BACKGROUND:
Low-titer group O whole blood (LTOWB) resuscitation is becoming common in both military and civilian settings and may represent the ideal resuscitation intervention. We sought to characterize the safety and efficacy of LTOWB resuscitation relative to blood component resuscitation.
STUDY DESIGN:
A prospective, multicenter, observational cohort study was performed using 7 trauma centers. Injured patients at risk of massive transfusion who required both blood transfusion and hemorrhage control procedures were enrolled. The primary outcome was 4-hour mortality. Secondary outcomes included 24-hour and 28-day mortality, achievement of hemostasis, death from exsanguination, and the incidence of unexpected survivors.
RESULTS:
A total of 1,051 patients in hemorrhagic shock met all enrollment criteria. The cohort was severely injured with >70% of patients requiring massive transfusion. After propensity adjustment, no significant 4-hour mortality difference across LTOWB and component patients was found (relative risk [RR] 0.90, 95% CI 0.59 to 1.39, p = 0.64). Similarly, no adjusted mortality differences were demonstrated at 24 hours or 28 days for the enrolled cohort. When patients with an elevated prehospital probability of mortality were analyzed, LTOWB resuscitation was independently associated with a 48% lower risk of 4-hour mortality (relative risk [RR] 0.52, 95% CI 0.32 to 0.87, p = 0.01) and a 30% lower risk of 28-day mortality (RR 0.70, 95% CI 0.51 to 0.96, p = 0.03).
CONCLUSIONS:
Early LTOWB resuscitation is safe but not independently associated with survival for the overall enrolled population. When patients were selected with an elevated probability of mortality based on prehospital injury characteristics, LTOWB was independently associated with a lower risk of mortality starting at 4 hours after arrival through 28 days after injury.
This study demonstrates the safety, feasibility, and efficacy of whole blood resuscitation in severely injured patients.
Hemorrhage remains the leading cause of potentially preventable death after injury.1,2 Despite major improvements in trauma resuscitation during the past 2 decades, patients continue to suffer high mortality due to uncontrolled hemorrhage in the first few hours after arrival.1,3,4 Interventions that provide outcome benefits that can be initiated early after injury have the potential to reduce morbidity and mortality and are essential to improving the care of the severely injured patient.5-7
Low-titer group O whole blood (LTOWB) resuscitation is increasingly common in both military and civilian settings and may represent the ideal early resuscitation intervention after injury. Recent studies demonstrate the safety of uncrossmatched LTOWB.6,8-11 Although early LTOWB resuscitation has increasingly been shown to be associated with improved outcomes, current high-level prospective, multicenter evidence supporting its pragmatic use is limited, specifically in the polytrauma patient with shock and concomitant traumatic brain injury (TBI).8,9,12 It may be in these complex injured patients where the character of early resuscitation matters most and where outcome benefits may be most evident.13-18
We sought to characterize the safety and efficacy of LTOWB in patients with hemorrhagic shock with and without concomitant TBI treated with early LTOWB resuscitation relative to patients who receive blood component resuscitation (COMPONENT) as their standard care. We hypothesized that LTOWB resuscitation would be associated with both survival and improved hemostasis.
METHODS
A prospective, multicenter, observational cohort study, with a planned enrollment time period of 3.5 years, was performed using 7 busy, level 1, trauma centers participating in the Linking Investigations in Trauma and Emergency Services (LITES, https://www.litesnetwork.org) clinical trials network. The cohort study was conducted and reported in accordance with the STROBE guidelines for observational studies.19 Ethical approval for the study was obtained using single IRB approval from the University of Pittsburgh and the Human Research Protection Office of the Department of Defense. The single IRB approved a waiver/alteration of the consent process and waiver of Health Insurance Portability and Accountability Act authorization spanning 36 hours.
Participating trauma centers were originally surveyed for the use of cold stored LTOWB in the early phase of injury as part of their standard care for patients in hemorrhagic shock. At study initiation, three of the original 7 participating trauma centers had existing early, in-hospital, cold-stored LTOWB resuscitation programs employed in their emergency department/trauma bay setting. Characteristics of each LTOWB program, including leukoreduction, titer levels used, and specific indications for LTOWB transfusion (eg childbearing age status), were at the discretion of each site’s local resuscitation protocol. A single LTOWB trauma center site also had the capability to provide LTOWB during the prehospital phase of care. The remaining COMPONENT sites used ratio-based blood component resuscitation strategies for hemorrhagic shock and similarly followed their respective local resuscitation protocols. Inclusion criteria for the cohort study were injured patients at risk of massive transfusion who met Assessment of Blood Consumption criteria20,21 (two or more of the following): hypotension (systolic blood pressure ≤ 90 mmHg); penetrating mechanism of injury; positive Focused Assessment for the Sonography of Trauma examination; heart rate ≥ 120; and who within 60 minutes of arrival required both blood/blood component transfusion and hemorrhage control procedures in the operating room or interventional radiology suite. Patients with qualifying vital signs and/or blood product transfusion that occurred in the prehospital phase of care also met inclusion criteria. A Focused Assessment for the Sonography of Trauma examination that was deferred due to the expedient transport to the operating room was considered as meeting one of the Assessment of Blood Consumption criteria.20 The presence of TBI for the study was designated by positive CT scan brain imaging after enrollment criteria were met. Exclusion criteria included age less than 15 years, penetrating brain injury, >5 minutes of consecutive CPR, death before initiation of transfer to the operating room/interventional radiology for hemorrhage control procedures, known prisoners, and known pregnancy.
Data were collected via a research electronic data capture (REDCap) online data repository for all participating sites, and all analyses were performed using SAS version 9.4. Measures included patient demographics, injury characteristics, prehospital and in-hospital vital signs, resuscitation interventions, transfusion volume totals, mortality outcomes, and laboratory assessments. Additional outcomes, including Rotterdam CT scores were collected for the TBI subgroup.22,23
The primary objectives of the study were to compare patient-level outcomes across early LTOWB and COMPONENT resuscitation groups. A LTOWB patient had to have received at least a single unit of LTOWB during the prehospital or early in-hospital phase of care. COMPONENT patients received only component blood products during their early resuscitation. Transfusion volume of any resuscitation type was based on patient need and the local site-specific transfusion practice. The prespecified primary outcome for the trial was 4-hour mortality. Secondary outcomes of interest included 24-hour mortality, 28-day mortality, achievement of hemostasis, adjudicated death from exsanguination/hemorrhage, and the incidence of unexpected survivors based on a prehospital probability of mortality >50% at 28 days.17,24-26 Unexpected survivor characterization was a post hoc subgroup analysis. Laboratory assessment of coagulation status and 4-hour and 24-hour transfusion requirements were also compared. Additionally, we assessed the incidence of multiple organ dysfunction, nosocomial infection, venous thromboembolism, deep venous thrombosis, pulmonary embolus, and laboratory markers of hemolysis for verification of safety. The presence of TBI was a prespecified subgroup for mortality outcomes. Rotterdam scores23 were determined by a single blinded neuroradiologist for all initial head CT scans and repeat head CT imaging when performed. Achievement of hemostasis was determined by reaching a nadir transfusion requirement of a single unit of whole blood or component red blood cells within a 60-minute period during the first 4 hours from arrival. Patients who did not achieve hemostasis or died within this 4-hour time frame were designated as not achieving hemostasis. Four-hour and 24-hour transfusion requirements were compared using units of product. Total transfusion volume requirements were compared by summing total volume in milliliters of transfusion across LTOWB and COMPONENT groups. The volume of each component transfused was estimated (red cell unit, 330 mL; plasma unit, 270 mL; single apheresis platelet unit, 250 mL) and the volume of a unit of LTOWB was estimated to equal to 500 mL.9 Massive transfusion was defined as the need for at least 3 units or more of any red blood cell–containing product (COMPONENT red blood cells or LTOWB) within a 60-minute time period during the first 4 hours from arrival.27-30 Causes of death due to exsanguination/hemorrhage were adjudicated by the enrolling site principal investigator.
