Abstract
This study aimed to evaluate the changes in Agaricus bisporus (white and brown) characteristics (colour and acidity parameters, lactic acid bacteria (LAB) and mould/yeast counts, biogenic amine content, fatty acid (FA) and volatile compound (VC) profiles, overall acceptability, and emotions induced for consumers) during a 48 h lactic acid fermentation with Lacticaseibacillus casei No. 210, Lactiplantibacillus plantarum No. 135, Lacticaseibacillus paracasei No. 244, and Pediococcus acidilactici No. 29 strains. Fermented white and brown A. bisporus showed higher LAB count and lower pH, lightness, redness, and yellowness than non-fermented ones. Yeast and fungi counts were similar between non-fermented and fermented samples. All samples contained spermidine (on average, 191.5 mg/kg) and some of the fermented samples had tyramine (on average, 80.7 mg/kg). Saturated FA was the highest in non-fermented brown A. bisporus. The highest monounsaturated and polyunsaturated FA contents were found in Lp. plantarum No. 135 fermented white and brown A. bisporus, respectively. For the first time, the VC profile of fermented A. bisporus was analysed. 1-Octen-3-ol content significantly decreased while benzyl alcohol, acetoin, and 2,3-butanediol increased in most fermented samples. Fermented A. bisporus received good acceptability scores. The emotional evaluation showed that the LAB strain and the interaction of the LAB strain and A. bisporus variety were significant on the intensity of emotions “happy” and “sad”, while all analysed factors and their interactions were significant on the intensity of “angry” and “disgusted” (p ≤ 0.05). The findings of this study show the potential of the selected LAB strains and contribute to the increasing body of research on fermented mushrooms.
Keywords: button mushrooms, lactic acid bacteria, fermentation, aroma compounds, fatty acids, emotions
1. Introduction
Edible mushrooms have been regarded as nutritional food, nutraceuticals, pharmaceuticals, and food flavour agents due to their rich chemical composition and health benefits [1]. Agaricus bisporus is the most prevalent commercially grown mushroom in the entire world and accounts for 35–45% of the global mushroom market, with Europe and North America being the primary producers [2]. Due to differences in cultivation substrates, phase of growth, and pre- and post-harvest circumstances, the nutrient profile of A. bisporus can vary [3]. A. bisporus consists of a high proportion of moisture and is high in protein (11.0–29.1%) and carbohydrate (51.1%) [4]. These mushrooms contain nine essential amino acids and are a source of non-starch polysaccharides (β-glucans, chitin, and mannans), oligosaccharides, minerals (K, P, Ca, Na, Zn, Mg, Fe, etc.), and vitamins D, C, B1, B2, and B3 [5]. Although A. bisporus has a low percentage of fat, it does contain some essential fatty acids including linoleic acid [4]. A. bisporus contains biologically active substances that have been proven to have anticancer, antioxidant, antidiabetic, antibacterial, and anti-inflammatory properties [4].
Immature A. bisporus with a white or brown cap (chestnut mushroom) and mature A. bisporus, so-called Portobello mushrooms, are supplied to the global market [3]. The popularity of brown A. bisporus is growing since most customers believe they taste better [6]. However, this type of A. bisporus is more susceptible to enzymatic browning during storage [6]. Because A. bisporus lacks a protective cuticle layer but has a high protein, moisture, respiration rate, and enzymatic activity, these mushrooms are difficult to keep stable during storage [7]. Various thermal (drying, cooling), chemical (coating, ozone, washing with antimicrobial agents), and physical (packaging, irradiation, ultrasound) preservation techniques are used to improve the storage stability of fresh A. bisporus, but they all have their limitations [3]. As processed food, mushrooms are usually sterilized, cooked, marinated, or fried. These treatments can have a considerable impact on the nutritional value (e.g., improved protein digestibility) and physical qualities of mushrooms, as well as an adverse effect on their bioactive compounds content (e.g., loss of vitamins, antioxidants, and other nutrients) [8].
Fermentation is one of the most ancient, time- and cost-effective ways of preserving food [9]. It was reported that lactic acid bacteria (LAB) are used for the production of fermented vegetables and juices (from fruits and vegetables), and this type of functional food is quite attractive for vegetarians and everyone who is seeking a healthy lifestyle [10,11]. Lactic acid fermentation of mushrooms is prevalent in Eastern European countries and Southeast Asia, but it is not widely employed on an industrial basis [12,13]. This method could be a sustainable biological way to not only preserve but also improve the functionality of edible mushrooms. LAB are safe microorganisms (labelled with GRAS status by the US Food and Drug Administration) and can possess antibacterial and antifungal activities, degrade anti-nutritional factors, improve digestibility and flavour of the fermented product, and enrich it with new volatile and bioactive (vitamins, peptides, exopolysaccharides, enzymes and etc.) compounds [14]. Moreover, each LAB strain may possess different technological, functional, and beneficial properties, which affect the attributes and safety of the final fermented product [15]. LAB are employed in a wide range of commercial food fermentations around the world [9]. Lactiplantibacillus plantarum is commonly employed in the food industry considering its ability to enhance antioxidant activity and to improve the flavour of food [16]. Lacticaseibacillus casei and Lacticaseibacillus paracasei are two of the most studied and used probiotic bacteria [17], while Pediococcus acidilactici is a potent producer of bacteriocins as well as a potential probiotic strain, used for the improvement of flavour and texture [18]. In contrast to spontaneous fermentation, the application of starter cultures prevents the proliferation of undesirable microorganisms, shortens the duration of the process, and simplifies product standardization [19]. However, attention should be paid to biogenic amine formation in fermented food because these compounds can be produced by microorganisms, including LAB, and, in high concentration, may elicit adverse effects on human health [20].
The changes in various mushroom characteristics during fermentation with LAB have been rarely reported. In the study of Jabłońska-Ryś et al. [19], changes in sugars and organic acid profile were found during A. bisporus fermentation with Lp. plantarum 299v and Lp. plantarum EK3, while the latter strain improved the organoleptic properties of fermented mushrooms. The same LAB strains were used in Jabłońska-Ryś et al. [21]’s studies for the fermentation of white and brown button mushrooms, where authors examined the colour, texture, and biogenic amine content. Skrzypczak et al. [13] used spontaneous fermentation of white button A. bisporus for LAB isolation. Radzki et al. [22] reported that Lp. plantarum fermentation of Pleurotus ostreatus mushroom polysaccharide resulted in considerable modifications in its chemical composition.
This study aimed to evaluate the changes in A. bisporus (white and brown varieties) mushroom characteristics (colour and acidity parameters, LAB and mould/yeast counts, biogenic amine content, fatty acid and volatile compound profiles, overall acceptability, and emotions induced for consumers) during the lactic acid fermentation with Lc. casei No. 210, Lp. plantarum No. 135, Lc. paracasei No. 244, and P. acidilactici No. 29 strains.
Most of the studies about fermented mushroom composition report changes in the main mushroom parameters without paying attention to the possible formation of undesirable compounds. Despite the fact that LAB has a GRAS status and lactic acid fermentation is an acceptable bio-preservation method for various foods, in this study, we would like to pay attention to the fact that some undesirable compounds can also be formed during this process. The main novelty of this study is based on the complex analysis, which includes both desirable and undesirable changes in the mushroom samples during fermentation. Additionally, as far as we know, No. 210, No. 135, No. 244, and No. 29 strains, previously isolated from spontaneously fermented cereals, were applied for the first time for A. bisporus mushroom fermentation. Taking into consideration that studies on biogenic amines in mushrooms are scarce and reported data on these compounds significantly differ among species, this study will lead to broader knowledge about the changes in edible mushrooms during lactic acid fermentation.
2. Materials and Methods
2.1. Edible Mushrooms and Lactic Acid Bacteria Strains Used for Fermentation
A. bisporus (white and brown varieties) mushrooms were obtained from the local supermarket in Kaunas (Lithuania). Fresh fruiting bodies were cleaned and boiled in water (200 g mushrooms in 500 mL of water) for 10 min with 6 g of salt. After boiling, mushrooms were cooled to room temperature (22 ± 2 °C) and used for fermentation. Non-fermented boiled mushrooms were analysed as control.
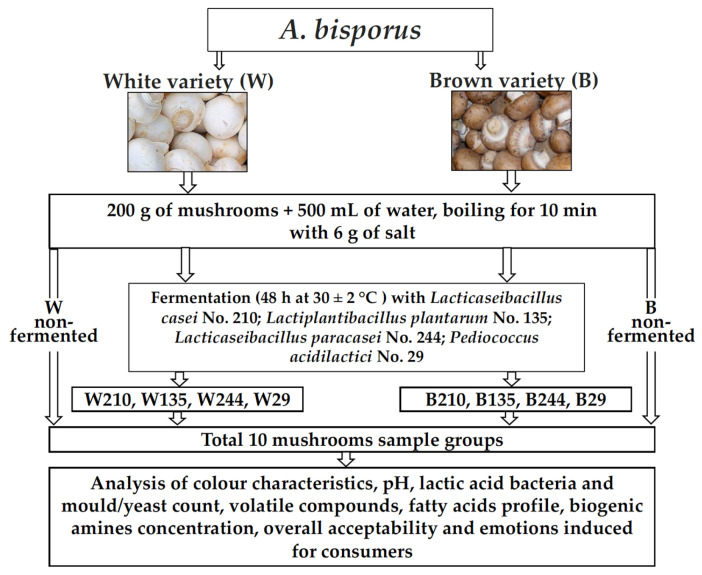
The LAB strains used in this study were previously isolated from spontaneously fermented cereal and showed a broad spectrum of antimicrobial and antifungal properties [23]. Lc. casei No. 210, Lp. plantarum No. 135, Lc. paracasei No. 244, and P. acidilactici No. 29 strains were multiplied in De Man, Rogosa, and Sharpe (MRS) broth culture medium (Biolife, Milan, Italy) at 30 °C under anaerobic conditions. Boiled mushrooms (200 g) were inoculated with 5 mL of LAB multiplied in MRS (average cell concentration 9.0 log10 CFU/mL) followed by fermentation for 48 h at 30 ± 2 °C. The principal scheme of the experiment is shown in Figure 1.
Figure 1.
Principal scheme of the experiment (W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain).
2.2. Analysis of the Mushrooms’ Colour and Acidity Characteristics, Lactic Acid Bacteria, and Mould/Yeast Counts
Before measuring the mushrooms’ colour characteristics and pH, samples were homogenized using a blender.
The colour coordinates (L*, a*, b*) were assessed using a CIELAB system (Chromameter CR-410, Konica Minolta, Tokyo, Japan) [24].
The pH was measured using a pH electrode (PP-15, Sartorius, Goettingen, Germany) [19].
The total titratable acidity (TTA, °N) was measured according to ISO 750:1998 standard [25]. TTA was determined for a 10 g of mushroom sample homogenized with 90 mL of distilled water and expressed as the volume, in mL, of 0.1 mol/L NaOH required to achieve a pH of 8.2.
The evaluation of the LAB count was performed according to ISO 15214:1998 standard [26]. 10 g of sample was homogenised with 90 mL of aqueous saline (9 g/L NaCl solution). Serial dilutions of 10−4 to 10−8 with the same saline were prepared for inoculation. Sterile MRS (Man, Rogosa, Sharpe) agar (CM0361, Oxoid, Basingstoke, UK) of 5 mm thickness was used for bacterial growth in Petri dishes. The dishes were separately seeded with the sample suspension using surface sowing and were incubated under anaerobic conditions at 30 °C for 72 h. All results were expressed in log10 of colony-forming units per gram (log10 CFU/g).
Yeasts and fungi count in edible mushroom samples were counted on chloramphenicol agar (CM0549, Oxoid, UK) after incubation at 25 °C for 5 days [27]. All experiments were carried out in triplicate, and the number of microorganisms was expressed in log10 CFU/g.
