Abstract
Chemerin is a novel adipokine that plays a major role in adipogenesis and lipid metabolism. It also induces inflammation and affects insulin signaling, steroidogenesis and thermogenesis. Consequently, it likely contributes to a variety of metabolic and cardiovascular diseases, including atherosclerosis, diabetes, hypertension and pre-eclampsia. This review describes its origin and receptors, as well as its role in various diseases, and subsequently summarizes how nutrition affects its levels. It concludes that vitamin A, fat, glucose and alcohol generally upregulate chemerin, while omega-3, salt and vitamin D suppress it. Dietary measures rather than drugs acting as chemerin receptor antagonists might become a novel tool to suppress chemerin effects, thereby potentially improving the aforementioned diseases. However, more detailed studies are required to fully understand chemerin regulation.
Keywords: chemerin, nutrients, cardiovascular disease, metabolic disease
1. Introduction
Over the last three decades, due to the obesity epidemic, attention has shifted to achieving an improved energy balance. The underlying concept is that a healthy lifestyle and well-controlled nutrition will avoid obesity, and consequently prevent the development of metabolic syndrome and any resulting cardiovascular disease [1].
Chemerin is a multifunctional protein that has recently been identified as an essential player in hypertension, myocardial infarction, preterm birth, diabetes, metabolic disease and liver cirrhosis [2,3]. In the two decades since its initial discovery, more than a thousand articles have been published on chemerin [4], but none reviewed its relationship with nutrition.
This review aims to comprehensively cover the physiology and pathological roles of chemerin from a nutritional point of view, an approach based on the literature search shown in Supplemental Figure S1. The underlying assumption is that by lowering chemerin levels through dietary interventions, novel therapeutic strategies may be identified for the prevention and treatment of various cardiovascular diseases associated with obesity and metabolic syndrome.
2. Chemerin and Its Receptor
2.1. Origin of Chemerin
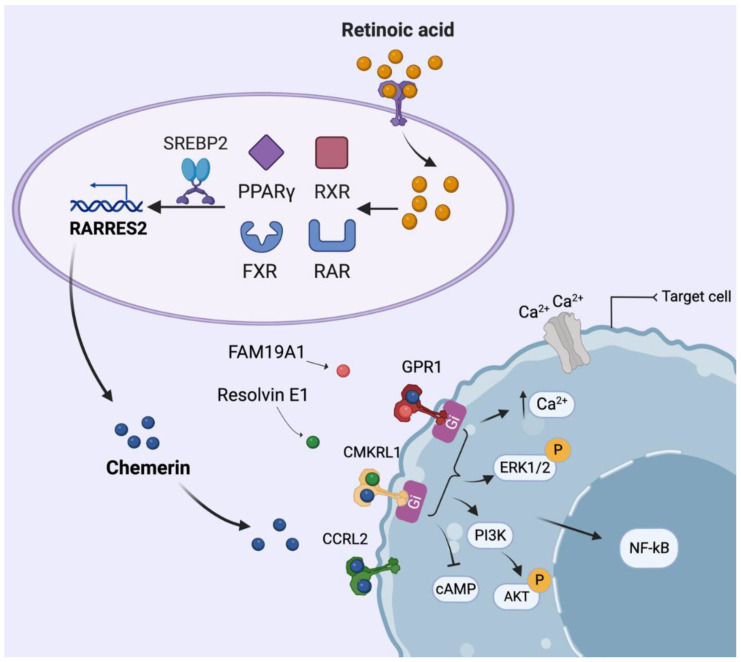
Chemerin was first identified in 1997 [5]. It was found in psoriatic lesions, and its expression increased after topical exposure to the retinoid tazarotene, hence its first name Tazarotene-induced Gene 2 (TIG2) [5]. Given this observation, the initial focus was on retinoic acid receptors (RARs) and retinoid X receptors (RXRs), with only the former resulting in TIG2 upregulation [5]. The gene then became known as retinoic acid receptor responder 2 (RARRES2) [6]. RARRES2 was believed to be a soluble ligand for a surface receptor involved in antiproliferative effects [7]. In 2003, the protein sequence of RARRES2 was unraveled, and it received the name chemerin, while simultaneously the G protein-coupled orphan receptor ChemR23 was confirmed to be its receptor [8]. Interestingly, two nuclear receptors heterodimerizing with RXR [9,10] and one nuclear regulatory factor [11] were also found to affect chemerin production (Figure 1). Indeed, the farnesoid X receptor (FXR) agonist GW4064 increased chemerin in HepG2 cells and primary hepatocytes, with this effect disappearing after FXR knockout [12]. Moreover, the RARRES2 promoter includes both a peroxisome proliferator-activated receptor γ (PPARγ)-binding sequence and a sterol regulatory element-binding protein 2 (SREBP2) binding site [13,14].
Figure 1.
Induction of chemerin synthesis with retinoic acid, the activation of its receptors, and the resulting second messenger cascade. Not only chemerin, but also FAM19A1 and resolvin E1 target these receptors. See text for further details. RARRES2, retinoic acid receptor responder 2; FXR, farnesoid X receptor; RAR, retinoic acid receptor; RXR, retinoid X receptor; PPARγ, peroxisome proliferator-activated receptor γ; SREBP2, sterol regulatory element-binding protein 2; CMKLR1, Chemerin-like receptor 1; CCRL2, CC-motif chemokine receptor-like 2; GPR1, chemerin type 2 receptor; ERK1/2, extracellular signal-regulated kinase 1/2; NFκB, nuclear factor-κB; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B.
Abundant chemerin levels occur in the liver, adipose tissue, and placenta [15,16]. Yet, its mechanism of secretion is poorly understood, and changes in its gene expression do not necessarily parallel changes in its secretion [17,18]. This implies that chemerin secretion is subject to additional regulation [17,18]. Its synthesis starts with preprochemerin [8]. This precursor has a conserved consensus amino-terminal signal sequence and is thought to be sorted via conventional cellular secretory pathways [19]. Preprochemerin is secreted as chemerin163S or prochemerin, following cleavage of its 20 amino acid signal peptides. Prochemerin can be detected in circulation blood [20,21]. Proteolytic removal of the C-terminal helical segment by plasmin or angiotensin-converting enzyme type 2 results in the generation of both chemerin157S and chemerin156F from prochemerin [19,22,23]. Hepatic as well as whole-body knockdown of chemerin yielded an almost complete disappearance of circulating chemerin. This suggests that the liver is the predominant source of chemerin in blood [24]. Nevertheless, chemerin produced locally (e.g., in adipocytes and placenta) plays an important role in lipid metabolism and vascular function [25,26,27]. Chemerin was initially reported to induce chemoattraction and inflammation [8] in a calcium-dependent manner [8,28,29]. Yet, following its identification in adipocytes, it became gradually known as a novel adipokine affecting adipogenesis and lipid metabolism. This resulted in its association with obesity, diabetes, and metabolic syndrome [30,31,32]. Simultaneously, it was observed to affect vascular contraction, paving the way for its association with hypertension [25,26]. Adipokines facilitate the interaction between adipose tissue and other tissues [33]. The most extensively investigated adipokines are adiponectin and leptin. In general, during the transition from lean to obese, leptin levels increase, while adiponectin levels decrease [33], thereby decreasing the adiponectin/leptin ratio. Hence, increasing this ratio now emerges as a therapeutic goal. To what degree the adiponectin/chemerin ratio might be used to a similar extent is currently being debated [34].
2.2. Chemerin Receptors
Chemerin-like receptor 1 (CMKLR1), also known as chemokine receptor-like 1, ChemR23, or chemerin1 [35], was first reported in 1996. This receptor is predominately expressed in dendritic cells, monocytes, macrophages, endothelial cells, the placenta, lungs, muscle, heart, adipose tissues, skin and spleen [2,35,36]. CMKLR1 is the most widely investigated chemerin receptor. Chemerin binding to CMKLR1 results in Gi activation, which decreases cyclic adenosine monophosphate (cAMP), thereby resulting in the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and nuclear factor kappa B (NFκB) activation [37,38] (Figure 1). Interestingly, the dietary supplement resolvin E1, a bioactive oxygenated product of eicosapentaenoic acid (EPA), exerted potent anti-inflammatory effects in a CMKLR1-dependent manner [39]. This suggests that resolving E1 competes with chemerin for CMKLR1 binding, thus preventing its inflammatory effects.
G protein-coupled receptor 1 (GPR1), also known as chemerin receptor 2 (chemerin2), was cloned in 1994 and identified as a chemerin receptor in 2008 [40,41]. It sequences homology with CMKLR1 is >40% [42]. Until today, as compared with CMKLR1, knowledge on GPR1 is limited. GPR1 occurs in the placenta, ovaries, testicles, skin, adipose tissue, skeletal muscle and brain [43,44]. GPR1 binds chemerin with high affinity, but this results in relatively weak biological signaling in a Gi-dependent manner [40,45]. GPR1 may have more agonists than chemerin, for e.g., FAM19A1, a member of the family with sequence similarity 19 that was recently reported as a novel ligand for GPR1 in the brain [40,46].
CC motif chemokine receptor-like 2 (CCRL2) is believed to function as a chaperone protein, concentrating chemerin locally and thereby allowing optimal chemerin–CMKLR1 interaction [22,47]. It neither internalizes chemerin nor transduces signals [2,20]. CCRL2 is expressed in various tissues, including adipose tissue, breasts, the placenta, lungs, macrophages, dendritic cells, neutrophils and microglia [20].
3. Nutrients and Chemerin
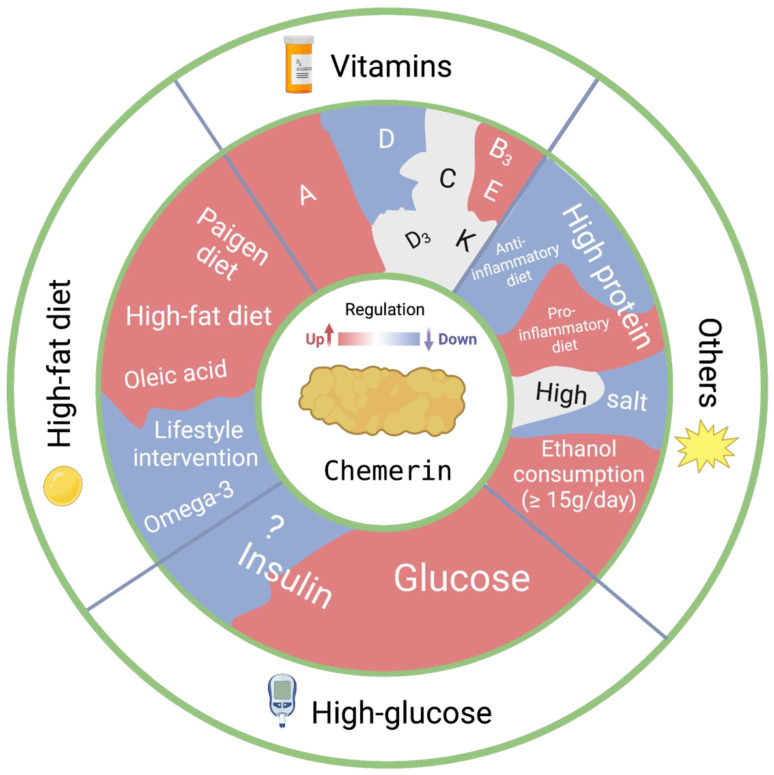
Nutrients and diet greatly affect chemerin production. Figure 2 summarizes the current knowledge.
