Abstract
Adverse childhood experiences (ACEs) are common and increase the risk of poor health outcomes. Resilience may offer protection against the impacts of ACEs. This study examined the association between maternal ACEs and mental/behavioral health outcomes during pregnancy overall and by resilience. The sample comprised pregnant patients in two pilot studies screened for eight ACEs and resilience during standard prenatal care in Kaiser Permanente Northern California from 1 March 2016 to 30 July 2016 (Study 1, medical centers A, B) and from 1 April 2018 to 31 March 2019 (Study 2, medical centers A, C). Early pregnancy outcomes included anxiety and depressive disorders, depression symptoms, intimate partner violence (IPV), and substance use. Multivariable logistic regression was used in this cross-sectional study to examine associations between maternal ACEs (0, 1–2, ≥3) and mental/behavioral health outcomes overall and among those with low and high resilience. Patients (n = 1084) averaged 30.8 years (SD 5.1); 41.7% were non-Hispanic White; 41.7% experienced ≥1 ACE, and 40.3% had low resilience. Patients with 1–2 ACEs or ≥3 ACEs (versus 0 ACEs) had higher odds of anxiety and depressive disorders, depressive symptoms, IPV, and any prenatal substance use (OR 1.44–4.40, p < 0.05). Each individual ACE was associated with ≥2 mental/behavioral health outcomes. In stratified analyses, having ≥1 ACE (vs. 0) was associated with a greater number of mental/behavioral health outcomes among patients with low versus high resilience. ACEs were associated with prenatal mental/behavioral health conditions, particularly in the context of low resilience, highlighting the importance of trauma-informed prenatal care and the need to study resilience-building interventions during pregnancy.
Keywords: pregnancy, perinatal health, mental health, substance use, adverse childhood experiences, resilience, screening
1. Introduction
Adverse childhood experiences (ACEs), or exposure to abuse, neglect, or household dysfunction prior to adulthood [1], are common: recent nationally representative surveys have estimated that 61% of adults have at least one ACE, and 25% have three or more ACEs [2]. ACEs are strongly associated with adverse medical and behavioral health outcomes in a dose–response fashion throughout the lifespan and intergenerationally [3,4,5,6,7,8]. ACEs have substantial economic costs due to factors such as chronic health problems, impaired educational achievement, reduced income and/or earning potential, and increased healthcare utilization [9,10].
Mental and behavioral health conditions, including depression, anxiety, exposure to violence/abuse, and substance use, are especially important to consider during pregnancy, as they can increase the risk of poor perinatal [11,12,13,14,15,16,17,18] and childhood outcomes [19,20,21,22,23,24]. Studies that have examined ACEs and mental health conditions in pregnancy have found associations between ACEs and prenatal depression/depressive symptoms [25,26,27,28,29,30], anxiety/anxiety symptoms [26,29], post-traumatic stress/post-traumatic stress disorder [25,26,29], and poor mental health [31]. Studies have also found an association between maternal ACEs and prenatal use of alcohol [26,27,32,33,34], tobacco or cannabis [26,27,31,32,35], and substance use or illicit drug use [26,27,31,32]. Fewer studies have examined the association between ACEs and intimate partner violence (IPV) during pregnancy. We previously found an association between ACEs and IPV [29], and Leeners et al. (2013) [35] found an association between child sexual abuse and physical, sexual, and emotional abuse in pregnancy.
The existing literature on ACEs and mental and behavioral health outcomes has been limited by small sample sizes, participants from a limited geographic area (e.g., within one city or patients at one hospital), or focusing only on one type (e.g., sexual abuse). Additionally, studies often collapsed maternal ACEs into categories without examining the associations between individual ACEs and maternal outcomes. Research that examines associations between individual ACEs and prenatal mental/behavioral health conditions is needed to better understand the utility of documenting specific ACEs in prenatal health care settings.
A growing body of literature has identified resilience, defined as the ability to adapt to and cope with stress and adversity [36,37], as a significant moderator of the health risks related to ACEs in the general population [38,39,40,41]. Recent studies have contributed to evidence of resilience as a psychological variable that is both a state and a trait [42,43]. It is critical to better understand the impact of resilience on the association between ACEs and perinatal health outcomes, as this can inform screening programs in clinical settings to identify patients in the highest-risk categories. Subsequently, interventions can be catered to address underlying psychological traits following childhood ACEs. Recent interventions have been developed for low-resilience patient populations [44], which could be modified for use in prenatal care settings.
Initial resilience research, generally including studies with small sample sizes, suggests that resilience may moderate associations between ACEs and prenatal and postpartum mental/behavioral health conditions [26,29,45]. In our prior study, we found that ACEs were associated with both mental and behavioral health conditions among pregnant patients, with the strongest associations among those with low levels of current resilience [29]. However, due to the small sample size, we were unable to examine the relationship between individual ACEs and mental/behavioral health outcomes or disaggregate the use of different substances during pregnancy. Additional studies with larger sample sizes are needed to better understand whether the associations between ACEs and key mental/behavioral health outcomes during pregnancy are different for those with high versus low resilience.
The current study builds on our previously published work by combining data from two pilot sites that tested the implementation of routine ACEs and resilience screening in obstetric care. The primary objective of the study was to: (1) examine whether the number of ACEs were associated with mental and behavioral health conditions during pregnancy, and (2) to conduct stratified analyses to examine associations between ACEs and mental and behavioral health conditions separately for pregnant patients with low versus high levels of resilience. A secondary objective of this study was to examine the associations between individual ACEs and mental and behavioral health conditions in pregnancy. We used the STROBE checklist for reporting this cross-sectional study.
2. Materials and Methods
2.1. Study Site
Kaiser Permanente Northern California (KPNC) is a nonprofit, multi-specialty healthcare delivery system with over 4.3 million members and approximately 45,000 live births annually across 21 hospitals. This study combines data from two KPNC pilot studies in three medical centers that screened English-speaking pregnant patients aged ≥18 for ACEs during standard prenatal care at their second or third prenatal visit (typically between 14–23 weeks gestation) from 1 March 2016 to 30 June 2016 (Study 1, medical centers A and B) and from 1 April 2018 to 31 March 2019 (Study 2, medical centers A and C). Medical assistants provided the ACEs screening questionnaire to patients to complete in the waiting room or exam room while waiting for their physician. Physicians reviewed the questionnaire responses with patients and provided an educational handout with relevant community and educational resources and, as needed, referrals for behavioral health services. Additional information about study methods has been published previously [29,46,47,48,49]. This study received approval from the KPNC Institutional Review Board with a waiver of informed consent.
2.2. Participants
The study sample comprised data from 1164 pregnancies in English-speaking patients (age >18) who completed the ACEs questionnaire during standard prenatal care in either Study 1 (N = 355) or Study 2 (N = 809). Fifty-eight pregnancies were excluded for incomplete data on ACEs (n = 8), prenatal alcohol screening (n = 29), median household income (n = 4), or depression diagnosis (n = 21). For the 18 patients with >1 pregnancy that met the inclusion criteria, only data from the latest pregnancy were retained (n = 18 pregnancies excluded). The final sample included 1084 patients.
