Abstract
Plant height is one of the key agronomic traits for improving the yield of sweet potato. Phytohormones, especially gibberellins (GAs), are crucial to regulate plant height. The enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) is the key enzyme for abscisic acid (ABA) biosynthesis signalling in higher plants. However, its role in regulating plant height has not been reported to date. Here, we cloned a new NCED gene, IbNCED1, from the sweet potato cultivar Jishu26. This gene encoded the 587-amino acid polypeptide containing an NCED superfamily domain. The expression level of IbNCED1 was highest in the stem and the old tissues in the in vitro-grown and field-grown Jishu26, respectively. The expression of IbNCED1 was induced by ABA and GA3. Overexpression of IbNCED1 promoted the accumulation of ABA and inhibited the content of active GA3 and plant height and affected the expression levels of genes involved in the GA metabolic pathway. Exogenous application of GA3 could rescue the dwarf phenotype. In conclusion, we suggest that IbNCED1 regulates plant height and development by controlling the ABA and GA signalling pathways in transgenic sweet potato.
Keywords: sweet potato, IbNCED1, plant height, ABA, GA
1. Introduction
Sweet potato, Ipomoea batatas (L.) Lam., is an important root crop worldwide [1,2]. In actual production, due to the genotype, excessive nitrogen application, uneven rainfall distribution, and improper irrigation, sweet potato is easily overgrown; this seriously impacts the yield, mechanization degree and the sustainable development of the sweet potato industry [3]. The ideal plant height of sweet potato helps to break through the bottleneck. However, research to date has yet to establish a clear genetic basis and the constituent elements in sweet potato.
Plant height is an agronomic trait with a complex genetic basis [4,5]. It is confined by stem elongation and plays important role in crop yield and quality [6]. With the rise of the green revolution, a large number of dwarf mutants, quantitative trait loci (QTLs), and genes have been identified to control plant height [7,8,9,10]. In wheat, the Reduced height (Rht) alleles, such as Rht-1, Rht-B1b, and Rht-D1b were introduced to reduce plant height, providing improved lodging resistance through interfering with the action or production of the gibberellins (GAs), plant hormones [11,12,13].
GAs are a class of tetracyclic diterpenoid phytohormones that mediate different processes of plant development including stem elongation, seed germination, trichome development, leaf expansion, induction of flowering, and pollen maturation [14,15]. More than 130 GAs have been identified, and GA1, GA3, GA4, and GA7 show capital biological activity that controls plant development [16,17,18]. Higher GA levels and more active GA biosynthesis were found to be correlated with plant height [19,20,21]. GA metabolism or signalling conferred grain productivity during the Green Revolution by reshaping plant stature [22,23]. Many genes have been identified relating to plant height through the GA signalling pathway. In rice, OsDREB2B, OsAP2-39, and OsWRKY21 reduce plant height development through the GA biosynthesis pathway [24]. TaLecRK-IV.1 and TaRht24 are regulators of plant height through the gibberellic acid and auxin-signalling pathways in wheat [25,26]. Overexpression of CmDRP resulted in a semi-dwarf phenotype with a significantly decreased active GA3 content, while reduced expression generated the opposite phenotype inchrysanthemum [27].
It has always been clear that GAs interact with other plant hormones [28]. GA and ABA usually play antagonistic roles in the regulation of germination, growth, and flowering in plants [29,30]. ABA affect the GA pathway through a different mechanism, such as an ABA-induced Ser/Thr protein kinase (PKABA1) and transcriptional regulators of ABA-induced WRKY, DELLA, and MYB [31,32,33,34,35]. Arabidopsis ABF2 and ABF4 transcription factors positively regulate potato tuber induction by regulating the expression of ABA- and GA-metabolism genes [36].
The enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) is the key enzyme for ABA biosynthesis signalling [37,38]. NCED genes are associated with development and tolerance by the ABA signalling pathway in plants. Overexpression of VaNCED1 delayed the development of transgenic Vitis vinifera [39]. OsNCED3 and OsNCED5 mediated seed dormancy, plant growth, abiotic stress tolerance, and leaf senescence by regulating ABA biosynthesis in rice [40,41]. LeNCED1 overexpression in tomato increased ABA concentration and prevented the induction of genes involved in ABA metabolism and the deactivations of GA and auxin that occurred in WT [42]. The expression of NCED genes in dwarf cotton accession was higher than that in taller ones, and GhNCED1-silenced cotton plants increased in height [43]. Up to now, NCED genes have not been identified in sweet potato. In this study, we cloned a new IbNCED1 gene for the 587aa from sweet potato. Functional analysis showed that IbNCED1 enhanced the accumulation of ABA and inhibited plant height, affected the expression levels of genes involved in the GA metabolic pathway, and affected the content of active GA.
2. Results
2.1. Cloning and Sequence Analysis of IbNCED1
The novel IbNCED1 gene was isolated from the sweet potato cultivar Jishu26. The 1764-bp ORF sequence of IbNCED1 encoded a protein of 587 aa with a molecular weight of 65.33 kDa and a predicted pI of 6.12, which belongs to the RPE65 superfamily (Figure 1A). Phylogenetic analysis of NCED proteins with a neighbor-joining method revealed that IbNCED1 has high homology with NCED proteins from Ipomoea triloba (ItNCED1, XP_031110150.1), Ipomoea nil (InNCED1, XP_019153780.1), and Solanum lycopersicum (SlNCED1, NP_001234455.1) (Figure 1B).
Figure 1.
Sequence alignment (A) and phylogenetic tree (B) of IbNCED1 with its homologs from other plants.
