Abstract
Background:
Traumatic brain injury (TBI) is an underrecognized public health threat. Survivors of TBI often suffer long-term neurocognitive deficits leading to the progressive onset of neurodegenerative disease. Recent data suggests that the gut-brain axis is complicit in this process. However, no study has specificallyaddressed whether fecal microbiota transfer (FMT) attenuates neurologic deficits after TBI.
Hypothesis:
We hypothesized that fecal microbiota transfer would attenuate neurocognitive, anatomic, and pathologic deficits after TBI.
Methods:
C57Bl/6 mice were subjected to severe TBI (n = 20) or sham-injury (n = 20) via an open-head controlled cortical impact. Post-injury, this cohort of mice underwent weekly oral gavage with a slurry of healthy mouse stool or vehicle alone beginning 1 h post-TBI followed by behavioral testing and neuropathologic analysis. 16S ribosomal RNA sequencing of fecal samples was performed to characterize gut microbial community structure pre- and post-injury. Zero maze and open field testing were used to evaluate post-traumatic anxiety, exploratory behavior, and generalized activity. 3D, contrast enhanced, magnetic resonance imaging was used to determine differences in cortical volume loss and white matter connectivity. Prior to euthanasia, brains were harvested for neuropathologic analysis.
Results:
Fecal microbiome analysis revealed a large variance between TBI, and sham animals treated with vehicle, while FMT treated TBI mice had restoration of gut dysbiosis back to levels of control mice. Neurocognitive testing demonstrated a rescue of normal anxiety-like and exploratory behavior in TBI mice treated with FMT. FMT treated TBI mice spent a greater percentage of time (22%, P = 0.0001) in the center regions of the Open Field as compared to vehicle treated TBI mice (13%). Vehicle-treated TBI animals also spent less time (19%) in the open areas of zero maze than FMT treated TBI mice (30%, P = 0.0001). Comparing in TBI mice treated with FMT, MRI demonstrated a marked attenuation in ventriculomegaly (P < 0.002) and a significant change in fractional anisotropy (i.e., loss of white matter connectivity) (P < 0.0001). Histologic analysis of brain sections revealed a FMT-injury dependent interaction in the microglia/macrophage-specific ionized calcium-binding protein, Iba1 (P = 0.002).
Conclusion:
These data suggest that restoring a pre-injury gut microbial community structure may be a promising therapeutic intervention after TBI.
Keywords: Traumatic brain injury, fecal microbiome transplantation, microbiome, dysbiosis, trauma, controlled cortical impact, behavior, anxiety
INTRODUCTION
Traumatic brain injury (TBI) is an underrecognized threat to public health. Approximately 2.8 million Americans sustain a TBI each year (1). Injurious inflammatory, hypoxic, ischemic, and excitotoxic mechanisms follow the initial insult increasing the secondary impact of the injury. Each year these secondary mechanisms of injury result in 80 to 90,000 additional American disabilities including acute neurocognitive deficits that progress to chronic neurodegenerative disease (2). Consequently, TBI has become a significant worldwide cause of death and disability (3). TBI is a heterogenous injury process including mechanical tissue disruption, free radical generation, disturbances in energy metabolism, neuronal excitotoxicity, and neuroinflammation (4). These various mechanisms of injury culminate in a gamut of behavioral, neurocognitive, and motor deficits (5). The gut is complicit in each of these processes via bidirectional communication with the brain via the gut-brain axis (6). In turn, the gut undergoes several pathologic changes as well including dysbiosis, bacterial translocation, sepsis, and eventually multi-system organ failure each of which generates a pathologic feedback loop with the injured brain.
Several groups have recently published on the pathologic feedback loop between the gut and the injured brain (7). For example, mice with innate and chemically induced dysbiosis display physiological, cognitive, and behavioral outcomes revealing an impaired immune response and neurogenesis after TBI (8, 9). In addition, microbial dysbiosis has been implicated in many physiological processes including poor cardiovascular health and inflammation in aged populations (10). In previous work, our group highlighted the deleterious effects of microbial dysbiosis after TBI (11). Follow-up studies also revealed changes in neurocognition correlating to divergent microbial community structure after TBI (12).
Fecal microbiota transfer (FMT) is widely accepted as an effective intervention in recurrent Clostridioides difficile infections (13). Furthermore, the literature has several examples of successful intervention with FMT in other neurologic disease and injury processes. For example, attenuation of neurologic deficits in preclinical models of stroke and spinal cord injury have been seen after FMT. Administration of FMT to human patients with autism spectrum disorders, multiple sclerosis, Parkinson’s disease, epilepsy, Tourette syndrome, and diabetic neuropathy have also shown positive clinical benefits (14). While the role of the gut microbiota community structure in the onset, progression, and resolution of neurologic disease is now recognized, it’s impact on TBI outcome has yet to be elucidated. No study to date has specifically addressed whether fecal microbiota transfer (FMT) can attenuate neurologic deficits after TBI. Therefore, we hypothesized that restoring a pre-injury gut microbial community structure via fecal microbiota transfer would attenuate neurocognitive, anatomic, and neuropathologic outcomes as compared to vehicle-treated mice after TBI.
METHODS
Experimental design
A2 × 2 experimental design was utilized with TBI, and Sham injury groups treated with either fecal microbiota transfer or vehicle. Mice were randomly assigned to experimental groups. Experimental TBI or sham injury (n = 20) was induced at 14 weeks of age. An a priori power calculation was performed (Gpower 3.1) determining that five animals per experimental group were necessary to detect a 20% difference between groups (utilizing the conventional values of 0.05 and 0.2 for α and β). TBI was induced via an open-head controlled cortical impact as we have previously published (15). Post-injury, sham injured and TBI mice underwent weekly oral gavage with either a slurry of healthy mouse stool or vehicle alone corresponding to their assigned group. 16S ribosomal RNA (rRNA) sequencing of fecal samples was performed to characterize gut microbiota at baseline and at 59 days post-injury. Zero maze (ZM) and Open field testing (OF) were used to evaluate post-traumatic anxiety, exploratory behavior, and generalized activity at 45-days post-injury (DPI). 3D, contrast-enhanced, MRI was used to assess cortical volume loss and white matter connectivity at 59-days post-injury. Brains were then harvested at the time of animal sacrifice for neuropathologic analysis. The duration of the study was 90 days. For greater within- and between-groups comparison, all experimental analyses were performed, on the indicated days, in the same cohort of animals.