All outcome models estimate a relative risk ratio by fitting a generalized linear model with a Poisson distribution, a log link function, and a robust variance adjustment.31 For the 4-hour mortality primary outcome across COMPONENT and LTOWB patients, an adjusted relative risk ratio was estimated using an inverse probability of treatment weight derived from a generalized boosted regression where treatment was regressed on a set of prehospital patient confounders, including vital signs, interventions/procedures, and measures of injury severity. For all other adjusted outcome comparisons, relative risk ratios were estimated controlling for age, sex, mechanism of injury (blunt vs penetrating), head Abbreviated Injury Score, Injury Severity Score (ISS), prehospital systolic blood pressure, and the need for prehospital blood product transfusion. The prehospital probability of mortality was estimated using logistic regression models using all relevant prehospital vital signs, prehospital interventions/procedures, and injury severity characteristics and was assessed using receiver operating characteristic curve analysis. Data from COMPONENT patients were used to fit the model, and the results were then applied across the entire enrolled cohort. Tests of association included Student‘s t-test when continuous measures were normally distributed, Mann-Whitney U when they were skewed, and chi-square when measures were categorical.
RESULTS
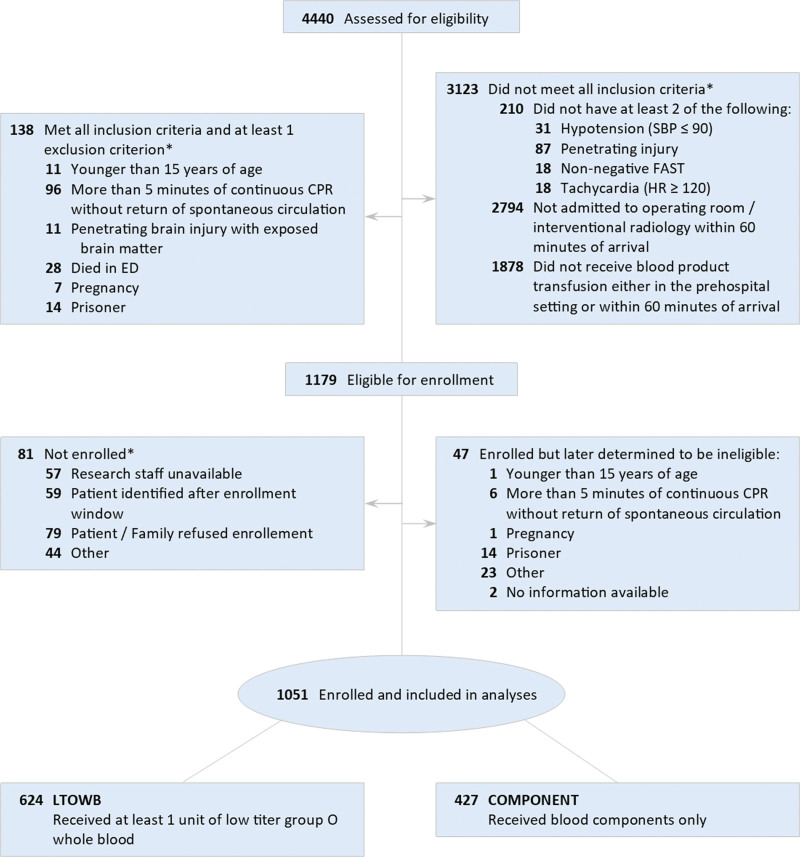
Over the planned 3.5-year enrollment period, 1,051 patients in hemorrhagic shock met all inclusion criteria and no exclusion criteria and were enrolled in the prospective cohort study (42 months; March 2018 to August 2021; Fig. 1). As early whole blood resuscitation became more accessible across the country, sites initially surveyed as COMPONENT sites initiated whole blood programs and became LTOWB-capable sites. More than 60% of patients had sustained a penetrating mechanism of injury (gunshot wound or stabbing). The enrolled study population was severely injured with a median injury ISS of 22 (interquartile range 13 to 30), and a 4-hour and a 28-day mortality rate of 8% and 17%, respectively. More than 70% of enrolled patients required massive transfusion..28 The enrolled cohort of patients had an incidence of radiographically documented TBI of 13.3%.
Figure 1.
CONSORT diagram for enrollment. ED, emergency department; FAST, Focused Assessment with Sonography in Trauma; HR, heart rate; LTOWB, low-titer group O whole blood; SBP, systolic blood pressure.
The prospective observational eligibility criteria attempted to select patients in hemorrhagic shock but did not stipulate any specific blood product resuscitation regimen. Enrolled patients at their respective trauma centers followed their standard resuscitation protocols. Only 66.3% of enrolled patients at LTOWB sites received LTOWB during their resuscitation. In those patients who received LTOWB, the median number of LTOWB units transfused was 2.0 interquartile range [1.0 to 3.5]. Of the subgroup of enrolled patients with TBI, 75% of patients at LTOWB sites received LTOWB resuscitation, with a median of 2.0 interquartile range [0.0 to 4.0] of LTOWB units being transfused.
When enrolled patients were compared by the early resuscitation regimen they received (LTOWB vs COMPONENT resuscitation), LTOWB and COMPONENT patients were similar in age, mechanisms of injury, and the need for prehospital blood transfusion. LTOWB and COMPONENT patients had similar ISS scores, but LTOWB patients were more likely to have an ISS >15. LTWOB patients were more likely male, were more commonly transferred from the scene of injury, had lower systolic blood pressures, had lower Glasgow Coma Scale scores, and were more likely to have concomitant TBI (Table 1).
Table 1.
