Abstract
Gliomas are the most common brain tumor in adults, and molecularly targeted therapies to treat gliomas are becoming a frequent topic of investigation. The current state of molecular targeted therapy research for adult-type diffuse gliomas has yet to be characterized, particularly following the 2021 WHO guideline changes for classifying gliomas using molecular subtypes. This systematic review sought to characterize the current state of molecular target therapy research for adult-type diffuse glioma to better inform scientific progress and guide next steps in this field of study. A systematic review was conducted in accordance with PRISMA guidelines. Studies meeting inclusion criteria were queried for study design, subject (patients, human cell lines, mice, etc.), type of tumor studied, molecular target, respective molecular pathway, and details pertaining to the molecular targeted therapy—namely the modality, dose, and duration of treatment. A total of 350 studies met the inclusion criteria. A total of 52 of these were clinical studies, 190 were laboratory studies investigating existing molecular therapies, and 108 were laboratory studies investigating new molecular targets. Further, a total of 119 ongoing clinical trials are also underway, per a detailed query on clinicaltrials.gov. GBM was the predominant tumor studied in both ongoing and published clinical studies as well as in laboratory analyses. A few studies mentioned IDH-mutant astrocytomas or oligodendrogliomas. The most common molecular targets in published clinical studies and clinical trials were protein kinase pathways, followed by microenvironmental targets, immunotherapy, and cell cycle/apoptosis pathways. The most common molecular targets in laboratory studies were also protein kinase pathways; however, cell cycle/apoptosis pathways were the next most frequent target, followed by microenvironmental targets, then immunotherapy pathways, with the wnt/β-catenin pathway arising in the cohort of novel targets. In this systematic review, we examined the current evidence on molecular targeted therapy for adult-type diffuse glioma and discussed its implications for clinical practice and future research. Ultimately, published research falls broadly into three categories—clinical studies, laboratory testing of existing therapies, and laboratory identification of novel targets—and heavily centers on GBM rather than IDH-mutant astrocytoma or oligodendroglioma. Ongoing clinical trials are numerous in this area of research as well and follow a similar pattern in tumor type and targeted pathways as published clinical studies. The most common molecular targets in all study types were protein kinase pathways. Microenvironmental targets were more numerous in clinical studies, whereas cell cycle/apoptosis were more numerous in laboratory studies. Immunotherapy pathways are on the rise in all study types, and the wnt/β-catenin pathway is increasingly identified as a novel target.
Keywords: glioma, glioblastoma, molecular targeted therapy, WHO brain tumor guideline, idh-mutant glioma, astrocytoma, idh-mutant astrocytoma, oligodendroglioma, protein kinase pathway, microenvironmental targets, immunotherapy, cell cycle, apoptosis, wnt/β-catenin pathway, molecular pathway, clinical studies, animal studies, systematic review, brain tumor
1. Introduction
As the most common brain tumor in adults, gliomas have sustained the focus of scientific research for the past several decades. Recently, more attention has been drawn to the diagnostic criteria of gliomas with the restructured 2021 WHO Classification of Tumors of the Central Nervous System, specifically focusing more on molecular biomarkers as a means of categorization [1]. Within this classification adult-type diffuse gliomas are the most prevalent tumor types, defined on the basis of molecular expression of isocitrate dehydrogenase (IDH) and the 1p/19q codeletion. These glioma subtypes include astrocytoma (IDH-mutant astrocytoma), oligodendroglioma (IDH-mutant and 1p19q-codeleted), and glioblastoma (GBM) (IDH-wildtype) [1]. The typical management of adult-type diffuse glioma begins with a resection or biopsy, followed by possible radiotherapy and/or chemotherapy with the alkylating agent, temozolomide, or the combination procarbazine, lomustine, and vincristine (PCV) [2]. Even with this regimen, recurrence is prevalent, and the prognosis is dismal, particularly in GBM, which has an average survival of 14–16 months [3].
As gliomas are becoming more molecularly defined, so too is their treatment progressing more towards the targeting of molecular pathways [4]. Compared with traditional chemotherapeutic drugs, molecularly targeted antitumor therapy has the advantage of strong specificity with minimal damage to normal tissues. Molecular-targeted glioma therapies have gained traction in the scientific literature, with many analyses centered on identifying mechanisms pertinent to glioma growth [5]. The Raf/MEK/Erk pathway has been of particular interest as a targetable pathway due to its preponderance among gliomas [5]. Additionally, a systematic review by Da Silva et al. highlighted the molecular targeted therapies in clinical trials for GBM, identifying four categories of targets: targeting the potential for unlimited replication, growth autonomy and migration, cell cycle and apoptosis, and angiogenesis [6].
To date, there has yet to be a systematic review of the literature characterizing molecular targeted therapy in adult-type diffuse gliomas. A comprehensive understanding of the progress in this field—both in terms of existing therapies and novel targets—is integral to guiding advancement in treatment development, integration into clinical trials, and more adequate treatment options for diffuse glioma patients.
2. Methods
A systematic review was performed, characterizing the current state of molecular targeted therapies for gliomas, to inform scientific progress and guide advancement in this area of study. The protocol was conducted in accordance with PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.
2.1. Search Strategy
A literature search of English-text articles was conducted through January 2023 using PubMed and Web of Science. Categories of concepts related to both molecular targeted therapy and glioma, both adhering to the 2021 WHO classification as well as prior classifications (including language such as low- or high-grade glioma), were searched; results were combined via Boolean operators (Appendix A).
Additionally, a search with the same search terms was conducted on clinicaltrials.gov to assess clinical trials relating to molecular targeted therapy for adult-type diffuse glioma.
2.2. Selection Criteria
Article titles and abstracts were screened for relevance by two authors (L.M. and N.K.G.), and duplicates were removed. The remaining articles were then screened in full text by three authors (L.M., N.K.G., and N.C.). Inclusion criteria used were: any clinical or laboratory studies testing molecular targeted therapies for glioma or laboratory studies identifying novel molecular targets for glioma with a target of adult-type diffuse glioma or its subtypes. Exclusion criteria used were: brain tumors other than adult-type diffuse glioma—such as medulloblastoma; any study of pediatric tumor focus, etc.; papers centering on the delivery technology rather than the molecular target; systemic therapies or adjuvants to molecular targeted therapies; studies with no molecular target identified; or papers that were correspondences, reviews, or commentaries. Conflict resolution at all stages of article selection was via discussion between authors.
2.3. Data Extraction
The data extraction for the systematic review included the following: first author, year of publication, design (multi-institutional retrospective analysis, in vivo, in vitro, and in vivo, etc.), study subject (patients, human cell lines, mice, etc.), ages of subjects, type of tumor studied, molecular target, respective molecular pathway, and the modality and results of the molecular targeted therapy investigated.
The data extraction for clinicaltrials.gov was limited to ongoing trials—defined as those with a status of completed, recruiting, or active, non-recruiting. The extracted variables included the following: title of the study, year started and year of most recent update, tumor type, NCT number, sponsoring or collaborating organization, molecular target of interest, intervention utilized, as well as the phase of the study (Phase 1, 2, or 3), status (active or recruiting), funding sources (NIH, industry, or other), and results, if available.
2.4. Data Categorization
Published studies were then divided into three main categories: clinical studies testing existing molecular targeted therapies; laboratory studies testing existing molecular targeted therapies; and laboratory studies identifying a novel molecular target.
Unless specifically stated otherwise in the study, tumor types were classified by molecular associations with respective cell lines in the literature. For instance, those classified under GBM included cell lines known to harbor wild-type IDH (U87, U251, T98G, and A172) and human tumors classified specifically as GBM [7,8,9,10,11]. In instances where molecular mutation information was not readily available or numeric glioma grading was utilized (grades I–IV), these were classified as simply “Glioma”.
To assess for 3-dimensional (3D) or spheroid technologies in laboratory studies of existing therapies, a full text search of terms related to these technologies—“sphere”, “spheroid”, “3D”, “3-D”, “3-Dimensional”—was also conducted.
Ongoing clinical trials were also queried in this manner and organized by tumor type.
2.5. Statistical Analysis
A meta-analysis was not conducted; therefore, descriptive data is reported for most variables in this study. To compare means by group, ANOVA testing was utilized. The chosen type 1 error rate was set to p < 0.05. All statistical analyses were performed via IBM SPSS Statistics for Macintosh (version 28.0.1.1) (Armonk, NY, USA).
2.6. Quality Assessment
The quality of evidence was determined by study design and graded using a level of evidence scheme adapted from Ackley et al. (Table 1) [12].
Table 1.
Level of Evidence and Quality Assessment.
| Level of Evidence (LoE) | Description |
|---|---|
| Level I | Evidence from a systematic review or meta-analysis of randomized control trials (RCTs) or evidence-based clinical practice guidelines based on RCTs. |
| Level II | Evidence obtained from at least one well-designed RCT (e.g., a large multi-site RCT). |
| Level III | Evidence obtained from well-designed controlled trials without randomization (i.e., quasi-experimental). |
| Level IV | Evidence from well-designed case-control or cohort studies. |
| Level V | Evidence from systematic reviews of descriptive and qualitative studies. |
| Level VI | Evidence from a single descriptive or qualitative study. |
| Level VII | Evidence from the opinions of authorities and/or reports of expert committees. |
Level of effectiveness rating scheme adapted from Ackley et al. 2007 [12].
3. Results
3.1. Search Results
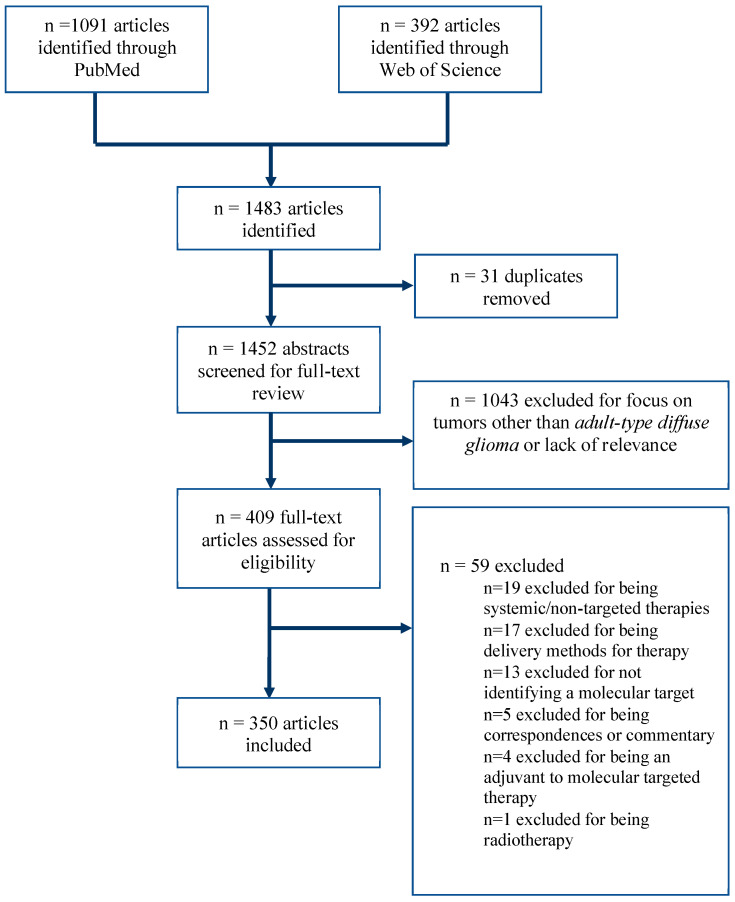
This study identified 350 articles for inclusion. (Figure 1) Data extraction for each respective category is detailed. Only 15% (52/350) of the total articles were clinical studies (Table 2). The majority of articles (54%, 190/350) were laboratory studies investigating existing molecularly targeted therapies (Table 3), and 31% (108/350) were laboratory studies identifying new molecular targets without testing an existing therapy (Table 4). Across these groups, clinical studies had a more recent median publication year (2017) compared to both laboratory studies testing existing therapies (2015) and laboratory studies identifying novel targets (2016; p < 0.05).
Figure 1.
PRISMA flow diagram, demonstrating search pathway results and included articles.
Table 2.
Summary of clinical studies implementing molecular targeted therapies in glioma.
| Study Author | Year | Tumor Type | Molecular Target | Intervention | Finding |
|---|---|---|---|---|---|
| Protein Kinase Pathways | |||||
| Berzero et al. [13] | 2021 | GBM, IDH-mutant Astrocytoma | RAF + MEK | Vemurafenib, Dabrafenib, Cobimetinib, Trametinib | The study highlights the long-term clinical benefits of RAFi/MEKi in adult patients with BRAF V600-mutant GGNTs |
| Butowski et al. [14] | 2010 | GBM | Protein kinase C-beta + PI3K/Akt | Enzastaurin + TMZ | Enzastaurin 250 mg/day given concomitantly with RT and temozolomide or adjuvantly with temozolomide was well tolerated |
| Chinnaiyan et al. [15] | 2013 | GBM | mTOR | Everolimus + TMZ + RT | Daily oral everolimus (10 mg) combined with both concurrent radiation and temozolomide, followed by adjuvant temozolomide, is well tolerated with an acceptable toxicity profile |
| Drobysheva et al. [16] | 2017 | IDH-mutant Astrocytoma | BRAF + MAPK | Dabrafenib + trametinib | PT1 and 2 were treated with MAPK and BRAF inhibitors and both showed marked responses, with PT1 only having a small residual abnormal signal at the primary tumor site and PT2 improving to stable disease |
| Franceschi et al. [17] | 2012 | GBM, IDH-mutant Astrocytoma | Src kinase | Dasatinib | Combination of CCNU and dasatinib showed significant hematological toxicities and led to suboptimal exposure to both agents |
| Fusco et al. [18] | 2021 | GBM, IDH-mutant Astrocytoma, Oligodendroglioma | BRAF + MEK | dabrafenib + Trametinib | Combination of BRAF/MEK inhibition has the potential to offer clinical benefit in both low-grade and high-grade gliomas |
| Hottinger et al. [19] | 2019 | Astrocytoma | MAPK + ERK | Dabrafenib + Trametinib | Reports and efficacy of dual BRAF/MEK inhibition in BRAF-mutated glioma |
| Johanns et al. [20] | 2018 | GBM | BRAF + MEK | Dabrafenib + Trametinib | PT1: 11mo therapy improved hemiparesis, speech, and functional status, after which the disease progressed and treatment was discontinued PT2: 3 mo therapy caused rapid response, allowing him to ambulate again, though he discontinued therapy and died shortly after |
| Kaley et al. [21] | 2018 | GBM, IDH-mutant Astrocytoma, and other gliomas | BRAF | Vemurafenib | BRAFv600 inhibition is a viable strategy, with a confirmed clinical benefit for 37.5% of patients and a best response of stable disease or better in 16/24 patients |
| Kanemaru et al. [22] | 2019 | Epithelioid GBM | BRAF + MEK | Dabrafenib and Trametinib | Dabrafenib and trametinib with radiation elicited a dramatic response in a patient with epithelioid GBM |
| Kebir et al. [23] | 2019 | GBM, IDH-mutant Astrocytoma | Multitarget kinase | Regorafenib | Study indicates a very poor performance of regorafenib in recurrent high-grade astrocytoma |
| Kleinschmidt-DeMasters et al. [24] | 2015 | GBM, IDH-mutant Astrocytoma | BRAF V600E kinase | Vemurafenib | E-GBMs can respond to targeted therapy |
| Lapointe et al. [25] | 2020 | GBM, IDH-mutant Astrocytoma | mTORC1/2 | Vistusertib + TMZ | Combination of vistusertib with TMZ in GBM patients at first recurrence demonstrated a favorable safety profile at the tested dose levels |
| Lee et al. [26] | 2012 | GBM | Multitarget kinase + mTOR | Sorafenib + Temsirolimus | Minimal activity in recurrent glioblastoma multiforme was seen at the MTD of the two combined agents |
| Lombardi et al. [27] | 2019 | GBM | Multitarget kinase + mTOR | Regorafenib | REGOMA showed an encouraging overall survival benefit of regorafenib in recurrent GBM |
| Mason et al. [28] | 2012 | GBM | mTOR1 | Everolimus + TMZ | Daily oral everolimus for 5 consecutive days every 28 days plus 150 mg/m2/day TMZ is an appropriate phase II dose for everolimus + TMZ |
| Migliorini et al. [29] | 2017 | Xanthoastrocytoma | BRAF + MEK | Dabrafenib + Trametinib | A patient with a refractory case of pleomorphic xanthoastrocytoma was treated with dual BRAF and MEK inhibition and exhibited a strong radiologic response |
| Rosenberg et al. [30] | 2022 | GBM, IDH-mutant Astrocytoma, and other gliomas | BRAF; BRAF + MEK | Vemurafenib + Dabrafenib + Trametinib | BRAF inhibition for BRAF-mutant glioma is a promising treatment paradigm; currently being evaluated prospectively in ACNS1723 clinical trial |
| Sanai et al. [31] | 2018 | GBM | Wee1K | AZD1775 | AZD1775 reaches therapeutic concentrations in contrast-enhancing areas of GBM in humans and is well tolerated |
| Schiff et al. [32] | 2015 | GBM or anaplastic astrocytoma | MET and VEGFR2 | Cabozantinib | Cabozantinib with TMZ and RT is well tolerated and warrants further evaluation |
| Shah et al. [33] | 2007 | Glioma | PDGFR | Imatinib and Hydroxyurea | Combining imatinib with hydroxyurea is effective in some glioma patients but is associated with dangerous myelosuppression |
| Shi et al. [34] | 2019 | IDH-wt, 1p19q co-deleted Glioma | BRAF V600E | Vemurafenib + Everolimus | Successfully treated a BRAF V600E-mutated anaplastic oligoastrocytoma with multiple extraneural metastases with vemurafenib and everolimus |
| Werner et al. [35] | 2022 | Glioma | Multitarget kinase | Regorafenib | Regorafenib is effective in recurrent grade III and IV gliomas, despite a high prevalence of level III and IV side effects |
| Wick et al. [36] | 2019 | GBM | ALK CDK4/6 mTOR MDM2 SHH |
Alectinib Palbociclib Temsirolimus Idasanutlin Vismodegib |
NCT Neuro Master Match (N2M2) trial Molecular signatures of GBM inform the treatment arm |
| Yau et al. [37] | 2020 | Ganglioglioma | BRAF + MEK | Vemurafenib and Cobimetinib | Combination BRAF and MEK inhibition is safe and feasible in a BRAF V600E unresectable ganglioglioma |
| Zustovich et al. [38] | 2013 | GBM | Multitarget kinase | Sorafenib | Combining sorafenib and temozolomide is feasible and safe and has activity in patients with relapsed GBM |
| Microenvironmental Targets (angiogenesis, cell-cell adhesion, iron/cation regulation) | |||||
| Badruddoja et al. [39] | 2017 | GBM | VEGF | Bevacizumab + TMZ | Bevacizumab plus bi-weekly temozolomide was well tolerated and may be a salvage regimen in recurrent glioblastoma |
| Brown et al. [40] | 2016 | GBM | VEGFR + EGFR | Cediranib + Gefitinib/placebo | Despite being underpowered with recruitment issues, this trial shows combining cediranib and gefitinib leads to increased PFS |
| Clarke et al. [41] | 2014 | GBM | VEGF + tyrosine kinase | Bevacizumab + Erlotinib | The combining of bevacizumab/erlotinib/ TMZ/radiotherapy appears to be well tolerated and improved progression-free survival but did not improve overall survival |
| D’Alessandris et al. [42] | 2013 | GBM | VEGF + EGFRvIII | Bevacizumab + Erlotinib | Obtained higher RR and PFS at 6 months (70%) than those reported in prior trials lacking molecular tumor analysis |
| Desjardins et al. [43] | 2012 | GBM | VEGF | Bevacizumab | Demonstrates that combined daily temozolomide and biweekly bevacizumab had some activity and was well tolerated |
| Hasselbalch et al. [44] | 2010 | GBM | EGFR, VEGF, topoisomerase I | Cetuximab + bevacizumab + irinotecan | None of the biomarkers tested alone or in combination could identify a patient population likely to benefit from bevacizumab and irinotecan, with or without the addition of cetuximab |
| Lassen et al. [45] | 2015 | GBM | Placental growth factor (PlGF) + VEGF | RO5323441 + Bevacizumab | Toxicity profile of RO5323441 plus bevacizumab was acceptable and manageable but not superior to bevacizumab alone |
| Lu et al. [46] | 2014 | GBM, Astrocytoma | VEGF | Bevacizumab + TMZ | After BEV treatment, most patients obtain more significant short-term responses with good toleration |
| Prados et al. [47] | 2009 | GBM or Gliosarcoma | EGFR | Erlotinib + TMZ + RT | Patients treated with erlotinib + TMZ + RT had improved survival |
| Vaccaro et al. [48] | 2014 | Glioma | VEGF | Bevacizumab | Bevacizumab and fotemustine showed anti-glioma activity and good tolerability among recurrent glioma patients |
| Vredenburgh et al. [49] | 2012 | GBM | VEGF | Bevacizumab + RT + TMZ | Addition of bevacizumab to the standard TMZ and RT regimen is associated with minimal toxicity |
| Wang et al. [50] | 2014 | GBM | EGFR | Nimotuzumab + TMZ + RT | Nimotuzumab, TMZ, and RT are safe therapeutic regimens, with similar survival times to other regimens |
| Wang et al. [51] | 2017 | Grade III and IV Glioma | VEGFR2 | Apatinib + Irinotecan | Apatinib plus irinotecan is a potentially useful combination therapy and should be further evaluated |
| Weller et al. [52] | 2017 | GBM | EGFR | TMZ +/− Rindopepimut | Rindopepimut did not reduce mortality as a monotherapy in newly diagnosed GBM, so it may be necessary to use it in combination therapy |
| Wick et al. [53] | 2020 | Glioma | TGF β | TMZ+RT +/− galunisertib | There was no difference in safety or efficacy between the standard therapy and the standard plus galunisertib |
| Immunotherapy Pathways | |||||
| Anghileri et al. [54] | 2021 | GBM | PD1 | Nivolumab | Nivolumab is useful for patients, despite a RCT failing to show overall benefits |
| Nayak et al. [55] | 2021 | GBM | PD1 + VEGF | Pembrolizumab + Bevacizumab | Pembrolizumab +/− bevacizumab is not an effective therapy |
| Reardon et al. [56] | 2020 | GBM | PD1 | Nivolumab | Nivolumab monotherapy in GBM was equally safe and effective as bevacizumab monotherapy |
| Cell Cycle/Apoptosis/Transcription Pathways | |||||
| Brachman et al. [57] | 2015 | GBM or Gliosarcoma | Thioredoxin + ribonucleotide reductases | Motexafin Gadolinium + TMZ + RT | Combining standard RT with TMZ and MGd did not achieve a significant survival advantage |
| Kubicek et al. [58] | 2009 | GBM, Astrocytoma | 26S Proteasome | Bortezomib | Bortezomib administered at its typical “systemic” dose (1.3 mg/m2) is well tolerated and safe in combination with TMZ and RT |
| Lin et al. [59] | 2020 | IDH-mutant Astrocytoma | CDK4 | Palbociclib | First case of spinal cord tumor reported to demonstrate an association between CDK4 amplification and response to Palbociclib-based combination therapy even after multiple recurrences |
| Other | |||||
| Desjardins et al. [43] | 2011 | GBM | Farnesyl transferase | SCH 66336 | The phase II dose of SCH 66336 when combined with standard 5-day temozolomide is 150 mg twice daily for patients on stratum A and 175 mg twice daily for patients on stratum B |
| Geletneky et al. [60] | 2017 | GBM | Protein NS1 | Rat H-1 parvovirus (H-1PV) | Confirms H-1PV safety, tolerability and ability to cross the blood-brain barrier; favorable PFS compared with controls |
| Hashimoto et al. [61] | 2015 | GBM | WT1 (Wilms Tumor 1) | WT1 peptide vaccination + TMZ | Safety profile of the combined Wilms tumor 1 peptide vaccination and temozolomide therapy approach for treating glioblastoma was confirmed |
| Patel et al. [62] | 2012 | Glioma | ER | Tamoxifen + TMZ | The maximum tolerated dose of tamoxifen + TMZ + RT was 100 mg/m2 |
| Sauter et al. [63] | 2022 | GBM | CSF1R, ABL, cKIT, PDGFR | Imatinib | Imatinib showed no effect on GBM |
Abbreviation: RCT, randomized control trial; RAF, rapamycin associated factor; MEK, mitogen-activated protein kinase kinase; TMZ, temozolomide; PI3K/Akt, phosphoinositide-3-kinase/protein kinase B; GBM, glioblastoma multiforme; RT, radiation therapy; mTOR, mechanistic target of rapamycin; PT1; BRAF, v-raf murine sarcoma viral oncogene homolog B; MAPK, mitogen-activated protein kinase; CCNU, lomustine; ERK, extracellular signal-regulated kinase; e-GBM, epithelioid glioblastoma multiforme; MET, mesenchymal-epithelial transition factor; VEGFR, vascular endothelial growth factor receptor; PFS, progression-free survival; PT, patient; RR, response rate; CDK, cyclin-dependent kinase; MDM2, mouse double minute 2 homolog; SHH, sonic hedgehog; TGF-β, transforming growth factor-beta; PD1, programmed cell death protein 1; cSF1R, colony-stimulating factor 1 receptor; ABL, Abelson tyrosine-protein kinase 1; cKIT, receptor tyro-sine-protein kinase Kit; PDGFR, platelet-derived growth factor receptor.
Table 3.
Summary of Laboratory Studies Implementing Molecular Targeted Therapies. All studies were level III evidence.