Animals
C57BL/6 male mice (Mus musculus) (28–30 g) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Animals were delivered at 12 weeks of age and given a 2-week facility acclimation period before the start of the experiment. Bedding transfer and mixing were also performed upon arrival. Mice were housed and maintained in a pathogen-free barrier facility located in the Northwestern University Center for Comparative Medicine. Standard chow (Harlan, Indianapolis IN) and water were provided for ad libitum feeding. The animals were housed on a 12:12 light-dark cycle for the duration of the study. Mice were treated in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. All experimental protocols were approved by Northwestern University Institutional Animal Care and Use Committee.
Traumatic brain injury
Mice received anesthesia using an intraperitoneal injection of 125 mg/kg ketamine (Ketaset, Fort Dodge, IA) and 10 mg/kg xylazine (Anased, Shenandoah, IA). Following anesthesia, TBI mice received a 1-cm scalp incision to reveal the sagittal and coronal sutures of the skull. The injury site was marked 2 mm rostral to the coronal suture and 2 mm left of the sagittal suture. The impact area (5 mm diameter) of the brain was exposed via a craniectomy leaving the dura mater intact. TBI mice were then stabilized within a stereotaxic operating frame. Impacts were delivered via a commercially available device (Impact One, Leica Biosystems, Des Planes IL) inducing a controlled cortical impact. A 3 mm diameter impacting rod was utilized. The rod impacted the brain at a velocity of 2.5 m/s penetrating to a depth of 2 mm with a dwell time of 0.1 s. Sham mice underwent anesthesia and scalp incision alone. All scalp incisions were sealed with VetBond (3 M) (Santa Cruz Animal Health, Dallas, TX) immediately following sham injury or TBI. Post-procedure analgesia with Buprenorphine SR (SR Veterinary Technologies, Windsor, CO) was administered to all animals via subcutaneous injection. Animals recovered in separate cages over a warming pad. Euthanasia of all mice was performed in accordance with AVMA guidelines. Mice were placed into a carbon dioxide chamber and euthanized by inhalation. The mice were then exsanguinated and perfused with phosphate buffered saline containing 4% paraformaldehyde followed by decapitation. Brains were harvested for analysis by immunohistochemistry.
Fecal microbiota transfer
Stool was collected daily, for 2 weeks from chow fed donor mice (10-week-old, male), placed in 1 mL cryogenic collection tubes, and flash frozen in liquid nitrogen before −80C storage. For each treatment frozen stool was diluted (1 g:1 mL) in sterile room temperature water, homogenized and mortared through a 100 μm mesh cell strainer to remove particulates. Mice were inoculated through oral gavage. The mice were given 200 uL of stool slurry or sterile water vehicle control once weekly for 4 weeks. The first treatment was administered 2 h post-TBI.
Fecal microbiome extraction and analysis
Stool processing, DNA extraction, and gene sequencing—
Mice were housed separately for 2 h on days of stool collection and fecal pellets were stored in collection vials. Fresh stool pellets were weighed and flash-frozen in liquid nitrogen and stored at −80°C until use. Frozen stool was diluted in 1:500 in lysis buffer (0.5 mol/L Tris-HCl, 20 mmol/L EDTA, 10 mmol/L NaCl, 0.1% SDS, pH = 9.0). Sample tubes were vortexed and homogenized. After a second 1:2 dilution and homogenization in lysis buffer. Particulate materials were removed by centrifugation with the supernatant being transferred to a new tube. DNAwas precipitated via the addition half the sample volume of ammonium acetate (7.5 mol/L) and two times the sample volume of ice-cold ethanol (95%−100%). Samples were incubated at −20°C for 30 to 45 min to increase precipitation. DNA was collected via centrifugation at room temperature. Genomic DNA was PCR amplified with primers CS1_515F and CS2_806R (modified from the primer set employed by the Earth Microbiome Project (EMP; GTGYCAGCMGCCGCGGTAA and GGACTACNVGGGTWTCTAAT) targeting the V4 regions of microbial small subunit ribosomal RNA genes. Repeated cycles of heating and cooling were used to make many copies of a specific region of DNA or amplicons. The amplicons were generated using a two-stage PCR amplification protocol as described previously (16). The primers contained 5’ common sequence tags (known as common sequence 1 and 2, CS1 and CS2) as described previously (17) First-stage PCR amplifications were performed in 10 microliter reactions in 96-well plates, using MyTaq HS 2X mastermix (Bioline). PCR conditions were 95°C for 5 minutes, followed by 28 cycles of 95°C for 30 minutes, 55°C for 45 minutes and 72°C for 60 minutes.
Subsequently, a second round of PCR amplification was performed in 10 μL reactions in 96-well plates. A mastermix for the entire plate was made using MyTaq HS 2X DNA Polymerase mastermix. Each well received a separate primer pair with a unique 10-base barcode, obtained from the Access Array Barcode Library for Illumina (Fluidigm, South San Francisco, CA; Item# 100–4876). These access array primers contained the CS1 and CS2 linker primers, supplied by Illumina Inc at the 3’ ends of the oligonucleotides. Cycling conditions were 95°C for 5 min, followed by eight cycles of 95°C for 30 min, 60°C for 30 min and 72°C for 30 min for maximum amplification of the genes contained within the samples. Samples were then pooled in equal volume using an EpMotion5075 liquid handling robot (Eppendorf, Hamburg, Germany). The pooled library was purified using an AMPure XP cleanup protocol (0.6X, vol/vol; Agencourt, Beckmann-Coulter) to remove fragments smaller than 300 bp. To provide quality and calibrate controls, the pooled libraries, with a 20% phiX spike-in, were loaded onto an Illumina MiniSeq mid-output flow cell (2×153 paired-end reads). The term for a sequenced DNA fragment detected within a sample is termed a read. Based on the distribution of reads per barcode, the amplicons (before purification) were pooled to generate a more balanced distribution of reads. The re-pooled library was purified using AMPure XP cleanup, as described above. The re-pooled libraries, with a 20% phiX spike-in, were loaded onto a Miniseq flow cell, and sequenced (2 × 153 paired-end reads). Fluidigm sequencing primers, targeting the CS1 and CS2 linker primer regions, were used to initiate sequencing. De-multiplexing of reads was performed on the instrument reconverting the detected signals within the samples and separating them for sample assignment. Library preparation, pooling, and sequencing were performed at the Genome Research Core (GRC) within the Research Resources Center (RRC) at the University of Illinois at Chicago (UIC).
