Abstract
Background and Objectives
It is critical to use validated instruments to diagnose and manage chewing and swallowing problems of persons living with dementia. The study aimed to synthesize the characteristics and psychometric quality of instruments that assess the chewing and swallowing abilities of persons living with dementia.
Research Design and Methods
The systematic review was used to conduct this study. We searched 5 electric databases for records published from January 1, 1980, to July 8, 2022. Records were eligible if they included any instrument to assess chewing ability or swallowing ability in the dementia population. Eight characteristics of eligible instruments were extracted from the records: (1) development process, (2) operationalized concept/construct, (3) sample and setting, (4) administration method, (5) items, (6) scoring format/interpretation, (7) reliability, and (8) validity. The psychometric assessment for self-report and observational tool was used to evaluate 12 psychometric properties of eligible instruments.
Results
In total, 11,074 records were reviewed. Thirty-five eligible instruments, including observational tools, self-report questionnaires, and physiological instruments, were identified from 60 records. All 8 instruments assessing chewing ability were evaluated as having low psychometric quality, and only 3 out of 27 instruments assessing swallowing ability were evaluated as having moderate psychometric quality. Fifteen instruments were tested for only 1 type of psychometric property, limiting the overall evaluation of psychometric evidence.
Discussion and Implications
The study findings inform the use and adaptation of appropriate instruments for practice and research. All existing instruments warrant further validation in larger samples to expand use in diverse care settings. This review described and evaluated current instruments measuring chewing and swallowing abilities and potential use in research and clinical practice to plan for and evaluate the effectiveness of mealtime and oral care practice and reduce health-related negative outcomes of persons living with dementia.
Keywords: Alzheimer’s disease, Measurement, Assessment, Psychometrics
Translational Significance: There is no previous synthesis of instruments assessing the chewing/swallowing abilities of persons living with dementia. This review identified 35 instruments, including observational tools, self-report questionnaires, and physiological instruments to assess chewing/swallowing ability. Only three instruments were evaluated as having moderate psychometric quality. This review provides directions for further psychometric testing to accumulate evidence and expand use of the instruments in research and practice. This review provides guidance to clinicians and formal/family caregivers to use psychometrically and conceptually appropriate instruments to screen, diagnose, and manage swallowing and chewing problems of persons living with dementia.
Background and Objectives
Nationwide, it has been reported that persons living with dementia often exhibit difficulties or disabilities in chewing and/or swallowing food as dementia progresses due to declining cognitive and physical function in diverse settings, such as long-term care (Chen et al., 2020; Park et al., 2013; Simões et al., 2020), home care (Sato et al., 2014; Takahashi et al., 2019), and primary care (Popman et al., 2018; Rösler et al., 2015). Chewing ability, including masticatory performance and chewing efficiency, is the ability to bite and masticate for appropriate times so food is of the proper size for individuals to swallow safely (Fontijn-Tekamp et al., 2000). Swallowing ability is the ability to pass solid or liquid food from the mouth to the throat safely and efficiently without leaving food in the airway or behind the pharynx (Rofes et al., 2010). Dysphagia is known as swallowing impairments or difficulties, a symptom of difficulty or discomfort during swallowing (Rofes et al., 2010).
Physical abilities such as lip opening and closing, tongue movement, rinsing and gargling, and storing food in the mouth as well as subjective evaluations of eating, such as changes in appetite, food preference, and eating habits, are significantly affected as dementia progresses (Simões et al., 2020). These changes in physical and subjective eating abilities are associated with declines in chewing and swallowing abilities, which are significant factors that affect the quality of life (Campos et al., 2018), malnutrition (Simões et al., 2020), and subsequent consequences including longer hospitalization or premature mortality of persons living with dementia (Ebihara et al., 2020; Hoshino et al., 2020).
Better understanding and early assessment of screening, detection, assessment, diagnosis, and management of chewing and swallowing abilities are critical to provide advanced care planning management and reducing complications and premature mortality in persons living with dementia (Hoshino et al., 2020; Simões et al., 2020). Chewing and swallowing difficulties become more challenging to manage when health care providers and family caregivers lack knowledge and skills in screening using appropriate tools. In addition, using psychometrically sound instruments to measure chewing and swallowing abilities informs the evaluation of the process, fidelity, and effectiveness of mealtime and nutrition care interventions and practices for persons living with dementia. Despite the high prevalence of the chewing and swallowing impairments and associated complications, characterizing and evaluating existing instruments that assess chewing and swallowing abilities of persons living with dementia has not been studied. The aim of this systematic review was to synthesize the characteristics and psychometric quality of existing instruments that were developed and/or used to measure chewing and/or swallowing abilities, including dysphagia, for persons living with dementia.
Research Design and Methods
Study Design, Data Sources, and Search Strategy
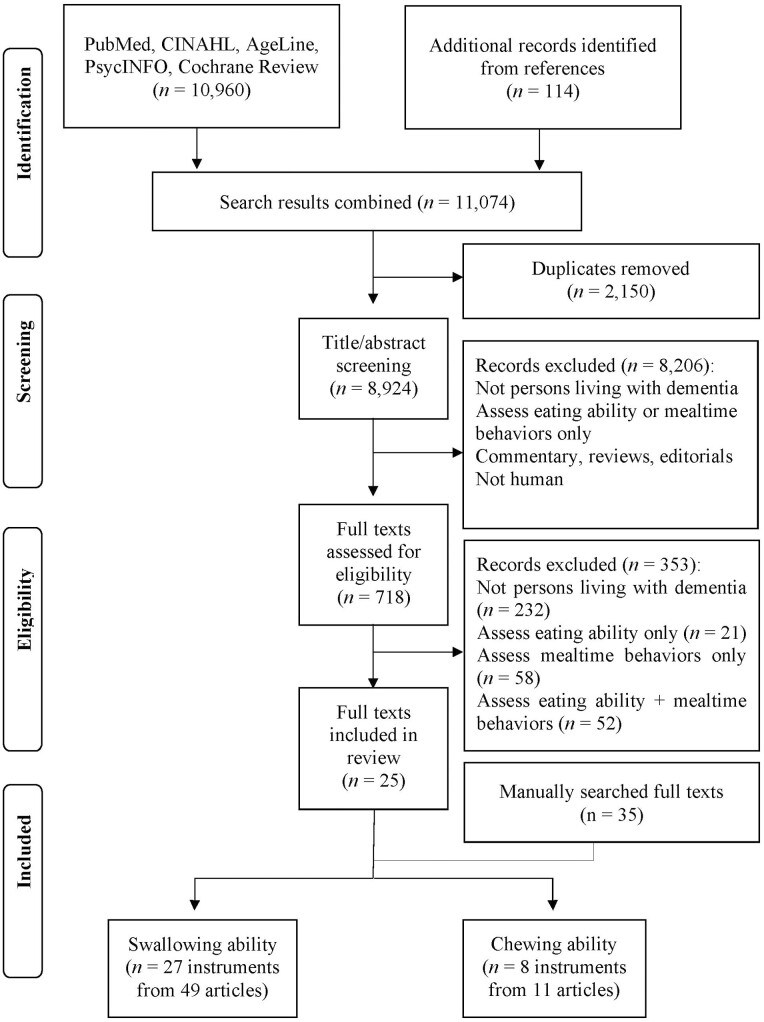
This systematic review was conducted following the preferred reporting items for systematic reviews and meta-analyses guideline: the PRISMA statement (Moher, 2010). We initially searched Pubmed, CINAHL, AgeLine, PsychInfo, and Cochrane Library to identify eligible peer-reviewed scholarly records published in English between January 1, 1980, and June 30, 2019. Keywords and matched subjects included dementia, Alzheimer*, feed*, eat*, meal*, and intake. An example of the search strategy in CINAHL was shown in Supplementary Table 1. Follow-up searches were conducted using the same five databases and search strategies between June 30, 2019, and February 15, 2022. Figure 1 shows the flow diagram of literature search and selection.
