Abstract
Trauma leading to severe hemorrhage and shock on average kills patients within 3 to 6 hours after injury. With average prehospital transport times reaching 1–6 hours in low- to middle-income countries, stopping the bleeding and reversing hemorrhagic shock is vital. First-generation intravenous hemostats rely on traditional drug delivery platforms, such as self-assembling systems, fabricated nanoparticles, and soluble polymers due to their active targeting, biodistribution, and safety. We discuss some challenges translating these therapies to patients, as very few have successfully made it through preclinical evaluation in large-animals, and none have translated to the clinic. Finally, we discuss the physiology of hemorrhagic shock, highlight a new low volume resuscitant (LVR) PEG-20k, and end with considerations for the rational design of LVRs.
1. INTRODUCTION
Trauma was responsible for more than 4 million deaths worldwide in 2019, a total greater than tuberculosis, HIV, and malaria combined, with road injuries being the leading cause of death for people aged 5 to 49.1 The WHO estimates that 90% of trauma-related deaths occur in low- and middleincome countries, where average prehospital transport times can range from 1–6 hours.2,3 During trauma-related severe hemorrhage (>30% total blood loss), hypovolemia and hypoperfusion of organs can lead to hemorrhagic shock.4 The reduced oxygen supply leads to anaerobic respiration, and results in lactate and acidosis. The severe blood loss and shock result in trauma-induced coagulopathy (TIC) impairing the body’s ability to clot, and perpetuating the vicious cycle (Fig 1 below).5 Multiple randomized control trials (RCTs) indicate the vast majority of hemorrhage-related fatalities occur within 3 to 6 hours after injury.6,7 Damage control resuscitation prioritizes surgical control to stop the bleeding, while simultaneously resuscitating to restore the intravascular space to reverse hemorrhagic shock in hopes of preventing TIC.8
Figure 1. Vicious cycle of severe hemorrhage from trauma.

Tissue damage and severe hemorrhage from trauma lead to hemorrhagic shock. All three of these result in trauma-induced coagulopathy (TIC) which leads to more hemorrhage. TIC is part of the triad of death where it is compounded by acidosis and hypothermia.
This review focuses on synthetic intravenous hemostats and fluid resuscitants (as opposed to purified or isolated natural products), that leverage polymer chemistry, bioengineering, and drug delivery principles to treat hemorrhage and hemorrhagic shock in the prehospital setting.
2. INTRAVENOUS HEMOSTATS
Hemostasis is driven by a quiescent circulating system of fully soluble proteins (vWf, fibrinogen) and colloidal particles (platelets, erythrocytes) that can be rapidly activated by blood factors (FII, FX, etc.) to self-assemble into an insoluble heterogenous hemostatic plug to halt bleeding.9 Furthermore, this system undergoes platelet-driven mechanical contraction and compaction of the fibrin network to stabilize the plug and fully close the wound. In primary hemostasis, platelets form an initial plug at the wound site. The activated platelets release coagulation factors and provide a surface to initiate secondary hemostasis that results in the formation of a fibrin network that reinforces the clot on the backside of the vessel.
Traditional drug delivery platforms, such as self-assembling systems (e.g., liposomes, peptide amphiphiles), fabricated nanoparticles (e.g. polymer micelles), and soluble polymers (e.g. polymer-peptide conjugates) were used as the basis of most first-generation intravenous hemostats. The active targeting and pharmacokinetic properties of these systems promoted accumulation at the active site of bleeding. More recent intravenous hemostats capitalize on the modularity of the parent platforms to recapitulate biophysical aspects of hemostasis and deliver payloads that augment the hemostatic plug.
2.1. Fabricated Nanoparticles (Polymer Micelles)
Nanoparticle hemostats target primary hemostasis and mimic one or more properties of platelets – biophysical, aggregation, adhesion, clot retraction, and activation.10,11 The Lavik group reported 400–500 nm particles consist of a degradable polyester core of poly(lactic-co-glycolic acid) (PLGA) or poly(lactic acid) (PLA) with poly(ethylene glycol) (PEG) arms functionalized with GRGDS peptides that bind to activated platelets and increase platelet aggregation. These particles increase survival in a lethal, non-coagulopathic rat blast trauma model (single bolus injection immediately after injury, dose = 5 mg/kg) and doses of 0.8 mg/kg, 2 mg/kg, and 3.3 mg/kg halt bleeding in a lethal, non-coagulopathic porcine grade III liver injury model (single bolus injection 5 minutes after injury), while 6.6 mg/kg led to rapid exsanguination.12,13 The platform is flexible; the polyester core has been replaced with chitosan succinate14 or polyurethane nanocapusles that can be used for drug delivery.15 Additionally, Gkikas et al. attached the fluorescent dye DiD and gold particles to hemostatic PLGA-PEG nanoparticles to image internal bleeding with nearinfrared imaging, and X-ray computed tomography.16 Hong et al. evaluated PLGA-PEG-GRGDS particles of various sizes and reported that particle size affected targeting of platelets, lung accumulation, platelet adhesion, and survivability in a lethal, non-coagulopathic rat inferior vena cava puncture model (single bolus injection immediately after injury, dose = ~67 – 89 mg/kg).17
The Barker group developed poly(N-isopropylacrylamide-co-acrylic acid) microgels decorated with fibrin-specific variable domain-like recognition motifs (sdFvs). Due to interactions between the soft microgels and polymerized fibrin network, the particles recapitulated platelet clot retraction by collapsing fibrin networks and increasing clot stiffness, which decreased bleeding times in a lethal, non-coagulopathic rat femoral bleed model (single bolus injection 5 min prior to injury, dose = 40 – 50 mg/kg).18,19 To decrease cost, the Brown group replaced the antibody fragments with peptides that mimic fibrin knob ‘B’ and targets fibrin hole ‘b’. These new “Fibrin-Affine Microgels with Clotting Yield” showed similar activity to the 1st generation antibody particles and decreased bleeding in a non-coagulopathic mouse liver laceration model (single bolus injection 5 minutes prior to injury, dose = 5 – 50 mg/kg).20 Fibrin-targeted poly(N-isopropylacrylamide) nanogels have been loaded with tissue-type plasminogen activator (tPA), for delivery of targeted therapeutics.21
