Abstract
Variety, geographical origin, and harvest season are important factors affecting the accumulation of polyphenols in Lycium barbarum. In this study, the effects of these factors on the polyphenolic components of this species were analyzed using ultra-performance liquid chromatography ion mobility quadrupole time-of-flight mass spectrometry. Moreover, the in vitro antioxidant activities of fruit extracts from this species were evaluated. The total polyphenolic contents of L. barbarum fruits from Jinghe County in Xinjiang and Zhongning County in Ningxia were 5.52–11.72 and 7.06–9.37 mg (gallic acid equivalent)/g dry weight, while the total flavonoid contents of L. barbarum fruits from these regions were 12.52–30.29 and 12.67–20.77 mg (rutin equivalent)/g dry weight, respectively. Overall, 39 types of polyphenols were identified in the fruit extracts, including 26 flavonoids, 10 phenolic acids, and three tannins. Of these, 11 polyphenols were quantitatively analyzed, which revealed rutin to be the most dominant polyphenolic component in fruits from Jinghe and Zhongning. There were significant differences (p < 0.05) in the polyphenolic contents and antioxidant activities of L. barbarum fruit extracts, depending on the geographical origin, variety, and harvest season. The antioxidant activity of this species was found to be significantly positively correlated with the polyphenolic contents. This study provided scientific guidance for comprehensive applications of polyphenols from different varieties of L. barbarum from separate geographical origins.
Keywords: Lycium barbarum, polyphenols, UPLC-IM-QTOF-MS, antioxidant activity, variety, geographical origin
1. Introduction
Lycium barbarum (Chinese wolfberry, Barbary wolfberry, or Chinese boxthorn) from the genus Lycium L. (Solanaceae) has received increased attention in recent years due to its traditional uses in Chinese herbal medicine and is listed in the “Pharmacopoeia of the People’s Republic of China” [1,2]. L. barbarum fruits are considered functional foods that have a large variety of beneficial effects. Recent studies have indicated that extracts of L. barbarum fruits possess a wide range of biological activities, including effects on aging, neuroprotection, anti-fatigue/endurance, anti-cancer activity, antioxidant properties, etc. [3,4]. At present, the species is distributed mainly in North and Northwest China, where it is widely cultivated as a high-yielding crop in the Qinghai, Ningxia, Gansu, Xinjiang, and Tianjin regions. L. barbarum fruit (wolfberry) is economically important and exported to more than 40 countries and regions. Moreover, Jinghe wolfberry and Zhongning wolfberry are listed in the China–EU Agreement on Cooperation and Protection of Geographical Indications [5]. Recently, L. barbarum fruits have become increasingly popular around the world because they contain diverse functional components, including amino acids, alkaloids, anthocyanins, polysaccharides, tocopherols, proteins, polyphenols, carotenoids, ascorbic acid, etc. [6,7,8,9,10,11,12].
Polyphenolic compounds, found abundantly in L. barbarum fruits, are secondary metabolites containing phenolic hydroxyl groups and include phenolic acids, flavonoids, and anthocyanins [13]. Polyphenols are known as the “seventh nutrient” for human health and have received much attention from researchers, due to their multifarious pharmacological properties, including hypoglycemic activity [14], antihyperlipidemic effects [15], activity in increasing metabolism [16,17], as well as antioxidant and anti-cancer properties [18,19,20,21]. Zhu et al. reported that phenolic amides extracted from L. barbarum fruits had better potential immunomodulatory activity than polysaccharides from this species and discovered three new phenolic amides and 12 analogues [22]. In a study of Goji berries, Bondia-Pons et al. identified 31 phenolic compounds and derivatives, including quercetin, coumaric acid, and caffeic acid and its derivatives, using non-targeted liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (LC-qTOF-MS) [23]. Increasing the CO2 concentration and appropriately raising the temperature has been shown to contribute to the accumulation of polyphenols in onions [24]. Although researchers have conducted many studies on the contents and factors that influence polyphenols in L. barbarum, questions such as what further factors influence the accumulation of polyphenols in the fruits remain.
Ecological conditions and tillage methods have a significant impact on the quality and bioactive constituents of fruit. High temperature, moderate soil moisture, strong sunlight, and low altitude are suitable conditions for cultivating L. barbarum fruits with a high content of nutritious metabolites [25,26,27]. Lu et al. found significant differences in the nutritional components and antioxidant activities of L. barbarum from different regions in China [28]. Furthermore, Nzeuwa et al. reported slight differences in the functional components of Lycium fruits from different areas, demonstrating that the phenolic contents of Lycium fruits from Nepal were higher than in those from China [29]. Liu et al. discriminated between the nutritional components of L. barbarum from different geographical origins and varieties using nuclear magnetic resonance (NMR) techniques, finding that the variety Ningqi 9 had more nutritional value than the variety Ningqi 7 [30]. Cheng et al. found that black wolfberries from different geographical origins could clearly be identified by their individual anthocyanin contents detected using ultra-high-performance liquid chromatography (UPLC)-TOF-MS [31]. Therefore, geographical conditions greatly affect the accumulation of polyphenols. Differences in the main nutrients and phytochemicals present in L. barbarum from different geographical origins have been widely reported, but many studies have not been specific about the variety sampled or have been inconsistent in the maturation, cultivation, and management strategies of the L. barbarum samples studied. Little is known about the antioxidant activities and overall differences in the polyphenolic components of L. barbarum fruits from different areas and varieties.
Ultra-performance liquid chromatography ion mobility quadrupole time-of-flight mass spectrometry (UPLC-IM-QTOF-MS) is a recently developed, qualitative and quantitative technique for analyzing active ingredients in natural products and has been widely used to identify plant polyphenol compounds. One of the benefits of this technique is that it can be used to analyze the structure of components in extracts without the requirement for reference materials. UPLC-IM-QTOF-MS has the advantages of high resolution, high sensitivity, and accurate molecular weight analysis and can be used to predict the molecular formulas of compounds using accurate mass numbers and ion fragments, which enables the accurate identification of polyphenolic structures.
In this study, L. barbarum fruits grown in Jinghe, Xinjiang Province and Zhongning, Ningxia Province were selected as research objects. The total polyphenolic and flavonoid contents of the fruits were determined using the Folin–Ciocalteau method and aluminum trichloride colorimetry. Thereafter, differences in the polyphenolic components of different varieties of L. barbarum fruits grown in two geographical regions and harvested during two seasons were compared using UPLC-IM-QTOF-MS. This study provided useful information for quality improvement and product development strategies.
