Abstract
Background: The prognosis of patients with chest pain after a negative exercise test is good, but some adverse events occur in this low-risk group. The aim of our study was to identify predictors of long-term adverse events after a negative exercise test in patients with chest pain and a lower intermediate (15–65%) pre-test probability of coronary artery disease (CAD) and to assess the prognostic value of exercise electrocardiography and exercise stress echocardiography in this group of patients. Methods: We identified from our stress test laboratory database 862 patients with chest pain without previously known CAD and with a pre-test probability of CAD ranging from 15 to 65% (mean 41 ± 14%) who underwent exercise testing. Patients were followed for the occurrence of death, non-fatal myocardial infarction (MI) and clinically guided revascularization. Results: During the median follow-up of 94 months, 87 patients (10.1%) had an adverse event (AE). A total of 30 patients died (3.5%), 23 patients suffered non-fatal MI (2.7%) and 34 patients (3.9%) had clinically guided revascularization (20 patients percutaneous and 14 patients surgical revascularizations). Male gender, age, the presence of diabetes and a slow heart rate recovery (HRR) in the first minute after exercise were independently related to the occurrence of AEs. Adverse events occurred in 10.3% of patients who were tested by exercise stress echocardiography and in 10.0% of those who underwent stress electrocardiography (p = 0.888). Conclusion: The risk of AEs after negative exercise testing in patients with a pre-test probability of CAD of 15–65% is low. Male patients with a history of diabetes and slow HRR in the first minute after exercise have an increased risk of an adverse outcome.
Keywords: chest pain, negative exercise testing, prognosis, predictors, heart rate recovery
1. Introduction
Exercise testing with or without imaging still has an important role in everyday clinical practice in patients with chest pain both for the diagnosis and risk stratification of coronary artery disease (CAD). It has been demonstrated that the presence of inducible ischemia carries a 5–10-fold increased risk for the occurrence of adverse events (AEs) [1,2,3].
However, in recent years, the number of tests positive for myocardial ischemia is decreasing and is currently relatively low (10–15%) [4]. A large meta-analysis including more than 11,000 patients showed that such a negative exercise test coupled with imaging (myocardial perfusion imaging or exercise stress echocardiography) carries good prognosis during a mean follow-up period of around 3 years [5]. A more recent meta-analysis confirmed previous findings showing an annual event risk of death and non-fatal myocardial infarction (MI) of 0.90% after negative exercise electrocardiography, and of 1.77% after negative exercise stress echocardiography after a median follow-up of around 2 years. The higher event rate in the group tested by exercise stress echocardiography can be attributed to the higher population event risk, which reflects common clinical practice where the patients with a higher probability of CAD are referred to exercise testing coupled with imaging [6]. Both meta-analyses had relatively short follow-up periods, included both patients with suspected and known CAD and were not able to identify predictors of adverse events. Data on long-term follow-up assessing the predictors of adverse outcome in patients without known CAD after a negative exercise test are relatively scarce. Adverse events after a negative exercise test may occur in patients with significant coronary artery stenosis that has not been identified by the test (false negative results). On the other hand, a large multicentric study comparing the prognostic value of coronary computed tomography angiography (CCTA) and functional testing showed that a significant number of adverse cardiac events occurred in patients with subclinical atherosclerosis and a negative exercise test [7] due to the presence of non-significant coronary artery stenosis, which cannot be identified by conventional exercise testing but may be responsible for the occurrence of acute events. These data suggest that some AEs occur in the low-risk group of patients and underscore the clinical need to further refine risk stratification in patients with a negative exercise test. Vulnerable patients prone to AEs, having lipid-rich atherosclerotic plaques with a thin cap, can be reliably identified using intracoronary imaging such as optical coherence tomography during invasive coronary angiography [8,9,10]. However, such an approach is related to radiation exposure and high costs. On the other hand, it has been shown that easily obtainable markers of autonomic nervous system activity, such as chronotropic incompetence [11,12] and heart rate recovery (HRR) after exercise [13,14], may identify patients with a pronounced risk of adverse outcome.
Therefore, the aim of our study was to identify predictors of long-term AEs after negative exercise in patients with chest pain and a lower intermediate (15–65%) pre-test probability of CAD. It was of additional interest to assess the prognostic value of negative exercise electrocardiography and exercise stress echocardiography in this group of patients.
2. Materials and Methods
2.1. Patient Population
We identified from our stress test laboratory database 1005 patients with chest pain without previous CAD (known significant coronary artery stenosis, previous MI and/or coronary revascularization) and with pre-test probability of CAD ranging from 15 to 65%, based on recommended clinical algorithm [15], who underwent exercise testing, stress electrocardiography or exercise stress echocardiography for the evaluation of chest pain from January 2007 to December 2008. Patients with uninterpretable electrocardiograms (ECGs) (left bundle branch block, Wolf–Parkinson–White syndrome and baseline ST-T abnormalities that preclude ECG interpretation), as well as patients with clinical complaints other than chest pain and those having non-cardiac conditions that affect ability to exercise were not considered eligible.
A detailed interview and clinical examination were performed prior to exercise testing in all patients for the assessment of nature of symptoms (typical vs. atypical chest pain) with the estimation of pre-test probability of CAD as previously described [15]. Diabetes mellitus [16], arterial hypertension [17] and hypercholesterolemia [18] were defined according to standard criteria. In addition, smoking status and family history of premature cardiovascular disease were assessed in all patients.
A total of 120 out of 1005 patients (11.9%) had positive exercise test defined as horizontal or down-sloping ST segment depression at 80 ms after the J point of at least 1 millimetre in at least 3 consecutive beats in 2 contiguous leads in the case of exercise ECG or as the development of new wall motion abnormality in at least 2 adjacent segments of left ventricle in the case of exercise stress echocardiography and were excluded from further analysis. After the exclusion of patients lost to follow-up (23 patients), final study population comprised 862 patients.
Patients were followed by phone contact for the occurrence of death, MI or clinically guided revascularization. To avoid misclassification of the cause of death, overall mortality was considered [19,20]. Myocardial infarction was defined by typical symptoms and electrocardiographic and cardiac enzyme changes and confirmed by discharge summary diagnosis.
