Abstract
Heart failure is the leading cause of morbidity and mortality and currently affects more than 60 million people worldwide. A key feature in the pathogenesis of almost all forms of heart failure is cardiac fibrosis, which is characterized by excessive accumulation of extracellular matrix components in the heart. Although cardiac fibrosis is beneficial in the short term after acute myocardial injury to preserve the structural and functional integrity of the heart, persistent cardiac fibrosis contributes to pathological cardiac remodeling, leading to mechanical and electrical dysfunction of the heart. Despite its high prevalence, standard therapies specifically targeting cardiac fibrosis are not yet available. Cell-based approaches have been extensively studied as potential treatments for cardiac fibrosis, but several challenges have been identified during clinical translation. The observation that extracellular vesicles (EVs) derived from stem and progenitor cells exhibit some of the therapeutic effects of the parent cells has paved the way to overcome limitations associated with cell therapy. However, to make EV-based products a reality, standardized methods for EV production, isolation, characterization, and storage must be established, along with concrete evidence of their safety and efficacy in clinical trials. This article discusses EVs as novel therapeutics for cardiac fibrosis from a translational perspective.
Keywords: heart failure, cardiac fibrosis, extracellular vesicles, therapy, clinical translation
1. Introduction
The cardiovascular field is in desperate need of translational success stories. Despite significant developments in pharmacological and device-based therapies to preserve cardiac output and delay disease progression in heart failure patients, there remains an enormous medical and financial burden, and innovative treatment strategies are urgently awaited [1]. Over the past twenty years, thousands of peer-reviewed articles and hundreds of pre-clinical and clinical trials have been published in the search for curative therapy for heart failure. However, they all have one thing in common: none of them have resulted in a clinical-grade product approved by a major regulatory authority.
A central factor in the progression from acute myocardial infarction to chronic or terminal heart failure is cardiac fibrosis, which is characterized by extensive remodeling of the myocardial extracellular matrix (ECM) [2]. Although this mechanism is essential to maintain the structural and functional integrity of the damaged heart, unrestrained cardiac fibrosis can result in tissue stiffening and decreased ventricular filling and contraction, ultimately contributing to the development of heart failure, arrhythmia, and sudden cardiac death [3,4]. Conventional therapies, such as renin–angiotensin–aldosterone system inhibitors and β-blockers, have been shown to effectively reduce ECM protein deposition in the injured myocardium, but they do not completely prevent the progression of cardiac fibrosis in patients with heart failure [5]. Unfortunately, to the best of our knowledge, there are currently no approved therapies that specifically and effectively target cardiac fibrosis. Barriers to the development of treatments specific to cardiac fibrosis include (i) the molecular mechanisms underlying cardiac fibrosis, which are complex and not fully understood yet [6], (ii) the limited regenerative potential of the adult human heart after myocardial infarction, which does not allow complete inhibition of cardiac fibrosis; otherwise, there is a high risk of cardiac rupture [7], (iii) the volatile microenvironment in the injured heart, which is associated with increased levels of inflammation and cell proliferation, may compromise efficient delivery of therapeutics [8], and (iv) the scarcity of suitable in vitro and in vivo models that robustly recapitulate cardiac fibrosis in humans.
In recent decades, cell-based approaches have been proposed as promising strategies to alleviate excessive cardiac fibrosis and improve heart function, but their clinical translation is complicated. Major challenges include the induction of innate or adaptive immune responses, the potential for tumor formation, and the low survival rate of transplanted cells at the targeted site [9,10]. Recognizing that the effect of administered stem/progenitor cells in myocardial injury is primarily mediated by the release of extracellular vesicles (EVs), EVs have attracted increasing attention due to their significant advantages in terms of stability, biocompatibility, and regulatory aspects [11]. The aim of this study is to outline the current prospects and challenges associated with cell-free EV-based products for the treatment of cardiac fibrosis, particularly from the perspective of clinical translation.
2. How Are EVs Defined and Where Do We Stand in the Regulatory Landscape?
The term EV, as coined by the International Society of Extracellular Vesicles (ISEV), includes all extracellular membrane-enclosed vesicles [12,13]. Structurally, EVs are nanoscale cell-derived particles with a lipid bilayer membrane that are secreted by most mammalian cells under both physiological and pathological conditions [14]. They can contain hundreds or thousands of bioactive molecules, including proteins, metabolites, lipids, and nucleic acids [15]. Currently, the EV field is one of the fastest-growing scientific areas, with more than 19,000 EV-focused articles found on PubMed, of which more than 80% have been published in the last 5 years. In addition, Clinicaltrials.gov lists more than 150 entries with “extracellular vesicles” as the search term. Due to their natural origin, EVs have several desirable properties, such as low immunogenicity and toxicity, high stability, excellent biocompatibility, inability to self-replicate, flexibility in dosing, and feasibility for pre- and post-isolation modification [16,17,18,19,20]. They can also overcome many of the limitations associated with current drug delivery systems, as they can cross biological barriers, travel long distances in body fluids, and deliver their cargo directly into the cytosol of recipient cells via membrane fusion and endocytosis [21]. In addition, EVs can be stored frozen for long periods of time to be available for immediate use in patients without significant loss of functional activity [22,23].
In order to successfully translate EV-based therapeutics to clinical practice, their quality, safety, and efficacy must be demonstrated, as is the case for any medicinal product. From a regulatory perspective, according to the guidelines of the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA), the classification of EVs depends on the specific therapeutic cargo they carry. In Europe, EVs are considered biologics if they are purified from non-modified cells or genetically engineered cells, but where the vesicles contain only functional transgenic protein. In contrast, when EVs are used as a delivery system for functional transgenic RNA with an intended therapeutic function in the patient, such a product is classified as an advanced therapy medicinal product and is subject to additional regulatory requirements [24]. The same criteria would apply in the United States [25]. Remarkably, while the EMA typically requires knowledge of a drug’s mechanism of action as part of the approval process [26], the FDA does not mandate such an understanding, only safety and some level of efficacy, meaning that entering direct clinical trials without this knowledge may be a potential path forward for EVs. However, a lack of understanding of EV-based products could lead to adverse outcomes, off-target effects, or ineffective dosing, which may explain why there is currently no EMA/FDA-approved clinical product containing eukaryotic EVs.
