Abstract
Thermoregulatory behavior in homeothermic animals is an innate behavior to defend body core temperature from environmental thermal challenges in coordination with autonomous thermoregulatory responses. In contrast to the progress in understanding the central mechanisms of autonomous thermoregulation, those of behavioral thermoregulation remain poorly understood. We have previously shown that the lateral parabrachial nucleus (LPB) mediates cutaneous thermosensory afferent signaling for thermoregulation. To understand the thermosensory neural network for behavioral thermoregulation, in the present study, we investigated the roles of ascending thermosensory pathways from the LPB in avoidance behavior from innocuous heat and cold in male rats. Neuronal tracing revealed two segregated groups of LPB neurons projecting to the median preoptic nucleus (MnPO), a thermoregulatory center (LPB→MnPO neurons), and those projecting to the central amygdaloid nucleus (CeA), a limbic emotion center (LPB→CeA neurons). While LPB→MnPO neurons include separate subgroups activated by heat or cold exposure of rats, LPB→CeA neurons were only activated by cold exposure. By selectively inhibiting LPB→MnPO or LPB→CeA neurons using tetanus toxin light chain or chemogenetic or optogenetic techniques, we found that LPB→MnPO transmission mediates heat avoidance, whereas LPB→CeA transmission contributes to cold avoidance. In vivo electrophysiological experiments showed that skin cooling-evoked thermogenesis in brown adipose tissue requires not only LPB→MnPO neurons but also LPB→CeA neurons, providing a novel insight into the central mechanism of autonomous thermoregulation. Our findings reveal an important framework of central thermosensory afferent pathways to coordinate behavioral and autonomous thermoregulation and to generate the emotions of thermal comfort and discomfort that drive thermoregulatory behavior.
SIGNIFICANCE STATEMENT Coordination of behavioral and autonomous thermoregulation is important for maintaining thermal homeostasis in homeothermic animals. However, the central mechanism of thermoregulatory behaviors remains poorly understood. We have previously shown that the lateral parabrachial nucleus (LPB) mediates ascending thermosensory signaling that drives thermoregulatory behavior. In this study, we found that one pathway from the LPB to the median preoptic nucleus mediates heat avoidance, whereas the other pathway from the LPB to the central amygdaloid nucleus is required for cold avoidance. Surprisingly, both pathways are required for skin cooling-evoked thermogenesis in brown adipose tissue, an autonomous thermoregulatory response. This study provides a central thermosensory network that coordinates behavioral and autonomous thermoregulation and generates thermal comfort and discomfort that drive thermoregulatory behavior.
Keywords: amygdala, autonomic, behavior, lateral parabrachial nucleus, preoptic area, thermoregulation
Introduction
Homeothermic animals use voluntary behavioral and involuntary autonomous thermoregulation in a coordinated manner to maintain body core temperature (Tcore) within a narrow range against changes in environmental temperature. Thermoregulatory behaviors, such as thermal comfort selection (i.e., avoidance of hot and cold temperatures), postural extension, grooming, and locomotion, enhance the effectiveness of autonomous thermoregulatory responses, such as sympathetic thermogenesis in brown adipose tissue (BAT), shivering thermogenesis in skeletal muscles, and cutaneous vasomotion. Despite the remarkable progress in understanding the central circuit mechanisms of autonomous thermoregulation (Morrison and Nakamura, 2019; K. Nakamura et al., 2022), those of behavioral thermoregulation are still under investigation.
Changes in environmental temperature are sensed by cutaneous warm and cold receptors and primary somatosensory fibers separately transmit the warm and cold sensory signals to lamina I neurons in the spinal and medullary dorsal horns, which then transmit the signals to the thalamus and to the lateral parabrachial nucleus (LPB) in the pons (Hylden et al., 1989; Li et al., 2006). The spinothalamic pathway delivers the sensory signals to the primary somatosensory cortex for the perception and discrimination of skin temperature (Tskin) (Craig et al., 1994; Craig, 2002). On the other hand, the spinoparabrachial pathway extends thermosensory signaling to the preoptic area (POA), a thermoregulatory center, to elicit immediate autonomous thermoregulatory responses to the changes in environmental temperature (Nakamura and Morrison, 2008, 2010). We have shown that the LPB, but not the thalamus, also mediates thermosensory signaling for avoidance of innocuous heat and cold (Yahiro et al., 2017). However, despite recent efforts (Norris et al., 2021; Jung et al., 2022), thermosensory neural pathways ascending from the LPB for behavioral thermoregulation are largely unknown.
In the LPB, two major groups of neurons expressing different transcription factors, forkhead box protein P2 (FoxP2) and LIM homeobox transcription factor 1β (Lmx1b), show segregated distributions (Miller et al., 2012). FoxP2-expressing LPB neurons project numerous axons to the POA and other hypothalamic regions (Huang et al., 2021a). In contrast, the Lmx1b-expressing group includes calcitonin gene-related peptide-expressing neurons that densely innervate the central amygdaloid nucleus (CeA) and other limbic regions (Huang et al., 2021b). It is hypothesized that these two projection groups transmit different thermosensory signals to the forebrain sites to drive coordinated behavioral and autonomous thermoregulatory responses. However, whether the POA is involved in behavioral thermoregulation has been controversial (Almeida et al., 2015). Whereas POA lesions do not affect thermal preference or operant thermoregulatory behavior (Lipton, 1968; Carlisle, 1969; Almeida et al., 2006), the POA is involved in body extension of rodents in a warm environment (Carlisle, 1969; Satinoff and Rutstein, 1970; Yu et al., 2016; Norris et al., 2021) and cold-seeking behavior (Tan et al., 2016). Although these findings implicate the POA in behavioral thermoregulation, the importance of LPB→POA thermosensory transmission in behavioral thermoregulation remains unclear.
The LPB→CeA pathway mediates nociceptive signaling to generate a fear memory that induces aversive behavior to pain (Bernard and Besson, 1990; Gauriau and Bernard, 2002; Han et al., 2015; Sato et al., 2015). However, whether the LPB→CeA pathway is involved in thermoregulatory avoidance of innocuous hot and cold is unknown. Because thermoregulatory behaviors are motivated by thermally evoked comfort and discomfort, it would be relevant to investigate whether emotion-related corticolimbic circuits, including the amygdala, are involved in behavioral thermoregulation.
In the present study, we investigated the roles of LPB neurons projecting to the median preoptic nucleus (MnPO) and those projecting to the CeA in behavioral thermoregulation and in coordination with autonomous thermoregulation in rats. We performed functional neuroanatomy to examine the distribution of collateral axons and the thermal responsiveness of the two projection neuron groups. Using tetanus toxin light chain (TeTxLC), designer receptors exclusively activated by designer drugs (DREADDs), and inhibitory optogenetic channels, we chronically or acutely inhibit LPB→MnPO or LPB→CeA transmission selectively, and performed two-floor innocuous thermal plate preference tests (TPPTs) to assess heat and cold avoidance behaviors and in vivo physiological recordings of autonomous thermoregulatory responses.
Materials and Methods
Animals
Male Wistar ST rats (280-400 g; 8-12 weeks old) were purchased from Japan SLC and housed with ad libitum access to food and water in a room air-conditioned at 25 ± 2°C with a standard 12 h light/dark cycle (light: 7:00-19:00; dark: 19:00-7:00) until they were used for surgery or experiments. All procedures conform to the guidelines of animal care by the Division of Experimental Animals, Nagoya University Graduate School of Medicine, and were approved by the Nagoya University Animal Care and Use Committee (approval #M220097-002).
Adeno-associated virus (AAV) vectors
Recombinant AAVs used in the present study except AAVrg-pgk-Cre were serotype 1 or 8 (see below). AAV-CMV-palGFP, which was used to transduce LPB neurons with a membrane-targeted form of green fluorescent protein (palGFP), was described in our earlier study (Kataoka et al., 2014). AAVs for Cre-dependent expression of iChloC-mCherry (AAV-EF1α-DIO-iChloC-mCherry) and palGFP (AAV-EF1α-DIO-palGFP) were also described previously (Kataoka et al., 2020). An AAV retrograde (AAVrg) for Cre expression (AAVrg-pgk-Cre) was purchased from Addgene (#24593, donated by Patrick Aebischer).
To produce AAVs for Cre-dependent expression of TeTxLC and EYFP (AAV-EF1α-DIO-TeTxLC-T2A-EYFP) and hM4Dinrxn and mCherry (AAV-EF1α-DIO-mCherry-T2A-hM4Dinrxn), we inserted a DNA cassette encoding TeTxLC-T2A-EYFP (VectorBuilder) or mCherry-T2A-hM4Dinrxn (Addgene, #52523, donated by Scott Sternson) into the plasmid, pAAV2-Ef1α-DIO-MCS, respectively. The produced plasmids, pAAV2-EF1α-DIO-TeTxLC-T2A-EYFP and pAAV2-Ef1α-DIO-mCherry-T2A-hM4Dinrxn, were used for the production and purification of the AAVs according to our methods (Kataoka et al., 2020; Takahashi et al., 2021).
The titrations of the AAVs used were 3.5 × 1011 GC/ml for AAV2/1-CMV-palGFP, 1.6 × 1014 GC/ml for AAV2/8-EF1α-DIO-iChloC-mCherry, 4.3 × 1014 GC/ml for AAV2/8-EF1α-DIO-palGFP, 9.5 × 1012 GC/ml for AAVrg-pgk-Cre, 6.0 × 1012 GC/ml for AAV2/8-EF1α-DIO-TeTxLC-T2A-EYFP, and 1.7 × 1014 GC/ml for AAV2/8-EF1α-DIO-mCherry-T2A-hM4Dinrxn.
Surgery
Rats were anesthetized by a subcutaneous injection with a combination anesthetic [medetomidine hydrochloride (0.15 mg/kg), midazolam (2.0 mg/kg), and butorphanol tartrate (2.5 mg/kg)] following gas anesthesia with 3% isoflurane. The depth of anesthesia was carefully checked by the absence of hindlimb withdrawal to a foot pinch and/or by the absence of eye blinking in response to a gentle touch to the cornea before the rats were positioned in a stereotaxic apparatus. During surgery, the appropriate depth of anesthesia was maintained by ensuring that no sign of pain or discomfort was observed.
A glass micropipette filled with a solution containing AAV or cholera toxin b-subunit (CTb) conjugated with AlexaFluor-488 (Alexa-488) or Alexa-594 (1 mg/ml; C22841 and C22842, Fisher Scientific) was perpendicularly inserted into the LPB, MnPO, or CeA. The solution was pressure-ejected using a Picospritzer III (Parker); and then, the micropipette was left in place for 5 min before being withdrawn. AAV injections were targeted at the following coordinates: LPB (9.0 mm caudal to bregma, 2.3 mm left and right to the midline, and 6.0 mm ventral to the brain surface; 200 nl/site), MnPO (on bregma, on the midline, and 7.4 and 7.7 mm ventral to the brain surface; 150 nl/site), and CeA (2.0-3.0 mm caudal to bregma, 4.7 mm left and right to the midline, and 7.6 mm ventral to the brain surface; 150 nl/site). CTb injection was made into the MnPO (coordinates as above; 150 nl/site) and bilaterally into the CeA (coordinates as above; 200 nl/site).
