Abstract
Background.
Surveillance for cases of acute flaccid paralysis (AFP) is a key strategy adopted for the eradication of polio. Detection of poliovirus circulation is often predicated on the ability to identify AFP cases and test their stool specimens for poliovirus infection in a timely manner. The Village Polio Volunteers (VPV) program was established in 2013 in a bid to strengthen polio eradication activities in Somalia, including AFP surveillance, given the country’s vulnerability to polio outbreaks.
Methods.
To assess the impact of the VPV program on AFP surveillance, we determined case counts, case-reporting sources, and nonpolio AFP rates in the years before and after program introduction (ie, 2011–2016). We also compared the stool specimen adequacy rates and timeliness of cases reported by VPVs to those reported by other sources.
Results.
In the years after program introduction, VPVs accounted for a high proportion of AFP cases reported in Somalia. AFP case counts rose from 148 cases in 2012, the year before program introduction, to 279 cases in 2015, when VPVs accounted for 40% of reported cases. Further, from 2012 to 2015, the nonpolio AFP rate improved from 2.8 to 4.8 cases per 100 000 persons aged <15 years. Stool specimen adequacy rates have been consistently high, and AFP cases have been detected in a timelier manner since the program was introduced.
Conclusions.
Given the impact of the VPV program on improving AFP surveillance indicators in Somalia, similar community-based programs could play a crucial role in enhancing surveillance activities in countries with limited healthcare infrastructure.
Keywords: surveillance, acute flaccid paralysis, polio, community-based surveillance, Somalia
Surveillance for cases of acute flaccid paralysis (AFP) is a key strategy adopted by the Global Polio Eradication Initiative (GPEI) for the eradication of poliomyelitis [1–3]. AFP mani-fests clinically as a syndrome characterized by sudden onset of weakness or paralysis affecting a limb or limbs. If the cause is poliomyelitis, this may result in permanent disability, and outcomes could sometimes be fatal [4, 5]. In addition to poliomyelitis, AFP cases may have several other causes, including Guillain-Barré syndrome and transverse myelitis [6].
Tracking and reporting AFP cases is an effective method for detecting poliovirus circulation in a specified geographic area, since paralysis due to poliovirus infection can only be confirmed by testing stool specimens taken from AFP cases [7]. Identification of ≥2 nonpolio AFP (NPAFP) cases per 100 000 persons aged <15 years is recommended by the World Health Organization (WHO) as a benchmark for surveillance activities in regions with active poliovirus transmission or places at significant risk of outbreaks [8, 9]. Even in regions that have been certified as polio free, such as Europe [10] and the Americas [11], AFP surveillance activities are undertaken as a way of maintaining certification standards [1, 7] and remaining vigilant in the event of reimportation of the virus as a result of migration from polio-infected areas [12–15]. Strengthening AFP surveillance systems is thus seen not just as essential to interrupting poliovirus circulation in areas with ongoing transmission but also for protecting the gains already achieved in places where the disease has been eliminated. This is why a country like Somalia, with a recent history of polio outbreaks, has been prioritized by GPEI for AFP surveillance strengthening.
Somalia has been embroiled in a protracted civil war since 1991 [16]. The country’s healthcare system has been severely weakened by political crisis, and, as a result, the delivery of immunization services lags considerably behind recommended global standards. Despite these challenges, the country successfully interrupted indigenous transmission of wild poliovirus (WPV) in 2002 [17]. The subsequent occurrence of 2 large polio outbreaks during 2005–2007 [18] and 2013–2014 [19, 20] underscores the continued vulnerability of the country to reintroduction of WPV. Although the country’s existing AFP surveillance system has mostly achieved recommended bench-marks for case detection during the past decade, subnational gaps have persisted, as evidenced by the delays in detecting some polio cases during the aforementioned outbreaks. Constraints due to insecurity coupled with bans on polio activities imposed by antigovernment elements have further hindered surveillance efforts in certain parts of the country. The aforementioned factors led to the establishment of the Village Polio Volunteers (VPV) program in September 2013.
The VPV program, a community-based program, was established to enhance polio eradication activities in Somalia by strengthening surveillance and improving vaccination coverage in local communities. Volunteers involved in the program, commonly referred to as VPVs, were recruited from local communities in nearly all districts of the country, with early priority given to districts designated as high risk based on predefined criteria. They were then trained by experienced polio program staff on key strategies for polio eradication, including AFP surveillance, and assigned to work in the communities from which they were recruited. More than 500 VPVs are currently opera-tional in all 4 geopolitical zones of Somalia (Central, Northeast, Northwest, and South).
