Abstract
In this study, we investigated urea glycerolysis over ZnAl2O4 catalysts that were prepared by using a citrate complex method and the influence of calcination temperatures on the surface properties of the prepared catalysts by varying the calcination temperature from 550 °C to 850 °C. As the reciprocal substitution between Al3+ and Zn2+ cations led to the formation of a disordered bulk ZnAl2O4 phase, different calcination temperatures strongly influenced the surface properties of the ZnAl2O4 catalysts, including oxygen vacancy. The increase in the calcination temperature from 550 °C to 650 °C decreased the inversion parameter of the ZnAl2O4 structure (from 0.365 to 0.222 for AlO4 and 0.409 to 0.358 for ZnO6). The disordered ZnAl2O4 structure led to a decrease in the surface acidity. The ZnAl2O4-550 catalyst had a large specific surface area, along with highly disordered surface sites, which increased surface acidity, resulting in a stronger interaction of the Zn NCO complex on its surface and an improvement in catalytic performance. Fourier transform infrared and thermogravimetric analysis results of the spent catalysts demonstrated the formation of a greater amount of a solid Zn NCO complex over ZnAl2O4-550 than ZnAl2O4-650. Consequently, the ZnAl2O4-550 catalyst outperformed the ZnAl2O4-650 catalyst in terms of glycerol conversion (72%), glycerol carbonate yield (33%), and byproduct formation.
Keywords: partially inverse spinel, polymeric citrate complex method, acidity, glycerol carbonate
1. Introduction
In recent years, climate change has become increasingly severe, which has led to the promotion of the development of the biodiesel industry [1,2]. Large amounts of crude glycerol as a byproduct generated from biodiesel production have an economic impact as a cheap and commercially viable renewable feedstock [3]. Among various glycerol reaction pathways, glycerolysis into glycerol carbonate (GC) is an interesting research direction. GC is an important glycerol derivative that is considered an interesting value-added chemical due to its excellent properties such as being a non-hazardous low-vapor pressure liquid, as well as its biodegradability, its low toxicity, its non-flammability, its high boiling point, and its low volatility [4,5,6]. Because of its outstanding properties, GC has a wide range of applications, including solvents, electrolytes, surfactants, wetting agents, and as a chemical intermediate in polymer synthesis [5,7].
Traditionally, the chemical synthesis of GC is by glycerol reacting with phosgene; however, this reaction is very dangerous and not environmentally friendly [8]. Alternatively, carbonate sources with milder reaction conditions such as dialkyl carbonate [9,10,11], alkylene carbonate [12], and CO2 [13,14] can be used. With special advantages such as being cheap, easy to obtain, and recyclable, urea is used, along with glycerol, to efficiently produce GC [15,16,17,18]. In our previous studies, catalytic performance was affected by the existence of Zn-containing intermediates, namely, zinc diamine diisocyanate (Zn(NH3)2(NCO)2, abbreviated as a Zn NCO complex). The formation of the solid Zn NCO complex on the ZnAl2O4 phase offered many benefits for increasing the GC yield: acting as active sites for the heterogeneous GC pathway and interacting with an insoluble ZnAl2O4 phase [17].
The ZnAl2O4 spinel structure is represented with the typical formula (Zn2+)[Al3+2]O4, where Zn2+ cations occupy one-eighth of the tetrahedral sites, and Al3+ cations occupy half of the octahedral sites. However, a normal ZnAl2O4 spinel structure can be partially disordered, replacing some Al3+ cations with Zn2+ cations in the octahedral sites and some Zn2+ cations with Al3+ cations in the tetrahedral sites [16]. It has been reported that the disordered structure of the ZnAl2O4 spinel structure affected the formation of the Zn NCO complex. Despite many studies on the various types of reactions or methods for preparing the ZnAl2O4 spinel structure, only a few studies have been conducted to investigate the influence of calcination temperatures on the structural properties of ZnAl2O4 catalysts. The formation of the disordered ZnAl2O4 structure is highly dependent on the calcination temperature.
Hence, in this work, ZnAl2O4 spinel catalysts with a disordered structure (denoted as ZnAl2O4-X, where X represents calcination temperature in °C) were prepared by using a polymeric citrate complex method and calcined at different temperatures ranging from 550 °C to 850 °C to investigate the influence of calcination temperatures on the ZnAl2O4 spinel lattice structure, the acidity of the catalysts, and the formation of a solid Zn NCO complex on the catalysts. We also used the prepared catalysts in the glycerolysis of urea under vacuum (3 kPa) at 140 °C for 5 h. Characterizations, including Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS), were employed to observe the disordered ZnAl2O4 spinel structure. Acidic sites were measured via the temperature-programmed desorption of NH3 (NH3-TPD), and the formation of a solid Zn NCO complex was demonstrated by FT-IR and thermogravimetric analysis (TGA).
2. Materials and Methods
2.1. Catalyst Preparation
Citrate complex ZnAl2O4 catalysts were prepared by using a modified citrate complex technique [19]. An aqueous solution of Zn(NO3)2.6H2O (Sigma-Aldrich Korea, Gyounggi, Republic of Korea) and Al(NO3)3·9H2O (Sigma-Aldrich Korea) with a molar ratio of Zn and Al in the precursor of 1:2 was prepared at room temperature, and the citric acid powder (citric acid to metal ratio of 2:1) (Sigma-Aldrich Korea) was added to the solution, followed by stirring with a magnetic bar. Stirring was continued at 70 °C to evaporate water until a yellow viscous gel solution was formed. The gel was further dried in an oven at 140 °C and spontaneously solidified by the emission of NOx gases. Finally, all catalysts were calcined under a set temperature (550 °C–850 °C) in a furnace for 4 h. The catalysts were denoted as ZnAl2O4-X, where X indicates the calcination temperature.
2.2. Reaction Test
The reaction was performed in a round-bottom, three-neck 100 mL flask, in which one neck was connected to a vacuum line through a water condenser. Glycerol (Sigma-Aldrich Korea) (0.2 mol) was added to the flask at 80 °C under stirring by a magnetic bar to reduce the high viscosity of the glycerol. The reactor was connected to a vacuum pump through a HNO3 (Sigma-Aldrich Korea) solution trap (to remove NH3) and a cold trap (to remove order volatiles). After 10 min, 0.2 mol of urea (Sigma-Aldrich Korea) was poured into the flask to mix with glycerol in the solution. When all the urea was dissolved completely in glycerol and the solution was transparent, a certain amount of catalyst (5 wt% compared with the initial glycerol amount) was added to the flask. The reaction was performed under vacuum pressure (3 kPa) at 140 °C with constant stirring.
After the reaction tests, ethanol (Sigma-Aldrich Korea) was poured into the final products, and the liquid products were separated from the spent catalyst via filtration. The liquid product was quantitatively analyzed via gas chromatography by using a gas chromatography machine (Acme 6100 GC, YL Instrument Co., Ltd., Dongangu, Anyang, Republic of Korea) (a schematic diagram of the catalyst activity test device is shown in Figure S1 in the Supplementary material) with a flame ionization detector and a capillary column DB-Wax (30 m × 0.25 mm × 0.25 µm). The molar amount of each component was calculated by using the internal standard method, with tetraethylene glycol (Sigma-Aldrich Korea) as the internal standard chemical. Glycerol conversion, GC selectivity, GC yield, and byproduct selectivity were calculated by using the equations below. The amount of each chemical is on the mole unit.
FT-IR spectra of the liquid products were obtained by using a Thermo Scientific™ Nicolet™ iS™5 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) by dropping a liquid sample between KBr (Sigma-Aldrich Korea) plates.
