Abstract
An analysis of ketamine and cocaine use in mice reveals that the drugs trigger release of the neurotransmitter dopamine through different mechanisms, and indicates that the risk of addiction to ketamine is low.
Ketamine is increasingly being used as a fast-acting antidepressant in research and in certain clinical settings1. But there are concerns around its therapeutic use, because it is classified as having low to moderate potential for physical or psychological dependence2. Substances are considered addictive if they lead to compulsive use despite negative consequences. On page 368, Simmler et al.3 investigate whether ketamine causes the behavioural and neuronal changes typically seen with highly addictive substances. They report that ketamine fails to establish key addiction-like behaviours in mice, and does not produce changes in the brain’s reward system that are linked to drug craving.
The authors began by comparing the behavioural effects of ketamine with those of a highly addictive substance, cocaine. Mice voluntarily took similar amounts of ketamine and cocaine, suggesting that ketamine is both rewarding and reinforcing (the latter meaning that a positive response to taking it once increases the inclination to take it again4).
These processes are controlled by the neurotransmitter dopamine, which is produced in the brain’s ventral tegmental area (VTA) — a region involved in reward processing5. During rewarding experiences, dopamine is released from the VTA and activates another brain region involved in reward, the nucleus accumbens (NAc)5. Simmler et al. therefore examined how ketamine alters dopamine levels in the NAc. They found that, although both ketamine and cocaine enhanced the release of dopamine from the VTA into the NAc, the rise in dopamine persisted for longer following cocaine exposure than with ketamine. The authors hypothesized that the mechanisms that drive these divergent dopamine responses might mean that ketamine has a reduced potential to be addictive.
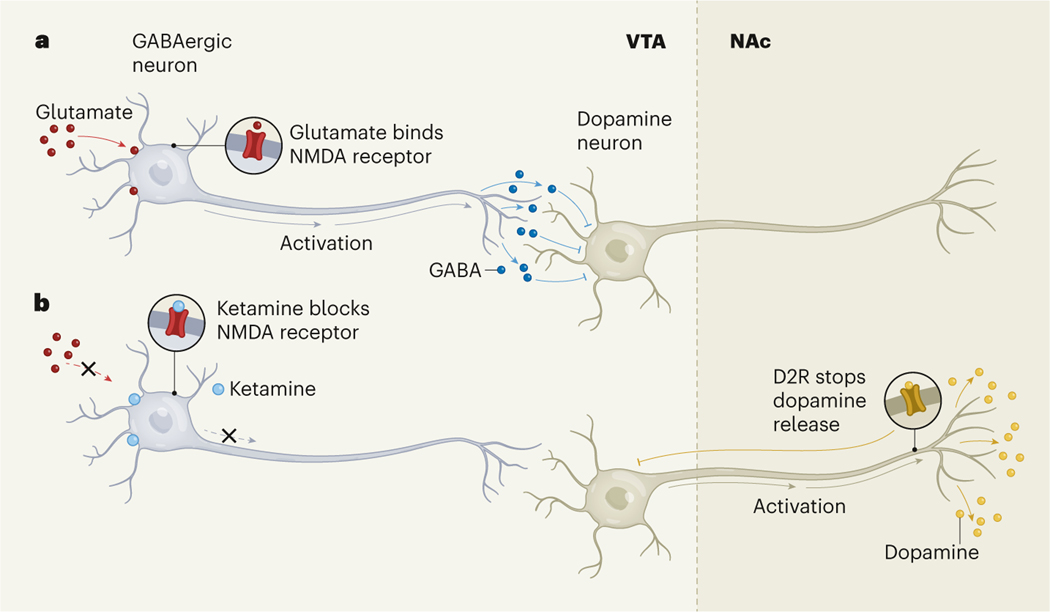
To understand why ketamine causes a short burst of dopamine in the NAc, the researchers examined ketamine’s effects on the VTA by implanting sensors in this region in mice, to measure changes in neuronal activity. They found that ketamine, but not cocaine, reduced the activity of neurons that release the inhibitory neurotransmitter GABA in the VTA. Because these ‘GABAergic’ neurons inhibit the VTA neurons that release dopamine, the result suggests that ketamine only briefly removes the brakes on dopamine release in the VTA, to induce a short burst of dopamine in the NAc (Fig. 1).
Figure 1 |. The effects of ketamine on dopamine release.
a, GABAergic neurons in the brain’s ventral tegmental area (VTA) are stimulated by binding of the neurotransmitter glutamate to NMDA receptor proteins. These neurons release the neurotransmitter GABA, which inhibits the activity of dopamine neurons that project to a reward centre called the nucleus accumbens (NAc). b, Ketamine binds to NMDA receptors to inhibit their activation. Simmler et al.3 show that this leads to activation of the dopamine neurons, triggering a short burst of dopamine release in the NAc. The release is rapidly quashed when dopamine binds to the D2-type receptor (D2R) protein on the dopamine neurons, inhibiting their activity. This mechanism of action differs from the pathways through which cocaine triggers sustained dopamine release (not shown), which might explain why ketamine is not highly addictive.
