Abstract
Injuries of the central (CNS) and peripheral nervous system (PNS) are a serious problem of the modern healthcare system. The situation is complicated by the lack of clinically effective neuroprotective drugs that can protect damaged neurons and glial cells from death. In addition, people who have undergone neurotrauma often develop mental disorders and neurodegenerative diseases that worsen the quality of life up to severe disability and death. Hydrogen sulfide (H2S) is a gaseous signaling molecule that performs various cellular functions in normal and pathological conditions. However, the role of H2S in neurotrauma and mental disorders remains unexplored and sometimes controversial. In this large-scale review study, we examined the various biological effects of H2S associated with survival and cell death in trauma to the brain, spinal cord, and PNS, and the signaling mechanisms underlying the pathogenesis of mental illnesses, such as cognitive impairment, encephalopathy, depression and anxiety disorders, epilepsy and chronic pain. We also studied the role of H2S in the pathogenesis of neurodegenerative diseases: Alzheimer’s disease (AD) and Parkinson’s disease (PD). In addition, we reviewed the current state of the art study of H2S donors as neuroprotectors and the possibility of their therapeutic uses in medicine. Our study showed that H2S has great neuroprotective potential. H2S reduces oxidative stress, lipid peroxidation, and neuroinflammation; inhibits processes associated with apoptosis, autophagy, ferroptosis and pyroptosis; prevents the destruction of the blood-brain barrier; increases the expression of neurotrophic factors; and models the activity of Ca2+ channels in neurotrauma. In addition, H2S activates neuroprotective signaling pathways in psychiatric and neurodegenerative diseases. However, high levels of H2S can cause cytotoxic effects. Thus, the development of H2S-associated neuroprotectors seems to be especially relevant. However, so far, all H2S modulators are at the stage of preclinical trials. Nevertheless, many of them show a high neuroprotective effect in various animal models of neurotrauma and related disorders. Despite the fact that our review is very extensive and detailed, it is well structured right down to the conclusions, which will allow researchers to quickly find the proper information they are interested in.
Keywords: hydrogen sulfide, neurotrauma, apoptosis, autophagy, ferroptosis, pyroptosis, neuron, glial cells, cognitive impairment, encephalopathy, depression, anxiety disorders, epilepsy, neurodegenerative diseases
1. Introduction
Neurotrauma is one of the leading causes of disability and death worldwide. It takes third position after cardiovascular and oncological diseases. Moreover, due to neurotrauma, the highest mortality and proportion of disability is observed among the young population. This situation is complicated due to the lack of effective clinical neuroprotective drugs that can protect neurons and glial cells from traumatic injury [1,2]. In addition, the heterogeneity of neurotrauma creates additional difficulties in their study and difficulties in developing and selecting a competent treatment strategy [3]. In addition, CNS and PNS injuries often lead to various mental disorders [4,5,6,7,8] and neurodegenerative diseases [9,10,11,12,13], which are accompanied by increased cell death of the nervous tissue [14,15,16,17,18,19]. It is worth noting that nerve cells are very sensitive to various influences, including microwave radiation, which can cause neurodegenerative diseases [20]. This confirms the presence of complex intermolecular interactions in the nervous tissue. To solve these problems, it is necessary to search for promising molecular targets and study the intracellular signaling processes associated with them [21].
Gasotransmitters are important signaling gaseous molecules that perform various functions in the body under normal and pathological conditions [22]. They play an important role in the processes of cell survival and death [23]. Although many signaling mechanisms of cytoprotection and cytotoxicity of these messengers are poorly understood and often contradictory, the S-gasotransmitter H2S remains of great interest to researchers, especially in conditions of traumatic damage to the nervous system [24,25,26], and mental [27] and neurodegenerative diseases [28].
H2S is produced endogenously in many tissues and is involved in various cellular processes: neurotransmission, apoptosis, inflammation, oxidative stress, angiogenesis, etc. [22]. Many scientific data indicate that H2S can act both as a neuroprotective agent and as a factor responsible for neurodegeneration [25,29,30,31]. Its role in neurotrauma is also ambiguous: some researchers point to its pronounced neuroprotective effect [29,32,33,34,35,36], while others associate it with cell death [37,38,39,40]. Of particular interest are the H2S-dependent signaling mechanisms of survival and death of nerve cells in mental disorders [4] and neurodegenerative diseases [41], which often develop with neurotrauma [4,5,6,7,8,9,10,11,12,13].
Thus, the purpose of this review is a comprehensive analysis of the current data on the role of H2S in the survival and death of neurons and glial cells in neurotrauma and related mental disorders and neurodegenerative diseases. This large-scale review study is a natural continuation of a recently published review in which we summarized scientific research on the role of gasotransmitters in apoptotic cell death in cardiovascular, renal, rheumatic, neurodegenerative diseases and mental disorders [42]. As a result, we decided to focus our attention on H2S as a potential cytoprotective signaling molecule. In this global review, the role of H2S-dependent signaling mechanisms in the survival and death of neurons and glial cells in injuries of the brain, spinal cord and PNS is comprehensively considered in detail and the signaling pathways underlying the pathogen of neurodegenerative diseases and mental disorders associated with neurotrauma are studied.
2. Materials and Methods
This large review study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [43]. The Technical Expert Group (TEG) consisted of 7 highly qualified specialists, including 3 molecular biologists (S.R., C.N., M.R.) and 4 clinical and psychiatric physicians (A.S., A.T., I.V., M.G.).
TEG sought to investigate the role of H2S in cell death in neurotrauma and related neurodegenerative and psychiatric illnesses. TEG searched for studies, assessed their reliability, and conceptualized and synthesized the findings.
2.1. Finding Sources
TEG searched for sources in PubMed (Public MedLine, managed by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine in Bethesda (Bethesda, MD, USA)), as well as in Web of Science, and Scopus. Our strategy search did not include restrictions on publication date, language and free full-text access in order to obtain the maximum coverage for the given parameters, namely keywords. In addition, to improve search efficiency, synonyms and terms associated with keywords and their phrases were used. We used logical operators such as “AND”, “OR” next to the keywords to create a logical string that allows us to refine our search and find the most relevant sources (Table 1). Additionally, we could use the logical operator “NOT” [44].
Table 1.
Sourcing strategy for each database, further information can be found in Supplementary Materials.
| Database | Search Strategy |
|---|---|
| PubMed | (hydrogen sulfide OR gasotransmitters OR cystathionine-β-synthase OR cystathionine-γ-lyase OR 3-mercaptopyruvate sulfurtransferase OR hydrogen sulfide donors) AND (neurotrauma OR traumatic brain injury OR spinal cord injury OR peripheral nerve injury OR axotomy OR apoptosis OR autophagy OR ferroptosis OR pyroptosis OR cell death OR axon OR dendrites OR dendritic spines OR cognitive impairment OR depression OR anxiety disorders OR epilepsy OR encephalopathy OR chronic pain OR oxidative stress OR inflammation OR neuroinflammation OR brain OR spinal cord OR neurotrophic factors OR cytokines OR neurodegeneration OR Alzheimer’s disease OR Parkinson’s disease OR protein folding) |
| Scopus | TITLE-ABS-KEY (“hydrogen sulfide” OR “gasotransmitters”) AND TITLE-ABS-KEY (“brain” OR “spinal cord” OR “nerves” OR “neurotrophic factors” OR “mental disorders” OR “neurodegenerative diseases”) |
| Web of Science | TOPIC (*hydrogen sulfide* OR *gasotransmitters*) AND TOPIC (*neurotrauma* OR *apoptosis* OR *autophagy* OR *pyroptosis* OR *ferroptosis* OR *axon* OR *dendrites* OR *donors* OR *mental disorders* OR *neurodegenerative diseases*) |
*—search by the root of the word; “”—exact keyword; ()—grouping of keywords; “AND”—both words; “OR”—either the first or second word.
2.2. Study Quality Assessment
At this stage, three authors (M.R., A.T., A.S.) independently checked the titles/abstracts of the sources that were obtained from the databases. In the case where one author recognized an article suitable for inclusion in a system analysis, the other authors analyzed it in detail. In addition, this process was controlled, and the first author (S.R.) acted as an independent arbiter. In case of a disagreement between the authors, the final decision on the inclusion or exclusion of an article was made by the first (S.R.) or the last author (M.G.).
The studies that were included in our systematic review had to meet the following criteria: (1) transparency of the results obtained; (2) competent statistical data analysis; (3) representative sample; competent choice of materials and methods for research; (4) correct interpretation of the data.
2.3. Conceptualization and Synthesis of the Received Data
The data obtained were subjected to detailed analysis by the entire TEG group. The obtained data were conceptualized and synthesized in the form of textual and graphic representation. The final evaluation of the results obtained was carried out by the first author (S.R.).
3. Classification and Molecular Mechanisms of Neurotrauma
Neurotrauma is damage to various structures of the CNS and PNS caused by external forces. It includes isolated and combined traumatic brain injury (TBI); isolated and combined spinal cord injury (SCI); and multiple limb injury with isolated or combined damage to bones, ligaments, blood vessels, and peripheral nerves [45]. Currently, it is known that neurotrauma can lead to various mental and neurodegenerative diseases. This can be based on both molecular mechanisms and direct mechanical damage to anatomically important structures of the CNS and PNS. However, before elucidating this issue in more detail, we will consider, in general, the classification of neurotrauma and the main molecular mechanisms that accompany this pathological condition.
TBI and SCI in young and middle-aged men is ahead of cardiovascular and oncological diseases. Along with this, injuries of the PNS are a major public health problem [1,2,46]. With mechanical damage in the nervous tissue, various pathological processes develop, leading to the death of neurons. The treatment of these neuropathological processes is a major public health problem worldwide. However, effective clinical neuroprotective drugs have not yet been found. Their search requires deep and comprehensive studies of the molecular mechanisms of neurodegeneration and neuroprotection in these pathological processes [2].
TBI is a type of damage where the skull suffers from mechanical effects, as well as intracranial formations—the brain, meninges, blood vessels, cranial nerves. TBI is a heterogeneous pathological condition [25,47,48,49]. The destruction of nervous tissue in TBI is due to primary and secondary mechanisms of brain damage. Primary damage is caused by the direct impact of mechanical energy on the substance of the brain. In the area of primary brain damage, necrosis of brain tissue, death of neurons and glial cells, axonal ruptures and vascular thrombosis occur [25,47,48,49,50]. As a result, a focus is formed, protected by a penumbra—a zone of moderate ischemia. Cell death in the penumbra region leads to the expansion of the zone of the necrotic focus of TBI [51,52].
Secondary brain damage develops in response to primary mechanical damage, which triggers a cascade of molecular cellular events: oxidative phosphorylation in the mitochondria is disrupted, intracellular Ca2+ concentration increases, free oxygen radicals and vasoactive metabolites of arachidonic acid are released, the mechanisms of the complement cascade and lipid peroxidation are activated, and so on [53]. A sharp activation of the metabolic processes in neurons leads to ATP pool depletion and a disruption of the functions of Ca2+ channels. As a result, there is an increase in the permeability of the cell membranes to Ca2+ ions and the release of Ca2+ from intracellular depots, which leads to the depolarization of neurons and the release of glutamate, which activates N-methyl-D-aspartic acid (NMDA) receptors (NMDARs). Intracellular overload of Ca2+ occurs, which triggers a whole cascade of reactions associated with the activation of phospholipases, proteases and nucleases, the lysis of structural proteins, the expression of pro-apoptotic genes, the release of cell death factors from mitochondria, hyper synthesis of nitric oxide, and oxidative stress [54,55]. Hence, leading to the apoptotic death of neurons and glial cells [47].
Another major traumatic injury to the central nervous system is SCI. This is characterized by compression, or partial or complete rupture of the spinal cord. This group of neurotraumas is characterized by high disability and mortality and is practically untreatable. [56,57]. Spinal cord injury can be characterized by the destruction of several, many, or all of the nerve fibers that pass through the injury site. Recovery after this type of neurotrauma is complicated by the extremely weak regenerative capabilities of the spinal cord and is usually possible only with mild damage; with slight death of nerve cells and slight destruction of spinal nerve fibers [58]. This type of injury is accompanied by primary and secondary injuries similar to TBI [59].
Furthermore, injuries to the PNS are of great danger, and often lead to a deterioration in the quality of life up to severe disability or death [60]. Peripheral nerve damage is the result of the destruction of nerves that extend from the spinal cord and brain to various parts of the body and are located outside of the CNS [61]. These nerves can be damaged as a result of various factors, such as trauma, disease, inflammation, etc. Of particular danger are injuries to peripheral nerves, often accompanied by their complete rupture, that is, axotomy (AT), which initiates a complex cascade of signaling and metabolic processes aimed at the death or survival of the neuron [62,63].
AT is characterized by three main molecular-cellular events: Wallerian degradation of the severed axon, death of the damaged neuron, or its regeneration with the regrowth of the axon and the restoration of nerve connections. The peculiarity of PNS neurons is their ability to regenerate a damaged axon, while CNS neurons degenerate and die as a result of AT [64]. About 30% of PNS motor and sensory neurons survive AT by restoring nerve connections. An important factor associated with the survival of nerve cells in AT is the distance from the site of the axon rupture to the soma. Generally, the larger it is, the higher the chances of neuron regeneration [65,66].
H2S plays an important role in pathological conditions and may also be involved in processes associated with inflammation [67,68], oxidative stress synthesis [32,34,69], apoptosis [39,40], and autophagy [25,50], etc.
4. The Role of Neurotrauma in the Pathogenesis of Mental Disorders
Neurotrauma of various origins can lead to cognitive impairment [4,5,6,7,8], depression and anxiety disorders [70,71,72], epilepsy [73,74,75,76], encephalopathy [77,78], neurodegenerative disorders [9,10,11,12,13] and chronic pain [79]. Moreover, all these pathological conditions can be accompanied by increased cell death, in particular, apoptosis [14,15,16,17,18,19], neuroinflammation [80,81,82,83], and oxidative stress [84,85,86,87], etc.
4.1. Traumatic Brain Injury
TBI often causes cognitive impairment [4,5], encephalopathy [77,78], depression and anxiety disorders [70,71], epilepsy [73,74], chronic pain [79], and increases the risk of developing neurodegenerative diseases [9,10].
For example, TBI can cause cognitive impairment as a result of damage to hippocampal neurons, which are especially vulnerable to trauma, and changes in the synaptic plasticity in this area. Impaired neurotransmission induced by activation of Ca2+-dependent phosphatases and proteases, loss of dendritic spines, Ca2+-excitotoxicity, increased levels of apoptosis, autophagy, necroptosis, and NMDAR activation, are also a key factor in the development of cognitive impairment in TBI [88].
TBI-induced depression may be the result of damage to the frontal lobe, white matter, amygdala, and disruption of the neuronal network between different areas of the brain [70]. Bruises and swelling of the brain, intracranial hematomas, and changes in the blood-brain barrier, are concomitant negative factors in TBI, which often lead to epileptic seizures [2]. The dysfunction of downstream inhibitory regulation, loss of neurons in noradrenergic centers, periaqueductal dysfunction gray substances, and dopamine deficiency often leads to chronic pain in TBI [89].
TBI can trigger the development of neurodegenerative diseases due to the formation of neurofibrillary tangle (NFT) and amyloid beta peptide (Aβ) plaques, activation of Aβ-containing microglia, increased levels of α-synuclein (a-Syn), amyloid-beta precursor protein (APP), beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), microtubule-associated protein tau (Tau), apolipoprotein E4 (ApoE4), presenilin-1 (PS1), and caspase-3 [9,10]. In addition, the deposition of hyper phosphorylated Tau in the depth of the sulci and a general lesion of the nervous tissue may underlie the pathogenesis of encephalopathy in TBI [90].
4.2. Spinal Cord Injury
Many studies have shown that SCI is often associated with the development of cognitive impairment [6,7,8], encephalopathy [91], depression and anxiety disorders [72,92], epilepsy [75,76], chronic pain [93,94], and neurodegenerative diseases [11,95].
SCI is associated with motor, sensory, and autonomic disturbances below the level of injury, resulting in severe mental and physical suffering. SCI causes hemodynamic disturbances and also affects areas of the brain associated with memory, emotions, and pain regulation as a result of pervasive neurodegenerative processes, which is a powerful inducer of cognitive impairment [96]. SCI can trigger gray matter atrophy in the sensorimotor cortex, cerebellum, medial prefrontal cortex, and anterior cingulate region, increase nerve cell death by triggering apoptotic signaling, and disrupt the neurotransmitter system. This negative front of molecular cellular events caused by SCI can lead to dementia, cognitive impairment, depression and anxiety disorders [97].
SCI increases the risk of developing neurodegenerative diseases. Parkinson’s disease can be caused as a result of neuroinflammation, microgliosis, accumulation of α-Syn and the subsequent loss of dopaminergic neurons in SCI [11]. The main mechanism for the development of neuropathic pain after SCI may be nerve root damage, neuroinflammation, activation of Na+ and NMDAR ion channels, and the inhibition of serotonergic, noradrenergic, opioid and gamma-aminobutyric receptors at the area of SCI [98].
4.3. Trauma of the Peripheral Nervous System
Peripheral nerve injury can lead to depression. It has been found that damage to peripheral nerves can increase the level of the pro-inflammatory cytokine interleukin-1β (IL-1β) in the frontal cortex and a simultaneous increase in the expression of glial fibrillary acidic protein (GFAP) in the periaqueductal gray (PAG). These negative molecular events may contribute to the development of depression-like behavior [99]. Peripheral nerve injury is reported to induce apoptosis in the dorsal horns of the spinal cord, and trigger mechanisms of excitotoxicity and death of GABAergic interneurons, leading to the development of chronic pain [100].
Of course, in the above sections, we have touched on only part of the large-scale molecular-cellular events during neurotrauma that can lead to certain mental disorders and neurodegenerative diseases. In all these pathological conditions, H2S plays an important role; so first, we will consider the main aspects of its endogenous synthesis, catabolism, storage, and a variety of biological effects.
5. Metabolism and Functions of H2S
5.1. Biosynthesis of H2S and Its Deposition
The third gasotransmitter, after nitric oxide (NO) and carbon monoxide (CO), is H2S. Its discovery as a signaling molecule dates back to 1996, when the endogenous formation of H2S in the brain tissue was established with the help of the enzyme cystathionine-β-synthase and its possible role in neuromodulation was assumed. In aqueous solutions, hydrogen sulfide dissociates into H+, HS− and S2−. Under physiological conditions, approximately 20% of this gas exists in the form of H2S, about 80% in the form of HS− and only traces in the form of S2− [101,102].
H2S mainly exists as gaseous molecules or sodium bisulfide (NaHS). H2S can bind to hemoglobin to form sulfhemoglobin. In addition, proteins containing the iron–sulfur complex and sulfane, which includes hydrosulfides/persulfides, are commonly recognized forms of H2S accumulation in the body [31].
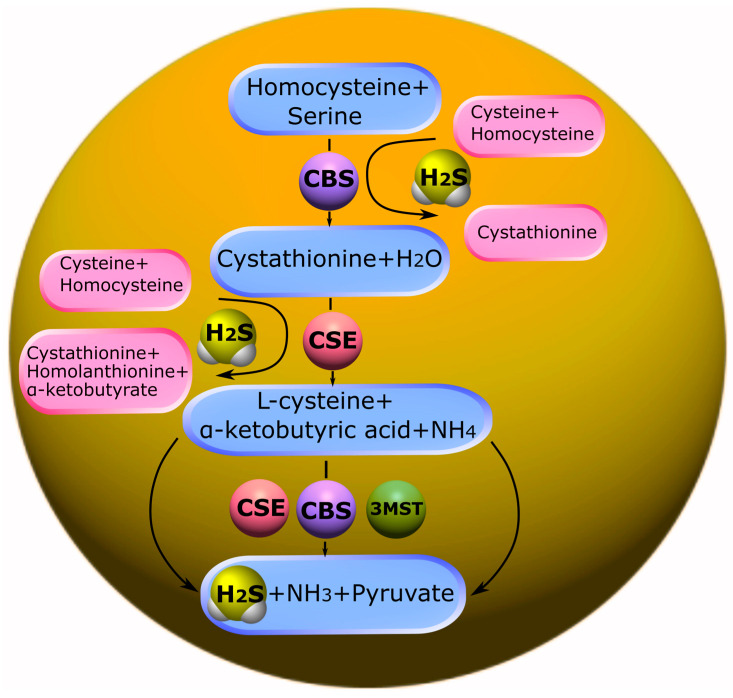
The main substrate for the production of H2S in humans and animals is L-cysteine, as well as its disulfide form—cysteine. The synthesis of hydrogen sulfide in the body occurs under the influence of the enzymes cystathionine-β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) (Figure 1), together with cysteine aminotransferase (CAT) [24,25,101,102].
Figure 1.
Biosynthesis of H2S in the body. Cystathionine-β-synthase (CBS) catalyzes the condensation of homocysteine (Hcy) with serine to form cystathionine, which cleaves cystathionine-γ-lyase (CSE). This results in the synthesis of H2S. CBS, CSE and 3-mercaptopyruvate sulfurtransferase (3-MST) catalyze the conversion of cysteine to H2S.
CBS is predominantly expressed in the brain, liver, kidneys, and pancreas. It is basically a cytosolic enzyme. However, in certain types of cells, it can be localized in the nucleus [103] and mitochondria [104]. CBS expression is controlled by various extracellular and intracellular mechanisms in normal and pathological conditions [105].
CBS catalyzes the condensation of homocysteine (Hcy) with serine to form cystathionine. Subsequently, cystathionine undergoes proteolysis by the enzyme CSE. This leads to the formation of cysteine, which is a precursor of glutathione. It should be noted that in addition to the canonical pathway, CBS is involved in desulfurization reactions that lead to the formation of endogenous H2S. The formation of H2S may be affected by a thiol-cysteine reaction catalyzed by CBS with the release of s-thiolate. Cysteine may also undergo hydrolysis by CSE to form H2S, as well as pyruvate and ammonia. Disruption of the mechanisms of regulation of CBS is associated with a change in the levels of Hcy and/or H2S and, inevitably, leads to various pathological conditions (Figure 1) [106,107].
CBS and CSE are jointly involved in the trans-sulfurization pathway, where homocysteine formed in the methionine cycle switches to cysteine synthesis (Figure 1) [108,109,110].
5.2. Catabolism of H2S
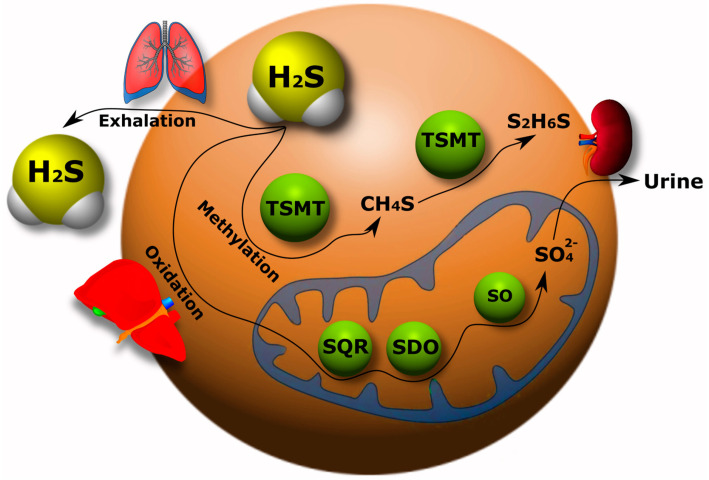
The catabolism of H2S has been studied much less than its synthesis in the body. Currently, three pathways of H2S catabolism are mainly known: oxidation, methylation and exhalation. The oxidation of H2S mainly occurs in hepatocytes. Here, H2S is oxidized by mitochondrial enzymes to sulfate with the intermediates persulfide (RSSH), sulfite (SO32−), and thiosulfate (S2O32−) [111,112].