| Study Author | Year | Tumor Type | Molecular Target | Intervention | Finding |
|---|---|---|---|---|---|
| Protein Kinase Pathways | |||||
| Aldea et al. [64] | 2014 | GBM | mTOR + RAF | Metformin + Sorafenib | Combining metformin and sorafenib is an effective treatment for TMZ-resistant glioblastoma cells |
| Aoki et al. [65] | 2013 | GBM | Ras | Nobiletin | Nobiletin inhibits Ras activity in C6 glioma cells |
| Arcella et al. [66] | 2013 | GBM | mTOR | Rapamycin | mTOR is upregulated in GBM and rapamycin represents a good inhibitor |
| Ariey-Bonnet et al. [67] | 2020 | GBM | MAPK14 | BMZ | BMZ (Benzimidazole) is a potent inhibitor of MAPK14, which would directly contribute to its anticancer properties |
| Balkhi et al. [68] | 2016 | GBM | Multitarget kinases | Caffeic Acid Phenethyl Ester (CAPE) + Dasatinib | Combinational therapy inhibits migration and invasiveness and decreases cell survival |
| Barbarisi et al. [11] | 2018 | GBM | CD44 | Quercetin + TMZ | CD44 targeted nanocarriers mediate site-specific delivery of quercetin via the CD44 receptor in GBM |
| Benezra et al. [69] | 2012 | GBM | Multitarget kinases | Dasatinib | Dasatinib has a significant survival benefit in vivo for mouse GBM |
| Camorani et al. [70] | 2015 | GBM | EGFRvIII | CL4 Aptamer + EGFR Tkis | CL4 and gefitinib cooperate with the anti-PDGFRβ Gint4.T aptamer in inhibiting cell proliferation. |
| Chen et al. [71] | 2019 | GBM | CD163 pathway (CK2, kinase) | TBB | By inhibiting CK2 with TBB (4,5,6,7-tetrabromo-1H-benzotriazole), it shows the CD163 pathway is crucial for tumor growth |
| Cheng et al. [72] | 2022 | GBM | CTSC | Piperlongumine + Scopoletin | CTSC (Cysteine cathepsin C) is a biomarker using the MAPK signaling pathway; inhibition with piperlongumine (more effective) and scopoletin decreases tumor growth |
| Ciesielski et al. [73] | 2018 | GBM | Src-kinase + tubulin polymerization inhibitory activity | Kx2-361 | The drug is active in vivo against orthotopic GL261 gliomas in syngeneic C57BL/6 mice |
| Cloninger et al. [74] | 2011 | GBM | SAPK2/p38 + mTORC1 | SB203580 + Rapamycin | Data support the combined use of SAPK2/p38 and mTORC1 inhibitors to achieve a synergistic antitumor therapeutic response |
| Combs et al. [75] | 2007 | GBM and Glioma | EGFR | Cetuximab | Triple combination of TMZ, RT, and cetuximab might be a promising multimodality treatment approach for patients with GBM |
| Dasgupta et al. [76] | 2015 | BRAF V600E GBM | BRAF V600E | Plx4720 + RT | Provide pre-clinical rationale for clinical trials of concurrent RT and BRAF V600E inhibitors |
| Dantas-Barbosa et al. [77] | 2015 | GBM and Ependymoma | mTOR | Γ-Secretase Inhibitor RO4929097 | RO4929097, through mTOR inhibition, potentiates cytotoxicity in vitro but does not enhance antitumor effects in vivo |
| Davare et al. [78] | 2018 | GBM and other cell types | ROS1 | Lorlatinib | ROS1 knockdown with lorlatinib resulted in powerful responses in mice |
| Di Stefano et al. [79] | 2015 | GBM | FGFR kinase | JNJ-42756493 | JNJ-42756493 elicited potent growth inhibition and significant tumor regression after two weeks |
| Dominguez et al. [80] | 2013 | GBM | DGK-α | R59022 + R59949 + siRNA | DGK-α is a potential therapeutic glioma target linked to multiple key pathways |
| Du et al. [81] | 2012 | GBM | Raf/MEK/ERK signaling pathway | Sorafenib + Vitamin K (VK1) | Combining sorafenib with VK1 induced apoptosis through downregulating proapoptotic proteins Bcl-2 and Mcl-1 |
| Emlet et al. [82] | 2014 | GBM | EGFRvIII + CD133 | Egfrviii + CD133 AB | EGFRvIII + CD133 BsAb allow for the specific targeting of cancer stem cells |
| Farrell et al. [83] | 2017 | GBM | MET | WO2010/019899A1 + PF04217903 + Crizotinib | Dual targeting of HGF and MET by combining extracellular ligand inhibitors with intracellular MET TKIs could be an effective intervention |
| Feng et al. [84] | 2010 | GBM | PI3K/Akt; JNK; ERK | Tamoxifen | Mechanism of TAM-induced apoptosis reveal PI3K/Akt, JNK, and ERK as potential targets |
| Glassman et al. [85] | 2021 | GBM, Oligodendroglioma | MAPK kinase | U0126 | Combining molecularly targeted therapies interferes more efficiently with glial tumor development and progression |
| Goker et al. [86] | 2020 | GBM | ALK | AZD3463 + TMZ | Combining TMZ with AZD3463 may increase the efficacy of a single TMZ treatment in GBM |
| Golubovskaya et al. [87] | 2013 | GBM | FAK | Y15 | Blockade of FAK autophosphorylation with the oral administration of a small-molecule inhibitor, Y15, has the potential to be an effective therapy approach for GBM |
| Grossauer et al. [88] | 2016 | Glioma | BRAF/MEK | Dabrafenib + Trametinib | BRAF and MEK combination therapy helps to prevent MAPK reactivation during treatment |
| Gursel et al. [89] | 2011 | GBM and IDH-mutant Astrocytoma | PI3K/Akt | PI103/Pcn | PI-103 and TCN are sensitive inhibitors of the PI3K/Akt/mTOR pathway |
| He et al. [90] | 2016 | GBM | MEK2 | MEK2 Antibody | MEK2 antagonists can be used as chemo-sensitizers to enhance the treatment efficacy of TMZ |
| Hjelmeland et al. [91] | 2007 | Astrocytoma | Raf + TOR | LBT613 + Everolimus | Combining LBT613 and RAD001 reduces glioma cell proliferation and invasion |
| Hong et al. [92] | 2014 | GBM | Aurora-A kinase | Alisertib | Inhibiting aurora-A kinase potentiatesthe effects of ionizing radiation on glioblastoma cells |
| Jiang et al. [93] | 2018 | GBM, other cell types | EGFR/EGFRvIII | EGFR/EGFRviii CAR T Cells | EGFR/EGFRvIII CAR T cells have strong anti-tumor and tumor-specific properties |
| Jin et al. [94] | 2013 | GBM | Akt + NOTCH | MRK003 + MK-2206 | Akt and NOTCH inhibition decrease glioma proliferation |
| Joel et al. [95] | 2015 | GBM | PBK/TOPK | Hi-Topk-032 | HITOPK-032 resulted in diminished tumor growth |
| Joshi et al. [96] | 2012 | GBM | Multitarget kinases | Gefitinib + Erlotinib + Sunitinib | Drug combinations containing sunitinib were most effective in vitro but not in vivo |
| Ju et al. [97] | 2016 | GBM | COX-2 | Celecoxib | Targeting epirubicin plus celecoxib liposomes was able to effectively destroy the glioma VM channels and exhibited significant efficacy in glioma |
| Junca et al. [98] | 2017 | GBM | ALK, ROS1, MET | Crizotinib | MET and ALK are overexpressed in glioma; crizotinib is a potential molecularly targeted strategy |
| Jung et al. [99] | 2014 | GBM | FOXO3A | Z-Ajoene | Z-ajoene specifically targets glioma CSCs through the FOXO3A pathway |
| Kawauchi et al. [100] | 2021 | GBM | ALK | Alectinib + Ceritinib | Treatment with the second-generation ALK inhibitors, alectinib and ceritinib, might serve as a potent therapeutic strategy against GBM |
| Kim et al. [101] | 2012 | GBM, Astrocytoma | Phosphoinositide 3-kinase/Akt + Ras/Raf | 5-Bromo-3-(3-Hydroxyprop-1-Ynyl)-2H-Pyran-2-One (BHP) | BHP targets GSCs and enhances their sensitivity to anticancer treatments |
| Koul et al. [102] | 2005 | GBM | Integrin-linked kinase | QLT0276 In DMSO | ILK inhibition down-regulates multiple pathways involved in proliferation and invasion |
| Koul et al. [103] | 2010 | GBM | PI3K/Akt | Px-866 | PX-866 inhibits growth, induces G1 arrest and apoptosis, and is safe and effective in mouse models |
| Liu et al. [104] | 2011 | GBM | bFGF | Anti bFGF siRNA | bFGF (basic fibroblast growth factor) siRNA is a possible treatment for glioma |
| Liu et al. [105] | 2014 | GBM | EGFR and PI3K/Akt | G19 | G19 acts on the EGFR and PI3K/Akt pathways and causes redox stress to kill glioma cells |
| Liu et al. [106] | 2014 | GBM | AMPK | Compound C | Compound C is an extremely potent antiglioma agent, though does not exclusively inhibit AMPK |
| Luchman et al. [107] | 2014 | GBM | mTOR1/2 | AZD8055 | Dual inhibition of mTOR1/2 with AZD8055 plus TMZ shows promise as a second-line treatment, especially in TMZ-resistant GBM |
| Ma et al. [108] | 2015 | GBM | STAT3 | Tetrandrine | Tetrandrine inhibits glioma growth dose-dependently while not affecting the development of chick embryos |
| Matsuda et al. [109] | 2012 | GBM | JNK | Sp600125 | JNK is involved in the development of stem-like potential in GBM cells and is an attractive target |
| Maxwell et al. [110] | 2021 | GBM | mTOR1/2 + MEK | TAK228 + Trametinib | Treatment with mTOR1/2 and MEK inhibitors induces various proteomic changes in gliomas |
| Nicolaides et al. [111] | 2011 | Astrocytoma | BRAF | Plx4720 | BRAF inhibition as a treatment for astrocytoma is highly supported by preclinical findings |
| Paternot et al. [112] | 2009 | GBM | mTOR1 + MEK1/2 | Rapamycin + PD184352 | Combined inhibition of mTOR1 and MEK1/2 should be considered in tumors with dysregulated CDK4 |
| Peng et al. [113] | 2013 | GBM | RACK1-PKC | siRNA | RACK1 is involved in glioma development via SRC/Akt activity |
| Pezuk et al. [114] | 2013 | GBM | PLK1 | Bi2536 + Tmz | PLK1 is a promising molecular target, and inhibition + TMZ is effective in vitro |
| Phillips et al. [115] | 2016 | GBM and epidermoid carcinoma | EGFR | Abt-414 | ABT-414 (antibody and MMAF fusion) is effective in treating a wide range of EGFR genotypes and can be advanced to phase I/II clinical trials |
| Premkumar et al. [116] | 2010 | GBM | IGF1R + Src | NVP-AEW541 + Dasatinib | Combined IGF1R and Src inhibition synergistically increased apoptosis in glioma cells without affecting normal astrocytes |
| Qin et al. [117] | 2014 | GBM | EMP2 | Anti-EMP2 antibodies/Anti-EMP2 Igg1 | EMP2 (epithelial membrane protein-2) promotes cell migration/invasion through protein kinases; inhibition kills tumor cells |
| Raub et al. [118] | 2015 | GBM | CDK4 + CDK6 | Abemaciclib Or Palbociclib + TMZ | Ademacicib with TMZ synergistically increased rat survival time |
| Salphati et al. [119] | 2012 | GBM | PI3K | Gne-317 | GNE-317 is a PI3K inhibitor designed to cross the blood brain barrier; represents a treatment option for GBM |
| Sathornsumetee et al. [120] | 2006 | GBM | BRAF, CRAF, VEGFR | AA1881 | AAL881 treatment showed tumor growth retardation in xenograft tumors and was well tolerated by mice |
| See et al. [121] | 2012 | GBM | MEK + PI3K/mTOR | Vemurafenib + PI103 | NF1-deficient GBM cell lines that are MEK inhibitor resistant respond well to dual therapy with MEK and PI3K/mTOR inhibition |
| Selvasaravanan et al. [122] | 2020 | GBM | MEK or PI3K | Trametinib + Pictilisib | MEK inhibition is not superior to PI3K inhibition, though MEK may have a use in combination therapy |
| Shingu et al. [123] | 2015 | GBM | MEK, EGFR, PI3K | Various Small Molecule Inhibitors | The most synergistic combinations of drugs affected RTKs and either MEK/ERK or PI3K |
| Siegelin et al. [124] | 2010 | GBM | BRAF | Sorafenib | sorafenib has potent in vivo and in vitro anti-glioma activity |
| Signore et al. [125] | 2014 | GBM | PDK1 + CHK1 | UCN-01 | UCN-01 downregulates PDK1 and CHK1, effectively killing tumor cells |
| Spino et al. [126] | 2019 | IDH-mutant Astrocytomas | DLL3 | Rovalpituzumab Tesirine | DLL3 is selectively and homogeneously expressed in IDH-mutant astrocytomas and can be targeted with available MABs |
| Thanasupawat et al. [127] | 2017 | GBM | FGFR | Dovitinib | Alternation of dovitinib and TMZ reduces GBM viability independent of MGMT and p53 status |
| Thompson et al. [128] | 2018 | PXA | Various | Various Antibodies + Kinase Inhibitors + Chemo Drugs | Bevacizumab, TMZ, and irinotecan should be considered as adjuvant therapies for PXA, though MEK and TK inhibitors should be investigated as well |
| Tsigelny et al. [129] | 2017 | GBM | OLIG2 | SKOG102 | SKOG102 exhibited potent anti-glioma activity in vivo and in vitro by downregulating OLIG2 |
| van den Heufel [130] | 2017 | PDX astrocytoma | MET | Compound A | Compound A prolonged survival of mice did not stop eventual progression |
| Wang J et al. [131] | 2013 | Glioma | MEK1 | Mir-181b + TMZ | miR181b enhances the sensitivity of glioma cells to TMZ by downregulating MEK1 |
| Wang et al. [132] | 2014 | GBM | RAS | Mir-143 | miR-143 is downregulated in glioma and involved in the inactivation of RAS |
| Wang et al. [133] | 2019 | Glioma Stem Cells | EGFR or PI3K and DHODH | Lapatinib + BKM120 + Teriflunomide | Combined targeting of intrinsic synthetic enzymes reduces pyrimidine synthesis; presents an effective glioma paradigm |
| Wichmann et al. [134] | 2015 | GBM | EGFR and HER2 | siRNA + Cetuximab + Trastuzumab | siRNA knock-down of EGFR and HER2 reduced the growth rate of GBM |
| Yan et al. [135] | 2017 | GBM | CSF-1R + cKIT + RTKs | PLX3397 + Vatalanib + Dovitinib | PLX3397 is an effective monotherapy and improves the efficacy of multiple tyrosine kinase inhibitors |
| Yang et al. [136] | 2008 | GBM | EGFR | Boronated EGFR MAB + Cetuximab | Both EGFR and EGFRvIII tumors must be targeted by a combination of boronated MAB and boronated cetuximab |
| Yao et al. [137] | 2015 | GBM | EGFR and BRAF | BRAF(V600E) Inhibitor PLX4720 | Inhibiting EGFR and BRAF(V600E) decreased tumor cell proliferation, increased apoptosis, and extended survival |
| Zavalhia et al. [138] | 2014 | Ependymomas and oligodendromas | cKIT | Imatinib | C117+ tumors are susceptible to imatinib, and its use in their treatment should be further investigated |
| Zhang et al. [139] | 2015 | GBM | mGluR1 | siRNA, Selective Antagonists Riluzole + BAY36-7620 | Anti-tumor activity of mGluR1 inhibition in vivo was demonstrated |
| Zhang et al. [140] | 2016 | GBM | HER2 | HER2 Specific NK Cells | Modified HER2-specific NK cells are effective against GBM |
| Zhang et al. [139] | 2017 | Glioma | BRAF V600E + MEK | PLX4032 + GDC0973 | Combined BRAF V600E and MEK inhibition prevents tumor rebound by MAPK activation in glioma |
| Cell Cycle/Apoptosis/Transcription Pathways | |||||
| Bychkov et al. [141] | 2020 | GBM | S100A9 (one of the heterodimers for calprotectin) | shRNA | Mambalgin-2 inhibits glioma and GBM cells but not normal astrocytes |
| Chen et al. [142] | 2013 | GBM and Glioma Stem Cells | IGFBP3 | IGFBP3 siRNA | S100A9 knockdown demonstrates a new anticancer strategy |
| Chen et al. [143] | 2019 | GBM | HDAC/EZH2 | Compound 26/UNC1999 | IGFBP3 depletion is a potential therapy through the induction of DNA damage and apoptosis |
| Grinshtein et al. [144] | 2016 | GBM | BAG3 | BAG3 siRNA | HDAC and EZH2 inhibition in combination lead to synergistic effects in vitro |
| Festa et al. [145] | 2011 | GBM and IDH-mutant Astrocytoma | miR-27a (FOXO3a) | Antagomir-27a | BAG3 is highly expressed in gliomas; effective therapeutic target |
| Ge et al. [146] | 2013 | GBM | Tumor checkpoint controller targeting microtubules | BAL101553 | MiR-27a may be up-regulated in human glioma, and antagomiR-27a of could inhibit proliferation and invasion ability |
| Genoud et al. [147] | 2021 | GBM | PAK5 | PAK5 shRNA | BAL101553 is a promising therapeutic agent for glioblastoma and could synergize with innate immune stimulation |
| Gu et al. [148] | 2015 | GBM | DR4/5 | TRAIL + Doxorubicin | PAK5 is overexpressed in glioma, and its inhibition blocks anti-apoptotic signals and promotes arrest |
| Guo et al. [149] | 2011 | GBM | CDK 4/6 + PDGFRα | Lenvatinib + Crenolanib + Abemaciclib + Palbociclib | TRAIL-LP and DOX-LP displayed stronger antiGBM effects than free drugs or liposomal drugs alone in vivo |
| Hamada et al. [150] | 2022 | Embryonic Kidney Cells | Procaspase-3 | PAC-1 (*Activating Molecule) | Inhibitors targeting PDGFRα and CDK 4/6 signaling can treat patients with the p.K455_N468delinsN splice variant |
| Joshi et al. [96] | 2017 | GBM | Phospholipase C | D609 | PAC1 + TMZ is feasible in a rodent model and a promising therapeutic regime |
| Kalluri et al. [151] | 2017 | Oligodendroglioma Stem Cells | NEK9 | NEK9-siRNA | Chronic D609 treatment leads to decreased biomarker (Olig2) levels and G1 arrest |
| Kaneta et al. [152] | 2013 | GBM | BMI-1 | Ptc-209 | NEK9 inhibition causes spindle inhibition and mitotic catastrophe |
| Kong et al. [153] | 2018 | GBM | OPN | shRNA | Tumor growth is attenuated by PTC-2009; proof-of-concept for BMI-1 oncogene inhibition |
| Lamour et al. [154] | 2015 | GBM | PLK1 | Bi2536 | Tumorigenic potential of U87-MG sphere cells was completely abrogated upon OPN (osteopontin) silencing |
| Lee et al. [26] | 2012 | GBM | Wee1K | Mk-1775 | PLK1 (polo-like kinase 1) is critical to survival of glioma cells; inhibition kills cells |
| Lescarbeau et al. [155] | 2016 | GBM | p53/MDM2 | D-PMNIbeta | Wee1K phosphorylation is an effective anti-tumor target site |
| Li et al. [156] | 2012 | GBM | miR-23a (APAF1) | Anti-mir-23a | D-PMIBeta is an effective inhibitor of p53 |
| Lian et al. [157] | 2013 | GBM | EGFR | AZD9291 | miR-23a is upregulated in gliomas; knockdown reduces tumor survivability |
| Liu et al. [158] | 2019 | GBM | STK17A | Anti-STK17A shRNA | AZD9291 demonstrated efficient preclinical activity in GBM in vitro and in vivo models |
| Mao et al. [159] | 2013 | GBM | MDM2/4 + α5β1/αvβ3 | Compound 9 | STK17A portends a worse prognosis; knockdown reduces tumor survivability |
| Merlino et al. [160] | 2018 | GBM | CDK 4/6 | PD-0332991 | Compound 9 has the potential to be a potent anti-glioma therapy via MDM2/4 and α5β1/αvβ3 inhibition |
| Michaud et al. [161] | 2010 | GBM | FOXM1 | Plumbagin | PD-0332991 inhibits glioma growth and increases survival |
| Niu et al. [162] | 2015 | GBM | XIAP + BCL-2 | RIST + ARIST | Plumbagin significantly inhibited glioma cell proliferation and induced cell apoptosis |
| Nonnenmacher et al. [163] | 2015 | GBM | MGMT | PRIMA-1MET | RIST (rapamycin, irinotecan, sunitinib, and temozolomide) and aRIST (alternative to rapamycin, GDC-0941) prolonged survival time and reduced tumor burden |
| Patyka et al. [164] | 2016 | GBM and IDH-mutant Astrocytoma | MDM2 | SP-141 | p53 is the probable target of PRIMA-1MET, making it an effective targeted therapy. |
| Punganuru et al. [165] | 2020 | GBM | HSP90 | BIIB021 + 17-AAG (HSP90 Inhibitor) + BRAFi +/Or MEKi | MDM2 inhibition by SP-141 can effectively curtail the growth of brain tumors in vitro and in vivo |
| Sasame J et al. [166] | 2022 | Embryonic Kidney Cells | HGFR/MET | Crizotinib | HSP90 inhibitor (plus BRAF or MEK inhibitors) overcome the limitations of current BRAFV600E mutant therapy |
| Tasaki et al. [167] | 2016 | IDH-mutant Astrocytoma and Glioma | IAPs | Gdc-0152 | HGFR/MET is highly expressed in GSCs and could be inhibited by crizotinib |
| Tchoghandjian et al. [168] | 2016 | GBM | EGFR | Afatinib + TMZ | Inhibitors of apoptosis proteins (IAPs) are associated with lower survival rates, and GDC-0152 increases survival |
| Vengoji et al. [169] | 2019 | GBM | Survivin | Survivin-siRNA/Transferrin Receptor Conjugate | Afatinib plus TMZ significantly delayed progression and growth in vivo and in vitro |
| Wang et al. [170] | 2011 | GBM | EZH2 | EZH2si-DMC | Conjugate decreases survivin expression and increases survival |
| Wang et al. [171] | 2019 | GBM | Carbamoyl-phosphate synthetase (CAD) | Teriflunomide | DMC nanoparticle-mediated EZH2-siRNA decreases tumor growth |
| Wang et al. [133] | 2023 | GBM | BCL6 | RI-BPi | Targeting pyrimidine synthesis may yield an improved clinical outcome |
| Xu et al. [172] | 2017 | GBM and other cell types | CUL7 | MIR-3940-5p | BCL6 is overexpressed in glioma and is associated with worse prognosis; RI-BPI reduces tumor growth |
| Xu et al. [173] | 2020 | Glioma | EGFRvIII | L8A4 | CUL7 promotes tumorigenesis via NF-kappa B activation and can be negatively regulated by miR-3940-5p |
| Yang et al. [136] | 2006 | GBM | EF2-kinase | EF2-siRNA | Show the therapeutic efficacy of molecular targeting of EGFRvIII |
| Zhang et al. [174] | 2011 | GBM | ID2 | Anti ID2 siRNA | EF2 (elongation factor 2) inhibits anoikis and regulates cell migration; knockdown inhibits these properties in tumor cells |
| Zhao et al. [175] | 2015 | GBM | CDK + Aurora (dual inhibitor) | Jnj-7706621 | ID2 upregulation decreases apoptosis in glioma; targeting increases apoptosis and drug sensitivity |
| Zhong et al. [176] | 2018 | GBM and other cell types | ATG9A | Bevacizumab + Chloroquine | JNJ-7706621 was a potential drug for the treatment of patients with glioblastoma |
| Microenvironmental Targets (angiogenesis, cell-cell adhesion, iron/cation regulation) | |||||
| Abdul Rahim et al. [177] | 2017 | GBM | Phosphatidylserine | SAPC-DOPS | ATG9A depletion leads to cell death; however, chloroquine was found ineffective at non-toxic doses |
| Angara et al. [178] | 2017 | GBM | Endothelial pigpen protein | Aptamer III.1 | HET0016 targets therapeutic resistance in glioma |
| Blanco et al. [179] | 2014 | GBM | NRP-1 | NRP-1 Mab | SAPc-DOPS selectively targets GBM, crosses the BBB, and may be an effective treatment |
| Blank et al. [180] | 2001 | GBM | O-acetyl GD2 ganglioside | Anti-GD2 Antibody | Aptamer III.1 found to selectively target GBM and is a potential treatment |
| Chen et al. [181] | 2013 | GBM | TFAM | Melatonin + TMZ | NRP-1Mab is an inhibitor of glioma growth and invasion and may be an effective treatment |
| Fleurence et al. [182] | 2016 | GBM | Pan-VEGF | Cediranib + TMZ | O-acetyl GD2 ganglioside represents a new molecular target to prevent glioma proliferation |
| Franco et al. [183] | 2018 | GBM | LTβR | Light-VTP | Melatonin causes cell death and potentiates TMZ effects by inhibiting TFAM (mitochondrial transcription factor A) |
| Grossman et al. [184] | 2013 | GBM | TRPV4 | Cannabidiol (CBD) | Intratumoral concentrations of TMZ in tumor ECF were slightly, but not statistically significantly, increased when compared to the treatment of TMZ alone |
| He et al. [185] | 2018 | GBM | VEGF + Src Family kinases | Bevacizumab + Dasatinib | LIGHT-VTP prevents angiogenesis, normalizes blood vessels, and promotes immune infiltration |
| Huang T et al. [186] | 2021 | GBM | Growth-Hormone Releasing Hormone | MIA-604 + MIA-690 | Antitumor effect of CBD in glioma is caused by lethal mitophagy, and we identified TRPV4 as a molecular target |
| Huveldt et al. [187] | 2013 | GBM | Nrf2 | siRNA | Dasatinib may block bevacizumab-induced invasion, and a phase II trial is being planned |
| Jaszberenyi et al. [188] | 2013 | GBM | MRP3 | Anti-MRP Antibody | GHRH antagonists have potent anti-cancer activity, which can augment standard chemotherapeutic treatments |
| Ji et al. [189] | 2013 | GBM | VEGFR | Axitinib | Nrf2 promotes glioma proliferation and is inversely correlated with prognosis; siRNA may be a potential drug |
| Kuan et al. [190] | 2010 | GBM | TfR (transferrin receptor) | T12 + B6 + T7 (Tfr-Targeting Peptides) | MRP3 is overexpressed in gliomas; antibodies used in the study are specific to the tumors and decrease growth |
| Lu et al. [191] | 2015 | GBM and Glioma Stem Cells | CX43 + miR-21 | B2 cAMP Agonist | Axitinib exhibits antiangiogenic activity and prolongs survival |
| Mojarad-Jabali et al. [192] | 2022 | GBM | Fibulin-3 | Mab428.2 | T7-modified liposomes (T7-LS) show BBB penetration capacity and demonstrate in vitro effectiveness |
| Mostafavi et al. [193] | 2015 | GBM and IDH-mutant Astrocytoma | LAT1 | BCH | CX43 and miR-21 modulation using B2 agonists is effective therapy for low- but not high-grade glioma |
| Nandhu et al. [194] | 2018 | GBM | NHE9 | Gold NEPTT | mAb428.2 inhibited fibulin-3, reduced tumor growth, and extended survival |
| Nawashiro et al. [195] | 2006 | GBM and Glioma | Lanosterol synthase | Mi-2 | LAT1 expression is inversely correlated with survival time, and BCH arrested growth and killed tumor cells |
| Pall et al. [196] | 2019 | GBM | HIF2α | PT2385 | Gold nanoparticle-enabled photothermal therapy (NEPTT) crosses the BBB, delivers the gold nanoparticles, and kills tumor cells |
| Phillips et al. [197] | 2019 | DIPG and GBM | EDB-FN | Docetaxel-Loaded EDB-FN Specific Micelles | Characterized pathway of MI-2 (menin inhibitor), existing glioma treatment |
| Renfrow et al. [198] | 2020 | GBM | VEGF | Anti-VEGF AB + Nimustine | HIF2α is a reasonable therapeutic target; PT2385 is an efficacious anti-tumor agent |
| Saw et al. [199] | 2021 | GBM, IDH-mutant Astrocytoma, and other cell types | tmTNFa | Recombinant IL2 or dsDNA | EDB-FN (extra domain B fibronectin) is a useful biomarker and has antitumor efficacy |
| Takano et al. [200] | 2003 | GBM | CTL1 (choline transporter-like protein 1) | AMB4269951 | Combination of antiangiogenic therapy with standard chemotherapy is a promising avenue for future therapy |
| Tyrinova et al. [201] | 2018 | Glioma | VEGFR2 | Apatinib | tmTNFa is upregulated by rIL-2 or dsDNA, which helps to restore dendritic cell anti-tumor activity |
| Watanabe et al. [202] | 2020 | GBM | Calmodulin, EGFR, aromatase | W-13 + Gefitinib + Exemestane | Amb4269951 has significant antitumor effects in glioma and was also without significant weight loss |
| Xia et al. [203] | 2022 | GBM | ITGA9 | miR-148a | Apatinib decreases tumor growth through the induction of ferroptosis via the VEGFR2/Nrf2/Keap1 pathway |
| Xiong et al. [204] | 2019 | GBM | STING | ASA404 | Identified three existing miRNA-based chemicals for use as therapy |
| Xu et al. [205] | 2019 | GBM | CD73 | Anti-CD73 | miR-148a can suppress the malignant phenotype of GBM by targeting ITGA9 |
| Immunotherapy Pathways | |||||
| Baehr et al. [206] | 2017 | GBM | ATX + LPA receptors | siRNA | ASA404, an inhibitor of STING (stimulator of interferon gene), demonstrates efficacy subcutaneously but has no relevant activity in orthotopic brain models |
| Goswami et al. [207] | 2020 | GBM | EMMPRIN | Icaritin | Propose a combination therapy to target CD73 plus blockade of PD1 and CTLA-4, suggesting anti-CD73 be tested |
| Merrill et al. [208] | 2004 | GBM and Glioma | NFkB | BAY117082 + MG132 | CD155 is highly expressed in glioma, and PVS-RIPO is highly effective in vitro |
| Schleicher et al. [209] | 2011 | GBM | FPR | F2 Procyanidins | ATX and LPA receptor downregulation radio-sensitizes tumor cells |
| Xu et al. [210] | 2015 | GBM | CXCR4 | POL5551 + MCR89 | Icaritin inhibits the invasion and EMT of GBM cells by targeting EMMPRIN (extracellular matrix metalloproteinase) |
| Zanotto-Filho et al. [211] | 2011 | GBM and Glioma | Site-1 protease | PF-429242 | NFkB inhibition helps defeat resistance mechanisms, decreases viability, and exhibits some toxicity |
| Zhang et al. [212] | 2009 | GBM and Glioma | CXCR4 | Tetramethylpyrazine | F2 procyanidins downregulates FPR (formyl peptide receptor) causing a cytotoxic effect |
| Other Pathways/Targets | |||||
| Barone et al. [213] | 2014 | GBM | Lactate (monocarboxylate) transporters | ACCA | Higher POL5551 tumor concentrations are associated with better survival, improving in combination with VEGF antagonism |
| Caruana et al. [214] | 2017 | GBM | APLNR | MM54 Or MM193 (APLNR Antagonists) | PF-429242 decreases viability, increases apoptosis and inflammation, and downregulates lipid synthesis |
| Chen et al. [215] | 2013 | GBM | Nestin | Anti-Nestin IGG | Tetramethylpyrazine’s effect on gliomas comes through the inhibition of CXCR4 |
| Chen et al. [216] | 2021 | GBM | EEF1A1 + RPL11 | Puromycin + Doxorubicin + Daunorubicin + Mitoxantrone | circ-ITCH inhibits tumor progression by regulating the miR-106a-5p/SASH1 axis |
| Colen et al. [217] | 2011 | GBM | MALAT1 | Nanocomplex Targeting MALAT1 + TMZ | ACCA (α-cyano-4-hydroxycinnamic acid) inhibits lactate transport and can be used to target brain tumors |
| Harford-Wright et al. [218] | 2017 | GBM | IDH1R132H | AGI-5198 (In Combo with HDACi) | Inhibition of APLNR (apelin G-protein coupled receptor) results in a significant reduction in tumor growth |
| Ishiwata et al. [219] | 2011 | GBM | hnRNP A1/B2 | Β-Asarone | Downregulating nestin is associated with decreased glioma proliferation, growth, and migration |
| Jiang et al. [220] | 2021 | Glioma | CRM1 | S109 | Database analysis comparing glioma and normal tissue resulted in the identification of two target genes and four possible drugs for glioma treatment |
| Kim et al. [221] | 2018 | GBM | LPAR1/3 | KI16425 | Concurrent treatment of TMZ and nanocomplex-mediated silencing of MALAT1 has a survival benefit |
| Kim et al. [222] | 2019 | IDH-mutant Astrocytoma | Dynamin 2 | Dynole 34-2 + Cydyn 4-36 | AGI-5198 attenuates histone deacetylase inhibitor (HDACi) resistance and presents a potential therapy combination |
| Li et al. [223] | 2018 | GBM and other cell types | c-Myb | Telomestatin | β-Asarone blocks the invasion and epithelial-mesenchymal transition of glioma cells via inhibiting hnRNP A1/B2 |
| Liu et al. [224] | 2016 | GBM and Glioma | miR-25 | miR-25 Inhibitor (Cat. No. 4464084) | CRM1 is a novel molecular target; S109 inhibits the proliferation of tumor cells |
| Loskutov et al. [225] | 2018 | GBM | PRC2 + BET bromodomain proteins | JQ1 + I-BET | LPA signaling knockdown reduced tumor growth |
| Luwor et al. [226] | 2019 | GBM | eIF-5A, DHS, DOHH (both eIF-5A activators) | Gc7 | Dynamin 2 inhibition via CyDyn 4-36 reduces tumor growth |
| Miyazaki et al. [227] | 2012 | GBM | TRAILR | Recombinant TRAIL + TMZ | Telomestatin impairs survival and growth via disrupting the c-myb protoconcogene |
| Peng et al. [228] | 2019 | GBM | EFTUD1 | EFTUD1 shRNA | miR-25, through wnt signaling, may serve as a promising molecular target for the treatment of glioma |
| Piunti et al. [229] | 2017 | DIPG and Glioma | PFK1 | Clotrimazole | Oncogenic properties of the histone point mutation H3K27M are reduced by inhibiting PRC2 and BET proteins |
| Preukschas et al. [230] | 2012 | GBM | YAP1 | Nsc682769 | eIF5-A is overexpressed in gliomas and its activator DHS represents a possible molecular target |
| Saito et al. [231] | 2004 | GBM | α7 nAChR | Rslurp-1 | TMZ + TRAIL have a synergistic effect on survival while being safe in tumor-bearing rats |
| Saito et al. [232] | 2014 | GBM | A1CF + FAM224A | shRNA | EFTUD1 (elongation factor such as GTPase 1) is overexpressed in glioma, and its downregulation induces arrest and apoptosis |
| Sanzey et al. [233] | 2015 | GBM | DLL3 | Rova-T | Clotrimazole inhibits PFK1 (phosphofructokinase 1) and increases survivability |
| Saunders et al. [234] | 2021 | GBM | Smoothened | Gdc-0449 | NSC682769 represents a new YAP1 (yes-associated protein 1) inhibitor that decreases glioma growth and proliferation |
| Shulepko et al. [235] | 2020 | GBM | KIF11 | Ipinesib | rSLURP-1 demonstrates antitumor activity through nAChR inhibition |
| Song Y et al. [236] | 2019 | GBM and other cell types | Brevican | Anti-Deglycosylated Brevican Peptide | A1CF/FAM224A/miR-590-3p/ZNF143 positive feedback loop regulates the malignant progression of tumor cells |
| Spino et al. [126] | 2019 | GBM and IDH-mutant Astrocytoma | miR-128 | Ginsenoside Rh2 | DLL3 (delta-like ligand 3) is selectively and homogeneously expressed in this tumor type; it is target with Rova-T (rovalpituzumab tesirine) |
| Tu et al. [237] | 2017 | GBM | 14-3-3 | siRNA | Smoothened is an effective prognostic biomarker, and GDC-0449 should be further evaluated as a potential drug |
| Venere et al. [238] | 2015 | GBM | IDH1R132H | Wm17 | Inhibition of KIF11 (kinesin family member 11) stopped tumor growth, impeded tumor initiation, and prolonged survival |
| von Spreckelsen et al. [239] | 2021 | GBM | FTO | SPI1 Inhibitor DB2313 | Deglycosylated Brevican is specific to high-grade gliomas; its knockdown by the BTP-7 peptide presents a new therapy |
| Wu et al. [240] | 2011 | GBM | Rh2 inhibits tumor proliferation via miR-128 upregulation | ||
| Yan et al. [241] | 2013 | GBM | mTOR + RAF | Metformin + Sorafenib | 14-3-3 downregulation causes decreased glioma survival |
| Zhang et al. [242] | 2021 | IDH-mutant Astrocytoma | Ras | Nobiletin | WM17 is a novel mutant IDH1 inhibitor that inhibits cell migration but not proliferation |
| Zhang et al. [243] | 2022 | GBM, IDH-mutant Astrocytoma, Oligodendroglioma | mTOR | Rapamycin | FTO (fat mass and obesity-associated protein) is a novel prognostic indicator and decreases tumor burden |
Abbreviations: RAF, rapidly accelerated fibrosarcoma; Ras, rat sarcoma; mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; GBM, glioblastoma multiforme; EGFR, epidermal growth factor receptor; SAPK2, stress-activated protein kinase 2; TMZ, temozolomide; RT, radiotherapy; ROS1, ROS proto-oncogene 1; FGFR, fibroblast growth factor receptor; MET, mesenchymal-epithelial transition factor; JNK, Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; ALK, anaplastic lymphoma kinase; FAK, focal adhesion kinase; TCN, tetra-cycline; MEK, mitogen-activated protein kinase kinase; NOTCH, neurogenic locus notch homolog protein; PBK/TOPK, PDZ-binding kinase/T-lymphokine-activated killer cell-originated protein ki-nase; COX-2, cyclooxygenase-2; FOXO3A, forkhead box O3a; CSCs, cancer stem cells; ILK, integrin-linked kinase; bFGF, basic fibroblast growth factor; AMPK, adenosine mono-phos-phate-activated protein kinase; STAT3, signal transducer and activator of transcription 3; PKC, protein kinase C; PLK1, polo-like kinase 1; EGF1R, epidermal growth factor receptor 1; EMP2, epithelial membrane protein-2; LAT, linker for activation of T-cells; HIF, hypoxia-inducible factor; TWIST, twist family BHLH transcription factor; CD, cluster of differentiation; PFK, phos-phofructokinase; PDK, pyruvate dehydrogenase kinase; ARHGAP15, Rho GTPase-activating protein 15.
Table 4.