For intracranial drug nanoinjections in DREADD experiments, a single sterile guide cannula (ID = 0.24 mm, OD = 0.46 mm; C315G; Plastics One) was inserted to target the MnPO (coordinates: 0.1 mm rostral to bregma, 1.4 mm left to the midline, and 6.6 mm ventral to the brain surface, tilted 10° laterally) or a paired sterile bilateral stainless guide cannula (ID = 0.24 mm, OD = 0.46 mm, intercannula distance = 1.6 mm; C235G-1.6/SPC, Plastics One) was perpendicularly inserted to target the DMH (coordinates: 2.8 mm caudal to bregma, 0.8 mm left and right to the midline, and 6.8 mm ventral to the brain surface) after AAV injections. The guide cannulae were then anchored with dental cement to stainless-steel screws attached to the skull. Dummy cannulae cut to the exact length of the guide cannulae were inserted into the guide cannulae to avoid clogging. Internal cannulae for injection with the thickness to fit the guide cannulae were cut to be long enough to allow the injector tip to protrude 1.0 mm below the tip of the guide cannulae.
For optogenetic experiments, fiber optic cannulae with a ceramic ferrule (200 μm core diameter, 0.39 NA; R-FOC-BL200C-39NA, RWD) were bilaterally inserted to target 0.5 mm dorsal to the CeA (coordinates: 2.5 mm caudal to bregma, 4.7 mm left and right to the midline, and 7.1 mm ventral to the brain surface), ventromedial hypothalamic nucleus (VMH; coordinates: 2.8 mm caudal to bregma, 2.1 mm left and right to the midline, and 8.9 mm ventral to the brain surface, tilted 10° laterally), or the bed nucleus of the stria terminalis (BNST; coordinates: 0.1 mm rostral to bregma, 2.9 mm left and right to the midline, and 6.3 mm ventral to the brain surface, tilted 12° laterally).
For telemetric monitoring of Tcore, the anesthetized rats were also implanted with a battery-operated telemetric transmitter (TA-F40, Data Science International) after the above procedures. The abdominal wall was incised and the telemetric transmitter was placed in the abdominal cavity.
At the end of surgery, the incised skin and abdominal wall were closed with sutures or wound clips and the surgical wound was treated with iodine. Ampicillin sodium solution (0.2 ml, 125 mg/ml) was injected into the left femoral muscle to prevent postoperative infection. Atipamezole solution (0.1 ml, 0.5 mg/ml) was injected subcutaneously to promote arousal. The rats were caged individually for at least 1 week to recover from the surgery with ad libitum access to food and water in a room air-conditioned at 24 ± 2°C under regular health checks.
Neural tracing and cold and heat exposure
Rats injected with AAV-CMV-palGFP or CTb were transcardially perfused as described below, >1 week after injection. For retrograde tracing with CTb combined with Fos study, CTb-injected, individually caged rats were exposed to 4°C, 25°C, or 36°C for 2 h in a climate chamber as described (Y. Nakamura et al., 2022), >1 week after CTb injection. Immediately after the exposure, the animals were subjected to transcardial perfusion below.
TPPT
The apparatus consisted of two black-painted metal thermal plates (19 cm × 30 cm) placed side by side, surrounded by black acrylic walls (40 cm high) with a slit for passage between the two plates (see Fig. 1F). The apparatus was placed in a climate chamber air-conditioned at 25 ± 0.5°C. A controller (Intercross) regulated the temperature of each plate within an error of ± 0.1°C using Peltier devices. For experiments with rats, one plate was set at 28°C (neutral) and the other at 15°C (innocuous cold) or 39°C (innocuous warm), as we performed previously (Yahiro et al., 2017). The temperature settings of the plates were randomly assigned, but not side-fixed. Immediately after a rat was placed on the plates, its behavior was monitored and recorded with a camera (C920 Pro HD Webcam, Logitech) for 20 or 30 min and was analyzed with the video-tracking software, ANY-maze (Stoelting). For each minute of the test period, the percentage of time spent on each plate, the times of transitions between plates, and the distance traveled were calculated and presented in a time course graph with a 1-min bin. The totals of these values for the entire test period were also calculated and presented in a bar graph. During testing, Tcore was also recorded every 1 min by a telemetry system (Ponemah, Data Science International).
Figure 1.

TeTxLC-mediated chronic suppression of LPB→MnPO neurons abolishes heat avoidance, but not cold avoidance. A, B, Nonselective AAV transduction of LPB neurons with palGFP (A) visualized their axons densely distributed in the MnPO and the CeA (B). Scale bars, 1.0 mm. ac, Anterior commissure; BLA, basolateral amygdaloid nucleus; CPu, caudate putamen; EP, entopeduncular nucleus; GP, globus pallidus; ic, internal capsule; LV, lateral ventricle; Me5, mesencephalic trigeminal nucleus; MPA, medial preoptic area; opt, optic tract; scp, superior cerebellar peduncle; VMPO, ventromedial preoptic nucleus; VPL, ventral posterolateral thalamic nucleus. C, Selective transduction of LPB→MnPO neurons with EYFP-T2A-TeTxLC using AAV and AAVrg. bGH-poly A, Bovine growth hormone polyadenylation signal; Ef1α, elongation factor 1-α promoter; ITR, inverted terminal repeat; WPRE, woodchuck hepatitis virus post-transcriptional regulatory element. D, EYFP- and/or Cre-immunoreactive neurons in the LPB. Scale bars, 500 µm. E, Selective transduction of LPB→MnPO neurons with palGFP visualized distributions of axons of LPB→MnPO neurons. Scale bars, 1 mm. 3V, Third ventricle. F, Thermal plate preference test. G, H, Effects of TeTxLC expression in LPB→MnPO neurons on heat (G) and cold (H) avoidance. Control rats expressed palGFP instead of EYFP-T2A-TeTxLC. Time course changes (left, every 1 min) in % duration of stay on 39°C or 15°C plate, times of entry into 39°C or 15°C plate, distance traveled, and Tcore. Right graphs represent % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and change in Tcore (ΔTcore, difference between value at time 0 and peak) for the 20 min test period. Data were analyzed by unpaired t tests (n = 7 per group; G: duration of stay, t(12) = 5.39; entries, t(12) = 0.70; distance, t(12) = 0.56; ΔTcore, t(12) = 2.80; H: duration of stay, t(12) = 0.57; entries, t(12) = 0.36; distance, t(12) = 0.74; ΔTcore, t(12) = 0.63). *p < 0.05. ***p < 0.001. Error bars indicate SEM.
In DREADD experiments, the rats that had undergone intracranial cannulation received bilateral nanoinjections of either saline or C21 into the MnPO or DMH through the cannulae before a TPPT. An internal cannula was connected to a Teflon tubing, and the inside of the cannula and tubing was filled with pyrogen-free 0.9% saline (Otsuka) or C21 (200 μg/ml, dissolved in saline; SML2392, Sigma-Aldrich). A Hamilton syringe (10 μl) filled with mineral oil was connected to the other end of the Teflon tubing and placed in a manually operated syringe manipulator (Narishige). The dummy cannulae inserted into the guide cannulae on the skull were gently removed, and the internal cannula was inserted into one of the guide cannulae. Then, the saline or C21 solution (50 nl/side) was slowly ejected through the cannula into the MnPO or DMH using the syringe manipulator. The injection volume was visually confirmed by the movement of the aqua–oil interface along the Teflon tubing. For bilateral injections into the DMH, immediately after injection on one side, another injection was made on the other side. Five minutes after completion of the bilateral nanoinjections, the rats were subjected to a 30-min TPPT.
For optogenetic experiments, the fiber optic cannulae implanted on the skull were connected with ferrule patch cables (M81L01, Thorlabs). The light source was a diode-pumped 445 nm blue laser (LuxX 445-100, Omicron) controlled by a function generator (AFG1022, Tektronix). The power output was measured at the fiber tip with a light meter beforehand and preset at 8 mW (when the laser was activated in a continuous mode). The target sites were illuminated with 50 ms light pulses at 10 Hz immediately after the rats were placed on the thermal plates, and illumination was continued for the 20 min of a TPPT period.
In vivo electrophysiology
In vivo physiological recordings followed our established procedure (Nakamura and Morrison, 2007, 2011). AAV-injected rats were anesthetized intravenously with urethane (0.8 g/kg) and α-chloralose (80 mg/kg) after cannulation of a femoral artery, a femoral vein, and the trachea under anesthesia with 3% isoflurane in 100% O2. The arterial cannula was attached to a pressure transducer to record arterial pressure and heart rate (HR). Tcore was monitored by a copper-constantan thermocouple (Physitemp) inserted into the rectum. The trunk was shaved and wrapped with a plastic water jacket, and Tskin was monitored from a thermocouple taped onto the skin 1 cm caudal to the xiphoid process. The water jacket was perfused with warm or cold water to maintain the Tcore between 36.0°C and 38.0°C. The rats were placed in a stereotaxic apparatus, artificially ventilated with 100% O2 through the tracheal cannula, and paralyzed with D-tubocurarine (0.6 mg, i.v., initial dose, supplemented with 0.3 mg/h) to stabilize BAT sympathetic nerve recording by preventing respiration-related movements. Mixed expired CO2 was monitored through the tracheal cannula using a capnometer to provide an index of changes in whole-body metabolism and was maintained between 3.5% and 4.5% under basal conditions. BAT temperature (TBAT) was measured from the left interscapular BAT pad with a needle-type copper-constantan thermocouple (0.33 mm diameter; Physitemp), and postganglionic BAT sympathetic nerve activity (SNA) was recorded from the central cut end of a nerve bundle isolated from the right interscapular BAT pad. BAT SNA was amplified (×5000-10,000) and filtered (1-300 Hz) using a CyberAmp 380 amplifier (Molecular Devices). All the physiological variables were digitized and recorded to a personal computer using Spike2 software (version 7.10; CED). BAT SNA amplitudes were quantified using Spike2 in sequential 4 s bins as the square root of the total power (root mean square) in the 0 to 20 Hz band of the autospectra of each 4 s segment of the BAT SNA traces. The “power/4 s” traces (see Figs. 2A,B,D,E, 7A,B,D,E) were used for quantification and statistical analyses of changes in BAT SNA.
Figure 2.

LPB→MnPO neurons mediate thermosensory signaling for BAT thermogenic and cardiovascular responses to skin cooling and body core cooling. A, B, Effects of palGFP and TeTxLC expression in LPB→MnPO neurons on BAT thermogenic and cardiovascular responses to skin cooling. AP, Arterial pressure; Exp. CO2, expired CO2. C, Skin cooling-evoked changes in physiological variables, which were analyzed by Mann–Whitney U tests (palGFP: n = 7, TeTxLC: n = 5; ΔBAT SNA: U = 0; ΔTBAT: U = 5; ΔExp. CO2: U = 4; ΔHR: U = 4; ΔMAP: U = 9). D, E, Effects of palGFP- and TeTxLC expression in LPB→MnPO neurons on BAT thermogenic and cardiovascular responses to reduction of Tcore. F, Body core cooling-evoked changes in BAT SNA, TBAT, Exp. CO2, HR, and MAP were compared with the values at 38°C. Data from control (palGFP) rats (n = 7) were analyzed by repeated-measures one-way ANOVA followed by Bonferroni's post hoc test (ΔBAT SNA: F(4,24) = 21.53, p < 0.001; ΔTBAT: F(4,24) = 1.74, p = 0.175; ΔExp. CO2: F(4,24) = 7.91, p < 0.001; ΔHR: F(4,24) = 0.99, p = 0.434; ΔMAP: F(4,24) = 5.39, p = 0.003). *p < 0.05; **p < 0.01; ***p < 0.001 (vs 38°C). Differences in cooling-evoked changes in the physiological variables between palGFP and TeTxLc groups were analyzed by repeated-measures two-way ANOVA followed by Bonferroni's post hoc test (palGFP, n = 7; TeTxLC, n = 5; ΔBAT SNA: group: F(1,10) = 10.84, p = 0.008, Tcore: F(4,40) = 15.34, p < 0.001, interaction: F(4,40) = 11.19, p < 0.001; ΔTBAT: group: F(1,10) = 31.39, p < 0.001, Tcore: F(4,40) = 14.60, p < 0.001, interaction: F(4,40) = 14.88, p < 0.001; ΔExp. CO2: group: F(1,10) = 16.87, p = 0.002, Tcore: F(4,40) = 1.02, p = 0.410, interaction: F(4,40) = 16.61, p < 0.001; ΔHR: group: F(1,10) = 0.98, p = 0.346, Tcore: F(4,40) = 1.97, p = 0.117, interaction: F(4,40) = 1.54, p = 0.208; ΔMAP: group: F(1,10) = 10.12, p < 0.001, Tcore: F(4,40) = 1.19, p = 0.332, interaction: F(4,40) = 5.44, p = 0.001). †p < 0.05; ††p < 0.01; †††p < 0.001 (vs palGFP). Error bars indicate SEM.