2.3. Catalyst Characterization
The Chemical Composition Zn/Al Molar Ratio was measured by using an Agilent Technologies 5110 ICP-OES (Agilent, Santa Clara, CA, USA) instrument. The surface characterizations were measured via N2 adsorption isotherm analysis on a Micromeritics ASAP 2020 (USA) apparatus. The surface area was calculated by using the Brunauer–Emmett–Teller (BET) method. X-ray diffraction (XRD) patterns for fresh catalysts were obtained by using a Rigaku RAD-3C diffractometer (Rigaku Corp., Tokyo, Japan) with Cu Ka radiation (λ = 1.5418 Å) at a scattering angle (2θ) scan rate of 2°/min, operating at 35 kV and 20 mA. The fresh and spent catalysts were analyzed by using a Thermo Scientific Nicolet iS5 FT-IR spectrometer (Thermo Fisher Scientific). XPS data were surveyed by using a Thermo Scientific K-Alpha XPS spectrometer (Thermo Fisher Scientific). The numbers of acidic and basic sites were measured on the basis of TPD-NH3/CO2 on a MicrotracBEL BELCAT-M instrument (MicrotracBEL Corp., Osaka, Japan). An amount of 100–200 mg of fresh catalysts was placed into the quartz sample tube of the instrument. Initially, the sample was pretreated under helium flow (100 mL/min) at 600 °C for 1 h and then cooled down to 50 °C. After that, a flow of NH3 or CO2 (50 mL/min) was injected for chemisorption. Finally, the temperature was increased to 600 °C with a ramping rate of 1.5 °C/min; the desorbed species were removed via helium flow (30 mL/min) and analyzed via thermal conductivity detection. The TGA of the spent catalysts was performed by using a TGA Q50 apparatus (TA Instruments, New Castle, DE, USA).
3. Results and Discussion
3.1. Characterizations of Fresh Catalysts
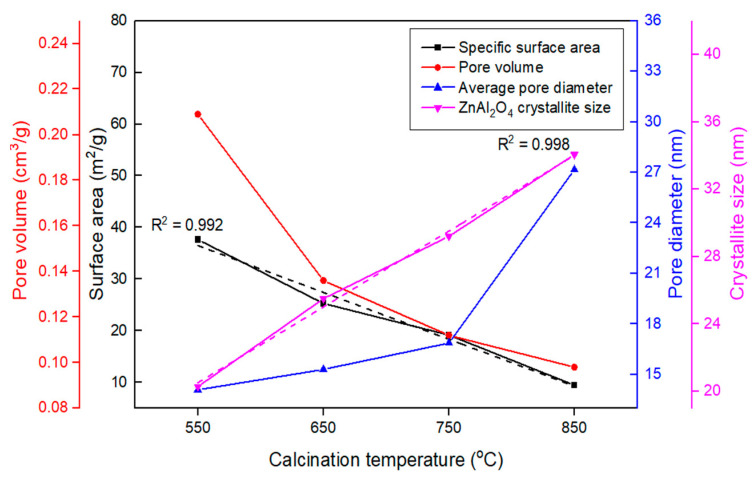
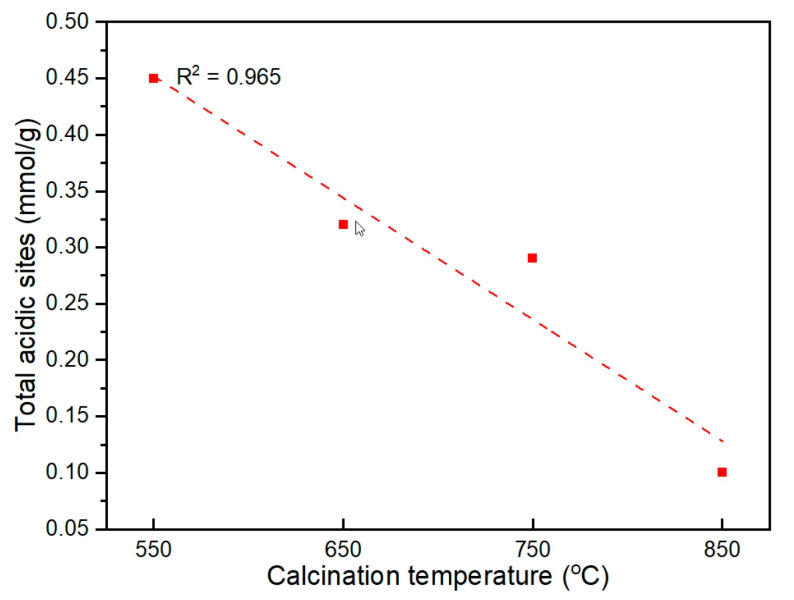
The textural properties of the fresh catalysts, which were calculated from N2 adsorption–desorption measurements, are summarized in Table 1, and the N2 adsorption–desorption isotherms and pore size distributions are shown in Figure S2. As indicated in Figure S2a, all ZnAl2O4 catalysts exhibited type IV isotherm and type H3 hysteresis loops (based on the IUPAC classification [20]), which is typical for mesoporous materials, indicating slit-shaped pores. As the calcination temperature increased, the hysteresis loop became narrower, with a lower BET surface area and a lower average pore volume in the order of ZnAl2O4-850 < ZnAl2O4-750 < ZnAl2O4-650 < ZnAl2O4-550. In contrast, the average pore diameter exhibited an opposite trend. Increasing the calcination temperature led to the collapse of small pores to generate bigger ones. The relationships between textural properties and calcination temperatures are depicted in Figure 1.
Table 1.
Textural properties, ZnAl2O4 crystallite sizes, and the degree of orderliness of the ZnAl2O4 spinel of fresh catalysts.
| Catalyst | Textural Properties | Chemical Composition |
Order Parameter c |
ZnAl2O4
Crystallite Size (nm) d |
||
|---|---|---|---|---|---|---|
| SBET (m2/g) a | Vpore (cm3/g) a | Dpore (nm) a | Zn/Al Molar Ratio b |
|||
| ZnAl2O4-550 | 37.65 | 0.21 | 14.09 | 2.07 | 0.4784 | 20.29 |
| ZnAl2O4-650 | 25.29 | 0.14 | 15.31 | 2.10 | 0.4922 | 25.50 |
| ZnAl2O4-750 | 19.17 | 0.11 | 16.87 | 2.11 | 0.5074 | 29.21 |
| ZnAl2O4-850 | 9.45 | 0.10 | 27.16 | 2.06 | 0.5153 | 34.06 |
a Measured via N2 isothermal adsorption–desorption at 77 K; surface area was calculated with BET method; pore volume and pore size were obtained with the BJH method. b Determined from ICP-OES. c Calculated via the fraction intensity of ZnAl2O4 (331) and [ZnAl2O4 (331) + ZnAl2O4 (400)] from XRD patterns. d Calculated via Debye–Scherrer equation applied to XRD ZnAl2O4 (311) peak.
Figure 1.
Relationship between textural properties and calcination temperatures.