2.2. Exercise Testing
All patients underwent maximal exercise test on treadmill using standard Bruce protocol. Immediately after exercise, patients lay down in a supine position. The decision to perform exercise ECG or exercise stress echocardiography was left to the discretion of physician performing exercise testing. Data on exercise duration, resting and peak heart rate, as well as on HRR in first minute after exercise, were recorded. Abnormal HRR was defined as ≤18 beats/min, as previously described and validated [21,22]. Chronotropic index, as a measure of chronotropic incompetence, was calculated by a formula [(peak heart rate–resting heart rate)/(220–age–resting heart rate)] and considered abnormal if <0.80 [23]. Blood pressure was monitored at baseline and at each stage of the exercise. Presence of symptoms during testing was assessed. Beta blockers were stopped for 48 h prior to exercise testing in all patients.
Exercise stress echocardiography was performed according to standard procedure. All echocardiographic images were obtained at rest and within 1 min after the peak exercise in recumbent (left lateral decubitus) position and digitally stored for analysis. Regional wall motion was assessed using 17-segment model as recommended [24].
2.3. Statistical Analysis
Continuous variables were reported as mean ± SD, and differences were assessed with the unpaired t test or Mann–Whitney U test as appropriate. Normal distribution of all continuous variables was confirmed by Kolmogorov–Smirnov test. Categorical variables were reported as percentages and compared between groups by chi-square test. Univariate analysis was used to evaluate the relation between various clinical and hemodynamic variables during exercise and occurrence of adverse events in the follow-up period. Event-free survival curves for adverse events were estimated by Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate (enter method) Cox proportional hazards models were used to assess predictors of adverse events. A significance of 0.05 was required for a variable to be included into the multivariate model. Hazard ratios with the corresponding 95% confidence intervals were estimated. Statistical significance was defined as p ˂ 0.05. Statistical Package for the Social Sciences (SPSS release 25.0, Chicago, IL, USA) was used for the analysis.
3. Results
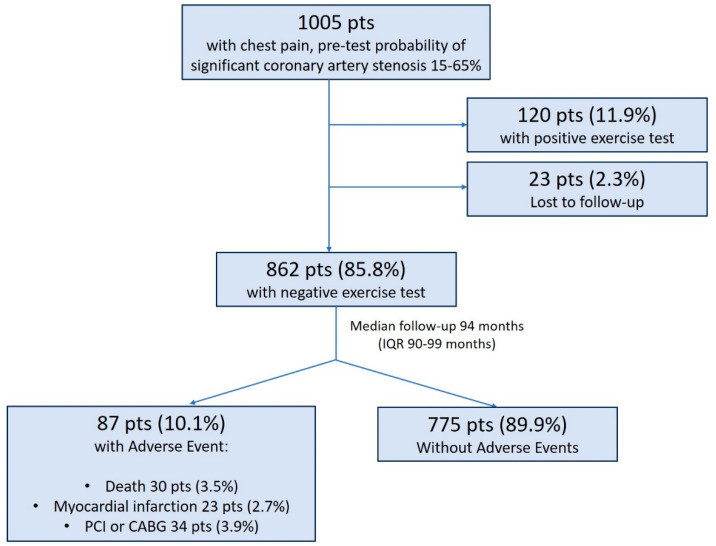
The final study population comprised 862 patients (mean age 56 ± 10 years, 42% of male patients). The mean pre-test probability of CAD was 41 ± 14%. A study flow chart with the main outcome results is presented in Figure 1.
Figure 1.
Study flow chart with main outcome results.
Baseline clinical characteristics of the study population and exercise data are summarized in Table 1. Patients were treated with statins (202 (24%)), acetylsalicylic acid (366 (44%)), ACE inhibitors/angiotensin receptor blockers (203 (24.4%)) and calcium channel blockers (181 (21%)).
Table 1.
Baseline clinical characteristics and exercise data.
| Variable | All Patients (n = 862) | Patients with AE a (n = 87, 10.1%) | Patients without AE a (n = 775, 89.9%) | p Value |
|---|---|---|---|---|
| Male gender | 364 (42.2%) | 56 (64.4%) | 308 (39.7%) | <0.001 |
| Age (years) | 56 ± 10 | 60 ± 10 | 55 ± 10 | <0.001 |
| Hypertension | 603 (70%) | 67 (77%) | 536 (69.2%) | 0.130 |
| Hyperlypoproteinemia | 488 (56.6%) | 50 (57.5%) | 438 (56.5%) | 0.865 |
| Smoker | 326 (37.9%) | 38 (44.2%) | 288 (37.2%) | 0.203 |
| Diabetes | 118 (13.7%) | 24 (27.6%) | 94 (12.1%) | <0.001 |
| Family history of CAD b | 509 (59%) | 49 (56.3%) | 460 (59.4%) | 0.585 |
| Typical chest pain | 403 (48.4%) | 71 (81.6%) | 498 (64.3%) | 0.001 |
| Duration of the test (minutes) | 7.4 ± 2.7 | 7.0 ± 2.7 | 7.5 ± 2.8 | 0.132 |
| Chronotropic index < 0.8 | 411 (47.7%) | 54 (62.1%) | 364 (47%) | 0.008 |
| Achieved target heart rate | 674 (78.2%) | 62 (71.3%) | 612 (79%) | 0.099 |
| Maximum achieved SBP c (mmHg) | 180 ± 21 | 183 ± 21 | 179 ± 22 | 0.126 |
| Maximum achieved DBP d (mmHg) | 100 ± 11 | 100 ± 11 | 100 ± 11 | 0.946 |
| Slow HRR e | 45 (5.2%) | 11 (12.6%) | 34 (4.4%) | 0.001 |
a AEs—adverse events (death + myocardial infarction + CABG + PCI), b CAD—coronary artery disease, c SBP—systolic blood pressure, d DBP—diastolic blood pressure, e HRR—heart rate recovery.
