Abstract
Much attention has been paid lately to harnessing the diagnostic and therapeutic potential of non-coding circular ribonucleic acids (circRNAs) and micro-RNAs (miRNAs) for the prevention and treatment of cardiovascular diseases. The genetic environment that contributes to atherosclerosis pathophysiology is immensely complex. Any potential therapeutic application of circRNAs must be assessed for risks, benefits, and off-target effects in both the short and long term. A search of the online PubMed database for publications related to circRNA and atherosclerosis from 2016 to 2022 was conducted. These studies were reviewed for their design, including methods for developing atherosclerosis and the effects of the corresponding atherosclerotic environment on circRNA expression. Investigated mechanisms were recorded, including associated miRNA, genes, and ultimate effects on cell mechanics, and inflammatory markers. The most investigated circRNAs were then further analyzed for redundant, disparate, and/or contradictory findings. Many disparate, opposing, and contradictory effects were observed across experiments. These include levels of the expression of a particular circRNA in atherosclerotic environments, attempted ascertainment of the in toto effects of circRNA or miRNA silencing on atherosclerosis progression, and off-target, cell-specific, and disease-specific effects. The high potential for detrimental and unpredictable off-target effects downstream of circRNA manipulation will likely render the practice of therapeutic targeting of circRNA or miRNA molecules not only complicated but perilous.
Keywords: circular RNA, micro RNA, atherosclerosis, cardiovascular disease, potential therapeutic treatments
1. Introduction
Much attention has been paid lately to harnessing the diagnostic and therapeutic potential of non-coding circular ribonucleic acids (circRNAs) and micro-RNAs (miRNAs) for the prevention and treatment of cardiovascular diseases. The advent of next-generation sequencing and the development of bioinformatics databases have facilitated the rapid expansion of circRNA research [1]. One recent review by Wang et al. [2] suggests that, although further clinical trials and basic scientific research are needed, targeting cardiovascular disease pathways via circRNA-mediated mechanisms may prove to be an efficacious strategy for preventing and diagnosing cardiovascular diseases [2]. A large body of ongoing research involves the roles of circRNA molecules in atherosclerosis, the underlying condition contributing to most cardiovascular diseases and the leading cause of death in the world.
The desire to attenuate the progression of atherosclerosis at the genetic level is appealing. Gene regulation likely plays a large role in the pathogenesis of atherosclerosis [3]. By understanding and manipulating the genetic pathways involved in atherosclerosis, we may be able to develop novel therapeutic targets, potentially including drugs for primary prevention. This would be a momentous development; given the recent demotion of chronic low-dose aspirin use, there are limited treatment options beyond those of lifestyle and risk factor modification for the primary prevention of cardiovascular disease. Still, 48% of adults older than 20 years of age have cardiovascular disease and would benefit from additional therapeutic approaches [4].
However, the genetic environment that contributes to atherosclerosis pathophysiology is immensely complex. A review by Siebert et al. [5], which summarized the role of non-coding RNAs, including miRNAs, in ischemic myocardial reperfusion injury, warned of a vast interplay between all non-coding RNAs, including circRNAs, that can have far-reaching effects throughout the entire body. Therefore, any potential therapeutic application of circRNAs must be assessed for risks, benefits, and off-target effects in both the short and long term [5]. Through a comprehensive review and analysis of the literature, this paper aims to evaluate the potential of circRNAs as therapeutic targets in atherosclerosis.
2. Methods
A search of the online PubMed database for publications from 2016 to 2022 was conducted using the following keywords: circular RNA, atherosclerosis, coronary artery disease, oxidized low-density lipoprotein, vascular smooth muscle cells, and endothelial cells. A total of 140 original research articles were included, and their findings are summarized in Table S1 [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145].
These studies were reviewed for their design, including methods for developing atherosclerosis and the effects of the corresponding atherosclerotic environment on circRNA expression. Investigated mechanisms were recorded, including associated miRNA, genes, and ultimate effects on cell mechanics, inflammatory markers, etc. Study findings were assessed for whether they were likely protective or promoted atherosclerosis development. This determination was made based on the association of known mechanisms associated with atherosclerosis pathogenesis. Accordingly, enhanced proliferation, migration, apoptosis, inflammation, oxidative stress, and pathogenic particle uptake were considered harmful, while the opposite was considered protective. Studies with both presumed protective and harmful effects were deemed to have equivocal findings. The most investigated circRNAs were then further analyzed for redundant, disparate, and/or contradictory findings.
3. Results
This review identified 140 studies conducted between 2016 and 2022 (Table S1). The majority employed in vitro models of human vascular smooth muscle cells (VSMCs) or endothelial cells (ECs). Atherosclerosis was simulated by stimulating cells with known pathogenic triggers, with oxidized low-density lipoprotein (ox-LDL) being the most common. Other triggers include platelet-derived growth factor-BB (PDGF-BB), high glucose, or high-fat diets in in vivo models. The effects of these pathogenic states on circRNA, miRNA, and their associated gene expression were then analyzed, as were their effects on cell proliferation, migration, apoptosis, inflammation, and oxidative stress. The interaction of these genetic molecules and their effects of expression on cell behavior and inflammation were elucidated via various assays, especially immunohistochemical staining techniques, after silencing the molecules of the presumed pathway. Only 19 (13.6%) studies performed an ancillary in vivo mouse/rat model. More commonly, the particular circRNA or miRNA level under investigation was measured in the serum of human subjects or mice with atherosclerosis to corroborate the experimental findings in vitro.
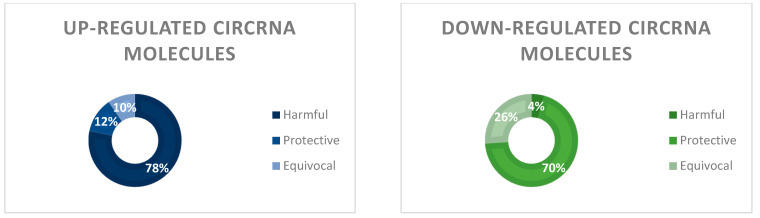
Of the 140 studies reviewed, 95 unique circRNA molecules were identified. The majority (76.8%) were up-regulated in in vitro atherosclerotic environments and/or the serum of patients with atherosclerosis. Of these, 79% correlated with mechanisms known to have pro-atherosclerotic effects in vivo, while 12.3% were associated with protective mechanisms. Of the 25.3% of cirRNAs found to be downregulated, overexpression of these molecules was more commonly associated with mitigation (69.6%) than propagation (4.3%) of atherosclerosis-associated mechanisms. One circRNA molecule, circHIPK3, was shown to be both up-regulated and downregulated across different studies [37,61,82,143]. Of note, at least 9.6% of circRNAs that were demonstrated to have increased expression had equivocal outcomes, i.e., they were unable to discern if the overall effects observed were pathogenic or protective. This percentage was even higher (26.1%) when analyzing only the molecules that were downregulated by atherosclerotic stimuli in vitro (Figure 1).
Figure 1.
The association between the overall effects of circRNA expression on the process of atherosclerosis. Up-regulation of circRNAs in atherosclerosis was found to be most associated with harmful (78%) effects, while downregulation was most associated with protective mechanisms (70%). Equivocal effects were demonstrated in 10% and 26% of studies in which circRNAs were observed to be up-regulated and downregulated, respectively.
The overwhelming majority of studies showed that circRNA molecules exerted their effects via sponging a cognate miRNA (Table S1). In turn, this led to increased expression of a particular gene and subsequent effects on cell mechanics and inflammatory pathways. Only 10 of the 140 studies demonstrated that circRNAs either executed their effects through a mechanism independent of miRNA sponging or failed to identify an associated miRNA [6,9,22,25,29,32,51,52,66,106]. Additionally, of the 133 miRNA molecules identified, 102 were unique, with the corollary being that 23.3% of miRNA molecules were found to interact downstream of multiple circRNAs. To highlight the redundancies and issues with replicability of these studies, the experimental findings of the most investigated molecules are discussed below, with attention paid to opposing or equivocal findings and mechanistic overlap.
3.1. CircANRIL
One of the first circRNAs discovered to play a role in atherosclerosis was circANRIL (Antisense non-coding RNA in the INK4 locus). It is located on chromosome 9p21, variants of which are known genetic risk factors for developing cardiovascular disease [146]. In 2010, Burd et al. [147] demonstrated that homozygous individuals for the atherosclerotic risk allele showed decreased expression of circANRIL and the coding INK4/ARF transcripts [147]. CircANRIL was later found to impair ribosome biogenesis, leading to activation of p53, which then resulted in decreased proliferation and increased apoptosis by directly binding to PES1 (pescadillo ribosomal biogenesis factor 1), an essential 60S-preribosomal assembly factor in VSMCs, ECs, and adventitial fibroblasts [6]. This represents one of the rare instances discovered in which circRNAs modulate atherosclerotic events via transcriptional regulation rather than indirectly through miRNA sponging. However, whether inhibition of cellular proliferation or apoptosis ultimately has positive or negative effects on atherosclerosis development depends on various factors that are difficult to determine with certainty [6].
Other studies attempted to corroborate the effects of circANRIL expression in an in vivo mouse model of atherosclerosis. Song et al. [9] showed that circANRIL overexpression was associated with the formation of atherosclerotic plaques and thombi in rats that were fed a high-fat diet and injected with a large dose of vitamin D3 to promote arterial calcification [9]. The study further supported the findings of increased rates of apoptosis demonstrated by Holdt et al. [6], but also showed higher levels of total cholesterol, triglycerides, LDL, and several pro-atherosclerotic and inflammatory markers, including interleukin (IL)-1, IL-6, matrix metallopeptidase-9 (MMP-9), c-reactive protein (CRP), BCL2 associated X (Bax), and caspase-3 [9]. Another investigation demonstrated that inhibition of circANRIL expression in a similar in vivo rat model reduced markers of vascular endothelial injury, oxidative stress, and inflammation [148]. These early studies suggested that in vivo models analyzing plaque development and inflammatory markers may produce reliable results to establish a causal link between circRNA expression and atherosclerosis development.
3.2. Circ_USP36/Circ_0003204
This review identified 11 different studies evaluating the role of circ_USP36 (ubiquitin specific peptidase 36)/circ_0003204 in the pathogenesis of atherosclerosis, establishing it as the most investigated circRNA molecule. All experiments were conducted in in vitro models of human VSMCs and ECs [29,42,55,72,74,75,78,81,92,98,139]. Liu et al. [29] showed that hsa_circ_0003204 was aberrantly overexpressed in ox-LDL-induced human umbilical vein ECs (HUVECs), while knockdown of this molecule promoted proliferation, migration, and invasion but reduced apoptosis [29]. Reduced expression of circ_0003204 also significantly correlated with lower E-cadherin but increased activity of N-cadherin and vimentin in oxLDL-induced HUVECs, findings that are associated with reduced cell mobility and plaque stability, respectively [149,150]. Thus, the knockdown of circ_0003204 was associated with both increased (harmful) and decreased (protective) cellular proliferation in this study. No associated miRNA was identified in this particular study.