3. How Can EV-Based Products Interrupt Cardiac Fibrosis?
In general, fibrotic processes in the heart can be broadly divided into two categories: reactive and reparative fibrosis [27]. Reactive fibrosis describes the excessive accumulation of ECM components in the interstitial or perivascular spaces, triggered by pressure or volume overload or other pathological stimuli, and can result in impaired relaxation and filling of the heart ventricles after contraction [28]. Reparative fibrosis is classically associated with acute myocardial injury, in which damaged cardiomyocytes are replaced by ECM components to prevent cardiac rupture while maintaining the contractile function of the heart [29,30]. Consequently, EV-based products that induce complete suppression of cardiac fibrosis in patients with acute myocardial infarction may lead to serious side effects, such as ventricular aneurysms or fatal heart rupture. In these patients, the initial cardiac fibrotic response in the infarct area is necessary, but avoiding cardiac fibrosis in the infarct border zone and the surrounding or even distant myocardial tissue is a critical step in preventing subsequent heart failure [31].
At the cellular and molecular levels, there are a number of signaling pathways and mediators involved in cardiac fibrosis that may provide suitable therapeutic targets for the treatment of heart failure, as reviewed elsewhere [5,32,33,34,35]. Overall, although disease progression is complex, dynamic, and patient-specific, immune responses and immune cell-mediated activation of cardiac fibroblasts are usually the first steps in initiating the ECM remodeling process after myocardial infarction. In detail, in response to heart injury, cardiomyocytes undergo apoptosis and release DNA and cellular proteins into the extracellular space that serve as damage-associated molecular patterns [36]. These signals are sensed by innate immune cells, which in turn produce and secrete a variety of pro-fibrotic factors to trigger fibroblast activation. Activated cardiac fibroblasts, referred to as myofibroblasts, are the central cellular effectors in cardiac fibrosis and are characterized by excessive production of ECM components and their smooth muscle cell-like contractile properties obtained by de novo synthesis of alpha-smooth muscle actin-containing stress fibers [37]. Intriguingly, recent studies have shown that different cardiac fibroblast subtypes are present in diseased tissue and undergo temporal variation at the time of injury [38,39,40,41,42]. For example, Ruiz-Villalba et al. identified a unique subset of cardiac fibroblasts that express high levels of collagen triple helix repeat containing 1 after myocardial infarction in mice [41], and Fu et al. have described a subpopulation of cardiac fibroblasts, the matrifibrocytes, that support the mature scar [42]. In addition to cardiac fibroblasts, a growing body of evidence suggests that macrophages are also key mediators of cardiac repair, playing a critical role in orchestrating pro-inflammatory processes immediately after injury (macrophage M1 phenotype) and participating in tissue remodeling by stimulating cardiac fibroblast activation (macrophage M2 phenotype) [43,44]. A fine regulation between the M1 and M2 subtypes is required to achieve a proper resolution of the initial inflammatory response and ensure effective cardiac remodeling. In the context of cardiac fibrosis therapy, EV-based products could act either early after myocardial infarction by stimulating pro-inflammatory M1 macrophages to differentiate into an anti-inflammatory M2 macrophage-like phenotype to attenuate chronic inflammation or at a later time by reducing pro-fibrotic M2 macrophages to alleviate progressive cardiac fibrosis [45,46]. In general, to be reasonable candidates for the treatment of cardiac fibrosis in clinical practice, we propose that EV-based products should meet at least some of the requirements listed in Table 1. Certain aspects have already been demonstrated, for example, for EVs derived from mesenchymal stromal cells [47,48,49,50,51,52]; however, due to our limited understanding of EV properties, it is currently challenging to satisfy all of these aspirations.
Table 1.
Proposed features of EV-based therapy for the treatment of cardiac fibrosis.
| (i) | Priming of immune cell phagocytic signaling for efficient clearance of dead cells |
| (ii) | Limiting cardiac fibrosis by reducing collagen deposition in the myocardium |
| (iii) | Inhibiting pro-fibrotic factors and their receptors |
| (iv) | Reduction in myofibroblast formation in the heart |
| (v) | Direct degradation of the fibrotic ECM in the myocardium |
| (vi) | Cardioprotection by reducing apoptosis of cardiomyocytes and other cell types |
| (vii) | Promotion of blood flow recovery by increasing microvascular density |
| (viii) | Improvement of cardiac function |
Among the variety of molecules encapsulated in EVs that can modulate cardiac fibrosis, regulatory microRNAs (miRs) have been of particular interest in recent years. For example, mesenchymal stromal cell-derived EVs carrying miR-19a, miR-22, miR-29, miR-133, and miR-210 have been shown to reduce cardiac fibrosis during heart regeneration and repair in pre-clinical trials [53,54,55]. Similarly, Ibrahim et al. reported that cardiosphere-derived EVs with enhanced levels of miR-92a attenuated cardiac fibrosis and improved survival in a mouse model of myocardial infarction [56]. However, unlike traditional pharmacological interventions that use single molecules with limited mechanisms of action, EVs deliver not just one miR, but a cocktail of multiple miRs that affect specific cells and tissues in numerous and coordinated ways. Therefore, a deeper understanding of their mechanism of action, potential targets, and possible side effects is desirable prior to the clinical implementation of EV-based products.
4. How Can the Therapeutic Efficacy of EV-Based Products Be Measured?
When evaluating the therapeutic efficacy of EV-based products for the treatment of cardiac fibrosis, a major challenge in translating clinical trials into practice is the reliance on so-called surrogate endpoints, such as a significant reduction in infarct size [57]. Other measurable parameters used as surrogate endpoints include left ventricular function, perfusion defects, patient functional status, and quality of life [58,59]. However, while they may not always be reliable indicators of more definitive endpoints, such as mortality, they can at least provide early insight into the therapeutic efficacy of EVs and streamline their development.