2.3. Measures
We assessed maternal ACEs using a modified version of the Behavioral Risk Factor Surveillance System Questionnaire [50] adapted to be appropriate for pregnant patients and easy to self-administer in a health care setting [29,46]. Patients responded “yes” or “no” to questions about whether 8 specific ACEs occurred prior to their 18th birthday. The questions were: (1) “Did you lose a parent through divorce, abandonment, death, or other reason?”, (2) “Did a parent or adult in your home ever swear at you, insult you, or put you down?”, (3) “Not including spanking, did a parent or adult in your home ever hit, beat, kick, or physically hurt you in any way?”, (4) “Did you experience unwanted sexual contact (such as fondling, or oral/anal/vaginal intercourse/penetration)?”, (5) “Did you live with anyone who had a problem with drinking or using drugs, including prescription medications?”, (6) “Did you live with anyone who was depressed, mentally ill, or attempted suicide?”, (7) “Did you live with anyone who went to jail or prison?”, (8) “Did your parents or adults in your home ever hit, punch, beat, or threaten to harm each other?”. Total possible ACEs counts ranged from 0 to 8.
Mental and behavioral health conditions during pregnancy were extracted from the electronic health record (EHR). Depression and anxiety disorder diagnoses during pregnancy were identified using the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9), and Tenth Revision (ICD-10) codes [29]. Depression symptoms were identified by the Patient Health Questionnaire-9 (PHQ-9), which is given to pregnant patients during standard prenatal care (depression is defined as a score >10) [51]. Intimate partner violence (IPV) was ascertained by ICD-9 and ICD-10 codes recorded in the EHR during the year before pregnancy or during pregnancy.
Any use of alcohol, nicotine, or cannabis during early pregnancy (including prior to pregnancy recognition) was based on universal screening via a self-reported questionnaire at the entry to prenatal care (at ~8 weeks gestation). Use of nicotine was additionally based on routine screening for patient-reported current tobacco smoking at the time of the ACEs screening; patients who self-reported nicotine use on the self-administered screening questionnaire and/or self-reported current tobacco smoking at the time of the ACEs screening were coded as yes for nicotine use. Prenatal cannabis use was additionally based on a positive urine toxicology test universally given to patients at the entrance to prenatal care, to which patients consent. Patients who self-reported any cannabis use during early pregnancy and/or had a positive urine toxicology test were coded as yes for cannabis use. Any prenatal substance use during early pregnancy was defined as being positive for prenatal alcohol, nicotine, and/or cannabis use. Prenatal nicotine use alone was not included as one of our outcomes due to low prevalence (<3%).
Patients were screened for resilience at the same time as ACEs screening using the 10-item Connor-Davidson Resilience Scale (CD-RISC 10) [52]. This questionnaire is a validated, self-reported measure of past-month resilience that has previously been used in research with pregnant and postpartum patients [29,52,53]. Questions address components of psychological resilience, such as the ability to bounce back after hardship, handle unpleasant or painful feelings, and adapt to change. Answer options are scored from 0 (“not at all true”) to 4 (“true nearly all the time”), with total scores ranging from 0 to 40 [45,54]. Scores were dichotomized into low (<32) and high resilience (>32) based on the national average [52], as conducted by our team previously [29].
Socio-demographics were obtained from the EHR and included the patient’s age at ACEs screening, race/ethnicity (Asian/Pacific Islander, Black, Hispanic, non-Hispanic White, other/unknown), and parity. Neighborhood median household income was based on census tract data and was divided into terciles: $0–$82,999, $83,000 to $105,999, and $106,000 or higher.
2.4. Statistical Analysis
Frequencies and percentages were used to describe socio-demographics (age, race/ethnicity, neighborhood median household income), clinical characteristics (parity, resilience), ACE count (0, 1–2, and ≥3), and individual ACEs. ACE count categories were consistent with what we have previously published [29] and provided clinically meaningful information about ACEs (i.e., none, some, and many). While >4 ACEs are generally considered to represent high-risk for adverse outcomes [6], only a small proportion of patients in our sample reported >4 ACEs, and, thus, our highest category is >3 ACEs. Seven multivariable logistic regression models were used to compare the odds of each of the mental/behavioral health conditions by ACE count and by individual ACEs and to compare the odds of mental/behavioral health conditions of interest by ACE count, stratified by patients with high versus low resilience [55,56]. We stratified by high/low resilience, as conducted in our prior work, due to our limited power to test for statistical significance of interaction and because stratification would provide clinically meaningful data to our clinicians and healthcare system to understand whether the combination of ACEs and low resilience is associated with highest odds of behavioral and mental health outcomes. All regression analyses were adjusted for maternal age categories, race/ethnicity, parity, and median neighborhood income categories based on previous literature and the availability of variables from the pilot study data and electronic medical records. To determine statistical significance, we first considered two-sided p-values of <0.05 statistically significant. Additionally, we applied the Benjamini-Hochberg procedure to decrease the potential of false positive results [57]. In brief, for this correction, we ranked the p-values of each of the seven mental and behavioral health outcomes for each category of ACE predictors (1–2 and >3 ACEs). We calculated the Benjamini-Hochberg critical value for each p-value using the formula ([i/m]*Q), where I = rank of p-value [ranging from 1 to 7], m = the total number of tests [7] and Q = the false discover rate [0.05]. Statistical analyses were performed in SAS 9.4. Odds ratio results are presented by effect size, with small effect >1.22, medium effect >1.86, and large effect >3.00 [58].
3. Results
The sample of pregnant patients (n = 1084) was primarily non-Hispanic White (41.7%), averaging 30.8 years (SD 5.1) (Table 1). Participants reported a mean of 1.0 ACE (SD 1.6); 58.2% reported 0 ACEs, 27.2% reported 1–2 ACEs, and 14.6% reported ≥3 ACEs. The most commonly reported ACEs were loss of a parent (23.4%), emotional abuse (15.6%), having lived with someone with a substance use problem (15.5%), and having lived with someone depressed, mentally ill, or suicidal (14.2%). Fewer patients had low resilience (40.3%) compared to high resilience (59.7%) based on CD-RISC scores (Table 2).
Table 1.
Patient Demographics (N = 1084).
| N (%) | |
|---|---|
| Age in years, categories | |
| 18–28 | 353 (32.6) |
| 29–33 | 400 (36.9) |
| 34–47 | 331 (30.5) |
| Age in years, mean (SD) | 30.8 (5.1) |
| Race/ethnicity | |
| Non-Hispanic White | 452 (41.7) |
| Hispanic | 260 (24.0) |
| Asian/Pacific Islander | 198 (18.3) |
| Black | 126 (11.6) |
| Multiple, other, or unknown | 48 (4.4) |
| Parity | |
| Nulliparous | 407 (37.6) |
| Primiparous | 406 (37.5) |
| Multiparous | 271 (24.0) |
| ACE count, categories | |
| 0 | 631 (58.2) |
| 1 | 202 (18.6) |
| 2 | 93 (8.6) |
| 3–8 | 158 (14.6) |
| ACE count, mean (SD) | 1.0 (1.6) |
Notes. ACE = Adverse childhood experience. SD = Standard deviation.