The Sen Gupta Group incorporates multiple peptide functionalities in their platelet-mimicking nanoparticles. SynthoPlate™ consists of 200 nm lipid vesicles with a PEG outer shell, decorated with three different peptides, vWF- and collagen-binding, and a fibrinogen mimic that binds active platelet integrin GPIIb-IIIa. SynthoPlate™ recapitulates both platelet adhesion and aggregation, improving “golden hour” survival in a lethal, non-coagulopathic porcine femoral artery traumatic bleed model (single bolus injection 1 minute after injury, dose = 1.7 × 1011 particles/kg).22–24 Their next-generation platelet-mimicking procoagulant nanoparticles (PPNs) recreate the procoagulant function of the platelet surface through diastearyol phosphatidylserine that is masked by plasmincleavable PEG. The anionic phospholipid is cloaked in circulation but unmasked at the site of injury, creating a surface to bind blood factors and results in the upregulation of thrombin generation. The PPNs decrease bleeding in a lethal, thrombocytopenic mouse liver transection model (single bolus injection 20 minutes before injury, dose = 2 mg/kg) and decreased bleeding in a lethal rat liver transection model (single bolus injection immediately after injury, dose = 2 mg/kg).25 Because the PPNs rely on thrombin generation that can be impaired in TIC, thrombin-loaded injury-site-targeted lipid nanoparticles (t-TLNPs) that release thrombin at the wound site were also recently reported. The t-TLNPs decrease bleeding and reverse severe coagulopathy in both a mouse tail-clip model (thrombocytopenia and heparin, single bolus injection 15 minutes before injury, targeted thrombin dose = 0.031 mg/kg) and a mouse liver transection (heparin, single bolus injection immediately after injection, targeted thrombin dose = 0.031 mg/kg).26
2.2. Synthetic Soluble Polymers
In contrast to the nanoparticle systems discussed previously, unimeric peptide-polymer conjugates do not form supramolecular structures in solution. Unlike nanoparticles which are recognized and rapidly cleared by the reticuloendothelial system, soluble polymers offer a more desirable biodistribution for intravenous hemostat applications. We have previously reported PolySTAT, a soluble polymer displaying multiple fibrin-binding peptides, that physically crosslinks and reinforces the fibrin network that forms during secondary hemostasis. PolySTAT reduces bleeding and improves survival in a lethal, non-coagulopathic rat femoral bleed model (single bolus injection immediately after injury, dose = 15 mg/kg).27–29 Recently, using a similar concept, the Mitragotri group developed HAPPI (hemostatic agent via polymer peptide infusion), hyaluronic acid polymer with grafted collagen-binding and von Willebrand factor-binding peptides. Their system exhibited selective binding to activated platelets, promoted platelet accumulation in vitro, and improved survival time in a lethal, non-coagulopathic rat inferior vena cava traumatic puncture model (single bolus injection immediately after injury, dose = 12 mg/kg).30
2.3. Polypeptides
Lastly, polypeptides have been developed to target and bind hemostatic factors, with some designed to self-assemble into hierarchical structures. Kearney et al. screened libraries of scaffold proteins to identify fibrinogen-binding proteins, and further showed that the proteins delay plasmin-mediated breakdown of clots by interfering with plasminogen-fibrin binding.31
Elastin-like polypeptides (ELPs) use a repeat pentapeptide (VPGXG) based off human tropoelastin that enables stimuli-responsive (pH, temp., light, salt) hydrophobic-driven collapse and aggregation in solution.32 Urosev et al. developed a recombinantly-produced 69 kDa hemostatic elastin-like polypeptide (hELP), that included an interspersed glutamine-based recognition sequence optimized for FXIIIa activity, and a lysine-donor block that enables hELPs to be covalently crosslinked into fibrin gels by FXIIIa. At physiologic temperature of 37 C, above the lower critical solution temperature of the ELP, the hydrophobic phase separation mechanically stiffened fibrin clots, and decreased clot porosity, which improved clotting kinetics and decreased fibrinolysis.33 The in vivo hemostatic efficacy of hELPs has not yet been reported.
Klein et al. developed a panel of tissue factor-targeting peptides attached to a generic peptide amphiphile (PA) sequence. When near each other, these 14–28 amino acids long (1.6–3.1 kDa) PAs self-assemble to form nanofibers of ~10–20 nm in diameter with lengths in the micrometer range. The best performing tissue factor-targeted PAs decreased blood loss by 35% - 59% compared to controls in a non-coagulopathic rat liver punch injury model (single bolus injection before injury, dose = ~5.6 – 7.1 mg/kg).34,35
2.4. A Key Challenge to clinical translation.
Pre-clinical synthetic injectable hemostatic agents have shown great promise in preclinical rodent models. However, significant challenges arise when moving to large animal models mainly due to a lack of models accurately reproducing human traumatic coagulopathy.36,37 Porcine models are a popular choice for trauma and resuscitation research and development given their larger size, blood volume, and similar cardiovascular system to humans. However, swine have distinct differences compared to humans when considering their blood coagulation and pulmonary systems. Pigs are hypercoagulable compared to humans and create very strong, robust blood clots with dense fibrin networks, that unlike humans, do not undergo significant fibrinolysis.38 Hemodilution can be used to create a dilutional coagulopathy; however, significant dilution is needed to make the clots hypocoagulable. Therefore, complex polytrauma with hemorrhagic shock alone is often insufficient to induce coagulopathy and hyperfibrinolysis like that seen with human traumatic coagulopathy.