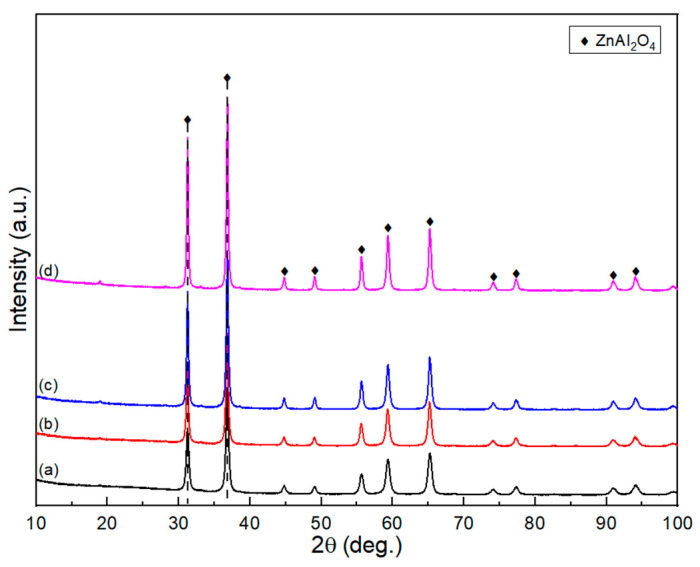
Figure 2 depicts the XRD patterns of the fresh ZnAl2O4-X catalysts. Characteristic peaks appearing at 2θ = 31.2°, 36.8°, 44.8°, 49.1°, 55.7°, 59.3°, 65.2°, 74.1°, and 77.3° can be attributed to the crystalline phase of ZnAl2O4. All XRD patterns show ZnAl2O4 peaks, which indicates the formation of ZnAl2O4 crystallite after high-temperature calcination by using the polymeric citrate complex method. The final decomposition temperature of the precursor in the derivative TGA (DTGA) result is below 550° (Figure S3), confirming that the ZnAl2O4 crystallite structure is completely formed at a calcination temperature above 550 °C. With increasing calcination temperature, the characteristic diffraction peaks become sharper, narrower, and more intensive, indicating an increase in the crystallinity and particle size of ZnAl2O4.
Figure 2.
XRD patterns of fresh catalysts: (a) ZnAl2O4-550, (b) ZnAl2O4-650, (c) ZnAl2O4-750, and (d) ZnAl2O4-850.
In addition, the average crystallite size (D) of single-phase spinel samples can be estimated by using the Debye–Scherrer equation [21,22]:
where D represents the average crystallite size, λ denotes the wavelength of the X-ray source (Cu Kα, 1.54 Å), β denotes the integral breadth of the (311) diffraction peak, and θ denotes the Bragg’s diffraction angle. The calculated results are presented in Table 1. In normally ordered spinel structures, the intensity of all odd reflections (e.g., 331) shows higher intensity than those of all even reflections (e.g., 400). The XRD peak intensities of (400) and (331) for various calcination temperatures are shown in Figure S4. It was observed that the peak intensities of (400) and (331) changed significantly with increasing calcination temperature. The degree of the order parameter in spinel samples can be calculated by using the following equation [23]:
where Io and Ie denote intensities corresponding to odd and even reflections, respectively. The disordered spinel phase can be formed because some fraction of Al3+ cations occupies the tetrahedral site, and Zn2+ cations occupy the octahedral site [24,25]. The degree of the order parameter in the spinel samples is shown in Table 1. The order parameters of ZnAl2O4-550 and ZnAl2O4-650 are 0.4784 and 0.4922, respectively. These values prove that the ZnAl2O4 spinel structure obtained at the calcination temperatures of 550 °C and 650 °C is a disordered structure, where Al3+ exists in tetrahedral positions and Zn2+ in octahedral positions. When the calcination temperature is raised above 750 °C, the degree of the order parameters is above 0.5, indicating that the ZnAl2O4 spinel is a normally ordered structure.
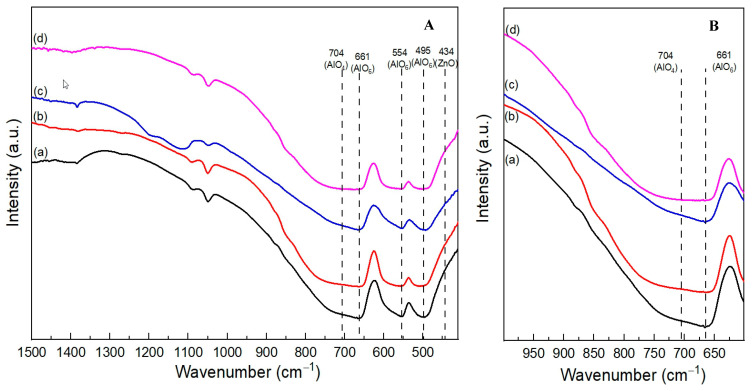
The FT-IR spectra of the fresh catalysts are shown in Figure 3A. The two vibration bands at 661 and 554 cm−1 can be attributed to the stretching mode of the octahedrally coordinated Al-O (AlO6), with another assignment of the peak at 495 cm−1 to the bending mode of AlO6. A broad shoulder band from 704 to 900 cm−1 can be assigned to tetrahedrally coordinated Al-O (AlO4) [16,26,27,28]. The enlarged spectra (from 600 to 1000 cm−1) focusing on the vibration bands of AlO4 and AlO6 are depicted in Figure 3B. The relative intensities of AlO4 to AlO6 decrease as the calcination temperature increases. The AlO4 vibration bands for the ZnAl2O4-750 and ZnAl2O4-850 catalysts are weak. In contrast, the shoulder of the AlO4 vibration for ZnAl2O4-550 and ZnAl2O4-650 is strong and observable. The presence of Al3+ in the tetrahedral position reflects a partial inversion of the ZnAl2O4 spinel structure. The FT-IR results are consistent with the XRD results and the inversion parameter obtained from the XPS measurement, which is shown later.
Figure 3.
(A)FT-IR spectra of fresh catalysts: (a) ZnAl2O4-550, (b) ZnAl2O4-650, (c) ZnAl2O4-750, and (d) ZnAl2O4-850. (B) Enlarged FT-IR spectra.