Figure 1.
Flow diagram of literature search.
After eligible instruments were identified from the five databases, we searched additional scholarly records that reported the use of eligible instruments until July 8, 2022, with full names and abbreviated names of the eligible instruments. We also searched for records that cited the original studies in which eligible instruments were developed and/or used to obtain a full list of scholarly records that reported the use of the eligible instruments. These exhaustive searches aimed to retrieve an inclusive list of scholarly records, including grey literature if relevant, that reported the use, modification, and/or testing of eligible instruments. The purpose was to extract all relevant data possible to allow for an intensive evaluation of the characteristics and psychometric quality of eligible instruments. The review protocol was predetermined and was not registered.
Inclusion and exclusion criteria
Scholarly records were included if they reported the development, testing, and/or use of any instruments that assess chewing ability or swallowing ability including dysphagia of persons living with dementia in any type of care settings (e.g., home care, long-term care, and primary care). Records were excluded if they were other types of publications than primary research (e.g., reviews, commentaries, and editorials).
Instruments were eligible if they were originally developed in persons living with dementia, or if they were originally developed in other populations and later modified and/or tested for use with persons living with dementia. If the instruments were used in both dementia and non-dementia populations, we only extracted data relevant to the dementia population.
Instruments were excluded if they: (1) only or mainly assess other constructs of interest that are relevant to mealtime than the chewing and swallowing abilities, such as eating ability (i.e., functional ability to start and finish a meal), nutritional status (e.g., mini nutritional assessment), general functional abilities (e.g., activities of daily living [ADL]), or mealtime behaviors/habits (e.g., agitated behavior, food satisfaction, food preference, and eating habits); (2) only used in patients without dementia diagnosis (i.e., other diagnoses relevant to dementia but not any type of dementia, e.g., Parkinson’s disease, stroke, and mild cognitive impairment [MCI]); or (3) only used in other populations (e.g., caregivers and caregiver-care recipient dyads).
Study selection and data extraction
After removing duplicated records, five research assistants including the first and second authors (S. Kim and K. Lee) screened and cross-checked eligible records and instruments by title, abstract, and full texts based on the inclusion and exclusion criteria to confirm eligibility (Figure 1). After cross-checking the eligibility of records, the first and second authors reviewed all full texts of eligible records for data extraction and discussed discrepancies until an agreement was reached. If the full texts of the original developer’s records were not available, information from the other scholarly records that contain the original development process was extracted. Eight characteristics of each eligible instrument were described using a data extraction worksheet: (1) the development process, (2) the concept or construct operationalized by the instrument, (3) the sample and setting the instrument was used or tested in, (4) the administration method, (5) description of items, (6) scoring format and interpretation, (7) reliability, and (8) validity.
Assessment of psychometric quality
The instrument’s psychometric quality was evaluated using the refined psychometric assessment for self-report and observational tools (PAT), a tool developed following the classical test theory (CTT) and the previous literature and published criteria including COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines and standards (Prinsen et al., 2018; Terwee et al., 2007, 2018; Zwakhalen et al., 2006). CTT was developed based on the assumption that the true score cannot be directly observed and can only be assessed indirectly through the observed score with random measurement error (Waltz et al., 2017).
The original PAT evaluates the ratio of sample size to the number of items, as well as a total of 11 CTT-based psychometric quality properties: (1) internal consistency, (2) test–retest reliability, (3) intrarater reliability, (4) interrater reliability, (5) content validity, (6) concurrent validity, (7) predictive validity, (8) divergent/discriminant validity, (9) convergent validity, (10) known group difference, and (11) structural validity. Each of the 11 quality properties and the ratio of a sample size to the number of items is scored from 0 to 2 and adds up to a total score to represent the level of psychometric quality of each instrument. Test–retest reliability assesses the subject’s consistency of responses to the same set of questions over time and therefore is only applicable for self-report instruments which do not involve raters or judges (Waltz et al., 2017). Intrarater and interrater reliability assess the consistency of responses to the same set of questions over time within the same rater and across different raters at the same period, respectively, and therefore are only applicable for observational instruments that require raters to complete. In this study, some physiological instruments do not have distinguishable variables that are specific to be assessed as an item. Therefore, they were not evaluated for the ratio of a sample size and number of items, content validity, and structural validity, all of which require information on items.
The original PAT did not include the evidence of sensitivity and specificity, which were reported in five included physiological instruments (i.e., Albertinen dementia dysphagia screening [ADDS], Rösler et al., 2015; 3-oz water swallow test. Suiter & Leder, 2007; Yale Swallow Protocol [YSP], Ward et al., 2020; standardized swallowing assessment [SSA], Park et al., 2014; volume–viscosity swallowing test [V–VST], Mokkink et al., 2018; Stamm et al., 1988). Therefore, we added criteria in the original PAT to evaluate evidence of sensitivity and specificity based on the literature and published criteria in the psychometric quality property of criterion validity. The refined PAT is shown in Supplementary Table 2.
The total scores range from: 0–20 (low quality: 0–6, moderate quality: 7–13, high quality: 14–20) for self-report instruments; 0–22 (low quality: 0–7, moderate quality: 8–15, high quality: 16–22) for observational instruments; and 0–16 (low quality: 0–5, moderate quality: 6–11, high quality: 12–16) for physiological instruments. The first author (S.K.) evaluated the psychometric properties of eligible instruments using PAT, and the second author (K.L.) reviewed and confirmed the results. The disagreements were discussed between the two authors to reach a consensus. We used a conservative rating (the lower or lowest scoring if there are multiple values for one psychometric quality property of an instrument) to prevent an overestimation of the overall quality of existing evidence (Liu & Kim, 2021; Zill et al., 2014).
The PAT has three advantages over previously published quality assessment criteria; PAT (1) includes the ratio of the sample size to the number of items, (2) separates each reliability and validity property, and (3) uses a numerical scoring system to facilitate calculation and comparison of overall psychometric quality across instruments. The PAT shows adequate feasibility and usability in prior systematic reviews of observational and self-report instruments (Liu & Kim, 2021; Liu et al., 2021).
Results
In total, 11,074 records were retrieved, among which 60 scholarly records were included in the review (Figure 1). From the 60 scholarly records, 35 eligible instruments were identified, of which eight assess chewing ability, and 27 assess swallowing ability (including dysphagia). Seven instruments are self-report questionnaires, 18 are direct observational instruments, and eight are physiological instruments that use specific tools or techniques to assess anatomic regions directly or indirectly (e.g., oral cavity, glottis, and pharynx). Two instruments were administered using both direct observation and physiological measures (ADDS and V–VST). Fourteen of the 35 instruments were reported in two or more full texts. Three of the 35 instruments were originally developed for persons living with dementia, and the other 32 were originally developed for other populations (i.e., stroke patients, healthy older adults, and patients with dysphagia) and later used with the dementia population. A detailed description of the included instruments is shown in Supplementary Table 3.