Figure 2.
Effect of nutrient or diet on chemerin synthesis.
3.1. Vitamins
Vitamin A is derived from carotenoids and retinyl esters. This vitamin is essential, among others, for maintaining embryogenesis, vision, immune regulation and the metabolism of glucose and lipids [48]. Retinoids and retinoic acids are the primary metabolites of vitamin A, and some of their actions have been reported to involve chemerin [49]. This is not surprising given the fact that retinoic acid acts via the RAR, which directly induces the transcription of RARRES2, i.e., chemerin [50]. Indeed, incubation of intestinal cells, bone marrow stromal cells, endothelial cells and brown adipose tissue with retinoic acid upregulated chemerin [51,52,53]. Moreover, both beta-carotene and all-trans retinoic acid supplementation increased CMKLR1 expression in vivo as well as in vitro [54,55]. Endothelial CCRL2 expression also displayed retinoid acid-sensitive regulation in vitro [56]. No such findings have been reported for GPR1.
Vitamin D supplementation led to improvement in rats with either pre-eclampsia or gestational diabetes mellitus, potentially because it lowered the elevated levels of chemerin in these models (see Section 4.3) [57,58]. While the protective effect of vitamin D on pre-eclampsia and gestational diabetes in humans is well established, to what degree this depends on chemerin lowering has not been investigated [59]. Additionally, both vitamin D-deficient obese children and type 2 diabetes mellitus patients display elevated chemerin levels [60,61], and circulating vitamin D levels negatively correlate with chemerin levels in breast cancer patients [62]. Yet, 1,25 dihydroxyvitamin D3, the active form of vitamin D, did not alter chemerin expression in renal tubular epithelial cells or endothelial cells [56,63]. One possibility is that the effects of vitamin D on chemerin are mediated via lipid lowering in vivo [64].
Vitamin C did not affect chemerin in adipocytes [65], and vitamin K absence in hepatocellular carcinoma patients did not alter chemerin [66]. Additionally, vitamin B3 increased chemerin mRNA levels in differentiated bovine preadipocytes [67], while vitamin E supplementation upregulated hepatic CMKLR1 mRNA expression [68].
3.2. High-Fat Diet and Glucose
A high-fat diet, resulting in obesity and nonalcoholic fatty liver (NAFLD) in rats and mice, generally upregulates chemerin in blood, adipose tissue and liver [69,70,71]. Similarly, higher chemerin levels are observed at these same sites in obese and NAFLD patients in comparison with healthy humans [72,73,74]. Interestingly, an intensive lifestyle intervention consisting of dietary changes and resistance exercise programs over the course of several months lowers chemerin in obese subjects [75,76]. The fat-induced chemerin upregulation likely involves PPARγ, since the PPARγ agonist pioglitazone suppressed chemerin while the antagonist GW9662 did the opposite [77]. Remarkably, both chemerin knockout in vivo and chemerin knockdown in adipocytes decreased PPARγ expression, suggesting that chemerin–PPARγ interaction may occur in two directions [13,14]. Furthermore, in differentiated 3T3-L1 cells, SREBP2 knockdown prevented the oleic acid-induced rise in chemerin [13], confirming that this transcription factor contributes to chemerin synthesis.
In mice, a high-fat diet upregulated CMKLR1 and CCRL2 in white adipose tissue and liver [78,79,80], while in rats chemerin knockout suppressed adipogenesis [81,82]. A high-fat diet also upregulated chemerin in pregnant mice, but decreased GPR1 [83]. Interestingly, GPR1 knockout mice exposed to a high-fat diet developed glucose intolerance with no change in body weight [84], while a lower body mass, body fat percentage and food intake was observed in CMKLR1 KO mice [85]. In apparent contrast with this latter finding, CMKLR1 and CCRL2 knockout mice exposed to a high-fat diet developed enhanced obesity [84,86], leading the authors to suggest that the net effect of the chemerin/CMKLR1 pathway might depend on the experimental setting.
A large cohort study has revealed a linear association between elevated levels of chemerin and the consumption of sugar-sweetened beverages [87]. Indeed, a high glucose challenge increased chemerin, both in 3T3-L1 cells and in mice in vivo, and this involved insulin [88]. Here, it is important to note that chemerin enhanced the insulin-stimulated glucose uptake in 3T3-L1 cells [89]. A similar chemerin upregulation, combined with increased CMKLR1 expression, was observed in human retinal pigment epithelium cells exposed to high glucose [90]. Yet, chemerin-mediated antagonism of insulin-induced signaling has also been observed, both in the vascular wall [91] and in human granulosa-lutein cells [92], although in the latter cells insulin still upregulated chemerin. Thus, while glucose upregulates chemerin in an insulin-dependent manner, chemerin may subsequently fine-tune the effects of insulin. Among others, this may involve the upregulation of pro-inflammatory cytokines via CMKLR1 [93], which will impair insulin signaling and promote insulin resistance [93]. In support of this concept, patients with proliferative diabetic retinopathy displayed higher serum chemerin and pro-inflammatory cytokine levels than patients with non-proliferative diabetic retinopathy [90].
Finally, omega-3 polyunsaturated fatty acids inhibit the secretion of chemerin from adipocytes [65,94]. This inhibition, which involved G-protein-coupled receptor 120, might contribute to the anti-inflammatory effects of omega-3 polyunsaturated fatty acids [95].
3.3. Protein, Salt and Alcohol
A healthy diet with a high protein and low carbohydrate content lowers chemerin, while the opposite occurs with a more pro-inflammatory (i.e., a low consumption of polyunsaturated and monounsaturated fats as well as fiber and high consumption of saturated fats) diet [96,97]. This was also true in patients with morbid obesity [98]. In contrast, a high intake of red meat, which associates with elevated levels of inflammatory markers, and a low intake of dairy, link to elevated chemerin levels [87].
Exposing Dahl salt-sensitive rats to a high-salt diet reduced circulating chemerin and increased its urinary secretion [99]. At the tissue level, high salt intake diminished chemerin particularly in adipocytes [100].
Chronic alcohol consumption upregulated chemerin in a dose-dependent manner, both in healthy humans (serum) [101,102] and in rats (serum and fat tissue) [101]. Chemerin mRNA levels were elevated in fat tissue in mice fed ethanol [103]. In patients with chronic pancreatitis, serum chemerin concentrations were higher in heavy drinkers compared with non-alcoholic patients [104]. The potential connection between alcohol, salt and chemerin levels may involve aldosterone. Notably, alcohol has been shown to increase aldosterone levels [105], whereas salt has been observed to decrease it [106]. Additionally, aldosterone has been found to elevate chemerin levels [107].
4. Potential Role of Chemerin in Metabolic and Cardiovascular Disease
4.1. Lipid Metabolism
Chemerin not only stimulates adipogenesis but also facilitates lipid accumulation in a wide variety of cells [29,108,109,110,111,112]. In agreement with this concept, its levels and receptors are upregulated in differentiating preadipocytes. Moreover, obesity, NAFLD and nonalcoholic steatohepatitis (NASH) are all accompanied by elevated chemerin levels, while attenuating these conditions lowers chemerin [72,77,113,114]. Table 1 summarizes the genes that are currently believed to be involved in the effects of chemerin on lipid metabolism. Here, it should be noted that a methionine–choline-deficient (MCD) diet (a classical dietary model of NASH) has also been reported to decrease CMKLR1 [114,115] and chemerin in the liver [12]. These opposing effects on chemerin might relate to sex, as increased chemerin levels were observed in male animals exposed to a MCD diet [116], while MCD-fed females displayed chemerin lowering [12]. Moreover, in hepatocytes or matured adipocyte cells, the fatty acids EPA, docosahexaenoic acid, palmitate acid and oleic acid all induced lipid accumulation, while only the latter increased chemerin expression, with the former three decreasing this expression [12,94,114]. In an oral lipid tolerance test, chemerin decreased when switching from fasting to lipid uptake, reaching its lowest level after 4 h [117].
Table 1.
Genes and proteins that are involved in the effect of chemerin on lipid metabolism.
| Related Genes or Proteins | Disease or Model | Sample Type | Species | Reference |
|---|---|---|---|---|
| CMKLR1; IL6 | NAFLD | Liver | Human | [72] |
| hsCRP | Obesity | Serum | Human | [73] |
| CMKLR1; PPARγ | T2D | Liver, gastrocnemius | Rat | [77] |
| ERK5; p-ERK5 | Obesity | Osteoclast | Mouse | [78] |
| PI3K; AKT; p-AKT | Obesity | Kupffer cells | Mouse | [79] |
| insulin; CCRL2; AKT; p-AKT; ERK; p-ERK | Obesity | Visceral adipose tissue | Mouse | [80] |
| CMKLR1; ERK1; ERK2; PPARγ; adiponectin; perilipin; FASN; HSL; GLUT4; IR; TNFα; IL6; leptin; UCP1 | Obesity; adipogenesis | Adipocytes (3T3-L1; brown adipose tissue) | Mouse | [81] |
| PPARγ; adiponectin; FAS; perilipin; leptin | Adipogenesis | Adipose tissue | Mouse | [82] |
| GPR1; GLUT3; AKT; p-AKT; PPARγ; FABP4 | GDM; obesity | Placenta | Human; Mouse | [83] |
| Insulin; AKT; p-AKT | Insulin challenge | Adipocytes (3T3-L1; primary human adipocytes) | Human; Mouse | [86] |
| Insulin; AKT; p-AKT | T2D; obesity | Human vascular smooth muscle cells, mouse aortas | Human; Mouse | [89] |
| CMKLR1; insulin; IRS1; p-IRS1 | T2D | Liver, adipose tissue | Mouse | [90] |
| HSL; LPL; leptin; PPARγ; CEBPα; FABP4 | Adipogenesis | Bovine intramuscular adipocytes | Bovine | [108] |
| Cyclophilin D; UCP1; UCP2; PRDM16; PEPCK; DGAT-2; DIO-2 | Obesity | Brown adipose tissue | Mouse | [109] |
| CMKLR1; TNFα; IL-1β; NFkB; PI3K; AKT; p-AKT | Pre-eclampsia | Placenta | Mouse | [110] |
| GPR1; SREBP1c; FASN; ACC1; DGAT-2; SCD-1; TNFα; IL6; SOCS3 | NAFLD | Human hepatoma cell line HepG2 | Human | [112] |
Abbreviations. CMKLR1, chemerin-like receptor 1; IL6, interleukin 6; hsCRP, high-sensitivity C-reactive protein; PPARγ, peroxisome proliferator-activated receptor γ; ERK, extracellular signal-regulated kinase; p-ERK, phosphate extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; p-AKT, phosphate protein kinase B; CCRL2, CC motif chemokine receptor-like 2; FASN, fatty acid synthase; HSL, hormone-sensitive lipase; GLUT4, glucose transporter type 4; GLUT3, glucose transporter type 3; IR, insulin receptor; TNFα, tumor necrosis factor alpha; UCP, uncoupling protein; GPR1, chemerin type 2 receptor; FABP4, fatty acid-binding protein 4; IRS1, insulin receptor substrate-1; p-IRS1, phosphate insulin receptor substrate-1; LPL, lipoprotein lipase; CEBPα, enhancer-binding protein alpha; PRDM16, positive regulatory domain zinc finger region protein 16; PEPCK, phosphoenolpyruvate carboxykinases; DGAT-2, diacylglycerol O-acyltransferase 2; DIO-2, type II iodothyronine deiodinase; NFκB, nuclear factor-κB; SREBP1c, sterol regulatory element-binding protein 1; ACC1, acetyl-CoA carboxylase 1; SCD-1, stearoyl-CoA-desaturase 1; SOCS3, suppressor of Cytokine Signaling-3; T2D, type 2 diabetes; GDM, gestational diabetes mellitus.