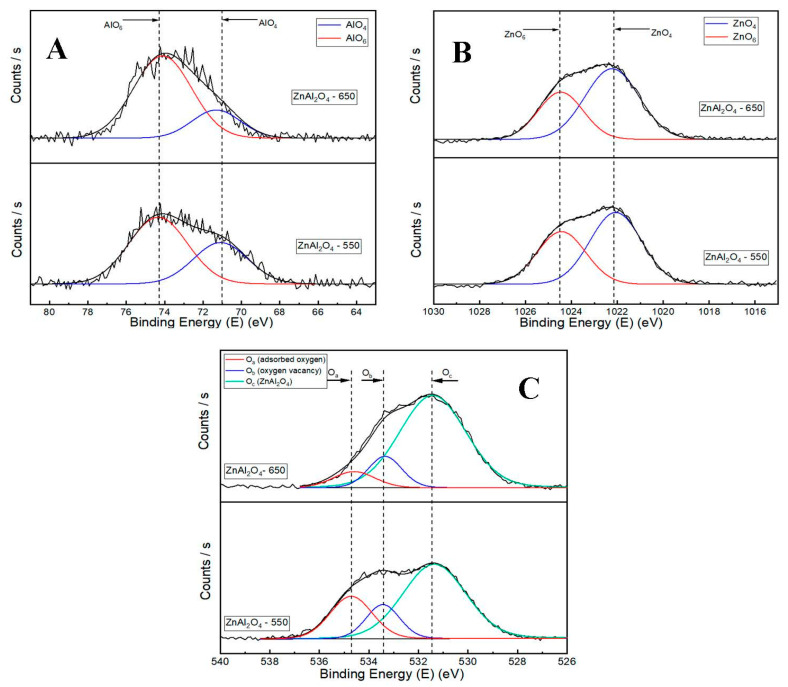
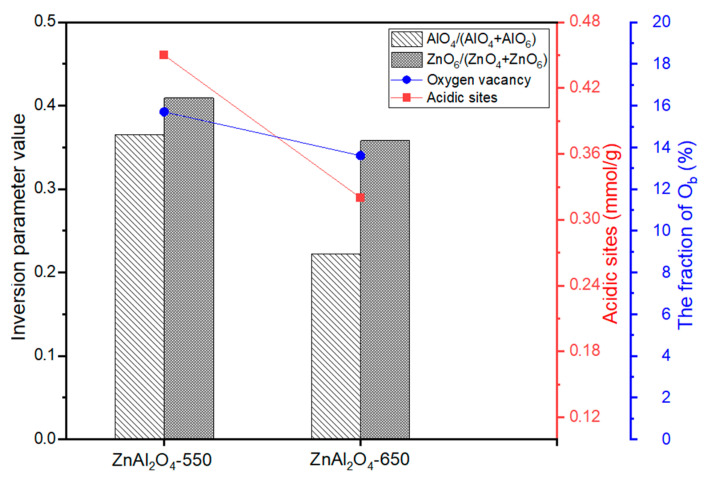
The deconvoluted XPS results of the fresh ZnAl2O4-550 and ZnAl2O4-650 catalysts are depicted in Figure 4. The Al2p XPS spectrum is illustrated in Figure 4A. The peak at a binding energy of 71.0 eV can be assigned to Al3+ occupying the tetrahedral sites (AlO4) [29], and a peak of approximately 74.2 eV can be assigned to Al3+ occupying the octahedral sites (AlO6) [29,30,31]. In Zn2p3/2 spectra, as shown in Figure 4B, the larger peak at a lower binding energy (approximately 1022–1022.2 eV) is attributed to Zn2+ occupying the tetrahedra sites (ZnO4), and a smaller peak at a higher binding energy (approximately 1024.5 eV) is attributed to Zn2+ occupying the octahedra sites (ZnO6) [29,30,32]. The normally ordered ZnAl2O4 spinel structure contains Al3+ cations at the octahedral sites and Zn2+ cations at the tetrahedral sites. When some Al3+ cations substitute Zn2+ cations in the tetrahedral positions and some Zn2+ cations substitute Al3+ cations in the octahedral positions, this results in the formation of a partially inversed spinel structure. The inversion parameter of a spinel structure can be defined by surface XPS measurements [30]. Table 2 shows the inversion parameter of the fresh ZnAl2O4-550 and ZnAl2O4-650 catalysts by calculating the ratio of AlO4/(AlO4 + AlO6) and ZnO6/(ZnO4 + ZnO6), and the inversion parameter values follow the order of ZnAl2O4-550 > ZnAl2O4-650. These results help us demonstrate that the degree of disorder of the spinel ZnAl2O4 structure decreases with increasing calcination temperature. The deconvoluted patterns of O1s can be fitted to three peaks. The peak Oa at the highest binding energy (approximately 543.5–534.8 eV) can be assigned to the oxygen weakly bonded with the surface of the catalysts (such as adsorbed H2O and O2 from the atmosphere) [16,33,34]. The peak at approximately 533.4–533.6 eV (Ob) can be assigned to the oxygen-deficient regions or oxygen vacancy (Ov) [33,34]. Finally, the peak at the lowest binding energy (approximately 531.4–531.7 eV) can be assigned to the lattice O in the ZnAl2O4 phase [16]. In a partially inversed spinel structure or a disordered structure, the substitution of a Zn2+ cation with an Al3+ cation to generate a Zn2+ octahedral site and Al3+ tetrahedral site is the main reason for the oxygen vacancy formation on the catalyst surface. An oxygen vacancy was formed to balance the positive charge of the cation caused by the substitution of Zn2+ for Al3+ at an octahedral site. The intensities of oxygen vacancy follow the order of ZnAl2O4-550 > ZnAl2O4-650, which are listed in Table 2.
Figure 4.
Deconvoluted XPS results of fresh catalysts: (A) Al2p XPS results, (B) Zn2p3/2 XPS results, and (C) O1s XPS results.
Table 2.
The change in the number of acidic sites and oxygen vacancy is based on the ratio of AlO4/(AlO4 + AlO6) and ZnO6/(ZnO4 + ZnO6).
| Catalyst | Intensity Ratio of AlO4/(AlO4 + AlO6) a | Intensity Ratio of ZnO6/(ZnO4 + ZnO6) b | Acidic Sites (mmol/g) |
The Fraction of Ob (%) c |
|---|---|---|---|---|
| ZnAl2O4-550 | 0.365 | 0.409 | 0.45 | 15.7 |
| ZnAl2O4-650 | 0.222 | 0.358 | 0.32 | 13.6 |
| ZnAl2O4-750 | - | - | 0.29 | - |
| ZnAl2O4-850 | - | - | 0.1 | - |
a Intensity ratios of the AlO4 (tetrahedral site) and AlO6 (octahedral site) were obtained from the deconvolution of XPS results by using the Origin software (OriginPro 2021 9.8.0.200). b Intensity ratios of the ZnO6 (octahedral site) and ZnO4 (tetrahedral site) were obtained from the deconvolution of XPS results by using the Origin software. c The fraction of Ob was calculated via the percentage of intensity of Ob in total intensity of deconvolution O1s XPS results.
The NH3-TPD measurements of the fresh catalysts are shown in Figure S5, and the acidic sites are summarized in Table 2. The number of acidic sites decreases in the order of ZnAl2O4-550 > ZnAl2O4-650 > ZnAl2O4-750 > ZnAl2O4-850. The relationship between the calcination temperature and the total number of acidic sites is shown in Figure 5. In this study, the presence of AlO4 and ZnO6 in the ZnAl2O4 spinel structure indicates the partially disordered structure of the catalyst. Furthermore, the partially disordered structure ZnAl2O4 spinel is capable of producing surface acidity on catalysts, which is related to the XPS intensity of Oa (in Figure 4) [16]. The high-intensity peak of Oa in the ZnAl2O4-550 catalyst and its dependency on the calcination temperature follow the same trend as the number of acidic sites.
Figure 5.
Relationship between total number of acidic sites and calcination temperatures.
The relationship between the number of acidic sites of the fresh catalysts and the inversion parameter (intensity ratio of AlO4/(AlO4 + AlO6)), the XPS intensity of oxygen vacancies (Ov), and the inversion parameter (intensity ratio of ZnO6/(ZnO4 + ZnO6)) are shown in Figure 6. In the normally ordered ZnAl2O4 spinel structure, Al3+ cations occupy the octahedral sites (AlO6) with low acidity. Meanwhile, Al3+ cations occupying tetrahedral sites (AlO4) in the partially inversed ZnAl2O4 spinel structure can result in higher surface acidity [16,27,35,36,37,38]. The higher Lewis acidity of AlO4 than AlO6 can be explained by the lower energy acceptor orbital of the AlO4 sites. When AlO4 contacts the surface, the removal of one of the four oxygen atoms results in the formation of three-coordinated Al with higher Lewis acidity strength [39]. Figure 6 depicts the positive relationship between the inversion parameter (AlO4/(AlO4 + AlO6)) and total surface acidity. With an increase in the calcination temperature, the disordered ZnAl2O4 structure decreases, indicating that there are fewer sites in which Al3+ cations occupy the tetrahedral position (AlO4), which decreases the total surface acidity. Another factor increasing surface acidity is the Lewis acidity of surface oxygen vacancies. Substitution of Zn2+ cations for Al3+ cations at the octahedral site and the formation of oxygen vacancies (Ov) to balance the positive charge of the cations can increase surface acidity. Mefford et al. [40] reported that the formation of surface hydroxyl groups was related to the Lewis acidity of surface oxygen vacancies, and evidence was provided from XPS Oa peaks of H2O or O2 adsorbed from the atmosphere. As the number of ZnO6 sites decreases with increasing calcination temperature, fewer oxygen vacancies are formed, lowering the surface acidity of the ZnAl2O4-650 catalyst compared with the ZnAl2O4-550 catalyst. From the above findings, we conclude that at the calcination temperature of 550 °C, a partially disordered ZnAl2O4 spinel structure is formed, in which some Al3+ cations occupy tetrahedral positions, and Zn2+ occupy octahedral positions, resulting in the formation of oxygen vacancies, which increases the surface acidity of the catalyst. With increasing calcination temperature, the structure of ZnAl2O4 gradually returns to normal, and the number of AlO4 and ZnO6 sites and oxygen vacancies decreases, lowering the surface acidity of the catalyst.
