Background and Aim:
Long-term maintenance of viral control, even HBsAg loss, remains a challenge for chronic hepatitis B (CHB) patients undergoing nucleos(t)ide analogue (NA) discontinuation. This study aimed to investigate the relationship between HBV-specific T-cell responses targeting peptides spanning the whole proteome and clinical outcomes in CHB patients after NA discontinuation.
Approach and Results:
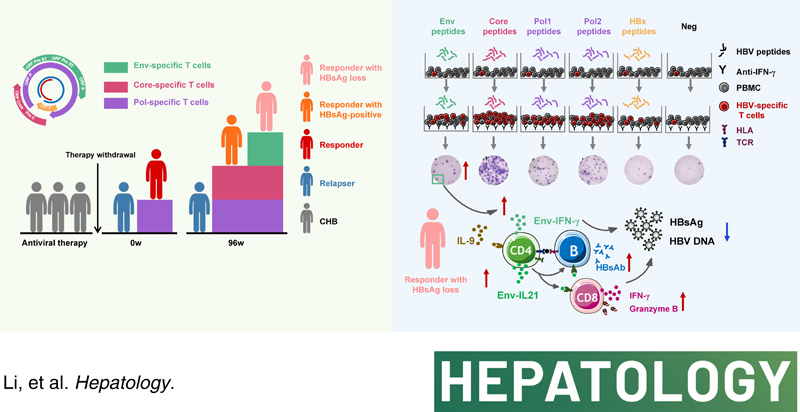
Eighty-eight CHB patients undergoing NA discontinuation were classified as responders (remained relapse-free up to 96 weeks) or relapsers (relapsed patients who underwent NA retreatment for up to 48 weeks and reachieved stable viral control). HBV-specific T-cell responses were detected at baseline and longitudinally throughout the follow-up. We found responders had a greater magnitude of HBV polymerase (Pol)-specific T-cell responses than relapsers at baseline. After long-term NA discontinuation, simultaneously enhanced HBV Core-induced and Pol-induced responses were observed in responders. Particularly, responders with HBsAg loss possessed enhanced HBV Envelope (Env)-induced responses after short-term and long-term follow-up. Notably, CD4+T cells accounted for the predominance of HBV-specific T-cell responses. Correspondingly, CD4-deficient mice showed attenuated HBV-specific CD8+T-cell responses, reduced HBsAb-producing B cells, and delayed HBsAg loss; in contrast, in vitro addition of CD4+T cells promoted HBsAb production by B cells. Besides, IL-9, rather than PD-1 blockade, enhanced HBV Pol-specific CD4+T-cell responses.
Conclusion:
HBV-specific CD4+T-cell responses induced by the targeted peptide possess specificities for long-term viral control and HBsAg loss in CHB patients undergoing NA discontinuation, indicating that CD4+T cells specific to distinct HBV antigens may endow with divergent antiviral potential.
INTRODUCTION
Chronic HBV infection remains a severe public health care problem. Current therapies for chronic hepatitis B (CHB) remain limited to pegylated interferon (IFN)-α or approved nucleos(t)ide analogue (NA).1 Considering the side effects and the inconvenience of pegylated IFN-α administration, well-tolerated NA with potent antiviral activity is the most commonly used. Nevertheless, NA therapy discontinuation can result in the relapse of HBV and the risk of liver failure.2 The overall goal of treating CHB is to achieve a functional cure characterized by prolonged HBsAg loss, which remains rare with the currently available antiviral therapies. It has been documented that HBsAg loss is associated with a reversion of liver fibrosis, a reduction in the risk of HCC, and a lower rate of HBV reactivation.1,3 Therefore, the achievement of sustained viral control, and even functional cure, remains a challenge for CHB patients after treatment withdrawal. To this end, investigating the factors that are closely related to sustained HBsAg loss after therapy withdrawal is warranted.
The HBV-specific cellular immune response plays a critical role in determining the outcome after NA discontinuation. It has been shown that patients with a higher frequency of HBV-specific T-cell responses are less likely to develop HBV flares when stopping NA therapy.4,5 HBV-specific T-cell responses targeting the Envelope (Env), Core, Polymerase (Pol), and HBx proteins can be induced. Data from HBV-infected patients reported that CD8+ T cells specific to Core and Pol exhibited distinct phenotypic, transcriptional, and functional features.6,7 Compared with Pol, Env-specific CD8+ T cells were more protective and capable of recognizing HBV-producing hepatocytes in HBV transgenic mice.8 Thus, T-cell immune response targeting different HBV antigens might possess a distinct ability against HBV and be associated with divergent outcomes of CHB patients after therapy withdrawal.
In recent years, the effects of HBV-specific CD4+ T cells have been increasingly valued. Previous studies have shown that HBV-specific CD4+ T cells serve as major regulators of the CD8+ T-cell–mediated adaptive immune response.9 A lack of CD4+ T cells may result in HBV persistence in patients with HBV/HCV coinfection.10 HBV-specific IFN-γ producing CD4+ T cells are associated with HBV clearance.11 These findings suggest that CD4+ T cells might also serve as an indispensable part in the functional cure of HBV infection. Apart from the well-established cellular immune response, an effective humoral arm is also essential for preventing HBV flare. Indeed, it has been shown that humoral immune responses mainly contribute to the prevention of HBV reactivation decades after HBsAg clearance.12 Since CD4+ T cells are essential for the differentiation and maturation of B cells, factors that promote CD4+ T-cell response may aid humoral immunity against HBV. However, reports have shown that B cells can enhance primary and memory CD4+ T-cell responses,13,14 suggesting the importance of B cells in conditioning T-cell responses. Thus, the influence of cooperation between specific CD4+ T cells and B cells in the functional cure of HBV infection needs to be further elucidated.
In this study, we longitudinally analyzed the function of HBV-specific T cells targeting different HBV peptides in CHB patients after NA therapy withdrawal in a single-center, noninterventional, retrospective, and prospective cohort. It was observed that HBV Core-specific and Pol-specific CD4+ T cells were mainly related to sustained viral control after therapy withdrawal. More importantly, we found HBV Env-specific CD4+ T-cell responses were associated with HBsAg loss. This report emphasizes a differential protective role of HBV-specific CD4+ T cells in sustained response and even functional cure after therapy withdrawal in CHB patients.