As a result, most of the H2S is excreted in the urine in the form of sulfate. It has been shown that the increase in sulfide oxidation in the kidneys, heart and liver upon administration of exogenous H2S is due to an increase in quinone oxidoreductase (SQR) (Figure 2). However, this effect was not observed in the brain tissue, which indicates a defect in the oxidation of sulfides in the nervous tissue of the brain [113].
Figure 2.
H2S catabolism pathways in the body: oxidation, methylation and exhalation. TSMT, thiol-S-methyl transferase; SQR, quinone oxidoreductase; SDO, sulfur deoxygenase; SO, sulfite oxidase.
Free H2S exists in low concentration in the blood and decays rapidly. Therefore, it will probably not all be transported to the liver for disposal. The question of H2S catabolism in the brain via alternative pathways independent of the liver and kidneys remains open [31].
The methylation of H2S occurs primarily in the cytoplasm in contrast to the oxidative catabolic pathway of this gasotransmitter. First, H2S is methylated to methane thiol, and then it is methylated to a non-toxic dimethyl sulfide by a thiol-S-methyl transferase (TSMT) (Figure 2). The methylation of sulfides has been found to be a significantly slower process than oxidation [114,115]. H2S can be excreted from the body through lung tissue (Figure 2) [31,116].
5.3. Various Biological Effects of Endogenous H2S
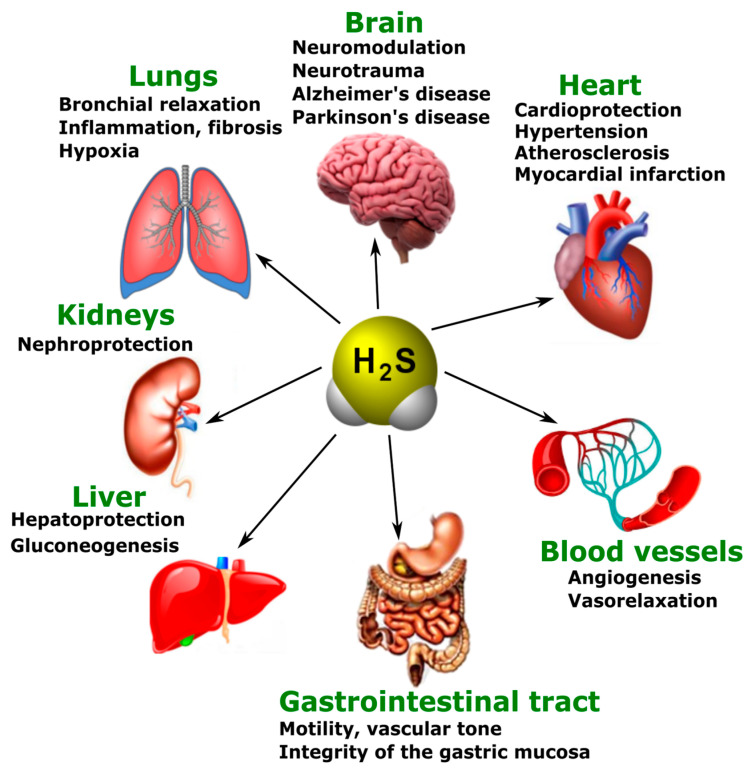
The main physiological effects of H2S are neuromodulation, regulation of vascular tone and oxidative stress, anti-inflammatory action, angiogenesis, and energy generation. However, these effects do not exhaust the diversity of the biological actions of H2S. Presently, the list of functions performed by this gaseous signaling agent is constantly expanding (Figure 3) [117].
Figure 3.
The participation of H2S in normal and pathological conditions in the brain, heart, blood vessels, gastrointestinal tract, liver, kidneys, and lungs.
Just like NO, H2S is a new generation neurotransmitter that has also been shown to have pronounced neuroprotective effects. In physiological conditions, H2S has a role in learning and memory processes. It facilitates long-term potentiation in the hippocampus by activating NMDARs associated with Ca2+ channels [118,119]. Disturbances in the endogenous production and metabolism of H2S have been observed in neurodegenerative diseases, such as Alzheimer’s (AD) and Parkinson’s disease (PD) [120,121]. Moreover, the use of exogenous H2S in these diseases has been proven to have a positive therapeutic effect [122,123].
Researches have shown that exogenous H2S reduces early brain injury in subarachnoid bleeding [124], increases motor function, and reduces cortical lesions after traumatic injury [67].
However, the biological effects of H2S are far from ambiguous. It has been proven that the neuroprotective effect of H2S is due to anti-oxidant, anti-inflammatory and anti-apoptotic properties [32]. However, under certain conditions, H2S can contribute to secondary damage to neurons, causing them to be overloaded with Ca2+ ions. In experiments on mice, it was found that inhibitors of enzymes for the synthesis of H2S can reduce early damage to the blood-brain barrier (BBB) after transient focal cerebral ischemia [125].
Now that we have considered the metabolism of H2S, as well as the molecular mechanisms of neurotrauma and its relationship with mental and neurodegenerative diseases, we can move on to the role of hydrogen sulfide in cell death in neurotrauma. This section will help us better understand the H2S-dependent signaling mechanisms that underlie mental disorders and neurodegenerative diseases.
6. Endogenous and Exogenous H2S in Neurotrauma
6.1. Endogenous H2S Levels in Neurotrauma
Recently, several experiments have been developed to study and detect changes in the concentration of H2S in neurotrauma in both animal and human models. The concentration of H2S has been shown to be a dynamic system. The level of the endogenous expression of H2S and CBS in the blood and brain tends to decrease after neurotrauma. Zhang M and colleagues demonstrated that CBS expression was suppressed in the cerebral cortex and hippocampus of mice in TBI. Furthermore, at first it gradually decreased, reaching the minimal values, and then increased. H2S demonstrated dynamic changes in TBI, in parallel with the expression of the key enzyme of its synthesis [126]. There was a significant decrease in CBS and CSE in the hypothalamus and brain stem. Furthermore, 3-MST decreased only in the hypothalamus. At the same time, regular intra-abdominal administration of NaHS restored the levels of CBS and CSE, and of 3-MST [127].
Interesting results were obtained on the TBI model in salmon fish, as the number of CBS+ cells in the telencephalon significantly increased after injury compared to the control group [128,129]. In a recent study, TBI was shown to significantly reduce H2S/CSE levels without significant changes in CBS expression [130]. In a porcine model of acute subdural hematoma, increased levels of CBS and CSE were observed in neurons, vessels, and parenchyma at the base of the cerebral gyri [131].
Changes in the level of H2S in the spinal cord have been reported in various neurodegenerative processes [132]. However, there is practically no information concerning changes in the level of hydrogen sulfide in the spinal cord during traumatic exposure. More scientific data is devoted to the study of the effect of hydrogen sulfide donors on the signaling mechanisms of survival and death of neurons and glial cells in SCI.
6.2. Exogenous H2S: Between Neuroprotection and Neurodegeneration
It is known that H2S is involved in the processes of neuroprotection and neurodegeneration in neurotrauma. The administration of H2S donors can protect neurons and prevent the development of hemodynamic disorders in TBI [126]. Increasing the concentration of H2S reduces cerebral edema, improves motor activity, and reduces apoptosis and autophagy in an animal model of TBI [133]. According to Jiang and co-authors, H2S leads to the activation of antioxidant enzymes, reducing the oxidative damage to nervous tissue cells in TBI [134]. The authors of another study indicate that H2S reduces mitochondrial dysfunction and autophagy in TBI (Figure 4) [135].
Figure 4.
The role of H2S in neuroprotection and neurodegeneration in neurotrauma. Arrows with a sharp end—positive regulation; arrows with a blunt end—negative regulation.
Increasing the concentration of H2S by using a ferrofluid hydrogel (FFH) with iron tetrasulfide (Fe3S4) significantly reduces activated microglial/macrophage levels and the expression of pro-inflammatory factors, and increases the rate of directional growth of axons in animal models of SCI [136]. The use of H2S-releasing silk fibroin hydrogel resulted in a decrease in the level of neuronal pyroptosis induced by TBI [137]. NaHS has been reported to reduce the area of spinal cord infarction in the ischemic-reperfusion model of injury [138]. CBS activation reduces the level of reactive oxygen species, lactate dehydrogenase expression, and apoptosis in the nervous tissue in massive cerebral infarction [139]. H2S donors reduce neurological disorders, nerve cell apoptosis, pro-inflammatory secretion, and oxidative stress in spinal injuries [140]. It is also reported that increasing H2S levels significantly reduces the permeability of the blood-spinal barrier, improving the recovery of damaged spinal cord neurons [57]. The use of H2S-bound nanoparticles promoted the regeneration of damaged spinal cords in rats via the TOR signaling pathway [141]. Intranasal administration of polysulfide prevented neurodegenerative changes in the injured spinal cord [142]. It has been shown that H2S is involved in neuroglial interaction in the spinal cord, regulating the survival and death of neurons [143]. This gasotransmitter showed a protective effect against the processes of demyelination in cauda equina fibers in cases of compression injury (Figure 4) [144].
In a mouse model of sciatic nerve damage, H2S was shown to significantly reduce neuropathic pain [145]. In addition, CSE and MST have been found to be present in normal nerves, and axotomy activates CSE in Schwann cells [113]. Inhibition of H2S production has been reported to improve the growth of regenerating axons and remyelination processes in peripheral nerve injuries (Figure 4) [146].
7. The Role of H2S in Cell Death in Neurotrauma
7.1. Participation of H2S in Oxidative Stress
Free radical processes are a vital and important link in metabolism, the violation of which leads to the development of oxidative stress. Violation of the dynamic equilibrium of the oxidant/anti-oxidant system towards free radical oxidation against the background of tension and violation of the coherence of the action of the antioxidant system components leads to the development of oxidative stress [35].
Recently, the main H2S-dependent biological effects in various neurotraumas are considered in the context of the regulation of oxidative stress. It is known that, at 37 °C and a pH of 7.4, more than 80% of H2S molecules dissolve in surface waters and dissociate into the ions H+, HS- and S2−. HS− is a powerful one-electron chemical reagent that effectively traps reactive oxygen species (ROS). Hydrosulfide anions are able to quench ROS by transferring a hydrogen atom or a single electron. The rate of this reaction is directly limited by diffusion. In this case, the reaction of hydrosulfide anions with molecular oxygen proceeds faster in the presence of divalent metal ions. H2S effectively interacts with hypochlorous acid (HClO), hydrogen peroxide (H2O2), lipid hydroperoxides and peroxynitrite (ONOO−), neutralizing their oxidative potential [147]. In addition, H2S itself is a reducing agent that can directly react and extinguish the superoxide anion (O2−), NO and its free radical products, as well as other ROS (Figure 5). In addition, H2S can act as a trigger molecular agent that triggers antioxidant defense processes [32]. It should be noted that the effectiveness of H2S and other simple SH-compounds in neutralizing free radicals is limited due to their low concentration in blood and tissues [148].
Figure 5.
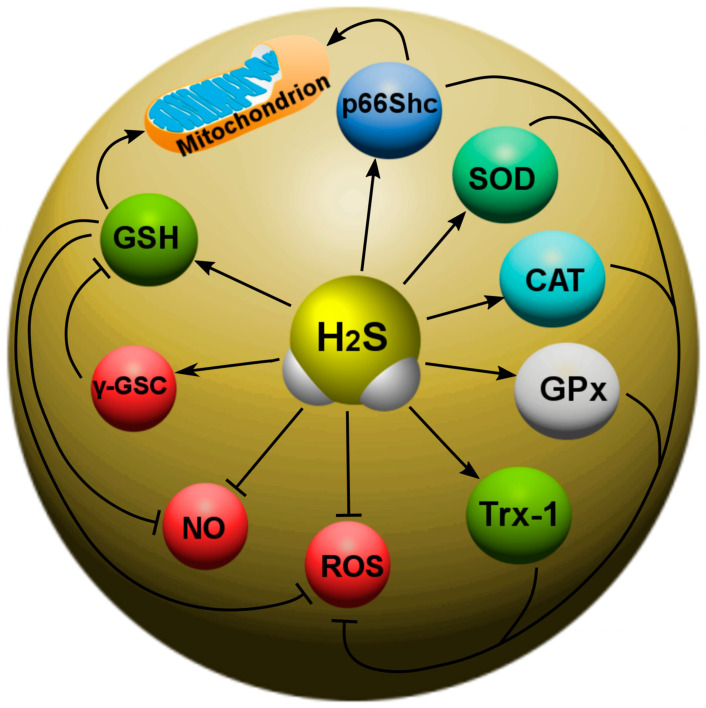
The role of H2S in oxidative stress in neurotrauma. H2S can directly react with and quench ROS and NO. In addition, H2S can increase the level of intracellular reduced glutathione (GSH), which is an antioxidant. However, H2S can activate γ-glutamylcysteine synthase (γ-GSC), which limits GSH synthesis. H2S is involved in the activation of a number of antioxidant defense enzymes: γ-glutamylcysteine synthase (γ-GSC), thioredoxin (Trx-1), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and p66Shc.
In addition, H2S can react with NO to form nitroxide (HNO), which is able to bind to the thiol groups of proteins, leading to the formation of disulfide bonds. HNO can modify GSH with the formation of GSH disulfide and sulfinamide, which can increase oxidative stress and inflammatory processes [149].
Studies have shown that H2S increases levels of intracellular reduced glutathione (GSH), which is a major antioxidant in the brain [33,34] and spinal cord [150]. Past studies have shown that the H2S donor promotes glutamate uptake in astrocytes by enhancing glial glutamate transporter GLT-1 delivery, enhancing cystine transport, and as a result, GSH synthesis [32]. Administration of H2S is also associated with elevated levels of GSH in the mitochondria [63]. On the other hand, H2S is able to enhance the activity of γ-glutamylcysteine synthase (γ-GSC), which acts as an enzyme that limits the rate of formation of GSH (Figure 5) [25].
Recently, Kimura et al. showed another mechanism of H2S effect on the intracellular production of GSH. They reported that the H2S produced in cells can be released into the extracellular space and restore cystine to cysteine, which will, thus, be efficiently imported into cells through a cysteine transporter other than the Xc− system and used to synthesize GSH (Figure 5). Meanwhile, Jane et al. also demonstrated that H2S increased intracellular GSH production by activating the glutamate-cysteine ligase catalytic subunit (GCLC) and the glutamate-cysteine ligase modifier subunit [32].
H2S increases thioredoxin (Trx-1), which is a small (12 kDa) molecule containing the characteristic Cys–Gly–Pro–Cys motif, and the oxidation–reduction of Trx-1 occurs from two of its cysteine residues. Trx-1 is a 12 kDa oxidoreductase enzyme containing a dithiol-disulfide active site that acts as an antioxidant, facilitating the reduction of other proteins by cysteine-thiol-disulfide [69]. Trx-1 has been reported to perform a variety of intracellular and extracellular functions, including capturing ROS and protecting the cell from oxidative stress. Trx-1 reduces hydrogen peroxide with peroxiredoxine (Prx), and oxidized Trx-1 is reduced with thioredoxine reductase. H2S has been shown to increase gene transcription and Trx-1 levels [34,69].
H2S can bind to the copper (Cu) catalytic center of superoxide dismutase (SOD), which leads to an increase in the rate of absorption of superoxide anions [151]. Recent studies have also shown that H2S can attenuate oxidative stress by increasing the activity of catalase (CAT) and glutathione peroxidase (GPx) (Figure 5). In addition, H2S can inhibit the mitochondrial production of ROS through p66Shc-dependent signaling. p66Shc is an adaptor protein. It has a negative effect on the ROS-mediated signaling pathway and is involved in the mitochondrial signaling of redox potential. Under oxidative stress, it travels to the mitochondria, binds to cytochrome c (Cyt c), and transfers electrons from Cyt c to molecular oxygen to form ROS. H2S interact with p66Shc through sulfhydration and reduces the formation of mitochondrial ROS [35]. However, a high level of H2S can, on the contrary, induce the formation of ROS and cause an increase in oxidative stress.
7.2. Modulation of the H2S Activity of NMDARs and Intracellular Ca2+ Homeostasis
H2S has been found to modulate NMDAR activity. Protein kinase A (PKA) is known to regulate NMDAR activity. Studies have shown that H2S can increase levels of cAMP, which, as a secondary messenger, activates PKA. As a result, the activity of the NMDAR increases. However, H2S can activate NMDARs in an independent way. Since NMDARs are extremely sensitive to oxidation and reduction reactions, the biological effects of H2S on these receptors may be due to the reduction of disulfide bonds [118]. H2S can directly interact with the cysteine residues of receptor subunits, modifying them by S-sulfhydration [119]. It has been established that the hyper activation of the NMDAR, which is an integral part of the pathogenesis of various neurotraumas, leads to Ca2+ excitotoxicity and cell death [54,55,118,119].
H2S can increase cytosolic Ca2+ in neurons by activating slow Ca2+ L-type channels. Ca2+ L-type channels are one of the main members of the family of potential-controlled calcium channels. The discovery of these channels occurs in response to a strong depolarization of the membrane and causes a prolonged current of Ca2+. Ca2+ L-type channels are expressed in many tissues, including the nervous system [152]. It is known that these Ca2+ channels are involved in the pathogenesis of various injuries of the central nervous system and the PNS [153,154,155]. Thus, it has been shown that H2S can increase the Ca2+ current in astrocytes, microglia and neurons through the activation of Ca2+ L-type channels [156,157]. These H2S effects can be significantly reduced by these channel antagonists [158,159]. The role of H2S in regulating fast T-type Ca-type CaV 3.2 channels is interesting. Studies have shown that the inhibition of CSE in sciatic nerve injury significantly weakened the activation of CaV3.2 in the ganglia of the posterior roots of the spinal cord. This effect is probably related to the redox H2S-dependent modulation of Ca2+ channels of this type [119,160]. It is worth noting that the amplification of the Ca2+ current through channels of the CaV3.2 type, activated by H2S, can participate in the regeneration of neurons [161].
7.3. Anti- and Pro-Inflammatory Effects of H2S
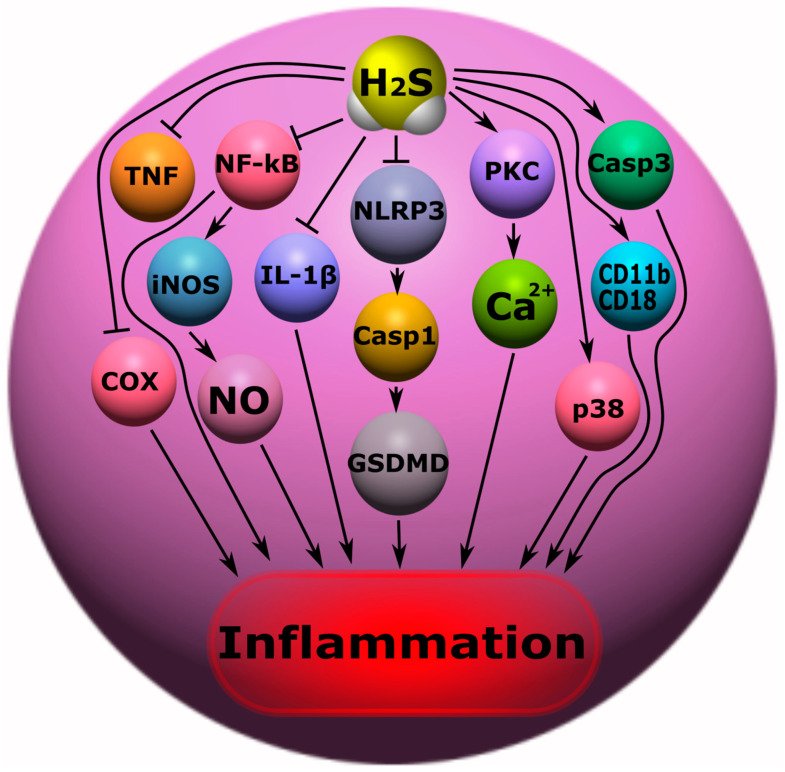
Neuro inflammation is an inflammatory response in the nervous tissue characterized by the activation of glial cells, the involvement of neutrophils and macrophages, and the increased synthesis of cytokines, chemokines, free radicals and secondary messengers. The neuroinflammatory response is characterized by a growing front of molecular cellular events underlying secondary damage to nervous tissue [162,163]. A number of studies have shown that H2S plays an important role in inflammatory processes in various pathological conditions, including neurotrauma. The use of ATB-346 (2-(6-methoxynapthalen-2-yl)-propionic acid 4-thiocarbamoyl-phenyl ester), a new H2S-releasing derivative of naproxen, in TBI, significantly reduced the inflammatory response, due to the inhibition of oxidative stress, nuclear NF-κB (factor kappa-light-chain-enhancer of activated B cells), leukocyte adhesion to the endothelium, tumor necrosis factor (TNF), and interleukin-1 β (IL-1 β) [67]. It is reported that H2S can reduce neuronal inflammation by inhibiting the NLRP3/caspase-1/GSDMD signaling pathway in ischemia/reperfusion brain injury [164]. H2S protects retinal ganglion cells in an ischemia/reperfusion injury animal model by regulating a range of signaling proteins involved in inflammation, oxidative stress, and mitochondrial homeostasis (Figure 6) [165].
Figure 6.
The role of H2S in inflammation in neurotrauma. TNF, tumor necrosis factor; COX, cyclooxygenase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; iNOS, inducible nitric oxide synthase; NO, nitric oxide; IL-1β, interleukin-1 beta; NLRP, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing; GSDMD, Gasdermin D; Casp1, caspase-1; Casp3, caspase-3; PKC, protein kinase C; Ca2+, calcium ions; CaM, calmodulin; p38, p38 mitogen-activated protein kinase; CD11b\CD18, Mac-1β2 integrin; CcO, cytochrome-c-oxidase. Arrows with a sharp end—positive regulation; arrows with a blunt end—negative regulation.
The family NF-κB is known to consist of transcription factors that play a complex role in immunity and inflammation. NF-κB regulates inflammation through nuclear translocation followed by the expression of pro-inflammatory factors [166]. H2S can modulate the activity of NF-κB activity through trans-sulfonation mechanisms, resulting in the inhibition of the nuclear translocation of NF-κB and a reduced inflammatory response [167,168]. In addition, H2S can inhibit the phosphorylation of the p65 NF-κB subunit, preventing the activation of this transcription factor [50]. It is worth noting that NF-κB is also a transcription factor for inducible NO synthase (iNOS) [169,170]. iNOS is a Ca2+-independent enzyme that generates high levels of NO, in contrast to the constitutive forms of NOS (eNOS/NOS3/endothelial NOS and nNOS/NOS1/neuronal NOS), and is responsible for the increase in oxidative stress and the progression of neuroinflammation [171]. H2S can significantly reduce inducible NO production through the inhibition of NF-kB. H2S can also inhibit iNOS expression through heme oxygenase (HO-1) activation in macrophages [68]. This gasotransmitter is capable of suppressing the activity of Ca2+-dependent NOS [172]. H2S has also been shown, in studies, to increase the production of NO in endothelial cells by activating eNOS. In addition, the anti-inflammatory effects of H2S in neurotrauma can be realized through the modulation of mitochondrial respiration due to the reversible inhibition of cytochrome-c-oxidase (CcO) (Figure 6) [173]. H2S has been reported to reduce inflammation in dorsal root ganglia during sciatic nerve transection [174].