The current gold standard for the diagnosis and evaluation of diffuse cardiac fibrosis is endomyocardial biopsy [60]. However, the invasive nature of the procedure, which is uncomfortable and risky for the patient, has a propensity for sampling error, and is not able to quantify the fibrotic burden of the entire myocardium, limits its use in daily clinical practice. Instead, cardiac fibrosis is more commonly assessed non-invasively and indirectly by measuring cardiac function or by visualizing macroscopic changes in the heart using echocardiography, computed tomography, and cardiac magnetic resonance (CMR) imaging [61]. In particular, late gadolinium enhancement on CMR imaging is a powerful technique for locating and quantifying regions of reparative fibrosis in the heart [62,63,64,65]. However, it is an expensive method that requires considerable skill in image acquisition and analysis and often suffers from poor image quality due to heart rate fluctuations and gadolinium washout during the relatively long acquisition time [66]. More recently, with the advent of novel T1 mapping techniques, reliable assessment of reactive cardiac fibrosis using CMR imaging has become possible [67]. However, the lack of standardization, leading to difficulties in inter-center comparisons, is a major barrier to its widespread adoption [68]. Blood biomarkers, while useful in the early detection of heart failure, remain the only indirect tools for the assessment of cardiac fibrosis [69,70]. For example, elevated serum levels of carboxyl-terminal pro-peptide of type I collagen and amino-terminal pro-peptides of types I and III collagen indicate increased collagen turnover, a marker of fibrotic changes and cardiac repair [71,72]. Unfortunately, none of the routinely used techniques meets all the requirements to determine the degree of cardiac fibrosis and monitor changes after treatment and, therefore, a combination of histological staining, imaging, and biomarker studies is usually needed [73]. Furthermore, it is important to note that the above methods reflect an increase in ECM components rather than the main drivers of cardiac fibrosis: myofibroblasts. Among others, one of their characteristics is the expression of fibroblast activation protein (FAP), a membrane-anchored peptidase [74]. Recently, FAP-targeting radiotracers have been developed that reliably bind and stain FAP, making FAP-specific positron emission tomography-computed tomography a promising non-invasive imaging technique to measure relative FAP density [75,76,77].
In summary, there is still a need for safe, reliable, and most importantly, non-invasive tools for routine use to monitor the progression of cardiac fibrosis in general and evaluate changes after administration of EV-based products, in particular in order to assess their therapeutic efficacy.
5. How to Deliver High-Quality EV-Based Products?
Despite the increasing attention on EV-based therapeutics and their potential for the treatment of cardiac fibrosis, there are still some limitations that hinder their clinical translation. One of the biggest challenges is the lack of reliable technologies for the large-scale production of EVs under good manufacturing practice (GMP) conditions that allow for high batch-to-batch consistency, purity, and performance. International groups such as the ISEV have established guidelines and protocols for standardized practices, but to date, there is no uniform approach [78,79,80,81,82,83].
The first step in the EV manufacturing process is to select the origin of EVs, as they can be derived from either cellular or non-cellular (e.g., body fluids) sources [84,85]. Numerous studies, including clinical trials, have focused on EVs derived from native, unmodified cells, and mesenchymal stromal cell-derived EVs have been at the forefront of these studies [86,87]. Aspects of cell culture that may affect cell status include the type of culture system, the media and supplements used, and the culture condition parameters. Alterations can result in changes in cell state and growth, thus potentially affecting the composition and therapeutic efficacy of the derived EVs. Therefore, culture conditions as well as the metabolic activity and cell number should be monitored regularly, for example by cell viability and proliferation assays [88,89].
The second step is EV secretion by the cultured cells into the surrounding cell culture medium, which can be either spontaneous or induced [90]. Spontaneous EV production is chosen to preserve the basal characteristics of the cells. In this case, the entire cell culture medium is replaced by an EV-depleted medium to obtain only the EVs secreted by the target cells under physiological conditions. In contrast, for induced EV production, cell culture under serum starvation conditions is the simplest strategy to increase EV yield, but it may affect cell behavior and, consequently, EV composition and quality. Other methods include pH value change, temperature shift, hypoxia, and additives in the culture medium, as well as chemical induction and physical stimulation, as discussed elsewhere [90]. In any case, all approaches to induce EV secretion from cultured cells must be proven to be safe and GMP-compliant.
In the third step, EVs are isolated from the cell culture medium; however, there is no consensus on the optimal isolation strategy. In fact, different research laboratories use different protocols to isolate EVs, including, for example, differential ultracentrifugation, size exclusion chromatography, polymer-based precipitation, and immunoaffinity separation [91,92]. Each of these methods has its own advantages and limitations, and there is wide variability in efficiency and purity [93]. From a regulatory perspective, purity concerns are of paramount importance, but EV-based products cannot currently be produced in a completely pure form. The end product of standard isolation methods is only referred to as an EV-enriched preparation that contains other components, such as protein aggregates [94,95]. In addition, regardless of the isolation method, a layer of biomolecules may be adsorbed on the surface of EVs, the so-called corona [96,97]. The corona cloaks the surface of EVs and can cover surface receptors, subsequently affecting their interactions with recipient cells [98]. Although research groups have shown that additional purification of EVs significantly reduces the number of proteins and nucleic acids in EV preparations, corona remodeling may have a major impact on downstream biological effects and thus the efficacy of EV-based products [99].
After isolation, in-depth characterization of EV preparations is an important aspect to ensure safe and effective clinical translation. Currently, EVs are identified using multiple complementary methods to determine particle number, size, morphology, surface markers, functionality, and cargo composition. It is generally recommended to perform nanoparticle tracking analysis for particle quantification and size estimation [100]. To evaluate the structure and distinguish EVs from non-EV particles, transmission electron microscopy is currently the most used method [101]. In addition, it is recommended to demonstrate the presence of commonly reported EV markers such as transmembrane proteins (CD9, CD63, CD81), heat shock proteins (Hsp70, Hsp90), or membrane fusion proteins (Annexin, TSG101) using standard antibody-based techniques (e.g., Western blot, enzyme-linked immunosorbent assay, or flow cytometry) [102,103]. In order to demonstrate EV functionality, it is important to test its uptake into recipient cells, for example, by using fluorescently labeled EVs [104,105]. EV cargo profiling is a key strategy for understanding the effects of EVs. Technologies central to this effort include targeted and untargeted mass spectrometry, proteomics, lipidomics, and high-throughput RNA sequencing [106,107,108,109]. Future developments in machine learning may further advance their use.
Another important consideration in the development of EV-based products is their preservation and storage [23,110]. Although there are no standardized storage protocols available, studies have shown that storage of EVs at −80 °C in single-dose aliquots for up to 7 months does not affect their potency and activity [83,111].
