Abstract
Specialized subpopulations of CD4+ T cells survey major histocompatibility complex class II–peptide complexes to control phagosomal infections, help B cells, regulate tissue homeostasis and repair or perform immune regulation. Memory CD4+ T cells are positioned throughout the body and not only protect the tissues from reinfection and cancer, but also participate in allergy, autoimmunity, graft rejection and chronic inflammation. Here we provide updates on our understanding of the longevity, functional heterogeneity, differentiation, plasticity, migration and human immunodeficiency virus reservoirs as well as key technological advances that are facilitating the characterization of memory CD4+ T cell biology.
CD4+ T cells play diverse roles in the immune system. Vaccinologists often emphasize their ability to promote class switching, somatic hypermutation and memory differentiation among B cells1,2. CD4+ T cells also provide help to CD8+ T cells by supporting their expansion and differentiation into functional memory T cells3. However, CD4+ T cells are also important effectors, killers and potent communicators, they regulate tissue homeostasis and wound healing and sound the alarm upon microbial invasion4–7. CD4+ T cells also have an essential role in restraining inflammation and constraining adaptive immune responses8. These diverse roles are accomplished by developmental specification events that result in unique CD4+ T cell lineages and differentiation states, which are considered as distinct subsets. The destruction of CD4+ T cells, as observed in untreated human immunodeficiency virus (HIV) infection, highlights the critical role of CD4+ T cells in maintaining functional immunity.
CD4+ T cells principally mediate surveillance through their T cell antigen receptor (TCR) and are restricted to major histocompatibility complex class II (MHC-II) molecules that present peptides of extracellular or phagosomal origin. MHC-II is constitutively expressed by professional antigen-presenting cells (APCs) including dendritic cells, macrophages and B cells. Upon receiving three signals from APCs (cognate antigen through the TCR, co-stimulation through co-stimulatory receptors and cytokines) for full activation, naive CD4+ T cells undergo a proliferative burst and differentiate into various effector subsets that each are tailored to different types of immune mechanisms or infections9–11 (Fig. 1). Most effector cells are short-lived, but a small fraction of T cells form long-lived memory cells that persist long beyond pathogen clearance. Memory CD4+ T cells mount an anamnestic response to reinfections that is quicker and of a higher magnitude than primary responses and can contribute to protective immunity12. We define CD4+ memory T cells as an antigen-experienced population that persists after antigen is presumably absent from the organism. This Review focuses on memory CD4+ T cells that persist after acute stimulations, such as infections or vaccines, with limited discussion of chronic stimulation contexts (tumors, autoimmunity) or memory regulatory T (Treg) cells.
Fig. 1 |. Dynamics of a CD4+ T cell response.
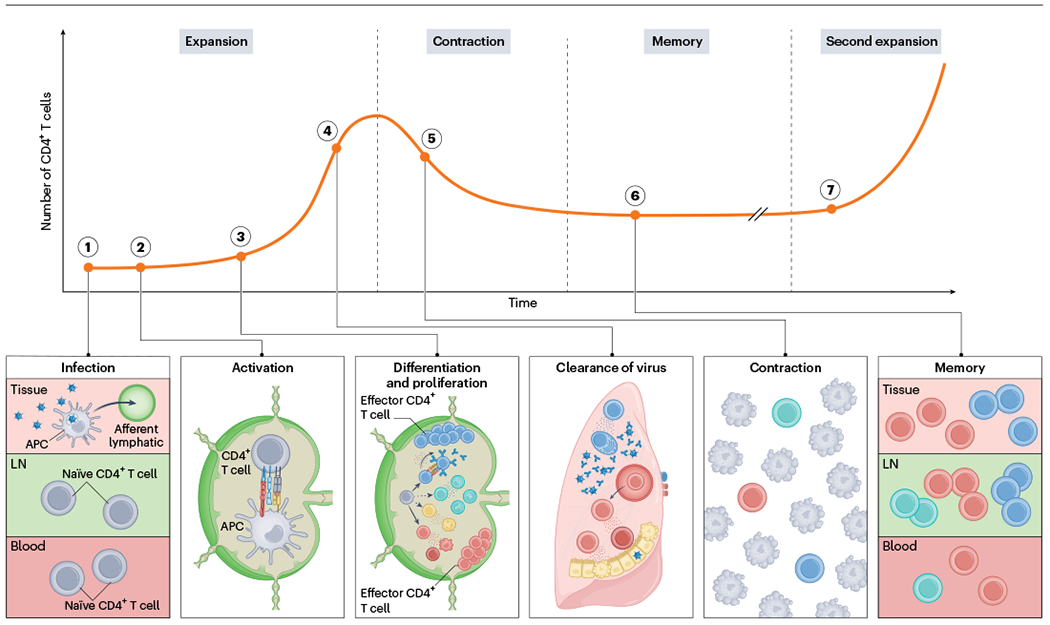
1. Naive CD4+ T cells quiescently recirculate through blood (dark red) and lymphoid (light green) tissues. Upon infection, for example by a respiratory pathogen, APCs migrate from infected barrier sites (magenta) to the draining lymph nodes through afferent lymphatics and present peptides from the pathogen on MHC-II molecules. 2. Recognition of the peptide–MHC-II complex through TCR in combination with co-stimulation and cytokine signals lead to the activation, differentiation and expansion of naive CD4+ lymph node T cells. 3. CD4+ T cells proliferate and differentiate into various effector subsets that each become poised to make specialized contributions to immunity. 4. Many proliferated T cells leave the lymph nodes and migrate to the infected tissue through blood to assist in pathogen control at sites of infection. 5. Once the infection is cleared, most pathogen-specific CD4+ T cells die resulting in contraction of the population. 6. However, a few survive to establish long-lived memory and stay widely distributed across the body. 7. Upon reinfection, memory CD4+ T cells can mount anamnestic responses that are quicker and of higher magnitude than a primary response.
Memory CD4+ T cell longevity
In humans, memory CD4+ T cells longevity is best documented after viral infections or replicating vaccines. The most informative scenarios are those in which the pathogen in question is unlikely to be encountered naturally, so that memory T cells would not have an opportunity to be boosted. In a defining study, blood samples were assessed for interferon-γ (IFNγ)-producing CD4+ T cells upon in vitro restimulation with ‘smallpox vaccine’-derived overlapping peptides using an ELISpot assay13,14. Memory CD4+ T cells could be detected 75 years after vaccination with an estimated t1/2 of 8–12 years. Memory CD4+ T cells can be detected for at least 34 years after measles vaccination, and measles is rarely encountered in societies with high vaccination rates that achieve herd immunity15. Total mumps-specific CD4+ T cell immunity to measles, mumps and rubella (MMR) vaccination lasts at least 21 years16. More recently, memory CD4+ T cells were reported to persist for at least 11 years after severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) infections, 2 years after Zika virus, and several months after SARS-CoV-2 infection or mRNA vaccination17–20.
Even with these landmark reports, we still have limited clinical information on memory CD4+ T cell longevity, despite the importance of the question. Complicating a general consensus, mouse studies suggest that CD4+ T cell memory may be less stable over time compared to CD8+ T cell memory21. Challenges in defining memory CD4+ T cell longevity may relate to the historic difficulty in quantifying antigen-specific T cells, the need for longitudinal or cross-sectional samples, the time it takes to acquire longitudinal samples and, in humans, the need to focus on infections that are not often reencountered. This latter issue arises, for example, when assessing the longevity of pertussis-vaccine specific immunity. While CD4+ T cell memory appears to persist for at least 5 years, periodic environmental exposures are likely22. Of note, all the above-mentioned longevity studies rely on cytokine secretion from peripheral blood mononuclear cells restimulated with peptides or virus to quantify the pathogen-specific memory CD4+ T cells. When considering the nature of certain memory CD4+ T cell subsets, this approach has its limitations and can only detect a small fraction of the pathogen-specific CD4+ T cell population. However, recent technological advances broaden the available tools to generate a more complete picture of the memory CD4+ T cell response.
Tools to study memory CD4+ T cells
Measuring pathogen-specific antibody titers has been feasible for about a century23. Quantifying antigen-specific T cell immunity is a recent innovation and rendered much more difficult due to MHC-restricted mechanisms of antigen detection, breadth of peptide epitopes that vary by MHC-II polymorphism and relevance of lymph nodes and tissue-localized T cells that are not represented within more easily sampled blood.