METHODS
Patients
Eighty-eight CHB patients were enrolled in an observational clinical trial (CON Study, ChiCTR-OOC-17013970) focused on the sustained response after discontinuation of NA therapy (Table S1 and S2, http://links.lww.com/HEP/E8). Patients were originally HBeAg-positive and received at least 48 weeks of NA therapy; subsequently, they would discontinue antiviral therapy if they achieved HBV DNA < 300 copies/mL and HBeAg seroconversion at 2 consecutive time points at least 6 months apart. After NA therapy discontinuation, patients were followed monthly during the initial 3 months and then were monitored at 3-month intervals for at least 96 weeks (0, 4, 8, 12, 24, 36, 48, 60, 72, 84, and 96 wk, respectively). According to clinical characteristics, patients were divided into 2 groups: responders and relapsers (Figure S1, http://links.lww.com/HEP/E8). Responders were patients who maintained HBV DNA ≤ 104 copies/mL, achieved alanine aminotransferase (ALT) normalization, and achieved HBeAg seroconversion for more than 96 weeks after therapy withdrawal. In addition, according to serum HBsAg levels after therapy withdrawal, responders were divided into responders with HBsAg loss and responders with HBsAg-positive. Relapsers were patients with confirmed relapse defined as HBV DNA > 104 copies/mL and ALT > 2× upper limit of normal detected at 2 consecutive time points within 1 month. Relapsers were subsequently treated with NA agents for at least 48 weeks and achieved clinical stability with HBV DNA < 300 copies/mL, ALT normalization, and HBeAg negativity. In adition, 88 patients with HBV infection from an observational clinical trial (SEARCH-B Study, NCT02167503) were recruited, including 60 HBsAg-positive and 28 HBsAg loss patients (Table S3, http://links.lww.com/HEP/E8). The exclusion criteria were HAV, HCV, HDV, HIV infection, autoimmune diseases, immunosuppressive conditions, and other severe illnesses or cancer. All experiments with human samples were approved by the Ethical Committee of Nanfang Hospital, and all subjects provided informed consent.
Synthetic peptides
A total of 393 15-mer overlapping single peptides (3D single peptides) covering the whole sequence of HBV were designed and synthesized. The overlapping peptides were assigned to a 2-dimensional (D) matrix system using a concept according to that previously described.15 Within a given protein matrix, each 3D single peptide was represented in 2 different peptide pools (2D matrix peptides), allowing for the identification of the respective peptide by responses in the 2 corresponding pools. As shown in Figure S2 (http://links.lww.com/HEP/E8), the Env 3D single peptides were mixed into 20 2D matrix peptides (9 or 10 3D single peptides in each Env 2D matrix peptide). Core 3D single peptides were mixed into 15 2D matrix peptides (6 or 9 3D single peptides in each Core 2D matrix peptide). Considering the length of the whole HBV Pol protein, it was divided equally into Pol1 and Pol2. Pol 3D single peptides were mixed into 21 2D matrix peptides (10 or 11 3D single peptides in each Pol 2D matrix peptide), while HBx 3D single peptides were mixed into 12 2D matrix peptides (6 3D single peptides in each HBx 2D matrix peptide). In addition, each 1D peptide pools (Env, Core, Pol1, Pol2, and HBx) contained all 3D single peptide mixtures (Env peptide pool contained 98 3D single peptides, Core peptide pool contained 51 3D single peptides, Pol1 peptide pool contained 104 3D single peptides, Pol2 peptide pool contained 104 3D single peptides, and HBx peptides pool contained 36 3D single peptides). HBV 1D peptide pools (Env, Core, Pol1, Pol2, and HBx) were used in peripheral blood mononuclear cells (PBMCs) in vitro expansion experiments (Figure S3, http://links.lww.com/HEP/E8).
In vitro expansion
PBMCs were isolated by the Ficoll-Hypaque centrifugation and used directly or cryopreserved. PBMCs were stimulated with 1D peptide pools (Env, Core, Pol1, Pol2, and HBx) in RPMI medium 1640 complete medium (Gibco; Thermo Fisher Scientific) supplemented with 10% AB (human serum AB). After PBMCs were pulsed with 1D peptide pools (4 μg/mL) for 1 hour at 37 °C in an incubator, IL-7 (25 ng/mL; PeproTech) was added. Cells were then cultured for 10 days with recombinant IL-2 (10 ng/mL; PeproTech) added on days 3 and 7 (Figure S3, http://links.lww.com/HEP/E8). Cells were collected, washed 6 times on day 10, and rested in a 37°C incubator for 24 hours for subsequent experiments. An enzyme-linked immunosorbent spot (ELISpot) assay was performed to screen responsive peptides, followed by intracellular cytokine staining confirmation with a single peptide.
Additional supporting information and reagents used in this study are included in Supplementary Materials and Methods (Table S10, http://links.lww.com/HEP/E8).
RESULTS
HBV Env-specific T-cell responses are associated with HBsAg loss after treatment withdrawal
First, we investigated the dynamic changes in the specific T-cell responses targeting peptides spanning the whole HBV proteome; a cultured ELISpot assay was performed to detect IFN-γ-producing HBV-specific T cells according to the procedure we have established previously (Figure 1A).16 It revealed that HBV Pol-specific T-cell response was higher in responders relative to relapsers at baseline (0 wk) of withdrawal (Figure 1B). Intriguingly, the frequency of HBV Pol-specific T cells at the flare time point (median: 20 wk) was decreased compared with that at baseline in relapsers (Figure S4, http://links.lww.com/HEP/E8), suggesting attenuated HBV-specific T-cell responses might not maintain viral control after therapy discontinuation. Notably, with the prolongation of NA discontinuation, responses against the Core and Pol proteins were markedly increased in responders compared with relapsers at 96 weeks of withdrawal, while similar patterns were not observed for the Env-induced or HBx-induced responses. These findings indicated that HBV Pol-specific T cells might associate with short-term response upon therapy discontinuation. In contrast, HBV Core-specific and Pol-specific T cells might associate with long-term virological control. Next, we explored whether patients with HBsAg loss during follow-up exhibited a distinct T-cell response. Of note, the frequency of Env-specific T cells at 4, 48, and 96 weeks of discontinuation, but not those of Pol-specific, or HBx-specific T cells, was significantly higher in responders with HBsAg loss than those with HBsAg-positive (Figure 1C). Further analysis showed that the frequency of HBV Env-specific IFN-γ-producing T cells at 4 weeks or 96 weeks of discontinuation was negatively correlated with HBsAg levels at 0 weeks of therapy withdrawal (Figure S5, http://links.lww.com/HEP/E8), suggesting that Env-specific T cells might associate with HBsAg loss after therapy withdrawal. Taken together, these data indicated that T cells specific to distinct HBV antigens might endow with divergent antiviral potential.
FIGURE 1.