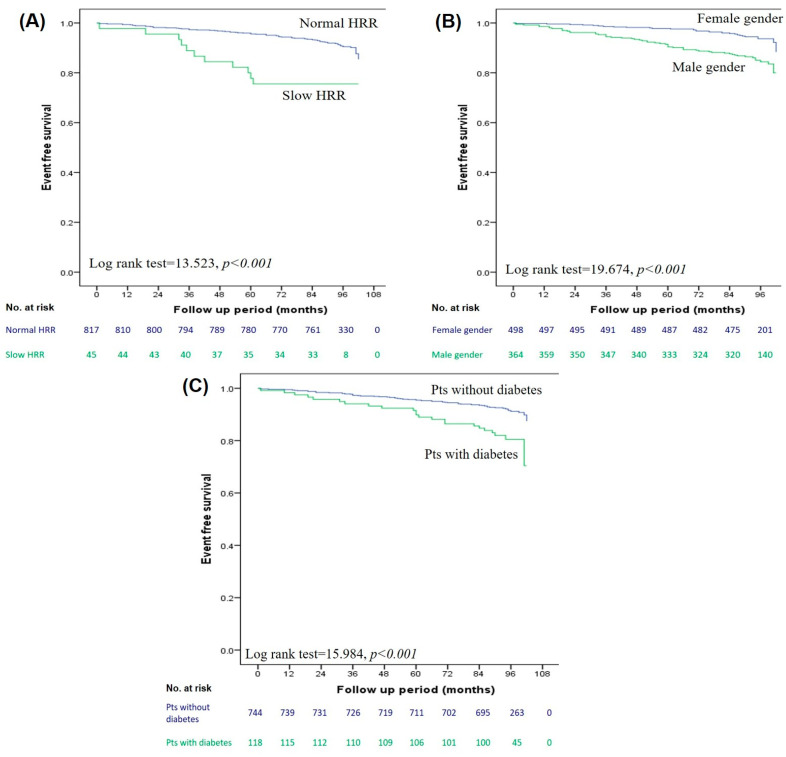
During the median follow-up of 94 months (IQR 90–99 months), 87 patients (10.1%) had an AE. A total of 30 patients died (3.5%), 23 patients suffered non-fatal MI (2.7%) and 34 patients (3.9%) had clinically guided revascularization (20 patients percutaneous and 14 patients surgical revascularizations). The annual rate of AEs was 1.3%. The median time to AE was 60 months (IQR 30–84 months). Patients with AEs were predominantly males, were older and had a significantly higher prevalence of diabetes and typical chest pain. Regarding exercise data, patients with AEs had a higher prevalence of slow HRR and impaired chronotropic index. A comparison of clinical and exercise data between patients with and without AEs is presented in Table 1. Univariate predictors of AEs are summarized in Table 2. Patients with a slow HRR after exercise had a significantly lower event-free survival time in comparison to patients with a preserved HRR (86.7 ± 4.2 vs. 97.9 ± 0.5 months, log-rank test 13.523, p < 0.001) (Figure 2A), as well as male patients (94.2 ± 1.1 vs. 99.6 ± 0.5 months for females, log-rank test 19.674, p < 0.001) (Figure 2B) and those with diabetes (92.9 ± 2.1 vs. 98.1 ± 0.6 months for non-diabetic, log-rank test 15.984, p < 0.001) (Figure 2C). Male gender, age, the presence of diabetes and a slow HRR were independently related to the occurrence of AE (Table 2).
Table 2.
Univariate and multivariate Cox proportional hazards analysis for AE a.
| Variable | HR h | Univariate Analysis | B | p Value | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI i | HR h | 95% CI i | p Value | ||||||
| Lower Level | Upper Level | Lower Level | Upper Level | ||||||
| Male gender | 2.600 | 1.676 | 4.032 | 0.955 | <0.001 | 2.525 | 1.441 | 4.425 | 0.001 |
| Age (years) | 1.045 | 1.021 | 1.070 | 0.044 | <0.001 | 1.042 | 1.017 | 1.067 | 0.001 |
| Hypertension | 1.542 | 0.935 | 2.543 | 0.433 | 0.089 | ||||
| Hyperlipoproteinemia | 1.086 | 0.698 | 1.634 | 0.066 | 0.762 | ||||
| Smoking | 1.395 | 0.910 | 2.139 | 0.333 | 0.126 | ||||
| Diabetes | 2.523 | 1.576 | 4.038 | 0.925 | <0.001 | 1.891 | 1.171 | 3.055 | 0.009 |
| Family history of CAD b | 0.894 | 0.585 | 1.365 | −0.113 | 0.603 | ||||
| Typical chest pain | 2.495 | 1.449 | 4.296 | 0.914 | 0.001 | 0.838 | 0.420 | 1.670 | 0.615 |
| SECHO c/SECG d | 0.989 | 0.641 | 1.525 | −0.011 | 0.959 | ||||
| Duration of the test (minutes) | 0.945 | 0.875 | 1.021 | −0.056 | 0.151 | ||||
| Achieved target heart rate | 0.682 | 0.429 | 1.086 | −0.382 | 0.107 | ||||
| Maximum achieved SBP e | 1.007 | 0.997 | 1.017 | 0.007 | 0.157 | ||||
| Maximum achieved DBP f | 1.000 | 0.982 | 1.019 | 0.000 | 0.976 | ||||
| Chronotropic index < 0.8 | 1.765 | 1.144 | 2.724 | −0.568 | 0.010 | 1.493 | 0.955 | 2.332 | 0.079 |
| Slow HRR g | 3.084 | 1.638 | 5.808 | 1.126 | <0.001 | 2.024 | 1.041 | 3.939 | 0.038 |
a AEs—adverse events (death + myocardial infarction + CABG + PCI), b CAD—coronary artery disease, c SECHO—stress echocardiography, d SECG—stress electrocardiography, e SBP—systolic blood pressure, f DBP—diastolic blood pressure, g HRR—heart rate recovery, h HR—hazard ratio, i CI—confidence interval.
Figure 2.
Kaplan–Meier analysis for adverse events according to heart rate recovery (A), gender (B), diabetes (C).
Overall, stress ECG was performed in 541 patients (63%), whereas exercise stress echocardiography was performed in 321 patients (37%). There was no significant difference in patient characteristics and exercise data between patients undergoing stress electrocardiography and those undergoing exercise stress echocardiography except for a somewhat higher pre-test probability of CAD in the latter group (Table 3). The rate of AEs was similar in two groups. Adverse events occurred in 10.3% of patients who were tested by exercise stress echocardiography and in 10.0% of those who underwent stress electrocardiography (p = 0.888).
Table 3.