Table 2.
Adverse Childhood Experiences (ACEs) Exposure and Prenatal Mental and Behavioral Health Conditions.
| Overall N (Col %) |
Prenatal Mental and Behavioral Health Conditions, N (Row %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Anxiety Disorder | Depressive Disorder | Depression Symptoms | IPV Diagnosis | Alcohol Use During Early Pregnancy | Cannabis Use During Early Pregnancy | Any Substance Use During Early Pregnancy | ||
| Total | 1084 | 133 (12.3) | 210 (19.4) | 112 (10.3) | 92 (8.5) | 117 (10.8) | 84 (7.7) | 201 (18.5) |
| Number of ACEs | ||||||||
| 0 | 631 (58.2) | 56 (8.9) | 88 (13.9) | 46 (7.3) | 29 (4.6) | 59 (9.4) | 37 (5.9) | 94 (14.9) |
| 1–2 | 295 (27.2) | 40 (13.6) | 69 (23.4) | 36 (12.2) | 32 (10.8) | 40 (13.6) | 26 (8.8) | 65 (22.0) |
| ≥3 | 158 (14.6) | 37 (23.4) | 53 (33.5) | 30 (19.0) | 31 (19.6) | 18 (11.4) | 21 (13.3) | 42 (26.6) |
| Resilience * | ||||||||
| Low (≤32) | 429 (40.3) | 70 (16.3) | 113 (26.3) | 78 (18.2) | 38 (8.9) | 43 (10.0) | 39 (9.1) | 81 (18.9) |
| High (>32) | 636 (59.7) | 62 (9.7) | 95 (14.9) | 33 (5.2) | 52 (8.2) | 74 (11.6) | 42 (6.6) | 117 (18.4) |
| Individual ACEs | ||||||||
| Loss of parent | ||||||||
| Yes | 254 (23.4) | 39 (15.4) | 68 (26.8) | 38 (15.0) | 36 (14.2) | 37 (14.6) | 23 (9.1) | 61 (24.0) |
| No | 830 (76.6) | 94 (11.3) | 142 (17.1) | 74 (8.9) | 56 (6.7) | 80 (9.6) | 61 (7.3) | 140 (16.9) |
| Emotional abuse | ||||||||
| Yes | 169 (15.6) | 37 (21.9) | 52 (30.8) | 35 (20.7) | 29 (17.2) | 19 (11.2) | 21 (12.4) | 43 (25.4) |
| No | 915 (84.4) | 96 (10.5) | 158 (17.3) | 77 (8.4) | 63 (6.9) | 98 (10.7) | 63 (6.9) | 158 (17.3) |
| Physical abuse | ||||||||
| Yes | 67 (6.2) | 13 (19.4) | 21 (31.3) | -- | 11 (16.4) | -- | -- | 16 (23.9) |
| No | 1017 (93.8) | 120 (11.8) | 189 (18.6) | -- | 81 (8.0) | -- | -- | 185 (18.2) |
| Sexual abuse | ||||||||
| Yes | 79 (7.3) | 16 (20.3) | 27 (34.2) | 13 (16.5) | 17 (21.5) | 14 (17.7) | 15 (19.0) | 29 (36.7) |
| No | 1005 (92.7) | 117 (11.6) | 183 (18.2) | 99 (9.9) | 75 (7.5) | 103 (10.2) | 69 (6.9) | 172 (17.1) |
| Lived with someone: | ||||||||
| -With substance use problem | ||||||||
| Yes | 168 (15.5) | 36 (21.4) | 54 (32.1) | 24 (14.3) | 30 (17.9) | 17 (10.1) | 20 (11.9) | 40 (23.8) |
| No | 916 (84.5) | 97 (10.6) | 156 (17.0) | 88 (9.6) | 62 (6.8) | 100 (10.9) | 64 (7.0) | 161 (17.6) |
| -Who was depressed, mentally ill, or suicidal | ||||||||
| Yes | 154 (14.2) | 36 (23.4) | 54 (35.1) | 33 (21.4) | 27 (17.5) | 14 (9.1) | 15 (9.7) | 31 (20.1) |
| No | 930 (85.8) | 97 (10.4) | 156 (16.8) | 79 (8.5) | 65 (7.0) | 103 (11.1) | 69 (7.4) | 170 (18.3) |
| -Who went to jail or prison | ||||||||
| Yes | 87 (8.0) | 18 (20.7) | 26 (29.9) | 12 (13.8) | 12 (13.8) | -- | 17 (19.5) | 27 (31.0) |
| No | 997 (92.0) | 115 (11.5) | 184 (18.5) | 100 (10.0) | 80 (8.0) | -- | 67 (6.7) | 174 (17.5) |
| -Who hit, punched, beat, or threatened to harm another adult in the home | ||||||||
| Yes | 91 (8.4) | 15 (16.5) | 24 (26.4) | 15 (16.5) | 18 (19.8) | 10 (11.0) | 15 (16.5) | 24 (26.4) |
| No | 993 (91.6) | 118 (11.9) | 186 (18.7) | 97 (9.8) | 74 (7.5) | 107 (10.8) | 69 (6.9) | 177 (17.8) |
Notes. ACE = Adverse childhood experience. IPV = Intimate partner violence diagnosis. Percentages may not add up to 100 due to rounding. Patients could have more than one prenatal mental or behavioral health condition. * Resilience was missing for 19 patients. Any substance use during early pregnancy was defined as being positive for alcohol, nicotine, and/or cannabis use during early pregnancy. Cells replaced with ‘--’ indicate cell counts of less than 10 patients or cell counts that could be used to derive cell counts with less than 10 patients; these cells were suppressed to protect patient identity.
In multivariable logistic regression models, having ≥3 vs. 0 ACEs was significantly associated with higher odds of all mental and behavioral health outcomes during pregnancy, with the exception of prenatal alcohol use (Table 3 and Table 4). The strength of the associations for ≥3 vs. 0 ACEs ranged from small for any substance use during early pregnancy to large for IPV. Further, having 1–2 vs. 0 ACEs was significantly associated with higher odds of mental and behavioral health conditions except for the use of substance use during early pregnancy (including alcohol, cannabis, and any substance use). The strength of the associations ranged from small for depressive disorder and depression symptoms to medium for IPV (Table 3 and Table 4). Upon applying the Benjamini-Hochberg procedure, only anxiety fell out of significance for having 1–2 ACEs.
Table 3.