Chewing Ability
Target population
A total of eight instruments assessing chewing ability were reported in 11 scholarly records. Only one instrument, the two-color chewing gum mixing ability test (Park et al., 2021; Shin et al., 2020; Weijenberg et al., 2015; Zenthöfer et al., 2021), was originally developed for use in the dementia population. Five instruments were originally developed for other populations and later used with the dementia population. For instance, masticatory efficiency (Campos et al., 2018) was originally developed to assess masticatory function in healthy and prosthodontic patients and later came to be used with persons living with mild Alzheimer’s disease. Subjective masticatory index (Komiyama et al., 2020), subjective mastication comfort analysis (Shin et al., 2020), subjective five food mastication ability (Shin et al., 2020), and chewing capacity assessment (Takehara et al., 2020) were all initially developed for healthy older adults. For maximum occlusal force (Komiyama et al., 2020) and palpation of masseter muscle tension ([PMMT], Hoshino et al., 2020), the original target populations were not specified in the studies that reported the original development process.
Development process
Among the eight instruments, the development process (including literature review and/or pilot testing) was only reported for masticatory efficiency and two-color chewing gum mixing ability test. The development process was not reported for the other six instruments.
Sample/setting and administration method
Of the eight instruments, six instruments were only used in community settings (masticatory efficiency, maximum occlusal force, subjective masticatory index, subjective mastication comfort analysis, subjective five food mastication ability, and chewing capacity assessment) and one was only used in institutionalized care settings (PMMT). The two-color chewing gum mixing ability test was used in both residential care and community settings. The sample sizes used for testing the eight instruments ranged from 16 to 210 individuals. Four of the eight instruments are self-report questionnaires (subjective masticatory index, subjective mastication comfort analysis, subjective five food mastication ability, and chewing capacity assessment), while the other four instruments are direct examinations or tests that use chewable test materials (masticatory efficiency), pressure measurement film (maximum occlusal force), color-changeable gum (two-color chewing gum mixing ability test), and evaluator’s palpation (PMMT).
Items and scoring
The four self-report instruments’ number of items ranges from 1 to 11. Three of them are questionnaires for which chewable food items, ranging from soft to hard (e.g., egg, meat, or carrot) are listed to assess chewing ability. The subjective mastication comfort analysis includes an additional item assessing the degree of the respondents’ perceived chewing comfort. The scoring methods are: (1) frequency, rate, and binary scoring (PMMT, subjective mastication comfort analysis, subjective five food mastication ability, and chewing capacity assessment), (2) rate of good and poor masticatory performance (subjective masticatory index), (3) weight percentage of comminuted test material (masticatory efficiency), and (4) maximum magnitude of bite force (maximum occlusal force). The two-color chewing gum mixing ability test was scored in two different formats across four studies: (1) frequency, rate, and binary scoring (Park et al., 2021; Zenthöfer et al., 2021), and (2) 5-points Likert for the changed gum color and/or Diffix score that indicates the amount of mixing (Shin et al., 2020; Weijenberg et al., 2015).
Swallowing Ability
Target population
The 27 instruments assessing swallowing ability were applied in 49 scholarly records. Among the 27 instruments, both ADDS and clinical swallowing examination (Horner et al., 1994) were originally developed for persons living with dementia, while the other 25 were not originally developed for but later used with persons living with dementia. Of those 25 instruments, food intake level scale ([FILS], Ebihara et al., 2020; Miyamoto et al., 2019), gugging swallowing screen ([GUSS], Salvato et al., 2018; Trapl et al., 2007), SSA, 3-oz water swallow test, food test ([FT], Takahashi et al., 2019) were developed for use with stroke patients. Eating assessment tool ([EAT-10], Chen et al., 2020; Özsürekci et al., 2020) and modified water swallow test ([MWST], Hoshino et al., 2020; Sato et al., 2014; Simões et al., 2020; Takahashi et al., 2019) were originally developed for use in patients with probable dysphagia. Timed water swallow test ([TWST], Borders et al., 2021) and V–VST were originally developed for patients with motor neuron diseases to assess neurogenic dysphagia. The test of masticating and swallowing solids ([TOMASS], Borders et al., 2021) was originally developed for use in patients with Parkinson’s disease. The Fiberoptic endoscopic examination of swallowing (FEES; Langmore et al., 2007; Leder et al., 2009; Rösler et al., 2015; Sheikhany et al., 2019; Suiter & Leder, 2007; Ward et al., 2020) was originally developed for patients whose level of dysphagia cannot be examined with traditional video-fluoroscopic method. For the remaining 14 instruments, the target populations in the original developer’s scholarly records could not be specified because information related to the development process, or the full texts of the developer’s records was not available.
Development process
The development process (i.e., literature reviews, expert reviews, developers’ clinical experiences, pilot testing, and psychometric testing) was specified for 13 of the 27 instruments. The use of specific frameworks or content validity index (CVI) scoring to evaluate content validity was not reported in the development of any instruments included in this review. ADDS and clinical swallowing examination (Horner et al., 1994) were developed by combining existing tools. For example, ADDS is developed by combining a water swallow test and GUSS. The development process of the other 12 instruments could not be specified because information related to the development process, or the full texts of the developer’s records was not available.
Sample/setting and administration method
The sample sizes used to test the 27 instruments varied from one to 346 individuals. Particularly, sample sizes of less than 20 were reported in 10 full texts. The instruments were tested in varied care settings: (1) home and/or community settings (n = 7); (2) acute-care settings (n = 6); (3) long-term care (LTC) settings (n = 2); (4) post-acute care settings (n = 1); and (5) mixed setting of hospital and LTC (n = 2). For the other six instruments, study settings varied across the included scholarly records: EAT-10 (LTC, outpatient); repetitive saliva swallow test ([RSST], home, outpatient; Nakamura et al., 2014; Takahashi et al., 2019); SSA (acute-care, LTC); SRS (acute-care, outpatient); FEES (acute care, post-acute care, outpatient); and MWST (home, acute-care, LTC). Settings were not specified in fMRI (Humbert et al., 2010), Measures of Bolus Flow Direction and Clearance (Humbert et al., 2010), and Measures of Range of Motion (Humbert et al., 2010).
Among the 27 instruments, EAT-10, a Questionnaire for Identifying the Risk of Oropharyngeal Dysphagia (Goes et al., 2014), and Seirei dysphasia screening questionnaire (Takahashi et al., 2019) are self-report and/or proxy-report questionnaires. Fourteen instruments are administered through direct observation by raters. For instance, GUSS assesses the swallowing ability of different food consistencies (i.e., liquid, semisolid, and solid) by a rater. MWST assesses the swallowing ability of 3 ml of cold water by a rater and was later combined with cervical auscultation to assess breath sound while the patient swallows. TWST and TOMASS were developed for direct observation but applied as a video-recorded swallowing examination via telehealth through Zoom in the included records.
Eight physiological instruments used different administration methods. Measures of Bolus flow direction and clearance (Humbert et al., 2010), measures of range of motion (Humbert et al., 2010), normalized residue ratio scale (NRRS) (Namasivayam-MacDonald & Riquelme, 2019), penetration aspiration scale (PAS; Humbert et al., 2010; Namasivayam-MacDonald & Riquelme, 2019; Namasivayam-MacDonald et al., 2021; Rösler et al., 2015), swallowing duration measures, and VFSS are administered using video-fluoroscopic observations. FEES is an endoscopic procedure using a camera for direct observation of the oral cavity, larynx, and vocal code. fMRI is an MRI imaging diagnostic instrument. In addition, ADDS and V–VST are administered using direct observation and physiologic assessment, which applies oxygen saturation using a pulse-oximeter before and after swallowing to identify signs of aspiration.