2.2. Expression Analysis of IbNCED1
To study the potential function of IbNCED1 in sweet potato, its expression in different tissues and treatments of Jishu26 was analyzed with qRT-PCR. The expression level of IbNCED1 was the highest in the stem of the in vitro-grown Jishu26 plants (Figure 2A). For the field-grown Jishu26 plants, the expression level of IbNCED1 was higher in the old stem, pencil root, and storage root tissues than in other young tissues (Figure 2B).
Figure 2.
Expression analysis of IbNCED1 in different tissues of Jishu26 plants. The expression analysis of IbNCED1 in different tissues of in vitro-grown (A) and field-grown (B) plants of Jishu26. LL: Leaflet; L: Leaf; PR: Pencil root; R: Root; S: Stem; SR: Storage root; YS: Young stem. The transcript levels of IbNCED1 in the leaf tissue or leaflet were set to 1. The expression analysis of IbNCED1 in different tissues of Jishu26 plants after different time points (h) in response to 100 mM ABA (C) and 100 mM GA (D), respectively. The expression level of IbNCED1 in the plant sampled at 0 h was set to 1. The data are presented as the means ± SEs (n = 3). * indicates significant differences from that of WT at p < 0.05, according to Student’s t-test.
The expression of IbNCED1 was downregulated in the leaf and upregulated in the stem and root after ABA and GA treatments. The expression level peaked at 3 h (4.520-fold in the stem and 4.56-fold in the root) after ABA treatment (Figure 2C), while it peaked at 12 h in the stem and at 6 h in the root (4.11- and 3.08-fold, respectively) after GA treatment (Figure 2D). These results suggest that IbNCED1 might be involved in ABA and GA response pathways.
2.3. Regeneration of the Transgenic Sweet Potato Plants
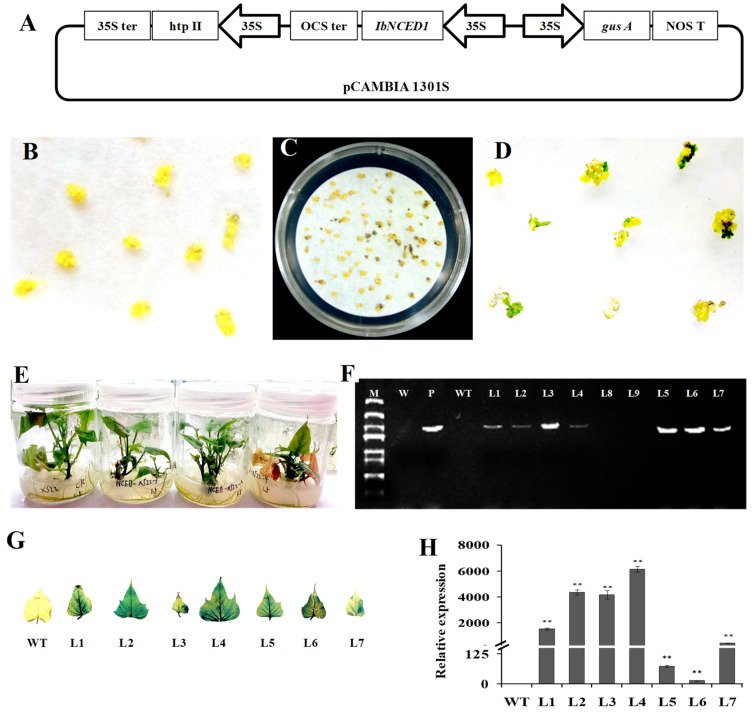
The overexpression vector pCAMBIA1301s-IbNCED1 was introduced into the Agrobacterium tumefaciens strain EHA105 (Figure 3A). Cell aggregates of Xushu22 (Figure 3B) co-cultivated with EHA105 carrying pCAMBIA1301-IbNCED1 were cultured on the selective MS medium with 2.0 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D), 100 mg L−1 carbenicillin (Carb), and 10 mg L−1 hygromycin (Hyg) (Figure 3C). Seventeen Hyg-resistant embryogenic calluses of 132 cell aggregates were obtained after 6 weeks. These Hyg-resistant embryogenic calluses were transferred to MS medium with 1.0 mg L−1 ABA and 100 mg L−1 Carb, and after 4 weeks of transfer, they formed plantlets (Figure 3D). Nine regenerated plants were transferred to MS medium and seven of them showed dwarf phenotype (Figure 3E). The seven regenerated plants were proved to be transgenic by PCR and GUS analyses, named L1, L2, …, L7, respectively (Figure 3F,G). qRT-PCR analysis revealed that the expression level of IbNCED1 was significantly increased in most of the transgenic plants compared with that of WT (Figure 3H).
Figure 3.
Production of the IbNCED1-overexpressing sweet potato plants. (A) Diagram of constitutive expression of the 35S promoter::IbNCED1 construct. (B) Embryogenic cultures proliferating in MS medium with 2.0 mg L−1 2,4-D. (C) Hyg-resistant calluses formedafter 4 weeks of selection on MS medium with 2.0 mg L−1 2,4-D, 100 mg L−1 Carb and 10 mg L−1 Hyg. (D) Germination of somatic embryos from Hyg-resistant calluses on MS medium with 1.0 mg L−1 ABA and 100 mg L−1 Carb. (E) Whole regenerated plantlets. (F) PCR analysis of the transgenic plants. Lane M: BL2000 plus DNA marker; Lane W: Water; Lane P: plasmid pCAMBI1301::IbNCED1 as a positive control; Lane WT: Xushu22 plant as a negative control. (G) GUS analysis of the transgenic plants. (H) qRT-PCR analysis of IbNCED1 in the transgenic plants. ** indicates a significant difference from that of WT at p < 0.01 according to Student’s t-test.