Figure 7.
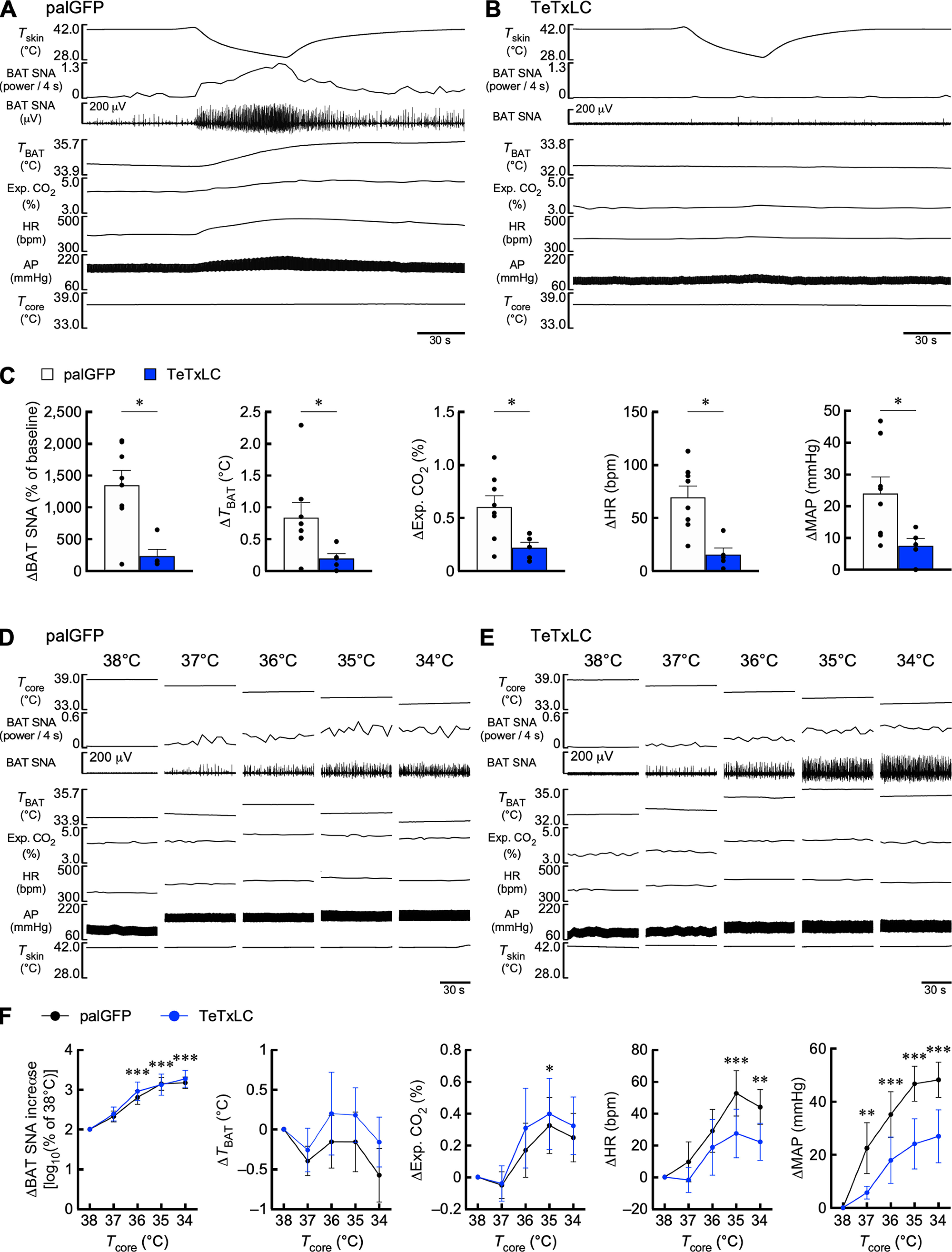
TeTxLC-mediated chronic suppression of LPB→CeA neurons abolishes BAT thermogenic and cardiovascular responses to skin cooling, but not responses to body core cooling. A, B, Effects of palGFP- and TeTxLC expression in LPB→CeA neurons on BAT thermogenic and cardiovascular responses to skin cooling. C, Skin cooling-evoked changes in physiological variables, which were analyzed by Mann–Whitney U tests (palGFP: n = 8, TeTxLC: n = 5; ΔBAT SNA: U = 5; ΔTBAT: U = 4; ΔExp. CO2: U = 4; ΔHR: U = 1; ΔMAP: U = 5). D, E, Effects of palGFP- and TeTxLC expression in LPB→CeA neurons on the BAT thermogenic and cardiovascular responses to reduction of Tcore. F, Body core cooling-evoked changes in BAT SNA, TBAT, Exp. CO2, HR, and MAP were compared with the values at 38°C. Data from control (palGFP) rats (n = 8) were analyzed by repeated-measures one-way ANOVA followed by Bonferroni's post hoc test (ΔBAT SNA: F(4,28) = 31.41, p < 0.001; ΔTBAT: F(4,28) = 1.72, p = 0.175; ΔExp. CO2: F(4,28) = 3.67, p = 0.016; ΔHR: F(4,28) = 7.16, p < 0.001; ΔMAP: F(4,28) = 18.25, p < 0.001). *p < 0.05; **p < 0.01; ***p < 0.001 (vs 38°C). Body cooling-evoked changes in the physiological variables in the TeTxLc group were not significantly different from those in the palGFP group, as determined by Bonferroni's post hoc test following repeated-measures two-way ANOVA (palGFP, n = 8; TeTxLC, n = 5; ΔBAT SNA: group: F(1,11) = 0.09, p = 0.770, Tcore: F(4,44) = 49.85, p < 0.001, interaction: F(4,44) = 0.25, p = 0.911; ΔTBAT: group: F(1,11) = 0.44, p = 0.519, Tcore: F(4,44) = 2.12, p = 0.095, interaction: F(4,44) = 0.41, p = 0.800; ΔExp. CO2: group: F(1,11) = 0.10, p = 0.754, Tcore: F(4,44) = 7.20, p < 0.001, interaction: F(4,44) = 0.18, p = 0.950; ΔHR: group: F(1,11) = 1.01, p = 0.336, Tcore: F(4,44) = 8.24, p < 0.001, interaction: F(4,44) = 0.67, p = 0.619; ΔMAP: group: F(1,11) = 3.21, p = 0.101, Tcore: F(4,44) = 19.68, p < 0.001, interaction: F(4,44) = 1.65, p = 0.180). Error bars indicate SEM.
To examine the effects of skin cooling on physiological parameters (see Figs. 2A–C, 7A–C), ice-cold water was perfused through the water jacket to lower Tskin from 40°C to 30°C. For data analyses in Figure 2C and 7C, baseline values of BAT SNA, TBAT, expired CO2, HR, and mean arterial pressure (MAP) were the averages during the 30 s period immediately before the initiation of skin cooling. Skin cooling-evoked changes in TBAT, expired CO2, HR, and MAP were the differences between the baseline values and their peak values within 30 s (60 s for TBAT) before the end of skin cooling. Skin cooling-evoked changes in BAT SNA were the average of the power/4 s value for the 30 s period before the end of skin cooling and expressed as % of the baseline values.
To examine the effects of Tcore on physiological parameters (see Figs. 2D–F, 7D–F), Tcore was gradually reduced from 38°C to 34°C by repeated skin cooling, and physiological parameters were measured for 60 s each time Tcore was reduced by 1°C and Tskin was at 40°C. In Figures 2F and 7F, 60 s average values of BAT SNA, TBAT, expired CO2, HR, and MAP at Tcore of 38°C were taken as baseline values, and differences from the baseline values in 60 s average values of TBAT, expired CO2, HR, and MAP at each Tcore were calculated. When the 60 s average values of the power/4 s value of BAT SNA were expressed as % of the baseline values, the data did not have a normal distribution required for two-way ANOVA. Therefore, the average value at each Tcore was expressed as log10% of the baseline value at Tcore of 38°C [log10 (% of 38°C BAT SNA)].
After all the recordings, the rats were transcardially perfused and the brain tissue was processed to confirm AAV injections and gene expression as described below.
Daily monitoring of Tcore and appetitive behaviors and thermal exposure
Diurnal changes in Tcore, activity, food intake, and water consumption of rats were measured in a cage equipped with an automated feeding behavior measuring device (Feedam, Melquest) placed in a climate chamber air-conditioned at 25 ± 0.5°C with a 12 h light/dark cycle (light: 7:00-19:00; dark: 19:00-7:00). Tcore and activity were measured by receiving signals from the implanted telemetry transmitter with receiver boards placed near the cage. Rats were individually housed in the cages from the day before the measurement to acclimate them to the environment, and measurements were performed over the next 2 d. Amounts of food and water consumed were measured every 10 min and used to calculate daily and hourly food intake and water consumption per body weight. To measure Tcore, activity, food intake, and water consumption in a cold or hot environment, the air temperature in the climate chamber was set at 4°C or 36°C, respectively, for 2 h (approximately between 9:00 and 15:00).
Immunohistochemistry
Immunohistochemical procedures followed our previous methods (Nakamura et al., 2000, 2004). Rats were deeply anesthetized and transcardially perfused with 4% formaldehyde in 0.1 m PB, pH 7.4. The brain was removed, postfixed in the fixative at 4°C for 2-3 h, and then cryoprotected in a 30% sucrose solution overnight. The tissue was cut into 30-μm-thick frontal sections on a freezing microtome.
For anterograde tracing of palGFP-labeled axons, sections were incubated overnight with an anti-GFP mouse antibody (1:200; A11120, Fisher Scientific) and then with an Alexa488-conjugated goat antibody to mouse IgG (10 μg/ml; A11029, Fisher Scientific) for 1 h.
For double immunofluorescence labeling of CTb and Fos, sections were incubated overnight with an anti-CTb goat serum (1:4000; #703, List Biological Labs) and an anti-Fos rabbit serum (1:10,000; PC38, Merck Millipore). After rinsing, the sections were incubated with an Alexa594-conjugated donkey antibody to goat IgG (5 μg/ml; A11058, Fisher Scientific) for 1 h and then blocked with 10% normal goat serum for 30 min. After a thorough wash, the sections were incubated with a biotinylated donkey antibody to rabbit IgG (10 μg/ml; AP182B, Merck Millipore) and then with Alexa488-conjugated streptavidin (2.5 μg/ml; S11223, Fisher Scientific).