The development of the ELISpot assay in the 1980s allowed the detection of pathogen-specific restimulated memory CD4+ T cells24. However, human memory CD4+ T cell subsets, which are limited cytokine producers, are poorly detected with this technique. Moreover, analyses are usually restricted to blood samples, which may underrepresent important subsets. Sampling of lymph nodes through fine-needle aspiration or tissues through biopsies has become increasingly common. In combination with a recently developed cytokine-independent approach to identify pathogen-specific memory CD4+ T cells, these advances facilitate the detection of a broader variety of CD4+ memory subsets25. The ‘activation-induced marker’ (AIM) assay takes advantage of the fact that OX40 and CD25 are specifically upregulated upon antigen stimulation and is especially useful in situations where the exact CD4+ T cell epitope is unknown. In such cases, for example, during the emergence of SARS-CoV-2, T cells can be restimulated with peptide megapools covering the whole breadth of possible epitopes26. A disadvantage of the AIM assay is that reactivation of memory CD4+ T cells induces transcriptional and phenotypic changes and thus impairs the characterization of long-lived memory CD4+ T cells in a quiescent state.
TCR-transgenic mice, in which monoclonal T cells express a TCR specific for an epitope of interest, have served as a valuable tool. Adoptive cell transfer of naive TCR-transgenic T cells allows tracking of pathogen-specific CD4+ T cell responses without the need for restimulation27. Curiously however, TCR-transgenic CD4+ T cells often fail to model important aspects of ‘normal’ polyclonal endogenous responses and do not recapitulate the breadth and heterogeneity of effector and memory CD4+ T cells28–30. Specific TCR-transgenic cells may exhibit intrinsic subset differentiation biases (OT-II and SM1 cells may be biased toward follicular helper T (TFH) cells, whereas TEa and SMARTA cells may be biased toward the TH1 subset of helper T cells) or differ from polyclonal endogenous populations with respect to longevity.
MHC-II-restricted tetramers enable the direct detection of endogenous polyclonal CD4+ T cells, without the need for restimulation and functional assays27,31. Tetramer reagents are composed of four monomeric biotinylated MHC-II molecules, which are oligomerized using fluorescent streptavidin and loaded with a peptide of interest. Of importance is the avidity afforded by the polyvalency of the reagent, as monomeric MHC-II–peptide complexes fail to identify epitope-specific T cells32. In contrast to MHC-I-restricted tetramers for CD8+ T cells, MHC-II-restricted tetramers have low sensitivity, detecting only 5–30% of total responding CD4+ T cells to a given antigen33. Possible explanations include decreased peptide–TCR affinity of CD4+ T cells compared to CD8+ T cells and/or decreased affinity of the co-receptor CD4+ to the MHC-II protein in comparison to the CD8–MHC-I interaction. The detection of low-affinity CD4+ T cells can be improved by further increasing the valency, as demonstrated by dodecamers34. Recently developed tetramers contain MHC-II molecules engineered for enhanced CD4 binding, allowing up to a fourfold increase in sensitivity of detecting antigen-specific CD4+ T cells35. An important advantage of tetramers over AIM assays is that tetramer-binding CD4+ T cells can be enriched for using magnetic beads, which facilitates detection of rare cell populations27.
With the rise of next-generation sequencing, new possibilities to identify epitope-specific CD4+ T cells will emerge. Algorithms for predicting dominant MHC-II-restricted epitopes contained with protein antigens, such as the Immune Epitope Database (IEDB; https://www.iedb.org/), have improved enough to be reasonably accurate. New approaches attempt to go even further by predicting epitope specificity based solely on TCR sequencing. This is based on structural predictions and may also leverage sequencing similarity of previously characterized clones36. As the databases of known epitopes and TCRs expand, the technology should only get more powerful. We anticipate that these newly developed tools will further enable research on CD4+ T cells.
Differentiation and memory
Following activation of naive CD4+ T cells, differentiation is informed by the nature of the pathogen and associated innate alarm signals9,11. Type 1 immune responses are elicited by intracellular infections that induce the expression of the transcription factor T-bet in CD4+ T cells, and subsequent TH1 cell differentiation through interleukin (IL)-12-mediated and IFN-γ-mediated STAT1 and STAT4 signaling (Fig. 2). TH1 cells are characterized by their capacity to secrete IFNγ. Type 2 immune responses are triggered by extracellular parasites, certain allergens, or weak immunogens that promote Gata3-expressing TH2 cells that are functionally defined by their ability to synthesize IL-4, IL-5 and IL-13 through IL-4-induced STAT6. Type 3 immune responses develop in response to fungal and extracellular bacterial infections and are driven by induction of the transcription factor RORγt, which promotes TH17 differentiation. TH17 cells secrete IL-17 through STAT3 signaling triggered by IL-6, IL-23, IL-1β or transforming growth factor-β. Type 1 cytokines promote CD8+ T cell and macrophage responses, type 2 cytokines attract mast cells, basophils and eosinophils, and type 3 responses preferentially induce the influx of neutrophils to the focus of inflammation (Fig. 2)9,11.
Fig. 2 |. CD4+ T cell differentiation is tailored to specific classes of immunogens.
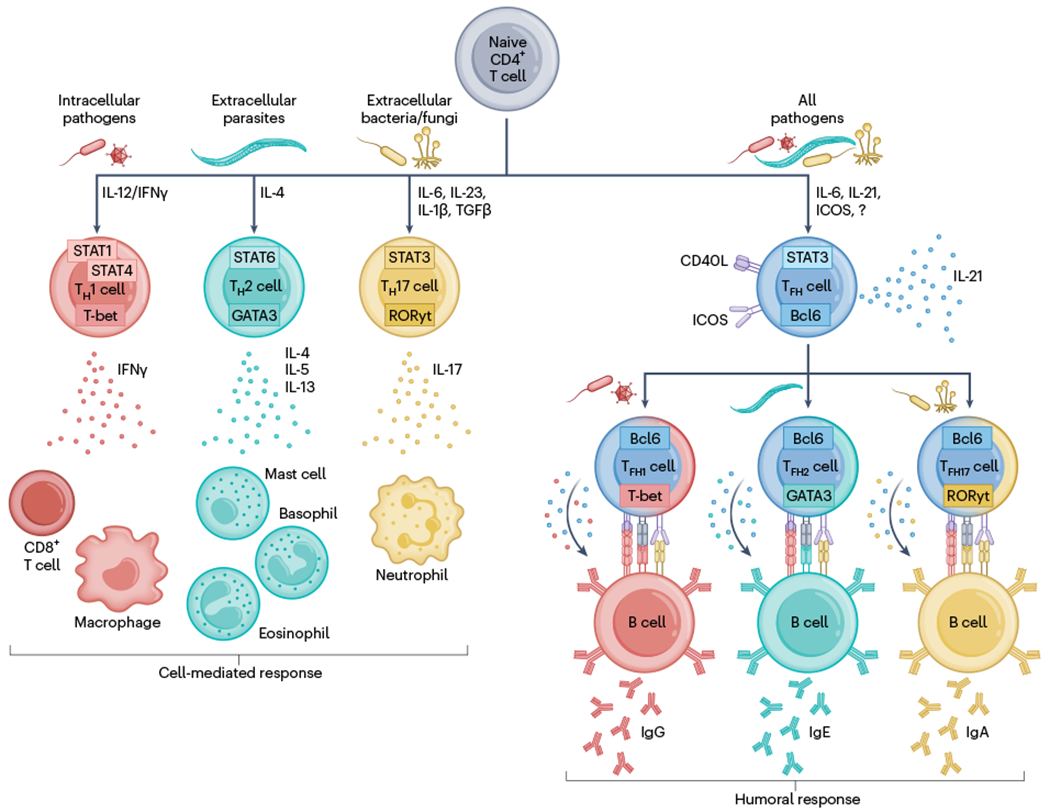
Naive CD4+ T cells differentiate into specialized effector subsets that support cell-mediated or humoral responses. Intracellular pathogens induce T-bet-expressing T helper (TH) 1 cells that secrete IFNγ to potentiate CD8+ T cell and macrophage responses. Extracellular parasites induce GATA3-expressing TH2 cells that secrete IL-4, IL-5 and IL-13 to recruit and activate mast cells, eosinophils or basophils. Extracellular bacteria or fungi trigger the formation of RORγt-expressing TH17 cells that produce IL-17 to trigger neutrophil responses. In parallel, immunogens induce Bcl6-expressing TFH cells that receive help from B cells through ICOS and in turn promote humoral responses by providing CD40L and cytokines to B cells through direct cell-to-cell interaction, triggering isotype switching, affinity maturation and differentiation into memory or antibody-secreting cells. TFH cells take on some characteristics of their cell-mediated response counterparts, expressing low levels of T-bet, GATA3 or RORγt, leading to biased secretion of IFNγ, IL-4 or IL-17 and skewing the antibody response toward specific isotypes.