2.4. Plant Height Assay
In vitro propagation of sweet potato is a basic step for routine gene bank and biotechnology research activities. The seven regenerated sweet potato lines were raised in plant numbers by vegetative propagation using an MS medium. The three transgenic sweet potato plants L1, L2, and L4, with high relative expression of IbNCED1 and stable dwarf phenotype, were selected to test plant height. The result show that overexpression of IbNCED1 conferred a reduction in height of in vitro-grown and greenhouse-grown transgenic plants (Figure 4A,B). The histological analysis of the longitudinal section showed that the pith cell length of the transgenic plants decreased in comparison to the WT (Figure 4C). All the results demonstrated that IbNCED1 demoted stem elongation primarily by reducing cell length.
Figure 4.
Plant height of transgenic sweet potato plants and WT. (A1,A2) Phenotypes and plant height of in vitro-grown transgenic sweet potato plants and WT cultured on MS medium for 4 weeks. The data are presented as the means ± SEs (n = 5). (B1,B2) Phenotypes and plant height of transgenic sweet potato plants and WT grown in transplanting boxes for 6 weeks. Bar = 10 cm. The data are presented as the means ± SEs (n = 5). (C1,C2)The histological analysis and cell length of in vitro-grown transgenic sweet potato plants and WT cultured on MS medium for 4 weeks. Bar = 100 μm. The data are presented as the means ± SEs (n = 20). The data are presented as the means ± SEs (n = 5). The different small letters indicate a significant difference at p < 0.05 according to Student’s t-test.
2.5. Underlying Mechanism of IbNCED1 in Plant Height
To explore the dwarfing mechanism and the dwarf genes of sweet potato, differentially expressed genes and metabolic pathways in transgenic sweet potato were analyzed by RNA sequencing (RNA-Seq) using 4-week-old in vitro-grown WT and transgenic line L2 (OE). After removing the adapter and low-quality reads, a total of 614,283,286 clean reads were obtained from two lines (three biological replicates per line), and the quality control and quality assessment of RNA-Seq data showed that the sample quality was reliable and can be analyzed later (Table S1 and Figure S1). Using WT as the control group and |log2 (Fold Change)| > 1 & q < 0.05 as the standard of gene differential expression, we obtained a total of 2938 differential expressed genes (DEGs), of which 1827 genes were downregulated, and 1111 genes were upregulated. KEGG enrichment analysis showed that the DEGs were primarily enriched metabolism, biosynthesis of secondary metabolites, plant MAPK signalling, and plant hormone signal transduction pathway (Figure 5A). The DEGs of GA biosynthesis and signal transduction pathway were downregulated (Figure 5B).
Figure 5.
The analysis of KEGG pathway in transgenic sweet potato (A) and the expression analysis of DEGs in GA signalling pathway (B).
To investigate the underlying mechanism of IbNCED1 in plant height, the phytohormone components of 4−week−old in vitro-grown sweet potato plants were measured. The results showed that the ABA and ABA−GE contents of the transgenic plants were significantly increased, while the GA3 content was significantly decreased compared with those of WT (Table 1). Exogenous GA3 treatment was performed on WT and transgenic sweet potato to determine the factors of height reduction. The WT plant and transgenic sweet potato could not grow on MS with 10 ng L−1 GA3 and 30 ng L−1 GA3, respectively (Figure S2). These results indicated that overexpression of IbNCED1 could reduce the GA sensitivity of transgenic sweet potato. To further prove the function of GA in plant height, we analyzed the plant height of transgenic sweet potato plants and WT after GA3 treatment, and the results showed that exogenous GA3 can restore the plant height of transgenic sweet potato (Figure 6). In conclusion, we suggest that IbNCED1 negatively regulates plant height by controlling the GA biosynthesis and signal transduction pathway.
Table 1.
The contents of ABA and GAs (mg g−1).
| Class | Index | WT | L2 |
|---|---|---|---|
| ABA | ABA | 7.60 ± 0.68 | 83.42 ± 1.77 |
| ABA-GE | 133.91 ± 4.82 | 355.59 ± 8.22 | |
| GA | GA1 | N/A | N/A |
| GA3 | 5.02 ± 0.16 | N/A | |
| GA4 | N/A | N/A | |
| GA7 | N/A | N/A | |
| GA9 | N/A | N/A | |
| GA15 | N/A | N/A | |
| GA19 | 21.15 ± 0.53 | 23.85 ± 0.84 | |
| GA20 | N/A | N/A | |
| GA24 | N/A | N/A | |
| GA53 | 6.36 ±0.68 | 11.34 ± 0.30 |
N/A—Not applicable.
Figure 6.
Plant height of transgenic sweet potato plants and WT after GA3 treatment. (A1–A3) Phenotypes and plant height of in vitro-grown transgenic sweet potato plants and WT cultured on MS medium for 6 weeks. (B1–B3) Phenotypes and plant height of in vitro-grown transgenic sweet potato plants and WT cultured on MS medium with 10 ng L−1 GA3 for 6 weeks. Bar = 10 cm. The data are presented as the means ± SEs (n = 3). The different small letters indicate a significant difference at p < 0.05 according to Student’s t-test.
3. Discussion
The Green Revolution has promoted a significant yield increase through the development of semi-dwarf plant architecture in rice, wheat, maize, and soybean [21,44,45,46]. The ideal architecture for sweet potato also could promote the mechanization degree and yield of storage roots. However, the dwarfing mechanism and the dwarf genes of sweet potato are still unclear. In this study, we cloned an IbNCED1 from the sweet potato cv. Jishu26 (Figure 1). The expression of IbNCED1 was downregulated in the leaf and upregulated in the stem and root after ABA and GA treatments (Figure 2). Its overexpression significantly conferred a reduction in the height of the transgenic sweet potato plants and promoted the accumulation of ABA and ABA−GE in transgenic sweet potato (Table 1). It is thought that IbNCED1 is key enzyme gene for ABA biosynthesis signalling in sweet potato.