2.3. Determination of the Mushrooms’ Volatile Compounds (VC)
The VC in edible mushrooms was analysed by gas chromatography–mass spectrometry (GC-MS) according to Bartkiene et al. [28] with some modifications. A solid phase microextraction (SPME) device with Stableflex™ fibre coated with a 50 µm PDMS-DVB-Carboxen™ layer (Supelco, Bellefonte, PA, USA) was used for analysis. Before the experiment, mushroom samples were homogenized using a blender. For headspace extraction, 1 g of homogenized mushrooms was added to the 20 mL extraction vial which was sealed with polytetrafluoroethylene septa and thermostatted at 60 °C for 15 min. The fibre was then exposed to the headspace of the vial for 10 min and desorbed in the injector liner for 2 min (splitless injection mode). Prepared samples were analysed with a GCMS-QP2010 gas chromatograph (Shimadzu, Japan) and a mass spectrometer. The following conditions were used for analysis: injector temperature 250 °C, ion source temperature 220 °C, interface temperature 280 °C. Helium (99.999% detector purity; AGA, Vilnius, Lithuania) was used as carrier gas at a flow rate of 0.97 mL/min. The Rxi®-5MS capillary column (0.25 mm ID, 0.25 μm film thickness, 30 m length; Restek, Centre County, PA, USA) was used for analysis. The temperature gradient was programmed from a start at 35 °C (5 min hold) to 200 °C (10 °C/min) up to 280 °C (25 °C/min) (5 min hold). The VC was identified according to mass spectra libraries (NIST11, NIST11S, FFNSC2).
2.4. Analysis of the Mushrooms’ Fatty Acid Profile
The extraction of lipids for fatty acids (FA) analysis was performed with chloroform/methanol (2:1 v/v), and FA methyl esters (FAME) were prepared according to Pérez-Palacios et al. [29] with some modifications. The FA composition of samples was identified using a gas chromatograph GC-2010 Plus (Shimadzu Europa GmbH, Duisburg, Germany) equipped with Mass Spectrometer GCMS-QP2010 (Shimadzu Europa GmbH, Duisburg, Germany). Separation was carried out on a Stabilwax-MS column (30 m length, 0.25 mmID, and 0.25 μm df) (Restek Corporation, Bellefonte, PA, USA). Oven temperature program started at 50 °C, then increased at a rate of 8 °C/min to 220 °C, held for 1 min at 220 °C, increased again at a rate of 20 °C/min to 240 °C and, finally, held throughout 10 min. The injector temperature was 240 °C, the interface −240 °C, and the ion source 240 °C. The carrier gas was helium at a flow rate of 0.91 mL/min. The individual FAME peaks were identified by comparing their retention times with FAME standards (Merck & Co., Inc., Kenilworth, NJ, USA).
2.5. Analysis of Biogenic Amine Content in Mushrooms
Sample preparation and determination of biogenic amines (BA) in mushroom samples were performed according to the method of Ben-Gigirey et al. [30], with some modifications described by Bartkiene et al. [31]. Before the experiment, mushroom samples were homogenized using a blender. The following BA were analysed: tryptamine, phenylethylamine, cadaverine, putrescine, histamine, tyramine, spermine, and spermidine. The standard BA solutions were prepared by dissolving known amounts of each BA (including internal standard—1.7-diamino-heptane) in 20 mL of deionised water. Briefly, 5 g of sample were extracted with 10 mL of perchloric acid (0.4 mol/L) twice. The derivatization of sample extracts and standards was performed using a dansyl chloride solution in acetonitrile (10 mg/mL) as a reagent. A Varian ProStar HPLC system (Varian Corp., Palo Alto, CA, USA) equipped with a ProStar 325 UV/VIS Detector and Galaxy software (Agilent, Santa Clara, CA, USA) was used for analysis. A Discovery® HS C18 column (150 × 4.6 mm, 5 µm; SupelcoTM Analytical, Bellefonte, PA, USA) was used to separate BA. Ammonium acetate (0.1 mol/L) and acetonitrile were used as the mobile phases at a flow rate of 0.8 mL/min. The sample volume injected was 20 µL and the amines were monitored at 254 nm. The BA were identified based on their retention times in comparison to their corresponding standards.
2.6. Evaluation of the Overall Acceptability and Emotions Induced for Consumers by the Edible Mushrooms
The overall acceptability of the mushroom samples was evaluated by 10 trained judges (from 30 to 50 years old, 7 females and 3 males), according to the International Standards Organization method 8586-1 [32], using a 10-point scale ranging from 0 (‘dislike extremely’) to 10 (‘like extremely’). Samples were also tested by applying FaceReader 8.0 software (Noldus Information Technology, Wageningen, The Netherlands), scaling eight emotion patterns (“neutral”, “happy”, “sad”, “angry”, “surprised”, “scared”, “disgusted”, “contempt”) according to the procedure described by Bartkiene et al. [28]. Each emotion’s intensity was represented on a scale of 0 (no emotion) to 1 (highest intensity of emotion). The valence was calculated as the intensity of “happy” minus the intensity of the negative emotion with the highest intensity. The valence scale ran from −1 to 1.
2.7. Statistical Analysis
Results of the microbiological and physicochemical analyses were expressed as the mean (n = 9) ± standard error (SE). Fermentation of mushrooms was performed once by fermenting three parallel samples; all analyses of the parallel samples were performed in triplicate. Results of the overall acceptability and emotions induced for judges by the edible mushrooms were expressed as the mean (n = 10) ± standard error (SE). Results were analysed using the statistical package SPSS for Windows V15.0 (SPSS Inc., Chicago, IL, USA, 2007). A linear Pearson’s correlation was used to quantify the strength of the relationship between the variables. Results were recognized as statistically significant at p ≤ 0.05.
3. Results and Discussion
3.1. Mushrooms’ Colour Characteristics, pH, Lactic Acid Bacteria, and Mould/Yeast Count
Colour characteristics and acidity parameters of the non-fermented and fermented mushroom samples are shown in Table 1. In comparison to non-fermented samples’ colour coordinates, the white variety of A. bisporus showed higher L* and b* coordinates (on average, by 14.7 and 3.24%, respectively), but lower a* coordinates (on average, by 20.5%), in comparison with brown-variety samples. In all cases (both white and brown varieties of mushroom), fermented samples showed lower L*, a*, and b* coordinates, in comparison with non-fermented ones. Multivariate analysis of variance showed that the variety of mushrooms is a significant factor in samples’ a* coordinates (p = 0.005). However, the LAB used for fermentation and mushroom variety, and the interaction of these factors were not significant on L* and b* coordinates.
Table 1.
Colour characteristics and acidity parameters of the non-fermented and fermented mushroom samples.
| Mushroom Samples | L* | a* | b* | pH | TTA, °N |
|---|---|---|---|---|---|
| NBS | |||||
| Wnon-f | 76.7 ± 1.25 j | 6.28 ± 0.59 e | 18.5 ± 0.09 g | 6.80 ± 0.01 i | 0.10 ± 0.03 a |
| Bnon-f | 65.4 ± 1.14 i | 7.90 ± 0.63 f | 17.9 ± 0.11 f | 6.66 ± 0.03 h | 0.10 ± 0.02 a |
| W210 | 55.3 ± 0.98 h | 3.47 ± 0.28 b | 17.8 ± 0.13 f | 4.90 ± 0.02 c | 0.40 ± 0.03 d |
| B210 | 33.0 ± 0.29 b | 4.88 ± 0.24 c | 9.36 ± 0.08 a | 5.44 ± 0.03 f | 0.20 ± 0.01 b |
| W135 | 48.2 ± 0.47 f | 3.06 ± 0.21 a,b | 15.2 ± 0.10 e | 5.33 ± 0.01 e | 0.30 ± 0.02 c |
| B135 | 39.6 ± 0.32 c | 6.07 ± 0.19 e | 12.6 ± 0.08 c | 5.72 ± 0.02 g | 0.20 ± 0.01 b |
| W244 | 51.2 ± 0.49 g | 3.13 ± 0.18 b | 17.9 ± 0.09 f | 4.24 ± 0.01 a | 0.40 ± 0.03 d |
| B244 | 32.1 ± 0.31 a | 5.43 ± 0.14 d | 9.84 ± 0.06 b | 4.33 ± 0.03 b | 0.20 ± 0.01 b |
| W29 | 45.7 ± 0.43 e | 2.83 ± 0.11 a | 12.9 ± 0.09 d | 5.44 ± 0.01 f | 0.20 ± 0.02 b |
| B29 | 43.7 ± 0.36 d | 4.54 ± 0.23 c | 12.8 ± 0.05 d | 5.27 ± 0.02 d | 0.30 ± 0.01 c |
W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; TTA—total titratable acidity; °N—Neiman degree; L* lightness; a* redness or −a* greenness; b* yellowness or −b* blueness; NBS—National Bureau of Standards units. Data expressed as mean values (n = 9) ± standard error (SE). a–j Mean values within the columns with different letters are significantly different (p ≤ 0.05).
This study’s results confirmed the data published by other authors, which indicate that lactic fermentation influences the colour parameters of mushrooms and reduces the lightness of mushroom fruiting bodies [12]. During lactic fermentation, also, significant changes in the a* and b* colour coordinates can be obtained [33]. LAB can produce a variety of metabolites, including various organic acids, short-chain fatty acids, amines, bacteriocins, vitamins, exopolysaccharides, etc. [34]. Additionally, LAB excretes a variety of enzymes [35], which are involved in fermentable substrate modification. Finally, these complex mechanisms can also be involved in the degradation of coloured compounds. It was reported that the decrease in the L* coordinate could be related to enzymatic and non-enzymatic browning in mushrooms [36].
Comparing the samples’ acidity parameters, the lowest pH was found in samples fermented with the Lc. paracasei No. 244 strain (white variety-sample pH was 4.24, brown-variety sample pH was 4.33). However, the analysed factors (variety of mushrooms and LAB strain used for fermentation) and their interactions were not significant on sample pH. The highest TTA was observed in white mushroom samples fermented with Lc. casei No. 210 and Lc. paracasei No. 244 strains (0.40° N). Despite the fact that a correlation between samples pH and TTA was not established, moderate positive correlations between samples TTA with L* and b* coordinates were found (r = 0.612, p ≤ 0.001 and r = 0.497, p = 0.005).
Fresh mushrooms’ pH is neutral [37]. The pH decrease during the fermentation process is associated with the accumulation of organic acids [38,39]. As well, the organic acid production of different microorganisms, including LAB strains, varies according to their characteristics [40]. The mushroom’s fruiting body can consist of 35 to 70% carbohydrates [41]. It was reported that LABs are able to ferment most of the mushroom carbohydrates, and lactic fermentation of mushroom fruiting bodies is possible without the addition of sugar [12]. Fungal carbohydrates include monosaccharides (i.e., glucose) and disaccharides (i.e., trehalose), sugar alcohols (i.e., mannitol), polysaccharides, glycogen, and chitin [42]. The use of selected LAB strains for mushroom fermentation ensures a correct process [12]. This study showed that the lowest pH of the mushrooms can be obtained by applying, for their fermentation, the Lc. paracasei No. 244 strain, which, in our previous studies, showed characteristics to ferment a broad spectrum of carbohydrates [23]. Additionally, the use of selected LAB increases the health-beneficial value of mushrooms due to the presence of viable LAB cells [43]. LAB, yeast, and fungi counts in non-fermented and fermented mushroom samples are shown in Table 2.
Table 2.
Lactic acid bacteria, yeast, and fungi counts (log10 CFU/g) in non-fermented and fermented mushroom samples.
| Mushroom Samples | LAB Count, log10 CFU/g | Yeasts and Fungi Count, log10 CFU/g |
|---|---|---|
| Wnon-f | 1.56 ± 0.16 a | 1.32 ± 0.27 a |
| Bnon-f | 1.48 ± 0.17 a | 1.45 ± 0.18 a |
| W210 | 7.82 ± 0.22 d | 1.14 ± 0.21 a |
| B210 | 7.79 ± 0.11 d | 1.12 ± 0.29 a |
| W135 | 7.53 ± 0.15 c,d | 1.19 ± 0.22 a |
| B135 | 7.41 ± 0.18 c | 1.25 ± 0.23 a |
| W244 | 6.53 ± 0.22 b | 1.36 ± 0.37 a |
| B244 | 6.49 ± 0.19 b | 1.28 ± 0.14 a |
| W29 | 6.51 ± 0.14 b | 1.47 ± 0.25 a |
| B29 | 6.47 ± 0.17 b | 1.33 ± 0.14 a |
W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; LAB—lactic acid bacteria; CFU—colony forming units. Data expressed as mean values (n = 9) ± standard error (SE). a–d Mean values within the columns with different letters are significantly different (p ≤ 0.05).