Figure 6.
Relationship between the number of acidic sites vs. inversion parameter (AlO4/(AlO4 + AlO6) and O1s XPS intensity (oxygen vacancy) vs. inversion parameter (ZnO6/(ZnO4 + ZnO6).
3.2. Catalytic Activity
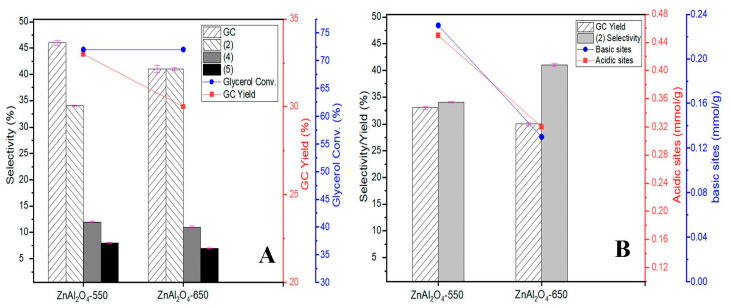
The reaction results (yield, conversion, and selectivity) calculated from gas chromatograms (a typical gas chromatogram is shown in Figure S6, see Supplementary Materials) for the glycerolysis of urea over the ZnAl2O4-550 and ZnAl2O4-650 catalysts are summarized in Table 3 and Figure 7A. During the reaction, besides the formation of the main product GC, there are also byproducts such as chemicals (2), (3), and (5). After the 5 h experiment, no other by-products are collected, indicating that the carbon balance between reagents and products is close to 100% (Table S1, see Supplementary Materials). The relationship between the number of acidic sites vs. GC yield and the number of basic sites (Figure S7, see Supplementary Materials) vs. byproduct (2) selectivity for each catalyst is depicted in Figure 7B. At a reaction time of 5 h, the ZnAl2O4-550 catalyst exhibited higher GC yield and lower (2) selectivity (GC yield = 46%, (2) selectivity = 34%) than the ZnAl2O4-650 catalyst with GC yield = 41% and (2) selectivity = 41%. Previous studies have reported the existence of the intermediate isocyanate (NCO) complex of Zn, which is an active site for the reaction [18,41]. Moreover, it has been proven that the Lewis acidic site acts as an active site for the ammonia (NH3) group of Zn NCO complex adsorption [16]. The ZnAl2O4-550 catalyst has a higher GC yield and more surface acidic sites than the ZnAl2O4-650 catalyst.
Table 3.
Analysis of liquid products obtained from the glycerolysis of urea over various mixed oxide catalysts prepared at different calcined temperatures. (Reaction temperature = 140 °C, reaction time = 5 h, reaction pressure = 3 kPa, glycerol/urea ratio = 1:1). (2): 2,3-dihydroxypropyl carbamate, (4): 4 (hydroxymethyl)oxazolidin-2-one, and (5): (2-oxo-1,3-dioxolan-4-yl) methylcarbamate.
| Catalyst | Glycerol Conversion (%) | GC Yield (%) | Selectivity (%) | Acidic Sites (mmol/g) | Basic Sites (mmol/g) | |||
|---|---|---|---|---|---|---|---|---|
| GC | (2) | (4) | (5) | |||||
| ZnAl2O4-550 | 72.2 ± 0.7 | 33 ± 0.3 | 45.8 ± 0.5 | 34.3 ± 0.1 | 12.4 ± 0.2 | 7.5 ± 0.1 | 0.45 | 0.23 |
| ZnAl2O4-650 | 72.2 ± 0.8 | 30 ± 0.3 | 40.9 ± 0.7 | 40.9 ± 0.3 | 10.9 ± 0.2 | 7.3 ± 0.1 | 0.32 | 0.13 |
Figure 7.
Catalytic performance of ZnAl2O4-550 and ZnAl2O4-650 catalysts in the glycerolysis of urea (A), and the relationship between the number of acidic sites vs. GC yield and the number of basic sites vs. (2) selectivity (B).
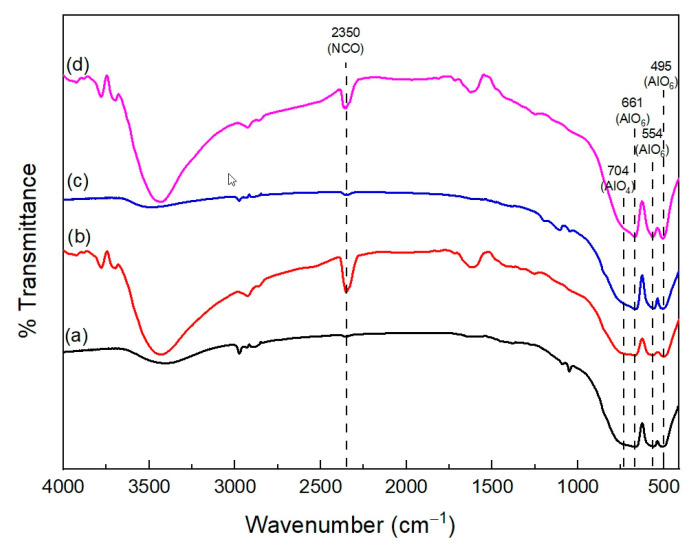
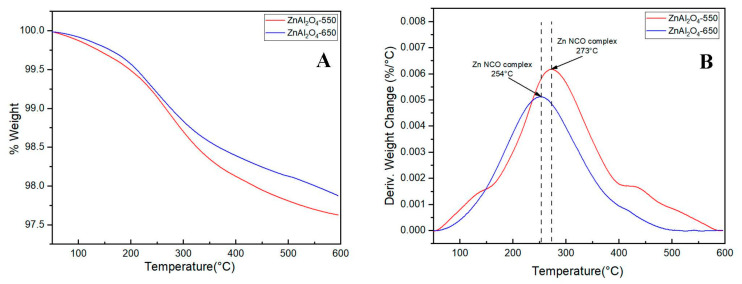
The formation of the Zn NCO complex on the solid phase can be confirmed by the FT-IR and TGA of the spent catalysts. The FT-IR spectra of the spent catalysts are shown in Figure 8. Besides the bands for AlO4 and AlO6 being detected at 704 and 661 cm−1, respectively, the vibration band of NCO in the Zn NCO complex on the solid phase is detected at 2350 cm−1 [42]. There is no information about the NCO band in the fresh catalysts. However, after a 5 h reaction, this band can be detected by FT-IR. The intensity of the NCO peak for ZnAl2O4-550 is higher than that for ZnAl2O4-650 because of the higher number of acidic sites in this catalyst. The same trend is presented by TGA and DTGA profiles in Figure 9. The DTGA profiles of the spent catalysts (Figure 9B) show the decomposition of the Zn NCO complex at 273 °C for ZnAl2O4-550 and 254 °C for ZnAl2O4-650 [16]. The higher decomposition temperature for the ZnAl2O4-550 catalyst can be attributed to a stronger crystalline Zn NCO complex with higher thermal stability. The DTGA results are consistent with the FT-IR results. The higher intensity of the Zn NCO decomposition peak in ZnAl2O4-550 demonstrated that the ability to adsorb the ammonia group in the Zn NCO of AlO4 (Lewis acidic site) is more significant in the ZnAl2O4-550 catalyst than in the ZnAl2O4-650 catalyst.