Summary of Laboratory Studies Identifying Novel Molecular Targets.
| Study Author | Year | Tumor Type | Molecular Target | Finding |
|---|---|---|---|---|
| Protein Kinase Pathways | ||||
| Chen et al. [244] | 2021 | GBM | ACTL6A | ACTL6A (actin-like 6A) knockdown inhibits tumor migration via suppressing the Akt pathway and increases sensitivity to TMZ |
| Edwards et al. [245] | 2006 | GBM | Phosphatidylinositol 3-kinase/Akt | Treatment of GBM cells with ILKAS can decrease ILK protein levels and downstream phosphorylation of the cell survival protein PKB/Akt on Ser473, the site specifically phosphorylated by ILK |
| Gabler et al. [246] | 2019 | BRAF V600E-mutated glioma | ETS1 | Concomitant BRAFV600E and TERT promoter mutations synergistically support cancer cell proliferation and immortalization through ETS1 (e-twenty-six transcription factor) |
| Gu et al. [247] | 2015 | GBM | ITSN1S | ITSN1 (Intersectin1-S) contributes to glioma growth through the Raf/MEK/ERK pathway; overexpression correlates with higher grade gliomas |
| Hou et al. [248] | 2015 | GBM | PERK | PERK (PKR-like kinase) silencing decreases tumor cell viability and ATP/lactate production; decreases tumor formation capacity |
| Iqbal et al. [249] | 2016 | GBM | PIM | Combination PIM (Proto-oncogene serine/threonine-protein kinase) and PI3K inhibition may be an effective regimen in treating heterogeneous tumors |
| Keating et al. [250] | 2010 | Astrocytoma | Mer and Axl RTKs | Mer and Axl RTK inhibition is a novel method to improve apoptotic response and chemosensitivity in astrocytoma |
| Kim et al. [251] | 2016 | Glioma Stem Cells | MLK4 | MLK4 regulates the mesenchymal identity of GSCs |
| Lerner et al. [252] | 2015 | GBM | PLK1 | PLK1 inhibition is especially effective against CD133+ GBM cell subpopulations |
| Liu et al. [253] | 2013 | GBM | EF-2 kinase | Targeting EF-2 kinase can enhance the anti-glioma activity of TMZ |
| Liu et al. [254] | 2015 | Glioma | GCN5 | GCN5 (general control of nucleotide synthesis 5) potentiates tumor proliferation and invasion via STAT3 and Akt signaling pathways |
| Mao et al. [159] | 2013 | GBM | STK17A | STK17A is a p53 target gene that is upregulated in GBM and associated with worse outcomes, while knockdown reduces proliferation, invasion, and migration |
| Martinez-Saez et al. [255] | 2016 | Glioma | peIF4E | peIF4E (eukaryotic translation initiation factor 4E), activated by the Ras-Raf-MAPK pathway, is an independent predictor of survival |
| Qin et al. [117] | 2014 | GBM | EMP2 | EMP2 is an activator of Src and represents a potential molecular target for glioma therapy |
| Shoshan et al. [256] | 1999 | Oligodendroma | NG2 and PDGFRa | NG2 and PDGFRa are both overexpressed in oligodendromas and may represent molecular target |
| Sulzmaier et al. [257] | 2016 | GBM | RSK2 | RSK2 serine/threonine-protein kinase is upregulated in glioma and is associated with decreased survival rates; knockdown reduces proliferation |
| Sun et al. [258] | 2020 | GBM | Nrf2 | Nrf2 inhibition leads to increased oxidative stress and decreased Ras/Raf/MEK activity |
| Thanasupawat et al. [127] | 2018 | GBM | CTRP8 | The CTRP8-STAT3 axis has strong anti-apoptotic properties involved in TMZ resistance |
| Tsuruta et al. [259] | 2011 | Glioma | PDGFRa and G-CSFR | Gliomas highly express PDGFRa (Platelet-derived growth factor receptor) and G-CSFR (colony stimulating factor receptor) |
| Wang et al. [133] | 2019 | GBM | Pyrimidine Synthesis Pathway | GSCs are vulnerable to inhibition of both the mutated enzyme and the rate-limiting (carbamoyl phosphate synthetase 2) |
| Yamanaka et al. [260] | 2006 | Glioma | DDR1 | DDR1 (discoidin domain receptor tyrosine kinase 1) is associated with glioma proliferation and a worsened prognosis |
| Zhang et al. [261] | 2016 | GBM | YAP1/TAZ-BIRC5 | The Hippo/YAP kinase pathway is abnormally activated by LATS downregulation and not affected by MST in glioma tissues |
| Zhang et al. [262] | 2022 | GBM | NDRG1 promoter | CW-type zinc finger 2 promotes the proliferation, invasion, migration, and EMT of glioma by regulating PTEN/PI3K/AKT signaling via binding to the N-myc downstream regulated gene 1 promoter (NDRG1) |
| Zhao et al. [263] | 2016 | GBM | PI3K/Akt and JNK | Combined inhibition of the PI3K p110β isoform and JNK may serve as a potent and promising therapeutic approach |
| Zhou et al. [264] | 2005 | GBM | FPR | FPR (Formyl Peptide receptor) acts through the JAK/STAT pathway and is highly expressed in GBM and other high-grade gliomas |
| Zhu et al. [265] | 2014 | GBM | Pyk2 or Orai1 | SOCE (store-operated Ca2+ entry) is enhanced in gliomas, and knockdown by either Pyk2 (proline-rich tyrosine kinase 2) or Orai 1 inhibition can act as a novel approach |
| Zohrabian et al. [266] | 2009 | GBM | MEK and ROCK | Rho/ROCK signaling is involved in GBM cell migration and proliferation and represents an ideal target |
| Cell Cycle/Apoptosis/Transcription Pathways | ||||
| Abe et al. [267] | 2019 | Glioma | CDK5 | CDK (cyclin-dependent kinase) 5 regulates lamellipodia and filopodia; blockade may decrease cell migration |
| Bai et al. [268] | 2014 | Glioma Stem Cells | TRF2 | TRF2 (telomeric repeat binding factor 2) inhibition blocks tumor proliferation and increases survival |
| Bai et al. [269] | 2020 | GBM | TTDA | TTDA (trichothiodystrophy group A protein) is an upstream regulator of p53-mediated apoptosis and acts as an oncogene |
| Cai et al. [270] | 2021 | GBM | TRIM32 | TRIM32 (tripartite motif protein 32) is overexpressed in glioma cells, and its knockdown decreases tumor growth and potentiates the TMZ response |
| Cao et al. [271] | 2010 | GBM and IDH-mutant Astrocytoma | 14-3-3-protein | 14-3-3 inhibition is associated with increased apoptosis, while 14-3-3 is upregulated in glioma cells |
| Chiang et al. [272] | 2012 | GBM | WOX1 | WOX1 overexpression inhibits p53 mutant glioma cells independent of the intrinsic apoptosis pathway |
| Feng et al. [273] | 2019 | GBM | TRIM14 | TRIM 14 (Tripartite motif-containing 14) tumor suppressor promotes EMT via ZEB2 (Zinc finger E-box-binding homeobox 2) |
| Godoy et al. [274] | 2021 | GBM | E2F1 | E2F1 suppression is associated with decreased growth, increased apoptosis and susceptibility to radiation, and delayed differentiation |
| Kang et al. [275] | 2019 | GBM | lncRNA RP11-732M18.3 | Inhibition of thelncRNA RP11-732M18.3, which promotes G1/S cell cycle transition, could provide a novel therapeutic target for glioma treatment |
| Kikuchi et al. [276] | 2017 | GBM | DEPDC1 | DEPDC1 (DEP domain containing 1) induced apoptosis through NF-κβ signaling |
| Klose et al. [277] | 2011 | GBM | BMP7 | BMP7 (Bone Morphogenetic Protein 7) is a potent tumor suppressor that induces G1/S cell cycle arrest via the BMP/TGF-β pathway |
| Lan et al. [278] | 2020 | GBM and other cell types | SNRPG | Downregulation of SNRPG (Small Nuclear Ribonucleoprotein Polypeptide G) induces cell cycle arrest and sensitizes tumor cells to TMZ by targeting Myc through a p53-dependent signaling pathway |
| Li et al. [279] | 2018 | GBM | CDK10 | CDK10 overexpression is associated with the inactivation of snail-mediated EMT |
| Luo et al. [280] | 2014 | Glioma and GBM | PAR2 | PAR2 (protease-activated receptor 2) is overexpressed in glioma cells and is involved in preventing apoptosis |
| Ma et al. [281] | 2017 | GBM | miR-96 | miR-96 suppresses the PDCD4 (programmed cell death protein 4) tumor suppressor and is associated with increased tumor growth |
| Meuth et al. [282] | 2008 | GBM | TASK3 | TASK1 and TASK3 (TWIK-related acid-sensitive K channel 3) are expressed in human glioma cells and are linked to glioma apoptosis |
| Tong et al. [283] | 2019 | GBM | YB-1 | YB-1 (Y-box binding protein 1) facilitates resistance of glioma cells to TMZ by activating MDM2/p53 signaling |
| Wirsching et al. [284] | 2014 | GBM and Glioma | TB4 | TB4 (thymosin beta 4) expression is correlated with glioma grade, and it modulates p53 and TGF-β |
| Yan et al. [285] | 2014 | Glioma | PRMT5 | PRMT5 (protein arginine methyltransferase 5) is a protein arginine methyltransferase that is overexpressed in gliomas; attenuation leads to cell-cycle arrest |
| Yuan et al. [286] | 2022 | GBM | HSP27 | HSP27 (heat shock protein 27) depletion promotes erastin-induced ferroptosis of tumor cells |
| Microenvironmental Targets (angiogenesis, cell-cell adhesion, cation regulation) | ||||
| Chung et al. [287] | 2018 | Glioma and GBM | EMP2 | EMP2 is a biomarker for glioma differentiation and correlates with decreased survival |
| Bao et al. [288] | 2016 | GBM and IDH-mutant Astrocytoma | CAP1 | CAP1 (adenylate cyclase-associated protein 1), a cytoskeleton regulator, significantly contributes to tumor proliferation, migration, and invasion |
| Haining et al. [289] | 2012 | Glioma | LAT1/4F2hc | LAT1/4F2hc amino acid transporter expression is correlated with proliferation, angiogenesis, and worsened outcomes |
| Ji et al. [189] | 2013 | GBM | Nrf2 and HIF1α | Nrf2 expression is directly correlated with HIF1α expression and is associated with worse outcomes |
| Kaur et al. [290] | 2012 | GBM | Cadherin-11 | cadherin-11 is associated with increased glioma survivability and mobility |
| Lan et al. [291] | 2014 | GBM and other cell types | miR-497 | Hypoxia-induced miR-497 is overexpressed in glioma and decreases glioma cell sensitivity to TMZ by inhibiting apoptosis |
| Li et al. [292] | 2017 | Glioma | miR-150 | miR-150 modulates the HIF1α pathway and upregulates glycolysis in glioma cells |
| Li et al. [293] | 2020 | Glioma | TWIST | TWIST transcription factor could be a predictor of poor prognosis in glioma patients; it shows a correlation with microvascular density |
| Liu et al. [294] | 2016 | Glioma and GBM | XBP1 | XBP1 (X-box binding protein 1) silencing reduces glioma cell viability and tumor formation capacity; it decreases glioma cell viability and ATP/lactate production |
| Ljubimova et al. [295] | 2004 | Glioma and Meningioma | Laminin-8 | Laminin-8 expression is highly correlated with tumor grades and inversely correlated with survival time |
| Martina et al. [296] | 2010 | GBM, IDH-mutant Astrocytoma, Oligodendroglioma | Tenascin-W | Tenascin-W is overexpressed in brain tumors and not in normal tissue; it is a marker for glioma-associated blood vessels and stimulates angiogenesis |
| Okubo et al. [297] | 2010 | Glioma | LAT1 | LAT1 (L-type amino acid transporter 1) expression corresponds with a higher density of microvessels in glioma |
| Pointer et al. [298] | 2017 | GBM | hERG | High hERG (human ether-à-go-go-related gene) potassium ion channel expression is correlated with decreased survival |
| Shi et al. [299] | 2019 | GBM | SLC2A1 | LINC00174 promotes cell invasion, migration, and upregulated SLC2A1(solute carrier family 2 member 1) |
| Wu et al. [300] | 2016 | GBM | 37LRP | 37LRP (37-kDa laminin receptor precursor) is a novel glioma target whose downregulation by siRNA is associated with decreased growth, invasion, and proliferation |
| Immunotherapy Pathways | ||||
| Han et al. [301] | 2019 | Glioma | HVEM | Immune checkpoint molecule herpesvirus entry mediator (HVEM) is overexpressed and associated with poor prognosis |
| Hong et al. [92] | 2014 | GBM and other tumor types | L1-CAM | The CE7 epitope of the L1-CAM adhesion molecule on tumors may be amenable to targeting by CE7R T cells, making it a promising target for adoptive immunotherapy |
| Ku et al. [302] | 2011 | GBM | CHI3L1 | CHI3L1 (Chitinase 3 like 1) contributes to glioma progression through invasion, resistance, and growth |
| Lou et al. [303] | 2017 | GBM | NUDT21 | NUDT21(nudix hydrolase 1) is an upstream regulator of the NF-κB pathway and a potential molecular target for the MES subtype of GBM |
| Saito et al. [304] | 2017 | GBM | KIF-20A | KIF-20A (kinesin family member 20A) is highly expressed in glioma cells but not normal brain tissue; its suppression blocks proliferation and reduces cytokinesis |
| Xu et al. [305] | 2020 | Glioma | PARP9 | PARP9 may serve as an unfavorable prognosis predictor for glioma |
| Yuan et al. [306] | 2019 | Glioma | CD204 | CD204 contributes to dysfunction of T cells in glioma |
| Yuan et al. [307] | 2022 | GBM | BACH1 | BACH1 (BTB Domain and CNC Homolog 1) attenuates the tumor-associated macrophage mediated immune response, therefore creating an immunosuppressive tumor environment |
| Zhang et al. [308] | 2021 | Oligodendroglioma and Glioma | S100A | Via databases, the S100A family was heavily involved in glioma immune infiltration and may represent an effective target |
| Zhu et al. [309] | 2022 | Glioma | PYGL | PYGL (Glycogen Phosphorylase L) can be used as a new biomarker and molecular target for evaluating the prognosis and immunotherapy of glioma |
| Wnt/β-catenin Pathways | ||||
| Chen et al. [310] | 2021 | Glioma | WTN5A | WNT5A gene, which expresses Wnt-5a, is overexpressed in gliomas; promotes EMT and angiogenesis |
| Di et al. [311] | 2021 | GBM | SPZ1, CXXC4 pathway | SPZ1 (Spermatogenic Leucine Zipper 1) stimulates glioma’s malignant progression via targeting CXXC4 |
| Friedmann-Morvinski et al. [312] | 2016 | GBM | OPN | OPN (osteopontin) plays a role in dedifferentiating glioma cells |
| Guo et al. [313] | 2020 | GBM | FRAT1 | FRAT1 (frequently rearranged in advanced T cell lymphomas-1) contributes to the tumorigenesis of glioma cells through wnt signaling |
| Lan et al. [314] | 2015 | GBM | PomGnT1 | Forced overexpression of PomGnT1 (peptide-O-linked mannose beta-1,2-N-acetylglucosaminyltransferase 1) promotes tumor progression via activation of beta-catenin |
| Mizobuchi et al. [315] | 2008 | GBM | REIC/Dkk-3 | REIC/Dkk-3 (reduced expression in immortalized cells /Dickkopf-related protein 3) is involved in Wnt-mediated apoptosis and is downregulated in glioma |
| Zhou et al. [316] | 2015 | GBM and IDH-mutant Astrocytoma | HOTAIR | High HOTAIR (HOX Transcript Antisense RNA) expression was associated with poor outcomes; depletion inhibits tumor cell migration/invasion |
| Other Pathways/Targets | ||||
| Borsics et al. [317] | 2010 | GBM | PRAF2 | PRAF2 (rab acceptor 1 domain family, member 2) downregulation reduces the invasiveness of tumor cells |
| Cui et al. [318] | 2019 | GBM | RHPN1-AS1 | Knockdown of RHPN1-AS1 inhibits the proliferation, migration, and invasion of tumor cells |
| Dong et al. [319] | 2021 | GBM | ANTXR1 | miR-381-3p could repress malignant behaviors in glioma by modulating ANTXR1 (anthrax toxin receptor 1) |
| Feve et al. [320] | 2014 | GBM | 13 different GPCRs | The transcriptome study shows 13 possible novel pathways that can be targeted by new drugs; refer to Table 1 of Feve et al., 2014 [320] |
| Han et al. [321] | 2017 | GBM | TAGLN2 | TAGLN2 (Transgelin-2) plays a role in promoting the development of human glioma |
| Hou et al. [322] | 2022 | Glioma Stem Cells | CircASPM | CircASPM is up-regulated in glioma tissues and is correlated with tumor progression and poor prognosis |
| Huang et al. [323] | 2020 | GBM | GAS5-AS1 | LncRNA GAS5-AS1 (growth arrest specific 5) inhibits glioma proliferation, migration, and invasion via miR-106b-5p/TUSC2 axis |
| Li et al. [324] | 2011 | GBM | DLL4-Notch | Combination therapy to block DLL4-Notch signaling may enhance the efficacy of VEGF inhibitors |
| Li et al. [325] | 2014 | GBM | miRNA network | There are 14 miRNAs and 5 pathways in the network that can represent glioma targets; refer to Figure 6A of Li et al., 2014 [325] |
| Li et al. [326] | 2019 | GBM | LINC00319 | LINC00319 (long intergenic non-protein coding RNA 319) is an oncogenic factor for glioma tumorigenesis; knockdown arrests the cell cycle and induces apoptosis |
| Li et al. [327] | 2021 | GBM and other cell types | IGF2BP2 | SUMOylation of IGF2BP2 (insulin-like growth factor 2 mRNA binding protein 2) regulated the OIP5-AS1/miR-495-3p axis to promote vasculogenic mimicry in tumor cells |
| Liu et al. [328] | 2015 | GBM and Glioma | miR-27b | miR-27b may promote glioma cell invasion through direct inhibition of Spry2 (sprouty homolog 2) expression |
| Liu et al. [329] | 2022 | GBM | LINC01094 | LINC01094 promotes glioma progression by modulating miR-224-5p/CHSY1 axis |
| Miller et al. [330] | 2017 | GBM | JMJD6 | JMJD6 (Jumonji Domain Containing 6) mediates tumor growth in vivo; targeting reduces glioma progression |
| Noorani et al. [331] | 2020 | GBM | 147 druggable genes | Whole genome sequencing of human tumors identified 147 druggable targets for EGFR-mutant GBM, refer to Table S8 in Noorani et al., 2020 [331] |
| Qiu et al. [332] | 2015 | GBM and Glioma | FoxJ2 | FoxJ2 (forkhead box J2) suppresses cell migration and invasion in glioma, so upregulating may be a strategy |
| Rose et al. [333] | 2021 | GBM and other tumor types | 11 surface proteins | Shotgun proteomics identified 11 new potential targets for glioma therapy; refer to Figure 2A of Rose et al., 2021 [333] |
| Sanzey et al. [233] | 2015 | GBM | PFK1 and PDK1 | Knockdown of PFK1 and PDK1, as well as some other glycolytic enzymes, acts an important enzyme in the metabolic escape pathways of GBM |
| Sharma et al. [334] | 2016 | IDH-mutant Astrocytoma | EZH2 | EZH2 (enhancer of zeste homologue 2) and miRNA reactors act as biomarkers for tumor progression |
| Sun et al. [335] | 2017 | GBM | FOXP3/ARHGAP15 | FOXP3 (forkhead box P3) and ARHGAP15 are both underexpressed in glioma tissues, and their absence plays a role in EMT |
| Visvanathan et al. [336] | 2018 | GBM and Glioma | METTL3 | METTL3 (methyltransferase-like 3) preserves stem-cell-like capabilities in glioma cells and mediates SOX2 radiation salvage |
| Wang et al. [337] | 2014 | GBM | TIP-1 | TIP1 (tax interacting protein 1) increases glioma invasion and angiogenesis; knockdown increases survivability |
| Wei et al. [338] | 2014 | GBM and Glioma | ADAR2 | The ADAR2 (adenosine deaminases acting on RNA 2) alternative splicing variant is upregulated in glioma cells and may contribute to the malignancy of gliomas |
| Weigle et al. [339] | 2005 | GBM and IDH-mutant Astrocytoma | SOX11 | SOX11 is highly and specifically expressed in glioma cells; it reactivates during tumorigenesis |
| Xin et al. [340] | 2020 | GBM | NFIA-AS2 | NFIA-AS2 (nuclear factor I A antisense RNA2 gene) could be a novel biomarker and therapeutic target for glioma patients |
| Zhang et al. [341] | 2022 | GBM and Oligodendroglioma | ANXA1 | ANXA1 is overexpressed in glioma tissues, plays a role in invasion and infiltration, and is an independent prognostic factor in glioma |
| Zhou et al. [342] | 2021 | GBM and Glioma | miR-190a-3p | miR-190a-3p contributes to glioma proliferation/migration and negatively regulates YOD1; can be suppressed by miR inhibition |
Abbreviations: ILK, integrin-linked kinase; TERT, telomerase reverse transcriptase; MLK, mammalian sterile 20-like kinase; PLK, polo-like kinase; EF, elongation factor; STK, serine/threonine kinase; EMP, epithelial membrane protein; PDGFR, platelet-derived growth factor receptor; RSK, ribosomal S6 kinase; CTRP, C1q/TNF-related protein; GSCs, glioma stem cells; PI3K/Akt, phosphatidylinositol 3-kinase/protein kinase B; JNK, Jun N-terminal kinase; MEK, mitogen-activated protein kinase kinase; ROCK, Rho-associated protein kinase; CDK, cyclin-dependent kinase; EMP, epithelial membrane protein; LAT, linker for activation of T cells; L1-CAM, L1 cell adhesion molecule; PARP9, poly(ADP-ribose) polymerase family member 9; CD, cluster of differentiation; CXXC, cysteine-rich CXXC domain-containing protein; RHPN1-AS1, RHPN1 antisense RNA 1; PFK, phosphofructokinase; PDK, pyruvate dehydrogenase kinase; ANXA, annexin A.
3.2. Clinical Studies Implementing Molecular Targeted Therapies
Fifty-two clinical studies implementing molecular targeted therapies for glioma were identified, with a median publication year of 2017 (Table 2) [343]. In terms of tumor type, 40/52 (77%) studied GBM (GBM), 10/52 (19%) studied IDH-mutant astrocytoma, and there was one study on an IDH-wt, 1p19q co-deleted glioma (2%). In terms of molecular targets, 26/52 (51%) targeted some form of protein kinase, 15/52 (29%) targeted angiogenesis or environmental pathways, 3/52 (6%) targeted immunotherapy pathways, and 3/52 (6%) targeted cell cycle or apoptosis pathways.
The level of evidence for the clinical studies varied. Eight studies had Level II evidence (15%), as they were multi-institutional clinical trials. Most published clinical studies had Level IV evidence (34/52; 65%), consisting of single institutional phase II or prospective trials. The rest of the clinical studies (10/52, 19%) were case reports or series, classifying them as Level VI studies (Table 1 and Table S1).
3.3. Laboratory Studies Implementing Molecular Targeted Therapies
There were 190 laboratory studies implementing existing molecularly targeted therapies for glioma (Table 3). An overwhelming majority of studies (167/190, 88%) focused on GBM, followed by studies of unspecified gliomas (17/190, 9%), then IDH-mutant astrocytomas (11/190, 6%), then oligodendrogliomas (3/190, 2%), with some studies covering multiple glioma types. The most prevalent molecular targets were those involving protein kinase pathways (79/190, 42%), particularly tyrosine kinase receptors. Out of the protein kinase pathways, PI3K/Akt/mTOR, Ras/BRAF/Mek/Erk, and upstream targets were found in the largest proportion (29/79, 37%). Additionally, nearly a quarter of all clinical studies targeted cycle/apoptosis/transcription-targeted pathways (41/190, 22%). The next most prevalent pathway targets were microenvironmental targets (30/190, 16%)—including angiogenesis, cell-cell adhesion molecules, and iron/cation regulation—followed by immunotherapy pathways (8/190, 4%) (Table 3). All studies were Level III evidence (Table 1).
Most studies (100/190, 53%) were conducted using combined in vitro and in vivo designs; the next most common were 61/190 (32%) in vitro studies, then 6/190 (3%) were combined in vivo and ex vivo studies (Table S2). The most frequent cell lines were U87 (105/190, 55%), U251 (51/190, 27%), T98 (22/190, 12%), A172 (19/190, 10%), or GBM patient samples (28/190, 15%) (Table S2).
Of the laboratory studies testing existing molecular targeted therapies, all were queried for whether or not they utilized spheroid or 3-dimensional (3D) technologies for cell culture as part of their methodology. Fifty-nine (31%) of studies adopted tumor sphere or 3D technology (Table S2).
3.4. Laboratory Studies Identifying Novel Molecular Targets
There were 108 laboratory studies identifying novel molecular targets for treating glioma (Table 4).
The majority of studies (82/108, 76%) focused on GBM, followed by 29/108 (27%) studying unspecified gliomas, 6/108 (6%) studying IDH-mutant astrocytoma, and lastly 3/108 (3%) studying oligodendroglioma, with some studies covering multiple glioma types. Twenty-seven (25%) studies targeted protein kinase pathways, 21/108 (19%) targeted cell cycle/apoptosis pathways, 16/108 (15%) studies targeted microenvironmental targets, 10/108 (9%) studies targeted immunotherapy pathways, and 7/108 (6%) targeted the wnt/beta catenin pathway (Figure 2). All studies were Level III evidence (Table 1).
Figure 2.
Summary of molecularly targeted pathways in adult-type diffuse glioma.
3.5. Ongoing Clinical Trials
A search of clincialtrials.gov yielded 341 clinical trials, of which 119 met our inclusion criteria for ongoing clinical trials investigating molecular targeted therapies for adult-type diffuse glioma and its subtypes (Table 5). The most prevalent targets involved protein kinase pathways (65/119, 55%), followed by angiogenesis or microenvironmental targets (33/119, 28%), then cell cycle/apoptosis (10/119, 8%), and immunotherapy pathways (10/119, 8%). For tumor types, 74/119 (62%) tested GBM, 5/119 (4%) tested IDH-mutant astrocytoma, and 2/119 (2%) tested oligodendroglioma, with many studies testing specific subcategories. The average start year was earlier in trials testing protein kinase targets (2009 ± 6), compared with trials testing cell cycle/apoptosis inhibitors (2016 ± 4) and immunotherapies (2018 ± 2), which occurred more recently on average (p < 0.001).
Table 5.
Summary of Ongoing Clinical trials Testing Molecular Targeted Therapies in Glioma.