Type 1, 2 and 3 responses are all associated with parallel differentiation of Bcl6-expressing TFH cells that help B cells and fine-tune humoral effector mechanisms (Fig. 2)2. TFH cells are induced in a two-step differentiation process37. Firstly, dendritic cells induce expression of CXCR5 on activated CD4+ T cells, allowing ‘pre-TFH’ cells to sense CXCL13 and to migrate to the T cell–B cell border of secondary lymphoid organs (SLOs). The signals that initiate the TFH cell program are still under investigation, but they include STAT3 signaling likely induced through IL-6, IL-21 and the co-stimulatory receptor inducible T cell co-stimulator (ICOS)1. Secondly, further stimulation by B cells reinforces the TFH program and enables their migration into germinal centers located in B cell follicles. Here, TFH cells select pathogen-reactive B cell clones to promote plasma cell and memory B cell differentiation, and long-lived antibody responses1. Germinal center B cells and their progeny with the highest affinity for antigen receive the most sustained TFH cell help, thus TFH cells promote affinity maturation1. TFH cells provide help to B cells through IL-21 secretion and cell-contact-dependent co-stimulation through CD40L, which interacts with CD40 on B cells and in return receive help through ICOS, which interacts with ICOSL on B cells10. Like non-TFH cells, TFH cells take on flavored characteristics based on the nature of the pathogenic insult and associated innate response mechanisms38. For this reason, a revised nomenclature has been proposed to refer to the three flavors of TFH cells as the TFH1, TFH2 or TFH17 subset38. All three TFH flavours promote some IgG. However, while TFH1 cells predominantly promote the generation of IgG responses,TFH2 cells can induce isotype class switching toward IgE and TFH17 cells are associated with antibody-skewing toward an IgA isotype38. This nomenclature is not rigid and may be influenced by the environment. For example, in response to lung influenza infection, TFH1 cells may also promote local IgA through IL-21 secretion to local B cells39,40.
The intrinsic factors or extrinsic cues that guide differentiation into TFH versus non-TFH effector cells have not been fully elucidated. Data suggest that this decision is initiated within the first two cell divisions37. Some findings have indicated a link between TH1 versus TFH cell differentiation and cumulative TCR signal strength29,41–50. Early in vitro work showed that TCR-transgenic cells stimulated with low antigen doses preferentially produce IL-4, which was associated with a TH2 response51–53. High antigen doses triggered IFNγ production, and thus TH1 differentiation. Yet, very high doses induced IL-4 production51,52. Several in vivo studies reported that cells receiving the strongest TCR signal preferentially differentiate into TFH cells29,43,46. However, others found that TFH cells can be induced by weak TCR signals, and that strong TCR stimulation preferentially induces TH1 cell differentiation41,42,44,45,47,48. The variable results might be explained by the use of different experimental models, antigen doses and persistence, and myriad other variables, so a clear picture has not yet emerged. Cues may be multifactorial, including exposure to distinct cytokine microenvironments or dendritic cell subsets, although a recent study found no evidence for the latter44. There may also be intrinsic regulation of diversity (for example, asymmetric division) contributing to the TFH and non-TFH effector fate54. A recent model suggests that the decision is centered around IL-2 signaling55.
Once pathogens are eliminated, most effector CD4+ T cells undergo cell death. However, ~5–10% of antigen-specific CD4+ T cells persist and form memory cells. In contrast to naive CD4+ T cells, memory CD4+ T cells do not require stimulation through MHC-II for survival, are more abundant for a given antigen and elicit an anamnestic response that is quicker and higher in magnitude12,56. Several mouse studies implied that memory CD4+ T cell numbers might be less stable over time compared to memory CD8+ T cells, or that certain subsets within the CD4+ T cell memory compartment are less stable21,57,58. However, this might depend on the nature of the pathogen in question rather than a generalizable phenomenon, as studies in humans indicate that while memory CD4+ T cells are less stable than their CD8+ T cell counterparts in response to measles vaccination, the opposite is true for smallpox vaccination13–15.
Memory CD4+ T cells in secondary lymphoid organs
In line with previously identified memory CD8+ T cell subsets, the CD4+ T cell memory compartment has traditionally been subdivided into CD62L+CCR7+ central memory T (TCM) cells and CD62L−CCR7− effector memory T (TEM) cells59. TEM cells are further partitioned into TH1, TH2 or TH17 memory cells and have been well described in mice and humans14,57,60–64. These populations are poised to secrete effector cytokines rapidly upon reactivation and foster enhanced secondary responses to reinfections. TCM cells are functionally defined by their ability to synthesize IL-2 and have less potential for rapid IFNγ or IL-4 secretion59. TCM cells also exhibit enhanced proliferation potential and provide a less differentiated backup reservoir for TEM cells. It has been debated whether TFH cells persist as a distinct memory cell subset, and what role memory TFH cells might play in humoral immunity65.
Addressing TFH cell memory has been hindered by phenotypic commonalities between TFH cells and TCM cells, including expression of TCF1, ICOS, Stat3 and ID3, and the apparent gradual waning in expression of the TFH cell hallmark proteins CXCR5 and PD-1 (refs. 57,66–69). Reactivation of primary memory CD4+ T cells results in accelerated antibody responses compared to naive mice and the formation of secondary TFH cells70. This suggested that TFH effector cells could feed into the TCM cell pool and, upon reactivation, differentiate into secondary TFH effector cells. Several differentiation schemes feature either TCM or TFH cell memory but often do not include both populations within the same model as two distinct subsets66,71,72 (Fig. 3). Increasing evidence supports the concept of memory TFH cells as a distinct memory population, and recent studies in mice identified the simultaneous presence of both TCM and TFH memory cell populations with distinct transcriptional programs (Fig. 3)28,68,69,73–75. Using a nanobody that prevents NAD-induced cell death during isolation from tissues, TFH cells were shown to persist for >400 d after an acute lymphocytic choriomeningitis (LCMV) infection, well after antigen is putatively cleared28. Here, long-lived TFH cells maintained elevated expression of CXCR5 and PD-1 compared to non-TFH cells (but lower than TFH effector cells) and high expression of a receptor that is also expressed during the effector phase, the folate receptor FR4 (ref. 76). FR4hi memory TFH cells are also generated upon Listeria monocytogenes infection or mRNA vaccination, but for unclear reasons are absent in TCR-transgenic memory CD4+ T cell populations28,77.
Fig. 3 |. Memory CD4+ T cell heterogeneity and ontogeny.
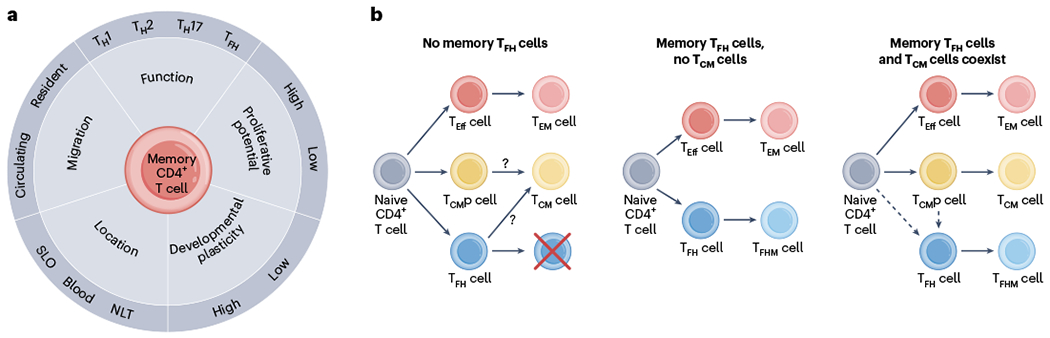
a, Memory CD4+ T cells exhibit heterogeneity in many parameters, including function, migration, location, developmental plasticity and proliferative potential. This makes categorization of defined subsets challenging. b, Existing memory CD4+ T cell differentiation schemes. Left, excludes memory TFH cells. Middle, specifies memory TFH cells, but excludes the concept of TCM cells. Right, includes both memory TFH cells and TCM cells. TFH cells might differentiate directly from activated naive CD4+ T cells, or come from a common TCM cell precursor.
