Abstract
Tics are unique from most movement disorders, in that they are partially suppressible. As part of the inhibitory motor network, the pre-supplementary motor area is engaged in motor control and may be involved in tic physiology. We used dual-site transcranial magnetic stimulation to assess inhibitory connectivity between right pre-supplementary motor area and left primary motor cortex, which has previously been demonstrated in healthy adults. We also used diffusion tensor imaging to investigate white matter connectivity in children with chronic tics. Twelve children with chronic tic disorder and fourteen typically developing controls underwent MRI with diffusion tensor imaging indices analysis followed by single and paired-pulse transcranial magnetic stimulation with conditioning pulse over the right pre-supplementary motor area followed by left motor cortex test pulse. Neurophysiologic and imaging data relationships to measures of tic severity and suppressibility were also evaluated in tic patients. Pre-supplementary motor area-mediated inhibition of left motor cortex was present in healthy control children but not in chronic tic disorder participants. Less inhibition correlated with worse tic suppressibility (ρ = − 0.73, p = 0.047). Imaging analysis showed increased fractional anisotropy in the right superior longitudinal fasciculus, corpus callosum, corona radiata and posterior limb of the internal capsule (p < 0.05) in tic participants, which correlated with lower self-reported tic suppressibility (ρ = − 0.70, p = 0.05). Physiologic data revealed impaired frontal-mediated motor cortex inhibition in chronic tic participants, and imaging analysis showed abnormalities in motor pathways. Collectively, the neurophysiologic and neuroanatomic data correlate with tic suppressibility, supporting the relevancy to tic pathophysiology.
Keywords: Transcranial magnetic stimulation, Diffusion tensor imaging, Tourette, Tics, Pre-supplementary motor area
Introduction
Motor and phonic tics are exceedingly common in childhood, affecting approximately 20% of children (Kurlan et al. 2001; Cubo 2012). They are the defining feature of chronic tic disorders (CTD), including Tourette Syndrome, which represent the most prevalent movement disorders in the pediatric population. Although there is a spectrum of symptom severity, many patients experience significant social and functional impairment (Conelea et al. 2011). The physiologic and structural mechanisms underlying tic generation and suppression, however, remain incompletely understood. Tics are somewhat unique amongst movement disorder phenomenology; in that they are partially suppressible. However, the ability to suppress tics requires the recognition of premonitory urge which lags behind the age of tic onset (Leckman et al. 1993). Both premonitory urge recognition and the ability to suppress tics improve as children mature (Banaschewski et al. 2003). The natural history of involuntary motor symptoms, sensory awareness and eventual tic suppression support the concept of neural network maturation within the motor system (Eichele and Plessen 2013).
Key amongst regions important for tic regulation, the supplementary motor complex (SMC) encompasses the supplementary motor area proper (SMA) as well as the contiguous pre-supplementary motor area (preSMA) and supplementary eye field (Nachev et al. 2008). Collectively, the SMC is understood to be involved in the regulation of motor actions, and serves as a node in the larger cortico-striato-thalamo-cortical network which is integral to human response inhibition (Nachev et al. 2008). The relationship of the SMC, motor control, and tics has been explored across numerous modalities. Functional MRI studies have demonstrated that the SMA is amongst the regions activated prior to tic onset and that greater SMA neuroactivation correlates with greater tic severity (Bohlhalter et al. 2006; Hampson et al. 2009; Wang et al. 2011). GABA levels in the SMA of Tourette Syndrome (TS) participants inversely correlate with SMA blood-oxygen-level-dependent (BOLD) signal changes during finger tapping and with cortical excitability in the primary motor cortex (M1) (Draper et al. 2014). Cortical physiology studies also demonstrate elevated coherence involving the SMC during intentional tic suppression as well as during inhibition of voluntary movements (Serrien et al. 2005; Franzkowiak et al. 2012).
Within the SMC, the preSMA is the rostral portion of the complex that may be even more critical for motor control (Nachev et al. 2008; Aron 2011). Anatomically, the preSMA and SMA proper have differential projections to the striatum and corticospinal tracts (Dum and Strick 1991; Picard and Strick 1996; Bozkurt et al. 2016). Functional imaging studies have revealed the role of preSMA in self-initiated, imaginary, complex motor movements and in motor inhibition (Cunnington et al. 2005; Chouinard and Paus 2010). Earlier dual-site transcranial magnetic stimulation (TMS) studies have explored the SMC’s modulatory function on the motor system. Without specifically mentioning the preSMA, TMS targeting different SMC regions may have already revealed inhibition of M1 excitability by the preSMA (Civardi et al. 2001). In this study, a conditioning TMS pulse delivered to a more anterior SMC region resulted in more consistent inhibition of M1 activity. Subsequently, more TMS studies in neurotypical adults specifically targeted the preSMA to demonstrate its inhibitory effect on M1 excitability at rest and during behavioral task (Picazio et al. 2014; Fiori et al. 2016). Although these data support the role of preSMA in motor inhibition, there have been no TMS studies in tic disorder patients investigating the associations between preSMA, M1 and the ability to suppress tics.
In parallel to the growing understanding of physiologic abnormalities in CTD, there has been expanding recognition of complex neuroanatomic differences in this clinical population. White matter analysis using diffusion tensor imaging (DTI) has demonstrated numerous disparities between tic patients and healthy controls (HC) (Greene et al. 2015). For instance, fractional anisotropy (FA) of the superior longitudinal fasciculus has been shown to be inversely related to intensity of premonitory urge (Draganski et al. 2010). Higher FA of the corpus callosum correlates with greater motor tic severity (Draper et al. 2014).
Although the preSMA is an important structure for motor inhibition, specific investigation of this specific sub-region of the SMC’s function and structural connectivity within the CTD population has been very limited. To address this knowledge gap, we aimed to examine the physiologic connectivity between the preSMA and M1 in typically developing, HC children as well as in CTD participants. Using an individualized neuronavigated dual-site, paired-pulse TMS paradigm, we tested the hypothesis that preSMA is important for inhibition of M1 cortical excitability and that this inhibition is abnormal in children with CTD. Moreover, we proposed to characterize white matter pathways of HC and CTD children and hypothesized that DTI would reveal differences in structural connectivity within the motor system. Finally, we explored the relationship of structural and physiologic differences with clinical ratings of tic severity and suppressibility.