2. Results and Discussion
2.1. The Polyphenolic and Flavonoid Contents of L. barbarum Fruits from Different Geographical Origins
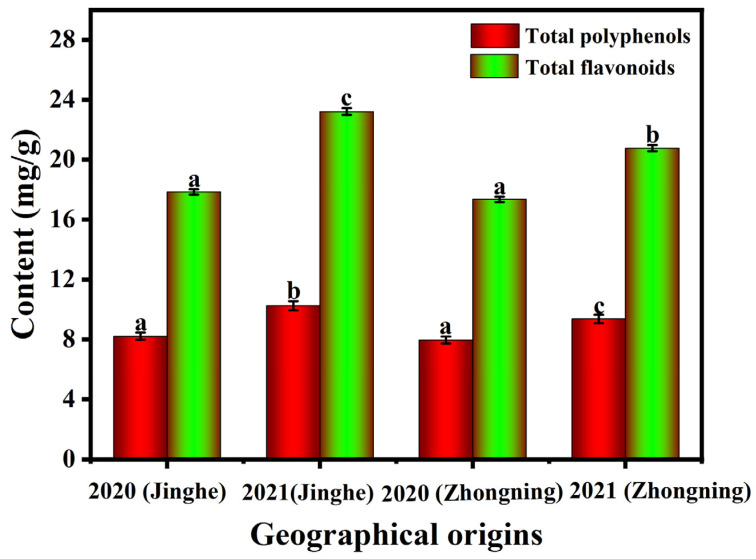
The total polyphenolic and flavonoid contents of L. barbarum fruits (varieties 5# and 7#) harvested during summer 2020 and 2021 from Jinghe and Zhongning are shown in Figure 1. The total polyphenolic and flavonoid contents of the samples ranged from 5.52 to 11.72 mg gallic acid equivalent (GAE)/g dry weight (DW) and 12.52 to 30.29 mg GAE/g DW, respectively. The total polyphenolic and flavonoid contents of the two varieties grown in Jinghe were generally higher than of those grown in Zhongning, even though both varieties originated from the Zhongning area. There was a significant difference (p < 0.05) between the polyphenolic contents of L. barbarum fruits harvested from the two regions in 2021. To explore the impact of meteorological factors on the accumulation of polyphenols in L. barbarum, the corresponding meteorological data for the samples grown in different geographical origins were analyzed statistically (Table S1). Altitude was taken as the average for all sample collection sites, and other meteorological data were obtained by the China Meteorological Administration. Temperature, difference in temperature, extreme temperature, annual precipitation, altitude, and hours of sunlight were recorded for the whole of the year in 2020. Comparing the meteorological conditions in Jinghe and Zhongning in 2020, Jinghe had a longer duration of sunlight, lower precipitation, and lower mean temperature, which might have been more conducive to the accumulation of polyphenolic compounds. In addition, Jinghe is surrounded by mountains on all sides, experiences drought with little rain, and is rich in sandy and saline–alkali soil, all of which are conducive to the healthy growth of L. barbarum.
Figure 1.
A comparison of the total polyphenolic and flavonoid contents of L. barbarum fruits harvested in summer 2020 and 2021 from different geographical origins. Different letters (a, b, c) across treatments indicate significant differences at p < 0.05.
2.2. The Polyphenolic and Flavonoid Contents in Four Varieties of L. barbarum
The total polyphenolic and flavonoid contents of L. barbarum samples from four varieties (5#, 7#, 9#, and 1801#) grown in Jinghe were measured according to the Folin–Ciocalteau method, and the results are shown in Table 1 and Table S2. In fruits harvested during 2020 and 2021, the total polyphenolic and flavonoid contents in 7# and 1801# were the highest, followed by those in 5# and 9#. The highest contents of total polyphenols and flavonoids were 11.72 and 30.29 GAE/g DW, respectively. There were significant differences among the total polyphenolic contents of the four varieties (p < 0.05). Among them, the differences seen in 1801# and 9# in different harvesting periods were more significant. The variation in the total flavonoid contents of the different varieties was similar to that of the total polyphenolic contents. Comprehensive analysis showed that the polyphenolic content of 7# was the highest and that of 9# harvested in summer was the lowest.
Table 1.
The total polyphenolic contents of different varieties of L. barbarum harvested during different seasons (X ± s, n = 3).
| Variety | Contents in the Summer of 2020 (mg GAE/g DW) | Contents in the Autumn of 2020 (mg GAE/g DW) | Contents in the Summer of 2021 (mg GAE/g DW) | Contents in the Autumn of 2021 (mg GAE/g DW) |
|---|---|---|---|---|
| 5# | 7.64 ± 0.24 b | 9.35 ± 0.29 c | 9.18 ± 0.88 bc | 9.63 ± 0.37 c |
| 7# | 8.21 ± 0.40 a | 10.88 ± 0.66 a | 10.25 ± 0.60 a | 10.08 ± 0.59 b |
| 9# | 5.52 ± 0.22 c | 9.54 ± 0.27 c | 8.70 ± 0.98 c | 9.90 ± 0.53 bc |
| 1801# | 8.14 ± 0.48 a | 10.24 ± 0.13 b | 9.70 ± 0.82 b | 11.72 ± 0.60 a |
Different lowercase letters (a, b, c) per column represent significant differences (p < 0.05).
2.3. The Polyphenolic Contents of L. barbarum Harvested during Different Seasons
Analyzing the total polyphenolic contents of L. barbarum revealed that fruits harvested in autumn had significantly higher levels than those harvested in summer (Table 1 and Table S2). Summer-harvested L. barbarum fruits are generally collected as four crops, while the autumn fruits are collected as two to three crops; thus, the ripening cycle of the autumn L. barbarum fruits is longer, and the temperature difference between day and night in autumn is relatively larger than that in summer, so the nutrients can fully accumulate in the fruits. Moreover, the polyphenolic contents of fruits collected in 2021 were obviously higher than those in 2020, which might have been caused by differences in the climate, sunlight duration, and temperature.
2.4. Comparison of Antioxidant Activity
2.4.1. Antioxidant Activity of L. barbarum Fruits Harvested from Different Geographical Origins
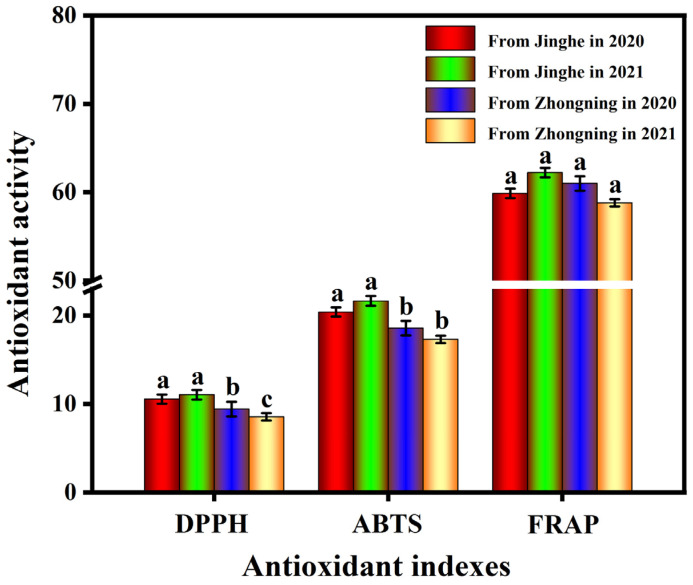
The antioxidant activity of L. barbarum fruits harvested from Jinghe and Zhongning in 2020 and 2021 was analyzed by measuring the DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging capacity and the ferric ion reducing antioxidant potential (FRAP). The DPPH method has good reproducibility due to its long free-radical half-life. The ABTS method can be used to measure the antioxidant capacity of hydrophilic and lipophilic substances; the reduced ABTS radical is colorless due to a color-quenching reaction. The principle of the FRAP method is that, with the antioxidant at low pH, ferric tripyridyltriazine (Fe(III)-TPTZ) is reduced to ferrous tripyridyltriazine (Fe(II)-TPTZ) and can be measured at 597 nm, accompanied by the appearance of blue color. The FRAP method can inhibit some endogenous interference factors. Therefore, the above three methods are widely used to evaluate the antioxidant activity of plant extracts. As shown in Figure 2, the antioxidant activity of L. barbarum extracts from fruits grown in different geographical origins had different capacities. The DPPH and ABTS radical scavenging and FRAP results from fruits grown in Jinghe were significantly higher than those in fruits grown in Zhongning in 2021 (p < 0.05). The DPPH and ABTS values of L. barbarum harvested from Jinghe were higher than those from Zhongning in 2020, but the FRAP value of L. barbarum harvested from Zhongning was higher than that in fruits harvested from Jinghe.
