Abstract
Drug development for Alzheimer’s disease, the leading cause of dementia, has been a long-standing challenge. Saponins, which are steroid or triterpenoid glycosides with various pharmacological activities, have displayed therapeutic potential in treating Alzheimer’s disease. In a comprehensive review of the literature from May 2007 to May 2023, we identified 63 references involving 40 different types of saponins that have been studied for their effects on Alzheimer’s disease. These studies suggest that saponins have the potential to ameliorate Alzheimer’s disease by reducing amyloid beta peptide deposition, inhibiting tau phosphorylation, modulating oxidative stress, reducing inflammation, and antiapoptosis. Most intriguingly, ginsenoside Rg1 and pseudoginsenoside-F11 possess these important pharmacological properties and show the best promise for the treatment of Alzheimer’s disease. This review provides a summary and classification of common saponins that have been studied for their therapeutic potential in Alzheimer’s disease, showcasing their underlying mechanisms. This highlights the promising potential of saponins for the treatment of Alzheimer’s disease.
Keywords: Alzheimer’s disease, saponin, amyloid beta, inflammation, oxidative stress, apoptosis
1. Introduction
Alzheimer’s disease (AD), the leading cause of dementia, is a progressive neurodegenerative disease [1] that is clinically characterized by memory loss, cognitive impairment, and behavioral disturbances [2]. Since its discovery in 1906, AD has emerged as one of the most costly, fatal, and burdensome diseases of this century [3]. The pathogenesis of AD involves various biological processes [4] involving the abnormal deposition of amyloid beta peptide (Aβ) [5], the accumulation of neurofibrillary tangles (NFTs) [6], neuroinflammation [7], neuronal apoptosis [8], neurotransmitter abnormities [9], and oxidative stress [10]. Despite considerable efforts, drug discovery for the treatment of AD has been slow, with only acetylcholinesterase (AChE)/butyrylcholinesterase (BChE) inhibitors [11] such as galantamine, donepezil, tacrine, and rivastigmine currently available as therapies [12]. However, these treatments only delay the onset of symptoms and cannot halt disease progression and are often associated with significant side effects [13]. Therefore, the development of new therapeutic drugs is urgently needed. Saponins, a type of natural compound, have been extensively studied for their various pharmacological properties [14]. Of particular interest is their potential to enhance learning and memory in individuals with AD [15].
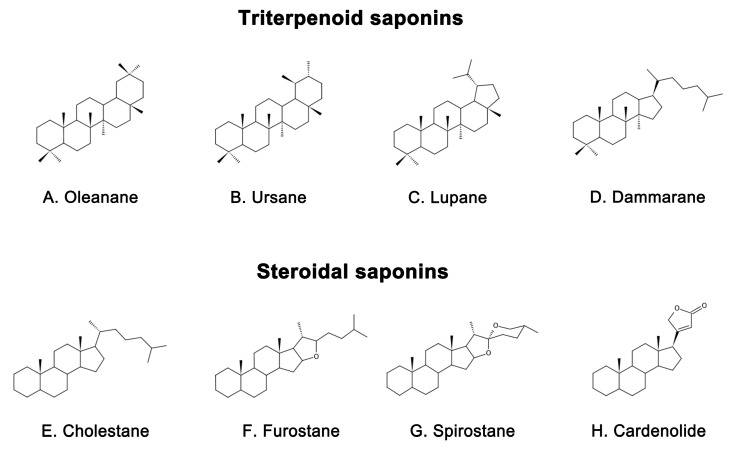
Saponins are naturally occurring compounds that are widely distributed in various plants [16], and they can be divided into two major groups based on their chemical structure: triterpenoid saponins and steroidal saponins [17]. Triterpenoid saponins are further subdivided into tetracyclic triterpenes and pentacyclic triterpenes and are mainly found in plants such as Pentaaceae, Leguminosae, Poria, and Platycodonaceae [18]. The main saponin skeletons of the triterpenoid saponins include dammarane, oleanane, ursane, and lupane (Figure 1A–D) [19]. Steroidal saponins, on the other hand, are mainly found in plants such as Dioscoreaceae, Liliaceae, and Scrophulariaceae [20]. The main saponin metaskeletons of steroidal saponins include spirostane, furostane, cholestane, and cardenolide (Figure 1E–H) [21]. Saponins possess multiple bioactivities, such as reduction of amyloid beta (Aβ) deposition [22], inhibition of tau protein phosphorylation [23], antioxidation [24], antiapoptosis [25], and anti-inflammation [26]. These properties make saponins promising therapeutic candidates for AD and other neurological disorders [27]. Meanwhile, the diversity of saponins found in different plants [28] makes them a valuable source of potential drugs for the treatment of AD. However, there is a current lack of comprehensive and systematic review in this field. To address this gap, we thoroughly searched the relevant literature in the major databases, including PubMed and Web of Science, using keywords “Alzheimer’s disease” or “AD” and “saponin”. By browsing all the relevant studies from May 2007 to May 2023, excluding review articles, 63 references were selected, involving 40 saponins extracted from different herbs. The selected references are known to be effective in the treatment of AD, present clear mechanisms, authentic and reliable information, and contain the latest findings in the field. The studies highlighted that saponins have exhibited various beneficial effects such as reducing Aβ levels, reducing NETs, exerting antioxidative, antiapoptotic, anti-inflammatory, and increased neurotransmitter-enhancing effects [25]. The underlying mechanisms behind these effects include reducing amyloid precursor protein (APP) production [29], improving tau protein phosphorylation [30], and reducing reactive oxygen species (ROS) generation [31], inhibiting the apoptotic and inflammatory signaling pathways [31,32], and increasing neurotransmitter expression [33]. This comprehensive review sheds light on the potential saponins as therapeutic agents for AD and related neurological disorders.
Figure 1.
Representative saponin skeleton structure of triterpenoid saponins include (A) Oleanane, (B) Ursane, (C) Lupane, (D) Dammarane and steroidal saponins include (E) Cholestane, (F) Furostane, (G) Spirostane, (H) Cardenolide.
2. Mechanism of Saponins in Treating Alzheimer’s Disease
2.1. Inhibition of Aβ Deposition and Neurotoxicity
Aβ is a peptide that is a major component of the senile plaques found in the brains of people with AD [34]. Its accumulation is highly neurotoxic and is considered a hallmark of AD [35], which can result in impaired cognitive function, including spatial memory [36]. Aβ is generated from the APP and is typically enclosed by microglia and dystrophic synapses that aggregate around neurons [37,38]. APP is a transmembrane protein located in the synapse of neurons, which can be cleaved by both amyloid and nonamyloid pathways [39]. In the nonamyloid pathway, APP is sequentially cleaved by α-secretase (mainly ADAM10) and γ-secretase [40], resulting in P3 peptide (P3), C83 carboxy-terminal fragment (C83), APP intracellular domain (AICD), and soluble amyloid precursor protein-α (sAPPα) with beneficial neurotrophic effects [41]. Conversely, in the amyloid pathway, APP is first cleaved by β-secretase and sAPPβ is secreted [42]. Subsequently, γ-secretase cleaves the C-terminal fragment (C99) of the residual APP and eventually leads to the release of peptides of different lengths [43]. The most prevalent of them are Aβ1-40 and Aβ1-42 [44], which are neurotoxic fragments capable of oligomerization, aggregation, and subsequent plaque formation [45]. In vivo, Aβ is degraded by a variety of proteases, most notably insulin-degrading enzymes (IDE) and neprilysin (NEP) [46]. However, the production or activity of these clearance enzymes may decrease with age, leading to a failure to clear Aβ in a timely manner [47]. This phenomenon has been linked to the reduced activity of the peroxisome proliferator-activated receptor γ (PPARγ) [48], which is a transcription factor that regulates the expression of IDE and Bace1, reduces Aβ production [49], promotes Aβ clearance [50], and exerts neuroprotective effects [51]. An imbalance between Aβ production and clearance is responsible for the accumulation of Aβ in the brain [52]. In order to develop novel treatments for AD, it may be beneficial to inhibit Aβ production, enhance its clearance, or directly combat its neurotoxicity [53].
Natural saponins have shown potential in affecting Aβ metabolism through different pathways [54]. For example, some natural saponins are able to inhibit the formation of Aβ by reducing APP production. Ginsenoside Rg1, a tetracyclic triterpenoid saponin extracted from ginseng [55], has been shown to reduce Aβ deposition in APP/presenilin 1(PS1) double transgenic AD model mice by lowering APP levels [56]. Pharmacokinetic studies have also shown that ginsenoside Rg1 can cross the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), which provides theoretical support for its ability to improve learning and memory abilities [57]. Xanthoceraside, another tetracyclic triterpenoid saponin, extracted from the bark of the Xanthoceras sorbifolia Bunge commonly used in traditional Chinese medicine (TCM) to treat rheumatism [58], has been shown to reduce APP protein levels and Aβ deposition in the cerebral cortex and hippocampus, thereby ameliorating cognitive function dysfunction in an AD mouse model induced by Aβ intracerebroventricular (ICV) injection [59]. In addition, ginsenoside Rh2, another ginseng derivative used in TCM, has been reported to regulate APP expression by reducing cholesterol and lipid raft levels. This ultimately led to an increase in sAPPα levels and a decrease in Aβ concentrations [60].
Most saponins have been shown to inhibit Aβ deposition by modulating the activity of APP-processing enzymes, such as ginsenoside Rg1 [61], RAPO-1-3 [62], onjisaponin B [62], pseudoginsenoside-F11 (PF11) [63], theasaponin E1 [64], anginsenoside (20S)-Rg3 [65], and ginsenoside C-K (CK) [66]. Ginsenoside Rg1 reduces the γ-secretase responsible for Aβ production by attenuating the Aβ-mediated inhibition of cAMP response element-binding protein (CREB) phosphorylation and protein kinase A (PKA) activity [61]. It also upregulates ADAM10 while reducing BACE1 in Wistar rat models of AD induced by ovariectomy (OVX) and D-galactose (D-gal) [67]. In sAPPα-transfected HT22 cells and neuroblastoma (SH-SY5Y) cells, ginsenoside Rg1 has been found to increase the levels of sAPPα and estrogen receptor (ER) α, elevate α-secretase activity, and decrease extracellular release of Aβ. Further studies have shown that these effects are mediated by the upregulation of phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) and phosphorylated protein kinase B (pAkt) [68] but can be reversed by ER antagonists and potentially attenuated by inhibitors of protein kinase C (PKC), MAPK, and phosphatidyl 3-kinase (PI3K) [68]. Yuan Zhi (RAPO) is a TCM formulation that is commonly used to treat dementia due to its neuroprotective effects [69]. Its active ingredient, RAPO-1-3, and onjisaponin B, an acyl saponin with a similar constituent to RAPO-1-3, have been found to reduce Aβ production by promoting the degradation of APP protein through interference with the PS1/BACE1 interaction [62]. Another compound, PF11, which is a pentacyclic triterpene abundant in ginseng [70], has also demonstrated efficacy in inhibiting APP amyloidogenic processing. By reducing the expression of BACE1, PF11 reduced Aβ deposition and ameliorated cognitive impairment and synaptic dysfunction in SAMP8 mice [63]. Theasaponin E1, a pentacyclic triterpene extracted from green tea seeds [71], has been shown to reduce the production of Aβ by increasing the activity of NEP and ADAM10 in APP (SweAPP N2a) cells while inhibiting the expression of BACE1, APP, and PS1 [64]. Furthermore, CK, which is produced through the degradation of protopanaxadiol saponins by the gut microbiota [72], has been studied for its neuroprotective properties. Currently, it is primarily obtained by glycosyl hydrolysis of proto-ginsenoside diol-type saponins [73]. In scopolamine-induced ICR mice, CK was found to reduce the expression of BACE1 and PS1, increase IDE activity, reduce Aβ expression, and improve memory function [66]. In addition, ginsenoside (20S)-Rg3, a component of heat-processed ginseng, has been found to reduce Aβ levels by increasing phosphatidylinositol 4-kinase IIα(PI4KIIα) activity and ultimately decrease the expression of γ-secretase by decreasing the association of PS1 fragments and lipid rafts in cultured primary neurons and in the brains of an AD mouse model [65].
Aside from inhibiting Aβ production, certain saponins have been found to promote the clearance of Aβ. For example, minor ginsenoside F1, a trace ginsenoside derived from Panax ginseng [74], has been shown to effectively reduce Aβ plaques and alleviate cognitive impairment in APP/PS1 mice by enhancing the expression of pCREB [75]. Similarly, Bacopaside I (BS-I), a tetracyclic triterpene and standardized extract of Bacopa monnieri [76], has been shown to be neuroprotective and improve cognitive function [77]. The aglycones of Bacopa monnieri and its derivatives have good intestinal absorption and BBB permeability [78]. Recent studies suggest that BS-I induces sufficient innate immune stimulation and phagocytosis to promote amyloid clearance, thereby reducing amyloid plaque burden in APP/PS1 mice [79]. In addition, Aβ deposition in the brain is linked to the dysfunction of the endosomal–lysosomal system dysfunction [80], which is regulated by the transcription factor EB (TFEB) [81]. PF11 has been observed to increase Aβ clearance by promoting the mammalian target of rapamycin (mTOR)-dependent TFEB-mediated lysosome biogenesis and alleviating endosomal–lysosomal system dysfunction through the conversion of Rab5 to Rab7 [82].
It is interesting to note that some natural saponins do not directly target key enzymes involved in Aβ metabolism, but instead act as natural agonists of peroxisome proliferator-activated receptor gamma (PPARγ) [83]. For example, in N2a/APP695 cells, ginsenoside Re, a major constituent of ginseng [84], has been shown to activate the PPARγ pathway, resulting in the inhibition of BACE1 at both the transcriptional and translational levels, as well as its activity. This, in turn, leads to the suppression of soluble polypeptide APPβ (sAPPβ) expression and the production of Aβ [85]. Similarly, Notoginsenoside R1 (NTR1), a tetracyclic triterpene present in the root of the popular Chinese medicine Panax ginseng [86], has been shown to inhibit the accumulation of Aβ in APP/PS1 mice and N2a-APP695sw cells, at least in part by activating the PPARγ pathway to increase IDE expression [87]. Meanwhile, it has been reported that ginsenoside Re and NTR1 exhibit improved bioavailability across the BBB when coadministered with Borneol [88]. Astragaloside IV (AS-IV), a tetracyclic triterpene from Astragalus membranaceus [89], has also been shown to be beneficial in neurodegenerative diseases with cognitive impairment [90]. Pharmacokinetic results showed that its elimination half-lives were 98.1, 67.2, and 71.8 min in male rats and 34.0, 66.9, and 131.6 min in female rats at AS-IV doses of 0.75, 1.5, and 3.0 mg/kg, respectively. Moreover, at least some of the AS-IV was able to cross the BBB [91]. AS-IV has been observed to act as a natural agonist for PPARγ, producing results comparable to those achieved by NTR1 in APP/PS1 mice and BACE1-transfected SH-SY5Y cells [92]. Jujuboside A (JuA), a triterpenoid saponin extracted from sour dates [93], has multiple biological activities including neuroprotective properties [94]. In Aβ-induced AD cellular models, JuA activates the Axl receptor tyrosine kinase (Axl)/heat shock protein 90 β (Hsp90β)/PPARγ signaling axis, which restores PPARγ levels and enhances Aβ42 clearance [95]. Finally, ginsenoside Rg1 inhibits the phosphorylation of PPARγ by downregulating the cyclin-dependent kinase (CDK5) pathway and ultimately reduces Aβ levels by affecting the expression of PPARγ target genes, including APP, BACE1, and IDE, in an AD model established using Aβ1-42-treated primary rat neurons [96].
It is noteworthy that certain saponins have been found to have neuroprotective properties, beyond their ability to modulate Aβ metabolism, through the inhibition of Aβ-induced neurotoxicity. For example, hederacolchiside-E, a triterpenoid saponin derived from the Phellodendron plant [97], has been shown to increase neuron activity [98] and prevent Aβ-induced neurotoxicity in both a scopolamine-induced rat AD model and Aβ1-42-induced human neuroblastoma SK-N-SH cells [99]. Similarly, polanoside A from the cactus polaskia chichipe backbg and chikusetsu saponin V, a pentacyclic triterpene saponin, protects SH-SY5Y cells from Aβ-induced cytotoxicity by inhibiting Aβ aggregation [100]. Notably, NTR1 has been found to prevent Aβ-induced neurotoxicity in APP/PS1 mice by increasing the membrane excitability of CA1 pyramidal neurons and reversing Aβ-induced hippocampal long-term potentiation damage [101]. In addition, akebia quinata, commonly known as the chocolate vine, is widely used in traditional medicine throughout East Asia and is also a popular food ingredient [102]. Fluorescence mapping studies have recently revealed the presence of a new pentacyclic triterpene, akequintoside F, mainly extracted from akebia quinata, which shows potential in inhibiting the formation of Aβ-42 plaques [103].
2.2. Inhibiting Aberrant Tau Protein Phosphorylation
NFTs, a well-known pathological hallmark of AD [104], are composed of excessively phosphorylated proteins linked by helical filaments known as tubulin-associated unit (tau) protein [105]. Tau protein, mainly found in neuronal axons, is a microtubule-associated protein involved in various functions such as axonal microtubule maintenance, cytoplasmic transport, and regulation of neuronal signaling [106,107]. Its optimal function depends on the low levels of normal phosphorylation [108]. However, overphosphorylation of tau protein leads to its detachment from microtubules, resulting in microtubule depolymerization, aggregation into NFTs, and ultimately neurodegeneration and cell death [109]. The correlation between NFT density and cognitive decline suggests that tau protein plays a central role in AD [110]. Furthermore, the prevalence of tau protein pathology positively correlates with cognitive impairment in AD, where a person with AD has increased levels of tau protein, hyperphosphorylated tau protein, and aggregated tau in their brain [111]. Abnormal tau protein phosphorylation plays a greater role in AD than the total tau protein [112]. As such, it is considered a critical pathogenic step in the development of NFTs and, subsequently, AD [113].
Several saponins have been found to inhibit the hyperphosphorylation of tau proteins, protect synaptic structures, and reduce neuronal damage. An octillol-type saponin, PF11, has been found to increase the activity of phosphatase-2A (PP2A) by enhancing leucine carboxyl methyltransferase-1 (LCMT-1) and improving cognitive impairment in SAMP/8 mice by inhibiting the hyperphosphorylation of tau protein at serine 396 and tyrosine 205 in the brain [63]. Streptozotocin (STZ) is a glucosamine–nitrosourea compound commonly used in AD studies to induce tau proteins’ hyperphosphorylation and cognitive impairment [114]. Tau protein phosphorylation occurs at multiple sites, usually associated with glycogen synthase kinase (GSK) 3 and cyclin-dependent kinase 5 (CDK5). PF11 was also shown to attenuate STZ-induced tau hyperphosphorylation in the brain of male Wistar rats by correcting the dysregulation of the insulin receptor substrate 1/PI3K/Akt/GSK-3β and calpain I/CDK5 signaling pathways [70]. Additionally, PF11 ameliorated STZ-induced learning and memory deficits, synaptic damage, and neuronal death [70]. Another active compound, Ginsenoside Rb1, found in ginseng [115], exhibits neuroprotective properties against different neurotoxins [116]. Research has shown that ginsenoside Rb1 counters cognitive impairment induced by aluminum oxide in ICR mice while reducing Ser396 tau phosphorylation by increasing PP2A levels and decreasing p-GSK levels [111,117]. Ginsenoside Rd, another compound found in ginseng [118], has been shown to have potential therapeutic applications in neurodegenerative diseases [119]. It has been demonstrated to reduce tau proteins’ hyperphosphorylation and neurotoxicity induced by okadaic acid (OA) by increasing PP2A activity in both rat and cortical neuronal models of AD [120].