In terms of LAB, in yeast and fungi counts in non-fermented mushrooms, significant differences were not established (on average, the LAB count in non-fermented mushrooms was 1.52 log10 CFU/g; yeast and fungi count in non-fermented mushrooms was 1.39 log10 CFU/g) (Table 2). Additionally, significant differences in non-fermented and fermented samples in yeast and fungi counts were not established; however, fermented samples showed significantly higher LAB counts in comparison with non-fermented ones. The highest LAB count was established in fermentation with Lc. casei No. 210 and in with Lp. plantarum No. 135 strains in white- and brown-variety mushroom samples (on average, 7.81 and 7.47 log10 CFU/g, respectively). The LAB count in samples fermented with Lc. paracasei No. 244 and P. acidilactici No. 29 strains was, on average, 6.50 log10 CFU/g.
It was reported that many LABs can be applied for mushroom fermentation, including, Lp. plantarum [43,44,45,46,47,48,49], Lactobacillus delbrueckii subsp. bulgaricus, Levilactobacillus brevis, Lc. casei, Lactobacillus helveticus, Lactiplantibacillus pentosus, Streptococcus lactis, Lactococcus lactis, Leuconostoc mesenteroides, and Propionibacterium freudenreichii [43,44,45,46,50]. Jabłonska-Ry’s et al. [33] reported that Lp. plantarum 299v strain contributed to a rapid reduction of the pH value of mushrooms, and the finished products showed a high number of viable LAB cells.
Venturini et al. [37] reported that in fresh mushrooms, the LAB count is lower, at 2.0 log10 CFU/g. However, yeast and mould co-existed with LAB in the process of fermentation [12]. The negative activity of yeast in the lactic fermentation process may lead to a decrease in fermentable substrate acidity [12]. However, these processes do not occur at pH 4.5 and below [51]. In our study, correlations between LAB count and acidity parameters, as well as between yeast and fungi count and acidity parameters were not found. However, the strain used for fermentation was a significant factor in LAB count in fermented samples (p = 0.004).
Microbial interactions play an important role in the diversity of microbial communities, which plays a key role in selecting functional microbiota and inhibiting the growth of non-desirable microorganisms. However, little is known about microbial interactions during the process of mushroom fermentation, which makes it difficult to control and manage. Although our previous studies showed that the LAB strains used in this experiment possess antifungal properties against some fungi (previously isolated from cereal), it can be that fungi that are present in mushroom masses are resistant to these LAB metabolites. Further studies are needed to indicate which fungi are predominant in mushrooms and to select the most appropriate LAB for their inhibition.
3.2. Biogenic Amine Formation in Fermented Mushroom Samples
Tryptamine, phenylethylamine, putrescine, cadaverine, and spermine were not found in mushroom samples (Table 3). However, in white- and brown-variety mushroom samples fermented with Lc. casei No. 210 strain and in white variety mushroom samples fermented with Lc. plantarum No. 135 strain, tyramine was formed (on average, tyramine content was 82.7 mg/kg). The main BA in non-fermented and fermented mushroom samples was spermidine. However, the analysed factors were not significant on spermidine content in mushroom samples.
Table 3.
Biogenic amines concentration (mg/kg) in mushroom samples.
| Mushroom Samples | Biogenic Amine, mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tryp-Tamine | Phenyl-Ethylamine | Putrescine | Cada-Verine | Histamine | Tyramine | Spermi-Dine | Spermine | Total Content | |
| Wnon-f | nd | nd | nd | nd | nd | nd | 183.9 ± 9.32 a,b | nd | 183.9 |
| Bnon-f | nd | nd | nd | nd | nd | nd | 181.2 ± 8.54 a | nd | 181.2 |
| W210 | nd | nd | nd | nd | nd | 75.1 ± 6.58 a | 184.9 ± 6.98 a | nd | 260.0 |
| B210 | nd | nd | nd | nd | nd | 85.5 ± 7.89 a | 203.3 ±10.5 b | nd | 288.7 |
| W135 | nd | nd | nd | nd | nd | 87.6 ± 7.74 a | 180.0 ± 8.69 a | nd | 267.6 |
| B135 | nd | nd | nd | nd | nd | nd | 222.3 ± 8.56 b | nd | 222.3 |
| W244 | nd | nd | nd | nd | nd | nd | 187.1 ± 9.54 a,b | nd | 187.1 |
| B244 | nd | nd | nd | nd | nd | nd | 186.9 ± 7.54 a,b | nd | 186.9 |
| W29 | nd | nd | nd | nd | nd | nd | 181.6 ± 9.76 a | nd | 181.6 |
| B29 | nd | nd | nd | nd | nd | nd | 203.3 ± 11.35 b | nd | 203.3 |
W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; nd—not determined. Data expressed as mean values (n = 9) ± standard error (SE). a–b Mean values within the columns with different letters are significantly different (p ≤ 0.05).
The amount of protein and the dry mass of fungal fruiting bodies could vary from 20 to 40% [12]. Our previous studies showed that fermentation as well as ultrasonication could increase BA content in wild mushrooms [31]. Additionally, the presence of putrescine and cadaverine in fresh fruiting bodies of fungi was reported [52,53]. In general, BA synthesis is related to the presence of decarboxylating microorganisms in all foods that contain proteins or free amino acids [20]. Usually, high concentrations of BA in non-fermented foods indicate undesired spoilage and microorganism activity. Despite the fact that LABs are generally recognized as safe microorganisms, BA can be formed by LAB decarboxylase in protein-rich fermented foods [54]. Therefore, the presence of slight quantities of BA in fermented foods is expected. In low concentrations, BA acts as neuromodulators, neurotransmitters, and antioxidants and is important for fertility and protein biosynthesis [20]. However, at higher concentrations, BA causes major food poisoning and other health issues such as headaches, allergies, diarrhoea, raised blood pressure, and cell proliferation [54]. The toxicological effects of BA are mainly associated with tyramine and histamine [55]. The concentration and type of BA in fermentable substrates are related to many factors, including amino acid content and composition, technological conditions, etc. [55]. The acidity of the substrate is also an important factor in the formation of BA [20]. In this study, it was found that the LAB strain used for fermentation is a significant factor in tyramine content in mushrooms (p ≤ 0.001). Positive moderate correlations were found between TTA and tyramine (r = 0.463, p = 0.010), as well as between TTA and spermidine (r = 0.710, p ≤ 0.001).
Certain BA, including tyramine, can be produced by LAB species in different fermented foods [20]. It was reported that spermidine could be formed from spermine and tyramine from tyrosine [56]. The total BA content in food should not exceed 750–900 mg/kg [57]. According to the results obtained in the present study, all tested samples were below this range. Studies on BA in mushrooms are scarce and reported concentrations of these compounds significantly differ among species [58,59,60]. Jabłonska-Ry’s et al. [59] reported that spermidine and putrescine are the most common BA in mushrooms. Reis et al. [60]’s study showed that commercial mushroom species (fresh) could contain spermidine, phenylethylamine, tyramine, and tryptamine. Jabłońska-Ryś et al. [21] only found spermidine, tyramine, and putrescine in button mushrooms fermented with Lp. plantarum 299v, while tyramine was not detected in samples fermented with Lp. plantarum EK3. Finally, this study showed that it is very important to select appropriate LAB strains for the fermentation of edible mushrooms to ensure their safety in the case of BA formation.
3.3. Fatty Acid Profile of the Non-Treated and Fermented Mushrooms
The fatty acid (FA) content (% of the total fat content) of the mushroom samples is shown in Table 4. The main FA in A. bisporus mushrooms was linoleic (C18:2), and the highest C18:2 content was established with Lp. plantarum No. 135 strain-fermented brown-variety samples (80.6% of the total fat content). Additionally, a positive moderate correlation between C18:2 and sample TTA was established (r = 0.777, p ≤ 0.001). Palmitic acid (C16:0) content in mushroom samples FA profile varied from 11.0 to 13.0% from the total fat content (in with Lp. plantarum No. 135 strain fermented brown variety samples and in with Lc. casei No. 210 strain fermented white variety samples, respectively). C16:0 also showed a positive moderate correlation with samples TTA (r = 0.781, p ≤ 0.001). The highest 9-octadecenoic acid (C18:1) content was found in Lp. plantarum No. 135 strain-fermented white-variety samples (10.6% of the total fat content). However, with the same strain-fermented brown-variety samples showed the lowest C18:1 content (3.44% of the total fat content). Between C18:1 content and LAB count in mushrooms, a negative weak correlation was established (r = −0.367, p = 0.046). Non-fermented brown-variety A. bisporus mushrooms showed the highest palmitoleic (C16:1) and stearic (C18:0) fatty acids content. C18:0 content in samples showed a positive weak correlation with yeast and fungi count (r = 0.372, p = 0.043). Eicosanoic (C20:0) and docosanoic (C22:0) FAs were found in 4 out of 10 tested mushroom samples, in non-fermented brown-variety mushrooms, in Lc. casei No. 210 strain-fermented white- and brown-variety mushrooms, and in Lp. plantarum No. 135 strain fermented brown variety samples. Multivariate analysis of variance showed that mushroom variety was a significant factor in C22:0 content (p ≤ 0.001); the LAB strain used for fermentation was a significant factor in C16:1, C20:0, and C22:0 content in mushrooms (p = 0.008, p = 0.001, p ≤ 0.001, respectively), and as the analysed factors’ interactions were significant on C20:0 and C22:0 content in mushrooms (p = 0.007 and p ≤ 0.001, respectively).
Table 4.
Fatty acid content (% of the total fat content) of the mushroom samples.
| Fatty Acids | Wnon-f | Bnon-f | W210 | B210 | W135 | B135 | W244 | B244 | W29 | B29 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid Content, % of the Total Fat Content | ||||||||||
| C16:0 | 12.3 ± 0.03 b | 12.3 ± 0.02 b | 13.0 ± 0.02 f | 12.4 ± 0.04 c | 12.4 ± 0.03 c | 11.0 ± 0.02 a | 12.5 ± 0.03 d | 12.3 ± 0.02 b | 12.7 ± 0.03 e | 12.4 ± 0.02 c |
| C16:1 | nd | 0.625 ± 0.005 c | 0.205 ± 0.002 a | 0.376 ± 0.004 b | nd | nd | nd | nd | nd | nd |
| C18:0 | 2.07 ± 0.05 b | 3.12 ± 0.06 g | 2.33 ± 0.03 d | 2.70 ± 0.06 f | 1.75 ± 0.04 a | 2.58 ± 0.06 e | 2.23 ± 0.04 c | 2.17 ± 0.07 b,c | 1.69 ± 0.02 a | 2.76 ± 0.06 f |
| C18:1 | 9.99 ± 0.05 h | 9.59 ± 0.07 g | 7.88 ± 0.05 f | 5.57 ± 0.04 b | 10.6 ± 0.09 i | 3.44 ± 0.05 a | 6.46 ± 0.07 d | 6.73 ± 0.06 e | 10.1 ± 0.09 h | 6.08 ± 0.05 c |
| C18:2 | 75.6 ± 0.31 c | 72.2 ± 0.28 a | 74.9 ± 0.33 b | 76.9 ± 0.19 d | 75.3 ± 0.24 b,c | 80.6 ± 0.43 f | 78.8 ± 0.29 e | 78.9 ± 0.31 e | 75.5 ± 0.42 b,c | 78.8 ± 0.27 e |
| C20:0 | nd | 1.11 ± 0.03 b | 0.830 ± 0.021 a | 1.04 ± 0.05 b | nd | 1.13 ± 0.04 b | nd | nd | nd | nd |
| C22:0 | nd | 1.13 ± 0.02 c | 0.905 ± 0.011 a | 1.05 ± 0.02 b | nd | 1.23 ± 0.01 d | nd | nd | nd | nd |
| Fatty acid profile (%) | ||||||||||
| SFA | 14.4 ± 0.04 b | 17.6 ± 0.05 h | 17.1 ± 0.03 f | 17.2 ± 0.04 g | 14.2 ± 0.01 a | 15.9 ± 0.03 e | 14.7 ± 0.04 c | 14.4 ± 0.06 b | 14.4 ± 0.04 b | 15.1 ± 0.03 d |
| MUFA | 9.99 ± 0.07 e | 10.2 ± 0.08 f | 8.09 ± 0.07 d | 5.94 ± 0.11 b | 10.6 ± 0.09 g | 3.44 ± 0.12 a | 6.46 ± 0.14 c | 6.73 ± 0.18 c | 10.1 ± 0.10 e,f | 6.08 ± 0.05 b |
| PUFA | 75.6 ± 0.25 c | 72.2 ± 0.32 a | 74.9 ± 0.41 b | 76.9 ± 0.38 d | 75.3 ± 0.29 b,c | 80.6 ± 0.52 f | 78.8 ± 0.47 e | 78.9 ± 0.33 e | 75.5 ± 0.48 b,c | 78.8 ± 0.36 e |
| Omega-3 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Omega-6 | 75.6 ± 0.48 b | 72.2 ± 0.31 a | 74.9 ± 0.47 b | 76.9 ± 0.44 c | 75.3 ± 0.38 b | 80.6 ± 0.51 e | 78.8 ± 0.39 d | 78.9 ± 0.61 d | 75.5 ± 0.52 b | 78.8 ± 0.54 d |
| Omega-9 | 9.99 ± 0.08 e | 10.2 ± 0.10 f | 8.09 ± 0.08 d | 5.94 ± 0.15 b | 10.6 ± 0.09 g | 3.44 ± 0.14 a | 6.46 ± 0.23 c | 6.73 ± 0.11 c | 10.1 ± 0.09 e,f | 6.08 ± 0.18 b,c |
W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; C16:0—Palmitic acid; C16:1—Palmitoleic acid; C18:0—Stearic acid; C18:1—9-Octadecenoic acid; C18:2—Linoleic acid; C20:0—eicosanoic acid; C22:0—Docosanoic acid; SFA—saturated fatty acids; MUFA—monounsaturated fatty acids; PUFA—polyunsaturated fatty acids; nd—not determined. Data expressed as mean values (n = 9) ± standard error (SE). a–i Mean values within the lines with different letters are significantly different (p ≤ 0.05).