16S rRNA gene data analysis—
QIIME 2 v2019.10 was used to process the reads (18). The input files used were the paired end reads in fastq format and a mapping file with the barcode sequence corresponding to each sample. Reads were split by sample-specific barcode, followed by denoising using the DADA2 plugin. Taxonomic classification was performed using the naive Bayes pre-trained QIIME2 classifier based on the Greengenes reference database 13_8. Samples with a low count of reads per sample were excluded and the rest were rarefied to a depth of 3,500 sequences per sample. 16S RNA gene statistics Alpha diversity (Faith’s PD, Shannon diversity, and observed operational taxonomic unit [OTU], richness) for various groups was generated and compared with a Kruskal–Wallis test. For beta diversity, pairwise unweighted and weighted UniFrac distances were generated and then the distances of the between-group differences were tested using PERMANOVA and permuted t tests in QIIME 2. The boxplots and the heatmap were produced using the relative abundances of the microbes at phyla and species level of taxonomic lineage using the package ggplot2 and heatmap.plus in R v3.6.1, respectively.
Behavioral phenotyping—
All experimental groups (N = 5/group) underwent behavioral phenotyping starting at 45 days post-injury in the Northwestern University Behavioral Phenotyping Core. Studies took place over 3 months and were conducted during the light phase cycle.
Elevated zero maze—
The elevated zero maze (ZM) test started 45 days post-injury (DPI) and was used to evaluate the anxiety response of mice (Crawley, 2009). The maze is grey and has a 45 cm inner diameter with a 6 cm wide track and is elevated 30 cm from the floor (Phenome Technologies Skokie, IL). The ZM has two open quadrants and two closed quadrants with 13.3 cm tall acrylic walls. The mice were placed inside the center of the closed quadrant and were exposed to white/yellow light. The animals were recorded for 10 min using Limelight 4 software (Actimetrics, Wilmette, IL) to track their movements. The maze was cleaned between trials using 70% ethanol.
Open field (OF) testing—
OF testing was conducted at 50 DPI and was used to assess anxiety, exploratory behavior, and locomotive activity (19). Mice were placed in the center of an enclosed 54.5 cm × 54.5 cm square box with 30 cm tall walls and exposed to white/yellow light. Each trial lasted 5 min and movement was recorded and tracked using LimeLight 4 software (Actimetrics, Wilmette, IL). The box was cleaned with 70% ethanol in between trials to minimize olfactory cues. Behavior was recorded with LimeLight 4 software.
Magnetic resonance imaging—
All MRI scans were performed using a 7 Tesla ClinScan MRI scanner (Bruker, Germany). Each mouse was anesthetized using a mixture of 100% oxygen and isoflurane and placed in a heated holder. A four-channel dedicated mouse brain surface coil was used to acquire the signal and generate the images. 3D anatomical brain images were obtained using a gradient echo sequence (TR=56 ms, TE=2 ms) with isotropic resolution. Diffusion MRI sequences using multi b-value, multi-direction diffusion gradients were employed to acquire diffusion weighted images later used to generate diffusion anisotropy data. Quantitative analysis was conducted on the MR images and on quantitative parametric maps using a combination of image processing software (ITK-SNAP, Xinapse JIM7.0) to segment the regions of interest and to extract quantitative imaging parameters.
Murine tissue microarray—
To construct tissue array blocks, mice were euthanized at day 93 DPI, and brains were paraffin-embedded. Five animals per group were euthanized and perfused with 4°C 1 × Hank’s Balanced Salt Solution (HBSS) followed by 4% paraformaldehyde in PBS. Whole brains were excised and fixed in 4% paraformaldehyde. Six cores (1.0 mm in diameter) were taken from identical areas of interest from each paraffin-embedded fixed brain. Areas of interest included three points on the ipsilateral side of the brain including the posterior, immediate, and anterior sites to the injury. Equal cores were taken from the contralateral side of the brain. Fixed brain cores were arranged into paraffin array blocks at room temperature and 4 mm sections were cut and slide-mounted. Sectioning, processing, and staining with Ionized calcium binding adaptor molecule 1 (Iba1) were performed at the Northwestern University Mouse Histology and Phenotyping Laboratory. Slides were imaged and saved using a TissueFax microscope (TissueGnostics, Vienna, Austria). Tissue images were analyzed using HistoQuest tissue analysis software (TissueGnostics).
Statistical analyses
Behavioral data are presented as mean ± SEM. Group means were compared by analysis of variance (ANOVA) and Tukey post hoc analysis. Significance was set at α < 0.05. ((P ≤ 0.05(*), 0.001(**), and 0.0001(***)). All analyses were conducted using GraphPad Prism (GraphPad, La Jola, Ca, version 6). For 16S alpha diversity faith’s PD, Shannon diversity, and observed operational taxonomic unit (OTU) richness for various groups were compared with a Kruskal–Wallis test. For beta diversity, pairwise unweighted and weighted UniFrac differences were tested using PERMANOVA and permuted t tests in QIIME 2. The boxplots and the heatmap were produced using the relative abundances of the microbes at phyla and species level of taxonomic lineage using the package ggplot2 and heatmap.plus in R v3.6.1 (R Core Team, Auckland, AU) respectively.
RESULTS
To determine whether fecal microbiome transfer would attenuate neurocognitive, anatomic, and pathologic outcomes after TBI, sham and TBI mice were administered FMT or vehicle control via oral gavage once weekly. To maintain clinical relevance, treatment started at 2 h post-TBI. Bacterial differences in stool collected at day 1 −TBI and +59 days post-TBI was correlated to neurocognitive testing, 3D contrast-enhanced MRI and neuropathology.
Fecal microbiome
Fresh stool pellets were collected 1-day prior to TBI, 1-day post-TBI, and 59-days post-TBI. After sequencing the conserved variable region within 16S rRNA genes, we identified, classified, and quantification the microbes contained within each stool pellet. Statistical comparisons were made between groups when bacterial reads reached a common threshold; other data points were excluded. Alpha diversity analysis was measured and is represented as box plots of species richness (Fig. 1A) and species evenness (Fig. 1B). Each data point represents the taxonomic assignment of 16S rRNA found in each sample and reveals the different distributions in relative species abundance. The data revealed a striking decline (P < 0.05) in species richness and evenness after TBI. Post-TBI treatment with FMT resulted in a marked increase (P < 0.001) in species richness and evenness as compared to vehicle treated TBI mice.
Fig. 1. Transfer of stool from healthy donor mice reverses the loss of species richness and species evenness after TBI in mice.