The other functions of NCED genes have been identified in different plants. GhNCED1 reduced plant height in cotton [47]. AtNCED3 and AtNCED5 contributed to ABA production affecting vegetative growth and drought tolerance in Arabidopsis [48,49]. OsNCED3 mediates seed dormancy, plant growth, abiotic stress tolerance, and leaf senescence by regulating ABA biosynthesis in rice [40]. In our study, overexpression of IbNCED1 reduced plant height and cell length of the stem in transgenic sweet potato (Figure 4). The results indicated that IbNCED1 plays an important role in reducing the growth of transgenic sweet potato by regulating ABA biosynthesis.
To date, many Rht genes have been identified in regulating plant height via participating in GA biosynthesis regulation in different plants [11,12,13]. The antagonistic regulations of GA and ABA have been reported in seed germination, cell development of the hypocotyls and plant height [50,51,52,53,54]. The miR528 and its target gene DWARF3 (D3) negatively regulate rice plant height by triggering a reduction of GA content and a significant increase in ABA accumulation in transgenic plants [55]. Overexpression of LeNCED1 limited biomass accumulation increased ABA concentration and prevented the induction of genes in ABA metabolism and GA deactivation [42]. GA20−oxidases (GA20oxs) that produce GA precursors, GA3−oxidases (GA3oxs) that produce bioactive GAs, and GA2−oxidases (GA2oxs) that deactivate precursors and bioactive GAs, were kay enzymes of the GA biosynthesis pathway [56,57]. GA20oxs are known to affect cell division and cell expansion, resulting in larger plants [58,59]. GA3ox1 and GA3ox2, which encode a GA3 beta-hydroxylase in GA biosynthesis, are significantly associated with cell lengths and plant height [60,61]. GA2oxs regulate plant growth by regulating endogenous bioactive Gas [62,63]. In the GA signal transduction pathway, the gibberellin receptor GIBBERELLIN INSENSITIVE DWAR (GID) was a putative candidate gene controlling plant height [64,65,66,67]. Interactions between GID1 and DELLAs mediated the GA signalling in land plants [68,69]. The plant-specific gibberellic acid-stimulated Arabidopsis (GASA) gene family plays roles in hormone response, promoted seedling germination and root extension, and plant development [70]. In this study, the expression of genes in GA biosynthesis and signal transduction pathways was downregulated in transgenic sweet potato (Figure 5B). Overexpression of IbNCED1 reduced the accumulation of GA3 and exogenous application of GA3 could rescue the dwarf phenotype (Table 1, Figure 6 and Figure S2). These results suggest that IbNCED1 regulates plant height and development by controlling the GA signalling pathway in transgenic sweet potato. All the analyses revealed that the occurrence of dwarfing in transgenic sweet potatoes with high ABA content was likely to be caused by the GA signalling pathway.
In wheat and rice, the Rht alleles were introduced to reduce plant height allowing the application of higher fertilizer rates to substantially increase grain yield [11]. The fertilizer rates of the dwarf transgenic sweet potato would impact the yield of storage roots. The main objective of future dwarf sweet potato research should be to optimize fertilizer rates.
4. Materials and Methods
4.1. Plant Material
Sweet potato cv. Jishu26 was used for isolation and expression analysis of the IbNCED1 gene. Sweet potato cv. Xushu22 was employed to characterize the function of IbNCED1.
4.2. Cloning and Sequence Analysis of IbNCED1
Total RNA from sweet potato cv. Jishu26 plants was extracted using the Trozol Up Kit (ET111, Transgen, Beijing, China). The first-strand cDNA was transcribed from the total RNA with the PrimeScriptTM RT reagent Kit with gDNA Eraser (PR047A, Takara, Beijing, China). Amino acid sequence alignment was analyzed using DNAMAN V6 software. The phylogenetic tree was constructed with MEGA 7.0 software with 1000 bootstrap replicates. The molecular weight and theoretical isoelectric point (pI) of IbNCED1 were calculated with ProtParam tool (https://web.expasy.org/protparam/) accessed on 9 May 2023.
4.3. Expression Analysis of IbNCED1
The transcript levels of IbNCED1 in leaf, stem and root tissues of the 4-week-old in vitro-grown plants and leaflet, leaf, stem, pencil root, and storage root tissues of the 80-day-old field-grown plants of Jishu26 were analyzed with qRT-PCR using SYBR Green Pro Taq HS kit (AG11701, ACCURATE BIOLOGY). Furthermore, the 4-week-old Jishu26 plants were stressed in Hoagland solution with 100 mM ABA and 100 mM GA, respectively, and sampled at 0, 3, 6, 12, and 24 h after stresses for analyzing the expression of IbNCED1. Ibactin (AY905538) was used to normalize the expression levels in sweet potato [71]. All the specific primers are shown in Supplementary Table S2.
4.4. Regeneration of the Transgenic Sweet Potato Plants
Embryogenic suspension cultures of sweetpotato cv. Xushu22 were prepared using MS medium with 2.0 mg L−1 2,4-D [72]. The overexpression vector pCAMBIA1301-IbNCED1 was introduced into the A. tumefaciens strain EHA105. The transformation and plant regeneration were performed as previously described [70]. The identification of the transgenic plants was conducted by PCR with specific primers (Supplementary Table S2). The expression levels of IbNCED1 in the in vitro-grown transgenic and WT plants were analyzed using specific primers designed in the non-conserved domain (Supplementary Table S2).