Items and scoring
The items of 14 out of 27 instruments ranged from 1 to 15. Item descriptions in t10 instruments are not available or specified in the records. The number of items of STAND, VFSS, and SSA varied across studies (e.g., 3–4 items).
Five instruments were scored using one format. For example, clinical swallowing examination and measures of Bolus flow direction and clearance were scored using the Likert format, 3-oz water swallow test and YSP were scored using the raw frequency of swallowing, and swallowing duration measures were scored using the raw duration of swallowing time. Eight instruments were scored using two different scoring methods; for example, four were scored using the frequency and rate of dysphagia risk (questionnaire for identifying risk of oropharyngeal dysphagia, STAND, GUSS, and SSA). Measures of range of motion were scored using a unique procedure that measures the range of motion of the larynx or hyoid bone by subtracting the mean coordinate from the peak coordinate of the larynx or hyoid bone before swallowing.
Thirteen instruments were scored using more than three scoring formats. The frequency, rate, and/or Likert-type scoring of swallowing ability were used for five instruments (Seirei dysphagia screening questionnaire, SRS, DSS, EAT-10, and PAS). Six instruments were scored using the frequency and rate as well as unique interpretation by instrument: the area ratio of the residue in epiglottic vallecula and pyriform sinuses after swallowing (NRRS); the number of swallows (FT); oxygen saturation level (V–VST); binary, and/or Likert-format (MWST); Likert-format, and/or the duration of oropharyngeal swallow response (VFSS); leakage time, bolus location, and/or the amount of food residue left after the swallow (FEES). TWST was scored using the number of swallows, volume consumed per swallow, time taken to swallow, and the presence of aspiration signs. TOMASS was scored using the number of bites, swallows, jaw movements, time taken to bite, and the presence of aspiration signs.
Psychometric Quality Assessment
Nearly half of the total 35 instruments (n = 15) were tested for only one type of psychometric property. All eight instruments assessing chewing ability were not tested for any type of reliability. Twenty-seven instruments assessing swallowing ability were tested for one to four types of psychometric properties. Among the 27 instruments, FEES, NRRS, and PAS were tested for both reliability and validity; TWST and TOMASS were tested for only inter and intrarater reliability. The other 22 instruments were tested for one or more types of validity, but not for any type of reliability.
The psychometric quality scores of included instruments were shown in Table 1 (see Supplementary Table 4 for the scoring details). All eight instruments assessing chewing ability were scored as having low psychometric quality (total scores range: 1–4). Masticatory efficiency, maximum occlusal force, PMMT, and chewing capacity assessment were scored as the highest among the eight instruments (total score = 4). Among 27 instruments assessing swallowing ability, RSST, SRS, and DSS were scored as the highest, with moderate psychometric quality (total score = 6). MWST, NRRS, STAND, and V–VST were scored as the second highest (total score = 5).
Table 1.
Psychometric Quality Assessment of Identified Instruments
| Concept/construct | No. | Instrument | Score (rating) |
|---|---|---|---|
| Swallowing ability | 1 | Albertinen Dementia dysphagia screening (ADDS) | 4 (low) |
| 2 | Clinical swallowing examination (CSE) | 1 (low) | |
| 3 | Eating assessment tool (EAT) | 3 (low) | |
| 4 | Fiberoptic endoscopic examination of swallowing (FEES) | 4 (low) | |
| 5 | Food intake level scale (FILS) | 4 (low) | |
| 6 | Functional magnetic resonance imaging (fMRI) | 2 (low) | |
| 7 | Measures of bolus flow direction and clearance | 0 (low) | |
| 8 | Measures of range of motion | 2 (low) | |
| 9 | 3-oz water swallow test | 3 (low) | |
| 10 | Modified water swallow test (MWST) | 5 (low) | |
| 11 | Normalized residue ratio scale (NRRS) | 5 (low) | |
| 12 | Penetration-aspiration scale (PAS) | 3 (low) | |
| 13 | Questionnaire for Identifying Risk of Oropharyngeal Dysphasia in Elderly Patients with Dementia | 1 (low) | |
| 14 | Repetitive saliva swallow test (RSST) | 6 (moderate) | |
| 15 | Screening tool for acute neurological dysphagia (STAND) | 5 (low) | |
| 16 | Seirei dysphasia screening questionnaire | 2 (low) | |
| 17 | Swallowing duration measures | 2 (low) | |
| 18 | Swallowing Rating Scale (SRS) | 6 (moderate) | |
| 19 | Timed water swallow test (TWST) | 3 (low) | |
| 20 | Video fluoroscopic evaluation of swallowing (VFSS) | 2 (low) | |
| 21 | Yale swallow protocol (YSP) | 1 (low) | |
| 22 | Test of masticating and swallowing solids (TOMASS) | 2 (low) | |
| 23 | Dysphagia severity scale (DDS) | 6 (moderate) | |
| 24 | Food test (FT) | 4 (low) | |
| 25 | Gugging swallowing screen (GSS) | 2 (low) | |
| 26 | Standardized swallowing assessment (SSA) | 2 (low) | |
| 27 | Volume-viscosity swallowing test (V–VST) | 5 (low) | |
| Biting/ chewing ability | 28 | Masticatory efficiency | 4 (low) |
| 29 | Maximum occlusal force | 4 (low) | |
| 30 | Palpation of masseter muscle tension (PMMT) | 4 (low) | |
| 31 | Subjective Masticatory Index | 3 (low) | |
| 32 | Subjective mastication comfort analysis | 2 (low) | |
| 33 | Subjective 5 food mastication ability | 3 (low) | |
| 34 | Two-color chewing gum mixing ability test | 1 (low) | |
| 35 | Chewing capacity assessment | 4 (low) |
Four out of the eight instruments assessing chewing ability and eight out of the 27 instruments assessing swallowing ability were evaluated as having moderate to adequate quality on the ratio of sample size to the number of items. The ratio was not obtained for 13 instruments (masticatory efficiency, subjective mastication comfort analysis, Two-color chewing gum mixing ability test, FEES, fMRI, measure of range of motion, MWST, NRRS, PAS, SRS, VFSS, YSP, and V–VST), in which the items or specific variables were not identified or reported.
Discussion
This systematic review is the first that described and evaluated observational, self-report, and physiological instruments that assess chewing and swallowing ability of persons living with dementia. The 35 eligible instruments assessing chewing and swallowing abilities were reported for use in 60 scholarly records, published between 1994 and 2021.
Ratio of Sample Size to the Number of Items
The use of an appropriate sample size to test an instrument is important to accurately estimate psychometric evidence depending on the number of items the instrument has (DeVellis, 2016; Waltz et al., 2017). In this review, only one-third of the 35 instruments were evaluated as having moderate or adequate quality based on the ratio of sample size to the number of items. Although there is no gold standard, it is recommended a sample size of at least 10 is needed per item, and a larger sample size is preferred, particularly for instruments with more response options (Likert-format vs binary) and when complex analysis is conducted, such as factor analysis or internal consistency (DeVellis, 2016; Waltz et al., 2017). Future testing of existing instruments is warranted in larger samples.