However, the role of H2S in inflammation is not so unambiguous. The over production of ROS by neutrophils can cause the oxidation of H2S to form sulfite, which leads to leukocyte adhesion and neutrophil activation through the activation of the Mac1 β 2 integrin (CD11b/CD18) and protein kinase C (PKC)/Ca2+ calmodulin pathway, respectively. In addition, H2S can inhibit the breakdown of caspase-3 and the activation of mitogen-activated protein kinase p38 (MAPK) in granulocytes, which is followed by an increase in the inflammatory response (Figure 6) [37,38].
7.4. The Effect of H2S on the Level of Neurotrophic Factors
Neurotrophic factors are high molecular weight polypeptides that play an important role in the survival, differentiation, and functioning of nerve and glial cells in the brain and spinal cord [175]. Different types of neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and glial cell-derived neurotrophic factor (GDNF), respectively, play a crucial role in neuronal regeneration in spinal injury, causing the growth of axons and dendrites and showing a neuroprotective effect in TBI neuron models [176,177,178].
It is known that H2S is able to modulate the level of neurotrophic factors in normal and pathological conditions [26,179,180]. Thus, in a mouse TBI model, the administration of an H2S donor restored GDNF and NGF levels in damaged neural tissue, preserving their neuroprotective effects [67]. The use of a mitochondria-targeted H2S donor in middle cerebral artery occlusion has been reported to increase BDNF and NGF expression, reducing ischemic neuronal damage [26]. The administration of NaHS, a donor of H2S, increased BDNF levels, probably through activation of the transcription factor cAMP response element-binding protein (CREB), which regulates the gene for this neurotrophic factor in brain damage [181].
H2S has also been found to regulate the expression of vascular endothelial growth factor (VEGF), which exhibits neurotrophic and neuroprotective effects in traumatic CNS injury. VEGF is involved in neovascularization, which is necessary for the repair of brain tissue and the regeneration of nerves after neurotrauma. Exogenous H2S significantly increased the level of VEGF in the affected area in TBI, which led to the restoration of the BBB [67].
7.5. Effects of H2S on the Blood-Brain Barrier and Cerebral Edema
Damage to the BBB is the most important pathological substrate of neurotrauma. It was found that H2S can participate in the restoration of the functional and anatomical integrity of the BBB [182,183], as well as reduce cerebral edema [25,184]. Thus, in a rat TBI model, it was shown that the use of NaHS, the classic H2S donor, reduced the excessive permeability of the BBB by activating the mitochondrial adenosine triphosphate-sensitive potassium channels and reducing oxidative stress. Positive H2S-dependent effects may be associated with the inhibition of PKC-α, β I, β II and δ and the activation of PKC-ε, as well as increased levels of Claudin-5, Occludin and ZO-1 [185]. In addition, H2S can reduce vascular dysfunction by modulating eNOS levels in TBI [186]. The administration of NaHS significantly reduced damage to the BBB in a spinal cord compression injury model. This H2S donor prevented the reduction of the proteins TJ (P120, β-catenin) and AJ (Occlusin), which are among the key components of the BBB [57]. It is known that H2S is able to reduce edema in traumatic injury to the naked brain. One of the mechanisms of this H2S-dependent effect may be to reduce the destruction of the BBB by suppressing the expression of aquaporin 4 (AQP4) on astrocytes and inhibition of matrix metalloproteinase-9 (MMP-9) [184].
7.6. The Role of H2S in Remyelination Processes
Remyelination is an important aspect of the recovery of damaged neurons in injuries of the brain [187], spinal cord [188], and peripheral nerves [189]. The processes associated with demyelination develop as a result of secondary damage, leading to a dysfunction of the neuronal network, neurodegeneration, and ultimately, to the death of neurons [188]. It is known that H2S can participate in this process [146].
It has been shown that the production of H2S in Schwann cells can lead to destruction of the myelin sheath and the recruitment of macrophages. H2S positively influences the dedifferentiation and proliferation of Schwann cells in Wallerian degeneration by regulating lysosomal-associated membrane protein 1 (LAMP1), p75 neurotrophin receptor (p75 NTR), c-Jun, and p-ERK1/2. The authors of the study suggest that inhibition of CSE expression may be a potential target in the treatment of pathological processes associated with demyelination [146]. However, there are other studies that show that H2S donor treatment leads to a decrease in the demyelination of cauda equina fibers and a decrease in glial cell apoptosis in compression injury [144]. NaHS is reported to promote axonal remyelination and repair by activating the PI3K/AKT/mTOR signaling pathway in TBI [135].
7.7. H2S-Associated Anti- and Pro-Apoptotic Signaling Mechanisms
H2S can modulate the apoptosis of neurons and glial cells in neurotrauma. It can act as an anti- and pro-oxidant, as described above, and can regulate the levels of anti- and pro-apoptotic groups of proteins in various traumatic injuries of the nervous system [29,30,31,133]. H2S can directly interact with proteins through S-sulfhydration or persulfhydration of cysteine residues on proteins [190], and bind to metalloproteins, modulating their activity and function [191]. In addition, H2S can realize its activity through the activation and inhibition of various signaling pathways [25,133].
The p53 protein is one of the key pro-apoptotic proteins. It is known as a tumor suppressor and a “guardian of the genome”. This protein controls the most important cellular functions: DNA repair, cell cycle, metabolism, apoptosis, etc. [192,193]. As a transcription factor, p53 is responsible for the expression of many genes, including genes associated with apoptotic cell death [194]. In addition, p53 can participate in transcription-independent processes, regulating mitochondrial functions and triggering the intracellular pathway of apoptosis [195,196]. We have shown the key role of this protein in cell death in neurotrauma in our studies using models of axotomy in vertebrate and invertebrate animals [197,198]. A number of scientific studies have also demonstrated the key role of p53 in the death of neurons and glial cells in various neurotraumas [199,200]. One of the signaling mechanisms for regulating the expression of p53 may be H2S (Figure 7). In a recent study, it was shown that H2S can inhibit the expression of the p53 protein in damaged neurons, exerting a neuroprotective effect in TBI. Moreover, as the authors suggest, this H2S-dependent effect was mediated through the pathway p53/glutaminase 2. The use of an H2S donor showed a significant decrease in TUNEL-positive neuronal and glial cells [29]. However, in other studies, H2S caused an increase in p53 expression and the initiation of apoptosis [39,40]. Another major pro-apoptotic protein, caspase-3, is also a target for H2S. Caspase-3 plays a central role in the cascade of caspases, proteolytic enzymes that sequentially activate each other and underlying proteases [201,202]. H2S has been shown to reduce the expression of caspase-3 in damaged neurons and their apoptosis in TBI [29,50]. H2S reduced levels of this proapoptotic enzyme in spinal cord injury models (Figure 7) [35,140]. However, there are studies in which H2S, on the contrary, induces the expression of caspase-3 and increases apoptotic cell death [203]. H2S can affect the activity of caspase-3 by regulating the ROS signaling pathway of activation of this enzyme [29,30]. In addition, the cysteine content of caspase-3 makes it a potential target for direct interaction with H2S. This gasotransmitter persulfides caspase-3 using cysteine 163, inhibiting its activity [204]. However, a single concept of the role of the H2S-dependent regulation of caspase-3 in survival or cell death still does not exist.
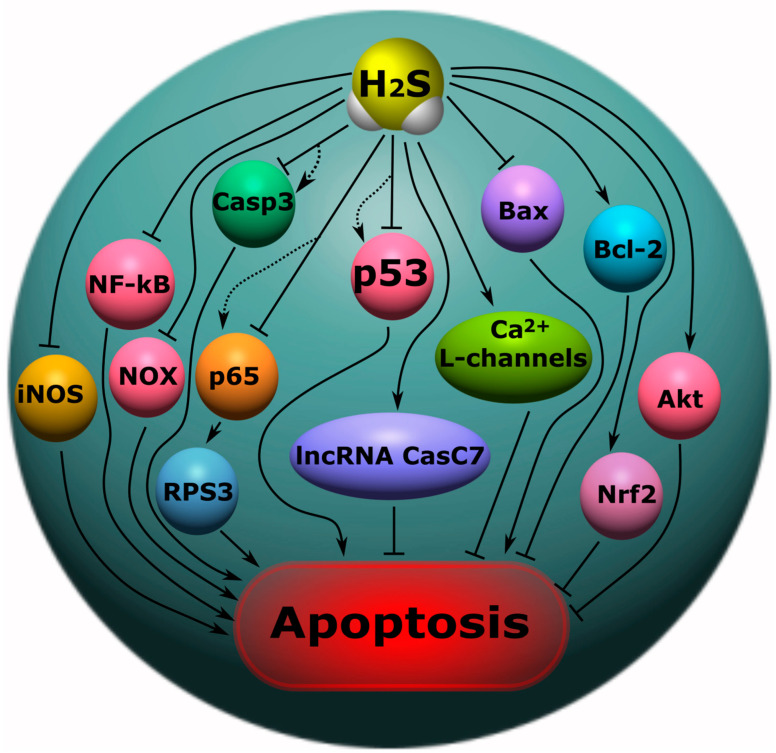
Figure 7.
The role of H2S in apoptosis in neurotrauma. NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; iNOS, inducible nitric oxide synthase; NO, nitric oxide; Casp1, caspase-1; Casp3, caspase-3; NOX, NADPH-oxidase; Bcl-2, B-cell lymphoma 2; Bax, bcl-2-like protein 4; Akt, protein kinase B; p53, tumor protein p53; Nfr2, nuclear factor erythroid 2–related factor 2; p65, RelA; RPS3, ribosomal protein S3; lncRNA CasC7, long non-coding RNA CasC7. Arrows with a sharp end—positive regulation; arrows with a blunt end—negative regulation; dotted line—alternative regulation.
Important proteins involved in the regulation of the permeability of the external mitochondrial membrane and in the regulation of apoptotic signaling are Bcl-2 (B-cell lymphoma 2) and Bax (bcl-2-like protein 4). Bax is known to activate cell death by causing the permeabilization of mitochondrial membranes. The Bcl-2 protein is a molecular antagonist of the proapoptotic effects of Bax. The balance between the levels of these proteins significantly affects cellular fate, leading to either survival or death [205]. A number of studies have shown that H2S can act as a modulator of the level of these proteins, regulating the mitochondrial pathway of apoptosis (Figure 7) [29,50]. The anti-apoptotic effect of H2S can be realized through the modulation of L-type Ca2+ channels and also through a decrease in the level of Bax and caspase-3 in the brain in a model of subarachnoid hemorrhage in rats [206]. It is also reported that H2S inhalation in ischemia/reperfusion injury of the brain increases Bcl-2 expression, reduces the level of NF-κB p65, and enhances Akt (protein kinase B) phosphorylation, which may lead to an anti-apoptotic effect. At the same time, exogenous H2S suppressed the expression of NOX4 (NADPH-oxidases 4) and CBS (Figure 7) [207].
H2S can interact with noncoding RNAs (ncRNAs), which are responsible, in particular, for cell death [208]. It is reported that H2S can reduce neuronal apoptosis through the activation of lncRNA CasC7, which has neuroprotective effects, in rat spinal cord ischemia-reperfusion injury (Figure 7) [138].
H2S regulates the activity of the NF-κB-dependent signaling pathway, which is a central mechanism in signaling between neurons and glial cells. H2S has been shown to reduce NF-kB levels by reducing the expression of iNOS, COX-2 (cyclooxygenases-2), and the cytokines responsible for the inflammatory response (Figure 7) [209,210]. However, H2S can sulfhydrate the p65 NF-κB subunit using cysteine-38, which promotes its binding to the coactivator of the ribosomal protein S3 (RPS3) and increases transcriptional activity [24,211]. H2S may regulate another transcription factor, Nrf2, associated with anti-apoptotic, anti-inflammatory, and antioxidant effects. H2S is reported to reduce nerve cell apoptosis through activation of an Nrf2-dependent signaling pathway in a model of spinal cord injury (Figure 7) [140].
7.8. H2S-Associated Mechanisms of Autophagy
Autophagy is the main physiological mechanism of intracellular degradation, by which cytoplasmic material is delivered to the lysosome and destroyed in it. It plays a crucial role in maintaining cell survival under various stressors and is a necessary link in the restoration of cellular homeostasis. However, autophagy can lead to cell death [212]. In neurotrauma, the natural process of autophagy is disturbed and can turn into a pathophysiological state, entailing the death of neurons and glial cells [213].
Autophagy plays an important role in the survival and death of neurons in brain injuries [214]. H2S has been shown to regulate autophagy-dependent cell death after TBI [25,50]. One of the mechanisms for blocking autophagic neuronal death in neurotrauma may be the H2S-dependent modulation of the PI3K/Akt/Nrf2 pathway and a reduction in oxidative stress [215]. It is known that PI3K/Akt/Nrf2 is a central mechanism involved in autophagy (Figure 8) [216,217].
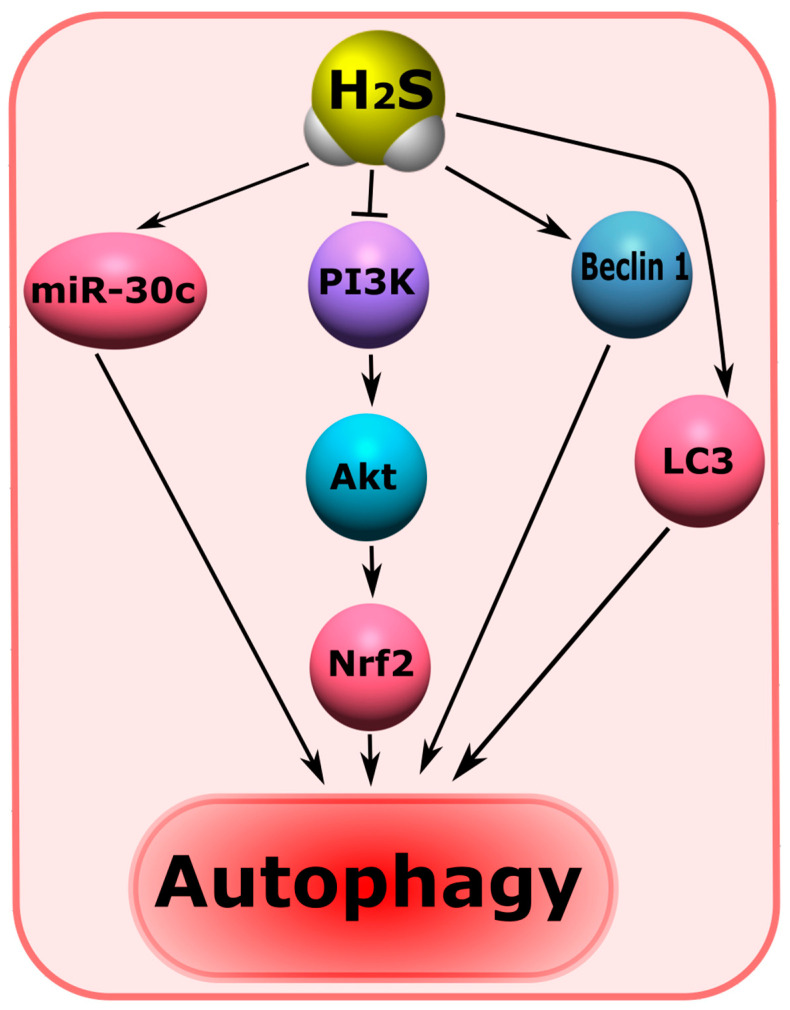
Figure 8.
The role of H2S in the regulation of autophagy in neurotrauma. miR-30c, micro-RNA 30c; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; Nfr2, nuclear factor erythroid 2–related factor 2; Beclin, the mammalian orthologue of yeast Atg6; LC3, Microtubule-associated protein 1A/1B-light chain 3. Arrows with a sharp end—positive regulation; arrows with a blunt end—negative regulation.
It is worth noting that autophagy plays a critical role in damage to nerve and glial cells in traumatic and ischemic-reperfusion injuries of the spinal cord [56]. H2S may regulate autophagy in spinal cord injuries [57,218]. In a model of ischemic-reperfusion spinal cord injuries, H2S has been shown to induce autophagy through the increased expression of miR-30c, Beclin 1, and LC3. miR-30c is a micro-RNA (miRNA) that has been shown to be actively involved in neuroprotection. This miRNA is known to regulate autophagy. In turn, Beclin 1 and LC3 are involved in autophagic processes (Figure 8) [218]. Another study reports that endogenous H2S, on the contrary, inhibits autophagy caused by endoplasmic reticulum stress in SCI [57].
7.9. H2S-Associated Mechanisms of Ferroptosis
Ferroptosis is another type of programmed necrotic cell death, which is characterized by Fe2+-dependent lipid peroxidation. It should be noted that, in its morpho-biochemical and molecular genetic mechanisms, ferroptosis differs from apoptosis, autophagy, and necroptosis [219]. In this type of cell death, the size of the mitochondria with condensed dense inner membranes decreases, their cristae undergo changes, up to their complete disappearance, and the mitochondrial membrane ruptures [220]. Traumatic action of the nervous tissue releases a large amount of Fe2+, which can lead to ferroptosis of neurons and glial cells [221].
H2S plays an important role in ferroptosis. This gasotransmitter can inhibit the process of ferroptosis by increasing the level of antioxidant enzymes, such as GSH, and via ROS uptake [222]. There are practically no data on the functions of H2S in the ferroptosis of neurons and glial cells in nervous tissues. For example, H2S protects the retinal-blood-brain barrier through the activation of the NRF2/KEAP1 signaling pathway, and via AMPK to p62 phosphorylation [223]. H2S is reported to protect BV2 microglial cells by reducing lactate dehydrogenase levels (LDH), oxidative stress, lipid peroxidation, and Fe2+ accumulation [224].
7.10. H2S-Associated Mechanisms of Pyroptosis
Pyroptosis is a type of programmed necrotic cell death that occurs as a result of caspase-1 activation and the disruption of the integrity of the plasma membrane. A feature of this cell death is the active release of IL-1β and IL-18, dependent on caspase-1, which determines the development of an inflammatory reaction [225]. To date, it has been proven that pyroptosis plays an important role in the pathogenesis of injuries of the brain and spinal cord [226]. It has been shown that H2S may be a key regulator of the processes associated with pyroptosis [164].
There are not many scientific data on the role of H2S in the pyroptosis of neurons and glial cells under conditions of traumatic injury. For example, studies have shown that the use of the new silk hydrogel fibroin (SF), which releases H2S, effectively reduces TBI-induced neuronal pyroptosis by inhibiting NOD-, LRR-, and pyrin domain-containing 3 (NLRP3), pyroptosis protein Gasdermin D (GSDMD), caspase-1, and apoptosis-associated speck-like protein containing a CARD (ASC or Picard). In addition, the authors demonstrated that H2S inhibits the expression of receptor-interacting serine/threonine-protein kinase 1 (RIPK-1), which is associated with necroptosis [137]. H2S may reduce neuronal pyroptosis after severe intracerebral hemorrhage, which often occurs in TBI [227]. NaHS use reduced the pyroptosis of retinal cells and brain neurons through inhibition of the NLRP3/caspase-1/GSDMD signaling pathway in an ischemia/reperfusion injury model [164].
8. The Role of H2S in Mental Disorders and Neurodegenerative Diseases
8.1. Cognitive Impairment
Endogenous H2S may reduce cognitive impairment through the reduction of endoplasmic reticulum stress and the inhibition of caspase-12 and C/EBP homologous protein (CHOP) levels [27]. It has been shown in a rat model of subarachnoid hemorrhage that H2S can reduce cognitive deficits by inhibiting the neuroinflammation induced by the TLR4/NF-κB signaling pathway that activates microglial cells (Figure 9) [228].
Figure 9.
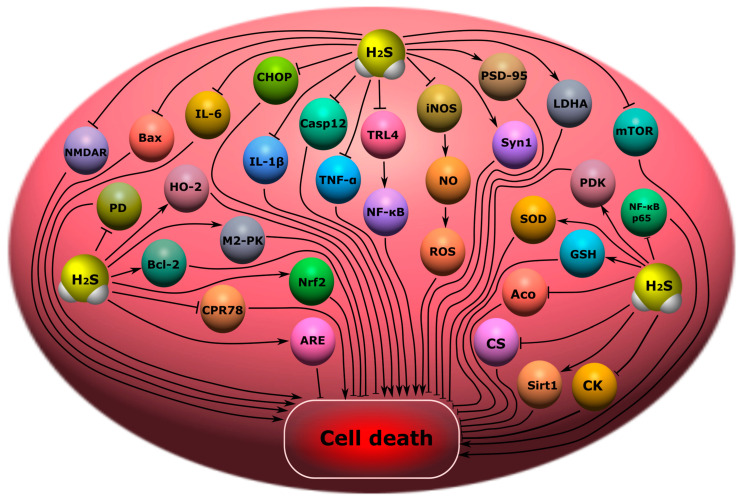
Possible H2S-dependent signaling mechanisms that regulate cell death in the nervous tissue in cognitive impairment and encephalopathy. H2S, hydrogen sulfide; CHOP, C/EBP homologous protein; Casp12, caspase-12; TRL4, toll like receptor 4; NF-ĸB, nuclear factor kappa-light-chain-enhancer of activated B cells; iNOS, inducible nitric oxide synthase; NO, nitric oxide; ROS, reactive oxygen species; PSD-9, postsynaptic density protein 95; Bax, bcl-2-like protein 4; NMDAR, N-methyl-D-aspartate receptor; IL-6, interleukin-6; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; Syn1, synapsin 1; LDHA, lactate dehydrogenase A; mTOR, mammalian target of rapamycin; PDK, pyruvate dehydrogenase kinase 1; SOD, superoxide dismutase; GSH, glutathione; Aco, aconitase; CS, citrate synthase; Sirt1, NAD-dependent deacetylase sirtuin-1; CK, creatine kinase; NF-ĸB p65, RelA; PD, pyruvate dehydrogenase; Bcl-2, B-cell lymphoma 2; HO-2, heme oxygenase 2; M2-PK, pyruvate kinase M2; CPR78, cuticular protein RR-2 motif 78; Nrf2, nuclear factor erythroid 2–related factor 2; ARE, antioxidant response element.
NaHS, the classic H2S donor, had a beneficial effect on the memory of TBI-surviving rats [4]. In a mouse model of surgical trauma accompanied by neuroinflammation, H2S improved orientation in the Morris water maze. At the same time, the neuroprotective effect of H2S was due to a decrease in the level of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in blood serum and in hippocampal cells, which is a key structure of learning and memory [229]. In another study, H2S-dependent cytoprotection was associated with a decrease in the level of NO and iNOS expression in hippocampal cells, as well as with the activation of the antioxidant defense system and a decrease in microglial activation (Figure 9) [230].