Each of the VPVs assigned to a district works closely with the district polio officer to actively search for and report AFP cases, in addition to other polio eradication activities, such as community sensitization ahead of vaccination campaigns. Through case search methods, such as house-to-house and healthcare facility visits, VPVs identify incident AFP cases using an active surveillance model to support routine reporting from public facilities and private healthcare providers. Once an AFP case is identified, VPVs immediately notify the district polio officer, and a case investigation is begun. In many areas, particularly those with access limitations, VPVs are actively involved in investigating the case with guidance from the district polio officer. A crucial step in the investigation is the collection of 2 stool specimens from the case patients ≥24 hours apart. Once collected, VPVs work closely with the district polio officer to ensure that the specimens are transported in a timely manner to the regional polio laboratory in Nairobi, Kenya, where testing is conducted. This article focuses on the contributions of VPVs to strengthening poliovirus surveillance in Somalia by assessing the impact of their activities on key AFP surveillance indicators.
METHODS
To assess the impact of the VPV program on AFP surveillance, we reviewed documents provided by the WHO Liaison Office for Somalia, the managing organization for the country’s polio program. Documents reviewed include program description documents outlining terms of reference for volunteers. We also reviewed and analyzed AFP surveillance data for all cases reported in Somalia from January 2011 to November 2016.
To determine the contributions of VPVs to AFP case detection, we calculated overall case counts and proportions during 2011–2016, categorized by reporting source. Categories of reporting sources included VPVs, public healthcare facilities, private healthcare providers, staff of the polio program (other than VPVs), and other sources within the community. We then calculated the NPAFP rates per 100 000 persons aged <15 years for each of the years under review. These NPAFP rates were derived from AFP cases from which neither wild nor vaccine-derived polioviruses were isolated. To assess the impact of inaccessibility on case detection and the performance of VPVs, we performed additional analyses for case counts, proportions, and NPAFP rates. We limited our analyses to districts with security and access limitations during the period under review. We compared the NPAFP rate in 2015, the year after the 2013–2014 polio outbreak, to the rate in 2012, the year before the outbreak, by calculating the incidence rate ratio using a Poisson regression model, adjusting for geopolitical zones. We did this to establish a statistical metric of performance of the surveillance system before and after the introduction of the VPV program.
In addition to AFP case counts and NPAFP rates, we evaluated the quality of surveillance activities undertaken by VPVs by examining 2 other key surveillance indicators: stool specimen adequacy rates and timeliness of reporting. Stool specimen adequacy rates assess the timeliness of investigation and the quality of the reverse cold chain system used for poliovirus isolation. For stool specimens taken from an AFP case to be considered adequate, 2 specimens need to be collected from the case-patient ≥24 hours apart and within 14 days of paralysis onset, and the specimens must arrive at the laboratory in good condition, that is, without leakage or desiccation and under cold conditions [9, 21]. WHO recommends that ≥80% of stool specimens arriving in the laboratory must be adequate.
Similarly, identification and reporting of an AFP case within 7 days of paralysis onset is an indicator of the timeliness of the surveillance system; hence, countries are required to identify and report ≥80% of AFP cases within 7 days of paralysis onset in order to meet the benchmark for timeliness [7]. To determine whether there were any differences in these key indicators by reporting source, we compared the adequacy of stool specimens and timeliness of reporting rates of AFP cases reported by VPVs with those reported by all other sources during 2014–2016, using χ2 tests. We stratified our results by year to decipher trends in timeliness of reporting when comparing AFP cases by reporting source. We computed the mean number days from paralysis onset to case reporting and 95% confidence intervals (CIs). Statistical analyses were performed using SAS software (version 9.3; SAS Institute), and statistical significance was defined as P < .05. This project was undertaken as part of the process of evaluating improvements in a public health surveillance system and was not considered to be human subjects research.
RESULTS
AFP Case Counts and NPAFP Rates
During 2011–2016, there was significant variation in AFP case counts and the NPAPF rates in Somalia. Before the 2013–2014 wild poliovirus outbreak, during which the VPV program was introduced, the number of AFP cases reported nationally declined from 172 cases in 2011 to 148 cases in 2012. None of the AFP cases reported during 2011–2012 were due to WPV infection, although 10 cases of circulating vaccine-derived poliovirus were reported during both years. Along with the decline in case reporting, the national NPAFP rate decreased from 3.2 to 2.8 cases per 100 000 persons aged <15 years when comparing case detection rates in 2011 to 2012 (Figure 1). AFP case counts spiked during the polio outbreak, increasing to 546 and 420 cases during 2013 and 2014, respectively.
Figure 1.