Fecal microbiome biodiversity was measured and is represented as box plots of species richness (A) and species evenness (B). Elements of alpha-diversity, measured by Simpson Index, which relates operational taxonomic unit (OTU) richness and evenness, are plotted with each box representing groups containing 5 mice (N = 5). TBI results in a marked decrease in the number of species present and their relative abundance. Post-TBI treatment with FMT abrogates TBI-induced decreases in richness and evenness. ((P ≤ 0.05(*), 0.001(**), and 0.0001(***)).
Next, we compared the gut microbial community structure of TBI, and sham-injured mice treated with FMT or vehicle control using a principal coordinates analysis (PCoA) diagram (Fig. 2A) and Atchison graph (Fig. 2B). 16S analysis of the fecal microbiome demonstrated that by post-injury day 59, all experimental groups had diverged markedly from their pre-injury gut microbial structures (Supplemental Figure 1, http://links.lww.com/SHK/B441). However, by post-injury day 59, the gut microbial community of TBI mice treated with FMT was most closely aligned with those of sham injured mice. Beta diversity analysis utilizing Principal Coordinates Analysis (PCoA), with each data point representing the taxonomic assignment of 16S rRNA, made evident the similarities of available samples (Fig. 2).
Fig. 2. Transfer of stool from health donor mice preserves the ratio between regional and local species diversity after TBI.

These graphs depict the relative distinctness and similarities of all groups. A principal coordinates analysis (PCoA) diagram (A) shows groups overall similarity of using relative placement along axis PC1 and PC2. Dots in the PCoA plot represent an individual sample. Colors indicate group (Red = Sham vehicle, Blue = TBI vehicle, Green = Sham-FMT, and Purple = TBI-FMT). In the chart, sham and TBI groups are separated by the most distance representing the greatest level of between-groups-variability. Despite distance, these two groups show similar clustering representing the least amount of within-groups-variability of microbial phenotypes. The Atchison graph depicts the internal group variability (mean) using nodes (polygons) of large or small sizes to represent the level of internal group diversity. Distance of nodes from a central point (centroid) reveals group similarity of group means. This Atchison plot reveals that sham-FMT samples show the greatest within group diversity while the small TBI polygon depicts the least. Similar sized Sham and FMT-TBI nodes have similar levels of internal group diversity.
To further characterize the interaction between FMT and TBI on gut microbial community structure, we delved into species-level changes over the course of injury. The heatmap generated shows thevariation of species that drove the injury and treatment-related changes observed in beta diversity (Fig. 3). At 59 DPI there were notable alterations in the species present secondary to both treatment and injury status. This post-injury status contrasted notably with the baseline data which showed few differences between the groups due to outside factors (i.e., cage effect) (Supplemental Figure 2, http://links.lww.com/SHK/B442).
Fig. 3. Murine fecal microbiome bacterial species post TBI and FMT treatment.

Heatmap plots were generated to represent the species-level differences in the microbial community of all groups at 59 days post TBI. Experimental groups are highlighted along the X-axis. Measured species are along the Y-axis. Color shaded lines represent the abundance of each species found with the most abundant species within a group in red and least abundant species shown with a yellow line. The graph reveals the presence of group dependent microbial networks due to treatment and injury.
Neurocognitive testing