In a postoperative trauma model, H2S reduced cognitive impairment in the Y-maze test; improved the recognition of new objects, and the Morris water maze, by increasing the expression of the synapsin-1 and PSD-95 proteins involved in the process of synaptic plasticity; and prevented a decrease in the density of synapses in the hippocampi of rats. In addition, H2S enhanced the Warburg effect, known as aerobic glycolysis, which promotes synaptic plasticity and has a neuroprotective effect [231], in the hippocampal cells of rats with a cognitive deficit against the background of an increase in the expression of hexokinase 2 (HO-2), pyruvate kinase M2 (M2-RK), lactate dehydrogenase A (LDH A), kinase pyruvate dehydrogenase 1 (PDK), increased levels of lactic acid, and reduced expression of pyruvate dehydrogenase (PD) (Figure 9) [232].
H2S is reported to improve object recognition and spatial orientation by decreasing Sirt1, oxidative stress, lipid peroxidation, CPR78, CHOP and caspase-12, and increasing GSH and SOD. At the same time, the H2S donor significantly reduced the level of apoptosis in the hippocampus, as evidenced by a decrease in TUNEL-positive cells and the Bax\Bcl-2 ratio (Figure 9) [15]. The use of H2S donors, ATB-346 and diallyl trisulfide, reduced memory deficits in rats by modulating neuroinflammation, oxidative stress, and the cholinergic system [233].
It is known that damage to the neuromodulatory system often occurs after TBI and underlies cognitive disorders [234]. It is indicated that exogenous H2S modulates the level of catecholamines and increases the endogenous synthesis of H2S in cognitive impairment [235].
8.2. Encephalopathy
H2S can significantly attenuate oxidative stress and apoptosis in encephalopathy through activation of the Nrf2/ARE signaling pathway [236]. However, a high content of H2S can lead to a decrease in the activity of citrate synthase (CS) and aconitase (Aco) in the mitochondria of neurons of the cerebral cortex, as well as creatine kinase (CK) in this brain structure, the striatum, and the hippocampus in encephalopathy. In addition, H2S can enhance lipid peroxidation and disrupt bioenergetic processes in mitochondria [237]. The use of NaHS or S-adenosylmethionine (SAMe), a CBS activator, reduces neuroinflammation and inhibits the expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), restores SIRT1 levels and the phosphorylation of mTOR and NF-κB p65 in neuronal HT-22 cells at high glucose levels, which may indicate H2S-dependent neuroprotective defense mechanisms in diabetic encephalopathy [238]. The cytoprotective effect of H2S in encephalopathy can be realized through the activation of the antioxidant defense system and a decrease in NMDARs expression (Figure 9) [239].
8.3. Depression and Anxiety Disorders
Studies have shown that the administration of NaHS for a week had an antidepressant and anxiolytic effect on mice and rats in various test procedures: forced swimming, tail hanging, and plus maze [240]. In a model of sleep deprivation in rats, H2S attenuated depressive and anxiety disorder through the increased expression of Sirt1 in the hippocampus and decreased levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and CC motif chemokine ligand 2 (CCL2). At the same time, H2S increased the expression of IL-4 and IL-10, which belong to the anti-inflammatory group of cytokines. The use of the Sirt1 inhibitor leveled the neuroprotective effects of H2S [241].
In another study, the use of NaHS resulted in a reduction in stress-induced depression-like behavior in rats, also via the H2S/Sirt1 signaling pathway, which inhibits endoplasmic reticulum stress in hippocampal neurons [242]. H2S reduced depressive and anxious behavior in mice by reducing ferroptosis in the H2S prefrontal cortex. This H2S-dependent effect was due to a decrease in Fe2+ deposition, oxidative stress, and an increase in GPX4 and SLC7A11 levels. In addition, the introduction of an H2S donor suppressed the activation of microglial cells, reduced the level of pro-inflammatory cytokines, and increased the expression of sirtuin 6 (Sirt6). Moreover, H2S increased the deacetylase activity of Sirt6 and reduced the level of acetylated histone H3 lysine 9 (H3K9ac), Notch1, ROS, and the activation of antioxidant enzymes (Figure 10) [243].
Figure 10.
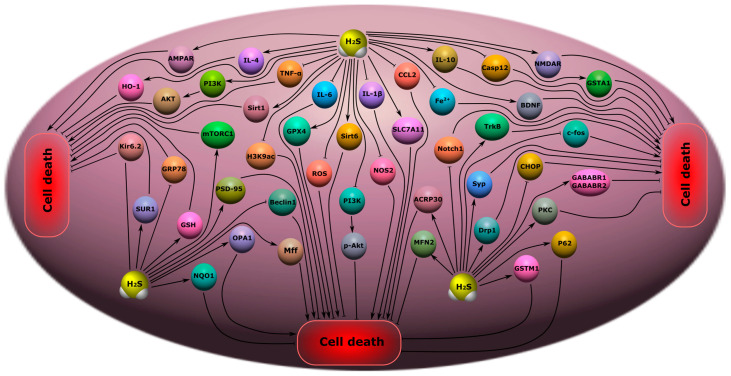
Possible H2S-dependent signaling mechanisms that regulate cell death in nervous tissue in depression, anxiety disorders, epilepsy, and chronic pain. H2S, hydrogen sulfide; AMPAR, AMPA-type glutamate receptor; IL-4, interleukin-4; IL-6, interleukin-6; IL-1β, interleukin-1β; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; HO-2, heme oxygenase 2; PI3K, phosphatidylinositol 3-kinase; AKT, RAC-alpha serine/threonine-protein kinase; Sirt1, NAD-dependent deacetylase sirtuin-1; mTORC1, mammalian target of rapamycin complex 1; H3K9ac, acetylated histone H3 lysine 9; GPX4, Glutathione peroxidase 4; Kir6.2, major subunit of the ATP-sensitive K+ channel; SUR1, subunit of the ATP-sensitive K+ channel; GRP78, glucose-regulated protein 78; GSH, glutathione; PSD-9, postsynaptic density protein 95; NQO1, NAD(P)H quinone dehydrogenase 1; OPA1, optic atrophy 1; Beclin, mammalian orthologue of yeast Atg6; Mff, mitochondrial fission factor; ROS, reactive oxygen species; Sirt6, NAD-dependent deacetylase sirtuin-6; p-Akt, phosphorylated RAC-alpha serine/threonine-protein kinase; NOS2, inducible nitric oxide synthase; SLC7A11, solute carrier family 7 member 11; CCL2, C-C motif ligand 2; Fe2+, iron ion; Casp12, caspase-12; NMDAR, N-methyl-D-aspartate receptor; GSTA1, glutathione S-transferase A1; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; c-fos, gene encoding c-fos protein; Notch1, Notch homolog 1; ACRP30, Adipocyte complement-related protein of 30 kDa; MFN2, Mitofusin-2; Syp, synaptophysin; Drp1, dynamin-related protein; CHOP, C\EBP homologous protein; PKC, protein kinase C; P62, sequestosome 1; GSTM1, glutathione s-transferase Mu 1; GABABR1\GABABR2, gamma-aminobutyric acid receptor subunits, GABABR1 and GABABR2.
Use of the donors H2S, llyl isothiocyanate (A-ITC), and phenyl isothiocyanate (P-ITC), reduced depressive behavior by activating the PI3K/p-Akt signaling pathway, as well as reducing oxidative stress, iNOS levels, and inflammatory response in hippocampal cells [244]. In a constraint-induced depression model, H2S has been shown to reduce synapse loss and autophagic cell death in the hippocampus via adiponectin activation. NaHS significantly reduced the number of autophagosomes and the level of Beclin 1. This H2S donor increased the expression of P62 and adipoxin in the hippocampus of rats with depression-like behavior (Figure 10). The use of Anti-acrp30, which inhibits adiponectin, neutralized the neuroprotective and antidepressant effects of NaHS [245].
It is reported that H2S prevents the decrease in the density of dendritic spines and increases the level of mTORC1, as well as the neurotrophic receptors, TrkB, in a rat model of chronic stress-induced depression. As a result of the H2S-dependent upregulation of the mTORC1/TrkB signaling pathway, the expression of synaptic proteins, such as PSD-95, synaptophysin, and the AMPA receptor GluR 1/2 subunit, in the hippocampus of rats with depression-like behavior, was increased [246]. H2S can realize its antidepressant and anxiolytic effects through the activation of the PI3K/AKT pathway and increased neurogenesis in the hippocampus [247]. Studies have shown that hippocampal levels of H2S, CBS, BDNF and PSD-95 are significantly reduced in chronic stress-induced depression in mice. However, the use of S-adenosylmethionine (SAM), which activates CBS, prevented these negative effects and improved the synapse ultrastructure [248]. H2S may reduce depressive symptoms through the inhibition of endoplasmic reticulum stress and lower levels of glucose-regulated protein 78 (GRP78), CCAAT/enhancer binding protein homologous protein (CHOP), and caspase-12, in rat hippocampal cells (Figure 10) [249].
8.4. Epilepsy
In a mouse model of electrically stimulated epileptic seizures, it has been shown that acute and recurrent seizures lead to a decrease in plasma H2S levels. The authors of the study suggest that H2S can be considered as a new candidate for the role of a biomarker of severe epileptic seizures. The level of thiocyanate, which is a product of cyanide metabolism via the trans-sulfonation pathway involving H2S, was also strongly reduced in the brain and plasma after convulsions. It is noted that the level of GSH did not change. The study yielded important data on the expression of a number of proteins. An increase in the anti-apoptotic protein optic atrophy 1 (OPA1) was observed, as well as mitochondrial fission factor (Mff), mitofusin 2 (MFN2), and dynamin-1-like protein (Drp1) after epileptic seizures (Figure 10) [250].
Studies have shown that a new H2S donor based on carbazole has an anti-convulsing effect. In addition, it reduced the level of aquaporin 4, 1β, IL-6 and TNF-α and increased the expression of protein kinase C (PKC). The use of a PKC inhibitor significantly attenuated these H2S-dependent effects. The authors suggest that the neuroprotective H2S signaling pathway in epilepsy may be mediated through PKC [251]. In addition, H2S synthesized on the basis of carbazole from aldehydes suppressed convulsions and increased the expression of the subunits of ATP-sensitive K+ channels, Kir6.2 and SUR1 [251]. In a febrile seizure model in rats, NaHS administration protected hippocampal cells, reduced c-fos expression, and increased the expression of gamma-aminobutyric acid receptor subunits, namely GABABR1 and GABABR2 [252]. The use of NaHS significantly increased the latency period between seizures, and suppressed the production of IL-1β and TNF-α in the hippocampus [253]. The inhibition of CBS with aminooxyacetate significantly increased the severity of seizures in rats with lindane-induced refractory generalized epilepsy (Figure 10) [254].
However, there is evidence that H2S may exacerbate seizure-like events in rats with entylenetetrazole-induced epilepsy and pilocarpine. Moreover, epileptic activity was recorded using the patch-clamp method in brain sections. The authors of the study suggest that this negative H2S effect was due to the activation of the NMDA and AMPA receptors, as well as the voltage-dependent Na+ channels, through signaling pathways associated with H2S [255]. The use of an H2S-sensitive fluorophore has been shown to increase H2S in mild epilepsy and significantly decrease in severe epilepsy due to neuronal damage [256]. H2S is reported to be elevated during seizures. The use of a CBS inhibitor reduced functional brain hyperemia in epilepsy. NaHS blocked ROS and reduced the cerebrovascular dysfunction caused by epileptic seizures (Figure 10) [257].
8.5. Chronic Pain
H2S inhalation can reduce neuropathic pain caused by sciatic nerve injury by reducing the expression of pro-inflammatory cytokines and the activation of IL-6-induced microglia in the spinal cord [145]. Using diallyl disulfide (DADS) and morpholin-4-ium 4-methoxyphenyl (morpholino), phosphinodithioate dichloromethane complex (GYY 4137), a sustained release of H2S, had an analgesic effect on chronic pain in mice by reducing oxidative stress and apoptosis in the amygdala and lowering the levels of phosphoinositide 3-kinase (PI3K) in this brain structure, gray matter, and the frontal part of the cingulate cortex. In addition, an H2S-dependent effect was manifested in the suppression of PKB activation in the infralimbic cortex of the cerebral hemispheres and in the amygdala [258]. Studies have shown that DADS increases the expression of Nrf2, HO-1, NQO1 and GSTM1 in dorsal root ganglia and the periaqueductal gray matter of the cerebral cortex in chronic pain (Figure 10). The authors suggest that H2S-induced activation of the antioxidant system in the CNS and PNS contributes to the reduction of pain sensations [259].
Introduction of the slowly releasing H2S donors, allyl isothiocyanate (A-ITC) and phenyl isothiocyanate (P-ITC), effectively reduced neuropathic pain, increased HO-1, GSTM1 and GSTA1 levels, and decreased neuroinflammation in the hippocampus and prefrontal brain [260]. NaHS also decreased hyperalgesia and allodynia through activation of the Nrf2/HO-1 signal pathways and decreased TNF-α, IL-1 β, IL-6 and high-mobility group protein B1 (HMGB1) in the dorsal brains of rats with neuropathic pain caused by compression of the ischial nerve (Figure 10) [261]. New σ 1 receptor antagonists, linked to H2S donors, have been developed and shown to be effective in pain relief [262].
9. Neurodegenerative Diseases
9.1. Alzheimer’s Disease
Studies have shown that there is a significant decrease in the level of H2S in the brain of patients suffering from AD [28]. Violation of H2S-homeostasis towards a decrease in the level of H2S is also observed in neurotrauma [126], which indicates the general mechanisms of dysfunction of the H2S-synthesizing system in these pathological conditions. H2S and its metabolites have been proposed as markers of cognitive impairment and vascular dysfunction in AD [120].
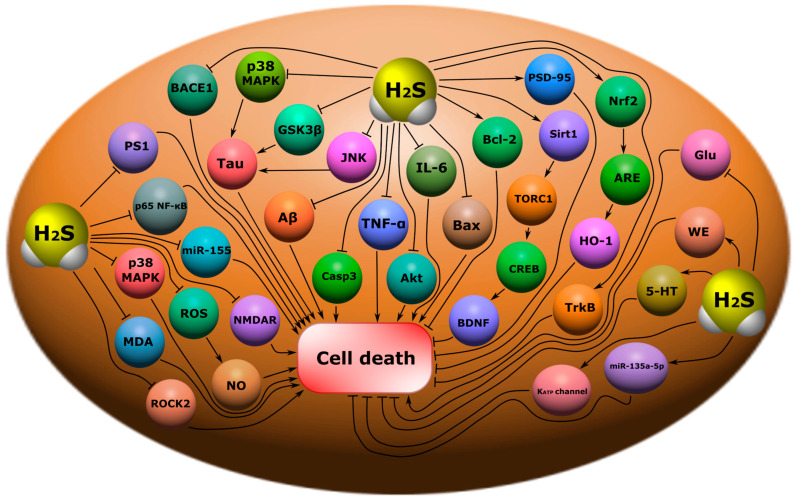
H2S is reported to inhibit the hyperphosphorylation of Tau by sulfhydration glycogen synthase kinase 3β (GSK3β), improving the motor and cognitive impairment caused by AD [41]. In a triple transgenic mouse model of AD (3×Tg-AD) demonstrating both Aβ and Tau disorders, the treatment for three months with H2S significantly protected learning and memory in 3×Tg-AD mice. At the same time, a decrease in amyloid β-plaques in the cortex and hippocampus was observed. These neuroprotective effects were due to the H2S-dependent downregulation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases, and p38, which play a key role in phosphorylation, Tau, inflammatory response, oxidative stress, and Ca2+-excitotoxicity (Figure 11) [263].
Figure 11.
Possible H2S-dependent signaling mechanisms that regulate cell death in nervous tissue in neurodegenerative diseases. BACE1, beta-site amyloid precursor protein cleaving enzyme 1; p38 MAPK, p38 mitogen-activated protein kinase; Tau, microtubule-associated protein tau; GSK3β, glycogen synthase kinase-3 beta; JNK, c-Jun N-terminal kinase; Aβ, amyloid beta; Casp3, caspase-3; TNF-α, tumor necrosis factor-α; Akt, RAC-alpha serine/threonine-protein kinase; IL-6, interleukin-6; Bax, bcl-2-like protein 4; Bcl-2, B-cell lymphoma 2; PSD-95, postsynaptic density protein 95; Sirt1, NAD-dependent deacetylase sirtuin-1; TORC1, target of rapamycin kinase complex 1; CREB, cAMP response element-binding protein; BDNF, brain-derived neurotrophic factor; Nrf2, nuclear factor erythroid 2–related factor 2; ARE, antioxidant response element; HO-1, heme oxygenase 1; TrkB, tropomyosin receptor kinase B; Glu, glutamic acid; WE, Warburg effect; 5-HT, serotonin; miR-133a-5p, microRNA 133a-5p; KATP channel, ATP-sensitive K+ channel; PS1, presenilin-1; p65 NF-ĸB, nuclear factor kappa-light-chain-enhancer of activated B cells; miR-155, microRNA 155; ROS, reactive oxygen species; NO, nitric oxide; MDA, malonic dialdehyde; NMDAR, N-methyl-D-aspartate receptor; ROCK2, Rho associated coiled-coil containing protein kinase 2.
H2S may decrease expression mRNA pro-inflammatory cytokines, TNF-α, IL-6 and IL-1β, and prevent synapse loss in AD by increasing synaptophysin and postsynaptic density protein 95 (PSD-95) [264]. The use of NaHS reduced TNF-α, miR-155, pAkt and inhibited apoptosis in AD-induced rats. The morphological picture in the hippocampus improved under the influence of this H2S donor [265]. The neuroprotective effects of H2S in AD may be due to a decrease in the level of TNF-α, BAX, caspase-3 and the activation of Bcl-2 [266]. It is reported that H2S can reduce disturbances in the blood-brain barrier, cerebral circulation, and also modulate NMDAR and synaptic plasticity in AD (Figure 11) [267].
NaHS administration improved spatial memory and learning while reducing apoptosis, microgliosis, and astrogliosis in the hippocampus. There was an H2S-dependent decrease in the level of Aβ1-40 phosphorylation of p38 MAPK and p65 NF-κB [268]. In another study, H2S showed a neuroprotective effect in AD, which was due to a decrease in the level of Aβ1-40 and Aβ42, as well as BACE1 (Beta-site APP-cleaving enzyme 1) and PS1. In addition, exogenous H2S led to a significant increase in ADAM 17 (a disintegrin and metalloproteinase domain 17) [268]. H2S exerts a protective effect against neurotoxins via sulfhydration of the transcription factors Sirt1 and Nrf2, which positively regulate gene expression for a number of antioxidant enzymes, and NF-ĸB, a key player in the inflammatory response. This leads to the regulation of downstream signaling pathways, such as SIRT1/TORC1/CREB/BDNF, TrkB, and Nrf2/ARE/HO-1, or other pathways. In addition, H2S can directly bind neurotoxic agents (Figure 11). Violation of these H2S-associated mechanisms may underlie the pathogenesis of AD [269].
9.2. Parkinson’s Disease
Abnormal levels of H2S and its metabolites have been identified in many studies in PD. Thus, it was shown that the level of endogenous H2S significantly decreased in the substantia nigra (SN) in PD, and the use of H2S donors contributed to the neuroprotective effect and reduced the death of dopaminergic neurons in this pathological condition [270]. The protective effect of H2S has been shown in various PD models in vitro and in vivo [271].
It was shown that the use of the H2S donor ACS84, a derivative of the compound L-Dopa, reduced motor dysfunction in mice with induced PD by reducing neuronal loss in the SN, via Nrf-2 activation, and through the increased expression of a number of antioxidant enzymes [272]. In addition, four L-Dopa hybrids associated with different H2S releasing compounds demonstrated efficacy in the treatment of PD, which could be due to a significant increase in the intracerebral dopamine and GSH reported in PD rats following administration of these drugs [273]. In addition, H2S donors can inhibit the ROS-NO cytotoxic pathway in PD (Figure 11) [121].
The H2S-dependent antiparkinsonism effect may be caused by a decrease in excess malonic production dialdehyde (MDA), a product of lipid peroxidation [274]. In a 6-hydroxydopamine (6-OHDA) induced PD model, NaHS significantly attenuated motor asymmetry, increased neuronal survival in the SN, and also increased striatal dopamine levels. However, the use of a blocker of ATP-sensitive K+-channels, glibenclamide, caused a decrease in the antiparkinsonian effects of NaHS, which indicates the importance of these channels in the implementation of the neuroprotective effects of H2S [274]. It is indicated that H2S attenuates cognitive impairment, promotes the polarization of microglia from M1 to M2, and enhances the Warburg effect in the hippocampus of rats with PD [245]. In addition, the neuroprotection of H2S may be due to the negative regulation of histone acetylation in PD [275]. H2S enhances the long-term potentiation of the hippocampus, inhibits the hyper activation of astrocytes, and reduces damage to the dopamine neurons in the striatum in rats with PD induced using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTP/p). In addition, exogenous H2S increased the level of serotonin and modulated the level of glutamate and γ-aminobutyric acid in the striatum (Figure 11) [276].
Recent studies have shown that an H2S-dependent neuroprotective effect may be mediated through activation of the BDNF-TrkB pathway in a mouse model of PD [277]. H2S significantly reduces the expression of Rho-associated protein kinase 2 (ROCK2), associated with neurodegenerative processes, by increasing the level of miR-135a-5p in an animal model of PD. It is known that miR-135a-5p inhibits the translation of ROCK2 mRNA in neurons and has a neuroprotective effect (Figure 11) [278].
10. Therapeutic Approaches Using H2S as a Neuroprotector
Many experimental animal studies have shown that H2S has a beneficial effect on the survival of neurons and glial cells in various pathological conditions, including neurotrauma, and psychiatric and neurodegenerative diseases. However, there is no H2S-associated neuroprotector that has undergone clinical trials, yet.
The complexity of the development of this drug lies in the fact that it is necessary to understand the molecular signaling mechanisms associated with H2S well, which are realized under the conditions of a particular pathological condition. This is not the end of the problem. The therapeutic effect of H2S largely depends on its concentration: a level of H2S close to the physiological level exhibits a neuroprotective effect, and a high level of H2S can lead to cytotoxicity. Therefore, this drug should release H2S at the required concentration for a long time and have a neurocumulative effect in order to accumulate precisely in the nervous tissue and not release H2S in non-target organs. Moreover, if there are already good developments on the first point, then the situation is more complicated regarding the second [279].
To date, the main H2S donors used in research are sodium sulfide (Na2S), calcium sulfide (CaS), and NaHS [280]. Their main drawback is the rapid release of H2S, which often leads to conflicting results. However, recent studies have increasingly used slow H2S donors, which are able to gradually increase the concentration of H2S over a long period of time. These donors include allyl isothiocyanate (A-ITC) and phenyl isothiocyanate (P-ITC) [260], as well as ferrofluid hydrogels (FFH) with iron tetrasulfide (Fe3S4), which have a beneficial effect on nerve and glial cells in pathological conditions [136].
A new water-soluble molecule that effectively but sustainably releases H2S is reported, namely GYY4137 (morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate). It has been shown that the intraperitoneal administration of GYY4137 reduces the negative manifestations of peripheral neuropathy by reducing the secretion of pro-inflammatory cytokines and the activation of microglia and astrocytes in the spinal cord [281]. In addition, GYY4137 protected retinal ganglion cells from oxidative stress and acute ischemic damage in experimental models of glaucoma [282]. In addition, this H2S donor affects immune cells involved in the pathogenesis of multiple sclerosis [283].