Comparison of baseline clinical characteristics, exercise data and outcome in patients tested with exercise stress echocardiography and stress electrocardiography.
| Variable | All Patients (n = 862) |
SECHO a
(n = 321, 37.2%) |
Stress ECG b
(n = 541, 62.8%) |
p Value |
|---|---|---|---|---|
| Male gender | 364 (42.2%) | 137 (42.7%) | 227 (42%) | 0.836 |
| Age | 56 ± 10 | 56 ± 9 | 56 ± 10 | 0.245 |
| Hypertension | 603 (70%) | 227 (70.7%) | 376 (69.5%) | 0.707 |
| Hyperlypoproteinemia | 488 (56.6%) | 190 (59.2%) | 298 (55.1%) | 0.240 |
| Smoker | 326 (37.9%) | 111 (34.7%) | 215 (39.7%) | 0.140 |
| Diabetes | 118 (13.7%) | 45 (14%) | 73 (13.5%) | 0.828 |
| Family history of CAD c | 509 (59%) | 190 (59.2%) | 319 (59%) | 0.948 |
| Typical chest pain | 293 (34%) | 119 (37.1%) | 174 (32.2%) | 0.141 |
| PTP d (%) | 40.7 ± 13.9 | 42.3 ± 14 | 39.7 ± 13.8 | 0.009 l |
| Duration of the test (minutes) | 7.4 ± 2.7 | 7.4 ± 2.9 | 7.5 ± 2.7 | 0.764 |
| Chronotropic index < 0.8 | 418 (48.5%) | 158 (49.2%) | 260 (48.1%) | 0.761 |
| Achieved target heart rate | 674 (78.2%) | 255 (79.4%) | 419 (77.4%) | 0.494 |
| Maximum achieved SBP e (mmHg) | 180 ± 21.1 | 180 ± 21.2 | 180.1 ± 21.1 | 0.814 |
| Maximum achieved DBP f (mmHg) | 100.4 ± 11.3 | 99.9 ± 12 | 100.5 ± 10.8 | 0.509 |
| Slow HRR g | 45 (5.2%) | 16 (5%) | 29 (5.4%) | 0.810 |
| AE h | 87 (10.1%) | 33 (10.3%) | 54 (10.0%) | 0.888 |
a SECHO—exercise stress echocardiography, b ECG—electrocardiogram, c CAD—coronary artery disease, d PTP—pre-test probability (Diamond–Forrester), e SBP—systolic blood pressure, f DBP—diastolic blood pressure, g HRR—heart rate recovery, h AEs—adverse events (death + myocardial infarction + CABG + PCI).
4. Discussion
The current study demonstrated that patients with chest pain and a pre-test probability of CAD of 15–65% have a low incidence of adverse events after a normal exercise test during long-term follow-up. The presence of diabetes and an impaired heart rate recovery in the first minute after exercise can further identify those with a poorer outcome.
A negative exercise test is a common finding in everyday clinical practice. The recent meta-analysis revealed a negative finding in 72% of exercise stress echocardiography tests (36 studies, including 28% of patients with known CAD), whereas the negativity rate was 75% for studies using stress electrocardiography (21 studies, including 23% of patients with known CAD) [6]. Similarly, a recent study from high-volume centers reported 80% of negative findings in exercise stress echocardiography after the 2000s in patients without previously known CAD [25]. The rate of the positive exercise test was even lower in our group of patients (11.9%). This difference probably reflects the fact that, in our study, we included only patients with a lower intermediate pre-test probability of CAD. The vast majority of our patients had a negative result in exercise testing, and such a finding carries an excellent prognosis. During a median follow-up of 94 months, 10.1% of patients with a negative test had an AE defined as death, MI or clinically guided revascularization with an annual event rate of 1.3%. When accounted for hard events (death, MI), the annual rate was 0.84%. A large meta-analysis evaluating the prognostic value of negative non-invasive cardiac investigations in patients with suspected or known CAD revealed annualized event rates of cardiac death and MI of 1.77% with exercise stress echocardiography and 0.9% with stress electrocardiography [6]. An adverse event rate, defined as an overall mortality or MI, of >2% was observed in negative exercise stress echocardiography tests after the 2000s in a recent study [25]. A study by Bangalore et al. confirmed that the negative exercise test also has a low hard cardiac event rate (less than 1% per year) in patients stratified according to different pre-test probabilities for the presence of CAD [26]. A significant proportion of AEs in our study were clinically guided revascularizations (percutaneous or surgical), with an annualized rate of 0.46%. In a previously published meta-analysis, the annual rate of unstable angina and revascularization was 0.95% after negative exercise stress echocardiography testing in patients with known or suspected CAD [5]. According to published data, even when both positive and negative test results are considered, the 60-day revascularization rate after an exercise test remains low (2.7%) in patients without previously known CAD [27], whereas the annual rate of revascularization after negative exercise stress echocardiography in patients without known CAD was reported to be around 3% [25]. Moreover, patients with severe to moderate ischemia, confirmed by different non-invasive tests, treated by optimal medical therapy have the same risk of ischemic cardiovascular events or death as those treated by revascularization, so a further decrease in revascularization procedures is expected [28]. A significantly lower revascularization rate after negative exercise testing in the current study confirms the low-risk nature of our study population. In our study, AEs occurred with a median of 60 months after negative exercise testing, with the range from 1 to 87 months. The early occurrence of AEs, especially clinically guided revascularizations, can be attributed to false negative test results. On the other hand, AEs in later stages of follow-up may reflect disease progression even in this group of low-risk patients. In addition, some of the AEs might be caused by the rupture of non-obstructive plaques that cannot be identified by exercise testing.
In this group of patients with negative exercise test results, the risk could be further stratified with the interaction of clinical characteristics (male gender, age, presence of diabetes) and a slow HRR in the first minute after exercise. Recently, the importance of classical risk factors for risk stratification has once more been emphasized in the contemporary patient population with chest pain and normal functional testing [7]. Namely, the addition of the Framingham Risk Score in a large-scale PROMISE trial significantly improved the discriminatory capacity of functional testing, which rendered the comparison to anatomic testing using CCTA non-significant [7]. Similarly, a study by Cortigiani et al. using exercise stress echocardiography for prognostic assessment in 14,140 patients, of whom 2835 were diabetics, showed that the prognosis after negative exercise stress echocardiography is far less benign in diabetic patients than in non-diabetic patients [29]. Our results confirm these findings and demonstrate that the presence of diabetes is a marker of a less favorable prognosis even in low-risk patients, i.e., younger patients without known CAD and without inducible ischemia.