Figure 2.
The antioxidant activity of L. barbarum fruits grown in different geographical origins. Different letters (a, b, c) across treatments indicate significant differences at p < 0.05.
2.4.2. Antioxidant Activity of Fruit Extracts from Different Varieties of L. barbarum
The antioxidant activity (DPPH, ABTS, and FRAP) of L. barbarum fruit extracts from varieties 5#, 7#, 9#, and 1801#, harvested from Jinghe, was measured. Table 2 shows that the antioxidant activities among the four varieties were significantly different (p < 0.05). In particular, 7# showed the highest DPPH radical scavenging ability and FRAP, with values of 12.09 mg Vc/g DW and 63.76 mmol Vc/g DW, respectively, while the ABTS radical scavenging ability of 7# was slightly lower than that of 1801#, with a value of 22.41 mmol Vc/g DW. The comprehensive analysis revealed that the antioxidant activities of 7# and 1801# were significantly higher than those of 5# and 9#. The order of antioxidant capacity in the different varieties was similar to that of the total polyphenolic and total flavonoid contents.
Table 2.
Antioxidant activity (DPPH, ABTS, and FRAP) of different L. barbarum (X ± s, n = 3).
| Variety | DPPH (mg VC/g DW) | ABTS (mmol VC/g DW) | FRAP (mmol VC/g DW) |
|---|---|---|---|
| 5# | 10.68 ± 1.10 b | 20.81 ± 0.63 bc | 59.15 ± 1.72 c |
| 7# | 12.09 ± 1.03 a | 21.76 ± 1.04 ab | 63.76 ± 1.91 a |
| 9# | 9.43 ± 1.38 c | 19.97 ± 1.80 c | 59.87 ± 2.01 c |
| 1801# | 11.60 ± 0.71 a | 22.41 ± 1.23 a | 61.43 ± 2.72 b |
Different lowercase letters (a, b, c) per column represent significant differences (p < 0.05).
The relationship between the total polyphenolic content, total flavonoid content, and antioxidant activity (DPPH, ABTS, and FRAP) of L. barbarum fruit extracts was evaluated using correlation analysis. As shown in Table S3, the total polyphenolic content was extremely significantly correlated with DPPH radical scavenging ability and FRAP (p < 0.01) and was significantly correlated with ABTS radical scavenging ability (p < 0.05). The correlation between total flavonoids and antioxidant activity (DPPH, ABTS, and FRAP) was extremely significant (p < 0.01). The results revealed that the amount of total polyphenols and total flavonoids had an important influence on the antioxidant activity of L. barbarum fruit extracts, which is consistent with a previous report [32].
Based on previous work and the results obtained from the present study, polyphenols are likely to be the important active compounds present in L. barbarum. A prior analysis of the antioxidant activity of phenolic acids and flavonoids in L. barbarum indicated that the flavonoid components such as rutin and quercetin were important for scavenging DPPH free radicals [33]. In addition, the abilities of L. barbarum to scavenge and prevent the formation of free radicals are closely related to the concentration and composition of the polyphenols. Thus, it was speculated that polyphenols may be the key active components contributing to the antioxidant activity of L. barbarum. The reason for the differences in antioxidant activity (DPPH, ABTS, and FRAP) of different varieties of L. barbarum and those from different regions might be due to the diversity of the polyphenols. The types and contents of polyphenolic compounds with antioxidant effects in L. barbarum showed certain differences in antioxidant activities.
2.5. Analysis of Polyphenolic Compounds in L. barbarum Fruit Extracts
The metabolomics method of UPLC-IM-QTOF-MS was established for the non-targeted qualitative and quantitative analysis of polyphenolic compounds in L. barbarum fruit extracts. UPLC-IM-QTOF-MS non-targeted screening showed a wide and chemically diverse profile of polyphenolic compounds in comparison to previous knowledge.
2.5.1. Qualitative Analysis of Polyphenols in L. barbarum Fruit Extract
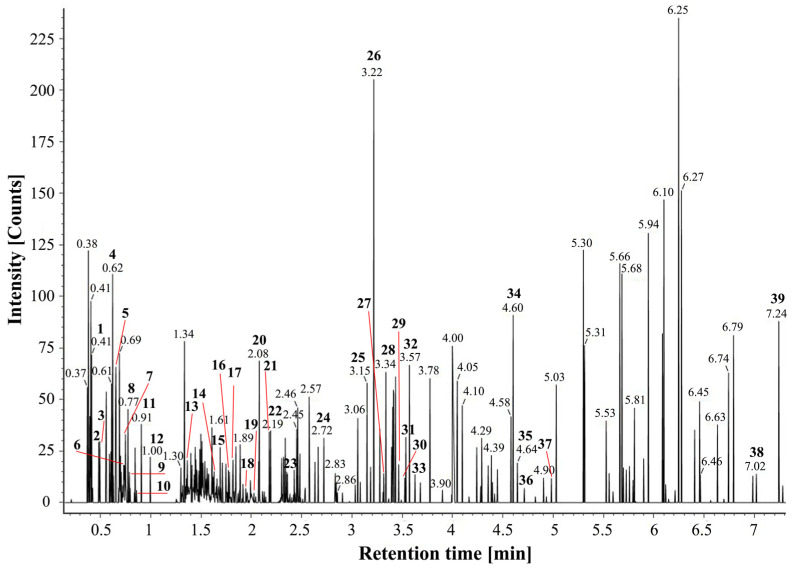
A total ion chromatogram for an L. barbarum fruit extract in positive-ion MSE mode is shown in Figure 3. This provided a relatively integrated picture of the metabolomic analysis and formed an analytical fingerprint for the identification and authentication of fruits or fruit-derived products. The identified polyphenols mainly peaked within 0.40–7.25 min. The exact m/z ratio, retention time, error, and fragment ions of characteristic peaks were shown in Table S4. By comparing the Waters Progenesis QI database (including natural product library, metabolite library, sugar metabolism library, and endogenous compound library) and related reports [6,34,35,36], a total of 39 polyphenolic compounds were identified in the L. barbarum fruit extract, including 26 flavonoids, 10 phenolic acids, and three tannins.
Figure 3.
Total ion chromatogram of L. barbarum fruit extract in positive-ion mode (Numbers 1–39 represent different characteristic peaks recognized).