Pigs are also distinct from humans in that their lungs that are laden with pulmonary macrophages. When exposed to therapies in the form of nanoparticles and polymers, pigs tend to exhibit complement activation-related pseudoallergy (CARPA) that mimics anaphylaxis. CARPA is mediated by massive release of vasoactive substances by pulmonary macrophages, leading to leading to significant pulmonary vasoconstriction. The Lavik group has mitigated CARPA response to their synthetic platelets through optimization of infusion rate, concentration, charge, PEG molecular weight (MW) and density, and have also developed in vitro screening tools to predict a therapy’s likelihood of triggering CARPA.12,13,15,39 Formulations that did not activate complement in vitro also avoided complement activation in vivo. However, unexpectedly, hard thrombi were observed in uninjured tissues of animals treated with both control and hemostatic nanoparticles, but not with saline, emphasizing the need for further safety studies with injected nanoparticles.40 To date, CARPA reactions to SynthoPlate™ have not been reported. In addition to reducing blood loss and prolonging acute survival in a lethal, non-coagulopathic porcine femoral artery traumatic bleed model, SynthoPlate™ has also been shown to be well-tolerated without clinically-relevant thromboembolic events after repeated dosing in healthy dogs where thrombocytopenia is common.24,41,42 However, due to the ethical constraints around clinical trials, the standard for demonstrating efficacy in preclinical trauma testing is high. The reader is encouraged to review a recent recommendation on end points for conducting trauma-related clinical trials.6,7
3. RESUSCITATION FLUIDS
3.1. Principles of Fluid Resuscitation
Severe hemorrhage (>30% total blood loss or >1.5 L of blood) results in hemorrhagic shock that requires fluid resuscitation to reduce mortality. The body replaces this lost volume through transcapillary refill, an auto-resuscitative process whereby 500 to 1500 mL of fluid are transferred from the interstitial and intracellular spaces to refill the intravascular space (Figure 3A below shows a breakdown of water in the human body). The fluid is believed to mainly come from skeletal muscle, which accounts for ~40% of total body mass and contains ~5 L and ~15 L of interstitial and intracellular fluid, respectively.43
Figure 3. Principles of fluid resuscitation.

A) Distribution of water between different compartments in the human body. B) Schematic of the Revised Starling principle that leads to fluid filtration across the capillary wall and three scenarios of filtration pressures (normal, transient capillary refill during severe hemorrhage, and reduced fluid filtration during shock). Figure 3A was modified from [78] by Lindsay M. Bigaet al. under the Creative Commons CC BY-SA 4.0 International License. Figure 3B was modified from [46] by Neil Herring and David J. Paterson, Copyright 2018 and reproduced by permission of Taylor and Francis Group, LLC, a division of Informa plc.
Water in the body is moved from compartment to compartment through the tight control of capillary walls and cell membranes. Since fluid resuscitants provide water, salts, and sometimes macromolecules, it is important to understand how the water and other constituents move across compartments. Volume kinetics applies pharmacokinetics fundamentals to analyze and simulate how the volume of intravenously administered fluids are distributed and eliminated in the body. The reader is directed to two excellent reviews that cover the application, math, and clinical implications.44,45
In the body, fluid flows out of the capillary beds into the interstitial space, which then drains into the lymphatic system and is returned to the intravascular space. The inside of blood vessels (intraluminal) is composed of endothelial cells connected by tight junctions and adherens that prevent plasma proteins from passing between cells. The intraluminal surface of endothelial cells is covered in a negatively charged layer of glycoproteins known as the endothelial glycocalyx (GCX), a structure that is crucial to modulating immune recognition, coagulation, and inflammation at the surface of endothelial cells. The GCX also serves as a semipermeable, filtration barrier for plasma proteins that creates a near protein-free ultrafiltrate of fluid that passes between endothelial cells from vasculature to interstitium (Figure 3B above).
The transport of fluid across the vessel wall was classically described by the Starling Equation which was updated after the development of the Michel-Weinbaum GCX model to the following:
The fluid flux, JV, is dictated by two opposing forces, i) the difference between the intravascular pressure in the capillary bed (Pc) and the interstitial space (Pi), which pushes fluid out of the capillary, and ii) the colloid osmotic pressure (COP), which is the difference between the plasma (πP) and the subglycocalyx space (πG) osmotic pressures, and drives water into the capillary. S is the capillary surface area, LP is the hydraulic conductance, or physically, the permeability of the vascular wall to water, and σd is the osmotic reflection coefficient, or physically, the permeability of the vascular wall to solutes.46–48
During normal steady state, pressure drops along the length of the capillary bed and plasma-free fluid is constantly flowing across the capillaries into the interstitial space, with fluid flux decreasing from the arterial to the venous side due to reduced intravascular pressure.
When severe hemorrhage occurs, blood pressure is reduced, coupled with arteriolar vasoconstriction and a decrease in precapillary resistance. Therefore, Pc is sharply decreased on the arterial side of capillaries. As a result, Pc is reduced below COP, leading to transient transcapillary refill that causes fluid to be reabsorbed into the intravascular space. Eventually, the revised Starling equation factors adjust and a steady state fluid flow outwards from the intravascular space to the interstitial returns (albeit at a much lower JV). Severe trauma is associated with sympathoadrenal activation, inflammation, and shedding of the endothelial glycocalyx and leaky capillary barriers, which significantly disrupts these finely tuned Starling forces.49
3.2. Clinical Use of Resuscitation Fluids
There have been many attempts to use non sanguineous resuscitation fluids, including crystalloids (normal saline, lactated ringers, hyperoncotic saline) or colloids (albumin, starches, dextrans, gelatin) for resuscitation of critical illness and injury. While these fluids can provide temporary intravascular volume expansion during resuscitation of shock, they have largely been replaced by blood products for use during hemorrhage due to their negative effects on blood coagulation and association with increased organ failure and mortality in trauma patients.50–53 However, new engineered colloids are in development that may provide new and interesting capabilities.
3.3. PEG-20k as a Novel Low Volume Resuscitant
A single low volume infusion (10% of estimated blood volume) of a concentrated, 10% w/v, 20 kDa PEG (PEG 20k) solution has shown remarkable potential as a new fluid resuscitant.54 The PEG-20k (also called a “low volume resuscitant” or LVR) acts both as a cell impermeant, drawing fluid out of the intracellular space and shrinking parenchymal and endothelial cells to alleviate compression of capillaries, and a traditional colloid increasing intravascular volume to reperfuse tisses.55 The LVR increases the tolerance of a patient’s organs to the low volume state of hemorrhagic shock, similar to organ preservation, improving a patient’s ability to cope with low blood pressure or the hypotensive state. To quantify the effect of a resuscitation fluid on the metabolic tolerance of animals to the low volume state, a metric known as “LVR time” is used. The LVR time reflects the duration of reduced lactate levels that is achieved after LVR administration.