Theasaponin E1, a vital compound found in green tea seeds, shows remarkable potential in reducing p-tau protein levels and inhibiting AD-promoting genes while activating AD-remitting genes [121]. This potential has been observed in AD models constructed from SHY5Y and glioblastoma (HTB2) cells. Notably, theasaponin E1 successfully reduced p-tau protein by inhibiting several enzymes, including GSK-3β, CDK5, c-Jun NH2-terminal kinase (JNK), MAPK, ERK1/MARK, and calmodulin-dependent protein kinase II alpha [121]. Similarly, Xanthoceraside, a triterpene saponin extracted from the shell of Xanthoceras sorbifolia Bunge, has been shown to exert neuroprotective effects by inhibiting the phosphorylation of tau protein [122]. In a study conducted on mice with AD, the administration of Xanthoceraside significantly decreased the expression of pGSK-3β and tau protein at Ser396 and Ser404, thereby effectively inhibiting the phosphorylation of tau protein [59].
2.3. Anti-Inflammatory Effect
In recent years, neuroinflammation has become recognized as a third neuropathological hallmark of AD, in addition to Aβ and NFTs [123]. Neuroinflammation is an inflammatory response to pathological insults that occur within the central nervous system (CNS), such as infection, trauma, ischemia, and toxin accumulation [124]. This response is characterized by the proliferation and activation of microglia and astrocytes in the brain [125]. Such activation is usually accompanied by the secretion of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) [126]. Microglia are innate immune cells that reside within the CNS and are thought to be important regulators in the inflammatory response within the brain [127]. In the early stages of AD, activated microglia can exert protective effects by phagocytosing and degrading Aβ [128,129]. However, when Aβ accumulates excessively, it binds various receptors on the surface of microglia such as cluster of differentiation (CD) 14, CD36 [130], Toll-like receptor (TLR) 4, and TLR2, to promote the release of inflammatory factors [131]. This response leads to neuroinflammation and further impairment of cognitive function. Similarly, astrocyte activation is also associated with Aβ [132]. Activated astrocytes produce various inflammatory mediators [133] and secrete small amounts of Aβ [134], resulting in a chronic vicious cycle [133]. These reactions can exacerbate neuroinflammation and damage neurons.
Multiple pathways are involved in microglia- and astrocyte-mediated neuroinflammation [135], one of which is the nuclear factor kappa-B (NF-κB) pathway [136]. This pathway activates the transcription of TNF-α, IL-1β, and IL-6, which exacerbates inflammation [137]. Microglial activation also depends on other signaling pathways such as the PI3K/Akt/mTOR pathway [138] which can promote the expression of the inflammatory mediators NO synthase (NOS) and cyclooxygenase-2 (Cox-2) [139,140]. Furthermore, MAPK/p38 [141], Janus kinase (JAK)/signal transducer, and activator of transcription (STAT) and other pathways also contribute to neuroinflammation [142]. Therefore, modulating inflammatory signaling pathways and inhibiting the production of proinflammatory factors may offer a promising approach to the treatment of AD.
There is mounting evidence to suggest that certain saponins can effectively inhibit neuroinflammation by blocking the NF-κB pathway. For example, theasaponin E1 has demonstrated anti-inflammatory properties in the treatment of AD by inhibiting the NF-κB pathway in a dose-dependent manner, ultimately leading to a reduction in levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α [121]. Another example is dioscin, a natural steroidal saponin derived from the rhizomes of dioscoreae nipponicae which has been found to possess a variety of pharmacological activities [143]. In a study involving H2O2-induced SH-SY5Y cells and an AD C57BL/6J mouse model established with AlCl3 and D-gal, dioscin exhibited the ability to inhibit the RAGE/NADPH oxidase 4 (NOX4) signaling axis, leading to a reduction in associated inflammatory factors, such as p-NF-κB/NF-κB, activator protein 1 (AP-1), IL-1β, IL-6, and TNF-α [144]. Additionally, Platycodigenin (PLA), a triterpenoid saponin from Platycodon grandifloras [145], has been shown to have anti-inflammatory and neuroprotective properties [146]. As a potential agonist of the PPARγ pathway, PLA promotes M2 microglial polarization by inhibiting MAPK and NF-κB signaling in Lipopolysaccharide (LPS)-induced BV2 and primary neuronal cells [147]. This leads to a significant reduction in the expression of the proinflammatory cytokines such as TNF-α, Cox2, IL-1β, and inducible nitric oxide synthase (iNOS), while increasing the expression of the anti-inflammatory cytokines such as IL-10, Interleukin-4 (IL-4), mannose receptor, arginase 1 (Arg1), and chitinase-like proteins [147]. As a result, PLA may be an effective modulator of microglial and neurosynaptic activity for the prevention of AD [147]. Furthermore, research has demonstrated that AS-IV possesses potent antineuroinflammatory properties both in vivo and in vitro [148]. Specifically, in experiments conducted on 5xFAD mice and LPS-induced AD models using BV2 cells, AS-IV was found to effectively suppress the activation of the NF-κB pathway and decrease the expression of proinflammatory mediators such as IL-1β, COX-2, iNOS, and TNF-α [148]. These results indicate that AS-IV may be an effective modulator of microglial and neurosynaptic activity for the prevention of AD.
Ginsenoside Rg5, a saponin extracted from ginseng [149], has demonstrated anti-inflammatory properties in BV2 cells by modulating the MAPK and PI3K/Akt pathways, as well as the downstream transcription factors NF-κB and AP-1 [150]. Furthermore, ginsenoside Rg5 has been shown to inhibit LPS-induced NO production and the expression of iNOS, TNF-α, IL-1β, COX-2, and matrix metalloproteinase-9 (MMP-9) in a time-dependent manner [150]. Another compound, CK, has been demonstrated to disrupt NF-κB p65 in Aβ-induced BV2 cells by upregulating low-density lipoprotein receptor-related protein 1 (LRP1) expression, leading to the reduced expression of multiple inflammatory mediators including TNF-α, IL-6, and IL-1β and resulting in a neuroprotective effect 46 [151]. Platycodon grandiflorum is a medicinal herb used in food and medicine [152], of which P. grandiflorum crude saponin (PGS) is its primary constituent with neuroprotective and cognitive-enhancing properties [153]. Notably, PGS has been shown to inhibit the activation of microglial and astrocyte in 5XFAD mice by modulating the NF-κB pathway, thereby reducing neuroinflammation and neuronal loss [153]. Platycodin D (PLD), a triterpene saponin found in Platycodon grandiflorum [145], possesses anticancer, anti-inflammatory, antioxidant, and neuroprotective properties [154]. In cellular assays, PLD has been shown to increase IκBα expressions and reduce TNF-α, IL-1β, and IL-6 levels by inhibiting the TLR4/NF-κB signaling pathway, thereby reducing Aβ-induced inflammation in BV2 cells [155]. Notably, there are pharmacokinetic studies showing that PGS has good BBB permeability, but the ability of Platycodonin D to cross the BBB requires further investigation [156]. Although previous research has suggested that Platycodonin D is able to cross the BBB by comparing its physicochemical parameters with other saponins such as saikosaponin A, glycyrrhizin, and ginsenoside, direct investigation is necessary to confirm its BBB permeability [157]. Moreover, notoginsenoside R2 (NTR2), a bioactive saponin found in Panax ginseng [158], has emerged as a promising treatment for AD [159]. NTR2 has been extensively studied for its ability to reduce COX-2 expression and attenuate Aβ-induced neuroinflammation in primary rat cortical neurons through the microRNA 27a (miR-27a)/SRY-Box Transcription Factor 8 (SOX8)/β-catenin axis [159]. Animal studies in SAMP8 mice have shown that NTR2 treatment or miR-27a antagonism can improve cognitive abilities, highlighting the potential of NTR2 as a treatment for AD [159].
Saponins have been shown to downregulate proinflammatory factors and upregulate anti-inflammatory factors, resulting in the inhibition of microglial or astrocyte activation and preventing neuroinflammation. However, the exact signaling pathways involved in this process are not fully understood. For example, bacopa floribunda (BF), a herb commonly used for memory enhancement and antiaging purposes, has been reported to have therapeutic potential in treating various CNS disorders linked to neuroinflammation [160]. Recent studies have revealed that saponin extracts from BF can inhibit microglial activation and decrease the expression of IL-1β and TNF-α, thereby alleviating neuroinflammation and protecting neuronal cells in the Aβ-induced AD BALB/c mouse model [161]. Additionally, AS-IV has been shown to inhibit the amelioration of Aβ-induced cognitive dysfunction by suppressing neuroinflammation [162]. In an Aβ-induced ICR mouse model of AD, the administration of AS-IV reduced neuroinflammation and neuronal damage by reversing the Aβ-induced increase in TNF-α, IL-1β, and IL-6 levels in a dose-dependent manner [162]. Importantly, ginsenoside Rg1 has also been reported to decrease the expression of NADPH oxidase 2 (NOX2) and NLR family pyrin domain containing 1(NLRP1) inflammasome in H2O2-induced primary hippocampal neuronal cells from SD rats [163]. The same effect was observed in ginsenoside Rg1-treated APP/PS1 mice, with ginsenoside Rg1 significantly reducing the levels of NLRP1, IL-1β, and TNF-α, alleviating neuroinflammation as well as cognitive dysfunction in the model mice [164]. Such effects hold great promise in the prevention of inflammation and plaque formation, and ultimately lead to the treatment of AD. Moreover, timosaponin BII, the primary constituent of rhizoma Anemarrhenae, a natural remedy used for its anti-inflammatory properties [165], has been demonstrated to effectively reverse LPS-induced neuroinflammation by reducing the expression of TNF-α and IL-1β in C57BL/6J mice and PC12 cells [166]. Ginsenoside Rb1 has been shown to reverse neuroinflammation in the Aβ ICV injection-induced AD Wistar rat model by reducing the expression of COX-2, IkB-α, neuronal nitric oxide synthase (nNOS), and Aβ1-42 [167]. These findings are similar to the anti-inflammatory effects of PF11 in SD rats with an OA-induced AD model [168], as well as the anti-inflammatory effects of cyclic Cycloastragenol in the Aβ-injected C57BL/6N model [169].
Another natural remedy, ginsenoside Rd, has been shown to alleviate neuroinflammation, reduce neuronal death in the hippocampal region, and enhance learning and memory in an Aβ-induced AD rat model [170]. This effect was achieved by decreasing the expression of ionized calcium-binding adapter molecule 1 (Iba-1), glial fibrillary acidic protein (GFAP), IL1-β, IL-6, and TNF-α, while increasing the expression of IL-10 and heat shock protein 70 (Hsp70) [170]. Another natural remedy with anti-inflammatory properties is Hyoscyamus niger, which has been shown to improve cognitive function and protect against neuroinflammatory damage [171]. Its main constituent, hyoscyamine (Hyo), has been shown to improve cognitive function and protect against neuroinflammatory damage [172]. When taken orally, Hyo reduces the expression of CDK11-P58, suppresses the proinflammatory cytokine IL-6, and increases the production of the anti-inflammatory cytokine IL-4 [173].
In general, natural saponins have anti-inflammatory physiological activity, thereby protecting nerve cells by modulating inflammation-related signaling pathways to inhibit the expression or release of inflammatory factors.
2.4. Improvement in Mitochondrial Function and Antioxidative Stress
Oxidative stress refers to an imbalance between oxidation and antioxidation in the body [174], which is mainly characterized by the increased production of reactive oxygen species and reduced ability of antioxidants to combat them [175]. The brain is susceptible to oxidative damage due to its high lipid content and lacks effective antioxidant defense mechanisms, despite high oxygen consumption [176,177]. It is now well established that the level of oxidation in the brain also increases with age [178]. Interestingly, extensive oxidative damage can be observed in the stage of mild cognitive impairment that precedes the typical clinical manifestations of AD, suggesting that oxidative stress may be a central mechanism in driving the disease [179].
Mitochondria, as essential organelles for aerobic metabolism, significantly contribute to the generation of oxidative stress [180]. Meanwhile, it has now been shown that mitochondrial oxidative stress can lead to axonal and neuronal damage and, ultimately, to neurological dysfunction [181]. When oxidative stress occurs within mitochondria, it can lead to mitochondrial swelling, rupture of the outer membrane, and loss of calcium ions, resulting in the further production and release of ROS [182]. Under physiological conditions, ROS can stimulate cell growth [183] and are eliminated by antioxidant enzymes and other antioxidant defense systems [184], such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) [185]. However, when a state of redox imbalance occurs, free radicals accumulate in large quantities, leading to lipid peroxidation, resulting in the production of malondialdehyde (MDA) [186], cell membrane damage, mitochondrial membrane damage, and even neuronal death [187]. Furthermore, oxidative stress may interact with Aβ aggregation in the brain [188], tau protein phosphorylation [189], and metal ion microenvironmental damage to amplify neuronal damage, ultimately contributing to the pathogenesis of AD [190,191].
Several natural saponins may act as exogenous antioxidants to combat AD by managing oxidative stress [192,193]. Ginsenoside Rh2, a rare ginsenoside with few reports on its neuroprotective effects compared to other similar molecules [194], has shown significant neuroprotective effects in a scopolamine-induced model of memory impairment in ICR mice. Ginsenoside Rh2 effectively managed MDA levels while increasing glutathione (GSH) levels and SOD activity in the brain, effectively inhibiting oxidative stress [195]. These findings suggest that ginsenoside Rh2 has great potential for the treatment of AD by inhibiting oxidative stress in the brain. Crude saponins from BF showed potent antioxidant effects in addition to anti-inflammatory effects. In addition, saponins from BF alleviated oxidative stress, reduced ROS, and MDA levels by restoring GSH-Px, GSH, and CAT levels in the Aβ ICV-induced BALB/c AD mouse model [161]. Similarly, ginsenoside Rd was found to reduce protein carbonyls and 4-hydroxy-2-nonenal (4-HNE) levels by decreasing the glutathione disulfide (GSSG)/GSH ratio, thereby counteracting oxidative stress in the brain of Aβ-induced AD mouse models [170]. Ginsenoside Rg3, a tetracyclic triterpenoid saponin, is the major bioactive component of ginseng and is widely used for the treatment of tumors [196]. Results from in vivo studies have shown that ginsenoside Rg3 can improve mitochondrial dysfunction and enhance ROS scavenging ability by increasing SOD, CAT, and GSH-Px levels, thus ameliorating oxidative stress in the brain of D-gal-induced AD rats [197]. PF11 was found to inhibit the production of both APP and Aβ in the cortex and hippocampus, while restoring the activity of SOD and GSH-Px in the brains of AD mice and reducing MDA levels in the cortex [198].
Ginsenoside Rg1 has also been found to protect primary cortical neurons against Aβ-induced increases in ROS and H2O2 levels by inhibiting oxidative stress [199]. This results in a decrease in mitochondrial membrane potential and an increase in cytochrome C (Cyt-c) oxidase activity, ultimately leading to a decrease in Cyt-c release and indicating improved mitochondrial function [199]. Another newly discovered saponin compound, huangjingsterol B from Polygonatum cyrtonema Hua, showed significant antioxidant activity [200] by reducing oxidative stress and protecting cells from Aβ-induced damage through modulating the activity of GSH-Px and SOD [200]. Similarly, researchers have found that a saponin from the fruit of Solanum anguivi can protect the P2 region of rat brain synaptosomes from oxidative damage induced by ferrous iron and sodium nitroprusside, reduce ROS generation, restore total thiol levels, and protect mitochondrial function [192]. This suggests that saponins from Solanum anguivi fruits may have antioxidant properties that benefit mitochondrial function [201]. PGS has a similar effect with its ability to upregulate intracellular heme oxygenase-1 (HO-1), SOD, CAT, and GSH-Px to inhibit Aβ-induced ROS production and reduce oxidative damage in AD brains [153]. Despite the demonstrated neuroprotective effects of Panax ginseng, the mechanisms underlying its antioxidant properties remain poorly understood. However, a recent study has shown that the primary bioactive compound in Panax ginseng, NTR1, is able to prevent the loss of mitochondrial membrane potential and reduce the accumulation of ROS induced by Aβ [202]. This suggests that NTR1 may exert neuroprotective effects by inhibiting oxidative stress. Furthermore, most previous experiments have focused on saponins extracted from Panax ginseng roots, and the pharmacological effects of other parts, such as saponins in P. notoginseng leaves (LPNS), are largely unknown [203]. LPNS, which mainly include the ginsenosides Rb1, Rb2, Rb3, and Rc, have been shown to have potent neuroprotective properties [203]. In H2O2- or oxygen and glucose deprivation (OGD)-induced astrocytes from the cortex of SD rats and SH-SY5Y cells, LPNS increased the levels of nuclear factor erythroid2-related factor 2 (Nrf2) and its downstream HO-1 and glutathione S-transferase pi 1 (GSTP1) in response to increased ROS, as well as significantly reducing lactate dehydrogenase (LDH) expression and increasing the cellular activity of astrocytes [203].
In other studies, saponins have been shown to alleviate oxidative stress by modulating oxidative stress-related pathways. In an Aβ-induced AD model, treatment with Cycloastragenol resulted in elevated levels of Nrf2 and HO-1 and a decrease in levels of ROS and lipid peroxidation (LPO) in the mouse brain, highlighting its potent antioxidant activity [169]. Similarly, Ginsenoside Rg1 responded to H2O2-induced PC12 cell injury by downregulating the NF-κB signaling pathway, as well as its upstream Akt and ERK1/2, thereby increasing the expression of inhibitor kappa B alpha (IκB-α) by inhibiting the phosphorylation of NF-κB/p65 and IκB-α [204]. Additionally, Dioscintn was found to improve physical coordination and enhance learning memory and spatial exploration in a mouse model by modulating the RAGE/NOX4 signaling axis, upregulating the expression of Nrf2, HO-1, and SOD in the oxidative stress pathway, and inhibiting the production of MDA and ROS [144]. Furthermore, recent research indicates that ginsenoside Rg1 enhances the antioxidant activity of SOD, CAT, and GSH-Px by modulating the wingless (Wnt)/GSK-3β/β-catenin signaling pathway [205], which leads to a decrease in oxidative products such as nitrotyrosine, 8-hydroxyguanosine (8-OHG), and MDA. This ameliorated oxidative stress damage, protected neurons, and ameliorated cognitive impairment in AD tree shrews [205]. Esculentoside A, a saponin from the roots of phytolaca esculenta [206], has been shown to reduce oxidative stress in 3 × Tg-AD mice [207]. This compound activates PPARγ, which decreases MDA and ROS and increases GSH and SOD levels, with its effects reversed by the PPARγ inhibitor GW9662 [207]. Additionally, in Aβ-induced PC12 cells, ginsenoside Rb1 can act as a PPARγ agonist to promote cholesterol efflux, thereby attenuating Aβ-induced cholesterol elevation, ROS accumulation and lipid peroxidation, and, finally, reducing cell death [208]. Furthermore, PLD was found to attenuate Aβ-induced oxidative stress in BV-2 cells by activating the Nrf2/HO-1 pathway [155]. This was evidenced by a reduction in ROS and MDA production, as well as an increase in SOD and NQO1 activity [155]. Furthermore, the antioxidant effect of PLD in Aβ-stimulated BV-2 cells was abolished by transfection with si-Nrf2, suggesting that the antioxidant activity of PLD is closely linked to the Nrf2/HO-1 signaling pathway [155]. Similarly, ginsenoside Re increased the expression of NAD (P) H dehydrogenase 1 and glutamate-cysteine through the activation of the Nrf/HO-1 pathway, as well as GSH levels, and the activities of SOD, glutamate-cysteine ligase regulatory subunit (GCLC) and GSH-Px [209]. These effects effectively reversed Aβ-induced ROS production in SH-SY5Y cells, thereby protecting them from oxidative stress damage [209]. Saikosaponin-D, the main component of Bupleurum falcatum [210], has been shown to cross the BBB directly to exert neuroprotective effects [211]. It has now been found that in glutamate-induced SH-SY5Y, Saikosaponin-D increases the activity of several endogenous antioxidant enzymes and the expression of Nrf2 and HO-1 through the activation of the PI3K pathway, thereby making SH-SY5Y cells free from glutamate neurotoxicity [212]. These findings demonstrate the potential of these compounds as effective antioxidants for the treatment of AD.