GABAergic neurons are stimulated when the neurotransmitter glutamate binds to proteins called NMDA receptors in the neuronal membrane. Ketamine binds to the NMDA receptor to prevent its activation by glutamate, thus preventing GABAergic neurons from becoming active6. Simmler and colleagues used CRISPR gene-editing technology to delete a subunit of the NMDA receptor protein to which ketamine binds. Preventing the GABAergic neurons from being inhibited in this way blocked the ketamine-induced dopamine burst. Interestingly, however, the deletion had only a minimal effect on the release of dopamine triggered by cocaine or by an opioid called fentanyl. These findings suggest that NMDA-receptor-dependent action is a ketamine-specific mechanism to promote dopamine release.
Next, the authors focused on why ketamine-evoked dopamine signalling decays so rapidly. When dopamine levels are high, its release is controlled through a negative feedback system — dopamine binds to D2-type dopamine receptor proteins located on VTA-derived neuronal projections in the NAc and prevents them from releasing dopamine7. And, indeed, when the authors blocked D2-type receptors, ketamine provoked prolonged release of dopamine.
Finally, Simmler et al. asked whether ketamine drives similar patterns of craving-related brain activity to those seen with other drugs, by testing how acute and long-term exposure to ketamine affects the electrical properties and neurochemical responses in the reward system of the mouse brain, compared with previous analyses of cocaine8,9. Neither acute nor long-term exposure to ketamine produced hallmark features of drug craving in the VTA or NAc. The authors also examined patterns of drug use when mice learnt to self-administer ketamine or cocaine. If extra effort was required to receive a drug, mice would make the effort to receive cocaine, but not ketamine. Mice would also not take ketamine if there was a negative consequence.
Together, these data demonstrate that ketamine does not promote the neural adaptations or uncontrolled use seen with addictive drugs. Simmler et al. propose that ketamine is rewarding and reinforcing, but has low liability to be addictive. The work therefore demonstrates the promise of ketamine as a viable treatment for depression.
Although this study is illuminating, more work is needed to characterize ketamine use further. This includes examining the potential for relapse following re-exposure to ketamine, and ketamine craving following long periods of abstinence, both of which can be studied in rodents10. Unlike Simmler et al., another study has reported escalation of and motivation for ketamine use11, suggesting that motivation to take ketamine might be dose dependent — a key consideration when selecting doses for therapeutic purposes12. Researchers should also assess the potential for ketamine addiction following chronic stress or experiences that provoke post-traumatic stress disorder, given that these phenomena will probably be common in people who might take ketamine therapeutically.
Ketamine’s lower likelihood of becoming addictive might, as Simmler and colleagues suggest, be due to its failure to produce physiological adaptations in the NAc and VTA similar to those triggered by cocaine. But the true picture is probably more complex. Other addictive drugs, such as opioids, produce electrochemical changes in the reward system that differ from those induced by both ketamine and cocaine, demonstrating that addiction does not arise through one pathway alone13. Communication between neurons and non-neuronal brain cells called glia relies heavily on glutamate signalling14 — as a result, ketamine is likely to have distinct effects on glia. Finally, ketamine is thought to restore impaired activity in brain regions that guide decision-making and memory formation in people who have depression15. However, long-term use of addictive drugs recruits these same brain areas to drive drug relapse16. It will be important to examine further the effects of long-term ketamine use on other brain regions and cell types, to ensure that there is no risk of addiction.
The need for such investigations not-withstanding, the current study presents compelling evidence that ketamine does not produce neurobiological and behavioural characteristics of addiction. The results therefore have the potential to remove the stigma associated with ketamine and expand its therapeutic use in people with depression.
Footnotes
The authors declare no competing interests.
References
- 1.Krystal JH, Abdallah CG, Sanacora G, Charney DS & Duman RS Neuron 101, 774–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan CJA, Curran HV & the Independent Scientific Committee on Drugs (ISCD). Addiction 107, 27–38 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Simmler LD et al. Nature 608, 368–373 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Dayan P. & Niv Y. Curr. Opin. Neurobiol. 18, 185–196 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Saunders BT, Richard JM, Margolis EB & Janak PH Nature Neurosci. 21, 1072–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autry AE et al. Nature 475, 91–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford CP Neuroscience 282, 13–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mameli M. et al. Nature Neurosci. 12, 1036–1041 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Pascoli V. et al. Nature 509, 459–464 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Venniro M, Banks ML, Heilig M, Epstein DH & Shaham Y. Nature Rev. Neurosci. 21, 625–643 (2020). [DOI] [PubMed] [Google Scholar]
- 11.De Luca MT & Badiani A. Psychopharmacology 214, 549–556 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Berman RM et al. Biol. Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Graziane NM et al. Nature Neurosci. 19, 915–925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruyer A, Kalivas PW & Scofield MD Neuropsychopharmacology 10.1038/s41386-022-01338-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price RB & Duman R. Mol. Psychiatry 25, 530–543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalivas PW & Volkow ND Am. J. Psychiatry 162, 1403–1413 (2005). [DOI] [PubMed] [Google Scholar]