In comparison to FA profiles of the mushroom samples, the highest saturated fatty acids (SFA) content was found in non-fermented brown variety mushroom samples (17.6% of the total fat content). However, the analysed factors and their interactions were not significant on SFA content in mushrooms. The highest monounsaturated fatty acid (MUFA) content was found in Lp. plantarum No. 135 strain-fermented white-variety samples (10.6% of the total fat content). The LAB strain used for fermentation, as well as the analysed factors’ interactions, were significant on MUFA content in mushroom samples (p = 0.035 and p = 0.030, respectively). The highest polyunsaturated fatty acid (PUFA) content was found in Lp. plantarum No. 135 strain-fermented brown-variety samples (80.6% of the total fat content); however, the analysed factors were not significant on PUFA content in mushrooms. Omega-3 FAs were not found in mushrooms. The highest omega-6 content was observed in brown-variety A. bisporus fermented with the Lp. plantarum No. 135 strain, and the highest omega-9 content was observed in white-variety mushrooms fermented with Lp. plantarum No. 135 strain. Mushroom variety was a significant factor in omega-9 content in samples (p = 0.001). Positive moderate correlations between samples TTA and SFA, PUFA, and omega-6 were established (r = 0.750, p ≤ 0.001; r = 0.777, p ≤ 0.001; and r = 0.777, p ≤ 0.001, respectively).
It was reported that mushroom lipids consist mainly of MUFAs and PUFAs, which are classified as healthy sources of lipids [61]. Although the crude fats in mushrooms are present only in small amounts, a considerable amount of linoleic acid is present in A. bisporus [62]. Stojković et al. [63] reported that A. bisporus lipids profile consists of myristic acid (C14:0) (0.58% of the total fat content), C16:0 (14.02% of the total fat content), C16:1 (0.42% of the total fat content), C18:0 (6.54% of the total fat content), C18:1n-9 (20.58% from the total fat content), C18:2n-6 (43.87% of the total fat content), α-linoleic acid (C18:3 n-3) (3.25% of the total fat content), C20:0 (3.16% of the total fat content), and C20:1 n-9 (0.08% of the total fat content).
According to Saiqa et al., the FA profile of A. bisporus comprises stearic acid, palmitic, linoleic, caprylic, oleic, erucic, and eicosanoic acids [64]. Additionally, it was reported that palmitic (12.67–14.71%) and linoleic (61.82–67.29%) FAs are the main FAs in A. bisporus [65]. As well, Shao et al. [66] reported that palmitic, linoleic, and stearic acids are the major FAs in A. bisporus’s lipid profile.
However, differences in FA profiles are obtained not just between the species but can also be related to a mushroom’s geographic origin [67]. However, mammals cannot synthesize linoleic and linolenic acids because they lack enzymes for ω-3 desaturation [62]. These FAs are essential and must be present in the human diet [68,69]. It was reported that A. bisporus from the Netherlands and Portugal showed the highest levels of omega-3 and -9 [63] and omega-6 [70], respectively. Muszyńska et al. [71] reported that in lyophilized mycelia of A. bisporus lipid profile, linoleic FA is predominant (43.9% of the total fat content). Therefore, mushrooms are good sources of essential FA omega-6 [72]. However, a complementary omega-3 source is required for a balanced diet [62].
It should be mentioned that the FA profile in cultivated and wild Agaricus sp. can vary [73] and the total FA contents can range from 180 to 5818 mg/kg DW. However, linoleic FA represents, on average, 90% of the A. bisporus fat content [74].
Additionally, the metabolism of LAB can favour lipid oxidation during fermentation or exert strong antioxidative effects [75]. Hydroperoxy linoleic FA is alternatively reduced to hydroxy-linoleic acid with concomitant oxidation of other substrate (in our study, mushrooms) constituents. In the presence of cysteine, peroxides are converted to the corresponding hydroxy-fatty acids [76]. Lactobacilli hydrate oleic, linoleic, and linoleic acids to hydroxyl fatty acids. Linoleic acid is converted to 13-hydroxy-9-octadecenoic acid or the antifungal 10-hydroxy-12-octadecenoic acid [76,77]. The reaction is catalysed by a fatty acid hydratase [78]. The results of this study can be valuable establishing a database about non-treated and fermented A. bisporus FA profiles, as well as their changes during fermentation.
3.4. Non-Treated and Fermented Mushrooms Volatile Compound Profiles
Volatile compound profiles of the mushroom samples (% of the total volatile compounds) are shown in Table 5. The main volatile compounds in mushroom samples were 1-octen-3-ol and benzyl alcohol. In comparison 1-octen-3-ol content, fermentation significantly reduced this volatile compound content in samples, and in Lp. plantarum No. 135 strain-fermented white-variety A. bisporus, 1-octen-3-ol was not detected. The LAB strain used for fermentation was a significant factor in 1-octen-3-ol content in mushrooms (p = 0.004). In comparing non-fermented white-variety mushrooms with fermented ones, different tendencies in the benzyl alcohol content were established. In white variety samples fermented with Lc. casei No. 210 and Lc. paracasei No. 244 strains, on average, 1.81 times higher benzyl alcohol content was found. However, in samples fermented with Lp. plantarum No. 135 strain, on average, 27.3% lower benzyl alcohol content was established in comparison with non-fermented samples. White-variety samples fermented with the P. acidilactici No. 29 strain showed similar benzyl alcohol content compared to non-fermented ones. When comparing non-fermented brown-variety samples with fermented ones, samples fermented with the Lc. casei No. 210 strain showed similar benzyl alcohol content compared to non-fermented ones; in samples fermented with the Lp. plantarum No. 135 strain, on average, 37.5% lower benzyl alcohol content was established; samples fermented with Lc. paracasei No. 244 and P. acidilactici No. 29 strains showed, on average, 34.1 and 41.3%, respectively, higher content of benzyl alcohol in comparison with non-fermented samples. The analysed factors and their interactions were not significant in benzyl alcohol content in mushroom samples.
Table 5.
Volatile compounds of the mushroom samples (% of the total volatile compounds).
| RT, min | Volatile Compounds | Mushroom Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wnon-f | Bnon-f | W210 | B210 | W135 | B135 | W244 | B244 | W29 | B29 | ||
| 4.41 | Acetoin | nd | nd | nd | 5.42 ± 0.48 a | 13.5 ± 1.14 b | 23.5 ± 2.22 c | nd | nd | nd | nd |
| 6.39 | 2,3-Butanediol | nd | nd | nd | 22.4 ± 2.25 b | 32.2 ± 3.29 c | 17.6 ± 0.16 a | nd | nd | 36.8 ± 2.41 c | nd |
| 11.78 | Benzaldehyde | 7.40 ± 0.41 e | 9.57 ± 0.52 f | 3.65 ± 0.34 d | 1.97 ± 0.15 b | 0.377 ± 0.041 a | 2.46 ± 0.25 c | 3.77 ± 0.38 d | 6.70 ± 0.54 e | nd | 4.14 ± 0.35 d |
| 12.43 | 1-Octen-3-ol | 33.6 ± 2.95 g | 20.8 ± 1.95 f | 0.819 ± 0.045 a | 2.89 ± 0.28 c,d | nd | 3.20 ± 0.27 d | 1.31 ± 0.12 b | 2.50 ± 0.22 c | 1.22 ± 0.11 b | 4.19 ± 0.36 e |
| 12.66 | 3-Octanone | 7.55 ± 0.53 f | 7.88 ± 0.47 f | 0.819 ± 0.056 b | 2.37 ± 0.25 d | 0.253 ± 0.018 a | 5.10 ± 0.49 e | 1.16 ± 0.15 c | 2.20 ± 0.21 d | 2.25 ± 0.23 d | 8.27 ± 0.41 f |
| 12.96 | 2-Ethylhexanol | 1.01 ± 0.10 a | 2.27 ± 0.23 b | nd | 0.871 ± 0.058 a | nd | 1.89 ± 0.17 b | nd | nd | nd | nd |
| 14.19 | Benzyl alcohol | 46.1 ± 3.44 b | 50.4 ± 4.39 b | 85.0 ± 5.32 d | 50.5 ± 4.11 b | 33.5 ± 2.98 a | 31.5 ± 2.72 a | 81.6 ± 6.35 d | 67.6 ± 4.74 c | 50.3 ± 4.11 b | 71.2 ± 5.69 c,d |
| 14.51 | Benzeneacetaldehyde | 1.47 ± 0.13 b | 5.79 ± 0.52 e | 1.88 ± 0.19 c | 7.23 ± 0.61 f | 0.782 ± 0.048 a | 4.21 ± 0.36 d | nd | 8.44 ± 0.53 g | 1.31 ± 0.10 b | 6.82 ± 0.52 e,f |
| 16.44 | Nonanal | 0.491 ± 0.036 b | 0.397 ± 0.035 a | 2.00 ± 0.19 f | 1.01 ± 0.09 d | 0.788 ± 0.069 c | 1.54 ± 0.14 e | 2.31 ± 0.22 f | 1.40 ± 0.13 e | 1.16 ± 0.11 d | 0.729 ± 0.041 c |
| 19.35 | Dodecane | 0.835 ± 0.071 a | 0.721 ± 0.069 a | 2.23 ± 0.21 d | 1.76 ± 0.16 c | 1.12 ± 0.11 b | 4.07 ± 0.28 f | 3.65 ± 0.34 e,f | 4.13 ± 0.39 f | 3.09 ± 0.25 e | 2.04 ± 0.19 c,d |
| 22.04 | Benzo-2,3-pyrrole | nd | nd | nd | nd | 15.5 ± 0.16 c | 1.32 ± 0.12 b | nd | 0.535 ± 0.043 a | nd | nd |
| 24.84 | Tetradecane | 0.732 ± 0.069 b | 0.599 ± 0.041 a | 2.40 ± 0.23 e | 1.60 ± 0.15 d | 0.977 ± 0.085 c | 2.88 ± 0.25 f | 3.71 ± 0.31 g | 4.01 ± 0.35 g | 3.10 ± 0.26 f | 1.47 ± 0.15 c |
| 27.74 | 2,4-bis(1,1-dimethylethyl)phenol | 0.828 ± 0.073 b | 1.56 ± 0.14 d | 1.21 ± 0.11 c | 1.97 ± 0.15 e | 1.09 ± 0.08 c | 0.694 ± 0.052 a | 2.46 ± 0.22 f | 2.54 ± 0.25 f | 0.770 ± 0.058 a,b | 1.12 ± 0.11 c |
RT—retention time; W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; RT—retention time; nd—not determined. Data expressed as mean values (n = 9) ± standard error (SE). a–g Mean values within the lines with different letters are significantly different (p ≤ 0.05).