Figure 8.
FT-IR results of fresh and spent catalysts: (a) ZnAl2O4-550 fresh, (b) ZnAl2O4-550 spent 5 h, (c) ZnAl2O4-650 fresh, and (d) ZnAl2O4-650 spent 5 h.
Figure 9.
(A) TGA and (B) DTGA of spent catalysts after 5 h reaction.
Another notable finding is the relationship between surface basicity and byproduct (2) selectivity. The basic site values are shown in Table 3 and Figure 7B, showing that the relationship between the number of basic sites and (2) selectivity is inversely proportional. That is, if the number of basic sites decreases, more byproducts (2) are formed, and the GC yield reduces. Fernandes et al. [43] explained that surface basicity acted as an active site for the (2) byproduct decomposition process. This study shows the same trend: when using ZnAl2O4-550 with more basic sites for the reaction, (2) selectivity (34%) is lower, whereas it is higher with ZnAl2O4-650 (41%).
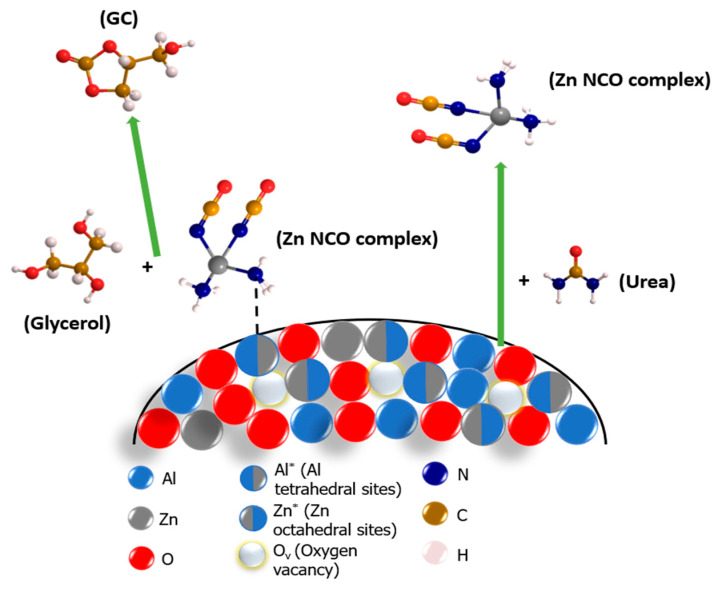
Catalytic reaction routes over a disordered ZnAl2O4 spinel structure are depicted in Scheme 1. As mentioned above, there are several disordered sites over a partially inverted ZnAl2O4 spinel: AlO4 (symbolized as Al*), ZnO6 (symbolized as Zn*) and oxygen vacancies (symbolized as Ov). ZnAl2O4 catalysis follows the heterogeneous reaction pathways. The catalysts form the Zn NCO complexes via the reaction with urea. The AlO4 site works as a Lewis acidic site where the ammonia (NH3) group of the Zn NCO complex can adsorb. The presence of the Zn NCO complex adsorbed on the solid phase is confirmed by the FT-IR and TGA measurements.
Scheme 1.
An illustration of the reaction pathways involved in the urea-glycerol carbonylation on the surface of the disordered ZnAl2O4 spinel structure.
In Table 4, the catalytic reaction results in this work are compared to those recently published in the literature. Because the ZnAl2O4 catalyst follows only the heterogeneous catalysis pathway, the glycerol conversion and GC yield values are relatively low. However, it can still be compared with some previous studies. Zhang et al. prepared the zinc glycerolate (ZMG) and ZnO from zinc glycerolate (ZnO from ZMG) via the calcination method. Glycerol conversion and GC yield of the ZMG are about 65 and 55%, respectively. The authors have demonstrated through reaction tests that catalysts with acidic and basic properties favor the synthesis of glycerol carbonate.
Table 4.
Summary of catalytic reaction performance of glycerol carbonylation with urea over several catalysts.
| Catalyst | Mol Ratio Glycerol/Urea | Weight % Catalyst/Glycerol |
Glycerol Conversion (%) | GC Yield (%) | Reference |
|---|---|---|---|---|---|
| ZnO pure | 1:1.5 | 5 | ~60 | ~35 | [44] |
| ZMG | 1:1.5 | 5 | ~65 | ~55 | [44] |
| Co3O4/ZnO | 1:1.5 | 5 | 69 | 97 | [45] |
| La2Cu0.5Fe0.5O4 | 1:1 | 5 | 49 | 82 | [46] |
| La(OH)3 | 1:1.5 | 5 | 42.3 | 55.6 | [47] |
| MoO3/SnO2 | 1:3 | 10 | 69 | 97 | [48] |
| MgO | 1:1.5 | - | 71 | 100 | [43] |
| ZnO | 1:1.5 | 5 | 67 | 49 | [49] |
| [HOEMIm][PF6] | 1:1.5 | - | 62 | 47 | [50] |
| ZnAl2O4-550 | 1:1.5 | 5 | 72.2 | 33 | This work |
| ZnAl2O4-650 | 1:1.5 | 5 | 72.2 | 30 | This work |
4. Conclusions
In this study, the disordered ZnAl2O4 catalysts were successfully prepared by using the citrate complex method at different temperatures ranging from 550 °C to 850 °C. The ZnAl2O4-550 catalyst exhibited a more disordered ZnAl2O4 structure than the other catalysts calcined at a higher calcination temperature, resulting in more disordered sites of AlO4 and more oxygen vacancies in ZnAl2O4-550. The ZnAl2O4-650 catalyst having Al3+ cations at the octahedral sites (AlO6) exhibited low acidity, whereas the AlO4 sites in ZnAl2O4-550 resulted in high surface acidity. From the XPS intensities of Oa (H2O or O2 adsorbed) and Ob (oxygen vacancies), ZnAl2O4-550 contained more surface oxygen vacancies than ZnAl2O4-650, which contributed to the increased surface acidity of ZnAl2O4-550. The high surface acidity of ZnAl2O4-550 resulted in strong interactions with the Zn NCO complex on its surface, improving catalytic performance. The intermediate product of urea glycerolysis was 2,3-dihydroxypropyl carbamate (2), which had higher selectivity for ZnAl2O4-650 than ZnAl2O4-550, lowering the GC yield.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano13131901/s1, Figure S1. A schematic diagram of the catalyst activity test device with the gas chromatographic analysis conditions; Figure S2: N2 adsorption–desorption isotherms (A) and pore size distribution curves (B): (a) ZnAl2O4-550, (b) ZnAl2O4-650, (c) ZnAl2O4-750, and (d) ZnAl2O4-850; Figure S3: DTGA curves of ZnAl2O4 xerogel precursor prepared by using polymeric citrate complex method; Figure S4: Enlarged XRD patterns of fresh catalysts: (a) ZnAl2O4-550, (b) ZnAl2O4-650, (c) Znl2O4-750, and (d) ZnAl2O4-850; Figure S5: NH3 profiles of fresh catalysts: (a) ZnAl2O4-550, (b) ZnAl2O4-650, (c) ZnAl2O4-750, and (d) ZnAl2O4-850; Figure S6: Typical gas chromatograms of ZnAl2O4-550 (A) and ZnAl2O4-650 (B). (2): 2,3-dihydroxypropyl carbamate, (4): 4-(hydroxymethyl) oxazolidine-2-one, and (5): (2-oxo-1,3-dioxolan-4-yl) methyl carbamate; Figure S7: CO2 profiles of fresh catalysts: (a) ZnAl2O4-550, (b) ZnAl2O4; Table S1: The initial amount of each reactant; the amount of glycerol and products after reaction and carbon balance.