Adjusted Odds Ratios of Prenatal Mental Health Conditions by Adverse Childhood Experiences (ACEs) Count and Individual ACE (N = 1084).
| Anxiety Disorder | Depressive Disorder | Depression Symptoms | IPV Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | p | aOR (95% CI) | p | aOR (95% CI) | p | aOR (95% CI) | p | |
| ACE count | ||||||||
| 1–2 vs. 0 ACEs | 1.56 (1.01–2.42) | 0.045 | 1.83 (1.28–2.61) | <0.001 * | 1.73 (1.08–2.77) | 0.02 * | 2.48 (1.46–4.22) | <0.001 * |
| ≥3 vs. 0 ACEs | 2.90 (1.81–4.66) | <0.001 * | 2.83 (1.88–4.27) | <0.001 * | 3.00 (1.78–5.03) | <0.001 * | 4.40 (2.52–7.67) | <0.001 * |
| Individual ACEs | ||||||||
| Loss of parent | 1.33 (0.88–2.00) | 0.18 | 1.69 (1.20–2.37) | 0.003 | 1.71 (1.11–2.63) | 0.02 | 2.01 (1.27–3.17) | 0.003 |
| Emotional abuse | 2.31 (1.50–3.56) | <0.001 | 2.01 (1.38–2.92) | <0.001 | 3.06 (1.94–4.81) | <0.001 | 2.61 (1.60–4.25) | <0.001 |
| Physical abuse | 1.79 (0.93–3.43) | 0.08 | 1.95 (1.12–3.39) | 0.02 | 1.46 (0.69–3.08) | 0.33 | 2.13 (1.05–4.34) | 0.04 |
| Sexual abuse | 1.91 (1.05–3.48) | 0.03 | 2.38 (1.44–3.95) | <0.001 | 1.62 (0.85–3.08) | 0.14 | 3.08 (1.68–5.67) | <0.001 |
| Lived with someone: | ||||||||
| -With substance use problem | 2.07 (1.34–3.21) | 0.001 | 2.03 (1.39–2.96) | <0.001 | 1.60 (0.96–2.64) | 0.07 | 2.83 (1.73–4.63) | <0.001 |
| -Who was depressed, mentally ill, or suicidal | 2.32 (1.50–3.61) | <0.001 | 2.38 (1.62–3.48) | <0.001 | 3.36 (2.09–5.40) | <0.001 | 2.76 (1.67–4.57) | <0.001 |
| -Who went to jail or prison | 1.97 (1.11–3.49) | 0.02 | 1.84 (1.11–3.03) | 0.02 | 1.17 (0.60–2.27) | 0.65 | 1.50 (0.77–2.93) | 0.24 |
| -Who hit, punched, beat, or threatened to harm another adult in the home | 1.41 (0.77–2.55) | 0.26 | 1.46 (0.88–2.41) | 0.14 | 1.84 (1.00–3.39) | 0.49 | 3.04 (1.69–5.46) | <0.001 |
Notes. aOR = Adjusted odds ratio. ACE = Adverse childhood experience. IPV = Intimate partner violence diagnosis. All models were adjusted for age, race/ethnicity, median neighborhood household income, and parity. * indicates statistical significance after applying the Benjamini-Hochberg adjustment for false discovery rates.
Table 4.
Adjusted Odds Ratios of Prenatal Behavioral Health Conditions by Adverse Childhood Experiences (ACEs) Count and Individual ACE (N = 1084).
| Alcohol Use During Early Pregnancy |
Cannabis Use During Early Pregnancy | Any Substance Use During Early Pregnancy | ||||
|---|---|---|---|---|---|---|
| aOR (95% CI) | p | aOR (95% CI) | p | aOR (95% CI) | p | |
| ACE count | ||||||
| 1–2 vs. 0 ACEs | 1.43 (0.93–2.21) | 0.11 | 1.28 (0.73–2.25) | 0.38 | 1.44 (1.00–2.07) | 0.053 |
| ≥3 vs. 0 ACEs | 1.13 (0.64–2.01) | 0.67 | 1.90 (1.03–3.51) | 0.039 * | 1.72 (1.12–2.65) | 0.01 * |
| Individual ACEs | ||||||
| Loss of parent | 1.49 (0.97–2.28) | 0.07 | 0.88 (0.51–1.52) | 0.65 | 1.29 (0.90–1.84) | 0.16 |
| Emotional abuse | 1.04 (0.61–1.76) | 0.89 | 1.93 (1.09–3.43) | 0.02 | 1.62 (1.08–2.42) | 0.02 |
| Physical abuse | 0.98 (0.43–2.24) | 0.97 | 1.27 (0.49–3.26) | 0.62 | 1.51 (0.82–2.79) | 0.18 |
| Sexual abuse | 1.81 (0.96–3.39) | 0.07 | 2.74 (1.40–5.36) | 0.003 | 2.60 (1.56–4.34) | <0.001 |
| Lived with someone: | ||||||
| -With substance use problem | 0.83 (0.48–1.45) | 0.52 | 1.64 (0.92–2.91) | 0.09 | 1.29 (0.85–1.94) | 0.23 |
| -Who was depressed, mentally ill, or suicidal | 0.72 (0.40–1.31) | 0.28 | 1.36 (0.72–2.57) | 0.35 | 1.04 (0.67–1.62) | 0.87 |
| -Who went to jail or prison | 0.54 (0.23–1.28) | 0.16 | 2.13 (1.11–4.05) | 0.02 | 1.65 (0.99–2.74) | 0.06 |
| -Who hit, punched, beat, or threatened to harm another adult in the home | 0.94 (0.47–1.89) | 0.86 | 2.54 (1.29–4.97) | 0.007 | 1.52 (0.91–2.55) | 0.11 |
Notes. aOR = Adjusted odds ratio. ACE = Adverse childhood experience. Adjusted for age, race/ethnicity, median neighborhood household income, and parity. For only the prenatal cannabis use outcome, the Asian race was combined with the multiple/other/unknown race category so the model would converge. Any substance use during early pregnancy was defined as being positive for alcohol, nicotine, and/or cannabis use during early pregnancy. * indicates statistical significance after applying the Benjamini-Hochberg adjustment for false discovery rates.
For the secondary objective, all individual ACEs were significantly associated with depressive disorder, and all except for living with someone who went to jail or prison were significantly associated with IPV. Childhood emotional abuse was associated with the greatest number of mental/behavioral health outcomes (6 outcomes), followed by sexual abuse (5 outcomes), and living with someone who was depressed, mentally ill, or suicidal (4 outcomes) (Table 3 and Table 4).