Acetoin was found in 3 out of 10 analysed samples and the LAB strain used for fermentation was a significant factor in this volatile compound content in mushrooms (p = 0.001). 2,3-Butanediol was established in the Lc. casei No. 210 strain and in the Lp. plantarum No. 135 strain-fermented brown-variety mushrooms, as well as in Lp. plantarum No. 135 strain- and in with P. acidilactici No. 29 strain-fermented brown variety samples. The analysed factors’ interactions were significant in 2,3-butanediol content in mushrooms (p = 0.027). Benzaldehyde was found in most of the mushroom samples, except in P. acidilactici No. 29 strain-fermented white-variety samples, and the LAB strain used for fermentation as well as the analysed factors’ interactions were significant in benzaldehyde content in mushroom samples (p = 0.001 and p = 0.012, respectively). In most of the samples, 3-octanone content was reduced after fermentation, except in P. acidilactici No. 29 strain-fermented brown-variety mushrooms. The LAB strain used for fermentation as well as the analysed factors’ interactions were significant in 3-octanone content in mushrooms (p ≤ 0.001). Ethylhexanol was found in both non-fermented samples as well as in brown-variety mushrooms fermented with Lc. casei No. 210 and Lp. plantarum No. 135 strains. All analysed factors and their interactions were significant in ethylhexanol content in samples (p ≤ 0.001). Benzeneacetaldehyde was identified in most of the mushroom samples, except in Lc. paracasei No. 244-fermented white-variety samples, and mushroom variety as well as the analysed factors’ interactions were significant in benzeneacetaldehyde content in samples (p ≤ 0.001 and p = 0.002, respectively). In all the cases, a higher content of nonanal was found in fermented samples, and all analysed factors and their interactions were significant in the volatile compound content in mushrooms (mushroom variety p = 0.022; LAB strain used for fermentation p ≤ 0.001; factors interaction p ≤ 0.001). Similar tendencies of dodecane content were established, and in all the cases, a higher content of dodecane was found in fermented samples in comparison with non-fermented ones. The LAB strain used for fermentation and the analysed factors’ interactions were significant on dodecane content in mushrooms (p ≤ 0.001 and p = 0.004, respectively). Benzo-2,3-pyrrole was found in 3 out of the 10 analysed sample groups, and all analysed factors and their interactions were significant on this volatile compound content in mushrooms (mushrooms variety p = 0.050; LAB strain used for fermentation p = 0.002; factors interaction p = 0.015). In all the cases, fermentation increased tetradecane content in mushrooms’ volatile compounds profiles, and all analysed factors and their interactions were significant in tetradecane content in samples (mushrooms variety p ≤ 0.001; LAB strain used for fermentation p ≤ 0.001; factors interaction p = 0.003). In comparison to 2,4-bis(1,1-dimethylethyl)phenol content in non-fermented and fermented samples, in most of the white-variety samples, this volatile compound content was increased (except samples fermented with P. acidilactici No. 29 strain). However, in brown-variety samples, after fermentation with Lp. plantarum No. 135 and P. acidilactici No. 29 strains, on average, 2.25 and 1.4 times, respectively, lower 2,4-bis(1,1-dimethylethyl)phenol content was found in comparison with non-fermented samples. The LAB strain used for fermentation and the analysed factors’ interactions were significant in 2,4-bis(1,1-dimethylethyl)phenol content in mushrooms (p = 0.016 and p ≤ 0.001, respectively).
The volatile compound profile of A. bisporus arises from FA and amino acid metabolism and can differ according to growth stages and conditions as well as genotype species [79]. During fermentation, LAB can produce a variety of volatile compounds, thereby giving fermented products a unique odour [16]. To the best of our knowledge, there are no data on the volatile compound profile of fermented A. bisporus. Only in the study of Wang et al. [80], the edible mushroom Pleurotus eryngii (boiled for 2 min) was fermented with Lp. plantarum. Compared to naturally fermented P. eryngii, similar contents of alcohols and higher content of aldehydes, ketones, and esters were found in P. eryngii fermented with Lp. plantarum.
Eight-carbon compound 1-octen-3-ol is a characteristic volatile of button mushrooms that provides a “mushroom-like, earthy, and buttery” odour and is generated during the enzymatic degradation of linoleic FA [81]. A significantly lower amount of this volatile was reported after the thermal treatment of mushrooms [82]. Similar to our study, the diminution of 1-octen-3-ol was also found in oats fermented with Lc. paracasei and after fermentation of Allomyrina dichotoma larvae with Lactobacillus acidophillus and Lp. plantarum [83,84].
Amino acid-derived benzyl alcohol, with its aromatic, floral, fruity, and sweet odour, is another dominant volatile in white and brown A. bisporus, whose content significantly decreases after heat treatment [5,81,85]. However, in our case, fermentation with LAB increased the content of this volatile, and this is related to the increased formation of alcohols during the process of LAB fermentation [86].
It was reported that the content of such ketones as 3-octanone (mushroom, herbal, lavender note), which is generated via enzymatic oxidation of linoleic or linolenic acids, reduces in A. bisporus after heat treatment [81]. Moreover, this volatile can also be degraded by LAB during fermentation [87]. The presence of 2,3-butanediol (fruity, creamy, and buttery notes) and acetoin in some of the fermented white and brown A. bisporus samples can be related to the LAB carbohydrate metabolism [88].
The amino acid phenylalanine oxidation metabolites are precursors of benzaldehyde (with the odour of almond, sweet, and phenolic) and benzene acetaldehyde (with the odour of green, sweat, and phenolic) [79]. Dodecane and tetradecane are specific volatiles and were also previously found in A. bisporus species [79]. Nonanal (waxy, aldehydic, rose note) and tetradecane (alkane) were previously identified in dried pine mushroom [89]. 2-ethylhexanol was detected in A. bisporus, Volariella volvacea and Cortinarius odorifer mushrooms and provides floral and cedrol woody notes [85,90,91]. 2,4-bis (1,1-dimethylethyl)- phenol was previously found in rare gilled mushrooms of India and Southern Ocean microalgae Chlorella sp. PR-1 and possesses biocidal activity [92,93].
3.5. Overall Acceptability and Emotions Induced for Judges by the Mushroom Samples
The overall acceptability and emotions induced for judges by mushroom samples are shown in Table 6. Despite that significant differences between mushroom samples’ overall acceptability and emotion “neutral” were not established, other fixed emotions’ intensities varied between the tested sample groups. In the intensity of the “happy” emotion, the highest expression was found with non-fermented white-variety mushroom samples (0.120). The lowest intensity of the emotion “happy” was expressed by judges with white and brown mushroom samples fermented with the Lp. plantarum No. 135 strain (on average, 0.013). The LAB strain used for fermentation and the interactions of the LAB strain used for fermentation and mushroom variety were significant in the intensity of the emotion “happy” induced in judges by mushroom samples (p ≤ 0.001 and p = 0.002, respectively). Additionally, between the intensity of the emotion “happy” and volatile compounds 1-octen-3-ol and 3-octanone, positive moderate correlations were found (r = 0.620, p ≤ 0.001 and r = 0.484, p = 0.007, respectively). Additionally, between the intensity of the emotion “happy” and the samples’ pH, a positive weak correlation was established (r = 0.398, p = 0.029).
Table 6.
Overall acceptability and emotions induced in judges by mushroom samples.
| Mushroom Samples | OA | Emotions Induced for Judges by Mushroom Samples (from 0 to 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | Valence | ||
| Wnon-f | 8.05 ± 1.48 a | 0.768 ± 0.039 a | 0.120 ± 0.011 e | 0.009 ± 0.002 a | 0.007 ± 0.001 b | 0.003 ± 0.001 a | nd | 0.020 ± 0.004 a,b | 0.001 ± 0.0005 a | 0.088 ± 0.024 d |
| Bnon-f | 7.42 ± 1.35 a | 0.769 ± 0.061 a | 0.037 ± 0.004 c | 0.034 ± 0.004 b | 0.011 ± 0.002 b,c | 0.005 ± 0.002 b | 0.001 ± 0.0003 a | 0.065 ± 0.009 d | 0.001 ± 0.0006 a | −0.065 ± 0.014 b |
| W210 | 6.06 ± 1.15 a | 0.763 ± 0.048 a | 0.022 ± 0.002 b | 0.055 ± 0.004 c,d | 0.004 ± 0.001 a | 0.004 ± 0.001 b | 0.001 ± 0.0004 a | 0.051 ± 0.007 d | 0.001 ± 0.0004 a | −0.068 ± 0.021 b |
| B210 | 5.89 ± 1.48 a | 0.760 ± 0.065 a | 0.038 ± 0.003 c | 0.064 ± 0.005 d | 0.007 ± 0.002 b | 0.009 ± 0.003 b | 0.001 ± 0.0002 a | 0.039 ± 0.004 c | 0.006 ± 0.0007 b | −0.055 ± 0.009 b |
| W135 | 5.25 ± 1.32 a | 0.768 ± 0.074 a | 0.011 ± 0.002 a | 0.057 ± 0.006 c,d | 0.015 ± 0.003 c | 0.009 ± 0.004 b | 0.002 ± 0.0005 b | 0.026 ± 0.003 b | 0.002 ± 0.0008 a | −0.077 ± 0.010 b |
| B135 | 5.29 ± 1.21 a | 0.712 ± 0.069 a | 0.014 ± 0.003 a | 0.091 ± 0.008 e | 0.007 ± 0.002 b | 0.003 ± 0.001 a | nd | 0.056 ± 0.005 d | 0.001 ± 0.0005 a | −0.119 ± 0.014 a |
| W244 | 6.69 ± 0.96 a | 0.718 ± 0.065 a | 0.028 ± 0.004 b | 0.063 ± 0.007 d | 0.003 ± 0.001 a | 0.002 ± 0.001 a | nd | 0.084 ± 0.007 e | 0.002 ± 0.0009 a | −0.096 ± 0.009 b |
| B244 | 6.87 ± 1.02 a | 0.770 ± 0.072 a | 0.021 ± 0.003 b | 0.048 ± 0.005 c | 0.016 ± 0.002 c | 0.007 ± 0.002 b | 0.001 ± 0.0004 a | 0.015 ± 0.003 a | 0.002 ± 0.0008 a | −0.048 ± 0.005 b |
| W29 | 5.88 ± 0.49 a | 0.699 ± 0.059 a | 0.028 ± 0.004 b | 0.094 ± 0.007 e | 0.004 ± 0.001 a | 0.008 ± 0.003 b | 0.003 ± 0.001 b | 0.047 ± 0.005 c,d | 0.002 ± 0.0006 a | −0.106 ± 0.012 a |
| B29 | 5.69 ± 0.65 a | 0.666 ± 0.063 a | 0.097 ± 0.005 d | 0.057 ± 0.006 c,d | 0.011 ± 0.002 b,c | 0.035 ± 0.005 c | 0.007 ± 0.002 c | 0.016 ± 0.002 a | 0.001 ± 0.0005 a | 0.021 ± 0.005 c |
W—A. bisporus white variety; B—A. bisporus brown variety; non-f—non-fermented samples; 210—fermented with Lc. casei No. 210 strain; 135—fermented with Lp. plantarum No. 135 strain; 244—fermented with Lc. paracasei No. 244 strain; 29—fermented with P. acidilactici No. 29 strain; OA—overall acceptability; nd—not determined. Data expressed as mean values (n = 10) ± standard error (SE). a–e Mean values within the columns with different letters are significantly different (p ≤ 0.05).