| Title | NCT # | Year Started | Last Update | Tumor Type | Molecular Target | Intervention |
|---|---|---|---|---|---|---|
| Protein Kinase Pathways | ||||||
| Imatinib Mesylate in Treating Patients with Recurrent Malignant Glioma or Meningioma | 00010049 | 2001 | 2018 | Recurrent Malignant Glioma or Meningioma | multiple tyrosine kinases | imatinib |
| Gefitinib in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | 00014170 | 2001 | 2013 | GBM | EGFR | Gefitinib |
| CCI-779 in Treating Patients with Recurrent Glioblastoma Multiforme | 00016328 | 2001 | 2013 | GBM or Gliosarcoma | mTOR | temsirolimus |
| Gefitinib in Treating Patients with Recurrent or Progressive CNS Tumors | 00025675 | 2001 | 2018 | GBM or Anaplastic Gliomas | EGFR | gefitinib |
| Erlotinib in Treating Patients with Solid Tumors and Liver or Kidney Dysfunction | 00030498 | 2001 | 2013 | Gliomas and Brain Metastases | EGFR | Erlotinib |
| Gefitinib and Radiation Therapy in Treating Patients with Glioblastoma Multiforme | 00052208 | 2002 | 2020 | GBM, Gliosarcoma | EGFR | Gefitinib |
| Imatinib Mesylate in Treating Patients with Gliomas | 00039364 | 2002 | 2012 | Glioma | Multiple tyrosine kinases | Imatinib |
| Erlotinib in Treating Patients with Recurrent Malignant Glioma or Recurrent or Progressive Meningioma | 00045110 | 2002 | 2017 | Glioma on EIADs | EGFR | erlotinib |
| Erlotinib and Temozolomide with Radiation Therapy in Treating Patients with Glioblastoma Multiforme or Other Brain Tumors | 00039494 | 2002 | 2013 | GBM or Gliosarcoma | EGFR | Erlotinib |
| A Phase II Exploratory, Multicentre, Open-label, Non-comparative Study of ZD1839 (Iressa) and Radiotherapy in the Treatment of Patients with Glioblastoma Multiforme | 00238797 | 2003 | 2011 | GBM | EGFR | Gefitinib |
| Imatinib Mesylate in Treating Patients with Recurrent Brain Tumor | 00049127 | 2003 | 2019 | Adult glioma | Multiple tyrosine kinases | Imatinib |
| Everolimus and Gefitinib in Treating Patients with Progressive Glioblastoma Multiforme or Progressive Metastatic Prostate Cancer | 00085566 | 2004 | 2016 | Progressive GBM | mTOR, EGFR | everolimus + gefinib |
| Erlotinib Compared with Temozolomide or Carmustine in Treating Patients with Recurrent Glioblastoma Multiforme | 00086879 | 2004 | 2017 | GBM | EGFR | erlotinib + carmustine + TMZ |
| Sorafenib in Treating Patients with Recurrent or Progressive Malignant Glioma | 00093613 | 2004 | 2014 | GBM | PDGFR | Sorafenib |
| Lapatinib in Treating Patients with Recurrent Glioblastoma Multiforme | 00099060 | 2004 | 2014 | Recurrent GBM | HER2, EGFR | lapatinib |
| GW572016 to Treat Recurrent Malignant Brain Tumors | 00107003 | 2005 | 2018 | GBM or gliosarcoma | EGFR/HER2 | lapatinib |
| Ph I Gleevec in Combo w RAD001 + Hydroxyurea for Pts w Recurrent MG | 613132 | 2005 | 2013 | Recurrent Malignant GBM | multiple tyrosine kinases, mTOR | imatinib + RAD001 + hydroxyurea |
| Phase II Imatinib + Hydroxyurea in Treatment of Patients with Recurrent/Progressive Grade II Low-Grade Glioma (LGG) | 00615927 | 2006 | 2013 | Astrocytomas or oligodendromas | Multiple tyrosine kinases | Imatinib + hydroxyurea |
| Oral Tarceva Study for Recurrent/Residual Glioblastoma Multiforme and Anaplastic Astrocytoma | 00301418 | 2006 | 2016 | GBM and Anaplastic Astrocytoma | EGFRvIII | Erlotinib |
| Sorafenib Tosylate and Temsirolimus in Treating Patients with Recurrent Glioblastoma | 00329719 | 2006 | 2018 | Recurrent GBM | multiple kinases, mTOR | sorafenib + temsirolimus |
| Sorafenib Combined with Erlotinib, Tipifarnib, or Temsirolimus in Treating Patients with Recurrent Glioblastoma Multiforme or Gliosarcoma | 00335764 | 2006 | 2018 | GBM or Gliosarcoma | PDGFR, EGFR, farnesyltransferase, mTOR | Sorafenib, erlotinib, tipifarnib, and temsirolimus |
| Temsirolimus, Temozolomide, and Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | 00316849 | 2006 | 2013 | GBM or Gliosarcoma | mTOR | temsirolimus + RT + TMZ |
| Tumor Tissue Analysis in Patients Receiving Imatinib Mesylate for Malignant Glioma | 00401024 | 2006 | 2018 | Glioma | Multiple tyrosine kinases | Imatinib |
| Erlotinib and Sorafenib in Treating Patients with Progressive or Recurrent Glioblastoma Multiforme | 00445588 | 2007 | 2016 | Recurrent GBM | ras-raf-MEK, mTOR | erlotinib + sorafenib |
| Dasatinib in Treating Patients with Recurrent Glioblastoma Multiforme or Gliosarcoma | 00423735 | 2007 | 2019 | GBM or Gliosarcoma | Multiple Kinases | Dasatinib |
| A Phase II Trial of Sutent (Sunitinib; SU011248) for Recurrent Anaplastic Astrocytoma and Glioblastoma | 00606008 | 2007 | 2012 | GBM or Anaplastic Astrocytoma | Multiple kinases | Sunitinib |
| Ph II Erlotinib + Sirolimus for Pts w Recurrent Malignant Glioma Multiforme | 00672243 | 2007 | 2013 | GBM | EGFR + IL2 | Erlotinib + sirolimus |
| Radiation Therapy and Temozolomide Followed by Temozolomide Plus Sorafenib for Glioblastoma Multiforme | 00544817 | 2007 | 2016 | GBM | PDGR | Sorafenib + TMZ + RT |
| Sunitinib Tumor Levels in Patients Not on Enzyme-Inducing Anti-Epileptic Drugs Undergoing Debulking Surgery for Recurrent Glioblastoma | 00864864 | 2007 | 2016 | Recurrent GBM | multiple tyrosine kinases | sunitinib |
| Sunitinib in Treating Patients with Recurrent Malignant Gliomas | 00499473 | 2007 | 2016 | Recurrent Malignant Gliomas | multiple kinases | sunitinib |
| Ph. 2 Sorafenib + Protracted Temozolomide in Recurrent GBM | 00597493 | 2007 | 2013 | Recurrent GBM | PDGFR | Sorafenib + TMZ |
| Ph I Dasatinib + Erlotinib in Recurrent MG | 00609999 | 2008 | 2014 | Recurrent Malignant Glioma | multiple kinases, EGFR | dasatinib + erlotinib |
| Ph I SU011248 + Irinotecan in Treatment of Pts w MG | 00611728 | 2008 | 2014 | GBM | Multiple kinases | Sunitinib + Irinotecan |
| BIBW 2992 (Afatinib) with or without Daily Temozolomide in the Treatment of Patients with Recurrent Malignant Glioma | 00727506 | 2008 | 2017 | Recurrent Grade III and IV glioma | ErbB | Afatinib |
| A Study of Temsirolimus and Bevacizumab in Recurrent Glioblastoma Multiforme | 00800917 | 2008 | 2010 | recurrent primary GBM | mTOR, VEGF | temsirolimus + bevacizumab |
| Everolimus in Treating Patients with Recurrent Low-Grade Glioma | 00823459 | 2009 | 2020 | Low-Grade Glioma | mTOR | everolimus |
| Sorafenib in Newly Diagnosed High Grade Glioma | 00884416 | 2009 | 2014 | Newly Diagnosed High Grade Glioma | Multiple Kinases | sorafenib + TMZ + RT |
| Everolimus, Temozolomide, and Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma | 00553150 | 2009 | 2020 | Grade IV gliomas | mTOR | everolimus + TMZ |
| Study of Sunitinib Before and During Radiotherapy in Newly Diagnosed Biopsy-only Glioblastoma Patients | 01100177 | 2009 | 2013 | GBM | Multiple kinases | Sunitinib |
| Dasatinib or Placebo, Radiation Therapy, and Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | 00869401 | 2009 | 2020 | GBM | Multiple kinases | TMZ + RT +/− dasatinib |
| Open Label Trial to Explore Safety of Combining Afatinib (BIBW 2992) and Radiotherapy with or without Temozolomide in Newly Diagnosed Glioblastoma Multiform | 00977431 | 2009 | 2019 | GBM | EGFR | afatinib + RT + TMZ |
| Radiation Therapy and Temsirolimus or Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma | 01019434 | 2009 | 2018 | GBM | mTOR | temsirolimus + TMZ |
| Temsirolimus and Perifosine in Treating Patients with Recurrent or Progressive Malignant Glioma | 01051557 | 2010 | 2021 | Glioma | mTOR | perifosine + temsirolimus |
| A Study in Subjects with Recurrent Malignant Glioma | 01137604 | 2010 | 2022 | Recurrent Malignant Gliomas | multiple tyrosine kinase inhibitor, VEGF | lenvatinib + bevacizumab |
| Bafetinib in Treating Patients with Recurrent High-Grade Glioma or Brain Metastases | 01234740 | 2010 | 2018 | Glioma or brain met | ABL1 | Bafetinib |
| Everolimus, Temozolomide, and Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | 01062399 | 2010 | 2022 | Newly Diagnosed GBM | mTOR | everolimus + RT + TMZ |
| EGFR Inhibition Using Weekly Erlotinib for Recurrent Malignant Gliomas | 01257594 | 2011 | 2023 | Glioma | EGFR | Erlotinib |
| AZD8055 for Adults with Recurrent Gliomas | 01316809 | 2011 | 2019 | Recurrent gliomas | mTOR | AZD8055 |
| Phase I-II Everolimus and Sorafenib in Recurrent High-Grade Gliomas | 01434602 | 2012 | 2022 | GBM or anaplastic gliomas | mTOR1/2 + PDGFR | everolimus + sorafenib |
| Lapatinib with Temozolomide and Regional Radiation Therapy for Patients with Newly-Diagnosed Glioblastoma Multiforme | 01591577 | 2012 | 2022 | Newly-Diagnosed GBM Multiforme | EGFR | lapatinib + TMZ |
| Sorafenib, Valproic Acid, and Sildenafil in Treating Patients with Recurrent High-Grade Glioma | 01817751 | 2013 | 2023 | Recurrent High-Grade Glioma PDGFRa+ | PDGFRA | kinase inhibitor Sorafenib + Valproic acid + Sildenafil |
| Lapatinib Ditosylate Before Surgery in Treating Patients with Recurrent High-Grade Glioma | 02101905 | 2014 | 2023 | EGFR Amplified Recurrent High-Grade Glioma | EGFR | Lapatinib |
| Study to Evaluate Safety and Activity of Crizotinib with Temozolomide and Radiotherapy in Newly Diagnosed Glioblastoma | 02270034 | 2014 | 2022 | GBM | ALK | crizotinib |
| Perifosine and Torisel (Temsirolimus) for Recurrent/Progressive Malignant Gliomas | 02238496 | 2014 | 2023 | Recurrent Glioma | mTOR | Temsirolimus, Perifostine |
| Study of LY2228820 with Radiotherapy Plus Concomitant TMZ in the Treatment of Newly Diagnosed Glioblastoma | 02364206 | 2015 | 2019 | GBM | p38 MAPK | LY2228820 |
| Study of Tesevatinib Monotherapy in Patients with Recurrent Glioblastoma | 02844439 | 2016 | 2021 | GBM | EGFR, VEGFR, HER2 | Tesevatinib |
| Dabrafenib and/or Trametinib Rollover Study | 03340506 | 2017 | 2023 | High Grade Glioma | B-Raf, MEKi | dabrafenib + trametinib |
| Ruxolitinib with Radiation and Temozolomide for Grade III Gliomas and Glioblastoma | 03514069 | 2018 | 2023 | Grade III Gliomas and GBM | JAK/STAT | Ruxolitinib + RT +TMZ |
| A Trial of Ipatasertib in Combination with Atezolizumab | 03673787 | 2018 | 2022 | GBM | AKT, PD-L1 | Ipatasertib, Atezolizumab |
| 18F-FDG PET and Osimertinib in Evaluating Glucose Utilization in Patients with EGFR Activated Recurrent Glioblastoma | 03732352 | 2018 | 2023 | EGFR Activated Recurrent GBM | EGFR | Osimertinib |
| 9-ING-41 in Patients with Advanced Cancers | 03678883 | 2019 | 2023 | Malignant glioma | GSK-3β | 9-ING-41 |
| Nedisertib and Radiation Therapy, Followed by Temozolomide for the Treatment of Patients with Newly Diagnosed MGMT Unmethylated Glioblastoma or Gliosarcoma | 04555577 | 2020 | 2022 | Newly Diagnosed MGMT Unmethylated GBM or Gliosarcoma | DNA-dependent protein kinase (DNA-PK) | Nedisertib + RT |
| Tofacitinib in Recurrent GBM Patients | 05326464 | 2022 | 2023 | Recurrent GBM | JAK | Tofacitinib |
| DETERMINE Trial Treatment Arm 5: Vemurafenib in Combination with Cobimetinib in Adult Patients with BRAF Positive Cancers. | 05768178 | 2023 | 2023 | Glioma | BRAF V600 | Vemurafenib + Cobimetinib |
| Superselective Intra-arterial Cerebral Infusion of Temsirolimus in HGG | 05773326 | 2023 | 2023 | recurrent high-grade glioma (grade 3 or 4 per WHO criteria) | mTOR | Temsirolimus |
| Microenvironmental Targets (angiogenesis, cell-cell adhesion, iron/cation regulation) | ||||||
| Gefitinib Plus Temozolomide in Treating Patients with Malignant Primary Glioma | 00027625 | 2002 | 2018 | Malignant Primary Glioma | EGFR | gefitinib + TMZ |
| Safety and Efficacy Study of Tarceva, Temodar, and Radiation Therapy in Patients with Newly Diagnosed Brain Tumors | 00187486 | 2004 | 2017 | GBM or Gliosarcoma | EGFR | erlotinib + TMZ |
| Erlotinib and Temsirolimus in Treating Patients with Recurrent Malignant Glioma | 00112736 | 2005 | 2015 | Recurrent Malignant Glioma | EGFR, mTOR | erlotinib + temsirolimus |
| Temozolomide and Radiation Therapy with or without Vatalanib in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | 00128700 | 2005 | 2012 | GBM | VEGFR | vatalanib + TMZ |
| Imatinib Mesylate, Vatalanib, and Hydroxyurea in Treating Patients with Recurrent or Relapsed Malignant Glioma | 00387933 | 2005 | 2015 | Recurrent or Relapsed Malignant Glioma | VEGF, multiple tyrosine kinases | imatinib + vatalanib + hydroxyurea |
| Cetuximab, Bevacizumab and Irinotecan for Patients with Malignant Glioblastomas | 00463073 | 2006 | 2008 | Malignant GBM | VEGF, EGFR | bevacizumab + cetuximab + irinotecan |
| PTK787/ZK 222584 in Combination with Temozolomide and Radiation in Patients with Glioblastoma Taking Enzyme-Inducing Anti-Epileptic Drugs | 00385853 | 2006 | 2013 | GBM | VEGF | PTK787/ZK (volitinib) + TMZ + RT |
| Pazopanib In Combination with Lapatinib in Adult Patients with Relapsed Malignant Glioma | 00350727 | 2006 | 2013 | Recurrent Glioma | VEGFR, HER2 | pazopanib and lapatinib |
| Phase (Ph) II Bevacizumab + Erlotinib for Patients (Pts) with Recurrent Malignant Glioma (MG) | 00671970 | 2007 | 2013 | Recurrent Malignant Gliomas | EGFR, VEGF | erlotinib + bevacizumab |
| Bevacizumab and Cediranib Maleate in Treating Patients with Metastatic or Unresectable Solid Tumor, Lymphoma, Intracranial Glioblastoma, Gliosarcoma or Anaplastic Astrocytoma | 00458731 | 2007 | 2014 | Metastatic GBM, Gliosarcoma, or Anaplastic Astrocytoma | VEGF | bevacizumab + cediranib maleate |
| Study of Bevacizumab Plus Temodar and Tarceva in Patients with Glioblastoma or Gliosarcoma | 00525525 | 2007 | 2014 | GBM or Gliosarcoma | VEGF + EGFR | bevacizumab + erlotinib + TMZ |
| Ph I Zactima + Imatinib Mesylate and Hydroxyurea for Pts w Recurrent Malignant Glioma | 00613054 | 2007 | 2012 | Recurrent Malignant Glioma | VEGFR, PI3KT, EGFR, PDGFR | Zactima + imatinib + hydroxyurea |
| Cediranib, Temozolomide, and Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma | 00662506 | 2008 | 2017 | GBM or Gliosarcoma | VEGFR | Cediranib |
| Bevacizumab and Sorafenib in Treating Patients with Recurrent Glioblastoma Multiforme | 00621686 | 2008 | 2018 | Recurrent GBM | VEGF + multiple tyrosine kinases | bevacizumab + sorafenib |
| RT, Temozolomide, and Bevacizumab Followed by Bevacizumab/Everolimus in First-line Treatment of GBM | 00805961 | 2009 | 2021 | GBM | VEGF, mTOR1/2 | Bevacizumab + Everolimus + RT + TMZ |
| Afatinib (BIBW 2992) QTcF Trial in Patients with Relapsed or Refractory Solid Tumours | 00875433 | 2009 | 2013 | Relapsed or Refractory Solid Tumours (GBM and brain metastases) | EGFR | Afatinib |
| Bevacizumab and Erlotinib After Radiation Therapy and Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma Multiforme or Gliosarcoma | 00720356 | 2009 | 2018 | GBM | VEGF + EGFR | Bevacizumab + Erlotinib |
| Dasatinib and Bevacizumab in Treating Patients with Recurrent or Progressive High-Grade Glioma or Glioblastoma Multiforme | 00892177 | 2009 | 2019 | GBM | VEGF + multiple kinases | Bevacizumab + dasatinib |
| Temozolomide and Radiation Therapy with or without Cediranib Maleate in Treating Patients with Newly Diagnosed Glioblastoma | 01062425 | 2010 | 2022 | GBM | VEGFR | TMZ + RT +/− cediranib |
| Cediranib Maleate and Cilengitide in Treating Patients with Progressive or Recurrent Glioblastoma | 00979862 | 2010 | 2015 | GBM or Gliosarcoma | VEGFR, integrins | Cediranib and Cilengitide |
| Gamma-Secretase Inhibitor RO4929097 and Cediranib Maleate in Treating Patients with Advanced Solid Tumors | 01131234 | 2010 | 2014 | Gliomas and Brain Mets | VEGFR and gamma secretase | Cediranib + RO4929097 |
| A Study of Avastin (Bevacizumab) and Irinotecan Versus Temozolomide Radiochemistry in Patients with Glioblastoma | 00967330 | 2010 | 2015 | Newly diagnosed GBM, non-methylated MGMT promoter | VEGF | bevacizumab + irinotecan + TMZ + RT |
| BIBF 1120 in Recurrent Glioblastoma Multiforme | 01251484 | 2011 | 2012 | Recurrent GBM | VEGFR | Cediranib |
| BIBF 1120 for Recurrent High-Grade Gliomas | 01380782 | 2012 | 2014 | GBM or Anaplastic Gliomas | VEGFR/PDGFR/FGFR | Nintedanib |
| CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients with Malignant Gliomas Expressing EGFRvIII | 01454596 | 2012 | 2019 | Malignant Gliomas Expressing EGFRvIII | EGFRvIII | CAR T cell targeting EGFRvIII |
| Tivozanib for Recurrent Glioblastoma | 01846871 | 2013 | 2019 | GBM | VEGFR | Tivozanib |
| A Randomized Phase II Clinical Trial on the Efficacy of Axitinib as a Monotherapy or in Combination with Lomustine for the Treatment of Patients with Recurrent Glioblastoma | 01562197 | 2014 | 2019 | GBM | VEGFR | Axitinib |
| Apatinib in Recurrent or Refractory Intracranial Central Nervous System Malignant Tumors | 03660761 | 2016 | 2019 | GBM | VEGFR2 | Apatinib + TMZ |
| Safety Study of Afatinib for Brain Cancer | 02423525 | 2016 | 2022 | Recurrent or Progressive Brain Cancer | VEGF | afatinib |
| Clinical Trial on the Combination of Avelumab and Axitinib for the Treatment of Patients with Recurrent Glioblastoma | 03291314 | 2017 | 2019 | Recurrent GBM | VEGFR, PD1L | Axitinib + Avelumab |
| Prediction of Therapeutic Response of Apatinib in Recurrent Gliomas | 04216550 | 2018 | 2021 | Recurrent Gliomas | VEGFR-2 | Apatinib |
| Ketoconazole Before Surgery in Treating Patients with Recurrent Glioma or Breast Cancer Brain Metastases | 03796273 | 2019 | 2022 | Recurrent Glioma or Breast Cancer Brain Metastases | tGLI1 | ketoconazole |
| Anlotinib Combined with STUPP for MGMT Nonmethylated Glioblastoma | 04725214 | 2021 | 2021 | MGMT nonmethylated GBM | VEGF | anlotinib |
| Cell Cycle/Apoptosis/Transcription Pathways | ||||||
| Study of the Poly (ADP-ribose) Polymerase-1 (PARP-1) Inhibitor BSI-201 in Patients with Newly Diagnosed Malignant Glioma | 00687765 | 2008 | 2022 | Newly diagnosed Malignant Glioma | PARP-1 | iniparib (BSI-201) + TMZ + RT |
| Virus DNX2401 and Temozolomide in Recurrent Glioblastoma | 01956734 | 2013 | 2017 | GBM | Rb | DNX2401 |
| Trial of Ponatinib in Patients with Bevacizumab-Refractory Glioblastoma | 02478164 | 2013 | 2018 | GBM | cKIT | Ponatinib |
| Safety and Efficacy of PD0332991 (Palbociclib), a Cyclin-dependent Kinase 4 and 6 Inhibitor, in Patients with Oligodendroglioma or Recurrent Oligoastrocytoma Anaplastic with the Activity of the Protein RB Preserved | 02530320 | 2015 | 2020 | Oligodendroma and oligoastrocytoma | CDK4/6 | Palbociclib |
| Zotiraciclib (TG02) Plus Dose-Dense or Metronomic Temozolomide Followed by Randomized Phase II Trial of Zotiraciclib (TG02) Plus Temozolomide Versus Temozolomide Alone in Adults with Recurrent Anaplastic Astrocytoma and Glioblastoma | 02942264 | 2016 | 2021 | Glioma | CDK9 | dinaciclib + TMZ |
| Phase I/IIa Study of Concomitant Radiotherapy with Olaparib and Temozolomide in Unresectable High Grade Gliomas Patients | 03212742 | 2017 | 2023 | Unresectable High Grade Glioma | poly(ADP-ribose) polymerase (PARP) inhibitor | olaparib + TMZ |
| A Phase 0/II Study of Ribociclib (LEE011) in Combination with Everolimus in Preoperative Recurrent High-Grade Glioma Patients Scheduled for Resection | 03834740 | 2018 | 2023 | Preoperative Recurrent High-Grade Glioma | CDK4/6, mTOR | ribociclib + everolimus |
| BGB-290 and Temozolomide in Treating Isocitrate Dehydrogenase (IDH)1/2-Mutant Grade I–IV Gliomas | 03749187 | 2019 | 2023 | Isocitrate Dehydrogenase (IDH)1/2-Mutant Grade I-IV Gliomas | Poly (ADP-Ribose) polymerase (PARP) inhibitor BGB-290 | BGB-29 + TMZ |
| Anticancer Therapeutic Vaccination Using Telomerase-derived Universal Cancer Peptides in Glioblastoma | 04280848 | 2020 | 2022 | Primary GBM | TERT | UCPVax + anti-cancer vaccine based on the telomerase-derived helper peptides |
| B7-H3 CAR-T for Recurrent or Refractory Glioblastoma | 04077866 | 2023 | 2022 | Recurrent or refractory GBM | B7-H3 | B7-H3 CAR-T |
| Immunotherapy Pathways | ||||||
| A Dose Escalation and Cohort Expansion Study of Anti-CD27 (Varlilumab) and Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors | 02335918 | 2015 | 2019 | Refractory GBM | CD27, PD-1 | varlilumab + nivolumab |
| Ipilimumab and/or Nivolumab in Combination with Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma or Gliosarcoma | 02311920 | 2015 | 2023 | Newly diagnosed GBM | CTLA-4, PD-1 | ipilimumab and/or nivolumab + TMZ |
| Study of Cabiralizumab in Combination with Nivolumab in Patients with Selected Advanced Cancers | 02526017 | 2015 | 2022 | Malignant Glioma | CSF1R TAMs, PD-1 | cabiralizumab + nivolumab |
| Intra-tumoral Ipilimumab Plus Intravenous Nivolumab Following the Resection of Recurrent Glioblastoma | 03233152 | 2016 | 2020 | Recurrent GBM | CTLA-4, PD1 | ipilimumab + nivolumab |
| Nivolumab for Recurrent or Progressive IDH Mutant Gliomas | 03557359 | 2018 | 2022 | Recurrent or Progressive IDH Mutant Gliomas | PD-1 | Nivolumab |
| Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) in Previously Treated Participants with Select Solid Tumors (MK-7902-005/E7080-G000-224/LEAP-005) | 03797326 | 2019 | 2022 | GBM | PD-1, multiple kinase inhibitors | Pembrolizumab, Lenvatinib |
| Efficacy of Nivolumab for Recurrent IDH Mutated High-Grade Gliomas | 03925246 | 2019 | 2021 | Recurrent IDH Mutated High-Grade Gliomas | PD-1 | nivolumab |
| Trial of Anti-Tim-3 in Combination with Anti-PD-1 and SRS in Recurrent GBM | 03961971 | 2020 | 2023 | Recurrent GBM | TIM-3, PD-1 | Sabatolimab, high-affinity, humanized, IgG4 (S228P) antibody + Spartalizumab + RT |
| Neoadjuvant Carilizumab and Apatinib for Recurrent High-Grade Glioma | 04588987 | 2020 | 2020 | Recurrent High-Grade Glioma | PD-1, TKI | carilizumab + apatinib |
| Ivosidenib (AG-120) with Nivolumab in IDH1 Mutant Tumors | 04056910 | 2021 | 2023 | IDH1 Mutant Tumors | IDH1, PD1 | ivosidenib |
| Other | ||||||
| A Phase 2b Clinical Study with a Combination Immunotherapy in Newly Diagnosed Patients with Glioblastoma | 04485949 | 2023 | 2023 | Newly diagnosed GBM | IGF1 | IGV-001 |
Abbreviations: GBM, glioblastoma multiforme; EGFR, epithelial growth factor receptor; mTOR, mammalian target of rapamycin; CNS, central nervous system; PDGFR, platelet-derived growth factor receptor; TMZ, temozolomide; HER2, human epidermal growth factor receptor 2; RT, radiation therapy; IL2, interleukin-2; erbB, erythroblastic leukemia viral oncogene homologue; ALK, anaplastic lymphoma kinase; MAPK, mitogen-activated protein kinase; MEKi, mitogen-activated protein kinase kinase inhibitor; JAK/STAT, janus kinase-signal transducer and activator of transcription; PD-L1, programmed cell death ligand 1; GSK-3β, glycogen synthase kinase 3β; PI3K, phosphoinositide-3-kinase; tGLI1, truncated glioma-associated oncogene homolog-1; PARP-1, poly(ADP-ribose)-polymerase 1; Rb, retinoblastoma tumor suppressor; CDK, cyclin-dependent kinase; TERT, Telomerase reverse transcriptase; CTLA-4, cytotoxic T lymphocyte antigen 4; CSF1R TAMs, CSF1R-expressing tumor-associated macrophages; TIM-3, T-cell immunoglobulin and mucin domain 3; IGF1, insulin-like growth factor 1.
The most common funding source was industry-related funding (54/119, 45%), followed by the National Institute of Health (NIH) (45/119, 38%) (Table S3). All ongoing clinical trials were in phase I or II.
4. Discussion
This systematic review examined the current evidence on molecular targeted therapy for adult-type diffuse glioma. The majority of clinical and laboratory studies focused on GBM, with few studies examining IDH-mutant astrocytomas, oligodendrogliomas, or unspecified gliomas. In both clinical and laboratory settings, protein kinase pathways—particularly PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk—were the most commonly targeted molecular pathways. The next most common molecular targets in published clinical studies and clinical trials were microenvironmental targets—including angiogenesis, cell-cell adhesion, or ion/cation regulation—followed by cell cycle/apoptosis pathways and immunotherapy. The second most common molecular targets in laboratory studies were cell cycle/apoptosis pathways, followed by microenvironmental targets, and then immunotherapy pathways. The wnt/β-catenin pathway was also prevalent in the studies identifying novel targets. The level of evidence for published clinical studies varied, with the majority being Level IV—consistent with early-phase, single-institution clinical trials; all laboratory studies were quasi-experimental designs. Published clinical studies testing molecular targeted therapies, in general, were published more recently than laboratory studies. Lastly, clinical trials on protein kinase pathways began earlier than other clinical trial types, particularly trials testing cell cycle/apoptosis targets or immunotherapy.
4.1. Adult-Type Diffuse Glioma Subtypes
Though the overwhelming majority of studies centered on GBM, the literature shows that adult gliomas found more frequently in practice tend to harbor IDH mutations [7,344]. The reason for the overrepresentation of GBM-focused studies and the underrepresentation of IDH-mutant astrocytoma or oligodendroglioma is multifactorial. First off, the updated WHO classification is a recent development as of 2021; because the majority of the works in this study occurred prior to the molecular subtype differentiation, there were likely studies that self-identified as GBM studies that may have included tumors with an IDH mutation or 1p19q co-deletion. To the best of our ability, we retroactively identified studies that specifically identified these molecular statuses, but those were few in number. Additionally, it is likely that GBM has received more research funding and scientific attention than other brain tumors, perhaps due to its more aggressive nature and mortality rates. Therefore, the funding for studies investigating IDH-mutant astrocytoma or oligodendroglioma may be less robust. Of note, the ongoing clinical trials for glioma vastly favor GBM as well, receiving the majority of funding from industry sources. Further studies to quantify the distribution of research funding between glioma subsets would be necessary to confirm this association. Lastly, the standard cell lines for all glioma research tend to be glioblastoma models, particularly U87, U373, and U251, as also reflected in our study [345] (Table S2).
4.2. Protein Kinase Pathways
In terms of molecular targets, protein kinase pathways—especially PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk—were the most prevalent in the clinical and laboratory studies analyzing existing therapies and novel targets to treat adult-type diffuse glioma. (Table 2, Table 3 and Table 4) These results are consistent with previous studies that have demonstrated a predominance in the PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk protein kinase pathways in molecularly targeted glioma treatment [5,6]. The importance of these pathways in glioma has been well-described in the literature; ultimately, these tumors harbor mutations that continuously activate these protein kinase signaling pathways, leading to increased tumorigenesis and progression [346,347,348].
Both the PI3K/Akt/mTOR and Ras/BRAF/Mek/Erk protein kinase pathways are also downstream of receptors such as EGFR, one of the most significant signaling pathways clinically implicated in glioma [349]. A systematic review of molecular targeted therapy clinical trials for GBM identified EGFR as the most prevalent molecular target [6]. Nonetheless, studies have demonstrated limited clinical benefit of anti-EGFR therapies, theorized to be secondary to PTEN-mediated resistance of GBM to this therapy type [350].
Similar to the published clinical studies on this topic, protein kinase pathways were by far the most predominant molecular targets tested in ongoing clinical trials. Interestingly, these therapeutics were also investigated much earlier on average. This finding is likely due to the fact that protein kinase inhibitors are some of the earliest molecular target therapies in the field of targeted oncologic interventions, thus being able to start clinical trials for the treatment of glioma as early as 2001 [351]. Perhaps, in the coming years, as the analysis of existing molecularly targeted therapies progresses from earlier stage clinical testing or laboratory testing, there will be a shift favoring more of the scientifically novel approaches—such as immunotherapeutics, cell cycle inhibitors, or more specifically localized targeting—in clinical trials.
Additional protein kinase pathways targeted in laboratory studies included HER2 receptors, epithelial membrane protein-2 (EMP2), and STAT3, to name a few [108,117,140]. HER2 expression tends to be low in GBM, and though one clinical trial examining a HER2 inhibitor has yet to show therapeutic gain, laboratory studies have promising evidence for efficacy [140,352]. EMP2 has been implicated in bevacizumab resistance and thus shows promise as a molecular target for preventing resistance in conjunction with this common therapeutic [117,353]. STAT3 plays a role in astrocyte development and has tumor suppressive roles in glial malignancies; this target shows promise in laboratory research using tetrandrine as an inhibitor [108]. Despite varying clinical evidence of efficacy, protein kinase-targeted therapies remain a prevalent area of study for both individual inhibitors and combined therapies.
4.3. Cell Cycle/Apoptosis Pathways
Interestingly, a prevalent molecular target in laboratory studies—both testing existing therapies and identifying novel targets—were cell cycle/apoptosis pathways. This difference may be attributed to the fact that clinical studies tend to focus on targets with existing FDA-approved therapies or targets that are more well-established in the literature, while studies with the goal of establishing new targets or testing newly developed therapies can explore a wider range of targets with less established evidence.
The use of cell cycle or apoptosis pathways as targets stems from the use of these pathways in the treatment of other tumors, in particular. In the present study, only four clinical studies included cell cycle/apoptosis pathway inhibitors, namely the cyclin-dependent kinase (CDK) 4/6 inhibitor palbociclib, the mouse double minute 2 (MDM2) inhibitor idasanutlin, the ribonucleotide reductase inhibitor Motexafin Gadolinium, and the 26S proteasome inhibitor bortezomib [36,57,58,59]. CDK and MDM2 inhibitors were also prevalent in laboratory studies testing existing therapies [150,156,160,161,165,176]. Two CDKs were identified as novel molecular targets—namely CDK 5 and 10—and other novel targets include other apoptosis regulators such as E2F1, trichothiodystrophy group A protein (TTDA), and protease activated receptor 2 (PAR2) [267,269,274,279,280].
4.4. Microenvironmental Pathways (Angiogenesis, Cell-Cell Adhesion, Ιron/Cation Regulation)
Anti-angiogenic therapies aim to compensate for the robust vascularity of gliomas, particularly GBM [354]. Specifically, vascular endothelial growth factor (VEGF) is overexpressed in GBM, providing rationale for the thirteen published clinical trials investigating VEGF inhibitors. Specific inhibitors studied include cediranib, cabozantinib, apatinib, and bevacizumab, which appear to be well-tolerated by patients and, in many cases, portend progression-free survival [32,39,40,41,42,43,45,46,48,49,50,55]. Many laboratory studies also tested VEGF inhibitors—namely bevacizumab, axitinib, and apatinib—and all found promising results in vivo [187,191,203]. Other microenvironmental targets included mitochondrial transcription factor A (TFAM), transient receptor potential cation channel subfamily V member 4 (TRPV4), and HIF2α. These targets were acted on by melatonin, cannabidiol, and PT2385, respectively, all of which demonstrated antitumor effects [183,186,198]. Promising novel microenvironmental targets include miR-497, TWIST transcription factor, and tenascin-W, among others [291,293,296].
4.5. Immunotherapy Pathways
The immune checkpoint blockade adopted in the glioma therapeutics model follows treatment paradigms for melanoma, lung cancer, colon cancer, and hepatocellular carcinoma; the therapies used to treat these tumors tend to block programmed cell death protein 1 (PD1), a protein known for attenuating the host immune response to tumor cells, or cytotoxic T lymphocyte antigen-4 (CTLA-4), a molecule that inhibits T-cell activation [355,356,357,358]. The clinical studies identified in the present study investigating immunotherapeutic pathways targeted PD1 using the inhibitor nivolumab [54,359]. Other immunotherapy targets that were found to be effective in vivo included the inhibition of CD73 with antibodies, extracellular matrix metalloproteinase (EMMPRIN) with icaritin, and NFκB with BAY117082 [207,210,211]. Novel immunotherapeutics for GBM and oligodendroglioma include cluster of differentiation 204 (CD204), S100A, and the CE7 epitope of the L1-CAM adhesion molecule [92,306,308].
4.6. Wnt/β-Catenin Pathway
The wnt/β-catenin pathway was much more prevalent in earlier stages of laboratory research identifying new targets, likely because the role of wnt/β-catenin in glioma progression is a more recent scientific advancement [310,311,312,313,314,315,316,349]. It is likely that in the upcoming years, the distribution of molecular targets may shift from protein kinase pathway-targeted therapies towards the wnt/β-catenin pathway or a combinatory approach of the two. Ongoing clinical trials have yet to target these pathways, but it is likely that this will soon change.
4.7. Study Design
The majority of laboratory studies utilized GBM cell lines or GBM patient samples. The frequent use of the U87 cell line in laboratory studies may be attributed to its widely accepted use as a model for GBM [360]. The use of technology such as spheroid or 3D cell culture is highly relevant in the context of therapies for gliomas. These technologies more accurately represent the tumor microenvironment and allow for better design of patient-specific treatments. Nearly one-third of laboratory studies testing existing therapies utilized this technology, implying that these studies are likely closer to translation to human studies.
The use of patient-derived GBM and glioma samples also highlights the importance of personalized medicine approaches in glioma treatment; nonetheless, this use also limits the generalizability of the conclusions, as most of these studies did not investigate molecular subtypes.
4.8. Implications
Molecular targeted therapy is predicted to revolutionize glioma therapy [361,362,363]. Particularly looking at the NCT Neuro Master Match (N2M2) trial, which uses molecular signatures of GBM to inform treatment, future studies will likely use the molecular identities of tumors to designate treatment [36]. These findings portend a shift in molecular targeted therapy research as well, wherein laboratory studies testing existing treatments will enter Phase I/II clinical trials and studies identifying novel targets will advance into the development and testing of therapies in a laboratory setting. Specifically, we will likely see a broadening of the current clinical studies and ongoing clinical trials—including more immunotherapeutics and microenvironmental pathway testing—in addition to testing of wnt/β-catenin pathway inhibitors in vitro and in vivo in the coming years.
4.9. Limitations
There are several limitations to our analysis that should be considered. First, there was significant heterogeneity in the patient populations, interventions, and outcomes reported across clinical studies. The quality of the studies included in our analysis also varied, with the majority having low levels of evidence due to being case reports or series. Notably, only 52 clinical studies were identified, which may be an underrepresentation of the true number of current clinical research studies investigating molecular targeted therapies for glioma. For instance, for GBM alone, a study analyzing the clinical trials related to molecular targeted therapy totaled 257 [6]. In contrast, the sum of published literature and ongoing clinical trials identified in this study totaled 171. This discrepancy is likely due to the fact that clinical trial titles may utilize specific drug names rather than the term “molecular targeted therapy” or broad names of categories within molecular targeted therapies.
Additional limitations include the fact that the studies had varying methodological quality and targeted different molecular pathways, making it difficult to draw definitive conclusions. The categorization of molecular targets is an imperfect model as well, for pathways such as STAT3 can simultaneously qualify as involving protein kinase inhibitors and angiogenesis, for instance [108]. The categorization of tumor types has also changed drastically since the WHO 2021 guideline change. This study retroactively reflects the updated tumor classification for these studies, using the literature to classify the mutation status of known cell lines. This may create a discrepancy between GBM literature released prior to 2021 and current models, but it more accurately reflects what these studies can add to future glioma literature. Our study, while comprehensive and broad in scope, is restricted by the vast variation, particularly in histological methodology and molecular marker identification capabilities.
Other limitations inherent to a systematic review are that of the search terms—for there may be studies about molecular targeted therapies that do not self-identify as such; publication bias from only including published studies; limiting the studies to only those available in English for full-text screen; and the lack of meta-analysis to quantify the data.
4.10. Future Directions
Future studies should aim to address these limitations by conducting larger multi-institutional clinical trials with standardized protocols and consistent reporting of outcomes. Studies should also consider investigating the effectiveness of combination therapies that target multiple molecular pathways simultaneously.
5. Conclusions
Here, we identify the current state of molecular target therapy research for adult-type diffuse gliomas, broadly found to be among one of three stages: validating molecular targeted therapies through published human clinical studies, testing existing therapies in a laboratory setting, and identifying novel molecular targets in a laboratory setting. We also queried clinicaltrials.gov for ongoing clinical trials on this topic. All studies predominantly investigated GBM, with few mentioning IDH-mutant astrocytomas or oligodendrogliomas. The most common molecular targets in all study types were protein kinase pathways such as PI3K/AKT/mTOR and Ras/BRAF/Mek/Erk. Microenvironmental targets were more numerous in clinical studies, whereas cell cycle/apoptosis were more numerous in laboratory studies. Immunotherapy pathways are few in number but on the rise in all study types, and the wnt/β-catenin pathway has been increasingly identified as a novel target.
Ultimately, these findings provide insight into the current state of molecular targeted therapy for glioma, highlighting the need for further investigation and the potential for this approach to improve patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310456/s1.