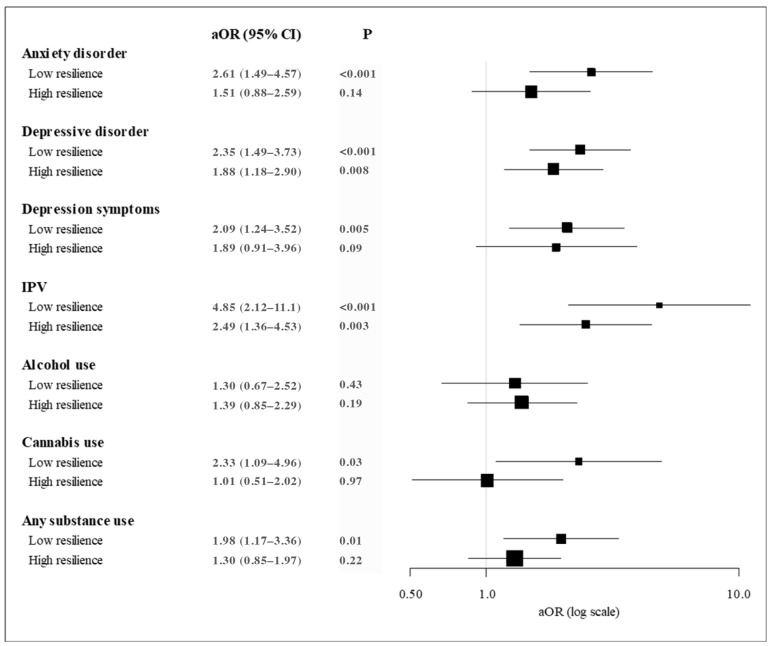
In multivariable models of outcomes stratified by low/high resilience, we dichotomized exposure to ACEs (1+ vs. 0 ACEs) due to the smaller sample sizes of patients with low (n = 429) and high resilience (n = 636). Among patients with low resilience, having 1+ ACE was associated with increased odds of 6 mental/behavioral health outcomes during pregnancy: anxiety disorder (medium effect), depressive disorder (medium effect), depressive symptoms (medium effect), IPV (large effect), cannabis use during early pregnancy (medium effect), and any substance use during early pregnancy (medium effect) (Figure 1). In contrast, among patients with high resilience, having 1+ ACE was significantly associated with only two mental/behavioral health outcomes during pregnancy: depressive disorder (medium effect) and IPV (medium effect).
Figure 1.
Adjusted Odds Ratios of Prenatal Mental and Behavioral Health Conditions by Exposure to One or More Adverse Childhood Experiences (ACEs) Stratified by Low (N = 429) and High (N = 636) Resilience; Notes. aOR = adjusted odds ratio. ACE = Adverse childhood experience. IPV = Intimate partner violence diagnosis. CI = confidence interval. 1+ vs. 0 ACEs for each outcome/category. Resilience was missing for 19 patients. Adjusted for age, race/ethnicity, median neighborhood household income, and parity. For only the prenatal cannabis use outcome, the Asian race was combined with the multiple/other/unknown race category so the model would converge. Any substance use was defined as being positive for any alcohol, nicotine, and/or cannabis use during early pregnancy.
4. Discussion
This study sought to: (1) examine whether the number of ACEs were associated with mental and behavioral health conditions during pregnancy, and (2) to conduct stratified analyses to examine associations between ACEs and mental and behavioral health conditions separately for pregnant patients with low versus high levels of resilience. Secondarily, we examined the associations between individual ACEs and mental and behavioral health conditions in pregnancy. Results from our diverse sample of pregnant patients screened for ACEs during standard prenatal care found evidence of a relationship between the number of ACEs and mental/behavioral health conditions in pregnancy, with the strongest relationships for the greatest category of ACEs, consistent with previous studies [26,29,34,59]. Compared to pregnant patients without ACEs, those with 1–2 ACEs had 1.5 to 2.5 times the odds of having an anxiety or depressive disorder, depression symptoms, IPV, or any substance use during early pregnancy, and those with ≥3 ACEs had 1.8 to 4.7 times the odds of having an anxiety or depressive disorder, depression symptoms, IPV, or any substance use during early pregnancy. Moreover, similar to our prior work [26,29,45], the strongest associations were between ACEs and IPV. Notably, many of the associations between the number of ACEs and mental/behavioral health conditions were stronger for pregnant patients with low versus high resilience. Finally, each individual ACE was associated with at least two prenatal mental/behavioral health conditions.
Results add support to recent efforts to assess maternal ACEs during pregnancy [46,48,60]. While a growing number of prenatal and obstetric healthcare settings are implementing ACEs screening, it remains unclear how best to record or report maternal ACEs. Many studies utilize ACE counts, categorize ACEs into none/low/high categories, or focus on one specific type of ACE (e.g., childhood sexual abuse) [27,32,33,34,59,61,62,63], while others aggregate certain ACEs into subtypes, such as “dysfunction” and “neglect” [31], “maltreatment” [25,26], or “violence” [27]. Findings from this study suggest that specific ACEs, including experiencing emotional abuse and having a parent in jail or prison, are particularly important predictors of mental/behavioral health conditions in pregnancy. Future studies are needed to continue to identify best practices for screening and recording maternal ACEs [64], and clinical perinatal settings may consider recording both the overall number of maternal ACEs, alongside individual ACE responses.
Resilience is an important consideration in prenatal care settings. This study adds to a small but growing body of literature demonstrating the impact of resilience on the relationship between ACEs and prenatal mental/behavioral health conditions [29]. There is evidence supporting resilience as a mixed state-trait psychological variable [65,66], suggesting that resilience-building interventions, particularly when tailored to an individual’s underlying psychological traits [67], may be an effective intervention strategy after ACE exposure. While efforts have been made to implement programs to improve patient resilience through strategies such as mindfulness and psychosocial skills training [68] or trauma-informed care during and after ACE screening [39], such interventions are limited in prenatal settings, and it remains unknown the extent to which maternal improvements in resilience may impact risk behaviors and outcomes for mothers and babies.
Nearly half of the patients in this pilot study experienced ACEs, highlighting the importance of implementing trauma-informed care in obstetric settings. Trauma-informed care involves the recognition of trauma on health and having staff employ practices to actively avoid re-traumatization of patients in order to promote healing [69]. One element of trauma-informed involves screening for ACEs during prenatal care [27,70]. Based on patient feedback, this screening should be conducted with provider empathy [64] and in a private exam room [60] by either a physician or midwife [60] or behavioral health counselor [64]. On the other hand, some clinical settings may opt for implementing universal trauma-informed care under the assumption that any patient may have experienced childhood or other neglect and/or abuse. While this approach may be particularly beneficial in limited-resource settings, we believe that this study demonstrates value in ACE and resilience screening and that patients with such positive screens may benefit from additional screening (e.g., substance use), referrals (e.g., mental health), and interventions (e.g., therapy, resilience-building).