For detection of EYFP and Cre recombinase coexpressed in cells AAV-transduced with TeTxLC, sections were incubated overnight with an anti-GFP rabbit antibody (0.5 μg/ml) (Tamamaki et al., 2000) and an anti-Cre recombinase mouse monoclonal antibody (1:1000; MAB3120, Merck Millipore). After a rinse, the sections were incubated for 1 h with an Alexa488-conjugated goat antibody to rabbit IgG (5 μg/ml; A11034, Fisher Scientific) and a biotinylated donkey antibody to mouse IgG (10 μg/ml; AP192B, Merck Millipore). The sections were further incubated with Alexa594-conjugated streptavidin (2.5 μg/ml; S11227, Fisher Scientific).
For detection of mCherry and Cre recombinase coexpressed in cells AAV-transduced with hM4Dinrxn or iChloC-mCherry, sections were incubated overnight with an anti-RFP rabbit antibody (0.5 μg/ml; R10367, Fisher Scientific) and an anti-Cre recombinase mouse monoclonal antibody (1:1000). After a rinse, the sections were incubated for 1 h with an Alexa594-conjugated goat antibody to rabbit IgG (5 μg/ml; A11012, Fisher Scientific) and a biotinylated donkey antibody to mouse IgG (10 μg/ml). The sections were further incubated with Alexa488-conjugated streptavidin (2.5 μg/ml). For detection of axon terminals of neurons transduced with iChloC-mCherry, sections were incubated overnight with an anti-monomeric RFP guinea pig antibody (0.1 μg/ml) (Hioki et al., 2010). After a rinse, the sections were incubated for 1 h with a biotinylated donkey antibody to guinea pig IgG (10 μg/ml; 706-065-148, Jackson ImmunoResearch Laboratories). The sections were further incubated with Alexa594-conjugated streptavidin (2.5 μg/ml).
Stained sections were mounted on glass slides and coverslipped with 50% glycerol/50% PBS containing 2.5% triethylenediamine. The sections were observed under an epifluorescence microscope (Eclipse 80i, Nikon) or a confocal laser scanning microscope (TCS SP8, Leica).
Anatomy and statistics
The anatomic nomenclature of most brain regions followed the brain atlas of Paxinos and Watson (2007).
Data are presented as mean ± SEM. Statistic comparison analyses were performed using a paired or unpaired t test, a Mann–Whitney U test, a repeated-measures one-way ANOVA followed by Bonferroni's multiple comparisons test, or a repeated-measures two-way ANOVA followed by Bonferroni's multiple comparisons test (Prism 9, GraphPad) as stated in the text and figure legends. All the statistical tests were two-sided. Statistical results with a p value of < 0.05 were considered significant.
Results
LPB→MnPO neurons are required for heat avoidance but not cold avoidance
To identify candidate forebrain areas to which LPB neurons transmit thermosensory signals, we performed anterograde neural tract tracing from the LPB in rats by unilaterally injecting AAV-CMV-palGFP into the LPB. Expression of palGFP in LPB neurons visualized their axon fibers innervating many, but specific forebrain regions, and particularly dense innervation was observed in the MnPO and CeA (Fig. 1A,B), consistent with earlier observations (Saper and Loewy, 1980; Fulwiler and Saper, 1984). We have previously shown that two segregated groups of neurons in the external lateral part (LPBel) and the dorsal part of the LPB (LPBd) are activated by cutaneous cold and warm sensory inputs and transmit the thermosensory signals to the POA to elicit cold-defensive and heat-defensive autonomous responses, respectively (Nakamura and Morrison, 2008, 2010). Therefore, in the present study, we first investigated whether LPB→MnPO projection neurons are involved in behavioral thermoregulation.
To this end, we chronically inhibited transmission of LPB→MnPO neurons in rats by pathway-selective transduction with TeTxLC (Fig. 1C), which blocks neurotransmitter release at axon terminals by cleaving v-SNARE proteins (Link et al., 1992; Schiavo et al., 1992). An AAV for Cre recombinase-dependent expression of TeTxLC and an AAVrg for Cre expression were injected into the LPB (bilateral) and MnPO, respectively, to selectively inhibit double-infected neurons (i.e., LPB→MnPO neurons) (Fig. 1C). As expected, numerous neurons in the LPBel and LPBd expressed EYFP (indicative of TeTxLC expression), and most of these EYFP-expressing neurons also expressed Cre recombinase [91 ± 2% (mean ± SEM of randomly chosen 3 rats) of EYFP-expressing neurons exhibited Cre immunoreactivity] (Fig. 1D), indicating selective TeTxLC expression in LPB→MnPO neurons. No EYFP-labeled cell bodies were found in areas other than the LPB. LPB→MnPO neurons in control rats were transduced with palGFP instead of TeTxLC. palGFP-labeled axons of LPB→MnPO neurons were densely distributed in the MnPO and adjacent medial preoptic area, but were not observed in the CeA (Fig. 1E).
The rats were subjected to a two-floor innocuous TPPT (Fig. 1F). During the test, the rats freely moved between the two thermal plate floors, one of which was set at 28°C, the thermoneutral temperature for laboratory rats, and the other was set at 39°C (warm) or 15°C (cold) under a room temperature of 25°C. Control rats preferred to stay on the 28°C plate rather than the 39°C or 15°C plate, exhibiting typical heat and cold avoidance behavior (Fig. 1G,H). In contrast, TeTxLC-transduced rats did not exhibit heat avoidance, but rather a slight heat preference (% of time spent on 28°C and 39°C plates: 34.5 ± 5.2% and 65.5 ± 5.2%, n = 7; paired t test, t(6) = 2.96, p = 0.025), resulting in a higher Tcore than control rats (Fig. 1G). However, TeTxLC-transduced rats did exhibit cold avoidance like control rats, and changes in Tcore were not different between the groups (Fig. 1H). In the tests with both temperature settings, the number of transition times and the distances the rats moved between the plates did not differ between control and TeTxLC-transduced rats (Fig. 1G,H). These results indicate that heat avoidance, but not cold avoidance, requires transmission by LPB→MnPO neurons.
Because we have shown that cold sensory signals through the LPB→MnPO pathway elicit autonomous cold defense responses (Nakamura and Morrison, 2008), the effect of TeTxLC-mediated blockade of LPB→MnPO neurons on skin cooling-evoked BAT thermogenesis was examined in an anesthetized preparation. In palGFP-transduced control rats, cooling the trunk skin evoked increases in BAT SNA, TBAT, expired CO2, and HR under the conditions of 37°C Tcore (Fig. 2A) (Nakamura and Morrison, 2007). However, these skin cooling-evoked physiological responses were abolished in TeTxLC-transduced rats under the same Tcore conditions (Fig. 2B,C). Skin cooling-evoked changes in MAP were comparable between the rat groups (Fig. 2C). These results are consistent with the notion that LPB→MnPO thermosensory transmission is required to elicit feedforward thermogenic and cardiac responses to environmental cold challenges (Nakamura and Morrison, 2008).
Next, we examined the effect of TeTxLC-mediated blockade of LPB→MnPO neurons on BAT thermogenesis evoked by body core cooling. In control rats, reduction of Tcore in 1°C decrements from 38°C to 34°C, while Tskin was controlled at 40°C, gradually increased BAT SNA, expired CO2 and MAP, but TBAT and HR were sustained (Fig. 2D,F). In TeTxLC-transduced rats, however, the same reduction in Tcore did not evoke BAT SNA and significantly reduced TBAT, expired CO2, and MAP compared with control rats (Fig. 2E,F). These results indicate that LPB→MnPO cold sensory transmission is required not only for physiological responses to environmental cooling, but also for cold defense responses to body cooling. The results also indicate that the AAV transduction with TeTxLC successfully blocked the cold sensory transmission by LPB→MnPO neurons, supporting our result from the TPPT that these neurons are not involved in cold avoidance.
We further investigated the effect of chronic blockade of LPB→MnPO transmission on the diurnal control of body temperature and appetitive behaviors. TeTxLC-transduced rats maintained a higher Tcore during the dark phase than control rats (Fig. 3A). Daily food intake of TeTxLC-transduced rats was also higher than that of control rats, although there was no obvious difference in circadian changes in hourly food intake (Fig. 3C). The level of activity and water intake were comparable between the rat groups (Fig. 3B,D). To test the ability of TeTxLC-transduced rats to defend Tcore against ambient heat and cold, they were exposed to an environment of 36°C or 4°C for 120 min. During exposure to 36°C, Tcore of TeTxLC-transduced rats showed a greater increase than that of control rats, reaching almost 40°C at the end of the exposure (Fig. 3E). Activity counts, food intake, and water intake during heat exposure were comparable between the rat groups (Fig. 3F–H). During exposure to 4°C, TeTxLC-transduced rats exhibited a significant reduction in Tcore compared with control rats, which were able to maintain their Tcore around 37°C (Fig. 3I). Control rats exhibited a cold-induced increase in food intake, which supplies energy for adaptive thermogenesis, during the second half of the cold exposure, whereas TeTxLC-transduced rats showed a reduced food intake (Fig. 3K), suggesting a role for LPB→MnPO neurons in the increased food intake associated with cold defense responses. Activity and water intake during the cold exposure did not differ between the rat groups (Fig. 3J,L). These results demonstrate that thermosensory afferent signals transmitted by LPB→MnPO neurons are essential for maintaining thermal homeostasis in hot and cold environments.
Figure 3.

TeTxLC-mediated suppression of LPB→MnPO neurons affects diurnal control of body temperature and appetitive behaviors and heat and cold tolerance. A-D, Effects of TeTxLC expression in LPB→MnPO neurons on diurnal changes in Tcore, activity counts, food intake, and water intake (left), which were analyzed by repeated-measures two-way ANOVA followed by Bonferroni's post hoc test [n = 7 per group; A: group: F(1,12) = 35.17, p < 0.001, time: F(287, 3444) = 38.10, p < 0.001, interaction: F(287, 3444) = 2.03, p < 0.001; B: group: F(1,12) = 1.65, p = 0.223, time: F(47, 564) = 20.87, p < 0.001, interaction: F(47, 564) = 0.84, p = 0.771; C: group: F(1,12) = 8.42, p = 0.013, time: F(47, 564) = 12.45, p < 0.001, interaction: F(47, 564) = 1.24, p = 0.139; D: group: F(1,12) = 0.52, p = 0.487, time: F(47, 564) = 13.75, p < 0.001, interaction: F(47, 564) = 1.47, p = 0.026]. †p < 0.05, time points with a significant difference between the groups. Right graphs represent intergroup differences in average Tcore and daily activity counts, food intake, and water intake, which were analyzed by unpaired t tests (n = 7 per group; A: t(12) = 5.92; B: t(12) = 1.29; C: t(12) = 2.90; D: t(12) = 0.72). *p < 0.05. ***p < 0.001. E-L, Rats with palGFP- or TeTxLC expression in LPB→MnPO neurons were exposed to 36°C (E-H) or 4°C (I-L). Time course changes in Tcore, activity counts, food intake, and water intake (left graphs) were analyzed by repeated-measures two-way ANOVA followed by Bonferroni's post hoc test [n = 7 per group; E: group: F(1,12) = 23.98, p < 0.001, time: F(140, 1680) = 141.0, p < 0.001, interaction: F(140, 1680) = 10.69, p < 0.001; F: group: F(1,12) = 0.00, p = 0.953, time: F(6,72) = 17.52, p < 0.001, interaction: F(6,72) = 0.71, p = 0.643; G: group: F(1,12) = 3.49, p = 0.087, time: F(7,84) = 2.03, p = 0.061, interaction: F(7,84) = 2.21, p = 0.041; H: group: F(1,12) = 0.78, p = 0.393, time: F(7,84) = 1.17, p = 0.331, interaction: F(7,84) = 0.81, p = 0.579; I: group: F(1,12) = 7.84, p = 0.016, time: F(140, 1680) = 4.296, p < 0.001, interaction: F(140, 1680) = 7.68, p < 0.001; J: group: F(1,12) = 1.473, p = 0.248, time: F(6,72) = 2.42, p = 0.034, interaction: F(6,72) = 0.36, p = 0.904; K: group: F(1,12) = 9.27, p = 0.010, time: F(7,84) = 2.69, p = 0.014, interaction: F(7,84) = 2.08, p = 0.055; L: group: F(1,12) = 2.11, p = 0.171, time: F(7,84) = 2.60, p = 0.018, interaction: F(7,84) = 0.61, p = 0.746]. †p < 0.05, time points with a significant difference between the groups. Right graphs represent intergroup differences in ΔTcore (difference between values at time 0 and 120 min), total activity counts, total food intake, and water intake, which were analyzed by unpaired t tests [n = 7 per group; E: t(12) = 4.51; F: t(12) = 0.03; G: t(12) = 1.90; H: t(12) = 1.39; I: t(12) = 4.96; J: t(12) = 1.23; K: t(12) = 3.01; L: t(12) = 1.38]. *p < 0.05. ***p < 0.001. Error bars indicate SEM.