Demographic and Injury Characteristics by Resuscitation Type
| Measure | LTOWB (n = 624) |
COMPONENT (n = 427) |
p Value |
|---|---|---|---|
| Age, y, median [IQR] | 35.0 [26.0–51.0] | 35.0 [25.0–47.0] | 0.15 |
| Sex, m, n (%) | 546 (87.5) | 297 (69.6) | <0.001 |
| Race, n (%) | 0.001 | ||
| White | 280 (44.9) | 235 (55.0) | |
| Black | 225 (36.1) | 139 (32.6) | |
| Other | 119 (19.1) | 53 (12.4) | |
| Injury mechanism, n (%) | |||
| Blunt | 252 (40.5) | 161 (37.7) | 0.36 |
| Fall | 29 (4.7) | 21 (4.9) | 0.85 |
| Machinery | 2 (0.3) | 1 (0.2) | 0.79 |
| MVC occupant ejected | 14 (2.3) | 15 (3.5) | 0.22 |
| MVC occupant not ejected | 93 (15.0) | 63 (14.8) | 0.93 |
| MVC motorcyclist | 53 (8.5) | 19 (4.4) | 0.010 |
| MVC cyclist | 4 (0.6) | 2 (0.5) | 0.71 |
| MVC pedestrian | 26 (4.2) | 23 (5.4) | 0.36 |
| MVC ATV | 5 (0.8) | 4 (0.9) | 0.82 |
| MVC not otherwise specified | 6 (1.0) | 3 (0.7) | 0.65 |
| Struck by or against | 14 (2.3) | 8 (1.9) | 0.68 |
| Other | 16 (2.6) | 8 (1.9) | 0.46 |
| Penetrating | 386 (62.1) | 271 (63.5) | 0.64 |
| Firearm | 281 (45.2) | 193 (45.2) | 0.99 |
| Impalement | 6 (1.0) | 2 (0.5) | 0.36 |
| Stabbing | 71 (11.4) | 52 (12.2) | 0.71 |
| Other | 31 (5.0) | 26 (6.1) | 0.44 |
| Transfer origin, n (%) | <0.001 | ||
| Scene | 526 (84.6) | 323 (75.6) | |
| Outside ED | 96 (15.4) | 104 (24.4) | |
| ISS, median [IQR] | 22.0 [14.0–33.0] | 21.0 [10.0–34.0] | 0.17 |
| >15, n (%) | 444 (72.8) | 281 (66.7) | 0.037 |
| Traumatic brain injury (CT diagnosed), n (%) | 99 (15.9) | 44 (10.3) | 0.010 |
| Head AIS, median [IQR] | 0.00 [0.00–2.00] | 0.00 [0.00–0.00] | 0.20 |
| >2, n (%) | 133 (21.8) | 76 (18.1) | 0.14 |
| Chest AIS, median [IQR] | 2.00 [0.00–3.00] | 2.00 [0.00–3.00] | 0.032 |
| >2, n (%) | 284 (46.6) | 175 (41.6) | 0.11 |
| Abdomen AIS, median [IQR] | 3.00 [0.00–4.00] | 3.00 [0.00–4.00] | 0.28 |
| >2, n (%) | 321 (52.6) | 230 (54.6) | 0.53 |
| Extremity AIS, median [IQR] | 2.00 [0.00–3.00] | 2.00 [0.00–3.00] | 0.66 |
| >2, n (%) | 220 (36.2) | 151 (35.9) | 0.92 |
| Glasgow Coma Scale, median [IQR] | 14.0 [3.00–15.0] | 15.0 [7.00–15.0] | 0.004 |
| <9, n (%) | 208 (35.4) | 109 (26.0) | 0.001 |
| Systolic blood pressure, mmHg, median [IQR] | 99.0 [80.0–120] | 105 [82.0–122] | 0.046 |
| <90 mmHg, n (%) | 176 (35.1) | 126 (32.1) | 0.36 |
| Heart rate, bpm, median [IQR] | 110 [88.0–130] | 108 [88.0–126] | 0.29 |
| >100 bpm, n (%) | 322 (58.7) | 248 (61.4) | 0.39 |
| Shock index, median [IQR] | 1.06 [0.81–1.37] | 1.00 [0.79–1.31] | 0.10 |
| Received any prehospital blood product, n (%) | 225 (36.2) | 138 (32.3) | 0.20 |
| Red blood cells | 128 (20.6) | 122 (28.6) | 0.003 |
| Plasma | 36 (5.8) | 32 (7.5) | 0.27 |
| Platelets | 12 (1.9) | 16 (3.7) | 0.07 |
| Whole blood | 118 (19.0) | 0 (0.0) | |
| Received any prehospital tranexamic acid, n (%) | 35 (5.6) | 15 (3.5) | 0.11 |
| Received any prehospital crystalloid, n (%) | 309 (49.7) | 227 (53.2) | 0.27 |
| Prehospital/ED intubation, n (%) | 225 (36.1) | 131 (30.7) | 0.07 |
| Prehospital/ED CPR, n (%) | 47 (7.5) | 29 (6.8) | 0.64 |
| Prehospital pelvic binder, n (%) | 47 (7.6) | 31 (7.3) | 0.86 |
AIS, Abbreviated Injury Scale; ATV, all-terrain vehicle; COMPONENT, blood component resuscitation; ED, emergency department; IQR, interquartile range; ISS, injury severity score; LTOWB, low-titer group O whole blood; MVC, motor vehicle collision.
When the primary 4-hour mortality outcome was compared across LTOWB and COMPONENT patients (Table 2), unadjusted mortality rates were similar (8.2% vs 7.5%, p = 0.71) After propensity adjustment, no significant 4-hour mortality difference across LTOWB and COMPONENT patients was found (RR 0.90, 95% CI 0.59 to 1.39, p = 0.64). Similarly, when 4-hour mortality was compared across the TBI subgroup, no significant mortality differences were found. When 24-hour mortality and 28-day mortality were compared, no unadjusted or adjusted mortality differences were demonstrated for the overall cohort or the TBI subgroup (Table 2).
Table 2.
Primary and Secondary Outcomes by Resuscitation Type
| Outcomes | LTOWB (n = 624) |
COMPONENT (n = 427) |
Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p Value | RR | 95% CI | p Value | |||
| Primary | ||||||||
| 4-h mortality† | 50 (8.2) | 32 (7.5) | 1.09 | (0.71–1.66) | 0.70 | 0.90 | (0.59–1.39) | 0.64 |
| TBI subgroup | 6 (6.4) | 2 (4.5) | 1.40 | (0.29–6.68) | 0.67 | 0.61 | (0.14–2.70) | 0.51 |
| Secondary | ||||||||
| 24-h mortality | 82 (13.4) | 49 (11.5) | 1.16 | (0.83–1.62) | 0.37 | 1.08 | (0.77–1.52) | 0.67 |
| TBI subgroup | 19 (20.2) | 6 (13.6) | 1.48 | (0.64–3.45) | 0.36 | 0.89 | (0.41–1.96) | 0.78 |
| 28-d mortality | 110 (17.9) | 66 (15.5) | 1.16 | (0.88–1.53) | 0.30 | 1.10 | (0.83–1.47) | 0.51 |
| TBI subgroup | 25 (26.6) | 11 (25.0) | 1.06 | (0.58–1.96) | 0.84 | 0.84 | (0.45–1.56) | 0.57 |
Adjusted for age, sex, injury type, head Abbreviated Injury Scale score, prehospital hypotension, receiving any prehospital blood product, and Injury Severity Score.
The adjusted model is weighted by the inverse probability of treatment (propensity score).
COMPONENT, blood component resuscitation; LTOWB, low-titer group O whole blood; RR, relative risk; TBI, traumatic brain injury.
When serial Rotterdam scores derived from head CT imaging of TBI patients were compared across LTOWB and COMPONENT patients, no significant differences were found in the scores derived from the initial head CT images or when subsequent head scan images (second) were compared. When the frequency of worsening head CT Rotterdam scores was compared across the groups, no significant differences were found (Table 3). International normalized ratio/prothrombin time at 4 hours and 24 hours were compared, there were no significant differences found between groups except a significantly lower median and lower percentage of abnormal clot lysis at 30 minutes (LY30) at the 24-hour period in LTOWB patients (Table 4).
Table 3.
Rotterdam CT Score Measures by Resuscitation Type
| Measure | LTOWB (n = 98) |
COMPONENT (n = 42) |
p Value |
|---|---|---|---|
| Rotterdam score | |||
| First CT scan, mean ± SD | 2.34 ± 0.90 | 2.33 ± 1.05 | 0.82 |
| Second CT scan, mean ± SD* | 2.78 ± 1.50 | 2.74 ± 1.52 | 0.95 |
| Difference, mean ± SD | 0.44 ± 1.05 | 0.40 ± 1.04 | 0.87 |
| Worsening, n (%)† | 21 (21.4) | 13 (31.0) | 0.23 |
19 patients died before they could be scanned for a second time. The scores for these patients have been set to 6.