2.5. Antiapoptotic Effect
It is widely accepted that neuronal apoptosis contributes significantly to the pathogenesis of AD [213] and is even a pathological hallmark of the disease [214]. Neuronal apoptosis has been found to be abundant in AD autopsy samples [215] and several AD models have successfully reproduced this apoptosis [216]. The biochemical signature of apoptosis is mainly the activation of cysteinase triggered by intrinsic or extrinsic apoptotic pathways [217]. Apoptosis triggered by external cellular signals is called the “death receptor“ pathway, which involves the activation of caspase-8 followed by cleavage of caspase-3, leading to apoptosis [218]. In contrast, the intrinsic apoptotic pathway is driven by the mitochondria [219]. Mitochondrial damage induces the release of Cyt-c and other apoptotic factors into the cytoplasm, which then activate caspase-9 and caspase-3, resulting in apoptosis [220]. The B-cell lymphoma-2 (Bcl-2) protein family includes proapoptotic proteins, BCL2-associated X (BAX), Bcl-2 antagonist killer 1 (BAK), Bcl-2-like protein 11 (BIM), p53 upregulated modulator of apoptosis (PUMA), and Bcl2 modifying factor (BMF), and antiapoptotic proteins, such as Bcl-2, lymphoma-extra-large (Bcl-xL), Bcl-2 like 2 (Bcl-w), and myeloid cell leukemia-1 (Mcl-1), which tightly regulates this apoptotic process by controlling the ratio of proapoptotic and antiapoptotic proteins [221,222], particularly the Bax/Bcl-2 ratio [223]. In AD patients, Bcl-2 expression is reduced in the hippocampus [224], while Bax accumulates near senile plaques and tau protein fiber tangles in an AD animal model, suggesting that mitochondria-mediated apoptosis is closely linked to the pathology of AD [225].
Numerous saponins, including astragaloside (AST), NTR2, PGS, AS-IV, NTR1, Ginsenoside Rg2, and Ginsenoside Rg1, have shown antiapoptotic effects in AD-associated models. AST, a small molecule saponin derived from Astragalus membranaceus, has been widely used in China for the treatment of chronic diseases [226]. In in vivo and in vitro experiments, AST can prevent Aβ-induced apoptosis in cortical neurons by modulating the PI3K/Akt and MAPK (or ERK) pathway [227]. This effect is reversed by the PI3K inhibitor LY294002 and mimicked by the inhibition of ERK and enhanced by its inhibitor U0126, suggesting that the neuroprotective effect of AST may also be related to the inhibition of the ERK pathways [227]. Similarly, NTR2 can reverse the Aβ-induced apoptosis of primary cortical neurons via miR-27a/SOX8/axis and ameliorate cognitive dysfunction in AD mice [159]. PGS may provide neuroprotection not only by reducing inflammation but also by inhibiting apoptosis. PGS increased the expression of Bcl-2 family proteins, resulting in a reduction in Cyt-c release and caspase-9 and -3 expression, thereby inhibiting Aβ-induced apoptosis in HT22 cells. PGS achieved this by inhibiting p38, ERK, and JNK activation, as evidenced by decreased levels of p-p38/p38, p-ERK/ERK, and p-JNK/JNK, and by downregulating MAPK signaling [153]. In vitro and in vivo experiments constructed in HT-22 cells and C57BL/6 induced by amyloid beta protein fragment 1-42 oligomers (AβO) have shown that AS-IV reduced the Aβ-induced inhibition of the brain-derived neurotrophic factor (BDNF)–tyrosine kinase receptor B (TrkB) signaling pathway by regulating PPARγ [228]. This mechanism leads to the reduction in the number and fluorescence density of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells and caspase-3 levels, ultimately alleviating hippocampal neuronal apoptosis and memory impairment [228]. In Aβ-induced PC12 cells, NTR1 has also been shown to exert an antiapoptotic effect, reducing the percentage of TUNEL-positive cells and the levels of caspase-3, Bax, and the Bax/Bcl-2 ratio, and exerted an antiapoptotic effect by suppressing the MAPK pathway [202].
Ginsenoside Rg2 and Rg1 have been found to inhibit apoptosis and improve cognitive function in AD rat models through different mechanisms. In the Aβ-induced SD rat model of AD, ginsenoside Rg2 increased the Bcl-2/Bax ratio and reduced caspase-3 activity by activating the PI3K/Akt signaling pathway, thereby reducing Aβ-induced apoptosis and improving cognitive function in a rat model of AD [229]. Ginsenoside Rg1 reduced the number of TUNEL-positive cells in the brains of AD rats, decreased the expression of glucose-regulated protein 78, and inhibited the ER stress-mediated apoptotic pathway of c-JNK [230]. Additionally, Ginsenoside Rg1 treatment reduced the expression of tumor necrosis factor receptor-associated factor 2 and inositol-requiring enzyme-1 and inhibited the activation of p-JNK. These findings suggest that Ginsenoside Rg1 has neuroprotective effects in AD rats [230]. Acori graminei Rhizoma (AGR) is a TCM used for AD with fewer side effects, but the underlying mechanism is unclear [231]. Recently, both in vivo and in vitro experiments have demonstrated that the combination of AGR and ginsenoside Rg1 is able to suppress HO-1 expression by increasing miR-873-5p expression, thereby attenuating miR-873-5p-mediated neuronal cell apoptosis and the cognitive dysfunction in mice with AD [232].
There are natural saponins that can function as natural inhibitors of apoptosis by regulating apoptosis-related targets directly. In an Aβ-induced AD rat model, pretreatment with ginsenoside Rd reduced the expression of caspase-3, suggesting both antiapoptotic and then neuroprotective effects [170]. Furthermore, ginsenoside Rg3 has been shown to increase the expression of Bcl-2 by reversing the abnormal increase in caspase-3, caspase-9, Bax, apoptosis-inducing factor (AIF), and Cyt-C induced by D-gal in AD models [197]. This resulted in a reduction in the number of TUNEL-positive cells, inhibition of D-gal-mediated apoptosis, and prevention of cognitive impairment in AD rats [197]. Similarly, PF11 significantly attenuated the expression of JNK 2, p53, and cleaved caspase-3 in the brains of APP/PS1 mice and Aβ-induced KM mice [198]. This suggests that the beneficial effects of PF11 on neuronal function may be due to its antiapoptotic properties, in addition to its previously mentioned inhibition of Aβ production [198]. Treatment with Cycloastragenol improved the learning and memory abilities of Aβ-induced mice, which was closely related to its reduction in Bax, caspase-3, and BIM expression and increased Bcl-2 expression in the brains of Aβ-induced mice [169]. Lychee seed is a TCM known to regulate blood sugar and lower blood lipids, as well as prevent liver damage, and have antioxidant, antiviral, and anticancer properties [233]. Recently, the saponin extract of lychee seed (LSS) was found to alleviate cognitive dysfunction by inhibiting cell apoptosis. In both Aβ-induced PC12 cells and AD rats, LSS can reduce the expression of homocysteine-3 and Bax, while increasing the expression of Bcl-2 and the ratio of Bcl-2/Bax, reversing apoptosis, and, ultimately, alleviating cognitive dysfunction in AD rats [234]. Polygalasaponin F (PGSF), a triterpenoid saponin monomer isolated from P. japonica, has been shown to improve hippocampal-dependent learning memory capacity [235]. Furthermore, it has also been reported to reduce the number of caspase3-positive cells, thereby reversing glutamate-induced apoptosis in primary hippocampal neurons [236].
3. Discussion
The summarized saponins, as shown in Table 1, are primarily categorized into monomeric saponins and total saponin extracts. Based on the sapogenin skeletons, monomeric saponins used for AD treatment can be divided into two categories: triterpenoid saponins and steroidal saponins. Triterpenoid saponins are the most common, with the majority derived from pentacyclic triterpenoids (oleanane) and tetracyclic triterpenoids (dammarane). On the other hand, most steroidal saponins are derived from spirostane. Accordingly, triterpenoid saponins and sapogenin skeletons such as oleanane, dammarane, and spirostane play a key role in AD treatment.
Table 1.
Saponins against AD. The table covers all the saponins that appear in this review. The specific information includes the saponin name, source, dose, and time of administration, mechanism, Specific Targets, pathway, and the location of its corresponding reference in this paper. Up arrows indicate upregulation, while the down arrows indicate downregulation.
| Compound and Original Source | Model | Dose | Drug Administration Time | Mechanism | Target | Signaling Pathway | Refs. |
|---|---|---|---|---|---|---|---|
| Ginsenoside Rg1, ginseng | Male APP/PS1 mice | 0.1, 1, 10 mg/kg | 30 days | Inhibition of Aβ deposition | APP, Aβ↓ | Unknown | [56] |
| Xanthoperidinol, husks of xanthoceras sorbifolia bunge | Male APP transgenic mice | 0.02, 0.08 or 0.32 mg/kg | 6 months | Inhibition of Aβ deposition | APP, Aβ↓ | Unknown | [59] |
| Ginsenoside Rh2, ginseng | Tg2576 mice | 10 mg/kg | 8 weeks | Inhibition of Aβ deposition | APP, Aβ↓ sAPPα↑ |
Unknown | [60] |
| Ginsenoside Rg1, ginseng | Male Tg mapp mice | 10 mg/kg | 3 months | Inhibition of Aβ deposition | Aβ, γ-secretase↓ | Unknown | [61] |
| Ginsenoside Rg1, ginseng | OVX and d-gal-induced female Wistar rats | 5, 10, 20 mg/kg | 6 weeks | Inhibition of Aβ deposition | Aβ, BACE1↓ ADAM10↑ |
Unknown | [67] |
| Ginsenoside Rg1, ginseng | HT22 cells and SH-SY5Y cells stably expressing the Swedish mutant APP Female Wistar rats |
In vitro: 2–10 μM In vivo: 10 mg/kg |
In vitro: 30 min In vivo: 8 weeks |
Inhibition of Aβ deposition | Aβ, sappα↓ α-secretase↑ |
ERK/MAPK PI3K/Akt |
[68] |
| RAPO-1-3 and onjisaponin B, radix polygalae | Male APP/PS1 mice | In vivo: 200 μL of Onjisaponin B (1 mg/mL), RAPO-1-3 (15 mg/mL) | 3 months | Inhibition of Aβ deposition | Aβ, APP↓ Interfered with the interaction between PS1 and BACE1 |
Unknown | [62] |
| Pseudoginsenoside-F11, ginseng | Male SAMP8 mice | 2, 8, 32 mg/kg | 3 months | Inhibition of Aβ deposition | Aβ, BACE1↓ | Unknown | [63] |
| Theasaponin E1, green tea seeds | Sweapp N2a cells | 5, 10, 15, 20, 25, and 30 μg/mL | 24 h | Inhibition of Aβ deposition | Aβ, γ-secretase, BACE1, APP↓ Neprilysin, ADAM10↑ |
Unknown | [64] |
| Ginsenoside compound K Protopanaxadiol saponin, panax notoginseng |
Scopolamine-induced male ICR mice | 20, 40 mg/kg | 14 days | Inhibition of Aβ deposition | BACE1, PS1, Aβ↓ IDE↑ |
Unknown | [66] |
| ginsenoside (20S)-Rg3, ginseng | Primary neuron APP/PS1 mice |
In vitro: 10, 30, 50 μM In vivo: 20 mg/kg |
In vitro: 6 h In vivo: 10 weeks |
Inhibition of Aβ deposition | Aβ, γ-secretase↓ PI4KIIα↑ |
Unknown | [65] |
| Minor ginsenoside F1, ginseng | Male APP/PS1 mice | 20 mg/kg | 8 weeks | Inhibition of Aβ deposition | Aβ↓ | Unknown | [75] |
| Bacopaside I, bacopa monniera | Male APP/PS1 mice | 15, 50 mg/kg | 2 months | Inhibition of Aβ deposition | Aβ↓ Innate immune stimulation and phagocytosis |
Unknown | [79] |
| Pseudoginsenoside-F11, ginseng | Primary rat microglial cells | 10, 30, and 100 μM | 6 h | Inhibition of Aβ deposition | Aβ↓ Cate, LAMP2, V-ATP, Rab5 and Rab7↑ |
Mtor/TFEB | [82] |
| Ginsenoside Re, ginseng | The n2a/APP695 cell line that stably expressed Swedish mutant human APP695 | 0, 25, 50, 100, 150, and 200 μM | 24 h | Inhibition of Aβ deposition | Sappβ, BACE1, Aβ↓ PPARγ↑ |
PPARγ | [84] |
| Notoginsenoside R1, panax notoginseng | N2a-APP695sw cells Male APP/PS1 mice |
In vitro: 1, 10, 100 μM In vivo: 5, 25 mg/kg |
In vitro: 48 h In vivo: 3 months |
Inhibition of Aβ deposition | Aβ↓ PPARγ, IDE↑ |
PPARγ | [84] |
| Astragaloside IV, astmgali Radix | SH-SY5Y cells transfected with BACE1 Male APP/PS1 mice |
In vitro: 250 μg/mL In vivo: 1, 10 mg/kg |
In vitro: 24 h In vivo: 3 months |
Inhibition of Aβ deposition | BACE1, Aβ↓ PPARγ↑ |
PPARγ | [92] |
| Jujuboside A, semen ziziphi spinosae | Aβ-induced BV2 cells Male APP/PS1 mice |
In vitro: 1, 5, 25 μM In vivo: 0.5, 1.5, or 5 mg/kg |
In vitro: 18 h In vivo: 7 days |
Inhibition of Aβ deposition | Aβ↓ PPARγ, Axl, HSP90β↑ |
Axl/HSP90/PPARγ | [95] |
| Ginsenoside Rg1, ginseng | Aβ-induced rat hippocampal neurons | 60 µM | 1 h | Inhibition of Aβ deposition | CDK5, BACE1, APP and Aβ↓ IDE↑ |
PPARγ | [96] |
| Hederacochiside-E, pulsatilla koreana | Aβ-induced human neuroblastoma SK-N-SH cells Scopolamine-induced rats |
In vitro: 1, 10, 30, 100 μM In vivo: 30, 60 mg/kg |
In vitro: 48 h In vivo: Unknown |
Inhibition of Aβ neurotoxicity | Neurotoxicity of Aβ↓ Neuronal cell activity↑ |
Unknown | [99] |
| Polanoside A and chikusetsusaponinv, polaskia chichipe backbg | SH-SY5Y cells | 25, 50 μM | 24 h | Inhibition of Aβ neurotoxicity | Neurotoxicity of Aβ↓ | Unknown | [100] |
| Notoginsenoside R1, panax notoginseng | C57BL/6J mice and APP/PS1 mice | 5 mg kg | 4 months | Inhibition of Aβ neurotoxicity | Neuronal excitability↑ | Unknown | [101] |
| Akequintoside F, akebia quinata | Aβ-induced E. Coli | 40 μM | 6 h | Inhibition of Aβ neurotoxicity | Inhibited Aβ-induced fibrosis | Unknown | [103] |
| Pseudoginsenoside-F11, ginseng | Male SAMP8 mice | 2, 8, 32 mg/kg | 3 months | Inhibiting aberrant tau-like protein phosphorylation | Tau hyperphosphorylation in serine 396 and tyrosine 205↓ m-PP2A, LCMT-1↑ | Unknown | [63] |
| Pseudoginsenoside-F11, ginseng | STZ-induced male Wistar rats | 2, 4, 8 mg/kg | 4 weeks | Inhibiting aberrant tau-like protein phosphorylation | The autolytic form of calpain, p35, p85, p25, CDK5, Hyperphosphorylation of tau protein at the specific sites Ser396 and Ser199/202 ↓ P-GSK-3β, phosphorylated AKT↑ |
Calpaini/CDK5 | [70] |
| Ginsenoside Rb1, ginseng | Alcl3-induced 32 female ICR mice | 20 mg/kg | 4 months | Inhibiting aberrant tau-like protein phosphorylation | Hyperphosphorylation of tau protein at the specific sites Ser396, p-GSK3 ↓ PP2A↑ |
Unknown | [117] |
| Ginsenoside Rd, ginseng | Cortical neurons cells OA-induced Sprague Dawley rats |
In vitro: 2.5, 5 μM In vivo: 10 mg/kg |
In vitro: 12 h In vivo: 7 days |
Inhibiting aberrant tau-like protein phosphorylation | Hyperphosphorylation of tau protein at ps199/S202 and ps396↓ PP2A↑ |
Unknown | [120] |
| Theasaponin E1, green tea seeds | SH-SY5Y human neuroblastoma cells and U-87 MG glioblastoma cells | 5, 10, 15, 20 μg/mL | 24 h | Inhibiting aberrant tau-like protein phosphorylation | GSK-3β, CDK5, JNK, MAPK, ERK1/MARK camkiiα, p-tau, APP, PS1, presenilin-2, apolipoprotein E4, and phosphatidylinositol binding clathrin assembly protein↓ TREM2 and IDE↑ |
Unknown | [121] |
| Xanthoceraside, husks of xanthoceras sorbifolia bunge | Male APP transgenic mice | 0.02, 0.08, 0.32 mg/kg | 6 months | Inhibiting aberrant tau-like protein phosphorylation | Tau protein at Ser396 and Ser404, pgsk-3β↓ | Unknown | [59] |
| Theasaponin E1, green tea seeds | SH-SY5Y human neuroblastoma cells and U-87 MG glioblastoma cells | 5, 10, 15, 20 μg/mL | 24 h | Anti-inflammatory effect | IL-1β, IL-6, and TNF-α↓ | NF-κB | [121] |
| Dioscin, dioscoreae nipponicae rhizomes | H2O2-induced SH-SY5Y cells Alcl3 combined with D-galactose-induced male C57BL/6 mice |