An obesogenic diet increases chemerin secretion from brown adipocytes, while cold stimulation caused the opposite [118,119]. Chemerin might contribute to temperature regulation, given that its overexpression decreased whole body and brown adipose tissue temperature in mice [120]. Chemerin overexpression additionally impaired metabolic homeostasis and induced glucose intolerance. These effects involved CMKLR1 and uncoupling protein 1. In addition, the chemerin–CMKLR1 axis is a physiological negative regulator of thermogenic beige fat, and targeting this pathway might be a novel strategy for obesity [121].
Circulating chemerin correlates positively with low-density lipoprotein (LDL) and negatively with high-density lipoprotein (HDL) [122,123]. Yet, the latter negative association particularly concerns large HDL, since a positive association was observed with both small and intermediate HDL. This suggests that chemerin is involved in the HDL maturing process [123,124]. LDL apheresis lowered circulating chemerin, implying that chemerin is bound, at least partly, to lipoproteins [125]. Future studies should investigate this possibility.
4.2. Cardiovascular Effects
Chemerin levels are elevated in multiple cardiovascular diseases (Table 2) [126,127,128,129]. Chemerin is not only an independent risk factor for arterial stiffness [130], but in chronic kidney disease it also is a predictive marker of atherosclerosis [131,132]. This relates to the above-described effects of chemerin on the atherogenic process, involving vascular remodeling, lipid deposition and inflammation [93,133,134,135]. Indeed, the expression of chemerin and its receptor CMKLR1 in periaortic and pericoronary fat and foam cells determines atherosclerosis severity [136,137] and correlates with carotid plaque instability [138].
Recent data suggest that chemerin also exerts effects in cardiomyocytes, vascular smooth muscle cells, endothelial cells and fibroblasts, and might even originate from some of these cells. Tumor necrosis factor-α upregulated chemerin in murine cardiomyocytes, and in these cells chemerin induced apoptosis by activating caspase 9 and reducing protein kinase B (AKT) [139]. In rat cardiac fibroblasts, chemerin promoted cell migration by increasing reactive oxygen species (ROS), AKT and ERK1/2 [140]. Aldosterone induced chemerin synthesis in cardiac fibroblasts via Rho/ROCK/JNK signaling [141]. In endothelial cells, chemerin promoted angiogenesis and ROS production and decreased insulin signaling and nitric oxide production [2,91,142].
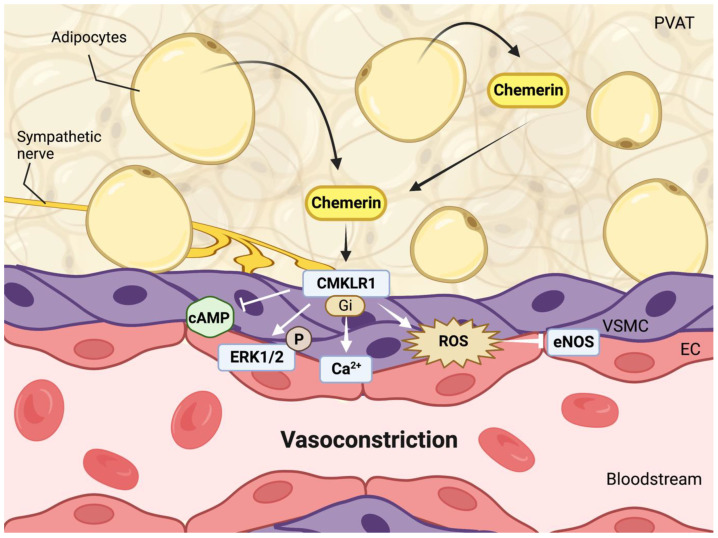
Vascular chemerin most likely originates from perivascular adipose tissue (PVAT), while CMKLR1 occurs in endothelial and vascular smooth muscle cells [26,143]. Exogenously added chemerin induced constriction via CMKLR1, Gi and calcium in isolated vessels (Figure 3), and this was enhanced after endothelial removal or during nitric oxide inhibition [26,28]. Without exogenous chemerin, endogenous chemerin derived from PVAT is also capable of inducing constriction, most likely by activating the sympathetic nervous system [143]. Remarkably, although both whole-body and hepatic chemerin knockdown abolished circulating chemerin [24], only whole-body knockdown also lowered blood pressure. This implies that chemerin from a non-hepatic source, most likely PVAT, contributes to blood pressure. To what degree the chemerin-induced upregulation of inflammatory cytokines in vascular smooth muscle cells [144] contributes to vessel contraction remains unknown.
Figure 3.
Chemerin-induced vasoconstriction involves both direct effects via its type 1 receptor (CMKLR1) on vascular smooth muscle cells (VSMCs), mediated via cyclic adenosine monophosphate (cAMP) reduction, upregulation of extracellular signal-regulated kinase 1/2 (ERK1/2) and reactive oxygen species (ROS), and indirect effects mediated via activation of the sympathetic nervous system. NO, generated by endothelial NO synthase (eNOS) in endothelial cells (EC), will counteract the effects of chemerin. Data are from references [24,26,28,35,143].
4.3. Pregnancy-Related Problems
Chemerin is also a major player during pregnancy. Circulating chemerin levels normally fall in the first and second trimesters of pregnancy, and then increase during the third trimester, reaching the highest levels at late gestation, to fall again to pre-pregnancy levels shortly after delivery [145,146,147]. The placenta is a major contributor to this rise in circulating chemerin [110]. Since cord blood chemerin levels exceed those in maternal blood [146], maternal and fetal chemerin levels may act independently. Yet, maternal obesity is associated with higher cord blood chemerin levels [148,149]. How chemerin upregulation during pregnancy is regulated and whether chemerin affects the fetus are unknown.
The high levels of chemerin in late pregnancy are suggestive of the possibility that they play a role in the preparation of delivery. This might require a delicate balance, given that overexpression of chemerin increases the risk of miscarriage [110]. Simultaneously, chemerin correlates positively with platelet count, which is relevant at the time of delivery to prevent hemorrhage [150,151,152]. Overall, excessively high maternal chemerin levels are indicative of a negative pregnancy outcome and a low birthweight, while cord blood chemerin levels associate positively with fetal birthweight [110,153,154]. In agreement with the former, intraperitoneal application of chemerin to pregnant mice with diabetes resulted in cognitive disorder in the offspring [155]. In the fetus, chemerin is expressed at the level of the intestine, where it peaks at 20–24 weeks of gestation to promote macrophage recruitment for gut development [52]. Thereafter intestinal chemerin expression returns to low levels.
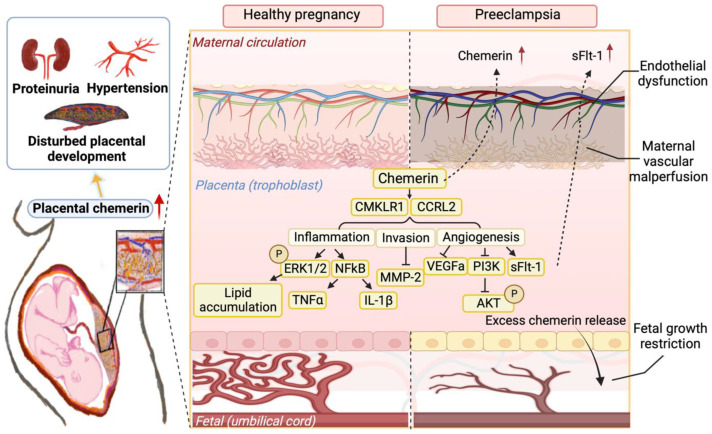
Serum chemerin is increased in pre-eclampsia, correlating with the severity of the disease and adverse neonatal outcomes [154,156]. In fact, its level in the first trimester may help to predict the occurrence of pre-eclampsia [157]. Importantly, the pre-eclamptic placenta releases more chemerin than a healthy placenta [110], supporting the concept that circulating chemerin in pregnancy is placenta-derived, and that the elevated chemerin levels in pre-eclamptic women originate in the placenta. Moreover, placental chemerin overexpression in mice induced a pre-eclampsia-like syndrome (Figure 4), characterized by high blood pressure, proteinuria, endothelial dysfunction and fetal growth restriction [110]. Placental chemerin overexpression simultaneously increased the circulating and placental levels of cholesterol, raising the possibility that chemerin might also contribute to dyslipidemia in pre-eclampsia [158]. A rat model of pre-eclampsia similarly displayed higher circulating chemerin levels [58]. In gestational diabetes mellitus (GDM), chemerin correlates with obesity and glucose homeostasis [50]. Yet, chemerin levels in the blood, adipose tissue and placenta are not necessarily elevated in GDM [159,160]—this may be limited to obese GDM women [161,162]. In such women, high cord blood chemerin levels were predictive for both maternal insulin resistance and large for gestational-age babies [148,149]. It is important to stress that adverse perinatal outcomes are linked to maternal cardiometabolic and neurocognitive outcomes [163,164]. This may represent the long-term consequences of inflammatory dysfunction, potentially involving chemerin.
Figure 4.