Nevertheless, the relationship between precursor TCM cells, TCM cells with TFH potential, TFH effector cells and memory TFH cells is far from clear. During CD4-dependent extrafollicular B cell responses initiated at the T cell–B cell border, CD4+ T cells that express intermediate levels of CXCR5 during an effector response have been referred to as TFH cells29,78. However, after LCMV infection, CXCR5+ cells include precursor CCR7+ TCM cells that depend on the transcription factor Thpok and are transcriptionally distinct, but closer to TH1 cells than TFH cells69. At a memory timepoint, CCR7+CD4+ T cells often express CXCR5, but in certain contexts can also be found within the CXCR5− fraction79. Transfer of CXCR5intCCR7+ cells 10 d after infection into naive mice revealed preferential homing to T cell zones, persistence, retention of a TCM cell phenotype and IL-2 production upon reactivation, thus meeting previous definitions of TCM cells, despite expression of CXCR5 (ref. 57). CXCR5 is a target of Bcl6, and Bcl6-deficient CD4+ T cells fail to generate CXCR5-expressing effectors at the peak of the response80. Bcl6 acts as a transcriptional suppressor and competes with Blimp1 for the induction or suppression of TFH and non-TFH programs81,82. That said, initiation of CXCR5 expression early in the immune response can be independent of Bcl6, and CD4+ T cells deficient for both Bcl6 and Blimp1 were unexpectedly able to form CXCR5+ T cells; however, they did otherwise lack much of the stereotypic TFH cell gene expression program73,80,83. Human circulating CXCR5+ memory TFH1 cells, but not circulating memory TFH2 or TFH17 cells were shown to be poor promoters of B cell responses84,85. Thus, CXCR5 expression is not the sole defining criterion for functional TFH cells, but resolving phenotypic and ontogenetic relationships remain challenges for the field.
Serum antibody is maintained by long-lived plasma cells in the bone marrow, which persist independently of CD4+ T cell help86. Therefore, a legitimate question is what are the memory TFH cells needed for. Evidence suggests that memory TFH cells might provide survival signals to long-lived plasma cells that localize in organs other than the bone marrow under homeostatic conditions28. Or upon reinfection, memory TFH cells might accelerate differentiation of memory B cells into plasma cells or promote secondary germinal center reactions70,87,88. Better characterization of where memory TFH cells localize and which cell subsets are in close proximity might better inform function. Speculatively, high expression of CXCR5 in combination with absence of CCR7 expression might allow some memory TFH cells to stay positioned within the B cell follicle or colocalize with memory B cells in the subcapsular sinus89,90. The existence of antigen-specific memory TFH cells in human SLOs has not been reported yet. While germinal centers in humans can persist for up to a year, the same microstructure is generally shorter-lived in mice, or persisting germinal centers seemingly change their antigen specificity over time91. This makes it more difficult to discriminate late effector TFH cells from bona fide memory TFH cells in the human setting. Perhaps sequencing of measles-specific and smallpox-specific memory CD4+ T cells from vaccine-draining lymph nodes will allow assessment of the persistence of human antigen-specific long-lived TFH cells in the likely absence of natural boosting, as has recently been executed for smallpox-specific memory B cells92. Lastly, it should be noted that the distinction of TEM, TCM and memory TFH cells underrepresents the heterogeneity of the CD4+ memory compartment in SLOs, as these three major subsets can be further subdivided. We discussed heterogeneity solely through the prism of functional and phenotypic attributes, but heterogeneity can be further expanded to different axes including localization, migration properties, proliferation potential and developmental plasticity (Fig. 3).
Developmental plasticity of memory CD4+ T cells
Epigenetic analyses revealed that memory TH1 cells exhibit increased IFNγ, but not IL-4 promoter accessibility, whereas the opposite was found for memory TH2 cells93. Although culturing in vitro differentiated TH2 cells in TH1 conditions can upregulate T-bet and IFNγ, ex vivo isolated effector memory TH2 cells display little plasticity93. In contrast, TH17 cells appear to have a less-fixed epigenetic state than memory TH1 or TH2 cells, potentially allowing differentiation into IFNγ-secreting TH1 cells94. Indeed, memory TH17 cells produce both IL-17 and IFNγ in nasal tissue upon reinfection with Streptococcus pyogenes95. Additionally, memory TH17 cells express stem-associated proteins like CD27 and TCF1, implying a less differentiated state61.
CD27 and TCF1 are also expressed on TCM cells, albeit not as highly as on TFH cells28,67,69. After LCMV infection, memory Ly6C−PSGL1+CD4+ T cells, which contain TCM cells, show greater expansion upon reactivation than Ly6C+ TEM cells, and generate both Ly6C− and Ly6C+ effector T cells67. When the Ly6C−PSGL1+CD4+ T cells and Ly6C−PSGL1− memory TFH cells were adoptively transferred and raced against each other, both generated similar numbers of TH1 cells and precursor TCM cells, but memory TFH cells were more efficient in generating secondary TFH effectors28. Influenza-specific memory TFH cells also exhibit exceptional plasticity65. In conclusion, memory TFH cells appear to retain considerable developmental plasticity and enhanced potential to produce TFH cell progeny.
Maintaining developmental plasticity within a response may be important. In the case of tuberculosis (TB), IFNγ-producing TH1 cells are crucial for providing protection96. To improve the existing Bacillus Calmette–Guerin (BCG) vaccine, a modified vaccinia Ankara vector expressing a TB antigen was generated97. Despite the successful establishment of memory TH1 cells, the clinical trial showed that the vaccine does not elicit any protection98 and left unclear if this was a failure of concept or execution. A mouse study showed that memory-like TH1 cells get efficiently reactivated but disappear quickly upon adoptive transfer and subsequent TB infection, indicative of a short-lived population99. In contrast, memory-like PD1+ICOS+CD4+ T cells seemed to persist longer, exhibited increased developmental plasticity and provided superior protection. One interpretation of these data is that an ideal TB vaccine would generate a balanced, heterogenous memory compartment consisting of terminally differentiated memory TH1 cells and memory CD4+ T cells that maintain developmental plasticity and proliferative capacity to optimally respond to TB over a prolonged period.
Memory CD4+ T cells in nonlymphoid tissues
Memory CD4+ T cells can be segregated by their migration properties. Under homeostatic conditions, TCM cells recirculate through blood, lymphatics and SLOs, whereas TEM cells are mostly restricted to the blood and upon reactivation preferentially migrate to inflamed tissues59. Based on their transcriptional profile, the majority of antigen-specific memory TFH cells within SLOs appear to be resident28. Nonlymphoid tissues (NLTs) are surveilled by abundant populations of memory CD4+ T cells, comprising largely tissue-resident memory T (TRM) cells but also equilibrating populations6,100–106 (Fig. 4). CD4+ TRM cells share properties with CD8+ TRM cells, including downregulation of the transcription factor KLF2 and the tissue egress molecules S1PR1 and CCR7, and upregulation of CD69 (refs. 6,107).
Fig. 4 |. Immunosurveillance by CD4+ T cells.