In vitro: 2.5, 5 μM In vivo: 20, 40, 80 mg/kg |
In vitro: 12 h In vivo: 4 weeks |
Anti-inflammatory effect | RAGE, NOX4, IL-1β, IL-6, TNF-α, p-NF-κB(p-p65/NF-κB(p65), and AP-1↓ | RAGE/NOX4 | [144] |
| Platycodigenin, platycodon grandifloras | Aβ-induced BV2 microglia and primary cortical microglia | 0.01–10 μM | 4 days | Anti-inflammatory effect | Inos, TNF-α, Cox2, IL-1β phosphorylated p65, and p38↓ IL-10, IL-4, mannose receptor, arg 1, and chitinase-like proteins↑ |
PPARγ | [147] |
| Astragaloside IV, astmgali Radix, | LPS-induced BV2 microglial cells Male 5xFAD mice |
In vitro: 10, 25, 50 μM In vivo: 10, 20 mg/kg |
In vitro: 6 h In vivo: 3 months |
Anti-inflammatory effect | IL-1β, COX-2, iNOS, and TNF-α↓ | Unknown | [148] |
| Ginsenoside Rg5, ginseng | LPS-induced BV2 microglial cells | 10, 30, 50 μM | 1 h | Anti-inflammatory effect | Inos, IL-1β, COX-2, MMP-9 NO, TNF-α, NF-κB, and AP-1↓ | MAPK and PI3K/Akt | [150] |
| Ginsenoside compound K, Panax notoginseng | LPS-induced BV2 microglial cells | 0, 1, 2, 4, 6, 8, and 10 μM | 2 h | Anti-inflammatory effect | TNF-α, IL-6, IL-1β↓ LRP1, NF-κB (p65)↑ |
NF-κB | [151] |
| P. Grandiflorum crude saponin, platycodon grandiflorum | Aβ-induced HT22 Hippocampal-derived neurons cells 5XFAD mouse |
In vitro: 5, 10, and 20 μg/mL In vivo: 50 mg/kg |
In vitro: 24 h In vivo: 3 weeks |
Anti-inflammatory effect | P-NF-κB/NF-κB, COX-2↓ P-IκBα↑ |
NF-κB | [153] |
| Platycodin D, platycodon grandiflorum | Aβ-induced BV2 cells | 0, 5, 10, 20, and 40 μM | 24 h | Anti-inflammatory effect | TNF-α, IL-1β, and IL-6↓ | TLR4/NF-κB | [155] |
| Notoginsenoside R2, Panax ginseng | Aβ-induced primary rat cortical neurons cells Male SAMP/8 mice Male SD rats |
In vitro: 30 μM In vivo: 250 mg/kg |
In vitro: Unknown In vivo: 8 weeks |
Anti-inflammatory effect | COX-2↓ | Mir-27a/SOX8/β-catenin | [159] |
| Saponins from bacopa floribunda, bacopa floribunda | Aβ-induced Eighty healthy BALB/c mice | 50, 100, and 200 mg/kg | 21 days | Anti-inflammatory effect | IL1β, TNF-α↓ | Unknown | [161] |
| Astragaloside IV, astmgali Radix | Aβ-induced male ICR mice | 20, 40, and 80 mg/kg | 3 weeks | Anti-inflammatory effect | TNF-α, IL-1β, and IL-6↓ | Unknown | [162] |
| Ginsenoside Rg1, ginseng | H2O2-inducedhippocampal neuron cells | 1, 5, and 10 µM | 24 h | Anti-inflammatory effect | NOX2, NLRP1↓ | Unknown | [163] |
| Ginsenoside Rg1, ginseng | Male APP/PS1 mice | 5, 10 mg/kg | 12 weeks | Anti-inflammatory effect | NLRP1, IL-1β, and TNF-α↓ | Unknown | [164] |
| Timosaponin BII, rhizoma anemarrhenae | LPS-induced PC12 cells LPS-induced C57BL/6J mice |
In vitro: 0.313, 0.625, 1.25, 2.50, and 5.00 mg/mL In vivo: 20 mg/kg |
In vitro: 12 h In vivo: 38 days |
Anti-inflammatory effect | TNF-α, IL-1β↓ | Unknown | [166] |
| Ginsenoside Rbl, ginseng | Aβ-induced male Wistar rats | 10 mg/kg | 4 weeks | Anti-inflammatory effect | COX-2, Ikb-α, Nnos↓ | Unknown | [167] |
| Pseudoginsenoside-F11, ginseng | OA-induced male SD rats | 2, 4, 8 mg/kg | 4 weeks | Anti-inflammatory effect | TNF-α, IL-1β↓ | Unknown | [168] |
| Cycloastragenol, astragalus radix | Aβ-induced male C57BL/6N mice | 20 mg/kg/d | 6 weeks | Anti-inflammatory effect | TNF-α and IL -1β↓ | MAPK | [169] |
| Ginsenoside Rd, ginseng | Aβ-induced Sprague Dawley rat | 10, 30 mg/kg | 30 days | Anti-inflammatory effect | IL-1β, IL-6, TNF-α, and S100β↓ IL-10 and HSP70↑ |
Unknown | [170] |
| Hyoscyamoside, hyoscymus niger | Aβ-induced male Wistar rats | 10 mg/kg | 28 days | Anti-inflammatory effect | CDK11-P58, IL-6↓ IL-4↑ |
Unknown | [173] |
| Ginsenoside Rh2, ginseng | Scop-induced male ICR mice | 12.5, 25 mg/kg | 14 days | Antioxidative stress | MDA↓ GSH, SOD↑ |
Unknown | [195] |
| Saponins from bacopa floribunda, bacopa floribunda | Aβ-induced Eighty healthy BALB/c mice | 50, 100, and 200 mg/kg | 21 days | Antioxidative stress | ROS, MDA↓ GSH-Px, GSH, and CAT↑ |
Unknown | [161] |
| Ginsenoside Rd, ginseng | Aβ-induced Sprague Dawley rat | 10, 30 mg/kg | 30 days | Antioxidative stress | GSSG/GSH, HNE↓ | Unknown | [170] |
| Ginsenoside Rg3, ginseng | D-gal-induced male wistar rats | 20 mg/kg/d | 60 days | Antioxidative stress | ROS↓ SOD, CAT, and GSH-Px↑ |
Unknown | [197] |
| Pseudoginsenoside-F11, ginseng | Aβ-induced male KM mice and APP/PS1 mice | 0.32, 1.6, 8 mg/kg | 4 weeks | Antioxidative stress | MDA↓ SOD, GSH-Px↑ |
Unknown | [198] |
| Ginsenoside Rg1, ginseng | Aβ-induced cortical neuron cells | 2.5–10 μM | 24 h | Antioxidative stress | ROS, H2O2↓ Cyt-c oxidase activity↑ |
Unknown | [199] |
| Huangjingsterol B, Stewed polygonatum cyrtonema hua. | Aβ-induced PC 12 cells | 0, 1.25, 12.5, 25, 50, and 100 mm | 2 h | Antioxidative stress | GSH-Px and SOD↑ | Unknown | [200] |
| Solanum anguivi, solanum anguivi lam | Fe [2]+ and SNP-induced Rat brain synaptosome P2 fractions | 10-200μg/mL | 30 min | Antioxidative stress | ROS↓ Total thiol↑ |
Unknown | [201] |
| P. Grandiflorum crude saponin, platycodon grandiflorum | Aβ-induced HT22 hippocampal-derived neurons cells 5XFAD mouse |
In vitro: 5, 10 and 20 μg/mL In vivo: 50 mg/kg |
In vitro: 24 h In vivo: 3 weeks |
Antioxidative stress | ROS↓ HO-1, SOD, CAT, and GSH-Px↑ |
Unknown | [153] |
| Notoginsenoside R1, panax notoginseng | Aβ-induced PC12 cells | 1, 5, 10, 50, 100 μM | 24 h | Antioxidative stress | ROS↓ | Unknown | [202] |
| Protopanoxadiol saponins, leaves of p. Notoginseng | H2O2 or OGD-induced astrocytes and SH-SY5Ycells | 2.5, 5, 10 μg/mL |
24 h | Antioxidative stress | ROS Nrf2, HO-1, glutathione S-transferase pi 1↑ |
Unknown | [203] |
| Cycloastragenol, astragalus radix | Aβ-induced male C57BL/6N mice | 20 mg/kg/d | 6 weeks | Antioxidative stress | ROS, LPO↓ Nrf2, HO-1↑ |
Nrf2/HO-1 | [169] |
| Ginsenoside Rg1, ginseng | H2O2-induced PC12 cells | 0.1–10 μM | 24 h | Antioxidative stress | Phosphorylation of NF-κB/p65 and IκB-α↓ IκB-α↑ |
NF-κB | [204] |
| Dioscin, dioscoreae nipponicae rhizomes | H2O2-induced SH-SY5Y cells Alcl3 combined with D-galactose-induced male C57BL/6 mice |
In vitro: 2.5, 5 μM In vivo: 20, 40, 80 mg/kg |
In vitro: 12 h In vivo: 4 weeks |
Antioxidative stress | MDA, ROS↓ Nrf2, HO-1, SOD↑ |
RAGE/NOX4 | [144] |
| Ginsenoside Rg1, ginseng | Aβ and D-gal-induced male tree shrews | 7.5, 15, 30 mg/kg | 8 weeks | Antioxidative stress | Nitrotyrosine, 8-OHG, and MDA↓ SOD, CAT, and GSH-Px↑ |
Wnt/GSK-3β/β-catenin | [205] |
| Esculentoside A, phytolaca esculenta | Triple transgenic AD mice | 5, 10 mg/kg | 8 weeks | Antioxidative stress | MDA, ROS↓ GSH, SOD↑ |
PPARγ | [207] |
| Ginsenoside Rb1, ginseng | Aβ-induced PC12 cells | 0, 10, 20, 30, 40, and 50 μM | 24 h | Antioxidative stress | MDA, ROS↓ cholesterol efflux↑ |
PPARγ | [208] |
| Platycodin D, platycodon grandiflorum | Aβ-induced BV2 cells | 0, 5, 10, 20, and 40 μM | 24 h | Antioxidative stress | ROS, MDA↓ SOD, NQO1↑ |
Nrf2/HO-1 | [155] |
| Ginsenoside Re, ginseng | Aβ-induced SH-SY5Y cells | 0, 5, 10, 20, 25, 30, 50, 75 μM | 24 h | Antioxidative stress | ROS↓ NAD(P)H dehydrogenase 1, glutamate-cysteine, GSH, SOD, GSH-Px, GCLC↑ |
Nrf2/HO-1 | [209] |
| Saikosaponin-D, Bupleurum falcatum | Glutamate-induced SH-SY5Y cells | 0.5, 1, 5, 10 μM | 6 h | Antioxidative stress | ROS, MDA↓ SO, gpx, CAT, Nrf2, and HO-1↑ |
PI3K | [212] |
| Astragaloside, Astragalus membranaceus | Aβ-induced primary cortical neuron Aβ-induced male Sprague Dawley rats |
In vitro: 0, 50, 100, 200, 400 μg/mL In vivo: 40 mg/kg |
In vitro: 24 h In vivo: 14 days |
Antiapoptotic | Caspase-3↓ | PI3K/AKT and MAPK (or ERK) | [227] |
| Notoginsenoside R2, Panax ginseng Male SAMP/8 mice Male SD rats |
Aβ-induced primary rat cortical neurons cells | In vitro: 30 μM In vivo: 250 mg/kg |
In vitro: Unknown In vivo: 8 weeks |
Antiapoptotic | Caspase-3↓ SOX8, β-catenin↑ |
Mir-27a/SOX8/β-catenin | [159] |
| P. Grandiflorum crude saponin, Platycodon grandiflorum | Aβ-induced HT22 hippocampal-derived neurons cells 5XFAD mouse |
In vitro: 5, 10 and 20 μg/mL In vivo: 50 mg/kg |
In vitro: 24 h In vivo: 3 weeks |
Antiapoptotic | Cyt-c release and caspase-9 and -3↓ Bcl-2 and Bcl-xl↑ |
MAPK | [153] |
| Astragaloside IV, Astmgali Radix | AβO-induced ht-22 cells AβO-induced Male C57BL/6 mice |
In vitro: 0.1 μM In vivo: 10, 20, 40 mg/kg |
In vitro: 24, 48 or 72 h In vivo: 1 week |
Antiapoptotic | Caspase-3↓ PPARγ, BDNF↑ |
PPARγ/BDNF | [228] |
| Notoginsenoside R1, Panax notoginseng | Aβ-induced PC12 cells | 1, 5, 10, 50, 100 μM | 24 h | Antiapoptotic | Caspase-3, Bax, Bax/Bcl-2↓ | MAPK | [202] |
| Ginsenoside Rg2, Ginseng | Aβ-induced SD rats | 25, 50, 100 mg/kg | 15 days | Antiapoptotic | Caspase-3↓ Bcl-2/Bax↑ |
PI3K/Akt | [229] |
| Ginsenoside Rg1, Ginseng | A double transgenic APP/PS1 rat model | AD rats were fed with 0.5% Rg1-enriched food | 2 months | Antiapoptotic | Caspase-3, Grp78, p-JNK↓ | JNK apoptotic pathway | [230] |
| Ginsenoside Rg1 and Acori graminei Rhizoma, Ginseng and Acori graminei Rhizoma | Aβ-induced primary hippocampal neurons Male SAMP8 mice |
In vitro: 10 µmol/L In vivo: Ginsenoside Rg1 (7.5 mg/kg/day) and 0.1 g AGR/10 g of body weight |
In vitro: 48 h In vivo: 3 weeks |
Antiapoptotic | HO-1↓ Mir-873-5p↑ |
Unknown | [232] |
| Ginsenoside Rd, Ginseng | Aβ-induced Sprague Dawley rat | 10, 30 mg/kg | 30 days | Antiapoptotic | Caspase-3↓ | Unknown | [170] |
| Ginsenoside Rg3, Ginseng | D-gal-induced male wistar rats | 20 mg/kg/d | 60 days | Antiapoptotic | Caspase-3, caspase-9, Bax, AIF, and Cyt C↓ Bcl-2↑ |
Unknown | [197] |
| Pseudoginsenoside-F11, Ginseng | Aβ-induced male KM mice and APP/PS1 mice | 0.32, 1.6, 8 mg/kg | 4 weeks | Antiapoptotic | JNK 2, p53, and cleaved caspase-3↓ | Unknown | [198] |
| Cycloastragenol, Astragalus radix | Aβ-induced male C57BL/6N mice | 20 mg/kg/d | 6 weeks | Antiapoptotic | Bax, caspase-3, BIM↓ Bcl-2↑ |
Unknown | [169] |
| Lychee Seed Saponins, Lychee seed | Aβ-induced PC12 cells Aβ-induced male SD rats |
In vitro: 0.95, 1.90, 3.80, 7.60 mg/L In vivo: 120, 240, 480 mg/kg |
In vitro: 12 h In vivo: 28 days |
Antiapoptotic | Bax, caspase-3↓ Bcl-2, Bcl-2/Bax↑ |
Unknown | [234] |
| Polygalasaponin F, Polygala japonica | Glutamate-induced primary culture of hippocampal neurons | 2, 4, 6, 8, 10 μM | 30 min | Antiapoptotic | Caspase-3↓ | Unknown | [236] |
When it comes to model selection, in vivo studies mostly rely on AD models induced by Aβ or transgenic mice such as SAMP/8 and APP/PS1, while in vitro studies usually employ Aβ-induced primary neuronal cells, PC12 cells, and SH-SY5Y cells. The duration of saponin administration ranges from 1 to 72 h for in vitro experiments and typically from 4 days to 3 months for in vivo experiments. This selection of models and duration of treatment is essential in ensuring the efficacy and safety of saponin-based therapies for AD, and the results of these studies can provide valuable insight into the potential effectiveness of saponins in clinical settings.
In terms of efficacy, saponins have been found to alleviate the symptoms of AD by inhibiting Aβ accumulation, aberrant tau phosphorylation, neuroinflammation, oxidative stress, and apoptosis. The anti-Aβ deposition effects of saponins, including ginsenoside Rg1, RAPO-1-3, and onjisaponin B, were achieved by regulating APP processing. They also inhibit Aβ neurotoxicity, demonstrating their good neuroprotective effects. Additionally, saponins such as PF11, Theasaponin E1, and Xanthoceraside can alleviate neurotoxicity caused by tau overphosphorylation, protecting neuronal cells from damage. Saponins also exhibit anti-inflammatory effects, with examples such as theasaponin E1, dioscin, and PLA working to reduce the levels of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, Cox2, iNOS, and MMP-9 while promoting the levels of anti-inflammatory cytokines IL-10 and Arg1 by regulating NF-κB, TLR4/NF-κB, and the miR-27a/SOX8/β-catenin, RAGE/NOX4, MAPK, PPARγ, and PI3K/Akt signaling pathways. Furthermore, saponins such as ginsenoside Rh2, crude saponins from BF, and ginsenoside Rd exhibit antioxidant properties by increasing neuronal activity and reducing the levels of ROS, MDA, NO, iNOS, and NOX4 by regulating the NF-κB, Akt/Nrf2, ERK1/2, RAGE/NOX4, Wnt/GSK-3β/β-linked protein, PPARγ, and Nrf2/HO-1 signaling pathways while increasing the activity of SOD, CAT, GPx, GSH, and GSH-Px. Finally, saponins such as AST, NTR2, and PGS are known for their antiapoptotic role by inhibiting the levels of proapoptotic proteins such as Bax, Cyt-c, caspase-8, and caspase-3 while increasing the level of Bcl-2. They achieve this by regulating the PI3K/Akt, MAPK, ERK, miR-27a/SOX8, MAPK, PPARγ, BDNF-TrkB, and mitochondrial apoptotic pathways. Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 provide a comprehensive overview of the signaling pathways and targets of saponin drugs against AD, indicating that different saponins act on similar signaling pathway targets to treat AD, while the same saponin drugs act on multiple targets across different signaling pathways. Collectively, saponins have been shown to have a positive impact on various mechanisms associated with the onset and progression of AD. However, their consistent effect is the enhancement of neuronal cell activity, leading to improved learning and memory abilities in experimental animals and ultimately alleviating cognitive dysfunction. Notably, in addition to invasive and pharmacological therapies, a number of noninvasive, nonpharmaceutical therapies are now demonstrating very high potential in the treatment of neurological disorders [237]. In clinical practice, a combination of pharmacological treatment and noninvasive brain stimulation techniques has been reported to modulate brain activity during the consolidation and fading of fear memories [238,239]. These findings not only deepen our understanding of AD but also provide new avenues for the treatment of AD.
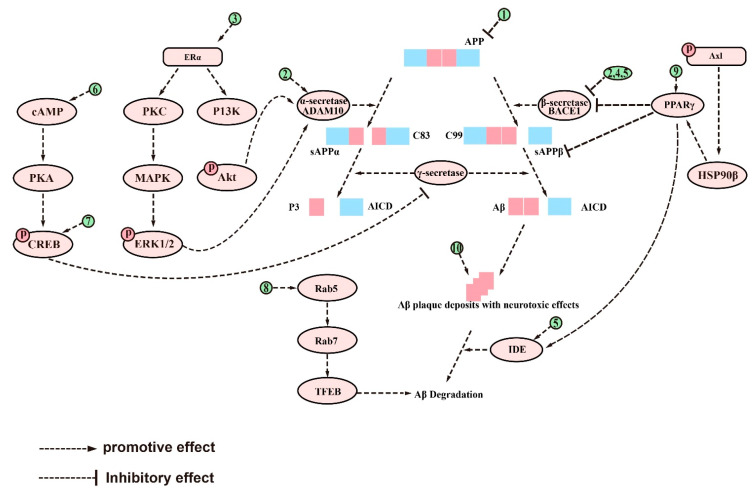
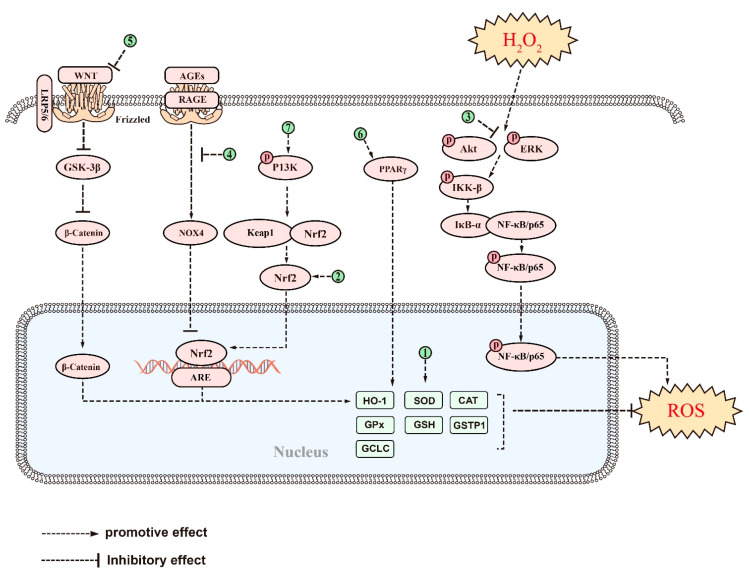
Figure 2.
The signaling pathways and targets of Aβ metabolism regulation by saponins. (1). Ginsenoside Rg1 and xanthoperidinol, to inhibit Aβ formation by reducing APP production; (2). Ginsenoside Rg1 and theasaponin E1, can enhance α-secretase activity and decrease β-secretase and γ-secretase activity; (3). Ginsenoside Rg1, regulates targets of PKC, MAPK, and PI3Ksignaling pathways; (4). RAPO-1-3, onjisaponin B, and PF11, can decrease β-secretase activity; (5). CK, can reduce the expression of BACE1 and PS1 and increase the activity of IDE; (6). Ginsenoside Rg1, regulates targets of PKA/pCREB signaling pathways; (7). Minor ginsenoside F1, can enhance the expression of pCREB; (8). PF11, can recover Rab conversions; (9). Ginsenoside Re, NTR1, AS-IV, Jujuboside A, and ginsenoside Rg1, regulate targets of PPARγ signaling pathways; (10). Hederacolchiside-E, polanoside A, chikusetsu saponin V, NTR1, and Akquintoside F, can directly inhibit the neurotoxicity of Aβ. The above-mentioned saponins inhibit the metabolism of Aβ by modulating the targets of these signaling pathways.