Placental trophoblast chemerin overexpression in mice induces a pre-eclampsia-like syndrome, involving hypertension and proteinuria, combined with diminished trophoblast invasion (by suppressing matrix metalloproteinase (MMP)-2), a disorganized labyrinth layer, and up-regulation of the anti-angiogenic factor soluble Fms-like tyrosine kinase-1 (sFlt-1) and the inflammation markers nuclear factor-κB (NFκB), tumor necrosis factor (TNF)-α and interleukin (IL)-1β, while downregulating vascular endothelial growth factor-a (VEGFa). The disturbed sFlt-1/VEGFa ratio involves the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway. Finally, extracellular signal-regulated kinase 1/2 (ERK1/2) upregulation might lead to lipid accumulation. Figure 4 summarizes the findings from references [110,158]. CMKLR1, chemerin-like receptor 1; CCRL2, CC motif chemokine receptor-like 2.
4.4. Sex Differences
Sex hormones likely contribute to the synthesis and effects of chemerin. In humans, serum chemerin increases with age, and chemerin levels are higher in females than in males [117,165]. However, in type 2 diabetes and obesity cohorts, serum chemerin in males was higher than in females [166,167]. In the deoxycorticosterone acetate–salt rat model, chemerin deletion decreased blood pressure in females while increasing blood pressure in males [168]. Furthermore, chemerin levels in white adipose tissue were downregulated in female rats and upregulated in male rats after gonadectomy [169]. The latter coincides with observations in differentiated 3T3-L1 adipocytes, where testosterone decreased chemerin release into the supernatant. Yet in these cells estradiol was without effect [117], and in lean women with polycystic ovarian syndrome (PCOS), chemerin levels were upregulated versus obese PCOS women [170]. Chemerin was observed to suppress follicular steroidogenesis and may thus contribute to PCOS [170,171]. Additionally, chemerin levels were low in subfertile males, most likely due to their elevated luteinizing hormone levels [172], and this was suggested to reflect a link between chemerin and reproductive function.
Table 2.
Circulating chemerin in various metabolic and cardiovascular diseases.
| Country | Population | Number of Included Patients (n) | Chemerin Levels (ng/mL) | BMI | Age | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Control | Diseased | Control | Diseased | Control | Diseased | ||||
| USA | Obesity | 10 | 37 | 76.2 | 147 | <25 | >25 | 54 | [21] |
| Hungary | Obesity | 50 | 50 | 405 | 590 | <25 | >25 | 43 | [122] |
| Mauritius | T2D | 142 | 114 | 249 | 250 | ≤25 | >25 | 49 | [31] |
| Saudi Arabia | T2D | 38 | 41 | 89 | 99 | >25 | >25 | 44 | [58] |
| Germany | T2D | 29 | 29 | 191 | 219 | >25 | >25 | 56 | [72] |
| USA | T2D | 969 | 173 | 180 | 191 | >25 | >25 | 45 | [162] |
| China | Atrial fibrillation | 146 | 256 | 107.74 | 133.24 | <25 | <25 | 60 | [126] |
| China | Coronary artery disease | 191 | 239 | 45.7 | 48.7 | ≤25 | ≤25 | 62 | [127] |
| China | Coronary artery disease | 56 | 132 | 90 | 111 | <25 | <25 | 62 | [128] |
| China | Coronary artery disease | 50 | 50 | 133 | 189 | ≤25 | ≤25 | 60 | [134] |
| Korea | Obesity and arterial stiffness | 35 | 33 | 106 | 120 | <25 | >25 | 52 | [129] |
| Canada | Stable and unstable carotid atherosclerotic plaque | 165 | 208 | >25 | 70 | [138] | |||
| Austria | Hypertension * | A total of 495 | 155 | 180 | >25 | 65 | [131] | ||
| T2D * | 170 | 192 | |||||||
| MetS * | 163 | 201 | |||||||
| Netherlands | Pre-eclampsia | 29 | 30 | 149 | 287 | ≤25 | ≤25 | 32 | [110] |
| Germany | Pre-eclampsia | 37 | 37 | 205 | 250 | <25 | <25 | 30 | [145] |
| Turkey | Pre-eclampsia | 46 | 88 | 200 | 358 | >25 | >25 | 27 | [154] |
| China | Pre-eclampsia | 477 | 41 | 181 | 312 | <25 | ≤25 | 26 | [157] |
| Germany | GDM | 80 | 40 | 218 | 230 | <25 | <25 | 30 | [160] |
Abbreviations. T2D, type 2 diabetes; MetS, metabolic syndrome; ACE, angiotensin-converting enzyme; AT1, angiotensin II type 1; GDM, gestational diabetes mellitus. * these three populations are from one cohort.
5. Conclusions
Chemerin is a novel player that might contribute to a wide variety of cardiovascular diseases, amongst others, by stimulating adipogenesis, inflammation and contraction, and by influencing thermogenesis, steroidogenesis and insulin signaling. Its concentrations vary widely, partly in a sex-dependent manner, and vitamin A, fat, glucose and alcohol generally upregulate it, while omega-3, salt and vitamin D suppress chemerin. Dietary measures rather than drugs acting as chemerin receptor antagonists might become novel tools to suppress chemerin effects, thereby potentially improving diseases such as atherosclerosis, diabetes, hypertension and pre-eclampsia. However, more detailed studies are required to fully understand chemerin regulation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15132878/s1, Figure S1: Flowchart of study selection.
Author Contributions
Conceptualization, L.T. and K.V.; methodology, L.T. and A.H.J.D.; software, L.T.; writing—original draft preparation, L.T., K.V., X.L. and A.H.J.D.; writing—review and editing, L.T., K.V., X.L. and A.H.J.D.; visualization, L.T.; supervision, K.V., X.L. and A.H.J.D.; funding acquisition, K.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. Figures with the credit “Created with BioRender.com”.
Funding Statement
Lunbo Tan and Koen Verdonk are supported by the Stichting Lijf en Leven.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Silveira Rossi J.L., Barbalho S.M., Reverete de Araujo R., Bechara M.D., Sloan K.P., Sloan L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022;38:e3502. doi: 10.1002/dmrr.3502. [DOI] [PubMed] [Google Scholar]
- 2.Ferland D.J., Mullick A.E., Watts S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020;33:975–986. doi: 10.1093/ajh/hpaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu M., Yang Y., Huang C., Ge L., Xue L., Xiao Z., Xiao T., Zhao H., Ren P., Zhang J.V. Chemerin: A Functional Adipokine in Reproductive Health and Diseases. Biomedicines. 2022;10:1910. doi: 10.3390/biomedicines10081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L., Leung L.L., Morser J. Chemerin Forms: Their Generation and Activity. Biomedicines. 2022;10:2018. doi: 10.3390/biomedicines10082018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagpal S., Patel S., Jacobe H., DiSepio D., Ghosn C., Malhotra M., Teng M., Duvic M., Chandraratna R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 1997;109:91–95. doi: 10.1111/1523-1747.ep12276660. [DOI] [PubMed] [Google Scholar]
- 6.Vinals C., Gaulis S., Coche T. Using in silico transcriptomics to search for tumor-associated antigens for immunotherapy. Vaccine. 2001;19:2607–2614. doi: 10.1016/S0264-410X(00)00500-4. [DOI] [PubMed] [Google Scholar]
- 7.Duvic M., Asano A.T., Hager C., Mays S. The pathogenesis of psoriasis and the mechanism of action of tazarotene. J. Am. Acad. Dermatol. 1998;39:S129–S133. doi: 10.1016/S0190-9622(98)70309-3. [DOI] [PubMed] [Google Scholar]
- 8.Wittamer V., Franssen J.D., Vulcano M., Mirjolet J.F., Le Poul E., Migeotte I., Brezillon S., Tyldesley R., Blanpain C., Detheux M., et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T.P., Rastinejad F. Structure of the intact PPAR-gamma-RXR—Nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claudel T., Staels B., Kuipers F. The Farnesoid X receptor: A molecular link between bile acid and lipid and glucose metabolism. Arter. Thromb. Vasc. Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 11.Tarling E.J., Ahn H., de Aguiar Vallim T.Q. The nuclear receptor FXR uncouples the actions of miR-33 from SREBP-2. Arter. Thromb. Vasc. Biol. 2015;35:787–795. doi: 10.1161/ATVBAHA.114.304179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng Y., Wang H., Lu Y., Liu S., Zhang Q., Huang J., Zhu R., Yang J., Zhang R., Zhang D., et al. Identification of chemerin as a novel FXR target gene down-regulated in the progression of nonalcoholic steatohepatitis. Endocrinology. 2013;154:1794–1801. doi: 10.1210/en.2012-2126. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S., Wanninger J., Schmidhofer S., Weigert J., Neumeier M., Dorn C., Hellerbrand C., Zimara N., Schaffler A., Aslanidis C., et al. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology. 2011;152:26–35. doi: 10.1210/en.2010-1157. [DOI] [PubMed] [Google Scholar]
- 14.Muruganandan S., Parlee S.D., Rourke J.L., Ernst M.C., Goralski K.B., Sinal C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J. Biol. Chem. 2011;286:23982–23995. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves K.B., Nguyen Dinh Cat A., Lopes R.A., Rios F.J., Anagnostopoulou A., Lobato N.S., de Oliveira A.M., Tostes R.C., Montezano A.C., Touyz R.M. Chemerin Regulates Crosstalk between Adipocytes and Vascular Cells through Nox. Hypertension. 2015;66:657–666. doi: 10.1161/HYPERTENSIONAHA.115.05616. [DOI] [PubMed] [Google Scholar]
- 16.Inci S., Aksan G., Dogan P. Chemerin as an independent predictor of cardiovascular event risk. Ther. Adv. Endocrinol. Metab. 2016;7:57–68. doi: 10.1177/2042018816629894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parlee S.D., Ernst M.C., Muruganandan S., Sinal C.J., Goralski K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-alpha. Endocrinology. 2010;151:2590–2602. doi: 10.1210/en.2009-0794. [DOI] [PubMed] [Google Scholar]
- 18.Sell H., Divoux A., Poitou C., Basdevant A., Bouillot J.L., Bedossa P., Tordjman J., Eckel J., Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 19.Parlee S.D., McNeil J.O., Muruganandan S., Sinal C.J., Goralski K.B. Elastase and tryptase govern TNFalpha-mediated production of active chemerin by adipocytes. PLoS ONE. 2012;7:e51072. doi: 10.1371/journal.pone.0051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer T.F., Beck-Sickinger A.G. Chemerin—Exploring a versatile adipokine. Biol. Chem. 2022;403:625–642. doi: 10.1515/hsz-2021-0409. [DOI] [PubMed] [Google Scholar]
- 21.Chang S.S., Eisenberg D., Zhao L., Adams C., Leib R., Morser J., Leung L. Chemerin activation in human obesity. Obesity. 2016;24:1522–1529. doi: 10.1002/oby.21534. [DOI] [PubMed] [Google Scholar]