Because 71.4% of patients experienced no change in scores, this measure distinguishes those whose score became worse compared with those whose score remained unchanged or improved (n = 1).
COMPONENT, blood component resuscitation; LTOWB, low-titer group O whole blood.
Table 4.
Coagulation Parameters and Transfusion Requirements by Resuscitation Type
| Measure | LTOWB (n = 624) |
COMPONENT (n = 427) |
p Value |
|---|---|---|---|
| Coagulation parameter | |||
| Within 4 h of arrival | |||
| International normalized ratio, median [IQR] | 1.21 [1.15–1.40] | 1.26 [1.16–1.40] | 0.20 |
| Prothrombin time, sec, median [IQR] | 350 [244–562] | 342 [228–539] | 0.44 |
| Rapid thromboelastography* | |||
| Kinetic time, min, median [IQR] | 2.00 [1.50–2.70] | 1.90 [1.50–2.50] | 0.14 |
| >2.5 min, n (%) | 128 (30.0) | 72 (24.2) | 0.09 |
| Alpha angle, degrees, median [IQR] | 69.1 [63.4–73.0] | 69.7 [64.3–73.5] | 0.19 |
| <60 degrees, n (%) | 72 (16.7) | 41 (13.8) | 0.29 |
| Maximum amplitude, mm, median [IQR] | 56.9 [51.3–61.2] | 57.6 [52.4–62.1] | 0.13 |
| <55 mm, n (%) | 176 (40.5) | 113 (37.9) | 0.49 |
| Clot lysis at 30 min, %, median [IQR] | 0.00 [0.00–0.40] | 0.00 [0.00–0.30] | 0.42 |
| >3%, n (%) | 8 (2.0) | 8 (2.9) | 0.43 |
| Activated clotting time, sec, median [IQR] | 113 [105–128] | 113 [105–128] | 0.69 |
| >128 sec, n (%) | 69 (18.5) | 48 (17.1) | 0.63 |
| Within 24 h of arrival | |||
| International normalized ratio, median [IQR] | 1.30 [1.20–1.40] | 1.31 [1.20–1.50] | 0.25 |
| Prothrombin time, sec, median [IQR] | 407 [281–619] | 377 [270–584] | 0.25 |
| Rapid thromboelastography* | |||
| Kinetic time, min, median [IQR] | 1.30 [1.10–1.80] | 1.30 [1.10–1.60] | 0.19 |
| >2.5 min, n (%) | 28 (7.3) | 12 (4.4) | 0.12 |
| Alpha angle, degrees, median [IQR] | 74.2 [71.1–77.0] | 74.6 [71.9–76.7] | 0.39 |
| <60 degrees, n (%) | 11 (2.9) | 3 (1.1) | 0.12 |
| Maximum amplitude, mm, median [IQR] | 63.8 [59.3–68.1] | 64.1 [60.3–67.9] | 0.61 |
| <55 mm, n (%) | 39 (10.1) | 18 (6.6) | 0.12 |
| Clot lysis at 30 min, %, median [IQR] | 0.20 [0.00–0.80] | 0.20 [0.00–1.30] | 0.04 |
| >3%, n (%) | 7 (1.8) | 12 (4.7) | 0.03 |
| Activated clotting time, sec, median [IQR] | 113 [105–128] | 113 [105–128] | 0.97 |
| >128 sec, n (%) | 67 (18.5) | 37 (14.4) | 0.18 |
| Transfusion requirement, median [IQR] | |||
| Within 4 h of arrival | |||
| Red blood cells, units | 4.00 [1.00–10.0] | 5.00 [2.00–11.0] | <0.001 |
| Plasma, units | 3.00 [0.00–9.00] | 3.00 [1.00–8.00] | 0.15 |
| Platelets, units | 0.00 [0.00–2.00] | 0.00 [0.00–1.00] | 0.56 |
| Whole blood, units | 2.00 [1.00–3.00] | — | — |
| Total, units | 10.0 [4.00–23.0] | 9.00 [4.00–19.0] | 0.12 |
| Total, mL† | 3170 [1330–6880] | 2695 [1210–5780] | <0.001 |
| Within 24 h of arrival | |||
| Red blood cells, units | 5.00 [1.00–12.0] | 5.00 [2.00–13.0] | <0.001 |
| Plasma, units | 4.00 [0.00–11.0] | 4.00 [1.00–9.00] | 0.36 |
| Platelets, units | 1.00 [0.00–2.00] | 0.00 [0.00–2.00] | 0.21 |
| Whole blood, units | 2.00 [1.00–3.00] | — | — |
| Total, units | 12.0 [4.00–27.0] | 10.0 [4.00–22.0] | 0.07 |
| Total, mL† | 4105 [1765–9290] | 2945 [1265–6935] | <0.001 |
Values are missing for n = 152 (14.5%) LTOWB patients and n = 103 (9.8%) COMPONENT patients.
Volume for each unit of red blood cells, plasma, platelets, and whole blood was estimated to be 330, 275, 250, and 500 mL, respectively.
COMPONENT, blood component resuscitation; IQR interquartile range; LTOWB low titer group O whole blood.
Considering 4-hour and 24-hour blood transfusion requirements compared across LTOWB and COMPONENT patients, there were no significant differences found in plasma or platelet transfusion and an expected reciprocal difference in the transfusion of LTOWB and component red blood cells (Table 4). There were no significant differences in total units of blood product transfused across comparison groups. When total transfusion volumes across the 2 groups were compared based on estimated volumes for a component and whole blood unit, the LTWOB demonstrated significantly greater volumes overall at both 4 hours and 24 hours after arrival.
When we compared the rate of massive transfusion across LTOWB and COMPONENT patients, LTOWB patients had a significantly higher rate of massive transfusion by 4 hours from admission (74.4% vs 64.8%, p < 0.01). When we compared mortality due to adjudicated death from exsanguination, there was no significant difference found between LTOWB and COMPONENT groups (8.8% vs 7.7%, p = 0.53). When we compared the rate of achieving hemostasis by 4 hours, LTOWB and COMPONENT patients were similar (82.9% vs 86.3%, p = 0.14).
When outcomes for safety were compared across LTOWB and COMPONENT groups, there were no differences found for the incidence of venous thromboembolism, multiple organ dysfunction, or nosocomial infection (Table 5). There was a significantly higher median of ICU and ventilator–free days for COMPONENT patients. When laboratory measurements for hemolysis were compared at 24 hours, no significant differences in haptoglobin or lactate dehydrogenase were seen, but LTOWB patients had elevated total bilirubin levels relative to component patients. Importantly, no hemolytic or transfusion reactions were reported in either group of the trial.
Table 5.