The drop in heart rate after exercise reflects the interplay between sympathetic withdrawal and parasympathetic reactivation [30,31], with the latter one being clinically more important since it has been shown that a reduced vagal activity has an adverse impact on mortality [32]. A slow HRR has been related to an increase in overall mortality [33,34,35] and increased incidence of cardiovascular events [13,14]. The prognostic value of HRR has been demonstrated in the whole spectrum of subjects: in apparently healthy and asymptomatic populations [13,14] and in patients with suspected and known CAD [21,36,37,38]. Our data extend previous knowledge by demonstrating that an impaired HRR, defined as the inability to decrease the heart rate for more than 18 beats in the first minute after exercise, retains the prognostic ability for the occurrence of AEs in the highly selected group of patients with chest pain, a lower intermediate pretest probability of CAD and clearly negative test results. An impaired HRR was associated with a higher incidence of overt and silent myocardial ischemia in long-term follow-up [39], suggesting its relation to CAD progression, a finding that might also explain some AEs that occurred late in our study.
From a pathophysiological point of view, a slow HRR after exercise has been linked to endothelial dysfunction [40], inflammation [41], increased arterial stiffness [42] and insulin resistance [43], factors that may accelerate the progression of atherosclerosis. Moreover, patients with an impaired HRR are more likely to have subclinical atherosclerosis, demonstrated by higher values of the coronary artery calcium score [44]. Additionally, patients with a slow HRR after exercise have impaired fibrinolysis expressed as elevated plasminogen activity inhibitor-1 activity, tissue plasminogen activator antigen and fibrinogen with a pronounced risk of atherothrombosis [45]. A higher incidence of AEs in patients with an impaired HRR observed in our study can be explained, on one hand, by a faster progression of atherosclerosis requiring revascularization. On the other hand, patients with an impaired HRR might have a higher prevalence of subclinical disease, which, together with endothelial dysfunction, inflammation and impaired fibrinolysis, may lead to MI and death even in the absence of obstructive CAD.
The last European Guidelines on chronic ischemic heart disease recommend the use of exercise testing coupled with imaging rather than exercise ECG alone for the detection of CAD [46]. Since other mechanisms than coronary artery stenosis may underly chest pain, multimodality imaging integrating “anatomical” and “functional” information is required to elucidate the exact mechanism of chest pain and myocardial ischemia [47].
In our study, we assessed the prognostic value of exercise electrocardiography and exercise stress echocardiography, two widely available tests in everyday clinical practice. Our data showed a similar rate of AEs in patients undergoing exercise stress electrocardiography and exercise stress echocardiography, similarly to some previously published data [48,49]. However, we were not able to compare the prognostic difference between the two diagnostic methodologies with current retrospective analysis. The decision to perform exercise electrocardiography or exercise stress echocardiography was left to the discretion of the physician performing exercise testing. A higher pre-test probability for the presence of obstructive CAD observed in patients referred to exercise stress echocardiography, although reflecting common clinical practice, might impact our study results. Additionally, the negativity of exercise stress echocardiography was based only on the absence of wall motion abnormalities. There is evidence of the declining prognostic value of a negative exercise stress echocardiography based only on regional wall motion abnormalities in contemporary populations [25]. In our study, we did not use a detailed echocardiography evaluation with the assessment of LV volumes, ejection fraction, LV contractile reserve, coronary flow velocity reserve in the left anterior descending artery and pulmonary congestion by the identification of lung B-lines. This ABCDE stress echo protocol is an effective predictor of survival in patients with chronic coronary syndrome, including those with suspected CAD who do not exhibit wall motion abnormalities during exercise or the pharmacological test [50]. At the time when the study was conducted, we routinely assessed only the wall motion abnormalities, so we could not identify all the patients prone to AEs. Moreover, the analysis of global longitudinal strain (GLS) at rest can better identify the presence of significant coronary artery stenosis, defined as the presence of a luminal narrowing of 50%, than stress vasodilator echocardiography by the analysis of the wall motion score index and coronary flow velocity reserve in the left anterior descending coronary artery in patients with a preserved left ventricular ejection fraction [51]. The number of patients with significant coronary artery stenosis would have increased in our study had we conducted an analysis of GLS during rest. The exclusion of these patients with significant but undiagnosed CAD from further analysis would have increased the prognostic value of negative exercise stress echocardiography in the current study.
Overall, patients with a negative exercise test and a lower intermediate pre-test probability of obstructive CAD have good prognosis. It has to be emphasized that a simple marker such as an impaired HRR has a considerable prognostic impact and, above all, is considerably cost saving, the latter being important nowadays [52,53]. Based on our results, we recommend that data on HRR should be included in every exercise testing report.
Study Limitations
The first limitation of our study is that it represents a single center experience; however, the data come from a high-volume certified center [54] experienced in performing and interpreting stress ECG and exercise stress echocardiography. The European Society of Cardiology Guidelines on chronic coronary syndrome recommended a new model for the assessment of pre-test probability for the presence of CAD that is mainly based on patients from countries with a low cardiovascular disease risk. However, our patient population comes from a country with a high prevalence of CAD; therefore, we used a previously recommended algorithm for pre-test probability assessment [15]. This tool evaluates the pre-test probability of a coronary artery stenosis of more than 50% during invasive angiography. Nevertheless, in our study, we considered the clinical outcome as the endpoint. These two measurements differ for several reasons. Firstly, the overall death could be unrelated to CAD. Moreover, angina may be caused by a stenosis of less than 50% with a hemodynamic significance or with a non-obstructive mechanism (i.e., angina with non-obstructive coronary arteries), such as microvascular dysfunction [55,56] or vasospasm [57]. In addition, it was highlighted that 6–8% of MIs may develop in the presence of non-significant coronary stenosis [58]. These patients may be identified by invasive coronary angiography or CCTA. However, we did not collect data on coronary angiography if it was not followed by revascularization. Finally, long-term outcomes were evaluated, so even if significant CAD might have been absent at the time of the exercise test, it could have developed during the follow-up period. Coronary revascularization was included as an outcome in the current study. The decision to perform coronary angiography and revascularization was made by referring physicians so we cannot exclude the possibility that some of the revascularizations were performed on intermediate stenosis without proof of ischemia. On the other hand, we were not aware of the data if additional functional testing that led to revascularization was performed during the follow-up period. In addition, we were not aware of the changes in medical therapy or life-style modification during the follow-up period so we cannot exclude the possibility that these changes might affect the outcome of the patients. We analyzed only patients undergoing exercise testing, so our data cannot be extrapolated to patients undergoing pharmacological testing. Therefore, patients unable to exercise, such as the elderly or patients with peripheral artery disease, who are at high risk for CAD, were not analyzed in the current study [59]. In this study, we could only analyze the data obtained on HRR in the first minute after exercise. Although it has been postulated that 2 min HRR might be more sensitive in predicting the risk of cardiovascular events than 1 min HRR [30], the latter has been previously validated in a study using exercise stress echocardiography for the detection of myocardial ischemia with patients in a supine position after exercise [11].