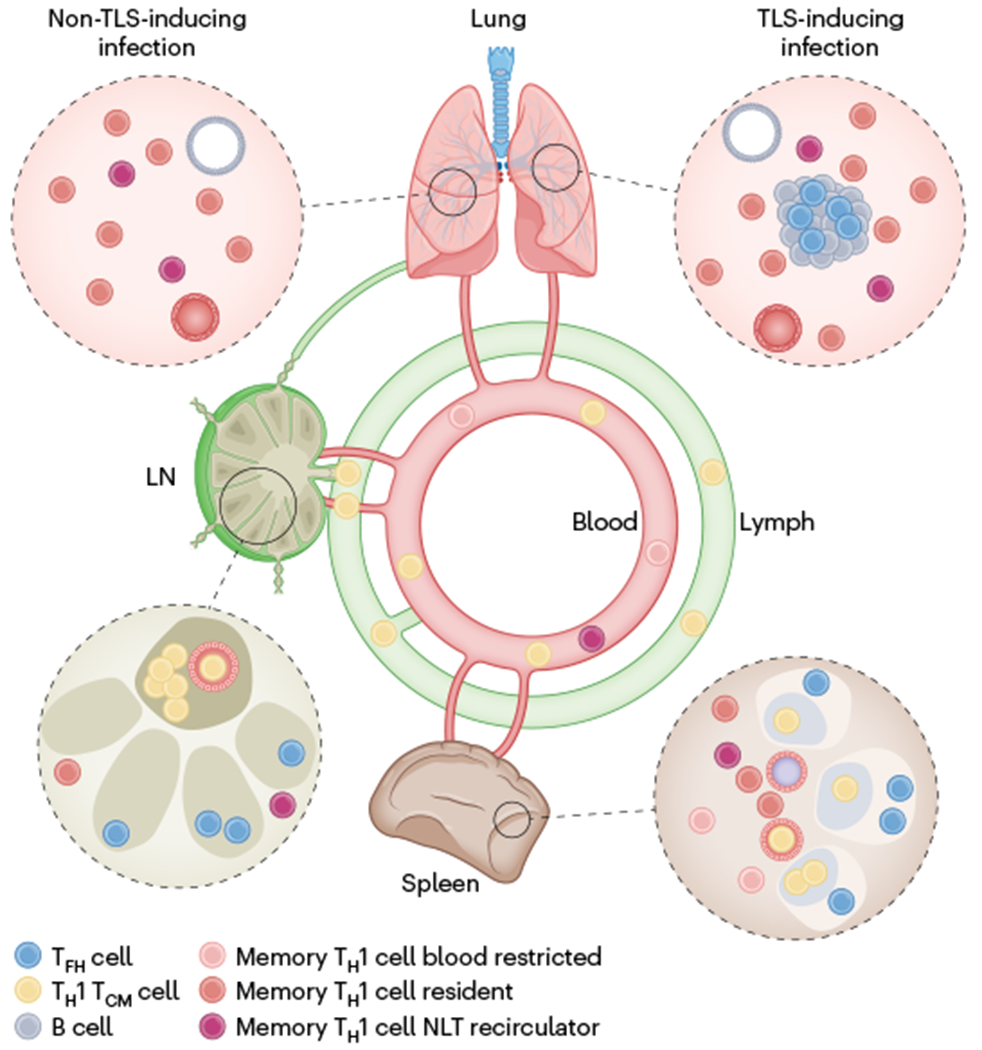
Memory CD4+ T cell immunosurveillance strategy after a respiratory viral infection that elicits a type I response. Although we focus on memory TH1 cells, both memory TH2 and memory TH17 cells have been well documented to take up permanent residence in NLTs. In contrast to TH1 TCM cells, which express CD62L, many memory TH1 cells cannot access lymph nodes (LN) through high endothelial venules. These memory TH1 cells in lymph nodes might represent TH1 cells that recirculate constitutively through NLTs and enter lymph nodes through afferent lymphatics (‘backdoor entry’) from upstream NLTs, or SLO-resident memory TH1 cells. Memory TH1 cells in the blood comprise blood restricted cells, NLT recirculators and TCM cells. FR4hi memory TFH cells populate SLOs and may reside at the outer boundaries of B cell follicles. TFH-like CD4+ TRM cells have been identified in lungs of influenza-infected mice. In contrast to resident TH1 cells, resident TFH cells seem locally restricted to TLSs.
CD4+ TRM cells in mice have been described in numerous tissues, including lung, skin, female reproductive tract, salivary glands, small intestine, large intestine, liver, kidneys, bone marrow and nasal tissue among others6,101,108–110. CD4+ TRM cells are generated in numerous infection modalities such as viral, bacterial, fungal, helminth or parasitic infection, or vaccination settings such as live attenuated vaccines, inactivated vaccines, acellular vaccines or mRNA vaccines, and can play protective roles77,105,109,111–120. CD4+ TRM cells are also engaged in pathogenic roles in autoimmune diseases such as asthma, inflammatory bowel disease or glomerulonephritis121–123. In humans, CD4+ TRM cells have been shown to persist for years in the small intestine, lung and liver upon organ transplantation124–126. Identification of human TRM cells in non-transplant situations is difficult and relies on surface markers, most prominently CD69. Of note, CD69 is an imperfect marker because in mice, parabiosis experiments revealed the existence of CD69− TRM cells and CD69 is also an early T cell activation marker127–129. Nevertheless, transcriptional profiling of human CD69+CD4+ T cells from tissues identified a partial overlap with mouse CD8+ TRM cells107. Combined TCR and single-cell RNA sequencing revealed expanded CD4+ T cell clones uniquely present in NLTs, providing further opportunities to refine TRM cell signatures130.
In contrast to CD8+ TRM cells, CD4+ TRM cells often lack expression of CD103 (ref. 6). CD103 facilitates interaction with E-cadherin-expressing epithelial cells and promotes T cell adhesion in epithelial layers. Ectopic expression of Runx3 in CD4+ T cells increased CD103 expression and preferential localization to epithelial layers in the skin and small intestine131. Thus, CD4+ TRM cells can adapt the Runx3-CD103 residency program, but why it is mainly restricted to CD8+ TRM cells is unclear. Indeed, epithelial cells in various NLTs including lungs, small intestine or skin can express MHC-II132–135. In a Streptococcus pneumoniae infection model, absence of MHC-II expression in lung epithelial cells resulted in a redistribution of CD4+ TRM cells and dysregulated barrier immunity upon reactivation135.
CD4+ TRM cells take on TH1, TH2 or TH17 effector functions, recruit and support various innate and adaptive cells at sites of infection and perform sensing and alarming functions upon reactivation6,7,30,79,105,109,111,113,117,121,136,137. But they do more than that. For example, CD4+ TRM cells can be involved in tissue remodeling via secretion of amphiregulin or can induce mucus metaplasia and airway hyperresponsiveness in an allergic asthma model114,121,138. However, compared to CD8+ TRM cells, CD4+ TRM cells are still understudied due to technical challenges.
An FR4+CXCR5+ TFH-like TRM cell population that promotes local antibody and CD8+ TRM cell responses through IL-21 secretion was identified in lungs of influenza-infected mice30,137,139. TFH-like TRM cells preferentially localize near B cells within inducible bronchus-associated lymphoid tissues, whereas TH1-like TRM cells are positioned at the outer boundaries of these structures30. Thus, the formation of TFH-like TRM cells in NLTs upon acute stimulations might require induction of tertiary lymphoid structures (TLSs). In humans, SARS-CoV-2-specific CD4+ TFH-like cells have been identified in lungs; however, their stimulation history is unknown140. Circulating IgG antibodies exudate into tissues and mucosal compartments. Nevertheless, local memory TFH cells might be important to induce rapid plasma cell differentiation upon reinfection to increase local antibody titers or fine-tune tissue-specific antibody responses, such as promoting dimeric IgA within mucosae141,142. Moreover, blood-borne antibodies poorly access some sites under homeostatic conditions, including olfactory tissues143. However, infections and vaccination with mucosal adjuvants can establish humoral protection of the olfactory epithelium through locally produced antibodies that are dependent on CD4+ T cell help. Conceptually, the decentralization of cellular immunity from SLOs to NLTs has become well established144–146. Identification of TFH-like TRM cells and resident memory B (BRM) cells in NLTs provides evidence that decentralization of immunity extends to the humoral arm as well.
Secondary lymphoid organ CD4+ TRM cells
Memory CD4+ T cells also take up permanent residence in SLOs. Data from human lymph nodes revealed that a surprisingly large proportion (~70%) of CD4+ T cells express CD69 without downregulating IL-7R, indicative of a resting memory population147. Using photoconvertible proteins, it was shown that 7 d after photoconversion, a substantial fraction of antigen-experienced CD4+ T cells in lymph nodes and Peyer’s patches retained the label, indicative of a resident population148. Tracking of antigen-specific memory CD4+ T cells using either a photoconvertible system or parabiosis further confirmed the existence of SLO TRM cells6,149,150. Of note, the resident population is not restricted to a TFH cell phenotype (TFH cells make up ~7%) but exhibits substantial heterogeneity148. Differences in migration attributes might allow for a refined subdivision of TEM cells. For example, Ly6C+ memory TH1 cells might be mostly restricted to the blood. However, a Ly6C− memory TH1 counterpart has been described with decreased expression of Klf2 and S1pr1 mRNA. This subset might take up permanent residence in a lymph node after leaving NLTs6,28. At this point, little is known about the ontogeny of CD4+ SLO TRM cells and their anatomic localization and function upon reactivation.