Safety Outcome Measures by Resuscitation Type
| Measure | LTOWB (n = 624) |
COMPONENT (n = 427) |
p Value |
|---|---|---|---|
| Outcomes | |||
| Deep vein thrombosis, n (%) | 49 (7.9) | 22 (5.2) | 0.09 |
| Pulmonary embolism, n (%) | 38 (6.1) | 26 (6.1) | >0.99 |
| Multiple organ failure, n (%) | 90 (33.7) | 48 (27.4) | 0.16 |
| Nosocomial infection, n (%) | 155 (24.8) | 95 (22.2) | 0.33 |
| ICU-free days, median [IQR]* | 21.0 [0.00–25.0] | 22.0 [4.00–26.0] | 0.05 |
| Ventilator free days, median [IQR]* | 24.0 [5.50–26.0] | 25.0 [13.0–27.0] | <0.01 |
| Hemolysis laboratory markers within 24 h of arrival,† median [IQR] | |||
| Total bilirubin, mg/dL | 0.95 [0.60–1.50] | 0.80 [0.60–1.30] | 0.03 |
| Haptoglobin, mg/dL | 70.0 [42.0–109] | 67.5 [38.2–113] | 0.33 |
| Lactate dehydrogenase, U/L | 407 [281–619] | 377 [270–584] | 0.25 |
Range is 0 to 28. Patients who died before day 28 are assigned a score of 0.
Values missing for n = 160 (15.2%) LTOWB patients and n = 130 (12.4%) COMPONENT patients.
COMPONENT, blood component resuscitation; IQR, interquartile range; LTOWB, low-titer group O whole blood.
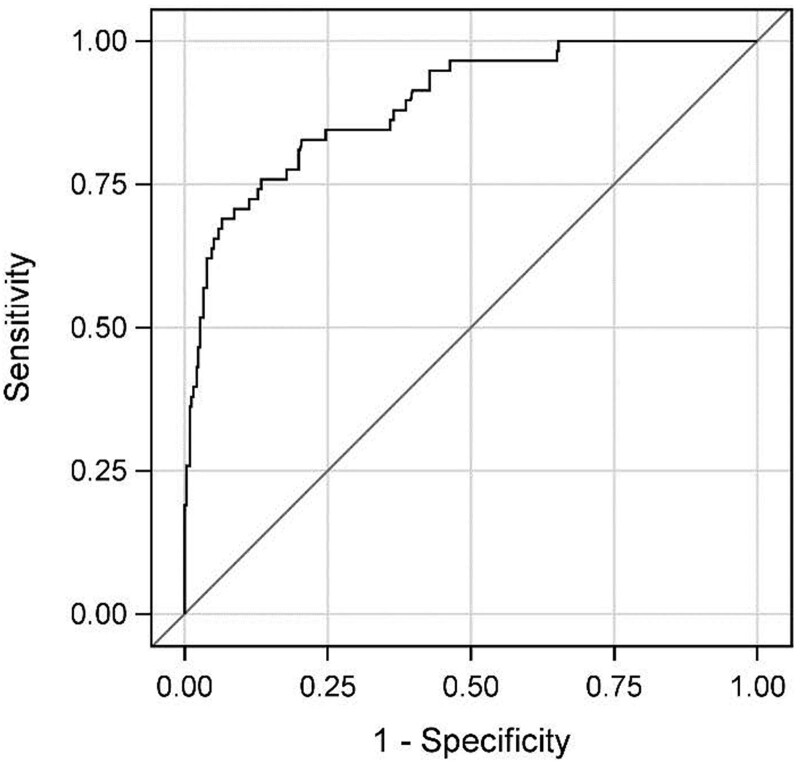
To compare the rate of unexpected survivors across the enrolled cohort, we first determined the individual patient prehospital predicted risk of 28-day mortality, using prehospital vital signs, prehospital interventions/procedures, and injury severity characteristics, and assessed its predictive capabilities via receiver operating characteristic curve analysis. Our prehospital mortality model was an excellent predictor of mortality with an Area Under the Curve = 0.89 (Fig. 2). When we selected those patients with a probability of mortality >50% and looked at the incidence of 28-day mortality across the comparison groups, the LTOWB group had a significantly lower rate of mortality than the COMPONENT group (unadjusted 39.3% vs 72.5%, p < 0.01). When we further characterized this unexpected survivor cohort, after controlling for all relevant confounders, regression analysis demonstrated LTOWB patients had >35% lower independent risk of 28-day mortality (RR 0.64, 95% CI 0.45 to 0.92, p = 0.02).
Figure 2.
Receiver operating characteristic (ROC) curve for prehospital probability of mortality regression model. Area under the curve = 0.8912.
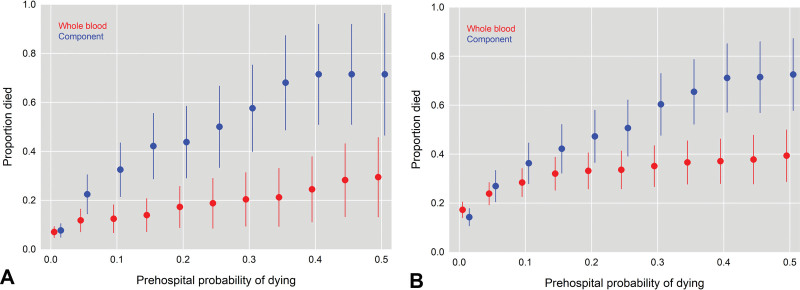
To further characterize the unexpected survivor relationship, we first tested to determine if there was an interaction between prehospital predicted mortality and any LTOWB benefit. We found that the prehospital probability of mortality of an individual patient significantly moderated the survival benefit attributable to LTOWB at 28 days. Based on these findings, we further explored this relationship and plotted the proportion of deaths at 4 hours and 28 days for LTOWB and COMPONENT patients by the predicted prehospital probability of mortality (Figs. 3A, 3B). These demonstrated a separation of LTOWB and COMPONENT patients as the probability of mortality increased. We then again performed our adjusted regression analyses for 4-hour mortality, 24-hour mortality, and 28-day mortality at increasing increments of prehospital predicted mortality probabilities (Table 6). These results demonstrated that, in those patients with a prehospital predicted mortality of 5% or greater, LTOWB was independently associated with >48% lower risk of 4-hour mortality (RR 0.52, 95% CI 0.32 to 0.87, p = 0.01). In those patients with a prehospital predicted mortality of 10% or greater, LTOWB was independently associated with >33% lower risk of 24-hour mortality (RR 0.67, 95% CI 0.47 to 0.97, p = 0.03). In those patients with a prehospital predicted mortality of 20% or greater, LTOWB was independently associated with a 30% lower risk of 28-day mortality (RR 0.70, 95% CI 0.51 to 0.96, p = 0.03).
Figure 3.
Proportion of 4-hour (A) and 28-day (B) deaths across low-titer group O whole blood and blood component resuscitation groups plotted against the prehospital probability of mortality.
Table 6.