5. Conclusions
The risk of adverse events after negative exercise testing in patients with a pre-test probability of CAD of 15–65% is low. However, male patients with a history of diabetes and slow HRR in the first minute after exercise have an increased risk of an adverse outcome and require special clinical attention.
Acknowledgments
This study was supported by Ministry of Science, Education and Technological Development of the Republic of Serbia (Project No. 41022), by technical support (equipment and materials).
Author Contributions
Conceptualization, V.G. and A.D.-D.; data analysis, V.G., A.D.-D., B.B. and N.B.; exercise test analysis, V.G., A.D.-D., N.B. and I.N.; clinical follow-up, M.T., I.J., I.P. and S.A. writing—original draft preparation, V.G. and A.D.-D.; review and editing, A.D.-D., B.B. and G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee od School of Medicine, Belgrade University for the Scientific Project No. 41022—Ministry of Science, Education and Technological Development of the Republic of Serbia.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hachamovitch R., Berman D.S., Shaw L.J., Kiat H., Cohen I., Cabico J.A., Friedman J., Diamond G.A. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.CIR.97.6.535. [DOI] [PubMed] [Google Scholar]
- 2.Marwick T.H., Case C., Vasey C., Allen S., Short L., Thomas J.D. Prediction of mortality by exercise echocardiography: A strategy for combination with the Duke treadmill score. Circulation. 2001;103:2566–2571. doi: 10.1161/01.CIR.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 3.Yao S.S., Qureshi E., Sherrid M.V., Chaudhry F.A. Practical applications in stress echocardiography: Risk stratification and prognosis in patients with known or suspectedischemic heart disease. J. Am. Coll. Cardiol. 2003;42:1084–1090. doi: 10.1016/S0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 4.Rozanski A., Gransar H., Hayes S.W., Min J., Friedman J.D., Thomson L.E., Berman D.S. Temporal Trends in the Frequency of Inducible Myocardial Ischemia During Cardiac Stress Testing: 1991 to 2009. J. Am. Coll. Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Metz L.D., Beattie M., Hom R., Redberg R.F., Grady D., Fleischmann K.E. The Prognostic Value of Normal Exercise Myocardial Perfusion Imaging and Exercise Echocardiography: A Meta-Analysis. J. Am. Coll. Cardiol. 2007;49:227–237. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Smulders M.W., Jaarsma C., Nelemans J.P., Bekkers S.C.A.M., Bucerius J., Leiner T., Crijns H.J.G.M., Wildberger J.E., Schalla S. Comparison of the prognostic value of negative non-invasive cardiac investigations in patients with suspected or known coronary arterydisease–a meta-analysis. Eur. Heart J. Cardiovasc. Imaging. 2017;18:980–987. doi: 10.1093/ehjci/jex014. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann U., Ferencik M., Udelson J.E., Picard M.H., Truong Q.A., Patel M.R., Huang M., Pencina M., Mark D.B., Heitner J.F., et al. PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) Circulation. 2017;135:2320–2332. doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguire A.D., Arbab-Zadeh A., Soeda T., Fuster V., Jang I.K. Optical Coherence Tomography of Plaque Vulnerability and Rupture: JACC Focus Seminar Part 1/3. J. Am. Coll. Cardiol. 2021;78:1257–1265. doi: 10.1016/j.jacc.2021.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo T., Ino Y., Mintz G.S., Shiono Y., Shimamura K., Takahata M., Terada K., Higashioka D., Emori H., Wada T., et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging. 2021;22:1376–1384. doi: 10.1093/ehjci/jeab028. [DOI] [PubMed] [Google Scholar]
- 10.Fabris E., Berta B., Roleder T., Hermanides R.S., Ijsselmuiden A.J., Kauer F., Alfonso F., von Birgelen C., Escaned J., Camaro C., et al. Thin-Cap Fibroatheroma Rather Than Any Lipid Plaques Increases the Risk of Cardiovascular Events in Diabetic Patients: Insights From the COMBINE OCT–FFR Trial. Circ. Cardiovasc. Interv. 2022;15:011728. doi: 10.1161/CIRCINTERVENTIONS.121.011728. [DOI] [PubMed] [Google Scholar]
- 11.Lauer M.S., Okin P.M., Larson M.G., Evans J.C., Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.CIR.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 12.Lauer M.S., Mehta R., Pashkow F.J., Okin P.M., Lee K., Marwick T.H. Association of chronotropic incompetence with echocardiographic ischemia and prognosis. J. Am. Coll. Cardiol. 1998;32:1280–1286. doi: 10.1016/S0735-1097(98)00377-5. [DOI] [PubMed] [Google Scholar]
- 13.Mora S., Redberg R.F., Cui Y., Whiteman M.K., Flaws J.A., Sharrett A.R., Blumenthal R.S. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 14.Morshedi-Meibodi A., Larson M.G., Levy D., O’donnell C.J., Vasan R.S. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study) Am. J. Cardiol. 2002;90:848–852. doi: 10.1016/S0002-9149(02)02706-6. [DOI] [PubMed] [Google Scholar]