However, the situation with slow H2S donors is also ambiguous. For example, GYY4137 is reported to be ineffective due to the low release of H2S, requiring high concentrations. In this connection, new, long-acting H2S donors based on GYY4137, such as AP67 and AP105, have been developed. They exhibit high biological activity [284]. FW1256 could be a promising H2S donor; it has demonstrated a good anti-inflammatory effect in a number of studies [285]. In addition, the use of ATB-346 (2-(6-methoxynapthalen-2-yl)-propionic acid 4-thiocarbamoyl-phenyl ester), releasing H2S, effectively reduced cell death in TBI and SCI by reducing the inflammatory response and oxidative stress [67]. There are also hybrid H2S donors that are capable of releasing not only H2S, but also another gasotransmitter. For example, NOSH-aspirin (NBS-1120) releases H2S and NO during its metabolism and has a pronounced anti-inflammatory and neuroprotective effect. Researchers suggest that it may become a new candidate for the treatment of neuropathological conditions [286]. Of course, there are a number of H2S donors that may have the potential to be new generation H2S-associated neuroprotectors.
It is possible to modulate the H2S level by using inhibitors of key enzymes of its synthesis. The best known inhibitors of CBS are aminooxyacetic acid (AOAA) and hydroxylamine (NH2OH), CSE–PAG (propargylglycine) and β-cyanolanine (BCA) [287], and 3 MST–2-[(4-hydroxy-6-methylpyrimidin-2-yl)sulfanyl]-1-(naphthalen-1-yl)ethan-1-one (HMPSNE) [288]. They can be used in cases where there is a dangerous neurotoxic level of H2S. However, it should be noted that the presented inhibitors are non-selective, inhibiting the activity of other enzymatic systems, which undoubtedly presents a big problem for their use in medicine and indicates the need to develop inhibitors directed to this class of enzymes [289].
Thus, neuropathology therapy using H2S donors/inhibitors is at the preclinical studies stage. However, even now, we can see the therapeutic potential of H2S, which has a wide range of biological effects. Further fundamental and practical research on this gasotransmitter is necessary for the effective treatment of many diseases, including pathological conditions associated with the CNS and PNS.
11. Conclusions
Injuries of the central and peripheral nervous system and associated neurodegenerative diseases and mental disorders are one of the main causes of disability and death worldwide after cardiovascular and oncological diseases. H2S can be considered as a potential molecular target for neuroprotective effects. Definitely, the positive role of H2S, manifested in the reduction of oxidative stress, inflammation, demyelination processes, excitotoxicity, apoptosis, autophagy, ferroptosis, and pyroptosis, prevail over its negative effects in the nervous tissue during traumatic damage to the CNS and PNS. In addition, the use of H2S donors effectively reduces the symptoms of mental disorders, such as cognitive impairment, encephalopathy, depression and anxiety disorders, epilepsy, chronic pain, and also inhibits the progression of neurodegenerative diseases (Table 2).
Table 2.
The role of H2S in cell death in neurotrauma and associated psychiatric and neurodegenerative diseases.
| The Role of H2S in Cell Death | ||
|---|---|---|
| Process | Effects of H2S on signaling pathways/molecular targets | Effect |
| Oxidative stress | Increasing GSH, Trx-1, COD, CAT, GPx, p66Shcis, and ROS uptake | Reducing oxidative stress levels |
| Ca2+-homeostasis | Modulation of NMDAR activity via PKA-dependent and independent pathways; regulation of L-type Ca2+ channels and fast T-type Ca-type CaV 3.2 channels | Increase/decrease in intracellular Ca2+ levels |
| Inflammation | Inhibition of ROS, NF-κB, leukocyte endothelial adhesion, TNF, IL-1 β, iNOS, NLRP3/caspase-1/GSDMD, cytochrome c oxidase | Reducing the level of inflammation |
| Oxidation of H2S to form sulfite leads to leukocyte adhesion and neutrophil activation via CD11b/CD18 and PKC/CaM; inhibition of H2S cleavage of caspase-3 and p38 in granulocytes | Increased levels of inflammation | |
| Remyelination | PI3K/AKT/mTOR activation | Remyelination and repair of axons |
| Regulation of LAMP1, p75NTR, c-Jun, and p-ERK1/2 | Increased dedifferentiation and proliferation of Schwann cells in Wallerian degeneration | |
| Apoptosis | Decreased expression of p53, caspase-3, Bax, NF-κB p65, NOX4, iNOS, COX-2; increased levels of Bcl-2, ncRNA CasC7 and Akt phosphorylation | Reducing the level of apoptosis |
| Autophagy | Inhibition of the PI3K/Akt/Nrf2 and ROS signaling pathways | Decreased autophagy |
| Increasing miR-30c, Beclin 1, and LC3 levels | Increase in autophagy | |
| Ferroptosis | Inhibition of ROS, LDH, and Fe2+ accumulation; increased GSH levels, NRF2/KEAP1 and AMPK; activation of p62 phosphorylation | Reducing ferroptosis |
| Pyroptosis | Inhibition of NOD-, LRR-, and NLRP3, GSDMD, caspase-1, and ASC | Reducing pyroptosis |
| The role of H2S in psychiatric disorders | ||
| Cognitive impairment | Inhibition of endoplasmic reticulum stress, caspase-12, CHOP and TLR4/NF-κB; decrease in the level of TNF-α, IL-1β and IL-6, Sirt1, ROS, LP, CPR78, CHOP, caspase-12, Bax; increase in synapsin-1 and PSD-95, Bcl-2, HO-2, M2-RK, LDHA, PDK in the hippocampus; catecholamine level modulation | Reducing the symptoms of cognitive impairment; improving spatial memory, learning, memorization; reducing the level of apoptosis in the cerebral cortex and hippocampus |
| Encephalopathy | Nrf2/ARE activation; decrease in IL-1β, IL-6, TNF-α levels; restoration of SIRT1 levels and phosphorylation of mTOR and NF-κB p65 | Reducing the symptoms of encephalopathy; reducing the level of neuroinflammation and apoptosis |
| Decreased activity of CS, Aco, and CK; increased LP in the brain with a high level of H2S | Increased neuroinflammation and apoptosis | |
| Depression and anxiety disorders | Increased expression of Sirt1, Sirt6, IL-4, IL-10, NOS2, PI3K/p-Akt, mTORC1, TrkB PSD-95, synaptophysin, and AMPA receptor GluR1/2 subunit; decreased levels of IL-1β, IL-6, TNF-α; deposition of Fe2+, ROS, H3K9ac, Notch1, Beclin 1, GRP78 | Antidepressant and anxiolytic effect; improvement of memory, learning ability; reduction of apoptosis; loss of dendritic spines |
| Epilepsy | Decreased levels of aquaporin 4, 1β, IL-6, TNF-α, c-fos; increased expression of PKC, Kir6.2 and SUR1, GABABR1 and GABABR2 | Reducing epileptic seizures |
| NMDAR and AMPAR activation | Increase in epileptic seizures | |
| Chronic pain | Decrease in PI3K, TNF-α, IL-1β, IL-6; increase in Nrf2, HO-1, NQO1 and GSTM1 | Reduced symptoms of chronic pain, neuroinflammation and apoptosis |
| The role of H2S in neurodegenerative disorders | ||
| Alzheimer’s disease | Inhibition of Tau hyperphosphorylation; expression of JNK, p38, TNF-α, IL-6, IL-1β, miR-155, pAkt, Bax, caspase-3, Aβ1-40, Aβ42; phosphorylation of p38 MAPK, p65 NF-κB, BACE1; increased levels of synaptophysin, PSD-95, Bcl-2, Sirt1, Nrf2 | Improving memory, learning; reducing the number of amyloid β-plaques in the hippocampal cortex, the level of neuroinflammation and oxidative stress |
| Parkinson’s disease | Increased expression of Nrf-2, dopamine, GSH; activation of BDNF/TrkB, miR-135a-5p; inhibition of ROS/NO, LP, ROCK2 | Reducing the progression of Parkinson’s disease; decreased death of dopaminergic neurons in the SN; neuroinflammation, oxidative stress |
Despite the progress achieved in this area, further research into H2S-dependent signaling mechanisms underlying the pathogenesis of neurotrauma, mental disorders, and neurodegenerative diseases is needed. This is of fundamental importance and should expand our knowledge of the H2S signaling mechanisms in the survival and death of neurons and glial cells. At the same time, these studies are of practical value and can become the basis for the development of new clinically effective H2S-associated neuroprotective drugs of a new generation and optimize the existing tactics for the treatment of the pathological conditions discussed above.
12. Main Conclusions
12.1. Effects of H2S in Neurons and Glial Cells in Injuries of the Central and Peripheral Nervous System
H2S reduces oxidative stress via uptake of ROS and increased levels of GSH, Trx-1, COD, CAT and GPx, and p66Shcis. However, high levels of H2S can induce oxidative stress;
H2S can modulate NMDAR activity through cAMP\PKA, and H2S can directly interact with cysteine residues of NMDAR subunits, modifying them by S-sulfhydration. H2S can activate slow L-type Ca2+ channels, as well as fast T-type Ca-type CaV 3.2 channels;
H2S can inhibit inflammation by reducing ROS, NF-κB, leukocyte adhesion to the endothelium, TNF, IL-1β, and NLRP3/caspase-1/GSDMD. However, excessive production of ROS can cause the oxidation of H2S to form sulfite, leading to leukocyte adhesion and neurophilic activation. H2S may increase inflammation through the inhibition of caspase-3 and the activation of p38 protein kinase;
H2S can increase the level of the neurotrophic factors GDNF, NGF, BDNF, and VEGF;
H2S is involved in maintaining the integrity of the blood-brain barrier via inhibition of PKC-α, β I, β II and δ, and activation of PKC-ε and increased levels of Claudin-5, Occlusin and ZO-1, as well as in the suppression of the expression of AQP4 on astrocytes and the inhibition of MMP-9 and NOS level modulation;
H2S can lead to both the destruction of the myelin sheath and the processes of remyelination and the repair of axons by activating the PI3K/AKT/mTOR signaling pathway;
H2S can regulate apoptosis by acting as an anti- or pro-oxidant, or by interacting with proteins involved in apoptotic signaling. H2S can reduce the expression of p53, caspase-3, Bax, NF-κB p65, NOX4, iNOS, COX-2, and increase Bcl-2, ncRNA CasC7 and the phosphorylation of Akt;
H2S can reduce autophagy by modeling the PI3K/Akt /Nrf2 and ROS signaling pathways. H2S may enhance autophagy by increasing miR-30c, Beclin 1, and LC3 levels;
H2S can inhibit ferroptosis by reducing the level of ROS, the LDH accumulation of Fe2+, and by increasing the antioxidant enzyme GSH, as well as through the activation of NRF2/KEAP1 and AMPK to phosphorylate p62;
H2S can reduce pyroptosis by inhibiting NOD-, LRR-, NLRP 3, GSDMD, caspase-1, and ASC.
12.2. Effects of H2S in Neurons and Glial Cells in Mental Disorders:
H2S may reduce cognitive impairment through the inhibition of endoplasmic reticulum stress, caspase-12, CHOP, and TLR4 /NF-κB; a decrease in the level of TNF-α, IL-1β and IL-6, Sirt1, ROS, LP, CPR78, CHOP, caspase-12, Bax; and an increase in synapsin-1 and PSD-95, Bcl-2, HO-2, M2-RK, LDHA, and PDK in the hippocampus. H2S modulates the level of catecholamines and reduces the level of apoptosis;
H2S may reduce apoptosis in encephalopathy through Nrf2/ARE activation. However, a high content of H2S reduces the activity of CS, Aco, and CK, and enhances LP in the brain. H2S reduces neuroinflammation through a decrease in the level of IL-1β, IL-6, TNF-α, and also restores the level of SIRT1 and phosphorylation mTOR and NF-κB p65 for encephalopathy;
H2S has an antidepressant and anxiolytic effect through an increase in the expression of Sirt1, Sirt6, IL-4, and IL-10, the activation of PI3K/p-Akt, and a decrease in the level of IL-1β, IL-6, TNF-α, Fe2+ deposition, ROS, NOS2, H3K9ac, Notch1, Beclin 1, and GRP78. H2S prevents the loss of dendritic spines and increases the level of mTORC1, TrkB PSD-95, synaptophysin, and the AMPA receptor GluR1/2 subunit in depression and anxiety disorders;
H2S reduces epileptic seizures. H2S reduces the level of aquaporin 4, 1β, IL-6, TNF-α, and c-fos, and increases the expression of PKC, Kir6.2 and SUR1, GABABR1 and GABABR2. However, H2S can also lead to seizures via activation of NMDARs and AMPARs;
H2S can reduce neuropathic pain through the inhibition of the expression of microglial activation, a decrease in the level of apoptosis, PI3K, TNF-α, IL-1β, and IL-6, and increased levels of Nrf2, HO-1, NQO1 and GSTM1.
12.3. Effects of H2S in Neurons and Glial Cells in Neurodegenerative Diseases:
H2S may reduce the progression of Alzheimer’s disease. H2S inhibits hyperphosphorylation Tau; reduces amyloid β-plaques in the hippocampal cortex, neuroinflammation, oxidative stress, JNK expression, p38, TNF-α, IL-6, IL-1β, miR-155, pAkt, Bax, caspase-3, Aβ1-40, Aβ42, the phosphorylation of p38 MAPK, p65 NF-κB, and BACE1; and increases synaptophysin levels, PSD-95, Bcl-2, Sirt1, and Nrf2;
H2S may reduce the progression of Parkinson’s disease. H2S reduces the death of dopaminergic neurons in the SN; increases the expression of Nrf-2, dopamine, and GSH; activates BDNF/TrkB, and miR-135a-5p; and inhibits ROS/NO, LP, and ROCK2.
Supplementary Materials
The following supporting information can be downloaded at: https://susy.mdpi.com/user/manuscripts/displayFile/120f7af664d45b2bd31fba7da53b59a5/supplementary.
Author Contributions
Conceptualization, S.R.; methodology, S.R.; validation, S.R.; data curation, S.R. and M.G.; writing—original draft preparation, S.R., C.N., A.S., M.R., A.T., I.V. and M.G.; writing—review and editing, S.R.; visualization, S.R.; supervision, S.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by a grant within the framework of the “Nauka-2030”, No. 1.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Furlan J.C., Gulasingam S., Craven B.C. Epidemiology of War-Related Spinal Cord Injury Among Combatants: A Systematic Review. Glob. Spine J. 2019;9:545–558. doi: 10.1177/2192568218776914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laskowitz D., Grant G.E. Translational Research in Traumatic Brain Injury. CRC Press; Boca Raton, FL, USA: 2016. [PubMed] [Google Scholar]
- 3.Rodkin S.V., Dzreyan V.A., Demyanenko S.V., Uzdensky A.B. The Role of p53-Dependent Signaling Pathways in Survival and Death of Neurons and Glial Cells after Peripheral Nerve Injury. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2021;15:334–347. doi: 10.1134/S199074782106009X. [DOI] [Google Scholar]
- 4.Karimi S.A., Hosseinmardi N., Janahmadi M., Sayyah M., Hajisoltani R. The protective effect of hydrogen sulfide (H2S) on traumatic brain injury (TBI) induced memory deficits in rats. Brain Res. Bull. 2017;134:177–182. doi: 10.1016/j.brainresbull.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Calvillo M., Irimia A. Neuroimaging and Psychometric Assessment of Mild Cognitive Impairment After Traumatic Brain Injury. Front. Psychol. 2020;11:1423. doi: 10.3389/fpsyg.2020.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachdeva R., Nightingale T.E., Krassioukov A.V. The Blood Pressure Pendulum following Spinal Cord Injury: Implications for Vascular Cognitive Impairment. Int. J. Mol. Sci. 2019;20:2464. doi: 10.3390/ijms20102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nightingale T.E., Zheng M.M.Z., Sachdeva R., Phillips A.A., Krassioukov A.V. Diverse cognitive impairment after spinal cord injury is associated with orthostatic hypotension symptom burden. Physiol. Behav. 2020;213:112742. doi: 10.1016/j.physbeh.2019.112742. [DOI] [PubMed] [Google Scholar]
- 8.Chiaravalloti N.D., Weber E., Wylie G., Dyson-Hudson T., Wecht J.M. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J. Spinal Cord Med. 2020;43:88–97. doi: 10.1080/10790268.2018.1543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R., Sen N. Traumatic brain injury: A risk factor for neurodegenerative diseases. Rev. Neurosci. 2016;27:93–100. doi: 10.1515/revneuro-2015-0017. [DOI] [PubMed] [Google Scholar]
- 10.Brett B.L., Gardner R.C., Godbout J., Dams-O’Connor K., Keene C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry. 2022;91:498–507. doi: 10.1016/j.biopsych.2021.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh T.-S., Huang Y.-P., Wang H.-I., Pan S.-L. Spinal cord injury and Parkinson’s disease: A population-based, propensity score-matched, longitudinal follow-up study. Spinal Cord. 2016;54:1215–1219. doi: 10.1038/sc.2016.74. [DOI] [PubMed] [Google Scholar]
- 12.Xu X.-J., Yang M.-S., Zhang B., Niu F., Dong J.-Q., Liu B.-Y. Glucose metabolism: A link between traumatic brain injury and Alzheimer’s disease. Chin. J. Traumatol. 2021;24:5–10. doi: 10.1016/j.cjtee.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White D.L., Kunik M.E., Yu H., Lin H.L., Richardson P.A., Moore S., Sarwar A.I., Marsh L., Jorge R.E. Post-Traumatic Stress Disorder is Associated with further Increased Parkinson’s Disease Risk in Veterans with Traumatic Brain Injury. Ann. Neurol. 2020;88:33–41. doi: 10.1002/ana.25726. [DOI] [PubMed] [Google Scholar]
- 14.Fox G.B., Fan L., Levasseur R.A., Faden A.I. Sustained Sensory/Motor and Cognitive Deficits With Neuronal Apoptosis Following Controlled Cortical Impact Brain Injury in the Mouse. J. Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 15.Li X.-N., Chen L., Luo B., Li X., Wang C.-Y., Zou W., Zhang P., You Y., Tang X.-Q. Hydrogen sulfide attenuates chronic restrain stress-induced cognitive impairment by upreglulation of Sirt1 in hippocampus. Oncotarget. 2017;8:100396–100410. doi: 10.18632/oncotarget.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu A.-H., Chu M., Wang Y.-P. Up-Regulation of Trem2 Inhibits Hippocampal Neuronal Apoptosis and Alleviates Oxidative Stress in Epilepsy via the PI3K/Akt Pathway in Mice. Neurosci. Bull. 2019;35:471–485. doi: 10.1007/s12264-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleem S. Apoptosis, Autophagy, Necrosis and Their Multi Galore Crosstalk in Neurodegeneration. Neuroscience. 2021;469:162–174. doi: 10.1016/j.neuroscience.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Ozdamar Unal G., Demirdas A., Nazıroglu M., Ovey I.S. Agomelatine attenuates calcium signaling and apoptosis via the inhibition of TRPV1 channel in the hippocampal neurons of rats with chronic mild stress depression model. Behav. Brain Res. 2022;434:114033. doi: 10.1016/j.bbr.2022.114033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H., Li N., Li Z., Li Y., Yu Y., Zhang L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022;13:1561. doi: 10.3389/fphar.2022.898574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mumtaz S., Rana J.N., Choi E.H., Han I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022;23:9288. doi: 10.3390/ijms23169288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-González A., Castañeda-Arriaga R., Guzmán-López E.G., Hernández-Ayala L.F., Galano A. Chalcone Derivatives with a High Potential as Multifunctional Antioxidant Neuroprotectors. ACS Omega. 2022;7:38254–38268. doi: 10.1021/acsomega.2c05518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali A., Wang Y., Wu L., Yang G. Gasotransmitter signaling in energy homeostasis and metabolic disorders. Free Radic. Res. 2021;55:83–105. doi: 10.1080/10715762.2020.1862827. [DOI] [PubMed] [Google Scholar]
- 23.Hendriks K.D., Maassen H., van Dijk P.R., Henning R.H., van Goor H., Hillebrands J.-L. Gasotransmitters in health and disease: A mitochondria-centered view. Curr. Opin. Pharmacol. 2019;45:87–93. doi: 10.1016/j.coph.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Sen N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017;429:543–561. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J., Zhang S., Shan H., Zhang M. Biologic Effect of Hydrogen Sulfide and Its Role in Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2020;2020:7301615. doi: 10.1155/2020/7301615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomierny B., Krzyżanowska W., Jurczyk J., Skórkowska A., Strach B., Szafarz M., Przejczowska-Pomierny K., Torregrossa R., Whiteman M., Marcinkowska M., et al. The Slow-Releasing and Mitochondria-Targeted Hydrogen Sulfide (H2S) Delivery Molecule AP39 Induces Brain Tolerance to Ischemia. Int. J. Mol. Sci. 2021;22:7816. doi: 10.3390/ijms22157816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv S., Wu N., Wang Q., Yang L. Endogenous hydrogen sulfide alleviates methotrexate-induced cognitive impairment by attenuating endoplasmic reticulum stress-induced apoptosis via CHOP and caspase-12. Fundam. Clin. Pharmacol. 2020;34:559–570. doi: 10.1111/fcp.12543. [DOI] [PubMed] [Google Scholar]
- 28.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 29.Sun J., Li X., Gu X., Du H., Zhang G., Wu J., Wang F. Neuroprotective effect of hydrogen sulfide against glutamate-induced oxidative stress is mediated via the p53/glutaminase 2 pathway after traumatic brain injury. Aging. 2021;13:7180–7189. doi: 10.18632/aging.202575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y., Yang X., Zhao S., Wei C., Yin Y., Liu T., Jiang S., Xie J., Wan X., Mao M., et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013;63:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Lu D., Wang L., Liu G., Wang S., Wang Y., Wu Y., Wang J., Sun X. Role of hydrogen sulfide in subarachnoid hemorrhage. CNS Neurosci. Ther. 2022;28:805–817. doi: 10.1111/cns.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z.-Z., Liu Y., Bian J.-S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majid A.S.A., Majid A.M.S.A., Yin Z.Q., Ji D. Slow Regulated Release of H2S Inhibits Oxidative Stress Induced Cell Death by Influencing Certain Key Signaling Molecules. Neurochem. Res. 2013;38:1375–1393. doi: 10.1007/s11064-013-1034-z. [DOI] [PubMed] [Google Scholar]