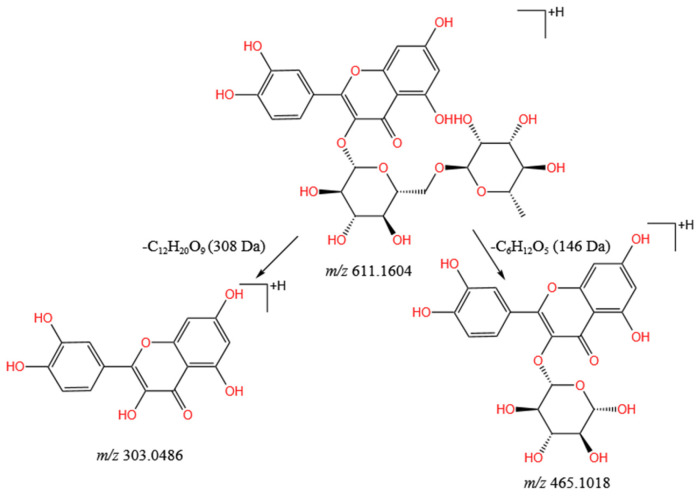
Flavonoids consists of three ring skeletons, C6-C3-C6, which are derived from the parent nucleus of flavone (2-phenylchromone) [37]. Twenty-six flavonoids were identified accurately in the L. barbarum fruit extract by comparing the Waters Progenesis QI database and previous literature reports, including flavanones (compound 15), flavones (compound 9), flavonols (compounds 2, 18, 21, 22, and 25–32), chalcone (compound 35), flavanes (compounds 7, 16, 17, and 33), and anthocyanidins (compounds 1, 3, 10, 13, 24, 36, and 37). Taking compound 25 in Table S4 as an example, the main cracking mechanism of this compound was analyzed. The retention time of compound 25 was 3.22 min, and it had a protonated molecular ion [M + H]+ at m/z 611.1604. Compound 25 generated MS/MS characteristic ions at m/z 465.1018 (isoquercetin [M + H]+) and at m/z 303.0486 (quercetin [M + H]+), because m/z 611.1604[M + H]+ lost 146 Da (rhamnoside) and 308 Da (rutinoside) [38]. The main cleavage pathway is shown in Figure 4. By comparing the retention time and MS/MS fragment information with the rutin reference substance, compound 25 was presumed to be rutin. This speculation was consistent with previous reports [39,40].
Figure 4.
Main cleavage pathway of rutin.
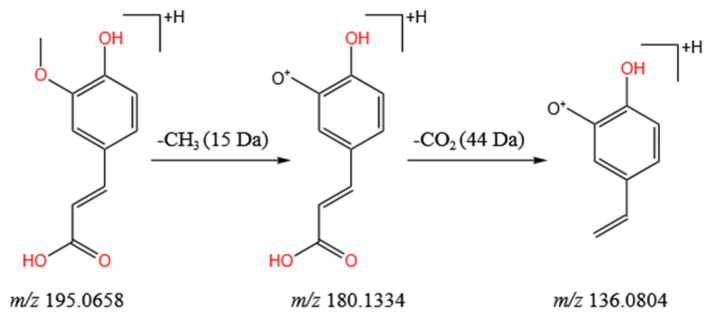
Phenolic acids are compounds with phenolic groups and organic carboxylic acid functional groups, and most of them are hydroxybenzoic acid derivatives with a C6-C1 skeleton and hydroxycinnamic acid derivatives with a C6-C3 skeleton [41,42]. They have potential biological properties such as antioxidation, antiviral, antibacterial, and anti-inflammatory activities [43]. A total of 11 phenolic acids were accurately identified in the L. barbarum fruit extract, including hydroxybenzoic acids (compounds 6, 11, 12, and 38) and hydroxycinnamic acids (compounds 4, 8, 14, 20, 23, and 34). Taking compound 20 in Table S4 as an example, the main cracking process of phenolic acid compounds was analyzed. The retention time of compound 20 was 2.08 min. By comparing MS/MS fragment information with the rutin reference substance and related literature [44], compound 20 with m/z at 195.0658 [M + H]+ was determined as ferulic acid, because it showed characteristic MS/MS ions at m/z 180.1334 [M−CH3 + H]+ and m/z 136.0804 [M−CH3−CO2 + H]+ (Figure 5).
Figure 5.
Main cleavage pathway of ferulic acid.
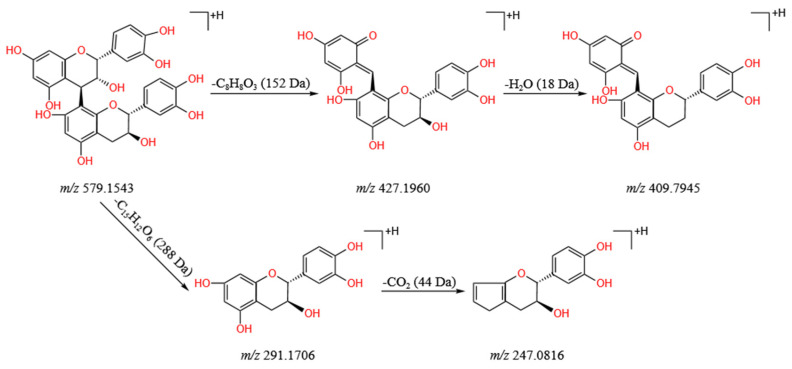
Tannins are water-soluble polyphenols with a relative molecular mass of between 500 and 3000, which exist widely in plants. Three tannins (compounds 5, 19, and 39) were accurately identified in the L. barbarum fruit extract. Taking compound 19 in Table S4 as an example, the main cracking mechanism of tannins was analyzed. The retention time of compound 19 was 2.03 min, and its protonated molecular ion was at m/z 579.1534 [M + H]+. As shown in Figure 6, compound 19 showed fragment ions at m/z 427.1960 [M−C8H8O3 + H]+; m/z 409.7945 (loss of C8H8O3 and H2O, respectively); m/z 291.1706 [M−288 + H]+; and 247.0816 [M−288−CO2 + H]+. By comparing the retention time, the Waters Progenesis QI database, and a previous report [45], compound 19 was inferred to be procyanidin B1.
Figure 6.
Main cleavage pathway of procyanidin B1.
2.5.2. Quantitative Analysis of Polyphenols in L. barbarum Fruit Extract
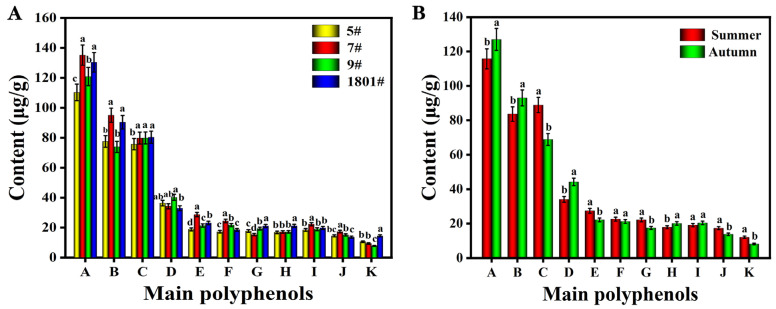
Based on the results of the qualitative analysis, the 11 main polyphenols in the L. barbarum fruit extract were quantitatively analyzed using the UPLC-IMS-QTOF-MS targeted metabolomics method and the external standard method. As shown in Table 3, the contents of rutin and p-coumaric acid were highest in the extract from L. barbarum fruit grown in Jinghe, at 125.38 μg/g and 90.05 μg/g, respectively, while the content of caffeic acid was the lowest, with a value of 10.44 μg/g. Most of the polyphenolic compounds in L. barbarum from Jinghe were found at higher levels than in fruit from Zhongning. In comparing varieties and harvest seasons (Figure 7), the contents of the main polyphenolic compounds in 7# and 1801# were generally higher than those in 5# and 9#, and the contents of rutin and p-coumaric acid found in autumn-harvested fruits were significantly higher than those in summer-harvested fruits, while ferulic acid was the opposite. The above analysis revealed that the polyphenolic compounds in fruits from different varieties of L. barbarum harvested in different seasons showed obvious differences, which provide favorable data for further targeted selection of L. barbarum for research purposes.