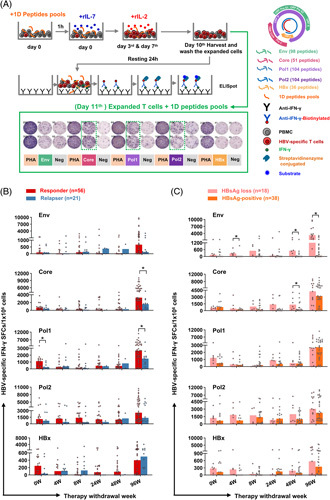
HBV-specific T-cell responses are related to viral control and HBsAg loss after treatment withdrawal. IFN-γ ELISpot assay was performed to assess HBV-specific T-cell responses. (A) Experimental procedures of the cultured ELISpot assays. (B and C) Dynamic view of the frequency of HBV Env-, Core-, Pol1-, Pol2-, and HBx-specific T cells in responders and relapsers, or in responders with HBsAg loss and responders with HBsAg-positive at 0, 4, 8, 24, 48, and 96 weeks after therapy withdrawal. (B and C) Mann–Whitney U test. *p < 0.05. Abbreviations: ELISpot, enzyme-linked immunosorbent spot; Env, Envelope; PBMCs, peripheral blood mononuclear cell; Pol, Polymerase; SFCs, spot-forming cells.
The breadth of HBV Env-specific T-cell responses is associated with HBsAg loss after NA discontinuation
To investigate the breadth of specific T-cell responses against the HBV peptides, we performed a systematic mapping of the single peptide to confirm specificity. A total of 393 single peptides spanning the whole HBV proteome were tested by T-cell ELISpot at 96 weeks of withdrawal. We found the coverage of responsive peptides targeting the Core (45%) was higher than that of the Env (20%), indicating that Core-specific T cells were more easily detected than Env-specific T cells (Figure 2A). Further analysis focusing on single responsive peptides in different groups revealed that responders and relapsers established a hierarchy of dominant and subdominant epitopes. As shown in Figure S6A (http://links.lww.com/HEP/E8), the frequencies of Pol026 (VNEKRRLKLIMPARF) and Pol105 (NLLSSNLSWLSLDVS) were elevated with marginal significance in relapsers. Notably, for responder subjects, the frequencies of Env87 (SWLSLLVPFVQWFVG) and Env95 (PSLYNILSPFLPLLP) were significantly higher in those with HBsAg loss than in HBsAg-positive subjects (Figure S6B, http://links.lww.com/HEP/E8). We next analyzed the responsive peptide coverage among sex-matched and age-matched patients. The coverage of responsive peptides was comparable in responders and relapsers (Figure 2B). Of particular interest was that the responsive peptide coverage of the Env, but not those of the Core, Pol, or HBx, was significantly higher in HBsAg loss than HBsAg-positive responders (Figure 2C), which indicated that, besides the magnitude, the breadth of Env-specific T-cell responses might also be related to HBsAg loss.
FIGURE 2.
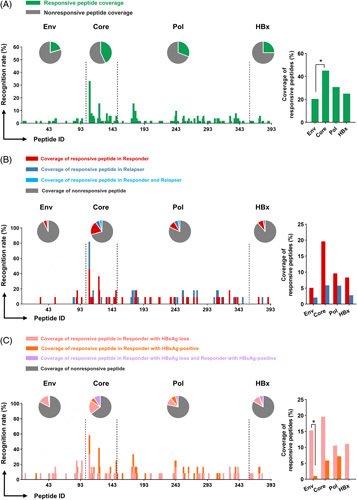
Analyzing the breadth of HBV-specific T-cell responses in patients with different outcomes after therapy withdrawal. A total of 393 single peptides were tested by T-cell ELISpot. The recognition rate was calculated as the number of patients who were responsive to one single peptide relative to all detected patients. The coverage of responsive peptides was the number of single responsive peptides divided by the number of corresponding peptides pool, including Env (98), Core (51), Pol (208), and HBx (36). (A) The recognition rate and the coverage of responsive peptides in all patients (n=51). The coverage of responsive peptides in (B) sex-matched and age-matched responders (n=11) and relapsers (n=11), or (C) HBsAg loss (n=12) and HBsAg-positive (n=12) responders at 96 weeks after therapy withdrawal. The number of patients with specific single responsive or nonresponsive peptides was noted in the bar. (A–C) Chi square test or Fisher test. *p < 0.05. Abbreviations: Env, Envelope; Pol, Polymerase.
HBV-specific CD4+ T cells account for the predominance of HBV-specific T cell responses and are closely related to HBsAg loss
We next explored which subset of HBV-specific T cells dominates after therapy withdrawal. As shown in Figure 3A and B, the coverage of single responsive peptides in CD4+ T cells targeting the Env was comparable to that in CD8+ T cells at 96 weeks of discontinuation. In contrast, the coverage of responsive peptides in CD4+ T cells targeting the Core, Pol, and HBx (79%, 65%, and 80%, respectively) was higher than that in CD8+ T cells. We then assessed the magnitude of HBV-specific CD4+ and CD8+ T-cell responses in responders and relapsers (Figure 3C and Figure S7, http://links.lww.com/HEP/E8). Compared with relapsers, responders showed an upregulated frequency of Core-specific CD4+ T cells at 96 weeks of discontinuation (Figure 3D). Especially, the frequencies of Env-specific, Core-specific, and Pol-specific CD4+ T cells were significantly higher than in CD8+ T cells in responders (Figure 3D). Combined with the above results, these findings suggested that Core-specific CD4+ T cells play a vital role in the sustained response after therapy withdrawal. Interestingly, the secretions of IFN-γ by Env-specific CD4+ T cells were upregulated in HBsAg loss compared with HBsAg-positive patients (Figure 3E). Furthermore, the frequencies of IFN-γ-producing HBV Env-specific, Core-specific, and Pol-specific CD4+ T cells were higher than the corresponding CD8+ T cells in patients with HBsAg loss (Figure 3E). These findings demonstrated that the majority of HBV-specific T-cell epitopes were related to CD4+ T-cell responses.
FIGURE 3.