4.5. Plant Height Analysis
The phenotypic of the 4-week-old in vitro-grown transgenic sweet potato plants and WT cultured on MS medium and the 6-week-old plants grown in transplanting boxes in the greenhouse were analyzed. At least 5 plants were measured for plant height. For paraffin section, the stem tissues were collected from WT and transgenic lines. The methods of paraffin section are dissected as described by Fang et al. (2021) [73]. At least 20 cells were measured in length.
4.6. RNA-Sequencing and Hormone Analysis
Due to the dwarf phenotype, total RNA was extracted from 4-week-old in vitro-grown sweet potato plant Xushu22 (WT) and transgenic lines L2 (OE) using a plant RNA kit (DP441, TIANGEN). The sequencing library was constructed using Ultra RNA sample preparation kit (Illumina) and then sequenced using an Illumina HiSeq 2500 according to the standard method (Illumina). Total reads were mapped to the I. Trifida genome (Sweet potato). Differentially expressed genes were identified using Cuffdiff with default criteria (fold change >1.5) and adjusted false discovery rate (p value < 0.05). Three independent biological replicates were used for the RNA-sequencing analysis. Analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was conducted according to database instructions (KEGG PATHWAY Database). The gene expression patterns were graphically represented in a heat map by cluster analysis using TBtools software (version number 1.108). The hormone contents of 4-week-old in vitro-grown WT and transgenic lines L2 plants were determined using high-performance liquid chromatography (HPLC).
4.7. Exogenous GA3 Treatment Analysis
In order to investigate the effect of GA3 on plants, the in vitro-grown transgenic and WT plants were cultured on MS medium with 0 (control), 5, 10, 20, 30, and 50 ng L−1 GA3 for 4 weeks. Furthermore, we measured the plant height of the in vitro-grown transgenic and WT plants culturing on MS medium with 0 (control) and 10 ng L−1 GA3 for 6 weeks.
4.8. Statistical Analysis
For cell length, at least 20 biological replicates were analysed. Data were presented as the mean ± SE and analyzed using Student’s t-test (two-tailed analysis). For biochemical and molecular biology analysis, all experiments were donefor at least three biological replicates. Significance levels at p < 0.05 and p < 0.01 are denoted by * (or different small letters) and **, respectively.
5. Conclusions
A novel 9-cis-epoxycarotenoid dioxygenase gene, IbNCED1, was isolated and characterized from sweet potato. Its overexpression in sweet potato led to a semi-dwarf phenotype, increased contents of ABA, decreased levels of GA3, and downregulated gene expression of the GA3 signal transduction pathway. IbNCED1 overexpression reduced sensitivity to GA3, and exogenous GA3 treatment rescued the dwarfism phenotype. It is suggested that IbNCED1 regulates plant height by the ABA and GA signalling pathways in transgenic sweet potato.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310421/s1.
Author Contributions
Y.Z., Q.W. and F.H. conceived and designed the experiments. Y.Z., C.Z. and T.D. performed the experiments. Y.Z., A.L. and Z.Q. analysed the data. Y.Z., C.Z., T.D., A.L., Z.Q., L.Z., S.D., Q.W. and F.H. contributed reagents, materials and analysis tools. Y.Z., Q.W. and F.H. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the China Agriculture Research System of Sweet potato (CARS-10-B06 and CARS-10-GW08), Taishan industry leading talents project (LJNY202002, LJNY202113), the Agricultural Seed Project of Shandong Province (2020LZGC004), and the Agricultural scientific and technological innovation project of Shandong Academy of Agricultural Sciences (CXGC2022E01).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.El Sheikha A.F., Ray R.C. Potential impacts of bioprocessing of sweet potato: Review. Crit. Rev. Food Sci. Nutr. 2017;57:455–471. doi: 10.1080/10408398.2014.960909. [DOI] [PubMed] [Google Scholar]
- 2.Escobar-Puentes A.A., Palomo I., Rodriguez L., Fuentes E., Villegas-Ochoa M.A., Gonzalez-Aguilar G.A., Olivas-Aguirre F.J., Wall-Medrano A. Sweet potato (Ipomoea batatas L.) phenotypes: From agroindustry to health effects. Foods. 2022;11:1058. doi: 10.3390/foods11071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gobena T.L., Asemie M.M., Firisa T.B. Evaluation of released sweet potato [Ipomoea batatas (L.) Lam] varieties for yield and yield-related attributes in Semen-Bench district of Bench-Sheko-Zone, South-Western Ethiopia. Heliyon. 2022;8:e10950. doi: 10.1016/j.heliyon.2022.e10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alqudah A.M., Koppolu R., Wolde G.M., Graner A., Schnurbusch T. The genetic architecture of barley plant stature. Front. Genet. 2016;7:117. doi: 10.3389/fgene.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wurschum T., Langer S.M., Longin C.F. Genetic control of plant height in European winter wheat cultivars. Theor. Appl. Genet. 2015;128:865–874. doi: 10.1007/s00122-015-2476-2. [DOI] [PubMed] [Google Scholar]
- 6.Mo Y., Vanzetti L.S., Hale I., Spagnolo E.J., Guidobaldi F., Al-Oboudi J., Odle N., Pearce S., Helguera M., Dubcovsky J. Identification and characterization of Rht25, a locus on chromosome arm 6AS affecting wheat plant height, heading time, and spike development. Theor. Appl. Genet. 2018;131:2021–2035. doi: 10.1007/s00122-018-3130-6. [DOI] [PubMed] [Google Scholar]
- 7.Greene A.D., Reay-Jones F., Kirk K.R., Peoples B.K., Greene J.K. Spatial associations of key lepidopteran pests with defoliation, NDVI, and plant height in soybean. Environ. Entomol. 2021;50:1378–1392. doi: 10.1093/ee/nvab098. [DOI] [PubMed] [Google Scholar]