To measure exploratory behavior and general activity post-TBI we assessed the animals in open field testing (OF) (Fig. 4A). We used measurements of time in the center of the open field and generalized activity within the first 5 min as indicators of anxiety. We found that sham animals with or without FMT treatment spent 19% to 21% of their time in open regions (Fig. 4C). FMT treated TBI mice spent a greater percentage of time (22%) in the center regions of the as compared to untreated TBI mice (13%, P < 0.0001). Zero maze (ZM) (Fig. 4B) was used as a follow-up measure of anxiety-like behavior. Sham-injured mice were used to establish a baseline, staying in open areas of the maze for nearly 26% of the measured time. After TBI, time in the open portion of the maze dropped significantly compared to both sham groups (19%, P < 0.001, 0.01) indicating a loss of normal murine anxiety-like behavior. However, post-injury treatment with FMT preserved this normal anxiety-like behavior (30%, P = 0.0001) (Fig. 4D). Taken together, these data show a FMT-related preservation of neurocognitive function after TBI.
Fig. 4. Fecal microbiome transfer alters anxiety and exploratory behavior in TBI mice.
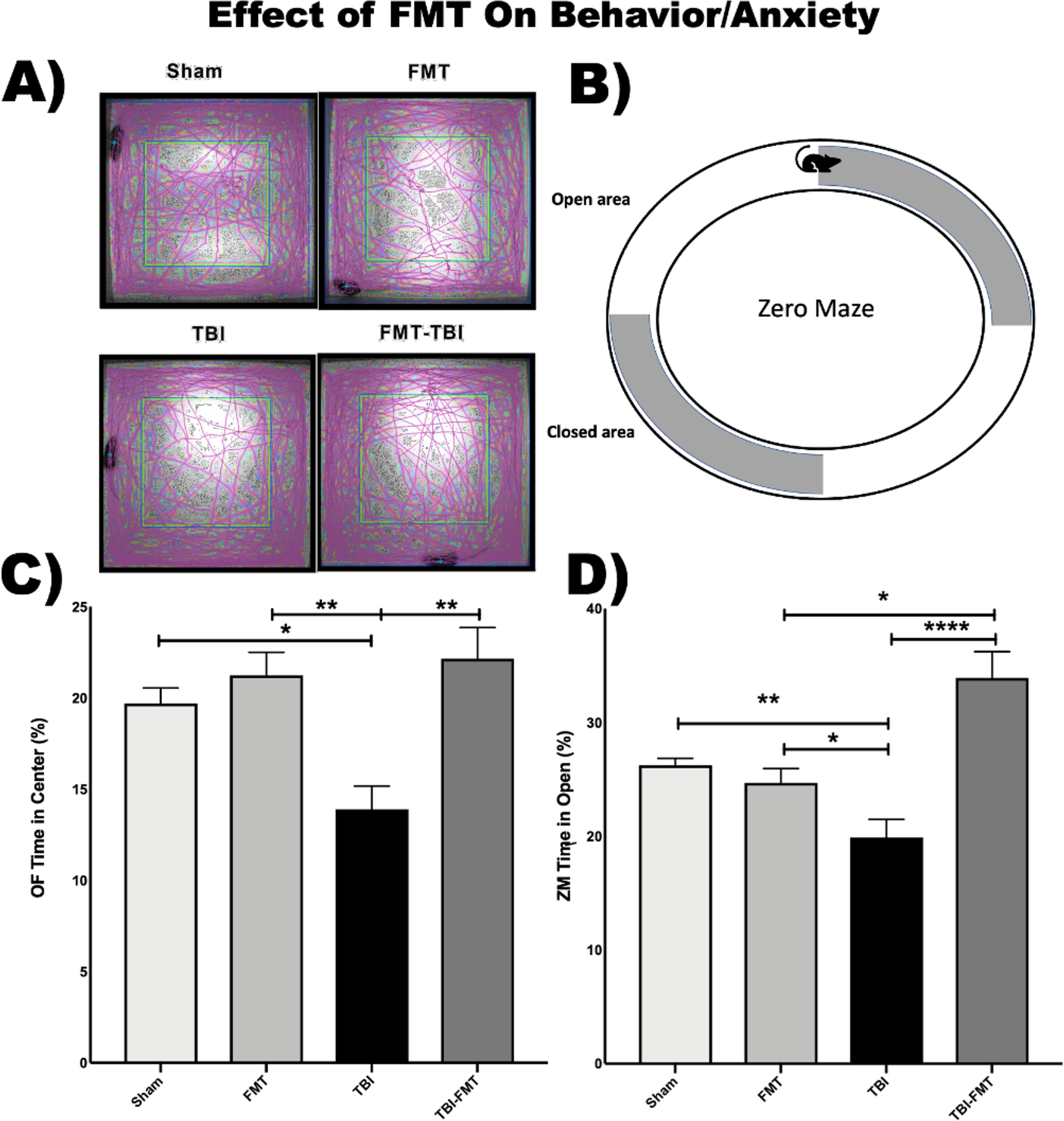
(A) Open Field (OF) was used to assess anxiety and exploratory behavior. The purple lines in this panel represent the path taken by mice in the five-minute testing period. (B) Elevated Zero Maze (ZM) was used to assess for post-injury anxiety. More time spent in the open areas indicates a higher inclination towards exploration and a lower level of less anxiety. (C) OF testing demonstrated preserved exploratory behavior in TBI mice post injury treatment as demonstrated by the increased percentage of time spent in the center of the open field compared to untreated injured animals (22% vs 19%: N = 5/group). (D) ZM demonstrated preservation of normal anxiety-like behavior in FMT-treated TBI mice as compared to vehicle-treated TBI mice with a significant increase in time spent in the open area (39% vs 19%; P = 0.0001; N = 5/group).
MRI
Brain ventricle size was used as a surrogate for cortical volume loss as we have previously published (Fig. 5A) (20). MRI analysis demonstrated a marked reduction in ventriculomegaly in FMT treated mice after TBI as compared to vehicle treated TBI mice (P < 0.002). The observed average ventricle size for each experimental group, plotted in Figure 5A and B is consistent with relative preservation of cortical volume in FMT-treated TBI mice and is consistent with the neurocognitive protection reported above. In addition, the diffusion MRI results are compared using a threshold modified fractional anisotropy (the relative degree of diffusion) approach which is used as a surrogate measurement of overall white-matter connectivity as we have described previously (20). The reduced fractional anisotropy (connectivity) in the vehicle treated TBI mice versus the FMT treated TBI mice (P = 0.039) are plotted in Figure 5C and D. Representative MR images are shown in Figure 6 reflecting the quantitative neuroanatomical findings. These data suggest a protective effect of FMT on the overall degree of anatomic damage post-TBI.
Fig. 5. FMT attenuates cortical volume loss and preserves white matter connectivity after TBI.
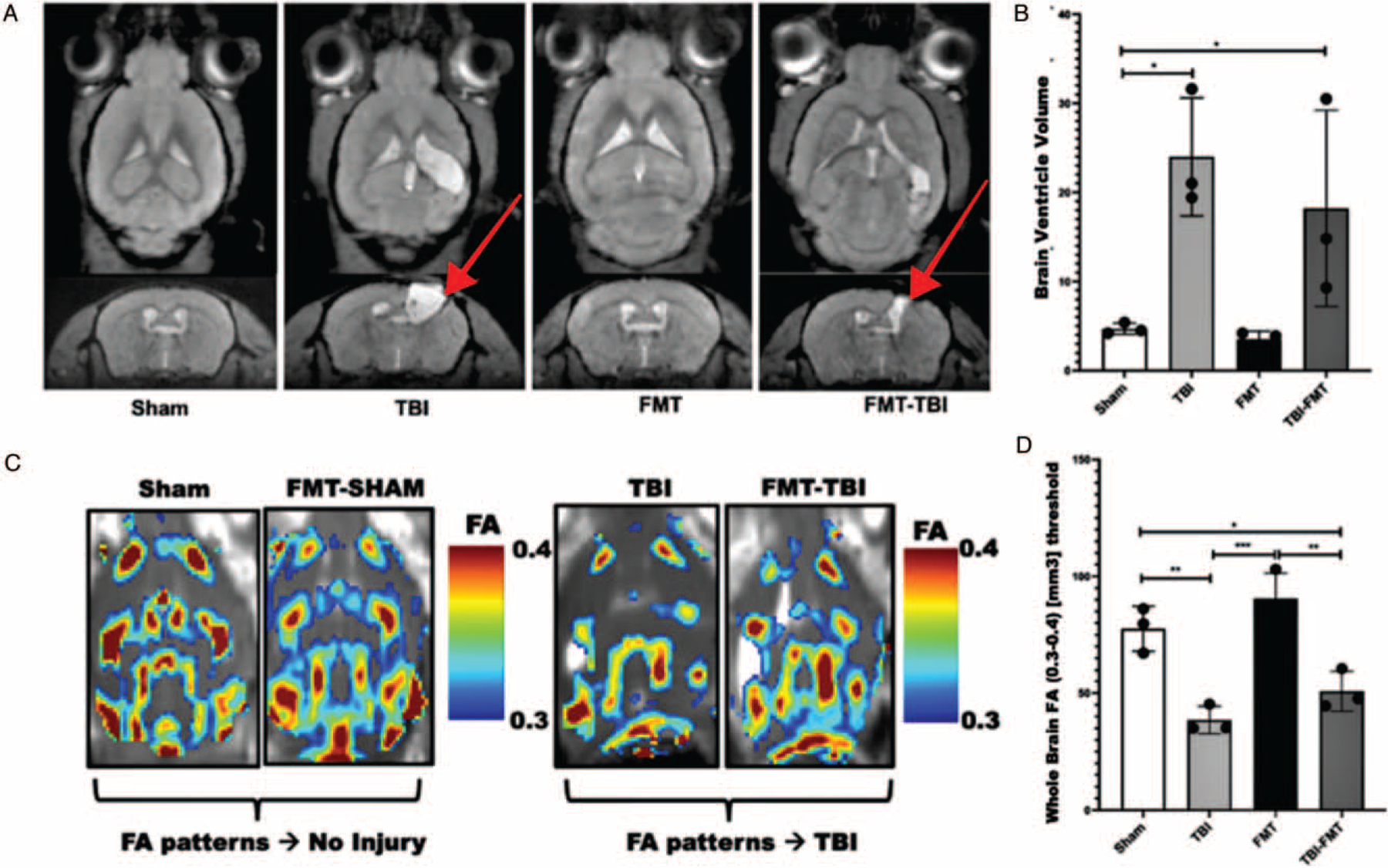
(A) Representative longitudinal and transverse MRI scans of animals (60DPI) with injury sites indicated with red arrows (N = 3). (B) FMT attenuates TBI-induced ventriculomegaly (red arrows) which is a well-described surrogate for cortical volume loss (P < 0.002). (C) Images ktrans level (measure of capillary permeability) were extracted from contrast-enhanced 3D-MRI. Fractional anisotropy (connectivity) maps were then extracted from the MRI images. (D) TBI induced a decrease in fractional anisotropy (white matter connectivity) compared to sham injury (P < 0.0001). FMT treatment attenuated this loss of connectively as compared to vehicle treated TBI mice (P = 0.04).