LPB→MnPO neurons mediate heat avoidance via synaptic inputs to the MnPO
It was possible that the TeTxLC-mediated chronic inhibition of LPB→MnPO neurons might have abolished heat avoidance by causing long-term circuit alternations. Moreover, axons of LPB→MnPO neurons were distributed not only in the POA, including the MnPO, but their collaterals were densely distributed in several other regions, including the DMH, paraventricular hypothalamic nucleus, lateral hypothalamic area, paraventricular thalamic nucleus, parasubthalamic nucleus, and lateral periaqueductal gray (Fig. 1E). Therefore, we performed acute local chemogenetic inhibition of synaptic release to examine whether direct synaptic inputs from the LPB to the MnPO mediate the thermosensory afferent signaling required to elicit heat avoidance. The Cre-dependent expression system with anterograde and retrograde AAVs was used to selectively transduce LPB→MnPO neurons with the inhibitory DREADD hM4Dinrxn, an axon-targeted variant of hM4Di conjugated with an intracellular domain of neurexin 1α (Stachniak et al., 2014) (Fig. 4A). Activation of hM4Dinrxn suppresses synaptic release probability (Stachniak et al., 2014). Similar to TeTxLC expression (Fig. 1C,D), many neurons in the LPBel and LPBd expressed mCherry (indicative of hM4Dinrxn expression) and most of these mCherry-expressing neurons also expressed Cre (Fig. 4B) [93 ± 2% (mean ± SEM of randomly chosen 3 rats) of mCherry-expressing neurons exhibited Cre immunoreactivity], indicating the high selectivity of hM4Dinrxn expression in LPB→MnPO neurons. LPB→MnPO neurons in control rats were transduced with palGFP instead of hM4Dinrxn.
Figure 4.

Local chemogenetic inhibition of presynaptic release from MnPO axon terminals of LPB→MnPO neurons abolishes heat avoidance. A, Selective transduction of LPB→MnPO neurons with hM4Dinrxn-T2A-mCherry using AAV and AAVrg. Control rats expressed palGFP instead of hM4Dinrxn-T2A-mCherry. B, mCherry- and/or Cre-immunoreactive neurons in the LPB. Scale bars, 500 µm. C, The AAV-injected rats were nanoinjected with saline or C21 into the MnPO (top). Bottom map represents injection sites in the MnPO of the rats AAV-transduced with palGFP (black dots) or mCherry-T2A-hM4Dinrxn (red dots). Scale bar, 1.0 mm. D, E, Effects of saline or C21 nanoinjection into the MnPO of rats expressing palGFP or hM4Dinrxn in LPB→MnPO neurons on heat (D) and cold (E) avoidance. Time course changes (left, every 1 min) in % duration of stay on 39°C or 15°C plate, times of entry into 39°C or 15°C plate, distance traveled, and Tcore. Right graphs represent % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and ΔTcore (difference between value at time 0 and peak) for the 30 min test period. Data were analyzed by repeated-measures one-way ANOVA followed by Bonferroni's post hoc test [D: palGFP, n = 5; hM4Dinrxn, n = 6; duration of stay: F(3,18) = 8.87, p = 0.001; entries: F(3,18) = 0.04, p = 0.989; distance: F(3,18) = 2.25, p = 0.117; ΔTcore: F(3,18) = 4.09, p = 0.022; E: palGFP, n = 5; hM4Dinrxn, n = 5; duration of stay: F(3,16) = 0.91, p = 0.456; entries: F(3,16) = 0.21, p = 0.889; distance: F(3,16) = 0.66, p = 0.588; ΔTcore: F(3,16) = 1.17, p = 0.353]. *p < 0.05. †p < 0.05. ††p < 0.01. F, Rats expressing mCherry-T2A-hM4Dinrxn in LPB→MnPO neurons received bilateral nanoinjections into the DMH with saline or C21 (top). Bottom map represents injection sites (red dots tied with a line). Scale bar, 500 µm. mt, Mammillothalamic tract. G, H, Effects of saline or C21 nanoinjections into the DMH on heat (G) and cold (H) avoidance. Graphs represent % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and ΔTcore (difference between value at time 0 and peak) for the 30 min test period. Data were analyzed by paired t tests [n = 7; G: duration of stay, t(6) = 1.30; entries, t(6) = 0.22; distance, t(6) = 0.50; ΔTcore, t(6) = 0.47; H: duration of stay, t(6) = 0.26; entries, t(6) = 0.00; distance, t(6) = 0.05; ΔTcore, t(6) = 0.83]. Error bars indicate SEM.
To locally inhibit synaptic release from LPB→MnPO axons in the MnPO, we nanoinjected the actuator, DREADD agonist 21 (C21), through a cannula preimplanted into the MnPO 5 min before TPPT (Fig. 4C). When tested for heat avoidance (39°C vs 28°C), hM4Dinrxn-transduced rats nanoinjected with C21 into the MnPO did not exhibit heat avoidance, but rather a slight heat preference (Fig. 4D; % of time spent on 28°C and 39°C plates: 38.4 ± 7.4% and 61.6 ± 7.4%, n = 6; paired t test, t(5) = 3.17, p = 0.025), similar to the rats with TeTxLC expression in LPB→MnPO neurons (Fig. 1G). In contrast, the same hM4Dinrxn-transduced rats following saline nanoinjection, as well as control rats following either saline or C21 nanoinjection into the MnPO, exhibited heat avoidance (Fig. 4D). Because of the lack of heat avoidance, hM4Dinrxn-transduced rats exhibited a significantly greater increase in Tcore following C21 nanoinjection than control rats, which also exhibited a mild elevation of Tcore because of injection stress (Fig. 4D). However, the increase in Tcore of hM4Dinrxn-transduced rats following C21 nanoinjection was not significantly higher than that following saline nanoinjection (Fig. 4D). The heat preference diminished in the last 10 min of the test period, possibly because of the hyperthermic state, which may activate the feedback mechanism involving activation of warm-sensitive POA neurons to avoid further hyperthermia (Morrison and Nakamura, 2019). C21 did not affect the number of transition times or the distances the rats moved between the plates (Fig. 4D). In the cold avoidance test (15°C vs 28°C), nanoinjection of C21 did not affect cold avoidance or changes in Tcore exhibited by hM4Dinrxn-transduced rats (Fig. 4E).
In another group of rats, we inhibited synaptic release in the DMH from collateral axons of LPB→MnPO neurons and examined the effect on heat and cold avoidance. Bilateral nanoinjections of C21 into the DMH of rats with hM4Dinrxn expression in LPB→MnPO neurons did not affect heat or cold avoidance behavior or Tcore changes (Fig. 4F–H). This result means that C21 injected locally at the DMH did not diffuse to the MnPO to affect the LPB→MnPO axon terminals, indicating a localized effect of C21. These results demonstrate that thermosensory synaptic inputs to the MnPO from LPB→MnPO neurons mediate heat avoidance.
LPB→CeA neurons are cold-activated and segregate from LPB→MnPO neurons
To investigate the involvement of LPB→CeA neurons in innocuous thermosensory transmission, we performed a functional retrograde tracing study. CTb, a retrograde neural tracer, was bilaterally injected into the CeA (Fig. 5A,B), and the rats were exposed to an environment of 4°C (cold exposure), 25°C (control exposure), or 36°C (heat exposure) for 2 h. Among the rat groups, comparable numbers of CTb-labeled cell bodies were observed in the parabrachial nucleus subdivisions: the central part of the LPB, the LPBd, the LPBel, the internal part of the LPB, and the medial parabrachial nucleus (Fig. 5C–E). Cold exposure significantly increased CTb-labeled neurons with expression of Fos, a marker for neuronal activation, in the LPBel, compared with control exposure (Fig. 5C,D,F). However, heat exposure did not increase Fos expression in CTb-labeled parabrachial neurons (Fig. 5C,D,F).
Figure 5.

LPB→CeA neurons are cold-activated and segregate from LPB→MnPO neurons. A, B, CTb was injected into the CeA. E, F, Injection sites in the right CeA for the group data are mapped in B (green: 25°C-exposed; blue: 4°C-exposed; red: 36°C-exposed). C, CTb- and Fos-immunoreactive cells in the LPBel following a 2 h exposure to ambient temperature of 25°C (control), 4°C (cold), or 36°C (heat). Solid and hollow arrowheads indicate CTb-labeled neuronal cell bodies with and without Fos immunoreactivity, respectively. Scale bar, 50 μm. D, Distributions of CTb-labeled and/or Fos-immunoreactive cells in the parabrachial nucleus following exposure to the respective temperature. Scale bar, 500 µm. LPBi, internal part of the LPB; MPB, medial parabrachial nucleus. E, F, Numbers of CTb-labeled cells (E) and Fos-immunoreactive CTb-labeled cells (F) in subareas of the parabrachial nucleus (counted in the right hemisphere; n = 4 rats per group). Data were analyzed by one-way ANOVA followed by Bonferroni's post hoc test [E: LPBc: F(2,9) = 0.72, p = 0.515; LPBd: F(2,9) = 0.11, p = 0.900; LPBel: F(2,9) = 0.77, p = 0.490; LPBi: F(2,9) = 0.19, p = 0.827; MPB: F(2,9) = 0.17, p = 0.849; F: LPBc: F(2,9) = 1.55, p = 0.264; LPBd: F(2,9) = 2.82, p = 0.112; LPBel: F(2,9) = 6.00, p = 0.022; LPBi: F(2,9) = 3.19, p = 0.090; MPB: F(2,9) = 1.87, p = 0.209]. *p < 0.05. Error bars indicate SEM. G, Double retrograde labeling of LPB→MnPO and LPB→CeA neurons with CTb conjugated with different fluorophores. H, Injection sites of CTb into the CeA and MnPO. Scale bars, 500 µm. I, Distribution of CTb-labeled cells in the parabrachial nucleus. Scale bar, 500 µm.
We next compared the distribution of LPB→MnPO neurons and LPB→CeA neurons within the LPB. Injections of CTb with different fluorophores into the MnPO and CeA of the same rat revealed adjacent but clearly segregated distributions of LPB→MnPO neurons and LPB→CeA neurons, and few neurons were double-labeled (Fig. 5G–I). This result is consistent with the lack of axon collaterals of LPB→MnPO neurons in the CeA (Fig. 1E). In the LPBel, its rostral part harbored LPB→MnPO neurons, which are activated by cutaneous cold sensory inputs (Nakamura and Morrison, 2008), whereas the caudal part was occupied by LPB→CeA neurons, which were also activated by cold exposure (Fig. 5D,I). These results indicate that distinct groups of LPB neurons innervate the MnPO and CeA and that CeA-projecting neurons in the LPBel are cold-activated.