The LAB strain used for fermentation and the interactions of the LAB strain used for fermentation and mushroom variety were significant in the intensity of the emotion “sad” induced in judges by mushroom samples (p ≤ 0.001), and the highest expression of the emotion “sad” was found with Lp. plantarum No. 135 strain-fermented brown-variety mushroom samples and with P. acidilactici No. 29 strain-fermented white-variety mushroom samples (on average, 0.093). Positive weak and moderate correlations between the intensity of the emotion “sad” and the volatile compounds acetoin and 2,3-butanediol were found (r = 0.387, p = 0.035 and r = 0.514, p = 0.004, respectively). Additionally, negative correlations between the intensity of the emotion “sad” and benzaldehyde; 1-octen-3-ol; 3-octanone; nonanal; dodecane; and tetradecane were established (r = −0.610, p ≤ 0.001; r = −0.568, p ≤ 0.001; r = −0.377, p = 0.040; r = −0.364, p = 0.048; r = −0.520, p = 0.003; and r = −0.391, p = 0.033, respectively).
All the analysed factors and their interactions were significant in the intensity of the emotion “angry” induced for judges by mushroom samples (p ≤ 0.001), and the lowest expression of the emotion “angry” was found with white-variety mushroom sample groups fermented with Lc. casei No. 210, Lc. paracasei No. 244, and P. acidilactici No. 29 strains. Positive correlations between the expression of the emotion “angry” intensity and the volatile compounds benzaldehyde; benzeneacetaldehyde; nonanal; benzo-2,3-pyrrole; and tetradecane were found (r = 0.370, p = 0.044; r = 0.587, p ≤ 0.001; r = 0.398, p = 0.029; r = 0.602, p ≤ 0.001; and r = 0.454, p = 0.012, respectively).
The highest intensity of the emotions “surprised” and “scared” were found with brown-variety mushroom sample groups fermented with P. acidilactici No. 29 strain. Correlations between the expression of the emotion “surprised“ intensity and the analysed physicochemical and microbiological parameters of the mushroom samples were not found. However, between the intensity of the emotion “scared” and some volatile compounds (2,3-butanediol; dodecane; benzo-2,3-pyrrole; and 2,4-bis(1,1-dimethylethyl)phenol), TTA, and tyramine content, significant correlations were established (r = 0.362, p = 0.050; r = −0.476, p = 0.008; r = 0.641, p ≤ 0.001; r = −0.475, p = 0.008; r = 0.426, p = 0.019; and r = 0.694, p ≤ 0.001, respectively). Additionally, the interaction of the analysed factors was significant in the intensity of the emotion “scared” induced in judges by mushroom samples (p = 0.016).
All the analysed factors and their interactions were significant in the intensity of the emotion “disgusted” induced for judges by mushroom samples (mushroom variety p = 0.013; LAB strain used for fermentation p = 0.001; factors interaction p ≤ 0.001), and the highest expression of the emotion “disgusted” was found with white-variety mushroom sample groups fermented with the Lc. paracasei No. 244 strain. Despite that the analysed factors were not significant in the intensity of emotion “contempt” induced in judges by mushroom samples, between the expression of the emotion “contempt” and some volatile compounds (2,3-butanediol; ethylhexanol; benzyl alcohol), TTA, and tyramine content, significant correlations were established (r = 0.504, p = 0.005; r = −0.554, p = 0.002; r = 0.454, p = 0.012; r = 0.424, p = 0.019; r = 0.575, p ≤ 0.001, respectively).
Finally, most of the tested samples’ valences were negative, except for the non-fermented white mushroom variety and P. acidilactici No. 29 strain-fermented brown mushroom samples, and the LAB strain used for fermentation and the analysed factors’ interactions were significant on the valence value (p ≤ 0.001).
Usually, mushrooms are incorporated into other food products, and their sensory profile and acceptability are evaluated [94]. There are only a few studies on the sensory analysis of fermented mushrooms. Liu et al. [50] reported that edible oyster mushrooms fermented with Lactiplantibacillus pentosus for 18 days received scores of 7–8 for overall acceptability. In the study of Jabłońska-Ryś et al. [19], 7 days of fermentation with Lp. plantarum 299v and Lp. plantarum EK3 was applied to A. bisporus and the scores of overall acceptability were lower compared to those obtained in our study. The evaluation of food-elicited emotions is an expanding field of interest in the food sector, as the emotional profile can be employed to differentiate foods with identical hedonic scores and plays an important role in estimating consumer purchase intentions [95,96]. Mushroom-elicited emotions were evaluated in the study of Tepsongkroh et al. [96], who found that sensory ratings and emotional response, measured with the Essense Profile®, of extruded snacks were notably affected when mushrooms Volvariella volvacea and Pleurotus pulmonarius were incorporated into the snack formula.
4. Conclusions
Fermentation with No. 210, No. 135, No. 244, and No. 29 strains induced changes in the physical properties of A. bisporus as well as volatile, BA, FA, and elicited emotion profiles. Fermented mushrooms had a darker colour and a higher count of viable LAB, but similar yeast and fungi counts compared to non-fermented ones. The total BA content in fermented A. bisporus was below the maximum level suggested for foods, and in most cases, fermented samples showed a higher content of polyunsaturated FA. The main volatile compounds in mushroom samples were 1-octen-3-ol and benzyl alcohol. The overall acceptability was similar for both non-fermented and fermented mushrooms. The LAB strain used for fermentation and the A. bisporus variety were significant factors in most of the elicited emotion intensities in the judges. This research provided beneficial information that can be further applied to the industrial production of fermented mushrooms. Furthermore, a wider spectrum of LAB for A. bisporus fermentation can be studied in the future to recommend the safest and most acceptable product for the consumer as well as the most appropriate LAB strain for the mushroom fermentation industry.
Acknowledgments
This work is based upon the work from COST Action 18101 SOURDOMICS—Sourdough biotechnology network towards novel, healthier and sustainable food and bioprocesses (https://sourdomics.com/, accessed on 29 April 2023; https://www.cost.eu/actions/CA18101/, accessed on 29 April 2023), where the author E.B. is the Vice-Chair and leader of the working group 6 “Project design and development innovative prototypes of products and small-scale processing technologies” and the author J.M.R. is the Chair and Grant Holder Scientific Representative and is supported by COST (European Cooperation in Science and Technology) (https://www.cost.eu/, accessed on 29 April 2023). COST is a funding agency for research and innovation networks.
Author Contributions
Conceptualization, E.B.; methodology, E.M., D.K., E.Z. and V.S.; software, D.K. and G.Z.; validation, D.K., E.Z. and V.S.; formal analysis, P.Z. and E.M.; investigation, D.K., E.M., V.S., E.Z., R.R. and P.Z.; resources, E.B.; data curation, E.B. and D.K.; writing—original draft preparation, D.K., V.S. and E.B.; writing—review and editing, E.B., D.K. and J.M.R.; visualization, E.Z., G.Z., E.M. and V.S.; supervision, E.B.; project administration, J.M.R. and E.B.; funding acquisition, E.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bakratsas G., Polydera A., Katapodis P., Stamatis H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Future Foods. 2021;4:100086. doi: 10.1016/j.fufo.2021.100086. [DOI] [Google Scholar]
- 2.Ramos M., Burgos N., Barnard A., Evans G., Preece J., Graz M., Ruthes A.C., Jiménez-Quero A., Martínez-Abad A., Vilaplana F. Agaricus bisporus and Its By-Products as a Source of Valuable Extracts and Bioactive Compounds. Food Chem. 2019;292:176–187. doi: 10.1016/j.foodchem.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K., Pu Y.-Y., Sun D.-W. Recent Advances in Quality Preservation of Postharvest Mushrooms (Agaricus bisporus): A Review. Trends Food Sci. Technol. 2018;78:72–82. doi: 10.1016/j.tifs.2018.05.012. [DOI] [Google Scholar]
- 4.Atila F., Owaid M.N., Shariati M.A. The Nutritional and Medical Benefits of Agaricus bisporus: A Review. J. Microbiol. Biotechnol. Food Sci. 2021;2021:281–286. doi: 10.15414/jmbfs.2017/18.7.3.281-286. [DOI] [Google Scholar]
- 5.Paulauskienė A., Tarasevičienė Ž., Šileikienė D., Česonienė L. The Quality of Ecologically and Conventionally Grown White and Brown Agaricus bisporus Mushrooms. Sustainability. 2020;12:6187. doi: 10.3390/su12156187. [DOI] [Google Scholar]
- 6.Bernaś E. Comparison of the Mechanism of Enzymatic Browning in Frozen White and Brown A. bisporus. Eur. Food Res. Technol. 2018;244:1239–1248. doi: 10.1007/s00217-018-3039-y. [DOI] [Google Scholar]
- 7.Vunduk J., Djekic I., Petrović P., Tomašević I., Kozarski M., Despotović S., Nikšić M., Klaus A. Challenging the Difference between White and Brown Agaricus bisporus Mushrooms: Science behind Consumers Choice. Br. Food J. 2018;120:1381–1394. doi: 10.1108/BFJ-10-2017-0550. [DOI] [Google Scholar]
- 8.Jaworska G., Pogoń K., Bernaś E., Duda-Chodak A. Nutraceuticals and Antioxidant Activity of Prepared for Consumption Commercial Mushrooms Agaricus bisporus and Pleurotus ostreatus. J. Food Qual. 2015;38:111–122. doi: 10.1111/jfq.12132. [DOI] [Google Scholar]
- 9.Sulieman A.A., Zhu K.-X., Peng W., Hassan H.A., Obadi M., Siddeeg A., Zhou H.-M. Rheological and Quality Characteristics of Composite Gluten-Free Dough and Biscuits Supplemented with Fermented and Unfermented Agaricus bisporus Polysaccharide Flour. Food Chem. 2019;271:193–203. doi: 10.1016/j.foodchem.2018.07.189. [DOI] [PubMed] [Google Scholar]
- 10.Szutowska J. Functional Properties of Lactic Acid Bacteria in Fermented Fruit and Vegetable Juices: A Systematic Literature Review. Eur. Food Res. Technol. 2020;246:357–372. doi: 10.1007/s00217-019-03425-7. [DOI] [Google Scholar]
- 11.Al-Sahlany S., Niamah A. Bacterial Viability, Antioxidant Stability, Antimutagenicity and Sensory Properties of Onion Types Fermentation by Using Probiotic Starter during Storage. Nutr. Food Sci. 2022;52:901–916. doi: 10.1108/NFS-07-2021-0204. [DOI] [Google Scholar]
- 12.Jabłońska-Ryś E., Skrzypczak K., Sławińska A., Radzki W., Gustaw W. Lactic Acid Fermentation of Edible Mushrooms: Tradition, Technology, Current State of Research: A Review. Compr. Rev. Food Sci. Food Saf. 2019;18:655–669. doi: 10.1111/1541-4337.12425. [DOI] [PubMed] [Google Scholar]
- 13.Skrzypczak K., Gustaw K., Jabłońska-Ryś E., Sławińska A., Gustaw W., Winiarczyk S. Spontaneously Fermented Fruiting Bodies of Agaricus bisporus as a Valuable Source of New Isolates of Lactic Acid Bacteria with Functional Potential. Foods. 2020;9:1631. doi: 10.3390/foods9111631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur H., Beresford T.P., Cotter P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients. 2020;12:1679. doi: 10.3390/nu12061679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R., Jeevaratnam K., Fatima A. Lactic Acid Bacteria: Probiotic Characteristic, Selection Criteria, and Its Role in Human Health (A Review) [(accessed on 29 April 2023)];Int. J. Emerg. Technol. Innov. Res. 2018 5:411–424. Available online: https://ssrn.com/abstract=3462244. [Google Scholar]
- 16.Chen Z., Fang X., Wu W., Chen H., Han Y., Yang H., Gao H. Effects of Fermentation with Lactiplantibacillus Plantarum GDM1. 191 on the Umami Compounds in Shiitake Mushrooms (Lentinus edodes) Food Chem. 2021;364:130398. doi: 10.1016/j.foodchem.2021.130398. [DOI] [PubMed] [Google Scholar]
- 17.Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R.P. The Lactobacillus Casei Group: History and Health Related Applications. Front. Microbiol. 2018;9:2107. doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Song Q., Wang M., Ren J., Liu S., Zhao S. Comparative Genomics Analysis of Pediococcus acidilactici Species. J. Microbiol. 2021;59:573–583. doi: 10.1007/s12275-021-0618-6. [DOI] [PubMed] [Google Scholar]
- 19.Jabłońska-Ryś E., Sławińska A., Skrzypczak K., Goral K. Dynamics of Changes in PH and the Contents of Free Sugars, Organic Acids and LAB in Button Mushrooms during Controlled Lactic Fermentation. Foods. 2022;11:1553. doi: 10.3390/foods11111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbieri F., Montanari C., Gardini F., Tabanelli G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods. 2019;8:17. doi: 10.3390/foods8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabłońska-Ryś E., Sławińska A., Skrzypczak K., Kowalczyk D., Stadnik J. Content of Biogenic Amines and Physical Properties of Lacto-Fermented Button Mushrooms. Appl. Sci. 2022;12:8957. doi: 10.3390/app12188957. [DOI] [Google Scholar]
- 22.Radzki W., Ziaja-Sołtys M., Nowak J., Rzymowska J., Topolska J., Sławińska A., Michalak-Majewska M., Zalewska-Korona M., Kuczumow A. Effect of Processing on the Content and Biological Activity of Polysaccharides from Pleurotus ostreatus Mushroom. LWT-Food Sci. Technol. 2016;66:27–33. doi: 10.1016/j.lwt.2015.10.016. [DOI] [Google Scholar]
- 23.Bartkiene E., Lele V., Ruzauskas M., Domig K.J., Starkute V., Zavistanaviciute P., Bartkevics V., Pugajeva I., Klupsaite D., Juodeikiene G., et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms. 2020;8:64. doi: 10.3390/microorganisms8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncero-Ramos I., Mendiola-Lanao M., Pérez-Clavijo M., Delgado-Andrade C. Effect of Different Cooking Methods on Nutritional Value and Antioxidant Activity of Cultivated Mushrooms. Int. J. Food Sci. Nutr. 2017;68:287–297. doi: 10.1080/09637486.2016.1244662. [DOI] [PubMed] [Google Scholar]
- 25.ISO 750:1998; Fruit and Vegetable Products—Determination of Titratable Acidity. [(accessed on 31 May 2023)]. Available online: https://www.iso.org/standard/22569.html.