Limitations
Pilot studies took place within three KPNC medical centers with English-speaking adult patients who were screened for ACEs at the start of their second trimester, and results may not be generalizable to non-English speaking, adolescent, or uninsured patient populations. As this study was conducted as a part of standard prenatal care, certain demographic variables that may be of interest were not available for use in statistical models (e.g., maternal education, marital status, employment). However, some of these variables may be on the causal pathway and may not be appropriate to include in analyses. Additionally, ACEs screening data did not include information about severity, duration, or age at exposure, neglect, or other important factors such as systematic racism, and additional studies are needed to examine the impact of these important variables on prenatal mental and behavioral health. Because of the distribution of ACE counts within our study population, our highest category of ACEs was >3, representing 14.6% of the participants. As many of our mental and behavioral outcomes were relatively rare, some of our non-significant findings may be due to Type 2 errors. The number of patients with certain ACEs was sometimes small when broken out by mental/behavioral health outcomes, which limited statistical power to detect differences in outcomes by individual ACEs. We were limited to substance use during early pregnancy and were unable to distinguish between substance use that occurred prior to versus after pregnancy recognition. Patients who endorsed any alcohol use during early pregnancy may have been reporting infrequent use that occurred only prior to pregnancy recognition; this may explain the lack of association between ACEs and prenatal alcohol use. Likewise, we were unable to include federally illicit substance use due to low rates of patient reports (<1%) and, therefore, are unable to address the relationships between ACEs and perinatal opioid, stimulant, and other illicit substance use. Additional studies that include data on continued substance use after pregnancy recognition are needed.
5. Conclusions
Using pilot data from an integrated healthcare delivery system with screening for ACEs during standard prenatal care in a diverse sample of pregnant patients, this study found that both count and individual ACEs were associated with mental and behavioral health conditions during pregnancy. Further, findings suggest that high resilience may buffer some of the deleterious effects of ACEs. Future research should focus on how resilience-based strategies can buffer the impact of ACEs on health outcomes. Our results suggest that clinicians and policymakers should support resilience-building clinical environments by supporting training and practice of trauma-informed care. Examples of action steps toward a trauma-informed care environment can be found through organizations, including The Substance Abuse and Mental Health Services Administration and ACEs Aware [71]. Results highlight the importance of trauma-informed prenatal care and underscore the need for future studies that investigate the efficacy of resilience-building interventions during the prenatal period.
Acknowledgments
We thank Carla Wicks, Fiona Sinclair, Krista Kotz, Diane Lott-Garcia, Dorothy Ferguson, Bridgid McCaw, Tracy Flanagan and Gina Smith-Anderson for their assistance with the pilot study implementation. Data were obtained through the Kaiser Permanente Northern California Division of Research’s Perinatal Research Unit’s Perinatal Obstetric Database.
Author Contributions
Conceptualization and methodology, all authors; data collection and formal analysis, T.R.F. and S.R.A.; writing—original draft preparation, T.R.F.; writing—review and editing, all authors; visualization, T.R.F., S.R.A. and K.C.Y.-W.; supervision, K.C.Y.-W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of KAISER PERMANENTE NORTHERN CALIFORNIA (protocol codes 1283129-13 approved 8 November 2018 and 1278211-22 approved 19 November 2015).
Informed Consent Statement
Patient consent was waived due to study procedures meeting Health Insurance Portability and Accountability Act requirements and all health system members being informed that their data may be used for research upon enrollment in the health plan.
Data Availability Statement
The data are not publicly available because authors do not have permission to share.
Conflicts of Interest
Authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This study was supported by grants from the Kaiser Permanente Community Benefits Program and an NIH NIDA K01 Award (DA043604). Tara Foti received funding from The Permanente Medical Group (TMPG) Delivery Science Fellowship Program.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Merrick M.T., Ford D.C., Ports K.A., Guinn A.S. Prevalence of adverse childhood experiences from the 2011-2014 Behavioral Risk Factor Surveillance System in 23 states. JAMA Pediatr. 2018;172:1038–1044. doi: 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell J.A., Walker R.J., Egede L.E. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am. J. Prev. Med. 2016;50:344–352. doi: 10.1016/j.amepre.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis C.H., Clohessy D.S., Stone A.L., Darnall B.D., Wilson A.C. Adverse childhood experiences in mothers with chronic pain and intergenerational impact on children. J. Pain. 2019;20:1209–1217. doi: 10.1016/j.jpain.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillis S.D., Anda R.F., Felitti V.J., Marchbanks P.A. Adverse childhood experiences and sexual risk behaviors in women: A retrospective cohort study. Fam. Plann. Perspect. 2001;33:206–211. doi: 10.2307/2673783. [DOI] [PubMed] [Google Scholar]
- 6.Hughes K., Bellis M.A., Hardcastle K.A., Sethi D., Butchart A., Mikton C., Jones L., Dunne M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 7.Lê-Scherban F., Wang X., Boyle-Steed K.H., Pachter L.M. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. 2018;141:e20174274. doi: 10.1542/peds.2017-4274. [DOI] [PubMed] [Google Scholar]
- 8.Pear V.A., Petito L.C., Abrams B. The role of maternal adverse childhood experiences and race in intergenerational high-risk smoking behaviors. Nicotine Tob. Res. 2017;19:623–630. doi: 10.1093/ntr/ntw295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Preventing Adverse Childhood Experiences (ACES): Leveraging the Best Available Experience. [(accessed on 23 June 2023)];2019 Available online: https://www.cdc.gov/violenceprevention/pdf/preventingACES.pdf.
- 10.Smith J.P., Smith G.C. Long-term economic costs of psychological problems during childhood. Soc. Sci. Med. 2010;71:110–115. doi: 10.1016/j.socscimed.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004;6((Suppl. 2)):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 12.Gold K.J., Marcus S.M. Effect of maternal mental illness on pregnancy outcomes. Expert Rev. Obstet. Gynecol. 2014;3:391–401. doi: 10.1586/17474108.3.3.391. [DOI] [Google Scholar]
- 13.Janssen P.A., Holt V.L., Sugg N.K., Emanuel I., Critchlow C.M., Henderson A.D. Intimate partner violence and adverse pregnancy outcomes: A population-based study. Community Ment. Health J. 2003;188:1341–1347. doi: 10.1067/mob.2003.274. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy F.P., O’Keeffe L.M., Khashan A.S., North R.A., Poston L., McCowan L.M.E., Baker P.N., Dekker G.A., Roberts C.T., Walker J.J., et al. Association between maternal alcohol consumption in early pregnancy and pregnancy outcomes. Obstet. Gynecol. 2013;122:830–837. doi: 10.1097/AOG.0b013e3182a6b226. [DOI] [PubMed] [Google Scholar]
- 15.Pinto S.M., Dodd S., Walkinshaw S.A., Siney C., Kakkar P., Mousa H.A. Substance abuse during pregnancy: Effect on pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010;150:137–141. doi: 10.1016/j.ejogrb.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar N.N. The impact of intimate partner violence on women’s reproductive health and pregnancy outcome. J. Obstet. Gynaecol. 2008;28:266–271. doi: 10.1080/01443610802042415. [DOI] [PubMed] [Google Scholar]
- 17.Schneid-Kofman N., Sheiner E., Levy A. Psychiatric illness and adverse pregnancy outcome. Int. J. Gynaecol. Obstet. 2008;101:53–56. doi: 10.1016/j.ijgo.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Wiencrot A., Nannini A., Manning S.E., Kennelly J. Neonatal outcomes and mental illness, substance abuse, and intentional injury during pregnancy. Matern. Child Health J. 2012;16:979–988. doi: 10.1007/s10995-011-0821-x. [DOI] [PubMed] [Google Scholar]