Mortality Outcomes by Time, Prehospital Probability of Death, and Resuscitation Type
| Mortality/probability* | LTOWB | COMPONENT | Model results† | ||
|---|---|---|---|---|---|
| RR | 95% CI | p Value | |||
| Hour 4, n/N (%) | |||||
| >0.05 | 24/204 (11.8) | 26/116 (22.4) | 0.52 | (0.32–0.87) | 0.01 |
| >0.10 | 18/145 (12.4) | 25/77 (32.5) | 0.38 | (0.22–0.67) | <0.01 |
| >0.20 | 15/87 (17.2) | 21/48 (43.8) | 0.39 | (0.21–0.71) | <0.01 |
| >0.35 | 11/52 (21.2) | 17/25 (68.0) | 0.26 | (0.14–0.46) | <0.001 |
| >0.50 | 10/34 (29.4) | 10/14 (71.4) | 0.35 | (0.19–0.66) | <0.01 |
| Hour 24, n/N (%) | |||||
| >0.05 | 56/257 (21.8) | 37/143 (25.9) | 0.84 | (0.59–1.20) | 0.35 |
| >0.10 | 49/181 (27.1) | 35/87 (40.2) | 0.67 | (0.47–0.97) | 0.03 |
| >0.20 | 36/112 (32.1) | 32/58 (55.2) | 0.52 | (0.35–0.76) | <0.01 |
| >0.35 | 24/68 (35.3) | 26/42 (61.9) | 0.51 | (0.33–0.80) | <0.01 |
| >0.50 | 18/43 (41.9) | 19/28 (67.9) | 0.57 | (0.35–0.93) | 0.02 |
| Day 28, n/N (%) | |||||
| >0.05 | 85/357 (23.8) | 53/197 (26.9) | 0.93 | (0.69–1.25) | 0.63 |
| >0.10 | 72/254 (28.3) | 50/138 (36.2) | 0.80 | (0.59–1.09) | 0.16 |
| >0.20 | 56/169 (33.1) | 43/91 (47.3) | 0.70 | (0.51–0.96) | 0.03 |
| >0.35 | 45/123 (36.6) | 36/55 (65.5) | 0.62 | (0.45–0.86) | <0.01 |
| >0.50 | 35/89 (39.3) | 29/40 (72.5) | 0.64 | (0.45–0.93) | 0.017 |
Prehospital probability of mortality was estimated by regressing mortality on demographics, injury type, Abbreviated Injury Scores, prehospital vital signs, prehospital medication, prehospital procedures, and receiving prehospital blood products on component-only patients. Parameter estimates were then applied to LTOWB patients for comparison.
Adjusted for age, sex, injury type, head Abbreviated Injury Scale score, prehospital hypotension, receiving any prehospital blood product, and Injury Severity Score.
c, concordance; COMPONENT, blood component resuscitation; LTOWB low-titer group O whole blood; RR relative risk.
Finally, due to the relatively low volume of LTOWB an individual patient received, we wanted to determine if there was a dose-response relationship regarding the quantity of LTOWB that was transfused. When we included the ratio of total LTOWB transfused relative to the total component product received in 24 hours in our regression models and further adjusted for the need for massive transfusion, this ratio was an independent predictor of survival in the LTOWB group of patients at 28 days (RR 0.60, 95% CI 0.42 to 0.85, p < 0.01). This demonstrates that, as the proportion of LTOWB increases during the early resuscitation period, irrespective of large volume transfusion, the independent risk of mortality decreases.
DISCUSSION
Despite major changes regarding when and how injured patients are resuscitated during the past 2 decades, mortality from hemorrhage continues to occur within hours of arrival at definitive trauma centers across the country.2-4 The tenets of “damage control resuscitation” improve outcomes after injury through balanced blood component resuscitation, minimization of crystalloid resuscitation, prevention of coagulopathy, and potential mitigation of downstream effects of shock and endothelial injury.1,32,33 Whole blood resuscitation, considered the definitive damage control resuscitation blood product, has been increasingly used in the civilian setting during the past 8 years, and low-titer anti-A and anti-B group O whole blood is considered the standard care at more than 80 high-volume trauma centers across the country.6,8,9,34,35
The documentation of outcome benefits attributable to whole blood resuscitation have lagged behind these resuscitation practice changes, and the specific injured patient cohorts who may benefit most from whole blood resuscitation are poorly characterized. The results of this prospective observational cohort study demonstrate that the LTOWB resuscitation is safe and adds important information to the growing literature on this practice. Whole blood resuscitation was not independently associated with a mortality benefit in the overall enrolled cohort or in the specific subgroup of brain-injured patients, yet a significant and robust survival advantage was afforded to patients with an elevated probability of death based on prehospital and injury characteristics. This survival advantage of LTOWB was observed for patients with a prehospital predicted mortality of 5% or greater.
The current results are similar to other recent prospective observational studies that have characterized whole blood resuscitation by demonstrating its safety, feasibility, and survival benefits.8,9,12 The current results differ in that survival benefit was only observed in those patients with an elevated probability of death based on prehospital characteristics. This may be due to differences in the specific inclusion and exclusion criteria used and/or the trauma centers selected for the study. Similarly, there may be injury characteristic differences of patients that are enrolled at a whole blood capable trauma center yet do not receive LTOWB resuscitation. It may be that the current cohort selected included a portion of patients where the quality or character of early resuscitation may not matter, specifically those with a low probability of death. Similar to previous studies,8,9,12 patients who received whole blood resuscitation in the current study were more severely injured, had lower Glasgow Coma Scale scores and lower presenting systolic blood pressures and a higher rate of massive transfusion.30 We found no major differences in coagulation parameters despite having a higher rate of massive transfusion and higher estimated total transfusion volume. We used a definition for massive transfusion that minimizes survival bias, incorporates both a rate and volume at early time points, and has been demonstrated to be associated with superior mortality prediction relative to historic definitions.28 After appropriate and robust confounder adjustments, despite these more severe injury characteristics, unadjusted and adjusted mortality rates were similar across LTWOB and COMPONENT groups for the entire cohort.
In the subgroup of patients with an elevated risk of mortality as predicted by our regression models, it is interesting that the proportion of deaths in the COMPONENT group rise in step with increasing predicted mortality, but the LTOWB group curve plateaus and remains relatively flat, despite increasing predicted mortality. This may explain the higher incidence of multiple organ dysfunction (nonsignificant) and lower ICU and ventilator–free days in the LTOWB group because the patients who survived with high predicated mortality, who otherwise may not have, will demonstrate significant organ dysfunction and high critical care needs.17
It was unexpected to find a lack of outcome benefit in patients with documented TBI. Previous studies demonstrate benefit in this cohort when plasma is provided soon after injury.13-16,18 It may be that the timing of an intervention, whether it is provided in the prehospital as compared with the in-hospital phase of care, is most relevant for the brain-injured population.36
The current study has limitations. First, it is an observational cohort study and patients who received LTOWB or COMPONENT early resuscitation had significant differences in injury characteristics and severity that may play a role in the results and conclusions demonstrated. The potential for unknown or unmeasured confounders exists and represents a major limitation in any observational study. The inclusion criteria did not specify the type of resuscitation (LTOWB vs COMPONENT), and during the time period of the study, whole blood resuscitation practice became increasingly common across the country. Enrolling sites had differences in resuscitation practice that may be important confounders. Multiple trauma centers used for the study who initially had only component resuscitation capabilities started whole blood programs after participation began. There may be differences in trauma centers who have recently changed their early resuscitation practice relative to those centers who have had whole blood capabilities for longer periods of time. Similarly, there may be relevant injury severity and outcome differences in a group of patients who are enrolled at a whole blood capable trauma center but do not receive LTOWB. The underlying reasons an enrolled patient at a trauma center with LTOWB capabilities did not receive LTOWB were not recorded in the dataset. The analysis focused on the specific resuscitation strategy individual patients received. There was a relatively high percentage of penetrating mechanism of injury enrolled, and, despite attempting to adjust for all important confounders, there may be differences in the response to LTOWB vs COMPONENT resuscitation based on mechanism of injury. Specific transfusion volumes of all components transfused were not able to be recorded and were estimated based on blood bank volume estimates and prior literature. Due to the observational design of the study, there was variability in resuscitation practice across LTOWB sites as in leukoreduction, titer levels, and specific indications for LTOWB transfusion (eg childbearing age status). Transfusion volumes for either group were based on patient need and site-specific transfusion practice. There was a relatively low median volume of LTOWB transfusion for the overall cohort, and attributing survival outcome differences from this strategy may be confounded. Similarly, some trauma centers had prehospital transfusion capabilities, but others did not. We controlled for this capability in our regression models but the potential for confounding remains. Importantly, ratios of blood components (red blood cell:plasma:platelet) were not protocolized for either the COMPONENT group or for the LTOWB group beyond the early resuscitation period, and this variability represents a major limitation. The laboratory measurements are associated with missingness due to the logistics of care management for severely injured patients. Although the missingness did not differ across comparison groups, this could lead to measurement differences and represents a significant limitation.