Essential roles of LPB→CeA neurons in behavioral and autonomous thermoregulation
To investigate the involvement of LPB→CeA neurons in behavioral thermoregulation, we transduced LPB→CeA neurons with TeTxLC or palGFP (control) by using the Cre-dependent expression system with anterograde and retrograde AAVs (Fig. 6A), and the rats were then subjected to TPPTs. Many neurons expressing EYFP (indicative of TeTxLC expression) were distributed in the LPBel (Fig. 6B), similar to neurons labeled with CTb injected into the CeA (Fig. 5D). High selectivity of TeTxLC expression in LPB→CeA neurons was demonstrated by Cre expression in most EYFP-expressing LPBel neurons [95 ± 0.6% (mean ± SEM of randomly chosen 3 rats) of EYFP-expressing neurons exhibited Cre immunoreactivity]. In TPPTs, control rats preferred the 28°C plate to the 39°C and 15°C plates, indicating avoidance of heat and cold, respectively (Fig. 6C,D). In contrast, TeTxLC-transduced rats exhibited neither heat nor cold avoidance (Fig. 6C,D; % of time spent on 28°C and 39°C plates: 42.5 ± 6.2% and 57.5 ± 6.2%, n = 13, paired t test, t(12) = 1.21, p = 0.25; % of time spent on 28°C and 15°C plates: 55.2 ± 5.8% and 44.8 ± 5.8%, n = 13, paired t test, t(12) = 0.90, p = 0.38). Because of the failure of heat avoidance, TeTxLC-transduced rats showed a greater increase in Tcore than control rats, but the failure of cold avoidance did not affect Tcore (Fig. 6C,D). The number of transition times and the distances the rats moved between the plates did not differ between control and TeTxLC-transduced rats (Fig. 6C,D). palGFP-labeled axons of LPB→CeA neurons were abundantly distributed not only in the CeA, but also in several other brain regions, including the lateral part of the BNST, paraventricular hypothalamic nucleus, VMH, paraventricular thalamic nucleus, parasubthalamic nucleus, and ventrolateral and lateral periaqueductal gray (Fig. 6E). Only sparse axon collaterals of LPB→CeA neurons were observed in the POA, consistent with their segregation from LPB→MnPO neurons. These results suggest that LPB→CeA neurons play an essential role in heat and cold avoidance by transmitting cold sensory signals to these projection sites.
Figure 6.

TeTxLC-mediated chronic suppression of LPB→CeA neurons abolishes heat and cold avoidance. A, Selective transduction of LPB→CeA neurons with EYFP-T2A-TeTxLC using AAV and AAVrg. B, EYFP- and/or Cre-immunoreactive neurons in the LPBel. Scale bar, 500 µm. C, D, Effects of the TeTxLC expression in LPB→CeA neurons on heat (C) and cold (D) avoidance. Control rats expressed palGFP instead of EYFP-T2A-TeTxLC. Time course changes (left, every 1 min) in % duration of stay on 39°C or 15°C plate, times of entry into 39°C or 15°C plate, distance traveled, and Tcore. Right graphs, % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and ΔTcore (difference between value at time 0 and peak) for the 20 min test period. Data were analyzed by unpaired t tests [palGFP: n = 9, TeTxLC: n = 13; C: duration of stay, t(20) = 3.25; entries, t(20) = 0.16; distance, t(20) = 0.32; ΔTcore, t(20) = 2.34; D: duration of stay, t(20) = 3.32; entries, t(20) = 0.04; distance, t(20) = 0.62; ΔTcore, t(20) = 0.89]. *p < 0.05. **p < 0.01. Error bars indicate SEM. E, Distribution of palGFP-labeled axons of LPB→CeA neurons in a control rat. Scale bars, 1.0 mm.
To investigate whether LPB→CeA neurons are also involved in autonomous thermoregulation, we performed in vivo electrophysiology in anesthetized rats expressing TeTxLC or palGFP (control) in LPB→CeA neurons. In response to trunk skin cooling under the Tcore condition of 37°C, control rats showed increases in BAT SNA, TBAT, expired CO2, HR, and MAP, which were, however, diminished in TeTxLC-transduced rats (Fig. 7A–C). In response to reduction of Tcore in 1°C decrements from 38°C to 34°C with Tskin controlled at 40°C, control rats showed gradual increases in BAT SNA, expired CO2, HR, and MAP (Fig. 7D,F). Interestingly, TeTxLC-transduced rats showed comparable increases in these variables in response to the same reduction in Tcore (Fig. 7E,F), unlike rats expressing TeTxLC in LPB→MnPO neurons (Fig. 2E,F). These results indicate that LPB→CeA neurons play an important role in feedforward autonomous responses to skin cooling as well as in behavioral thermoregulation, but not in cold defense responses to body cooling.
Further investigation of the effect of chronic blockade of LPB→CeA neurons with TeTxLC on the diurnal control of body temperature and appetitive behaviors revealed that TeTxLC-transduced rats exhibited an ∼0.5°C higher Tcore than palGFP-transduced control rats throughout the 2 d recording period (Fig. 8A). Daily food intake of TeTxLC-transduced rats was also higher, whereas their activity and water intake were comparable with those of control rats (Fig. 8B–D). Next, we examined whether the TeTxLC-transduced rats could maintain their Tcore in a 36°C or 4°C environment. During exposure to 36°C, TeTxLC-transduced rats showed a greater increase in Tcore than control rats, reaching almost 40°C at the end of the 120 min exposure (Fig. 8E). Activity counts and food and water intake were not different between the two groups of rats (Fig. 8F–H). During exposure to 4°C, TeTxLC-transduced rats showed a 1.6°C drop in Tcore and consumed less food and water than control rats, but had comparable activity levels (Fig. 8I–L). These results show that LPB→CeA neurons play an essential role in the maintenance of body temperature under environmental thermal challenges and in the expression of consummatory behaviors in a cold environment.
Figure 8.

TeTxLC-mediated suppression of LPB→CeA neurons affects diurnal control of body temperature and appetitive behaviors and heat and cold tolerance. A-D, Effects of TeTxLC expression LPB→CeA neurons on diurnal changes in Tcore, activity counts, food intake, and water intake (left), which were analyzed by repeated-measures two-way ANOVA followed by Bonferroni's post hoc test [palGFP, n = 6; TeTxLC, n = 7; A: group: F(1,11) = 51.41, p < 0.001, time: F(287, 3157) = 35.67, p < 0.001, interaction: F(287, 3157) = 1.66, p < 0.001; B: group: F(1,11) = 0.54, p = 0.476, time: F(47, 517) = 20.63, p < 0.001, interaction: F(47, 517) = 1.16, p = 0.230; C: group: F(1,11) = 11.27, p = 0.006, time: F(47, 517) = 10.54, p < 0.001, interaction: F(47, 517) = 0.83, p = 0.784; D: group: F(1,11) = 0.08, p = 0.786, time: F(47, 517) = 8.41, p < 0.001, interaction: F(47, 517) = 1.20, p = 0.182]. †p < 0.05, time points with a significant difference between the groups. Right graphs represent intergroup differences in average Tcore and daily activity counts, food intake, and water intake, which were analyzed by unpaired t tests (palGFP, n = 6; TeTxLC, n = 7; A: t(11) = 7.114; B: t(11) = 0.737; C: t(11) = 5.144; D: t(11) = 0.317). ***p < 0.001. E-L, Rats with palGFP- or TeTxLC expression in LPB→CeA neurons were exposed to 36°C (E-H) or 4°C (I-L). Time course changes in Tcore, activity counts, food intakes, and water intake (left graphs) were analyzed by repeated-measures two-way ANOVA followed by Bonferroni's post hoc test [palGFP, n = 6; TeTxLC, n = 7; E: group: F(1,11) = 23.73, p = 0.005, time: F(140, 1540) = 104.3, p < 0.001, interaction: F(140, 1540) = 4.76, p < 0.001; F: group: F(1,11) = 0.20, p = 0.665, time: F(6,66) = 22.41, p < 0.001, interaction: F(6,66) = 1.14, p = 0.348; G: group: F(1,11) = 2.46, p = 0.145, time: F(7,77) = 2.98, p = 0.008, interaction: F(7,77) = 1.12, p = 0.358; H: group: F(1,11) = 1.07, p = 0.324, time: F(7,77) = 1.31, p = 0.258, interaction: F(7,77) = 1.06, p = 0.401; I: group: F(1,11) = 5.32, p = 0.042, time: F(140, 1540) = 15.72, p < 0.001, interaction: F(140, 1540) = 15.45, p < 0.001; J: group: F(1,11) = 2.01, p = 0.184, time: F(6,66) = 3.46, p = 0.005, interaction: F(6,66) = 1.08, p = 0.387; K: group: F(1,11) = 6.48, p = 0.027, time: F(7,77) = 5.678, p < 0.001, interaction: F(7,77) = 2.30, p = 0.035; L: group: F(1,11) = 6.32, p = 0.029, time: F(7,77) = 3.61, p = 0.002, interaction: F(7,77) = 3.57, p = 0.002]. †p < 0.05, time points with a significant difference between the groups. Right graphs represent intergroup differences in ΔTcore (difference between values at time 0 and 120 min), total activity counts, food intake, and water intake, which were analyzed by unpaired t tests (palGFP, n = 6; TeTxLC, n = 7; E: t(11) = 2.80; F: t(11) = 0.43; G: t(11) = 1.54; H: t(11) = 1.01; I: t(11) = 5.96; J: t(11) = 1.41; K: t(11) = 2.55; L: t(11) = 2.51). *p < 0.05. ***p < 0.001. Error bars indicate SEM.
LPB→CeA neurons mediate cold avoidance via axonal inputs to the CeA
Because LPB→CeA neurons project their collateral axons to several brain sites other than the CeA (Fig. 6E), we determined whether their axonal inputs to the CeA mediate heat and cold avoidance behavior by selectively suppressing their CeA axon terminals. Because our attempt at local chemogenetic inhibition of CeA axon terminals failed because of damage to LPB→CeA axons passing through the stria terminalis by the guide cannula inserted into the CeA, we performed optogenetic inhibition of CeA axon terminals using optical fibers thinner and less invasive than the cannula. The Cre-dependent expression system with anterograde and retrograde AAVs was used to selectively transduce LPB→CeA neurons with iChloC, a chloride-conducting channelrhodopsin for photoinhibition of neuronal activity (Wietek et al., 2015) (Fig. 9A). Neurons expressing mCherry-conjugated iChloC were predominantly distributed in the LPBel and most of them expressed Cre [94 ± 4% (mean ± SEM of randomly chosen 3 rats) of mCherry-expressing neurons exhibited Cre immunoreactivity] (Fig. 9B), demonstrating the high selectivity of iChloC expression in LPB→CeA neurons. Many mCherry-labeled axon terminals were found in the CeA and some formed basket-like terminals surrounding a cell body (Fig. 9B), but no cell bodies in the CeA were labeled with mCherry. Even with bilateral local photoinhibition of CeA axon terminals of LPB→CeA neurons over the 20 min period of TPPT (Fig. 9C), iChloC-mCherry-transduced rats exhibited heat avoidance and Tcore changes similar to control rats expressing palGFP instead of iChloC-mCherry (Fig. 9D). In the cold avoidance test, however, local photoinhibition of LPB→CeA axon terminals abolished cold avoidance behavior of iChloC-mCherry-transduced rats (% of time spent on 28°C and 15°C plates: 57.9 ± 9.7% and 42.1 ± 9.7%, n = 8; paired t test, t(7) = 0.81, p = 0.44), although their Tcore changes and the number of transition times and the distances they moved between the plates were comparable to those of control rats (Fig. 9E).