In a lethal, severe hemorrhagic shock rat model, where ~50 – 60% of total blood volume was removed, PEG-20k registered the maximum possible LVR time of 240 minutes, and fully rescued MAP back to baseline levels (single bolus infusion of 10% of estimated blood volume of 10% w/v PEG-20k, dose = ~625 mg/kg).56 In a preclinical porcine model of lethal hemorrhagic shock, PEG-20k had superior survival rates (100%, n=6) at 24 hours compared to whole blood (16.7%, n=6) and Hextend (0%, n=5), all treatments were given via a single bolus infusion of 10% of estimated total blood volume.57 In a lethal, non-coagulopathic hybrid-model of controlled (tail bleed) and uncontrolled (splenic bleed) hemorrhage in rats, PEG-20k had an LVR time of 240 minutes (100% survival), and PEG-20k-treated rats did not show any signs of clot rupturing, even though their average MAP nearly returned to baseline after infusion.54 Additional mechanistic studies revealed that PEG-20k did not affect the extrinsic or intrinsic coagulation pathways, but did disrupt plateletfibrin(ogen) interactions leading to a mild thrombocytopenia.58–61 Measurements of capillary blood flow in rats during resuscitation showed PEG-20k increased blood flow from baseline much more than normal saline.55 To better understand the ratio of PEG-20k in the intravascular space versus the interstitial space, the osmotic reflection coefficient (σd) was measured in rats and found to be 0.65, which means ~65% of the PEG molecules stay in the intravascular space while ~35% migrate to the interstitial space.62 This ratio may be key to the favorable action of PEG-20k by enabling an oncotic gradient moving fluid from cellular to interstitial and intravascular spaces, thus preventing cytotoxic cellular edema. Additional in vivo studies exploring the effect of MW demonstrated PEG-20k and PEG-40k had nearly identical efficacy in a lethal hemorrhagic shock rat model, whereas PEG-8k and PEG-100k, while still increasing the LVR time compared to the saline control, did not perform as well.54 In a mouse biodistribution study, it was reported only ~4% of radiolabeled PEG-20k remained in the mouse at 72 hours post injection.63
3.4. Considerations for future polymer-based LVRs
PEG-based LVRs have highlighted the potential of polymer engineering to open the door to new rationally designed low volume resuscitants. Reversible addition-fragmentation chain-transfer (RAFT) (Figure 4A), atom transfer radical polymerization (ATRP), and ring-opening metathesis polymerization (ROMP) are all controlled polymerization techniques frequently used in drug delivery.64,65 RAFT, ATRP, and ROMP allow the precise control of length (degree of polymerization and MW), composition (statistical or block copolymers, single-unit insertion), dispersity, architecture (linear, comb, radiant star, hyperbranched), degradability (enzymatic, hydrolytic, thermal), stimuli-responsive linkers, and end-group functionalization across a widerange of monomers (neutral, anionic, cationic, zwitterionic, peptides, DNA, sugars).66–72 The field of drug delivery has leveraged these techniques to create polymer vehicles (peptide conjugates, micelles) that can target, circulate, and release therapies to control biodistribution, pharmacokinetics, and degradation in the body (Figure 4B and 4C below).73,74
Figure 4. Overview of the flexibility of common drug delivery vehicles.

A) RAFT allows for the precise control of MW even with complex block copolymers with multiple monomer types. B) Pros and cons of polymeric and lipid nanoparticle systems. C) Different targeting, biophysical, and architecture characteristics used in drug delivery systems. Figure 2A was reprinted from [63] by Nghia P. Truong et al., Copyright 2021 with permission from Copyright Clearance Center: Spring Nature. Figures 2B and 2C was reprinted from [73] by Michael J. Mitchell et al., Copyright 2020 with permission from Copyright Clearance Center: Spring Nature.
Appropriate tailoring of MW of polymers is crucial to balancing half-life, distribution to peripheral tissues, and excretion to control COP while minimizing tissue accumulation which could lead to unintended toxicity and/or tissue edema.75 A distribution study in mice with radio-labeled PEGs of MWs from 3k to 190k indicates circulation half-life increases an order of magnitude from PEG-6k to PEG-20k, and another order of magnitude moving to PEG ≥ 50k, with clearance closely following an inverse relationship to half-life. The urinary clearance of PEG abruptly decreases around ~30k, and liver accumulation increases with MW above a MW of 50k driven by Kupffer cell uptake. The authors concluded PEG-50k exhibited the longest half-life with low organ accumulation.63
Although PEG-based LVRs have shown promise in preclinical testing, there have been recent instances of anaphylaxis from COVID-19 vaccines with PEG as the suspected culprit. Since osmotic pressure is a colligative property (proportional to the number of moles), other neutral, water-soluble polymers will all have similar osmolarities in solution. Poly(vinyl alcohol) for instance has similar biodistribution to PEG, and could potentially be used as an alternative.76 Additionally, the clinical use of fluid resuscitation has revealed that the amount of administered water is important to avoiding tissue edema. Two ways to limit the amount of water administered, are i) to increase the concentration of LVR or ii) increase the osmotic potential of the polymer. Moving away from linear polymers to new architectures can help reduce solution viscosity and increase water holding capacity, which has been shown recently with hyperbranched polyglycerols (HPGs). HPGs have also shown increased biodistribution and improved blood compatibility compared to their linear counterparts of similar MWs.77,78
To increase the osmotic potential, charged polymers (anionic, cationic, zwitterionic) can attract counter ions in solution through the Gibbs-Donan effect, resulting in a non-linear osmotic effect. This is displayed by albumin which has 17 negative charges at physiologic conditions.46 However, cationic polymers are known to bind to negatively charged surfaces (e.g. GCX) and show higher rates of hemolysis. Anionic polymers will be repelled by the GCX, unless appropriately sized, but show rapid kidney accumulation, and can act as a surface to activate FXII in the blood. Zwitterionic polymers display high blood compatibility, without any effect on blood coagulation, and can coordinate higher amounts of water compared to the glycerol units of PEG.77,79 Recently, Kumar et al. functionalized 6 kDa dextran with amine (N)-oxide-based zwitterionic groups (100DS1NOx). The incorporation of zwitterionic groups, doubled the osmolarity of dextran, and created a low-fouling colloid that decreased cell accumulation, showed no adverse effect on coagulation in vitro, and was rapidly cleared via renal filtration and hepatic circulation showing very little organ accumulation. In a lethal, severe hemorrhagic shock rat model 100DS1NOx increases survival time compared to commercial 6% hydroxyethyl starch (HES 130/0.4) and performs similar to plasma (five bolus injections given five minutes apart, dose = 64 mg/kg per bolus [320 mg/kg total]).80
3.5. Conclusions
Prehospital care is vital to ensuring the patient makes it to the ER. In preclinical animal models, fully synthetic intravenous hemostats and LVRs directly treat TIC to stop bleeding and refill the vascular space to reverse hemorrhagic shock, respectively. First-generation therapies continue to highlight design constraints and needs, refine animal models, and elucidate biophysical aspects of clotting. We believe drug delivery platforms provide the design flexibility necessary to continue to innovate in the field of trauma care.