Materials and methods
Patient recruitment, diagnosis, and clinical assessment
CTD patients (8–17 years of age) with Tourette Syndrome or chronic motor tic disorder based on DSM-5 criteria (American Psychiatric Association 2013) were recruited from the Tourette Syndrome Clinic at the Cincinnati Children’s Hospital Medical Center (CCHMC). Children with CTD plus co-occurring conditions such as Attention Deficit Hyperactivity Disorder (ADHD) and Obsessive–Compulsive Disorder (OCD) who were taking stable doses of CNS medications were eligible. Typically developing children in the same age range were recruited as controls from the community through online and posted advertisements. Exclusion was based on a standard questionnaire proposed for potential TMS participants (Rossi et al. 2011). Clinical symptoms for tics, ADHD, and OCD were assessed using validated scales—Yale Global Tic Severity Scale (YGTSS; total possible tic score of 50), Premonitory Urge for Tics Scale (PUTS; total possible score of 40), Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS; total possible score of 40) and DuPaul ADHD Rating Scale (total possible score of 54) (Leckman et al. 1989; Scahill et al. 1997; DuPaul 1998; Woods et al. 2005). Although YGTSS (Leckman et al. 1989) and PUTS (Woods et al. 2005) are both clinical scales used to assess tic disorder patients, each scale addresses different aspects of tic phenomenology. The YGTSS is a clinician-administered scale that assesses symptom severity by scoring tic counts, frequency, intensity, complexity and interference whereas PUTS is a self-reported 10-question scale primarily quantifying sensory symptoms relating to tics. The first nine PUTS questions ask patients about their premonitory urge awareness and relief from premonitory urge, capturing sensory phenomenology. The final PUTS question asks patients how well they can suppress their tics. For this question, participants answered “not at all,” “a little,” “pretty much,” or “very much” in response to “I am able to stop my tics, even if only for a short period of time.” These answers correspond to ordinal numeric scores of 1, 2, 3 and 4, respectively. Thus, question 10 addresses motor control.
A parent or legal guardian of pediatric participants gave written informed consent, which was approved by the CCHMC Institutional Review Board. Children also gave written assent for the study.
Brain MRI
All participants underwent brain MRI with 3 T Philips Ingenia MRI scanner (Philips Medical Systems, Best, the Netherlands) equipped with dStream 32 channel head coil to obtain T1-weighted images for localization of the right preSMA. Prior anatomical and functional imaging studies have shown that the preSMA and SMA are approximately separated by the perpendicular line intersecting with the anterior–posterior commissure line at the anterior commissure (VCA line) (Picard and Strick 1996; Kim et al. 2010; Zhang et al. 2012; Bozkurt et al. 2016). We identified the right preSMA as the cortical gyrus just anterior to the VCA line.
Transcranial magnetic stimulation
TMS was performed with a Magstim® BiStim2 stimulator and two D40 (40 mm) Alpha B.I. coils (Magstim Co., Wales, UK). The smaller coil ensures fitting two coils on children’s heads and avoiding activation of right M1, which may lead to unintended M1-M1 interhemispheric inhibition (IHI) (Ferbert et al. 1992). Bilateral first dorsal interossei (FDI) electromyography signals were amplified and filtered (100/1000 Hz) (Coulbourn Instruments, Allentown, PA) before being digitized at 2 kHz and stored for analysis using Signal® software and a Micro1401 interface (Cambridge Electronic Design, Cambridge, UK). Participants were seated comfortably, with both arms and hands fully supported on a pillow. TMS data was collected while participants were awake but at rest. Muscle relaxation was maintained during the TMS session through visual and electrophysiologic monitoring. The coil was placed over the left M1 at the optimal site and angle for obtaining motor evoked potentials (MEP) in the right FDI to determine the resting motor threshold (RMT) (Mills and Nithi 1997; Conforto et al. 2004).
PreSMA-M1 paired-pulse TMS data acquisition
Using BrainSight® Neuronavigation System (Rogue Research, Montreal, Canada), we placed the TMS coil over the right preSMA such that the induced current flowed from right hemisphere towards mid-sagittal line, based on a prior study comparing different coil positions for stimulation of the SMC to modulate M1 excitability (Arai et al. 2012). The stimulation intensity for the preSMA conditioning pulse was 110% of RMT, chosen because of previous studies showing that higher intensity resulted in more significant IHI (Kukaswadia et al. 2005), more robust SMA-mediated modulation of M1 (Arai et al. 2011) and higher resting-state fMRI connectivity index (Xu et al. 2016). The test pulse intensity over left M1 was set at 120% of RMT. We chose three different interstimulus intervals (ISI) for the preSMA-M1 paired-pulse protocol: 6, 8, and 10 ms. The minimum ISI required for an M1 conditioning pulse to inhibit the contralateral M1 is 6 ms (Ferbert et al. 1992). Therefore, most adult TMS studies examining SMC-M1 interaction have utilized 6 ms (Civardi et al. 2001; Neubert et al. 2010; Arai et al. 2011, 2012; Shirota et al. 2012; Picazio et al. 2014). Since this procedure has not been studied in children, we included 8 and 10 ms to increase the probability of finding a condition which would generate robust preSMA-mediated M1 inhibition.
Diffusion tensor imaging
DTI data were acquired with a 61-direction spin echo EPI sequence. The following parameters were used: TR/TE = 8593/97 ms; field of view = 256 × 256 mm; acquisition matrix: 128 × 128; slice thickness = 2 mm (voxel resolution = 2 × 2 × 2 mm); number of slices = 68; SENSE factor = 2; number of average = 1; diffusion weighting factor b value = 1000 s/mm2. Seven interleaved frames of images without diffusion weighting (b0) were acquired. A high-resolution 3D T1-weighted anatomical data set (voxel size = 1 × 1 × 1 mm) was acquired in the sagittal direction for image registration and review. Fractional anisotropy (FA), as well as, mean, axial, and radial diffusivity (MD, AD, RD) were calculated and analyzed using the Tract Based Spatial Statistics (TBSS) approach in FSL software (Smith et al. 2004). For analysis of group difference, DTI indices were tested voxel-by-voxel using two-sample t test with age adjusted as a covariate. Due to the high number of voxel-by-voxel tests, threshold-free cluster enhancement (TFCE) method (Smith and Nichols 2009) was used to correct for multiple comparisons. Localization of the anatomical regions of the white matter structures was determined based on the Johns Hopkins University white matter tractography atlas (Hua et al. 2008).
Statistical analysis
All statistical analyses were performed using the SAS® (SAS Institute, Inc., Cary, NC). Age and RMT between groups were compared using Wilcoxon–Mann–Whitney test, while sex proportion was analyzed using Fisher’s exact test. Two-tailed α of 0.05 was used to define statistical significance.
For the TMS data, trials with electromyographic artifact before TMS pulse were excluded. Peak-peak MEP amplitude (mV) was analyzed in a linear mixed model using proc glim-mix with an unstructured covariance model. The independent variables included age, diagnosis (HC vs CTD) and TMS condition (6, 8 and 10 ms ISI). We included all second- and third-order interactions between the variables. False Discovery Rate (Benjamini and Hochberg 1995) was used to account for multiple comparisons between pulse conditions and groups. There were ten of these relevant comparisons—A) six within-group paired- vs single-pulse comparisons (i.e. HC 6/8/10 ms paired-pulse vs HC single-pulse condition; CTD 6/8/10 ms paired-pulse vs CTD single-pulse condition) and B) four between-group/within-pulse condition comparisons (i.e. HC single-pulse vs CTD single-pulse, HC 6 ms paired-pulse vs CTD 6 ms paired-pulse, HC 8 ms paired-pulse vs CTD 8 ms paired-pulse, HC 10 ms paired-pulse vs CTD 10 ms paired-pulse).