- 8.Hassan M.A., Yang M., Fu L., Rasheed A., Zheng B., Xia X., Xiao Y., He Z. Accuracy assessment of plant height using an unmanned aerial vehicle for quantitative genomic analysis in bread wheat. Plant Methods. 2019;15:37. doi: 10.1186/s13007-019-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sera B. Simple traits among diaspore weight/number, plant height and ability of vegetative propagation. J. Integr. Plant Biol. 2008;50:1563–1569. doi: 10.1111/j.1744-7909.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 10.Trini J., Maurer H.P., Neuweiler J.E., Wurschum T. Identification and fine-mapping of quantitative trait loci controlling plant height in central european winter Triticale (×Triticosecale Wittmack) Plants. 2021;10:1592. doi: 10.3390/plants10081592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedden P. The genes of the Green Revolution. Trends Genet. 2003;19:5–9. doi: 10.1016/S0168-9525(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 12.Borojevic K., Borojevic K. The transfer and history of “reduced height genes” (Rht) in wheat from Japan to Europe. J. Hered. 2005;96:455–459. doi: 10.1093/jhered/esi060. [DOI] [PubMed] [Google Scholar]
- 13.Van De Velde K., Thomas S.G., Heyse F., Kaspar R., Van Der Straeten D., Rohde A. N-terminal truncated RHT-1 proteins generated by translational reinitiation cause semi-dwarfing of wheat Green Revolution alleles. Mol. Plant. 2021;14:679–687. doi: 10.1016/j.molp.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Olszewski N., Sun T.P., Gubler F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell. 2002;14((Suppl. S1)):S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel R. Gibberellin signaling in plants: Entry of a new MicroRNA player. Plant Physiol. 2020;183:5–6. doi: 10.1104/pp.20.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedden P. A novel gibberellin promotes seedling establishment. Nat. Plants. 2019;5:459–460. doi: 10.1038/s41477-019-0427-7. [DOI] [PubMed] [Google Scholar]
- 17.Hedden P., Sponsel V. A century of gibberellin research. J. Plant Growth Regul. 2015;34:740–760. doi: 10.1007/s00344-015-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binenbaum J., Weinstain R., Shani E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018;23:410–421. doi: 10.1016/j.tplants.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Ni Z., Yao Y., Nie X., Sun Q. Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.) BMC Genet. 2007;8:40. doi: 10.1186/1471-2156-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong J., Peng Z., Peng Q., Cai Q., Peng W., Chen M., Yao J. Regulation of plant height in rice by the polycomb group genes OsEMF2b, OsFIE2 and OsCLF. Plant Sci. 2018;267:157–167. doi: 10.1016/j.plantsci.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Moon S., Jang S., Lee S., An G., Jung K.H., Park S.K. OsbHLH073 negatively regulates internode elongation and plant height by modulating GA homeostasis in rice. Plants. 2020;9:547. doi: 10.3390/plants9040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S., Wang Y. Harnessing hormone gibberellin knowledge for plant height regulation. Plant Cell Rep. 2022;41:1945–1953. doi: 10.1007/s00299-022-02904-8. [DOI] [PubMed] [Google Scholar]
- 23.Siekmann D., Jansen G., Zaar A., Kilian A., Fromme F.J., Hackauf B. A genome-wide association study pinpoints quantitative trait genes for plant height, heading date, grain quality, and yield in rye (Secale cereale L.) Front. Plant Sci. 2021;12:718081. doi: 10.3389/fpls.2021.718081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Z.M., Jin Y.M., Wu T., Hu L.J., Zhang Y., Jiang W.Z., Du X.L. OsDREB2B, an AP2/ERF transcription factor, negatively regulates plant height by conferring GA metabolism in rice. Front. Plant Sci. 2022;13:1007811. doi: 10.3389/fpls.2022.1007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saidou M., Zhang Z.Y. The L-Type Lectin-like receptor kinase gene TaLecRK-IV.1 regulates the plant height in wheat. Int. J. Mol. Sci. 2022;23:8208. doi: 10.3390/ijms23158208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X.L., Xia X.C., Xu D.G., Liu Y.Q., Xie L., Hassan M.A., Song J., Li F.J., Wang D.S., Zhang Y., et al. Rht24b, an ancient variation of TaGA2ox-A9, reduces plant height without yield penalty in wheat. New Phytol. 2022;233:738–750. doi: 10.1111/nph.17808. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X., Ding L., Song A.P., Li S., Liu J.Y., Zhao W.Q., Jia D.W., Guan Y.X., Zhao K.K., Chen S.M., et al. DWARF and Robust Plant regulates plant height via modulating gibberellin biosynthesis in chrysanthemum. Plant Physiol. 2022;190:2484–2500. doi: 10.1093/plphys/kiac437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss D., Ori N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007;144:1240–1246. doi: 10.1104/pp.107.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abley K., Formosa-Jordan P., Tavares H., Chan E.Y., Afsharinafar M., Leyser O., Locke J.C. An ABA-GA bistable switch can account for natural variation in the variability of Arabidopsis seed germination time. Elife. 2021;10:e59485. doi: 10.7554/eLife.59485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lando A.P., Viana W.G., Vale E.M., Santos M., Silveira V., Steiner N. Cellular alteration and differential protein profile explain effects of GA3 and ABA and their inhibitor on Trichocline catharinensis (Asteraceae) seed germination. Physiol. Plant. 2020;169:258–275. doi: 10.1111/ppl.13076. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z.L., Powers S.J., Gong F., Phillips A.L., Hedden P., Sun T.P., et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Cadenas A., Zentella R., Walker-Simmons M.K., Ho T.H. Gibberellin/abscisic acid antagonism in barley aleurone cells: Site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. Plant Cell. 2001;13:667–679. doi: 10.1105/tpc.13.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achard P., Herr A., Baulcombe D.C., Harberd N.P. Modulation of floral development by a gibberellin-regulated microRNA. Development. 2004;131:3357–3365. doi: 10.1242/dev.01206. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z., Zhang Z.L., Zou X.L., Yang G.X., Komatsu S., Shen Q.X.J. Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J. 2006;46:231–242. doi: 10.1111/j.1365-313X.2006.02694.x. [DOI] [PubMed] [Google Scholar]
- 35.Lang J., Fu Y.X., Zhou Y., Cheng M.P., Deng M., Li M.L., Zhu T.T., Yang J., Guo X.J., Gui L.X., et al. Myb10-D confers PHS-3D resistance to pre-harvest sprouting by regulating NCED in ABA biosynthesis pathway of wheat. New Phytol. 2021;230:1940–1952. doi: 10.1111/nph.17312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García M.N.M., Stritzler M., Capiati D.A. Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta. 2014;239:615–631. doi: 10.1007/s00425-013-2001-2. [DOI] [PubMed] [Google Scholar]
- 37.Chen K., Li G.J., Bressan R.A., Song C.P., Zhu J.K., Zhao Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020;62:25–54. doi: 10.1111/jipb.12899. [DOI] [PubMed] [Google Scholar]
- 38.Iuchi S., Kobayashi M., Taji T., Naramoto M., Seki M., Kato T., Tabata S., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 39.He R.R., Zhuang Y., Cai Y.M., Aguero C.B., Liu S.L., Wu J., Deng S.H., Walker M.A., Lu J., Zhang Y.L. Overexpression of 9-cis-epoxycarotenoid dioxygenase cisgene in grapevine increases drought tolerance and results in pleiotropic effects. Front. Plant Sci. 2018;9:970. doi: 10.3389/fpls.2018.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y., Guo Y.M., Liu Y.T., Zhang F., Wang Z.K., Wang H.Y., Wang F., Li D.P., Mao D.D., Luan S., et al. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front. Plant Sci. 2018;9:162. doi: 10.3389/fpls.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y., Jiao Y., Xie N.K., Guo Y.M., Zhang F., Xiang Z.P., Wang R., Wang F., Gao Q.M., Tian L.F., et al. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019;287:110188. doi: 10.1016/j.plantsci.2019.110188. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Andujar C., Martinez-Perez A., Ferrandez-Ayela A., Albacete A., Martinez-Melgarejo P.A., Dodd I.C., Thompson A.J., Perez-Perez J.M., Perez-Alfocea F. Impact of overexpression of 9-cis-epoxycarotenoid dioxygenase on growth and gene expression under salinity stress. Plant Sci. 2020;295:110268. doi: 10.1016/j.plantsci.2019.110268. [DOI] [PubMed] [Google Scholar]
- 43.Iuchi S., Kobayashi M., Yamaguchi-Shinozaki K., Shinozaki K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal P., Balyan H.S., Gupta P.K. Identification of modifiers of the plant height in wheat using an induced dwarf mutant controlled by RhtB4c allele. Physiol. Mol. Biol. Plants. 2020;26:2283–2289. doi: 10.1007/s12298-020-00904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teng F., Zhai L.H., Liu R.X., Bai W., Wang L.Q., Huo D.G., Tao Y.S., Zheng Y.L., Zhang Z.X. ZmGA3ox2, a candidate gene for a major QTL, qPH3.1, for plant height in maize. Plant J. 2013;73:405–416. doi: 10.1111/tpj.12038. [DOI] [PubMed] [Google Scholar]
- 46.Bhat J.A., Karikari B., Adeboye K.A., Ganie S.A., Barmukh R., Hu D., Varshney R.K., Yu D. Identification of superior haplotypes in a diverse natural population for breeding desirable plant height in soybean. Theor. Appl. Genet. 2022;135:2407–2422. doi: 10.1007/s00122-022-04120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pei X.X., Wang X.Y., Fu G.Y., Chen B.J., Nazir M.F., Pan Z., He S.P., Du X.M. Identification and functional analysis of 9-cis-epoxy carotenoid dioxygenase (NCED) homologs in G. hirsutum. Int. J. Biol. Macromol. 2021;182:298–310. doi: 10.1016/j.ijbiomac.2021.03.154. [DOI] [PubMed] [Google Scholar]
- 48.Frey A., Effroy D., Lefebvre V., Seo M., Perreau F., Berger A., Sechet J., To A., North H.M., Marion-Poll A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012;70:501–512. doi: 10.1111/j.1365-313X.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- 49.Behnam B., Iuchi S., Fujita M., Fujita Y., Takasaki H., Osakabe Y., Yamaguchi-Shinozaki K., Kobayashi M., Shinozaki K. Characterization of the promoter region of an Arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013;20:315–324. doi: 10.1093/dnares/dst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S.A., Jang S., Yoon E.K., Heo J.O., Chang K.S., Choi J.W., Dhar S., Kim G., Choe J.E., Heo J.B., et al. Interplay between ABA and GA modulates the timing of asymmetric cell divisions in the Arabidopsis root ground tissue. Mol. Plant. 2016;9:870–884. doi: 10.1016/j.molp.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Murcia G., Fontana A., Pontin M., Baraldi R., Bertazza G., Piccoli P.N. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry. 2017;135:34–52. doi: 10.1016/j.phytochem.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Kozaki A., Aoyanagi T. Molecular aspects of seed development controlled by gibberellins and abscisic acids. Int. J. Mol. Sci. 2022;23:1876. doi: 10.3390/ijms23031876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuan P.A., Kumar R., Rehal P.K., Toora P.K., Ayele B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018;9:668. doi: 10.3389/fpls.2018.