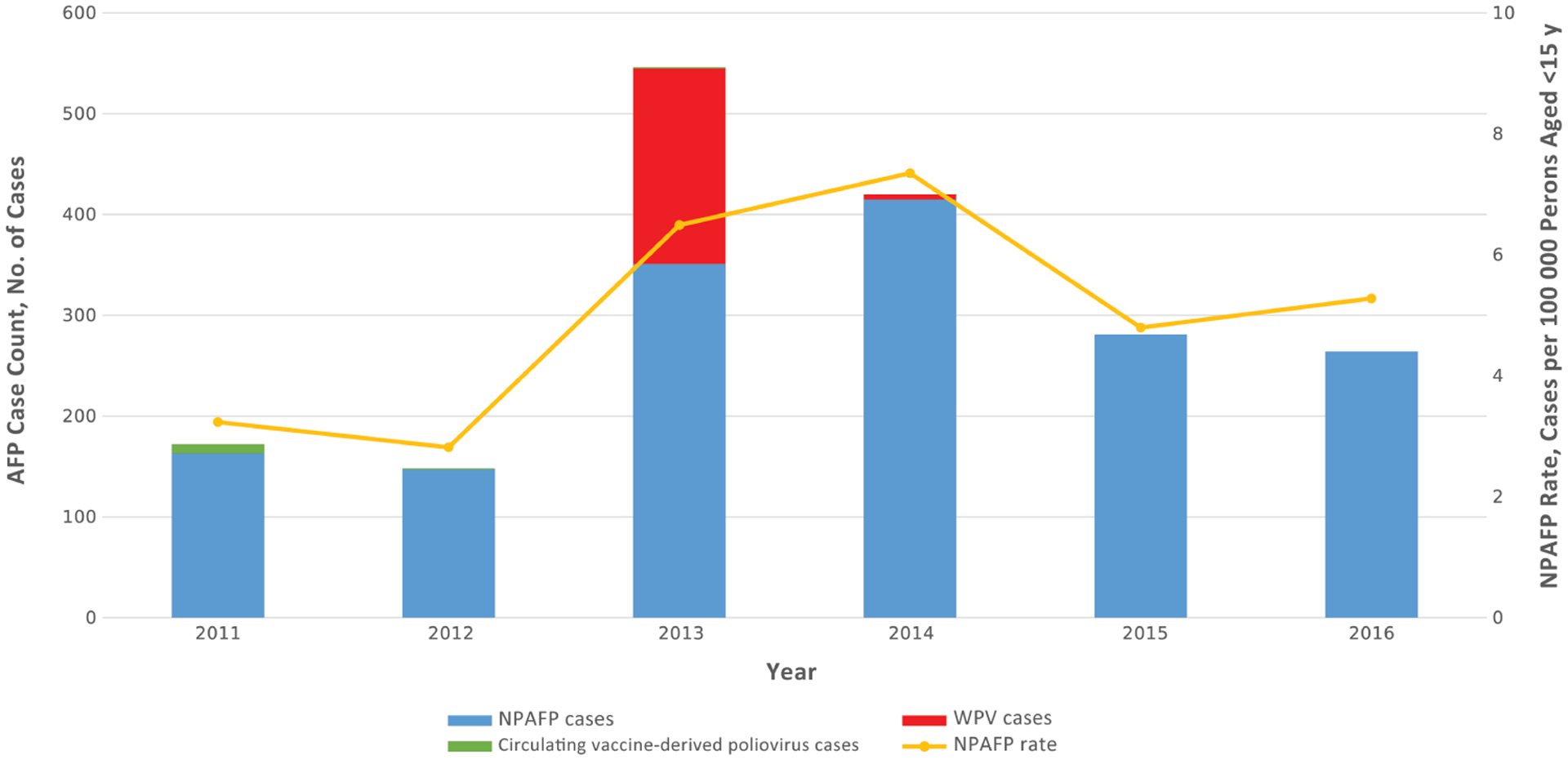
AFP case counts by type and nonpolio AFP (NPAF) rates in Somalia, 2011–2016. Abbreviations: AFP, acute flaccid paralysis; WPV, wild poliovirus.
Of the 546 AFP cases reported in 2013, 194 (36%) were subsequently sconfirmed to be WPV cases. Five additional WPV cases were confirmed in 2014, the last of which was identified by a VPV working in Hobyo district in the Northeast zone. In line with improvements in case detection, NPAFP rates rose considerably during the outbreak, increasing to 6.5 and 7.4 cases per 100 000 persons aged <15 years during 2013 and 2014, respectively. Only a small proportion (2%) of NPAFP cases reported during the outbreak were considered clinically compatible with polio after expert review. NPAFP rates decreased from levels during the outbreak to 4.8 and 5.3 cases per 100 000 persons aged <15 years during 2015 and 2016, respectively, but remained significantly above preoutbreak reporting levels. Using a Poisson regression model adjusting for geopolitical zone, the incidence rate ratio for NPAFP cases was 1.7 (95% CI, 1.4–2.1; P < .001) when comparing the NPAFP rate in 2015, the year after the outbreak, to that of 2012, the year before outbreak onset.