Separate correlation analyses were conducted to examine the relationship of TMS, DTI measures and clinical ratings (YGTSS total tic score and PUTS). Both the YGTSS and PUTS are ordinal scales; therefore, Spearman rank correlation was used. We also decided a priori to conduct correlation analysis specifically with the PUTS question 10. The rationale for this analysis is that this question distinctly pertains to tic suppressibility (self perceived), which relates to motor control. Thus, this question, unlike the other PUTS questions, corresponds to the putative role of prefrontal cortex in controlling tics and is therefore intrinsically related to the aim of this study and its neuroanatomical methods. Corrections for multiple comparisons were not performed for these exploratory clinical/physiological correlations. Age was included as a covariate to conduct a partial Spearman correlation for two reasons. First, the ability to suppress tics improves with age (Banaschewski et al. 2003). Second, the age matching process for this sample was not based on each individual participant. Therefore, we included this covariate to account for any potential age-based developmental differences in motor control. For these correlation analyses, the TMS measures were calculated as a ratio (i.e. average paired-pulse condition amplitude divided by average single-pulse condition amplitude). False discovery rate was used to correct for correlations with each distinct clinical measure (i.e., correction for 3 TMS time-points vs. YGTSS comparisons; correction for 3 TMS time-points vs. PUTS comparisons).
Finally, we performed correlation analysis between DTI index and clinical scores acquired from the CTD group. Average DTI value was extracted from voxels within the region that presented significant group difference in DTI. Since we only found significant group difference in FA values (see results), only FA was tested in the correlation analysis.
Results
Participants
There were 26 participants in total, 19 males and 7 females. All were right-handed except one CTD and one HC participant. Due to high left M1 RMT and dental hardware, not all participants completed both TMS and DTI. Of the 12 HC (13.3 ± 2.7 years; range 10–17 years) and 11 CTD (13.2 ± 1.9 years; range 11–16 years) participants who completed TMS, there was no difference in age (p = 1.0) or sex ratio (p = 0.32). There was also no difference in age (p = 0.71) or sex (p = 0.35) for those who completed DTI sequence: 10 HC (13.1 ± 3.0 years; range 10–17 years) and 9 CTD (13.3 ± 2.1 years; range 11–16 years). All participants tolerated MRI acquisition and/or TMS without any significant adverse effects.
Clinical ratings
CTD participants had YGTSS total tic scores ranging from 8 to 42, with median of 21.5 and mean ± SD of 22.4 ± 9.8. PUTS scores ranged from 11 to 29, with median of 19 and mean ± SD of 20.3 ± 5.4. Online Resource 1 provides detailed demographic and clinical data.
PreSMA-M1 paired-pulse inhibition
RMT was not different (p = 0.46) between participants with CTD (60.5 ± 10.3%) and HCs (64.1 ± 10.1%). In the regression model, three independent variables (age, diagnosis, TMS pulse condition) were included to examine their effects on MEP amplitudes. Our primary interest is the third-order interaction among these three variables, which reached statistical significance (p = 0.016; Online Resource 2). False Discovery Rate was used for post hoc analysis to examine the MEP amplitudes between HC vs CTD across different TMS conditions while controlling for age. This analysis revealed only one significant comparison, showing that a preSMA conditioning pulse 8 ms prior to M1 test pulse reduced MEP in HCs (p = 0.025; Fig. 1; Online Resource 3). There was trend level significance for 6 and 10 ms trials in HCs (p = 0.068). None of the paired-pulse conditions were significantly different from single pulse condition in the CTD group. There was no significant difference in single-pulse conditions between CTD and HC groups.
Fig. 1.
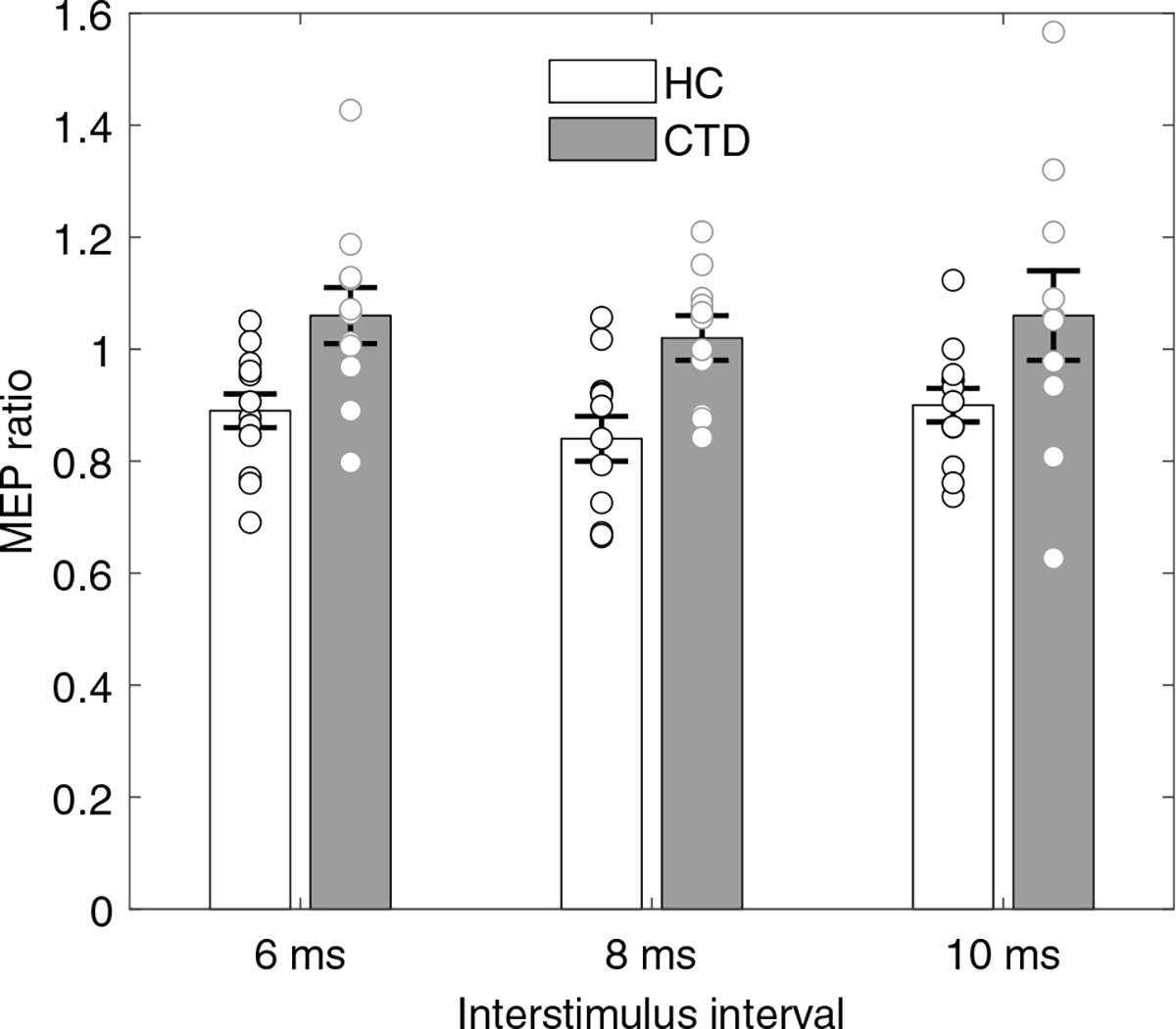
MEP ratio was expressed as paired-pulse MEP means divided by single-pulse MEP means; thus, lower ratio represents more robust M1 inhibition. Significant preSMA-mediated M1 inhibition occurred in 8-ms inter-stimulus interval trials in controls (p = 0.025; corrected using false discovery rate) but not CTD participants. Error bars represent standard error of mean; individual MEP ratios are also shown.