Reliability
Although all self- or proxy-report questionnaires and observational and physiological instruments require reliability testing, only five out of the 35 instruments were tested for internal consistency, intra and interrater reliability, and/or test–retest reliability. Although internal consistency is a critical psychometric property used to estimate the homogeneity of an instrument with more than one item (DeVellis, 2016; Waltz et al., 2017), none of the eligible instruments were tested for internal consistency. Internal consistency can be ensured through a well-defined development process of an instrument and is usually estimated using Cronbach’s alpha or Omega (DeVellis, 2016; Hayes & Coutts, 2020; McNeish, 2018; Terwee et al., 2007; Waltz et al., 2017).
Five of all 35 instruments were tested for and showed moderate to adequate evidence of intrarater reliability and interrater reliability. All observational and physiological instruments that require observations by raters should be tested for stability among raters (interrater reliability) and/or over time (intrarater reliability; DeVellis, 2016; Waltz et al., 2017; Zwakhalen et al., 2006). Stability of self- or proxy-report questionnaires is recommended through estimates of test–retest reliability (i.e., the consistency of item responses over time; DeVellis, 2016; Waltz et al., 2017; Zwakhalen et al., 2006). In this review, none of the seven self- or proxy-report instruments were tested for test–retest reliability. Intra and interrater reliability, and test–retest reliability can be estimated by calculating the reliability coefficient (e.g., Cohen’s Kappa, percentage agreement, and intraclass correlation coefficient [ICC]). Future testing of existing instruments is greatly needed to accumulate evidence of reliability.
Validity
Validity is defined as the extent to which an instrument assesses a construct of interest that the instrument is intended to measure (DeVellis, 2016; Waltz et al., 2017). In this review, all instruments were tested for one to three types of validity except TWST and TOMASS (only tested for intra and interrater reliability, respectively). All instruments were evaluated as having moderate to adequate quality on the tested type of validity, except four instruments which were evaluated as having low quality on specific types of validity (i.e., two-color chewing gum mixing ability test [predictive validity], EAT-10 [convergent validity], PAS [known group difference validity], measures of bolus [known group difference validity]).
The included instruments were tested for only certain types of validity rather than all types of validity. Specifically, content validity, divergent validity, and structural validity were not tested for any instruments, while concurrent validity, predictive validity, convergent validity, and/or known group difference validity were tested for one or more instruments.
Content validity is an essential property that aims to establish item appropriateness during the early stage of the instrument development process. Content validity can be estimated using the CVI, a matrix to quantify the quality of items, including relevance, specificity, clarity, feasibility, and/or representativeness of the items that measure the construct of interest (Terwee et al., 2007). However, none of the included instruments was reported with CVI. In addition, we could not find any detailed development process for over half of the 35 instruments, including conceptual framework, original target population, and the item selection process such as an expert review. The use of conceptual frameworks is critical to provide dimensions of the concept and validation of the items (Waltz et al., 2017). Expert review is recommended for item selection and refinement to establish face and content validity (Liu et al., 2020; Waltz et al., 2017).
Divergent validity is one type of construct validity evaluating to what extent independent instruments of theoretically unrelated or distinct concepts diverge or are not correlated (Waltz et al., 2017; Zwakhalen et al., 2006). One possible reason for the lack of testing of divergent validity is that assessments of constructs not correlated with or distinct from the construct of interest (chewing and/or swallowing ability) are less likely to be included in the studies, unless the studies aimed to assess psychometric properties of instruments, particularly divergent validity. All included scholarly records in this review were cross-sectional or longitudinal studies that aimed to assess chewing and swallowing ability and relationships with other constructs, rather than measurement development and validation studies that specifically aimed to establish psychometric properties of the instruments. Additional assessments of constructs that are not or less likely to be related to the target construct of interest (chewing and/or swallowing ability) may not be considered in the design because the addition of such assessments can increase response burden, decrease response rate, increase measurement errors due to respondent fatigue, and increase cost and time (Waltz et al., 2017).
Structural validity is the most straightforward type of construct validity that aims to estimate how the items of an instrument that assesses the construct of interest are consistent with a theoretical hypothesis (Terwee et al., 2007). Exploratory and confirmatory factor analysis can be used to empirically justify the item selection during the initial stage of instrument development (Waltz et al., 2017). However, factor analysis is recommended when a relatively larger sample (N > 100) is available (Waltz et al., 2017). Only 13 instruments were tested in a sample size of 100 or more individuals in this review. The other instruments tested in small samples would not allow for testing of structural validity.
Concurrent validity was tested for two physiological instruments and three direct observational instruments using sensitivity and specificity estimates. Sensitivity and specificity values are newly added criteria in the PAT and are often used to evaluate criterion validity (i.e., concurrent validity and predictive validity) of physiological and direct observational instruments (Trevethan, 2017). All five physiological and direct observational instruments were compared with their “gold standards” (i.e., reference standard). However, the consistency of these values is closely related to the characteristics of target instruments, gold standards, and target population (Trevethan, 2017). Because there are concerns about the validity of gold standards, these values should be reported for both the target instrument and gold standards with sample characteristics to adequately evaluate the concurrent validity of the instrument. In this review, only the included instruments (i.e., target instrument), rather than their gold standards, were reported for sensitivity and specificity values. Other statistics used to assess criterion validity include correlation coefficient (i.e., r, rs), area under the curve (AUC), or 95% CI, if there is no gold standard for a given instrument.
Predictive validity, convergent validity, and known group difference validity were tested for most of the included instruments. Specifically, known group difference validity was tested for over 80% of the included instruments (n = 29), which were evaluated as having moderate to adequate quality except for two instruments. The design of the included cross-sectional or longitudinal studies that aimed to examine differences in chewing or swallowing ability between groups, based on estimates of p value or 95% CI, allowed for examination of known group difference validity. Future research on measurement quality for the included instruments is needed to accumulate psychometric evidence for reliability and validity which were not tested for or had low psychometric quality.
Psychometric Quality
In this review, we used the PAT to evaluate the psychometric quality of the included instruments. The PAT was used in our prior systematic reviews of instruments and showed evidence of feasibility and ease of use (Kim & Liu, 2021; Kim et al., 2022; Liu & Kim, 2021; Liu et al., 2021). Overall, included instruments were evaluated as having low psychometric quality except for RSST, SRS, and DSS, which assess swallowing ability and were scored as having moderate psychometric quality. However, these three instruments were not tested for any type of reliability and were only tested for two or three types of validity (i.e., predictive, convergent, and known group difference validity). The eight instruments assessing chewing ability were evaluated as having moderate or adequate psychometric quality, the overall score of those instruments was evaluated as low. Similarly, with prior reviews of instruments in mealtime research, reasons for low psychometric quality for most instruments included in this review are the use of a small sample size and lack of testing on specific types of psychometric properties.
Implications and Directions for Future Research
In this review of 35 instruments published in 60 scholarly records until July 2022, only three instruments assessing swallowing ability (i.e., RSST, SRS, and DSS) were evaluated as having moderate psychometric quality in persons living with dementia. These three instruments were evaluated as having high psychometric quality in predicting swallowing ability/dysphagia and discriminating between high and low severity groups. Especially, RSST has only one item with simple scoring that can be easy to implement by formal and family caregivers, and even by the patient. With further testing of additional psychometric properties (i.e., reliability, content validity, and other criterion validity if there is a “gold standard”) in larger samples of persons living with dementia, health care providers would apply and test these instruments to screen and manage possible swallowing problems as well as accumulate evidence and expand the use of psychometrically sound instruments in the primary, long-term, and home care settings.