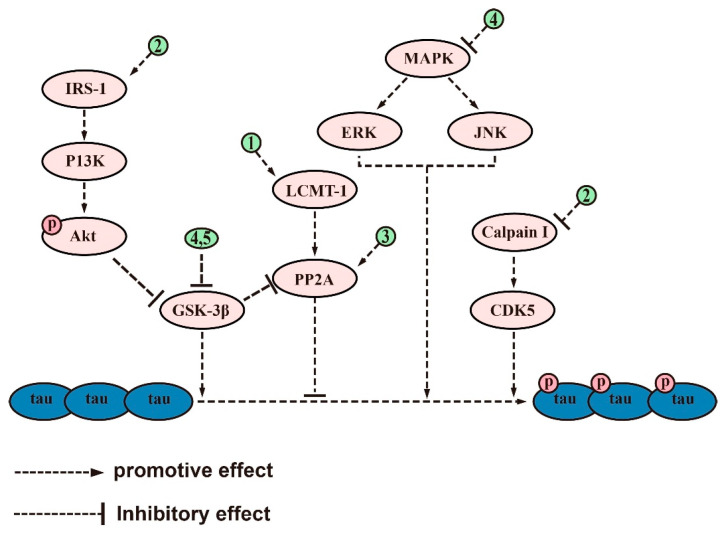
Figure 3.
The signaling pathways and targets of saponin-regulated tau protein phosphorylation. (1). PF11 and xanthoperidinol, regulate targets of LCMT-1/PP2A signaling pathways; (2). PF11, regulates targets of IRS-1/P13K/Akt/GSK-3β and calpain I/CDK5 signaling pathways; (3). Ginsenoside Rb1 and Ginsenoside Rd, regulate the activity of PP2A; (4). Theasaponin E1, inhibits tau protein phosphorylation by regulating GSK-3β, CDK5, c-Jun JNK, MAPK, ERK1/MARK, and PP2A; (5). Xanthoceraside, regulates the activity of GSK-3β. The above-mentioned saponins inhibit tau protein phosphorylation by modulating the targets of these signaling pathways.
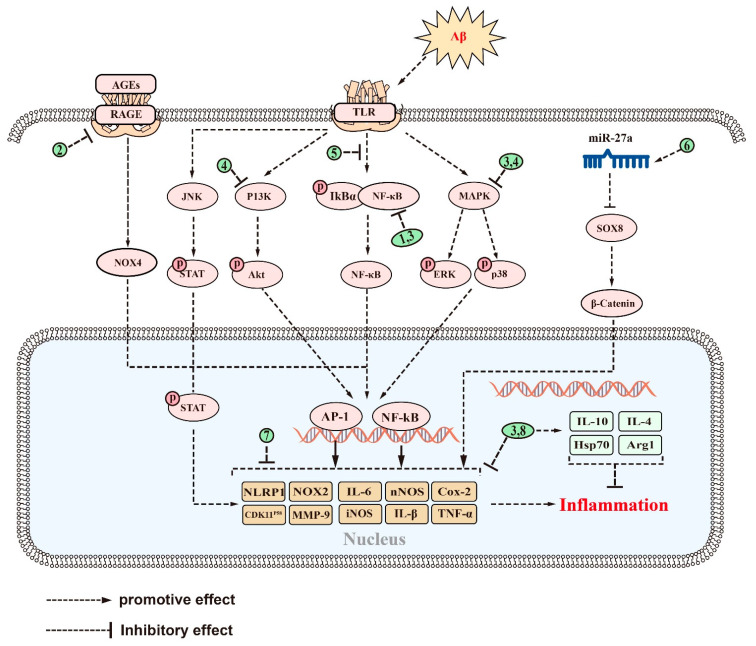
Figure 4.
The signaling pathways and targets of saponins against neuroinflammation in AD. (1). Theasaponin E1, CK, PGS, and AS-IV, regulate targets of NF-κB signaling pathway; (2). Dioscin, regulates targets of RAGE/NOX4 signaling pathway; (3). Platycodigenin, regulates targets of MAPK and NF-κB signaling pathways; (4). Ginsenoside Rg5, regulates targets of MAPK and PI3K/Akt signaling pathways; (5). PLD, regulates targets of TLR4/NF-κB signaling pathway; (6). NTR2, regulates targets of miR-27a/SOX8/β-catenin signaling pathways; (7). BF, AS-IV, Ginsenoside Rg1, Timosaponin BII, ginsenoside Rb1, and PF11, regulate various inflammatory factors, such as IL-16, IL-1β, etc.; (8). Ginsenosides Rd and hyo, enhance the expression of many anti-inflammatory factors, such as IL-10 and Hsp70, in addition to reducing inflammatory factors. The above saponins suppress neuroinflammation in AD through regulating targets of these signaling pathways.
Figure 5.
The signaling pathways and targets of saponins against oxidative stress in AD. (1). Ginsenoside Rh2, Crude saponins from BF, ginsenoside Rd, ginsenoside Rg3, PF11, ginsenoside Rg1, huangjingsterol B, saponin from the fruit of Solanum anguivi, and PGS, increased expression of many antioxidant factors such as GSH-Px, SOD, CAT, etc.; (2). LPNS, Cycloastragenol, PLD, and ginsenoside Re, regulate targets of Nrf2/HO-1 signaling pathway; (3). Ginsenoside Rg1, regulates targets of NF-κB signaling pathways; (4). Dioscintn, regulates targets of RAGE/NOX4signaling pathways; (5). Ginsenoside Rg1, regulates targets of Wnt/GSK-3β/β-catenin signaling pathway; (6). Esculentoside A and Ginsenoside Rb1, regulate targets of PPARγ signaling pathways; (7). Saikosaponin-D, regulates targets of PI3Ksignaling pathway.T above saponins suppress oxidative stress in AD through regulating targets of these signaling pathways.
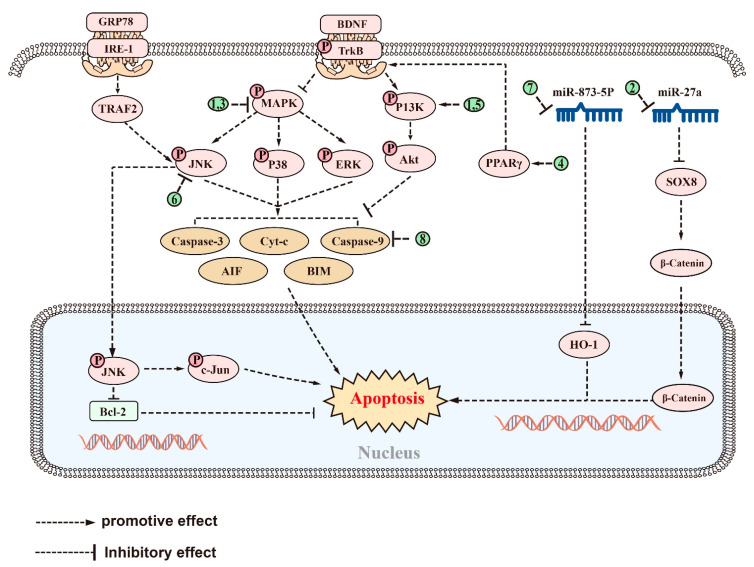
Figure 6.
The signaling pathways and targets of saponins against apoptosis in AD. (1). AST, regulates targets of PI3K/Akt and MAPK (orERK) signaling pathways; (2). NTR2, regulates targets of miR-27a/SOX8 signaling pathway; (3). PGS and NTR1, regulate targets of MAPK signaling pathways; (4). AS-IV, regulates targets of PPARγ/BDNF signaling pathways; (5). Ginsenoside Rg2, regulates targets of PI3K/Akt signaling pathway; (6). Ginsenoside Rg1, inhibits the activation of p-JNK; (7). AGR, regulates targets of miR-873-5p signaling pathways; (8). Ginsenosides Rd, ginsenoside Rg3, PF11, Cycloastragenol, LSS, and PGSF, can directly regulate apoptosis-related targets such as caspase-3, Bax, and AIF, etc. The above saponins suppress apoptosis in AD through regulating targets of these signaling pathways.
Taken together, saponins represent a promising class of natural compounds with neuroprotective effects found in various plants, showing considerable potential in the prevention and treatment of AD. In contrast to drugs that elevate acetylcholine levels, saponins are able to exert positive therapeutic effects by inhibiting Aβ accumulation and tau protein phosphorylation, reducing oxidative stress and inflammation, and decreasing apoptosis. Furthermore, their natural origin makes them readily available and generally safe, albeit occasional side effects have been reported. For example, some animal studies have found that saponins can interact with cell membranes and form pores, which can destabilize cell membranes [240] and cause hemolytic toxicity, leading to anemia [241]. Secondly, long-term use of ginsenosides can lead to estrogen-like effects, resulting in symptoms such as vaginal bleeding and breast pain in some patients [242]. In addition, it should be noted that taking ginsenosides in large quantities for extended periods can lead to insomnia and high blood pressure [243]. At the same time, ginsenosides can also cause abdominal pain, diarrhea, and vomiting due to potential intestinal wall irritation and damage [244]. Furthermore, there is some evidence indicating that high doses of saikosaponins can lead to increased serum transaminase activity and liver SOD activity in mice and rats, leading to liver damage [245]. Despite these potential side effects, their great promise as a treatment for AD remains unchanged, although more research is needed to fully understand the safety and efficacy of saponins for the treatment of AD.
4. Conclusions and Perspective
Current studies suggest that saponins, including both triterpenoid and steroidal saponins, have the potential to treat AD by reducing Aβ deposition, inhibiting tau protein phosphorylation, regulating oxidative stress, reducing inflammation, and promoting neuronal survival. The detailed signaling pathways through which saponins exert their effects have been described above and in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. However, there are several issues that require further investigation. Firstly, most of the studies have been carried out on animals or cells, whereas human clinical trials have been rare. Secondly, most of the saponins are derived from natural drugs, and their extraction or isolation presents some difficulties [246]. Thirdly, although natural saponins are effective, future chemical modifications and synthesizing may improve their activity and yield while reducing adverse effects [247]. Despite these challenges, saponins hold potential as therapeutic agents for the treatment of AD, but further studies are necessary to fully comprehend their mechanisms and potential benefits.
5. Limitations and Future Directions
Every research project has limitations, and this review is no exception. Therefore, there are several issues which need to be addressed in further studies. Firstly, the saponins discussed in this review for AD treatment are mostly total saponins, rather than specific individual components. Thus, further exploration of saponins’ activity, isolation of their individual components, and elucidation of their mechanisms of action are necessary. Secondly, although numerous animal or cellular studies have been conducted, data from human clinical trials are scarce. Future studies must focus on clinical trials to better understand the therapeutic effects and side effects of saponins. Furthermore, there have been few pharmacokinetic studies on saponins, limited information on their blood–brain barrier permeability and plasma half-life. Therefore, in future research, more attention should be paid to the study of pharmacokinetics. Finally, current studies have overlooked the synergistic effects between saponins. Although this is very challenging, further research should focus on investigating the deeper mechanisms of the synergistic effects between saponins to better understand AD.
Author Contributions
Writing—original draft preparation, R.Z. and M.Z.; writing—review and editing, X.Z. and Y.Z.; visualization, N.L. and L.W.; supervision, J.G. and Y.L.; project administration, X.J.; funding acquisition, L.Y. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
National Key R&D Program of China (No. 2021YFE0200300), National Natural Science Foundation of China (No. 82174470), Natural Science Foundation of Tianjin City, China: 21JCQNJC01170, Tianjin Research Innovation Project for Postgraduate Students: 2021YJSS172 and Graduate Research Innovation Project of Tianjin University of Traditional Chinese Medicine: YJSKC-20212012.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vossel K., Ranasinghe K.G., Beagle A.J., La A., Ah P.K., Castro M., Mizuiri D., Honma S.M., Venkateswaran N., Koestler M., et al. Effect of Levetiracetam on Cognition in Patients with Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial. JAMA Neurol. 2021;78:1345–1354. doi: 10.1001/jamaneurol.2021.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balupuri A., Choi K.E., Kang N.S. Aggregation Mechanism of Alzheimer’s Amyloid β-Peptide Mediated by α-Strand/α-Sheet Structure. Int. J. Mol. Sci. 2020;21:1094. doi: 10.3390/ijms21031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm A., Biliouris E.E., Lang U.E., Götz J., Mensah-Nyagan A.G., Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell. Mol. Life Sci. 2016;73:201–215. doi: 10.1007/s00018-015-1988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng H., Fridkin M., Youdim M. From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals. 2014;7:113–135. doi: 10.3390/ph7020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamayev R., Matsuda S., Arancio O., D’Adamio L. β- but not γ-secretase proteolysis of APP causes synaptic and memory deficits in a mouse model of dementia. EMBO Mol. Med. 2012;4:171–179. doi: 10.1002/emmm.201100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra S., Roy A., Jana M., Pahan K. Cinnamic acid activates PPARα to stimulate Lysosomal biogenesis and lower Amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol. Dis. 2019;124:379–395. doi: 10.1016/j.nbd.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polis B., Srikanth K.D., Elliott E., Gil-Henn H., Samson A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics. 2018;15:1036–1054. doi: 10.1007/s13311-018-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffen J., Krohn M., Schwitlick C., Brüning T., Paarmann K., Pietrzik C.U., Biverstål H., Jansone B., Langer O., Pahnke J. Expression of endogenous mouse APP modulates β-amyloid deposition in hAPP-transgenic mice. Acta Neuropathol. Commun. 2017;20:49. doi: 10.1186/s40478-017-0448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindpani K., Turner C., Waldvogel H.J., Faull R.L.M., Kwakowsky A. Impaired Expression of GABA Signaling Components in the Alzheimer’s Disease Middle Temporal Gyrus. Int. J. Mol. Sci. 2020;21:8704. doi: 10.3390/ijms21228704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruthirakuhan M., Herrmann N., Vieira D., Gallagher D., Lanctot K.L. The Roles of Apathy and Depression in Predicting Alzheimer Disease: A Longitudinal Analysis in Older Adults With Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry. 2019;27:873–882. doi: 10.1016/j.jagp.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mphahlele M.J., Gildenhuys S., Agbo E.N. In Vitro Evaluation and Docking Studies of 5-oxo-5H-furo [3,2-g] chromene-6-carbaldehyde Derivatives as Potential Anti-Alzheimer’s Agents. Int. J. Mol. Sci. 2019;20:5451. doi: 10.3390/ijms20215451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleem F., Mehmood R., Mehar S., Khan M.T.J., Khan Z.U., Ashraf M., Ali M.S., Abdullah I., Froeyen M., Mirza M.U., et al. Bioassay Directed Isolation, Biological Evaluation and in Silico Studies of New Isolates from Pteris cretica L. Antioxidants. 2019;8:231. doi: 10.3390/antiox8070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee B.D., Yoo J.M., Baek S.Y., Li F.Y., Sok D.E., Kim M.R. 3,3’-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants. 2019;9:3. doi: 10.3390/antiox9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Zhu H., Kang J., Warusawitharana H.K., Chen S., Wang K., Yu F., Wu Y., He P., Tu Y., et al. Comparative Transcriptome and Phytochemical Analysis Provides Insight into Triterpene Saponin Biosynthesis in Seeds and Flowers of the Tea Plant (Camellia sinensis) Metabolites. 2022;12:204. doi: 10.3390/metabo12030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon S., Iqbal H., Kim S.M., Jin M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol. Ther. 2023;31:148–160. doi: 10.4062/biomolther.2022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guclu-Ustundag O., Mazza G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Morales E., de la Fuente G., Duval S., Wehrli C., Bouillon M., Lahmann M., Preskett D., Braganca R., Newbold C.J. Antiprotozoal Effect of Saponins in the Rumen Can Be Enhanced by Chemical Modifications in Their Structure. Front. Microbiol. 2017;8:399. doi: 10.3389/fmicb.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiulai R., Roşca O.J., Antal D.S., Mioc M., Mioc A., Racoviceanu R., Macaşoi I., Olariu T., Dehelean C., Creţu O.M., et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules. 2020;25:5557. doi: 10.3390/molecules25235557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dashbaldan S., Pączkowski C., Szakiel A. Variations in Triterpenoid Deposition in Cuticular Waxes during Development and Maturation of Selected Fruits of Rosaceae Family. Int. J. Mol. Sci. 2020;21:9762. doi: 10.3390/ijms21249762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massana-Codina J., Schnee S., Allard P.M., Rutz A., Boccard J., Michellod E., Cleroux M., Schurch S., Gindro K., Wolfender J.L. Insights on the Structural and Metabolic Resistance of Potato (Solanum tuberosum) Cultivars to Tuber Black Dot (Colletotrichum coccodes) Front. Plant Sci. 2020;11:1287. doi: 10.3389/fpls.2020.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sy L.K., Lok C.N., Wang J.Y., Liu Y., Cheng L., Wan P.K., Leung C.T., Cao B., Kwong W.L., Chang R.C., et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016;7:3206–3214. doi: 10.1039/C5SC02377G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y.W., Zhu X., Lu Q., Wang J.Y., Li W., Wei Y.Q., Yin X.X. Total saponins from Rhizoma Anemarrhenae ameliorate diabetes-associated cognitive decline in rats: Involvement of amyloid-beta decrease in brain. J. Ethnopharmacol. 2012;139:194–200. doi: 10.1016/j.jep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Abduljawad A.A., Elawad M.A., Elkhalifa M., Ahmed A., Hamdoon A., Salim L., Ashraf M., Ayaz M., Hassan S., Bungau S. Alzheimer’s Disease as a Major Public Health Concern: Role of Dietary Saponins in Mitigating Neurodegenerative Disorders and Their Underlying Mechanisms. Molecules. 2022;27:6804. doi: 10.3390/molecules27206804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao A.V., Gurfinkel D.M. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metabol. Drug Interact. 2000;17:211–235. doi: 10.1515/DMDI.2000.17.1-4.211. [DOI] [PubMed] [Google Scholar]
- 25.Esmeeta A., Adhikary S., Dharshnaa V., Swarnamughi P., Ummul Maqsummiya Z., Banerjee A., Pathak S., Duttaroy A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022;153:113384. doi: 10.1016/j.biopha.2022.113384. [DOI] [PubMed] [Google Scholar]
- 26.Dandawate P.R., Subramaniam D., Padhye S.B., Anant S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016;14:81–100. doi: 10.1016/S1875-5364(16)60002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalini V.T., Neelakanta S.J., Sriranjini J.S. Neuroprotection with Bacopa monnieri—A review of experimental evidence. Mol. Biol. Rep. 2021;48:2653–2668. doi: 10.1007/s11033-021-06236-w. [DOI] [PubMed] [Google Scholar]
- 28.Bahrami Y., Zhang W. MMFC Distribution of Saponins in the Sea Cucumber Holothuria lessoni; the Body Wall Versus the Viscera, and Their Biological Activities. Mar. Drugs. 2018;16:423. doi: 10.3390/md16110423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujihara K., Shimoyama T., Kawazu R., Sasaki H., Koyama K., Takahashi K., Kinoshita K. Amyloid β aggregation inhibitory activity of triterpene saponins from the cactus Stenocereus pruinosus. J. Nat. Med. 2021;75:284–298. doi: 10.1007/s11418-020-01463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin S.J., Park Y.H., Jeon S.G., Kim S., Nam Y., Oh S.M., Lee Y.Y., Moon M. Red Ginseng Inhibits Tau Aggregation and Promotes Tau Dissociation In Vitro. Oxid. Med. Cell. Longev. 2020;2020:7829842. doi: 10.1155/2020/7829842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan M.I., Karima G., Khan M.Z., Shin J.H., Kim J.D. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int. J. Mol. Sci. 2022;23:10065. doi: 10.3390/ijms231810665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng M., Zhang R., Yang Q., Guo L., Zhang X., Yu B., Gan J., Yang Z., Li H., Wang Y., et al. Pharmacological therapy to cerebral ischemia-reperfusion injury: Focus on saponins. Biomed. Pharmacother. 2022;155:113696. doi: 10.1016/j.biopha.2022.113696. [DOI] [PubMed] [Google Scholar]
- 33.Rudakewich M., Ba F., Benishin C.G. Neurotrophic and neuroprotective actions of ginsenosides Rb (1) and Rg (1) Planta Med. 2001;67:533–537. doi: 10.1055/s-2001-16488. [DOI] [PubMed] [Google Scholar]