Fig. 6. Ionized calcium binding adaptor molecule 1 (IBA1) stained sections of ipsilateral mouse cortex.
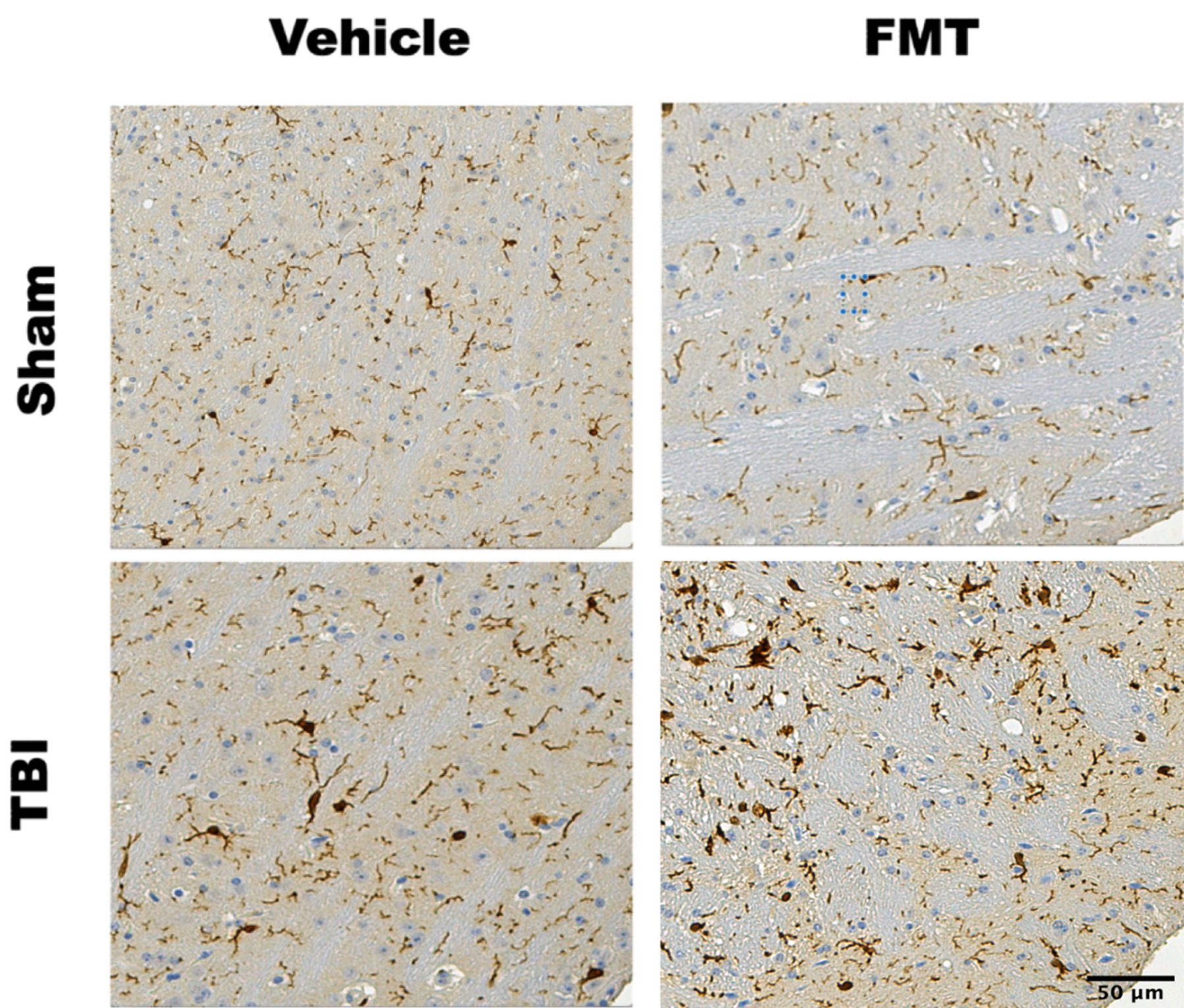
IBA1 stained tissue from the ipsilateral cortex at the impact site 90 days after localized CCI brain injury. Ten micrometers of samples from six equalized locations within the ipsilateral cortex of TBI and Sham-TBI brains +/− FMT treatment at the 90DPI timepoint. Sham injured mice show no appreciable increase in IBA1 staining whether treated with FMT or vehicle. TBI resulted in a marked increase in IBA1 staining as compared to sham injury. Post injury treatment with FMT resulted in a further increase in IBA1 staining as compared to vehicle treatment TBI mice (P = 0.002).