CONCLUSIONS
In conclusion, LTOWB resuscitation was safe but not independently associated with survival benefits in the overall enrolled cohort of this observational study. When patients were selected with an elevated probability of mortality based on prehospital injury characteristics, LTOWB was independently associated with a lower risk of mortality starting at 4 hours after arrival through 28 days after injury. Further high-level, randomized clinical trials are needed to appropriately characterize the injured population that benefits most from this valuable transfusion resource.
Appendix
Members of the SWAT study group: Laura Vincent, MS, RN, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Mark H Yazer, MD, Department of Pathology, University of Pittsburgh, Pittsburgh, PA; David O Okonkwo, MD, PhD, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA; Ava M Puccio, RN, PhD, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA; Vikas Agarwal MD, Department of Radiology, University of Pittsburgh, Pittsburgh, PA; Erin E Fox, PhD, Department of Surgery, University of Texas Health Science Center, Houston, TX; Charles E Wade, PhD, Department of Surgery, University of Texas Health Science Center, Houston, TX; Benjamin S Abella, MD, MPhil, Department of Surgery, University of Pennsylvania, Philadelphia, PA; Sean Van Walchren, BS, Department of Surgery, Oregon Health & Science University, Portland, OR; Roman Dudaryk MD, Department of Surgery, University of Miami/Jackson Memorial Hospital, Miami, FL; Joshua B Brown, MD MSc, FACS, Department of Surgery, University of Pittsburgh, Pittsburgh, PA; Matthew D Neal, MD, FACS, Department of Surgery, University of Pittsburgh, Pittsburgh, PA
Author Contributions
Conceptualization: Sperry, Cotton, Vincent, Cannon, Schreiber, Moore, Namias, Minei, Yazer, Okonkwo, Puccio, Agarwal, Fox, Wade, Brown, Neal, Wisniewski, Guyette
Data curation: Sperry, Luther, Vincent, Cannon, Agarwal, Van Walchren, Dudaryk, Wisniewski, Guyette
Methodology: Sperry, Cotton, Luther, Vincent, Cannon, Schreiber, Minei, Yazer, Okonkwo, Puccio, Agarwal, Fox, Wade, Abella, Van Walchren, Dudaryk, Brown
Supervision: Sperry, Vincent
Writing – original draft: Sperry, Cotton, Cannon, Schreiber, Moore, Namias, Minei, Yazer, Okonkwo, Puccio, Agarwal, Fox, Wade, Abella, Van Walchren, Dudaryk, Brown, Neal, Wisniewski, Guyette
Writing – review & editing: Sperry, Cotton, Luther, Vincent, Cannon, Schreiber, Moore, Namias, Minei, Yazer, Okonkwo, Puccio, Agarwal, Fox, Wade, Abella, Van Walchren, Dudaryk, Brown, Neal, Wisniewski, Guyette
Funding acquisition: Cotton, Vincent, Cannon, Schreiber, Moore, Namias, Minei, Okonkwo, Puccio, Fox, Wade, Abella, Van Walchren, Wisniewski, Guyette
Investigation: Cotton, Vincent, Schreiber, Moore, Namias, Minei, Neal, Wisniewski
Formal analysis: Luther, Cannon, Moore, Yazer, Okonkwo, Van Walchren, Brown, Wisniewski, Guyette
Validation: Luther, Vincent, Brown, Neal
Project administration: Vincent, Wisniewski
Resources: Yazer
Acknowledgment:
University of Pittsburgh, Presbyterian Hospital; Clinical & Data Coordinating Center and enrolling site: Barbara J Early-Young, Meghan L Buck, Peter W Adams, Rachel L Molinaro, Alexandra Merti, Ashely M Harner, Elizabeth A Gimbel, Logan Owens, Hannah Hayes, Alan Jackson, Laurie Silfies, Lisa Over, Steve Knopf, Melody Macey-Kalcevic, Angela Pattison, Megan E Buhay, Brianna J Higginbottom, Marissa L Marcin, MACRO Research Specialists, MACRO Clinical Trials Research Associates, and Cara Battistella. McGovern Medical School at UTHealth: Yu Bai, Kandice L Motley, Yao-Wei Wang, Victoria Herrick; Garrett Woodruff, Veda Pa, Rhonda Hobbs, Jeanette Podbielski, Laura Vincent, Christy Allen, Subin Alexander, Natolie Hamilton, Symantha Lopez, Selina Hernandez Gonzalez, Jason Rashall, James Seymour, and Nicole Zarate. University of Pennsylvania: Alea Zone, Sarah Joergensen, Liam Forsythe, Daria Zaitseva, Paul Callahan, Komal Khan, Olivia Doran, Sarah Gamblin, Lydia Fisher, Daniela Schmulevich, Steve Balian, Carrie Diamond, Jonathan Kolansky, and Dena Torrente. Oregon Health & Science University: Sean Van Walchren, Diane Lape. Denver Health Medical Center: Angela Sauaia, Jason Haukoos, Lee Anne Ammons, James Chandler, Marcela Fitzpatrick, Emmalee Vittatoe, Nick Brant, Stephanie Kennedy, and Megan Swope. University of Miami: Ronald Manning, Cristina Botero Fonnegra, Sebastian Brito, Vivian Calderon, Majid Chammas, Anthony Dure, Chelsea Ferreira, Allison Ferreira, Richard Guerra, Ivonne Guzman, Aaliyah Jolly, and Rajan Ramdev. University of Texas Southwestern: Shreedhar Reddy, Nadia Nassaj.
Abbreviations and Acronyms
- COMPONENT
- blood component resuscitation
- ISS
- Injury Severity Score
- LTOWB
- low-titer group O whole blood
- RR
- relative risk
- TBI
- traumatic brain injury
Members of the SWAT study group who coauthored this article are listed in the Appendix.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Cannon receives royalty payments from UpToDate and is a paid consultant to CSL Behring. Dr Schrieber is a paid consultant to and receives grant support from CSL Behring and Haemonetics and is a paid consultant to Tricot. Dr Nahmias was a paid advisory board member at Merck and CSL Behring. Dr Yazer receives lecture payments from Grifols and is a paid advisory board member at Hemanext and Verax Biomedical. Dr Wade’s institute receives funding from Grifols and Athersys. Dr Abella is a paid consultant to Becton Dickinson. Dr Dudaryk receives speaker fees from Haemonetics and is a paid consultant to Phillips Healthcare. Dr Neal is a paid board member at Haima Therapeutics, receives grant funding and honorarium payments from Haemonetics, honorarium payments from Takeda, and grant funding from Instrumentation Laboratories. Dr Moore receives research support from Haemonetics, Werfen, Hemosonics, Stago, and Diapharma. Other authors have nothing to disclose.
Support: This work is supported by the US Army Medical Research and Materiel Command under Contract No. W81XWH-16-D-0024-0002. Dr Neal is supported by NIH grant [R35GM119526].
Disclaimer: The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
Presented at the Military Health System Research Symposium, Kissimmee, FL, September 2022.