Several other in vitro experiments demonstrated suppressed cell viability and promotion of apoptosis, inflammation, oxidative stress, and cell migration and invasion associated with increased expression of circ_USP36 and subsequent miRNA sponging in response to ox-LDL-stimulated ECs. Specifically, these effects were attributed to circ_USP36/circ_0003204 inhibition of miR-20a-5p, miR-98-5p, and miR-188-3p leading to increased ROCK2, vascular cell adhesion protein-1 (VCAM-1), and TRP6 gene expression, respectively [72,74,75,81,92]. On the other hand, Huang et al. [55] observed that, through increased expression of WNT4 from sponging of miR-637, circ_USP36 overexpression was associated with suppressed proliferation and migration of human aortic ECs treated with ox-LDL in vitro [55]. While largely agreeing with the effects that circ_USP36/circ_0003204 promotes inflammation and oxidative stress, a recent study also found decreased tube formation in HUVECs stimulated with ox-LDL through sponging of miR-491-5p and increased expression of intercellular adhesion molecule-1 (ICAM-1) [139]. Taken together, these studies present contradictory results regarding the effects of circ_USP36/circ_0003204 on VSMC and EC proliferation, migration, and invasion, suggesting that their regulation is complex and clinical significance challenging to capture.
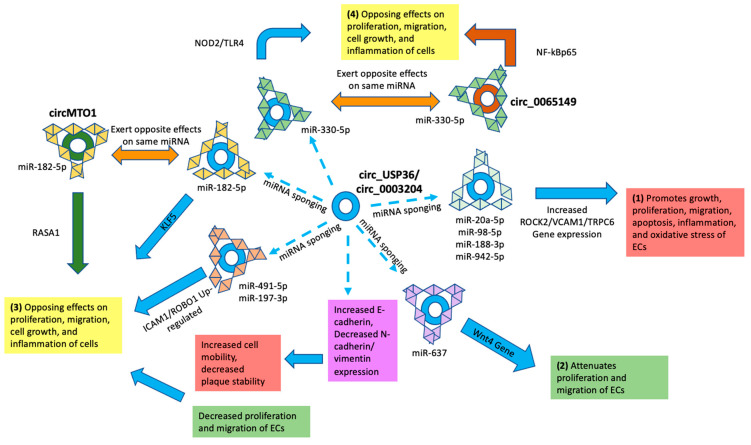
Another study showed that the expression of circ_USP36 was also increased in ox-LDL-treated human umbilical vein VSMCs via sponging of miR-182-5p [42]. This led to increased activity of the KLF5 gene, which induced VSMC proliferation and metastasis. Circ_USP36 knockdown inhibited this proliferation and metastasis by up-regulating miR-182-5p [42]. However, lower levels of circMTO1 in the serum of humans with atherosclerosis coincided with augmentation of miR-182-5p, increased proliferation, and reduced apoptosis in an in vitro analysis of ox-LDL-stimulated VSMCs. Overexpression of circMTO1 led to less inhibition of miR-182-5p and subsequently greater activation of the RASA1 gene, reduced proliferation, and increased apoptosis of VSMCs [57]. Similarly, while lower levels of circ_0065149 were observed in a model of ox-LDL human umbilical vein ECs in vitro, overexpression was associated with miR-330-5p sponging and associated effects of increased cell viability, proliferation, and migration, but reduced apoptosis and inflammation [62]. These outcomes are opposed to those of increased inflammation with miR-330-5p sponging observed by Su et al. [78]. These studies demonstrate that different circRNA molecules can exhibit both protective and detrimental effects on the development of atherosclerosis via sponging of the same miRNA. This suggests that targeting a specific circRNA for therapeutic purposes could possibly result in unintended pathogenic consequences in opposition to the objective of such manipulation (Figure 2).
Figure 2.
The role of circ_0003204/USP36 in the pathogenesis of atherosclerosis. Sponging of miRNAs by up-regulated circ_0003204/USP36 has been shown to lead to (1) promotion of growth, proliferation, migration, apoptosis, inflammation, and oxidative stress of ECs [72,74,75], but also (2) attenuation of proliferation and migration of ECs, known to be protective from further intimal hyperplasia [55]. Furthermore, some miRNAs are inhibited by multiple circRNAs, the effects of which have (3) and (4) contradictory outcomes on cell growth, proliferation, migration, and inflammation [29,42,78,81,92,98,139]. These opposing effects make it difficult to predict the overall effects of targeting a specific circRNA or miRNA for therapeutic purposes.
3.3. CircCHFR
Six in vitro experiments examined circCHFR [21,43,85,112,117,128]. All studies showed consistent results of upregulation of circCHFR in atherosclerotic environments simulated by treating cells with ox-LDL or PDGF-BB. Subsequent miRNA sponging and overexpression of various genes were further associated with pro-atherosclerotic mechanisms. In one model, sponging of miR-370 led to increased FOXO1/Cyclin D1 expression, facilitating VSMC proliferation and migration [21]. Another in vitro study linked these findings with increased markers of inflammation via miR-214-3p inhibition and increased Wnt4 expression [43]. Increased circCHFR activity was also associated with the augmentation of apoptosis and proinflammatory cytokine secretion. Reciprocally, silencing of circCHFR increased cell survival and reduced apoptosis in ECs [112]. When analyzed at the level of circRNA expression alone, these studies appear to show that up-regulation of circCHFR in models of atherosclerosis consistently and reliably leads to pathogenic progression.
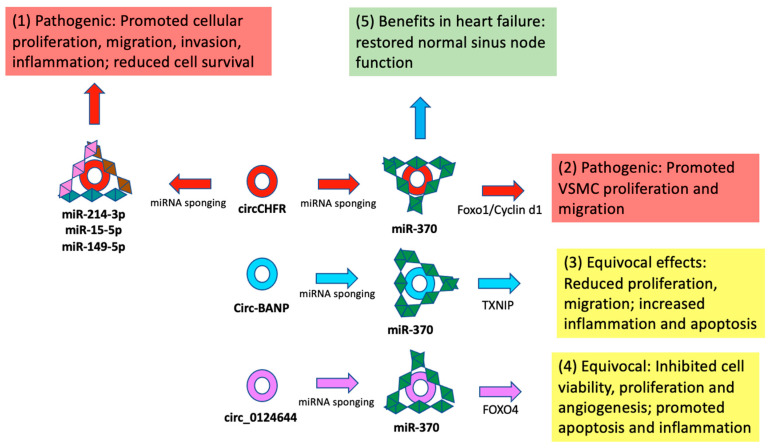
However, miR-370 has been found to be regulated by other circRNA molecules with opposing downstream consequences. For example, sponging of miR-370 by circ-BANP has been associated with reduced proliferation and migration of HUVECs, the opposite of that found through the interaction of cricCHFR and miR-370 [21,45]. Overexpression of circ_0124644 leading to inhibition of miR-370 similarly had equivocal outcomes on atherosclerosis progression by inhibiting cell viability, proliferation, and angiogenesis but promoting apoptosis and inflammation [121]. Silencing of miR-370 has also been associated with sinus node function recovery in patients with heart failure [151]. These studies suggest contradictory effects via similar mechanisms of miR-370 inhibition (Figure 3).
Figure 3.
The role of circCHFR in the pathogenesis of atherosclerosis. Sponging of several miRNAs by circCHFR has been shown to lead to (1) increased cellular proliferation, migration, invasion, inflammation, and reduced cell cycle survival—all mechanisms known to contribute to atherosclerosis development [43,85,128]. However, circCHFR has also been demonstrated to (2) reduce the expression of miR-370 [21], the inhibition of which has also been shown to have opposing effects compared to circCHFR via sponging by (3) circ-BANP and (4) circ_0124644 [45,121]. (5) Disinhibition of mir-370 also likely adversely affects sinus node function in patients with heart failure [151].
Thus, therapeutic interventions aimed at reducing circCHFR expression may lead to conflicting results via downstream gene regulation and disparate effects on VSMC and EC proliferation and migration. Even if these effects ultimately reduce atherosclerosis progression, disinhibition of miR-370 may promote life-threatening arrhythmias in patients with heart failure, suggesting that cardiovascular pathologies other than atherosclerosis may also be negatively affected [151]. Furthermore, since these studies were not corroborated in in vivo models, it is hard to determine the ultimate effects of the mechanisms elucidated on the process of atherosclerosis. While most studies also observed that circCHFR was up-regulated in the serum of patients with atherosclerosis, this up-regulation may lead to the promotion of some genes that foster protective effects against atherosclerosis.
3.4. CircHIPK3
Four in vitro models studied the effects of circHIPK3 in atherosclerosis pathogenesis [37,61,82,143]. Two of these investigations showed higher levels of circHIPK3 in pro-atherosclerotic in vitro environments, while two demonstrated attenuated activity (Table S1). Wang et al. [82] showed that increased levels of circHIPK3 in mice aortic EC-secreted exosomes in response to high glucose levels correlated with more significant proliferation and inhibition of apoptosis of VSMCs and VCAM-1 expression and uptake of glucose-rich exosomes by VSMCs. This occurred via sponging of miR-106a-5p and amplified expression of FOXO1 and VCAM-1 [82]. Similar effects were seen in human aortic and umbilical artery VSMCs through a mechanism involving inhibition of miR-637, leading to increased expression of cyclin-dependent kinase 6 (CDK6) [61]. In opposition to these findings, sponging of miR-637 by circ_0002194 correlated with reduced angiogenesis and increased apoptosis rates of ox-LDL-treated vascular ECs [122].