Neuropathology
Ionized calcium-binding adaptor molecule 1 (IBA1) staining of tissue microarray (TMA) samples from animals was assessed to determine whether the FMT-associated attenuation of neurocognitive deficits correlated to microglial activation. IBA1 is a pan-microglia marker that stains both resting and activated cells. Staining was quantified using Histoquest software (from where). Two-way analysis of variance (ANOVA) revealed a statistical interaction of FMT treatment and TBI (P = 0.002) in IBA1 stained sections. Because expression of IBA1 increases with microglial activation but not the absolute number of either resting or activated microglia we reasoned that the substantial increase in IBA1 coverage in FMT-treated mice post-TBI was attributable to increased microglia activation (Fig. 6) (21).
DISCUSSION
There is growing evidence that the gut microbiome plays an important role in brain health both under conditions of homeostasis as well as in neurologic disease and injury (22). In the case of TBI, injury-induced neuroinflammation and neurodegeneration results in secondary injury to the gastrointestinal (GI) system with increased mucosal permeability and dysmotility of the intestinal wall through the bidirectional brain-gut axis (23). These events can further contribute to the pathogenesis of GI disorders and inflammation (22). Emerging studies have indicated that gut dysbiosis is an important mediator in these secondary GI insults which can feedback on the index brain injury resulting in worse neurocognitive, anatomic, and pathologic outcomes (23).
While the precise mechanism(s) underlying the role of the gut microbial community in brain health have yet to be fully elucidated, the data from this study suggest that the composition of the gut microbiota may represent a novel therapeutic target after TBI. We hypothesized that restoring a pre-injury gut microbial community structure via fecal microbiota transfer would attenuate neurocognitive deficits as compared to vehicle-treated mice after TBI. To test this hypothesis, we treated TBI and sham TBI mice with fecal microbiota transfer or vehicle control in a post-injury treatment paradigm. We observed that FMT can restore pre-injury levels of gut microbial community structure which correlated to marked improvements in neurocognitive outcomes. Furthermore, in vivo MRI demonstrated attenuated cortical volume loss and preserved white matter connectively in FMT-treated TBI mice as compared those treated with vehicle alone. These in vivo findings correlated with postmortem neuropathology showing increased microglia activation in the brains of TBI mice treated with FMT up to 90 days post-injury.
Fecal microbiota transfer with stool from healthy donors has been shown in a variety of models to have clinical benefits (24). For example, fecal transfer has been shown to reduce colonic inflammation and activate various immune-mediated pathways leading to altered IL-10 production by CD4+ T cells, invariant natural killer T (iNKT) cells and Antigen Presenting Cells (APC) in models of intestinal and neurological disease (25). These alterations in intestinal homeostasis can affect the neuroinflammation that triggers dysfunction in neurological disorders like Alzheimer’s disease, Parkinson’s disease, Huntington’ disease, amyotrophic lateral sclerosis, and stroke (14). While theorizing a mechanism for FMT’s beneficial effects, previous studies point to the ability of fecal transfer to balance bacterial diversity thereby decreasing competition for nutrients, increasing the presence of antimicrobial peptides, and increasing the bile-mediated inhibition of fungal spore germination and growth (26). All of these effects have the ability to alter immune cell defenses (26). Another highly referenced mechanism is the ability of FMT to increase the production of short-chain fatty acids (SCFA) like acetate, propionate, and butyrate which have been linked to increased gut and brain health (27). Due to the ability of FMT to directly alter several elements within the microbiome, future studies are needed to elucidate specific mechanisms underlying FMT’s beneficial effects in neurologic injury and disease. Our data points to similar protective neuroimmune effects of FMT after TBI with the restoration of gut microbial community structure, preservation of species-normal behavior traits, reduction of cortical volume loss, preservation of white matter connectivity, and activation of microglia up to 3 months post-TBI.
In vivo neuroanatomic data obtained through quantitative MRI in our study suggests preservation of white matter connectivity between brain regions such as the amygdala, hippocampus, and prefrontal cortex. This preservation may be mediated by the recruitment and activation of microglia throughout the injured brain as evidenced by the increase in IBA1 staining seen in our study. While we could not identify increased GFAP staining in the brains of FMT mice, increased neuroimmune cell staining at the site of injury may represent yet another mechanism by which FMT exerts its neuroprotective effects. This is corroborated by our own data in the current study as well as evidence in the literature showing that FMT modulates the recruitment and activation of astrocytes and microglia in mouse models of Alzheimer’s (Alz) and multiple sclerosis (MS) (28, 29).
Limitations of the current study include the inherent limits in mechanistic data that can be gleaned from a whole stool FMT study. While dominated by bacteria, the microbiome also includes commensal populations of fungi, viruses, archaea, and protists, each filling a niche and producing possibly influential bioactive substances through a host of metabolic processes. FMT does not provide information on specific taxa that may be the primary drivers of noted beneficial effects. Short chain fatty acid production/metabolism and the relative abundance/reduction of specific taxa may be some of the putative mechanisms underlying the observed neuroprotective effects of FMT, follow up experiments to specifically interrogate these potential mechanisms are necessary. Future studies will also need to assess targeted microbiota interventions such as mono or poly association with specific taxa known to produce SCFA as published studies have shown the ability of an external injury to decrease butyrate and butyrate-producing bacterial species in the gut (30). We will assess whether the administration of SCFA alone or dietary fiber supplementation in a clinically relevant manner would therapeutically maintain gut microbial structure and decrease neurologic damage after TBI. As an additional consideration, clinical TBI is a disparate injury process ranging from mild to severe, diffuse to focal, and single to repeated exposures (31). Whether FMT can be generalized to the entire spectrum of TBI remains to be further investigated. Lastly, the roles of age, sex, and weight differences in the context of FMT treatment and TBI represent additional area for future investigation.
CONCLUSION
In conclusion, our data shows that post injury FMT has the potential to restore gut microbial community structure, attenuate neurocognitive deficits, preserve white matter connectivity between brain regions, and activate the resident innate immune cells of the brain. Taken together these data suggest that FMT may represent a novel treatment paradigm for TBI. Given that FMT is already an established treatment for some disease processes, such as chronic clostridium difficile colitis, translation of FMT to TBI may not be as far away from clinical practice as other potential therapies. Additionally, these data point towards a role of FMT in in the activation of microglia which will be the target of future studies in our laboratory.
Supplementary Material
Acknowledgments
NIH Grant 1R01GM130662 and NIH Grant 1R01GM130662 - S1.
Footnotes
Ethical Approval and Consent to participate: This article does not contain any studies with human participants. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animals were treated and cared for in accordance with the National Institutes of Health Guidelines for the Use of Laboratory Animals. The Northwestern University Institutional Animal Care and Use Committee approved the experimental protocol.