Family caregivers provide most of the care for persons living with dementia in the community settings including mealtime care (Alzheimer’s Association [AA], 2022). Therefore, early detection of swallowing problems in patients by caregivers is essential to manage symptoms and avoid complications, comorbidity, and mortality. Based on this review, there are two proxy-report questionnaires assessing swallowing ability (i.e., EAT-10, Questionnaire for Identifying Risk of Oropharyngeal Dysphasia in Elderly Patients with Dementia). However, these instruments were evaluated as having overall low psychometric quality with no reliability testing. Reliability testing is essential for self- or proxy-report questionnaires to ensure consistency of the subject’s responses over time (Waltz et al., 2017). To establish psychometric evidence and more practical application of these instruments, testing on reliability among family caregivers is warranted. Further testing will also ensure family caregivers use these instruments as a screening tool to detect signs of swallowing difficulties.
For the eight instruments assessing chewing ability, only one or two types of validity were tested and none of the types of reliability were tested between 2015 and 2021, indicating these instruments are still in the early stages of the development process. Masticatory efficiency, maximum occlusal force, PMMT, and chewing capacity assessment were evaluated as having the highest psychometric quality. However, they were tested in only one study, respectively. On the other hand, the two-color chewing gum mixing ability test was tested in four records, however, only for predictive validity and known group difference validity with low to moderate psychometric quality. Further efforts are warranted for the validity and reliability testing of these instruments in various samples, including health care providers and family caregivers. Specifically, the subjective masticatory index, subjective mastication comfort analysis, subjective five food mastication ability, and chewing capacity assessment are self-report questionnaires, which can be applied by family caregivers in a community setting as a proxy-report tool to screen early signs of chewing problems of persons living with dementia. However, all these instruments were evaluated as having low overall psychometric quality in this review and require further testing in this population for more practical use. In addition, refinement, or modification of items of these instruments are suggested to improve the quality of fundamental psychometric properties to apply family caregivers as a proxy.
The instrument development and validation process requires extensive and reiterative refinements and testing of items. We found all the instruments were recently applied in populations with dementia and had low psychometric quality, except for RSST, SRS, and DSS with moderate quality. Further testing of essential psychometric properties is recommended for all instruments to accumulate evidence and expand use in future research and clinical practice.
Limitations
We only searched and screened published literature that have an English title and abstract due to the large volume of scholarly records obtained which may result in selection bias. This review focused on synthesizing psychometric evidence of eligible instruments in dementia populations. Some of the included instruments were applied and tested in other populations than people living with dementia with reported psychometric evidence (e.g., FEES, fMRI, 3-oz water swallow test), and such evidence was not synthesized in this review. Therefore, the findings of the review mainly generalize to the dementia population.
Relevance to Clinical Practice
Chewing and swallowing problems are one of the problematic concerns in persons living with dementia, that directly affect mealtime performance (Takizawa et al., 2016). Measuring chewing and swallowing abilities using valid and reliable instruments is critical in clinical practice, to plan for mealtime care and reduce health-related negative outcomes and premature mortality (Hoshino et al., 2020; Simões et al., 2020). Psychometrically sound instruments can be used to evaluate the process, fidelity, and effectiveness of mealtime care or oral health clinical practice. In this review, we suggested the instruments that were evaluated as having moderate to adequate quality on the tested properties. They can be applied in the dementia population by healthcare providers to diagnose and manage chewing and swallowing abilities for care planning. Other self- and proxy-report instruments with low psychometric quality can be applied to use with family caregivers to screen early signs of chewing/swallowing problems in persons living with dementia after additional psychometric testing. Further application of those instruments in a larger sample of persons living with dementia will also accumulate evidence and expand the use of psychometrically sound instruments in clinical practice and community settings.
Supplementary Material
Acknowledgments
We acknowledge the developers of the included instruments who shared the full version of the instruments. The authors also acknowledge assistance from Oladipupo A. Amao, Lena J. Hughes, and Madison G. Mann for literature screening and data extraction.
Contributor Information
Sohyun Kim, College of Nursing and Health Innovation, University of Texas at Arlington, Arlington, Texas, USA.
Kyuri Lee, College of Nursing, The University of Iowa, Iowa City, Iowa, USA.
Wen Liu, College of Nursing, The University of Iowa, Iowa City, Iowa, USA.
Funding
This study was partly supported by the National Institute on Aging at the National Institutes of Health, grant number K23AG066856 (PI: W. Liu). The sponsor was not involved in study design, data collection and analysis, interpretation of findings, and manuscript preparation.
Conflict of Interest
We have no commercial or other resource relationships to disclose.
Author Contributions
Study conceptualization and design: W. Liu., S. Kim. Acquisition of data: S. Kim, K. Lee, W. Liu. Analysis and interpretation of data S. Kim, K. Lee, W. Liu. Drafting of the manuscript: S. Kim, K. Lee. Critical revision of the manuscript for important intellectual content: S. Kim, K. Lee, W. Liu.
Registration and Protocol
The protocol of this review was not registered in any register repository.
References
- Alzheimer’s Association. (2022). 2022 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 19(4). doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- Borders, J. C., Sevitz, J. S., Malandraki, J. B., Malandraki, G. A., & Troche, M. S. (2021). Objective and subjective clinical swallowing outcomes via telehealth: Reliability in outpatient clinical practice. American Journal of Speech-Language Pathology, 30(2), 598–608. doi: 10.1044/2020_AJSLP-20-00234 [DOI] [PubMed] [Google Scholar]
- Campos, C. H., Ribeiro, G. R., & Rodrigues Garcia, R. C. M. (2018). Mastication and oral health–related quality of life in removable denture wearers with Alzheimer disease. Journal of Prosthetic Dentistry, 119(5), 764–768. doi: 10.1016/j.prosdent.2017.07.010 [DOI] [PubMed] [Google Scholar]
- Chen, S., Cui, Y., Ding, Y., Sun, C., Xing, Y., Zhou, R., & Liu, G. (2020). Prevalence and risk factors of dysphagia among nursing home residents in eastern China: A cross-sectional study. BMC Geriatrics, 20(1), 1–352. doi: 10.1186/s12877-020-01752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVellis, R. F. (2016). Scale development: Theory and applications (Vol. 26). Sage Publications. [Google Scholar]