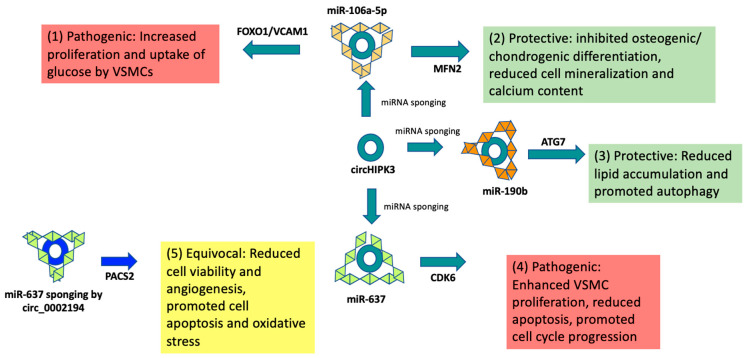
Zhang W-B et al. [143] found lower levels of circHIPK3 in the serum and tissues of patients with atherosclerosis. This was associated with osteogenic and chondrogenic differentiation and increased cell mineralization and calcium content in VSMCs in vitro. In fact, overexpression of circHIPK3 led to sponging of miR-106a-5p and subsequent activation of the MFN2 gene, which inhibited osteogenic and chondrogenic differentiation, ultimately leading to less calcium accumulation in VSMCs [143]. In this case, miR-106a-5p sponging had beneficial effects, which contradicts the findings that miR-106a-5p inhibition facilitated pathogenic proliferation and migration of VSMCs [82]. Another study showed that downregulation of circHIPK3 led to disinhibition of miiR-190b, decreased activity of the ATG7 signal pathway, and subsequently lower rates of autophagy and higher rates of lipid accumulation in both mice in vivo and ox-LDL-treated human umbilical vein ECs in vitro [37]. On the other hand, overexpression of circHIPK3 resulted in sponging of miR-190b and increased activity of the ATG7 pathway, which correlated with reduced lipid accumulation and promoted autophagy (Figure 4).
Figure 4.
Studies investigating the effects of in vitro atherosclerosis environments on circHIPK3 demonstrate both (1) and (4) harmful effects of cellular proliferation, migration, and apoptosis [61,82], and (2) and (3) protective effects of inhibited osteogenic differentiation, mineralization, and calcium deposition, reduced lipid accumulation, and increased rates of autophagy [37,143]. Both (1) harmful and (2) protective effects were seen from sponging of miR-106a-5p [82]. Opposing effects on angiogenesis were also demonstrated via sponging of miR-637 by (4) circHIPK3 and [61] (5) circ_0002194 [122], the latter of which suggests equivocal effects on the pathogenesis of atherosclerosis due to the observed impaired angiogenesis but enhanced oxidative stress.
Analysis of the results of the circHIPK3 investigations illustrates three major points of contention: 1. In similar proxies for atherosclerotic environments, crcHIPK3 expression was found to be both increased and decreased. 2. Analysis downstream of circHIPK3 expression, i.e., of miR-637, showed opposing effects when it was inhibited by other circRNA molecules, i.e., circ_0002194. 3. The ultimate effects of overexpression of circHIPK3 were found to be both pathogenic (increased proliferation, apoptosis, and glucose uptake) and protective (reduced angiogenesis, apoptosis, osteogenic differentiation, and lipid accumulation) in regard to atherosclerosis development and progression. It is possible disparate effects were seen due to different tissue types and methods of atherosclerosis stimulation in vitro. However, similar results would have been expected regardless of the method used to simulate atherosclerosis. Furthermore, the possibility that intervening in one tissue type to halt atherosclerosis progression could promote atherosclerosis in a different tissue type is alarming. Alternatively, these findings could point to issues with the general replicability of these studies in vitro. Regardless, they underscore the complexity of the genetic milieu of atherosclerosis and the likely unintended negative consequences of circRNA manipulation.
4. Discussion
Several issues have been raised with using circRNAs as potential therapeutic targets to modify disease processes. Some systemic problems include the toxicity of nanoparticles, mis-spliced byproducts, and synthetic circRNA immunogenicity [152]. Highlighted by this review—with respect to in vitro models evaluating atherosclerosis—are also questions of study design, interpretation of overall results, contradictory effects caused by off-target RNA silencing, and cell-specific and disease-specific effects. No two studies that used a supplemental in vivo model studied the same circRNA. Thus, we cannot comment on the redundancy or reproducibility of the effects of a particular circRNA within in vivo models.
4.1. Study Design and Issues with Interpretation
As previously discussed, the majority of the circRNA molecules that were studied were upregulated in the serum of subjects with atherosclerosis and in in vitro models of atherosclerosis induced via established pathogenic triggers. This is likely because circRNA molecules with increased levels are easier to identify than ones with decreased expression, which represents a kind of ascertainment bias in identifying potential circRNA targets for investigation. The majority of experiments, which found increased expression of circRNA molecules in states of atherosclerosis, also found an association with mechanisms known to promote atherosclerosis in vivo—most commonly proliferation and apoptosis of ECs or VSCMS—while silencing the circRNA under investigation and promoting its cognate miRNA resulted in opposite effects. However, whether angiogenesis and apoptosis in atherosclerosis are beneficial or harmful depends on their effects on intimal hyperplasia, plaque stability, plaque content, phenotypic switching, and the stage of atherosclerosis [153,154]. Thus, it is difficult to determine the clinical significance of atherosclerotic mechanisms in vitro.
Even when conceding the benefit of the doubt that a particular mechanism known to promote atherosclerosis in vivo, e.g., proliferation and migration of EC and VSMCs, has similar effects in vitro, a significant percentage of studies yielded equivocal results. Often, seemingly opposing effects were observed in response to upregulation or downregulation of a particular circRNA in vitro. For example, Chen et al. [45] demonstrated that while circ-BANP was associated with apoptosis and inflammation and promoted cell viability, it also correlated with increased migration, invasion, and tube formation of ECs [45]. Antagonistic effects on proliferation, migration, and promotion of calcification of VSMCs by circHIPK3 sponging of miR-106a-5p were also observed (Figure 4). Whether these mechanisms, which have seemingly oppositive effects on atherosclerosis development, lead to the progression or attenuation of atherosclerosis in toto, it is difficult to ascertain via in vitro analyses alone. EC dysfunction present in the early stages of atherosclerosis is associated with chronic inflammatory changes in the arteries [155]. Alternatively, the results could have been inaccurate, pointing to potential issues with the general replicability of the results of these study designs.
Ancillary in vivo studies often investigate different pathogenic processes or stages of atherosclerosis and therefore do not effectively corroborate the in vitro findings. For example, one study that evaluated the effects of circGSE1 expression on EC senescence also looked at the effects of angiogenesis on limb ischemia in mice via femoral artery ligation [125]. Few studies have analyzed the in vivo formation of atherosclerosis. Song et al. [9] showed that circANRIL overexpression was associated with the formation of atherosclerotic plaques and thrombi in rats that were fed a high-fat diet and were injected with a large dose of vitamin D3 (to promote arterial calcification) [9]. However, as extensively demonstrated in this review, circRNA molecule expression can correlate with either promotion or attenuation of atherosclerosis and therefore does not establish a causative relationship. Min et al. [123] showed increased expression of ciPVT1 in the senescent umbilical vein and coronary artery ECs, while silencing ciPVT1 led to delayed senescence, promoted proliferation, and increased the angiogenic activity of ECs. A correlative in vivo mouse study using a plug assay found that plugs mixed with silenced ciPVT1-transfected HUVECs showed less new vessel formation macroscopically [123]. This study shows the potential of the findings of in vivo studies to corroborate those of in vitro analyses of circRNA interactions and their effects on atherosclerosis [123]. However, such a model was rarely used in these investigations.
4.2. Off-Target RNA Silencing
This review identified a significant overlap of circRNA and miRNA interactions, resulting in disparate and opposing effects on mechanisms associated with the development of atherosclerosis. As seen in Figure 2, Figure 3 and Figure 4, the most investigated circRNA molecules, circ_USP36/circ_0003204, circCHFR, and circHIPK3, were found to have disparate, opposing, and often contradictory results across studies. While the majority of miRNAs inhibited by circ_USP36/circ_0003204 led to the regulation of genes that promoted pathogenic mechanisms such as increased proliferation, migration of cells, and inflammation, the sponging of others was found to be correlated with the opposite effects (Figure 2). Similar findings of harmful, protective, and equivocal effects on atherosclerosis development were seen when analyzing the mechanisms of circCHFR (Figure 3) and circHIPK3 (Figure 4) across studies.
Sponging the same miRNA by different circRNAs also had contradictory effects on cell proliferation, apoptosis, and inflammation. For example, miR-182-5p was demonstrated to be affected downstream of four different circRNA molecules: circ_USP36, circMTO1, hsa_circ_0004831, and Circ_0050486 [42,57,77,142]. While sponging of miR-182-5p by circ_USP36 led to increased activity of the KLF5 gene, which induced VSMC proliferation and metastasis, inhibition of miR-182-5p via overexpression of circMTO1 and subsequent RASA1 gene activation had the opposite effects of decreased VSMC proliferation and decreased apoptosis (Table S1). As another example, silencing of overexpressed circCHFR molecules led to de-inhibition of miR-370, allowing it to prevent expression of FOXO1/cyclin D1 genes, resulting in decreased proliferation and migration of VSMCs [21]. Circ-BANP silencing, which similarly resulted in increased levels of miR-370, however, was ultimately associated with the opposite finding: increased EC migration, invasion, and tube formation [45]. Similar results were seen when looking at the different effects of circHIPK3 and circ_0002194 on the sponging of mir-637 (Figure 4). All these cases underline the ubiquitous collateral off-target downstream and lateral effects of targeting a particular circRNA or miRNA for therapeutic purposes.
4.3. Differential Effects across Cell Types and Diseases
There were also significant cell-specific effects observed on the process of atherosclerosis development. Ox-LDL-treated HUVECs were associated with reduced expression of circHIPK3 in vitro, while overexpression correlated with reduced lipid accumulation and the promotion of autophagy [37]. In contrast, increased proliferation and reduced apoptosis of VSMCs, most likely a pathogenic mechanism, were observed in conjunction with increased circHIPK3 expression of aortic and umbilical artery VSMCs in vitro [61]. In response to a high-glucose environment, mouse aortic EC-secreted exosomes also promoted proliferation and inhibited apoptosis of VSMCs while promoting VCAM-1 expression and uptake of exosomes by VSMCs [82]. In human VSMCs, circHIPK3 was downregulated in tissues and blood samples of atherosclerosis patients and VSMCs with osteogenic and cartilage differentiation. Concordantly, overexpression of circHIPK3 was associated with the athero-protective effects of inhibited osteogenic and chondrogenic differentiation and reduced cell mineralization and calcium content [143].
Thus, increased expression of circHIPK3 was associated with both protective and detrimental mechanisms in the context of atherosclerosis development. The effects likely depend on particular cell types tested, e.g., VSMCs, ECs, and/or atherosclerosis-inducing agents, and overall milieus. Opposing effects in different cells further complicate the selection of cricRNA molecules such as circHIPK3. In this particular case, these studies suggest that silencing of circHPIK3 would lead to the negative effects of increased lipid accumulation in ECs and calcification of VSMCs but the positive effects of reduced proliferation and increased apoptosis in VSMCs, as well as decreased VCAM-1 expression and VSMC adhesion, indicating contrasting effects across different cell types.