- 22.Zabel B.A., Allen S.J., Kulig P., Allen J.A., Cichy J., Handel T.M., Butcher E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 23.Schultz S., Saalbach A., Heiker J.T., Meier R., Zellmann T., Simon J.C., Beck-Sickinger A.G. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangement. Biochem. J. 2013;452:271–280. doi: 10.1042/BJ20121880. [DOI] [PubMed] [Google Scholar]
- 24.Ferland D.J., Seitz B., Darios E.S., Thompson J.M., Yeh S.T., Mullick A.E., Watts S.W. Whole-Body But Not Hepatic Knockdown of Chemerin by Antisense Oligonucleotide Decreases Blood Pressure in Rats. J. Pharmacol. Exp. Ther. 2018;365:212–218. doi: 10.1124/jpet.117.245456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobato N.S., Neves K.B., Filgueira F.P., Fortes Z.B., Carvalho M.H., Webb R.C., Oliveira A.M., Tostes R.C. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 2012;91:600–606. doi: 10.1016/j.lfs.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Watts S.W., Dorrance A.M., Penfold M.E., Rourke J.L., Sinal C.J., Seitz B., Sullivan T.J., Charvat T.T., Thompson J.M., Burnett R., et al. Chemerin connects fat to arterial contraction. Arter. Thromb. Vasc. Biol. 2013;33:1320–1328. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanan Y., Yi J., Xiaojing L., Jing Q., Xiaohui W. Adipo-specific chemerin knockout alters the metabolomic profile of adipose tissue under normal and high-fat diet conditions: Application of an untargeted liquid chromatography-tandem mass spectrometry metabolomics method. Biomed. Chromatogr. 2021;35:e5220. doi: 10.1002/bmc.5220. [DOI] [PubMed] [Google Scholar]
- 28.Ferland D.J., Darios E.S., Neubig R.R., Sjogren B., Truong N., Torres R., Dexheimer T.S., Thompson J.M., Watts S.W. Chemerin-induced arterial contraction is G(i)- and calcium-dependent. Vascul. Pharmacol. 2017;88:30–41. doi: 10.1016/j.vph.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roh S.G., Song S.H., Choi K.C., Katoh K., Wittamer V., Parmentier M., Sasaki S. Chemerin—A new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 2007;362:1013–1018. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 30.MacDougald O.A., Burant C.F. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–161. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Bozaoglu K., Bolton K., McMillan J., Zimmet P., Jowett J., Collier G., Walder K., Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 32.Leniz A., Gonzalez M., Besne I., Carr-Ugarte H., Gomez-Garcia I., Portillo M.P. Role of chemerin in the control of glucose homeostasis. Mol. Cell. Endocrinol. 2022;541:111504. doi: 10.1016/j.mce.2021.111504. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S., Kusminski C.M., Scherer P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021;128:136–149. doi: 10.1161/CIRCRESAHA.120.314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A., Choubey M., Bora P., Krishna A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018;25:1462–1473. doi: 10.1177/1933719118770547. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy A.J., Davenport A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin(1)) and GPR1 (Chemerin(2)) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018;70:174–196. doi: 10.1124/pr.116.013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gantz I., Konda Y., Yang Y.K., Miller D.E., Dierick H.A., Yamada T. Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet. Cell Genet. 1996;74:286–290. doi: 10.1159/000134436. [DOI] [PubMed] [Google Scholar]
- 37.Hu S., Shao Z., Zhang C., Chen L., Mamun A.A., Zhao N., Cai J., Lou Z., Wang X., Chen J. Chemerin facilitates intervertebral disc degeneration via TLR4 and CMKLR1 and activation of NF-kB signaling pathway. Aging. 2020;12:11732–11753. doi: 10.18632/aging.103339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czerniak A.S., Kretschmer K., Weiss T., Beck-Sickinger A.G. The Chemerin Receptor CMKLR1 Requires Full-Length Chemerin for High Affinity in Contrast to GPR1 as Demonstrated by a New Nanoluciferase-Based Binding Assay. ChemMedChem. 2022;17:e202200413. doi: 10.1002/cmdc.202200413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnea G., Strapps W., Herrada G., Berman Y., Ong J., Kloss B., Axel R., Lee K.J. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchese A., Docherty J.M., Nguyen T., Heiber M., Cheng R., Heng H.H., Tsui L.C., Shi X., George S.R., O’Dowd B.F. Cloning of human genes encoding novel G protein-coupled receptors. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- 42.Kisielewska K., Rytelewska E., Gudelska M., Kiezun M., Dobrzyn K., Bogus-Nowakowska K., Kaminska B., Smolinska N., Kaminski T. Expression of chemerin receptors CMKLR1, GPR1 and CCRL2 in the porcine pituitary during the oestrous cycle and early pregnancy and the effect of chemerin on MAPK/Erk1/2, Akt and AMPK signalling pathways. Theriogenology. 2020;157:181–198. doi: 10.1016/j.theriogenology.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Li J., Xiang L., Jiang X., Teng B., Sun Y., Chen G., Chen J., Zhang J.V., Ren P.G. Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice. J. Orthop. Transl. 2017;10:42–51. doi: 10.1016/j.jot.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L., Ma P., Huang C., Liu Y., Zhang Y., Gao C., Xiao T., Ren P.G., Zabel B.A., Zhang J.V. Expression of chemerin and its receptors in rat testes and its action on testosterone secretion. J. Endocrinol. 2014;220:155–163. doi: 10.1530/JOE-13-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster S.R., Hauser A.S., Vedel L., Strachan R.T., Huang X.P., Gavin A.C., Shah S.D., Nayak A.P., Haugaard-Kedstrom L.M., Penn R.B., et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell. 2019;179:895–908.e821. doi: 10.1016/j.cell.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng C., Chen D., Zhang Y., Bai Y., Huang S., Zheng D., Liang W., She S., Peng X., Wang P., et al. FAM19A1 is a new ligand for GPR1 that modulates neural stem-cell proliferation and differentiation. FASEB J. 2018;32:5874–5890. doi: 10.1096/fj.201800020RRR. [DOI] [PubMed] [Google Scholar]
- 47.Monnier J., Lewen S., O’Hara E., Huang K., Tu H., Butcher E.C., Zabel B.A. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J. Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeed A., Dullaart R.P.F., Schreuder T., Blokzijl H., Faber K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2017;10:29. doi: 10.3390/nu10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song Y., Yin W., Dan Y., Sheng J., Zeng Y., He R. Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology. 2019;157:248–256. doi: 10.1111/imm.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helfer G., Wu Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018;238:R79–R94. doi: 10.1530/JOE-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zdanowicz K., Bobrus-Chociej A., Lebensztejn D.M. Chemerin as Potential Biomarker in Pediatric Diseases: A PRISMA-Compliant Study. Biomedicines. 2022;10:591. doi: 10.3390/biomedicines10030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maheshwari A., Kurundkar A.R., Shaik S.S., Kelly D.R., Hartman Y., Zhang W., Dimmitt R., Saeed S., Randolph D.A., Aprahamian C., et al. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G1–G10. doi: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabel B.A., Silverio A.M., Butcher E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 54.Martensson U.E., Bristulf J., Owman C., Olde B. The mouse chemerin receptor gene, mcmklr1, utilizes alternative promoters for transcription and is regulated by all-trans retinoic acid. Gene. 2005;350:65–77. doi: 10.1016/j.gene.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Razny U., Polus A., Kiec-Wilk B., Wator L., Hartwich J., Stachura J., Tomaszewska R., Dyduch G., Laidler P., Schmitz G., et al. Angiogenesis in Balb/c mice under beta-carotene supplementation in diet. Genes Nutr. 2010;5:9–16. doi: 10.1007/s12263-009-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalvo-Feo S., Del Prete A., Pruenster M., Salvi V., Wang L., Sironi M., Bierschenk S., Sperandio M., Vecchi A., Sozzani S. Endothelial cell-derived chemerin promotes dendritic cell transmigration. J. Immunol. 2014;192:2366–2373. doi: 10.4049/jimmunol.1302028. [DOI] [PubMed] [Google Scholar]
- 57.Abeer A., Said M.D., Suzan M.M., Moursi M.D. Vitamin D Supplementation Reduces Serum Chemerin Level in Gestational Diabetes Mellitus Rat Model. Med. J. Cairo Univ. 2019;87:3069–3080. doi: 10.21608/mjcu.2019.59509. [DOI] [Google Scholar]
- 58.Nassar S.Z., Badae N.M. Protective effect of vitamin D supplementation in a rat modal of preeclampsia: A possible implication of chemerin. Hypertens Pregnancy. 2019;38:149–156. doi: 10.1080/10641955.2019.1597108. [DOI] [PubMed] [Google Scholar]
- 59.Palacios C., Kostiuk L.K., Pena-Rosas J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019;7:CD008873. doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Askary A.E., Gharib A.F., Almehmadi M., Bakhuraysah M.M., Hajjiahmed A.A.A., Al-Hejji L.I., Alharthi M.S., Shafie A. The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus. Open Chem. 2021;19:1174–1183. doi: 10.1515/chem-2021-0107. [DOI] [Google Scholar]
- 61.Reyman M., Verrijn Stuart A.A., van Summeren M., Rakhshandehroo M., Nuboer R., de Boer F.K., van den Ham H.J., Kalkhoven E., Prakken B., Schipper H.S. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int. J. Obes. 2014;38:46–52. doi: 10.1038/ijo.2013.75. [DOI] [PubMed] [Google Scholar]
- 62.Gharib A.F., El Askary A., Almehmadi M., Alhuthali H.M., Elsawy W.H., Allam H.H., Elsayyad L.K., Ayoub M.A., Shafie A. Association of vitamin D deficiency and inflammatory cytokines with the clinicopathological features of breast cancer in female Saudi patients. Eur. J. Inflamm. 2022;20:1721727X221106507. doi: 10.1177/1721727X221106507. [DOI] [Google Scholar]
- 63.De Palma G., Castellano G., Del Prete A., Sozzani S., Fiore N., Loverre A., Parmentier M., Gesualdo L., Grandaliano G., Schena F.P. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011;79:1228–1235. doi: 10.1038/ki.2011.32. [DOI] [PubMed] [Google Scholar]