- Ebihara, T., Miyamoto, T., & Kozaki, K. (2020). Prognostic factors of 90-day mortality in older people with healthcare-associated pneumonia. Geriatrics & Gerontology International, 20(11), 1036–1043. doi: 10.1111/ggi.14036 [DOI] [PubMed] [Google Scholar]
- Fontijn-Tekamp, F. A., Slagter, A. P., Bilt, A., Hof, M. A., Witter, D. J., Kalk, W., & Jansen, J. A. (2000). Biting and chewing in overdentures, full dentures, and natural dentitions. Journal of Dental Research, 79(7), 1519–1524. doi: 10.1177/00220345000790071501 [DOI] [PubMed] [Google Scholar]
- Goes, V. F., Mello-Carpes, P. B., Oliveira, L. O., Hack, J., Magro, M., & Bonini, J. S. (2014). Evaluation of dysphagia risk, nutritional status and caloric intake in elderly patients with Alzheimer’s. Revista Latino-Americana de Enfermagem, 22, 317–324. doi: 10.1590/0104-1169.3252.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F., & Coutts, J. J. (2020). Use Omega rather than Cronbach’s alpha for estimating reliability. But…. Communication Methods and Measures, 14(3), 1–24. doi: 10.1080/19312458.2020.1718629 [DOI] [Google Scholar]
- Horner, J., Alberts, M. J., Dawson, D. V., & Cook, G. M. (1994). Swallowing in Alzheimer’s disease. Alzheimer Disease and Associated Disorders, 8(3), 177–189. doi: 10.1097/00002093-199408030-00004 [DOI] [PubMed] [Google Scholar]
- Hoshino, D., Watanabe, Y., Edahiro, A., Kugimiya, Y., Igarashi, K., Motokawa, K., Ohara, Y., Hirano, H., Myers, M., Hironaka, S., & Maruoka, Y. (2020). Association between simple evaluation of eating and swallowing function and mortality among patients with advanced dementia in nursing homes: 1-Year prospective cohort study. Archives of Gerontology and Geriatrics, 87, 103969–103969. doi: 10.1016/j.archger.2019.103969 [DOI] [PubMed] [Google Scholar]
- Humbert, I. A., McLaren, D. G., Kosmatka, K., Fitzgerald, M., Johnson, S., Porcaro, E., Kays, S., Umoh, E. -O., & Robbins, J. (2010). Early deficits in cortical control of swallowing in Alzheimer’s disease. Journal of Alzheimer’s Disease, 19(4), 1185–1197. doi: 10.3233/jad-2010-1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., & Liu, W. (2021). Psychometric properties of instruments measuring dyadic communication and environment in dementia care: A systematic review. Gerontologist, 63(1), 52–70. doi: 10.1093/geront/gnab178 [DOI] [PubMed] [Google Scholar]
- Kim, S., Liu, W., Daack-Hirsch, S., & Williams, K. N. (2022). Development and psychometric testing of the dyadic communication observational coding scheme in DEmentia care (DCODE): Family dyadic communication in dementia. Aging & Mental Health. doi: 10.1080/13607863.2022.2126819 [DOI] [PubMed] [Google Scholar]
- Komiyama, T., Ohi, T., Hiratsuka, T., Miyoshi, Y., Tomata, Y., Zhang, S., Tsuji, I., Watanabe, M., & Hattori, Y. (2020). Cognitive impairment and depressive symptoms lead to biases in self-evaluated masticatory performance among community-dwelling older Japanese adults: The Tsurugaya Project. Journal of Dentistry, 99, 103403. doi: 10.1016/j.jdent.2020.103403 [DOI] [PubMed] [Google Scholar]
- Langmore, S. E., Olney, R. K., Lomen-Hoerth, C., & Miller, B. L. (2007). Dysphagia in patients with frontotemporal lobar Dementia. Archives of Neurology, 64(1), 58–62. doi: 10.1001/archneur.64.1.58 [DOI] [PubMed] [Google Scholar]
- Leder, S. B., Suiter, D. M., & Lisitano Warner, H. (2009). Answering orientation questions and following single-step verbal commands: Effect on aspiration status. Dysphagia, 24(3), 290–295. doi: 10.1007/s00455-008-9204-x [DOI] [PubMed] [Google Scholar]
- Liu, W., Batchelor, M., & Williams, K. (2020). Ease of use, feasibility and inter-rater reliability of the refined Cue Utilization and Engagement in Dementia (CUED) mealtime video-coding scheme. Journal of Advanced Nursing (John Wiley & Sons, Inc.), 76(12), 3609–3622. doi: 10.1111/jan.14548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., & Kim, S. (2021). Dyadic interactions and physical and social environment in dementia mealtime care: A systematic review of instruments. Annals of the New York Academy of Sciences, 1505(1), 23–39. doi: 10.1111/nyas.14667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W., Kim, S., & Alessio, H. (2021). Mealtime caregiving knowledge, attitudes, and behaviors for persons living with dementia: A systematic review of psychometric properties of instruments. International Journal of Nursing Studies, 114, 103824–103824. doi: 10.1016/j.ijnurstu.2020.103824 [DOI] [PubMed] [Google Scholar]
- McNeish, D. (2018). Thanks coefficient alpha, we’ll take it from here. Psychological Methods, 23(3), 412–433. doi: 10.1037/met0000144 [DOI] [PubMed] [Google Scholar]
- Miyamoto, T., Ebihara, T., & Kozaki, K. (2019). The association between eating difficulties and biliary sludge in the gallbladder in older adults with advanced dementia, at end of life. PLoS One, 14(7), e0219538–e0219538. doi: 10.1371/journal.pone.0219538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D. (2010). Corrigendum to: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International journal of surgery (London, England), 8(8), 658. doi: 10.1016/j.ijsu.2010.07.299 [DOI] [PubMed] [Google Scholar]
- Mokkink, L. B., de Vet, H. C. W., Prinsen, C. A. C., Patrick, D. L., Alonso, J., Bouter, L. M., & Terwee, C. B. (2018). COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Quality of Life Research, 27(5), 1171–1179. doi: 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K., Watanabe, N., Ohkawa, H., Ando, M., Ogura, Y., Funabiki, S., Kume, A., Urano, K., Osada, T., & Yamamura, K. (2014). Effects on caregiver burden of a donepezil hydrochloride dosage increase to 10 mg/day in patients with Alzheimer’s disease. Patient Preference and Adherence, 2014(8), 1223–1228. doi: 10.2147/PPA.S69750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam-MacDonald, A. M., Alomari, N., Attner, L., Benjamin, R. D., Chill, A., Doka, S., Guastella, R., Marchese, J., Oppedisano, S., Ressa, K., Rider, B. E., Sandoval, G. K., Soyfer, A., Thompson, R., Walshe, C. M., & Riquelme, L. F. (2021). A retrospective analysis of swallowing function and physiology in patients living with DEmentia. Dysphagia, 37(4), 900–908. doi: 10.1007/s00455-021-10350-z [DOI] [PubMed] [Google Scholar]
- Namasivayam-MacDonald, A. M., & Riquelme, L. F. (2019). Quantifying airway invasion and pharyngeal residue in patients with dementia. Geriatrics (Basel), 4(1), 13. doi: 10.3390/geriatrics4010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özsürekci, C., Arslan, S. S., Demir, N., Çalışkan, H., Şengül Ayçiçek, G., Kılınç, H. E., Yaşaroğlu, F., Kızılarslanoğlu, C., Tuna Doğrul, R., Balcı, C., Sümer, F., Karaduman, A., Yavuz, B. B., Cankurtaran, M., & Halil, M. G. (2020). Timing of dysphagia screening in Alzheimer’s dementia. Journal of Parenteral and Enteral Nutrition, 44(3), 516–524. doi: 10.1002/jpen.1664 [DOI] [PubMed] [Google Scholar]
- Park, T., Jung, Y. S., Son, K., Bae, Y. C., Song, K. B., Amano, A., & Choi, Y. H. (2021). More teeth and posterior balanced occlusion are a key determinant for cognitive function in the elderly. International Journal of Environmental Research and Public Health, 18(4), 1996. doi: 10.3390/ijerph18041996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y., Han, H., Oh, S., & Chang, H. (2014). Validation of the Korean version of the standardized swallowing assessment among nursing home residents. Journal of Gerontological Nursing, 40(2), 26–35; quiz 36. doi: 10.3928/00989134-20131220-08 [DOI] [PubMed] [Google Scholar]