Figure 2. Synthetic intravenous hemostats and low volume resuscitants.
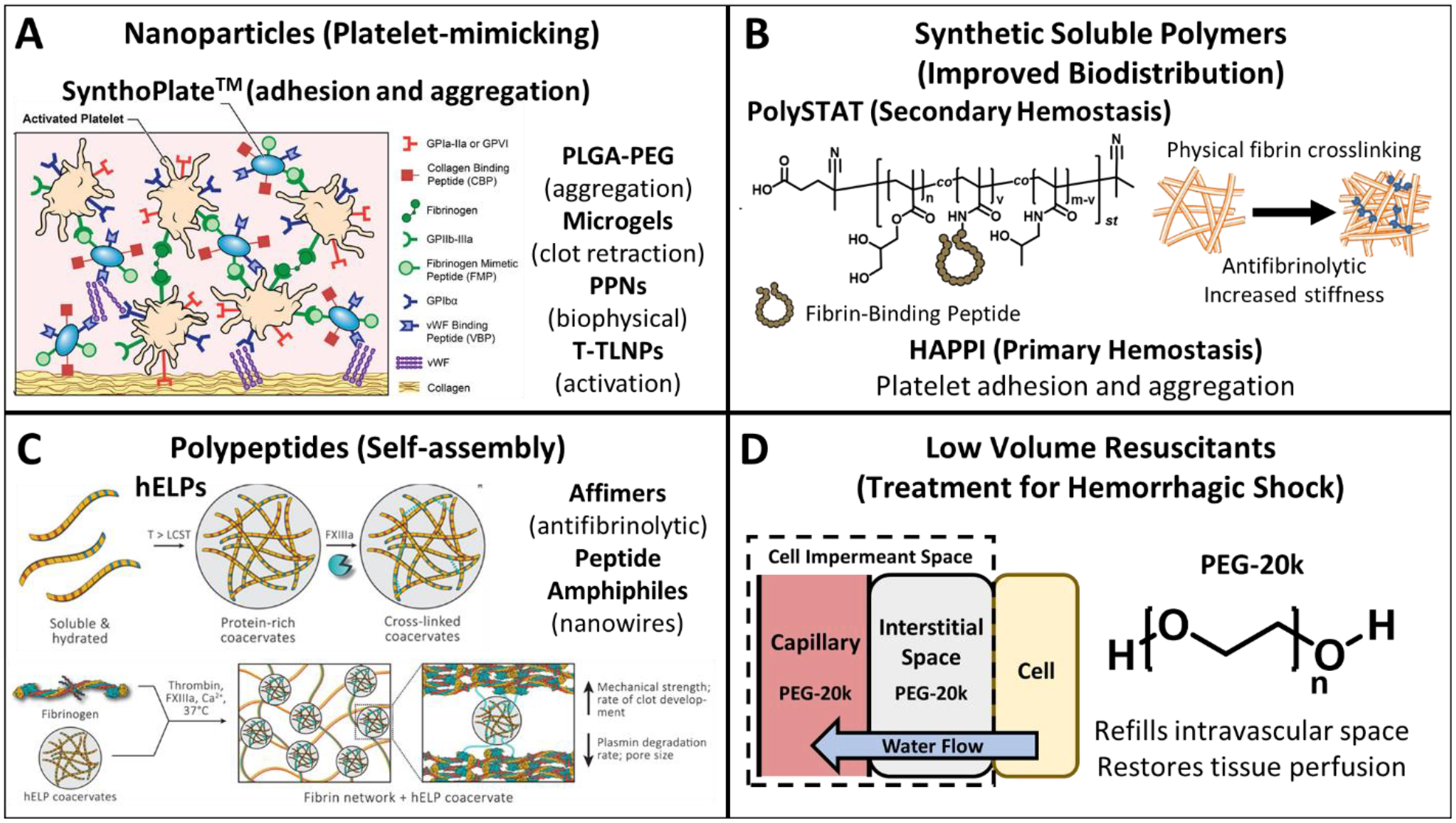
A) Nanoparticle systems recapitulate one or multiple characteristics of platelets - adhesion, aggregation, clot retraction, activation, and biophysical. B) Synthetic soluble polymer systems improve biodistribution (compared to nanoparticles), and target both primary and secondary hemostasis. C) Polypeptides self-assemble at the wound site to increase the mechanical strength of clots or disrupt native enzymatic activity. D) Low volume resuscitants consisting of PEG-20k act as both a cell impermeant and a traditional colloid which drives water from the intracellular space and interstitial space to the intravascular space. Figure 2A was modified from [24] by under the Creative Commons Attribution 4.0 International license. Figure 2C was modified from [33] under the Creative Commons CC BY-NC-ND 4.0 International License.
Funding Sources
This work was supported by NIH 1R01HL139007. TJP was supported by an NSF Graduate Fellowship (DGE-1762114).