DTI measures for white matter integrity
FA was found to be increased in CTD participants compared to HCs in the right superior longitudinal fasciculus, corpus callosum (genu, body, splenium), superior and posterior corona radiata, and posterior limb of the internal capsule (adjusted for age, corrected p < 0.05, Fig. 2). There was no significant group difference in diffusivity (MD, AD or RD).
Fig. 2.
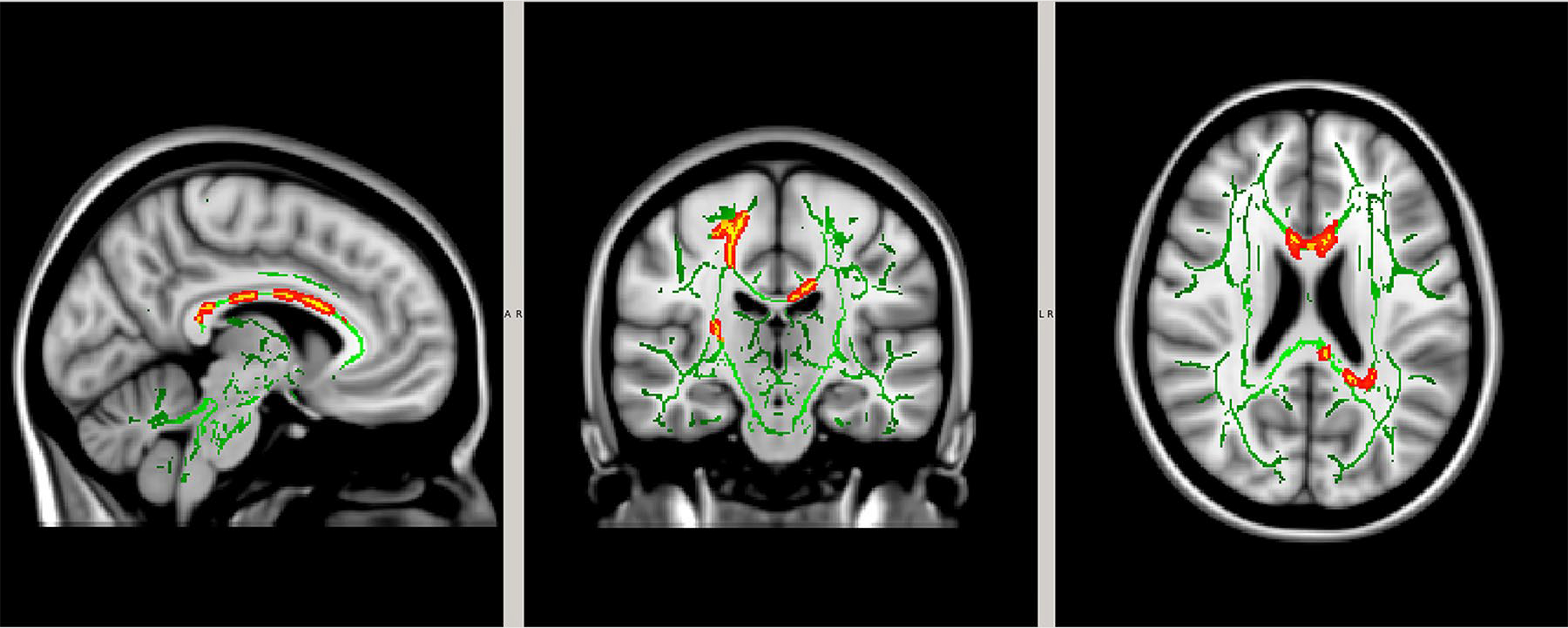
White matter tracts (green) were compared between controls and CTD participants. Regions of significant differences in fractional anisotropy are shown in yellow outlined by red (p < 0.05).
Correlation analyses of physiologic, imaging and clinical data
When exploring the relationship between preSMA-M1 paired-pulse inhibition and tic severity (YGTSS total tic score), age-adjusted Spearman correlation analysis was not significant after correcting for multiple comparisons. However, there was trend significance between 8 ms condition and YGTSS (ρ = 0.68, p = 0.089; Fig. 3a). Although there was no significant correlation between the preSMA-M1 inhibition and total PUTS score, higher MEP ratio for the 6 ms condition (i.e. less preSMA-mediated M1 inhibition) correlated with worse self-reported tic suppressibility (i.e. less patient-perceived capacity to inhibit tics) (ρ = − 0.73, p = 0.047; Fig 3b).
Fig. 3.
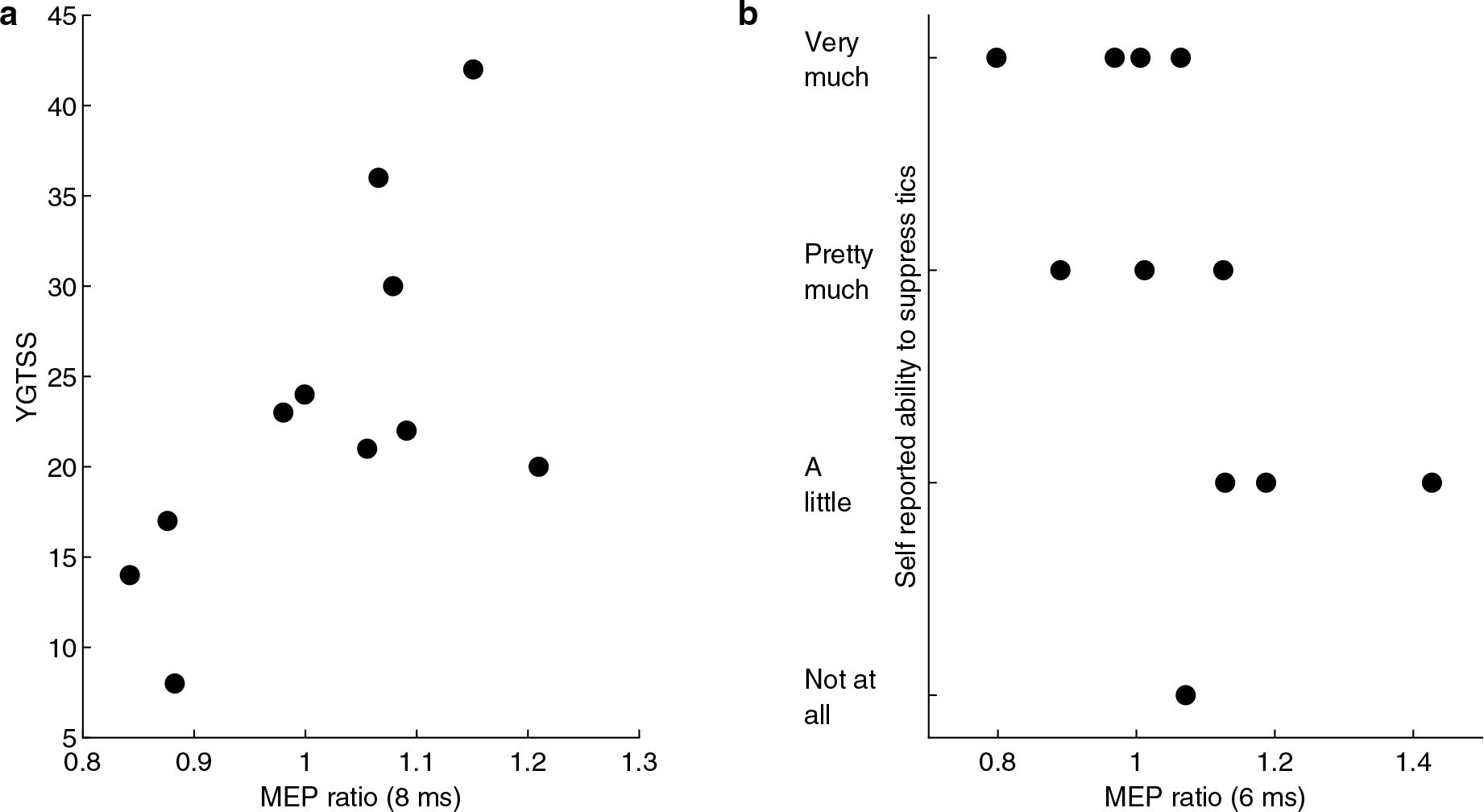
a Age-adjusted Spearman rank correlation between YGTSS and 8 ms MEP ratio demonstrated trend significance (ρ = 0.68, p = 0.089) after correcting for multiple comparisons using false discovery rate. b Self-reported tic suppressibility inversely correlated with 6 ms MEP ratio (ρ = − 0.73, p = 0.047) after adjusting for age and correcting for multiple comparisons using false discovery rate. Therefore, those CTD participants with less preSMA-mediated M1 inhibition are less able to suppress tics.