In summary, due to the complexity of EVs, commercialization of large-scale manufacturing of EV-based therapeutics requires a technologically superior facility, a robust quality management system, and a GMP-compliant technology in order to deliver high-quality, well-characterized products to patients.
6. What Safety Issues Must Be Considered for EV-Based Products?
In addition to efficacy and quality aspects, safety is of paramount importance for the clinical implementation of EV-based products. In fact, in early clinical trials, when EVs are first used in humans, safety is the priority. Potential risks associated with EV-based therapeutics include (i) undesired distribution in the body, (ii) unwanted immune reactions, such as allergy and rejection, (iii) side effects of components other than EVs administered concomitantly, (iv) involvement in cancer progression and metastasis, especially when applied multiple times over a long period of time, (v) transmission of infectious diseases through microbial contamination of EV preparations, and (vi) variability in efficacy and quality.
Overall, there are two methods of EVs delivery: intramyocardial injection, which is efficient but invasive, and intravenous injection, which is less invasive but results in low cardiac retention. Previous research has shown that intravenously applied EVs have a limited half-life in the blood and are rapidly cleared in the liver, lungs, and spleen [112,113]. In fact, excessive retention of EVs in the liver not only affects their bioavailability but also increases the risk of developing liver damage. A better understanding of the pharmacokinetics and pharmacodynamics of EV-based products is key to their clinical translation but is hampered by the limitations of our current animal models. First, the animals used are typically young, whereas heart failure patients with cardiac fibrosis are older. Second, methods to detect EVs in tissues and organs require a substantial accumulation of fluorescent or radiolabeled EVs, which is a challenging task and may not correspond to physiological conditions. Third, the biodistribution of EVs varies depending on the isolation method used; for example, intravenously administered EVs isolated by ultracentrifugation plus liquid chromatography show less accumulation in the lungs than EVs isolated by ultracentrifugation alone [114]. Compared to intravenous delivery, intramyocardial administration of EVs may prolong the lifespan of EVs in the heart, but the procedure is more complex and carries a higher risk of complications.
Numerous studies have shown that EVs are unlikely to induce an immune response, but only a few research groups have thoroughly evaluated their potential for toxic or immunogenic effects. However, this is particularly important because therapeutic EVs, mostly derived from human cell lines, are initially tested in animal models throughout pre-clinical development. In one of the most thorough investigations into the immune response of human EVs in animal models, Zhu et al. showed in a mouse model that human embryonic kidney 293 T cell-derived EVs had no toxic effects, and immune markers were not significantly altered over a 3-week period [115].
Another safety issue with EVs is related to the lack of clarity about their cargo. A recent study of known miR targets has revealed that miRs found in mesenchymal stromal cell-derived EVs may also play a critical role in the tumor biology of various cancers [116]. Given that EVs have a half-life of less than 24 h [117], a single administration of EV-based products may not be sufficient to target cardiac fibrosis, and multiple doses would be required. However, this approach could lead to the accumulation of oncogenic miRs in patients with early-stage cancers that were not detected prior to treatment. It would, therefore, be important to screen patients in advance to avoid effects that could favor or even worsen existing tumors.
In addition to the characterization of EVs, the absence of microbial contamination is an important issue before EV-based products can be utilized in clinical settings. Due to the relatively small volume of EV preparations, filtration sterilization could be performed at the end of the isolation process [118], but standard measures to ensure product sterility are still lacking. Regarding viral safety, given the similarities between EVs and viral particles in terms of size and composition, EV-producing cells should be carefully monitored for a viral infection at the beginning of EV production, and EV preparations should be tested at all relevant manufacturing steps.
In conclusion, future studies should focus on conducting a thorough and long-term safety evaluation of EVs, which could also help to determine their safe and therapeutic doses for clinical use.
7. Conclusions
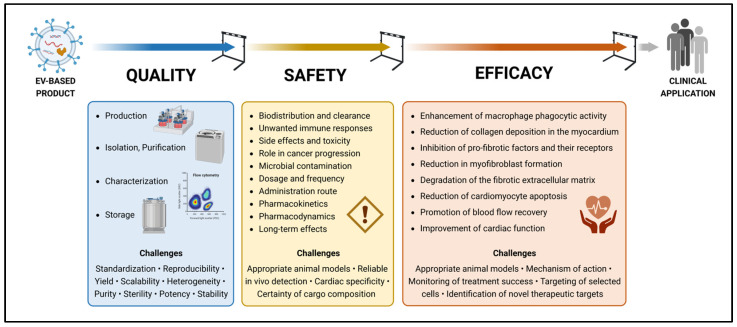
We believe that EVs represent the next frontier in cell-free therapy; however, this research is still in its infancy, and there is a long way to go before clinical application (Figure 1). One of the most critical challenges for EV-based therapy in heart failure patients is the cardiac specificity and retention of EVs. Most of the currently reported therapeutic effects of EVs on cardiac diseases are based on the direct administration of EVs into the myocardium or pericardial cavity, which is too invasive for routine clinical application. To achieve the therapeutic effects of EVs by intravenous administration, further research is needed to develop efficient EV delivery methods. In addition to that, there are more hurdles to overcome. First, standardized and quality-controlled GMP-compliant methods must be optimized on an industrial scale for the reproducible production of homogeneous EV-based therapeutics. Second, gold standards must be defined to characterize the composition and purity of EV preparations. However, given the difficulties in isolating a uniform EV population, a possible strategy would be to prioritize therapeutic efficacy over purity. Downgrading the regulation of regenerative medicine products could accelerate their clinical implementation, but it is imperative that their safety profile is fully evaluated. Third, appropriate pre-clinical in vivo models must be developed to optimize EV dosing, including the definition of appropriate timing and frequency of application. In particular, in vivo imaging of EVs is required to quantify the number of EVs delivered to damaged cardiac tissue. Fourth, innovative tools must be developed to monitor the progression of cardiac fibrosis after the administration of EV-based products. Fifth, novel therapeutic targets must be identified to selectively reduce or even reverse cardiac fibrosis without any side effects. Timely interdisciplinary studies are needed to address all these challenges. Otherwise, EVs will remain on the laboratory bench, where they show great promise in reducing cardiac fibrosis, and never make it to the bedside.