- 19.Cohodes E.M., Gee D.G., Lieberman A.F. Associations between prenatal substance exposure, prenatal violence victimization, unintended pregnancy, and trauma exposure in childhood in a clinical setting. Infant Ment. Health J. 2019;40:786–798. doi: 10.1002/imhj.21815. [DOI] [PubMed] [Google Scholar]
- 20.Flak A.L., Su S., Bertrand J., Denny C.H., Kesmodel U.S., Cogswell M.E. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: A meta-analysis. Alcohol. Clin. Exp. Res. 2014;38:214–226. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- 21.Luoma I., Tamminen T., Kaukonen P., Laippala P., Puura K., Salmelin R., Almqvist F. Longitudinal study of maternal depressive symptoms and child well-being. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Oken E., Huh S.Y., Taveras E.M., Rich-Edwards J.W., Gillman M.W. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes. Res. 2005;13:2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S.E., Hatoum A.S., Fine J.D., Johnson E.C., Hansen I., Karcher N.R., Moreau A.L., Bondy E., Qu Y., Carter E.B., et al. Associations between prenatal cannabis exposure and childhood outcomes: Results from the ABCD Study. JAMA Psychiatry. 2021;78:64–76. doi: 10.1001/jamapsychiatry.2020.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood B., Delaney-Black V., Covington C., Nordstrom-Klee B., Ager J., Templin T., Janisse J., Martier S., Sokol R.J. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108:E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- 25.Atzl V.M., Narayan A.J., Rivera L.M., Lieberman A.F. Adverse Childhood Experiences and prenatal mental health: Type of ACEs and age of maltreatment onset. J. Fam. Psychol. 2019;33:304–314. doi: 10.1037/fam0000510. [DOI] [PubMed] [Google Scholar]
- 26.Osofsky J.D., Osofsky H.J., Frazer A.L., Fields-Olivieri M.A., Many M., Selby M., Holman S., Conrad E. The importance of Adverse Childhood Experiences during the perinatal period. Am. Psychol. 2021;76:350–363. doi: 10.1037/amp0000770. [DOI] [PubMed] [Google Scholar]
- 27.Racine N., Byles H., Killam T., Ereyi-Osas W., Madigan S. Asking about childhood adversity in the prenatal care setting: Cross-sectional associations with maternal health and mental health Outcomes. Matern. Child Health J. 2022;26:994–1004. doi: 10.1007/s10995-021-03301-5. [DOI] [PubMed] [Google Scholar]
- 28.Wajid A., van Zanten S.V., Mughal M.K., Biringer A., Austin M.P., Vermeyden L., Kingston D. Adversity in childhood and depression in pregnancy. Arch. Womens Ment. Health. 2020;23:169–180. doi: 10.1007/s00737-019-00966-4. [DOI] [PubMed] [Google Scholar]
- 29.Young-Wolff K.C., Alabaster A., McCaw B., Stoller N., Watson C., Sterling S., Ridout K.K., Flanagan T. Adverse childhood experiences and mental and behavioral health conditions during pregnancy: The role of resilience. J. Women’s Health. 2019;28:452–461. doi: 10.1089/jwh.2018.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corona K., Chavez T., Stewart K., Toledo-Corral C.M., Farzan S.F., Habre R., Grubbs B., Al-Marayati L., Lurvey N., Lerner D., et al. Adverse Childhood Experiences and prenatal depression in the maternal and development risks from environmental and social stressors pregnancy cohort. J. Obstet. Gynaecol. 2022;42:3014–3020. doi: 10.1080/01443615.2022.2125298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasthi D.L., Nagle-Yang S., Frank S., Masotya M., Huth-Bocks A. Associations between adverse childhood experiences and prenatal mental health and substance use among urban, low-income women. Community Ment. Health J. 2022;58:595–605. doi: 10.1007/s10597-021-00862-1. [DOI] [PubMed] [Google Scholar]
- 32.Chung E.K., Nurmohamed L., Mathew L., Elo I.T., Coyne J.C., Culhane J.F. Risky health behaviors among mothers-to-be: The impact of adverse childhood experiences. Acad. Pediatr. 2010;10:245–251. doi: 10.1016/j.acap.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Currie C.L., Sanders J.L., Swanepoel L.M., Davies C.M. Maternal adverse childhood experiences are associated with binge drinking during pregnancy in a dose-dependent pattern: Findings from the All Our Families cohort. Child Abuse Negl. 2020;101:104348. doi: 10.1016/j.chiabu.2019.104348. [DOI] [PubMed] [Google Scholar]
- 34.Frankenberger D.J., Clements-Nolle K., Yang W. The association between adverse childhood experiences and alcohol use during pregnancy in a representative sample of adult women. Women’s Health Issues. 2015;25:688–695. doi: 10.1016/j.whi.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leeners B., Stiller R., Block E., Gorres G., Rath W., Tschudin S. Prenatal care in adult women exposed to childhood sexual abuse. J. Perinat. Med. 2013;41:365–374. doi: 10.1515/jpm-2011-0086. [DOI] [PubMed] [Google Scholar]
- 36.Bonanno G.A. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 37.Connor K.M., Davidson J.R. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC) Depress. Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 38.Delgado C., Roche M., Fethney J., Foster K. Mental health nurses’ psychological well-being, mental distress, and workplace resilience: A cross-sectional survey. Int. J. Ment. Health Nurs. 2021;30:1234–1247. doi: 10.1111/inm.12874. [DOI] [PubMed] [Google Scholar]
- 39.Leitch L. Action steps using ACEs and trauma-informed care: A resilience model. Health Justice. 2017;5:5. doi: 10.1186/s40352-017-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q., Jiang M., Li S., Yang Y. Social support, resilience, and self-esteem protect against common mental health problems in early adolescence: A nonrecursive analysis from a two-year longitudinal study. Medicine. 2021;100:e24334. doi: 10.1097/MD.0000000000024334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macia P., Barranco M., Gorbena S., Alvarez-Fuentes E., Iraurgi I. Resilience and coping strategies in relation to mental health outcomes in people with cancer. PLoS ONE. 2021;16:e0252075. doi: 10.1371/journal.pone.0252075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De La Rosa G.M., Webb-Murphy J.A., Johnston S.L. Development and validation of a brief measure of psychological resilience: An adaptation of the response to stressful experiences scale. Mil. Med. 2016;181:202–208. doi: 10.7205/MILMED-D-15-00037. [DOI] [PubMed] [Google Scholar]
- 43.Ye Z.J., Zhang Z., Zhang X.Y., Tang Y., Chen P., Liang M.Z., Sun Z., Yu Y.L. State or trait? Measuring resilience by generalisability theory in breast cancer. Eur. J. Oncol. Nurs. 2020;46:101727. doi: 10.1016/j.ejon.2020.101727. [DOI] [PubMed] [Google Scholar]
- 44.Liu J.J.W., Ein N., Gervasio J., Battaion M., Reed M., Vickers K. Comprehensive meta-analysis of resilience interventions. Clin. Psychol. Rev. 2020;82:101919. doi: 10.1016/j.cpr.2020.101919. [DOI] [PubMed] [Google Scholar]
- 45.Sexton M.B., Hamilton L., McGinnis E.W., Rosenblum K.L., Muzik M. The roles of resilience and childhood trauma history: Main and moderating effects on postpartum maternal mental health and functioning. J. Affect. Disord. 2015;174:562–568. doi: 10.1016/j.jad.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flanagan T., Alabaster A., McCaw B., Stoller N., Watson C., Young-Wolff K.C. Feasibility and acceptability of screening for adverse childhood experiences in prenatal care. J. Women’s Health. 2018;27:903–911. doi: 10.1089/jwh.2017.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson C., Wei J., Varnado N., Rios N., Flanagan T., Alabaster A., Staunton M., Sterling S.A., Gunderson E.P., Young-Wolff K.C. Adverse childhood experiences and early and continued breastfeeding: Findings from an integrated health care delivery system. J. Women’s Health. 2021;30:367–376. doi: 10.1089/jwh.2020.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson C., Wei J., Varnado N., Rios N., Staunton M., Ferguson D., Young-Wolff K.C. Pregnant women’s perspectives on screening for adverse childhood experiences and resilience during prenatal care. Psychol. Trauma. 2022;14:1299–1303. doi: 10.1037/tra0001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson C., Young-Wolff K. The Impact of ACES and Resilience on Obstetric Health Outcomes. Women’s Health Research Collaborative; New York, NY, USA: 2022. [Google Scholar]
- 50.Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System Survey ACE Data, 2009–2020. [(accessed on 23 June 2023)];2020 Available online: https://www.cdc.gov/violenceprevention/aces/ace-brfss.html.