CD4+ TRM cells as a reservoir for HIV
CD4+ T cells are the major reservoir of HIV. Antiretroviral therapy (ART) suppresses replication of HIV in CD4+ T cells from the blood to undetectable levels but does not eradicate the virus in infected cells. Thus, infected people undergo lifelong therapy. Subsequent viral rebound is thought to occur in a small fraction of latently infected long-lived memory CD4+ T cells151. A drug concentration-dependent spatial model argues that ongoing viral replication may occur in pharmacologic sanctuaries within lymphoid tissues because ART drug levels are below the threshold to efficiently suppress replication in non-activated CD4+ T cells152. Importantly, viral DNA molecules in CD4+ T cells were increased in the cervix, ileum and bronchoalveolar lavage compared to blood samples years after suppressive treatment, suggesting that NLTs might be sites of viral persistence too153–155. Analysis of cellular reservoirs in the cervix of aviremic women revealed that CD4+ TRM cells express markers associated with susceptibility for HIV infection and make up >95% of infected CD4+ T cells154,156. Furthermore, the overall frequency of CD4+ TRM cells decreased in ART-treated versus uninfected women. Studies in macaques have shown that initiating ART 3 d after infection is too late to prevent viral rebound after 24 weeks of continued therapy, suggesting that the viral reservoir is seeded in nonlymphoid tissues as early as 1–2 d after infection157,158. In addition, limited tissue penetration of antiretroviral drugs might lead to subtherapeutic concentrations at the sites of infection159. Quantitative studies in mice indicate that CD8+ TRM cells constitute perhaps the most abundant subset of antigen-experienced CD8+ T cells, and the same may be true for CD4+ T cells127. Thus, CD4+ TRM cells may represent a quantitatively relevant HIV reservoir that will not be sampled in blood and could in theory provide sanctuaries for reemerging infection.
Memory Treg cells
There have been increasing investigations into the durability of Treg cells, many of which are specific for constitutively expressed self-antigens and non-self-antigens, and whether Treg cell maintenance exclusively depends on tonic TCR stimulation. Analyses of putative memory Treg cells are technically challenging due to limitations in defining antigen specificity, low abundance and lack of unstimulated memory-specific markers160–162.
By using an elegant mouse model in which expression of a self-antigen in the skin was turned off, established Treg cells were shown to persist in the absence of antigen163. Subsequently, fetus-specific Treg cells were identified in the SLOs of pregnant mice, and these Treg cells persisted after delivery164. In a viral infection model, Treg cells were shown to persist in the lung, rapidly expand upon reinfection, and potently suppress effector CD4+ T cells to mitigate tissue damage without negatively impacting viral clearance, providing further evidence for memory165,166. In contrast, inducible genetic tracing approaches indicated that inflammation-experienced Treg cells lack functional memory, potentially avoiding generalized host immunosuppression167. Thus, memory Treg cells may be contextual, although more investigations are needed.
Chronic antigen stimulation and exhaustion
The persistence of antigen has a profound effect on CD4+ T cell differentiation, although this varies by the type of infection, tumor or autoimmune context. Compared to mouse naive CD4+ T cells, memory CD4+ T cells that persist after clearance of acute LCMV Armstrong infection largely share patterns of gene expression with CD4+ T cells exposed to chronic LCMV Cl13 (ref. 168). However, persistent LCMV antigen stimulation eventually induces CD4+ T cell exhaustion, which is characterized by a lower magnitude of cells, decreased cytokine production and upregulation of inhibitory receptors including PD-1, Lag3 and CTLA-4. These inhibitory markers are also expressed by TFH cells, and CD4+ T cell differentiation upon chronic LCMV infection is skewed toward a TFH phenotype168–170. However, exhaustion markers, such as TOX, are much higher on a per-cell basis in chronically stimulated TH1 and TFH cells compared to acutely stimulated TFH effectors42. A memory-like TCF-1+Bcl6lo progenitor T cell population that shares similarities with TCM or TFH precursor cells was proposed to sustain both TFH and TH1 effector responses during chronic viral infection171. However, a shift toward TFH cell differentiation is not a generalizable feature of chronic antigen stimulation. For instance, TH1 cells become the dominant subset in response to chronic phagosomal infections, such as Mycobacterium tuberculosis or Salmonella enterica172,173. Tumor-infiltrating CD4+ T cells kill cancer cells, destroy tumor vessels and sustain leukocyte responses mainly through the secretion of IFNγ, TNF and IL-2 (ref. 174). While most CD4+ T cells within the tumor environment are TH1-like cells, tumor-draining lymph nodes and tumor-associated TLSs also contain TFH-like cells, reminiscent of the heterogeneity and spatial distribution of influenza-induced pulmonary CD4+ TRM cells175 (Fig. 4). However, in contrast to the well-established role of TFH cells in vaccination and infection, the role of TFH in antitumor immunity is less clear, and might include contributions to forming TLS through CXCL13 secretion, promotion of CD8+ T cell and B cell responses through production of IL-21 (ref. 175).
CD4+ T cells participate in numerous autoimmune conditions including allergic asthma, inflammatory bowel disease, psoriasis, multiple sclerosis and rheumatoid arthritis176,177. Although TH2-like cells are implicated in asthma, autoreactive CD4+ T cells often display a TH17 or a TH1–TH17 hybrid phenotype79,176,177. In comparison to CD4+ T cells responding to foreign antigen, the affinity of self-reactive CD4+ T cells is typically lower, because high-affinity clones usually get deleted in development or differentiate into Treg cells178. In an elegant mouse study, engineered CAR T cells eliminated specific autoimmune-reactive CD4+ T cells179. CAR T cells that only depleted high-affinity self-reactive CD4+ T cells failed to ameliorate established experimental autoimmune encephalomyelitis, a model of multiple sclerosis179. However, CAR T cells that also depleted low-affinity self-reactive CD4+ T cells reversed disease179. It seems likely that low-affinity CD4+ T cells may better maintain function in contexts of chronic antigen stimulation, whether it be derived from self, tumor or infection.
Conclusion
The diverse biology of memory CD4+ T cells, the sequestration of subsets in tissues outside of blood, the complexity of assays for their identification and scarcity of antigen-specific populations all provide challenges to their study. However, substantial strides have recently been made, resulting in increasingly sophisticated models that describe divisions of labor and ontogenetic relationships among highly specialized memory CD4+ T cell subsets. Issues that are critical to address in the future include a better description of memory CD4+ T cell heterogeneity, including transcriptional regulation, migration properties and spatial distribution of memory CD4+ T cells within lymphoid and nonlymphoid tissues. A better understanding of how memory CD4+ T cells communicate with various immune and non-immune cellular networks within distinct microenvironments could help inform strategies to manipulate harmful autoimmune-reactive memory CD4+ T cells, reprogram memory CD4+ T cells to fight cancer and to foster new vaccination strategies that induce the right subsets at the right location to efficiently harness the protective potential of memory CD4+ T cells. Thus, gaining a more complete grasp of memory CD4+ T cell biology should have broad implications.