Figure 1.
Hurdles to clinical application of EV-based products for the treatment of cardiac fibrosis. Created with BioRender.com.
Author Contributions
Conceptualization, S.N. and T.Z.N.-S.; investigation, S.N.; literature research, S.N., M.R.E. and T.Z.N.-S.; writing—original draft preparation, S.N. and T.Z.N.-S.; writing—review and editing, S.N., M.R.E., M.Y.E. and T.Z.N.-S.; visualization, S.N.; supervision, M.Y.E. and T.Z.N.-S.; funding acquisition, S.N. and M.Y.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
We acknowledge financial support from the Open Access Publication Fund of Charité–Universitätsmedizin Berlin and the German Research Foundation (DFG). This work is part of a project funded by the DFG (project number 510857108).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc. Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frangogiannis N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Silva A.C., Pereira C., Fonseca A.C.R.G., Pinto-do-Ó P., Nascimento D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2020;8:621644. doi: 10.3389/fcell.2020.621644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L., Murphy A.J., Dart A.M. A Clinical Perspective of Anti-Fibrotic Therapies for Cardiovascular Disease. Front. Pharmacol. 2017;8:186. doi: 10.3389/fphar.2017.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y., Iyer R.P., Jung M., Czubryt M.P., Lindsey M.L. Cardiac Fibroblast Activation Post-Myocardial Infarction: Current Knowledge Gaps. Trends Pharmacol. Sci. 2017;38:448–458. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabé-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H., et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S., Qiu Y., Tao J. The challenges and optimization of cell-based therapy for cardiovascular disease. J. Transl. Intern. Med. 2021;9:234–238. doi: 10.2478/jtim-2021-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passier R., van Laake L.W., Mummery C.L. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 11.Nazari-Shafti T.Z., Neuber S., Falk V., Emmert M.Y. Toward next-generation advanced therapies: Extracellular vesicles and cell therapy—Partners or competitors? Regen. Med. 2021;16:215–218. doi: 10.2217/rme-2020-0138. [DOI] [PubMed] [Google Scholar]
- 12.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witwer K.W., Goberdhan D.C., O’Driscoll L., Théry C., Welsh J.A., Blenkiron C., Buzás E.I., Di Vizio D., Erdbrügger U., Falcón-Pérez J.M., et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12182. doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleh A.F., Lázaro-Ibáñez E., Forsgard M.A.-M., Shatnyeva O., Osteikoetxea X., Karlsson F., Heath N., Ingelsten M., Rose J., Harris J., et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019;11:6990–7001. doi: 10.1039/C8NR08720B. [DOI] [PubMed] [Google Scholar]
- 17.Somiya M., Yoshioka Y., Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles. 2018;7:1440132. doi: 10.1080/20013078.2018.1440132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabani B., Brand M., Albert I., Inderbitzin J., Eichenseher F., Schmelcher M., Rohrer J., Riedl R., Lehmann S. A novel surface functionalization platform to prime extracellular vesicles for targeted therapy and diagnostic imaging. Nanomedicine. 2022;47:102607. doi: 10.1016/j.nano.2022.102607. [DOI] [PubMed] [Google Scholar]
- 19.Görgens A., Corso G., Hagey D.W., Jawad Wiklander R., Gustafsson M.O., Felldin U., Lee Y., Bostancioglu R.B., Sork H., Liang X., et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles. 2022;11:e12238. doi: 10.1002/jev2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Joshi B.S., de Beer M.A., Giepmans B.N.G., Zuhorn I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano. 2020;14:4444–4455. doi: 10.1021/acsnano.9b10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10:295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeyaram A., Jay S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017;20:1. doi: 10.1208/s12248-017-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. [(accessed on 2 May 2023)]. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:311:0067:0128:en:PDF.
- 25.Insights G.T., Stapleton S. Exosomes as therapeutics and drug delivery vehicles: Global regulatory perspectives. Cell Gene Ther. Insights. 2020;6:1561–1569. doi: 10.18609/cgti.2020.172. [DOI] [Google Scholar]
- 26.European Medicines Agency How EMA Evaluates Medicines for Human Use. [(accessed on 5 February 2023)]. Available online: https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines/how-ema-evaluates-medicines.
- 27.Sweeney M., Corden B., Cook S.A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: Mirage or miracle? EMBO Mol. Med. 2020;12:e10865. doi: 10.15252/emmm.201910865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara H., Takeda N., Komuro I. Pathophysiology and therapeutic potential of cardiac fibrosis. Inflamm. Regen. 2017;37:13. doi: 10.1186/s41232-017-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinde A.V., Frangogiannis N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Borne S.W.M., Diez J., Blankesteijn W.M., Verjans J., Hofstra L., Narula J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat. Rev. Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 31.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travers J.G., Tharp C.A., Rubino M., McKinsey T.A. Therapeutic targets for cardiac fibrosis: From old school to next-gen. J. Clin. Investig. 2022;132:e148554. doi: 10.1172/JCI148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raziyeva K., Kim Y., Zharkinbekov Z., Temirkhanova K., Saparov A. Novel Therapies for the Treatment of Cardiac Fibrosis Following Myocardial Infarction. Biomedicines. 2022;10:2178. doi: 10.3390/biomedicines10092178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y., Zhou H., Liu X., Li J., Xu K., Fu X., Ye L., Li G. Applications of Single-Cell RNA Sequencing in Cardiovascular Research. Front. Cell Dev. Biol. 2021;9:810232. doi: 10.3389/fcell.2021.810232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parichatikanond W., Luangmonkong T., Mangmool S., Kurose H. Therapeutic Targets for the Treatment of Cardiac Fibrosis and Cancer: Focusing on TGF-β Signaling. Front. Cardiovasc. Med. 2020;7:34. doi: 10.3389/fcvm.2020.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs) J. Mol. Cell. Cardiol. 