- 64.Melguizo-Rodriguez L., Costela-Ruiz V.J., Garcia-Recio E., De Luna-Bertos E., Ruiz C., Illescas-Montes R. Role of Vitamin D in the Metabolic Syndrome. Nutrients. 2021;13:830. doi: 10.3390/nu13030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prieto-Hontoria P.L., Perez-Matute P., Fernandez-Galilea M., Lopez-Yoldi M., Sinal C.J., Martinez J.A., Moreno-Aliaga M.J. Effects of alpha-lipoic acid on chemerin secretion in 3T3-L1 and human adipocytes. Biochim. Biophys. Acta. 2016;1861:260–268. doi: 10.1016/j.bbalip.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Imai K., Takai K., Hanai T., Shiraki M., Suzuki Y., Hayashi H., Naiki T., Nishigaki Y., Tomita E., Shimizu M., et al. Impact of serum chemerin levels on liver functional reserves and platelet counts in patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2014;15:11294–11306. doi: 10.3390/ijms150711294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopp C., Hosseini A., Singh S.P., Regenhard P., Khalilvandi-Behroozyar H., Sauerwein H., Mielenz M. Nicotinic acid increases adiponectin secretion from differentiated bovine preadipocytes through G-protein coupled receptor signaling. Int. J. Mol. Sci. 2014;15:21401–21418. doi: 10.3390/ijms151121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding X.M., Mu Y.D., Zhang K.Y., Wang J.P., Bai S.P., Zeng Q.F., Peng H.W. Vitamin E improves antioxidant status but not lipid metabolism in laying hens fed a aged corn-containing diet. Anim. Biosci. 2021;34:276–284. doi: 10.5713/ajas.19.0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amirpour M., Fanaei H., Karajibani M., Montazerifar F., Dashipour A. Beneficial effect of symbiotic supplementation during pregnancy in high fat diet-induced metabolic disorder in rats: Role of chemerin. Obes. Med. 2020;19:100247. doi: 10.1016/j.obmed.2020.100247. [DOI] [Google Scholar]
- 70.Ernst M.C., Issa M., Goralski K.B., Sinal C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- 71.Yun H., Dumbell R., Hanna K., Bowen J., McLean S.L., Kantamneni S., Pors K., Wu Q.F., Helfer G. The Chemerin-CMKLR1 Axis is Functionally important for Central Regulation of Energy Homeostasis. Front. Physiol. 2022;13:897105. doi: 10.3389/fphys.2022.897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Docke S., Lock J.F., Birkenfeld A.L., Hoppe S., Lieske S., Rieger A., Raschzok N., Sauer I.M., Florian S., Osterhoff M.A., et al. Elevated hepatic chemerin mRNA expression in human non-alcoholic fatty liver disease. Eur. J. Endocrinol. 2013;169:547–557. doi: 10.1530/EJE-13-0112. [DOI] [PubMed] [Google Scholar]
- 73.Chakaroun R., Raschpichler M., Kloting N., Oberbach A., Flehmig G., Kern M., Schon M.R., Shang E., Lohmann T., Dressler M., et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61:706–714. doi: 10.1016/j.metabol.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Landgraf K., Friebe D., Ullrich T., Kratzsch J., Dittrich K., Herberth G., Adams V., Kiess W., Erbs S., Korner A. Chemerin as a mediator between obesity and vascular inflammation in children. J. Clin. Endocrinol. Metab. 2012;97:E556–E564. doi: 10.1210/jc.2011-2937. [DOI] [PubMed] [Google Scholar]
- 75.Marti A., Martinez I., Ojeda-Rodriguez A., Azcona-Sanjulian M.C. Higher Lipopolysaccharide Binding Protein and Chemerin Concentrations Were Associated with Metabolic Syndrome Features in Pediatric Subjects with Abdominal Obesity during a Lifestyle Intervention. Nutrients. 2021;13:289. doi: 10.3390/nu13020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S.H., Lee S.H., Ahn K.Y., Lee D.H., Suh Y.J., Cho S.G., Choi Y.J., Lee D.H., Lee S.Y., Hong S.B., et al. Effect of lifestyle modification on serum chemerin concentration and its association with insulin sensitivity in overweight and obese adults with type 2 diabetes. Clin. Endocrinol. 2014;80:825–833. doi: 10.1111/cen.12249. [DOI] [PubMed] [Google Scholar]
- 77.Lin X., Yang Y., Qu J., Wang X. Aerobic exercise decreases chemerin/CMKLR1 in the serum and peripheral metabolic organs of obesity and diabetes rats by increasing PPARgamma. Nutr. Metab. 2019;16:17. doi: 10.1186/s12986-019-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramos-Junior E.S., Leite G.A., Carmo-Silva C.C., Taira T.M., Neves K.B., Colon D.F., da Silva L.A., Salvador S.L., Tostes R.C., Cunha F.Q., et al. Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice. J. Bone. Miner. Res. 2017;32:974–984. doi: 10.1002/jbmr.3072. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W., Liu Y., Wu M., Zhu X., Wang T., He K., Li P., Wu X. PI3K inhibition protects mice from NAFLD by down-regulating CMKLR1 and NLRP3 in Kupffer cells. J. Physiol. Biochem. 2017;73:583–594. doi: 10.1007/s13105-017-0589-6. [DOI] [PubMed] [Google Scholar]
- 80.Xu M., Wang Y.M., Li W.Q., Huang C.L., Li J., Xie W.H., Zeng H.X., Tao L.F., Li X. Ccrl2 deficiency deteriorates obesity and insulin resistance through increasing adipose tissue macrophages infiltration. Genes Dis. 2022;9:429–442. doi: 10.1016/j.gendis.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goralski K.B., McCarthy T.C., Hanniman E.A., Zabel B.A., Butcher E.C., Parlee S.D., Muruganandan S., Sinal C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 82.Ferland D.J., Garver H., Contreras G.A., Fink G.D., Watts S.W. Chemerin contributes to in vivo adipogenesis in a location-specific manner. PLoS ONE. 2020;15:e0229251. doi: 10.1371/journal.pone.0229251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang B., Huang C., Zhao H., Zhu W., Wang B., Wang H., Chen J., Xiao T., Niu J., Zhang J. Impact of GPR1 signaling on maternal high-fat feeding and placenta metabolism in mice. Am. J. Physiol. Endocrinol. Metab. 2019;316:E987–E997. doi: 10.1152/ajpendo.00437.2018. [DOI] [PubMed] [Google Scholar]
- 84.Rourke J.L., Muruganandan S., Dranse H.J., McMullen N.M., Sinal C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 2014;222:201–215. doi: 10.1530/JOE-14-0069. [DOI] [PubMed] [Google Scholar]
- 85.Ernst M.C., Haidl I.D., Zuniga L.A., Dranse H.J., Rourke J.L., Zabel B.A., Butcher E.C., Sinal C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology. 2012;153:672–682. doi: 10.1210/en.2011-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouger L., Denis G.R., Luangsay S., Parmentier M. ChemR23 knockout mice display mild obesity but no deficit in adipocyte differentiation. J. Endocrinol. 2013;219:279–289. doi: 10.1530/JOE-13-0106. [DOI] [PubMed] [Google Scholar]
- 87.Koelman L., Reichmann R., Bornhorst C., Schulze M.B., Weikert C., Biemann R., Isermann B., Fritsche A., Aleksandrova K. Determinants of elevated chemerin as a novel biomarker of immunometabolism: Data from a large population-based cohort. Endocr. Connect. 2021;10:1200–1211. doi: 10.1530/EC-21-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauer S., Bala M., Kopp A., Eisinger K., Schmid A., Schneider S., Neumeier M., Buechler C. Adipocyte chemerin release is induced by insulin without being translated to higher levels in vivo. Eur. J. Clin. Invest. 2012;42:1213–1220. doi: 10.1111/j.1365-2362.2012.02713.x. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi M., Takahashi Y., Takahashi K., Zolotaryov F.N., Hong K.S., Kitazawa R., Iida K., Okimura Y., Kaji H., Kitazawa S., et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–578. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 90.Wang L., Zhang Y., Guo Y., Ding W., Chang A., Wei J., Li X., Qian H., Zhu C. Chemerin/CMKLR1 Axis Promotes the Progression of Proliferative Diabetic Retinopathy. Int. J. Endocrinol. 2021;2021:4468625. doi: 10.1155/2021/4468625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neves K.B., Nguyen Dinh Cat A., Alves-Lopes R., Harvey K.Y., Costa R.M.D., Lobato N.S., Montezano A.C., Oliveira A.M., Touyz R.M., Tostes R.C. Chemerin receptor blockade improves vascular function in diabetic obese mice via redox-sensitive and Akt-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1851–H1860. doi: 10.1152/ajpheart.00285.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X., Zhu Q., Wang W., Qi J., He Y., Wang Y., Lu Y., Wu H., Ding Y., Sun Y. Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients. FASEB J. 2019;33:11303–11313. doi: 10.1096/fj.201802829R. [DOI] [PubMed] [Google Scholar]
- 93.Ernst M.C., Sinal C.J. Chemerin: At the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Sainz N., Fernandez-Galilea M., Costa A.G.V., Prieto-Hontoria P.L., Barraco G.M., Moreno-Aliaga M.J. n-3 polyunsaturated fatty acids regulate chemerin in cultured adipocytes: Role of GPR120 and derived lipid mediators. Food Funct. 2020;11:9057–9066. doi: 10.1039/D0FO01445A. [DOI] [PubMed] [Google Scholar]
- 95.Giacobbe J., Benoiton B., Zunszain P., Pariante C.M., Borsini A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry. 2020;11:122. doi: 10.3389/fpsyt.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mirmajidi S., Izadi A., Saghafi-Asl M., Vahid F., Karamzad N., Amiri P., Shivappa N., Hebert J.R. Inflammatory Potential of Diet: Association with Chemerin, Omentin, Lipopolysaccharide-Binding Protein, and Insulin Resistance in the Apparently Healthy Obese. J. Am. Coll. Nutr. 2019;38:302–310. doi: 10.1080/07315724.2018.1504348. [DOI] [PubMed] [Google Scholar]