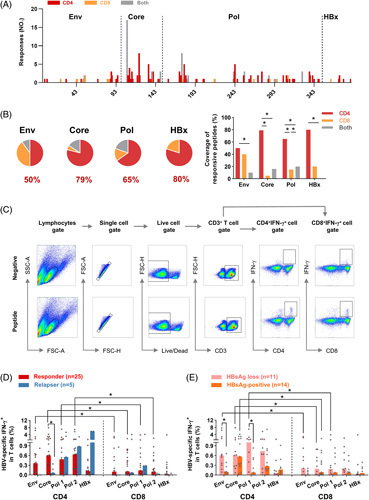
HBV-specific T-cell responses are predominantly mediated by CD4+ T cells. (A) The number of CD4+ and CD8+ T-cell responses targeting all HBV proteins was displayed for patients at 96 weeks of withdrawal. (B) The coverages of indicated peptides (Env, Core, Pol, and HBx) responding to CD4+ T cells (red), CD8+ T cells (orange), and both CD4+ and CD8+ T cells (gray) are shown and compared. Annotation represents the coverage of responsive peptides targeting CD4+ T cells. The coverage of responsive peptides was calculated as the number of single peptides responding to CD4+ or CD8+ T cells divided by the number of single responsive peptides. (C) Representative FACS plots showed the gating strategy and the staining of IFN-γ-producing HBV-specific T cells. The frequency of IFN-γ-producing HBV-specific T cells in CD4+ or CD8+ T cells in (D) responders and relapsers at 96 weeks of withdrawal, or (E) HBsAg loss and HBsAg-positive patients. (B) Chi square test or Fisher test. (D and E) Mann–Whitney U test. *p < 0.05. Abbreviations: Env, Envelope; Pol, Polymerase.
Since the interaction of CD4+ T lymphocytes with MHC II molecules is crucial for T-cell activation, we next investigated the relationship between human leukocyte antigen (HLA) alleles and outcomes after NA discontinuation. The frequencies of the C*03:04 and DPB1*02:02 alleles showed a higher trend in responders (Table S4, http://links.lww.com/HEP/E8). Patients with HBsAg loss showed higher frequencies of HLA class II DQB1*05:01, DRB1*14:05, and DRB1*15:02 alleles, while the frequency of the HLA class I A*11:01 allele was lower (Table S5, http://links.lww.com/HEP/E8). Besides, similar results were observed in Table S6 and Table S7 (http://links.lww.com/HEP/E8) by performing the Cochran-Armitage trend test to test for a trend in patients with different genotypes (wild type, heterozygous, and homozygous allele). In addition, we also found a close association between genetic variation in the CD4 or HLA-DR region (Table S8 and Table S9, http://links.lww.com/HEP/E8) and the relapse or HBsAg loss, suggesting that CD4-associated genetic markers might serve as potential predictors of the clinical outcomes when considering anti-HBV therapy discontinuation.
CD4+ T cell deficiency resulted in attenuated HBV-specific CD8+ T-cell responses
The above-mentioned results highlighted the importance of HBV-specific CD4+ T cells in maintaining HBsAg loss after therapy discontinuation; we then investigated whether CD4+ T cells could favor HBsAg loss by affecting CD8+ T-cell–mediated immune responses. We first found that HBsAg loss patients possessed a higher frequency of IL-21-producing Env-specific CD4+ T cells than HBsAg-positive patients (Figure 4A). In vitro addition of IL-21 augmented IFN-γ-producing Env-specific CD8+ T cells (Figure 4B). Furthermore, IL-21R-KO mice with the HBV model showed reduced Env-specific CD8+ T cells compared with the WT mice (Figure 4C). Thus, we speculated that CD4+ T cells might affect CD8+ T cells in an IL-21-involved manner. We next investigated the effect of CD4+ T cells on CD8+ T cells; we performed in vitro assays in the context of CD4+ T-cell depletion, as shown in Figure 4D, decreased effector memory CD8+ T cells, CD69 expression, and elevated naïve CD8+ T cells were found in patients with therapy discontinuation, indicating that CD4+ T cells induced a strong effector phenotype on CD8+ T cells. In addition, CD4+ T-cell–depleted PBMCs showed reduced Granzyme B+CD8+ T cells and HBV-specific IFN-γ+CD8+ T cells. We next analyzed the impact of different CD4+ T cell subsets on CD8+ T cells. Compared with relapsers, responders had a higher frequency of effector memory CD4+ T cells but a lower frequency of naïve CD4+ T cells at 4 and 8 weeks after treatment discontinuation (Figure S8A, http://links.lww.com/HEP/E8). Transcriptome analysis from liver tissue revealed that, compared with CHB patients without memory-activated CD4+ T cells infiltration, upregulated CD8+ T-cell activation and differentiation were detected in those patients with infiltrating memory-activated CD4+ T cells (Figure 4E, upper panel). In comparison, hepatic infiltrating naïve CD4+ T cells downregulated the activation and differentiation of CD8+ T cells (Figure 4E, lower panel). Notably, CD4+ T-cell–deficient mice showed delayed HBsAg clearance and reduced IFN-γ-producing HBV-specific CD8+ T cells (Figure 4F). These data indicated that CD4+ T cells promote CD8+ T-cell–mediated immune responses.
FIGURE 4.
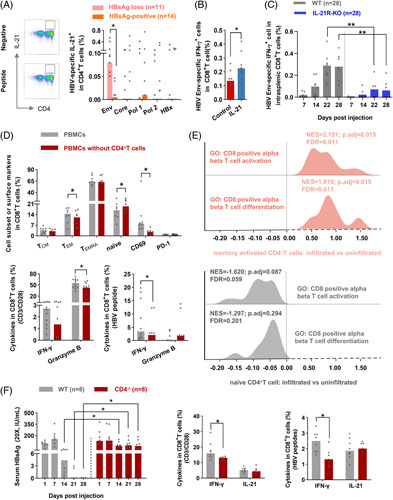
CD4+ T cells favor CD8+ T-cell–mediated cellular immune responses. Representative FACS plots showed the staining of IL-21-producing HBV-specific CD4+ T cells. The frequency of IL-21-producing HBV-specific CD4+ T cells in HBsAg loss and HBsAg-positive patients with 96 weeks of therapy discontinuation. (B) The frequency of IFN-γ-producing HBV Env-specific CD8+ T cells from patients with chronic HBV infection after stimulated with recombination human IL-21 (IL-21) and PBS (control), respectively (n=8) for 10 days. (C) WT and IL-21R-KO mice were injected with pAAV-HBV1.2 HDI. Splenic lymphocytes were stimulated with HBV Env peptide pools for 6 hours; the frequency of IFN-γ-producing HBV-specific intrasplenic CD8+ T cells was detected at indicated time points. (D) Bulk PBMCs and corresponding PBMCs with CD4+ T-cell depletion from patients with therapy withdrawal (n=12) were cultured without a stimulant for 5 days, or stimulated with anti-CD3/CD28 for 3 days, or stimulated with HBV peptides for 10 days. The cell subset, CD69, PD-1, granzyme B, and IFN-γ expression on CD8+ T cells in bulk PBMCs and PBMCs without CD4+ T cells were compared. (E) The data analysis from NCBI’s Gene Expression Omnibus (GSE83148) included 122 CHB patients. Hepatic infiltrated immune cells (memory-activated CD4+ T cells and naïve CD4+ T cells) and uninfiltrated immune cells were compared in liver tissues using the CIBERSORT web portal. GSEA was conducted to evaluate the effect of memory-activated CD4+ T cells (pink, upper panel) and naïve CD4+ T cells (gray, lower panel) on CD8+ T-cell activation and differentiation gene ontology pathway. (F) WT and CD4-/- mice were injected with pAAV-HBV1.2 HDI. Concentrations of HBsAg (20×, IU/mL) were measured. The frequency of total and HBV-specific IFN-γ+CD8+ and IL-21+CD8+ T cells were detected in WT and CD4-/- mice. (A–D, and F) Mann–Whitney U test or paired Wilcoxon test. *p < 0.05, **p < 0.01. Abbreviations: Env, Envelope; GO, gene ontology; GSEA, gene set enrichment analysis; HDI, hydrodynamic injection; PBMCs, peripheral blood mononuclear cell; Pol, Polymerase; TCM, central memory T; TEM, effector memory T; WT, wild type.