Acknowledgements
This work was supported by the Swiss National Science Foundation (P2B-SP3-200187 to M.K.) and the National Institutes of Health grants (R01CA238439, R01AI146032, R01AI084913 and R01AI150600 to D.M.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Vinuesa CG, Linterman MA, Yu D & MacLennan IC Follicular helper T cells. Annu Rev. Immunol 34, 335–368 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Crotty S T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laidlaw BJ, Craft JE & Kaech SM The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat. Rev. Immunol 16, 102–111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain SL, McKinstry KK & Strutt TM Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol 12, 136–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A & Rudra D Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. 9, 883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beura LK et al. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med 216, 1214–1229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glennie ND, Volk SW & Scott P Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog. 13, e1006349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josefowicz SZ, Lu LF & Rudensky AY Regulatory T cells: mechanisms of differentiation and function. Annu Rev. Immunol 30, 531–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annunziato F, Romagnani C & Romagnani S The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol 135, 626–635 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Crotty S Follicular helper CD4 T cells (TFH). Annu Rev. Immunol 29, 621–663 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Ahmed R & Gray D Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Hammarlund E et al. Duration of antiviral immunity after smallpox vaccination. Nat. Med 9, 1131–1137 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Walker JM & Slifka MK Longevity of T cell memory following acute viral infection. Adv. Exp. Med. Biol 684, 96–107 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Naniche D et al. Decrease in measles virus-specific CD4+ T cell memory in vaccinated subjects. J. Infect. Dis 190, 1387–1395 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Jokinen S, Osterlund P, Julkunen I & Davidkin I Cellular immunity to mumps virus in young adults 21 years after measles-mumps-rubella vaccination. J. Infect. Dis 196, 861–867 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Terahara K et al. SARS-CoV-2-specific CD4+ T cell longevity correlates with TH17-like phenotype. iScience 25, 104959 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel RR et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badolato-Correa J et al. Differential longevity of memory CD4 and CD8 T Cells in a cohort of the mothers with a history of ZIKV infection and their children. Front Immunol. 12, 610456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng OW et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 34, 2008–2014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homann D, Teyton L & Oldstone MB Differential regulation of antiviral T cell immunity results in stable CD8+ but declining CD4+ T cell memory. Nat. Med 7, 913–919 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Fedele G, Cassone A & Ausiello CM T cell immune responses to Bordetella pertussis infection and vaccination. Pathog. Dis 73, ftv051 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Hirst GK The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med 75, 49–64 (1942). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czerkinsky C et al. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J. Immunol. Methods 110, 29–36 (1988). [DOI] [PubMed] [Google Scholar]
- 25.Dan JM et al. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J. Immunol 197, 983–993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grifoni A et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon JJ et al. Tracking epitope-specific T cells. Nat. Protoc 4, 565–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunzli M et al. Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci. Immunol 5, eaay5552 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Tubo NJ et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153, 785–796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swarnalekha N et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol 6, eabb6808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford F, Kozono H, White J, Marrack P & Kappler J Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity 8, 675–682 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Altman JD et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996). [PubMed] [Google Scholar]
- 33.Martinez RJ, Andargachew R, Martinez HA & Evavold BD Low-affinity CD4+ T cells are major responders in the primary immune response. Nat. Commun 7, 13848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J et al. Detection, phenotyping, and quantification of antigen-specific T cells using a peptide–MHC dodecamer. Proc. Natl Acad. Sci. USA 113, E1890–E1897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dileepan T et al. MHC class II tetramers engineered for enhanced binding to CD4 improve detection of antigen-specific T cells. Nat. Biotechnol 39, 943–948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogorelyy MV et al. Resolving SARS-CoV-2 CD4+ T cell specificity via reverse epitope discovery. Cell Rep. Med 3, 100697 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi YS et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenbarth SC et al. CD4+ T cells that help B cells—a proposal for uniform nomenclature. Trends Immunol. 42, 658–669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh JE et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol 6, eabj5129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R & Tangye SG IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol 181, 1767–1779 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Keck S et al. Antigen affinity and antigen dose exert distinct influences on CD4 T cell differentiation. Proc. Natl Acad. Sci. USA 111, 14852–14857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunzli M, Reuther P, Pinschewer DD & King CG Opposing effects of T cell receptor signal strength on CD4 T cells responding to acute versus chronic viral infection. Elife 10, e61869 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fazilleau N, McHeyzer-Williams LJ, Rosen H & McHeyzer-Williams MG The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol 10, 375–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotov DI et al. TCR affinity biases TH cell differentiation by regulating CD25, Eef1e1, and Gbp2. J. Immunol 202, 2535–2545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snook JP, Kim C & Williams MA TCR signal strength controls the differentiation of CD4+ effector and memory T cells. Sci. Immunol 3, eaas9103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiToro D et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361, eaao2933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamoorthy V et al. The IRF4 gene regulatory module functions as a read–write integrator to dynamically coordinate T helper cell fate. Immunity 47, 481–497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ploquin MJ, Eksmond U & Kassiotis G B cells and TCR avidity determine distinct functions of CD4+ T cells in retroviral infection. J. Immunol 187, 3321–3330 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanguri V, Govern CC, Smith R & Huseby ES Viral antigen density and confinement time regulate the reactivity pattern of CD4 T cell responses to vaccinia virus infection. Proc. Natl Acad. Sci. USA 110, 288–293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govern CC, Paczosa MK, Chakraborty AK & Huseby ES Fast on-rates allow short dwell time ligands to activate T cells. Proc. Natl Acad. Sci. USA 107, 8724–8729 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Constant S, Pfeiffer C, Woodard A, Pasqualini T & Bottomly K Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med 182, 1591–1596 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosken NA, Shibuya K, Heath AW, Murphy KM & O’Garra A The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J. Exp. Med 182, 1579–1584 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruterbusch M, Pruner KB, Shehata L & Pepper M In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol 38, 705–725 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Arsenio J, Metz PJ & Chang JT Asymmetric cell division in T lymphocyte fate diversification. Trends Immunol. 36, 670–683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osum KC & Jenkins MK Toward a general model of CD4+ T cell subset specification and memory cell formation. Immunity 56, 475–484 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swain SL, Hu H & Huston G Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ & Jenkins MK Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pepper M et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol 11, 83–89 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sallusto F, Lenig D, Forster R, Lipp M & Lanzavecchia A Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Kryczek I et al. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med 3, 104ra100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muranski P et al. TH17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mojtabavi N, Dekan G, Stingl G & Epstein MM Long-lived TH2 memory in experimental allergic asthma. J. Immunol 169, 4788–4796 (2002). [DOI] [PubMed] [Google Scholar]
- 63.McGeachy MJ TH17 memory cells: live long and proliferate. J. Leukoc. Biol 94, 921–926 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Nakayama T et al. TH2 cells in health and disease. Annu. Rev. Immunol 35, 53–84 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Luthje K et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol 13, 491–498 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Hale JS et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity 38, 805–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall HD et al. Differential expression of Ly6C and T-bet distinguish effector and memory TH1 CD4+ cell properties during viral infection. Immunity 35, 633–646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andreatta M et al. A CD4+ T cell reference map delineates subtype-specific adaptation during acute and chronic viral infections. Elife 11, e76339 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciucci T et al. The emergence and functional fitness of memory CD4+ T cells require the transcription factor Thpok. Immunity 50, 91–105 e104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacLeod MK et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol 186, 2889–2896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pepper M & Jenkins MK Origins of CD4+ effector and central memory T cells. Nat. Immunol 12, 467–471 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen QP, Deng TZ, Witherden DA & Goldrath AW Origins of CD4+ circulating and tissue-resident memory T cells. Immunology 157, 3–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ciucci T et al. Dependence on Bcl6 and Blimp1 drive distinct differentiation of murine memory and follicular helper CD4+ T cells. J. Exp. Med 219, e20202343 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson AM et al. Evolution of antigen-specific follicular helper T cell transcription from effector function to memory. Sci. Immunol 7, eabm2084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krueger PD, Osum KC & Jenkins MK CD4+ memory T cell formation during type 1 immune responses. Cold Spring Harb. Perspect. Biol 13, a038141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyer SS et al. Identification of novel markers for mouse CD4+ T follicular helper cells. Eur. J. Immunol 43, 3219–3232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kunzli M et al. Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells. Sci. Immunol 7, eadd3075 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee SK et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med 208, 1377–1388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hondowicz BD et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity 44, 155–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi J et al. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol 21, 777–789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnston RJ et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oestreich KJ, Mohn SE & Weinmann AS Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol 13, 405–411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med 209, 1841–1852 (2012). S1841-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morita R et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Locci M et al. Human circulating PD-1+CXCR3−CXCR5+ memory TFH cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slifka MK, Matloubian M & Ahmed R Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol 69, 1895–1902 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ise W et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc. Natl Acad. Sci. USA 111, 11792–11797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asrir A, Aloulou M, Gador M, Perals C & Fazilleau N Interconnected subsets of memory follicular helper T cells have different effector functions. Nat. Commun 8, 847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moran I et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat. Commun 9, 3372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suan D et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42, 704–718 (2015). [DOI] [PubMed] [Google Scholar]
