.pone.0187590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank I.; Trotter B.; Dummert J.; Scholz-Böttcher B. M.; Löder M. G. J.; Laforsch C. Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna. Environ. Pollut. 2019, 255, 113233 10.1016/j.envpol.2019.113233. [DOI] [PubMed] [Google Scholar]
- Karakolis E. G.; Nguyen B.; You J. B.; Rochman C. M.; Sinton D. Fluorescent Dyes for Visualizing Microplastic Particles and Fibers in Laboratory-Based Studies. Environ. Sci. Technol. Lett. 2019, 6, 334–340. 10.1021/acs.estlett.9b00241. [DOI] [Google Scholar]
- Stock V.; Fahrenson C.; Thuenemann A.; Dönmez M. H.; Voss L.; Böhmert L.; Braeuning A.; Lampen A.; Sieg H. Impact of artificial digestion on the sizes and shapes of microplastic particles. Food Chem. Toxicol. 2020, 135, 111010 10.1016/j.fct.2019.111010. [DOI] [PubMed] [Google Scholar]
- Kelpsiene E.; Ekvall M. T.; Lundqvist M.; Torstensson O.; Hua J.; Cedervall T. Review of ecotoxicological studies of widely used polystyrene nanoparticles. Environ. Sci.: Processes Impacts 2022, 24, 8–16. 10.1039/d1em00375e. [DOI] [PubMed] [Google Scholar]
- Hesler M.; Aengenheister L.; Ellinger B.; Drexel R.; Straskraba S.; Jost C.; Wagner S.; Meier F.; Von Briesen H.; Büchel C.; Wick P.; Buerki-Thurnherr T.; Kohl Y. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, 104610 10.1016/j.tiv.2019.104610. [DOI] [PubMed] [Google Scholar]
- Lithner D.; Nordensvan I.; Dave G. Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile–butadiene–styrene, and epoxy to Daphnia magna. Environ. Sci. Pollut. Res. 2012, 19, 1763–1772. 10.1007/s11356-011-0663-5. [DOI] [PubMed] [Google Scholar]
- Leibman K. C. Metabolism and toxicity of styrene. Environ. Health Perspect. 1975, 11, 115–119. 10.1289/ehp.7511115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan S.; Sadacharan C. M.; Rostama B. Polystyrene and Polyethylene Microplastics Decrease Cell Viability and Dysregulate Inflammatory and Oxidative Stress Markers of MDCK and L929 Cells In Vitro. Exposure Health 2022, 14, 75–85. 10.1007/s12403-021-00419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier V.; Duval J.-L.; Hindie M.; Pouletaut P.; Nagel M.-D. Comparative particle-induced cytotoxicity toward macrophages and fibroblasts. Cell Biol. Toxicol. 2003, 19, 145–159. 10.1023/A:1024723326036. [DOI] [PubMed] [Google Scholar]
- Prokić M. D.; Gavrilović B. R.; Radovanović T. B.; Gavrić J. P.; Petrović T. G.; Despotović S. G.; Faggio C. Studying microplastics: Lessons from evaluated literature on animal model organisms and experimental approaches. J. Hazard. Mater. 2021, 414, 125476 10.1016/j.jhazmat.2021.125476. [DOI] [PubMed] [Google Scholar]
- Tkaczyk A.; Bownik A.; Dudka J.; Kowal K.; Ślaska B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Sci. Total Environ. 2021, 763, 143038 10.1016/j.scitotenv.2020.143038. [DOI] [PubMed] [Google Scholar]
- Elendt B.-P.; Bias W.-R. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing. Effects of the optimization of culture conditions on life history parameters of D. magna. Water Res. 1990, 24, 1157–1167. 10.1016/0043-1354(90)90180-E. [DOI] [Google Scholar]
- Rodebush W. H.; Feldman I. Ultraviolet Absorption Spectra of Organic Molecules. III. Mechanical Interference of Substituent Groups with Resonance Configurations. J. Am. Chem. Soc. 1946, 68, 896–899. 10.1021/ja01209a059. [DOI] [PubMed] [Google Scholar]
- Garrigós M. C.; Marin M. L.; Cantó A.; Sánchez A. Determination of residual styrene monomer in polystyrene granules by gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1061, 211–216. 10.1016/j.chroma.2004.10.102. [DOI] [PubMed] [Google Scholar]
- Petha N. H.; Lokhande R. S.; Patil R. M.; Seshadri D. T. Determination of residual (free) monomer in water based polymer emulsions by head space gas chromatography mass spectrometry. Polym. Test. 2017, 63, 462–466. 10.1016/j.polymertesting.2017.09.002. [DOI] [Google Scholar]
- Mueller M. T.; Fueser H.; Trac L. N.; Mayer P.; Traunspurger W.; Höss S. Surface-Related Toxicity of Polystyrene Beads to Nematodes and the Role of Food Availability. Environ. Sci. Technol. 2020, 54, 1790–1798. 10.1021/acs.est.9b06583. [DOI] [PubMed] [Google Scholar]