- 34.Morroni F., Sita G., Graziosi A., Turrini E., Fimognari C., Tarozzi A., Hrelia P. Protective Effects of 6-(Methylsulfinyl) hexyl Isothiocyanate on Abeta (1-42)-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and Apoptosis in Mice. Int. J. Mol. Sci. 2018;19:2083. doi: 10.3390/ijms19072083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mucke L., Selkoe D.J. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., Gallagher M., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 37.Mumford P., Tosh J., Anderle S., Gkanatsiou W.E., Lau G., Noy S., Cleverley K., Saito T., Saido T.C., Yu E., et al. Genetic Mapping of APP and Amyloid-beta Biology Modulation by Trisomy 21. J. Neurosci. 2022;42:6453–6468. doi: 10.1523/JNEUROSCI.0521-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao P., Xu Y., Jiang L.L., Fan X., Ku Z., Li L., Liu X., Deng M., Arase H., Zhu J.J., et al. LILRB2-mediated TREM2 signaling inhibition suppresses microglia functions. Mol. Neurodegener. 2022;17:44. doi: 10.1186/s13024-022-00550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee E.K., Kim H.H., Kuwano Y., Abdelmohsen K., Srikantan S., Subaran S.S., Gleichmann M., Mughal M.R., Martindale J.L., Yang X., et al. hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nat. Struct. Mol. Biol. 2010;17:732–739. doi: 10.1038/nsmb.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachdev R., Kappes-Horn K., Paulsen L., Duernberger Y., Pleschka C., Denner P., Kundu B., Reimann J., Vorberg I. Endoplasmic Reticulum Stress Induces Myostatin High Molecular Weight Aggregates and Impairs Mature Myostatin Secretion. Mol. Neurobiol. 2018;55:8355–8373. doi: 10.1007/s12035-018-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mockett B.G., Guévremont D., Elder M.K., Parfitt K.D., Peppercorn K., Morrissey J., Singh A., Hintz T.J., Kochen L., Tom Dieck S., et al. Glutamate Receptor Trafficking and Protein Synthesis Mediate the Facilitation of LTP by Secreted Amyloid Precursor Protein-α. J. Neurosci. 2019;39:3188–3203. doi: 10.1523/JNEUROSCI.1826-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox P.A., Davis D.A., Mash D.C., Metcalf J.S., Banack S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. R. Soc. B. Biol. Sci. 2016;283:20152317. doi: 10.1098/rspb.2015.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samaey C., Schreurs A., Stroobants S., Balschun D. Early Cognitive and Behavioral Deficits in Mouse Models for Tauopathy and Alzheimer’s Disease. Front. Aging Neurosci. 2019;11:335. doi: 10.3389/fnagi.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koon N.A., Itokazu Y., Yu R.K. Ganglioside-Dependent Neural Stem Cell Proliferation in Alzheimer’s Disease Model Mice. ASN Neuro. 2015;7:1759091415618916. doi: 10.1177/1759091415618916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hongo N., Takamura Y., Nishimaru H., Matsumoto J., Tobe K., Saito T., Saido T.C., Nishijo H. Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer’s Disease-Related Pathological Progression in the Hippocampus of App (NL-G-F/NL-G-F) Mice. Front. Pharmacol. 2020;11:307. doi: 10.3389/fphar.2020.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaguri H., Hashimoto S., Watamura N., Sato K., Takamura R., Nagata K., Tsubuki S., Ohshima T., Yoshiki A., Sato K., et al. Recent Advances in the Modeling of Alzheimer’s Disease. Front. Neurosci. 2022;16:807473. doi: 10.3389/fnins.2022.807473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X.Y., Huang S., Chen Q.B., Zhang D., Li W., Ao R., Leung F.C., Zhang Z., Huang J., Tang Y., et al. Metformin Ameliorates Aβ Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020;2020:2315106. doi: 10.1155/2020/2315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moosecker S., Gomes P., Dioli C., Yu S., Sotiropoulos I., Almeida O. Activated PPARγ Abrogates Misprocessing of Amyloid Precursor Protein, Tau Missorting and Synaptotoxicity. Front. Cell. Neurosci. 2019;13:239. doi: 10.3389/fncel.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medrano-Jiménez E., Jiménez-Ferrer Carrillo I., Pedraza-Escalona M., Ramírez-Serrano C.E., Álvarez-Arellano L., Cortés-Mendoza J., Herrera-Ruiz M., Jiménez-Ferrer E., Zamilpa A., Tortoriello J., et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflammation. 2019;16:143. doi: 10.1186/s12974-019-1515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinda B., Dinda M., Kulsi G., Chakraborty A., Dinda S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019;169:185–199. doi: 10.1016/j.ejmech.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A.P., Prabhakaran P., Kumar B.R.P., Jeyarani V., Dhanabal S.P., Justin A. Glitazones, PPAR-γ and Neuroprotection. Mini Rev. Med. Chem. 2021;21:1457–1464. doi: 10.2174/1389557521666210304112403. [DOI] [PubMed] [Google Scholar]
- 52.Mohamed L.A., Qosa H., Kaddoumi A. Age-Related Decline in Brain and Hepatic Clearance of Amyloid-β is Rectified by the Cholinesterase Inhibitors Donepezil and Rivastigmine in Rats. ACS Chem. Neurosci. 2015;6:725–736. doi: 10.1021/acschemneuro.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagano K., Tomaselli S., Molinari H., Ragona L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front. Neurosci. 2020;14:619667. doi: 10.3389/fnins.2020.619667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alolga R.N., Nuer-Allornuvor G.F., Kuugbee E.D., Yin X., Ma G. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol. Res. 2020;152:104630. doi: 10.1016/j.phrs.2020.104630. [DOI] [PubMed] [Google Scholar]
- 56.Li F., Wu X., Li J., Niu Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol. Med. Rep. 2016;13:4904–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 57.Xue W., Liu Y., Qi W.Y., Gao Y., Li M., Shi A.X., Li K.X. Pharmacokinetics of ginsenoside Rg1 in rat medial prefrontal cortex, hippocampus, and lateral ventricle after subcutaneous administration. J. Asian Nat. Prod. Res. 2016;18:587–595. doi: 10.1080/10286020.2016.1177026. [DOI] [PubMed] [Google Scholar]
- 58.Kunisawa K., Shan J., Lu Q., Yang Y., Kosuge A., Kurahashi H., Saito K., Zou L., Nabeshima T., Mouri A. Loureirin C and Xanthoceraside Attenuate Depression-Like Behaviors and Expression of Interleukin-17 in the Prefrontal Cortex Induced by Chronic Unpredictable Mild Stress in Mice. Neurochem. Res. 2022;47:2880–2889. doi: 10.1007/s11064-022-03692-z. [DOI] [PubMed] [Google Scholar]
- 59.Jin G., Wang L.H., Ji X.F., Chi T.Y., Qi Y., Jiao Q., Xu Q., Zhou X.Y., Zhang R., Zou L.B. Xanthoceraside rescues learning and memory deficits through attenuating β-amyloid deposition and tau hyperphosphorylation in APP mice. Neurosci. Lett. 2014;573:58–63. doi: 10.1016/j.neulet.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 60.Qiu J., Li W., Feng S.H., Wang M., He Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet. Mol. Res. 2014;13:3586–3598. doi: 10.4238/2014.May.9.2. [DOI] [PubMed] [Google Scholar]
- 61.Fang F., Chen X., Huang T., Lue L.F., Luddy J.S., Yan S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta. 2012;1822:286–292. doi: 10.1016/j.bbadis.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X., Cui J., Yu Y., Li W., Hou Y., Wang X., Qin D., Zhao C., Yao X., Zhao J., et al. Traditional Chinese Nootropic Medicine Radix Polygalae and Its Active Constituent Onjisaponin B Reduce β-Amyloid Production and Improve Cognitive Impairments. PLoS ONE. 2016;11:e151147. doi: 10.1371/journal.pone.0151147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., Yang J., Liu C., Xie J., Qiu S., Yang X., Wu C. Pseudoginsenoside-F11 alleviates cognitive deficits and Alzheimer’s disease-type pathologies in SAMP8 mice. Pharmacol. Res. 2019;139:512–523. doi: 10.1016/j.phrs.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 64.Khan M.I., Shin J.H., Kim M.Y., Shin T.S., Kim J.D. Green Tea Seed Isolated Theasaponin E1 Ameliorates AD Promoting Neurotoxic Pathogenesis by Attenuating Aβ Peptide Levels in SweAPP N2a Cells. Molecules. 2020;25:2334. doi: 10.3390/molecules25102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang M.S., Baek S.H., Chun Y.S., Moore A.Z., Landman N., Berman D., Yang H.O., Morishima-Kawashima M., Osawa S., Funamoto S., et al. Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013;288:20868–20882. doi: 10.1074/jbc.M112.445734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Q., Lin J., Zhang H., Liu Y., Kan M., Xiu Z., Chen X., Lan X., Li X., Shi X., et al. Ginsenoside Compound K Regulates Amyloid β via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J. Mol. Neurosci. 2019;67:62–71. doi: 10.1007/s12031-018-1210-3. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X., Wang J., Xing Y., Gong L., Li H., Wu Z., Li Y., Wang J., Wang Y., Dong L., et al. Effects of ginsenoside Rg1 or 17β-estradiol on a cognitively impaired, ovariectomized rat model of Alzheimer’s disease. Neuroscience. 2012;220:191–200. doi: 10.1016/j.neuroscience.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Shi C., Zheng D.D., Fang L., Wu F., Kwong W.H., Xu J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim. Biophys. Acta. 2012;1820:453–460. doi: 10.1016/j.bbagen.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Lin Z., Gu J., Xiu J., Mi T., Dong J., Tiwari J.K. Traditional Chinese medicine for senile dementia. Evid. Based Complement. Altern. Med. 2012;2012:692621. doi: 10.1155/2012/692621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu L., Hou X.J., Che X.H., Zhou T.S., Liu X.Q., Wu C.F., Yang J.Y. Pseudoginsenoside-F11 attenuates cognitive dysfunction and tau phosphorylation in sporadic Alzheimer’s disease rat model. Acta Pharmacol. Sin. 2021;42:1401–1408. doi: 10.1038/s41401-020-00562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.D., Chaudhary N., Seo H.J., Kim M.Y., Shin T.S. Theasaponin E1 as an effective ingredient for anti-angiogenesis and anti-obesity effects. Biosci. Biotechnol. Biochem. 2014;78:279–287. doi: 10.1080/09168451.2014.893183. [DOI] [PubMed] [Google Scholar]
- 72.Oh J., Kim J.S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016;7:4506–4515. doi: 10.1039/C6FO01077F. [DOI] [PubMed] [Google Scholar]
- 73.Lee S., Kwon M.C., Jang J.P., Sohng J.K., Jung H.J. The ginsenoside metabolite compound K inhibits growth, migration and stemness of glioblastoma cells. Int. J. Oncol. 2017;51:414–424. doi: 10.3892/ijo.2017.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim J.K., Cui C.H., Yoon M.H., Kim S.C., Im W.T. Bioconversion of major ginsenosides Rg1 to minor ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii and enzyme characterization. J. Biotechnol. 2012;161:294–301. doi: 10.1016/j.jbiotec.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 75.Han J., Oh J.P., Yoo M., Cui C.H., Jeon B.M., Kim S.C., Han J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol. Brain. 2019;12:77. doi: 10.1186/s13041-019-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakravarty A.K., Sarkar T., Masuda K., Shiojima K., Nakane T., Kawahara N. Bacopaside I and II: Two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry. 2001;58:553–556. doi: 10.1016/S0031-9422(01)00275-8. [DOI] [PubMed] [Google Scholar]
- 77.Pham H.T.N., Tran H.N., Nguyen P.T., Le X.T., Nguyen K.M., Phan S.V., Yoneyama M., Ogita K., Yamaguchi T., Folk W.R., et al. Bacopa monnieri (L.) Wettst. Extract Improves Memory Performance via Promotion of Neurogenesis in the Hippocampal Dentate Gyrus of Adolescent Mice. Int. J. Mol. Sci. 2020;21:3365. doi: 10.3390/ijms21093365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramasamy S., Chin S.P., Sukumaran S.D., Buckle M.J., Kiew L.V., Chung L.Y. In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri. PLoS ONE. 2015;10:e0126565. doi: 10.1371/journal.pone.0126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y., Yuan X., Shen Y., Zhao J., Yue R., Liu F., He W., Wang R., Shan L., Zhang W. Bacopaside I ameliorates cognitive impairment in APP/PS1 mice via immune-mediated clearance of β-amyloid. Aging. 2016;8:521–533. doi: 10.18632/aging.100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seyfried N.T., Dammer E.B., Swarup V., Nandakumar D., Duong D.M., Yin L., Deng Q., Nguyen T., Hales C.M., Wingo T., et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst. 2017;4:60–72. doi: 10.1016/j.cels.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown R.A., Voit A., Srikanth M.P., Thayer J.A., Kingsbury T.J., Jacobson M.A., Lipinski M.M., Feldman R.A., Awad O. mTOR hyperactivity mediates lysosomal dysfunction in Gaucher’s disease iPSC-neuronal cells. Dis. Model. Mech. 2019;12:dmm038596. doi: 10.1242/dmm.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao X.C., Xue X., Zhang H.T., Zhu M.M., Yang X.W., Wu C.F., Yang J.Y. Pseudoginsenoside-F11 alleviates oligomeric β-amyloid-induced endosome-lysosome defects in microglia. Traffic. 2019;20:61–70. doi: 10.1111/tra.12620. [DOI] [PubMed] [Google Scholar]
- 83.Villarroel-Vicente C., Gutierrez-Palomo S., Ferri J., Cortes D., Cabedo N. Natural products and analogs as preventive agents for metabolic syndrome via peroxisome proliferator-activated receptors: An overview. Eur. J. Med. Chem. 2021;221:113535. doi: 10.1016/j.ejmech.2021.113535. [DOI] [PubMed] [Google Scholar]
- 84.Kim S., Oh M.H., Kim B.S., Kim W.I., Cho H.S., Park B.Y., Park C., Shin G.W., Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J. Ginseng Res. 2015;39:365–370. doi: 10.1016/j.jgr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao G., Su P., Zhang S., Guo L., Zhang H., Liang Y., Qin C., Zhang W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016;793:101–108. doi: 10.1016/j.ejphar.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Limsuwanchote S., Wungsintaweekul J., Yusakul G., Han J.Y., Sasaki-Tabata K., Tanaka H., Shoyama Y., Morimoto S. Preparation of a monoclonal antibody against notoginsenoside R1, a distinctive saponin from Panax notoginseng, and its application to indirect competitive ELISA. Planta Med. 2014;80:337–342. doi: 10.1055/s-0033-1360394. [DOI] [PubMed] [Google Scholar]
- 87.Li Z., Li H., Zhao C., Lv C., Zhong C., Xin W., Zhang W. Protective Effect of Notoginsenoside R1 on an APP/PS1 Mouse Model of Alzheimer’s Disease by Up-Regulating Insulin Degrading Enzyme and Inhibiting Aβ Accumulation. CNS Neurol. Disord. Drug Targets. 2015;14:360–369. doi: 10.2174/1871527314666150225141521. [DOI] [PubMed] [Google Scholar]
- 88.Shao L., Sun C., Lu W., Chen J., Su D., Gao S., Chen S., Fang W., Liu Y., Wang B., et al. Effects of Borneol on the Release of Compound Danshen Colon-Specific Osmotic Pump Capsule In Vitro and Pharmacokinetics Study in Beagle Dogs. AAPS PharmSciTech. 2020;21:316. doi: 10.1208/s12249-020-01840-8. [DOI] [PubMed] [Google Scholar]
- 89.Kasetti R.B., Maddineni P., Kodati B., Nagarajan B., Yacoub S. Astragaloside IV Attenuates Ocular Hypertension in a Mouse Model of TGFβ2 Induced Primary Open Angle Glaucoma. Int. J. Mol. Sci. 2021;22:12508. doi: 10.3390/ijms222212508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tohda C., Tamura T., Matsuyama S., Komatsu K. Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br. J. Pharmacol. 2006;149:532–541. doi: 10.1038/sj.bjp.0706865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W.D., Zhang C., Liu R.H., Li H.L., Zhang J.T., Mao C., Moran S., Chen C.L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006;79:808–815. doi: 10.1016/j.lfs.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 92.Wang X., Wang Y., Hu J.P., Yu S., Li B.K., Cui Y., Ren L., Zhang L.D. Astragaloside IV, a Natural PPARγ Agonist, Reduces Aβ Production in Alzheimer’s Disease Through Inhibition of BACE1. Mol. Neurobiol. 2017;54:2939–2949. doi: 10.1007/s12035-016-9874-6. [DOI] [PubMed] [Google Scholar]
- 93.Oda K., Matsuda H., Murakami T., Katayama S., Ohgitani T., Yoshikawa M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000;381:67–74. doi: 10.1515/BC.2000.009. [DOI] [PubMed] [Google Scholar]
- 94.Tabassum S., Misrani A., Tang B.L., Chen J., Yang L., Long C. Jujuboside A prevents sleep loss-induced disturbance of hippocampal neuronal excitability and memory impairment in young APP/PS1 mice. Sci. Rep. 2019;9:4512. doi: 10.1038/s41598-019-41114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang M., Qian C., Zheng Z.G., Qian F., Wang Y., Thu P.M., Zhang X., Zhou Y., Tu L., Liu Q., et al. Jujuboside A promotes Aβ clearance and ameliorates cognitive deficiency in Alzheimer’s disease through activating Axl/HSP90/PPARγ pathway. Theranostics. 2018;8:4262–4278. doi: 10.7150/thno.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quan Q., Li X., Feng J., Hou J., Li M., Zhang B. Ginsenoside Rg1 reduces &β-amyloid levels by inhibiting CDΚ5-induced PPAR&γ; phosphorylation in a neuron model of Alzheimer’s disease. Mol. Med. Rep. 2020;22:3277–3288. doi: 10.3892/mmr.2020.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee W., Choi H.J., Sim H., Choo S., Song G.Y., Bae J.S. Barrier protective functions of hederacolchiside-E against HMGB1-mediated septic responses. Pharmacol. Res. 2021;163:105318. doi: 10.1016/j.phrs.2020.105318. [DOI] [PubMed] [Google Scholar]
- 98.Seo J.S., Kim T.K., Leem Y.H., Lee K.W., Park S.K., Baek I.S., Kim K.S., Im G.J., Lee S.M., Park Y.H., et al. SK-PC-B70M confers anti-oxidant activity and reduces Aβ levels in the brain of Tg2576 mice. Brain Res. 2009;1261:100–108. doi: 10.1016/j.brainres.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 99.Han C.K., Choi W.R., Oh K.B. Cognition-enhancing and neuroprotective effects of hederacolchiside-E from Pulsatilla koreana. Planta Med. 2007;73:665–669. doi: 10.1055/s-2007-981530. [DOI] [PubMed] [Google Scholar]
- 100.Fujihara K., Koike S., Ogasawara Y., Takahashi K., Koyama K., Kinoshita K. Inhibition of amyloid β aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe. Bioorg. Med. Chem. 2017;25:3377–3383. doi: 10.1016/j.bmc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 101.Yan S., Li Z., Li H., Arancio O., Zhang W. Notoginsenoside R1 increases neuronal excitability and ameliorates synaptic and memory dysfunction following amyloid elevation. Sci. Rep. 2014;4:6352. doi: 10.1038/srep06352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S.G., Lee E., Chae J., Kim J.S., Lee H.S., Lim Y.M., So J.H., Hahn D., Nam J.O. Bioconverted Fruit Extract of Akebia Quinata Exhibits Anti-Obesity Effects in High-Fat Diet-Induced Obese Rats. Nutrients. 2022;14:4683. doi: 10.3390/nu14214683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chowdhury M.A., Ko H.J., Lee H., Aminul H.M., Park I.S., Lee D.S., Woo E.R. Oleanane triterpenoids from Akebiae Caulis exhibit inhibitory effects on Aβ42 induced fibrillogenesis. Arch. Pharm. Res. 2017;40:318–327. doi: 10.1007/s12272-016-0885-7. [DOI] [PubMed] [Google Scholar]
- 104.Usenovic M., Niroomand S., Drolet R.E., Yao L., Gaspar R.C., Hatcher N.G., Schachter J., Renger J.J., Parmentier-Batteur S. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. 2015;35:14234–14250. doi: 10.1523/JNEUROSCI.1523-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubenstein R., Chang B., Grinkina N., Drummond E., Davies P., Ruditzky M., Sharma D., Wang K., Wisniewski T. Tau phosphorylation induced by severe closed head traumatic brain injury is linked to the cellular prion protein. Acta Neuropathol. Commun. 2017;5:30. doi: 10.1186/s40478-017-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Papanikolopoulou K., Roussou I.G., Gouzi J.Y., Samiotaki M., Panayotou G., Turin L., Skoulakis E.M.C. Drosophila Tau Negatively Regulates Translation and Olfactory Long-Term Memory, but Facilitates Footshock Habituation and Cytoskeletal Homeostasis. J. Neurosci. 2019;39:8315–8329. doi: 10.1523/JNEUROSCI.0391-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lacovich V., Espindola S.L., Alloatti M., Pozo D.V., Cromberg L.E., Carna M.E., Forte G., Gallo J.M., Bruno L., Stokin G.B., et al. Tau Isoforms Imbalance Impairs the Axonal Transport of the Amyloid Precursor Protein in Human Neurons. J. Neurosci. 2017;37:58–69. doi: 10.1523/JNEUROSCI.2305-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barini E., Antico O., Zhao Y., Asta F., Tucci V., Catelani T., Marotta R., Xu H., Gasparini L. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol. Neurodegener. 2016;11:16. doi: 10.1186/s13024-016-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Candia R.F., Cohen L.S., Morozova V., Corbo C., Alonso A.D. Importin-Mediated Pathological Tau Nuclear Translocation Causes Disruption of the Nuclear Lamina, TDP-43 Mislocalization and Cell Death. Front. Molec. Neurosci. 2022;15:888420. doi: 10.3389/fnmol.2022.888420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noel A., Foveau B., LeBlanc A.C. Caspase-6-cleaved Tau fails to induce Tau hyperphosphorylation and aggregation, neurodegeneration, glial inflammation, and cognitive deficits. Cell Death Dis. 2021;12:227. doi: 10.1038/s41419-021-03506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Kant R., Goldstein L.S.B., Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020;21:21–35. doi: 10.1038/s41583-019-0240-3. [DOI] [PubMed] [Google Scholar]