In the years before the VPV program was introduced (ie, 2011 and 2012), the majority of AFP cases were reported through public healthcare facilities, such as hospitals and maternal and child health centers. Such facilities accounted for 43% and 33% of case reporting in 2011 and 2012 (Figure 2). Other reporting sources during this period included regular polio program staff, which accounted for 30% of reporting in 2012, and private healthcare providers, including traditional healers, which accounted for 21% of reporting in 2012. The patterns of reporting held in 2013, the first year of the polio outbreak, with public healthcare facilities again being the main reporting source for AFP cases. VPVs accounted for only 2% of reported cases in 2013, having begun their activities in September of the same year. By 2014, however, VPVs were responsible for reporting approximately a quarter of all AFP cases, comparable to other major reporting sources, such as public healthcare facilities (24%) and regular polio program staff (29%). The contributions of VPVs to case reporting further improved in the following years. Among AFP cases with known reporting sources, VPVs accounted for 40% of the 279 cases reported in 2015, a high among reporting sources. They again accounted for the highest proportion of AFP cases reported in 2016 when compared with other reporting sources.
Figure 2.
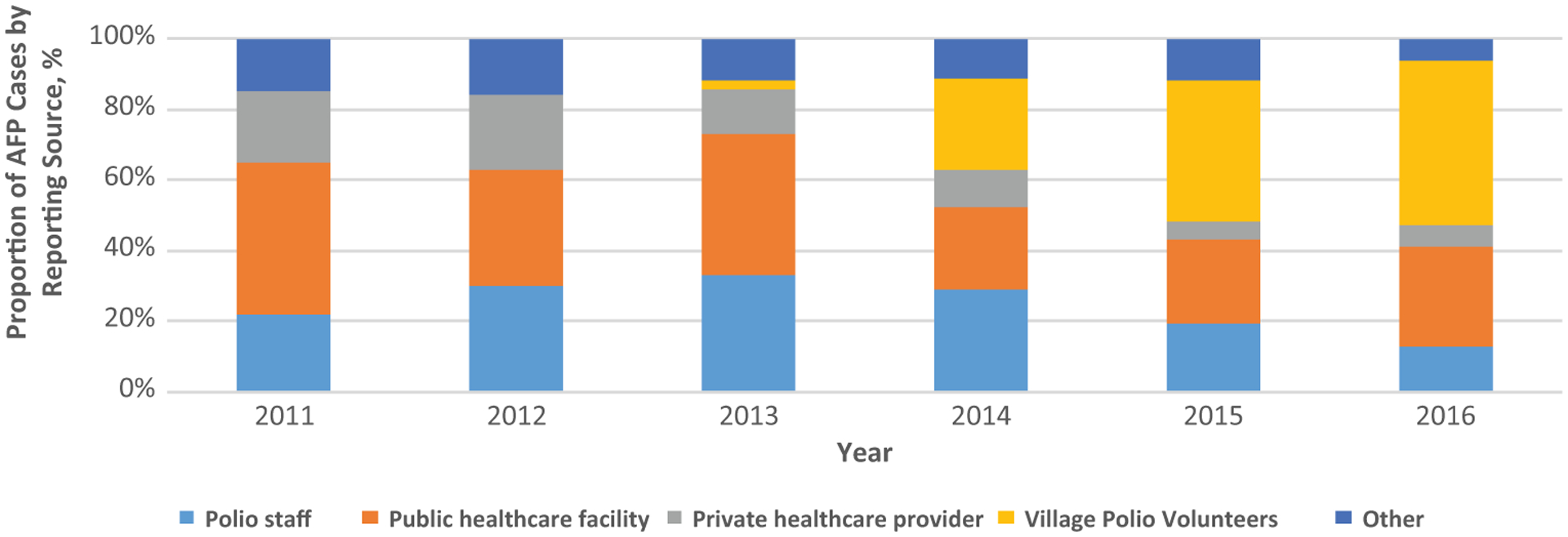
AFP case-reporting sources in Somalia, 2011–2016. Abbreviation: AFP, acute flaccid paralysis.