Furthermore, it is likely that targeting a specific disease process, such as atherosclerosis in this case, may have unintended effects on other cardiovascular pathologies. While sponging of miR-370 by circCHFR led to increased FOXO1/Cyclin D activity which enhanced VSMC proliferation and migration, inhibition of miR-370 was also associated with beneficial effects on sinus node function in an in vitro mouse model of heart failure [21,151]. Thus, therapy aimed at silencing circCHFR to mitigate atherosclerosis development would likely lead to increased miR-370 expression, which may have pathogenic effects on sinus rhythm function in patients with heart failure. In addition, there are numerous extra-cardiac disease processes that may be affected by such genetic manipulation, the effects of which are hard to account for. For example, miR-370 has also been shown to play a regulatory role in various cancers, including cervical, ovarian, lung, gastric, and hepatocellular, among many others [156,157,158,159,160].
In summary, silencing of a particular circRNA leading to disinhibition of its related miRNA could result in the intended effect of halting atherosclerosis. However, several other pathways would need to be accounted for to mitigate the unintended consequences of amplifying atherosclerosis or other disease progressions. These include disparate, opposing, and contradictory downstream and lateral effects of silencing a particular circRNA in different tissue types and varying disease processes. Any risk-benefit analysis aimed at evaluating the adoption of such a therapeutic approach would ultimately be limited by the sheer scope of genetic interactions and their effects, as well as the discovery and knowledge of those mechanisms and effects.
5. Conclusions
This review represents the largest and most systematic review of studies evaluating the role of circRNA in the pathogenesis of atherosclerosis. With a focus on the most studied molecules, many disparate, opposing, and contradictory effects were observed across experiments. These include levels of the expression of a particular circRNA in atherosclerotic environments, attempted ascertainment of the in toto effects of circRNA or miRNA silencing on atherosclerosis progression, and off-target, cell-specific, and disease-specific effects. Accordingly, many of these studies conclude that a specific circular RNA regulates atherosclerosis. This review shows that this regulation is a complex orchestration more akin to directing traffic with multiple moving vehicles and intersections than a linear assembly line. Given the high potential for detrimental and unpredictable off-target effects downstream of circRNA manipulation, the practice of therapeutic targeting of circRNA or miRNA molecules appears too complex at the current level of knowledge. Future studies need to pay attention to the mechanisms being examined and manipulated in the context of stages of atherosclerosis, cell type, and downstream and lateral effects of circRNA manipulation. In this regard, we need more correlative in vivo studies designed to investigate the role of circRNAs in atherosclerosis development and progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12134446/s1, Table S1: A summary of the mechanisms of the 140 studies investigating the role of circular RNA in the pathogenesis of atherosclerosis from the years 2016 to 2022, as identified in PubMed.
Author Contributions
Conceptualization, Y.B. and J.T.; Methodology, Y.B., J.T., Y.Z. and Y.E.C.; Software, J.T.; Validation, Y.B., Y.E.C. and Y.Z.; Formal Analysis, J.T., Y.B. and C.M.; Investigation, J.T. and C.M.; Data Curation, J.T. and C.M.; Writing—Original Draft Preparation, J.T. and C.M.; Writing—Review and Editing, J.T, Y.B., Y.Z. and Y.E.C.; Visualization, J.T. and C.M.; Supervision, Y.B. and Y.E.C.; Project Administration, Y.B. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was funded by in part by a grant from the John S. Dunn Chair in Cardiology Research and Education. Award number: B2017/BMD-3738.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yu C.Y., Kuo H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019;26:29. doi: 10.1186/s12929-019-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K., Gao X.Q., Wang T., Zhou L.Y. The Function and Therapeutic Potential of Circular RNA in Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2023;37:181–198. doi: 10.1007/s10557-021-07228-5. [DOI] [PubMed] [Google Scholar]
- 3.Björkegren J.L.M., Lusis A.J. Atherosclerosis: Recent developments. Cell. 2022;185:1630–1645. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 5.Siebert V., Allencherril J., Ye Y., Wehrens X.H.T., Birnbaum Y. The Role of Non-coding RNAs in Ischemic Myocardial Reperfusion Injury. Cardiovasc. Drugs Ther. 2019;33:489–498. doi: 10.1007/s10557-019-06893-x. [DOI] [PubMed] [Google Scholar]
- 6.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., Kohlmaier A., Herbst A., Northoff B.H., Nicolaou A., et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Cui L., Yuan J., Zhang Y., Sang H. Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle cells proliferation and migration by sponging miR-124. Biochem. Biophys. Res. Commun. 2017;494:126–132. doi: 10.1016/j.bbrc.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 8.Li C.Y., Ma L., Yu B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother. 2017;95:1514–1519. doi: 10.1016/j.biopha.2017.09.064. [DOI] [PubMed] [Google Scholar]
- 9.Song C.L., Wang J.P., Xue X., Liu N., Zhang X.H., Zhao Z., Liu J.G., Zhang C.P., Piao Z.H., Liu Y., et al. Effect of Circular ANRIL on the Inflammatory Response of Vascular Endothelial Cells in a Rat Model of Coronary Atherosclerosis. Cell Physiol. Biochem. 2017;42:1202–1212. doi: 10.1159/000478918. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y., Yang Z., Zheng B., Zhang X.H., Zhang M.L., Zhao X.S., Zhao H.Y., Suzuki T., Wen J.K. A Novel Regulatory Mechanism of Smooth Muscle α-Actin Expression by NRG-1/circACTA2/miR-548f-5p Axis. Circ. Res. 2017;121:628–635. doi: 10.1161/CIRCRESAHA.117.311441. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y.Y., Wang J.Q., Guo X.X., Bi Y., Wang C.X. Circ-SATB2 upregulates STIM1 expression and regulates vascular smooth muscle cell proliferation and differentiation through miR-939. Biochem. Biophys. Res. Commun. 2018;505:119–125. doi: 10.1016/j.bbrc.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 12.Pan L., Lian W., Zhang X., Han S., Cao C., Li X., Li M. Human circular RNA-0054633 regulates high glucose-induced vascular endothelial cell dysfunction through the microRNA-218/roundabout 1 and microRNA-218/heme oxygenase-1 axes. Int. J. Mol. Med. 2018;42:597–606. doi: 10.3892/ijmm.2018.3625. [DOI] [PubMed] [Google Scholar]
- 13.Yang L., Han B., Zhang Y., Bai Y., Chao J., Hu G., Yao H. Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy. 2018;14:404–418. doi: 10.1080/15548627.2017.1414755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng J., Liu Q., Hu N., Zheng F., Zhang X., Ni Y., Liu J. Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene. 2019;709:1–7. doi: 10.1016/j.gene.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Hall I.F., Climent M., Quintavalle M., Farina F.M., Schorn T., Zani S., Carullo P., Kunderfranco P., Civilini E., Condorelli G., et al. Circ_Lrp6, a Circular RNA Enriched in Vascular Smooth Muscle Cells, Acts as a Sponge Regulating miRNA-145 Function. Circ. Res. 2019;124:498–510. doi: 10.1161/CIRCRESAHA.118.314240. [DOI] [PubMed] [Google Scholar]
- 16.Kong P., Yu Y., Wang L., Dou Y.Q., Zhang X.H., Cui Y., Wang H.Y., Yong Y.T., Liu Y.B., Hu H.J., et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019;47:3580–3593. doi: 10.1093/nar/gkz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang L., Quan A., Sun H., Xu Y., Sun G., Cao P. MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene. 2019;711:143948. doi: 10.1016/j.gene.2019.143948. [DOI] [PubMed] [Google Scholar]
- 18.Shen L., Hu Y., Lou J., Yin S., Wang W., Wang Y., Xia Y., Wu W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019;19:3923–3932. doi: 10.3892/mmr.2019.10011. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Zhang Z., Yang S. Circ_RUSC2 upregulates the expression of miR-661 target gene. Biochem. Cell Biol. 2019;97:709–714. doi: 10.1139/bcb-2019-0031. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y., Zhang S., Yue M., Li Y., Bi J., Liu H. Angiotensin II inhibits apoptosis of mouse aortic smooth muscle cells through regulating the circNRG-1/miR-193b-5p/NRG-1 axis. Cell Death Dis. 2019;10:362. doi: 10.1038/s41419-019-1590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Yang F., Zhao H., Wang M., Zhang Y. Circular RNA circCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via miR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids. 2019;16:434–441. doi: 10.1016/j.omtn.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W., Lin J., Li B., Cao S., Li H., Zhao J., Liu K., Li Y., Sun S. Screening and functional prediction of differentially expressed circRNAs in proliferative human aortic smooth muscle cells. J. Cell. Mol. Med. 2020;24:4762–4772. doi: 10.1111/jcmm.15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding P., Ding Y., Tian Y., Lei X. Circular RNA circ_0010283 regulates the viability and migration of oxidized low-density lipoprotein-induced vascular smooth muscle cells via an miR-370-3p/HMGB1 axis in atherosclerosis. Int. J. Mol. Med. 2020;46:1399–1408. doi: 10.3892/ijmm.2020.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Z., Zhu Y., Zhang J., Yang W., Chen Z., Li B. Hsa-circ_0010283 Regulates Oxidized Low-Density Lipoprotein-Induced Proliferation and Migration of Vascular Smooth Muscle Cells by Targeting the miR-133a-3p/Pregnancy-Associated Plasma Protein A Axis. Circ. J. 2020;84:2259–2269. doi: 10.1253/circj.CJ-20-0345. [DOI] [PubMed] [Google Scholar]