Abbreviations
- LVR
low volume resuscitant
- TIC
trauma-induced coagulopathy
- RCT
randomized control trial
- vWf
von Willebrand factor
- FII
coagulation factor II or prothrombin
- FX
coagulation factor X or Stuart-Prower factor
- PLGA
poly(lactic-co-glycolic acid)
- PLA
poly(lactic acid)
- PEG
poly(ethylene glycol)
- DiD
dioctadecyl-3,3,3 3 tetramethylindodicarbocyanine
- GRGDS
glycine-arginine-glycine-aspartic acid-serine
- sdFvs
fibrin-specific variable domain-like recognition motifs
- tPA
tissue-type plasminogen activator
- GPIIb-IIIa
- PPNs
platelet-mimicking procoagulant nanoparticles
- t-TLNPs
thrombin-loaded injury-site-targeted lipid nanoparticles
- HAPPI
hemostatic agent via polymer peptide infusion
- ELP
elastin-like polypeptide
- VPGXG
valine-proline-glycine-“interchangeable amino acid”-glycine
- hELP
hemostatic elastin-like polypeptide
- FXIIIa
activated factor XIII or activated fibrin stabilizing factor
- PA
peptide amphiphile
- CARPA
complement activation-related pseudoallergy
- MW
molecular weight
- GCX
endothelial glycocalyx
- Pc
capillary bed pressure
- Pi
interstitial space pressure
- COP
colloid osmotic pressure
- πp
plasma osmotic pressure
- πG
subglycocalyx osmotic pressure
- S
capillary surface area
- Lp
hydraulic conductance
- σd
osmotic reflection coefficient
- Jv
fluid flux
- PEG-20k
poly(ethylene glycol) of 20,000 g/mol molecular weight
- RAFT
reversible addition-fragmentation chain-transfer
- ATRP
atom transfer radical polymerization
- ROMP
ring-opening metathesis polymerization
- DNA
deoxyribonucleic acid
- HPG
hyperbranched poly(glycerol)
- FXII
coagulation factor XII or Hageman factor
- PLA
poly(lactic acid)
- FSN
fibrin-specific nanogel
- 100DS1NOx
6 kDa dextran with amine (N)-oxide-based zwitterionic groups
- HES (130/0.4)
6% Hydroxyethyl starch 130 kDa with 0.4 molar substitution ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vos T et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 396, 1204–1222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World health statistics 2022: monitoring health for the SDGs, sustainable development goals. https://www.who.int/publications-detail-redirect/9789240051157.
- 3.Kironji AG et al. Identifying barriers for out of hospital emergency care in low and lowmiddle income countries: a systematic review. BMC Health Serv Res 18, 291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon JW Hemorrhagic Shock. N Engl J Med 378, 370–379 (2018). [DOI] [PubMed] [Google Scholar]
- **5.Moore EE et al. Trauma-induced coagulopathy. Nat Rev Dis Primers 7, 1–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of trauma-induced coagulopathy including mechanisms and pathophysiology at the cellular-, tissue-, and organ-level. Clinical data on diagnosis, screening, prevention, and management (prehospital and hospital). A gap assessment and outlook of TIC research.
- 6.Mayer SA et al. Recommended Primary Outcomes for Clinical Trials Evaluating Hemostatic Agents in Patients With Intracranial Hemorrhage: A Consensus Statement. JAMA Network Open 4, e2123629 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Spinella PC et al. Recommended primary outcomes for clinical trials evaluating hemostatic blood products and agents in patients with bleeding: Proceedings of a National Heart Lung and Blood Institute and US Department of Defense Consensus Conference. Journal of Trauma and Acute Care Surgery 91, S19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibner E, Andreae M, Galvagno SM & Scalea T Damage control resuscitation. Clin Exp Emerg Med 7, 5–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Risser F, Urosev I, López-Morales J, Sun Y & Nash MA Engineered Molecular Therapeutics Targeting Fibrin and the Coagulation System: a Biophysical Perspective. Biophys Rev 14, 427–461 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of biophysical mechanisms that enable coagulation with a special focus on fibrinogen, the biophysical properties of fibrin networks, and ways to target it in vivo.
- 10.Luc NF et al. Bioinspired artificial platelets: past, present and future. Platelets 33, 35–47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghunathan S, Rayes J & Sen Gupta A Platelet-inspired nanomedicine in hemostasis thrombosis and thromboinflammation. J of Thrombosis Haemost 20, 1535–1549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onwukwe C et al. Engineering Intravenously Administered Nanoparticles to Reduce Infusion Reaction and Stop Bleeding in a Large Animal Model of Trauma. Bioconjugate Chem. 29, 2436–2447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbard WB et al. Hemostatic nanoparticles increase survival, mitigate neuropathology and alleviate anxiety in a rodent blast trauma model. Sci Rep 8, 10622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P et al. GRGDS-functionalized chitosan nanoparticles as a potential intravenous hemostat for traumatic hemorrhage control in an animal model. Nanomedicine: Nanotechnology, Biology and Medicine 14, 2531–2540 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Maisha N, Rubenstein M, Bieberich CJ & Lavik E Getting to the Core of It All: Nanocapsules to Mitigate Infusion Reactions Can Promote Hemostasis and Be a Platform for Intravenous Therapies. Nano Lett. 21, 9069–9076 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Gkikas M et al. Systemically Administered Hemostatic Nanoparticles for Identification and Treatment of Internal Bleeding. ACS Biomater. Sci. Eng 5, 2563–2576 (2019). [DOI] [PubMed] [Google Scholar]
- **17.Hong C et al. Modulating Nanoparticle Size to Understand Factors Affecting Hemostatic Efficacy and Maximize Survival in a Lethal Inferior Vena Cava Injury Model. ACS Nano 16, 2494–2510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using well-characterized PEG-PLGA-GRDS nanoparticles over multiple size ranges from <100 nm to 500 nm, the researchers demonstrate differences in platelet binding, aggregation, and accumulation in vitro. Followed by differences in biodistribution, tissue accumulation, and survival in vivo with a lethal inferior vena cava puncture rat model. This is the first study to clearly show control of nanoparticle size can be crucial to the efficacy of an intravenous hemostat.
- 18.Brown AC et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nature Mater 13, 1108–1114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsch N, Brown AC, Barker TH & Lyon LA Enhancing clot properties through fibrin-specific self-cross-linked PEG side-chain microgels. Colloids and Surfaces B: Biointerfaces 166, 89–97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandi S et al. Synthetic Platelet Microgels Containing Fibrin Knob B Mimetic Motifs Enhance Clotting Responses. Advanced Therapeutics 4, 2100010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Mihalko EP et al. Fibrin-specific poly(N-isopropylacrylamide) nanogels for targeted delivery of tissue-type plasminogen activator to treat thrombotic complications are well tolerated in vivo. Bioengineering & Translational Medicine 7, e10277 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using tPA-loaded fibrin-specific poly(N-isopropylacrylamide) nanogels (FSNs), the researchers demonstrate the ability to target and release tPA at the site of systemic microthrombi in a mouse model of disseminated intravascular coagulation. They evaluate safety and optimize dose through in vitro assays, histological analysis, biodistribution, and real-time in vivo intravital imaging of coagulation.