When limited by accessibility status, case reporting levels and sources in areas with security and access limitations mirrored national trends during 2011–2016. Among the 25 districts that were either partially or completely inaccessible for polio immunization activities during 2011–2012, NPAFP rates were similar to those reported nationally. From 2011 to 2012, rates of case reporting in these areas decreased from 3.2 to 2.6 cases per 100 000 persons aged <15 years in 2012. The NPAFP rates in areas with access limitations increased during the outbreak years to 5.4 and 7.1 in 2013 and 2014, respectively, and remained above preoutbreak levels in the succeeding years, with rates of 4.6 and 5.2 being reported in 2015 and 2016, respectively. In terms of reporting sources, public healthcare facilities, private healthcare providers, and regular polio program staff each reported 28% of the 32 AFP cases identified in districts with access limitations in 2011 (Figure 3). These traditional reporting sources accounted for the bulk of AFP cases reported from these areas over the next 2 years, with VPVs responsible for only 3% of AFP cases reported from districts with access limitations in 2013. In 2014, however, VPVs accounted for just over a third (37%) of the 157 AFP cases identified in 41 districts with access limitations. The proportion of AFP cases detected by VPVs in these areas further increased to 52% in 2015 and 56% in 2016.
Figure 3.
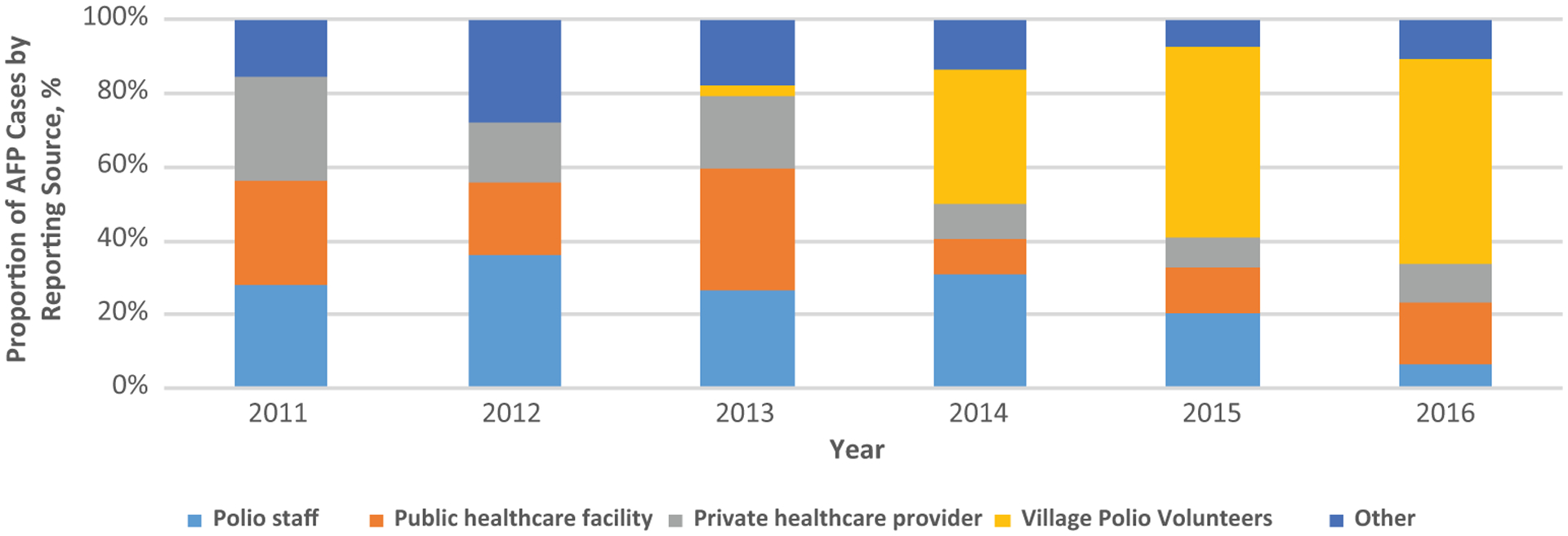
AFP case-reporting in areas in Somalia with access limitations, 2011–2016. Abbreviation: AFP, acute flaccid paralysis.
Stool Specimen Adequacy Rates and Timeliness of Reporting
Stool specimen adequacy rates were consistently >95% during 2011–2016 except in 2013, when the rate dropped to 86.8%. During 2014–2016, no statistically significant difference was found between adequacy of stool specimens reported by VPVs (98.3%) and those reported by other sources (96.8%). Approximately 90% of AFP cases were notified within 7 days of paralysis of onset during 2011–2012. The proportion of AFP cases notified within 7 days dipped to 70.3%, below the recommended benchmark of ≥80%, during 2013, but steadily improved in the following years, increasing from 81.6% in 2014 to 83.3% in 2015 and 92.8% in 2016. No significant difference was found when comparing the timeliness of AFP cases reported within 7 days by VPVs (86.0%) and those reported by other sources (84.8%) during 2014–2016.