Within the white matter regions exhibiting significant FA group difference (Fig. 2), correlation analyses were performed between FA values and clinical data in CTD participants. FA values did not correlate with YGTSS total tic score or total PUTS score. However, FA values inversely correlated with self-reported tic suppressibility (ρ = − 0.70, p = 0.05; Fig. 4). In other words, higher FA values correlated with lower self-reported ability to suppress tics.
Fig. 4.
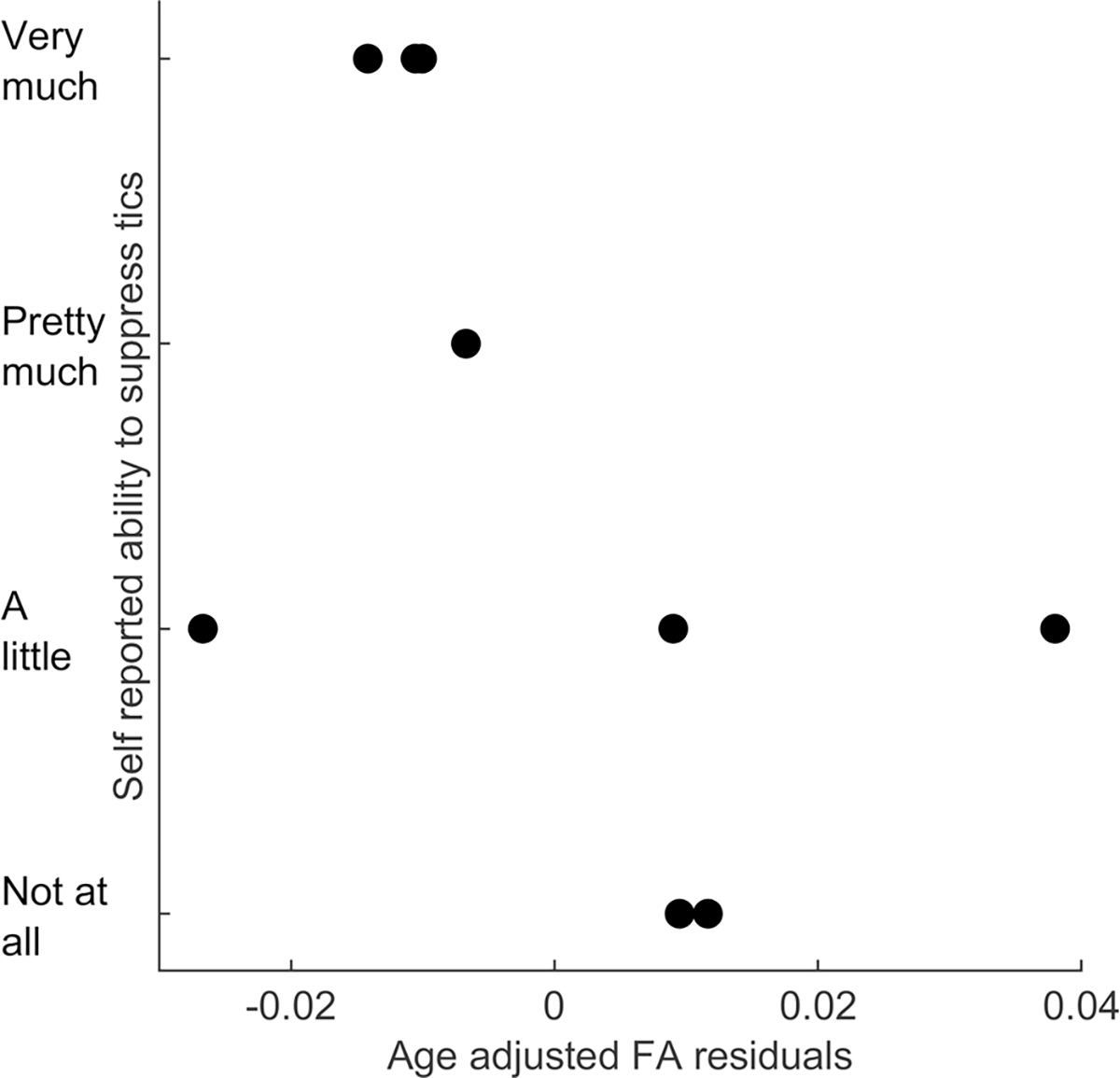
FA values in regions of difference inversely correlated with self-reported tic suppressibility (ρ = − 0.70, p=0.05), indicating those with higher FA are less able to suppress tics. Figure shows relationship between tic suppressibility and age-adjusted FA residual values.
Of seven CTD participants who completed both DTI and TMS, Spearman correlation analysis was not significant between FA and preSMA-M1 paired-pulse inhibition.
Discussion
PreSMA-mediated motor inhibition abnormalities in CTD
Given the supplementary motor complex is critically involved in the pathophysiology of chronic tic disorders and the rostral preSMA is important for motor inhibition, we employed a unique dual-site, paired-pulse TMS paradigm to explore preSMA functional connectivity with M1. To our knowledge, this is the first report documenting prefrontal-M1 inhibition in typically developed children. Notably, this preSMA-mediated M1 inhibition did not reliably occur in chronic tic disorder participants. Additionally, less inhibition correlated with worse self-reported tic suppressibility for CTD participants.