- 97.Markova M., Koelman L., Hornemann S., Pivovarova O., Sucher S., Machann J., Rudovich N., Thomann R., Schneeweiss R., Rohn S., et al. Effects of plant and animal high protein diets on immune-inflammatory biomarkers: A 6-week intervention trial. Clin. Nutr. 2020;39:862–869. doi: 10.1016/j.clnu.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Koelman L., Markova M., Seebeck N., Hornemann S., Rosenthal A., Lange V., Pivovarova-Ramich O., Aleksandrova K. Effects of High and Low Protein Diets on Inflammatory Profiles in People with Morbid Obesity: A 3-Week Intervention Study. Nutrients. 2020;12:3636. doi: 10.3390/nu12123636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abais-Battad J.M., Lund H., Fehrenbach D.J., Dasinger J.H., Alsheikh A.J., Mattson D.L. Parental Dietary Protein Source and the Role of CMKLR1 in Determining the Severity of Dahl Salt-Sensitive Hypertension. Hypertension. 2019;73:440–448. doi: 10.1161/HYPERTENSIONAHA.118.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferland D.J., Flood E.D., Garver H., Yeh S.T., Riney S., Mullick A.E., Fink G.D., Watts S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol. Genom. 2019;51:553–561. doi: 10.1152/physiolgenomics.00050.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ren R.Z., Zhang X., Xu J., Zhang H.Q., Yu C.X., Cao M.F., Gao L., Guan Q.B., Zhao J.J. Chronic ethanol consumption increases the levels of chemerin in the serum and adipose tissue of humans and rats. Acta Pharmacol. Sin. 2012;33:652–659. doi: 10.1038/aps.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou H.H., Teng M.S., Hsu L.A., Er L.K., Wu S., Ko Y.L. Circulating chemerin level is associated with metabolic, biochemical and haematological parameters-A population-based study. Clin. Endocrinol. 2021;94:927–939. doi: 10.1111/cen.14441. [DOI] [PubMed] [Google Scholar]
- 103.McCullough R.L., McMullen M.R., Poulsen K.L., Kim A., Medof M.E., Nagy L.E. Anaphylatoxin Receptors C3aR and C5aR1 Are Important Factors That Influence the Impact of Ethanol on the Adipose Secretome. Front. Immunol. 2018;9:2133. doi: 10.3389/fimmu.2018.02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adrych K., Stojek M., Smoczynski M., Sledzinski T., Sylwia S.W., Swierczynski J. Increased serum chemerin concentration in patients with chronic pancreatitis. Dig. Liver Dis. 2012;44:393–397. doi: 10.1016/j.dld.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 105.Aoun E.G., Jimenez V.A., Vendruscolo L.F., Walter N.A.R., Barbier E., Ferrulli A., Haass-Koffler C.L., Darakjian P., Lee M.R., Addolorato G., et al. A relationship between the aldosterone-mineralocorticoid receptor pathway and alcohol drinking: Preliminary translational findings across rats, monkeys and humans. Mol. Psychiatry. 2018;23:1466–1473. doi: 10.1038/mp.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lastra G., Dhuper S., Johnson M.S., Sowers J.R. Salt, aldosterone, and insulin resistance: Impact on the cardiovascular system. Nat. Rev. Cardiol. 2010;7:577–584. doi: 10.1038/nrcardio.2010.123. [DOI] [PubMed] [Google Scholar]
- 107.Ma J., Sun F., Wang J., Jiang H., Lu J., Wang X., Zhang J., Shi C., You W., Li X., et al. Effects of Aldosterone on Chemerin Expression and Secretion in 3T3-L1 Adipocytes. Exp. Clin. Endocrinol. Diabetes. 2018;126:187–193. doi: 10.1055/s-0043-118749. [DOI] [PubMed] [Google Scholar]
- 108.Fu Y.Y., Chen K.L., Li H.X., Zhou G.H. The adipokine Chemerin induces lipolysis and adipogenesis in bovine intramuscular adipocytes. Mol. Cell Biochem. 2016;418:39–48. doi: 10.1007/s11010-016-2731-0. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y., Shen W.J., Qiu S., Yang P., Dempsey G., Zhao L., Zhou Q., Hao X., Dong D., Stahl A., et al. Chemerin regulates formation and function of brown adipose tissue: Ablation results in increased insulin resistance with high fat challenge and aging. FASEB J. 2021;35:e21687. doi: 10.1096/fj.202100156R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan L., Chen Z., Sun F., Zhou Z., Zhang B., Wang B., Chen J., Li M., Xiao T., Neuman R.I., et al. Placental trophoblast-specific overexpression of chemerin induces preeclampsia-like symptoms. Clin. Sci. 2022;136:257–272. doi: 10.1042/CS20210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang H., Li F., Kong X., Yuan X., Wang W., Huang R., Li T., Geng M., Wu G., Yin Y. Chemerin regulates proliferation and differentiation of myoblast cells via ERK1/2 and mTOR signaling pathways. Cytokine. 2012;60:646–652. doi: 10.1016/j.cyto.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 112.Zhu L., Huang J., Wang Y., Yang Z., Chen X. Chemerin causes lipid metabolic imbalance and induces passive lipid accumulation in human hepatoma cell line via the receptor GPR1. Life Sci. 2021;278:119530. doi: 10.1016/j.lfs.2021.119530. [DOI] [PubMed] [Google Scholar]
- 113.Hatziagelaki E., Herder C., Tsiavou A., Teichert T., Chounta A., Nowotny P., Pacini G., Dimitriadis G., Roden M. Serum Chemerin Concentrations Associate with Beta-Cell Function, But Not with Insulin Resistance in Individuals with Non-Alcoholic Fatty Liver Disease (NAFLD) PLoS ONE. 2015;10:e0124935. doi: 10.1371/journal.pone.0124935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krautbauer S., Wanninger J., Eisinger K., Hader Y., Beck M., Kopp A., Schmid A., Weiss T.S., Dorn C., Buechler C. Chemerin is highly expressed in hepatocytes and is induced in non-alcoholic steatohepatitis liver. Exp. Mol. Pathol. 2013;95:199–205. doi: 10.1016/j.yexmp.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Pohl R., Feder S., Haberl E.M., Rein-Fischboeck L., Weiss T.S., Spirk M., Bruckmann A., McMullen N., Sinal C.J., Buechler C. Chemerin Overexpression in the Liver Protects against Inflammation in Experimental Non-Alcoholic Steatohepatitis. Biomedicines. 2022;10:132. doi: 10.3390/biomedicines10010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rein-Fischboeck L., Haberl E.M., Pohl R., Feder S., Liebisch G., Krautbauer S., Buechler C. Variations in hepatic lipid species of age-matched male mice fed a methionine-choline-deficient diet and housed in different animal facilities. Lipids Health Dis. 2019;18:172. doi: 10.1186/s12944-019-1114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmid A., Bala M., Leszczak S., Ober I., Buechler C., Karrasch T. Pro-inflammatory chemokines CCL2, chemerin, IP-10 and RANTES in human serum during an oral lipid tolerance test. Cytokine. 2016;80:56–63. doi: 10.1016/j.cyto.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 118.Hansen I.R., Jansson K.M., Cannon B., Nedergaard J. Contrasting effects of cold acclimation versus obesogenic diets on chemerin gene expression in brown and brite adipose tissues. Biochim. Biophys. Acta. 2014;1841:1691–1699. doi: 10.1016/j.bbalip.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Villarroya F., Cereijo R., Villarroya J., Giralt M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Z., Liu S., Qian B., Zhou L., Shi J., Liu J., Xu L., Yang Z. CMKLR1 senses chemerin/resolvin E1 to control adipose thermogenesis and modulate metabolic homeostasis. Fundam. Res. 2022 doi: 10.1016/j.fmre.2022.06.014. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin Y., Xiao L., Cai Q., Zhu C., Li S., Li B., Liu T., Zhang Q., Wang Y., Li Y., et al. The chemerin-CMKLR1 axis limits thermogenesis by controlling a beige adipocyte/IL-33/type 2 innate immunity circuit. Sci. Immunol. 2021;6:eabg9698. doi: 10.1126/sciimmunol.abg9698. [DOI] [PubMed] [Google Scholar]
- 122.Maghsoudi Z., Kelishadi R., Hosseinzadeh-Attar M.J. The comparison of chemerin, adiponectin and lipid profile indices in obese and non-obese adolescents. Diabetes Metab. Syndr. 2016;10:S43–S46. doi: 10.1016/j.dsx.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 123.Lorincz H., Katko M., Harangi M., Somodi S., Gaal K., Fulop P., Paragh G., Seres I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin. Endocrinol. 2014;81:370–377. doi: 10.1111/cen.12363. [DOI] [PubMed] [Google Scholar]
- 124.Navab M., Reddy S.T., Van Lenten B.J., Fogelman A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 125.Varga V.E., Lorincz H., Zsiros N., Fulop P., Seres I., Paragh G., Balla J., Harangi M. Impact of selective LDL apheresis on serum chemerin levels in patients with hypercholesterolemia. Lipids Health Dis. 2016;15:182. doi: 10.1186/s12944-016-0353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eichelmann F., Schulze M.B., Wittenbecher C., Menzel J., Weikert C., di Giuseppe R., Biemann R., Isermann B., Fritsche A., Boeing H., et al. Chemerin as a Biomarker Linking Inflammation and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2019;73:378–379. doi: 10.1016/j.jacc.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 127.Zhang G., Xiao M., Zhang L., Zhao Y., Yang Q. Association of serum chemerin concentrations with the presence of atrial fibrillation. Ann. Clin. Biochem. 2017;54:342–347. doi: 10.1177/0004563216664367. [DOI] [PubMed] [Google Scholar]
- 128.Yan Q., Zhang Y., Hong J., Gu W., Dai M., Shi J., Zhai Y., Wang W., Li X., Ning G. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine. 2012;41:281–288. doi: 10.1007/s12020-011-9550-6. [DOI] [PubMed] [Google Scholar]
- 129.Xiaotao L., Xiaoxia Z., Yue X., Liye W. Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron. Artery Dis. 2012;23:412–416. doi: 10.1097/MCA.0b013e3283576a60. [DOI] [PubMed] [Google Scholar]
- 130.Yoo H.J., Choi H.Y., Yang S.J., Kim H.Y., Seo J.A., Kim S.G., Kim N.H., Choi K.M., Choi D.S., Baik S.H. Circulating chemerin level is independently correlated with arterial stiffness. J. Atheroscler. Thromb. 2012;19:59–66. doi: 10.5551/jat.9647. [DOI] [PubMed] [Google Scholar]
- 131.Kaur J., Mattu H.S., Chatha K., Randeva H.S. Chemerin in human cardiovascular disease. Vascul. Pharmacol. 2018;110:1–6. doi: 10.1016/j.vph.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 132.Leiherer A., Muendlein A., Kinz E., Vonbank A., Rein P., Fraunberger P., Malin C., Saely C.H., Drexel H. High plasma chemerin is associated with renal dysfunction and predictive for cardiovascular events—Insights from phenotype and genotype characterization. Vascul. Pharmacol. 2016;77:60–68. doi: 10.1016/j.vph.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 133.Sun J.X., Zhang C., Cheng Z.B., Tang M.Y., Liu Y.Z., Jiang J.F., Xiao X., Huang L. Chemerin in atherosclerosis. Clin. Chim. Acta. 2021;520:8–15. doi: 10.1016/j.cca.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 134.Gao X., Mi S., Zhang F., Gong F., Lai Y., Gao F., Zhang X., Wang L., Tao H. Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc. Diabetol. 2011;10:87. doi: 10.1186/1475-2840-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Q., Chen Y., Chen S., Wu X., Nong W. Correlation between adiponectin, chemerin, vascular endothelial growth factor and epicardial fat volume in patients with coronary artery disease. Exp. Ther. Med. 2020;19:1095–1102. doi: 10.3892/etm.2019.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spiroglou S.G., Kostopoulos C.G., Varakis J.N., Papadaki H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 137.Kostopoulos C.G., Spiroglou S.G., Varakis J.N., Apostolakis E., Papadaki H.H. Chemerin and CMKLR1 expression in human arteries and periadventitial fat: A possible role for local chemerin in atherosclerosis? BMC Cardiovasc Disord. 2014;14:56. doi: 10.1186/1471-2261-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yanofsky R., Sancho C., Gasbarrino K., Zheng H., Doonan R.J., Jaunet F., Steinmetz-Wood S., Veinot J.P., Lai C., Daskalopoulou S.S. Expression of Resistin, Chemerin, and Chemerin’s Receptor in the Unstable Carotid Atherosclerotic Plaque. Stroke. 2021;52:2537–2546. doi: 10.1161/STROKEAHA.120.030228. [DOI] [PubMed] [Google Scholar]