2016;94:189–200. doi: 10.1016/j.yjmcc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Czubryt M.P. Cardiac Fibroblast to Myofibroblast Phenotype Conversion-An Unexploited Therapeutic Target. J. Cardiovasc. Dev. Dis. 2019;6:28. doi: 10.3390/jcdd6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Villalba A., Simón A.M., Pogontke C., Castillo M.I., Abizanda G., Pelacho B., Sánchez-Domínguez R., Segovia J.C., Prósper F., Pérez-Pomares J.M. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J. Am. Coll. Cardiol. 2015;65:2057–2066. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 39.Kanisicak O., Khalil H., Ivey M.J., Karch J., Maliken B.D., Correll R.N., Brody M.J., J Lin S.-C., Aronow B.J., Tallquist M.D., et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang L., Lu L., Zhang R., Chen K., Yan X. Comprehensive Integration of Single-Cell Transcriptional Profiling Reveals the Heterogeneities of Non-cardiomyocytes in Healthy and Ischemic Hearts. Front. Cardiovasc. Med. 2020;7:615161. doi: 10.3389/fcvm.2020.615161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Villalba A., Romero J.P., Hernández S.C., Vilas-Zornoza A., Fortelny N., Castro-Labrador L., San Martin-Uriz P., Lorenzo-Vivas E., García-Olloqui P., Palacio M., et al. Single-Cell RNA Sequencing Analysis Reveals a Crucial Role for CTHRC1 (Collagen Triple Helix Repeat Containing 1) Cardiac Fibroblasts After Myocardial Infarction. Circulation. 2020;142:1831–1847. doi: 10.1161/CIRCULATIONAHA.119.044557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu X., Khalil H., Kanisicak O., Boyer J.G., Vagnozzi R.J., Maliken B.D., Sargent M.A., Prasad V., Valiente-Alandi I., Blaxall B.C., et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y., Nurakhayev S., Nurkesh A., Zharkinbekov Z., Saparov A. Macrophage Polarization in Cardiac Tissue Repair Following Myocardial Infarction. Int. J. Mol. Sci. 2021;22:2715. doi: 10.3390/ijms22052715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lis-López L., Bauset C., Seco-Cervera M., Cosín-Roger J. Is the Macrophage Phenotype Determinant for Fibrosis Development? Biomedicines. 2021;9:1747. doi: 10.3390/biomedicines9121747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J., Li X., Hu J., Chen F., Qiao S., Sun X., Gao L., Xie J., Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019;115:1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Couto G., Gallet R., Cambier L., Jaghatspanyan E., Makkar N., Dawkins J.F., Berman B.P., Marbán E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation. 2017;136:200–214. doi: 10.1161/CIRCULATIONAHA.116.024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil M., Saheera S., Dubey P.K., Kahn-Krell A., Kumar Govindappa P., Singh S., Tousif S., Zhang Q., Lal H., Zhang J., et al. Novel Mechanisms of Exosome-Mediated Phagocytosis of Dead Cells in Injured Heart. Circ. Res. 2021;129:1006–1020. doi: 10.1161/CIRCRESAHA.120.317900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J.-G., Li H.-R., Han J.-X., Li B.-B., Yan D., Li H.-Y., Wang P., Luo Y. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci. Rep. 2018;8:9047. doi: 10.1038/s41598-018-27435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bian S., Zhang L., Duan L., Wang X., Min Y., Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 50.Deng S., Zhou X., Ge Z., Song Y., Wang H., Liu X., Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Biol. 2019;114:105564. doi: 10.1016/j.biocel.2019.105564. [DOI] [PubMed] [Google Scholar]
- 51.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y., Sun X., Cao W., Ma J., Sun L., Qian H., Zhu W., Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015;2015:761643. doi: 10.1155/2015/761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun S.-J., Wei R., Li F., Liao S.-Y., Tse H.-F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep. 2021;16:1662–1673. doi: 10.1016/j.stemcr.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Zhao Y., Chen W., Xie L., Zhao Z.-A., Yang J., Chen Y., Lei W., Shen Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017;8:268. doi: 10.1186/s13287-017-0722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., Lin Y., Xiang P., Tang Y., Hu X., et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018;46:1659–1670. doi: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim A.G.E., Li C., Rogers R., Fournier M., Li L., Vaturi S.D., Antes T., Sanchez L., Akhmerov A., Moseley J.J., et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat. Biomed. Eng. 2019;3:695–705. doi: 10.1038/s41551-019-0448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 58.Hess A., Thackeray J.T., Wollert K.C., Bengel F.M. Radionuclide Image-Guided Repair of the Heart. JACC. Cardiovasc. Imaging. 2020;13:2415–2429. doi: 10.1016/j.jcmg.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Hamo C.E., Gheorghiade M., Butler J. Novel Endpoints for Heart Failure Clinical Trials. Curr. Heart Fail. Rep. 2017;14:210–216. doi: 10.1007/s11897-017-0334-z. [DOI] [PubMed] [Google Scholar]
- 60.Cunningham K.S., Veinot J.P., Butany J. An approach to endomyocardial biopsy interpretation. J. Clin. Pathol. 2006;59:121–129. doi: 10.1136/jcp.2005.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassan S., Barrett C.J., Crossman D.J. Imaging tools for assessment of myocardial fibrosis in humans: The need for greater detail. Biophys. Rev. 2020;12:969–987. doi: 10.1007/s12551-020-00738-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akkaya M., Higuchi K., Koopmann M., Burgon N., Erdogan E., Damal K., Kholmovski E., McGann C., Marrouche N.F. Relationship between left atrial tissue structural remodelling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace. 2013;15:1725–1732. doi: 10.1093/europace/eut147. [DOI] [PubMed] [Google Scholar]