Appendix A. Search Terms
| Glioma [Mesh] OR “Glioma/drug therapy” OR “Glioblastoma/drug therapy” OR Ganglioglioma/drug therapy [MeSH] OR “adult-type diffuse glioma” OR “oligodendroglioma” OR “astrocytoma” |
| AND |
| Molecular targeted therapy [MeSH] OR Protein Kinase Inhibitors/administration and dosage [MeSH] OR “Antineoplastic Combined Chemotherapy Protocols/administration and dosage” OR “Receptor Protein-Tyrosine Kinases/analysis” OR “Multikinase inhibitor” OR MAP Kinases/antagonists and inhibitors [MeSH] OR “Mitogen-Activated Protein Kinase Kinases/antagonists and inhibitors” OR “Immune Checkpoint Inhibitors/therapeutic use” ** |
| ** Format adapted for PubMed, Web of Science-Medline, and clinicaltrials.gov advanced searches, respectively, from 1 January 1900 through 1 January 2023. |
Author Contributions
Conceptualization, L.M. and M.T.K.; methodology: L.M., N.K.G. and M.T.K.; software, L.M.; validation, L.M., N.K.G. and M.T.K.; formal analysis, L.M.; investigation, L.M., N.K.G. and N.C.; resources, L.M., N.K.G. and N.C.; data curation, L.M., N.K.G. and N.C.; writing—original draft preparation, L.M.; writing—reviewing and editing, L.M., N.K.G., N.C. and M.T.K.; visualization, N.K.G. and L.M.; supervision: M.T.K.; project administration, M.T.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This requirement was not applicable due to the study not involving human subjects or animals aside from already-published sources.
Informed Consent Statement
No consent for publication was required, as papers used in this study that used individual patient information were from already published sources.
Data Availability Statement
Due to the nature of the research, there was no primary data collected. Materials were obtained from searches of the PubMed and Web of Science databases.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakola A.S., Skjulsvik A.J., Myrmel K.S., Sjåvik K., Unsgård G., Torp S.H., Aaberg K., Berg T., Dai H.Y., Johnsen K., et al. Surgical Resection versus Watchful Waiting in Low-Grade Gliomas. Ann. Oncol. 2017;28:1942–1948. doi: 10.1093/annonc/mdx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai R., Fang S., Pang B., Liu Y., Wang Y., Zhang W., Jiang T. Molecular Pathology and Clinical Implications of Diffuse Glioma. Chin. Med. J. 2022;135:2914–2925. doi: 10.1097/CM9.0000000000002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitfield B.T., Huse J.T. Classification of Adult-type Diffuse Gliomas: Impact of the World Health Organization 2021 Update. Brain Pathol. 2022;32:e13062. doi: 10.1111/bpa.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang K., Wu Z., Zhang H., Zhang N., Wu W., Wang Z., Dai Z., Zhang X., Zhang L., Peng Y., et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer. 2022;21:39. doi: 10.1186/s12943-022-01513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz Da Silva E., Mercier M.-C., Etienne-Selloum N., Dontenwill M., Choulier L. A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials. Cancers. 2021;13:1795. doi: 10.3390/cancers13081795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichimura K., Pearson D.M., Kocialkowski S., Bäcklund L.M., Chan R., Jones D.T.W., Collins V.P. IDH1 Mutations Are Present in the Majority of Common Adult Gliomas but Rare in Primary Glioblastomas. Neuro-Oncology. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinzen D., Divé I., Lorenz N.I., Luger A.-L., Steinbach J.P., Ronellenfitsch M.W. Second Generation MTOR Inhibitors as a Double-Edged Sword in Malignant Glioma Treatment. Int. J. Mol. Sci. 2019;20:4474. doi: 10.3390/ijms20184474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett M., Fujii Y., Osaka N., Ito D., Hirota Y., Sasaki A. Gliomas. Exon Publications; Brisbane, Australia: 2021. Emerging Roles of Wild-Type and Mutant IDH1 in Growth, Metabolism and Therapeutics of Glioma; pp. 61–78. [DOI] [PubMed] [Google Scholar]
- 10.Miyata S., Tominaga K., Sakashita E., Urabe M., Onuki Y., Gomi A., Yamaguchi T., Mieno M., Mizukami H., Kume A., et al. Comprehensive Metabolomic Analysis of IDH1R132H Clinical Glioma Samples Reveals Suppression of β-Oxidation Due to Carnitine Deficiency. Sci. Rep. 2019;9:9787. doi: 10.1038/s41598-019-46217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbarisi M., Iaffaioli R.V., Armenia E., Schiavo L., De Sena G., Tafuto S., Barbarisi A., Quagliariello V. Novel Nanohydrogel of Hyaluronic Acid Loaded with Quercetin Alone and in Combination with Temozolomide as New Therapeutic Tool, CD44 Targeted Based, of Glioblastoma Multiforme. J. Cell. Physiol. 2018;233:6550–6564. doi: 10.1002/jcp.26238. [DOI] [PubMed] [Google Scholar]
- 12.Ackley B.J., Ladwig G.B., Swan B.A., Tucker S.J. Evidence-Based Nursing Care Guidelines—E-Book: Medical-Surgical Interventions. Elsevier Health Sciences; Amsterdam, The Netherlands: 2007. [Google Scholar]
- 13.Berzero G., Bellu L., Baldini C., Ducray F., Guyon D., Eoli M., Silvani A., Dehais C., Idbaih A., Younan N., et al. Sustained Tumor Control With MAPK Inhibition in BRAF V600-Mutant Adult Glial and Glioneuronal Tumors. Neurology. 2021;97:e673–e683. doi: 10.1212/WNL.0000000000012330. [DOI] [PubMed] [Google Scholar]
- 14.Butowski N., Chang S.M., Lamborn K.R., Polley M.Y., Parvataneni R., Hristova-Kazmierski M., Musib L., Nicol S.J., Thornton D.E., Prados M.D. Enzastaurin plus Temozolomide with Radiation Therapy in Glioblastoma Multiforme: A Phase I Study. Neuro-Oncology. 2010;12:608–613. doi: 10.1093/neuonc/nop070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinnaiyan P., Won M., Wen P.Y., Rojiani A.M., Wendland M., Dipetrillo T.A., Corn B.W., Mehta M.P. RTOG 0913: A Phase I Study of Daily Everolimus (RAD001) In Combination with Radiation Therapy and Temozolomide in Patients with Newly Diagnosed Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:880–884. doi: 10.1016/j.ijrobp.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobysheva A., Klesse L.J., Bowers D.C., Rajaram V., Rakheja D., Timmons C.F., Wang J., Koral K., Gargan L., Ramos E., et al. Targeted MAPK Pathway Inhibitors in Patients With Disseminated Pilocytic Astrocytomas. J. Natl. Compr. Cancer Netw. JNCCN. 2017;15:978–982. doi: 10.6004/jnccn.2017.0139. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi E., Stupp R., Van Den Bent M.J., Van Herpen C., Laigle Donadey F., Gorlia T., Hegi M., Lhermitte B., Strauss L.C., Allgeier A., et al. EORTC 26083 Phase I/II Trial of Dasatinib in Combination with CCNU in Patients with Recurrent Glioblastoma. Neuro-Oncology. 2012;14:1503–1510. doi: 10.1093/neuonc/nos256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusco M.J., Piña Y., Macaulay R.J., Sahebjam S., Forsyth P.A., Peguero E., Walko C.M. Durable Progression-Free Survival With the Use of BRAF and MEK Inhibitors in Four Cases With BRAF V600E-Mutated Gliomas. Cancer Control J. Moffitt Cancer Cent. 2021;28:10732748211040012. doi: 10.1177/10732748211040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hottinger A.F., Bensaid D., De Micheli R., Moura B., Mokhtari K., Cardoso E., Idbaih A., Stupp R. Leptomeningeal Tumor Response to Combined MAPK/ERK Inhibition in V600E-Mutated Gliomas despite Undetectable CSF Drug Levels. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:155–156. doi: 10.1093/annonc/mdy468. [DOI] [PubMed] [Google Scholar]
- 20.Johanns T.M., Ferguson C.J., Grierson P.M., Dahiya S., Ansstas G. Rapid Clinical and Radiographic Response With Combined Dabrafenib and Trametinib in Adults With BRAF-Mutated High-Grade Glioma. J. Natl. Compr. Cancer Netw. JNCCN. 2018;16:4–10. doi: 10.6004/jnccn.2017.7032. [DOI] [PubMed] [Google Scholar]
- 21.Kaley T., Touat M., Subbiah V., Hollebecque A., Rodon J., Lockhart A.C., Keedy V., Bielle F., Hofheinz R.D., Joly F., et al. BRAF Inhibition in BRAF(V600)-Mutant Gliomas: Results From the VE-BASKET Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanemaru Y., Natsumeda M., Okada M., Saito R., Kobayashi D., Eda T., Watanabe J., Saito S., Tsukamoto Y., Oishi M., et al. Dramatic Response of BRAF V600E-Mutant Epithelioid Glioblastoma to Combination Therapy with BRAF and MEK Inhibitor: Establishment and Xenograft of a Cell Line to Predict Clinical Efficacy. Acta Neuropathol. Commun. 2019;7:119. doi: 10.1186/s40478-019-0774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebir S., Rauschenbach L., Radbruch A., Lazaridis L., Schmidt T., Stoppek A.-K., Pierscianek D., Stuschke M., Forsting M., Sure U., et al. Regorafenib in Patients with Recurrent High-Grade Astrocytoma. J. Cancer Res. Clin. Oncol. 2019;145:1037–1042. doi: 10.1007/s00432-019-02868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinschmidt-DeMasters B.K., Aisner D.L., Foreman N.K. BRAF VE1 Immunoreactivity Patterns in Epithelioid Glioblastomas Positive for BRAF V600E Mutation. Am. J. Surg. Pathol. 2015;39:528–540. doi: 10.1097/PAS.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapointe S., Mason W., MacNeil M., Harlos C., Tsang R., Sederias J., Luchman H.A., Weiss S., Rossiter J.P., Tu D., et al. A Phase I Study of Vistusertib (Dual MTORC1/2 Inhibitor) in Patients with Previously Treated Glioblastoma Multiforme: A CCTG Study. Investig. New Drugs. 2020;38:1137–1144. doi: 10.1007/s10637-019-00875-4. [DOI] [PubMed] [Google Scholar]
- 26.Lee C., Fotovati A., Triscott J., Chen J., Venugopal C., Singhal A., Dunham C., Kerr J.M., Verreault M., Yip S., et al. Polo-like Kinase 1 Inhibition Kills Glioblastoma Multiforme Brain Tumor Cells in Part through Loss of SOX2 and Delays Tumor Progression in Mice. Stem Cells. 2012;30:1064–1075. doi: 10.1002/stem.1081. [DOI] [PubMed] [Google Scholar]
- 27.Lombardi G., De Salvo G.L., Brandes A.A., Eoli M., Rudà R., Faedi M., Lolli I., Pace A., Daniele B., Pasqualetti F., et al. Regorafenib Compared with Lomustine in Patients with Relapsed Glioblastoma (REGOMA): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2019;20:110–119. doi: 10.1016/S1470-2045(18)30675-2. [DOI] [PubMed] [Google Scholar]
- 28.Mason W.P., Macneil M., Kavan P., Easaw J., Macdonald D., Thiessen B., Urva S., Lwin Z., McIntosh L., Eisenhauer E. A Phase I Study of Temozolomide and Everolimus (RAD001) in Patients with Newly Diagnosed and Progressive Glioblastoma Either Receiving or Not Receiving Enzyme-Inducing Anticonvulsants: An NCIC CTG Study. Investig. New Drugs. 2012;30:2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 29.Migliorini D., Aguiar D., Vargas M.-I., Lobrinus A., Dietrich P.-Y. BRAF/MEK Double Blockade in Refractory Anaplastic Pleomorphic Xanthoastrocytoma. Neurology. 2017;88:1291–1293. doi: 10.1212/WNL.0000000000003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg T., Yeo K.K., Mauguen A., Alexandrescu S., Prabhu S.P., Tsai J.W., Malinowski S., Joshirao M., Parikh K., Farouk Sait S., et al. Upfront Molecular Targeted Therapy for the Treatment of BRAF-Mutant Pediatric High-Grade Glioma. Neuro-Oncology. 2022;24:1964–1975. doi: 10.1093/neuonc/noac096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanai N., Li J., Boerner J., Stark K., Wu J., Kim S., Derogatis A., Mehta S., Dhruv H.D., Heilbrun L.K., et al. Phase 0 Trial of AZD1775 in First-Recurrence Glioblastoma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:3820–3828. doi: 10.1158/1078-0432.CCR-17-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiff D., Desjardins A., Cloughesy T., Mikkelsen T., Glantz M., Chamberlain M.C., Reardon D.A., Wen P.Y. Phase 1 Dose Escalation Trial of the Safety and Pharmacokinetics of Cabozantinib Concurrent with Temozolomide and Radiotherapy or Temozolomide after Radiotherapy in Newly Diagnosed Patients with High-Grade Gliomas. Cancer. 2016;122:582–587. doi: 10.1002/cncr.29798. [DOI] [PubMed] [Google Scholar]
- 33.Shah G.D., Silver J.S., Rosenfeld S.S., Gavrilovic I.T., Abrey L.E., Lassman A.B. Myelosuppression in Patients Benefiting from Imatinib with Hydroxyurea for Recurrent Malignant Gliomas. J. Neurooncol. 2007;85:217–222. doi: 10.1007/s11060-007-9408-1. [DOI] [PubMed] [Google Scholar]
- 34.Shi L., Zou Z., Ding Q., Liu Q., Zhou H., Hong X., Peng G. Successful Treatment of a BRAF V600E-Mutant Extracranial Metastatic Anaplastic Oligoastrocytoma with Vemurafenib and Everolimus. Cancer Biol. Ther. 2019;20:431–434. doi: 10.1080/15384047.2018.1529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner J.-M., Wolf L., Tscherpel C., Bauer E.K., Wollring M., Ceccon G., Deckert M., Brunn A., Pappesch R., Goldbrunner R., et al. Efficacy and Tolerability of Regorafenib in Pretreated Patients with Progressive CNS Grade 3 or 4 Gliomas. J. Neurooncol. 2022;159:309–317. doi: 10.1007/s11060-022-04066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wick W., Dettmer S., Berberich A., Kessler T., Karapanagiotou-Schenkel I., Wick A., Winkler F., Pfaff E., Brors B., Debus J., et al. N2M2 (NOA-20) Phase I/II Trial of Molecularly Matched Targeted Therapies plus Radiotherapy in Patients with Newly Diagnosed Non-MGMT Hypermethylated Glioblastoma. Neuro-Oncology. 2019;21:95–105. doi: 10.1093/neuonc/noy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yau W.H., Ameratunga M. Combination of BRAF and MEK Inhibition in BRAF V600E Mutant Low-Grade Ganglioglioma. J. Clin. Pharm. Ther. 2020;45:1172–1174. doi: 10.1111/jcpt.13112. [DOI] [PubMed] [Google Scholar]
- 38.Zustovich F., Landi L., Lombardi G., Porta C., Galli L., Fontana A., Amoroso D., Galli C., Andreuccetti M., Falcone A., et al. Sorafenib plus Daily Low-Dose Temozolomide for Relapsed Glioblastoma: A Phase II Study. Anticancer Res. 2013;33:3487–3494. doi: 10.1200/jco.2011.29.15_suppl.2080. [DOI] [PubMed] [Google Scholar]
- 39.Badruddoja M.A., Pazzi M., Sanan A., Schroeder K., Kuzma K., Norton T., Scully T., Mahadevan D., Ahmadi M.M. Phase II Study of Bi-Weekly Temozolomide plus Bevacizumab for Adult Patients with Recurrent Glioblastoma. Cancer Chemother. Pharmacol. 2017;80:715–721. doi: 10.1007/s00280-017-3405-7. [DOI] [PubMed] [Google Scholar]
- 40.Brown N., McBain C., Nash S., Hopkins K., Sanghera P., Saran F., Phillips M., Dungey F., Clifton-Hadley L., Wanek K., et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE. 2016;11:e0156369. doi: 10.1371/journal.pone.0156369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke J.L., Molinaro A.M., Phillips J.J., Butowski N.A., Chang S.M., Perry A., Costello J.F., DeSilva A.A., Rabbitt J.E., Prados M.D. A Single-Institution Phase II Trial of Radiation, Temozolomide, Erlotinib, and Bevacizumab for Initial Treatment of Glioblastoma. Neuro-Oncology. 2014;16:984–990. doi: 10.1093/neuonc/nou029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Alessandris Q.G., Montano N., Cenci T., Martini M., Lauretti L., Bianchi F., Larocca L.M., Maira G., Fernandez E., Pallini R. Targeted Therapy with Bevacizumab and Erlotinib Tailored to the Molecular Profile of Patients with Recurrent Glioblastoma. Preliminary Experience. Acta Neurochir. 2013;155:33–40. doi: 10.1007/s00701-012-1536-5. [DOI] [PubMed] [Google Scholar]
- 43.Desjardins A., Reardon D.A., Coan A., Marcello J., Herndon II J.E., Bailey L., Peters K.B., Friedman H.S., Vredenburgh J.J. Bevacizumab and Daily Temozolomide for Recurrent Glioblastoma. Cancer. 2012;118:1302–1312. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 44.Hasselbalch B., Eriksen J.G., Broholm H., Christensen I.J., Grunnet K., Horsman M.R., Poulsen H.S., Stockhausen M.-T., Lassen U. Prospective Evaluation of Angiogenic, Hypoxic and EGFR-Related Biomarkers in Recurrent Glioblastoma Multiforme Treated with Cetuximab, Bevacizumab and Irinotecan. APMIS. 2010;118:585–594. doi: 10.1111/j.1600-0463.2010.02631.x. [DOI] [PubMed] [Google Scholar]
- 45.Lassen U., Chinot O.L., McBain C., Mau-Sørensen M., Larsen V.A., Barrie M., Roth P., Krieter O., Wang K., Habben K., et al. Phase 1 Dose-Escalation Study of the Antiplacental Growth Factor Monoclonal Antibody RO5323441 Combined with Bevacizumab in Patients with Recurrent Glioblastoma. Neuro-Oncology. 2015;17:1007–1015. doi: 10.1093/neuonc/nov019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Y., Qi S., Ouyang H., Li Z., Yin Y., Shi J., Qiu X., Mo Y. Preliminary clinical evaluations of bevacizumab for recurrent malignant glioma in Chinese patients. Zhonghua Yi Xue Za Zhi. 2014;94:1165–1168. [PubMed] [Google Scholar]
- 47.Prados M.D., Byron S.A., Tran N.L., Phillips J.J., Molinaro A.M., Ligon K.L., Wen P.Y., Kuhn J.G., Mellinghoff I.K., de Groot J.F., et al. Toward Precision Medicine in Glioblastoma: The Promise and the Challenges. Neuro-Oncology. 2015;17:1051–1063. doi: 10.1093/neuonc/nov031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaccaro V., Fabi A., Vidiri A., Giannarelli D., Metro G., Telera S., Vari S., Piludu F., Carosi M.A., Villani V., et al. Activity and Safety of Bevacizumab plus Fotemustine for Recurrent Malignant Gliomas. BioMed Res. Int. 2014;2014:351252. doi: 10.1155/2014/351252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vredenburgh J.J., Desjardins A., Kirkpatrick J.P., Reardon D.A., Peters K.B., Herndon J.E., Marcello J., Bailey L., Threatt S., Sampson J., et al. Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated with Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:58–66. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 50.Wang F., Huang Q., Zhou L.-Y. Analysis of the Treatment of Gliomas with SEC Therapy Combined with Radiochemotherapy. Eur. Rev. Med. Pharmacol. Sci. 2015;19:2400–2405. [PubMed] [Google Scholar]
- 51.Wang L., Liang L., Yang T., Qiao Y., Xia Y., Liu L., Li C., Lu P., Jiang X. A Pilot Clinical Study of Apatinib plus Irinotecan in Patients with Recurrent High-Grade Glioma: Clinical Trial/Experimental Study. Medicine. 2017;96:e9053. doi: 10.1097/MD.0000000000009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wick A., Desjardins A., Suarez C., Forsyth P., Gueorguieva I., Burkholder T., Cleverly A.L., Estrem S.T., Wang S., Lahn M.M., et al. Phase 1b/2a Study of Galunisertib, a Small Molecule Inhibitor of Transforming Growth Factor-Beta Receptor I, in Combination with Standard Temozolomide-Based Radiochemotherapy in Patients with Newly Diagnosed Malignant Glioma. Investig. New Drugs. 2020;38:1570–1579. doi: 10.1007/s10637-020-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anghileri E., Di Ianni N., Paterra R., Langella T., Zhao J., Eoli M., Patanè M., Pollo B., Cuccarini V., Iavarone A., et al. High Tumor Mutational Burden and T-Cell Activation Are Associated with Long-Term Response to Anti-PD1 Therapy in Lynch Syndrome Recurrent Glioblastoma Patient. Cancer Immunol. Immunother. CII. 2021;70:831–842. doi: 10.1007/s00262-020-02769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nayak L., Molinaro A.M., Peters K., Clarke J.L., Jordan J.T., de Groot J., Nghiemphu L., Kaley T., Colman H., McCluskey C., et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021;27:1048–1057. doi: 10.1158/1078-0432.CCR-20-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reardon D.A., Brandes A.A., Omuro A., Mulholland P., Lim M., Wick A., Baehring J., Ahluwalia M.S., Roth P., Bähr O., et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brachman D.G., Pugh S.L., Ashby L.S., Thomas T.A., Dunbar E.M., Narayan S., Robins H.I., Bovi J.A., Rockhill J.K., Won M., et al. Phase 1/2 Trials of Temozolomide, Motexafin Gadolinium, and 60-Gy Fractionated Radiation for Newly Diagnosed Supratentorial Glioblastoma Multiforme: Final Results of RTOG 0513. Int. J. Radiat. Oncol. 2015;91:961–967. doi: 10.1016/j.ijrobp.2014.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubicek G.J., Werner-Wasik M., Machtay M., Mallon G., Myers T., Ramirez M., Andrews D., Curran W.J., Dicker A.P. A Phase I Trial Using the Proteasome Inhibitor Bortezomib and Concurrent Temozolomide and Radiotherapy for CNS Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2009;74:433–439. doi: 10.1016/j.ijrobp.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J., Yu L., Fu Y., Chen H., Zheng X., Wang S., Gao C., Cao Y., Lin L. A Refractory Case of CDK4-Amplified Spinal Astrocytoma Achieving Complete Response upon Treatment with a Palbociclib-Based Regimen: A Case Report. BMC Cancer. 2020;20:630. doi: 10.1186/s12885-020-07061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geletneky K., Hajda J., Angelova A.L., Leuchs B., Capper D., Bartsch A.J., Neumann J.O., Schöning T., Hüsing J., Beelte B., et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25:2620–2634. doi: 10.1016/j.ymthe.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto N., Tsuboi A., Kagawa N., Chiba Y., Izumoto S., Kinoshita M., Kijima N., Oka Y., Morimoto S., Nakajima H., et al. Wilms Tumor 1 Peptide Vaccination Combined with Temozolomide against Newly Diagnosed Glioblastoma: Safety and Impact on Immunological Response. Cancer Immunol. Immunother. CII. 2015;64:707–716. doi: 10.1007/s00262-015-1674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel S., DiBiase S., Meisenberg B., Flannery T., Patel A., Dhople A., Cheston S., Amin P. Phase I Clinical Trial Assessing Temozolomide and Tamoxifen with Concomitant Radiotherapy for Treatment of High-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:739–742. doi: 10.1016/j.ijrobp.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 63.Sautter L., Hofheinz R., Tuettenberg J., Grimm M., Vajkoczy P., Groden C., Schmieder K., Hochhaus A., Wenz F., Giordano F.A. Open-Label Phase II Evaluation of Imatinib in Primary Inoperable or Incompletely Resected and Recurrent Glioblastoma. Oncology. 2020;98:16–22. doi: 10.1159/000502483. [DOI] [PubMed] [Google Scholar]
- 64.Aldea M.D., Petrushev B., Soritau O., Tomuleasa C.I., Filip A.G., Chereches G., Cenariu M., Tatomir C., Florian I.-S., Crivii C.B., et al. Metformin plus Sorafenib Highly Impacts Temozolomide Resistant Glioblastoma Stem-like Cells. J. BUON. 2014;19:502–511. [PubMed] [Google Scholar]
- 65.Aoki K., Nakamura H., Suzuki H., Matsuo K., Kataoka K., Shimamura T., Motomura K., Ohka F., Shiina S., Yamamoto T., et al. Prognostic Relevance of Genetic Alterations in Diffuse Lower-Grade Gliomas. Neuro-Oncology. 2018;20:66–77. doi: 10.1093/neuonc/nox132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arcella A., Biagioni F., Oliva M.A., Bucci D., Frati A., Esposito V., Cantore G., Giangaspero F., Fornai F. Rapamycin Inhibits the Growth of Glioblastoma. Brain Res. 2013;1495:37–51. doi: 10.1016/j.brainres.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 67.Ariey-Bonnet J., Carrasco K., Le Grand M., Hoffer L., Betzi S., Feracci M., Tsvetkov P., Devred F., Collette Y., Morelli X., et al. In Silico Molecular Target Prediction Unveils Mebendazole as a Potent MAPK14 Inhibitor. Mol. Oncol. 2020;14:3083–3099. doi: 10.1002/1878-0261.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balkhi H.M., Gul T., Haq E. Anti-Neoplastic and Calcium Modulatory Action of Caffeic Acid Phenethyl Ester and Dasatinib in C6 Glial Cells: A Therapeutic Perspective. CNS Neurol. Disord.-Drug Targets. 2016;15:54–63. doi: 10.2174/1871527315666151110125435. [DOI] [PubMed] [Google Scholar]
- 69.Benezra M., Hambardzumyan D., Penate-Medina O., Veach D.R., Pillarsetty N., Smith-Jones P., Phillips E., Ozawa T., Zanzonico P.B., Longo V., et al. Fluorine-Labeled Dasatinib Nanoformulations as Targeted Molecular Imaging Probes in a PDGFB-Driven Murine Glioblastoma Model. Neoplasia. 2012;14:1132–1143. doi: 10.1593/neo.121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camorani S., Crescenzi E., Colecchia D., Carpentieri A., Amoresano A., Fedele M., Chiariello M., Cerchia L. Aptamer Targeting EGFRvIII Mutant Hampers Its Constitutive Autophosphorylation and Affects Migration, Invasion and Proliferation of Glioblastoma Cells. Oncotarget. 2015;6:37570–37587. doi: 10.18632/oncotarget.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen T., Chen J., Zhu Y., Li Y., Wang Y., Chen H., Wang J., Li X., Liu Y., Li B., et al. CD163, a Novel Therapeutic Target, Regulates the Proliferation and Stemness of Glioma Cells via Casein Kinase 2. Oncogene. 2019;38:1183–1199. doi: 10.1038/s41388-018-0515-6. [DOI] [PubMed] [Google Scholar]
- 72.Cheng X., Ren Z., Liu Z., Sun X., Qian R., Cao C., Liu B., Wang J., Wang H., Guo Y., et al. Cysteine Cathepsin C: A Novel Potential Biomarker for the Diagnosis and Prognosis of Glioma. Cancer Cell Int. 2022;22:53. doi: 10.1186/s12935-021-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ciesielski M.J., Bu Y., Munich S.A., Teegarden P., Smolinski M.P., Clements J.L., Lau J.Y.N., Hangauer D.G., Fenstermaker R.A. KX2-361: A Novel Orally Bioavailable Small Molecule Dual Src/Tubulin Inhibitor That Provides Long Term Survival in a Murine Model of Glioblastoma. J. Neurooncol. 2018;140:519–527. doi: 10.1007/s11060-018-2992-4. [DOI] [PubMed] [Google Scholar]
- 74.Cloninger C., Bernath A., Bashir T., Holmes B., Artinian N., Ruegg T., Anderson L., Masri J., Lichtenstein A., Gera J. Inhibition of SAPK2/P38 Enhances Sensitivity to MTORC1 Inhibition by Blocking IRES-Mediated Translation Initiation in Glioblastoma. Mol. Cancer Ther. 2011;10:2244–2256. doi: 10.1158/1535-7163.MCT-11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Combs S.E., Schulz-Ertner D., Roth W., Herold-Mende C., Debus J., Weber K.-J. In Vitro Responsiveness of Glioma Cell Lines to Multimodality Treatment with Radiotherapy, Temozolomide, and Epidermal Growth Factor Receptor Inhibition with Cetuximab. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:873–882. doi: 10.1016/j.ijrobp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Dasgupta T., Olow A.K., Yang X., Hashizume R., Nicolaides T.P., Tom M., Aoki Y., Berger M.S., Weiss W.A., Stalpers L.J.A., et al. Survival Advantage Combining a BRAF Inhibitor and Radiation in BRAF V600E-Mutant Glioma. J. Neurooncol. 2016;126:385–393. doi: 10.1007/s11060-015-1939-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dantas-Barbosa C., Bergthold G., Daudigeos-Dubus E., Blockus H., Boylan J.F., Ferreira C., Puget S., Abely M., Vassal G., Grill J., et al. Inhibition of the NOTCH Pathway Using γ-Secretase Inhibitor RO4929097 Has Limited Antitumor Activity in Established Glial Tumors. Anticancer Drugs. 2015;26:272–283. doi: 10.1097/CAD.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 78.Davare M.A., Henderson J.J., Agarwal A., Wagner J.P., Iyer S.R., Shah N., Woltjer R., Somwar R., Gilheeney S.W., DeCarvalo A., et al. Rare but Recurrent ROS1 Fusions Resulting From Chromosome 6q22 Microdeletions Are Targetable Oncogenes in Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:6471–6482. doi: 10.1158/1078-0432.CCR-18-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Stefano A.L., Fucci A., Frattini V., Labussiere M., Mokhtari K., Zoppoli P., Marie Y., Bruno A., Boisselier B., Giry M., et al. Detection, Characterization, and Inhibition of FGFR-TACC Fusions in IDH Wild-Type Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015;21:3307–3317. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dominguez C.L., Floyd D.H., Xiao A., Mullins G.R., Kefas B.A., Xin W., Yacur M.N., Abounader R., Lee J.K., Wilson G.M., et al. Diacylglycerol Kinase α Is a Critical Signaling Node and Novel Therapeutic Target in Glioblastoma and Other Cancers. Cancer Discov. 2013;3:782–797. doi: 10.1158/2159-8290.CD-12-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du W., Zhou J., Wang D., Gong K., Zhang Q. Vitamin K1 Enhances Sorafenib-Induced Growth Inhibition and Apoptosis of Human Malignant Glioma Cells by Blocking the Raf/MEK/ERK Pathway. World J. Surg. Oncol. 2012;10:60. doi: 10.1186/1477-7819-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Emlet D.R., Gupta P., Holgado-Madruga M., Del Vecchio C.A., Mitra S.S., Han S.Y., Li G., Jensen K.C., Vogel H., Xu L.W., et al. Targeting a Glioblastoma Cancer Stem-Cell Population Defined by EGF Receptor Variant III. Cancer Res. 2014;74:1238–1249. doi: 10.1158/0008-5472.CAN-13-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farrell P.J., Matuszkiewicz J., Balakrishna D., Pandya S., Hixon M.S., Kamran R., Chu S., Lawson J.D., Okada K., Hori A., et al. MET Tyrosine Kinase Inhibition Enhances the Antitumor Efficacy of an HGF Antibody. Mol. Cancer Ther. 2017;16:1269–1278. doi: 10.1158/1535-7163.MCT-16-0771. [DOI] [PubMed] [Google Scholar]
- 84.Feng Y., Huang J., Ding Y., Xie F., Shen X. Tamoxifen-Induced Apoptosis of Rat C6 Glioma Cells via PI3K/Akt, JNK and ERK Activation. Oncol. Rep. 2010;24:1561–1567. doi: 10.3892/or_00001018. [DOI] [PubMed] [Google Scholar]