The unique role of the preSMA
The SMC is an important node in the cortico-striato-thalamo-cortical network that is activated prior to tics (Bohlhalter et al. 2006; Hampson et al. 2009; Wang et al. 2011). Consequently, numerous attempts to modulate this cortical region in CTD using repetitive TMS (rTMS) have been undertaken. Initial efforts seemed promising in open label studies, but randomized sham-controlled rTMS trials targeting the SMC failed to reduce tics (Wu et al. 2014; Landeros-Weisenberger et al. 2015). Although several methodological factors may explain the negative results, one potential explanation is target selection; thus, providing the rationale for examining a different target within the supplementary motor complex. To place the results of our study in context, it is important to understand SMC anatomy as well as techniques used for localization in TMS studies. Here, we focused on studying the physiology of the preSMA, because this rostral region of the SMC has greater connectivity with prefrontal regions and striatum, whereas the SMA-proper predominantly projects to the corticospinal system (Nachev et al. 2008). Human cortical physiology studies have also demonstrated different activity patterns between preSMA vs SMA (Ikeda et al. 1999; Kunieda et al. 2000; Yazawa et al. 2000; Arai et al. 2011). Given the preSMA and SMA-proper exhibit these unique neurophysiologic properties, prior techniques for TMS target site identification are important. Most studies have used the vertex (Cz) as a reference point to identify SMA-proper (4 cm anterior) and preSMA (6 cm anterior) based on scalp distance (Civardi et al. 2001; Arai et al. 2011, 2012; Shirota et al. 2012). Civardi et al. found that stimulation 6 cm anterior to Cz resulted in greater inhibition of M1 MEP as compared to 4 cm (Civardi et al. 2001), suggesting that the more rostrally stimulated site, probable preSMA, exerts a greater inhibitory effect on M1. However, using this scalp landmark-based approach has overall yielded mixed results (Civardi et al. 2001; Arai et al. 2012). More recent studies have used individualized brain MRI images for localization (Picazio et al. 2014; Xu et al. 2016). Picazio et al. used the VCA line to localize preSMA for delivery of conditioning pulse and demonstrated preSMA-M1 inhibition during Go/NoGo task (Picazio et al. 2014). Since children’s head circumference can vary with age, our anatomically based approach provided a more precise TMS method to target and quantify preSMA-mediated M1 inhibition.
Very few studies have examined preSMA specifically in the CTD population (Carvalho et al. 2015; Behler et al. 2018). Although the role of preSMA in tic generation and suppression is not yet well understood, there is an abundance of information about the importance of the preSMA in motor control (Nachev et al. 2008). Both preSMA and SMA proper show BOLD signal activity in self-initiated as well as in externally cued movements, but preSMA activation occurs earlier in the self-initiated movements (Cunnington et al. 2002). During cognitive control tasks (e.g. stop signal, task switching tasks), preSMA activation occurs during successful trials (Rushworth et al. 2002; Aron and Poldrack 2006; Li et al. 2006) and negatively correlates with stop signal reaction time (Li et al. 2006). Electrophysiology studies employing direct electrical stimulation of the preSMA led to inhibition of ongoing movements (Filevich et al. 2012). Electrocorticographic and electroencephalographic studies have demonstrated preSMA activity during motor execution and inhibition (Ikeda et al. 1999; Kunieda et al. 2000; Yazawa et al. 2000; Wagner et al. 2018). Collectively, these findings along with the known anatomic connectivity, support the notion that the preSMA is an essential node within the SMC for motor inhibition. Furthermore, our findings of 1) reduced preSMA-mediated M1 inhibition in CTD and 2) less inhibition correlating with more severe tics and worse tic suppressibility, in the context of this unique role of the preSMA in motor control, suggests that preSMA likely also plays an important role in tic physiology.
White matter abnormalities in CTD
As a complementary method of examining connectivity, we also used DTI to evaluate the white matter pathways of CTD participants. Prior structural studies in CTD participants have produced variable findings across multiple neuroanatomic regions (Makki et al. 2008; Thomalla et al. 2009; Draganski et al. 2010; Govindan et al. 2010; Neuner et al. 2010; Saporta et al. 2010; Jackson et al. 2013; Liu et al. 2013; Muller-Vahl et al. 2014; Debes et al. 2015; Wen et al. 2016; Wolff et al. 2016; Sigurdsson et al. 2018). Our study found that CTD participants had greater FA than HCs, most prominently in the corpus callosum, but also in the right superior longitudinal fasciculus, corona radiata, and posterior limb of the internal capsule, supporting the idea of multifocal and likely network-based differences in CTD participants. Further, those CTD participants with the greatest deviation in FA from the HCs were also those who reported lowest subjective ability to suppress tics. Our DTI data did not correlate with YGTSS total tic score, although a prior mixed adult and pediatric study showed that higher callosal FA values correlated with more motor tics (Draper et al. 2014).
The abnormally higher FA in CTD participants in our study corroborates the findings in several other reports (Thomalla et al. 2009; Draganski et al. 2010; Martino et al. 2017), but conflicts with other studies where decrease in FA was found in motor system tracts and other white matter microstructures (Neuner et al. 2010; Saporta et al. 2010; Jackson et al. 2013; Wen et al. 2016). The discrepancy of findings in DTI indices and the varying locations that present DTI abnormalities may be attributable to several factors including sample size, cohort age range, severity and chronicity of the disease, and exposure to medication and other therapeutic interventions. In addition, methodological differences in analysis, such as region of interest approach (hypothesis driven) vs a whole brain voxel-based analysis approach (data driven) also limits interpretation and comparison.
The findings in our DTI analysis appear to hold potential analogies to abnormalities seen in our TMS evaluation. The corpus callosum, as the major site of axonal crossing between hemispheres, and which has recurrently emerged as a site of white matter abnormality in chronic tic patients, is likely integral to the functional interhemispheric inhibition which was found to be deficient in CTD participants (Baumer et al. 2010). A prior study utilizing TMS and white matter analysis, indeed, found an inverse relationship between callosal FA and M1-M1 interhemispheric inhibition in their control participants (Baumer et al. 2010). Thus, our finding that CTD participants demonstrate FA abnormalities in these regions may corroborate the finding of impaired preSMA driven inhibition of primary motor cortex seen in our TMS data.