CD4+ T-cell–involved humoral immune response favors HBsAg loss
We then investigated whether CD4+ T cells could favor HBsAg loss by affecting B-cell–mediated humoral immune response; we first quantified HBV-specific B cells. As shown in Figure 5A, HBsAg loss patients showed an elevated frequency of HBsAg-specific memory B cells and decreased HBsAg-specific atypical memory B cells. In addition, we found HBsAg-specific B cells had downregulated the expression of inhibitory molecular CD32 and upregulated IL-6 expression in HBsAg loss patients (Figure 5B). Of note, the frequency of HBsAg-specific memory B cells was inversely correlated with serum HBsAg quantification and HBV DNA levels (Figure 5C). Further analysis of B cell function revealed an increased frequency of HBsAb-producing B cells in HBsAg loss patients at 96 weeks after therapy withdrawal (Figure 5D). These observations indicated that HBsAg-specific memory B cells might play an essential role in HBsAg loss.
FIGURE 5.
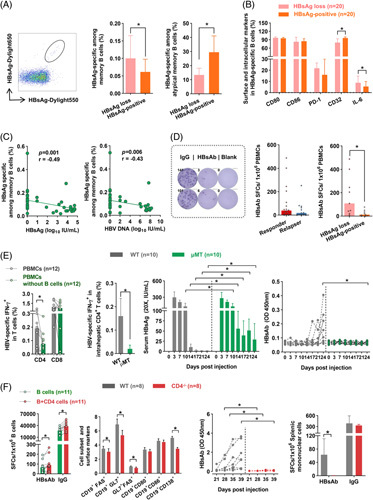
CD4+ T-cell-involved humoral immune response favors HBsAg loss. (A) Representative plots of HBsAg-specific B cells. The frequency of (A) HBsAg-specific memory B cells and atypical memory B cells, (B) surface markers and IL-6 of HBsAg-specific B cells in HBsAg loss (n=20) and HBsAg-positive (n=20) patients (n=20). (C) Spearman correlation analysis of HBsAg-specific memory B cells with HBsAg or HBV DNA levels (n=40). (D) Representative plots of B cell ELISpot assays. HBsAb-producing B cells in responders (n=18) and relapsers (n=13) or HBsAg loss (n=9) and HBsAg-positive patients (n=9) at 96 weeks of discontinuation. (E, left panel) Bulk PBMCs or B cell-depleted PBMCs were stimulated with HBV peptides for 10 days; subsequently, IFN-γ-producing HBV-specific T cells in CD4+ and CD8+ T cells were assessed. (E, right panel) WT and μMT mice were injected with pAAV-HBV1.2 HDI. HBV-specific IFN-γ+ intrahepatic CD4+ T cells in WT and μMT mice were detected. Dynamic changes in concentrations of HBsAg (20×, IU/mL) and the OD values of HBsAb in WT and μMT mice were measured. (F, left panel) B cells were cocultured with or without CD4+ T cells, and HBsAb-producing and IgG-producing cells were detected by ELISpot. (F, right panel) WT and CD4-/- mice were injected with pAAV-HBV1.2 HDI. The activation markers (FAS, GL7, CD80, and CD86) of B cells, GC (FAS+GL7+), plasma cells (CD19+CD138+), and the OD values of HBsAb were measured. HBsAb-producing and IgG-producing splenic mononuclear cells were detected by ELISpot. (A–F) Mann–Whitney U test or paired Wilcoxon test. *p < 0.05. Abbreviations: HDI, hydrodynamic injection; OD, optical density; PBMCs, peripheral blood mononuclear cells; SFCs, spot-forming cells; WT, wild type.
We next evaluated the cognate CD4+ T cell/B cell interactions. To investigate whether B cells were involved in specific CD4+ T-cell responses, B cells were depleted from PBMCs. We observed that HBV-specific CD4+ T-cell responses were significantly lower in B cell-depleted PBMCs than in integrated PBMCs. In contrast, the levels of specific CD8+ T cells were comparable in the 2 groups (Figure 5E). Furthermore, B cell-deficient μMT mice showed markedly reduced production of IFN-γ by intrahepatic HBV-specific CD4+ T cells, and B cell deficiency caused a delay in HBsAg clearance and a lack of HBsAb production (Figure 5E). Next, we assessed the effect of CD4+ T cells on HBV-specific B cell responses; it was observed that IgG- and HBsAb-secreting B cells in autologous B and CD4+ T-cell cocultures were significantly enhanced compared with those in B cell monocultures (Figure 5F). As expected, CD4+ T-cell–deficient mice showed downregulated expression of activation markers of B cells, plasma cells, and production of HBsAb (Figure 5F). These findings indicated that CD4+ T cells favor HBsAg loss by contributing to B cell–mediated humoral immune response.