- 22.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL & Gupta AS In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials 34, 3031–3041 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Dyer MR et al. Intravenous administration of synthetic platelets (SynthoPlate) in a mouse liver injury model of uncontrolled hemorrhage improves hemostasis: Journal of Trauma and Acute Care Surgery 84, 917–923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickman DA et al. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Sci Rep 8, 3118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **25.Sekhon UDS et al. Platelet-mimicking procoagulant nanoparticles augment hemostasis in animal models of bleeding. Science Translational Medicine 14, eabb8975 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using the phospholipid phosphatidylserine, the researchers demonstrate for the first time a liposome-based platelet-mimicking particle that recapitulates adhesion, aggregation, and the procoagulant function of the platelet surface in vivo. A plasmin cleavable linker is used to hide the negative surface during circulation, then reveal it at the site of bleeding.
- 26.Girish A et al. Platelet-Inspired Intravenous Nanomedicine for Injury-Targeted Direct Delivery of Thrombin to Augment Hemostasis in Coagulopathies. ACS Nano (2022) doi: 10.1021/acsnano.2c05306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan LW et al. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Science translational medicine 7, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamm RJ et al. Peptide valency plays an important role in the activity of a synthetic fibrin-crosslinking polymer. Biomaterials 132, 96–104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamm RJ et al. Optimizing the Polymer Chemistry and Synthesis Method of PolySTAT, an Injectable Hemostat. ACS Biomater. Sci. Eng 6, 7011–7020 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Gao Y et al. A polymer-based systemic hemostatic agent. Science Advances 6, eaba0588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearney KJ et al. Affimer proteins as a tool to modulate fibrinolysis, stabilize the blood clot, and reduce bleeding complications. Blood 133, 1233–1244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash MA Elastin-like polypeptides: protein-based polymers for biopharmaceutical development: Medicinal Chemistry and Chemical Biology Highlights. CHIMIA 76, 478–478 (2022). [Google Scholar]
- 33.Urosev I, Morales JL & Nash MA Phase Separation of Intrinsically Disordered Protein Polymers Mechanically Stiffens Fibrin Clots. Advanced Functional Materials 30, 2005245 (2020). [Google Scholar]
- 34.Morgan CE et al. Tissue-Factor Targeted Peptide Amphiphile Nanofibers as an Injectable Therapy To Control Hemorrhage. ACS Nano 10, 899–909 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Klein MK et al. Development of Optimized Tissue-Factor-Targeted Peptide Amphiphile Nanofibers to Slow Noncompressible Torso Hemorrhage. ACS Nano 14, 6649–6662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Ask A et al. Spotlight on animal models of acute traumatic coagulopathy: an update. Transfusion and Apheresis Science 61, 103412 (2022). [DOI] [PubMed] [Google Scholar]; Review of the current landscape of animal models used to investigate trauma-induced coagulopathy. Identifies some growing consensus of animal models using defined trauma, hemorrhage, shock, and resuscitation periods, along with gaps still present due to model variability, animal physiology differences, and difficulty reproducing TIC pathophysiology.
- 37.Parr MJ et al. Traumatic Coagulopathy: Where are the Good Experimental Models? Journal of Trauma and Acute Care Surgery 65, 766–771 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Velik-Salchner C et al. Normal values for thrombelastography (ROTEM®) and selected coagulation parameters in porcine blood. Thrombosis Research 117, 597–602 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Maisha N et al. Engineering PEGylated Polyester Nanoparticles to Reduce Complement-Mediated Infusion Reaction. Bioconjugate Chem. 32, 2154–2166 (2021). [DOI] [PubMed] [Google Scholar]
- **40.Maisha N et al. PEGylated Polyester Nanoparticles Trigger Adverse Events in a Large Animal Model of Trauma and in Naïve Animals: Understanding Cytokine and Cellular Correlations with These Events. ACS Nano 16, 10566–10580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; While evaluating their PEG-PLA-GRGDS nanoparticles in a porcine polytrauma model, the researchers observed hard thrombi in tissues of uninjured control animals for both the active hemostatic nanoparticles and the unfunctionalized controls (PEG-PLA). In previous studies, these nanoparticles had been optimized to mitigate their CARPA response, and indeed there were no traditional signs of CARPA present in the swine and the hemostatic nanoparticles did lead to a reduction in blood loss. They used data science methods to identify correlations between the thrombi and changes in IL-6, INF-alpha, lymphocytes, and neutrophils. This study highlights some of the challenges translating intravenous hemostats through preclinical large-animal models.
- 41.Insight N Haima Therapeutics Receives Phase II TVSF Award from the State of Ohio to Develop its SynthoPlate Product for Veterinary Use. https://www.pharmasalmanac.com/articles/haima-therapeutics-receives-phase-ii-tvsf-awardfrom-the-state-of-ohio-to-develop-its-synthoplate-product-for-veterinary-use.
- 42.Guillaumin J, Satchell PW, Yaxley PE, Bruckman MA & Gupta AS Safety profile of repeated infusion of platelet-like nanoparticles in healthy dogs. American Journal of Veterinary Research 83, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Dull RO & Hahn RG Transcapillary refill: The physiology underlying fluid reabsorption. Journal of Trauma and Acute Care Surgery 90, e31 (2021). [DOI] [PubMed] [Google Scholar]; Reviews clinical data supporting transcapillary refill, and time course of physiologic changes that occur and lead to fluid reabsorption.