- 34.Tyagi N., Moshal K.S., Sen U., Vacek T.P., Kumar M., Hughes W.M., Kundu S., Tyagi S.C. H2S Protects Against Methionine–Induced Oxidative Stress in Brain Endothelial Cells. Antioxid. Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L., Yu S., Yang K., Li C., Liang Y. Hydrogen Sulfide Inhibits Autophagic Neuronal Cell Death by Reducing Oxidative Stress in Spinal Cord Ischemia Reperfusion Injury. Oxid. Med. Cell. Longev. 2017;2017:8640284. doi: 10.1155/2017/8640284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng G., Muqadas M., Adlat S., Zheng H., Li G., Zhu P., Nasser M.I. Protective Effect of Hydrogen Sulfide on Cerebral Ischemia–Reperfusion Injury. Cell. Mol. Neurobiol. 2023;43:15–25. doi: 10.1007/s10571-021-01166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia M. Role of Hydrogen Sulfide in the Pathology of Inflammation. Scientifica. 2012;2012:159680. doi: 10.6064/2012/159680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y., Li Y., Zhao Z., Li P., Xie Y. Hydrogen Sulfide Overproduction Is Involved in Acute Ischemic Cerebral Injury Under Hyperhomocysteinemia. Front. Neurosci. 2020;14:582851. doi: 10.3389/fnins.2020.582851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calenic B., Yaegaki K., Ishkitiev N., Kumazawa Y., Imai T., Tanaka T. p53-Pathway activity and apoptosis in hydrogen sulfide-exposed stem cells separated from human gingival epithelium. J. Periodontal Res. 2013;48:322–330. doi: 10.1111/jre.12011. [DOI] [PubMed] [Google Scholar]
- 40.Calenic B., Yaegaki K., Kozhuharova A., Imai T. Oral Malodorous Compound Causes Oxidative Stress and p53-Mediated Programmed Cell Death in Keratinocyte Stem Cells. J. Periodontol. 2010;81:1317–1323. doi: 10.1902/jop.2010.100080. [DOI] [PubMed] [Google Scholar]
- 41.Giovinazzo D., Bursac B., Sbodio J.I., Nalluru S., Vignane T., Snowman A.M., Albacarys L.M., Sedlak T.W., Torregrossa R., Whiteman M., et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA. 2021;118:e2017225118. doi: 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodkin S., Nwosu C., Sannikov A., Tyurin A., Chulkov V.S., Raevskaya M., Ermakov A., Kirichenko E., Gasanov M. The Role of Gasotransmitter-Dependent Signaling Mechanisms in Apoptotic Cell Death in Cardiovascular, Rheumatic, Kidney, and Neurodegenerative Diseases and Mental Disorders. Int. J. Mol. Sci. 2023;24:6014. doi: 10.3390/ijms24076014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 44.Martin S., Hussain Z., Boyle J. A beginner’s guide to the literature search in medical education. Scott. Med. J. 2017;62:58–62. doi: 10.1177/0036933017707163. [DOI] [PubMed] [Google Scholar]
- 45.Smith C. Neurotrauma. Handb. Clin. Neurol. 2018;145:115–132. doi: 10.1016/B978-0-12-802395-2.00008-0. [DOI] [PubMed] [Google Scholar]
- 46.Chang W.-T.W., Badjatia N. Neurotrauma. Emerg. Med. Clin. N. Am. 2014;32:889–905. doi: 10.1016/j.emc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Khatri N., Thakur M., Pareek V., Kumar S., Sharma S., Datusalia A.K. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord.–Drug Targets. 2018;17:689–695. doi: 10.2174/1871527317666180627120501. [DOI] [PubMed] [Google Scholar]
- 48.Sussman E.S., Pendharkar A.V., Ho A.L., Ghajar J. Mild Traumatic Brain Injury and Concussion: Terminology and Classification. Elsevier; Amsterdam, The Netherlands: 2018. pp. 21–24. [DOI] [PubMed] [Google Scholar]
- 49.Robinson C.P. Moderate and Severe Traumatic Brain Injury. Contin. Lifelong Learn. Neurol. 2021;27:1278–1300. doi: 10.1212/CON.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M., Shan H., Chang P., Wang T., Dong W., Chen X., Tao L. Hydrogen Sulfide Offers Neuroprotection on Traumatic Brain Injury in Parallel with Reduced Apoptosis and Autophagy in Mice. PLoS ONE. 2014;9:e87241. doi: 10.1371/journal.pone.0087241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K., Liu B., Ma J. Research progress in traumatic brain penumbra. Chin. Med. J. 2014;127:1964–1968. [PubMed] [Google Scholar]
- 52.Sun G., Gao F., Zhao Z., Sun H., Xu W., Wu L., He Y. Endoplasmic reticulum stress-induced apoptosis in the penumbra aggravates secondary damage in rats with traumatic brain injury. Neural Regen. Res. 2016;11:1260–1266. doi: 10.4103/1673-5374.189190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killen M.J., Giorgi-Coll S., Helmy A., Hutchinson P.J., Carpenter K.L. Metabolism and inflammation: Implications for traumatic brain injury therapeutics. Expert Rev. Neurother. 2019;19:227–242. doi: 10.1080/14737175.2019.1582332. [DOI] [PubMed] [Google Scholar]
- 54.Zong P., Feng J., Yue Z., Li Y., Wu G., Sun B., He Y., Miller B., Yu A.S., Su Z., et al. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron. 2022;110:1944–1958.e8. doi: 10.1016/j.neuron.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moojen V.K.M., Damiani-Neves M., Bavaresco D.V., Pescador B.B., Comim C.M., Quevedo J., Boeck C.R. NMDA preconditioning prevents object recognition memory impairment and increases brain viability in mice exposed to traumatic brain injury. Brain Res. 2012;1466:82–90. doi: 10.1016/j.brainres.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 56.Zhou K., Sansur C., Xu H., Jia X. The Temporal Pattern, Flux, and Function of Autophagy in Spinal Cord Injury. Int. J. Mol. Sci. 2017;18:466. doi: 10.3390/ijms18020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Wu Y., Han W., Li J., Xu K., Li Z., Wang Q., Xu K., Liu Y., Xie L., et al. Hydrogen Sulfide Ameliorates Blood-Spinal Cord Barrier Disruption and Improves Functional Recovery by Inhibiting Endoplasmic Reticulum Stress-Dependent Autophagy. Front. Pharmacol. 2018;9:858. doi: 10.3389/fphar.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eckert M.J., Martin M.J. Trauma. Surg. Clin. N. Am. 2017;97:1031–1045. doi: 10.1016/j.suc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Anjum A., Yazid M.D., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goulart C., Martinez A.B. Tubular conduits, cell-based therapy and exercise to improve peripheral nerve regeneration. Neural Regen. Res. 2015;10:565–567. doi: 10.4103/1673-5374.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhandari P.S. Management of peripheral nerve injury. J. Clin. Orthop. Trauma. 2019;10:862–866. doi: 10.1016/j.jcot.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renthal W., Tochitsky I., Yang L., Cheng Y.-C., Li E., Kawaguchi R., Geschwind D.H., Woolf C.J. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron. 2020;108:128–144.e9. doi: 10.1016/j.neuron.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hart A.M., Terenghi G., Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol. Res. 2008;30:999–1011. doi: 10.1179/174313208X362479. [DOI] [PubMed] [Google Scholar]
- 64.Navarro X., Vivó M., Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Rishal I., Fainzilber M. Axon–soma communication in neuronal injury. Nat. Rev. Neurosci. 2014;15:32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- 66.Patodia S., Raivich G. Role of Transcription Factors in Peripheral Nerve Regeneration. Front. Mol. Neurosci. 2012;5:8. doi: 10.3389/fnmol.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campolo M., Esposito E., Ahmad A., Di Paola R., Paterniti I., Cordaro M., Bruschetta G., Wallace J.L., Cuzzocrea S. Hydrogen sulfide-releasing cyclooxygenase inhibitor ATB-346 enhances motor function and reduces cortical lesion volume following traumatic brain injury in mice. J. Neuroinflamm. 2014;11:196. doi: 10.1186/s12974-014-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh G.-S., Pae H.-O., Lee B.-S., Kim B.-N., Kim J.-M., Kim H.-R., Jeon S.B., Jeon W.K., Chae H.-J., Chung H.-T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 69.Corsello T., Komaravelli N., Casola A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants. 2018;7:129. doi: 10.3390/antiox7100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medeiros G.C., Twose C., Weller A., Dougherty J.W., Goes F.S., Sair H.I., Smith G.S., Roy D. Neuroimaging Correlates of Depression after Traumatic Brain Injury: A Systematic Review. J. Neurotrauma. 2022;39:755–772. doi: 10.1089/neu.2021.0374. [DOI] [PubMed] [Google Scholar]
- 71.Delmonico R.L., Theodore B.R., Sandel M.E., Armstrong M.A., Camicia M. Prevalence of depression and anxiety disorders following mild traumatic brain injury. PM R. 2022;14:753–763. doi: 10.1002/pmrj.12657. [DOI] [PubMed] [Google Scholar]
- 72.Kaur J., Ghosh S., Singh P., Dwivedi A.K., Sahani A.K., Sinha J.K. Cervical Spinal Lesion, Completeness of Injury, Stress, and Depression Reduce the Efficiency of Mental Imagery in People With Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2022;101:513–519. doi: 10.1097/PHM.0000000000001955. [DOI] [PubMed] [Google Scholar]
- 73.Mariajoseph F.P., Chen Z., Sekhar P., Rewell S.S., O’Brien T.J., Antonic-Baker A., Semple B.D. Incidence and risk factors of posttraumatic epilepsy following pediatric traumatic brain injury: A systematic review and meta-analysis. Epilepsia. 2022;63:2802–2812. doi: 10.1111/epi.17398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pease M., Gonzalez-Martinez J., Puccio A., Nwachuku E., Castellano J.F., Okonkwo D.O., Elmer J. Risk Factors and Incidence of Epilepsy after Severe Traumatic Brain Injury. Ann. Neurol. 2022;92:663–669. doi: 10.1002/ana.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J., Liu Z., Liu G., Gao K., Zhou H., Zhao Y., Wang H., Zhang L., Liu S. Spinal cord injury and its underlying mechanism in rats with temporal lobe epilepsy. Exp. Ther. Med. 2020;19:2103–2112. doi: 10.3892/etm.2020.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kruitbosch J.M., Schouten E.J., Tan I.Y., Veendrick-Meekes M.J.B.M., de Vocht J.W.M.M. Cervical spinal cord injuries in patients with refractory epilepsy. Seizure. 2006;15:633–636. doi: 10.1016/j.seizure.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Hay J., Johnson V.E., Smith D.H., Stewart W. Chronic Traumatic Encephalopathy: The Neuropathological Legacy of Traumatic Brain Injury. Annu. Rev. Pathol. Mech. Dis. 2016;11:21–45. doi: 10.1146/annurev-pathol-012615-044116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lucke-Wold B.P., Turner R.C., Logsdon A.F., Bailes J.E., Huber J.D., Rosen C.L. Linking Traumatic Brain Injury to Chronic Traumatic Encephalopathy: Identification of Potential Mechanisms Leading to Neurofibrillary Tangle Development. J. Neurotrauma. 2014;31:1129–1138. doi: 10.1089/neu.2013.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffman J., Curran M., Lucas S., Zumsteg J. Collaborative Care to Treat Chronic Pain after Traumatic Brain Injury: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2022;103:e57. doi: 10.1016/j.apmr.2022.08.573. [DOI] [Google Scholar]
- 80.Bradburn S., Murgatroyd C., Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 82.Troubat R., Barone P., Leman S., Desmidt T., Cressant A., Atanasova B., Brizard B., El Hage W., Surget A., Belzung C., et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021;53:151–171. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 83.Vergne-Salle P., Bertin P. Chronic pain and neuroinflammation. Jt. Bone Spine. 2021;88:105222. doi: 10.1016/j.jbspin.2021.105222. [DOI] [PubMed] [Google Scholar]
- 84.Praticò D., Clark C.M., Liun F., Lee V.Y.-M., Trojanowski J.Q. Increase of Brain Oxidative Stress in Mild Cognitive Impairment. Arch. Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 85.Black C.N., Bot M., Scheffer P.G., Penninx B.W.J.H. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol. Med. 2017;47:936–948. doi: 10.1017/S0033291716002828. [DOI] [PubMed] [Google Scholar]
- 86.Geronzi U., Lotti F., Grosso S. Oxidative stress in epilepsy. Expert Rev. Neurother. 2018;18:427–434. doi: 10.1080/14737175.2018.1465410. [DOI] [PubMed] [Google Scholar]
- 87.Kaushik A.S., Strath L.J., Sorge R.E. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain Ther. 2020;9:487–498. doi: 10.1007/s40122-020-00200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker K.R., Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 2013;5:29. doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Irvine K.-A., Clark J.D. Chronic Pain After Traumatic Brain Injury: Pathophysiology and Pain Mechanisms. Pain Med. 2018;19:1315–1333. doi: 10.1093/pm/pnx153. [DOI] [PubMed] [Google Scholar]
- 90.Ghajari M., Hellyer P.J., Sharp D.J. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain. 2017;140:333–343. doi: 10.1093/brain/aww317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjelakovic B., Dimitrijevic L., Lukic S., Golubovic E. Hypertensive encephalopathy as a late complication of autonomic dysreflexia in a 12-year-old boy with a previous spinal cord injury. Eur. J. Pediatr. 2014;173:1683–1684. doi: 10.1007/s00431-014-2281-y. [DOI] [PubMed] [Google Scholar]
- 92.Kennedy P., Rogers B.A. Anxiety and depression after spinal cord injury: A longitudinal analysis. Arch. Phys. Med. Rehabil. 2000;81:932–937. doi: 10.1053/apmr.2000.5580. [DOI] [PubMed] [Google Scholar]
- 93.Hunt C., Moman R., Peterson A., Wilson R., Covington S., Mustafa R., Murad M.H., Hooten W.M. Prevalence of chronic pain after spinal cord injury: A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2021;46:328–336. doi: 10.1136/rapm-2020-101960. [DOI] [PubMed] [Google Scholar]
- 94.Kang J., Cho S.S., Kim H.Y., Lee B.H., Cho H.J., Gwak Y.S. Regional Hyperexcitability and Chronic Neuropathic Pain Following Spinal Cord Injury. Cell. Mol. Neurobiol. 2020;40:861–878. doi: 10.1007/s10571-020-00785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Del Tredici K., Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 96.Alcántar-Garibay O., Incontri-Abraham D., Ibarra A. Spinal cord injury-induced cognitive impairment: A narrative review. Neural Regen. Res. 2022;17:2649–2654. doi: 10.4103/1673-5374.339475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Cao T., Ritzel R.M., He J., Faden A.I., Wu J. Dementia, Depression, and Associated Brain Inflammatory Mechanisms after Spinal Cord Injury. Cells. 2020;9:1420. doi: 10.3390/cells9061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rekand T., Hagen E., Grønning M. Chronic pain following spinal cord injury. Tidsskr. Nor. Legeforening. 2012;132:974–979. doi: 10.4045/tidsskr.11.0794. [DOI] [PubMed] [Google Scholar]
- 99.Norman G.J., Karelina K., Zhang N., Walton J.C., Morris J.S., DeVries A.C. Stress and IL-1β contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inquimbert P., Moll M., Latremoliere A., Tong C.-K., Whang J., Sheehan G.F., Smith B.M., Korb E., Athié M.C.P., Babaniyi O., et al. NMDA Receptor Activation Underlies the Loss of Spinal Dorsal Horn Neurons and the Transition to Persistent Pain after Peripheral Nerve Injury. Cell Rep. 2018;23:2678–2689. doi: 10.1016/j.celrep.2018.04.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao Q., Ying J., Xiang L., Zhang C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine. 2018;97:e13065. doi: 10.1097/MD.0000000000013065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khattak S., Rauf M.A., Khan N.H., Zhang Q.-Q., Chen H.-J., Muhammad P., Ansari M.A., Alomary M.N., Jahangir M., Zhang C.-Y., et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules. 2022;27:3389. doi: 10.3390/molecules27113389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kabil O., Zhou Y., Banerjee R. Human Cystathionine β-Synthase Is a Target for Sumoylation. Biochemistry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- 104.Bhattacharyya S., Saha S., Giri K., Lanza I.R., Nair K.S., Jennings N.B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A.L., et al. Cystathionine Beta-Synthase (CBS) Contributes to Advanced Ovarian Cancer Progression and Drug Resistance. PLoS ONE. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Omorou M., Liu N., Huang Y., Al-Ward H., Gao M., Mu C., Zhang L., Hui X. Cystathionine beta-Synthase in hypoxia and ischemia/reperfusion: A current overview. Arch. Biochem. Biophys. 2022;718:109149. doi: 10.1016/j.abb.2022.109149. [DOI] [PubMed] [Google Scholar]
- 106.Zhu H., Blake S., Chan K.T., Pearson R.B., Kang J. Cystathionine β-Synthase in Physiology and Cancer. Biomed. Res. Int. 2018;2018:3205125. doi: 10.1155/2018/3205125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu Z., Zhao T., Tao L., Yu Q., Yang Y., Cheng J., Lu S., Ding Q. Cystathionine β-Synthase-Derived Hydrogen Sulfide Correlates with Successful Aging in Mice. Rejuvenation Res. 2019;22:513–520. doi: 10.1089/rej.2018.2166. [DOI] [PubMed] [Google Scholar]
- 108.Jurkowska H., Kaczor-Kamińska M., Bronowicka-Adamska P., Wróbel M. Cystathionine γ-lyase. Postepy Hig. Med. Dosw. 2014;68:1–9. doi: 10.5604/17322693.1085372. [DOI] [PubMed] [Google Scholar]
- 109.Zhao K. Regulation of cystathionine gamma-lyase/H2S system and its pathological implication. Front. Biosci. 2014;19:1355–1369. doi: 10.2741/4286. [DOI] [PubMed] [Google Scholar]
- 110.Szijártó I.A., Markó L., Filipovic M.R., Miljkovic J.L., Tabeling C., Tsvetkov D., Wang N., Rabelo L.A., Witzenrath M., Diedrich A., et al. Cystathionine γ-Lyase–Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension. 2018;71:1210–1217. doi: 10.1161/HYPERTENSIONAHA.117.10562. [DOI] [PubMed] [Google Scholar]
- 111.Kabil O., Banerjee R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang M.Y., Dugbartey G.J., Juriasingani S., Sener A. Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021;22:6452. doi: 10.3390/ijms22126452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park B.S., Kim H.-W., Rhyu I.J., Park C., Yeo S.G., Huh Y., Jeong N.Y., Jung J. Hydrogen sulfide is essential for Schwann cell responses to peripheral nerve injury. J. Neurochem. 2015;132:230–242. doi: 10.1111/jnc.12932. [DOI] [PubMed] [Google Scholar]
- 114.Lupoli R., Di Minno A., Spadarella G., Franchini M., Sorrentino R., Cirino G., Di Minno G. Methylation Reactions, the Redox Balance and Atherothrombosis: The Search for a Link with Hydrogen Sulfide. Semin. Thromb. Hemost. 2015;41:423–432. doi: 10.1055/s-0035-1549848. [DOI] [PubMed] [Google Scholar]
- 115.Lajin B., Francesconi K.A. The hydrogen sulfide metabolite trimethylsulfonium is found in human urine. Sci. Rep. 2016;6:27038. doi: 10.1038/srep27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Insko M.A., Deckwerth T.L., Hill P., Toombs C.F., Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br. J. Pharmacol. 2009;157:944–951. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang R. Hydrogen Sulfide: The Third Gasotransmitter in Biology and Medicine. Antioxid. Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 118.Yakovlev A.V., Kurmasheva E.D., Ishchenko Y., Giniatullin R., Sitdikova G.F. Age-Dependent, Subunit Specific Action of Hydrogen Sulfide on GluN1/2A and GluN1/2B NMDA Receptors. Front. Cell. Neurosci. 2017;11:375. doi: 10.3389/fncel.2017.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munaron L., Avanzato D., Moccia F., Mancardi D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 2013;53:77–84. doi: 10.1016/j.ceca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Disbrow E., Stokes K.Y., Ledbetter C., Patterson J., Kelley R., Pardue S., Reekes T., Larmeu L., Batra V., Yuan S., et al. Plasma hydrogen sulfide: A biomarker of Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2021;17:1391–1402. doi: 10.1002/alz.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu L., Wang J., Wang H. Hydrogen sulfide alleviates oxidative stress injury and reduces apoptosis induced by MPP+ in Parkinson’s disease cell model. Mol. Cell. Biochem. 2020;472:231–240. doi: 10.1007/s11010-020-03801-y. [DOI] [PubMed] [Google Scholar]
- 122.Tabassum R., Jeong N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019;16:1386–1396. doi: 10.7150/ijms.36516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tabassum R., Jeong N., Jung J. Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases. Neural Regen. Res. 2020;15:653–662. doi: 10.4103/1673-5374.266911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui Y., Duan X., Li H., Dang B., Yin J., Wang Y., Gao A., Yu Z., Chen G. Hydrogen Sulfide Ameliorates Early Brain Injury Following Subarachnoid Hemorrhage in Rats. Mol. Neurobiol. 2016;53:3646–3657. doi: 10.1007/s12035-015-9304-1. [DOI] [PubMed] [Google Scholar]
- 125.Jiang Z., Li C., Manuel M.L., Yuan S., Kevil C.G., McCarter K.D., Lu W., Sun H. Role of Hydrogen Sulfide in Early Blood-Brain Barrier Disruption following Transient Focal Cerebral Ischemia. PLoS ONE. 2015;10:e0117982. doi: 10.1371/journal.pone.0117982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang M., Shan H., Wang T., Liu W., Wang Y., Wang L., Zhang L., Chang P., Dong W., Chen X., et al. Dynamic Change of Hydrogen Sulfide After Traumatic Brain Injury and its Effect in Mice. Neurochem. Res. 2013;38:714–725. doi: 10.1007/s11064-013-0969-4. [DOI] [PubMed] [Google Scholar]
- 127.Huerta de la Cruz S., Rodríguez-Palma E.J., Santiago-Castañeda C.L., Beltrán-Ornelas J.H., Sánchez-López A., Rocha L., Centurión D. Exogenous hydrogen sulfide restores CSE and CBS but no 3-MST protein expression in the hypothalamus and brainstem after severe traumatic brain injury. Metab. Brain Dis. 2022;37:1863–1874. doi: 10.1007/s11011-022-01033-1. [DOI] [PubMed] [Google Scholar]