Table 3.
Main polyphenolic contents of L. barbarum fruits harvested from different geographical origins (n = 50).
| Compounds | Linear Equation | Linear Range (μg/L) | R2 | Contents in Jinghe (μg/g) | Contents in Zhongning (μg/g) |
|---|---|---|---|---|---|
| Rutin | y = 16.283x + 205.61 | 1500–90,000 | 0.9978 | 125.38 ± 21.89 | 98.59 ± 23.76 |
| p-coumaric acid | y = 8.9186x − 15058 | 3300–12,000 | 0.9973 | 90.05 ± 11.46 | 70.38 ± 6.97 |
| Ferulic acid | y = 3.7786x − 5304 | 5000–20,000 | 0.9957 | 78.75 ± 3.41 | 62.01 ± 5.65 |
| Quercetin | y = 12.129x − 21.878 | 450–6500 | 0.9954 | 38.62 ± 3.19 | 41.32 ± 8.23 |
| Kaempferol-3-O-rutinoside | y = 29.865x + 732.33 | 30–1500 | 0.9957 | 25.12 ± 3.60 | 18.22 ± 3.12 |
| Isoquercetin | y = 18.515x + 802.05 | 100–900 | 0.9946 | 21.54 ± 2.98 | 13.39 ± 2.40 |
| Isorhamnetin-3-glucoside | y = 28.776x + 586.64 | 10–180 | 0.9929 | 21.13 ± 2.82 | 17.56 ± 2.53 |
| Chlorogenic acid | y = 4.7343x − 767.2 | 400–3600 | 0.9960 | 18.65 ± 2.80 | 10.06 ± 2.71 |
| Isorhamnetin-3-O-rutinoside | y = 31.045x + 672.94 | 40–360 | 0.9962 | 18.17 ± 1.20 | 15.34 ± 1.91 |
| Isorhamnetin | y = 32.993x + 487.01 | 50–450 | 0.9939 | 15.30 ± 2.17 | 11.59 ± 3.61 |
| Caffeic acid | y = 1.3820x − 1179.7 | 10,000–70,000 | 0.9931 | 10.44 ± 2.62 | 9.06 ± 2.15 |
Figure 7.
Comparison of the main polyphenolic contents of fruits from different varieties of L. barbarum (A) harvested during different seasons (B). (A, rutin; B, p-coumaric acid; C, ferulic acid; D, quercetin; E, kaempferol-3-O-rutinoside; F, isoquercetin; G, chlorogenic acid; H, isorhamnetin-3-O-rutinoside; I, isorhamnetin-3-glucoside; J, isorhamnetin; K, caffeic acid). Different letters across treatments (a, b, c, d) indicate significant differences at p < 0.05.
3. Materials and Methods
3.1. Chemicals and Reagents
Leucine enkephalin was purchased from Waters Corporation (Milford, MA, USA). Chromatography-grade methanol, mass-spectrometry-grade acetonitrile, and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Folin & Ciocalteu phenol reagent was bought from Sigma-Aldrich, Co., Ltd. (St. Louis, MO, USA). Ferrous sulfate, ferric chloride, and analytical-grade ethyl alcohol were procured from Tianjin Xinbo Chemical Co., Ltd. (Tianjin, China). Analytical-grade anhydrous sodium carbonate (Na2CO3), aluminum chloride (AlCl3), potassium acetate, and gallic acid monohydrate (purity 99.0%) were obtained from Tianjin Yongsheng Fine Chemical Co., Ltd. (Tianjin, China). The phenolic standards such as rutin, quercetin, ferulic acid, caffeic acid, isorhamnetin, kaempferol 3-O-beta-rutinoside, p-coumaric acid, chlorogenic acid, and isoquercetin were purchased from Anpel Laboratory Technologies Inc. (Shanghai, China). Hydrochloric acid was purchased from Xi’an Chemical Reagent Factory (Xi’an, China). The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), and sodium acetate were provided by Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Ultrapure water was purchased from Watsons Group Ltd. (Hong Kong, China).
3.2. Instruments
The polyphenolic compounds were determined with an ultra-performance liquid chromatography ion mobility quadrupole time-of-flight mass spectrometer (UPLC-IM QTOF-MS) (Waters, Milford, MA, USA). The absorbance was measured using an ultraviolet and visible spectrophotometer (UV-2700) for the determination of total polyphenols and total flavonoids (Shimadzu, Kyoto, Japan). An XSE 204 balance was used to weigh L. barbarum samples (Mettler-Toledo, Greinfesee, Switzerland). The samples were mixed using an MS3 vortex mixer (IKA, Staufen, Germany) and centrifuged using a Sorvall biofuge Stratos system (Thermo Fisher Scientific, Waltham, MA, USA). The L. barbarum extracting solution was concentrated using a Hei-VAP Precision rotary evaporator (Heidolph, Schwabach, Germany).
3.3. Sample Collection
Four varieties of fresh L. barbarum fruit samples were collected from Jinghe County, Boertala Mongol Autonomous Prefecture, Xinjiang (E 82°17′–83°51′, N 44°32′–45°10′; altitude: 270–390 m) and Zhongning County, Zhongwei City, NingXia (E 105°33′–106°22′, N 37°17′–38°49′; altitude: 1080–1372 m) from June to September in 2020 and 2021, respectively, including “Ningqi 5#” (5#), “Ningqi 7#” (7#), “Ningqi 9#” (9#), and “Xinjiang local variety 1801#” (1801#) collected from Jinghe County (Figure 8A) and “Ningqi 5#” and “Ningqi 7#” collected from Zhongning County (Figure 8B). The L. barbarum fruit samples were at the same stage of maturity, with complete fruit grains and no diseases or pests. The fresh L. barbarum fruits were stored at −20 °C for further use. Overall, 150 samples were collected.
Figure 8.
(A) Four varieties (1801#, 7#, 9#, and 5#) of fresh L. barbarum fruits from Jinghe Xinjiang and (B) two varieties (5# and 7#) of fresh L. barbarum fruits from Zhongning.
3.4. Extract Collection
The fresh L. barbarum fruits were freeze-dried in a vacuum, ground into powder, and stored in brown glass bottles at −4 °C. Briefly, the dry ground L. barbarum samples (1.0 g) were added to 20 mL of 70% methanol solution containing 1.0% hydrochloric acid in 50 mL plastic centrifuge tubes. After mixing thoroughly, the samples were ultrasonicated at 40 °C for 30 min and then centrifuged at 10,000 rpm for 10 min using a high-speed refrigerated centrifuge. The supernatant was collected, and the extraction process was repeated twice. Then, the supernatants were combined and filtered. The mixture was adjusted to 35 mL with methanol and stored at −4 °C for further experiment.
3.5. Measurement of Total Polyphenols
The concentration of total polyphenols was detected using the Folin–Ciocalteau method according to a previous report with some modifications [46]. Gallic acid was regarded as an equivalent weight. The data were expressed as mg gallic acid equivalents (GAE)/g of dry weight (DW). Absorbance was measured at 765 nm. As shown in Figure S1, the content of total polyphenols was measured according to a standard calibration curve (y = 0.8861x + 0.0151) with a linear range from 0 to 1.75 mg/mL (R2 = 0.9997).