- 85.Glassmann A., Reichmann K., Scheffler B., Glas M., Veit N., Probstmeier R. Pharmacological Targeting of the Constitutively Activated MEK/MAPK-Dependent Signaling Pathway in Glioma Cells Inhibits Cell Proliferation and Migration. Int. J. Oncol. 2011;39:1567–1575. doi: 10.3892/ijo.2011.1165. [DOI] [PubMed] [Google Scholar]
- 86.Goker Bagca B., Ozates N.P., Asik A., Caglar H.O., Gunduz C., Biray Avci C. Temozolomide Treatment Combined with AZD3463 Shows Synergistic Effect in Glioblastoma Cells. Biochem. Biophys. Res. Commun. 2020;533:1497–1504. doi: 10.1016/j.bbrc.2020.10.058. [DOI] [PubMed] [Google Scholar]
- 87.Golubovskaya V.M., Huang G., Ho B., Yemma M., Morrison C.D., Lee J., Eliceiri B.P., Cance W.G. Pharmacologic Blockade of FAK Autophosphorylation Decreases Human Glioblastoma Tumor Growth and Synergizes with Temozolomide. Mol. Cancer Ther. 2013;12:162–172. doi: 10.1158/1535-7163.MCT-12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grossen A., Gavula T., Chrusciel D., Evans A., McNall-Knapp R., Taylor A., Fossey B., Brakefield M., Carter C., Schwartz N., et al. Multidisciplinary Neurocutaneous Syndrome Clinics: A Systematic Review and Institutional Experience. Neurosurg. Focus. 2022;52:E2. doi: 10.3171/2022.2.FOCUS21776. [DOI] [PubMed] [Google Scholar]
- 89.Gürsel D.B., Connell-Albert Y.S., Tuskan R.G., Anastassiadis T., Walrath J.C., Hawes J.J., Amlin-Van Schaick J.C., Reilly K.M. Control of Proliferation in Astrocytoma Cells by the Receptor Tyrosine Kinase/PI3K/AKT Signaling Axis and the Use of PI-103 and TCN as Potential Anti-Astrocytoma Therapies. Neuro-Oncology. 2011;13:610–621. doi: 10.1093/neuonc/nor035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He H., Yao M., Zhang W., Tao B., Liu F., Li S., Dong Y., Zhang C., Meng Y., Li Y., et al. MEK2 Is a Prognostic Marker and Potential Chemo-Sensitizing Target for Glioma Patients Undergoing Temozolomide Treatment. Cell. Mol. Immunol. 2016;13:658–668. doi: 10.1038/cmi.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hjelmeland A.B., Lattimore K.P., Fee B.E., Shi Q., Wickman S., Keir S.T., Hjelmeland M.D., Batt D., Bigner D.D., Friedman H.S., et al. The Combination of Novel Low Molecular Weight Inhibitors of RAF (LBT613) and Target of Rapamycin (RAD001) Decreases Glioma Proliferation and Invasion. Mol. Cancer Ther. 2007;6:2449–2457. doi: 10.1158/1535-7163.MCT-07-0155. [DOI] [PubMed] [Google Scholar]
- 92.Hong H., Stastny M., Brown C., Chang W.C., Ostberg J.R., Forman S.J., Jensen M.C. Diverse Solid Tumors Expressing a Restricted Epitope of L1-CAM Can Be Targeted by Chimeric Antigen Receptor Redirected T Lymphocytes. J. Immunother. 2014;37:93–104. doi: 10.1097/CJI.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 93.Jiang H., Gao H., Kong J., Song B., Wang P., Shi B., Wang H., Li Z. Selective Targeting of Glioblastoma with EGFRvIII/EGFR Bitargeted Chimeric Antigen Receptor T Cell. Cancer Immunol. Res. 2018;6:1314–1326. doi: 10.1158/2326-6066.CIR-18-0044. [DOI] [PubMed] [Google Scholar]
- 94.Jin R., Nakada M., Teng L., Furuta T., Sabit H., Hayashi Y., Demuth T., Hirao A., Sato H., Zhao G., et al. Combination Therapy Using Notch and Akt Inhibitors Is Effective for Suppressing Invasion but Not Proliferation in Glioma Cells. Neurosci. Lett. 2013;534:316–321. doi: 10.1016/j.neulet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Joel M., Mughal A.A., Grieg Z., Murrell W., Palmero S., Mikkelsen B., Fjerdingstad H.B., Sandberg C.J., Behnan J., Glover J.C., et al. Targeting PBK/TOPK Decreases Growth and Survival of Glioma Initiating Cells in Vitro and Attenuates Tumor Growth in Vivo. Mol. Cancer. 2015;14:121. doi: 10.1186/s12943-015-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joshi A.D., Botham R.C., Schlein L.J., Roth H.S., Mangraviti A., Borodovsky A., Tyler B., Joslyn S., Looper J.S., Podell M., et al. Synergistic and Targeted Therapy with a Procaspase-3 Activator and Temozolomide Extends Survival in Glioma Rodent Models and Is Feasible for the Treatment of Canine Malignant Glioma Patients. Oncotarget. 2017;8:80124–80138. doi: 10.18632/oncotarget.19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ju R.-J., Zeng F., Liu L., Mu L.-M., Xie H.-J., Zhao Y., Yan Y., Wu J.-S., Hu Y.-J., Lu W.-L. Destruction of Vasculogenic Mimicry Channels by Targeting Epirubicin plus Celecoxib Liposomes in Treatment of Brain Glioma. Int. J. Nanomed. 2016;11:1131–1146. doi: 10.2147/IJN.S94467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Junca A., Villalva C., Tachon G., Rivet P., Cortes U., Guilloteau K., Balbous A., Godet J., Wager M., Karayan-Tapon L. Crizotinib Targets in Glioblastoma Stem Cells. Cancer Med. 2017;6:2625–2634. doi: 10.1002/cam4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung Y., Park H., Zhao H.Y., Jeon R., Ryu J.H., Kim W.Y. Systemic Approaches Identify a Garlic-Derived Chemical, Z-Ajoene, as a Glioblastoma Multiforme Cancer Stem Cell-Specific Targeting Agent. Mol. Cells. 2014;37:547–553. doi: 10.14348/molcells.2014.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawauchi D., Takahashi M., Satomi K., Yamamuro S., Kobayashi T., Uchida E., Honda-Kitahara M., Narita Y., Iwadate Y., Ichimura K., et al. The ALK Inhibitors, Alectinib and Ceritinib, Induce ALK-Independent and STAT3-Dependent Glioblastoma Cell Death. Cancer Sci. 2021;112:2442–2453. doi: 10.1111/cas.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim R.-K., Kim M.-J., Yoon C.-H., Lim E.-J., Yoo K.-C., Lee G.-H., Kim Y.-H., Kim H., Jin Y.B., Lee Y.-J., et al. A New 2-Pyrone Derivative, 5-Bromo-3-(3-Hydroxyprop-1-Ynyl)-2H-Pyran-2-One, Suppresses Stemness in Glioma Stem-Like Cells. Mol. Pharmacol. 2012;82:400–407. doi: 10.1124/mol.112.078402. [DOI] [PubMed] [Google Scholar]
- 102.Koul D., Shen R., Bergh S., Lu Y., de Groot J., Liu T., Mills G., Yung W. Targeting Integrin-Linked Kinase Inhibits Akt Signaling Pathways and Decreases Tumor Progression of Human Glioblastoma. Mol. Cancer Ther. 2005;4:1681–1688. doi: 10.1158/1535-7163.MCT-05-0258. [DOI] [PubMed] [Google Scholar]
- 103.Koul D., Shen R., Kim Y.-W., Kondo Y., Lu Y., Bankson J., Ronen S.M., Kirkpatrick D.L., Powis G., Yung W.K.A. Cellular and in Vivo Activity of a Novel PI3K Inhibitor, PX-866, against Human Glioblastoma. Neuro-Oncology. 2010;12:559–569. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J., Xu X., Feng X., Zhang B., Wang J. Adenovirus-Mediated Delivery of BFGF Small Interfering RNA Reduces STAT3 Phosphorylation and Induces the Depolarization of Mitochondria and Apoptosis in Glioma Cells U251. J. Exp. Clin. Cancer Res. 2011;30:80. doi: 10.1186/1756-9966-30-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu H., Zhou L., Shi S., Wang Y., Ni X., Xiao F., Wang S., Li P., Ding K. Oligosaccharide G19 Inhibits U-87 MG Human Glioma Cells Growth in Vitro and in Vivo by Targeting Epidermal Growth Factor (EGF) and Activating P53/P21 Signaling. Glycobiology. 2014;24:748–765. doi: 10.1093/glycob/cwu038. [DOI] [PubMed] [Google Scholar]
- 106.Liu X., Chhipa R.R., Nakano I., Dasgupta B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Anti-Glioma Agent. Mol. Cancer Ther. 2014;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luchman H.A., Stechishin O.D., Dang N.H., Blough M.D., Chesnelong C., Kelly J.J., Nguyen S.A., Chan J.A., Weljie A.M., Cairncross J.G., et al. An in Vivo Patient-Derived Model of Endogenous IDH1-Mutant Glioma. Neuro-Oncology. 2012;14:184–191. doi: 10.1093/neuonc/nor207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma J., Zhang Y., Li R., Ye J., Li H., Zhang Y., Ma Z., Li J., Zhong X., Yang X. Tetrandrine Suppresses Human Glioma Growth by Inhibiting Cell Survival, Proliferation and Tumour Angiogenesis through Attenuating STAT3 Phosphorylation. Eur. J. Pharmacol. 2015;764:228–239. doi: 10.1016/j.ejphar.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 109.Matsuda K., Sato A., Okada M., Shibuya K., Seino S., Suzuki K., Watanabe E., Narita Y., Shibui S., Kayama T., et al. Targeting JNK for Therapeutic Depletion of Stem-like Glioblastoma Cells. Sci. Rep. 2012;2:516. doi: 10.1038/srep00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maxwell M.J., Arnold A., Sweeney H., Chen L., Lih T.-S.M., Schnaubelt M., Eberhart C.G., Rubens J.A., Zhang H., Clark D.J., et al. Unbiased Proteomic and Phosphoproteomic Analysis Identifies Response Signatures and Novel Susceptibilities After Combined MEK and MTOR Inhibition in BRAFV600E Mutant Glioma. Mol. Cell. Proteom. 2021;20:100123. doi: 10.1016/j.mcpro.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolaides T.P., Li H., Solomon D.A., Hariono S., Hashizume R., Barkovich K., Baker S.J., Paugh B.S., Jones C., Forshew T., et al. Targeted Therapy for BRAFV600E Malignant Astrocytoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paternot S., Roger P.P. Combined Inhibition of MEK and Mammalian Target of Rapamycin Abolishes Phosphorylation of Cyclin-Dependent Kinase 4 in Glioblastoma Cell Lines and Prevents Their Proliferation. Cancer Res. 2009;69:4577–4581. doi: 10.1158/0008-5472.CAN-08-3260. [DOI] [PubMed] [Google Scholar]
- 113.Peng R., Jiang B., Ma J., Ma Z., Wan X., Liu H., Chen Z., Cheng Q., Chen R. Forced Downregulation of RACK1 Inhibits Glioma Development by Suppressing Src/Akt Signaling Activity. Oncol. Rep. 2013;30:2195–2202. doi: 10.3892/or.2013.2723. [DOI] [PubMed] [Google Scholar]
- 114.Pezuk J.A., Brassesco M.S., Morales A.G., de Oliveira J.C., de Paula Queiroz R.G., Machado H.R., Carlotti C.G., Neder L., Scrideli C.A., Tone L.G. Polo-like Kinase 1 Inhibition Causes Decreased Proliferation by Cell Cycle Arrest, Leading to Cell Death in Glioblastoma. Cancer Gene Ther. 2013;20:499–506. doi: 10.1038/cgt.2013.46. [DOI] [PubMed] [Google Scholar]
- 115.Phillips A.C., Boghaert E.R., Vaidya K.S., Mitten M.J., Norvell S., Falls H.D., DeVries P.J., Cheng D., Meulbroek J.A., Buchanan F.G., et al. ABT-414, an Antibody-Drug Conjugate Targeting a Tumor-Selective EGFR Epitope. Mol. Cancer Ther. 2016;15:661–669. doi: 10.1158/1535-7163.MCT-15-0901. [DOI] [PubMed] [Google Scholar]
- 116.Premkumar D.R., Jane E.P., Pollack I.F. Co-Administration of NVP-AEW541 and Dasatinib Induces Mitochondrial-Mediated Apoptosis through Bax Activation in Malignant Human Glioma Cell Lines. Int. J. Oncol. 2010;37:633–643. doi: 10.3892/ijo_00000712. [DOI] [PubMed] [Google Scholar]
- 117.Qin Y., Fu M., Takahashi M., Iwanami A., Kuga D., Rao R.G., Sudhakar D., Huang T., Kiyohara M., Torres K., et al. Epithelial Membrane Protein-2 (EMP2) Activates Src Protein and Is a Novel Therapeutic Target for Glioblastoma. J. Biol. Chem. 2014;289:13974–13985. doi: 10.1074/jbc.M113.543728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raub T.J., Wishart G.N., Kulanthaivel P., Staton B.A., Ajamie R.T., Sawada G.A., Gelbert L.M., Shannon H.E., Sanchez-Martinez C., De Dios A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. Biol. Fate Chem. 2015;43:1360–1371. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 119.Salphati L., Heffron T.P., Alicke B., Nishimura M., Barck K., Carano R.A., Cheong J., Edgar K.A., Greve J., Kharbanda S., et al. Targeting the PI3K Pathway in the Brain—Efficacy of a PI3K Inhibitor Optimized to Cross the Blood-Brain Barrier. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:6239–6248. doi: 10.1158/1078-0432.CCR-12-0720. [DOI] [PubMed] [Google Scholar]
- 120.Sathornsumetee S., Hjelmeland A.B., Keir S.T., McLendon R.E., Batt D., Ramsey T., Yusuff N., Rasheed B.K.A., Kieran M.W., Laforme A., et al. AAL881, a Novel Small Molecule Inhibitor of RAF and Vascular Endothelial Growth Factor Receptor Activities, Blocks the Growth of Malignant Glioma. Cancer Res. 2006;66:8722–8730. doi: 10.1158/0008-5472.CAN-06-0284. [DOI] [PubMed] [Google Scholar]
- 121.See W.L., Tan I.-L., Mukherjee J., Nicolaides T., Pieper R.O. Sensitivity of Glioblastomas to Clinically Available MEK Inhibitors Is Defined by Neurofibromin 1 Deficiency. Cancer Res. 2012;72:3350–3359. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selvasaravanan K.D., Wiederspohn N., Hadzalic A., Strobel H., Payer C., Schuster A., Karpel-Massler G., Siegelin M.D., Halatsch M.-E., Debatin K.-M., et al. The Limitations of Targeting MEK Signalling in Glioblastoma Therapy. Sci. Rep. 2020;10:7401. doi: 10.1038/s41598-020-64289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shingu T., Holmes L., Henry V., Wang Q., Latha K., Gururaj A.E., Gibson L.A., Doucette T., Lang F.F., Rao G., et al. Suppression of RAF/MEK or PI3K Synergizes Cytotoxicity of Receptor Tyrosine Kinase Inhibitors in Glioma Tumor-Initiating Cells. J. Transl. Med. 2016;14:46. doi: 10.1186/s12967-016-0803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siegelin M.D., Raskett C.M., Gilbert C.A., Ross A.H., Altieri D.C. Sorafenib Exerts Anti-Glioma Activity in Vitro and in Vivo. Neurosci. Lett. 2010;478:165–170. doi: 10.1016/j.neulet.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Signore M., Pelacchi F., di Martino S., Runci D., Biffoni M., Giannetti S., Morgante L., De Majo M., Petricoin E.F., Stancato L., et al. Combined PDK1 and CHK1 Inhibition Is Required to Kill Glioblastoma Stem-like Cells in Vitro and in Vivo. Cell Death Dis. 2014;5:e1223. doi: 10.1038/cddis.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spino M., Kurz S.C., Chiriboga L., Serrano J., Zeck B., Sen N., Patel S., Shen G., Vasudevaraja V., Tsirigos A., et al. Cell Surface Notch Ligand DLL3 Is a Therapeutic Target in Isocitrate Dehydrogenase-Mutant Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:1261–1271. doi: 10.1158/1078-0432.CCR-18-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thanasupawat T., Glogowska A., Burg M., Krcek J., Beiko J., Pitz M., Zhang G.J., Hombach-Klonisch S., Klonisch T. C1q/TNF-Related Peptide 8 (CTRP8) Promotes Temozolomide Resistance in Human Glioblastoma. Mol. Oncol. 2018;12:1464–1479. doi: 10.1002/1878-0261.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson E.M., Landi D., Ashley D., Keir S.T., Bigner D. Bevacizumab, Irinotecan, Temozolomide, Tyrosine Kinase Inhibition, and MEK Inhibition Are Effective against Pleomorphic Xanthoastrocytoma Regardless of V600E Status. J. Neurooncol. 2018;140:261–268. doi: 10.1007/s11060-018-2975-5. [DOI] [PubMed] [Google Scholar]
- 129.Tsigelny I.F., Mukthavaram R., Kouznetsova V.L., Chao Y., Babic I., Nurmemmedov E., Pastorino S., Jiang P., Calligaris D., Agar N., et al. Multiple Spatially Related Pharmacophores Define Small Molecule Inhibitors of OLIG2 in Glioblastoma. Oncotarget. 2017;8:22370–22384. doi: 10.18632/oncotarget.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van den Heuvel C.N.A.M., Navis A.C., de Bitter T., Amiri H., Verrijp K., Heerschap A., Rex K., Dussault I., Caenepeel S., Coxon A., et al. Selective MET Kinase Inhibition in MET-Dependent Glioma Models Alters Gene Expression and Induces Tumor Plasticity. Mol. Cancer Res. MCR. 2017;15:1587–1597. doi: 10.1158/1541-7786.MCR-17-0177. [DOI] [PubMed] [Google Scholar]
- 131.Wang J., Sai K., Chen F., Chen Z. MiR-181b Modulates Glioma Cell Sensitivity to Temozolomide by Targeting MEK1. Cancer Chemother. Pharmacol. 2013;72:147–158. doi: 10.1007/s00280-013-2180-3. [DOI] [PubMed] [Google Scholar]
- 132.Wang L., Shi Z.M., Jiang C.F., Liu X., Chen Q.D., Qian X., Li D.M., Ge X., Wang X.F., Liu L.Z., et al. MiR-143 Acts as a Tumor Suppressor by Targeting N-RAS and Enhances Temozolomide-Induced Apoptosis in Glioma. Oncotarget. 2014;5:5416–5427. doi: 10.18632/oncotarget.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X., Yang K., Wu Q., Kim L.J.Y., Morton A.R., Gimple R.C., Prager B.C., Shi Y., Zhou W., Bhargava S., et al. Targeting Pyrimidine Synthesis Accentuates Molecular Therapy Response in Glioblastoma Stem Cells. Sci. Transl. Med. 2019;11:eaau4972. doi: 10.1126/scitranslmed.aau4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wichmann H., Güttler A., Bache M., Taubert H., Rot S., Kessler J., Eckert A.W., Kappler M., Vordermark D. Targeting of EGFR and HER2 with Therapeutic Antibodies and SiRNA: A Comparative Study in Glioblastoma Cells. Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft Al. 2015;191:180–191. doi: 10.1007/s00066-014-0743-9. [DOI] [PubMed] [Google Scholar]
- 135.Yan D., Kowal J., Akkari L., Schuhmacher A.J., Huse J.T., West B.L., Joyce J.A. Inhibition of Colony Stimulating Factor-1 Receptor Abrogates Microenvironment-Mediated Therapeutic Resistance in Gliomas. Oncogene. 2017;36:6049–6058. doi: 10.1038/onc.2017.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang W., Barth R.F., Wu G., Kawabata S., Sferra T.J., Bandyopadhyaya A.K., Tjarks W., Ferketich A.K., Moeschberger M.L., Binns P.J., et al. Molecular Targeting and Treatment of EGFRvIII-Positive Gliomas Using Boronated Monoclonal Antibody L8A4. Clin. Cancer Res. 2006;12:3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 137.Yao T.W., Zhang J., Prados M., Weiss W.A., James C.D., Nicolaides T. EGFR Blockade Prevents Glioma Escape from BRAFV600E Targeted Therapy. Oncotarget. 2015;6:21993–22005. doi: 10.18632/oncotarget.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zavalhia L.S., Romitti M., Netto G.C., dos Santos G.T., Meurer R.T., Hilbig A., Michalowski M.B., Barbosa Coutinho L.M., de Castro Ribeiro M. Evaluation of the Expression of C-Kit (CD117) in Ependymomas and Oligodendrogliomas. Dis. Markers. 2012;33:61–68. doi: 10.1155/2012/167405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang C., Yuan X.R., Li H.Y., Zhao Z.J., Liao Y.W., Wang X.Y., Su J., Sang S.S., Liu Q. Anti-Cancer Effect of Metabotropic Glutamate Receptor 1 Inhibition in Human Glioma U87 Cells: Involvement of PI3K/Akt/MTOR Pathway. Cell. Physiol. Biochem. 2015;35:419–432. doi: 10.1159/000369707. [DOI] [PubMed] [Google Scholar]
- 140.Zhang C., Burger M.C., Jennewein L., Genßler S., Schönfeld K., Zeiner P., Hattingen E., Harter P.N., Mittelbronn M., Tonn T., et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016;108:djv375. doi: 10.1093/jnci/djv375. [DOI] [PubMed] [Google Scholar]
- 141.Bychkov M., Shulepko M., Osmakov D., Andreev Y., Sudarikova A., Vasileva V., Pavlyukov M.S., Latyshev Y.A., Potapov A.A., Kirpichnikov M., et al. Mambalgin-2 Induces Cell Cycle Arrest and Apoptosis in Glioma Cells via Interaction with ASIC1a. Cancers. 2020;12:1837. doi: 10.3390/cancers12071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen S., Zhao H., Deng J., Liao P., Xu Z., Cheng Y. Comparative Proteomics of Glioma Stem Cells and Differentiated Tumor Cells Identifies S100A9 as a Potential Therapeutic Target. J. Cell. Biochem. 2013;114:2795–2808. doi: 10.1002/jcb.24626. [DOI] [PubMed] [Google Scholar]
- 143.Chen C.H., Chen P.Y., Lin Y.Y., Feng L.Y., Chen S.H., Chen C.Y., Huang Y.C., Huang C.Y., Jung S.M., Chen L.Y., et al. Suppression of Tumor Growth via IGFBP3 Depletion as a Potential Treatment in Glioma. J. Neurosurg. 2019;132:168–179. doi: 10.3171/2018.8.JNS181217. [DOI] [PubMed] [Google Scholar]
- 144.Grinshtein N., Rioseco C.C., Marcellus R., Uehling D., Aman A., Lun X., Muto O., Podmore L., Lever J., Shen Y., et al. Small Molecule Epigenetic Screen Identifies Novel EZH2 and HDAC Inhibitors That Target Glioblastoma Brain Tumor-Initiating Cells. Oncotarget. 2016;7:59360–59376. doi: 10.18632/oncotarget.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Festa M., Del Valle L., Khalili K., Franco R., Scognamiglio G., Graziano V., De Laurenzi V., Turco M.C., Rosati A. BAG3 Protein Is Overexpressed in Human Glioblastoma and Is a Potential Target for Therapy. Am. J. Pathol. 2011;178:2504–2512. doi: 10.1016/j.ajpath.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ge Y.F., Sun J., Jin C.J., Cao B.Q., Jiang Z.F., Shao J.F. AntagomiR-27a Targets FOXO3a in Glioblastoma and Suppresses U87 Cell Growth in Vitro and in Vivo. Asian Pac. J. Cancer Prev. APJCP. 2013;14:963–968. doi: 10.7314/APJCP.2013.14.2.963. [DOI] [PubMed] [Google Scholar]
- 147.Genoud V., Espinoza F.I., Marinari E., Rochemont V., Dietrich P.-Y., McSheehy P., Bachmann F., Lane H.A., Walker P.R. Treating ICB-Resistant Glioma with Anti-CD40 and Mitotic Spindle Checkpoint Controller BAL101553 (Lisavanbulin) JCI Insight. 2021;6:e142980. doi: 10.1172/jci.insight.142980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gu X., Wang C., Wang X., Ma G., Li Y., Cui L., Chen Y., Zhao B., Li K. Efficient Inhibition of Human Glioma Development by RNA Interference-Mediated Silencing of PAK5. Int. J. Biol. Sci. 2015;11:230–237. doi: 10.7150/ijbs.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guo L., Fan L., Pang Z., Ren J., Ren Y., Li J., Chen J., Wen Z., Jiang X. TRAIL and Doxorubicin Combination Enhances Anti-Glioblastoma Effect Based on Passive Tumor Targeting of Liposomes. J. Control. Release Off. J. Control. Release Soc. 2011;154:93–102. doi: 10.1016/j.jconrel.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 150.Hamada T., Akahane T., Yokoyama S., Higa N., Kirishima M., Matsuo K., Shimokawa M., Yoshimoto K., Tanimoto A. An Oncogenic Splice Variant of PDGFRα in Adult Glioblastoma as a Therapeutic Target for Selective CDK4/6 Inhibitors. Sci. Rep. 2022;12:1275. doi: 10.1038/s41598-022-05391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kalluri H.S.G., Kuo J.S., Dempsey R.J. Chronic D609 Treatment Interferes with Cell Cycle and Targets the Expression of Olig2 in Glioma Stem like Cells. Eur. J. Pharmacol. 2017;814:81–86. doi: 10.1016/j.ejphar.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 152.Kaneta Y., Ullrich A. NEK9 Depletion Induces Catastrophic Mitosis by Impairment of Mitotic Checkpoint Control and Spindle Dynamics. Biochem. Biophys. Res. Commun. 2013;442:139–146. doi: 10.1016/j.bbrc.2013.04.105. [DOI] [PubMed] [Google Scholar]
- 153.Kong Y., Ai C., Dong F., Xia X., Zhao X., Yang C., Kang C., Zhou Y., Zhao Q., Sun X., et al. Targeting of BMI-1 with PTC-209 Inhibits Glioblastoma Development. Cell Cycle. 2018;17:1199–1211. doi: 10.1080/15384101.2018.1469872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lamour V., Henry A., Kroonen J., Nokin M.J., von Marschall Z., Fisher L.W., Chau T.L., Chariot A., Sanson M., Delattre J.Y., et al. Targeting Osteopontin Suppresses Glioblastoma Stem-like Cell Character and Tumorigenicity in Vivo. Int. J. Cancer. 2015;137:1047–1057. doi: 10.1002/ijc.29454. [DOI] [PubMed] [Google Scholar]
- 155.Lescarbeau R.S., Lei L., Bakken K.K., Sims P.A., Sarkaria J.N., Canoll P., White F.M. Quantitative Phosphoproteomics Reveals Wee1 Kinase as a Therapeutic Target in a Model of Proneural Glioblastoma. Mol. Cancer Ther. 2016;15:1332–1343. doi: 10.1158/1535-7163.MCT-15-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li C., Shen J., Wei X., Xie C., Lu W. Targeted Delivery of a Novel Palmitylated D-Peptide for Antiglioblastoma Molecular Therapy. J. Drug Target. 2012;20:264–271. doi: 10.3109/1061186X.2011.645162. [DOI] [PubMed] [Google Scholar]
- 157.Lian S., Shi R., Bai T., Liu Y., Miao W., Wang H., Liu X., Fan Y. Anti-MiRNA-23a Oligonucleotide Suppresses Glioma Cells Growth by Targeting Apoptotic Protease Activating Factor-1. Curr. Pharm. Des. 2013;19:6382–6389. doi: 10.2174/13816128113199990509. [DOI] [PubMed] [Google Scholar]
- 158.Liu X., Chen X., Shi L., Shan Q., Cao Q., Yue C., Li H., Li S., Wang J., Gao S., et al. The Third-Generation EGFR Inhibitor AZD9291 Overcomes Primary Resistance by Continuously Blocking ERK Signaling in Glioblastoma. J. Exp. Clin. Cancer Res. CR. 2019;38:219. doi: 10.1186/s13046-019-1235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mao P., Hever-Jardine M.P., Rahme G.J., Yang E., Tam J., Kodali A., Biswal B., Fadul C.E., Gaur A., Israel M.A., et al. Serine/Threonine Kinase 17A Is a Novel Candidate for Therapeutic Targeting in Glioblastoma. PLoS ONE. 2013;8:e81803. doi: 10.1371/journal.pone.0081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Merlino F., Daniele S., La Pietra V., Di Maro S., Di Leva F.S., Brancaccio D., Tomassi S., Giuntini S., Cerofolini L., Fragai M., et al. Simultaneous Targeting of RGD-Integrins and Dual Murine Double Minute Proteins in Glioblastoma Multiforme. J. Med. Chem. 2018;61:4791–4809. doi: 10.1021/acs.jmedchem.8b00004. [DOI] [PubMed] [Google Scholar]
- 161.Michaud K., Solomon D.A., Oermann E., Kim J.-S., Zhong W.-Z., Prados M.D., Ozawa T., James C.D., Waldman T. Pharmacologic Inhibition of Cyclin-Dependent Kinases 4 and 6 Arrests the Growth of Glioblastoma Multiforme Intracranial Xenografts. Cancer Res. 2010;70:3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Niu M., Cai W., Liu H., Chong Y., Hu W., Gao S., Shi Q., Zhou X., Liu X., Yu R. Plumbagin Inhibits Growth of Gliomas in Vivo via Suppression of FOXM1 Expression. J. Pharmacol. Sci. 2015;128:131–136. doi: 10.1016/j.jphs.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 163.Nonnenmacher L., Westhoff M.A., Fulda S., Karpel-Massler G., Halatsch M.E., Engelke J., Simmet T., Corbacioglu S., Debatin K.M. RIST: A Potent New Combination Therapy for Glioblastoma. Int. J. Cancer. 2015;136:E173–E187. doi: 10.1002/ijc.29138. [DOI] [PubMed] [Google Scholar]
- 164.Patyka M., Sharifi Z., Petrecca K., Mansure J., Jean-Claude B., Sabri S. Sensitivity to PRIMA-1(MET) Is Associated with Decreased MGMT in Human Glioblastoma Cells and Glioblastoma Stem Cells Irrespective of P53 Status. Oncotarget. 2016;7:60245–60269. doi: 10.18632/oncotarget.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Punganuru S.R., Artula V., Zhao W., Rajaei M., Deokar H., Zhang R., Buolamwini J.K., Srivenugopal K.S., Wang W. Targeted Brain Tumor Therapy by Inhibiting the MDM2 Oncogene: In Vitro and In Vivo Antitumor Activity and Mechanism of Action. Cells. 2020;9:1592. doi: 10.3390/cells9071592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sasame J., Ikegaya N., Kawazu M., Natsumeda M., Hayashi T., Isoda M., Satomi K., Tomiyama A., Oshima A., Honma H., et al. HSP90 Inhibition Overcomes Resistance to Molecular Targeted Therapy in BRAF(V600E)-Mutant High-Grade Glioma. Clin. Cancer Res. 2022;28:2425–2439. doi: 10.1158/1078-0432.CCR-21-3622. [DOI] [PubMed] [Google Scholar]
- 167.Tasaki T., Fujita M., Okuda T., Yoneshige A., Nakata S., Yamashita K., Yoshioka H., Izumoto S., Kato A. MET Expressed in Glioma Stem Cells Is a Potent Therapeutic Target for Glioblastoma Multiforme. Anticancer Res. 2016;36:3571–3577. [PubMed] [Google Scholar]
- 168.Tchoghandjian A., Soubéran A., Tabouret E., Colin C., Denicolaï E., Jiguet-Jiglaire C., El-Battari A., Villard C., Baeza-Kallee N., Figarella-Branger D. Inhibitor of Apoptosis Protein Expression in Glioblastomas and Their in Vitro and in Vivo Targeting by SMAC Mimetic GDC-0152. Cell Death Dis. 2016;7:e2325. doi: 10.1038/cddis.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vengoji R., Macha M.A., Nimmakayala R.K., Rachagani S., Siddiqui J.A., Mallya K., Gorantla S., Jain M., Ponnusamy M.P., Batra S.K., et al. Afatinib and Temozolomide Combination Inhibits Tumorigenesis by Targeting EGFRvIII-CMet Signaling in Glioblastoma Cells. J. Exp. Clin. Cancer Res. 2019;38:266. doi: 10.1186/s13046-019-1264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang F., Bai H.R., Wang J., Bai Y.Z., Dou C.W. Glioma Growth Inhibition in Vitro and in Vivo by Single Chain Variable Fragments of the Transferrin Receptor Conjugated to Survivin Small Interfering RNA. J. Int. Med. Res. 2011;39:1701–1712. doi: 10.1177/147323001103900512. [DOI] [PubMed] [Google Scholar]
- 171.Wang X., Hua Y., Xu G., Deng S., Yang D., Gao X. Targeting EZH2 for Glioma Therapy with a Novel Nanoparticle-SiRNA Complex. Int. J. Nanomed. 2019;14:2637–2653. doi: 10.2147/IJN.S189871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu L., Chen Y., Dutra-Clarke M., Mayakonda A., Hazawa M., Savinoff S.E., Doan N., Said J.W., Yong W.H., Watkins A., et al. BCL6 Promotes Glioma and Serves as a Therapeutic Target. Proc. Natl. Acad. Sci. USA. 2017;114:3981–3986. doi: 10.1073/pnas.1609758114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu J., Zhang Z., Qian M., Wang S., Qiu W., Chen Z., Sun Z., Xiong Y., Wang C., Sun X., et al. Cullin-7 (CUL7) Is Overexpressed in Glioma Cells and Promotes Tumorigenesis via NF-Kappa B Activation. J. Exp. Clin. Cancer Res. 2020;39:59. doi: 10.1186/s13046-020-01553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang L., Zhang Y., Liu X.Y., Qin Z.H., Yang J.M. Expression of Elongation Factor-2 Kinase Contributes to Anoikis Resistance and Invasion of Human Glioma Cells. Acta Pharmacol. Sin. 2011;32:361–367. doi: 10.1038/aps.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao Z., He H., Wang C., Tao B., Zhou H., Dong Y., Xiang J., Wang L., Luo C., Lu Y., et al. Downregulation of Id2 Increases Chemosensitivity of Glioma. Tumor Biol. 2015;36:4189–4196. doi: 10.1007/s13277-015-3055-5. [DOI] [PubMed] [Google Scholar]