Figure 9.

Local optogenetic inhibition of CeA axon terminals of LPB→CeA neurons abolishes cold avoidance. A, Selective transduction of LPB→CeA neurons with iChloC-mCherry using AAV and AAVrg. Control rats expressed palGFP instead of iChloC-mCherry. B, iChloC-mCherry- and/or Cre-immunoreactive neurons in the LPB (top). Their axon terminals with mCherry immunoreactivity were distributed in the CeA (bottom) and formed basket-like structures (inset). Scale bars: top, 200 µm; bottom, 100 µm; inset, 10 µm. C, CeA axon terminals of LPB→CeA neurons were illuminated. D, E, Effects of illumination (light blue) of CeA axon terminals in rats expressing palGFP or iChloC-mCherry in LPB→CeA neurons on heat (D) and cold (E) avoidance. Time course changes (left, every 1 min) in % duration of stay on 39°C or 15°C plate, times of entry into 39°C or 15°C plate, distance traveled, and Tcore. Right graphs represent % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and ΔTcore (difference between value at time 0 and peak) for the 20 min test period. Data were analyzed by repeated-measures one-way ANOVA followed by Bonferroni's post hoc test [palGFP, n = 6, iChloC-mCherry: n = 8; D: duration of stay: F(3,24) = 1.31, p = 0.294; entries: F(3,24) = 2.00, p = 0.141; distance: F(3,24) = 1.17, p = 0.342; ΔTcore: F(3,24) = 1.73, p = 0.187; E: duration of stay: F(3,24) = 6.79, p = 0.002; entries: F(3,24) = 0.51, p = 0.679; distance: F(3,24) = 0.43, p = 0.736; ΔTcore: F(3,24) = 3.17, p = 0.043). **p < 0.01. ††p < 0.01. F–K, Rats expressing iChloC-mCherry in LPB→CeA neurons received bilateral illumination of the VMH (F–H) or BNST (I–K). F, I, Bottom images represent VMH and BNST axon terminals of LPB→CeA neurons expressing iChloC-mCherry. Scale bars, 100 µm. Graphs represent effects of illumination of VMH or BNST axon terminals in rats expressing iChloC-mCherry in LPB→CeA neurons on heat (G,J) and cold (H,K) avoidance: % duration of stay on 39°C or 15°C plate, total times of entry into 39°C or 15°C plate, total distance traveled, and ΔTcore (difference between value at time 0 and peak) for the 20 min test period. The data were analyzed by paired t tests [G: n = 5; duration of stay, t(4) = 0.43; entries, t(4) = 1.93; distance, t(4) = 2.20; ΔTcore, t(4) = 0.63; H: n = 6; duration of stay, t(5) = 0.71; entries, t(5) = 0.13; distance, t(5) = 0.44; ΔTcore, t(5) = 0.70; J: n = 5; duration of stay, t(4) = 0.93; entries, t(4) = 1.72; distance, t(4) = 0.99; ΔTcore, t(4) = 1.53; K: n = 5; duration of stay, t(4) = 0.79; entries, t(4) = 0.88; distance, t(4) = 0.55; ΔTcore, t(4) = 0.81]. Error bars indicate SEM.
We also photoinhibited VMH or BNST collateral axon terminals of LPB→CeA neurons, as the VMH has recently been shown to contain cold-responsive neurons linked to autonomous thermoregulation (Feng et al., 2022) and the BNST, which is considered part of the extended amygdala, had the second densest distribution of collaterals of LPB→CeA neurons (Fig. 6E). Bilateral photoinhibition of LPB→CeA neuronal axons in the VMH or BNST had no effect on heat or cold avoidance or Tcore changes during the TPPTs (Fig. 9F–K). These results raise the notion that LPB→CeA neurons mediate cold avoidance via their cold sensory transmission to the CeA, but not via their transmission to the VMH and BNST.
Discussion
This study provides new insights into the importance of LPB-mediated ascending thermosensory pathways in behavioral thermoregulation by demonstrating that synaptic transmission at MnPO axon terminals of LPB→MnPO neurons mediates heat avoidance and that cold sensory transmission to the CeA by LPB→CeA neurons mediates cold avoidance. This study also unexpectedly shows that LPB→CeA neurons, as well as LPB→MnPO neurons, play an important role in autonomous thermoregulation. These findings provide an important framework of the LPB-mediated central thermosensory network that elicits behavioral and autonomous thermoregulatory responses in a coordinated manner to defend thermal homeostasis against environmental thermal challenges (Fig. 10).
Figure 10.
A model for the innocuous thermosensory afferent network that drives behavioral and autonomous thermoregulatory responses. The LPB→POA and LPB→CeA pathways mediate innocuous heat and cold avoidance, respectively, whereas both pathways are required for autonomous thermoregulation. Both LPB→POA and LPB→CeA pathways transmit thermosensory information from the skin. However, only the LPB→POA pathway receives thermosensory information from body core structures to integrate with (Tskin) information.
We focused on heat and cold avoidance among thermoregulatory behaviors because it is recruited in the first phase of behavioral thermoregulation in response to changes in ambient temperature. Most previous studies of thermoregulatory behavior have performed operant behavior experiments (Lipton, 1968; Carlisle, 1969; Jung et al., 2022). However, because operant behaviors require a learning process before they are acquired, studying heat and cold avoidance, innate behaviors, is considered more suitable for understanding the fundamental central circuit mechanisms of behavioral thermoregulation.
We identified LPB→MnPO neurons and LPB→CeA neurons as segregated subgroups of LPB neurons, which likely belong to the FoxP2- and Lmx1b-expressing groups of LPB neurons, respectively (Huang et al., 2021a,b). Because both groups of LPB neurons are predominantly glutamatergic (Miller et al., 2012; Geerling et al., 2016; Sun et al., 2020), the two groups of LPB neurons we focused on probably provide excitatory inputs to the projection sites.
Although both LPB and MnPO are pivotal brain sites for autonomous thermoregulation (Morrison and Nakamura, 2019; K. Nakamura et al., 2022), whether cutaneous thermosensory monosynaptic inputs from the LPB to the MnPO drive behavioral thermoregulation has been unclear. A recent study showed that optogenetic stimulation of POA axons from Pdyn-positive LPB neurons, which are activated by heat exposure, induces postural extension as seen in hot environments (Norris et al., 2021). Because Pdyn-positive LPB neurons project to multiple forebrain regions in addition to the POA (Norris et al., 2021), the induction of postural extension by optogenetic stimulation of their POA axons may be because of backfiring to activate collateral axons to another projection site. Moreover, postural extension is elicited by an increase in Tcore rather than Tskin (Roberts and Martin, 1974), and stimulation of POA axons of Pdyn-positive LPB neurons had no effect on thermal preference (Norris et al., 2021). In the present study, chemogenetic presynaptic inhibition of MnPO axon terminals of LPB→MnPO neurons abolished heat avoidance, clearly demonstrating that LPB→MnPO monosynaptic transmission of cutaneous thermosensory information drives heat avoidance. The contribution of Pdyn-positive LPB→MnPO neurons to behavioral thermoregulation seems to be limited, and further studies are awaited to identify the molecular marker that labels the neuronal group mediating the LPB→MnPO monosynaptic warm sensory transmission for heat avoidance.
Curiously, suppression of LPB→MnPO neurons had no effect on cold avoidance. However, if LPB→MnPO neurons make a subsidiary contribution to cold avoidance, the effect of blockade of these neurons could be masked by stronger driving signals mediated by other thermosensory pathways, such as the LPB→CeA pathway. More research is needed to understand the role of LPB→MnPO cold sensory transmission in behavioral thermoregulation.
Suppression of LPB→MnPO neurons with TeTxLC abolished sympathetic thermogenic and cardiovascular responses to skin cooling and impaired the animals' ability to defend Tcore in cold and hot environments. Interestingly, it also reduced the feeding response associated with the autonomous cold defense responses. These results strongly support the notion that cutaneous cold and warm sensory signaling through the LPB→MnPO pathway elicits a variety of feedforward autonomous thermoregulatory responses to defend thermal homeostasis from environmental thermal challenges (Nakamura and Morrison, 2008, 2010). The thermosensory inputs to the MnPO are considered to alter the tonic GABAergic inhibitory efferent signaling from prostaglandin EP3 receptor-expressing POA neurons, which controls sympathetic outflow through the DMH and rostral medullary raphe region to thermoregulatory effectors, such as BAT and skin blood vessels, for thermal homeostasis (Y. Nakamura et al., 2022). Suppression of LPB→MnPO neurons also abolished BAT thermogenesis evoked by Tcore reduction. Because this thermogenic response was likely elicited by activation of cold-sensitive afferents from body core structures, including the abdomen (Gupta et al., 1979), our result suggests that LPB→MnPO neurons transmit afferent signals of temperatures in the peripheral body core as well as the skin (Fig. 10). The LPB neurons mediating autonomous heat defense responses have been proposed to include those expressing Pdyn or Cck (Geerling et al., 2016; Yang et al., 2020; Norris et al., 2021), whereas no specific molecular markers have been identified for the LPB neurons mediating cold defense.
The upward shift in Tcore caused by suppression of LPB→MnPO neurons at room temperature (Fig. 3A), which probably blocked both cold and warm sensory inputs to the POA, suggests that warm sensory signals to prevent hyperthermia have a more important effect on the POA circuit mechanism for thermal homeostasis than cold sensory signals to prevent hypothermia. Consistently, rats with suppression of LPB→MnPO neurons or LPB→MnPO axon terminals exhibited a slight preference for warmth (Figs. 1G, 4D). Our results suggest that when the POA is isolated from thermosensory information from the skin and body core structures, it regulates Tcore in a higher-than-normal set-point range under thermoneutral conditions, presumably by enhancing warmth seeking and reducing heat loss. LPB→MnPO thermosensory inputs appear to be important in controlling Tcore within a normal range.
LPB→CeA neurons have been shown to respond to nociceptive stimuli (Bernard and Besson, 1990; Gauriau and Bernard, 2002). Our present study showed that LPB→CeA neurons are activated in response to cold exposure and mediate cold avoidance. Because the temperatures used in the cold exposure and cold avoidance test in our study were innocuous, our results indicate that LPB→CeA neurons transmit innocuous cold sensory signals. Consistent with this view, LPB-projecting lamina I neurons in the spinal cord respond polymodally to hot, innocuous cold, and nociceptive stimuli (Chisholm et al., 2021). However, it remains unknown whether the innocuous cold-responsive group of LPB→CeA neurons also responds to other modalities of sensory signals, including pain.
Intriguingly, heat avoidance was abolished by chronic suppression of LPB→CeA neurons with TeTxLC, but not by acute photoinhibition of CeA axon terminals of LPB→CeA neurons. Neither did photoinhibition of VMH or BNST axon terminals of LPB→CeA neurons affect heat avoidance. These results suggest that heat avoidance requires transmission via axon collaterals of these neurons to a projection site other than the CeA, VMH, and BNST. Further investigation by photoinhibition of each collateral projection site of LPB→CeA neurons may identify the pathway responsible for heat avoidance. Alternatively, it is possible that a long-term alteration of the neural circuitry by the TeTxLC-mediated chronic suppression of LPB→CeA neurons might have diminished heat avoidance.