The mean duration from paralysis onset to notification was the same (4.5 days) for AFP cases reported by both VPVs and other reporting sources during 2014–2016. Overall reporting trends during 2014–2016 suggested more rapid identification of AFP cases over time by both VPVs and other reporting sources. Among AFP cases reported by VPVs, the mean duration from paralysis onset to notification improved from 5.4 (95% CI, 4.84–5.97) days in 2014 to 3.7 (3.32–4.14) days in 2016 (Table 1). Similarly, the mean duration to case notification decreased from 4.8 (95% CI, 4.32–5.21) days in 2014 to 3.8 (3.3–4.34) days in 2016 among all other reporting sources.
Table 1.
Duration from Paralysis Onset to Notification of Acute Flaccid Paralysis Cases by Year and Reporting Source, Somalia, 2014–2016
| Measure of Duration | Reporting Source by Year | |||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | ||||
| VPVs | Others | VPVs | Others | VPVs | Others | |
| AFP cases, No. | 109 | 307 | 111 | 168 | 122 | 142 |
| Duration from paralysis onset to notification, d | ||||||
| Mean (SD) | 5.4 (2.99) | 4.76 (3.98) | 4.6 (2.82) | 4.82 (5.7) | 3.73 (2.29) | 3.82 (3.13) |
| 95% CI | 4.84–5.97 | 4.32–5.21 | 4.07–5.13 | 3.95–5.68 | 3.32–4.14 | 3.3–4.34 |
| Range | 0–18 | 0–31 | 0–14 | 0–63 | 0–13 | 0–28 |
Abbreviations: AFP, acute flaccid paralysis; CI, confidence interval; SD, standard deviation; VPVs, Village Polio Volunteers.
DISCUSSION
The success of GPEI in drastically reducing the number of polio cases reported worldwide has been well documented [22–25]. Much of this success is predicated on the ability of countries to track and detect the emergence of new polio cases in places with active circulation of WPV based on surveillance for AFP cases. Systems for AFP surveillance are particularly crucial in countries like Somalia, where the dilapidated healthcare infrastructure makes for increased vulnerability to polio outbreaks despite the elimination of indigenous WPV transmission. The establishment of the VPV program to supplement existing structures in place for AFP surveillance has proved to be of vital importance in enhancing the country’s capacity to mount an effective response to outbreaks and maintain its polio-free status.
Introduction of the VPV program in the middle of the 2013–2014 polio outbreak in Somalia not only helped facilitate efforts to interrupt the outbreak, which ended in August 2014 [26], it has also ensured sustenance of a number of measures taken to strengthen AFP surveillance activities by the national polio program. Before the outbreak, the national NPAFP rate had hovered around 3 cases per 100 000 persons aged <15 years, above the recommended benchmark of ≥2 cases but masking subnational gaps that resulted in delayed detection of some of the cases identified during the outbreak. Intensified surveillance activities during the outbreak led to a 132% increase in the NPAFP rate in 2013. Although much of the improvement in case detection observed during the outbreak was the direct result of increased resources for response activities provided by GPEI, as recommended in the guidelines for outbreak response [27], the role of VPVs during and after the response cannot be overstated. Compared with the 194 laboratory-confirmed WPV cases identified in 2013, only 5 WPV cases were identified in 2014. However, despite the recession and eventual cessation of the outbreak in 2014, the NPAFP rate rose from levels in 2013, largely owing to the contributions of the newly introduced VPV program. Of note, the last WPV case identified during the outbreak was reported by a VPV working in the Northeast zone of the country.
After the end of the outbreak, NPAFP rates have declined from levels during the outbreak, but they have remained significantly above preoutbreak levels. The NPAFP rate for 2015 (the year after the outbreak) was 1.7 times that in 2012, the year before outbreak onset. Although we could not determine from our data whether AFP cases identified by VPVs could have otherwise been detected by other reporting sources, our findings strongly suggest that much of the improvement in surveillance has been driven by the activities of VPVs. Despite an expansion in the number of reporting sites by the national polio program, VPVs have accounted for the majority of AFP cases detected in Somalia over the past 3 years, that is, from 2014 to 2016. This effect is even more pronounced when we limited our analysis to districts with security and access limitations, where VPVs have accounted for more than half of the AFP cases identified during 2015–2016.
With respect to other key surveillance indicators, stool specimen adequacy rates remained stable and above the recommended level during the period under review. No significant differences were observed in the adequacy of samples collected from cases reported by VPVs compared with those reported by other sources, including more experienced polio program staff. Timeliness of reporting standards, pegged at ≤7 days from paralysis onset, declined steeply from 90% to 70% in 2013, mostly owing to intensified surveillance activities and late reporting of cases that were previously undetected by the surveillance system. Notification speeds have since improved, with >80% of cases being reported within 7 days during 2014–2016. More importantly, cases are being reported faster by both VPVs and other sources, as the mean interval from paralysis onset to reporting of a case improved from 5.4 days in 2014 to 3.7 days in 2016 for VPVs and from 4.8 to 3.8 days for other sources.