IL-9 augments HBV-specific CD4+ T-cell responses in patients after therapy discontinuation
Since HBV-specific CD4+ T cells were crucial for sustained viral control and HBsAg loss after therapy discontinuation, we sought to explore the potential factors that influence HBV-specific CD4+ T-cell responses. We first performed the MILLIPLEX assay to detect the serum levels of 16 checkpoint proteins at the baseline of therapy withdrawal in responders and relapsers. There was no statistical significance in responders and relapsers (Figure 6A). Higher expressions of PD-1, 2B4, and CD160 on total T cells and Eomes on CD8+ T cells were observed in relapsers than in responders at 8 or 12 weeks after treatment discontinuation (Figure S8B, http://links.lww.com/HEP/E8). These observations indicated that persistent exposure to HBV might gradually lead to the evolution of exhausted phenotypes. Nevertheless, PD-1 blockade did not restore HBV-specific T-cell response (Figure 6B).
FIGURE 6.
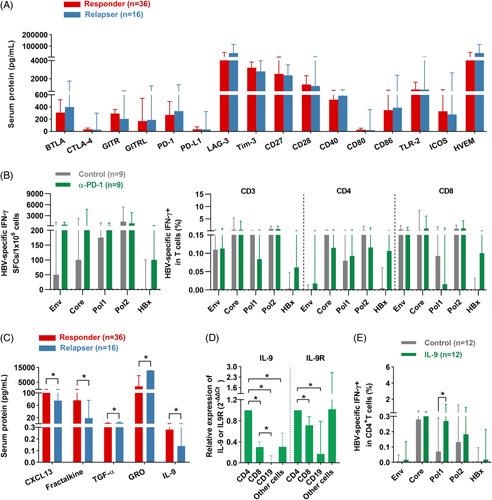
IL-9 augments HBV-specific CD4+ T-cell responses after therapy discontinuation. (A) Concentrations of checkpoint proteins in the serum at the baseline (0 wk) of therapy withdrawal in responders and relapsers. (B) The frequency of IFN-γ-producing specific T cells in the presence/absence of PD-1 blockade in patients at 96 weeks of therapy withdrawal. (C) Concentrations of serum CXCL13, Fractalkine, TGF-α, GRO, and IL-9 in responders and relapsers at the baseline (0 wk) of therapy withdrawal. (D) Quantitative real-time PCR for IL-9 and IL-9R expression (n=6) was performed among CD4+ T cells, CD8+ T cells, CD19+ cells, and other cells (activated PBMCs with CD4+, CD8+, and CD19+ cell depletion). (E) The frequency of IFN-γ-producing specific CD4+ T cells was assessed after in vitro expansion for 10 days in the presence/absence of IL-9 in patients at 96 weeks of therapy withdrawal. (B and E) Paired Wilcoxon test; (A, C, and D) Mann–Whitney U test. *p < 0.05. Abbreviations: Env, Envelope; IL-9R, IL-9 receptor; Pol, Polymerase; rhIL-9, recombinant human IL-9; SFCs, spot-forming cells.
We next evaluated the expressions of 38 cytokines and chemokines at the baseline of therapy withdrawal by the MILLIPLEX assay. CXCL13, Fractalkine, and IL-9 levels were significantly higher in responders than relapsers, while the levels of TGF-α and GRO were lower in responders (Figure 6C). Of note, CD4+ T cells were the primary source of IL-9 and showed elevated expression of IL-9 receptors (Figure 6D). As IL-9 has been reported to promote the growth of a T helper cell line and the expansion of antigen-specific T cells,17 we assessed the effect of IL-9 on HBV-specific CD4+ T cells. We found IL-9 augmented Pol-specific CD4+ T-cell responses (Figure 6E). These findings indicated that elevated IL-9 might maintain HBV-specific CD4+ T-cell responses after anti-HBV treatment interruption.
DISCUSSION
The maintenance of viral control and HBsAg loss remains a challenge in CHB patients after discontinuation of antiviral treatment. In this study, using a series of samples from a longitudinal cohort of CHB patients with NA discontinuation and peptides spanning the whole HBV genome, we found boosted HBV Pol-specific CD4+ T cells at baseline withdrawal; Core-specific and Pol-specific CD4+ T-cell responses are related to sustained viral control after long-term treatment withdrawal. Importantly, Env-specific CD4+ T cells and Env-specific memory B cells are associated with HBsAg loss, and CD4+ T cells can dominate the production of anti-HBs from B cells. These findings suggest that robust HBV-specific CD4+ T-cell responses are critical for maintaining favorable outcomes in CHB patients after antiviral treatment withdrawal.
It is widely accepted that HBV-specific T cells are associated with HBV elimination. Nevertheless, a small number of HBV-specific effector T cells could be detected in ex vivo ELISpot. After in vitro expansion, central memory T cells were activated and underwent proliferation. Cultured ELISpot is more sensitive to HBV-specific T-cell detection (Figure S3B, http://links.lww.com/HEP/E8). Thus, we speculated that a more significant pre-expansion effector T-cell population and greater expansion capacity of central memory T cells might account for the higher numbers in cultured ELISpot. Besides, it has been documented that HBV-specific T cells targeting different proteins possess distinct features. Herein, we found that Core-specific and Pol-specific T cells exhibited a more robust response than Env-specific T cells both in responders and relapsers (Figure S9, http://links.lww.com/HEP/E8). More importantly, we also verified that the magnitude of Core-specific and Pol-specific T cells were associated with the sustainability of viral control, which is in line with previous research showing increased Core-specific and Pol-specific T cells in nonflare patients compared with flare patients receiving NA.4 It should be noted that relative to 0 weeks of withdrawal, Core-specific and Pol-specific T cells in relapsers had a higher trend at the last time point. We speculated that NA retreatment for those patients might partially restore the T-cell response. For another, the duration of cell preservation should also be considered. At this point, we mainly focused on the difference between the responder and the relapser at corresponding time points. Although no significant difference was found among patients of different ages (Figure S10A and B, http://links.lww.com/HEP/E8), responders with strong HBV Core-specific and Pol-specific T-cell responses showed a decreased trend of age (Table S1, http://links.lww.com/HEP/E8); this finding indicates age might be an important factor that influences HBV-specific T cells. Indeed, a new perspective regarding age, in other words, the duration of HBV exposure exerts a major effect on HBV-specific immunity has been proposed by different research groups.18–20 In addition, sex is also a factor that affects HBV-specific T cells (Figure S10C and D, http://links.lww.com/HEP/E8); therefore, young female CHB patients should be considered as the optimal candidates for anti-HBV therapy and might have a greater chance of achieving safe discontinuation of antiviral therapy.