- 26.ISO 15214; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. [(accessed on 11 August 2022)]. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/02/68/26853.html.
- 27.ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. [(accessed on 31 March 2022)]. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/82/38276.html.
- 28.Bartkiene E., Mockus E., Mozuriene E., Klementaviciute J., Monstaviciute E., Starkute V., Zavistanaviciute P., Zokaityte E., Cernauskas D., Klupsaite D. The Evaluation of Dark Chocolate-Elicited Emotions and Their Relation with Physico Chemical Attributes of Chocolate. Foods. 2021;10:642. doi: 10.3390/foods10030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez-Palacios T., Ruiz J., Ferreira I.M.P.L.V.O., Petisca C., Antequera T. Effect of Solvent to Sample Ratio on Total Lipid Extracted and Fatty Acid Composition in Meat Products within Different Fat Content. Meat Sci. 2012;91:369–373. doi: 10.1016/j.meatsci.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Gigirey B., Vieites Baptista de Sousa J.M., Villa T.G., Barros-Velazquez J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus Alalunga) J. Food Prot. 1999;62:933–939. doi: 10.4315/0362-028X-62.8.933. [DOI] [PubMed] [Google Scholar]
- 31.Bartkiene E., Zokaityte E., Starkute V., Mockus E., Klupsaite D., Lukseviciute J., Bogomolova A., Streimikyte A., Ozogul F. Biopreservation of Wild Edible Mushrooms (Boletus Edulis, Cantharellus, and Rozites Caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods. 2022;11:1800. doi: 10.3390/foods11121800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO; [(accessed on 15 October 2021)]. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/53/45352.html. [Google Scholar]
- 33.Jabłońska-Ryś E., Sławińska A., Radzki W., Gustaw W. Evaluation of the Potential Use of Probiotic Strain Lactobacillus Plantarum 299v in Lactic Fermentation of Button Mushroom Fruiting Bodies. Acta Sci. Pol. Technol. Aliment. 2016;15:399–407. doi: 10.17306/J.AFS.2016.4.38. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Wu J., Lv M., Shao Z., Hungwe M., Wang J., Bai X., Xie J., Wang Y., Geng W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021;9:612285. doi: 10.3389/fbioe.2021.612285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filannino P., Bai Y., Di Cagno R., Gobbetti M., Gänzle M.G. Metabolism of Phenolic Compounds by Lactobacillus Spp. during Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015;46:272–279. doi: 10.1016/j.fm.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Van Bennekom E.O., Zhang Y., Abee T., Smid E.J. Long-Chain Vitamin K2 Production in Lactococcus Lactis Is Influenced by Temperature, Carbon Source, Aeration and Mode of Energy Metabolism. Microb. Cell Factories. 2019;18:1–14. doi: 10.1186/s12934-019-1179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venturini M.E., Reyes J.E., Rivera C.S., Oria R., Blanco D. Microbiological Quality and Safety of Fresh Cultivated and Wild Mushrooms Commercialized in Spain. Food Microbiol. 2011;28:1492–1498. doi: 10.1016/j.fm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Lao Y., Zhang M., Li Z., Bhandari B. A Novel Combination of Enzymatic Hydrolysis and Fermentation: Effects on the Flavor and Nutritional Quality of Fermented Cordyceps Militaris Beverage. LWT. 2020;120:108934. doi: 10.1016/j.lwt.2019.108934. [DOI] [Google Scholar]
- 39.Palmqvist E., Hahn-Hägerdal B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000;74:25–33. doi: 10.1016/S0960-8524(99)00161-3. [DOI] [Google Scholar]
- 40.Janßen D., Eisenbach L., Ehrmann M.A., Vogel R.F. Assertiveness of Lactobacillus Sakei and Lactobacillus Curvatus in a Fermented Sausage Model. Int. J. Food Microbiol. 2018;285:188–197. doi: 10.1016/j.ijfoodmicro.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Guillamón E., García-Lafuente A., Lozano M., D’Arrigo M., Rostagno M.A., Villares A., Martínez J.A. Edible Mushrooms: Role in the Prevention of Cardiovascular Diseases. Fitoterapia. 2010;81:715–723. doi: 10.1016/j.fitote.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Kalbarczyk J., Radzki W. Uprawiane Grzyby Wyższe Jako Cenny Składnik Diety Oraz Źródło Substancji Aktywnych Biologicznie. Herba Pol. J. 2009;55:224–232. [Google Scholar]
- 43.Skapska S., Owczarek L., Jasinska U., Halasinska A., Danielczuk J., Sokolowska B., Rolno-Spozywczego I.B.P. Changes in the Antioxidant Capacity of Edible Mushrooms during Lactic Acid Fermentation. Zywnosc Nauka Technol. Jakosc Pol. 2008;4:243–250. [Google Scholar]
- 44.Jabłońska-Ryś E., Sławińska A. Possibilities of Use of Lactic Fermentation in Bio Conservation of Edible Mushrooms Fruit Bodies; Proceedings of the Abstract: 2nd International Conference and Workshop, Plant—The Source of Research Material; Lublin, Poland. 21–24 June 2012; pp. 18–20. [Google Scholar]
- 45.Jabłońska-Ryś E., Kalbarczyk J., Sztaba A. Zastosowanie Kultur Starterowych Bakterii Mlekowych i Propionowych w Procesie Kwaszenia Owocników Pieczarki; Proceedings of the Doniesienie na V Konferencję PTTŻ nt. “Jakość i bezpieczeństwo żywności”, Białobrzegi nad Zalewem Zegrzyńskim; Poland. 17–18 November 2005; pp. 17–18. [Google Scholar]
- 46.Joshi V.K., Kaur M., Thakur N.S. Lactic Acid Fermentation of Mushroom (Agaricus bisporus) for Preservation and Preparation of Sauce. Acta Aliment. Bp. 1996;25:1–11. [Google Scholar]
- 47.Milanovič N., Davidovič A., Savič A. Lactic Acid Fermentation of Mushroom (Agaricus bisporus) with Lactobacillus Plantarum; Proceedings of the 9th Savjetovanje Hemicara i Tehnologa Republike Srpske (338–345); Banja Luka, Bosnia and Herzegovina. 12–13 November 2010; pp. 12–13. [Google Scholar]
- 48.Niksic M., Stojanovic M., Zivanovic S., Veljic S. Ecological Approach in Preservation of Edible Mushrooms by Lactic Acid Fermentation. Poslovna Zajednica Vrenje; Vrnjacka Banja, Yugoslavia: 1997. [Google Scholar]
- 49.Zheng H.-G., Chen J.-C., Ahmad I. Preservation of King Oyster Mushroom by the Use of Different Fermentation Processes. J. Food Process. Preserv. 2018;42:e13396. doi: 10.1111/jfpp.13396. [DOI] [Google Scholar]
- 50.Liu Y., Xie X., Ibrahim S.A., Khaskheli S.G., Yang H., Wang Y., Huang W. Characterization of Lactobacillus Pentosus as a Starter Culture for the Fermentation of Edible Oyster Mushrooms (Pleurotus spp.) LWT-Food Sci. Technol. 2016;68:21–26. doi: 10.1016/j.lwt.2015.12.008. [DOI] [Google Scholar]
- 51.Franco W., Pérez-Díaz I.M. Role of Selected Oxidative Yeasts and Bacteria in Cucumber Secondary Fermentation Associated with Spoilage of the Fermented Fruit. Food Microbiol. 2012;32:338–344. doi: 10.1016/j.fm.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Kalač P., Křížek M. Formation of Biogenic Amines in Four Edible Mushroom Species Stored under Different Conditions. Food Chem. 1997;58:233–236. doi: 10.1016/S0308-8146(96)00170-7. [DOI] [Google Scholar]
- 53.Yen G.-C. Effects of Heat Treatment and Storage Temperature on the Biogenic Amine Content of Straw Mushroom (Volvariella volvacea) J. Sci. Food Agric. 1992;58:59–61. doi: 10.1002/jsfa.2740580111. [DOI] [Google Scholar]
- 54.Tittarelli F., Perpetuini G., Di Gianvito P., Tofalo R. Biogenic Amines Producing and Degrading Bacteria: A Snapshot from Raw Ewes’ Cheese. LWT. 2019;101:1–9. doi: 10.1016/j.lwt.2018.11.030. [DOI] [Google Scholar]
- 55.Özogul Y., Özogul F. Biogenic Amines in Food. Royal Society of Chemistry; London, UK: 2019. Biogenic Amines Formation, Toxicity, Regulations in Food. [Google Scholar]
- 56.Özogul F., Hamed I. The Importance of Lactic Acid Bacteria for the Prevention of Bacterial Growth and Their Biogenic Amines Formation: A Review. Crit. Rev. Food Sci. Nutr. 2018;58:1660–1670. doi: 10.1080/10408398.2016.1277972. [DOI] [PubMed] [Google Scholar]
- 57.Ladero V., Calles-Enriquez M., Fernandez M., Alvarez M.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010;6:145–156. doi: 10.2174/157340110791233256. [DOI] [Google Scholar]
- 58.Makhamrueang N., Sirilun S., Sirithunyalug J., Chaiyana W., Wangcharoen W., Peerajan S., Chaiyasut C. Effect of Pretreatment Processes on Biogenic Amines Content and Some Bioactive Compounds in Hericium Erinaceus Extract. Foods. 2021;10:996. doi: 10.3390/foods10050996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jabłońska-Ryś E., Sławińska A., Stachniuk A., Stadnik J. Determination of Biogenic Amines in Processed and Unprocessed Mushrooms from the Polish Market. J. Food Compos. Anal. 2020;92:103492. doi: 10.1016/j.jfca.2020.103492. [DOI] [Google Scholar]
- 60.Reis G.C.L., Custódio F.B., Botelho B.G., Guidi L.R., Gloria M.B.A. Investigation of Biologically Active Amines in Some Selected Edible Mushrooms. J. Food Compos. Anal. 2020;86:103375. doi: 10.1016/j.jfca.2019.103375. [DOI] [Google Scholar]
- 61.Abugri D., McElhenney W., Willian K. Fatty Acid Profiling in Selected Cultivated Edible and Wild Medicinal Mushrooms in Southern United States. J. Exp. Food Chem. 2016;2:1–7. doi: 10.4172/2472-0542.1000108. [DOI] [Google Scholar]
- 62.Sande D., de Oliveira G.P., e Moura MA F., de Almeida Martins B., Lima M.T.N.S., Takahashi J.A. Edible Mushrooms as a Ubiquitous Source of Essential Fatty Acids. Food Res. Int. 2019;125:108524. doi: 10.1016/j.foodres.2019.108524. [DOI] [PubMed] [Google Scholar]