Author Contributions
H.N.-P.: methodology, validation, investigation; N.P.-N.: data curation, writing—original draft preparation; E.W.S.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Core Research Institute Basic Science Research Program (2021R1A6A1A03038858) and “Regional Innovation Strategy program” (No. 2021RIS-003) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2021R1A2B5B01001448).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Abbaszaadeh A., Ghobadian B., Omidkhah M.R., Najafi G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012;63:138–148. doi: 10.1016/j.enconman.2012.02.027. [DOI] [Google Scholar]
- 2.Ambat I., Srivastava V., Sillanpää M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018;90:356–369. doi: 10.1016/j.rser.2018.03.069. [DOI] [Google Scholar]
- 3.Fan X., Burton R., Zhou Y. Glycerol (byproduct of biodiesel production) as a source for fuels and chemicals mini review. Open Fuels Ener. Sci. J. 2010;3:17–22. doi: 10.2174/1876973X01003010017. [DOI] [Google Scholar]
- 4.Ochoa-Gómez J.R., Gómez-Jiménez-Aberasturi O., Ramirez-Lopez C., Belsué M. A brief review on industrial alternatives for the manufacturing of glycerol carbonate, a green chemical. Org. Process Res. Dev. 2012;16:389–399. doi: 10.1021/op200369v. [DOI] [Google Scholar]
- 5.Christy S., Noschese A., Lomeli-Rodriguez M., Greeves N., Lopez-Sanchez J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018;14:99–107. doi: 10.1016/j.cogsc.2018.09.003. [DOI] [Google Scholar]
- 6.Teng W.K., Ngoh G.C., Yusoff R., Aroua M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014;88:484–497. doi: 10.1016/j.enconman.2014.08.036. [DOI] [Google Scholar]
- 7.Sonnati M.O., Amigoni S., de Givenchy E.P.T., Darmanin T., Choulet O., Guittard F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013;15:283–306. doi: 10.1039/C2GC36525A. [DOI] [Google Scholar]
- 8.Ochoa-Gómez J.R., Gómez-Jiménez-Aberasturi O., Maestro-Madurga B., Pesquera-Rodríguez A., Ramírez-López C., Lorenzo-Ibarreta L., Torrecilla-Soria J., Villarán-Velasco M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009;366:315–324. doi: 10.1016/j.apcata.2009.07.020. [DOI] [Google Scholar]
- 9.Song X., Pan D., Wu Y., Cheng P., Wei R., Gao L., Zhang J., Xiao G. Synthesis of glycerol carbonate over porous La-Zr based catalysts: The role of strong and super basic sites. J. Alloys Compd. 2018;750:828–837. doi: 10.1016/j.jallcom.2018.03.392. [DOI] [Google Scholar]
- 10.Devarajan A., Thiripuranthagan S., Radhakrishnan R., Kumaravel S. Solvent free transesterification of glycerol into glycerol carbonate over nanostructured CaAl hydrotalcite catalyst. J. Nanosci. Nanotechnol. 2018;18:4588–4599. doi: 10.1166/jnn.2018.15265. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Liu J., He D. Catalytic synthesis of glycerol carbonate from biomass-based glycerol and dimethyl carbonate over Li-La2O3 catalysts. Appl. Catal. A Gen. 2018;564:234–242. doi: 10.1016/j.apcata.2018.07.032. [DOI] [Google Scholar]
- 12.Li Q., Zhang W., Zhao N., Wei W., Sun Y. Synthesis of cyclic carbonates from urea and diols over metal oxides. Catal. Today. 2006;115:111–116. doi: 10.1016/j.cattod.2006.02.033. [DOI] [Google Scholar]
- 13.Park C.-y., Nguyen-Phu H., Shin E.W. Glycerol carbonation with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: Preferred reaction route depending on crystalline structure. Mol. Catal. 2017;435:99–109. doi: 10.1016/j.mcat.2017.03.025. [DOI] [Google Scholar]
- 14.Song X., Wu Y., Pan D., Zhang J., Xu S., Gao L., Wei R., Xiao G. Functionalized DVB-based polymer catalysts for glycerol and CO2 catalytic conversion. J. CO2 Util. 2018;28:326–334. doi: 10.1016/j.jcou.2018.10.015. [DOI] [Google Scholar]
- 15.Nguyen-Phu H., Park C.-y., Eun W.S. Activated red mud-supported Zn/Al oxide catalysts for catalytic conversion of glycerol to glycerol carbonate: FTIR analysis. Catal. Commun. 2016;85:52–56. doi: 10.1016/j.catcom.2016.07.012. [DOI] [Google Scholar]
- 16.Nguyen-Phu H., Shin E.W. Disordered structure of ZnAl2O4 phase and the formation of a Zn NCO complex in ZnAl mixed oxide catalysts for glycerol carbonylation with urea. J. Catal. 2019;373:147–160. doi: 10.1016/j.jcat.2019.03.043. [DOI] [Google Scholar]
- 17.Nguyen-Phu H., Park C.-y., Shin E.W. Dual catalysis over ZnAl mixed oxides in the glycerolysis of urea: Homogeneous and heterogeneous reaction routes. Appl. Catal. A Gen. 2018;552:1–10. doi: 10.1016/j.apcata.2017.12.018. [DOI] [Google Scholar]
- 18.Fujita S.-i., Yamanishi Y., Arai M. Synthesis of glycerol carbonate from glycerol and urea using zinc-containing solid catalysts: A homogeneous reaction. J. Catal. 2013;297:137–141. doi: 10.1016/j.jcat.2012.10.001. [DOI] [Google Scholar]
- 19.Chen L., Sun X., Liu Y., Zhou K., Li Y. Porous ZnAl2O4 synthesized by a modified citrate technique. J. Alloys Compd. 2004;376:257–261. [Google Scholar]
- 20.Sotomayor F.J., Cychosz K.A., Thommes M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018;3:34–50. [Google Scholar]
- 21.Tangcharoen T., T-Thienprasert J., Kongmark C. Effect of calcination temperature on structural and optical properties of MAl2O4 (M = Ni, Cu, Zn) aluminate spinel nanoparticles. J. Adv. Ceram. 2019;8:352–366. doi: 10.1007/s40145-019-0317-5. [DOI] [Google Scholar]
- 22.Holzwarth U., Gibson N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011;6:534. doi: 10.1038/nnano.2011.145. [DOI] [PubMed] [Google Scholar]
- 23.Dwibedi D., Murugesan C., Leskes M., Barpanda P. Role of annealing temperature on cation ordering in hydrothermally prepared zinc aluminate (ZnAl2O4) spinel. Mater. Res. Bull. 2018;98:219–224. doi: 10.1016/j.materresbull.2017.10.010. [DOI] [Google Scholar]
- 24.Simeone D., Dodane-Thiriet C., Gosset D., Daniel P., Beauvy M. Order–disorder phase transition induced by swift ions in MgAl2O4 and ZnAl2O4 spinels. J. Nucl. Mater. 2002;300:151–160. doi: 10.1016/S0022-3115(01)00749-8. [DOI] [Google Scholar]
- 25.Barpanda P., Behera S., Gupta P., Pratihar S., Bhattacharya S. Chemically induced order disorder transition in magnesium aluminium spinel. J. Eur. Ceram. Soc. 2006;26:2603–2609. doi: 10.1016/j.jeurceramsoc.2005.04.032. [DOI] [Google Scholar]