- 25.Guo M., Yan R., Ji Q., Yao H., Sun M., Duan L., Xue Z., Jia Y. IFN regulatory Factor-1 induced macrophage pyroptosis by modulating m6A modification of circ_0029589 in patients with acute coronary syndrome. Int. Immunopharmacol. 2020;86:106800. doi: 10.1016/j.intimp.2020.106800. [DOI] [PubMed] [Google Scholar]
- 26.Huang H.S., Huang X.Y., Yu H.Z., Xue Y., Zhu P.L. Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-κB axis in endothelial cells. Biochem. Biophys. Res. Commun. 2020;525:512–519. doi: 10.1016/j.bbrc.2020.02.109. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z., Li P., Wu L., Zhang D., Du B., Liang C., Gao L., Zhang Y., Yao R. Hsa_circ_0029589 knockdown inhibits the proliferation, migration and invasion of vascular smooth muscle cells via regulating miR-214-3p and STIM1. Life Sci. 2020;259:118251. doi: 10.1016/j.lfs.2020.118251. [DOI] [PubMed] [Google Scholar]
- 28.Liu H., Ma X., Wang X., Mao Z., Zhu J., Chen F. Hsa_circ_0000345 regulates the cellular development of ASMCs in response to oxygenized low-density lipoprotein. J. Cell. Mol. Med. 2020;24:11849–11857. doi: 10.1111/jcmm.15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Ma X., Mao Z., Shen M., Zhu J., Chen F. Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell. Signal. 2020;70:109595. doi: 10.1016/j.cellsig.2020.109595. [DOI] [PubMed] [Google Scholar]
- 30.Ma C., Gu R., Wang X., He S., Bai J., Zhang L., Zhang J., Li Q., Qu L., Xin W., et al. circRNA CDR1as Promotes Pulmonary Artery Smooth Muscle Cell Calcification by Upregulating CAMK2D and CNN3 via Sponging miR-7-5p. Mol. Ther. Nucleic Acids. 2020;22:530–541. doi: 10.1016/j.omtn.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W., Li T., Pi S., Huang L., Liu Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020;529:753–759. doi: 10.1016/j.bbrc.2020.06.067. [DOI] [PubMed] [Google Scholar]
- 32.Qin M., Wang W., Zhou H., Wang X., Wang F., Wang H. Circular RNA circ_0003645 silencing alleviates inflammation and apoptosis via the NF-κB pathway in endothelial cells induced by oxLDL. Gene. 2020;755:144900. doi: 10.1016/j.gene.2020.144900. [DOI] [PubMed] [Google Scholar]
- 33.Tian D., Xiang Y., Tang Y., Ge Z., Li Q., Zhang Y. Circ-ADAM9 targeting PTEN and ATG7 promotes autophagy and apoptosis of diabetic endothelial progenitor cells by sponging mir-20a-5p. Cell Death Dis. 2020;11:526. doi: 10.1038/s41419-020-02745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G., Li Y., Liu Z., Ma X., Li M., Lu Q., Lu Z., Niu L., Fan Z., Lei Z. Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol. Cell. Biochem. 2020;471:51–61. doi: 10.1007/s11010-020-03764-0. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Zhan J., Lin X., Wang Y., Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem. Funct. 2020;38:1056–1068. doi: 10.1002/cbf.3543. [DOI] [PubMed] [Google Scholar]
- 36.Wei H., Cao C., Wei X., Meng M., Wu B., Meng L., Gu S., Li H. Circular RNA circVEGFC accelerates high glucose-induced vascular endothelial cells apoptosis through miR-338-3p/HIF-1α/VEGFA axis. Aging. 2020;12:14365–14375. doi: 10.18632/aging.103478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei M.Y., Lv R.R., Teng Z. Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020;24:12849–12858. doi: 10.26355/eurrev_202012_24187. [DOI] [PubMed] [Google Scholar]
- 38.Wei Z., Ran H., Yang C. CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sci. 2020;259:118241. doi: 10.1016/j.lfs.2020.118241. [DOI] [PubMed] [Google Scholar]
- 39.Yu H., Zhao L., Zhao Y., Fei J., Zhang W. Circular RNA circ_0029589 regulates proliferation, migration, invasion, and apoptosis in ox-LDL-stimulated VSMCs by regulating miR-424-5p/IGF2 axis. Vasc. Pharmacol. 2020;135:106782. doi: 10.1016/j.vph.2020.106782. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L.L. CircRNA-PTPRA promoted the progression of atherosclerosis through sponging with miR-636 and upregulating the transcription factor SP1. Eur. Rev. Med. Pharmacol. Sci. 2020;24:12437–12449. doi: 10.26355/eurrev_202012_24039. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W., Sui Y. CircBPTF knockdown ameliorates high glucose-induced inflammatory injuries and oxidative stress by targeting the miR-384/LIN28B axis in human umbilical vein endothelial cells. Mol. Cell. Biochem. 2020;471:101–111. doi: 10.1007/s11010-020-03770-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Q., Lu Y.H., Wang X., Zhang X.J. Circ_USP36/miR-182-5p/KLF5 axis regulates the ox-LDL-induced injury in human umbilical vein smooth muscle cells. Am. J. Transl. Res. 2020;12:7855–7869. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang J.B., Li T., Hu X.M., Ning M., Gao W.Q., Lang Y.H., Zheng W.F., Wei J. Circ_CHFR expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3282–3292. doi: 10.26355/eurrev_202003_20696. [DOI] [PubMed] [Google Scholar]
- 44.Bai Y., Liu F., Yang Z. CircRNA LRP6 promotes high-glucose induced proliferation and migration of vascular smooth muscle cells through regulating miR-545-3p/HMGA1 signaling axis. Am. J. Transl. Res. 2021;13:8909–8920. [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G., Li Y., Zhang A., Gao L. Circular RNA Circ-BANP Regulates Oxidized Low-density Lipoprotein-induced Endothelial Cell Injury Through Targeting the miR-370/Thioredoxin-interacting Protein Axis. J. Cardiovasc. Pharmacol. 2021;77:349–359. doi: 10.1097/FJC.0000000000000964. [DOI] [PubMed] [Google Scholar]
- 46.Ding Y., Tang T., Lu J., Wang J. Circ_UBR4 Knockdown Alleviates Oxidized Low-Density Lipoprotein-Provoked Growth and Migration of Human Vascular Smooth Muscle Cells by Acting on the miR-637/FOXO4 Pathway. J. Cardiovasc. Pharmacol. 2021;78:534–543. doi: 10.1097/FJC.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 47.Fan K., Ruan X., Wang L., Lu W., Shi Q., Xu Y. Circ_0004872 promotes platelet-derived growth factor-BB-induced proliferation, migration and dedifferentiation in HA-VSMCs via miR-513a-5p/TXNIP axis. Vasc. Pharmacol. 2021;140:106842. doi: 10.1016/j.vph.2021.106842. [DOI] [PubMed] [Google Scholar]
- 48.Fu X., Niu T., Yang T., Li X. CircMAPK1 promotes the proliferation and migration of vascular smooth muscle cells through miR-22-3p/ methyl-CpG binding protein 2 axis. Nutr. Metab. Cardiovasc. Dis. 2021;31:2189–2198. doi: 10.1016/j.numecd.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y., Li G., Fan S., Wang Y., Wei H., Li M., Li X. Circ_0093887 upregulates CCND2 and SUCNR1 to inhibit the ox-LDL-induced endothelial dysfunction in atherosclerosis by functioning as a miR-876-3p sponge. Clin. Exp. Pharmacol. Physiol. 2021;48:1137–1149. doi: 10.1111/1440-1681.13504. [DOI] [PubMed] [Google Scholar]
- 50.Ge Y., Liu W., Yin W., Wang X., Wang J., Zhu X., Xu S. Circular RNA circ_0090231 promotes atherosclerosis in vitro by enhancing NLR family pyrin domain containing 3-mediated pyroptosis of endothelial cells. Bioengineered. 2021;12:10837–10848. doi: 10.1080/21655979.2021.1989260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong X., Tian M., Cao N., Yang P., Xu Z., Zheng S., Liao Q., Chen C., Zeng C., Jose P.A., et al. Circular RNA circEsyt2 regulates vascular smooth muscle cell remodeling via splicing regulation. J. Clin. Investig. 2021;131:e147031. doi: 10.1172/JCI147031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han L., Li D., Hang Y., Zong X., Lv J., Bai X., Lu Y., Zhang P., Zhou M., Wu Z., et al. Downregulation of hsa_circ_0004543 Activates oxLDL-Induced Vascular Endothelial Cell Proliferation and Angiogenesis. Front. Genet. 2021;12:632164. doi: 10.3389/fgene.2021.632164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Q., Shao D., Hao S., Yuan Y., Liu H., Liu F., Mu Q. CircSCAP Aggravates Oxidized Low-density Lipoprotein-induced Macrophage Injury by Upregulating PDE3B by miR-221-5p in Atherosclerosis. J. Cardiovasc. Pharmacol. 2021;78:e749–e760. doi: 10.1097/FJC.0000000000001118. [DOI] [PubMed] [Google Scholar]
- 54.Hu F., Chen X., Gao J., Shen Y., Yang J. CircDIP2C ameliorates oxidized low-density lipoprotein-induced cell dysfunction by binding to miR-556-5p to induce TET2 in human umbilical vein endothelial cells. Vasc. Pharmacol. 2021;139:106887. doi: 10.1016/j.vph.2021.106887. [DOI] [PubMed] [Google Scholar]