As persistent exposure to HBsAg may cause the deletion of Env-specific T cells,21 Env responses are particularly compromised in CHB patients. Interestingly, data from our cohort has shown that HBV Env-specific T-cell responses were closely related to baseline HBsAg levels after therapy withdrawal (Figure S5 and Figure S10F, http://links.lww.com/HEP/E8). A previous report has shown that patients with baseline lower HBsAg levels are more likely to achieve functional cure,5,22 which is in line with our results (Table S2, http://links.lww.com/HEP/E8). These observations might explain a close association between Env-specific T-cell responses and HBsAg loss in CHB patients after treatment withdrawal and highlight HBsAg levels as a key point for the clinical decision of therapy withdrawal. Indeed, although HBsAg-positive patients possessed lower Env-specific T-cell responses than Core-specific and Pol-specific T-cell responses, the magnitude of specific T-cell response was comparable among Env, Core, and Pol in HBsAg loss in CHB patients (Figure S9, http://links.lww.com/HEP/E8). The divergent capacity of distinct HBV-specific T cells might relate to their affinity for the target antigen and the quantity of HBV protein produced by infected hepatocytes. A report from a model of HBV transgenic mice showed that Env-specific CD8+ T cells had a higher affinity and were more capable of recognizing antigens than Pol-specific CD8+ T cells.8,23 Thus, we speculated that T cells from HBsAg loss in CHB patients could recognize more epitopes derived from the Env, and the multispecificity of T-cell response may favor HBsAg clearance. Our research highlights the critical role of different peptides in sustained response or functional cure. In addition, individual HBV epitopes might be beneficial to engineer HBV-specific T cells utilizing a classical T-cell receptor, which might provide a functionally enhanced population of HBV-specific T cells.24,25 Thus, it is also critical to investigate how specific T-cell responses targeting individual HBV epitopes impact clinical outcomes. This study found that patients with HBsAg loss showed an enhanced response to Env87 (Env 334–348) and Env95 (Env 366–380) exclusively. These observations might provide clues for designing and synthesizing pentamers and subsequent T-cell receptor clones, which are expected to be used for T-cell receptor T-cell immunotherapy of chronic HBV infection.
The function of HBV-specific T cells is closely related to cytokines or chemokines. Herein, we found increased expressions of serum CXCL13, fractalkine (CX3CL1), and IL-9 in responders at the baseline of therapy withdrawal. IL-9 was initially reported as a T-cell growth factor because it promotes the growth of a T helper cell line.17,26 Its expression is related to the expansion of antigen-specific Th2 cell populations. Previous studies have reported that HBV-specific IL-9-producing CD4+ T cells are downregulated in CHB and HCC patients.27 Our findings found that IL-9 augmented HBV-specific CD4+ T cells in patients after treatment discontinuation, indicating that IL-9 may play an essential role in promoting adaptive immunity against HBV. It should be noted that HBV-specific CD8+ T cells present in the blood of CHB patients, who did not suffer from relapse after treatment withdrawal were selectively enriched for a PD-1+ T-cell population and these cells were functional,4 and blockade of PD-L1 enhanced HBV-specific T-cell responses, especially after discontinuation of therapy.28 Nevertheless, we found that the PD-1 blockade did not improve HBV-specific T-cell responses (Figure 6C). This divergence may be related to population heterogeneity and the duration of therapy discontinuation. Besides, as HBV-specific T cells can be more easily rescued by PD-L1 blockade in CHB patients with low levels of HBsAg and HBcrAg,18 a more precise stratification of CHB patients might help reach a consistent conclusion.
It is commonly recognized that cytotoxic CD8+ T cells play a significant role in HBV clearance; in contrast, the effect of HBV-specific CD4+ T cells has been underestimated. Our data demonstrated that most HBV-specific T cells present in the blood of CHB patients who stopped antiviral therapy were CD4+ T cells. A recent study in HBV transgenic mice has revealed that peripheral HBsAg clearance does not promote in vivo HBV-specific CD8+ T-cell responses,29 and CD8+ T-cell response against HBV Core could continuously decline after HBsAg clearance in HBV-infected patients.12 Thus, we speculated that HBV-specific CD4+ T or CD8+ T cells might perform a predisposed role in different stages. CD4-aided humoral immune responses may mainly contribute to the prevention of HBV reactivation after HBsAg clearance. More importantly, Env-specific CD4+ T cells were associated with HBsAg loss upon therapy withdrawal. Similarly, patients with HBsAg loss undergoing NA therapy show a higher trend in CD4+ T cells specific to the HBV Env than HBsAg-positive patients.30 Thus, a therapeutic intervention targeting the improvement of the Env-specific CD4+ T-cell responses might favor achieving a higher rate of HBsAg clearance. Previous research has shown that HBV-specific CD4+ T-cell responses primarily target epitopes of the Pol, Core, and X proteins in HBeAg-negative patients,11 which support our data. Moreover, their research revealed overlapping Tfh and Th1 lineage commitments of HBV-specific CD4+ T cells. This finding might explain why the levels of IL-21 secreted by Env-specific CD4+ T cells in the HBsAg loss group were significantly higher than those in the HBsAg-positive group. IL-21, a cytokine mainly produced by activated CD4+ T cells, plays a vital role in promoting B cell responses and reinvigorates the antiviral activity of specific CD8+ T cells in CHB patients.31 Furthermore, CD4+ T cells facilitated HBV-specific CD8 responses and the production of HBsAb by B cells, which is in line with a previous report that anti-HBs production is thought to be T-cell–dependent.23,32 Our study indicated synergistic effects of cellular immunity (specific CD4+ T cells), and humoral immunity (specific B cells) might promote HBsAg clearance. Therapeutic intervention targeting the improvement of Env-specific CD4+ T-cell responses might aid in the functional cure of HBV.
In summary, our study highlights the relationship between CD4+ T-cell responses targeting different HBV peptides and outcomes of CHB patients after therapy discontinuation. HBV Core-specific and Pol-specific CD4+ T cells are mainly related to the sustainability of response after drug withdrawal, while Env-specific CD4+ T cells are associated with HBsAg loss. These results may contribute to a better understanding of the mechanism of sustained response and functional cure after discontinuation of anti-HBV therapy and help identify more effective immunotherapeutic approaches.
Supplementary Material
AUTHOR CONTRIBUTIONS
Yongyin Li, Chris Kafai Li, Libo Tang, and Jinlin Hou designed the study. Chunhua Wen, Shuqin Gu, Weibin Wang, Ling Guo, Xuan Yi, Yang Zhou, Zheyu Dong, and Xin Fu performed most of the experiments. Yongyin Li, Chunhua Wen, Shuqin Gu, Weibin Wang, Ling Guo, Libo Tang, Yuhao Wang, and Haitao Chen analyzed the data. Chunhua Wen, Shuqin Gu, Weibin Wang, Ling Guo, Shihong Zhong, Kuiyuan Huang, Junhua Yin, Chunxiu Zhong, Xieer Liang, Rong Fan, and Jie Peng collected the study data. Junhua Yin, Chunxiu Zhong, Xieer Liang, Rong Fan, Deke Jiang, Xiaoyong Zhang, Jian Sun, and Jie Peng provided materials. Chunhua Wen, Libo Tang, and Yongyin Li wrote the manuscript. Yongyin Li, Jie Peng, and Jinlin Hou reviewed the manuscript. Yongyin Li and Jinlin Hou supervised the study.