Limitations
While we used a unique TMS paradigm along with DTI white matter analysis to demonstrate clinically relevant connectivity changes in CTD, there are several limitations. First, the sample size is small and consists of participants who have co-occurring psychiatric diagnoses and were prescribed CNS active medications. Second, while participants were asked to stay awake and still during data collection, state dependency of preSMA activity (Mars et al. 2009; Neubert et al. 2010) may affect signal to noise ratio. Furthermore, state dependency of brain activity in the CTD population is particularly important because tics often decrease during focused attention (Misirlisoy et al. 2015). Therefore, it may be possible to detect preSMA-mediated M1 inhibition in CTD patients if they are engaged in a behavioral task, as opposed to lack of inhibition while they are resting comfortably without any cognitive demand. Third, the inclusion of control condition(s) may strengthen the interpretation of our data. Options for delivering the conditioning pulse could involve either using a sham coil over the preSMA or using an active pulse over a region not expected to modulate M1. Furthermore, a positive control could involve delivering the conditioning pulse over the right inferior frontal gyrus which has been shown to downregulate M1 (Fiori et al. 2016). Fourth, there are also challenges that stem from the TMS technique. RMT is higher with the smaller 40 mm coil and children have higher RMT, therefore the use of this smaller coil limits data collection in younger children. Finally, we utilized a conservative approach for DTI analysis by using TBSS analysis combined with the TFCE method for multiple comparison correction. Therefore, we cannot rule out the possibility of false negative results.
Conclusions
Here, we report the first pediatric TMS study to examine frontal-mediated inhibition of primary motor cortex in typically developing children and in those with chronic tic disorders. Our physiologic data revealed reduction of preSMA-mediated M1 inhibition in CTD participants. Our imaging analysis found differences in pathways that may be involved in the preSMA-M1 inhibition. Collectively, the neurophysiologic and neuroanatomic data correlate with tic suppressibility, suggesting that these findings may be relevant to disease mechanism behind CTD. Future studies with larger sample size along with concurrent TMS and behavioral task paradigms, as well as the use of complimentary imaging modalities such as neurotransmitter evaluation, may help further delineate the role of the preSMA in motor regulation and inhibition and provide a means for more effective treatment targeting in chronic tic disorders.
Supplementary Material
Acknowledgements
We appreciate participants and families who took time to participate in this study.
Funding
This research was supported in part by The Sage Foundation (Brighton, MI), Cincinnati Children’s Research Foundation, Tourette Association of America and National Institutes of Health.
Footnotes
Conflict of interest DG has received honoraria and travel support from the Tourette Association of America and has received research support from the National Institutes of Health. PH has received research support through the National Institutes of Health. SW received funding support from Cincinnati Children’s Research Foundation, has received salary support from Tourette Association of America and the National Institute of Health, and received funding for equipment used in this study from The Sage Foundation (Brighton, MI). The rest of the authors have no disclosures relevant to this study. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Compliance of ethical standards
Ethics approval This study was approved by the CCHMC Institutional Review Board.
Consent to participate A parent or legal guardian of pediatric participants gave written informed consent. Children also gave written assent for the study.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00221-020-06017-0.
Availability of data and material
The data that support the findings of this study are available upon reasonable request.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Arai N, Muller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U (2011) State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosci 31:15376–15383. 10.1523/JNEUROSCI.2271-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Lu MK, Ugawa Y, Ziemann U (2012) Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res 220:79–87. 10.1007/s00221-012-3117-5 [DOI] [PubMed] [Google Scholar]
- Aron AR (2011) From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–68. 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006) Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26:2424–2433. 10.1523/JNEUROSCI.4682-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Woerner W, Rothenberger A (2003) Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol 45:700–703 [DOI] [PubMed] [Google Scholar]
- Baumer T, Thomalla G, Kroeger J et al. (2010) Interhemispheric motor networks are abnormal in patients with Gilles de la Tourette syndrome. Mov Disord 25:2828–2837. 10.1002/mds.23418 [DOI] [PubMed] [Google Scholar]
- Behler N, Leitner B, Mezger E et al. (2018) Cathodal tDCS over motor cortex does not improve Tourette syndrome: lessons learned from a case series. Front Behav Neurosci 12:194. 10.3389/fnbeh.2018.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S et al. (2006) Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain 129:2029–2037 [DOI] [PubMed] [Google Scholar]
- Bozkurt B, Yagmurlu K, Middlebrooks EH et al. (2016) Microsurgical and tractographic anatomy of the supplementary motor area complex in humans. World Neurosurg 95:99–107. 10.1016/j.wneu.2016.07.072 [DOI] [PubMed] [Google Scholar]
- Carvalho S, Goncalves OF, Soares JM, Sampaio A, Macedo F, Fregni F, Leite J (2015) Sustained effects of a neural-based intervention in a refractory case of Tourette syndrome. Brain Stimul 8:657–659. 10.1016/j.brs.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T (2010) What have we learned from “perturbing” the human cortical motor system with transcranial magnetic stimulation? Front Hum Neurosci 4:173. 10.3389/fnhum.2010.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC (2001) Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage 14:1444–1453. 10.1006/nimg.2001.0918 [DOI] [PubMed] [Google Scholar]
- Conelea CA, Woods DW, Zinner SH et al. (2011) Exploring the impact of chronic tic disorders on youth: results from the Tourette syndrome impact survey. Child Psychiatry Hum Dev 42:219–242. 10.1007/s10578-010-0211-4 [DOI] [PubMed] [Google Scholar]
- Conforto AB, Z’Graggen WJ, Kohl AS, Rosler KM, Kaelin-Lang A (2004) Impact of coil position and electrophysiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clin Neurophysiol 115:812–819. 10.1016/j.clinph.2003.11.010 [DOI] [PubMed] [Google Scholar]
- Cubo E (2012) Review of prevalence studies of tic disorders: methodological caveats. Tremor Other Hyperkinet Mov (N Y) 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E (2002) The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 15:373–385. 10.1006/nimg.2001.0976 [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E (2005) Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum Mov Sci 24:644–656. 10.1016/j.humov.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Debes N, Jeppesen S, Raghava JM, Groth C, Rostrup E, Skov L (2015) Longitudinal magnetic resonance imaging (MRI) analysis of the developmental changes of Tourette syndrome reveal reduced diffusion in the cortico-striato-thalamo-cortical pathways. J Child Neurol 30:1315–1326. 10.1177/0883073814560629 [DOI] [PubMed] [Google Scholar]
- Draganski B, Martino D, Cavanna AE et al. (2010) Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain 133:3661–3675. 10.1093/brain/awq300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A, Stephenson MC, Jackson GM, Pepes S, Morgan PS, Morris PG, Jackson SR (2014) Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr Biol 24:2343–2347. 10.1016/j.cub.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (1991) The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11:667–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ (1998) ADHD rating scale-IV: checklists, norms, and clinical interpretation. Guilford Press, New York [Google Scholar]