- 91.de Carvalho RVH et al. Clonal replacement sustains long-lived germinal centers primed by respiratory viruses. Cell 186, 131–146 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chappert P et al. Human anti-smallpox long-lived memory B cells are defined by dynamic interactions in the splenic niche and long-lasting germinal center imprinting. Immunity 55, 1872–1890 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Messi M et al. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nat. Immunol 4, 78–86 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Wei G et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dileepan T et al. Robust antigen specific TH17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog. 7, e1002252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper AM et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med 178, 2243–2247 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scriba TJ et al. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J. Infect. Dis 203, 1832–1843 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Tameris MD et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moguche AO et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med 212, 715–728 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reinhardt RL, Khoruts A, Merica R, Zell T & Jenkins MK Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Gebhardt T et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Park CO et al. Staged development of long-lived T cell receptor alphabeta TH17 resident memory T cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol 142, 647–662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bromley SK, Yan S, Tomura M, Kanagawa O & Luster AD Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J. Immunol 190, 970–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe R et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med 7, 279ra239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glennie ND et al. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med 212, 1405–1414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins N et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun 7, 11514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar BV et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20, 2921–2934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Hara JM et al. Generation of protective pneumococcal-specific nasal resident memory CD4+ T cells via parenteral immunization. Mucosal Immunol. 13, 172–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teijaro JR et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187, 5510–5514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siracusa F et al. Nonfollicular reactivation of bone marrow resident memory CD4 T cells in immune clusters of the bone marrow. Proc. Natl Acad. Sci. USA 115, 1334–1339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zens KD, Chen JK & Farber DL Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, e85832 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith NM et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 11, 220–235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amezcua Vesely MC et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell 178, 1176–1188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kabat AM et al. Resident TH2 cells orchestrate adipose tissue remodeling at a site adjacent to infection. Sci. Immunol 7, eadd3263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Braverman J et al. Staphylococcus aureus specific lung resident memory CD4+ Th1 cells attenuate the severity of influenza virus induced secondary bacterial pneumonia. Mucosal Immunol. 15, 783–796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iijima N & Iwasaki A T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirchner FR & LeibundGut-Landmann S Tissue-resident memory Th17 cells maintain stable fungal commensalism in the oral mucosa. Mucosal Immunol. 14, 455–467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stary G et al. VACCINES. a mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348, aaa8205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allen AC et al. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 11, 1763–1776 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Wilk MM et al. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with Bordetella pertussis. J. Immunol 199, 233–243 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI & Luster AD Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J. Exp. Med 217, e20190865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zundler S et al. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat. Immunol 20, 288–300 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Krebs CF et al. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol 5, eaba4163 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Bartolome-Casado R et al. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol. 14, 402–410 (2021). [DOI] [PubMed] [Google Scholar]
- 125.Snyder ME et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol 4, eaav5581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pallett LJ et al. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. J. Exp. Med 217, e20200050 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Steinert EM et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ziegler SF, Ramsdell F & Alderson MR The activation antigen CD69. Stem Cells 12, 456–465 (1994). [DOI] [PubMed] [Google Scholar]
- 129.Beura LK et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48, 327–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poon MML et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat. Immunol 24, 309–319 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fonseca R et al. Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells. Nat. Immunol 23, 1236–1245 (2022). [DOI] [PubMed] [Google Scholar]
- 132.Cunningham AC et al. Constitutive expression of MHC and adhesion molecules by alveolar epithelial cells (type II pneumocytes) isolated from human lung and comparison with immunocytochemical findings. J. Cell Sci 107, 443–449 (1994). [DOI] [PubMed] [Google Scholar]
- 133.Biton M et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 175, 1307–1320 e1322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tamoutounour S et al. Keratinocyte-intrinsic MHCII expression controls microbiota-induced TH1 cell responses. Proc. Natl Acad. Sci. USA 116, 23643–23652 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shenoy AT et al. Antigen presentation by lung epithelial cells directs CD4+ TRM cell function and regulates barrier immunity. Nat. Commun 12, 5834 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shenoy AT et al. Lung CD4+ resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal Immunol. 13, 334–343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Son YM et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol 6, eabb6852 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morimoto Y et al. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity 49, 134–150 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Tan HX et al. Inducible bronchus-associated lymphoid tissues (iBALT) serve as sites of B cell selection and maturation following influenza infection in mice. Front Immunol. 10, 611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poon MML et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol 6, eabl9105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kutteh WH, Prince SJ & Mestecky J Tissue origins of human polymeric and monomeric IgA. J. Immunol 128, 990–995 (1982). [PubMed] [Google Scholar]
- 142.Naderi W, Schreiner D & King CG T cell-B cell collaboration in the lung. Curr. Opin. Immunol 81, 102284 (2023). [DOI] [PubMed] [Google Scholar]
- 143.Wellford SA et al. Mucosal plasma cells are required to protect the upper airway and brain from infection. Immunity 55, 2118–2134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosato PC, Wijeyesinghe S, Stolley JM & Masopust D Integrating resident memory into T cell differentiation models. Curr. Opin. Immunol 63, 35–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wijeyesinghe S et al. Expansible residence decentralizes immune homeostasis. Nature 592, 457–462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gray JI & Farber DL Tissue-resident immune cells in humans. Annu Rev. Immunol 40, 195–220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sathaliyawala T et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ugur M, Schulz O, Menon MB, Krueger A & Pabst O Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nat. Commun 5, 4821 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Marriott CL, Dutton EE, Tomura M & Withers DR Retention of Ag-specific memory CD4+ T cells in the draining lymph node indicates lymphoid tissue resident memory populations. Eur. J. Immunol 47, 860–871 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Durand A et al. Profiling the lymphoid-resident T cell pool reveals modulation by age and microbiota. Nat. Commun 9, 68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murray AJ, Kwon KJ, Farber DL & Siliciano RF The latent reservoir for HIV-1: how immunologic memory and clonal expansion contribute to HIV-1 persistence. J. Immunol 197, 407–417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lorenzo-Redondo R et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530, 51–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yukl SA et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J. Infect. Dis 208, 1212–1220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cantero-Perez J et al. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun 10, 4739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Costiniuk CT et al. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS 32, 2279–2289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Joag VR et al. Identification of preferential CD4+ T cell targets for HIV infection in the cervix. Mucosal Immunol. 9, 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Whitney JB et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Whitney JB et al. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun 9, 5429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Devanathan AS & Cottrell ML Pharmacology of HIV cure: site of action. Clin. Pharmacol. Ther 109, 841–855 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rosenblum MD, Way SS & Abbas AK Regulatory T cell memory. Nat. Rev. Immunol 16, 90–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khantakova JN, Bulygin AS & Sennikov SV The regulatory T cell memory phenotype: what we know. Cells 11, 1687 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu T, Soong L, Liu G, Konig R & Chopra AK CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol. Direct 4, 40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosenblum MD et al. Response to self antigen imprints regulatory memory in tissues. Nature 480, 538–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rowe JH, Ertelt JM, Xin L & Way SS Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brincks EL et al. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J. Immunol 190, 3438–3446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sanchez AM, Zhu J, Huang X & Yang Y The development and function of memory regulatory T cells after acute viral infections. J. Immunol 189, 2805–2814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van der Veeken J et al. Memory of inflammation in regulatory T cells. Cell 166, 977–990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Crawford A et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fahey LM et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med 208, 987–999 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Snell LM et al. Overcoming CD4 TH1 cell fate restrictions to sustain antiviral CD8 T cells and control persistent virus infection. Cell Rep. 16, 3286–3296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xia Y et al. BCL6-dependent TCF-1+ progenitor cells maintain effector and helper CD4+ T cell responses to persistent antigen. Immunity 55, 1200–1215 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morgan J et al. Classical CD4 T cells as the cornerstone of antimycobacterial immunity. Immunol. Rev 301, 10–29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tubo NJ & Jenkins MK CD4+ T cells: guardians of the phagosome. Clin. Microbiol. Rev 27, 200–213 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Speiser DE, Chijioke O, Schaeuble K & Munz C CD4+ T cells in cancer. Nat. Cancer 4, 317–329 (2023). [DOI] [PubMed] [Google Scholar]
- 175.Gutierrez-Melo N & Baumjohann D T follicular helper cells in cancer. Trends Cancer 9, 309–325 (2023). [DOI] [PubMed] [Google Scholar]
- 176.Oja AE, van Lier RAW & Hombrink P Two sides of the same coin: protective versus pathogenic CD4+ resident memory T cells. Sci. Immunol 7, eabf9393 (2022). [DOI] [PubMed] [Google Scholar]
- 177.Raphael I, Joern RR & Forsthuber TG Memory CD4+ T cells in immunity and autoimmune diseases. Cells 9, 531 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koehli S, Naeher D, Galati-Fournier V, Zehn D & Palmer E Optimal T cell receptor affinity for inducing autoimmunity. Proc. Natl Acad. Sci. USA 111, 17248–17253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yi J et al. Antigen-specific depletion of CD4+ T cells by CAR T cells reveals distinct roles of higher- and lower-affinity TCRs during autoimmunity. Sci. Immunol 7, eabo0777 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]