- Moradi Z.; Kiarostami V.; Amini M. Rapid analysis of styrene in drinking water and tea samples using dispersive liquid-liquid microextraction combined with liquid chromatography-ultraviolet detection. Food Anal. Methods 2017, 10, 41–48. 10.1007/s12161-016-0547-x. [DOI] [Google Scholar]
- Flanjak J.; Sharrad J. Quantitative analysis of styrene monomer in foods. A limited East Australian Survey. J. Sci. Food Agric. 1984, 35, 457–462. 10.1002/jsfa.2740350416. [DOI] [Google Scholar]
- Geraldo M. D.; Montenegro M. I.; Pletcher D. An electrochemical method for the determination of residual styrene in polystyrene. Talanta 1995, 42, 1725–1729. 10.1016/0039-9140(95)01643-0. [DOI] [PubMed] [Google Scholar]
- Newell J. Residual monomer in polystyrene. Anal. Chem. 1951, 23, 445–447. 10.1021/ac60051a015. [DOI] [Google Scholar]
- Maxwell I. A.; Kurja J.; van Doremaele G. H. J.; German A. L.; Morrison B. R. Partial swelling of latex particles with monomers. Makromol. Chem. 1992, 193, 2049–2063. 10.1002/macp.1992.021930823. [DOI] [Google Scholar]
- Yalkowsky S. H.; He Y.; Jain P.. Handbook of aqueous solubility data; CRC press, 2019; p 468. [Google Scholar]
- Nestor J.; Esquena J.; Solans C.; Levecke B.; Booten K.; Tadros T. F. Emulsion polymerization of styrene and methyl methacrylate using a hydrophobically modified inulin and comparison with other surfactants. Langmuir 2005, 21, 4837–4841. 10.1021/la047018y. [DOI] [PubMed] [Google Scholar]
- Nishi C.; Nakajima N.; Ikada Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. 1995, 29, 829–834. 10.1002/jbm.820290707. [DOI] [PubMed] [Google Scholar]
- Di Veroli G. Y.; Fornari C.; Goldlust I.; Mills G.; Koh S. B.; Bramhall J. L.; Richards F. M.; Jodrell D. I. An automated fitting procedure and software for dose-response curves with multiphasic features. Sci. Rep. 2015, 5, 14701. 10.1038/srep14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C. M.Hansen solubility parameters: a user’s handbook; CRC press, 2007; pp 55–56. [Google Scholar]
- Harvilchuck J. A.; Carlson G. P. Comparison of styrene and its metabolites styrene oxide and 4-vinylphenol on cytotoxicity and glutathione depletion in Clara cells of mice and rats. Toxicology 2006, 227, 165–172. 10.1016/j.tox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Xu M.; Halimu G.; Zhang Q.; Song Y.; Fu X.; Li Y.; Li Y.; Zhang H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- An C. Y.; Zhu B. S.; Lu Q. H.. Cytotoxicity of polystyrene nanospheres internalization in mouse fibroblast cells. In 2nd IEEE International Nanoelectronics Conference, 2008; pp 1087–1092.
- Shao X.-R.; Wei X. Q.; Song X.; Hao L. Y.; Cai X. X.; Zhang Z. R.; Peng Q.; Lin Y. F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Proliferation 2015, 48, 465–474. 10.1111/cpr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Hu Y.; Yin L.; Tang C.; Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Dash B. C.; Réthoré G.; Monaghan M.; Fitzgerald K.; Gallagher W.; Pandit A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials 2010, 31, 8188–8197. 10.1016/j.biomaterials.2010.07.067. [DOI] [PubMed] [Google Scholar]
- Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD, 2004. [Google Scholar]
- Cushman J. R.; Rausina G. A.; Cruzan G.; Gilbert J.; Williams E.; Harrass M. C.; Sousa J. V.; Putt A. E.; Garvey N. A.; Laurent J. R.; Machado M. W. Ecotoxicity hazard assessment of styrene. Ecotoxicol. Environ. Saf. 1997, 37, 173–180. 10.1006/eesa.1997.1540. [DOI] [PubMed] [Google Scholar]
- Baumann J.; Sakka Y.; Bertrand C.; Köser J.; Filser J. Adaptation of the Daphnia sp. acute toxicity test: miniaturization and prolongation for the testing of nanomaterials. Environ. Sci. Pollut. Res. 2014, 21, 2201–2213. 10.1007/s11356-013-2094-y. [DOI] [PubMed] [Google Scholar]
- Wolf M. A.; Rowe V. K.; McCollister D. D.; Hollingsworth R. L.; Oyen F. Toxicological Studies of Certain Alkylated Benzenes and Benzene. Experiments on Laboratory Animals. AMA Arch. Ind. Health 1956, 14, 387–398. [PubMed] [Google Scholar]
- Grossman R. F.; Lutz J. T. Jr.. Polymer modifiers and additives; CRC press, 2018; pp ix–xii. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.