- Eichele H, Plessen KJ (2013) Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol 27:33–45. 10.3233/BEN-120294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546. 10.1113/jphysiol.1992.sp019243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich E, Kuhn S, Haggard P (2012) Negative motor phenomena in cortical stimulation: implications for inhibitory control of human action. Cortex 48:1251–1261. 10.1016/j.cortex.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Fiori F, Chiappini E, Soriano M, Paracampo R, Romei V, Borgomaneri S, Avenanti A (2016) Long-latency modulation of motor cortex excitability by ipsilateral posterior inferior frontal gyrus and pre-supplementary motor area. Sci Rep 6:38396. 10.1038/srep38396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzkowiak S, Pollok B, Biermann-Ruben K et al. (2012) Motor-cortical interaction in Gilles de la Tourette syndrome. PLoS ONE 7:e27850. 10.1371/journal.pone.0027850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Makki MI, Wilson BJ, Behen ME, Chugani HT (2010) Abnormal water diffusivity in corticostriatal projections in children with Tourette syndrome. Hum Brain Mapp 31:1665–1674. 10.1002/hbm.20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Schlaggar BL, Black KJ (2015) Neuroimaging in Tourette syndrome: research highlights From 2014–2015. Curr Dev Disord Rep 2:300–308. 10.1007/s40474-015-0062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF (2009) Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry 65:594–599. 10.1016/j.biopsych.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S et al. (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T et al. (1999) Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain 122(Pt 5):915–931. 10.1093/brain/122.5.915 [DOI] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Manfredi V, Millon G, Hollis C, Jackson GM (2013) Motor excitability is reduced prior to voluntary movements in children and adolescents with Tourette syndrome. J Neuropsychol 7:29–44. 10.1111/j.1748-6653.2012.02033.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ et al. (2010) Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage 49:2375–2386. 10.1016/j.neuroimage.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R (2005) Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol 563:915–924. 10.1113/jphysiol.2004.080010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunieda T, Ikeda A, Ohara S et al. (2000) Different activation of presupplementary motor area, supplementary motor area proper, and primary sensorimotor area, depending on the movement repetition rate in humans. Exp Brain Res 135:163–172. 10.1007/s002210000519 [DOI] [PubMed] [Google Scholar]
- Kurlan R, McDermott MP, Deeley C et al. (2001) Prevalence of tics in schoolchildren and association with placement in special education. Neurology 57:1383–1388 [DOI] [PubMed] [Google Scholar]
- Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, Lisanby SH (2015) Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimul 8:574–581. 10.1016/j.brs.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ (1989) The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28:566–573 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, Cohen DJ (1993) Premonitory urges in Tourette’s syndrome. Am J Psychiatry 150:98–102 [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R (2006) Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 26:186–192. 10.1523/JNEUROSCI.3741-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Miao W, Wang J et al. (2013) Structural abnormalities in early Tourette syndrome children: a combined voxel-based morphometry and tract-based spatial statistics study. PLoS ONE 8:e76105. 10.1371/journal.pone.0076105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki MI, Behen M, Bhatt A, Wilson B, Chugani HT (2008) Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov Disord 23:2349–2356. 10.1002/mds.22264 [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, Rushworth MF (2009) Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci 29:6926–6931. 10.1523/JNEUROSCI.1396-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino D, Delorme C, Pelosin E, Hartmann A, Worbe Y, Avanzino L (2017) Abnormal lateralization of fine motor actions in Tourette syndrome persists into adulthood. PLoS ONE 12:e0180812. 10.1371/journal.pone.0180812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Nithi KA (1997) Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve 20:570–576 [DOI] [PubMed] [Google Scholar]
- Misirlisoy E, Brandt V, Ganos C, Tubing J, Munchau A, Haggard P (2015) The relation between attention and tic generation in Tourette syndrome. Neuropsychology 29:658–665. 10.1037/neu0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Vahl KR, Riemann L, Bokemeyer S (2014) Tourette patients’ misbelief of a tic rebound is due to overall difficulties in reliable tic rating. J Psychosom Res 76:472–476. 10.1016/j.jpsychores.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF (2010) Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A 107:13240–13245. 10.1073/pnas.1000674107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Kupriyanova Y, Stocker T et al. (2010) White-matter abnormalities in Tourette syndrome extend beyond motor pathways. Neuroimage 51:1184–1193. 10.1016/j.neuroimage.2010.02.049 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996) Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6:342–353 [DOI] [PubMed] [Google Scholar]
- Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, Koch G (2014) Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol 24:2940–2945. 10.1016/j.cub.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686. 10.1016/j.clinph.2010.12.037 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002) Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87:2577–2592. 10.1152/jn.2002.87.5.2577 [DOI] [PubMed] [Google Scholar]
- Saporta AS, Chugani HT, Juhasz C, Makki MI, Muzik O, Wilson BJ, Behen ME (2010) Multimodality neuroimaging in Tourette syndrome: alpha-[11C] methyl-L-tryptophan positron emission tomography and diffusion tensor imaging studies. J Child Neurol 25:336–342. 10.1177/0883073809339394 [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M et al. (1997) Children’s Yale-Brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852 [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P (2005) Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain 128:116–125. 10.1093/brain/awh318 [DOI] [PubMed] [Google Scholar]
- Shirota Y, Hamada M, Terao Y, Ohminami S, Tsutsumi R, Ugawa Y, Hanajima R (2012) Increased primary motor cortical excitability by a single-pulse transcranial magnetic stimulation over the supplementary motor area. Exp Brain Res 219:339–349. 10.1007/s00221-012-3095-7 [DOI] [PubMed] [Google Scholar]
- Sigurdsson HP, Pepes SE, Jackson GM, Draper A, Morgan PS, Jackson SR (2018) Alterations in the microstructure of white matter in children and adolescents with Tourette syndrome measured using tract-based spatial statistics and probabilistic tractography. Cortex 104:75–89. 10.1016/j.cortex.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Thomalla G, Siebner HR, Jonas M et al. (2009) Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain 132:765–777. 10.1093/brain/awn339 [DOI] [PubMed] [Google Scholar]
- Wagner J, Wessel JR, Ghahremani A, Aron AR (2018) Establishing a right frontal beta signature for stopping action in scalp EEG: implications for testing inhibitory control in other task contexts. J Cogn Neurosci 30:107–118. 10.1162/jocn_a_01183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS (2011) The neural circuits that generate tics in Tourette’s syndrome. Am J Psychiatry 168:1326–1337. 10.1176/appi.ajp.2011.09111692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Liu Y, Wang J et al. (2016) Combining tract- and atlas-based analysis reveals microstructural abnormalities in early Tourette syndrome children. Hum Brain Mapp 37:1903–1919. 10.1002/hbm.23146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N, Luehr I, Sender J, Ehrlich S, Schmidt-Samoa C, Dechent P, Roessner V (2016) A DTI study on the corpus callosum of treatment-naive boys with “pure” Tourette syndrome. Psychiatry Res Neuroimaging 247:1–8. 10.1016/j.pscychresns.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S (2005) Premonitory Urge for Tics Scale (PUTS): initial psychometric results and examination of the premonitory urge phenomenon in youths with Tic disorders. J Dev Behav Pediatr 26:397–403 [DOI] [PubMed] [Google Scholar]
- Wu SW, Maloney T, Gilbert DL et al. (2014) Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul 7:212–218. 10.1016/j.brs.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Xu B, Sandrini M, Wang WT et al. (2016) PreSMA stimulation changes task-free functional connectivity in the fronto-basal-ganglia that correlates with response inhibition efficiency. Hum Brain Mapp 37:3236–3249. 10.1002/hbm.23236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kunieda T et al. (2000) Human presupplementary motor area is active before voluntary movement: subdural recording of Bereitschaftspotential from medial frontal cortex. Exp Brain Res 131:165–177. 10.1007/s002219900311 [DOI] [PubMed] [Google Scholar]
- Zhang S, Ide JS, Li CS (2012) Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex 22:99–111. 10.1093/cercor/bhr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.