- 55.Huang J.G., Tang X., Wang J.J., Liu J., Chen P., Sun Y. A circular RNA, circUSP36, accelerates endothelial cell dysfunction in atherosclerosis by adsorbing miR-637 to enhance WNT4 expression. Bioengineered. 2021;12:6759–6770. doi: 10.1080/21655979.2021.1964891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y., Liang B., Chen X. Exosomal circular RNA circ_0074673 regulates the proliferation, migration, and angiogenesis of human umbilical vein endothelial cells via the microRNA-1200/MEOX2 axis. Bioengineered. 2021;12:6782–6792. doi: 10.1080/21655979.2021.1967077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji N., Wang Y., Gong X., Ni S., Zhang H. CircMTO1 inhibits ox-LDL-stimulated vascular smooth muscle cell proliferation and migration via regulating the miR-182-5p/RASA1 axis. Mol. Med. 2021;27:73. doi: 10.1186/s10020-021-00330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji P., Song X., Lv Z. Knockdown of circ_0004104 Alleviates Oxidized Low-Density Lipoprotein-Induced Vascular Endothelial Cell Injury by Regulating miR-100/TNFAIP8 Axis. J. Cardiovasc. Pharmacol. 2021;78:269–279. doi: 10.1097/FJC.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X., Chen L., Wu H., Chen Y., Lu W., Lu K. Knockdown of Circular Ubiquitin-specific Peptidase 9 X-Linked Alleviates Oxidized Low-density Lipoprotein-induced Injuries of Human Umbilical Vein Endothelial Cells by Mediating the miR-148b-3p/KLF5 Signaling Pathway. J. Cardiovasc. Pharmacol. 2021;78:809–818. doi: 10.1097/FJC.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 60.Jiewei Y., Jingjing Z., Jingjing X., Guilan Z. Downregulation of circ-UBAP2 ameliorates oxidative stress and dysfunctions of human retinal microvascular endothelial cells (hRMECs) via miR-589-5p/EGR1 axis. Bioengineered. 2021;12:7508–7518. doi: 10.1080/21655979.2021.1979440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang L., Jia H., Huang B., Lu S., Chen Z., Shen J., Zou Y., Wang C., Sun Y. Identification of Differently Expressed mRNAs in Atherosclerosis Reveals CDK6 Is Regulated by circHIPK3/miR-637 Axis and Promotes Cell Growth in Human Vascular Smooth Muscle Cells. Front. Genet. 2021;12:596169. doi: 10.3389/fgene.2021.596169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li D., Jin W., Sun L., Wu J., Hu H., Ma L. Circ_0065149 Alleviates Oxidized Low-Density Lipoprotein-Induced Apoptosis and Inflammation in Atherosclerosis by Targeting miR-330-5p. Front. Genet. 2021;12:590633. doi: 10.3389/fgene.2021.590633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li R., Jiang Q., Zheng Y. Circ_0002984 induces proliferation, migration and inflammation response of VSMCs induced by ox-LDL through miR-326-3p/VAMP3 axis in atherosclerosis. J. Cell. Mol. Med. 2021;25:8028–8038. doi: 10.1111/jcmm.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S., Huang T., Qin L., Yin L. Circ_0068087 Silencing Ameliorates Oxidized Low-Density Lipoprotein-Induced Dysfunction in Vascular Endothelial Cells Depending on miR-186-5p-Mediated Regulation of Roundabout Guidance Receptor 1. Front. Cardiovasc. Med. 2021;8:650374. doi: 10.3389/fcvm.2021.650374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Li L., Dong X., Ding J., Ma H., Han W. Circ_GRN Promotes the Proliferation, Migration, and Inflammation of Vascular Smooth Muscle Cells in Atherosclerosis Through miR-214-3p/FOXO1 Axis. J. Cardiovasc. Pharmacol. 2021;77:470–479. doi: 10.1097/FJC.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 66.Liang G., Chen S., Xin S., Dong L. Overexpression of hsa_circ_0001445 reverses oxLDL-induced inhibition of HUVEC proliferation via SRSF1. Mol. Med. Rep. 2021;24:507. doi: 10.3892/mmr.2021.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin D.S., Zhang C.Y., Li L., Ye G.H., Jiang L.P., Jin Q. Circ_ROBO2/miR-149 Axis Promotes the Proliferation and Migration of Human Aortic Smooth Muscle Cells by Activating NF-κB Signaling. Cytogenet. Genome Res. 2021;161:414–424. doi: 10.1159/000517294. [DOI] [PubMed] [Google Scholar]
- 68.Liu J., Wang Y., Liao Y., Zhou Y., Zhu J. Circular RNA PPP1CC promotes. J. Int. Med. Res. 2021;49:300060521996564. doi: 10.1177/0300060521996564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo Y., Huang C. CircSFMBT2 facilitates vascular smooth muscle cell proliferation by targeting miR-331-3p/HDAC5. Life Sci. 2021;264:118691. doi: 10.1016/j.lfs.2020.118691. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y., Zheng B., Zhang X.H., Nie Z.Y., Yu J., Zhang H., Wang D.D., Shi B., Bai Y., Yang Z., et al. circACTA2 mediates Ang II-induced VSMC senescence by modulation of the interaction of ILF3 with CDK4 mRNA. Aging. 2021;13:11610–11628. doi: 10.18632/aging.202855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mei X., Cui X.B., Li Y., Chen S.Y. CircSOD2: A Novel Regulator for Smooth Muscle Proliferation and Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2021;41:2961–2973. doi: 10.1161/ATVBAHA.121.316911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miao J., Wang B., Shao R., Wang Y. CircUSP36 knockdown alleviates oxidized low-density lipoprotein-induced cell injury and inflammatory responses in human umbilical vein endothelial cells via the miR-20a-5p/ROCK2 axis. Int. J. Mol. Med. 2021;47:40. doi: 10.3892/ijmm.2021.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng H., Sun J., Li Y., Zhang Y., Zhong Y. Circ-USP9X Inhibition Reduces Oxidized Low-density Lipoprotein-induced Endothelial Cell Injury via the microRNA 599/Chloride Intracellular Channel 4 Axis. J. Cardiovasc. Pharmacol. 2021;78:560–571. doi: 10.1097/FJC.0000000000001104. [DOI] [PubMed] [Google Scholar]
- 74.Peng K., Jiang P., Du Y., Zeng D., Zhao J., Li M., Xia C., Xie Z., Wu J. Oxidized low-density lipoprotein accelerates the injury of endothelial cells via circ-USP36/miR-98-5p/VCAM1 axis. IUBMB Life. 2021;73:177–187. doi: 10.1002/iub.2419. [DOI] [PubMed] [Google Scholar]
- 75.Peng W., Li S., Chen S., Yang J., Sun Z. Hsa_circ_0003204 Knockdown Weakens Ox-LDL-Induced Cell Injury by Regulating miR-188-3p/TRPC6 Axis in Human Carotid Artery Endothelial Cells and THP-1 Cells. Front. Cardiovasc. Med. 2021;8:731890. doi: 10.3389/fcvm.2021.731890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shao X., Liu Z., Liu S., Lin N., Deng Y. Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/miR-135a-5p/CLIC4 axis in AS cell model in vitro. Mol. Cell. Biochem. 2021;476:1783–1795. doi: 10.1007/s11010-020-04035-8. [DOI] [PubMed] [Google Scholar]
- 77.Su G., Sun G., Lv J., Zhang W., Liu H., Tang Y., Su H. Hsa_circ_0004831 downregulation is partially responsible for atorvastatinalleviated human umbilical vein endothelial cell injuries induced by ox-LDL through targeting the miR-182-5p/CXCL12 axis. BMC Cardiovasc. Disord. 2021;21:221. doi: 10.1186/s12872-021-01998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su Q., Dong X., Tang C., Wei X., Hao Y., Wu J. Knockdown of circ_0003204 alleviates oxidative low-density lipoprotein-induced human umbilical vein endothelial cells injury: Circulating RNAs could explain atherosclerosis disease progression. Open Med. 2021;16:558–569. doi: 10.1515/med-2021-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun C., Li J., Li Y., Li L., Huang G. Circular RNA circUBR4 regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells through miR-185-5p/FRS2 axis. Mol. Cell. Biochem. 2021;476:3899–3910. doi: 10.1007/s11010-021-04207-0. [DOI] [PubMed] [Google Scholar]
- 80.Tiliwaldi H., Tursun A., Tohti A., Mamatzunun M., Wu Z. Circ_0000345 Protects Endothelial Cells from Oxidized Low-Density Lipoprotein-Induced Injury by miR-129-5p/Ten-Eleven Translocation Axis. J. Cardiovasc. Pharmacol. 2021;77:603–613. doi: 10.1097/FJC.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 81.Wan H., You T., Luo W. circ_0003204 Regulates Cell Growth, Oxidative Stress, and Inflammation in ox-LDL-Induced Vascular Endothelial Cells via Regulating miR-942-5p/HDAC9 Axis. Front. Cardiovasc. Med. 2021;8:646832. doi: 10.3389/fcvm.2021.646832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S., Shi M., Li J., Zhang Y., Wang W., Xu P., Li Y. Endothelial cell-derived exosomal circHIPK3 promotes the proliferation of vascular smooth muscle cells induced by high glucose via the miR-106a-5p/Foxo1/Vcam1 pathway. Aging. 2021;13:25241–25255. doi: 10.18632/aging.203742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X., Bai M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc. Disord. 2021;21:51. doi: 10.1186/s12872-020-01800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen Y., Chun Y., Lian Z.Q., Yong Z.W., Lan Y.M., Huan L., Xi C.Y., Juan L.S., Qing Z.W., Jia C., et al. circRNA-0006896-miR1264-DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol. Med. Rep. 2021;23:311. doi: 10.3892/mmr.2021.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu S., Yang S., Qu H. circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells. Open Life Sci. 2021;16:1053–1063. doi: 10.1515/biol-2021-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu W.P., Zhou M.Y., Liu D.L., Min X., Shao T., Xu Z.Y., Jing X., Cai M.Y., Xu S., Liang X., et al. circGNAQ, a circular RNA enriched in vascular endothelium, inhibits endothelial cell senescence and atherosclerosis progression. Mol. Ther. Nucleic Acids. 2021;26:374–387. doi: 10.1016/j.omtn.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong F., Mao R., Zhang L., Zhao R., Tan K., Liu C., Xu J., Du G., Zhang T. CircNPHP4 in monocyte-derived small extracellular vesicles controls heterogeneous adhesion in coronary heart atherosclerotic disease. Cell Death Dis. 2021;12:948. doi: 10.1038/s41419-021-04253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu F., Shen L., Chen H., Wang R., Zang T., Qian J., Ge J. circDENND1B Participates in the Antiatherosclerotic Effect of IL-1β Monoclonal Antibody in Mouse by Promoting Cholesterol Efflux via miR-17-5p/Abca1 Axis. Front. Cell Dev. Biol. 2021;9:652032. doi: 10.3389/fcell.2021.652032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu H., Pan Y., Dai M., Xu H., Li J. Circ_0003423 Alleviates ox-LDL-Induced Human Brain Microvascular Endothelial Cell Injury via the miR-589-5p/TET2 Network. Neurochem. Res. 2021;46:2885–2896. doi: 10.1007/s11064-021-03387-x. [DOI] [PubMed] [Google Scholar]
- 90.Yu F., Zhang Y., Wang Z., Gong W., Zhang C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics. 2021;11:5404–5417. doi: 10.7150/thno.48389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng Z., Xia L., Fan S., Zheng J., Qin J., Fan X., Liu Y., Tao J., Li K., Ling Z., et al. Circular RNA CircMAP3K5 Acts as a MicroRNA-22-3p Sponge to Promote Resolution of Intimal Hyperplasia Via TET2-Mediated Smooth Muscle Cell Differentiation. Circulation. 2021;143:354–371. doi: 10.1161/CIRCULATIONAHA.120.049715. [DOI] [PubMed] [Google Scholar]