Community-based surveillance programs, such as the VPV program, encourage a paradigm shift from passive reporting to active detection of diseases of public health significance. The success of these programs in improving AFP case detection has been demonstrated in countries such as Niger and Tanzania [28, 29]. Given the increasing threat posed by insecurity to polio eradication efforts in places like Nigeria [30] and Afghanistan [9, 31], such programs will play an increasingly prominent role in bringing the goal of disease eradication within reach. Although approaches such as using the military in polio vaccination activities have been adopted in countries like Angola [32] and Pakistan [33], community-based programs are better suited to building trust [34] and ensuring sustainability in countries with weak healthcare systems [28]. Building trust at the grassroots level through community-based programs is beneficial on multiple levels because programs like the VPV program often evolve to encompass other disease surveillance and prevention activities. Just as GPEI manpower resources have played a crucial role in strengthening laboratory, disease surveillance, and outbreak response capacity in many countries in Africa [29, 35–37], VPV program assets could eventually transition into broader roles aimed at surveilling and preventing other vaccine-preventable and endemic diseases, such as measles and malaria.
The VPV program has contributed significantly to improved surveillance for AFP cases in Somalia. In the near term, this improvement enhances the ability of the country’s polio program to detect and respond to new polio outbreaks, albeit limited by the reliance of AFP surveillance on the emergence of paralytic polio cases to determine the circulation of poliovirus in an area. Improved AFP surveillance will also serve as a key factor for certification of the country and continent as polio free when the disease is eventually eliminated from Africa. In the long run, such a program could help ameliorate deficiencies in the country’s healthcare system for the prevention and control of other diseases of public health significance.
Acknowledgments.
We acknowledge the contributions of the national polio program staff and the Village Polio Volunteers in Somalia to the success of this project.
Financial support.
This project was funded and supported by the US Centers for Disease Control and Prevention.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Global Polio Eradication Initiative (GPEI). Polio eradication and endgame strategic plan 2013–2018. Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed 2 January 2017.
- 2.Birmingham ME, Linkins RW, Hull BP, Hull HF. Poliomyelitis surveillance: the compass for eradication. J Infect Dis 1997; 175(suppl 1):S146–50. [DOI] [PubMed] [Google Scholar]
- 3.Wassilak SG, Oberste MS, Tangermann RH, Diop OM, Jafari HS, Armstrong GL. Progress toward global interruption of wild poliovirus transmission, 2010–2013, and tackling the challenges to complete eradication. J Infect Dis 2014; 210:S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010; 172:1213–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Momen AA, Shakurnia A. An epidemiological analysis of acute flaccid paralysis in Khuzestan Province, southwest Iran, from 2006 to 2010. Epidemiol Health 2016; 38:e2016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx A, Glass JD, Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiol Rev 2000; 22:298–316. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO). Recommended standards for surveillance of selected vaccine preventable diseases: vaccines and biologicals. Geneva, Switzerland: World Health Organization, 2003:31–34. [Google Scholar]
- 8.Morales M, Tangermann RH, Wassilak SG. Progress toward polio eradication—worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:470–3. [DOI] [PubMed] [Google Scholar]
- 9.Mbaeyi C, Saatcioglu A, Tangermann RH, Hadler S, Ehrhardt D. Progress toward poliomyelitis eradication—Afghanistan, January 2014–August 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1166–70. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). Certification of poliomyelitis eradication. Fifteenth meeting of the European Regional Certification Commission, Copenhagen, 19–21 June 2002. Available at: http://www.euro.who.int/__data/assets/pdf_file/0003/79374/E88105.pdf. Accessed 2 January 2017. [Google Scholar]
- 11.Pan American Health Organization. Weekly Poliovirus Surveillance Bulletin. Vol 9. Washington, DC: Pan American Health Organization, 20 August 1994. [Google Scholar]
- 12.Sousa IP Jr, Burlandy FM, Oliveira SS, et al. Acute flaccid paralysis laboratorial surveillance in a polio-free country: Brazil, 2005–2014. Hum Vaccin Immunother 2016; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan P, O’Lorcain P, Cotter S, et al. Reporting of acute flaccid paralysis in children under 15 years of age: improving surveillance, January 2009–December 2014. Ir Med J 2016; 109:357. [PubMed] [Google Scholar]