- 44.Hahn RG & Warner DS Volume Kinetics for Infusion Fluids. Anesthesiology 113, 470–481 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Hahn RG Understanding volume kinetics. Acta Anaesthesiologica Scandinavica 64, 570–578 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Herring N & Paterson DJ Levick’s Introduction to Cardiovascular Physiology. (CRC Press, 2018). doi: 10.1201/9781351107754. [DOI] [Google Scholar]
- 47.Woodcock TE & Woodcock TM Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. BJA: British Journal of Anaesthesia 108, 384–394 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Woodcock TE & Michel CC Advances in the Starling Principle and Microvascular Fluid Exchange; Consequences and Implications for Fluid Therapy. Frontiers in Veterinary Science 8, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson P, Stensballe J & Ostrowski S Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Critical Care 21, 25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada MY et al. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. International Journal of Surgery 38, 78–82 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Jones DG et al. Crystalloid resuscitation in trauma patients: deleterious effect of 5L or more in the first 24h. BMC Surgery 18, 93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarychanski R et al. Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A Systematic Review and Meta-analysis. JAMA 309, 678–688 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Kashy BK et al. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 121, 730–739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangino MJ Polyethylene Glycol Polymers in Low Volume Resuscitation. https://apps.dtic.mil/sti/citations/AD1105416 (2020).
- 55.Plant V et al. Low-Volume Resuscitation for Hemorrhagic Shock: Understanding the Mechanism of PEG-20k. J Pharmacol Exp Ther 361, 334–340 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Parrish D, Lindell SL, Reichstetter H, Aboutanos M & Mangino MJ Cell impermeant based low volume resuscitation in hemorrhagic shock: A biological basis for injury involving cell swelling. Ann Surg 263, 565–572 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **57.Khoraki J et al. Superior Survival Outcomes of a Polyethylene Glycol-20k Based Resuscitation Solution in a Preclinical Porcine Model of Lethal Hemorrhagic Shock. Annals of Surgery 275, e716–e724 (2022). [DOI] [PubMed] [Google Scholar]; Using a porcine model of lethal hemorrhagic shock (controlled hemorrhage), the authors show PEG-20k has superior survival (4 hours and 24 hours), LVR time, and restoration of intravascular volume compared to whole blood and hextend. This study showed that LVRs are able to reverse hemorrhagic shock even though there is a large decrease in oxygen-carrying capacity of the LVR treated animals due to significant volume expansion.
- 58.Liebrecht LK et al. Thromboelastographic analysis of novel polyethylene glycol based low volume resuscitation solutions. PLOS ONE 13, e0207147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebrecht LK et al. Effects of a novel low volume resuscitation solutions on coagulation and platelet function. PLOS ONE 14, e0215386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wickramaratne N et al. Acute resuscitation with polyethylene glycol-20k: A thromboelastographic analysis. Journal of Trauma and Acute Care Surgery 87, 322–330 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Bakaltcheva I, Ganong JP, Holtz BL, Peat RA & Reid T Effects of high-molecularweight cryoprotectants on platelets and the coagulation system. Cryobiology 40, 283–293 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Parrish D et al. New Low Volume Resuscitation Solutions Containing PEG-20k. J Trauma Acute Care Surg 79, 22–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaoka T, Tabata Y & Ikada Y Distribution and Tissue Uptake of Poly(ethylene glycol) with Different Molecular Weights after Intravenous Administration to Mice. Journal of Pharmaceutical Sciences 83, 601–606 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Truong NP, Jones GR, Bradford KGE, Konkolewicz D & Anastasaki A A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat Rev Chem 5, 859–869 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Sutthasupa S, Shiotsuki M & Sanda F Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym J 42, 905–915 (2010). [Google Scholar]
- 66.University CM Preparation of Functional Materials - Matyjaszewski Polymer Group - Carnegie Mellon University. http://www.cmu.edu/maty/materials/index.html.
- 67.Moad CL & Moad G Fundamentals of reversible addition–fragmentation chain transfer (RAFT). Chemistry Teacher International 3, 3–17 (2021). [Google Scholar]
- 68.Blosch SE, Scannelli SJ, Alaboalirat M & Matson JB Complex Polymer Architectures Using Ring-Opening Metathesis Polymerization: Synthesis, Applications, and Practical Considerations. Macromolecules 55, 4200–4227 (2022). [Google Scholar]
- 69.Kammeyer JK, Blum AP, Adamiak L, Hahn ME & Gianneschi NC Polymerization of protecting-group-free peptides via ROMP. Polym. Chem 4, 3929–3933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elling BR, Su JK, Feist JD & Xia Y Precise Placement of Single Monomer Units in Living Ring-Opening Metathesis Polymerization. Chem 5, 2691–2701 (2019). [Google Scholar]
- 71.Tanaka J, Archer NE, Grant MJ & You W Reversible-Addition Fragmentation Chain Transfer Step-Growth Polymerization. J. Am. Chem. Soc 143, 15918–15923 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Hakobyan K, McErlean CSP & Müllner M Activating ATRP Initiators to Incorporate End-Group Modularity into Photo-RAFT Polymerization. Macromolecules 53, 10357–10365 (2020). [Google Scholar]
- 73.Ekladious I, Colson YL & Grinstaff MW Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 18, 273–294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell MJ et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20, 101–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adamik K-N & Yozova ID Colloids Yes or No? - a “Gretchen Question” Answered. Frontiers in Veterinary Science 8, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamaoka T, Tabata Y & Ikada Y Comparison of Body Distribution of Poly(vinyl alcohol) with Other Water-soluble Polymers after Intravenous Administration. Journal of Pharmacy and Pharmacology 47, 479–486 (1995). [DOI] [PubMed] [Google Scholar]
- 77.Jawanda M, Lai BFL, Kizhakkedathu JN, Ishihara K & Narain R Linear and hyperbranched phosphorylcholine based homopolymers for blood biocompatibility. Polym. Chem 4, 3140–3146 (2013). [Google Scholar]
- 78.Imran ul-haq M, Lai BFL, Chapanian R & Kizhakkedathu JN Influence of architecture of high molecular weight linear and branched polyglycerols on their biocompatibility and biodistribution. Biomaterials 33, 9135–9147 (2012). [DOI] [PubMed] [Google Scholar]
- 79.Wen J, Weinhart M, Lai B, Kizhakkedathu J & Brooks DE Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials 86, 42–55 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Kumar R et al. Low-Fouling Zwitterionic Polymeric Colloids as Resuscitation Fluids for Hemorrhagic Shock. Advanced Materials 34, 2207376 (2022). [DOI] [PubMed] [Google Scholar]