- 15.Genders T.S., Steyerberg E.W., Alkadhi H., Leschka S., Desbiolles L., Nieman K., Galema T.W., Meijboom W.B., Mollet N.R., de Feyter P.J., et al. A clinical prediction rule for the diagnosis of coronary artery disease: Validation, updating, and extension. Eur. Heart J. 2011;32:1316–1330. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 16.Handelsman Y., Bloomgarden Z., Grunberger G., Umpierrez G., Zimmerman R.S., Bailey T.S., Blonde L., Bray G.A., Cohen A.J., Dagogo-Jack S., et al. American Association of Clinical Endocrinologist and American College of Endocrinology-a clinical practical guideline for developing a diabetes melitus comprehensive care plan-2015. AACE/ACE Diabetes Guidel. Endocr. Pract. 2015;21((Suppl. 1)):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur. Heart J. 2007;28:1462–1536. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 18.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., Hoes A.W., Jennings C.S., Landmesser U., Pedersen T.R., et al. ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 19.Lauer M.S., Blackstone E.H., Young J.B., Topol E.J. Cause of death in clinical research: Time for a reassessment? J. Am.Coll. Cardiol. 1999;34:618–620. doi: 10.1016/S0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb S.S. Dead is dead—Artificial definitions are no substitute. Lancet. 1997;349:662–663. doi: 10.1016/S0140-6736(97)22010-6. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J., Thamilarasan M., Blackstone E.H., Thomas J.D., Lauer M.S. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: The case of stress echocardiography. Circulation. 2001;104:1911–1916. doi: 10.1161/circ.104.16.1911. [DOI] [PubMed] [Google Scholar]
- 22.Vivekananthan D.P., Blackstone E.H., Pothier C.E., Lauer M.S. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J. Am. Coll. Cardiol. 2003;42:831–838. doi: 10.1016/S0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 23.Wilkoff B.L., E Miller R. Exercise testing for chronotropic assessment. Cardiol. Clin. 1992;10:705–717. doi: 10.1016/S0733-8651(18)30211-X. [DOI] [PubMed] [Google Scholar]
- 24.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A., Picard M.H., Roman M.J., Seward J., Shanewise J.S., et al. American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Cortigiani L., Urluescu M.-L., Coltelli M., Carpeggiani C., Bovenzi F., Picano E. Apparent Declining Prognostic Value of a Negative Stress Echocardiography Based on Regional Wall Motion Abnormalities in Patients With Normal Resting Left Ventricular Function Due to the Changing Referral Profile of the Population Under Study. Circ. Cardiovasc. Imaging. 2019;12:e008564. doi: 10.1161/CIRCIMAGING.118.008564. [DOI] [PubMed] [Google Scholar]
- 26.Bangalore S., Gopinath D., Yao S.S., Chaudhry F.A. Risk Stratification Using Stress Echocardiography: Incremental Prognostic Value over Historic, Clinical, and Stress Electrocardiographic Variables Across a Wide Spectrum of Bayesian Pretest Probabilities for Coronary Artery Disease. J. Am. Soc. Echocardiogr. 2007;20:244–252. doi: 10.1016/j.echo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Mudrick D.W., Cowper P.A., Shah B.R., Patel M.R., Jensen N.C., Peterson E.D., Douglas P.S. Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. Am. Heart J. 2012;163:454–461. doi: 10.1016/j.ahj.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnur S.S.K., Achim A., Toth G.G. Clinical application of results of the ISCHEMIA trial. Trends Cardiovasc. Med. 2023;33:125–130. doi: 10.1016/j.tcm.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Cortigiani L., Borelli L., Raciti M., Bovenzi F., Picano E., Molinaro S., Sicari R. Prediction of Mortality by Stress Echocardiography in 2835 Diabetic and 11 305 Nondiabetic Patients. Circ. Cardiovasc. Imaging. 2015;8:e002757. doi: 10.1161/circimaging.114.002757. [DOI] [PubMed] [Google Scholar]
- 30.Pecanha T., Silva-Junior N.D., Forjaz C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging. 2014;34:327–339. doi: 10.1111/cpf.12102. [DOI] [PubMed] [Google Scholar]
- 31.Pierpont G.L., Stolpman D.R., Gornick C.C. Heart rate recovery post-exercise as an index of parasympathetic activity. J. Auton. Nerv. Syst. 2000;80:169–174. doi: 10.1016/S0165-1838(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 32.La Rovere M.T., Pinna G.D., Hohnloser S.H. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.CIR.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 33.Cole C.R., Blackstone E.H., Pashkow F.J., Snader C.E., Lauer M.S. Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality. N. Engl. J. Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 34.Nishime E.O., Cole C.R., Blackstone E.H., Pashkow F.J., Lauer M.S. Heart Rate Recovery and Treadmill Exercise Score as Predictors of Mortality in Patients Referred for Exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 35.Cole C.R., Foody J.M., Blackstone E.H., Lauer M.S. Heart Rate Recovery after Submaximal Exercise Testing as a Predictor of Mortality in a Cardiovascularly Healthy Cohort. Ann. Intern. Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 36.Diaz L.A., Brunken R.C., Blackstone E.H., Snader C.E., Lauer M.S. Independent contribution of myocardial perfusion defects to exercise capacity and heart rate recovery for prediction of all-cause mortality in patients with known or suspected coronary heart disease. J. Am. Coll. Cardiol. 2001;37:1558–1564. doi: 10.1016/S0735-1097(01)01205-0. [DOI] [PubMed] [Google Scholar]
- 37.Gayda M., Bourassa M.G., Tardif J.-C., Fortier A., Juneau M., Nigam A. Heart Rate Recovery After Exercise and Long-term Prognosis in Patients with Coronary Artery Disease. Can. J. Cardiol. 2012;28:201–207. doi: 10.1016/j.cjca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Qiu S., Cai X., Sun Z., Li L., Zuegel M., Steinacker J.M., Schumann U. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017;6:e005505. doi: 10.1161/JAHA.117.005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.-I., Shin S.-Y., Park S.K., Barrett-Connor E. Usefulness of the Integrated Scoring Model of Treadmill Tests to Predict Myocardial Ischemia and Silent Myocardial Ischemia in Community-Dwelling Adults (from the Rancho Bernardo Study) Am. J. Cardiol. 2015;115:1049–1055. doi: 10.1016/j.amjcard.2015.01.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang P.-H., Leu H.-B., Chen J.-W., Cheng C.-M., Huang C.-Y., Tuan T.-C., Ding P.Y.-A., Lin S.-J. Usefulness of attenuated heart rate recovery immediately after exercise to predict endothelial dysfunction in patients with suspected coronary artery disease. Am. J. Cardiol. 2004;93:10–13. doi: 10.1016/j.amjcard.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Jae S.Y., Ahn E.S., Heffernan K.S., Woods J.A., Lee M.-K., Park W.H., Fernhall B. Relation of Heart Rate Recovery After Exercise to C-Reactive Protein and White Blood Cell Count. Am. J. Cardiol. 2007;99:707–710. doi: 10.1016/j.amjcard.2006.09.121. [DOI] [PubMed] [Google Scholar]