- 63.Schelbert E.B., Hsu L.-Y., Anderson S.A., Mohanty B.D., Karim S.M., Kellman P., Aletras A.H., Arai A.E. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ. Cardiovasc. Imaging. 2010;3:743–752. doi: 10.1161/CIRCIMAGING.108.835793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kali A., Cokic I., Tang R.L.Q., Yang H.-J., Sharif B., Marbán E., Li D., Berman D.S., Dharmakumar R. Determination of location, size, and transmurality of chronic myocardial infarction without exogenous contrast media by using cardiac magnetic resonance imaging at 3 T. Circ. Cardiovasc. Imaging. 2014;7:471–481. doi: 10.1161/CIRCIMAGING.113.001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta S., Ge Y., Singh A., Gräni C., Kwong R.Y. Multimodality Imaging Assessment of Myocardial Fibrosis. JACC. Cardiovasc. Imaging. 2021;14:2457–2469. doi: 10.1016/j.jcmg.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Yang G., Zhuang X., Khan H., Haldar S., Nyktari E., Ye X., Slabaugh G., Wong T., Mohiaddin R., Keegan J., et al. Segmenting Atrial Fibrosis from Late Gadolinium-Enhanced Cardiac MRI by Deep-Learned Features with Stacked Sparse Auto-Encoders. In: Valdés Hernández M., González-Castro V., editors. Medical Image Understanding and Analysis. Springer International Publishing; Cham, Switzerland: 2017. pp. 195–206. [Google Scholar]
- 67.Diao K.-Y., Yang Z.-G., Xu H.-Y., Liu X., Zhang Q., Shi K., Jiang L., Xie L.-J., Wen L.-Y., Guo Y.-K. Histologic validation of myocardial fibrosis measured by T1 mapping: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2016;18:92. doi: 10.1186/s12968-016-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popescu I.A., Werys K., Zhang Q., Puchta H., Hann E., Lukaschuk E., Ferreira V.M., Piechnik S.K. Standardization of T1-mapping in cardiovascular magnetic resonance using clustered structuring for benchmarking normal ranges. Int. J. Cardiol. 2021;326:220–225. doi: 10.1016/j.ijcard.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 69.Ding Y., Wang Y., Zhang W., Jia Q., Wang X., Li Y., Lv S., Zhang J. Roles of Biomarkers in Myocardial Fibrosis. Aging Dis. 2020;11:1157–1174. doi: 10.14336/AD.2020.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González A., Richards A.M., de Boer R.A., Thum T., Arfsten H., Hülsmann M., Falcao-Pires I., Díez J., Foo R.S.Y., Chan M.Y., et al. Cardiac remodelling—Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022;24:927–943. doi: 10.1002/ejhf.2493. [DOI] [PubMed] [Google Scholar]
- 71.Querejeta R., Varo N., López B., Larman M., Artiñano E., Etayo J.C., Martínez Ubago J.L., Gutierrez-Stampa M., Emparanza J.I., Gil M.J., et al. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.CIR.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 72.López B., González A., Ravassa S., Beaumont J., Moreno M.U., San José G., Querejeta R., Díez J. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J. Am. Coll. Cardiol. 2015;65:2449–2456. doi: 10.1016/j.jacc.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 73.Díez J., de Boer R.A. Management of cardiac fibrosis is the largest unmet medical need in heart failure. Cardiovasc. Res. 2022;118:e20–e22. doi: 10.1093/cvr/cvab228. [DOI] [PubMed] [Google Scholar]
- 74.Nagaraju C.K., Dries E., Popovic N., Singh A.A., Haemers P., Roderick H.L., Claus P., Sipido K.R., Driesen R.B. Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction. Sci. Rep. 2017;7:10801. doi: 10.1038/s41598-017-09790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altmann A., Haberkorn U., Siveke J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2021;62:160–167. doi: 10.2967/jnumed.120.244806. [DOI] [PubMed] [Google Scholar]
- 76.Heckmann M.B., Reinhardt F., Finke D., Katus H.A., Haberkorn U., Leuschner F., Lehmann L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging. 2020;13:e010628. doi: 10.1161/CIRCIMAGING.120.010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Langer L.B.N., Hess A., Korkmaz Z., Tillmanns J., Reffert L.M., Bankstahl J.P., Bengel F.M., Thackeray J.T., Ross T.L. Molecular imaging of fibroblast activation protein after myocardial infarction using the novel radiotracer [(68)Ga]MHLL1. Theranostics. 2021;11:7755–7766. doi: 10.7150/thno.51419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lener T., Gimona M., Aigner L., Borger V., Buzas E., Camussi G., Chaput N., Chatterjee D., Court F.A., Del Portillo H.A., et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 80.Silva A.K.A., Morille M., Piffoux M., Arumugam S., Mauduit P., Larghero J., Bianchi A., Aubertin K., Blanc-Brude O., Noël D., et al. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs—EVOLVE France”. Adv. Drug Deliv. Rev. 2021;179:114001. doi: 10.1016/j.addr.2021.114001. [DOI] [PubMed] [Google Scholar]
- 81.Sluijter J.P.G., Davidson S.M., Boulanger C.M., Buzás E.I., de Kleijn D.P.V., Engel F.B., Giricz Z., Hausenloy D.J., Kishore R., Lecour S., et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andriolo G., Provasi E., Brambilla A., Lo Cicero V., Soncin S., Barile L., Turchetto L., Radrizzani M. GMP-Grade Methods for Cardiac Progenitor Cells: Cell Bank Production and Quality Control. Methods Mol. Biol. 2021;2286:131–166. doi: 10.1007/7651_2020_286. [DOI] [PubMed] [Google Scholar]
- 83.Andriolo G., Provasi E., Lo Cicero V., Brambilla A., Soncin S., Torre T., Milano G., Biemmi V., Vassalli G., Turchetto L., et al. Exosomes from human cardiac progenitor cells for therapeutic applications: Development of a GMP-grade manufacturing method. Front. Physiol. 2018;9:1169. doi: 10.3389/fphys.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Banerjee A., Jain S.M., Abrar S.S., Kumar M.M., Mathew C., Pathak S. Sources, isolation strategies and therapeutic outcome of exosomes at a glance. Regen. Med. 2020;15:2361–2378. doi: 10.2217/rme-2020-0077. [DOI] [PubMed] [Google Scholar]
- 85.Liang B., He X., Zhao Y.-X., Zhang X.-X., Gu N. Advances in Exosomes Derived from Different Cell Sources and Cardiovascular Diseases. BioMed Res. Int. 2020;2020:7298687. doi: 10.1155/2020/7298687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pachler K., Lener T., Streif D., Dunai Z.A., Desgeorges A., Feichtner M., Öller M., Schallmoser K., Rohde E., Gimona M. A Good Manufacturing Practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles. Cytotherapy. 2017;19:458–472. doi: 10.1016/j.jcyt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Bari E., Perteghella S., Di Silvestre D., Sorlini M., Catenacci L., Sorrenti M., Marrubini G., Rossi R., Tripodo G., Mauri P., et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells. 2018;7:190. doi: 10.3390/cells7110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riss T.L., Moravec R.A., Niles A.L., Duellman S., Benink H.A., Worzella T.J., Minor L. Cell Viability Assays. In: Markossian S., Grossman A., Brimacombe K., Arkin M., Auld D., Austin C., Baell J., Chung T.D.Y., Coussens N.P., Dahlin J.L., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD, USA: 2004. [Google Scholar]