3.6. Measurement of Total Flavonoids
The concentration of total flavonoids was determined according to an aluminum trichloride colorimetric method, as reported by Wu et al. [47]. Rutin was used as the equivalent weight. The total flavonoid content was expressed as mg rutin equivalent (RE)/g of DW. Absorbance was measured at 415 nm. The content of total flavonoids was measured according to the standard calibration curve (y = 0.2823x + 0.0274) with a linear range from 0 to 4 mg/mL (R2 = 0.9997) (Figure S2).
3.7. Antioxidant Activity Analysis
3.7.1. Determination of DPPH Radical Scavenging Ability
The DPPH radical scavenging ability was determined using a DPPH radical scavenging capacity assay kit. Absorbance was measured with a multiscan spectrum microplate spectrophotometer (Bio Tek, Winooski, VT, USA) at 515 nm. First, different concentrations of positive control vitamin C (VC) standard solution (0.01–0.3 mg/mL) were prepared. The standard calibration curve was obtained (y = 0.7421x + 0.1428, R2 = 0.9922) by calculating the DPPH radical scavenging rate of VC. The DPPH radical scavenging ability of each L. barbarum sample was calculated, and the results were expressed as VC equivalent per gram of L. barbarum sample (mg VC/g DW). The radical scavenging rates of the positive control (1) and DPPH (2) were calculated as follows.
| Radical scavenging rate of positive control (%) = [(A0 − Acontrol)/A0] × 100 | (1) |
| Radical scavenging rate of DPPH (%) = [A0 − (A1 − A2)/A0] × 100 | (2) |
where A0 is the absorbance of the blank solution; Acontrol is the absorbance of VC; A1 is the absorbance of the added sample; and A2 is the absorbance of the sample control.
3.7.2. Determination of ABTS Radical Scavenging Ability
The ABTS radical scavenging ability was measured using an ABTS radical scavenging capacity assay kit. Absorbance was measured with a multiscan spectrum microplate spectrophotometer (Bio Tek, Winooski, VT, USA) at 405 nm. Different concentrations of positive-control VC standard solution (0.1–1 mmol/L) were prepared. The standard calibration curve was obtained (y = 0.7045x − 0.0076, R2 = 0.9962) by calculating the ABTS radical scavenging rate of VC. The ABTS radical scavenging ability was expressed as VC equivalents per gram of L. barbarum sample (mmol VC/g DW). The formulae used to calculate the radical scavenging rate of the positive control and ABTS were the same as in Section 3.7.1.
3.7.3. Determination of Ferric Ion Reducing Antioxidant Power
The FRAP was determined based on a previously reported method with some modifications [48]. FeSO4 standard solutions were prepared at different concentrations (0.5−4.0 mmol/mL). The FRAP working solutions consisted of 10 mmol/L TPTZ solution, 0.3 mol/L sodium acetate (pH 3.6), and 20 mmol/L FeCl3 solution in the ratio of 1:10:1. The mixture was placed in a water bath at 35 °C for 30 min. Then, 0.1 mL of FeSO4 standard solution and 0.9 mL of FRAP working solution were added to 6 mL of ultrapure water. After mixing them well and placing in a water bath at 35 °C for 30 min, the absorbance was measured at 597 nm. The standard calibration curve was obtained as y = 0.1356x + 0.0899 (R2 = 0.9994). The FRAP value was expressed as Fe2+ equivalents per gram of L. barbarum sample (mmol Fe2+/g DW).
3.8. UPLC-IM-QTOF-MS Analysis
UPLC-IM-QTOF-MS was used to detect the phenolic compounds in the L. barbarum samples. Firstly, the L. barbarum fruit extract was filtered through a 0.22 μm filtration membrane and injected into the UPLC-IM-QTOF-MS system. The polyphenolic compounds in the prepared samples were separated in an ACQUITY UPLC-BEN C18 column (2.1 × 50 mm, 1.7 μm; Waters, Milford, MA, USA) at 35 °C. The mobile phase was water (A) and acetonitrile (B). The gradient elution was as follows: 0−1 min, 99% A; 1−10 min, A from 99% to 0%; 10−11 min, 0% A; and 11−13 min, A from 0% to 99%. The flow rate was 0.3 mL/min, and the injection volume was 5 μL.
Ion source: electrospray ionization (ESI) was used in positive-ion mode with the following parameters: source temperature, 100 °C; capillary voltage, 3 kV; desolvation temperature, 350 °C; gas flow, 900 L/h; low collision energy, 6 eV; and higher collision energy, 10 to 40 eV.
3.9. Statistical Analysis
All data are presented as the mean ± standard deviation. SPSS 26.0 software was used to perform statistical analysis. Significant differences in the polyphenol concentrations in L. barbarum fruits from different geographical origins and varieties were calculated using a one-way ANOVA, along with the Duncan test. UNIFI 1.8 software and the Waters Progenesis QI database were used for qualitative and quantitative analysis of polyphenolic compounds in L. barbarum. Origin 2019b software was used to compile charts.
4. Conclusions
The total polyphenol and flavonoid contents of different L. barbarum fruit varieties grown in two geographical origins, Jinghe and Zhongning, and harvested during different seasons were analyzed. The polyphenol contents of L. barbarum fruits harvested in autumn were generally higher than those of fruits harvested in summer. The polyphenolic contents and antioxidant capacities (DPPH, ABTS, and FRAP) of fruits from different geographical origins and varieties of L. barbarum were significantly different (p < 0.05). A UPLC-IM-QTOF-MS metabolomics-based method was established for the qualitative and quantitative analysis of the polyphenolic compounds in L. barbarum. A total of 39 polyphenolic compounds were identified, including 26 flavonoids, 10 phenolic acids, and three tannins. It could be seen that the polyphenols in Jinghe-sourced L. barbarum were mainly flavonoids, followed by phenolic acids. The contents of all kinds of polyphenols of L. barbarum grown in Jinghe were generally higher than in those harvested from Zhongning, which revealed that more hours of sunlight and an appropriate amount of water shortage were more conducive to the accumulation of polyphenols. In addition, the contents of polyphenolic compounds in different varieties of L. barbarum and in fruits harvested during different seasons showed obvious differences. This study explored the polyphenolic components of L. barbarum, providing scientific and technological support for further high-value development and utilization of L. barbarum.
Acknowledgments
Thanks to Jinghexian Tianshanguoye Agricultural Science and Technology Co. Ltd. for providing the materials used in the experiments.
Supplementary Materials
The following supporting information can be downloaded from: https://www.mdpi.com/article/10.3390/molecules28134930/s1. Table S1: The climatic conditions of the different geographical origins at which L. barbarum samples grew in 2020; Table S2: The total flavonoid contents of different L. barbarum varieties harvested during summer and autumn (X ± s, n = 3); Table S3: Correlation of total polyphenolic content, total flavonoid content, and antioxidant capacity of L. barbarum; Table S4: The polyphenolic compounds identified in L. barbarum; Figure S1: The regression equation of gallic acid; Figure S2: The regression equation of rutin.
Author Contributions
Conceptualization, D.Z.; Data curation, Y.J., Y.W. and X.M.; Formal analysis, Y.J., L.K. and X.M.; Funding acquisition, Y.J., Y.W. and D.Z.; Investigation, L.M.; Methodology, Y.J. and D.Z.; Project administration, H.L.; Supervision, L.M. and D.Z.; Validation, L.M. and H.L.; Visualization, Y.W. and L.K.; Writing—original draft, Y.J.; Writing—review and editing, D.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of L. barbarum used in this study are available from the authors.