Consent for publication: All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria. This article is original, has not already been published in a journal, and is not currently under consideration by another journal
The authors report no conflict(s) of interest.
The data that support the findings in this manuscript are available from the corresponding author upon request. The 16S rRNA gene data have been deposited to the Illumina Basespace data repository with the following dataset identifier: ‘‘Project MINI667_Steve-Schwulst_Brooker-Davis_38696’’.
Reviewer link: https://basespace.illumina.com/projects/286888607
he data that support the findings in this manuscript are available from the corresponding author upon request. The 16S rRNA gene data have been deposited to the Illumina Basespace data repository with the following dataset identifier: ‘‘Project MINI667_Steve-Schwulst_Brooker-Davis_38696’’.
Reviewer link: https://basespace.illumina.com/projects/286888607
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.shockjournal.com).
REFERENCES
- 1.Nittayasoot N, Peterson AB, Thammawijaya P, Parker EM, Sathawornwiwat A, Boonthanapat N, Chantian T, Voradetwitaya L, Jiraphongsa C, Sagarasaeranee O, et al. : Evaluation of a hospital-based injury surveillance system for monitoring road traffic deaths in Phuket, Thailand. Traffic Inj Prev 20(4):365–371, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeKosky ST, Asken BM: Injury cascades in TBI-related neurodegeneration. Brain Inj 31(9):1177 –1182, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faul M, Coronado V. Chapter 1: Epidemiology of traumatic brain injury In: Grafman J, Salazar AM, eds. Vol. 127. Elsevier, 2015, 3–13. [DOI] [PubMed] [Google Scholar]
- 4.Makinde HM, Cuda CM, Just TB, Perlman HR, Schwulst SJ: Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol 199(10):3583 –3591, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister TW: Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 13(3):287–300, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanscom M, Loane DJ, Shea-Donohue T: Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest 131(12):e143777, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson SE, Watts LT, Burmeister DM, Merrill D, Scroggins S, Zou Y, Lai Z, Grandhi R, Lewis AM, Newton LM, et al. : Moderate traumatic brain injury alters the gastrointestinal microbiome in a time-dependent manner. SHOCK 52 (2):240–248, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Almeida C, Oliveira R, Soares R, Barata P: Influence of gut microbiota dysbiosis on brain function: a systematic review. Porto Biomed J 5(2):1–8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celorrio M, Abellanas MA, Rhodes J, Goodwin V, Moritz J, Vadivelu S, Wang L, Rodgers R, Xiao S, Anabayan I, et al. : Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol Commun 9(1):40, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamo T, Akazawa H, Suda W, Saga-Kamo A, Shimizu Y, Yagi H, Liu Q, Nomura S, Naito AT, Takeda N, et al. : Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PloS One 12(3): e0174099–e1174099, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BTt Islam MBAR, Das P Gilbert JA, Ho KJ, Schwulst SJ: Differential fecal microbiome dysbiosis after equivalent traumatic brain injury in aged versus young adult mice. J Exp Neurol 2(3):120–130, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam M, Davis BTt, Kando MJ, Mao Q, Procissi D, Weiss C, Schwulst SJ: Differential neuropathology and functional outcome after equivalent traumatic brain injury in aged versus young adult mice. Exp Neurol 341:113714, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar V, Fischer M: Expert opinion on fecal microbiota transplantation for the treatment of Clostridioides difficile infection and beyond. Expert Opin Biol Ther 20(1):73–81, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, Ducarmon QR, Keller JJ, Kuijper EJ, Contarino MF: Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol 10:98–198, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makinde HM, Just TB, Gadhvi GT, Winter DR, Schwulst SJ: Microglia adopt longitudinal transcriptional changes after traumatic brain injury. J Surg Res 246:113–122, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naqib A, Poggi S, Wang W, Hyde M, Kunstman K, Green SJ: Making and sequencing heavily multiplexed, high-throughput 16S ribosomal RNA gene amplicon libraries using a flexible, two-stage PCR protocol. Methods Mol Biol 1783:149–169, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Moonsamy PV, Williams T, Bonella P, Holcomb CL, Höglund BN, Hillman G, Goodridge D, Turenchalk GS, Blake LA, Daigle DA, et al. : High throughput HLA genotyping using 454 sequencing and the Fluidigm Access Array( System for simplified amplicon library preparation. Tissue Antigens 81(3):141–149, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. : Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37(8):852–857, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould TD, Dao DT, Kovacsics CE: In: Gould TD, editor. The Open Field Test Totowa, NJ: Humana Press; p. 1–20, 2009. [Google Scholar]
- 20.Makinde HM, Just TB, Cuda CM, Bertolino N, Procissi D, Schwulst SJ: Monocyte depletion attenuates the development of posttraumatic hydrocephalus and preserves white matter integrity after traumatic brain injury. PLoS One 13 (11):e0202722, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP: Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry 23(2):177–198, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanscom M, Loane DJ, Shea-Donohue T: Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest 131(12.):e143777, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice MW, Pandya JD, Shear DA: Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries. Front Neurol 10:875, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, Pierini S, Tanes C, Ganetsky A, Morgan MA, Gill S, Tanyi JL, et al. : Gut microbiota modulates adoptive cell therapy via CD8a dendritic cells and IL-12. Vol.3. JCI Insight 3(4):e94952, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assimakopoulos SF, Papadopoulou I, Bantouna D, de Lastic AL, Rodi M, Mouzaki A, Gogos CA, Zolota V, Maroulis I: Fecal microbiota transplantation and hydrocortisone ameliorate intestinal barrier dysfunction and improve survival in a rat model of cecal ligation and puncture-induced sepsis. Shock 55(5):666–675, 2021. [DOI] [PubMed] [Google Scholar]
- 26.Khoruts A, Sadowsky MJ: Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 13(9):508–516, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva YP, Bernardi A, Frozza RL: The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol 11(25), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, Ye S, Ye K, Wei D, Song Z, et al. : Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry 9(1):189, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, Peng X, Cao X, Huang Z, Zhou H, et al. : Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediat Inflam 2020:2058272, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuethe JW, Armocida SM, Midura EF, Rice TC, Hildeman DA, Healy DP, Caldwell CC: Fecal microbiota transplant restores mucosal integrity in a murine model of burn injury. Shock 45(6):647–652, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR: Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant 26 (7):1118–1130, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