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golldack D., Li C., Mohan H., Probst N. Gibberellins and abscisic acid signal crosstalk: Living and developing under unfavorable conditions. Plant Cell Rep. 2013;32:1007–1016. doi: 10.1007/s00299-013-1409-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Liu X., Wang M., Xie L.J., Wu Z.X., Yu J.M., Wang Y.C., Zhang Z.Q., Jia Y.F., Liu Q.P. The miR528-D3 module regulates plant height in rice by modulating the gibberellin and abscisic acid metabolisms. Rice. 2022;15:27. doi: 10.1186/s12284-022-00575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katyayini N.U., Rinne P., Tarkowska D., Strnad M., van der Schoot C. Dual role of gibberellin in perennial shoot branching: Inhibition and activation. Front. Plant Sci. 2020;11:736. doi: 10.3389/fpls.2020.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearce S., Huttly A.K., Prosser I.M., Li Y.D., Vaughan S.P., Gallova B., Patil A., Coghill J.A., Dubcovsky J., Hedden P., et al. Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biol. 2015;15:130. doi: 10.1186/s12870-015-0520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voorend W., Nelissen H., Vanholme R., De Vliegher A., Van Breusegem F., Boerjan W., Roldan-Ruiz I., Muylle H., Inze D. Overexpression of GA20-OXIDASE1 impacts plant height, biomass allocation and saccharification efficiency in maize. Plant Biotechnol. J. 2016;14:997–1007. doi: 10.1111/pbi.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filo J., Wu A., Eliason E., Richardson T., Thines B.C., Harmon F.G. Gibberellin driven growth in elf3 mutants requires PIF4 and PIF5. Plant Signal. Behav. 2015;10:e992707. doi: 10.4161/15592324.2014.992707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh H., Ueguchi-Tanaka M., Sentoku N., Kitano H., Matsuoka M., Kobayashi M. Cloning and functional analysis of two gibberellin 3 beta-hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl. Acad. Sci. USA. 2001;98:8909–8914. doi: 10.1073/pnas.141239398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mares D., Derkx A., Cheong J., Zaharia I., Asenstorfer R., Mrva K. Gibberellins in developing wheat grains and their relationship to late maturity alpha-amylase (LMA) Planta. 2022;255:119. doi: 10.1007/s00425-022-03899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo S.F., Yang S.Y., Chen K.T., Hsing Y.I., Zeevaart J.A., Chen L.J., Yu S.M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell. 2008;20:2603–2618. doi: 10.1105/tpc.108.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C., Ma J.J., Zhang Y.H., Yang S.X., Feng X.Z., Yan J. The miR166 mediated regulatory module controls plant height by regulating gibberellic acid biosynthesis and catabolism in soybean. J. Integr. Plant Biol. 2022;64:995–1006. doi: 10.1111/jipb.13253. [DOI] [PubMed] [Google Scholar]
- 64.Liu X.L., Yang W.C., Wang J., Yang M.X., Wei K., Liu X.Y., Qiu Z.K., van Giang T., Wang X.X., Guo Y.M., et al. SlGID1a is a putative candidate gene for qtph1.1, a major-effect quantitative trait locus controlling tomato plant height. Front. Genet. 2020;11:881. doi: 10.3389/fgene.2020.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gazara R.K., Moharana K.C., Bellieny-Rabelo D., Venancio T.M. Expansion and diversification of the gibberellin receptor gibberellin insensitive dwarf1 (GID1) family in land plants. Plant Mol. Biol. 2018;97:435–449. doi: 10.1007/s11103-018-0750-9. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida H., Tanimoto E., Hirai T., Miyanoiri Y., Mitani R., Kawamura M., Takeda M., Takehara S., Hirano K., Kainosho M., et al. Evolution and diversification of the plant gibberellin receptor GID1. Proc. Natl. Acad. Sci. USA. 2018;115:E7844–E7853. doi: 10.1073/pnas.1806040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueguchi-Tanaka M., Nakajima M., Motoyuki A., Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 2007;58:183–198. doi: 10.1146/annurev.arplant.58.032806.103830. [DOI] [PubMed] [Google Scholar]
- 68.Ito T., Okada K., Fukazawa J., Takahashi Y. DELLA-dependent and -independent gibberellin signaling. Plant Signal. Behav. 2018;13:e1445933. doi: 10.1080/15592324.2018.1445933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daviere J.M., Wild M., Regnault T., Baumberger N., Eisler H., Genschik P., Achard P. Class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr. Biol. 2014;24:1923–1928. doi: 10.1016/j.cub.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Roxrud I., Lid S.E., Fletcher J.C., Schmidt E.D., Opsahl-Sorteberg H.G. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 2007;48:471–483. doi: 10.1093/pcp/pcm016. [DOI] [PubMed] [Google Scholar]
- 71.Liu D.G., Wang L.J., Zhai H., Song X.J., He S.Z., Liu Q.C. A novel alpha/beta-hydrolase gene IbMas enhances salt tolerance in transgenic sweet potato. PLoS ONE. 2014;9:e115128. doi: 10.1371/journal.pone.0115128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Q.C., Zhai H., Wang Y., Zhang D.P. Efficient plant regeneration from embryogenic suspension cultures of sweet potato. Vitr. Cell. Dev. Biol.-Plant. 2001;37:564–567. doi: 10.1007/s11627-001-0098-7. [DOI] [Google Scholar]
- 73.Fang X., Bo C., Wang M.J., Yuan H.T., Li W., Chen H.W., Ma Q., Cai R.H. Overexpression of the maize WRKY114 gene in transgenic rice reduce plant height by regulating the biosynthesis of GA. Plant Signal. Behav. 2021;16:1967635. doi: 10.1080/15592324.2021.1967635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.