- 89.Adan A., Kiraz Y., Baran Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016;17:1213–1221. doi: 10.2174/1389201017666160808160513. [DOI] [PubMed] [Google Scholar]
- 90.Syromiatnikova V., Prokopeva A., Gomzikova M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022;23:10522. doi: 10.3390/ijms231810522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jia Y., Yu L., Ma T., Xu W., Qian H., Sun Y., Shi H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 2022;12:6548–6575. doi: 10.7150/thno.74305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liangsupree T., Multia E., Riekkola M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A. 2021;1636:461773. doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 93.Brennan K., Martin K., FitzGerald S.P., O’Sullivan J., Wu Y., Blanco A., Richardson C., Mc Gee M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020;10:1039. doi: 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang P., Yeo J.C., Lim C.T. Advances in Technologies for Purification and Enrichment of Extracellular Vesicles. SLAS Technol. 2019;24:477–488. doi: 10.1177/2472630319846877. [DOI] [PubMed] [Google Scholar]
- 95.Coumans F.A.W., Brisson A.R., Buzas E.I., Dignat-George F., Drees E.E.E., El-Andaloussi S., Emanueli C., Gasecka A., Hendrix A., Hill A.F., et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 96.Monopoli M.P., Aberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 97.Buzas E.I. Opportunities and challenges in studying the extracellular vesicle corona. Nat. Cell Biol. 2022;24:1322–1325. doi: 10.1038/s41556-022-00983-z. [DOI] [PubMed] [Google Scholar]
- 98.Tóth E.Á., Turiák L., Visnovitz T., Cserép C., Mázló A., Sódar B.W., Försönits A.I., Petővári G., Sebestyén A., Komlósi Z., et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles. 2021;10:e12140. doi: 10.1002/jev2.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S.Y., Khanal D., Tharkar P., Kalionis B., Chrzanowski W. None of us is the same as all of us: Resolving the heterogeneity of extracellular vesicles using single-vesicle, nanoscale characterization with resonance enhanced atomic force microscope infrared spectroscopy (AFM-IR) Nanoscale Horiz. 2018;3:430–438. doi: 10.1039/C8NH00048D. [DOI] [PubMed] [Google Scholar]
- 100.Comfort N., Cai K., Bloomquist T.R., Strait M.D., Ferrante A.W.J., Baccarelli A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021 doi: 10.3791/62447-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pascucci L., Scattini G. Imaging extracelluar vesicles by transmission electron microscopy: Coping with technical hurdles and morphological interpretation. Biochim. Biophys. Acta Gen. Subj. 2021;1865:129648. doi: 10.1016/j.bbagen.2020.129648. [DOI] [PubMed] [Google Scholar]
- 102.Suárez H., Gámez-Valero A., Reyes R., López-Martín S., Rodríguez M.J., Carrascosa J.L., Cabañas C., Borràs F.E., Yáñez-Mó M. A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles. Sci. Rep. 2017;7:11271. doi: 10.1038/s41598-017-11249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., Liebler D.C., Ping J., Liu Q., Evans R., et al. Reassessment of Exosome Composition. Cell. 2019;177:428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dehghani M., Gaborski T.R. Fluorescent labeling of extracellular vesicles. Methods Enzymol. 2020;645:15–42. doi: 10.1016/bs.mie.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Roberts-Dalton H.D., Cocks A., Falcon-Perez J.M., Sayers E.J., Webber J.P., Watson P., Clayton A., Jones A.T. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale. 2017;9:13693–13706. doi: 10.1039/C7NR04128D. [DOI] [PubMed] [Google Scholar]
- 106.Choi D.-S., Kim D.-K., Kim Y.-K., Gho Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2015;34:474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 107.Kreimer S., Belov A.M., Ghiran I., Murthy S.K., Frank D.A., Ivanov A.R. Mass-spectrometry-based molecular characterization of extracellular vesicles: Lipidomics and proteomics. J. Proteome Res. 2015;14:2367–2384. doi: 10.1021/pr501279t. [DOI] [PubMed] [Google Scholar]
- 108.Kim K.M., Abdelmohsen K., Mustapic M., Kapogiannis D., Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA. 2017;8 doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Balkom B.W.M., Gremmels H., Giebel B., Lim S.K. Proteomic Signature of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Proteomics. 2019;19:e1800163. doi: 10.1002/pmic.201800163. [DOI] [PubMed] [Google Scholar]
- 110.Kusuma G.D., Barabadi M., Tan J.L., Morton D.A.V., Frith J.E., Lim R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018;9:1199. doi: 10.3389/fphar.2018.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., Yang S., Blanko E.V.R., Peng Q., Ma X., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263. doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang M., Jordan V., Blenkiron C., Chamley L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles. 2021;10:e12085. doi: 10.1002/jev2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T., Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Nordin J.Z., Lee Y., Vader P., Mäger I., Johansson H.J., Heusermann W., Wiklander O.P.B., Hällbrink M., Seow Y., Bultema J.J., et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Zhu X., Badawi M., Pomeroy S., Sutaria D.S., Xie Z., Baek A., Jiang J., Elgamal O.A., Mo X., Perle K.L., et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles. 2017;6:1324730. doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nazari-Shafti T.Z., Neuber S., Duran A.G., Exarchos V., Beez C.M., Meyborg H., Krüger K., Wolint P., Buschmann J., Böni R., et al. MiRNA Profiles of Extracellular Vesicles Secreted by Mesenchymal Stromal Cells-Can They Predict Potential Off-Target Effects? Biomolecules. 2020;10:1353. doi: 10.3390/biom10091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marzi M.J., Ghini F., Cerruti B., de Pretis S., Bonetti P., Giacomelli C., Gorski M.M., Kress T., Pelizzola M., Muller H., et al. Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome Res. 2016;26:554–565. doi: 10.1101/gr.198788.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gimona M., Pachler K., Laner-Plamberger S., Schallmoser K., Rohde E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017;18:1190. doi: 10.3390/ijms18061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.