Contributor Information
Laura Vincent, Department of Surgery, University of Pittsburgh.
Mark H Yazer, Department of Pathology, University of Pittsburgh.
David O Okonkwo, Department of Neurological Surgery, University of Pittsburgh.
Ava M Puccio, Department of Neurological Surgery, University of Pittsburgh.
Vikas Agarwal, Department of Radiology, University of Pittsburgh.
Erin E Fox, Department of Surgery, University of Texas Health Science Center.
Charles E Wade, Department of Surgery, University of Texas Health Science Center.
Benjamin S Abella, Department of Surgery, University of Pennsylvania.
Sean Van Walchren, Department of Surgery, Oregon Health & Science University.
Roman Dudaryk, Department of Surgery, University of Miami/Jackson Memorial Hospital.
Joshua B Brown, Department of Surgery, University of Pittsburgh.
Matthew D Neal, Department of Surgery, University of Pittsburgh, Pittsburgh, PA.
Collaborators: Laura Vincent, Mark H Yazer, David O Okonkwo, Ava M Puccio, Vikas Agarwal, Erin E Fox, Charles E Wade, Benjamin S Abella, Sean Van Walchren, Roman Dudaryk, Joshua B Brown, and Matthew D Neal
REFERENCES
- 1.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB, Moore EE, Sperry JL, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273:395–401. [DOI] [PubMed] [Google Scholar]
- 3.Sperry JL, Guyette FX, Adams PW. Prehospital plasma during air medical transport in trauma patients. N Engl J Med. 2018;379:1783. [DOI] [PubMed] [Google Scholar]
- 4.Fox EE, Holcomb JB, Wade CE, et al. ; PROPPR Study Group. Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyette FX, Sperry JL, Peitzman AB, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the Prehospital Air Medical Plasma Trial. Ann Surg. 2021;273:358– 364. [DOI] [PubMed] [Google Scholar]
- 6.Guyette FX, Zenati M, Triulzi DJ, et al. Prehospital low titer group O whole blood is feasible and safe: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2022;92:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperry JL, Brown JB. Whole-blood resuscitation following traumatic injury and hemorrhagic shock-should it be standard care? JAMA Surg. 2023;158:540. [DOI] [PubMed] [Google Scholar]
- 8.Brill JB, Tang B, Hatton G, et al. Impact of incorporating whole blood into hemorrhagic shock resuscitation: analysis of 1,377 consecutive trauma patients receiving emergency-release uncrossmatched blood products. J Am Coll Surg. 2022;234:408–418. [DOI] [PubMed] [Google Scholar]
- 9.Hazelton JP, Ssentongo AE, Oh JS, et al. Use of cold-stored whole blood is associated with improved mortality in hemostatic resuscitation of major bleeding: a multicenter study. Ann Surg. 2022;276:579–588. [DOI] [PubMed] [Google Scholar]
- 10.Seheult JN, Bahr M, Anto V, et al. Safety profile of uncrossmatched, cold-stored, low-titer, group O+ whole blood in civilian trauma patients. Transfusion. 2018;58:2280–2288. [DOI] [PubMed] [Google Scholar]
- 11.Gaines BA, Yazer MH, Triulzi DJ, et al. Low titer group O Whole blood in injured children requiring massive transfusion. Ann Surg. 2023;277:e919–e924. [DOI] [PubMed] [Google Scholar]
- 12.Torres CM, Kent A, Scantling D, et al. Association of whole blood with survival among patients presenting with severe hemorrhage in US and Canadian dult civilian trauma centers. JAMA Surg. Published online January 18, 2023;e226978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Moheimani H, Li S, et al. High dimensional multiomics reveals unique characteristics of early plasma administration in polytrauma patients with TBI. Ann Surg. 2022;276:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Cyr A, Gruen DS, et al. ; PAMPer study group. Lipidomic signatures align with inflammatory patterns and outcomes in critical illness. Nat Commun. 2022;13:6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Vodovotz Y, Abdelhamid S, et al. ; PAMPer study group. Multi-omic analysis in injured humans: patterns align with outcomes and treatment responses. Cell Rep Med. 2021;2:100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruen DS, Guyette FX, Brown JB, et al. Association of prehospital plasma with survival in patients with traumatic brain injury: a secondary analysis of the PAMPer Cluster Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruen DS, Guyette FX, Brown JB, et al. Characterization of unexpected survivors following a prehospital plasma randomized trial. J Trauma Acute Care Surg. 2020;89:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruen DS, Brown JB, Guyette FX, et al. Prehospital plasma is associated with distinct biomarker expression following injury. JCI Insight. 2020;5:e135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. [DOI] [PubMed] [Google Scholar]
- 20.Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(Suppl 1):S33–S39. [DOI] [PubMed] [Google Scholar]
- 21.Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–352. [DOI] [PubMed] [Google Scholar]
- 22.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. [DOI] [PubMed] [Google Scholar]
- 23.Huang YH, Deng YH, Lee TC, Chen WF. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery. 2012;71:80–85. [DOI] [PubMed] [Google Scholar]
- 24.Russell RJ, Hodgetts TJ, McLeod J, et al. The role of trauma scoring in developing trauma clinical governance in the Defence Medical Services. Philos Trans R Soc Lond B Biol Sci. 2011;366:171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champion HR, Copes WS, Sacco WJ, et al. The Major Trauma Outcome Study: establishing national norms for trauma care. J Trauma. 1990;30:1356–1365. [PubMed] [Google Scholar]
- 26.Norris R, Woods R, Harbrecht B, et al. TRISS unexpected survivors: an outdated standard? J Trauma. 2002;52:229–234. [DOI] [PubMed] [Google Scholar]
- 27.Sim ES, Guyette FX, Brown JB, et al. ; PAMPer study group. Massive transfusion and the response to prehospital plasma: it is all in how you define it. J Trauma Acute Care Surg. 2020;89:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage SA, Sumislawski JJ, Zarzaur BL, et al. The new metric to define large-volume hemorrhage: results of a prospective study of the critical administration threshold. J Trauma Acute Care Surg. 2015;78:224–9; discussion 229–230. [DOI] [PubMed] [Google Scholar]
- 29.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Time matters in 1: 1 resuscitations: concurrent administration of blood: plasma and risk of death. J Trauma Acute Care Surg. 2014;77:833–837; discussion 837–838. [DOI] [PubMed] [Google Scholar]
- 30.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74:396–400; discussion 400–402. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang R, Kerby JD, Kalkwarf KJ, et al. ; PROPPR Study Group. Earlier time to hemostasis is associated with decreased mortality and rate of complications: results from the Pragmatic Randomized Optimal Platelet and Plasma Ratio trial. J Trauma Acute Care Surg. 2019;87:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardenas JC, Zhang X, Fox EE, et al. ; PROPPR Study Group. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeper CM, Yazer MH, Neal MD. Whole-blood resuscitation of injured patients: innovating from the past. JAMA Surg. 2020;155:771–772. [DOI] [PubMed] [Google Scholar]
- 35.Leeper CM, Yazer MH, Neal MD. Whole-blood resuscitation of injured patients’ plasma. JAMA Surg. 2021;156:101–102. [DOI] [PubMed] [Google Scholar]
- 36.Lewis RE, Muluk SL, Reitz KM, et al. ; PAMPer Study Group. Prehospital plasma is associated with survival principally in patients transferred from the scene of injury: A secondary analysis of the PAMPer trial. Surgery. 2022;172:1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]