- 92.Zhang B., Zhang Y., Li R., Li Y., Yan W. Knockdown of circular RNA hsa_circ_0003204 inhibits oxidative stress and apoptosis through the miR-330-5p/Nod2 axis to ameliorate endothelial cell injury induced by low-density lipoprotein. Cent. Eur. J. Immunol. 2021;46:140–151. doi: 10.5114/ceji.2021.108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang C., Wang L., Shen Y. Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/TRIM14 axis in atherosclerosis. BMC Cardiovasc. Disord. 2021;21:207. doi: 10.1186/s12872-021-02012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang P., Wang W., Li M. Circ_0010283/miR-377-3p/Cyclin D1 Axis Is Associated with Proliferation, Apoptosis, Migration, and Inflammation of Oxidized Low-density Lipoprotein-Stimulated Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2021;78:437–447. doi: 10.1097/FJC.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Q., Long J., Li N., Ma X., Zheng L. Circ_CLASP2 Regulates High Glucose-Induced Dysfunction of Human Endothelial Cells Through Targeting miR-140-5p/FBXW7 Axis. Front. Pharmacol. 2021;12:594793. doi: 10.3389/fphar.2021.594793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X., Lu J., Zhang Q., Luo Q., Liu B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 2021;54:11. doi: 10.1186/s40659-021-00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X., Wang P., Yuan K., Li M., Shen Y., Que H., Wang Y., Liang W. Hsa_circ_0024093 accelerates VSMC proliferation via miR-4677-3p/miR-889-3p/USP9X/YAP1 axis in. Mol. Ther. Nucleic Acids. 2021;26:511–522. doi: 10.1016/j.omtn.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Li W., Li H., Zhou M., Zhang J., Fu Y., Zhang C., Sun X. Circ_USP36 Silencing Attenuates Oxidized Low-Density Lipoprotein-Induced Dysfunction in Endothelial Cells in Atherosclerosis Through Mediating miR-197-3p/ROBO1 Axis. J. Cardiovasc. Pharmacol. 2021;78:e761–e772. doi: 10.1097/FJC.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Zhang C., Chen Z., Wang M. Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis. Open Life Sci. 2021;16:419–430. doi: 10.1515/biol-2021-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng X., Liu J., Gong X., Zhang X., Ma S. Circ_0002984 Enhances Growth, Invasion, and Migration in PDGF-bb-Induced Vascular Smooth Muscle Cells Through miR-379-5p/FRS2 Axis. J. Cardiovasc. Pharmacol. 2021;78:875–884. doi: 10.1097/FJC.0000000000001143. [DOI] [PubMed] [Google Scholar]
- 101.Cai Y., Xu L., Xu C., Wang Y., Fan C. Hsa_circ_0001445 inhibits ox-LDL-induced HUVECs inflammation, oxidative stress and apoptosis by regulating miRNA-640. Perfusion. 2022;37:86–94. doi: 10.1177/0267659120979472. [DOI] [PubMed] [Google Scholar]
- 102.Chen M., Li F., Jiang Q., Zhang W., Li Z., Tang W. Role of miR-181b/Notch1 Axis in circ_TNPO1 Promotion of Proliferation and Migration of Atherosclerotic Vascular Smooth Muscle Cells. J. Healthc. Eng. 2022;2022:4086935. doi: 10.1155/2022/4086935. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Chen S., Sun L., Zhang J., Zhang L., Liu X. Oxygenized Low-Density Lipoprotein-Induced ASMC Dysregulation Depends on circ_0000345-Mediated Regulatory Mechanism. J. Atheroscler. Thromb. 2022;29:1849–1863. doi: 10.5551/jat.63327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen T., Li L., Ye B., Chen W., Zheng G., Xie H., Guo Y. Knockdown of hsa_circ_0005699 attenuates inflammation and apoptosis induced by ox-LDL in human umbilical vein endothelial cells through regulation of the miR-450b-5p/NFKB1 axis. Mol. Med. Rep. 2022;26:290. doi: 10.3892/mmr.2022.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du N., Li M., Yang D. Hsa_circRNA_102541 regulates the development of atherosclerosis by targeting miR-296-5p/PLK1 pathway. Ir. J. Med. Sci. 2022;191:1153–1159. doi: 10.1007/s11845-021-02708-x. [DOI] [PubMed] [Google Scholar]
- 106.Heumüller A.W., Jones A.N., Mourão A., Klangwart M., Shi C., Wittig I., Fischer A., Muhly-Reinholz M., Buchmann G.K., Dieterich C., et al. Locus-Conserved Circular RNA cZNF292 Controls Endothelial Cell Flow Responses. Circ. Res. 2022;130:67–79. doi: 10.1161/CIRCRESAHA.121.320029. [DOI] [PubMed] [Google Scholar]
- 107.Hou X., Dai H., Zheng Y. Circular RNA hsa_circ_0008896 accelerates atherosclerosis by promoting the proliferation, migration and invasion of vascular smooth muscle cells via hsa-miR-633/CDC20B (cell division cycle 20B) axis. Bioengineered. 2022;13:5987–5998. doi: 10.1080/21655979.2022.2039467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jing B., Hui Z. Circular RNA_0033596 aggravates endothelial cell injury induced by oxidized low-density lipoprotein via microRNA-217-5p /chloride intracellular channel 4 axis. Bioengineered. 2022;13:3410–3421. doi: 10.1080/21655979.2022.2027062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kou L., Yang N., Dong B., Yang J., Song Y., Li Y., Qin Q. Circular RNA testis-expressed 14 overexpression induces apoptosis and suppresses migration of ox-LDL-stimulated vascular smooth muscle cells via regulating the microRNA 6509-3p/thanatos-associated domain-containing apoptosis-associated protein 1 axis. Bioengineered. 2022;13:13150–13161. doi: 10.1080/21655979.2022.2070582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lei X., Yang Y. Oxidized low-density lipoprotein contributes to injury of endothelial cells via the circ_0090231/miR-9-5p/TXNIP axis. Cent. Eur. J. Immunol. 2022;47:41–57. doi: 10.5114/ceji.2021.112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X., Kang X., Di Y., Sun S., Yang L., Wang B., Ji Z. CircCHMP5 Contributes to Ox-LDL-induced Endothelial Cell Injury Through the Regulation of MiR-532-5p/ROCK2 axis. Cardiovasc. Drugs Ther. 2022 doi: 10.1007/s10557-022-07316-0. [DOI] [PubMed] [Google Scholar]
- 112.Li Y., Wang B. Circular RNA circCHFR downregulation protects against oxidized low-density lipoprotein-induced endothelial injury via regulation of microRNA-15b-5p/growth arrest and DNA damage inducible gamma. Bioengineered. 2022;13:4481–4492. doi: 10.1080/21655979.2022.2032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin J., Liu C., Xu J., Li S., Dai D., Zhang L., Yonghui P. Circ_0021155 can participate in the phenotypic transformation of human vascular smooth muscle cells via the miR-4459/TRPM7 axis. Biochem. Biophys. Res. Commun. 2022;630:133–142. doi: 10.1016/j.bbrc.2022.08.065. [DOI] [PubMed] [Google Scholar]
- 114.Liu F., Gao B., Wang Y. CircIRAK1 aggravates ox-LDL-induced endothelial cell injury in atherosclerosis via TRIM14 upregulation by binding to miR-330-5p1. Clin. Hemorheol. Microcirc. 2022 doi: 10.3233/CH-221551. Pre-press . [DOI] [PubMed] [Google Scholar]
- 115.Liu J., Zhang X., Yu Z., Zhang T. Circ_0026218 ameliorates oxidized low-density lipoprotein-induced vascular endothelial cell dysfunction by regulating miR-188-3p/TLR4/NF-κB pathway. Cardiovasc. Drugs Ther. 2022 doi: 10.1007/s10557-022-07416-x. [DOI] [PubMed] [Google Scholar]
- 116.Liu S., Wang L., Wu X., Wu J., Liu D., Yu H. Overexpression of hsa_circ_0022742 suppressed hyperglycemia-induced endothelial dysfunction by targeting the miR-503-5p/FBXW7 axis. Microvasc. Res. 2022;139:104249. doi: 10.1016/j.mvr.2021.104249. [DOI] [PubMed] [Google Scholar]
- 117.Lu Q., Li Y., Lou J., Li P., Gu Y., Wang X. Circ-CHFR modulates the proliferation, migration, and invasion of ox-LDL-induced human aorta vascular smooth muscle cells through the miR-214-3p/PAPPA axis. Clin. Hemorheol. Microcirc. 2022;80:399–412. doi: 10.3233/CH-211288. [DOI] [PubMed] [Google Scholar]
- 118.Luo X., Zhou X. CircRNA-PTPRA Knockdown Inhibits Atherosclerosis Progression by Repressing ox-LDL-Induced Endothelial Cell Injury via Sponging of miR-671-5p. Biochem. Genet. 2023;61:187–201. doi: 10.1007/s10528-022-10256-x. [DOI] [PubMed] [Google Scholar]
- 119.Ma J., Liu J., Li T., Ren J. Hsa_circ_0030042 Facilitates the Proliferation and Migration of Vascular Smooth Muscle Cells via the miR-514a-3p/FOXO1 Axis. J. Endovasc. Ther. 2022;29:611–622. doi: 10.1177/15266028211057086. [DOI] [PubMed] [Google Scholar]
- 120.Ma W., Wei D., Li X., Shan L., Fan H., Jin H., Song B., Zhang B. CircPCNX Promotes PDGF-BB-Induced Proliferation and Migration of Human Aortic Vascular Smooth Muscle Cells Through Regulating miR-1278/DNMT1 Axis. Cardiovasc. Drugs Ther. 2022 doi: 10.1007/s10557-022-07342-y. [DOI] [PubMed] [Google Scholar]
- 121.Mao X., Wang L., Chen C., Tao L., Ren S., Zhang L. Circ_0124644 enhances ox-LDL-induced cell damages in human umbilical vein endothelial cells through upregulating FOXO4 by sponging miR-370-3p. Clin. Hemorheol. Microcirc. 2022;81:135–147. doi: 10.3233/CH-211375. [DOI] [PubMed] [Google Scholar]