- 128.Pushchina E.V., Stukaneva M.E., Varaksin A.A. Hydrogen Sulfide Modulates Adult and Reparative Neurogenesis in the Cerebellum of Juvenile Masu Salmon, Oncorhynchus masou. Int. J. Mol. Sci. 2020;21:9638. doi: 10.3390/ijms21249638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pushchina E.V., Zharikova E.I., Varaksin A.A. Mechanical Brain Injury Increases Cells’ Production of Cystathionine β-Synthase and Glutamine Synthetase, but Reduces Pax2 Expression in the Telencephalon of Juvenile Chum Salmon, Oncorhynchus keta. Int. J. Mol. Sci. 2021;22:1279. doi: 10.3390/ijms22031279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J., Cao L., Feng X., Zhou B., Li L. Octreotide-mediated neurofunctional recovery in rats following traumatic brain injury. Role of H2S, Nrf2 and TNF-α. Acta Cirúrgica Bras. 2021;36:e361204. doi: 10.1590/acb361204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Denoix N., Merz T., Unmuth S., Hoffmann A., Nespoli E., Scheuerle A., Huber-Lang M., Gündel H., Waller C., Radermacher P., et al. Cerebral Immunohistochemical Characterization of the H2S and the Oxytocin Systems in a Porcine Model of Acute Subdural Hematoma. Front. Neurol. 2020;11:649. doi: 10.3389/fneur.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Greco V., Neri C., Pieragostino D., Spalloni A., Persichilli S., Gastaldi M., Mercuri N.B., Longone P., Urbani A. Investigating Different Forms of Hydrogen Sulfide in Cerebrospinal Fluid of Various Neurological Disorders. Metabolites. 2021;11:152. doi: 10.3390/metabo11030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang X., Bian J.-S. Hydrogen Sulfide: A Neuromodulator and Neuroprotectant in the Central Nervous System. ACS Chem. Neurosci. 2014;5:876–883. doi: 10.1021/cn500185g. [DOI] [PubMed] [Google Scholar]
- 134.Jiang X., Huang Y., Lin W., Gao D., Fei Z. Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate–sensitive potassium channels and reduction of oxidative stress. J. Surg. Res. 2013;184:e27–e35. doi: 10.1016/j.jss.2013.03.067. [DOI] [PubMed] [Google Scholar]
- 135.Xu K., Wu F., Xu K., Li Z., Wei X., Lu Q., Jiang T., Wu F., Xu X., Xiao J., et al. NaHS restores mitochondrial function and inhibits autophagy by activating the PI3K/Akt/mTOR signalling pathway to improve functional recovery after traumatic brain injury. Chem. Biol. Interact. 2018;286:96–105. doi: 10.1016/j.cbi.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 136.Wang R., Wu X.-X., Tian Z., Hu T., Cai C., Wu G.-P., Jiang G.-B., Liu B. Sustained release of hydrogen sulfide from anisotropic ferrofluid hydrogel for the repair of spinal cord injury. Bioact. Mater. 2023;23:118–128. doi: 10.1016/j.bioactmat.2022.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X., Huang X., Liu C., Li S., Yang Z., Zhang F., Chen X., Shan H., Tao L., Zhang M. Surface-fill H2S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 2022;154:259–274. doi: 10.1016/j.actbio.2022.11.021. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y., Pan L., Jiang A., Yin M. Hydrogen sulfide upregulated lncRNA CasC7 to reduce neuronal cell apoptosis in spinal cord ischemia-reperfusion injury rat. Biomed. Pharmacother. 2018;98:856–862. doi: 10.1016/j.biopha.2017.12.079. [DOI] [PubMed] [Google Scholar]
- 139.Wang F., Zhou H., Zhang X. SAM, a cystathionine beta-synthase activator, promotes hydrogen sulfide to promote neural repair resulting from massive cerebral infarction induced by middle cerebral artery occlusion. Metab. Brain Dis. 2022;37:1641–1654. doi: 10.1007/s11011-022-00976-9. [DOI] [PubMed] [Google Scholar]
- 140.Xu C., Zhang M., Zhang G., Yan S., Yan W. Hydrogen Sulfide Improves Functional Recovery in Rat Traumatic Spinal Cord Injury Model by Inducing Nuclear Translocation of NF-E2-Related Factor 2. Biol. Pharm. Bull. 2021;44:b21-00259. doi: 10.1248/bpb.b21-00259. [DOI] [PubMed] [Google Scholar]
- 141.Liu H., Tong K., Zhong Z., Wang G. Mechanism of Hydrogen Sulfide Drug-Loaded Nanoparticles Promoting the Repair of Spinal Cord Injury in Rats Through Mammalian Target of Rapamycin/Signal Transducer and Activator of Transcription 3 Signaling Pathway. Sci. Adv. Mater. 2021;13:1691–1698. doi: 10.1166/sam.2021.4109. [DOI] [Google Scholar]
- 142.Kanemaru E., Miyazaki Y., Marutani E., Ezaka M., Goto S., Ohshima E., Bloch D.B., Ichinose F. Intranasal administration of polysulfide prevents neurodegeneration in spinal cord and rescues mice from delayed paraplegia after spinal cord ischemia. Redox Biol. 2023;60:102620. doi: 10.1016/j.redox.2023.102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nii T., Eguchi R., Yamaguchi S., Otsuguro K. Hydrogen sulfide induces Ca2+ release from the endoplasmic reticulum and suppresses ATP-induced Ca2+ signaling in rat spinal cord astrocytes. Eur. J. Pharmacol. 2021;891:173684. doi: 10.1016/j.ejphar.2020.173684. [DOI] [PubMed] [Google Scholar]
- 144.Liu X., Fu Z., Wu Y., Hu X., Zhu T., Jin C. Neuroprotective effect of hydrogen sulfide on acute cauda equina injury in rats. Spine J. 2016;16:402–407. doi: 10.1016/j.spinee.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 145.Kida K., Marutani E., Nguyen R.K., Ichinose F. Inhaled hydrogen sulfide prevents neuropathic pain after peripheral nerve injury in mice. Nitric Oxide. 2015;46:87–92. doi: 10.1016/j.niox.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jung J., Jeong N. Hydrogen sulfide controls peripheral nerve degeneration and regeneration: A novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases. Neural Regen. Res. 2014;9:2119–2121. doi: 10.4103/1673-5374.147940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Predmore B.L., Lefer D.J., Gojon G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Olson K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta–Bioenerg. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 149.Bruce King S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic. Biol. Med. 2013;55:21–34. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kesherwani V., Nelson K.S., Agrawal S.K. Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res. 2013;1527:222–229. doi: 10.1016/j.brainres.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 151.Searcy D.G., Whitehead J.P., Maroney M.J. Interaction of Cu, Zn Superoxide Dismutase with Hydrogen Sulfide. Arch. Biochem. Biophys. 1995;318:251–263. doi: 10.1006/abbi.1995.1228. [DOI] [PubMed] [Google Scholar]
- 152.Vega-Vela N.E., Osorio D., Avila-Rodriguez M., Gonzalez J., García-Segura L.M., Echeverria V., Barreto G.E. L-Type Calcium Channels Modulation by Estradiol. Mol. Neurobiol. 2017;54:4996–5007. doi: 10.1007/s12035-016-0045-6. [DOI] [PubMed] [Google Scholar]
- 153.Jiang M.C., Birch D.V., Heckman C.J., Tysseling V.M. The Involvement of CaV1.3 Channels in Prolonged Root Reflexes and Its Potential as a Therapeutic Target in Spinal Cord Injury. Front. Neural Circuits. 2021;15:642111. doi: 10.3389/fncir.2021.642111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alles S.R., Garcia E., Balasubramanyan S., Jones K., Tyson J.R., Joy T., Snutch T.P., Smith P.A. Peripheral nerve injury increases contribution of L-type calcium channels to synaptic transmission in spinal lamina II: Role of α2δ–1 subunits. Mol. Pain. 2018;14:174480691876580. doi: 10.1177/1744806918765806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ihbe N., Le Prieult F., Wang Q., Distler U., Sielaff M., Tenzer S., Thal S.C., Mittmann T. Adaptive Mechanisms of Somatostatin-Positive Interneurons after Traumatic Brain Injury through a Switch of α Subunits in L-Type Voltage-Gated Calcium Channels. Cereb. Cortex. 2022;32:1093–1109. doi: 10.1093/cercor/bhab268. [DOI] [PubMed] [Google Scholar]
- 156.Tang G., Wu L., Wang R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 157.Nagai Y., Tsugane M., Oka J., Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- 158.Yong Q.C., Choo C.H., Tan B.H., Low C.-M., Bian J.-S. Effect of hydrogen sulfide on intracellular calcium homeostasis in neuronal cells. Neurochem. Int. 2010;56:508–515. doi: 10.1016/j.neuint.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 159.García-Bereguiaín M.A., Samhan-Arias A.K., Martín-Romero F.J., Gutiérrez-Merino C. Hydrogen Sulfide Raises Cytosolic Calcium in Neurons Through Activation of L-Type Ca2+ Channels. Antioxid. Redox Signal. 2008;10:31–42. doi: 10.1089/ars.2007.1656. [DOI] [PubMed] [Google Scholar]
- 160.Okubo K., Takahashi T., Sekiguchi F., Kanaoka D., Matsunami M., Ohkubo T., Yamazaki J., Fukushima N., Yoshida S., Kawabata A. Inhibition of T-type calcium channels and hydrogen sulfide-forming enzyme reverses paclitaxel-evoked neuropathic hyperalgesia in rats. Neuroscience. 2011;188:148–156. doi: 10.1016/j.neuroscience.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 161.Nagasawa K., Tarui T., Yoshida S., Sekiguchi F., Matsunami M., Ohi A., Fukami K., Ichida S., Nishikawa H., Kawabata A. Hydrogen sulfide evokes neurite outgrowth and expression of high-voltage-activated Ca2+ currents in NG108-15 cells: Involvement of T-type Ca2+ channels. J. Neurochem. 2009;108:676–684. doi: 10.1111/j.1471-4159.2008.05808.x. [DOI] [PubMed] [Google Scholar]
- 162.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016;139:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Woodburn S.C., Bollinger J.L., Wohleb E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021;18:258. doi: 10.1186/s12974-021-02309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang K.-L., Li W.-H., Liu Y.-J., Wei Y.-J., Ren Y.-K., Mai C.-D., Zhang S.-Y., Zuo Y., Sun Z.-Z., Li D.-L., et al. Hydrogen Sulfide Attenuates Neuroinflammation by Inhibiting the NLRP3/Caspase-1/GSDMD Pathway in Retina or Brain Neuron following Rat Ischemia/Reperfusion. Brain Sci. 2022;12:1245. doi: 10.3390/brainsci12091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu H., Perumal N., Manicam C., Mercieca K., Prokosch V. Proteomics Reveals the Potential Protective Mechanism of Hydrogen Sulfide on Retinal Ganglion Cells in an Ischemia/Reperfusion Injury Animal Model. Pharmaceuticals. 2020;13:213. doi: 10.3390/ph13090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Éva Sikura K., Combi Z., Potor L., Szerafin T., Hendrik Z., Méhes G., Gergely P., Whiteman M., Beke L., Fürtös I., et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating RUNX2 by NF-κB, a link between inflammation and mineralization. J. Adv. Res. 2021;27:165–176. doi: 10.1016/j.jare.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rose P., Zhu Y.-Z., Moore P.K. Hydrogen Sulfide and the Immune System. Adv. Exp. Med. Biol. 2021;1315:99–128. doi: 10.1007/978-981-16-0991-6_5. [DOI] [PubMed] [Google Scholar]
- 169.Singh A.K., Awasthi D., Dubey M., Nagarkoti S., Kumar A., Chandra T., Barthwal M.K., Tripathi A.K., Dikshit M. High oxidative stress adversely affects NFκB mediated induction of inducible nitric oxide synthase in human neutrophils: Implications in chronic myeloid leukemia. Nitric Oxide. 2016;58:28–41. doi: 10.1016/j.niox.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 170.Rodkin S.V., Kovaleva V.D., Berezhnaya E.V., Neginskaya M.A., Uzdensky A.B. Ca2+- and NF-κB-dependent generation of NO in the photosensitized neurons and satellite glial cells. J. Photochem. Photobiol. B Biol. 2019;199:111603. doi: 10.1016/j.jphotobiol.2019.111603. [DOI] [PubMed] [Google Scholar]
- 171.Qu W., Cheng Y., Peng W., Wu Y., Rui T., Luo C., Zhang J. Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022;59:3124–3139. doi: 10.1007/s12035-022-02788-5. [DOI] [PubMed] [Google Scholar]
- 172.Kubo S., Kurokawa Y., Doe I., Masuko T., Sekiguchi F., Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241:92–97. doi: 10.1016/j.tox.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 173.Kondo K., Bhushan S., King A.L., Prabhu S.D., Hamid T., Koenig S., Murohara T., Predmore B.L., Gojon G., Gojon G., et al. H2S Protects Against Pressure Overload–Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bai X., Batallé G., Balboni G., Pol O. Hydrogen Sulfide Increases the Analgesic Effects of µ- and δ-Opioid Receptors during Neuropathic Pain: Pathways Implicated. Antioxidants. 2022;11:1321. doi: 10.3390/antiox11071321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Côté M.-P., Azzam G.A., Lemay M.A., Zhukareva V., Houlé J.D. Activity-Dependent Increase in Neurotrophic Factors Is Associated with an Enhanced Modulation of Spinal Reflexes after Spinal Cord Injury. J. Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.DeKosky S.T., Goss J.R., Miller P.D., Styren S.D., Kochanek P.M., Marion D. Upregulation of Nerve Growth Factor Following Cortical Trauma. Exp. Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- 177.Goss J.R., O’Malley M.E., Zou L., Styren S.D., Kochanek P.M., DeKosky S.T. Astrocytes Are the Major Source of Nerve Growth Factor Upregulation Following Traumatic Brain Injury in the Rat. Exp. Neurol. 1998;149:301–309. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- 178.Hermann D.M., Kilic E., Kügler S., Isenmann S., Bähr M. Adenovirus-Mediated Glial Cell Line-Derived Neurotrophic Factor (GDNF) Expression Protects against Subsequent Cortical Cold Injury in Rats. Neurobiol. Dis. 2001;8:964–973. doi: 10.1006/nbdi.2001.0448. [DOI] [PubMed] [Google Scholar]
- 179.Li X., Zhuang Y.-Y., Wu L., Xie M., Gu H.-F., Wang B., Tang X.-Q. Hydrogen Sulfide Ameliorates Cognitive Dysfunction in Formaldehyde-Exposed Rats: Involvement in the Upregulation of Brain-Derived Neurotrophic Factor. Neuropsychobiology. 2020;79:119–130. doi: 10.1159/000501294. [DOI] [PubMed] [Google Scholar]
- 180.Mohseni F., Bagheri F., Rafaiee R., Norozi P., Khaksari M. Hydrogen sulfide improves spatial memory impairment via increases of BDNF expression and hippocampal neurogenesis following early postnatal alcohol exposure. Physiol. Behav. 2020;215:112784. doi: 10.1016/j.physbeh.2019.112784. [DOI] [PubMed] [Google Scholar]
- 181.Li T., Liu H., Xue H., Zhang J., Han X., Yan S., Bo S., Liu S., Yuan L., Deng L., et al. Neuroprotective Effects of Hydrogen Sulfide Against Early Brain Injury and Secondary Cognitive Deficits Following Subarachnoid Hemorrhage. Brain Pathol. 2017;27:51–63. doi: 10.1111/bpa.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Y., Jia J., Ao G., Hu L., Liu H., Xiao Y., Du H., Alkayed N.J., Liu C.-F., Cheng J. Hydrogen sulfide protects blood-brain barrier integrity following cerebral ischemia. J. Neurochem. 2014;129:827–838. doi: 10.1111/jnc.12695. [DOI] [PubMed] [Google Scholar]
- 183.Kumar M., Sandhir R. Hydrogen sulfide attenuates hyperhomocysteinemia-induced blood-brain barrier permeability by inhibiting MMP-9. Int. J. Neurosci. 2022;132:1061–1071. doi: 10.1080/00207454.2020.1860967. [DOI] [PubMed] [Google Scholar]
- 184.Cai S., Li Q., Fan J., Zhong H., Cao L., Duan M. Therapeutic Hypothermia Combined with Hydrogen Sulfide Treatment Attenuated Early Blood–Brain Barrier Disruption and Brain Edema Induced by Cardiac Arrest and Resuscitation in Rat Model. Neurochem. Res. 2022;48:967–979. doi: 10.1007/s11064-022-03824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li H., Zhu L., Feng J., Hu X., Li C., Zhang B. Hydrogen Sulfide Decreases Blood-Brain Barrier Damage via Regulating Protein Kinase C and Tight Junction After Cardiac Arrest in Rats. Cell. Physiol. Biochem. 2018;47:994–1006. doi: 10.1159/000490166. [DOI] [PubMed] [Google Scholar]
- 186.López-Preza F.I., Huerta de la Cruz S., Santiago-Castañeda C., Silva-Velasco D.L., Beltran-Ornelas J.H., Tapia-Martínez J., Sánchez-López A., Rocha L., Centurión D. Hydrogen sulfide prevents the vascular dysfunction induced by severe traumatic brain injury in rats by reducing reactive oxygen species and modulating eNOS and H2S-synthesizing enzyme expression. Life Sci. 2023;312:121218. doi: 10.1016/j.lfs.2022.121218. [DOI] [PubMed] [Google Scholar]
- 187.Haber M., James J., Kim J., Sangobowale M., Irizarry R., Ho J., Nikulina E., Grin’kina N.M., Ramadani A., Hartman I., et al. Minocycline plus N-acteylcysteine induces remyelination, synergistically protects oligodendrocytes and modifies neuroinflammation in a rat model of mild traumatic brain injury. J. Cereb. Blood Flow Metab. 2018;38:1312–1326. doi: 10.1177/0271678X17718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mekhail M., Almazan G., Tabrizian M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: A review. Prog. Neurobiol. 2012;96:322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 189.Svennigsen Å., Dahlin L. Repair of the Peripheral Nerve—Remyelination that Works. Brain Sci. 2013;3:1182–1197. doi: 10.3390/brainsci3031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Iqbal I.K., Bajeli S., Sahu S., Bhat S.A., Kumar A. Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2-SIRT1 interaction to initiate autophagy. Autophagy. 2021;17:3511–3529. doi: 10.1080/15548627.2021.1876342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fukuto J.M., Vega V.S., Works C., Lin J. The chemical biology of hydrogen sulfide and related hydropersulfides: Interactions with biologically relevant metals and metalloproteins. Curr. Opin. Chem. Biol. 2020;55:52–58. doi: 10.1016/j.cbpa.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 192.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Simabuco F.M., Morale M.G., Pavan I.C.B., Morelli A.P., Silva F.R., Tamura R.E. p53 and metabolism: From mechanism to therapeutics. Oncotarget. 2018;9:23780–23823. doi: 10.18632/oncotarget.25267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36:3943–3956. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang D.B., Kinoshita C., Kinoshita Y., Morrison R.S. p53 and mitochondrial function in neurons. Biochim. Biophys. Acta–Mol. Basis Dis. 2014;1842:1186–1197. doi: 10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dai C.-Q., Luo T.-T., Luo S.-C., Wang J.-Q., Wang S.-M., Bai Y.-H., Yang Y.-L., Wang Y.-Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016;48:337–347. doi: 10.1007/s10863-016-9669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rodkin S., Dzreyan V., Bibov M., Ermakov A., Derezina T., Kirichenko E. NO-Dependent Mechanisms of p53 Expression and Cell Death in Rat’s Dorsal Root Ganglia after Sciatic-Nerve Transection. Biomedicines. 2022;10:1664. doi: 10.3390/biomedicines10071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rodkin S., Khaitin A., Pitinova M., Dzreyan V., Guzenko V., Rudkovskii M., Sharifulina S., Uzdensky A. The Localization of p53 in the Crayfish Mechanoreceptor Neurons and Its Role in Axotomy-Induced Death of Satellite Glial Cells Remote from the Axon Transection Site. J. Mol. Neurosci. 2020;70:532–541. doi: 10.1007/s12031-019-01453-2. [DOI] [PubMed] [Google Scholar]
- 199.Rachmany L., Tweedie D., Rubovitch V., Yu Q.-S., Li Y., Wang J.-Y., Pick C.G., Greig N.H. Cognitive Impairments Accompanying Rodent Mild Traumatic Brain Injury Involve p53-Dependent Neuronal Cell Death and Are Ameliorated by the Tetrahydrobenzothiazole PFT-α. PLoS ONE. 2013;8:e79837. doi: 10.1371/journal.pone.0079837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang J., Cui Z., Feng G., Bao G., Xu G., Sun Y., Wang L., Chen J., Jin H., Liu J., et al. RBM5 and p53 expression after rat spinal cord injury: Implications for neuronal apoptosis. Int. J. Biochem. Cell Biol. 2015;60:43–52. doi: 10.1016/j.biocel.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 201.Jiang M., Qi L., Li L., Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. doi: 10.1038/s41420-020-00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dzreyan V., Rodkin S., Nikul V., Pitinova M., Uzdensky A. The Expression of E2F1, p53, and Caspase 3 in the Rat Dorsal Root Ganglia After Sciatic Nerve Transection. J. Mol. Neurosci. 2021;71:826–835. doi: 10.1007/s12031-020-01705-6. [DOI] [PubMed] [Google Scholar]
- 203.Yang G., Sun X., Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 204.Ye X., Li Y., Lv B., Qiu B., Zhang S., Peng H., Kong W., Tang C., Huang Y., Du J., et al. Endogenous Hydrogen Sulfide Persulfidates Caspase-3 at Cysteine 163 to Inhibit Doxorubicin-Induced Cardiomyocyte Apoptosis. Oxid. Med. Cell. Longev. 2022;2022:6153772. doi: 10.1155/2022/6153772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khodapasand E., Jafarzadeh N., Farrokhi F., Kamalidehghan B., Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015;19:69–75. doi: 10.6091/ibj.1366.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Duan H.-Z., Wu C.-W., Shen S.-L., Zhang J.-Y., Li L. Neuroprotective Effects of Early Brain Injury after Subarachnoid Hemorrhage in Rats by Calcium Channel Mediating Hydrogen Sulfide. Cell. Mol. Neurobiol. 2021;41:1707–1714. doi: 10.1007/s10571-020-00940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Scheid S., Goeller M., Baar W., Wollborn J., Buerkle H., Schlunck G., Lagrèze W., Goebel U., Ulbrich F. Hydrogen Sulfide Reduces Ischemia and Reperfusion Injury in Neuronal Cells in a Dose- and Time-Dependent Manner. Int. J. Mol. Sci. 2021;22:10099. doi: 10.3390/ijms221810099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lu Q.-B., Ding Y., Fu X., Sun H., Zhang J.-R. Hydrogen sulfide in health and diseases: Crosstalk with noncoding RNAs. Am. J. Physiol. Physiol. 2023;324:C856–C877. doi: 10.1152/ajpcell.00507.2022. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Q., Yuan L., Liu D., Wang J., Wang S., Zhang Q., Gong Y., Liu H., Hao A., Wang Z. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol. Res. 2014;84:32–44. doi: 10.1016/j.phrs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 210.Zhang J., Ding Y., Wang Z., Kong Y., Gao R., Chen G. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med. Gas Res. 2017;7:113–119. doi: 10.4103/2045-9912.208517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yin Y., Sun G., Li E., Kiselyov K., Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res. Rev. 2017;34:3–14. doi: 10.1016/j.arr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wu J., Lipinski M.M. Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells. 2019;8:693. doi: 10.3390/cells8070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sarkar C., Zhao Z., Aungst S., Sabirzhanov B., Faden A.I., Lipinski M.M. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10:2208–2222. doi: 10.4161/15548627.2014.981787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang J., Shi C., Wang H., Gao C., Chang P., Chen X., Shan H., Zhang M., Tao L. Hydrogen sulfide protects against cell damage through modulation of PI3K/Akt/Nrf2 signaling. Int. J. Biochem. Cell Biol. 2019;117:105636. doi: 10.1016/j.biocel.2019.105636. [DOI] [PubMed] [Google Scholar]