Funding Statement
This research was funded by the Project of Renovation Capacity Building for the Young Sci-Tech Talents Sponsored by Xinjiang Academy of Agricultural Sciences (grant no. xjnkq-2020013), Xinjiang Academy of Agricultural Sciences Science and Technology Innovation Platform Capacity Improvement Construction Project (grant no. xnypt006), the Project of Renovation Capacity Building for the Young Sci-Tech Talents Sponsored by Xinjiang Academy of Agricultural Sciences (grant no. xjnkq-2023018), Local Sci-Tech Development Guided by the Central Government of China, and Innovation Environment (Talent, Base) Construction Special Project of Xinjiang Uygur Autonomous Region-Resource Sharing Platform Construction (PT2312).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhao D., Kai J., Zhao T.B., Qiao C. Study on optimization of extraction process of Lycium barbarum L. polyphenols by response surface methodology. IOP Conf. Ser. Earth Environ. Sci. 2020;531:12069. doi: 10.1088/1755-1315/531/1/012069. [DOI] [Google Scholar]
- 2.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia (Volume 1) China Medical Science Press; Beijing, China: 2020. pp. 128–260. [Google Scholar]
- 3.Yu M.S., Leung S.K.Y., Lai S.W., Che C.M., Zee S.Y., So K.F., Yuen W.H., Chang R.C.C. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against b-amyloid peptide neurotoxicity. Exp. Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L.F. Preparation and antioxidant activity of Lycium barbarum oligosaccharides. Carbohydr. Polym. 2014;99:646–648. doi: 10.1016/j.carbpol.2013.08.084. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S.S., Wei Y.M., Wei S., Liu H.Y., Guo B.L. Authentication of Zhongning wolfberry with geographical indication by mineral profile. Int. J. Food Sci. Technol. 2017;52:457–463. doi: 10.1111/ijfs.13301. [DOI] [Google Scholar]
- 6.Zhang G., Chen S., Zhou W., Meng J., Deng K., Zhou H., Hu N., Suo Y. Rapid qualitative and quantitative analyses of eighteen phenolic compounds from Lycium ruthenicum Murray by UPLC-Q-Orbitrap MS and their antioxidant activity. Food Chem. 2018;269:150–156. doi: 10.1016/j.foodchem.2018.06.132. [DOI] [PubMed] [Google Scholar]
- 7.Wen S., Xu Y., Niu C., Liu Q., Zhang L., Xiang Y., Wang W., Sun Z. Chemical constituents of the fruits of Lycium barbarum and their neuroprotective activity. Chem. Nat. Compd. 2020;56:923–926. doi: 10.1007/s10600-020-03188-8. [DOI] [Google Scholar]
- 8.Kan X., Yan Y., Ran L., Lu L., Cao Y. Ultrasonic-assisted extraction and high-speed counter-current chromatography purification of zeaxanthin dipalmitate from the fruits of Lycium barbarum L. Food Chem. 2019;310:125854. doi: 10.1016/j.foodchem.2019.125854. [DOI] [PubMed] [Google Scholar]
- 9.Wang P., Wang J., Zhang H., Wang C., Zhao L., Huang T., Qing K. Chemical composition, crystal morphology, and key gene expression of the cuticular waxes of Goji (Lycium barbarum L.) berries. J. Agr. Food Chem. 2021;69:7874–7883. doi: 10.1021/acs.jafc.1c02009. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Guo S., Zhou J., Zhu Y., Zhang F., Zeng F., Duan R., Xu M., Duan J. Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021;97:104292. doi: 10.1016/j.bse.2021.104292. [DOI] [Google Scholar]
- 11.Liu W., Xia M., Bai J., Yang L., Wang Z., Wang R., Shi Y. Chemical characterization and 5α-reductase inhibitory activity of phenolic compounds in goji berries. J. Pharm. Biomed. Anal. 2021;201:114119. doi: 10.1016/j.jpba.2021.114119. [DOI] [PubMed] [Google Scholar]
- 12.Mocan A., Cairone F., Locatelli M., Cacciagrano F., Carradori S., Vodnar D.C., Crișan G., Simonetti G., Cesa S. Polyphenols from Lycium barbarum (Goji) fruit European cultivars at different maturation steps: Extraction, HPLC-DAD analyses, and biological evaluation. Antioxidants. 2019;8:562. doi: 10.3390/antiox8110562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mocan A., Moldovan C., Zengin G., Bender O., Locatelli M., Simirgiotis M., Atalay A., Vodnar D.C., Rohn S., Crișan G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018;115:414–424. doi: 10.1016/j.fct.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W., Zhang Y., Shi Y. Visualizing the spatial distribution of endogenous molecules in wolfberry fruit at different development stages by matrix-assisted laser desorption/ionization mass spectrometry imaging. Talanta. 2021;234:122687. doi: 10.1016/j.talanta.2021.122687. [DOI] [PubMed] [Google Scholar]
- 15.Liu J., Meng J., Du J., Liu X., Pu Q., Di D., Chen C. Preparative separation of flavonoids from Goji berries by mixed-mode macroporous adsorption resins and effect on Aβ-expressing and anti-aging genes. Molecules. 2020;25:3511. doi: 10.3390/molecules25153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olech M., Kasprzak K., Wójtowicz A., Oniszczuk T., Nowak R., Waksmundzka-Hajnos M., Combrzyński M., Gancarz M., Kowalska I., Krajewska A., et al. Polyphenol composition and antioxidant potential of instant gruels enriched with Lycium barbarum L. fruit. Molecules. 2020;25:4538. doi: 10.3390/molecules25194538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y., Pang X., Zhu X., Meng Z., Chen X., Zhang J., Ding Q., Li Q., Dou G., Ma B. Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites. Biomed. Pharmacother. 2021;139:111654. doi: 10.1016/j.biopha.2021.111654. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Ma Z., Wang J., Wang P., Lu D., Deng S., Lei H., Gao Y., Tao Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT. 2021;140:110837. doi: 10.1016/j.lwt.2020.110837. [DOI] [Google Scholar]
- 19.Yang T., Hu Y., Yan Y., Zhou W., Chen G., Zeng X., Cao Y. Characterization and evaluation of antioxidant and anti-inflammatory activities of flavonoids from the fruits of Lycium barbarum. Foods. 2022;11:306. doi: 10.3390/foods11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocan A., Vlase L., Vodnar D.C., Bischin C., Hanganu D., Gheldiu A.M., Oprean R., Silaghi-Dumitrescu R., Crișan G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules. 2014;19:10056–10073. doi: 10.3390/molecules190710056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q., Chen W., Zhao J., Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–236. doi: 10.1016/j.foodchem.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Zhu P., Zhao Y., Dai Z., Qin X., Yuan H., Jin Q., Wang Y., Liu Y., Luo X. Phenolic amides with immunomodulatory activity from the nonpolysaccharide fraction of Lycium barbarum fruits. J. Agr. Food Chem. 2020;68:3079–3087. doi: 10.1021/acs.jafc.9b07499. [DOI] [PubMed] [Google Scholar]
- 23.Bondia-Pons I., Savolainen O., Törrönen R., Martinez J.A., Poutanen K., Hanhineva K. Metabolic profiling of Goji berry extracts for discrimination of geographical origin by non-targeted liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2014;63:132–138. doi: 10.1016/j.foodres.2014.01.067. [DOI] [Google Scholar]