- 176.Zhong S., Wu B., Dong X., Han Y., Jiang S., Zhang Y., Bai Y., Luo S.X., Chen Y., Zhang H., et al. Identification of Driver Genes and Key Pathways of Glioblastoma Shows JNJ-7706621 as a Novel Antiglioblastoma Drug. World Neurosurg. 2018;109:e329–e342. doi: 10.1016/j.wneu.2017.09.176. [DOI] [PubMed] [Google Scholar]
- 177.Abdul Rahim S.A., Dirkse A., Oudin A., Schuster A., Bohler J., Barthelemy V., Muller A., Vallar L., Janji B., Golebiewska A., et al. Regulation of Hypoxia-Induced Autophagy in Glioblastoma Involves ATG9A. Br. J. Cancer. 2017;117:813–825. doi: 10.1038/bjc.2017.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Angara K., Rashid M.H., Shankar A., Ara R., Iskander A., Borin T.F., Jain M., Achyut B.R., Arbab A.S. Vascular Mimicry in Glioblastoma Following Anti-Angiogenic and Anti-20-HETE Therapies. Histol. Histopathol. 2017;32:917–928. doi: 10.14670/HH-11-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Blanco V.M., Chu Z., Vallabhapurapu S.D., Sulaiman M.K., Kendler A., Rixe O., Warnick R.E., Franco R.S., Qi X. Phosphatidylserine-Selective Targeting and Anticancer Effects of SapC-DOPS Nanovesicles on Brain Tumors. Oncotarget. 2014;5:7105–7118. doi: 10.18632/oncotarget.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blank M., Weinschenk T., Priemer M., Schluesener H. Systematic Evolution of a DNA Aptamer Binding to Rat Brain Tumor Microvessels—Selective Targeting of Endothelial Regulatory Protein Pigpen. J. Biol. Chem. 2001;276:16464–16468. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- 181.Chen L., Miao W., Tang X., Zhang H., Wang S., Luo F., Yan J. Inhibitory Effect of Neuropilin-1 Monoclonal Antibody (NRP-1 MAb) on Glioma Tumor in Mice. J. Biomed. Nanotechnol. 2013;9:551–558. doi: 10.1166/jbn.2013.1623. [DOI] [PubMed] [Google Scholar]
- 182.Fleurence J., Cochonneau D., Fougeray S., Oliver L., Geraldo F., Terme M., Dorvillius M., Loussouarn D., Vallette F., Paris F., et al. Targeting and Killing Glioblastoma with Monoclonal Antibody to O-Acetyl GD2 Ganglioside. Oncotarget. 2016;7:41172–41185. doi: 10.18632/oncotarget.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Franco D.G., Moretti I.F., Marie S.K.N. Mitochondria Transcription Factor A: A Putative Target for the Effect of Melatonin on U87MG Malignant Glioma Cell Line. Molecules. 2018;23:1129. doi: 10.3390/molecules23051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grossman R., Tyler B., Rudek M.A., Kim E., Zadnik P., Khan U., Blakeley J.O., Pathak A.P., Brem H. Microdialysis Measurement of Intratumoral Temozolomide Concentration after Cediranib, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, in a U87 Glioma Model. Cancer Chemother. Pharmacol. 2013;72:93–100. doi: 10.1007/s00280-013-2172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He B., Jabouille A., Steri V., Johansson-Percival A., Michael I.P., Kotamraju V.R., Junckerstorff R., Nowak A.K., Hamzah J., Lee G., et al. Vascular Targeting of LIGHT Normalizes Blood Vessels in Primary Brain Cancer and Induces Intratumoural High Endothelial Venules. J. Pathol. 2018;245:209–221. doi: 10.1002/path.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang T., Xu T., Wang Y., Zhou Y., Yu D., Wang Z., He L., Chen Z., Zhang Y., Davidson D., et al. Cannabidiol Inhibits Human Glioma by Induction of Lethal Mitophagy through Activating TRPV4. Autophagy. 2021;17:3592–3606. doi: 10.1080/15548627.2021.1885203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huveldt D., Lewis-Tuffin L.J., Carlson B.L., Schroeder M.A., Rodriguez F., Giannini C., Galanis E., Sarkaria J.N., Anastasiadis P.Z. Targeting Src Family Kinases Inhibits Bevacizumab-Induced Glioma Cell Invasion. PLoS ONE. 2013;8:e56505. doi: 10.1371/journal.pone.0056505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jaszberenyi M., Schally A.V., Block N.L., Zarandi M., Cai R.Z., Vidaurre I., Szalontay L., Jayakumar A.R., Rick F.G. Suppression of the Proliferation of Human U-87 MG Glioblastoma Cells by New Antagonists of Growth Hormone-Releasing Hormone in Vivo and in Vitro. Target. Oncol. 2013;8:281–290. doi: 10.1007/s11523-013-0264-y. [DOI] [PubMed] [Google Scholar]
- 189.Ji X., Wang H., Zhu J., Tang Y., Zhou Y., Zhu L., Gao C., Li W., You W., Yu B., et al. Correlation of Nrf2 and HIF-1 Alpha in Glioblastoma and Their Relationships to Clinicopathologic Features and Survival. Neurol. Res. 2013;35:1044–1050. doi: 10.1179/1743132813Y.0000000251. [DOI] [PubMed] [Google Scholar]
- 190.Kuan C.T., Wakiya K., Herndon J.E., Lipp E.S., Pegram C.N., Riggins G.J., Rasheed A., Szafranski S.E., McLendon R.E., Wikstrand C.J., et al. MRP3: A Molecular Target for Human Glioblastoma Multiforme Immunotherapy. BMC Cancer. 2010;10:468. doi: 10.1186/1471-2407-10-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lu L., Saha D., Martuza R.L., Rabkin S.D., Wakimoto H. Single Agent Efficacy of the VEGFR Kinase Inhibitor Axitinib in Preclinical Models of Glioblastoma. J. Neurooncol. 2015;121:91–100. doi: 10.1007/s11060-014-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mojarad-Jabali S., Farshbaf M., Hemmati S., Sarfraz M., Motasadizadeh H., Mojarrad J.S., Atyabi F., Zakeri-Milani P., Valizadeh H. Comparison of Three Synthetic Transferrin Mimetic Small Peptides to Promote the Blood-Brain Barrier Penetration of Vincristine Liposomes for Improved Glioma Targeted Therapy. Int. J. Pharm. 2022;613:121395. doi: 10.1016/j.ijpharm.2021.121395. [DOI] [PubMed] [Google Scholar]
- 193.Mostafavi H., Khaksarian M., Joghataei M.T., Yoosefee S., Soleimannejad M., Gholamzadeh R., Bahnamiri S.S., Hadjighassem M.R. CAMP-Epac Pathway Stimulation Modulate Connexin-43 and MicroRNA-21 Expression in Glioma Cells. Basic Clin. Neurosci. 2015;6:52–57. [PMC free article] [PubMed] [Google Scholar]
- 194.Nandhu M.S., Behera P., Bhaskaran V., Longo S.L., Barrera-Arenas L.M., Sengupta S., Rodriguez-Gil D.J., Chiocca E.A., Viapiano M.S. Development of a Function-Blocking Antibody Against Fibulin-3 as a Targeted Reagent for Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:821–833. doi: 10.1158/1078-0432.CCR-17-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nawashiro H., Otani N., Shinomiya N., Fukui S., Ooigawa H., Shima K., Matsuo H., Kanai Y., Endou H. L-Type Amino Acid Transporter 1 as a Potential Molecular Target in Human Astrocytic Tumors. Int. J. Cancer. 2006;119:484–492. doi: 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- 196.Pall A.E., Juratli L., Guntur D., Bandyopadhyay K., Kondapalli K.C. A Gain of Function Paradox: Targeted Therapy for Glioblastoma Associated with Abnormal NHE9 Expression. J. Cell. Mol. Med. 2019;23:7859–7872. doi: 10.1111/jcmm.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Phillips R.E., Yang Y., Smith R.C., Thompson B.M., Yamasaki T., Soto-Feliciano Y.M., Funato K., Liang Y., Garcia-Bermudez J., Wang X., et al. Target Identification Reveals Lanosterol Synthase as a Vulnerability in Glioma. Proc. Natl. Acad. Sci. USA. 2019;116:7957–7962. doi: 10.1073/pnas.1820989116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Renfrow J.J., Soike M.H., West J.L., Ramkissoon S.H., Metheny-Barlow L., Mott R.T., Kittel C.A., D’Agostino R.B.J.r., Tatter S.B., Laxton A.W., et al. Attenuating Hypoxia Driven Malignant Behavior in Glioblastoma with a Novel Hypoxia-Inducible Factor 2 Alpha Inhibitor. Sci. Rep. 2020;10:15195. doi: 10.1038/s41598-020-72290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Saw P.E., Xu X., Kang B.R., Lee J., Lee Y.S., Kim C., Kim H., Kang S.-H., Na Y.J., Moon H.J., et al. Extra-Domain B of Fibronectin as an Alternative Target for Drug Delivery and a Cancer Diagnostic and Prognostic Biomarker for Malignant Glioma. Theranostics. 2021;11:941–957. doi: 10.7150/thno.44948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takano S., Tsuboi K., Matsumura A., Nose T. Anti-Vascular Endothelial Growth Factor Antibody and Nimustine as Combined Therapy: Effects on Tumour Growth and Angiogenesis in Human Glioblastoma Xenografts. Neuro-Oncology. 2003;5:1–7. doi: 10.1215/S1522851702000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tyrinova T., Leplina O., Mishinov S., Tikhonova M., Kalinovskiy A., Chernov S., Dolgova E., Stupak V., Voronina E., Bogachev S., et al. Defective Dendritic Cell Cytotoxic Activity of High-Grade Glioma Patients’ Results from the Low Expression of Membrane TNF and Can Be Corrected In Vitro by Treatment with Recombinant IL-2 or Exogenic Double-Stranded DNA. J. Interferon Cytokine Res. 2018;38:298–310. doi: 10.1089/jir.2017.0084. [DOI] [PubMed] [Google Scholar]
- 202.Watanabe S., Nishijima N., Hirai K., Shibata K., Hase A., Yamanaka T., Inazu M. Anticancer Activity of Amb4269951, a Choline Transporter-Like Protein 1 Inhibitor, in Human Glioma Cells. Pharmaceuticals. 2020;13:104. doi: 10.3390/ph13050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xia L., Gong M., Zou Y., Wang Z., Wu B., Zhang S., Li L., Jin K., Sun C. Apatinib Induces Ferroptosis of Glioma Cells through Modulation of the VEGFR2/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2022;2022:9925919. doi: 10.1155/2022/9925919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xiong D.D., Xu W.Q., He R.Q., Dang Y.W., Chen G., Luo D.Z. In Silico Analysis Identified MiRNA-based Therapeutic Agents against Glioblastoma Multiforme. Oncol. Rep. 2019;41:2194–2208. doi: 10.3892/or.2019.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu T.J., Qiu P., Zhang Y.B., Yu S.Y., Xu G.M., Yang W. MiR-148a Inhibits the Proliferation and Migration of Glioblastoma by Targeting ITGA9. Hum. Cell. 2019;32:548–556. doi: 10.1007/s13577-019-00279-9. [DOI] [PubMed] [Google Scholar]
- 206.Baehr O., Gross S., Harter P.N., Kirches E., Mawrin C., Steinbach J.P., Mittelbronn M. ASA404, a Vascular Disrupting Agent, as an Experimental Treatment Approach for Brain Tumors. Oncol. Lett. 2017;14:5443–5451. doi: 10.3892/ol.2017.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Goswami S., Walle T., Cornish A.E., Basu S., Anandhan S., Fernandez I., Vence L., Blando J., Zhao H., Yadav S.S., et al. Immune Profiling of Human Tumors Identifies CD73 as a Combinatorial Target in Glioblastoma. Nat. Med. 2020;26:39–46. doi: 10.1038/s41591-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Merrill M., Bernhardt G., Sampson J., Wikstr C.J., Bigner D., Gromeier M. Poliovirus Receptor CD155-Targeted Oncolysis of Glioma. Neuro-Oncology. 2004;6:208–217. doi: 10.1215/S1152851703000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schleicher S.M., Thotala D.K., Linkous A.G., Hu R., Leahy K.M., Yazlovitskaya E.M., Hallahan D.E. Autotaxin and LPA Receptors Represent Potential Molecular Targets for the Radiosensitization of Murine Glioma through Effects on Tumor Vasculature. PLoS ONE. 2011;6:e22182. doi: 10.1371/journal.pone.0022182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu B., Jiang C., Han H., Liu H., Tang M., Liu L., Ji W., Lu X., Yang X., Zhang Y., et al. Icaritin Inhibits the Invasion and Epithelial-to-Mesenchymal Transition of Glioblastoma Cells by Targeting EMMPRIN via PTEN/AKt/HIF-1α Signalling. Clin. Exp. Pharmacol. Physiol. 2015;42:1296–1307. doi: 10.1111/1440-1681.12488. [DOI] [PubMed] [Google Scholar]
- 211.Zanotto-Filho A., Braganhol E., Schröder R., de Souza L.H., Dalmolin R.J., Pasquali M.A., Gelain D.P., Battastini A.M., Moreira J.C. NFκB Inhibitors Induce Cell Death in Glioblastomas. Biochem. Pharmacol. 2011;81:412–424. doi: 10.1016/j.bcp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 212.Zhang F.-J., Yang J.-Y., Mou Y.-H., Sun B.-S., Ping Y.-F., Wang J.-M., Bian X.-W., Wu C.-F. Inhibition of U-87 Human Glioblastoma Cell Proliferation and Formyl Peptide Receptor Function by Oligomer Procyanidins (F2) Isolated from Grape Seeds. Chem. Biol. Interact. 2009;179:419–429. doi: 10.1016/j.cbi.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 213.Barone A., Sengupta R., Warrington N.M., Smith E., Wen P.Y., Brekken R.A., Romagnoli B., Douglas G., Chevalier E., Bauer M.P., et al. Combined VEGF and CXCR4 Antagonism Targets the GBM Stem Cell Population and Synergistically Improves Survival in an Intracranial Mouse Model of Glioblastoma. Oncotarget. 2014;5:9811–9822. doi: 10.18632/oncotarget.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Caruana B.T., Skoric A., Brown A.J., Lutze-Mann L.H. Site-1 Protease, a Novel Metabolic Target for Glioblastoma. Biochem. Biophys. Res. Commun. 2017;490:760–766. doi: 10.1016/j.bbrc.2017.06.114. [DOI] [PubMed] [Google Scholar]
- 215.Chen Z., Pan X., Georgakilas A.G., Chen P., Hu H., Yang Y., Tian S., Xia L., Zhang J., Cai X., et al. Tetramethylpyrazine (TMP) Protects Cerebral Neurocytes and Inhibits Glioma by down Regulating Chemokine Receptor CXCR4 Expression. Cancer Lett. 2013;336:281–289. doi: 10.1016/j.canlet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 216.Chen W., Wu M., Cui S.-T., Zheng Y., Liu Z., Luo L.-S. CircRNA Circ-ITCH Inhibits the Proliferation and Invasion of Glioma Cells Through Targeting the MiR-106a-5p/SASH1 Axis. Cell Transplant. 2021;30:0963689720983785. doi: 10.1177/0963689720983785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Colen C.B., Shen Y., Ghoddoussi F., Yu P., Francis T.B., Koch B.J., Monterey M.D., Galloway M.P., Sloan A.E., Mathupala S.P. Metabolic Targeting of Lactate Efflux by Malignant Glioma Inhibits Invasiveness and Induces Necrosis: An in Vivo Study. Neoplasia. 2011;13:620–632. doi: 10.1593/neo.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Harford-Wright E., Andre-Gregoire G., Jacobs K.A., Treps L., Le Gonidec S., Leclair H.M., Gonzalez-Diest S., Roux Q., Guillonneau F., Loussouarn D., et al. Pharmacological Targeting of Apelin Impairs Glioblastoma Growth. Brain J. Neurol. 2017;140:2939–2954. doi: 10.1093/brain/awx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ishiwata T., Teduka K., Yamamoto T., Kawahara K., Matsuda Y., Naito Z. Neuroepithelial Stem Cell Marker Nestin Regulates the Migration, Invasion and Growth of Human Gliomas. Oncol. Rep. 2011;26:91–99. doi: 10.3892/or.2011.1267. [DOI] [PubMed] [Google Scholar]
- 220.Jiang Z., Shi Y., Tan G., Wang Z. Computational Screening of Potential Glioma-Related Genes and Drugs Based on Analysis of GEO Dataset and Text Mining. PLoS ONE. 2021;16:e0247612. doi: 10.1371/journal.pone.0247612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim S.S., Harford J.B., Moghe M., Rait A., Pirollo K.F., Chang E.H. Targeted Nanocomplex Carrying SiRNA against MALAT1 Sensitizes Glioblastoma to Temozolomide. Nucleic Acids Res. 2018;46:1424–1440. doi: 10.1093/nar/gkx1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim G.-H., Choi S.Y., Oh T.-I., Kan S.-Y., Kang H., Lee S., Oh T., Ko H.M., Lim J.-H. IDH1(R132H) Causes Resistance to HDAC Inhibitors by Increasing NANOG in Glioblastoma Cells. Int. J. Mol. Sci. 2019;20:2679. doi: 10.3390/ijms20112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li L., Wu M., Wang C., Yu Z., Wang H., Qi H., Xu X. Beta-Asarone Inhibits Invasion and EMT in Human Glioma U251 Cells by Suppressing Splicing Factor HnRNP A2/B1. Molecules. 2018;23:671. doi: 10.3390/molecules23030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu X., Chong Y., Tu Y., Liu N., Yue C., Qi Z., Liu H., Yao Y., Liu H., Gao S., et al. CRM1/XPO1 Is Associated with Clinical Outcome in Glioma and Represents a Therapeutic Target by Perturbing Multiple Core Pathways. J. Hematol. Oncol. 2016;9:108. doi: 10.1186/s13045-016-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Loskutov Y.V., Griffin C.L., Marinak K.M., Bobko A., Margaryan N.V., Geldenhuys W.J., Sarkaria J.N., Pugacheva E.N. LPA Signaling Is Regulated through the Primary Cilium: A Novel Target in Glioblastoma. Oncogene. 2018;37:1457–1471. doi: 10.1038/s41388-017-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Luwor R., Morokoff A.P., Amiridis S., D’Abaco G., Paradiso L., Stylli S.S., Nguyen H.P.T., Tarleton M., Young K.A., O’Brien T.J., et al. Targeting Glioma Stem Cells by Functional Inhibition of Dynamin 2: A Novel Treatment Strategy for Glioblastoma. Cancer Investig. 2019;37:144–155. doi: 10.1080/07357907.2019.1582060. [DOI] [PubMed] [Google Scholar]
- 227.Miyazaki T., Pan Y., Joshi K., Purohit D., Hu B., Demir H., Mazumder S., Okabe S., Yamori T., Viapiano M., et al. Telomestatin Impairs Glioma Stem Cell Survival and Growth through the Disruption of Telomeric G-Quadruplex and Inhibition of the Proto-Oncogene, c-Myb. Clin. Cancer Res. 2012;18:1268–1280. doi: 10.1158/1078-0432.CCR-11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Peng G., Yang C., Liu Y., Shen C. MiR-25-3p Promotes Glioma Cell Proliferation and Migration by Targeting FBXW7 and DKK3. Exp. Ther. Med. 2019;18:769–778. doi: 10.3892/etm.2019.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Piunti A., Hashizume R., Morgan M.A., Bartom E.T., Horbinski C.M., Marshall S.A., Rendleman E.J., Ma Q., Takahashi Y.H., Woodfin A.R., et al. Therapeutic Targeting of Polycomb and BET Bromodomain Proteins in Diffuse Intrinsic Pontine Gliomas. Nat. Med. 2017;23:493–500. doi: 10.1038/nm.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Preukschas M., Hagel C., Schulte A., Weber K., Lamszus K., Sievert H., Pällmann N., Bokemeyer C., Hauber J., Braig M., et al. Expression of Eukaryotic Initiation Factor 5A and Hypusine Forming Enzymes in Glioblastoma Patient Samples: Implications for New Targeted Therapies. PLoS ONE. 2012;7:e43468. doi: 10.1371/journal.pone.0043468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Saito R., Bringas J.R., Panner A., Tamas M., Pieper R.O., Berger M.S., Bankiewicz K.S. Convection-Enhanced Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand with Systemic Administration of Temozolomide Prolongs Survival in an Intracranial Glioblastoma Xenograft Model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 232.Saito K., Iizuka Y., Ohta S., Takahashi S., Nakamura K., Saya H., Yoshida K., Kawakami Y., Toda M. Functional Analysis of a Novel Glioma Antigen, EFTUD1. Neuro-Oncology. 2014;16:1618–1629. doi: 10.1093/neuonc/nou132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sanzey M., Abdul Rahim S.A., Oudin A., Dirkse A., Kaoma T., Vallar L., Herold-Mende C., Bjerkvig R., Golebiewska A., Niclou S.P. Comprehensive Analysis of Glycolytic Enzymes as Therapeutic Targets in the Treatment of Glioblastoma. PLoS ONE. 2015;10:e0123544. doi: 10.1371/journal.pone.0123544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saunders J.T., Holmes B., Benavides-Serrato A., Kumar S., Nishimura R.N., Gera J. Targeting the YAP-TEAD Interaction Interface for Therapeutic Intervention in Glioblastoma. J. Neurooncol. 2021;152:217–231. doi: 10.1007/s11060-021-03699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shulepko M.A., Bychkov M.L., Lyukmanova E.N., Kirpichnikov M.P. Recombinant Analogue of the Human Protein SLURP-1 Inhibits the Growth of U251 MG and A172 Glioma Cells. Dokl. Biochem. Biophys. 2020;493:211–214. doi: 10.1134/S1607672920040134. [DOI] [PubMed] [Google Scholar]
- 236.Song Y., Shao L., Xue Y., Ruan X., Liu X., Yang C., Zheng J., Shen S., Chen J., Li Z., et al. Inhibition of the Aberrant A1CF-FAM224A-MiR-590-3p-ZNF143 Positive Feedback Loop Attenuated Malignant Biological Behaviors of Glioma Cells. J. Exp. Clin. Cancer Res. 2019;38:248. doi: 10.1186/s13046-019-1200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tu Y., Niu M., Xie P., Yue C., Liu N., Qi Z., Gao S., Liu H., Shi Q., Yu R., et al. Smoothened Is a Poor Prognosis Factor and a Potential Therapeutic Target in Glioma. Sci. Rep. 2017;7:42630. doi: 10.1038/srep42630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Venere M., Horbinski C., Crish J.F., Jin X., Vasanji A., Major J., Burrows A.C., Chang C., Prokop J., Wu Q., et al. The Mitotic Kinesin KIF11 Is a Driver of Invasion, Proliferation, and Self-Renewal in Glioblastoma. Sci. Transl. Med. 2015;7:304ra143. doi: 10.1126/scitranslmed.aac6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.von Spreckelsen N., Fadzen C.M., Hartrampf N., Ghotmi Y., Wolfe J.M., Dubey S., Yang B.Y., Kijewski M.F., Wang S., Farquhar C., et al. Targeting Glioblastoma Using a Novel Peptide Specific to a Deglycosylated Isoform of Brevican. Adv. Ther. 2021;4:2000244. doi: 10.1002/adtp.202000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu N., Wu G.C., Hu R., Li M., Feng H. Ginsenoside Rh2 Inhibits Glioma Cell Proliferation by Targeting MicroRNA-128. Acta Pharmacol. Sin. 2011;32:345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yan Y., Xu Y., Gao Y.-Y., Zong Z.-H., Zhang Q., Li C., Wang H.-Q. Implication of 14-3-3 Epsilon and 14-3-3 Theta/Tau in Proteasome Inhibition-Induced Apoptosis of Glioma Cells. Cancer Sci. 2013;104:55–61. doi: 10.1111/cas.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang N., Zheng B., Yao X., Huang X., Du J., Shen Y., Huang Z., Chen J., Lin Q., Lan W., et al. Identification and Characterization of a Novel Mutant Isocitrate Dehydrogenase 1 Inhibitor for Glioma Treatment. Biochem. Biophys. Res. Commun. 2021;551:38–45. doi: 10.1016/j.bbrc.2021.02.112. [DOI] [PubMed] [Google Scholar]
- 243.Zhang S., Zhao S., Qi Y., Li B., Wang H., Pan Z., Xue H., Jin C., Qiu W., Chen Z., et al. SPI1-Induced Downregulation of FTO Promotes GBM Progression by Regulating Pri-MiR-10a Processing in an M6A-Dependent Manner. Mol. Ther.-Nucleic Acids. 2022;27:699–717. doi: 10.1016/j.omtn.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen X., Xiang Z., Li D., Zhu X., Peng X. ACTL6A Knockdown Inhibits Cell Migration by Suppressing the AKT Signaling Pathway and Enhances the Sensitivity of Glioma Cells to Temozolomide. Exp. Ther. Med. 2021;21:175. doi: 10.3892/etm.2020.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Edwards L.A., Verreault M., Thiessen B., Dragowska W.H., Hu Y., Yeung J.H.F., Dedhar S., Bally M.B. Combined Inhibition of the Phosphatidylinositol 3-Kinase/Akt and Ras/Mitogen-Activated Protein Kinase Pathways Results in Synergistic Effects in Glioblastoma Cells. Mol. Cancer Ther. 2006;5:645–654. doi: 10.1158/1535-7163.MCT-05-0099. [DOI] [PubMed] [Google Scholar]
- 246.Gabler L., Lötsch D., Kirchhofer D., van Schoonhoven S., Schmidt H.M., Mayr L., Pirker C., Neumayer K., Dinhof C., Kastler L., et al. TERT Expression Is Susceptible to BRAF and ETS-Factor Inhibition in BRAFV600E/TERT Promoter Double-Mutated Glioma. Acta Neuropathol. Commun. 2019;7:128. doi: 10.1186/s40478-019-0775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gu F., Zhang H., Qin F., Liu X., Li W., Fu L., Ying G., Li B., Zhang M., Ma Y. Intersectin1-s, A Multidomain Adapter Protein, Is Essential for Malignant Glioma Proliferation. Glia. 2015;63:1595–1605. doi: 10.1002/glia.22830. [DOI] [PubMed] [Google Scholar]
- 248.Hou X., Liu Y., Liu H., Chen X., Liu M., Che H., Guo F., Wang C., Zhang D., Wu J., et al. PERK Silence Inhibits Glioma Cell Growth under Low Glucose Stress by Blockage of P-AKT and Subsequent HK2’s Mitochondria Translocation. Sci. Rep. 2015;5:9065. doi: 10.1038/srep09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Iqbal A., Eckerdt F., Bell J., Nakano I., Giles F.J., Cheng S.Y., Lulla R.R., Goldman S., Platanias L.C. Targeting of Glioblastoma Cell Lines and Glioma Stem Cells by Combined PIM Kinase and PI3K-P110α Inhibition. Oncotarget. 2016;7:33192–33201. doi: 10.18632/oncotarget.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Keating A.K., Kim G.K., Jones A.E., Donson A.M., Ware K., Mulcahy J.M., Salzberg D.B., Foreman N.K., Liang X., Thorburn A., et al. Inhibition of Mer and Axl Receptor Tyrosine Kinases in Astrocytoma Cells Leads to Increased Apoptosis and Improved Chemosensitivity. Mol. Cancer Ther. 2010;9:1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim S.-H., Ezhilarasan R., Phillips E., Gallego-Perez D., Sparks A., Taylor D., Ladner K., Furuta T., Sabit H., Chhipa R., et al. Serine/Threonine Kinase MLK4 Determines Mesenchymal Identity in Glioma Stem Cells in an NF-Kappa B-Dependent Manner. Cancer Cell. 2016;29:201–213. doi: 10.1016/j.ccell.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lerner T.N., Shilyansky C., Davidson T.J., Evans K.E., Beier K.T., Zalocusky K.A., Crow A.K., Malenka R.C., Luo L., Tomer R., et al. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu X., Zhang L., Wu J., Zhou L., Ren Y.-J., Yang W.-Q., Ming Z.-J., Chen B., Wang J., Zhang Y., et al. Inhibition of Elongation Factor-2 Kinase Augments the Antitumor Activity of Temozolomide against Glioma. PLoS ONE. 2013;8:e81345. doi: 10.1371/journal.pone.0081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu K., Zhang Q., Lan H., Wang L., Mou P., Shao W., Liu D., Yang W., Lin Z., Lin Q., et al. GCN5 Potentiates Glioma Proliferation and Invasion via STAT3 and AKT Signaling Pathways. Int. J. Mol. Sci. 2015;16:21897–21910. doi: 10.3390/ijms160921897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Martínez-Sáez E., Peg V., Ortega-Aznar A., Martínez-Ricarte F., Camacho J., Hernández-Losa J., Ferreres Piñas J.C., Ramón YCajal S. PeIF4E as an Independent Prognostic Factor and a Potential Therapeutic Target in Diffuse Infiltrating Astrocytomas. Cancer Med. 2016;5:2501–2512. doi: 10.1002/cam4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shoshan Y., Nishiyama A., Chang A., Mörk S., Barnett G.H., Cowell J.K., Trapp B.D., Staugaitis S.M. Expression of Oligodendrocyte Progenitor Cell Antigens by Gliomas: Implications for the Histogenesis of Brain Tumors. Proc. Natl. Acad. Sci. USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sulzmaier F.J., Young-Robbins S., Jiang P., Geerts D., Prechtl A.M., Matter M.L., Kesari S., Ramos J.W. RSK2 Activity Mediates Glioblastoma Invasiveness and Is a Potential Target for New Therapeutics. Oncotarget. 2016;7:79869–79884. doi: 10.18632/oncotarget.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sun W., Zhang W., Yu J., Lu Z., Yu J. Inhibition of Nrf2 Might Enhance the Anti-Tumor Effect of Temozolomide in Glioma Cells via Inhibition of Ras/Raf/MEK Signaling Pathway. Int. J. Neurosci. 2021;131:975–983. doi: 10.1080/00207454.2020.1766458. [DOI] [PubMed] [Google Scholar]
- 259.Tsuruta T., Aihara Y., Kanno H., Funase M., Murayama T., Osawa M., Fujii H., Kubo O., Okada Y. Shared Molecular Targets in Pediatric Gliomas and Ependymomas. Pediatr. Blood Cancer. 2011;57:1117–1123. doi: 10.1002/pbc.23009. [DOI] [PubMed] [Google Scholar]
- 260.Yamanaka R., Arao T., Yajima N., Tsuchiya N., Homma J., Tanaka R., Sano M., Oide A., Sekijima M., Nishio K. Identification of Expressed Genes Characterizing Long-Term Survival in Malignant Glioma Patients. Oncogene. 2006;25:5994–6002. doi: 10.1038/sj.onc.1209585. [DOI] [PubMed] [Google Scholar]
- 261.Zhang H., Geng D., Gao J., Qi Y., Shi Y., Wang Y., Jiang Y., Zhang Y., Fu J., Dong Y., et al. Expression and Significance of Hippo/YAP Signaling in Glioma Progression. Tumor Biol. 2016;37:15665–15676. doi: 10.1007/s13277-016-5318-1. [DOI] [PubMed] [Google Scholar]
- 262.Zhang J., Yang Y., Dong Y., Liu C. Microrchidia Family CW-Type Zinc Finger 2 Promotes the Proliferation, Invasion, Migration and Epithelial-Mesenchymal Transition of Glioma by Regulating PTEN/PI3K/AKT Signaling via Binding to N-Myc Downstream Regulated Gene 1 Promoter. Int. J. Mol. Med. 2022;49:16. doi: 10.3892/ijmm.2021.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhao H.-F., Wang J., Jiang H.-R., Chen Z.-P., To S.-S.T. PI3K P110β Isoform Synergizes with JNK in the Regulation of Glioblastoma Cell Proliferation and Migration through Akt and FAK Inhibition. J. Exp. Clin. Cancer Res. 2016;35:78. doi: 10.1186/s13046-016-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhou Y., Bian X., Le Y., Gong W., Hu J., Zhang X., Wang L., Iribarren P., Salcedo R., Howard O., et al. Formylpeptide Receptor FPR and the Rapid Growth of Malignant Human Gliomas. JNCI-J. Natl. Cancer Inst. 2005;97:823–835. doi: 10.1093/jnci/dji142. [DOI] [PubMed] [Google Scholar]
- 265.Zhu M., Chen L., Zhao P., Zhou H., Zhang C., Yu S., Lin Y., Yang X. Store-Operated Ca2+ Entry Regulates Glioma Cell Migration and Invasion via Modulation of Pyk2 Phosphorylation. J. Exp. Clin. Cancer Res. 2014;33:98. doi: 10.1186/s13046-014-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zohrabian V.M., Forzani B., Chau Z., Murali R., Jhanwar-Uniyal M. Rho/ROCK and MAPK Signaling Pathways Are Involved in Glioblastoma Cell Migration and Proliferation. Anticancer Res. 2009;29:119–123. [PubMed] [Google Scholar]