- 63.Stojković D., Reis F.S., Glamočlija J., Ćirić A., Barros L., Griensven L.J.L.D.V., Ferreira I.C.F.R., Soković M. Cultivated Strains of Agaricus bisporus and A. Brasiliensis: Chemical Characterization and Evaluation of Antioxidant and Antimicrobial Properties for the Final Healthy Product—Natural Preservatives in Yoghurt. Food Funct. 2014;5:1602–1612. doi: 10.1039/c4fo00054d. [DOI] [PubMed] [Google Scholar]
- 64.Saiqa S., Haq N.B., Muhammad A.H., Muhammad A.A., Rehman A. Studies on Chemical Composition and Nutritive Evaluation of Wild Edible Mushrooms. Iran. J. Chem. Chem. Eng. 2008;27:151–154. doi: 10.30492/ijcce.2008.6981. [DOI] [Google Scholar]
- 65.Öztürk M., Duru M.E., Kivrak Ş., Mercan-Doğan N., Türkoglu A., Özler M.A. In Vitro Antioxidant, Anticholinesterase and Antimicrobial Activity Studies on Three Agaricus Species with Fatty Acid Compositions and Iron Contents: A Comparative Study on the Three Most Edible Mushrooms. Food Chem. Toxicol. 2011;49:1353–1360. doi: 10.1016/j.fct.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Shao S., Hernandez M., Kramer J.K.G., Rinker D.L., Tsao R. Ergosterol Profiles, Fatty Acid Composition, and Antioxidant Activities of Button Mushrooms as Affected by Tissue Part and Developmental Stage. J. Agric. Food Chem. 2010;58:11616–11625. doi: 10.1021/jf102285b. [DOI] [PubMed] [Google Scholar]
- 67.Hossain S., Hashimoto M., Choudhury E.K., Alam N., Hussain S., Hasan M., Choudhury S.K., Mahmud I. Dietary Mushroom (Pleurotus ostreatus) Ameliorates Atherogenic Lipid in Hypercholesterolaemic Rats. Clin. Exp. Pharmacol. Physiol. 2003;30:470–475. doi: 10.1046/j.1440-1681.2003.03857.x. [DOI] [PubMed] [Google Scholar]
- 68.Silva Figueiredo P., Carla Inada A., Marcelino G., Maiara Lopes Cardozo C., De Cássia Freitas K., De Cássia Avellaneda Guimarães R., Pereira de Castro A., Aragão do Nascimento V., Aiko Hiane P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients. 2017;9:1158. doi: 10.3390/nu9101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simopoulos A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reis F.S., Barros L., Martins A., Ferreira I.C.F.R. Chemical Composition and Nutritional Value of the Most Widely Appreciated Cultivated Mushrooms: An Inter-Species Comparative Study. Food Chem. Toxicol. 2012;50:191–197. doi: 10.1016/j.fct.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 71.Muszynska B., Kala K., Rojowski J., Grzywacz A., Opoka W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies—A Review. Pol. J. Food Nutr. Sci. 2017;67:173–181. doi: 10.1515/pjfns-2016-0032. [DOI] [Google Scholar]
- 72.Morales P., Ferreira I.C.F.R., Carvalho A.M., Sánchez-Mata M.C., Cámara M., Tardío J. Fatty Acids Profiles of Some Spanish Wild Vegetables. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2012;18:281–290. doi: 10.1177/1082013211427798. [DOI] [PubMed] [Google Scholar]
- 73.Barros L., Cruz T., Baptista P., Estevinho L.M., Ferreira I.C.F.R. Wild and Commercial Mushrooms as Source of Nutrients and Nutraceuticals. Food Chem. Toxicol. 2008;46:2742–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 74.Baars J.J.P., Sonnenberg A.S.M., Mumm R., Stijger I., Wehrens H.R.M.J. Metabolites Contributing to Taste in Agaricus bisporus. Plant Research International; Wageningen, The Netherlands: 2016. [Google Scholar]
- 75.Gänzle M.G. Enzymatic and Bacterial Conversions during Sourdough Fermentation. Food Microbiol. 2014;37:2–10. doi: 10.1016/j.fm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 76.Shahzadi T., Abbasi M.A., Riaz T., Rehman A.-U., Siddiqui S.Z., Ajaib M. Caryopteris odorata: A rich source of antioxidants for protection against chronic diseases and food products. J. Chil. Chem. Soc. 2011;56:678–681. doi: 10.4067/S0717-97072011000200012. [DOI] [Google Scholar]
- 77.Ogawa J., Matsumura K., Kishino S., Omura Y., Shimizu S. Conjugated Linoleic Acid Accumulation via 10-Hydroxy-12-Octadecaenoic Acid during Microaerobic Transformation of Linoleic Acid by Lactobacillus Acidophilus. Appl. Environ. Microbiol. 2001;67:1246–1252. doi: 10.1128/AEM.67.3.1246-1252.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volkov A., Liavonchanka A., Kamneva O., Fiedler T., Goebel C., Kreikemeyer B., Feussner I. Myosin Cross-Reactive Antigen of Streptococcus Pyogenes M49 Encodes a Fatty Acid Double Bond Hydratase That Plays a Role in Oleic Acid Detoxification and Bacterial Virulence. J. Biol. Chem. 2010;285:10353–10361. doi: 10.1074/jbc.M109.081851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng T., Yang M., Ma B., Zhao Y., Zhuang H., Zhang J., Chen D. Volatile Profiles of Two Genotype Agaricus bisporus Species at Different Growth Stages. Food Res. Int. Ott. Ont. 2021;140:109761. doi: 10.1016/j.foodres.2020.109761. [DOI] [PubMed] [Google Scholar]
- 80.Wang B., Zhao N., Li J., Xu R., Wang T., Guo L., Ma M., Fan M., Wei X. Selenium-Enriched Lactobacillus Plantarum Improves the Antioxidant Activity and Flavor Properties of Fermented Pleurotus Eryngii. Food Chem. 2021;345:128770. doi: 10.1016/j.foodchem.2020.128770. [DOI] [PubMed] [Google Scholar]
- 81.Du X., Sissons J., Shanks M., Plotto A. Aroma and Flavor Profile of Raw and Roasted Agaricus bisporus Mushrooms Using a Panel Trained with Aroma Chemicals. LWT. 2021;138:110596. doi: 10.1016/j.lwt.2020.110596. [DOI] [Google Scholar]
- 82.Selli S., GUCLU G., Sevindik O., Kelebek H. Variations in the Key Aroma and Phenolic Compounds of Champignon (Agaricus bisporus) and Oyster (Pleurotus ostreatus) Mushrooms after Two Cooking Treatments as Elucidated by GC-MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021;354:129576. doi: 10.1016/j.foodchem.2021.129576. [DOI] [PubMed] [Google Scholar]
- 83.Lee H.E., Kim J., Kim Y., Bang W.Y., Yang J., Lee S.J., Jung Y.H. Identification and Improvement of Volatile Profiles of Allomyrina Dichotoma Larvae by Fermentation with Lactic Acid Bacteria. Food Biosci. 2021;43:101257. doi: 10.1016/j.fbio.2021.101257. [DOI] [Google Scholar]
- 84.Lee S.M., Oh J., Hurh B.-S., Jeong G.-H., Shin Y.-K., Kim Y.-S. Volatile Compounds Produced by Lactobacillus paracasei during Oat Fermentation. J. Food Sci. 2016;81:C2915–C2922. doi: 10.1111/1750-3841.13547. [DOI] [PubMed] [Google Scholar]
- 85.Davila M., Muniz A., Du X. The Impact of Roasting and Steaming on Savory Flavors Contributed by Amino Acids, 5′-Nucleotides, and Volatiles in Agaricus bisporus Mushrooms. Int. J. Gastron. Food Sci. 2022;30:100590. doi: 10.1016/j.ijgfs.2022.100590. [DOI] [Google Scholar]
- 86.Wu C., Li T., Qi J., Jiang T., Xu H., Lei H. Effects of Lactic Acid Fermentation-Based Biotransformation on Phenolic Profiles, Antioxidant Capacity and Flavor Volatiles of Apple Juice. LWT. 2020;122:109064. doi: 10.1016/j.lwt.2020.109064. [DOI] [Google Scholar]
- 87.Liang Z., Yang C., He Z., Lin X., Chen B., Li W. Changes in Characteristic Volatile Aroma Substances during Fermentation and Deodorization of Gracilaria Lemaneiformis by Lactic Acid Bacteria and Yeast. Food Chem. 2023;405:134971. doi: 10.1016/j.foodchem.2022.134971. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y., Zhang C., Liu F., Jin Z., Xia X. Ecological Succession and Functional Characteristics of Lactic Acid Bacteria in Traditional Fermented Foods. Crit. Rev. Food Sci. Nutr. 2022:1–15. doi: 10.1080/10408398.2021.2025035. [DOI] [PubMed] [Google Scholar]
- 89.Guo Y., Chen D., Dong Y., Ju H., Wu C., Lin S. Characteristic Volatiles Fingerprints and Changes of Volatile Compounds in Fresh and Dried Tricholoma Matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018;1099:46–55. doi: 10.1016/j.jchromb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 90.Xu X., Xu R., Jia Q., Feng T., Huang Q., Ho C.-T., Song S. Identification of Dihydro-β-Ionone as a Key Aroma Compound in Addition to C8 Ketones and Alcohols in Volvariella volvacea Mushroom. Food Chem. 2019;293:333–339. doi: 10.1016/j.foodchem.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Kleofas V., Popa F., Fraatz M.A., Rühl M., Kost G., Zorn H. Aroma Profile of the Anise-like Odour Mushroom Cortinarius Odorifer. Flavour Fragr. J. 2015;30:381–386. doi: 10.1002/ffj.3250. [DOI] [Google Scholar]
- 92.Borthakur M., Gurung A.B., Bhattacharjee A., Joshi S.R. Analysis of the Bioactive Metabolites of the Endangered Mexican Lost Fungi Campanophyllum—A Report from India. Mycobiology. 2020;48:58–69. doi: 10.1080/12298093.2020.1723388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta P., Banerjee A., Castillo A., Bandopadhyay R. Novel Phenolic Compound from Southern Ocean Microalgae Chlorella Sp. PR-1 and Its Antibacterial Activity. Gayana Bot. 2021;78:29–37. doi: 10.4067/S0717-66432021000100029. [DOI] [Google Scholar]
- 94.Chun S., Chambers E., Han I. Development of a Sensory Flavor Lexicon for Mushrooms and Subsequent Characterization of Fresh and Dried Mushrooms. Foods. 2020;9:980. doi: 10.3390/foods9080980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaneko D., Toet A., Brouwer A.-M., Kallen V., van Erp J.B.F. Methods for Evaluating Emotions Evoked by Food Experiences: A Literature Review. Front. Psychol. 2018;9:911. doi: 10.3389/fpsyg.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tepsongkroh B., Jangchud K., Jangchud A., Chonpracha P., Ardoin R., Prinyawiwatkul W. Consumer Perception of Extruded Snacks Containing Brown Rice and Dried Mushroom. Int. J. Food Sci. Technol. 2020;55:46–54. doi: 10.1111/ijfs.14220. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.