- 122.Mei R., Wu M., Ren F. Knockdown of circ_0002194 protects against oxidized low-density lipoprotein-induced cell damage via the regulation of the miR-637/PACS2 axis in human vascular endothelial cells. Interact. Cardiovasc. Thorac. Surg. 2022;35:ivac210. doi: 10.1093/icvts/ivac210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Min X., Cai M.Y., Shao T., Xu Z.Y., Liao Z., Liu D.L., Zhou M.Y., Wu W.P., Zhou Y.L., Mo M.H., et al. A circular intronic RNA ciPVT1 delays endothelial cell senescence by regulating the miR-24-3p/CDK4/pRb axis. Aging Cell. 2022;21:e13529. doi: 10.1111/acel.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng H., Liu S., Li Y., Wang C., Zhong Y. A Novel circUBR4/miR-491-5p/NRP2 ceRNA Network Regulates Oxidized Low-density Lipoprotein-induced Proliferation and Migration in Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2022;79:512–522. doi: 10.1097/FJC.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 125.Qiu J., Chen R., Zhao L., Lian C., Liu Z., Zhu X., Cui J., Wang S., Wang M., Huang Y., et al. Circular RNA circGSE1 promotes angiogenesis in ageing mice by targeting the miR-323-5p/NRP1 axis. Aging. 2022;14:3049–3069. doi: 10.18632/aging.203988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryu J., Choe N., Kwon D.H., Shin S., Lim Y.H., Yoon G., Kim J.H., Kim H.S., Lee I.K., Ahn Y., et al. Circular RNA circSmoc1-2 regulates vascular calcification by acting as a miR-874-3p sponge in vascular smooth muscle cells. Mol. Ther. Nucleic Acids. 2022;27:645–655. doi: 10.1016/j.omtn.2021.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian Y., Zheng G., Xie H., Guo Y., Zeng H., Fu Y., Liu X. Study on the Mechanism of circRNA-0024103 Reducing Endothelial Cell Injury by Regulating miR-363/MMP-10. Contrast Media Mol. Imaging. 2022;2022:1709325. doi: 10.1155/2022/1709325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang M., Li C., Cai T., Zhang A., Cao J., Xin H. Circ_CHFR Promotes Platelet-Derived Growth Factor-BB-Induced Proliferation, Invasion, and Migration in Vascular Smooth Muscle Cells via the miR-149-5p/NRP2 Axis. J. Cardiovasc. Pharmacol. 2022;79:e94–e102. doi: 10.1097/FJC.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 129.Wang P., Zhang H., Wang Y. Circ_0003423 Alleviates Oxidized Low-Density Lipoprotein-Induced Endothelial Cell Injury by Sponging miR-142-3p and Activating Sirtuin 3/Superoxide Dismutase 2 Pathway. J. Surg. Res. 2022;277:384–397. doi: 10.1016/j.jss.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 130.Wang X., Ma C., Hou X., Zhang G., Huang Y. Circular RNA circ_0002984 Promotes Cell Proliferation and Migration by Regulating miR-181b-5p/Vascular Endothelial Growth Factor Axis and PI3K-AKT Signaling Pathway in Oxidized Low-Density Lipoprotein-Treated Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2022;79:501–511. doi: 10.1097/FJC.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y., Chen X., Lu Z., Lai C. Circ_0093887 regulated ox-LDL induced human aortic endothelial cells viability, apoptosis, and inflammation through modulating miR-758-3p/BAMBI axis in atherosclerosis. Clin. Hemorheol. Microcirc. 2022;81:343–358. doi: 10.3233/CH-221445. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y., Pei W., Lu P. Circ_ARHGAP32 acts as miR-665 sponge to upregulate FGF2 to promote ox-LDL induced vascular smooth muscle cells proliferation and migration. Clin. Hemorheol. Microcirc. 2022;82:169–182. doi: 10.3233/CH-221469. [DOI] [PubMed] [Google Scholar]
- 133.Wang Z., Wang H., Guo C., Yu F., Zhang Y., Qiao L., Zhang H., Zhang C. Role of hsa_circ_0000280 in regulating vascular smooth muscle cell function and attenuating neointimal hyperplasia via ELAVL1. Cell. Mol. Life Sci. 2022;80:3. doi: 10.1007/s00018-022-04602-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang J. Mmu_circ_0000271 regulated the growth of ox-LDL-stimulated mouse vascular smooth muscle cells via sponging miR-5123. Genes Genom. 2022;44:1099–1108. doi: 10.1007/s13258-022-01268-3. [DOI] [PubMed] [Google Scholar]
- 135.Yang Y., Mao W., Wang L., Lu L., Pang Y. Circular RNA circLMF1 regulates PDGF-BB-induced proliferation and migration of human aortic smooth muscle cells by regulating the miR-125a-3p/VEGFA or FGF1 axis. Clin. Hemorheol. Microcirc. 2022;80:167–183. doi: 10.3233/CH-211166. [DOI] [PubMed] [Google Scholar]
- 136.Ye B., Liang X., Zhao Y., Cai X., Wang Z., Lin S., Wang W., Shan P., Huang W., Huang Z. Hsa_circ_0007478 aggravates NLRP3 inflammasome activation and lipid metabolism imbalance in ox-LDL-stimulated macrophage via miR-765/EFNA3 axis. Chem. Biol. Interact. 2022;368:110195. doi: 10.1016/j.cbi.2022.110195. [DOI] [PubMed] [Google Scholar]
- 137.Ye Q., Ju C., Ye Z., Tong J. Circ_ROBO2/miR-186-5p/TRIM14 axis regulates oxidized low-density lipoprotein-induced cardiac microvascular endothelial cell injury. Regen. Ther. 2022;20:138–146. doi: 10.1016/j.reth.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu L., Ma W., Song B., Wang S., Li X., Wang Z. Hsa_circ_0030042 Ameliorates Oxidized Low-Density Lipoprotein-Induced Endothelial Cell Injury via the MiR-616-3p/RFX7 Axis. Int. Heart J. 2022;63:763–772. doi: 10.1536/ihj.22-065. [DOI] [PubMed] [Google Scholar]
- 139.Zhang D., Zhang G., Yu K., Zhang X., Jiang A. Circ_0003204 knockdown protects endothelial cells against oxidized low-density lipoprotein-induced injuries by targeting the miR-491-5p-ICAM1 pathway. J. Thromb. Thrombolysis. 2022;53:302–312. doi: 10.1007/s11239-021-02606-0. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H., Zhang B., Chen C., Chen J. Circular RNA circLIFR regulates the proliferation, migration, invasion and apoptosis of human vascular smooth muscle cells via the miR-1299/KDR axis. Metab. Brain Dis. 2022;37:253–263. doi: 10.1007/s11011-021-00853-x. [DOI] [PubMed] [Google Scholar]
- 141.Zhang M., Zhu Y., Zhu J., Xie Y., Wu R., Zhong J., Qiu Z., Jiang L. circ_0086296 induced atherosclerotic lesions via the IFIT1/STAT1 feedback loop by sponging miR-576-3p. Cell. Mol. Biol. Lett. 2022;27:80. doi: 10.1186/s11658-022-00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang P., Wang W., Li M. Role and mechanism of circular RNA circ_0050486 in regulating oxidized low-density lipoprotein-induced injury in endothelial cells. Clin. Hemorheol. Microcirc. 2022;82:107–124. doi: 10.3233/CH-211259. [DOI] [PubMed] [Google Scholar]
- 143.Zhang W.B., Qi Y.F., Xiao Z.X., Chen H., Liu S.H., Li Z.Z., Zeng Z.F., Wu H.F. CircHIPK3 Regulates Vascular Smooth Muscle Cell Calcification Via the miR-106a-5p/MFN2 Axis. J. Cardiovasc. Transl. Res. 2022;15:1315–1326. doi: 10.1007/s12265-022-10247-8. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y., Wang S., Guo S., Zhang X., Yang C., Su G., Wan J. Circ_0004104 participates in the regulation of ox-LDL-induced endothelial cells injury via miR-942-5p/ROCK2 axis. BMC Cardiovasc. Disord. 2022;22:517. doi: 10.1186/s12872-022-02959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu J., Chen Z., Peng X., Zheng Z., Le A., Guo J., Ma L., Shi H., Yao K., Zhang S., et al. Extracellular Vesicle-Derived circITGB1 Regulates Dendritic Cell Maturation and Cardiac Inflammation via miR-342-3p/NFAM1. Oxid. Med. Cell. Longev. 2022;2022:8392313. doi: 10.1155/2022/8392313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel R.S., Asselbergs F.W., Quyyumi A.A., Palmer T.M., Finan C.I., Tragante V., Deanfield J., Hemingway H., Hingorani A.D., Holmes M.V. Genetic variants at chromosome 9p21 and risk of first versus subsequent coronary heart disease events: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2014;63:2234–2245. doi: 10.1016/j.jacc.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Burd C.E., Jeck W.R., Liu Y., Sanoff H.K., Wang Z., Sharpless N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi P., Ji H., Zhang H., Yang J., Guo R., Wang J. circANRIL reduces vascular endothelial injury, oxidative stress and inflammation in rats with coronary atherosclerosis. Exp. Ther. Med. 2020;20:2245–2251. doi: 10.3892/etm.2020.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lyon C.A., Johnson J.L., Williams H., Sala-Newby G.B., George S.J. Soluble N-cadherin overexpression reduces features of atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2009;29:195–201. doi: 10.1161/ATVBAHA.108.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bobryshev Y.V., Lord R.S., Watanabe T., Ikezawa T. The cell adhesion molecule E-cadherin is widely expressed in human atherosclerotic lesions. Cardiovasc. Res. 1998;40:191–205. doi: 10.1016/S0008-6363(98)00141-2. [DOI] [PubMed] [Google Scholar]
- 151.Yanni J., D’Souza A., Wang Y., Li N., Hansen B.J., Zakharkin S.O., Smith M., Hayward C., Whitson B.A., Mohler P.J., et al. Silencing miR-370-3p rescues funny current and sinus node function in heart failure. Sci. Rep. 2020;10:11279. doi: 10.1038/s41598-020-67790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He A.T., Liu J., Li F., Yang B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal. Transduct. Target. Ther. 2021;6:185. doi: 10.1038/s41392-021-00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grootaert M.O.J., Bennett M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021;117:2326–2339. doi: 10.1093/cvr/cvab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kockx M.M., Herman A.G. Apoptosis in atherosclerosis: Beneficial or detrimental? Cardiovasc. Res. 2000;45:736–746. doi: 10.1016/S0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- 155.Gimbrone M.A., García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu Y., Li G., Ma Y., Luo G., Wang Q., Zhang S. Effect of Exosomal lncRNA MALAT1/miR-370-3p/STAT3 Positive Feedback Loop on PI3K/Akt Pathway Mediating Cisplatin Resistance in Cervical Cancer Cells. J. Oncol. 2023;2023:6341011. doi: 10.1155/2023/6341011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma H., Qu S., Zhai Y., Yang X. circ_0025033 promotes ovarian cancer development via regulating the hsa_miR-370-3p/SLC1A5 axis. Cell. Mol. Biol. Lett. 2022;27:94. doi: 10.1186/s11658-022-00364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Long X., Wang D.G., Wu Z.B., Liao Z.M., Xu J.J. Circular RNA hsa_circ_0004689 (circSWT1) promotes NSCLC progression via the miR-370-3p/SNAIL axis by inducing cell epithelial-mesenchymal transition (EMT) Cancer Med. 2023;12:8289–8305. doi: 10.1002/cam4.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ning T., Zhang H., Wang X., Li S., Zhang L., Deng T., Zhou L., Liu R., Bai M., Ge S., et al. [Corrigendum] miR-370 regulates cell proliferation and migration by targeting EGFR in gastric cancer. Oncol. Rep. 2022;48:194. doi: 10.3892/or.2022.8409. [DOI] [PubMed] [Google Scholar]
- 160.Peng S., Chen Y., Li T., Mao J., Yang P., Zou B., Luo L., Zhang W., Wang W., Xie R., et al. Hsa-microRNA-370-3p targeting Snail and Twist1 suppresses IL-8/STAT3-driven hepatocellular carcinoma metastasis. Cancer Sci. 2022;113:4120–4134. doi: 10.1111/cas.15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.