- 139.Rodriguez-Penas D., Feijoo-Bandin S., Garcia-Rua V., Mosquera-Leal A., Duran D., Varela A., Portoles M., Rosello-Lleti E., Rivera M., Dieguez C., et al. The Adipokine Chemerin Induces Apoptosis in Cardiomyocytes. Cell Physiol. Biochem. 2015;37:176–192. doi: 10.1159/000430343. [DOI] [PubMed] [Google Scholar]
- 140.Yamamoto A., Sagara A., Otani K., Okada M., Yamawaki H. Chemerin-9 stimulates migration in rat cardiac fibroblasts in vitro. Eur. J. Pharmacol. 2021;912:174566. doi: 10.1016/j.ejphar.2021.174566. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y., Jin X., Qiao L., Li Y., Yang R., Wang M., Zhang G., Zhang Y., Liu T., Rao M., et al. Aldosterone upregulates chemerin via chemokinelike receptor 1-Rho-ROCK-JNK signaling in cardiac fibroblasts. Int. J. Clin. Exp. Med. 2017;10:6756–6762. [Google Scholar]
- 142.Bozaoglu K., Curran J.E., Stocker C.J., Zaibi M.S., Segal D., Konstantopoulos N., Morrison S., Carless M., Dyer T.D., Cole S.A., et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Darios E.S., Winner B.M., Charvat T., Krasinksi A., Punna S., Watts S.W. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H498–H507. doi: 10.1152/ajpheart.00998.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie Y., Liu L. Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction. J. Transl. Med. 2022;20:141. doi: 10.1186/s12967-021-03220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stepan H., Philipp A., Roth I., Kralisch S., Jank A., Schaarschmidt W., Lossner U., Kratzsch J., Bluher M., Stumvoll M., et al. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul. Pept. 2011;168:69–72. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Kasher-Meron M., Mazaki-Tovi S., Barhod E., Hemi R., Haas J., Gat I., Zilberberg E., Yinon Y., Karasik A., Kanety H. Chemerin concentrations in maternal and fetal compartments: Implications for metabolic adaptations to normal human pregnancy. J. Perinat. Med. 2014;42:371–378. doi: 10.1515/jpm-2013-0166. [DOI] [PubMed] [Google Scholar]
- 147.Garces M.F., Sanchez E., Ruiz-Parra A.I., Rubio-Romero J.A., Angel-Muller E., Suarez M.A., Bohorquez L.F., Bravo S.B., Nogueiras R., Dieguez C., et al. Serum chemerin levels during normal human pregnancy. Peptides. 2013;42:138–143. doi: 10.1016/j.peptides.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 148.Barker G., Lim R., Rice G.E., Lappas M. Increased chemerin concentrations in fetuses of obese mothers and correlation with maternal insulin sensitivity. J. Fetal. Neonatal. Med. 2012;25:2274–2280. doi: 10.3109/14767058.2012.686540. [DOI] [PubMed] [Google Scholar]
- 149.Boutsikou T., Briana D.D., Boutsikou M., Kafalidis G., Stamati L., Baka S., Hassiakos D., Gourgiotis D., Malamitsi-Puchner A. Cord blood chemerin and obestatin levels in large for gestational age infants. J. Matern. Fetal. Neonatal. Med. 2013;26:123–126. doi: 10.3109/14767058.2012.728648. [DOI] [PubMed] [Google Scholar]
- 150.Antony K.M., Mansouri R., Arndt M., Rocky Hui S.K., Jariwala P., McMullen V.M., Teruya J., Aagaard K. Establishing thromboelastography with platelet-function analyzer reference ranges and other measures in healthy term pregnant women. Am. J. Perinatol. 2015;32:545–554. doi: 10.1055/s-0034-1396700. [DOI] [PubMed] [Google Scholar]
- 151.Hsu L.A., Chou H.H., Teng M.S., Wu S., Ko Y.L. Circulating chemerin levels are determined through circulating platelet counts in nondiabetic Taiwanese people: A bidirectional Mendelian randomization study. Atherosclerosis. 2021;320:61–69. doi: 10.1016/j.atherosclerosis.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 152.Du X.Y., Zabel B.A., Myles T., Allen S.J., Handel T.M., Lee P.P., Butcher E.C., Leung L.L. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J. Biol. Chem. 2009;284:751–758. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mazaki-Tovi S., Kasher-Meron M., Hemi R., Haas J., Gat I., Lantsberg D., Hendler I., Kanety H. Chemerin is present in human cord blood and is positively correlated with birthweight. Am. J. Obstet. Gynecol. 2012;207:412.e1–412.e10. doi: 10.1016/j.ajog.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Cetin O., Kurdoglu Z., Kurdoglu M., Sahin H.G. Chemerin level in pregnancies complicated by preeclampsia and its relation with disease severity and neonatal outcomes. J. Obstet. Gynaecol. 2017;37:195–199. doi: 10.1080/01443615.2016.1233947. [DOI] [PubMed] [Google Scholar]
- 155.Liang Z., Han L., Sun D., Chen Y., Wu Q., Zhang L., Zhou M., Chen D. Chemerin-induced macrophages pyroptosis in fetal brain tissue leads to cognitive disorder in offspring of diabetic dams. J. Neuroinflammation. 2019;16:226. doi: 10.1186/s12974-019-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ferland D.J., Watts S.W. Chemerin: A comprehensive review elucidating the need for cardiovascular research. Pharmacol. Res. 2015;99:351–361. doi: 10.1016/j.phrs.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Q.L., Zhu M., Jin Y., Wang N., Xu H.X., Quan L.M., Wang S.S., Li S.S. The predictive value of the first-trimester maternal serum chemerin level for pre-eclampsia. Peptides. 2014;62:150–154. doi: 10.1016/j.peptides.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 158.Tan L., Ouyang Z., Chen Z., Sun F., Guo H., Wang F., Mulder M., Sun Y., Lu X., Zhang J.V., et al. Adipokine chemerin overexpression in trophoblasts leads to dyslipidemia in pregnant mice: Implications for preeclampsia. Lipids Health Dis. 2023;22:12. doi: 10.1186/s12944-023-01777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun J., Ren J., Zuo C., Deng D., Pan F., Chen R., Zhu J., Chen C., Ye S. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2020;19:26. doi: 10.1186/s12944-020-01209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pfau D., Stepan H., Kratzsch J., Verlohren M., Verlohren H.J., Drynda K., Lossner U., Bluher M., Stumvoll M., Fasshauer M. Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Horm. Res. Paediatr. 2010;74:56–61. doi: 10.1159/000282114. [DOI] [PubMed] [Google Scholar]
- 161.van Poppel M.N., Zeck W., Ulrich D., Schest E.C., Hirschmugl B., Lang U., Wadsack C., Desoye G. Cord blood chemerin: Differential effects of gestational diabetes mellitus and maternal obesity. Clin. Endocrinol. 2014;80:65–72. doi: 10.1111/cen.12140. [DOI] [PubMed] [Google Scholar]
- 162.Liang Z., Zhou M., Xu X.K., Qu F., Chen D. Is Chemerin associated with gestational diabetes mellitus? An evidence-based clinical research from Chinese women. J. Obstet. Gynaecol. 2018;38:482–487. doi: 10.1080/01443615.2017.1385596. [DOI] [PubMed] [Google Scholar]
- 163.O’Kelly A.C., Michos E.D., Shufelt C.L., Vermunt J.V., Minissian M.B., Quesada O., Smith G.N., Rich-Edwards J.W., Garovic V.D., El Khoudary S.R., et al. Pregnancy and Reproductive Risk Factors for Cardiovascular Disease in Women. Circ. Res. 2022;130:652–672. doi: 10.1161/CIRCRESAHA.121.319895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Basit S., Wohlfahrt J., Boyd H.A. Pre-eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ. 2018;363:k4109. doi: 10.1136/bmj.k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bozaoglu K., Segal D., Shields K.A., Cummings N., Curran J.E., Comuzzie A.G., Mahaney M.C., Rainwater D.L., VandeBerg J.L., MacCluer J.W., et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J. Clin. Endocrinol. Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi M., Inomata S., Okimura Y., Iguchi G., Fukuoka H., Miyake K., Koga D., Akamatsu S., Kasuga M., Takahashi Y. Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: Sex dimorphism. Endocr. J. 2013;60:37–44. doi: 10.1507/endocrj.EJ12-0201. [DOI] [PubMed] [Google Scholar]
- 167.Daxer J., Herttrich T., Zhao Y.Y., Vogel M., Hiemisch A., Scheuermann K., Korner A., Kratzsch J., Kiess W., Quante M. Nocturnal levels of chemerin and progranulin in adolescents: Influence of sex, body mass index, glucose metabolism and sleep. J. Pediatr. Endocrinol. Metab. 2017;30:57–61. doi: 10.1515/jpem-2016-0378. [DOI] [PubMed] [Google Scholar]
- 168.Watts S.W., Darios E.S., Mullick A.E., Garver H., Saunders T.L., Hughes E.D., Filipiak W.E., Zeidler M.G., McMullen N., Sinal C.J., et al. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension. FASEB J. 2018;32:6596–6614. doi: 10.1096/fj.201800479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sanchez-Rebordelo E., Cunarro J., Perez-Sieira S., Seoane L.M., Dieguez C., Nogueiras R., Tovar S. Regulation of Chemerin and CMKLR1 Expression by Nutritional Status, Postnatal Development, and Gender. Int. J. Mol. Sci. 2018;19:2905. doi: 10.3390/ijms19102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lima P.D.A., Nivet A.L., Wang Q., Chen Y.A., Leader A., Cheung A., Tzeng C.R., Tsang B.K. Polycystic ovary syndrome: Possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages. Biol. Reprod. 2018;99:838–852. doi: 10.1093/biolre/ioy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Q., Kim J.Y., Xue K., Liu J.Y., Leader A., Tsang B.K. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology. 2012;153:5600–5611. doi: 10.1210/en.2012-1424. [DOI] [PubMed] [Google Scholar]
- 172.Bobjer J., Katrinaki M., Dermitzaki E., Margioris A.N., Giwercman A., Tsatsanis C. Serum chemerin levels are negatively associated with male fertility and reproductive hormones. Hum. Reprod. 2018;33:2168–2174. doi: 10.1093/humrep/dey310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.