- Park, Y. H., Han, H. R., Oh, B. M., Lee, J., Park, J., Yu, S. J., & Chang, H. (2013). Prevalence and associated factors of dysphagia in nursing home residents. Geriatric Nursing, 34(3), 212–217. doi: 10.1016/j.gerinurse.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Popman, A., Richter, M., Allen, J., & Wham, C. (2018). High nutrition risk is associated with higher risk of dysphagia in advanced age adults newly admitted to hospital. Nutrition & Dietetics, 75(1), 52–58. doi: 10.1111/1747-0080.12385 [DOI] [PubMed] [Google Scholar]
- Prinsen, C. A. C., Mokkink, L. B., Bouter, L. M., Alonso, J., Patrick, D. L., de Vet, H. C. W., & Terwee, C. B. (2018). COSMIN guideline for systematic reviews of patient-reported outcome measures. Quality of Life Research, 27, 1147–1157. doi: 10.1007/s11136-018-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofes, L., Arreola, V., Almirall, J., Cabré, M., Campins, L., García-Peris, P., Speyer, R., & Clavé, P. (2010). Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterology Research and Practice, 2011, 1–13. doi: 10.1155/2011/818979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler, A., Pfeil, S., Lessmann, H., Höder, J., Befahr, A., & von Renteln-Kruse, W. (2015). Dysphagia in Dementia: Influence of Dementia severity and food texture on the prevalence of aspiration and latency to swallow in hospitalized geriatric patients. Journal of the American Medical Directors Association, 16(8), 697–701. doi: 10.1016/j.jamda.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Salvato, G., Mercurio, M., Sberna, M., Paulesu, E., & Bottini, G. (2018). A very light lunch: Interoceptive deficits and food aversion at onset in a case of behavioral variant frontotemporal dementia . Alzheimer’s & Dementia, 10(1), 750–754. doi: 10.1016/j.dadm.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, E., Hirano, H., Watanabe, Y., Edahiro, A., Sato, K., Yamane, G., & Katakura, A. (2014). Detecting signs of dysphagia in patients with Alzheimer’s disease with oral feeding in daily life. Geriatrics & Gerontology International, 14(3), 549–555. doi: 10.1111/ggi.12131 [DOI] [PubMed] [Google Scholar]
- Sheikhany, A. R., Hady, A. F. A., & Farag, S. (2019). Oropharyngeal dysphagia profile in early versus late stage dementia. Egyptian Journal of Otolaryngology, 35(1), 103–109. doi: 10.4103/ejo.ejo_98_18 [DOI] [Google Scholar]
- Shin, H. E., Cho, M. J., Amano, A., Song, K. B., & Choi, Y. H. (2020). Association between mastication-related factors and the prevalence of dementia in Korean elderly women visiting senior centres. Gerodontology, 37(2), 177–184. doi: 10.1111/ger.12453 [DOI] [PubMed] [Google Scholar]
- Simões, A. L. S., Oliva Filho, A., & Hebling, E. (2020). Signs for early detection of dysphagia in older adults with severe Alzheimer’s disease. Journal of Nutrition, Health and Aging, 24(6), 659–664. doi: 10.1007/s12603-020-1382-8 [DOI] [PubMed] [Google Scholar]
- Stamm, J. W., Disney, J. A., Graves, R. C., Bohannan, H. M., & Abernathy, J. R. (1988). The university of North Carolina caries risk assessment Study I: Rationale and content. Journal of Public Health Dentistry, 48(4), 225–232. doi: 10.1111/j.1752-7325.1988.tb03203.x [DOI] [PubMed] [Google Scholar]
- Suiter, D. M., & Leder, S. B. (2007). Clinical utility of the 3-ounce Water Swallow Test. Dysphagia, 23(3), 244–250. doi: 10.1007/s00455-007-9127-y [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Amemiya, K., Nakatsuka, M., Nakamura, K., Kasai, M., & Meguro, K. (2019). Impaired eating and swallowing function in older adults in the community: The Kurihara project. International Journal of Environmental Research and Public Health, 16(20), 4040. doi: 10.3390/ijerph16204040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara, S., Wright, F. A. C., Waite, L. M., Naganathan, V., Hirani, V., Blyth, F. M., Le Couteur, D. G., Seibel, M. J., Handelsman, D. J., & Cumming, R. G. (2020). Oral health and cognitive status in the Concord Health and Ageing in Men Project: A cross-sectional study in community-dwelling older Australian men. Gerodontology, 37(4), 353–360. doi: 10.1111/ger.12469 [DOI] [PubMed] [Google Scholar]
- Takizawa, C., Gemmell, E., Kenworthy, J., & Speyer, R. (2016). A systematic review of the prevalence of oropharyngeal Dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia, 31(3), 434–441. doi: 10.1007/s00455-016-9695-9 [DOI] [PubMed] [Google Scholar]
- Terwee, C. B., Bot, S. D., de Boer, M. R., van der Windt, D. A., Knol, D. L., Dekker, J., Bouter, L. M., & de Vet, H. C. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology, 60(1), 34–42. doi: 10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Terwee, C. B., Prinsen, C. A. C., Chiarotto, A., Westerman, M. J., Patrick, D. L., Alonso, J., Bouter, L. M., de Vet, H. C. W., & Mokkink, L. B. (2018). COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A Delphi study. Quality of Life Research, 27(5), 1159–1170. doi: 10.1007/s11136-018-1829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapl, M., Enderle, P., Nowotny, M., Teuschl, Y., Matz, K., Dachenhausen, A., & Brainin, M. (2007). Dysphagia bedside screening for acute-stroke patients: The gugging swallowing screen. Stroke, 38(11), 2948–2952. doi: 10.1161/STROKEAHA.107.483933 [DOI] [PubMed] [Google Scholar]
- Trevethan, R. (2017). Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Frontiers in Public Health, 5, 307–307. doi: 10.3389/fpubh.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz, C. F., Strickland, O., & Lenz, E. R. (2017). Measurement in nursing and health research (5th ed.). Springer. doi: 10.1891/9780826170620 [DOI] [Google Scholar]
- Ward, M., Skelley-Ashford, M., Brown, K., Ashford, J., & Suiter, D. (2020). Validation of the Yale Swallow Protocol in post-acute care: A prospective, double-blind, multirater study. American Journal of Speech-Language Pathology, 29(4), 1937–1943. doi: 10.1044/2020_AJSLP-19-00147 [DOI] [PubMed] [Google Scholar]
- Weijenberg, R. A. F., Lobbezoo, F., Visscher, C. M., & Scherder, E. J. A. (2015). Oral mixing ability and cognition in elderly persons with dementia: A cross-sectional study. Journal of Oral Rehabilitation, 42(7), 481–486. doi: 10.1111/joor.12283 [DOI] [PubMed] [Google Scholar]
- Zenthöfer, A., Ehret, J., Zajac, M., Kilian, S., Kostunov, J., Rammelsberg, P., & Klotz, A.-L. (2021). How do changes in oral health and chewing efficiency affect the changes of oral-health-related quality of life of nursing-home residents in the short term? Clinical Interventions in Aging, 16, 789–798. doi: 10.2147/CIA.S303197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill, J. M., Christalle, E., Muller, E., Harter, M., Dirmaier, J., & Scholl, I. (2014). Measurement of physician-patient communication—A systematic review. PLoS One, 9(12), e112637. doi: 10.1371/journal.pone.0112637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwakhalen, S. M., Hamers, J. P., Abu-Saad, H. H., & Berger, M. P. (2006). Pain in elderly people with severe dementia: A systematic review of behavioural pain assessment tools. BMC Geriatrics, 6(1), 3. doi: 10.1186/1471-2318-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.