The extensive collateral projections from LPB→CeA neurons to emotion-related brain regions suggest that the ascending network of these neurons underlies the generation of the emotions of thermal comfort and discomfort that drive thermoregulatory behavior. Among the emotion-related regions, the amygdala has been recognized as an important site for the memory of aversive stimuli, such as fear and pain, to elicit aversive (avoidance) behavior from the stimuli based on experience (Chiang et al., 2020). However, whether the amygdala stores memories of innocuous thermal sensations has been unknown. As shown in this study, avoidance behavior was usually enhanced in the later period of TPPTs, by which time a thermosensory memory might be stored in the CeA, whereas blockade of LPB→CeA monosynaptic inputs abolished cold avoidance, possibly by blocking the formation of a memory of cold sensation on the plate. The reason why the amygdala stores the memory of cold sensation, but not of warm sensation, may be that, for small mammals, cold sensation, potentially leading to hypothermia, is more aversive than warm sensation, since moderate heat gain often has a positive value in the systemic energy balance. If thermosensory inputs to the CeA generate the unpleasant emotions that underlie innate thermoregulatory behaviors, an important future question is how the unpleasant thermosensory memories stored in the amygdala are integrated with the POA-driven commands to develop thermal avoidance behaviors.
Unexpectedly, suppression of LPB→CeA neurons with TeTxLC abolished skin cooling-evoked BAT thermogenesis and cardiovascular responses and also impaired the ability to defend Tcore against environmental thermal challenges. It also diminished the feeding and drinking responses during cold defense. These present results are the first to implicate the amygdala in autonomous responses for thermal homeostasis. Because LPB→CeA neurons projected few collaterals to the POA, it is unlikely that these neurons directly influence the thermoregulatory mechanism in the POA. Unlike suppression of LPB→MnPO neurons, suppression of LPB→CeA neurons did not inhibit BAT thermogenesis evoked by Tcore reduction, suggesting that LPB→CeA neurons do not transmit thermosensory signals from body core structures (Fig. 10). In the present study, which focused more on the mechanism of behavioral thermoregulation, we did not test whether optogenetic local inhibition of CeA axon terminals of LPB→CeA neurons alters BAT thermogenesis and other autonomous responses. Further studies are needed to understand how LPB→CeA neurons contribute to autonomous thermoregulation.
Footnotes
This work was supported by Japan Science and Technology Agency Moonshot R&D JPMJMS2023 to K.N.; Ministry of Education, Culture, Sports, Science and Technology of Japan Grants-in-Aid for Scientific Research JP22K06470 and JP19K06954 to N.K. and JP23H00398 and JP20H03418 to K.N.; and Japan Agency for Medical Research and Development JP23wm0525002 to N.K. T.Y. was supported by the Takeda Science Foundation scholarship. We thank Patrick Aebischer and Scott Sternson for sharing plasmids; Misako Takemoto for technical assistance for AAV production; and Akihiro Fukushima, Manami Oya, and Yoshiko Nakamura for helpful discussion.
The authors declare no competing financial interests.
References
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA (2006) Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One 1:e1. 10.1371/journal.pone.0000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Vizin RC, Carrettiero DC (2015) Current understanding on the neurophysiology of behavioral thermoregulation. Temperature (Austin) 2:483–490. 10.1080/23328940.2015.1095270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Besson JM (1990) The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63:473–490. 10.1152/jn.1990.63.3.473 [DOI] [PubMed] [Google Scholar]
- Carlisle HJ (1969) Effect of preoptic and anterior hypothalamic lesions on behavioral thermoregulation in the cold. J Comp Physiol Psychol 69:391–402. 10.1037/h0028170 [DOI] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald AM, Ross SE (2020) Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106:927–939.e5. 10.1016/j.neuron.2020.03.014 [DOI] [PubMed] [Google Scholar]
- Chisholm KI, Lo Re L, Polgár E, Gutierrez-Mecinas M, Todd AJ, McMahon SB (2021) Encoding of cutaneous stimuli by lamina I projection neurons. Pain 162:2405–2417. 10.1097/j.pain.0000000000002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A (1994) A thalamic nucleus specific for pain and temperature sensation. Nature 372:770–773. 10.1038/372770a0 [DOI] [PubMed] [Google Scholar]
- Feng C, Wang Y, Zha X, Cao H, Huang S, Cao D, Zhang K, Xie T, Xu X, Liang Z, Zhang Z (2022) Cold-sensitive ventromedial hypothalamic neurons control homeostatic thermogenesis and social interaction-associated hyperthermia. Cell Metab 34:888–901.e5. 10.1016/j.cmet.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319:229–259. 10.1016/0165-0173(84)90012-2 [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF (2002) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87:251–258. 10.1113/eph8702357 [DOI] [PubMed] [Google Scholar]
- Geerling JC, Kim M, Mahoney CE, Abbott SB, Agostinelli LJ, Garfield AS, Krashes MJ, Lowell BB, Scammell TE (2016) Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 310:R41–R54. 10.1152/ajpregu.00094.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta BN, Nier K, Hensel H (1979) Cold-sensitive afferents from the abdomen. Pflugers Arch 380:203–204. 10.1007/BF00582158 [DOI] [PubMed] [Google Scholar]
- Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD (2015) Elucidating an affective pain circuit that creates a threat memory. Cell 162:363–374. 10.1016/j.cell.2015.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T (2010) Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol 518:668–686. 10.1002/cne.22237 [DOI] [PubMed] [Google Scholar]
- Huang D, Grady FS, Peltekian L, Geerling JC (2021a) Efferent projections of Vglut2, Foxp2, and Pdyn parabrachial neurons in mice. J Comp Neurol 529:657–693. 10.1002/cne.24975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Grady FS, Peltekian L, Laing JJ, Geerling JC (2021b) Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J Comp Neurol 529:2911–2957. 10.1002/cne.25136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Anton F, Nahin RL (1989) Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience 28:27–37. 10.1016/0306-4522(89)90229-7 [DOI] [PubMed] [Google Scholar]
- Jung S, Lee M, Kim DY, Son C, Ahn BH, Heo G, Park J, Kim M, Park HE, Koo DJ, Park JH, Lee JW, Choe HK, Kim SY (2022) A forebrain neural substrate for behavioral thermoregulation. Neuron 110:266–279.e9. 10.1016/j.neuron.2021.09.039 [DOI] [PubMed] [Google Scholar]
- Kataoka N, Hioki H, Kaneko T, Nakamura K (2014) Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab 20:346–358. 10.1016/j.cmet.2014.05.018 [DOI] [PubMed] [Google Scholar]
- Kataoka N, Shima Y, Nakajima K, Nakamura K (2020) A central master driver of psychosocial stress responses in the rat. Science 367:1105–1112. 10.1126/science.aaz4639 [DOI] [PubMed] [Google Scholar]
- Li J, Xiong K, Pang Y, Dong Y, Kaneko T, Mizuno N (2006) Medullary dorsal horn neurons providing axons to both the parabrachial nucleus and thalamus. J Comp Neurol 498:539–551. 10.1002/cne.21068 [DOI] [PubMed] [Google Scholar]
- Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, Baumert M, Südhof TC, Niemann H, Jahn R (1992) Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun 189:1017–1023. 10.1016/0006-291x(92)92305-h [DOI] [PubMed] [Google Scholar]
- Lipton JM (1968) Effects of preoptic lesions on heat-escape responding and colonic temperature in the rat. Physiol Behav 3:165–169. [Google Scholar]
- Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD (2012) Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience 218:110–125. 10.1016/j.neuroscience.2012.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K (2019) Central mechanisms for thermoregulation. Annu Rev Physiol 81:285–308. 10.1146/annurev-physiol-020518-114546 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2007) Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292:R127–R36. 10.1152/ajpregu.00427.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2008) Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol 586:2611–2620. 10.1113/jphysiol.2008.152686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2010) A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107:8848–8853. 10.1073/pnas.0913358107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2011) Central efferent pathways for cold-defensive and febrile shivering. J Physiol 589:3641–3658. 10.1113/jphysiol.2011.210047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M (2000) Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol 421:543–569. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T (2004) Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24:5370–5380. 10.1523/JNEUROSCI.1219-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nakamura Y, Kataoka N (2022) A hypothalamomedullary network for physiological responses to environmental stresses. Nat Rev Neurosci 23:35–52. 10.1038/s41583-021-00532-x [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yahiro T, Fukushima A, Kataoka N, Hioki H, Nakamura K (2022) Prostaglandin EP3 receptor-expressing preoptic neurons bidirectionally control body temperature via tonic GABAergic signaling. Sci Adv 8:eadd5463. 10.1126/sciadv.add5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Shaker JR, Cone AL, Ndiokho IB, Bruchas MR (2021) Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. Elife 10:e60779. 10.7554/eLife.60779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. 6th edition, London, UK: Academic Press. [Google Scholar]
- Roberts WW, Martin JR (1974) Peripheral thermoreceptor control of thermoregulatory responses of the rat. J Comp Physiol Psychol 87:1109–1118. 10.1037/h0037584 [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD (1980) Efferent connections of the parabrachial nucleus in the rat. Brain Res 197:291–317. 10.1016/0006-8993(80)91117-8 [DOI] [PubMed] [Google Scholar]
- Satinoff E, Rutstein J (1970) Behavioral thermoregulation in rats with anterior hypothalamic lesions. J Comp Physiol Psychol 71:77–82. 10.1037/h0028959 [DOI] [PubMed] [Google Scholar]
- Sato M, Ito M, Nagase M, Sugimura YK, Takahashi Y, Watabe AM, Kato F (2015) The lateral parabrachial nucleus is actively involved in the acquisition of fear memory in mice. Mol Brain 8:22. 10.1186/s13041-015-0108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359:832–835. 10.1038/359832a0 [DOI] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM (2014) Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82:797–808. 10.1016/j.neuron.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Liu R, Guo F, Wen MQ, Ma XL, Li KY, Sun H, Xu CL, Li YY, Wu MY, Zhu ZG, Li XJ, Yu YQ, Chen Z, Li XY, Duan S (2020) Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat Commun 11:5974. 10.1038/s41467-020-19767-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ishida Y, Kataoka N, Nakamura K, Hioki H (2021) Efficient labeling of neurons and identification of postsynaptic sites using adeno-associated virus vector. In: Receptor and ion channel detection in the brain (Lujan R, Ciruela F, eds), pp 323–341. New York: Springer. [Google Scholar]
- Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T (2000) Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res 38:231–236. 10.1016/s0168-0102(00)00176-0 [DOI] [PubMed] [Google Scholar]
- Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, Knight ZA (2016) Warm-sensitive neurons that control body temperature. Cell 167:47–59.e15. 10.1016/j.cell.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Wiegert JS (2015) An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci Rep 5:14807. 10.1038/srep14807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiro T, Kataoka N, Nakamura Y, Nakamura K (2017) The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci Rep 7:5031. 10.1038/s41598-017-05327-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WZ, Du X, Zhang W, Gao C, Xie H, Xiao Y, Jia X, Liu J, Xu J, Fu X, Tu H, Fu X, Ni X, He M, Yang J, Wang H, Yang H, Xu XH, Shen WL (2020) Parabrachial neuron types categorically encode thermoregulation variables during heat defense. Sci Adv 6:eabb9414. 10.1126/sciadv.abb9414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Qualls-Creekmore E, Rezai-Zadeh K, Jiang Y, Berthoud HR, Morrison CD, Derbenev AV, Zsombok A, Münzberg H (2016) Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci 36:5034–5046. 10.1523/JNEUROSCI.0213-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]