- 267.Abe T., La T.M., Miyagaki Y., Oya E., Wei F.-Y., Sumida K., Fujise K., Takeda T., Tomizawa K., Takei K., et al. Phosphorylation of Cortactin by Cyclin-Dependent Kinase 5 Modulates Actin Bundling by the Dynamin 1-Cortactin Ring-like Complex and Formation of Filopodia and Lamellipodia in NG108-15 Glioma-Derived Cells. Int. J. Oncol. 2019;54:550–558. doi: 10.3892/ijo.2020.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bai Y., Lathia J.D., Zhang P., Flavahan W., Rich J.N., Mattson M.P. Molecular Targeting of TRF2 Suppresses the Growth and Tumorigenesis of Glioblastoma Stem Cells. Glia. 2014;62:1687–1698. doi: 10.1002/glia.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bai H.-L., Kang C.-M., Sun Z.-Q., Li X.-H., Dai X.-Y., Huang R.-Y., Zhao J.-J., Bei Y.-R., Huang X.-Z., Lu Z.-F., et al. TTDA Inhibited Apoptosis by Regulating the P53-Bax/Bcl2 Axis in Glioma. Exp. Neurol. 2020;331:113380. doi: 10.1016/j.expneurol.2020.113380. [DOI] [PubMed] [Google Scholar]
- 270.Cai Y., Gu W.T., Cheng K., Jia P.F., Li F., Wang M., Zhang W.F., Qiu J.T., Wu Z.B., Zhao W.G. Knockdown of TRIM32 Inhibits Tumor Growth and Increases the Therapeutic Sensitivity to Temozolomide in Glioma in a P53-Dependent and -Independent Manner. Biochem. Biophys. Res. Commun. 2021;550:134–141. doi: 10.1016/j.bbrc.2021.02.098. [DOI] [PubMed] [Google Scholar]
- 271.Cao W., Yang X., Zhou J., Teng Z., Cao L., Zhang X., Fei Z. Targeting 14-3-3 Protein, Difopein Induces Apoptosis of Human Glioma Cells and Suppresses Tumor Growth in Mice. Apoptosis. 2010;15:230–241. doi: 10.1007/s10495-009-0437-4. [DOI] [PubMed] [Google Scholar]
- 272.Chiang M.F., Yeh S.T., Liao H.F., Chang N.S., Chen Y.J. Overexpression of WW Domain-Containing Oxidoreductase WOX1 Preferentially Induces Apoptosis in Human Glioblastoma Cells Harboring Mutant P53. Biomed. Pharmacother. 2012;66:433–438. doi: 10.1016/j.biopha.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 273.Feng S., Cai X., Li Y., Jian X., Zhang L., Li B. Tripartite Motif-Containing 14 (TRIM14) Promotes Epithelial-Mesenchymal Transition via ZEB2 in Glioblastoma Cells. J. Exp. Clin. Cancer Res. 2019;38:57. doi: 10.1186/s13046-019-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 274.Godoy P.R.D.V., Donaires F.S., Montaldi A.P.L., Sakamoto-Hojo E.T. Anti-Proliferative Effects of E2F1 Suppression in Glioblastoma Cells. Cytogenet. Genome Res. 2021;161:372–381. doi: 10.1159/000516997. [DOI] [PubMed] [Google Scholar]
- 275.Kang C.M., Bai H.L., Li X.H., Huang R.Y., Zhao J.J., Dai X.Y., Zheng L., Qiu Y.R., Hu Y.W., Wang Q. The Binding of LncRNA RP11-732M18.3 with 14-3-3 β/α Accelerates P21 Degradation and Promotes Glioma Growth. EBioMedicine. 2019;45:58–69. doi: 10.1016/j.ebiom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kikuchi R., Sampetrean O., Saya H., Yoshida K., Toda M. Functional Analysis of the DEPDC1 Oncoantigen in Malignant Glioma and Brain Tumor Initiating Cells. J. Neurooncol. 2017;133:297–307. doi: 10.1007/s11060-017-2457-1. [DOI] [PubMed] [Google Scholar]
- 277.Klose A., Waerzeggers Y., Monfared P., Vukicevic S., Kaijzel E.L., Winkeler A., Wickenhauser C., Lowik C.W.G.M., Jacobs A.H. Imaging Bone Morphogenetic Protein 7 Induced Cell Cycle Arrest in Experimental Gliomas. Neoplasia. 2011;13:276-U123. doi: 10.1593/neo.101540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lan Y., Lou J., Hu J., Yu Z., Lyu W., Zhang B. Downregulation of SNRPG Induces Cell Cycle Arrest and Sensitizes Human Glioblastoma Cells to Temozolomide by Targeting Myc through a P53-Dependent Signaling Pathway. Cancer Biol. Med. 2020;17:112. doi: 10.20892/j.issn.2095-3941.2019.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li H., You Y., Liu J. Cyclin-Dependent Kinase 10 Prevents Glioma Metastasis via Modulation of Snail Expression. Mol. Med. Rep. 2018;18:1165–1170. doi: 10.3892/mmr.2018.9059. [DOI] [PubMed] [Google Scholar]
- 280.Luo R., Wang X., Dong Y., Wang L., Tian C. Activation of Protease-Activated Receptor 2 Reduces Glioblastoma Cell Apoptosis. J. Biomed. Sci. 2014;21:25. doi: 10.1186/1423-0127-21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ma Q., Huang J., Xiong Y., Yang X., Han R., Zhu W. MicroRNA-96 Regulates Apoptosis by Targeting PDCD4 in Human Glioma Cells. Technol. Cancer Res. Treat. 2017;16:92–98. doi: 10.1177/1533034616629260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Meuth S.G., Herrmann A., Ip C.W., Kanyshkova T., Bittner S., Weishaupt A., Budde T., Wiendl H. The Two-Pore Domain Potassium Channel TASK3 Functionally Impacts Glioma Cell Death. J. Neurooncol. 2008;87:263–270. doi: 10.1007/s11060-008-9517-5. [DOI] [PubMed] [Google Scholar]
- 283.Tong H., Zhao K., Zhang J., Zhu J., Xiao J. YB-1 Modulates the Drug Resistance of Glioma Cells by Activation of MDM2/P53 Pathway. Drug Des. Devel. Ther. 2019;13:317–326. doi: 10.2147/DDDT.S185514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wirsching H.-G., Krishnan S., Florea A.-M., Frei K., Krayenbuehl N., Hasenbach K., Reifenberger G., Weller M., Tabatabai G. Thymosin Beta 4 Gene Silencing Decreases Stemness and Invasiveness in Glioblastoma. Brain. 2014;137:433–448. doi: 10.1093/brain/awt333. [DOI] [PubMed] [Google Scholar]
- 285.Yan F., Alinari L., Lustberg M.E., Martin L.K., Cordero-Nieves H.M., Banasavadi-Siddegowda Y., Virk S., Barnholtz-Sloan J., Bell E.H., Wojton J., et al. Genetic Validation of the Protein Arginine Methyltransferase PRMT5 as a Candidate Therapeutic Target in Glioblastoma. Cancer Res. 2014;74:1752–1765. doi: 10.1158/0008-5472.CAN-13-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yuan F., Sun Q., Zhang S., Ye L., Xu Y., Xu Z., Liu B., Zhang S., Chen Q. HSP27 Protects against Ferroptosis of Glioblastoma Cells. Hum. Cell. 2022;35:238–249. doi: 10.1007/s13577-021-00645-6. [DOI] [PubMed] [Google Scholar]
- 287.Chung L.K., Pelargos P.E., Chan A.M., Demos J.V., Lagman C., Sheppard J.P., Nguyen T., Chang Y.-L., Hojat S.A., Prins R.M., et al. Tissue Microarray Analysis for Epithelial Membrane Protein-2 as a Novel Biomarker for Gliomas. Brain Tumor Pathol. 2018;35:1–9. doi: 10.1007/s10014-017-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bao Z., Qiu X., Wang D., Ban N., Fan S., Chen W., Sun J., Xing W., Wang Y., Cui G. High Expression of Adenylate Cyclase-Associated Protein 1 Accelerates the Proliferation, Migration and Invasion of Neural Glioma Cells. Pathol. Res. Pract. 2016;212:264–273. doi: 10.1016/j.prp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 289.Haining Z., Kawai N., Miyake K., Okada M., Okubo S., Zhang X., Fei Z., Tamiya T. Relation of LAT1/4F2hc Expression with Pathological Grade, Proliferation and Angiogenesis in Human Gliomas. BMC Clin. Pathol. 2012;12:4. doi: 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kaur H., Phillips-Mason P.J., Burden-Gulley S.M., Kerstetter-Fogle A.E., Basilion J.P., Sloan A.E., Brady-Kalnay S.M. Cadherin-11, a Marker of the Mesenchymal Phenotype, Regulates Glioblastoma Cell Migration and Survival In Vivo. Mol. Cancer Res. 2012;10:293–304. doi: 10.1158/1541-7786.MCR-11-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lan J., Xue Y., Chen H., Zhao S., Wu Z., Fang J., Han C., Lou M. Hypoxia-Induced MiR-497 Decreases Glioma Cell Sensitivity to TMZ by Inhibiting Apoptosis. FEBS Lett. 2014;588:3333–3339. doi: 10.1016/j.febslet.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 292.Li S.-J., Liu H.-L., Tang S.-L., Li X.-J., Wang X.-Y. MicroRNA-150 Regulates Glycolysis by Targeting von Hippel-Lindau in Glioma Cells. Am. J. Transl. Res. 2017;9:1058–1066. [PMC free article] [PubMed] [Google Scholar]
- 293.Li C., Chen Y., Zhang Q., Guo C., Chen F., Xi S., Zeng J., Ke C., Sharma H.S., Chen Z. International Review of Neurobiology. Volume 151. Academic Press Ltd.—Elsevier Science Ltd.; London, UK: 2020. Expression of Twist Associated to Microcirculation Patterns of Human Glioma Correlated with Progression and Survival of the Patient—Novel Therapeutic Advances In Glioblastoma. [DOI] [PubMed] [Google Scholar]
- 294.Liu Y., Hou X., Liu M., Yang Z., Bi Y., Zou H., Wu J., Che H., Li C., Wang X., et al. XBP1 Silencing Decreases Glioma Cell Viability and Glycolysis Possibly by Inhibiting HK2 Expression. J. Neurooncol. 2016;126:455–462. doi: 10.1007/s11060-015-2003-y. [DOI] [PubMed] [Google Scholar]
- 295.Ljubimova J., Fugita M., Khazenzon N., Das A., Pikul B., Newman D., Sekiguchi K., Sorokin L., Sasaki T., Black K. Association between Laminin-8 and Glial Tumor Grade, Recurrence, and Patient Survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 296.Martina E., Degen M., Rueegg C., Merlo A., Lino M.M., Chiquet-Ehrismann R., Brellier F. Tenascin-W Is a Specific Marker of Glioma-Associated Blood Vessels and Stimulates Angiogenesis in Vitro. FASEB J. 2010;24:778–787. doi: 10.1096/fj.09-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Okubo S., Zhen H.-N., Kawai N., Nishiyama Y., Haba R., Tamiya T. Correlation of L-Methyl-C-11-Methionine (MET) Uptake with l-Type Amino Acid Transporter 1 in Human Gliomas. J. Neurooncol. 2010;99:217–225. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- 298.Pointer K.B., Clark P.A., Eliceiri K.W., Salamat M.S., Robertson G.A., Kuo J.S. Administration of Non-Torsadogenic Human Ether-à-Go-Go-Related Gene Inhibitors Is Associated with Better Survival for High HERG-Expressing Glioblastoma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:73–80. doi: 10.1158/1078-0432.CCR-15-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shi J., Zhang Y., Qin B., Wang Y., Zhu X. Long Non-Coding RNA LINC00174 Promotes Glycolysis and Tumor Progression by Regulating MiR-152-3p/SLC2A1 Axis in Glioma. J. Exp. Clin. Cancer Res. 2019;38:395. doi: 10.1186/s13046-019-1390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wu H., Li J., Xu D., Jv D., Meng X., Qiao P., Cui T., Shi B. The 37-KDa Laminin Receptor Precursor Regulates the Malignancy of Human Glioma Cells. Cell Biochem. Funct. 2016;34:516–521. doi: 10.1002/cbf.3225. [DOI] [PubMed] [Google Scholar]
- 301.Han M.-Z., Wang S., Zhao W.-B., Ni S.-L., Yang N., Kong Y., Huang B., Chen A.-J., Li X.-G., Wang J., et al. Immune Checkpoint Molecule Herpes Virus Entry Mediator Is Overexpressed and Associated with Poor Prognosis in Human Glioblastoma. Ebiomedicine. 2019;43:159–170. doi: 10.1016/j.ebiom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ku B.M., Lee Y.K., Ryu J., Jeong J.Y., Choi J., Eun K.M., Shin H.Y., Kim D.G., Hwang E.M., Yoo J.C., et al. CHI3L1 (YKL-40) Is Expressed in Human Gliomas and Regulates the Invasion, Growth and Survival of Glioma Cells. Int. J. Cancer. 2011;128:1316–1326. doi: 10.1002/ijc.25466. [DOI] [PubMed] [Google Scholar]
- 303.Lou J.-C., Lan Y.-L., Gao J.-X., Ma B.-B., Yang T., Yuan Z.-B., Zhang H.-Q., Zhu T.-Z., Pan N., Leng S., et al. Silencing NUDT21 Attenuates the Mesenchymal Identity of Glioblastoma Cells via the NF-Kappa B Pathway. Front. Mol. Neurosci. 2017;10:420. doi: 10.3389/fnmol.2017.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Saito K., Ohta S., Kawakami Y., Yoshida K., Toda M. Functional Analysis of KIF20A, a Potential Immunotherapeutic Target for Glioma. J. Neurooncol. 2017;132:63–74. doi: 10.1007/s11060-016-2360-1. [DOI] [PubMed] [Google Scholar]
- 305.Xu H., Chai S., Wang Y., Wang J., Xiao D., Li J., Xiong N. Molecular and Clinical Characterization of PARP9 in Gliomas: A Potential Immunotherapeutic Target. CNS Neurosci. Ther. 2020;26:804–814. doi: 10.1111/cns.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yuan Y., Zhao Q., Zhao S., Zhang P., Zhao H., Li Z., Du Y., Tian X., Lu J. Characterization of Transcriptome Profile and Clinical Features of a Novel Immunotherapy Target CD204 in Diffuse Glioma. Cancer Med. 2019;8:3811–3821. doi: 10.1002/cam4.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yuan F., Cong Z., Cai X., Zhu J., Yuan L., Wang Y., Tang C., Ma C. BACH1 as a Potential Target for Immunotherapy in Glioblastomas. Int. Immunopharmacol. 2022;103:108451. doi: 10.1016/j.intimp.2021.108451. [DOI] [PubMed] [Google Scholar]
- 308.Zhang Y., Yang X., Zhu X.L., Bai H., Wang Z.Z., Zhang J.J., Hao C.Y., Duan H.B. S100A Gene Family: Immune-Related Prognostic Biomarkers and Therapeutic Targets for Low-Grade Glioma. Aging. 2021;13:15459–15478. doi: 10.18632/aging.203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhu Y., Liu Z., Lv D., Cheng X., Wang J., Liu B., Han Z., Wangs Y., Liu R., Gao Y. Identification of PYGL as a Key Prognostic Gene of Glioma by Integrated Bioinformatics Analysis. Future Oncol. 2022;18:579–596. doi: 10.2217/fon-2021-0759. [DOI] [PubMed] [Google Scholar]
- 310.Chen T., Zhang F., Liu J., Huang Z., Zheng Y., Deng S., Liu Y., Wang J., Sun X. Dual Role of WNT5A in Promoting Endothelial Differentiation of Glioma Stem Cells and Angiogenesis of Glioma Derived Endothelial Cells. Oncogene. 2021;40:5081–5094. doi: 10.1038/s41388-021-01922-2. [DOI] [PubMed] [Google Scholar]
- 311.Di C., Liang J., Wang Y., Zhao G., Zhao Y. SPZ1 Promotes Glioma Aggravation via Targeting CXXC4. J. BUON. 2021;26:373–379. [PubMed] [Google Scholar]
- 312.Friedmann-Morvinski D., Bhargava V., Gupta S., Verma I.M., Subramaniam S. Identification of Therapeutic Targets for Glioblastoma by Network Analysis. Oncogene. 2016;35:608–620. doi: 10.1038/onc.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Guo G., Liu J., Ren Y., Mao X., Hao Y., Zhong C., Chen X., Wang X., Wu Y., Lian S., et al. FRAT1 Enhances the Proliferation and Tumorigenesis of CD133+ Nestin+ Glioma Stem Cells In Vitro and In Vivo. J. Cancer. 2020;11:2421–2430. doi: 10.7150/jca.37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lan J., Guo P., Lin Y., Mao Q., Guo L., Ge J., Li X., Jiang J., Lin X., Qiu Y. Role of Glycosyltransferase PomGnT1 in Glioblastoma Progression. Neuro-Oncology. 2015;17:211–222. doi: 10.1093/neuonc/nou151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mizobuchi Y., Matsuzaki K., Kuwayama K., Kitazato K., Mure H., Kageji T., Nagahiro S. REIC/Dkk-3 Induces Cell Death in Human Malignant Glioma. Neuro-Oncology. 2008;10:244–253. doi: 10.1215/15228517-2008-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhou X., Ren Y., Zhang J., Zhang C., Zhang K., Han L., Kong L., Wei J., Chen L., Yang J., et al. HOTAIR Is a Therapeutic Target in Glioblastoma. Oncotarget. 2015;6:8353–8365. doi: 10.18632/oncotarget.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Borsics T., Lundberg E., Geerts D., Koomoa D.-L.T., Koster J., Wester K., Bachmann A.S. Subcellular Distribution and Expression of Prenylated Rab Acceptor 1 Domain Family, Member 2 (PRAF2) in Malignant Glioma: Influence on Cell Survival and Migration. Cancer Sci. 2010;101:1624–1631. doi: 10.1111/j.1349-7006.2010.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cui P., Su J., Li Q., Xu G., Zhu N. LncRNA RHPN1-AS1 Targeting MiR-625/REG3A Promotes Cell Proliferation And Invasion Of Glioma Cells. Oncotargets Ther. 2019;12:7911–7921. doi: 10.2147/OTT.S209563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dong Z., Zhang J., Niu L., Hou G., Gao Z., Yang Q. MiR-381-3p Involves in Glioma Progression by Suppressing Tumor-Promoter Factor ANTXR1. Comput. Math. Methods Med. 2021;2021:4883509. doi: 10.1155/2021/4883509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 320.Fève M., Saliou J.M., Zeniou M., Lennon S., Carapito C., Dong J., Van Dorsselaer A., Junier M.P., Chneiweiss H., Cianférani S., et al. Comparative Expression Study of the Endo-G Protein Coupled Receptor (GPCR) Repertoire in Human Glioblastoma Cancer Stem-like Cells, U87-MG Cells and Non Malignant Cells of Neural Origin Unveils New Potential Therapeutic Targets. PLoS ONE. 2014;9:e91519. doi: 10.1371/journal.pone.0091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Han M.-Z., Xu R., Xu Y.-Y., Zhang X., Ni S.-L., Huang B., Chen A.-J., Wei Y.-Z., Wang S., Li W.-J., et al. TAGLN2 Is a Candidate Prognostic Biomarker Promoting Tumorigenesis in Human Gliomas. J. Exp. Clin. Cancer Res. 2017;36:155. doi: 10.1186/s13046-017-0619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hou D., Wang Z., Li H., Liu J., Liu Y., Jiang Y., Lou M. Circular RNA CircASPM Promotes the Progression of Glioblastoma by Acting as a Competing Endogenous RNA to Regulate MiR-130b-3p/E2F1 Axis. J. Cancer. 2022;13:1664–1678. doi: 10.7150/jca.57691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Huang W., Shi Y., Han B., Wang Q., Zhang B., Qi C., Liu F. LncRNA GAS5-AS1 Inhibits Glioma Proliferation, Migration, and Invasion via MiR-106b-5p/TUSC2 Axis. Hum. Cell. 2020;33:416–426. doi: 10.1007/s13577-020-00331-z. [DOI] [PubMed] [Google Scholar]
- 324.Li J.-L., Sainson R.C.A., Oon C.E., Turley H., Leek R., Sheldon H., Bridges E., Shi W., Snell C., Bowden E.T., et al. DLL4-Notch Signaling Mediates Tumor Resistance to Anti-VEGF Therapy in Vivo. Cancer Res. 2011;71:6073–6083. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- 325.Li R., Li X., Ning S., Ye J., Han L., Kang C., Li X. Identification of a Core MiRNA-Pathway Regulatory Network in Glioma by Therapeutically Targeting MiR-181d, MiR-21, MiR-23b, β-Catenin, CBP, and STAT3. PLoS ONE. 2014;9:e101903. doi: 10.1371/journal.pone.0101903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Li Q., Wu Q., Li Z., Hu Y., Zhou F., Zhai Z., Yue S., Tian H. LncRNA LINC00319 Is Associated with Tumorigenesis and Poor Prognosis in Glioma. Eur. J. Pharmacol. 2019;861:172556. doi: 10.1016/j.ejphar.2019.172556. [DOI] [PubMed] [Google Scholar]
- 327.Li H., Wang D., Yi B., Cai H., Wang Y., Lou X., Xi Z., Li Z. SUMOylation of IGF2BP2 Promotes Vasculogenic Mimicry of Glioma via Regulating OIP5-AS1/MiR-495-3p Axis. Int. J. Biol. Sci. 2021;17:2912–2930. doi: 10.7150/ijbs.58035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Liu C., Liang S., Xiao S., Lin Q., Chen X., Wu Y., Fu J. MicroRNA-27b Inhibits Spry2 Expression and Promotes Cell Invasion in Glioma U251 Cells. Oncol. Lett. 2015;9:1393–1397. doi: 10.3892/ol.2015.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Liu L., Xu Q., Xiong Y., Deng H., Zhou J. LncRNA LINC01094 Contributes to Glioma Progression by Modulating MiR-224-5p/CHSY1 Axis. Hum. Cell. 2022;35:214–225. doi: 10.1007/s13577-021-00637-6. [DOI] [PubMed] [Google Scholar]
- 330.Miller T.E., Liau B.B., Wallace L.C., Morton A.R., Xie Q., Dixit D., Factor D.C., Kim L.J.Y., Morrow J.J., Wu Q., et al. Transcription Elongation Factors Represent in Vivo Cancer Dependencies in Glioblastoma. Nature. 2017;547:355–359. doi: 10.1038/nature23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Noorani I., de la Rosa J., Choi Y.H., Strong A., Ponstingl H., Vijayabaskar M.S., Lee J., Lee E., Richard-Londt A., Friedrich M., et al. PiggyBac Mutagenesis and Exome Sequencing Identify Genetic Driver Landscapes and Potential Therapeutic Targets of EGFR-Mutant Gliomas. Genome Biol. 2020;21:181. doi: 10.1186/s13059-020-02092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Qiu X., Ji B., Yang L., Huang Q., Shi W., Ding Z., He X., Ban N., Fan S., Zhang J., et al. The Role of FoxJ2 in the Migration of Human Glioma Cells. Pathol. Res. Pract. 2015;211:389–397. doi: 10.1016/j.prp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 333.Rose M., Cardon T., Aboulouard S., Hajjaji N., Kobeissy F., Duhamel M., Fournier I., Salzet M. Surfaceome Proteomic of Glioblastoma Revealed Potential Targets for Immunotherapy. Front. Immunol. 2021;12:746168. doi: 10.3389/fimmu.2021.746168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sharma V., Purkait S., Takkar S., Malgulwar P.B., Kumar A., Pathak P., Suri V., Sharma M.C., Suri A., Kale S.S., et al. Analysis of EZH2: Micro-RNA Network in Low and High Grade Astrocytic Tumors. Brain Tumor Pathol. 2016;33:117–128. doi: 10.1007/s10014-015-0245-1. [DOI] [PubMed] [Google Scholar]
- 335.Sun Z., Zhang B., Wang C., Fu T., Li L., Wu Q., Cai Y., Wang J. Forkhead Box P3 Regulates ARHGAP15 Expression and Affects Migration of Glioma Cells through the Rac1 Signaling Pathway. Cancer Sci. 2017;108:61–72. doi: 10.1111/cas.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Visvanathan A., Patil V., Arora A., Hegde A.S., Arivazhagan A., Santosh V., Somasundaram K. Essential Role of METTL3-Mediated m(6)A Modification in Glioma Stem-like Cells Maintenance and Radioresistance. Oncogene. 2018;37:522–533. doi: 10.1038/onc.2017.351. [DOI] [PubMed] [Google Scholar]
- 337.Wang H., Han M., Whetsell W.J.r., Wang J., Rich J., Hallahan D., Han Z. Tax-Interacting Protein 1 Coordinates the Spatiotemporal Activation of Rho GTPases and Regulates the Infiltrative Growth of Human Glioblastoma. Oncogene. 2014;33:1558–1569. doi: 10.1038/onc.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wei J., Li Z., Du C., Qi B., Zhao X., Wang L., Bi L., Wang G., Zhang X., Su X., et al. Abnormal Expression of an ADAR2 Alternative Splicing Variant in Gliomas Downregulates Adenosine-to-Inosine RNA Editing. Acta Neurochir. 2014;156:1135–1142. doi: 10.1007/s00701-014-2004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Weigle B., Ebner R., Temme A., Schwind S., Schmitz M., Kiessling A., Rieger M., Schackert G., Schackert H., Rieber E. Highly Specific Overexpression of the Transcription Factor SOX11 in Human Malignant Gliomas. Oncol. Rep. 2005;13:139–144. doi: 10.3892/or.13.1.139. [DOI] [PubMed] [Google Scholar]
- 340.Xin J., Zhao Y.H., Zhang X.Y., Tian L.Q. LncRNA NFIA-AS2 Promotes Glioma Progression through Modulating the MiR-655-3p/ZFX Axis. Hum. Cell. 2020;33:1273–1280. doi: 10.1007/s13577-020-00408-9. [DOI] [PubMed] [Google Scholar]
- 341.Zhang D., Wang W., Zhou H., Su L., Han X., Zhang X., Han W., Wang Y., Xue X. ANXA1: An Important Independent Prognostic Factor and Molecular Target in Glioma. Front. Genet. 2022;13:851505. doi: 10.3389/fgene.2022.851505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhou L., Li L., Chen Y., Chen C., Zhi Z., Yan L., Wang Y., Liu B., Zhai Q. MiR-190a-3p Promotes Proliferation and Migration in Glioma Cells via YOD1. Comput. Math. Methods Med. 2021;2021:3957738. doi: 10.1155/2021/3957738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 343.Kumthekar P. A Phase 0 First-In-Human Study Using NU-0129: A Spherical Nucleic Acid (SNA) Gold Nanoparticle Targeting BCL2L12 in Recurrent Glioblastoma Multiforme or Gliosarcoma Patients. [(accessed on 29 May 2023)];2022 Available online: clinicaltrials.gov.
- 344.Miller J.J., Gonzalez Castro L.N., McBrayer S., Weller M., Cloughesy T., Portnow J., Andronesi O., Barnholtz-Sloan J.S., Baumert B.G., Berger M.S., et al. Isocitrate Dehydrogenase (IDH) Mutant Gliomas: A Society for Neuro-Oncology (SNO) Consensus Review on Diagnosis, Management, and Future Directions. Neuro-Oncology. 2023;25:4–25. doi: 10.1093/neuonc/noac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Allen M., Bjerke M., Edlund H., Nelander S., Westermark B. Origin of the U87MG Glioma Cell Line: Good News and Bad News. Sci. Transl. Med. 2016;8:354re3. doi: 10.1126/scitranslmed.aaf6853. [DOI] [PubMed] [Google Scholar]
- 346.Kenesei Á., Volkó J., Szalóki N., Mocsár G., Jambrovics K., Balajthy Z., Bodnár A., Tóth K., Waldmann T.A., Vámosi G. IL-15 Trans-Presentation Is an Autonomous, Antigen-Independent Process. J. Immunol. 2021;207:2489–2500. doi: 10.4049/jimmunol.2100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Cole D.E., Lester-McCully C.M., Widemann B.C., Warren K.E. Plasma and Cerebrospinal Fluid Pharmacokinetics of the Akt Inhibitor, Perifosine, in a Non-Human Primate Model. Cancer Chemother. Pharmacol. 2015;75:923–928. doi: 10.1007/s00280-015-2711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Britten C.D. PI3K and MEK Inhibitor Combinations: Examining the Evidence in Selected Tumor Types. Cancer Chemother. Pharmacol. 2013;71:1395–1409. doi: 10.1007/s00280-013-2121-1. [DOI] [PubMed] [Google Scholar]
- 349.Paul I., Bhattacharya S., Chatterjee A., Ghosh M.K. Current Understanding on EGFR and Wnt/β-Catenin Signaling in Glioma and Their Possible Crosstalk. Genes Cancer. 2013;4:427–446. doi: 10.1177/1947601913503341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mellinghoff I.K., Wang M.Y., Vivanco I., Haas-Kogan D.A., Zhu S., Dia E.Q., Lu K.V., Yoshimoto K., Huang J.H.Y., Chute D.J., et al. Molecular Determinants of the Response of Glioblastomas to EGFR Kinase Inhibitors. N. Engl. J. Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 351.National Institute of Health (NIH) 219 Studies Found for: Molecular Targeted Therapy OR Molecular Target OR Protein Kinase Inhibitors/Administration OR Mitogen-Activated Protein Kinase Kinases/Antagonists and Inhibitors OR Antineoplastic Combined Chemotherapy Protocols/Administration and Dosage|Glioma Glioblastoma Multiforme. Clinicaltrials.Gov. [(accessed on 29 May 2023)]; Available online: https://clinicaltrials.gov/ct2/results?cond=Glioma+Glioblastoma+Multiforme&term=molecular+targeted+therapy+OR+molecular+target+OR+Protein+Kinase+Inhibitors%2Fadministration+OR+Mitogen-Activated+Protein+Kinase+Kinases%2Fantagonists+and+inhibitors+OR+Antineoplastic+Combined+Chemotherapy+Protocols%2Fadministration+and+dosage&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=
- 352.Thiessen B., Stewart C., Tsao M., Kamel-Reid S., Schaiquevich P., Mason W., Easaw J., Belanger K., Forsyth P., McIntosh L., et al. A Phase I/II Trial of GW572016 (Lapatinib) in Recurrent Glioblastoma Multiforme: Clinical Outcomes, Pharmacokinetics and Molecular Correlation. Cancer Chemother. Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 353.Patel K.S., Kejriwal S., Thammachantha S., Duong C., Murillo A., Gordon L.K., Cloughesy T.F., Liau L., Yong W., Yang I., et al. Increased Epithelial Membrane Protein 2 Expression in Glioblastoma after Treatment with Bevacizumab. Neuro-Oncol. Adv. 2020;2:vdaa112. doi: 10.1093/noajnl/vdaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wirsching H.-G., Weller M. The Role of Molecular Diagnostics in the Management of Patients with Gliomas. Curr. Treat. Options Oncol. 2016;17:51. doi: 10.1007/s11864-016-0430-4. [DOI] [PubMed] [Google Scholar]
- 355.Wang J.Y., Bettegowda C. Genetics and Immunotherapy: Using the Genetic Landscape of Gliomas to Inform Management Strategies. J. Neurooncol. 2015;123:373–383. doi: 10.1007/s11060-015-1730-4. [DOI] [PubMed] [Google Scholar]
- 356.Xiong W., Zhao Y., Du H., Guo X. Current Status of Immune Checkpoint Inhibitor Immunotherapy for Lung Cancer. Front. Oncol. 2021;11:704336. doi: 10.3389/fonc.2021.704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Han J.W., Park S.-H. Advances in Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. J. Liver Cancer. 2021;21:139–145. doi: 10.17998/jlc.2021.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhang H., Dai Z., Wu W., Wang Z., Zhang N., Zhang L., Zeng W.-J., Liu Z., Cheng Q. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J. Exp. Clin. Cancer Res. 2021;40:184. doi: 10.1186/s13046-021-01987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Restrepo P., Yong R., Laface I., Tsankova N., Nael K., Akturk G., Sebra R., Gnjatic S., Hormigo A., Losic B. Tumoral and Immune Heterogeneity in an Anti-PD-1-Responsive Glioblastoma: A Case Study. Cold Spring Harb. Mol. Case Stud. 2020;6:a004762. doi: 10.1101/mcs.a004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ostrom Q.T., Gittleman H., Farah P., Ondracek A., Chen Y., Wolinsky Y., Stroup N.E., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology. 2013;15:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Penas-Prado M., Gilbert M.R. Molecularly Targeted Therapies for Malignant Gliomas: Advances and Challenges. Expert Rev. Anticancer Ther. 2007;7:641–661. doi: 10.1586/14737140.7.5.641. [DOI] [PubMed] [Google Scholar]
- 362.Roesler R., Brunetto A.T., Abujamra A.L., de Farias C.B., Brunetto A.L., Schwartsmann G. Current and Emerging Molecular Targets in Glioma. Expert Rev. Anticancer Ther. 2010;10:1735–1751. doi: 10.1586/era.10.167. [DOI] [PubMed] [Google Scholar]
- 363.Huang T.T., Sarkaria S.M., Cloughesy T.F., Mischel P.S. Targeted Therapy for Malignant Glioma Patients: Lessons Learned and the Road Ahead. Neurotherapeutics. 2009;6:500–512. doi: 10.1016/j.nurt.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the nature of the research, there was no primary data collected. Materials were obtained from searches of the PubMed and Web of Science databases.