- 42.Fei D.-Y., Arena R., Arrowood J.A., Kraft K.A. Relationship between arterial stiffness and heart rate recovery in apparently healthy adults. Vasc. Health Risk Manag. 2005;1:85–89. doi: 10.2147/vhrm.1.1.85.58938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo H.-K., Gore J.M. Relation of heart rate recovery after exercise to insulin resistance and chronic inflammation in otherwise healthy adolescents and adults: Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Clin. Res. Cardiol. 2015;104:764–772. doi: 10.1007/s00392-015-0843-2. [DOI] [PubMed] [Google Scholar]
- 44.Jae S.Y., Kurl S., Laukkanen J., Yoon E.S., Choi Y.-H., Fernhall B., Franklin B.A. Relation of heart rate recovery after exercise testing to coronary artery calcification. Ann. Med. 2017;49:404–410. doi: 10.1080/07853890.2017.1292044. [DOI] [PubMed] [Google Scholar]
- 45.Jae S.Y., Carnethon M.R., Ahn E.S., Heffernan K.S., Choi Y.-H., Lee M.-K., Fernhall B. Association between heart rate recovery after exercise testing and plasminogen activator inhibitor 1, tissue plasminogen activator, and fibrinogen in apparently healthy men. Atherosclerosis. 2008;197:415–419. doi: 10.1016/j.atherosclerosis.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 47.Morrone D., Gentile F., Aimo A., Cameli M., Barison A., Picoi M.E., Guglielmo M., Villano A., DeVita A., Mandoli G.E., et al. Perspectives in noninvasive imaging for chronic coronary syndromes. Int. J. Cardiol. 2022;365:19–29. doi: 10.1016/j.ijcard.2022.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Shaw L.J., Veledar E., Wenger N.K., Mieres J.H., Hendel R.H., Boden W.E., Gulati M., Hachamovitch R., Arrighi J.A., Merz C.N.B., et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: Results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) Trial. Circulation. 2012;125:e934–e935. doi: 10.1161/circulationaha.112.092270. [DOI] [PubMed] [Google Scholar]
- 49.Elhendy A., Shub C., McCully R.B., Mahoney D.W., Burger K.N., A Pellikka P.A. Exercise echocardiography for the prognostic stratification of patients with low pretest probability of coronary artery disease. Am. J. Med. 2001;111:18–23. doi: 10.1016/S0002-9343(01)00746-X. [DOI] [PubMed] [Google Scholar]
- 50.Ciampi Q., Zagatina A., Cortigiani L., Wierzbowska-Drabik K., Kasprzak J.D., Haberka M., Djordjevic-Dikic A., Beleslin B., Boshchenko A., Ryabova T., et al. Prognostic value of stressechocardiography assessed by ABCDE protocol. Eur. Heart J. 2021;42:3869–3878. doi: 10.1093/eurheartj/ehab493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaibazzi N., Bergamaschi L., Pizzi C., Tuttolomondo D. Resting global longitudinal strain and stress echocardiography to detect coronary artery disease burden. Eur. Heart J. Cardiovasc. Imaging. 2023;24:e86–e88. doi: 10.1093/ehjci/jead046. [DOI] [PubMed] [Google Scholar]
- 52.Pletscher M., Walker S., Moschetti K., Pinget C., Wasserfallen J.-B., Greenwood J.P., Schwitter J., Girardin F.R. Cost-effectiveness of functional cardiac imaging in the diagnostic work-up of coronary heart disease. Eur. Heart J. Qual. Care Clin. Outcomes. 2016;2:201–207. doi: 10.1093/ehjqcco/qcw008. [DOI] [PubMed] [Google Scholar]
- 53.Bedetti G., Pasanisi E.M., Pizzi C., Turchetti G., Lore C. Economic analysis including long-term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardiovasc. Ultrasound. 2008;6:21. doi: 10.1186/1476-7120-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picano E., Ciampi Q., Citro R., D’andrea A., Scali M.C., Cortigiani L., Olivotto I., Mori F., Galderisi M., Costantino M.F., et al. Stress echo 2020: The international stress echo study in ischemic and non-ischemic heart disease. Cardiovasc. Ultrasound. 2017;15:3. doi: 10.1186/s12947-016-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanza G.A., Morrone D., Pizzi C., Tritto I., Bergamaschi L., De Vita A., Villano A., Crea F. Diagnostic approach for coronary microvascular dysfunction in patients with chest pain and no obstructive coronary artery disease. Trends Cardiovasc. Med. 2022;32:448–453. doi: 10.1016/j.tcm.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Kenkre T.S., Malhotra P., Johnson B.D., Handberg E.M., Thompson D.V., Marroquin O.C., Rogers W.J., Pepine C.J., Merz C.N.B., Kelsey S.F., et al. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation) Circ. Cardiovasc. Qual. Outcomes. 2017;10:e003863. doi: 10.1161/CIRCOUTCOMES.116.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woudstra J., Vink C.E.M., Schipaanboord D.J.M., Eringa E.C., Ruijter H.M.D., Feenstra R.G.T., Boerhout C.K.M., Beijk M.A.M., de Waard G.A., Ong P., et al. Meta-analysis and systematic review of coronary vasospasm in ANOCA patients: Prevalence, clinical features and prognosis. Front. Cardiovasc. Med. 2023;10:1129159. doi: 10.3389/fcvm.2023.1129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasupathy S., Air T., Dreyer R.P., Tavella R., Beltrame J.F. Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 59.Achim A., Stanek A., Homorodean C., Spinu M., Onea H.L., Lazăr L., Marc M., Ruzsa Z., Olinic D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Health. 2022;19:9801. doi: 10.3390/ijerph19169801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.