- 51.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connor K.M., Davidson J.R. The Connor-Davidson Resilience Scale (CD-RISC) 2017. [(accessed on 7 April 2023)]. Available online: http://www.cd-risc.com/index.php.
- 53.Kohler S., Hofmann A. Can motivational interviewing in emergency care reduce alcohol consumption in young people? A systematic review and meta-analysis. Alcohol Alcohol. 2015;50:107–117. doi: 10.1093/alcalc/agu098. [DOI] [PubMed] [Google Scholar]
- 54.Li G., Kong L., Zhou H., Kang X., Fang Y., Li P. Relationship between prenatal maternal stress and sleep quality in Chinese pregnant women: The mediation effect of resilience. Sleep Med. 2016;25:8–12. doi: 10.1016/j.sleep.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Brunton R., Wood T., Dryer R. Childhood abuse, pregnancy-related anxiety and the mediating role of resilience and social support. J. Health Psychol. 2022;27:868–878. doi: 10.1177/1359105320968140. [DOI] [PubMed] [Google Scholar]
- 56.Howell K.H., Miller-Graff L.E., Schaefer L.M., Scrafford K.E. Relational resilience as a potential mediator between Adverse Childhood Experiences and prenatal depression. J. Health Psychol. 2020;25:545–557. doi: 10.1177/1359105317723450. [DOI] [PubMed] [Google Scholar]
- 57.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 58.Olivier J., May W.L., Bell M.L. Relative effect sizes for measures of risk. Commun. Stat. Theory Methods. 2017;46:6774–6781. doi: 10.1080/03610926.2015.1134575. [DOI] [Google Scholar]
- 59.Currie C.L., Tough S.C. Adverse childhood experiences are associated with illicit drug use among pregnant women with middle to high socioeconomic status: Findings from the All Our Families Cohort. BMC Pregnancy Childbirth. 2021;21:133. doi: 10.1186/s12884-021-03591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olsen J.M., Galloway E.G., Guthman P.L. Exploring women’s perspectives on prenatal screening for adverse childhood experiences. Public Health Nurs. 2021;38:997–1008. doi: 10.1111/phn.12956. [DOI] [PubMed] [Google Scholar]
- 61.Downing N.R., Akinlotan M., Thornhill C.W. The impact of childhood sexual abuse and adverse childhood experiences on adult health related quality of life. Child Abuse Negl. 2021;120:105181. doi: 10.1016/j.chiabu.2021.105181. [DOI] [PubMed] [Google Scholar]
- 62.Fergusson D.M., McLeod G.F., Horwood L.J. Childhood sexual abuse and adult developmental outcomes: Findings from a 30-year longitudinal study in New Zealand. Child Abuse Negl. 2013;37:664–674. doi: 10.1016/j.chiabu.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Irish L., Kobayashi I., Delahanty D.L. Long-term physical health consequences of childhood sexual abuse: A meta-analytic review. J. Pediatr. Psychol. 2010;35:450–461. doi: 10.1093/jpepsy/jsp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson C., Kathryn R.K., Nancy G., Kelly Y.C. Promising practices for implementing adverse childhood experiences and resilience screening in obstetric care. J. Women’s Health. 2022;31:1377–1379. doi: 10.1089/jwh.2022.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lock S., Rees C.S., Heritage B. Development and validation of a brief measure of psychological resilience: The state–trait assessment of resilience scale. Aust. Psychol. 2020;55:10–25. doi: 10.1111/ap.12434. [DOI] [Google Scholar]
- 66.American Association of Neurological Surgeons. American Society of Neuroradiology. Cardiovascular and Interventional Radiology Society of Europe. Canadian Interventional Radiology Association. Congress of Neurological Surgeons. European Society of Minimally Invasive Neurological Therapy. European Society of Neuroradiology. European Stroke Organization. Society for Cardiovascular Angiography and Interventions. Society of Interventional Radiology et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke. 2018;13:612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 67.Maltby J., Day L., Hall S. Refining trait resilience: Identifying engineering, ecological, and adaptive facets from extant measures of resilience. PLoS ONE. 2015;10:e0131826. doi: 10.1371/journal.pone.0131826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox S., Lydon S., Byrne D., Madden C., Connolly F., O’Connor P. A systematic review of interventions to foster physician resilience. Postgrad. Med. J. 2018;94:162–170. doi: 10.1136/postgradmedj-2017-135212. [DOI] [PubMed] [Google Scholar]
- 69.Substance Abuse and Mental Health Services Administration SAMHSA’s Concept of Trauma and Guidance for a Trauma-Informed Approach. [(accessed on 7 April 2023)];2014 Available online: https://ncsacw.acf.hhs.gov/userfiles/files/SAMHSA_Trauma.pdf.
- 70.Sperlich M., Seng J.S., Li Y., Taylor J., Bradbury-Jones C. Integrating trauma-informed care into maternity care practice: Conceptual and practical issues. J. Midwifery Women’s Health. 2017;62:661–672. doi: 10.1111/jmwh.12674. [DOI] [PubMed] [Google Scholar]
- 71.Aces Aware Screen. Treat. Heal. Provide Treatment & Healing. 2023. [(accessed on 7 April 2023)]. Available online: https://www.acesaware.org/provide-treatment-healing/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available because authors do not have permission to share.