- 14.Pellegrinelli L, Bubba L, Primache V, et al. Surveillance of poliomyelitis in Northern Italy: results of acute flaccid paralysis surveillance and environmental surveillance, 2012–2015. Hum Vaccin Immunother 2016; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogka V, Labropoulou S, Emmanouil M, et al. Laboratory surveillance of polio and other enteroviruses in high-risk populations and environmental samples. Appl Environ Microbiol 2017; 83:e02872–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünewald F Aid in a city at war: the case of Mogadishu, Somalia. Disasters 2012; 36(suppl 1):S105–25. [DOI] [PubMed] [Google Scholar]
- 17.Mbaeyi C, Kamadjeu R, Mahamud A, Webeck J, Ehrhardt D, Mulugeta A. Progress toward polio eradication—Somalia, 1998–2013. J Infect Dis 2014; 210(suppl 1):S173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO). Somalia is again polio-free. Wkly Epidemiol Rec 2008; 83:117–8. [PubMed] [Google Scholar]
- 19.Kamadjeu R, Mahamud A, Webeck J, et al. Polio outbreak investigation and response in Somalia, 2013. J Infect Dis 2014; 210(suppl 1):S181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahamud A, Kamadjeu R, Webeck J, et al. Effectiveness of oral polio vaccination against paralytic poliomyelitis: a matched case-control study in Somalia. J Infect Dis 2014; 210(suppl 1):S187–93. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CH, Mahamud A, Safdar RM, et al. Progress toward poliomyelitis eradication—Pakistan, January 2015–September 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1295–9. [DOI] [PubMed] [Google Scholar]
- 22.Cochi SL, Jafari HS, Armstrong GL, et al. A world without polio. J Infect Dis 2014; 210(suppl 1):S1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallansch MA, Sandhu HS. The eradication of polio—progress and challenges. N Engl J Med 2006; 355:2508–11. [DOI] [PubMed] [Google Scholar]
- 24.Moturi EK, Porter KA, Wassilak SG, et al. ; EIS officer, Centers for Disease Control and Prevention. Progress toward polio eradication—worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep 2014; 63:468–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine 2011; 29(suppl 4):D80–5. [DOI] [PubMed] [Google Scholar]
- 26.Hagan JE, Wassilak SG, Craig AS, et al. ; Centers for Disease Control and Prevention (CDC). Progress toward polio eradication—worldwide, 2014–2015. MMWR Morb Mortal Wkly Rep 2015; 64:527–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Global Polio Eradication Initiative (GPEI). Responding to a poliovirus outbreak: standard operating procedures for a new polio outbreak in a polio-free country. Available at: http://polioeradication.org/wp-content/uploads/2016/07/9.5_13IMB.pdf. Accessed 2 January 2017.
- 28.Ndiaye SM, Quick L, Sanda O, Niandou S. The value of community participation in disease surveillance: a case study from Niger. Health Promot Int 2003; 18:89–98. [DOI] [PubMed] [Google Scholar]
- 29.Kamso J, Mvika ES, Ota MO, Okeibunor J, Mkanda P, Mihigo R. The contribution of the polio eradication initiative to narrowing the gaps in the health workforce in the African Region. Vaccine 2016; 34:5150–4. [DOI] [PubMed] [Google Scholar]
- 30.Bigna JJ. Polio eradication efforts in regions of geopolitical strife: the Boko Haram threat to efforts in sub-Saharan Africa. Afr Health Sci 2016; 16:584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mbaeyi C, Shukla H, Smith P, et al. Progress toward poliomyelitis eradication—Afghanistan, January 2015–August 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1195–9. [DOI] [PubMed] [Google Scholar]
- 32.Fekadu L, Okeibunor J, Nsubuga P, Kipela JM, Mkanda P, Mihigo R. Reaching the unreached with polio vaccine and other child survival interventions through partnership with military in Angola. Vaccine 2016; 34:5155–8. [DOI] [PubMed] [Google Scholar]
- 33.Hussain SF, Boyle P, Patel P, Sullivan R. Eradicating polio in Pakistan: an analysis of the challenges and solutions to this security and health issue. Global Health 2016; 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee LM, Thacker SB. Public health surveillance and knowing about health in the context of growing sources of health data. Am J Prev Med 2011; 41:636–40. [DOI] [PubMed] [Google Scholar]
- 35.Kouadio K, Okeibunor J, Nsubuga P, Mihigo R, Mkanda P. Polio infrastructure strengthened disease outbreak preparedness and response in the WHO African Region. Vaccine 2016; 34:5175–80. [DOI] [PubMed] [Google Scholar]
- 36.Mwengee W, Okeibunor J, Poy A, et al. Polio Eradication Initiative: contribution to improved communicable diseases surveillance in WHO African Region. Vaccine 2016; 34:5170–4. [DOI] [PubMed] [Google Scholar]
- 37.Gumede N, Coulibaly SO, Yahaya AA, et al. Polio Eradication Initiative (PEI) contribution in strengthening public health laboratories systems in the African Region. Vaccine 2016; 34:5164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