- 216.Zhao G., Qi L., Wang Y., Li X., Li Q., Tang X., Wang X., Wu C. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol. Environ. Saf. 2021;222:112529. doi: 10.1016/j.ecoenv.2021.112529. [DOI] [PubMed] [Google Scholar]
- 217.Luo L.-F., Qin L.-Y., Wang J.-X., Guan P., Wang N., Ji E.-S. Astragaloside IV Attenuates the Myocardial Injury Caused by Adriamycin by Inhibiting Autophagy. Front. Pharmacol. 2021;12:669782. doi: 10.3389/fphar.2021.669782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li L., Jiang H., Li Y., Guo Y. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J. Biomed. Sci. 2015;22:50. doi: 10.1186/s12929-015-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhou J., Jin Y., Lei Y., Liu T., Wan Z., Meng H., Wang H. Ferroptosis Is Regulated by Mitochondria in Neurodegenerative Diseases. Neurodegener. Dis. 2020;20:20–34. doi: 10.1159/000510083. [DOI] [PubMed] [Google Scholar]
- 221.Cheng H., Wang P., Wang N., Dong W., Chen Z., Wu M., Wang Z., Yu Z., Guan D., Wang L., et al. Neuroprotection of NRF2 against Ferroptosis after Traumatic Brain Injury in Mice. Antioxidants. 2023;12:731. doi: 10.3390/antiox12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jin R., Yang R., Cui C., Zhang H., Cai J., Geng B., Chen Z. Ferroptosis due to Cystathionine γ Lyase/Hydrogen Sulfide Downregulation under High Hydrostatic Pressure Exacerbates VSMC Dysfunction. Front. Cell Dev. Biol. 2022;10:829316. doi: 10.3389/fcell.2022.829316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yu M., Wang W., Dang J., Liu B., Xu J., Li J., Liu Y., He L., Ying Y., Cai J., et al. Hydrogen sulfide protects retinal pigment epithelium cells against ferroptosis through the AMPK- and p62-dependent non-canonical NRF2-KEAP1 pathway. Exp. Cell Res. 2023;422:113436. doi: 10.1016/j.yexcr.2022.113436. [DOI] [PubMed] [Google Scholar]
- 224.Yu Y., Li X., Wu X., Li X., Wei J., Chen X., Sun Z., Zhang Q. Sodium hydrosulfide inhibits hemin-induced ferroptosis and lipid peroxidation in BV2 cells via the CBS/H2S system. Cell. Signal. 2023;104:110594. doi: 10.1016/j.cellsig.2023.110594. [DOI] [PubMed] [Google Scholar]
- 225.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hu X., Chen H., Xu H., Wu Y., Wu C., Jia C., Li Y., Sheng S., Xu C., Xu H., et al. Role of Pyroptosis in Traumatic Brain and Spinal Cord Injuries. Int. J. Biol. Sci. 2020;16:2042–2050. doi: 10.7150/ijbs.45467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang J., Li S., Yang Z., Liu C., Chen X., Zhang Y., Zhang F., Shi H., Chen X., Tao L., et al. Implantation of injectable SF hydrogel with sustained hydrogen sulfide delivery reduces neuronal pyroptosis and enhances functional recovery after severe intracerebral hemorrhage. Biomater. Adv. 2022;135:212743. doi: 10.1016/j.bioadv.2022.212743. [DOI] [PubMed] [Google Scholar]
- 228.Duan H., Li L., Shen S., Ma Y., Yin X., Liu Z., Yuan C., Wang Y., Zhang J. Hydrogen Sulfide Reduces Cognitive Impairment in Rats After Subarachnoid Hemorrhage by Ameliorating Neuroinflammation Mediated by the TLR4/NF-κB Pathway in Microglia. Front. Cell. Neurosci. 2020;14:210. doi: 10.3389/fncel.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chu Q.-J., He L., Zhang W., Liu C.-L., Ai Y.-Q., Zhang Q. Hydrogen sulfide attenuates surgical trauma-induced inflammatory response and cognitive deficits in mice. J. Surg. Res. 2013;183:330–336. doi: 10.1016/j.jss.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 230.Yin L., Gao S., Li C. Exogenous hydrogen sulfide alleviates surgery-induced neuroinflammatory cognitive impairment in adult mice by inhibiting NO signaling. BMC Anesthesiol. 2020;20:12. doi: 10.1186/s12871-019-0927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bas-Orth C., Tan Y.-W., Lau D., Bading H. Synaptic Activity Drives a Genomic Program That Promotes a Neuronal Warburg Effect. J. Biol. Chem. 2017;292:5183–5194. doi: 10.1074/jbc.M116.761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen S.-M., Li M., Xie J., Li S., Xiang S.-S., Liu H.-Y., Chen Z., Zhang P., Kuang X., Tang X.-Q. Hydrogen sulfide attenuates postoperative cognitive dysfunction through promoting the pathway of Warburg effect-synaptic plasticity in hippocampus. Toxicol. Appl. Pharmacol. 2020;409:115286. doi: 10.1016/j.taap.2020.115286. [DOI] [PubMed] [Google Scholar]
- 233.Mostafa D.K., El Azhary N.M., Nasra R.A. The hydrogen sulfide releasing compounds ATB-346 and diallyl trisulfide attenuate streptozotocin-induced cognitive impairment, neuroinflammation, and oxidative stress in rats: Involvement of asymmetric dimethylarginine. Can. J. Physiol. Pharmacol. 2016;94:699–708. doi: 10.1139/cjpp-2015-0316. [DOI] [PubMed] [Google Scholar]
- 234.Jenkins P.O., Mehta M.A., Sharp D.J. Catecholamines and cognition after traumatic brain injury. Brain. 2016;139:2345–2371. doi: 10.1093/brain/aww128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kumar M., Modi M., Sandhir R. Hydrogen sulfide attenuates homocysteine-induced cognitive deficits and neurochemical alterations by improving endogenous hydrogen sulfide levels. BioFactors. 2017;43:434–450. doi: 10.1002/biof.1354. [DOI] [PubMed] [Google Scholar]
- 236.Jin X., Chen D., Wu F., Zhang L., Huang Y., Lin Z., Wang X., Wang R., Xu L., Chen Y. Hydrogen Sulfide Protects Against Ammonia-Induced Neurotoxicity Through Activation of Nrf2/ARE Signaling in Astrocytic Model of Hepatic Encephalopathy. Front. Cell. Neurosci. 2020;14:573422. doi: 10.3389/fncel.2020.573422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cardoso G.M.F., Pletsch J.T., Parmeggiani B., Grings M., Glanzel N.M., Bobermin L.D., Amaral A.U., Wajner M., Leipnitz G. Bioenergetics dysfunction, mitochondrial permeability transition pore opening and lipid peroxidation induced by hydrogen sulfide as relevant pathomechanisms underlying the neurological dysfunction characteristic of ethylmalonic encephalopathy. Biochim. Biophys. Acta–Mol. Basis Dis. 2017;1863:2192–2201. doi: 10.1016/j.bbadis.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 238.Li X., Yu P., Yu Y., Xu T., Liu J., Cheng Y., Yang X., Cui X., Yin C., Liu Y. Hydrogen sulfide ameliorates high glucose-induced pro-inflammation factors in HT-22 cells: Involvement of SIRT1-mTOR/NF-κB signaling pathway. Int. Immunopharmacol. 2021;95:107545. doi: 10.1016/j.intimp.2021.107545. [DOI] [PubMed] [Google Scholar]
- 239.Kwon K.W., Nam Y., Choi W.S., Kim T.W., Kim G.M., Sohn U.D. Hepatoprotective effect of sodium hydrosulfide on hepatic encephalopathy in rats. Korean J. Physiol. Pharmacol. 2019;23:263–270. doi: 10.4196/kjpp.2019.23.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen W.-L., Xie B., Zhang C., Xu K.-L., Niu Y.-Y., Tang X.-Q., Zhang P., Zou W., Hu B., Tian Y. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in behavioral models of depression and anxiety. Behav. Pharmacol. 2013;24:590–597. doi: 10.1097/FBP.0b013e3283654258. [DOI] [PubMed] [Google Scholar]
- 241.Kang X., Jiang L., Lan F., Tang Y.-Y., Zhang P., Zou W., Chen Y.-J., Tang X.-Q. Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res. Bull. 2021;177:194–202. doi: 10.1016/j.brainresbull.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 242.Liu S.-Y., Li D., Zeng H.-Y., Kan L.-Y., Zou W., Zhang P., Gu H.-F., Tang X.-Q. Hydrogen Sulfide Inhibits Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior by Upregulation of Sirt-1: Involvement in Suppression of Hippocampal Endoplasmic Reticulum Stress. Int. J. Neuropsychopharmacol. 2017;20:867–876. doi: 10.1093/ijnp/pyx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang Y., Wang S., Xin Y., Zhang J., Wang S., Yang Z., Liu C. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021;278:119551. doi: 10.1016/j.lfs.2021.119551. [DOI] [PubMed] [Google Scholar]
- 244.Batallé G., Cabarga L., Pol O. The Inhibitory Effects of Slow-Releasing Hydrogen Sulfide Donors in the Mechanical Allodynia, Grip Strength Deficits, and Depressive-Like Behaviors Associated with Chronic Osteoarthritis Pain. Antioxidants. 2019;9:31. doi: 10.3390/antiox9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tian Q., Tang H.-L., Tang Y.-Y., Zhang P., Kang X., Zou W., Tang X.-Q. Hydrogen Sulfide Attenuates the Cognitive Dysfunction in Parkinson’s Disease Rats via Promoting Hippocampal Microglia M2 Polarization by Enhancement of Hippocampal Warburg Effect. Oxid. Med. Cell. Longev. 2022;2022:2792348. doi: 10.1155/2022/2792348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hou X.-Y., Hu Z.-L., Zhang D.-Z., Lu W., Zhou J., Wu P.-F., Guan X.-L., Han Q.-Q., Deng S.-L., Zhang H., et al. Rapid Antidepressant Effect of Hydrogen Sulfide: Evidence for Activation of mTORC1-TrkB-AMPA Receptor Pathways. Antioxid. Redox Signal. 2017;27:472–488. doi: 10.1089/ars.2016.6737. [DOI] [PubMed] [Google Scholar]
- 247.Jiang W., Tang Y.-Y., Zhu W.-W., Li C., Zhang P., Li R.-Q., Chen Y.-J., Zou W., Tang X.-Q. PI3K/AKT pathway mediates the antidepressant- and anxiolytic-like roles of hydrogen sulfide in streptozotocin-induced diabetic rats via promoting hippocampal neurogenesis. Neurotoxicology. 2021;85:201–208. doi: 10.1016/j.neuro.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 248.Luoa X., Fanga T.Y., Yanga X., Panga Y.P., Rena J., Newellc K.A., Yubc Y., Huangbc X.-F., Liuac Y. S-adenosyl methionine improves depression-like behaviours and synaptic markers by elevating the expression of endogenous hydrogen sulfide in the hippocampus. Neuropsychiatry. 2018;8:495–504. doi: 10.4172/Neuropsychiatry.1000371. [DOI] [Google Scholar]
- 249.Tan H., Zou W., Jiang J., Tian Y., Xiao Z., Bi L., Zeng H., Tang X. Disturbance of hippocampal H2S generation contributes to CUMS-induced depression-like behavior: Involvement in endoplasmic reticulum stress of hippocampus. Acta Biochim. Biophys. Sin. 2015;47:285–291. doi: 10.1093/abbs/gmv009. [DOI] [PubMed] [Google Scholar]
- 250.Cho C., Zeigler M., Mizuno S., Morrison R.S., Totah R.A., Barker-Haliski M. Reductions in Hydrogen Sulfide and Changes in Mitochondrial Quality Control Proteins Are Evident in the Early Phases of the Corneally Kindled Mouse Model of Epilepsy. Int. J. Mol. Sci. 2022;23:1434. doi: 10.3390/ijms23031434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhu Z., He Y., Liu Z., Zhang W., Kang Q., Lin Y., Qiu J., Zhang Y., Xu P., Zhu X. A hydrogen sulfide donor suppresses pentylenetetrazol-induced seizures in rats via PKC signaling. Eur. J. Pharmacol. 2021;898:173959. doi: 10.1016/j.ejphar.2021.173959. [DOI] [PubMed] [Google Scholar]
- 252.Han Y., Qin J., Chang X., Yang Z., Bu D., Du J. Modulating effect of hydrogen sulfide on gamma-aminobutyric acid B receptor in recurrent febrile seizures in rats. Neurosci. Res. 2005;53:216–219. doi: 10.1016/j.neures.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 253.Zhuang F., Zhou X., Li H., Yang X., Dong Z., Zhou W., Chen J. Hydrogen Sulfide Promotes Learning and Memory and Suppresses Proinflammatory Cytokines in Repetitive Febrile Seizures. Neuroimmunomodulation. 2016;23:271–277. doi: 10.1159/000449504. [DOI] [PubMed] [Google Scholar]
- 254.Sutulovic N., Rasic-Markovic A., Grubac Ž., Duric E., Hrncic D. The effects of hydrogen sulfide synthesis inhibition in lindane-induced seizures in rats: A behavioral and EEG study. Arch. Biol. Sci. 2020;72:457–463. doi: 10.2298/ABS200802039S. [DOI] [Google Scholar]
- 255.Luo Y., Wu P.-F., Zhou J., Xiao W., He J.-G., Guan X.-L., Zhang J.-T., Hu Z.-L., Wang F., Chen J.-G. Aggravation of Seizure-like Events by Hydrogen Sulfide: Involvement of Multiple Targets that Control Neuronal Excitability. CNS Neurosci. Ther. 2014;20:411–419. doi: 10.1111/cns.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li J., Zhou Y., Song L., Yang S., Wang Q., Zhou Y., Zhang X.-B., Qing Z., Yang R. Brain-targeted Near-Infrared Nanobeacon for In Situ Monitoring H2S Fluctuation during Epileptic Seizures. Anal. Chem. 2022;94:15085–15092. doi: 10.1021/acs.analchem.2c03254. [DOI] [PubMed] [Google Scholar]
- 257.Liu J., Pourcyrous M., Fedinec A.L., Parfenova H. Cerebroprotective actions of hydrogen sulfide in the epileptic brain in newborn pigs. Pediatr. Res. 2023 doi: 10.1038/s41390-023-02486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Batallé G., Bai X., Pouso-Vázquez E., Roch G., Rodríguez L., Pol O. The Recovery of Cognitive and Affective Deficiencies Linked with Chronic Osteoarthritis Pain and Implicated Pathways by Slow-Releasing Hydrogen Sulfide Treatment. Antioxidants. 2021;10:1632. doi: 10.3390/antiox10101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Batallé G., Bai X., Pol O. The Interaction between Carbon Monoxide and Hydrogen Sulfide during Chronic Joint Pain in Young Female Mice. Antioxidants. 2022;11:1271. doi: 10.3390/antiox11071271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cabarga L., Batallé G., Pol O. Treatment with slow-releasing hydrogen sulfide donors inhibits the nociceptive and depressive-like behaviours accompanying chronic neuropathic pain: Endogenous antioxidant system activation. J. Psychopharmacol. 2020;34:737–749. doi: 10.1177/0269881120913154. [DOI] [PubMed] [Google Scholar]
- 261.Chen H., Xie K., Chen Y., Wang Y., Wang Y., Lian N., Zhang K., Yu Y. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int. Immunopharmacol. 2019;75:105746. doi: 10.1016/j.intimp.2019.105746. [DOI] [PubMed] [Google Scholar]
- 262.Dichiara M., Artacho-Cordón A., Turnaturi R., Santos-Caballero M., González-Cano R., Pasquinucci L., Barbaraci C., Rodríguez-Gómez I., Gómez-Guzmán M., Marrazzo A., et al. Dual Sigma-1 receptor antagonists and hydrogen sulfide-releasing compounds for pain treatment: Design, synthesis, and pharmacological evaluation. Eur. J. Med. Chem. 2022;230:114091. doi: 10.1016/j.ejmech.2021.114091. [DOI] [PubMed] [Google Scholar]
- 263.Vandini E., Ottani A., Zaffe D., Calevro A., Canalini F., Cavallini G.M., Rossi R., Guarini S., Giuliani D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology. 2019;103:50–60. doi: 10.1159/000494113. [DOI] [PubMed] [Google Scholar]
- 264.Cheng X., Gu J., Pang Y., Liu J., Xu T., Li X., Hua Y., Newell K.A., Huang X.-F., Yu Y., et al. Tacrine–Hydrogen Sulfide Donor Hybrid Ameliorates Cognitive Impairment in the Aluminum Chloride Mouse Model of Alzheimer’s Disease. ACS Chem. Neurosci. 2019;10:3500–3509. doi: 10.1021/acschemneuro.9b00120. [DOI] [PubMed] [Google Scholar]
- 265.Aboulhoda B.E., Rashed L.A., Ahmed H., Obaya E.M.M., Ibrahim W., Alkafass M.A.L., Abd El-Aal S.A., ShamsEldeen A.M. Hydrogen sulfide and mesenchymal stem cells-extracted microvesicles attenuate LPS-induced Alzheimer’s disease. J. Cell. Physiol. 2021;236:5994–6010. doi: 10.1002/jcp.30283. [DOI] [PubMed] [Google Scholar]
- 266.Giuliani D., Ottani A., Zaffe D., Galantucci M., Strinati F., Lodi R., Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 267.Kamat P.K., Kyles P., Kalani A., Tyagi N. Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer’s Disease-Like Pathology, Blood–Brain Barrier Disruption, and Synaptic Disorder. Mol. Neurobiol. 2016;53:2451–2467. doi: 10.1007/s12035-015-9212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xuan A., Long D., Li J., Ji W., Zhang M., Hong L., Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer’s disease. J. Neuroinflamm. 2012;9:687. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Aschner M., Skalny A.V., Ke T., da Rocha J.B., Paoliello M.M., Santamaria A., Bornhorst J., Rongzhu L., Svistunov A.A., Djordevic A.B., et al. Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants. Curr. Neuropharmacol. 2022;20:1908–1924. doi: 10.2174/1570159X20666220302101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cao X., Cao L., Ding L., Bian J. A New Hope for a Devastating Disease: Hydrogen Sulfide in Parkinson’s Disease. Mol. Neurobiol. 2017;55:3789–3799. doi: 10.1007/s12035-017-0617-0. [DOI] [PubMed] [Google Scholar]
- 271.Xue X., Bian J.-S. Neuroprotective Effects of Hydrogen Sulfide in Parkinson’s Disease Animal Models. Methods Enzymol. 2015;554:169–186. doi: 10.1016/bs.mie.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 272.Xie L., Hu L.-F., Teo X.Q., Tiong C.X., Tazzari V., Sparatore A., del Soldato P., Dawe G.S., Bian J.-S. Therapeutic Effect of Hydrogen Sulfide-Releasing L-Dopa Derivative ACS84 on 6-OHDA-Induced Parkinson’s Disease Rat Model. PLoS ONE. 2013;8:e60200. doi: 10.1371/journal.pone.0060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee M., Tazzari V., Giustarini D., Rossi R., Sparatore A., Del Soldato P., McGeer E., McGeer P.L. Effects of Hydrogen Sulfide-releasing l-DOPA Derivatives on Glial Activation. J. Biol. Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sarukhani M., Haghdoost-Yazdi H., Sarbazi Golezari A., Babayan-Tazehkand A., Dargahi T., Rastgoo N. Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: Behavioral, histological and biochemical studies. Neurol. Res. 2018;40:525–533. doi: 10.1080/01616412.2017.1390903. [DOI] [PubMed] [Google Scholar]
- 275.Sun Y., Li D., Su Y., Zhao H., Pang W., Zhao W., Wu S. Protective effect of hydrogen sulfide is mediated by negative regulation of epigenetic histone acetylation in Parkinson’s disease. Arch. Med. Sci. 2020;19 doi: 10.5114/aoms.2020.93121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wang M., Zhu J., Pan Y., Dong J., Zhang L., Zhang X., Zhang L. Hydrogen sulfide functions as a neuromodulator to regulate striatal neurotransmission in a mouse model of Parkinson’s disease. J. Neurosci. Res. 2015;93:487–494. doi: 10.1002/jnr.23504. [DOI] [PubMed] [Google Scholar]
- 277.Hacioglu G., Cirrik S., Tezcan Yavuz B., Tomruk C., Keskin A., Uzunoglu E., Takir S. The BDNF-TrkB signaling pathway is partially involved in the neuroprotective effects of hydrogen sulfide in Parkinson’s disease. Eur. J. Pharmacol. 2023;944:175595. doi: 10.1016/j.ejphar.2023.175595. [DOI] [PubMed] [Google Scholar]
- 278.Liu Y., Liao S., Quan H., Lin Y., Li J., Yang Q. Involvement of microRNA-135a-5p in the Protective Effects of Hydrogen Sulfide Against Parkinson’s Disease. Cell. Physiol. Biochem. 2016;40:18–26. doi: 10.1159/000452521. [DOI] [PubMed] [Google Scholar]
- 279.Huang Y., Omorou M., Gao M., Mu C., Xu W., Xu H. Hydrogen sulfide and its donors for the treatment of cerebral ischaemia-reperfusion injury: A comprehensive review. Biomed. Pharmacother. 2023;161:114506. doi: 10.1016/j.biopha.2023.114506. [DOI] [PubMed] [Google Scholar]
- 280.Liu J., Mesfin F.M., Hunter C.E., Olson K.R., Shelley W.C., Brokaw J.P., Manohar K., Markel T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants. 2022;11:1788. doi: 10.3390/antiox11091788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shayea A.M.F., Mousa A.M.A., Renno W.M., Nadar M.S., Qabazard B., Yousif M.H.M. Chronic Treatment With Hydrogen Sulfide Donor GYY4137 Mitigates Microglial and Astrocyte Activation in the Spinal Cord of Streptozotocin-Induced Diabetic Rats. J. Neuropathol. Exp. Neurol. 2020;79:1320–1343. doi: 10.1093/jnen/nlaa127. [DOI] [PubMed] [Google Scholar]
- 282.Liu H., Anders F., Thanos S., Mann C., Liu A., Grus F.H., Pfeiffer N., Prokosch-Willing V. Hydrogen Sulfide Protects Retinal Ganglion Cells Against Glaucomatous Injury In Vitro and In Vivo. Investig. Opthalmology Vis. Sci. 2017;58:5129–5141. doi: 10.1167/iovs.17-22200. [DOI] [PubMed] [Google Scholar]
- 283.Lazarević M., Battaglia G., Jevtić B., Djedovic N., Bruno V., Cavalli E., Miljković Đ., Nicoletti F., Momčilović M., Fagone P. Upregulation of Tolerogenic Pathways by the Hydrogen Sulfide Donor GYY4137 and Impaired Expression of H2S-Producing Enzymes in Multiple Sclerosis. Antioxidants. 2020;9:608. doi: 10.3390/antiox9070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Whiteman M., Perry A., Zhou Z., Bucci M., Papapetropoulos A., Cirino G., Wood M.E. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Springer; Berlin/Heidelberg, Germany: 2015. Phosphinodithioate and Phosphoramidodithioate Hydrogen Sulfide Donors; pp. 337–363. [DOI] [PubMed] [Google Scholar]
- 285.Huang C.W., Feng W., Peh M.T., Peh K., Dymock B.W., Moore P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016;113:533–546. doi: 10.1016/j.phrs.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 286.Lee M., McGeer E., Kodela R., Kashfi K., McGeer P.L. NOSH-aspirin (NBS-1120), a novel nitric oxide and hydrogen sulfide releasing hybrid, attenuates neuroinflammation induced by microglial and astrocytic activation: A new candidate for treatment of neurodegenerative disorders. Glia. 2013;61:1724–1734. doi: 10.1002/glia.22553. [DOI] [PubMed] [Google Scholar]
- 287.Asimakopoulou A., Panopoulos P., Chasapis C.T., Coletta C., Zhou Z., Cirino G., Giannis A., Szabo C., Spyroulias G.A., Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br. J. Pharmacol. 2013;169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Casili G., Randi E., Panagaki T., Zuhra K., Petrosino M., Szabo C. Inhibition of the 3-mercaptopyruvate sulfurtransferase—Hydrogen sulfide system promotes cellular lipid accumulation. GeroScience. 2022;44:2271–2289. doi: 10.1007/s11357-022-00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Petrosino M., Zuhra K., Kopec J., Hutchin A., Szabo C., Majtan T. H2S biogenesis by cystathionine beta-synthase: Mechanism of inhibition by aminooxyacetic acid and unexpected role of serine. Cell. Mol. Life Sci. 2022;79:438. doi: 10.1007/s00018-022-04479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.