- 112.Pîrşcoveanu D.F.V., Pirici I., Tudorică V., Bălşeanu T.A., Albu V.C., Bondari S., Bumbea A.M., Pîrşcoveanu M. Tau protein in neurodegenerative diseases—A review. Rom. J. Morphol. Embryol. 2017;58:1141–1150. [PubMed] [Google Scholar]
- 113.Voss K., Gamblin T.C. GSK-3β phosphorylation of functionally distinct tau isoforms has differential, but mild effects. Mol. Neurodegener. 2009;4:18. doi: 10.1186/1750-1326-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahmoudi N., Kiasalari Z., Rahmani T., Sanaierad A., Afshin-Majd S., Naderi G., Baluchnejadmojarad T., Roghani M. Diosgenin Attenuates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats: Underlying Mechanisms. Neuropsychobiology. 2021;80:25–35. doi: 10.1159/000507398. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed T., Raza S.H., Maryam A., Setzer W.N., Braidy N., Nabavi S.F., de Oliveira M.R., Nabavi S.M. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res. Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 116.Shalaby A.M., Alnasser S.M., Ahmed Khairy D., Alabiad M.A., Alorini M., Jaber F.A., Tawfeek S.E. The neuroprotective effect of ginsenoside Rb1 on the cerebral cortex changes induced by aluminium chloride in a mouse model of Alzheimer’s disease: A histological, immunohistochemical, and biochemical study. J. Chem. Neuroanat. 2023;129:102248. doi: 10.1016/j.jchemneu.2023.102248. [DOI] [PubMed] [Google Scholar]
- 117.Zhao H.H., Di J., Liu W.S., Liu H.L., Lai H., Lu Y.L. Involvement of GSK3 and PP2A in ginsenoside Rb1’s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013;241:228–234. doi: 10.1016/j.bbr.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 118.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J. Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adams M.E., Li D.K., McConkey J.P., Davidson R.G., Day B., Duncan C.P., Tron V. Evaluation of cartilage lesions by magnetic resonance imaging at 0.15 T: Comparison with anatomy and concordance with arthroscopy. J. Rheumatol. 1991;18:1573–1580. [PubMed] [Google Scholar]
- 120.Li L., Liu J., Yan X., Qin K., Shi M., Lin T., Zhu Y., Kang T., Zhao G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011;138:135–141. doi: 10.1016/j.jep.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 121.Khan M.I., Khan M.Z., Shin J.H., Shin T.S., Lee Y.B., Kim M.Y., Kim J.D. Neuroprotective Effects of Green Tea Seed Isolated Saponin Due to the Amelioration of Tauopathy and Alleviation of Neuroinflammation: A Therapeutic Approach to Alzheimer’s Disease. Molecules. 2022;27:2079. doi: 10.3390/molecules27072079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou H., Tai J., Xu H., Lu X., Meng D. Xanthoceraside Could Ameliorate Alzheimer’s Disease Symptoms of Rats by Affecting the Gut Microbiota Composition and Modulating the Endogenous Metabolite Levels. Front. Pharmacol. 2019;10:1035. doi: 10.3389/fphar.2019.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li F., Eteleeb A.M., Buchser W., Sohn C., Wang G., Xiong C., Payne P.R., McDade E., Karch C.M., Harari O., et al. Weakly activated core neuroinflammation pathways were identified as a central signaling mechanism contributing to the chronic neurodegeneration in Alzheimer’s disease. Front. Aging Neurosci. 2022;14:935279. doi: 10.3389/fnagi.2022.935279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Howe A.M., Burke S., O’Reilly M.E., McGillicuddy F.C., Costello D.A. Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages. Mol. Neurobiol. 2022;59:2348–2362. doi: 10.1007/s12035-022-02756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kurtys E., Casteels C., Real C.C., Eisel U., Verkuyl J.M., Broersen L.M., Klein H.C., Dierckx R., Doorduin J., de Vries E. Therapeutic effects of dietary intervention on neuroinflammation and brain metabolism in a rat model of photothrombotic stroke. CNS Neurosci. Ther. 2019;25:36–46. doi: 10.1111/cns.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alkhatip A.A.A.M.M., Kamel M.G., Hamza M.K., Farag E.M., Yassin H.M., Elayashy M., Naguib A.A., Wagih M., Abd-Elhay F.A., Algameel H.Z., et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021;21:505–514. doi: 10.1080/14737159.2021.1915773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar P., Mishra J., Kumar N. Mechanistic Role of Jak3 in Obesity-Associated Cognitive Impairments. Nutrients. 2022;14:3715. doi: 10.3390/nu14183715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Merighi S., Nigro M., Travagli A., Gessi S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022;23:12990. doi: 10.3390/ijms232112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tiwari S., Atluri V., Kaushik A., Yndart A., Nair M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu Y., Ye R.D. Microglial Aβ receptors in Alzheimer’s disease. Cell. Mol. Neurobiol. 2015;35:71–83. doi: 10.1007/s10571-014-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gambuzza M.E., Sofo V., Salmeri F.M., Soraci L., Marino S., Bramanti P. Toll-like receptors in Alzheimer’s disease: A therapeutic perspective. CNS Neurol. Disord. Drug Targets. 2014;13:1542–1558. doi: 10.2174/1871527313666140806124850. [DOI] [PubMed] [Google Scholar]
- 132.Abubakar M.B., Sanusi K.O., Ugusman A., Mohamed W., Kamal H., Ibrahim N.H., Khoo C.S., Kumar J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022;14:742408. doi: 10.3389/fnagi.2022.742408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Subedi L., Venkatesan R., Kim S.Y. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling. Int. J. Mol. Sci. 2017;18:1423. doi: 10.3390/ijms18071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Radenovic L., Nenadic M., Ulamek-Koziol M., Januszewski S., Czuczwar S.J., Andjus P.R., Pluta R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging. 2020;12:12251–12267. doi: 10.18632/aging.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaur D., Sharma V., Deshmukh R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 136.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim Y., Lee H., Kim S.Y., Lim Y. Effects of Lespedeza Bicolor Extract on Regulation of AMPK Associated Hepatic Lipid Metabolism in Type 2 Diabetic Mice. Antioxidants. 2019;8:599. doi: 10.3390/antiox8120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Urasaki Y., Beaumont C., Talbot J.N., Hill D.K., Le T.T. Akt3 Regulates the Tissue-Specific Response to Copaiba Essential Oil. Int. J. Mol. Sci. 2020;21:2851. doi: 10.3390/ijms21082851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tyagi A., Kamal M.A., Poddar N.K. Integrated Pathways of COX-2 and mTOR: Roles in Cell Sensing and Alzheimer’s Disease. Front. Neurosci. 2020;14:693. doi: 10.3389/fnins.2020.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dhapola R., Hota S.S., Sarma P., Bhattacharyya A., Medhi B., Reddy D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology. 2021;29:1669–1681. doi: 10.1007/s10787-021-00889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Falcicchia C., Tozzi F., Arancio O., Watterson D.M., Origlia N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020;21:5624. doi: 10.3390/ijms21165624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jain M., Singh M.K., Shyam H., Mishra A., Kumar S., Kumar A., Kushwaha J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021;28:191–200. doi: 10.1177/09727531211070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Azam S., Haque M.E., Cho D.Y., Kim J.S., Jakaria M., Kim I.S., Choi D.K. Dioscin-Mediated Autophagy Alleviates MPP+-Induced Neuronal Degeneration: An In Vitro Parkinson’s Disease Model. Molecules. 2022;27:2827. doi: 10.3390/molecules27092827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guan L., Mao Z., Yang S., Wu G., Chen Y., Yin L., Qi Y., Han L., Xu L. Dioscin alleviates Alzheimer’s disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed. Pharmacother. 2022;152:113248. doi: 10.1016/j.biopha.2022.113248. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y., Tian S., Yi B., Feng Z., Chu T., Liu J., Zhang C., Zhang S., Wang Y. Platycodin D sensitizes KRAS-mutant colorectal cancer cells to cetuximab by inhibiting the PI3K/Akt signaling pathway. Front. Oncol. 2022;12:1046143. doi: 10.3389/fonc.2022.1046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Son I.H., Park Y.H., Lee S.I., Yang H.D., Moon H.I. Neuroprotective activity of triterpenoid saponins from Platycodi radix against glutamate-induced toxicity in primary cultured rat cortical cells. Molecules. 2007;12:1147–1152. doi: 10.3390/12051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang Z., Liu B., Yang L.E., Zhang C. Platycodigenin as Potential Drug Candidate for Alzheimer’s Disease via Modulating Microglial Polarization and Neurite Regeneration. Molecules. 2019;24:3207. doi: 10.3390/molecules24183207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He L., Sun J., Miao Z., Chen S., Yang G. Astragaloside IV attenuates neuroinflammation and ameliorates cognitive impairment in Alzheimer’s disease via inhibiting NF-κB signaling pathway. Heliyon. 2023;9:e13411. doi: 10.1016/j.heliyon.2023.e13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim S.N., Ha Y.W., Shin H., Son S.H., Wu S.J., Kim Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng C.A. Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharm. Biomed. Anal. 2007;45:164–170. doi: 10.1016/j.jpba.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 150.Lee Y.Y., Park J.S., Jung J.S., Kim D.H., Kim H.S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Sci. 2013;14:9820–9833. doi: 10.3390/ijms14059820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiao H., Jia J. Ginsenoside compound K acts via LRP1 to alleviate Amyloid β (42)-induced neuroinflammation in microglia by suppressing NF-κB. Biochem. Biophys. Res. Commun. 2022;590:14–19. doi: 10.1016/j.bbrc.2021.12.071. [DOI] [PubMed] [Google Scholar]
- 152.Nyakudya E., Jeong J.H., Lee N.K., Jeong Y.S. Platycosides from the Roots of Platycodon grandiflorum and Their Health Benefits. Prev. Nutr. Food Sci. 2014;19:59–68. doi: 10.3746/pnf.2014.19.2.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ji Y.J., Kim S., Kim J.J., Jang G.Y., Moon M., Kim H.D. Crude Saponin from Platycodon grandiflorum Attenuates Aβ-Induced Neurotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Signaling Pathways. Antioxidants. 2021;10:1968. doi: 10.3390/antiox10121968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo Q., Wei G., Wu X., Tang K., Xu M., Wu Y., Liu Y., Li X., Sun Z., Ju W., et al. Platycodin D inhibits platelet function and thrombus formation through inducing internalization of platelet glycoprotein receptors. J. Transl. Med. 2018;16:311. doi: 10.1186/s12967-018-1688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang J., Song N., Liu Y., Guo J. Platycodin D Inhibits β-Amyloid-Induced Inflammation and Oxidative Stress in BV-2 Cells Via Suppressing TLR4/NF-κB Signaling Pathway and Activating Nrf2/HO-1 Signaling Pathway. Neurochem. Res. 2021;46:638–647. doi: 10.1007/s11064-020-03198-6. [DOI] [PubMed] [Google Scholar]
- 156.Stępnik K. Biomimetic Chromatographic Studies Combined with the Computational Approach to Investigate the Ability of Triterpenoid Saponins of Plant Origin to Cross the Blood-Brain Barrier. Int. J. Mol. Sci. 2021;22:3573. doi: 10.3390/ijms22073573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fu Y., Xin Z., Liu B., Wang J., Wang J., Zhang X., Wang Y., Li F. Platycodin D Inhibits Inflammatory Response in LPS-Stimulated Primary Rat Microglia Cells through Activating LXRα-ABCA1 Signaling Pathway. Front. Immunol. 2018;8:1929. doi: 10.3389/fimmu.2017.01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.González-Burgos E., Fernandez-Moriano C., Gómez-Serranillos M.P. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2015;10:14–29. doi: 10.1007/s11481-014-9569-6. [DOI] [PubMed] [Google Scholar]
- 159.Hu Y., Wu L., Jiang L., Liang N., Zhu X., He Q., Qin H., Chen W. Notoginsenoside R2 reduces Aβ25-35-induced neuronal apoptosis and inflammation via miR-27a/SOX8/β-catenin axis. Hum. Exp. Toxicol. 2021;40:S347–S358. doi: 10.1177/09603271211041996. [DOI] [PubMed] [Google Scholar]
- 160.Jeyasri R., Muthuramalingam P., Suba V., Ramesh M., Chen J.T. Bacopa monnieri and Their Bioactive Compounds Inferred Multi-Target Treatment Strategy for Neurological Diseases: A Cheminformatics and System Pharmacology Approach. Biomolecules. 2020;10:536. doi: 10.3390/biom10040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oyeleke M.B., Owoyele B.V. Saponins and flavonoids from Bacopa floribunda plant extract exhibit antioxidant and anti-inflammatory effects on amyloid β 1-42-induced Alzheimer’s disease in BALB/c mice. J. Ethnopharmacol. 2022;288:114997. doi: 10.1016/j.jep.2022.114997. [DOI] [PubMed] [Google Scholar]
- 162.Chen F., Yang D., Cheng X.Y., Yang H., Yang X.H., Liu H.T., Wang R., Zheng P., Yao Y., Li J. Astragaloside IV Ameliorates Cognitive Impairment and Neuroinflammation in an Oligomeric Aβ Induced Alzheimer’s Disease Mouse Model via Inhibition of Microglial Activation and NADPH Oxidase Expression. Biol. Pharm. Bull. 2021;44:1688–1696. doi: 10.1248/bpb.b21-00381. [DOI] [PubMed] [Google Scholar]
- 163.Xu T.Z., Shen X.Y., Sun L.L., Chen Y.L., Zhang B.Q., Huang D.K., Li W.Z. Ginsenoside Rg1 protects against H2O2-induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 2019;43:717–726. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Huang L., Kong L., Su Y., Zhou H., Ji P., Sun R., Wang C., Li W., Li W. Ginsenoside Rg1 alleviates learning and memory impairments and Aβ disposition through inhibiting NLRP1 inflammasome and autophagy dysfunction in APP/PS1 mice. Mol. Med. Rep. 2023;27:6. doi: 10.3892/mmr.2022.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Passos F.R.S., Araújo-Filho H.G., Monteiro B.S., Shanmugam S., Araújo A.A.S., Almeida J.R.G.D.S., Thangaraj P., Júnior L.J.Q., Quintans J.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine. 2022;96:153842. doi: 10.1016/j.phymed.2021.153842. [DOI] [PubMed] [Google Scholar]
- 166.Chen W., Li R., Zhu S., Ma J., Pang L., Ma B., Du L., Jin Y. Nasal timosaponin BII dually sensitive in situ hydrogels for the prevention of Alzheimer’s disease induced by lipopolysaccharides. Int. J. Pharm. 2020;578:119115. doi: 10.1016/j.ijpharm.2020.119115. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y., Liu J., Zhang Z., Bi P., Qi Z., Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci. Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 168.Chu J., Wang J., Cui L., Liu S., An N., Han J., Che X., Wu C., Yang J. Pseudoginsenoside-F11 ameliorates okadiac acid-induced learning and memory impairment in rats via modulating protein phosphatase 2A. Mech. Ageing Dev. 2021;197:111496. doi: 10.1016/j.mad.2021.111496. [DOI] [PubMed] [Google Scholar]
- 169.Ikram M., Jo M.H., Choe K., Khan A., Ahmad S., Saeed K., Kim M.W., Kim M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells. 2021;10:2719. doi: 10.3390/cells10102719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu J., Yan X., Li L., Zhu Y., Qin K., Zhou L., Sun D., Zhang X., Ye R., Zhao G. Ginsennoside rd attenuates cognitive dysfunction in a rat model of Alzheimer’s disease. Neurochem. Res. 2012;37:2738–2747. doi: 10.1007/s11064-012-0866-2. [DOI] [PubMed] [Google Scholar]