- 26.Da Silva A.A., Gonçalves A.d.S., Davolos M.R. Characterization of nanosized ZnAl2O4 spinel synthesized by the sol–gel method. J. Sol-Gel Sci. Technol. 2009;49:101–105. doi: 10.1007/s10971-008-1833-x. [DOI] [Google Scholar]
- 27.Busca G., Lorenzelli V., Escribano V.S., Guidetti R. FT-113 study of the surface properties of the spinels NiAl2O4 and CoAl2O4 in relation to those of transitional aluminas. J. Catal. 1991;131:167–177. doi: 10.1016/0021-9517(91)90333-Y. [DOI] [Google Scholar]
- 28.Morterra C., Magnacca G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today. 1996;27:497–532. doi: 10.1016/0920-5861(95)00163-8. [DOI] [Google Scholar]
- 29.Menon S.G., Choudhari K.S., Shivashankar S.A., Chidangil S., Kulkarni S.D. Microwave solution route to ceramic ZnAl2O4 nanoparticles in 10 minutes: Inversion and photophysical changes with thermal history. New J. Chem. 2017;41:5420–5428. doi: 10.1039/C7NJ01006K. [DOI] [Google Scholar]
- 30.Duan X., Yuan D., Yu F. Cation distribution in Co-doped ZnAl2O4 nanoparticles studied by X-ray photoelectron spectroscopy and 27Al solid-state NMR spectroscopy. Inorg. Chem. 2011;50:5460–5467. doi: 10.1021/ic200433r. [DOI] [PubMed] [Google Scholar]
- 31.Tshabalala K., Cho S.-H., Park J.-K., Pitale S.S., Nagpure I., Kroon R., Swart H., Ntwaeaborwa O. Luminescent properties and X-ray photoelectron spectroscopy study of ZnAl2O4: Ce3+, Tb3+ phosphor. J. Alloys Compd. 2011;509:10115–10120. doi: 10.1016/j.jallcom.2011.08.054. [DOI] [Google Scholar]
- 32.Druska P., Steinike U., Šepelák V. Surface structure of mechanically activated and of mechanosynthesized zinc ferrite. J. Solid State Chem. 1999;146:13–21. doi: 10.1006/jssc.1998.8284. [DOI] [Google Scholar]
- 33.Jayathilake D., Peiris T.N., Sagu J.S., Potter D.B., Wijayantha K., Carmalt C., Southee D. Microwave-assisted synthesis and processing of Al-doped, Ga-doped, and Al, Ga codoped ZnO for the pursuit of optimal conductivity for transparent conducting film fabrication. ACS Sustain. Chem. Eng. 2017;5:4820–4829. doi: 10.1021/acssuschemeng.7b00263. [DOI] [Google Scholar]
- 34.Chen M., Wang X., Yu Y., Pei Z., Bai X., Sun C., Huang R., Wen L. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000;158:134–140. doi: 10.1016/S0169-4332(99)00601-7. [DOI] [Google Scholar]
- 35.Murthy I., Swamy C. Catalytic decomposition of 2-propanol on Co1+xAl2−xO4 spinel system. Catal. Lett. 1994;27:103–112. doi: 10.1007/BF00806983. [DOI] [Google Scholar]
- 36.Busca G., Lorenzelli V., Ramis G., Willey R.J. Surface sites on spinel-type and corundum-type metal oxide powders. Langmuir. 1993;9:1492–1499. doi: 10.1021/la00030a012. [DOI] [Google Scholar]
- 37.Lundie D.T., McInroy A.R., Marshall R., Winfield J.M., Jones P., Dudman C.C., Parker S.F., Mitchell C., Lennon D. Improved description of the surface acidity of η-alumina. J. Phys. Chem. B. 2005;109:11592–11601. doi: 10.1021/jp0405963. [DOI] [PubMed] [Google Scholar]
- 38.Trombetta M., Busca G., Lenarda M., Storaro L., Ganzerla R., Piovesan L., Lopez A.J., Alcantara-Rodrìguez M., Rodríguez-Castellón E. Solid acid catalysts from clays: Evaluation of surface acidity of mono-and bi-pillared smectites by FT-IR spectroscopy measurements, NH3-TPD and catalytic tests. Appl. Catal. A Gen. 2000;193:55–69. [Google Scholar]
- 39.Sohlberg K., Pantelides S.T., Pennycook S.J. Surface Reconstruction and the Difference in Surface Acidity between γ- and η-Alumina. J. Am. Chem. Soc. 2001;123:26–29. doi: 10.1021/ja002095a. [DOI] [PubMed] [Google Scholar]
- 40.Mefford J.T., Hardin W.G., Dai S., Johnston K.P., Stevenson K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014;13:726–732. doi: 10.1038/nmat4000. [DOI] [PubMed] [Google Scholar]
- 41.Turney T.W., Patti A., Gates W., Shaheen U., Kulasegaram S. Formation of glycerol carbonate from glycerol and urea catalysed by metal monoglycerolates. Green Chem. 2013;15:1925–1931. doi: 10.1039/c3gc37028c. [DOI] [Google Scholar]
- 42.Lu F., Song B., He P., Wang Z., Wang J. Electrochemical impedance spectroscopy (EIS) study on the degradation of acrylic polyurethane coatings. RSC Adv. 2017;7:13742–13748. doi: 10.1039/C6RA26341K. [DOI] [Google Scholar]
- 43.Fernandes G.P., Yadav G.D. Selective glycerolysis of urea to glycerol carbonate using combustion synthesized magnesium oxide as catalyst. Catal. Today. 2018;309:153–160. doi: 10.1016/j.cattod.2017.08.021. [DOI] [Google Scholar]
- 44.Zhang P., Liu L., Fan M., Dong Y., Jiang P. The value-added utilization of glycerol for the synthesis of glycerol carbonate catalyzed with a novel porous ZnO catalyst. RSC Adv. 2016;6:76223–76230. doi: 10.1039/C6RA14288E. [DOI] [Google Scholar]
- 45.Rubio-Marcos F., Calvino-Casilda V., Bañares M.A., Fernandez J.F. Control of the interphases formation degree in Co3O4/ZnO catalysts. ChemCatChem. 2013;5:1431–1440. doi: 10.1002/cctc.201200620. [DOI] [Google Scholar]
- 46.Zhang J., He D. Lanthanum-based mixed oxides for the synthesis of glycerol carbonate from glycerol and urea. React. Kinet. Mech. Catal. 2014;113:375–392. doi: 10.1007/s11144-014-0739-6. [DOI] [Google Scholar]
- 47.Procopio D., Di Gioia M.L. An overview of the latest advances in the catalytic synthesis of glycerol carbonate. Catalysts. 2022;12:50. doi: 10.3390/catal12010050. [DOI] [Google Scholar]
- 48.Mallesham B., Rangaswamy A., Rao B.G., Rao T.V., Reddy B.M. Solvent-free production of glycerol carbonate from bioglycerol with urea over nanostructured promoted SnO2 catalysts. Catal. Lett. 2020;150:3626–3641. doi: 10.1007/s10562-020-03241-9. [DOI] [Google Scholar]
- 49.Nguyen-Phu H., Shin E.W. Investigating time-dependent Zn species over Zn-based catalysts in glycerol carbonylation with urea and their roles in the reaction mechanism. Appl. Catal. A Gen. 2018;561:28–40. doi: 10.1016/j.apcata.2018.05.016. [DOI] [Google Scholar]
- 50.Chen J., Wang C., Dong B., Leng W., Huang J., Ge R., Gao Y. Ionic liquids as eco-friendly catalysts for converting glycerol and urea into high value-added glycerol carbonate. Chin. J. Catal. 2015;36:336–343. doi: 10.1016/S1872-2067(14)60257-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.