- 24.Gouda M., Nassarawa S.S., Gupta S.D., Sanusi N.I.I., Nasiru M.M. Evaluation of carbon dioxide elevation on phenolic compounds and antioxidant activity of red onion (Allium cepa L.) during postharvest storage. Plant Physiol. Biochem. 2023;200:107752. doi: 10.1016/j.plaphy.2023.107752. [DOI] [PubMed] [Google Scholar]
- 25.Duan W., Zhang Z., Zhu J., Zhang D., Qian D., Teng F., Zhao Y., Chen F., Li R., Yang J. Comparative analysis of the phenolic profile of Lycium barbarum L. fruits from different regions in China. Molecules. 2022;27:5842. doi: 10.3390/molecules27185842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y.J., Liang X.J., Guo S.J., Li Y.K., Zhang B., Yin Y., An W., Cao Y.L., Zhao J.H. Evaluation of nutrients and related environmental factors for wolfberry (Lycium barbarum) fruits grown in the different areas of China. Biochem. Syst. Ecol. 2019;86:103916. [Google Scholar]
- 27.Wang Y., Liang X., Li Y., Fan Y., Li Y., Cao Y., An W., Shi Z., Zhao J., Guo S. Changes in metabolome and nutritional quality of Lycium barbarum fruits from three typical growing areas of China as revealed by widely targeted metabolomics. Metabolites. 2020;10:46. doi: 10.3390/metabo10020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y.Y., Guo S., Zhang F., Yan H., Qian D., Wang H., Jin L., Duan J. Comparison of functional components and antioxidant activity of Lycium barbarum L. fruits from different regions in China. Molecules. 2019;24:2228. doi: 10.3390/molecules24122228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yossa Nzeuwa I.B., Guo B., Zhang T., Wang L., Ji Q., Xia H., Sun G. Comparative metabolic profiling of Lycium fruits (Lycium barbarum and Lycium chinense) from different areas in China and from Nepal. J. Food Qual. 2019;2019:4396027. doi: 10.1155/2019/4396027. [DOI] [Google Scholar]
- 30.Liu J., Shi X., Lin H., He C., Li Q., Shen G., Feng J. Geographical origin identification and quality comparison of Ningxia goji berries (Lycium barbarum L.) by NMR-based techniques. J. Food Compos. Anal. 2023;119:105258. doi: 10.1016/j.jfca.2023.105258. [DOI] [Google Scholar]
- 31.Cheng H., Wu W., Chen J., Pan H., Xu E., Chen S., Ye X., Chen J. Establishment of anthocyanin fingerprint in black wolfberry fruit for quality and geographical origin identification. LWT. 2022;157:113080. doi: 10.1016/j.lwt.2022.113080. [DOI] [Google Scholar]
- 32.Lin S., Guo H., Gong J.D.B., Lu M., Lu M., Wang L., Zhang Q., Qin W., Wu D. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cereal Sci. 2018;81:69–75. doi: 10.1016/j.jcs.2018.04.001. [DOI] [Google Scholar]
- 33.Magiera S., Zaręba M. Chromatographic determination of phenolic acids and flavonoids in Lycium barbarum L. and evaluation of antioxidant activity. Food Anal. Methods. 2015;8:2665–2674. doi: 10.1007/s12161-015-0166-y. [DOI] [Google Scholar]
- 34.Wu T., Lv H.Y., Wang F.Z., Wang Y. Characterization of polyphenols from Lycium ruthenicum fruit by UPLC-Q-TOF/MSE and their antioxidant activity in Caco-2 cells. J. Agric. Food Chem. 2016;64:2280–2288. doi: 10.1021/acs.jafc.6b00035. [DOI] [PubMed] [Google Scholar]
- 35.Rocchetti G., Chiodelli G., Giuberti G., Ghisoni S., Baccolo G., Blasi F., Montesano D., Trevisan M., Lucini L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods. 2018;40:564–572. doi: 10.1016/j.jff.2017.11.042. [DOI] [Google Scholar]
- 36.Lv J.M., Gouda M., Zhu Y.Y., Ye X.Q., Chen J.C. Ultrasound-assisted extraction optimization of proanthocyanidins from kiwi (Actinidia chinensis) leaves and evaluation of its antioxidant activity. Antioxidants. 2021;10:1317. doi: 10.3390/antiox10081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan A.U., Dagur H.S., Khan M., Malik N., Alam M., Mushtaque M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur. J. Med. Chem. Rep. 2021;3:100010. doi: 10.1016/j.ejmcr.2021.100010. [DOI] [Google Scholar]
- 38.Jiang S., Chen C., Dong Q., Shao Y., Zhao X., Tao Y., Yue H. Alkaloids and phenolics identification in fruit of Nitraria tangutorum Bobr. By UPLC-Q-TOF-MS/MS and their a-glucosidase inhibitory effects in vivo and in vitro. Food Chem. 2021;364:130412. doi: 10.1016/j.foodchem.2021.130412. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y.Y., Lu H.L., Wang Q., Liu H.L., Shen H.T., Xu W.B., Ge J., He D.J. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021;44:1404–1420. doi: 10.1002/jssc.202000962. [DOI] [PubMed] [Google Scholar]
- 40.Kroslakova I., Pedrussio S., Wolfram E. Direct coupling of HPTLC with MALDI-TOF MS for qualitative detection of flavonoids on phytochemical fingerprints. Phytochem. Anal. 2016;27:222–228. doi: 10.1002/pca.2621. [DOI] [PubMed] [Google Scholar]
- 41.Goufo P., Pereira J., Moutinho-Pereira J., Correia C.M., Figueiredo N., Carranca C., Rosa E., Trindade H. Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ. Exp. Bot. 2014;99:28–37. doi: 10.1016/j.envexpbot.2013.10.021. [DOI] [Google Scholar]
- 42.Thomas M., Badr A., Desjardins Y., Gosselin A., Angers P. Characterization of industrial broccoli discards (Brassica oleracea var. Italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018;245:1204–1211. doi: 10.1016/j.foodchem.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y., Wang M., Jiang N., Wang Y., Feng X. Use of ultra–performance liquid chromatography-tandem mass spectrometry on sweet cherries to determine phenolic compounds in peel and flesh. J. Sci. Food Agr. 2019;99:3555–3562. doi: 10.1002/jsfa.9576. [DOI] [PubMed] [Google Scholar]
- 44.Biesaga M., Pyrzynska K. Liquid chromatography/tandem mass spectrometry studies of the phenolic compounds in honey. J. Chromatogr. A. 2009;1216:6620–6626. doi: 10.1016/j.chroma.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Jiménez J., Torres J.L. Analysis of proanthocyanidins in almond blanch water by HPLC-ESI-QqQ-MS/MS and MALDI-TOF/TOF MS. Food Res. Int. 2012;49:798–806. doi: 10.1016/j.foodres.2012.09.005. [DOI] [Google Scholar]
- 46.Chen Z., Zhong B., Barrow C.J., Dunshea F.R., Suleria H.A.R. Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Arab. J. Chem. 2021;14:103151. doi: 10.1016/j.arabjc.2021.103151. [DOI] [Google Scholar]
- 47.Wu S., Shen D., Wang R., Li Q., Mo R., Zheng Y., Zhou Y., Liu Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021;350:129217. doi: 10.1016/j.foodchem.2021.129217. [DOI] [PubMed] [Google Scholar]
- 48.Liang Y., Li Y., Zhang L., Liu X. Phytochemicals and antioxidant activity in four varieties of head cabbages commonly consumed in China. Food Prod. Process. Nutr. 2019;1:1–9. doi: 10.1186/s43014-019-0003-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.