- 171.Begum S., Saxena B., Goyal M., Ranjan R., Joshi V.B., Rao C., Krishnamurthy S., Sahai M. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and isolation of a new coumarinolignan. Fitoterapia. 2010;81:178–184. doi: 10.1016/j.fitote.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 172.Banjari I., Marcek T., Tomic S., Waisundara V.Y. Forestalling the Epidemics of Parkinson’s Disease Through Plant-Based Remedies. Front. Nutr. 2018;5:95. doi: 10.3389/fnut.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soureshjani F.H., Kheirollahi M., Yaghmaei P., Fattahjadnematalahi S. Possible Preventive Effect of Donepezil and Hyoscyamoside by Reduction of Plaque Formation and Neuroinflammation in Alzheimer’s Disease. Int. J. Prevent. Med. 2021;12:66. doi: 10.4103/ijpvm.IJPVM_143_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ayalon I., de Barros Marangoni L.F., Benichou J.I.C., Avisar D., Levy O. Red Sea corals under Artificial Light Pollution at Night (ALAN) undergo oxidative stress and photosynthetic impairment. Glob. Chang. Biol. 2019;25:4194–4207. doi: 10.1111/gcb.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gargouri B., Yousif N.M., Bouchard M., Fetoui H., Fiebich B.L. Inflammatory and cytotoxic effects of bifenthrin in primary microglia and organotypic hippocampal slice cultures. J. Neuroinflamm. 2018;15:159. doi: 10.1186/s12974-018-1198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pousa P.A., Souza R.M., Melo P., Correa B., Mendonca T., Simoes-E-Silva A.C., Miranda D.M. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells. 2021;10:1423. doi: 10.3390/cells10061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muke S., Kaikini A., Peshattiwar V., Bagle S., Dighe V., Sathaye S. Neuroprotective Effect of Coumarin Nasal Formulation: Kindling Model Assessment of Epilepsy. Front. Pharmacol. 2018;9:992. doi: 10.3389/fphar.2018.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ward S.M., Himmelstein D.S., Ren Y., Fu Y., Yu X.W., Roberts K., Binder L.I., Sahara N. TOC1: A valuable tool in assessing disease progression in the rTg4510 mouse model of tauopathy. Neurobiol. Dis. 2014;67:37–48. doi: 10.1016/j.nbd.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ohashi H., Tsuji M., Oguchi T., Momma Y., Nohara T., Ito N., Yamamoto K., Nagata M., Kimura A.M., Kiuchi Y., et al. Combined Treatment with Curcumin and Ferulic Acid Suppressed the Aβ-Induced Neurotoxicity More than Curcumin and Ferulic Acid Alone. Int. J. Mol. Sci. 2022;23:9685. doi: 10.3390/ijms23179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim J.I., Lee S.Y., Park M., Kim S.Y., Kim J.W., Kim S.A., Kim B.N. Peripheral Mitochondrial DNA Copy Number is Increased in Korean Attention-Deficit Hyperactivity Disorder Patients. Front. Psychiatry. 2019;10:506. doi: 10.3389/fpsyt.2019.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Polyák H., Galla Z., Nánási N., Cseh E.K., Rajda C., Veres G., Spekker E., Szabó Á., Klivényi P., Tanaka M., et al. The Tryptophan-Kynurenine Metabolic System Is Suppressed in Cuprizone-Induced Model of Demyelination Simulating Progressive Multiple Sclerosis. Biomedicines. 2023;11:945. doi: 10.3390/biomedicines11030945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hayes H.M., Angerosa J., Piers A.T., White J.D., Koleff J., Thurgood M., Moody J., Cheung M.M., Pepe S. Preserved Left Ventricular Function despite Myocardial Fibrosis and Myopathy in the Dystrophin-Deficient D2.B10-Dmd mdx/J Mouse. Oxid. Med. Cell. Longev. 2022;2022:5362115. doi: 10.1155/2022/5362115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rezaei T., Rezaei M., Karimifard S., Mahmoudi B.F., Dakkali M.S., Heydari M., Afshari-Behbahanizadeh S., Mostafavi E., Bokov D.O., Ansari M.J., et al. Folic Acid-Decorated pH-Responsive Nanoniosomes With Enhanced Endocytosis for Breast Cancer Therapy: In Vitro Studies. Front. Pharmacol. 2022;13:851242. doi: 10.3389/fphar.2022.851242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nataraj A., Govindan S., Ramani P., Subbaiah K.A., Sathianarayanan S., Venkidasamy B., Thiruvengadam M., Rebezov M., Shariati M.A., Lorenzo J.M., et al. Antioxidant, Anti-Tumour, and Anticoagulant Activities of Polysaccharide from Calocybe indica (APK2) Antioxidants. 2022;11:1694. doi: 10.3390/antiox11091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abbasi-Oshaghi E., Mirzaei F., Pourjafar M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: In vivo and in vitro study. Int. J. Nanomed. 2019;14:1919–1936. doi: 10.2147/IJN.S192382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Robinson R., Srinivasan M., Shanmugam A., Ward A., Ganapathy V., Bloom J., Sharma A., Sharma S. Interleukin-6 trans-signaling inhibition prevents oxidative stress in a mouse model of early diabetic retinopathy. Redox Biol. 2020;34:101574. doi: 10.1016/j.redox.2020.101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Valeri A., Chiricosta L., Calcaterra V., Biasin M., Cappelletti G., Carelli S., Zuccotti G.V., Bramanti P., Pelizzo G., Mazzon E., et al. Transcriptomic Analysis of HCN-2 Cells Suggests Connection among Oxidative Stress, Senescence, and Neuron Death after SARS-CoV-2 Infection. Cells. 2021;10:2189. doi: 10.3390/cells10092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alavi Naini S.M., Soussi-Yanicostas N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell. Longev. 2015;2015:151979. doi: 10.1155/2015/151979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Merelli A., Repetto M., Lazarowski A., Auzmendi J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021;82:S109–S126. doi: 10.3233/JAD-201074. [DOI] [PubMed] [Google Scholar]
- 191.Radbruch H., Bremer D., Guenther R., Cseresnyes Z., Lindquist R., Hauser A.E., Niesner R. Ongoing Oxidative Stress Causes Subclinical Neuronal Dysfunction in the Recovery Phase of EAE. Front. Immunol. 2016;7:92. doi: 10.3389/fimmu.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cui Y., Li F., Zhu X., Xu J., Muhammad A., Chen Y., Li D., Liu B., Wang C., Wang Z., et al. Alfalfa saponins inhibit oxidative stress-induced cell apoptosis through the MAPK signaling pathway. Redox Rep. 2022;27:1–8. doi: 10.1080/13510002.2021.2017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yoo K.Y., Park S.Y. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules. 2012;17:3524–3538. doi: 10.3390/molecules17033524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hsieh Y.H., Deng J.S., Chang Y.S., Huang G.J. Ginsenoside Rh2 Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 Signaling Pathways in Mice. Nutrients. 2018;10:1208. doi: 10.3390/nu10091208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lv J., Lu C., Jiang N., Wang H., Huang H., Chen Y., Li Y., Liu X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021;35:337–345. doi: 10.1002/ptr.6804. [DOI] [PubMed] [Google Scholar]
- 196.Lee S.J., Lee W.J., Chang S.E., Lee G.Y. Antimelanogenic effect of ginsenoside Rg3 through extracellular signal-regulated kinase-mediated inhibition of microphthalmia-associated transcription factor. J. Ginseng Res. 2015;39:238–242. doi: 10.1016/j.jgr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Y., Yang X., Wang S., Song S. Ginsenoside Rg3 Prevents Cognitive Impairment by Improving Mitochondrial Dysfunction in the Rat Model of Alzheimer’s Disease. J. Agric. Food Chem. 2019;67:10048–10058. doi: 10.1021/acs.jafc.9b03793. [DOI] [PubMed] [Google Scholar]
- 198.Wang C.M., Liu M.Y., Wang F., Wei M.J., Wang S., Wu C.F., Yang J.Y. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2013;106:57–67. doi: 10.1016/j.pbb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 199.Huang T., Fang F., Chen L., Zhu Y., Zhang J., Chen X., Yan S.S. Ginsenoside Rg1 attenuates oligomeric Aβ (1-42)-induced mitochondrial dysfunction. Curr. Alzheimer Res. 2012;9:388–395. doi: 10.2174/156720512800107636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang L., Wu Y., Yin H., Yang X., Yuan M., Ning H. Two new compounds from the stewed Polygonatum cyrtonema Hua and their protective effect against Aβ (25–35) induced cytotoxicity and oxidative stress damage. Nat. Prod. Res. 2021;35:4945–4952. doi: 10.1080/14786419.2020.1753735. [DOI] [PubMed] [Google Scholar]
- 201.Elekofehinti O.O., Kamdem J.P., Meinerz D.F., Kade I.J., Adanlawo I.G., Rocha J.B. Saponin from the fruit of Solanum anguivi protects against oxidative damage mediated by Fe (2+) and sodium nitroprusside in rat brain synaptosome P2 fraction. Arch. Pharm. Res. 2015;10 doi: 10.1007/s12272-014-0536-9. [DOI] [PubMed] [Google Scholar]
- 202.Ma B., Meng X., Wang J., Sun J., Ren X., Qin M., Sun J., Sun G., Sun X. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014;22:151–159. doi: 10.1016/j.intimp.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 203.Zhou N., Tang Y., Keep R.F., Ma X., Xiang J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine. 2014;21:1189–1195. doi: 10.1016/j.phymed.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem. Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 205.Yang Y., Wang L., Zhang C., Guo Y., Li J., Wu C., Jiao J., Zheng H. Ginsenoside Rg1 improves Alzheimer’s disease by regulating oxidative stress, apoptosis, and neuroinflammation through Wnt/GSK-3β/β-catenin signaling pathway. Chem. Biol. Drug Des. 2022;99:884–896. doi: 10.1111/cbdd.14041. [DOI] [PubMed] [Google Scholar]
- 206.Momenah M.A., Almutairi L.A., Alqhtani H.A., Al-Saeed F.A., Syaad K.M.A., Alhag S.K., Al-Qahtani M.A., Hakami Z.H., Mallick J., Ahmed A.E. Esculentoside A Inhibits Proliferation, Colony Formation, Migration, and Invasion of Human Colorectal Cancer Cells. Evid. Based Complement. Alternat. Med. 2023;2023:7530725. doi: 10.1155/2023/7530725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.He Z., Li X., Wang Z., Tu S., Feng J., Du X., Ni J., Li N., Liu Q. Esculentoside A alleviates cognitive deficits and amyloid pathology through peroxisome proliferator-activated receptor γ-dependent mechanism in an Alzheimer’s disease model. Phytomedicine. 2022;98:153956. doi: 10.1016/j.phymed.2022.153956. [DOI] [PubMed] [Google Scholar]
- 208.Changhong K., Peng Y., Yuan Z., Cai J. Ginsenoside Rb1 protected PC12 cells from Aβ25-35-induced cytotoxicity via PPARγ activation and cholesterol reduction. Eur. J. Pharmacol. 2021;893:173835. doi: 10.1016/j.ejphar.2020.173835. [DOI] [PubMed] [Google Scholar]
- 209.Liu M., Bai X., Yu S., Zhao W., Qiao J., Liu Y., Zhao D., Wang J., Wang S. Ginsenoside Re Inhibits ROS/ASK-1 Dependent Mitochondrial Apoptosis Pathway and Activation of Nrf2-Antioxidant Response in β-Amyloid-Challenged SH-SY5Y Cells. Molecules. 2019;24:2687. doi: 10.3390/molecules24152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Manoharan S., Deivendran B., Perumal E. Chemotherapeutic Potential of Saikosaponin D: Experimental Evidence. J. Xenobiot. 2022;12:378–405. doi: 10.3390/jox12040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao X., Li Y., Meng M., Wang P., Feng Y., Jia J., Qin X. Exploration of chemical composition and absorption characteristics of Chaigui granules based on UHPLC-Q-orbitrap-MS/MS. J. Pharm. Biomed. Anal. 2020;187:113293. doi: 10.1016/j.jpba.2020.113293. [DOI] [PubMed] [Google Scholar]
- 212.Du J., Song D., Li Y., Liu J., Huang X., Li B., Li L. Saikosaponin-D Mitigates Oxidation in SH-SY5Y Cells Stimulated by Glutamate Through Activation of Nrf2 Pathway: Involvement of PI3K. Neurotox. Res. 2022;40:230–240. doi: 10.1007/s12640-021-00438-7. [DOI] [PubMed] [Google Scholar]
- 213.Okouchi M., Ekshyyan O., Maracine M., Aw T.Y. Neuronal apoptosis in neurodegeneration. Antioxid. Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 214.Sharma V.K., Singh T.G., Singh S., Garg N., Dhiman S. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem. Res. 2021;46:3103–3122. doi: 10.1007/s11064-021-03418-7. [DOI] [PubMed] [Google Scholar]
- 215.Mufson E.J., He B., Nadeem M., Perez S.E., Counts S.E., Leurgans S., Fritz J., Lah J., Ginsberg S.D., Wuu J., et al. Hippocampal proNGF signaling pathways and β-amyloid levels in mild cognitive impairment and Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012;71:1018–1029. doi: 10.1097/NEN.0b013e318272caab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Roth K.A. Caspases, apoptosis, and Alzheimer disease: Causation, correlation, and confusion. J. Neuropathol. Exp. Neurol. 2001;60:829–838. doi: 10.1093/jnen/60.9.829. [DOI] [PubMed] [Google Scholar]
- 217.Castro M.A., Dalmolin R.J., Moreira J.C., Mombach J.C., de Almeida R.M. Evolutionary origins of human apoptosis and genome-stability gene networks. Nucleic Acids Res. 2008;36:6269–6283. doi: 10.1093/nar/gkn636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Park K.R., Lee H., Cho M., Yun H.M. A Phytochemical Constituent, (E)-Methyl-Cinnamate Isolated from Alpinia katsumadai Hayata Suppresses Cell Survival, Migration, and Differentiation in Pre-Osteoblasts. Int. J. Mol. Sci. 2020;21:3700. doi: 10.3390/ijms21103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Karousi P., Artemaki P.I., Sotiropoulou C.D., Christodoulou S., Scorilas A., Kontos C.K. Identification of Two Novel Circular RNAs Deriving from BCL2L12 and Investigation of Their Potential Value as a Molecular Signature in Colorectal Cancer. Int. J. Mol. Sci. 2020;21:8867. doi: 10.3390/ijms21228867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Asara Y., Marchal J.A., Carrasco E., Boulaiz H., Solinas G., Bandiera P., Garcia M.A., Farace C., Montella A., Madeddu R. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int. J. Mol. Sci. 2013;14:16600–16616. doi: 10.3390/ijms140816600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lanning M.E., Yu W., Yap J.L., Chauhan J., Chen L., Whiting E., Pidugu L.S., Atkinson T., Bailey H., Li W., et al. Structure-based design of N-substituted 1-hydroxy-4-sulfamoyl-2-naphthoates as selective inhibitors of the Mcl-1 oncoprotein. Eur. J. Med. Chem. 2016;113:273–292. doi: 10.1016/j.ejmech.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Valentin R., Grabow S., Davids M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood. 2018;132:1248–1264. doi: 10.1182/blood-2018-02-791350. [DOI] [PubMed] [Google Scholar]
- 223.Öztaş E., Kara M., Boran T., Bişirir E., Karaman E.F., Kaptan E., Özhan G. Cellular Stress Pathways Are Linked to Acetamiprid-Induced Apoptosis in SH-SY5Y Neural Cells. Biology. 2021;10:820. doi: 10.3390/biology10090820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nagy Z.S., Esiri M.M. Apoptosis-related protein expression in the hippocampus in Alzheimer’s disease. Neurobiol. Aging. 1997;18:565–571. doi: 10.1016/S0197-4580(97)00157-7. [DOI] [PubMed] [Google Scholar]
- 225.MacGibbon G.A., Lawlor P.A., Sirimanne E.S., Walton M.R., Connor B., Young D., Williams C., Gluckman P., Faull R.L., Hughes P., et al. Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res. 1997;750:223–234. doi: 10.1016/S0006-8993(96)01351-0. [DOI] [PubMed] [Google Scholar]
- 226.Costa I.M., Lima F.O.V., Fernandes L.C.B., Norrara B., Neta F.I., Alves R.D., Cavalcanti J.R.L.P., Lucena E.E.S., Cavalcante J.S., Rego A.C.M., et al. Astragaloside IV Supplementation Promotes A Neuroprotective Effect in Experimental Models of Neurological Disorders: A Systematic Review. Curr. Neuropharmacol. 2019;17:648–665. doi: 10.2174/1570159X16666180911123341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chang C.P., Liu Y.F., Lin H.J., Hsu C.C., Cheng B.C., Liu W.P., Lin M.T., Hsu S.F., Chang L.S., Lin K.C. Beneficial Effect of Astragaloside on Alzheimer’s Disease Condition Using Cultured Primary Cortical Cells Under β-amyloid Exposure. Mol. Neurobiol. 2016;53:7329–7340. doi: 10.1007/s12035-015-9623-2. [DOI] [PubMed] [Google Scholar]
- 228.Wang X., Xu W., Chen H., Li W., Li W., Zhu G. Astragaloside IV prevents Aβ (1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020;1747:147041. doi: 10.1016/j.brainres.2020.147041. [DOI] [PubMed] [Google Scholar]
- 229.Cui J., Shan R., Cao Y., Zhou Y., Liu C., Fan Y. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats. J. Ethnopharmacol. 2021;266:113466. doi: 10.1016/j.jep.2020.113466. [DOI] [PubMed] [Google Scholar]
- 230.Mu J.S., Lin H., Ye J.X., Lin M., Cui X.P. Rg1 exhibits neuroprotective effects by inhibiting the endoplasmic reticulum stress-mediated c-Jun N-terminal protein kinase apoptotic pathway in a rat model of Alzheimer’s disease. Mol. Med. Rep. 2015;12:3862–3868. doi: 10.3892/mmr.2015.3853. [DOI] [PubMed] [Google Scholar]
- 231.Nho J.H., Kim A.H., Jung H.K., Lee M.J., Jang J.H., Yang B.D., Lee H.J., Lee K.H., Woo K.W., Cho H.W. Water Extract of Acori Graminei Rhizoma Attenuates Features of Rheumatoid Arthritis in DBA/1 Mice. Evid. Based Complement. Alternat. Med. 2019;2019:3637453. doi: 10.1155/2019/3637453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shi R., Zhang S., Cheng G., Yang X., Zhao N., Chen C. Ginsenoside Rg1 and Acori Graminei Rhizoma Attenuates Neuron Cell Apoptosis by Promoting the Expression of miR-873-5p in Alzheimer’s Disease. Neurochem. Res. 2018;43:1529–1538. doi: 10.1007/s11064-018-2567-y. [DOI] [PubMed] [Google Scholar]
- 233.Contreras-Castro A.I., Oidor-Chan V.H., Bustamante-Camilo P., Pelayo-Zaldívar C., Díaz de León-Sánchez F., Mendoza-Espinoza J.A. Chemical Characterization and Evaluation of the Antihyperglycemic Effect of Lychee (Litchi chinensis Sonn.) cv. Brewster. J. Med. Food. 2021;25:61–69. doi: 10.1089/jmf.2021.0098. [DOI] [PubMed] [Google Scholar]
- 234.Wang X., Wu J., Yu C., Tang Y., Liu J., Chen H., Jin B., Mei Q., Cao S., Qin D. Lychee Seed Saponins Improve Cognitive Function and Prevent Neuronal Injury via Inhibiting Neuronal Apoptosis in a Rat Model of Alzheimer’s Disease. Nutrients. 2017;9:105. doi: 10.3390/nu9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shin J.W., Park H., Cho Y., Lee S., Yoon J., Maeng S. Reduced Consolidation, Reinstatement, and Renewal of Conditioned Fear Memory by Repetitive Treatment of Radix Polygalae in Mice. Front. Psychiatry. 2017;8:97. doi: 10.3389/fpsyt.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sun C., Cao X.C., Liu Z.Y., Ma C.L., Li B.M. Polygalasaponin F protects hippocampal neurons against glutamate-induced cytotoxicity. Neural Regen. Res. 2022;17:178–184. doi: 10.4103/1673-5374.314321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Battaglia S., Nazzi C., Thayer J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci. Biobehav. Rev. 2023;149:105163. doi: 10.1016/j.neubiorev.2023.105163. [DOI] [PubMed] [Google Scholar]
- 238.Battaglia S., Di Fazio C., Vicario C.M., Avenanti A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023;24:5926. doi: 10.3390/ijms24065926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tanaka M., Diano M., Battaglia S. Editorial: Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry. 2023;2023:1068. doi: 10.3389/fpsyt.2023.1225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen Z., Duan H., Tong X., Hsu P., Han L., Morris-Natschke S.L., Yang S., Liu W., Lee K.H. Cytotoxicity, Hemolytic Toxicity, and Mechanism of Action of Pulsatilla Saponin D and Its Synthetic Derivatives. J. Nat. Prod. 2018;81:465–474. doi: 10.1021/acs.jnatprod.7b00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.El Hazzam K., Hafsa J., Sobeh M., Mhada M., Taourirte M., El Kacimi K., Yasri A. An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules. 2020;25:1059. doi: 10.3390/molecules25051059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Greenspan E.M. Ginseng and vaginal bleeding. JAMA. 1983;249:2018. doi: 10.1001/jama.1983.03330390026012. [DOI] [PubMed] [Google Scholar]
- 243.Siegel R.K. Ginseng and high blood pressure. JAMA. 1980;243:32. doi: 10.1001/jama.1980.03300270020020. [DOI] [PubMed] [Google Scholar]
- 244.Coon J.T., Ernst E. Panax ginseng: A systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 245.Zhou P., Shi W., He X.Y., Du Q.Y., Wang F., Guo J. Saikosaponin D: Review on the antitumour effects, toxicity and pharmacokinetics. Pharm. Biol. 2021;59:1480–1489. doi: 10.1080/13880209.2021.1992448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lee W.S., Lee H.J., Yang J.Y., Shin H.L., Choi S.W., Kim J.K., Seo W.D., Kim E.H. The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease. Nutrients. 2022;14:4103. doi: 10.3390/nu14194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Juang Y.P., Liang P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules. 2020;25:4974. doi: 10.3390/molecules25214974. [DOI] [PMC free article] [PubMed] [Google Scholar]