ACKNOWLEDGEMENTS
The authors express their gratitude to Professor Antonio Bertoletti for his critical comments and suggestions. The authors also express their gratitude to Johnson & Johnson for generously providing HBV overlapping peptides. The authors also thank Gilead Sciences for providing DyLight 550- and DyLight 650-labeled HBsAg (HBsAgD550/D650).
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program of China (2022YFC2303600 and 2022YFC2304800), the National Natural Science Foundation of China (81971933 and 82022007), and the Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University (2020J003).
CONFLICTS OF INTEREST
Jinlin Hou has received consulting fees from AbbVie, Arbutus, Bristol Myers Squibb, Gilead Sciences, Johnson & Johnson, Roche, and grants from Bristol Myers Squibb and Johnson & Johnson. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine aminotransferase; CHB, chronic hepatitis B; ELISpot, Enzyme-linked immunosorbent spot; Env, Envelope; HLA, human leukocyte antigen; IFN, interferon; NA, nucleos(t)ide analogue; PBMCs, peripheral blood mononuclear cells; Pol, Polymerase; TCM, central memory T; TEM, effector memory T; SFCs, spot-forming cells.
Yongyin Li, Chunhua Wen, Shuqin Gu, Weibin Wang, and Ling Guo share co-first authorship.
Yongyin Li, Libo Tang, Jie Peng, and Jinlin Hou share co-corresponding authors.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Yongyin Li, Email: yongyinli@foxmail.com.
Chunhua Wen, Email: 1354278062@qq.com.
Shuqin Gu, Email: shuqingu@foxmail.com.
Weibin Wang, Email: 991698375@qq.com.
Ling Guo, Email: 81974340@qq.com.
Chris Kafai Li, Email: kli35@ITS.JNJ.com.
Xuan Yi, Email: 153445158@qq.com.
Yang Zhou, Email: zhouyang.smu@qq.com.
Zheyu Dong, Email: 949410849@qq.com.
Xin Fu, Email: 502911992@qq.com.
Shihong Zhong, Email: shihongzhong2021@foxmail.com.
Yuhao Wang, Email: 1139575379@qq.com.
Kuiyuan Huang, Email: kuiyhuang@foxmail.com.
Junhua Yin, Email: 576334096@qq.com.
Chunxiu Zhong, Email: zhongchx@qq.com.
Xieer Liang, Email: freeliang@163.com.
Rong Fan, Email: hope273@163.com.
Haitao Chen, Email: chenhaitao0919@126.com.
Deke Jiang, Email: dekejiang17@smu.edu.cn.
Xiaoyong Zhang, Email: xiaoyzhang@smu.edu.cn.
Jian Sun, Email: doctorsunjian@qq.com.
Libo Tang, Email: tanglibosmu@foxmail.com.
Jie Peng, Email: pjie138@163.com.
Jinlin Hou, Email: jlhousmu@163.com.
REFERENCES
- 1.Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827–44. [DOI] [PubMed] [Google Scholar]
- 2.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–17. [DOI] [PubMed] [Google Scholar]
- 3.Cornberg M, Honer Zu Siederdissen C. HBsAg seroclearance with NUCs: rare but important. Gut. 2014;63:1208–9. [DOI] [PubMed] [Google Scholar]
- 4.Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-López M, Lens S, Pallett LJ, Testoni B, Rodríguez-Tajes S, Mariño Z, et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogeveen RC, Robidoux MP, Schwarz T, Heydmann L, Cheney JA, Kvistad D, et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut. 2019;68:893–904. [DOI] [PubMed] [Google Scholar]
- 7.Schuch A, Salimi Alizei E, Heim K, Wieland D, Kiraithe MM, Kemming J, et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut. 2019;68:905–15. [DOI] [PubMed] [Google Scholar]
- 8.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci U S A. 2010;107:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbani S, Boni C, Amadei B, Fisicaro P, Cerioni S, Valli MA, et al. Acute phase HBV-specific T cell responses associated with HBV persistence after HBV/HCV coinfection. Hepatology. 2005;41:826–31. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Luo H, Wan X, Fu X, Mao Q, Xiang X, et al. TNF-α/IFN-γ profile of HBV-specific CD4 T cells is associated with liver damage and viral clearance in chronic HBV infection. J Hepatol. 2020;72:45–56. [DOI] [PubMed] [Google Scholar]
- 12.Kefalakes H, Jochum C, Hilgard G, Kahraman A, Bohrer AM, El Hindy N, et al. Decades after recovery from hepatitis B and HBsAg clearance the CD8+ T cell response against HBV core is nearly undetectable. J Hepatol. 2015;63:13–19. [DOI] [PubMed] [Google Scholar]
- 13.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois Cauwelaert N, Baldwin SL, Orr MT, Desbien AL, Gage E, Hofmeyer KA, et al. Antigen presentation by B cells guides programing of memory CD4(+) T-cell responses to a TLR4-agonist containing vaccine in mice. Eur J Immunol. 2016;46:2719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan AT, Loggi E, Boni C, Chia A, Gehring AJ, Sastry KS, et al. Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J Virol. 2008;82:10986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Jiang X, Liu X, Guo L, Wang W, Gu S, et al. Identification of the association between HBcAg-specific T cell and viral control in chronic HBV infection using a cultured ELISPOT assay. J Leukoc Biol. 2021;109:455–65. [DOI] [PubMed] [Google Scholar]
- 17.Angkasekwinai P, Dong C. IL-9-producing T cells: potential players in allergy and cancer. Nat Rev Immunol. 2021;21:37–48. [DOI] [PubMed] [Google Scholar]
- 18.Aliabadi E, Urbanek-Quaing M, Maasoumy B, Bremer B, Grasshoff M, Li Y, et al. Impact of HBsAg and HBcrAg levels on phenotype and function of HBV-specific T cells in patients with chronic hepatitis B virus infection. Gut. 2022;71:2300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bert N, Gill US, Hong M, Kunasegaran K, Tan DZM, Ahmad R, et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology. 2020;159:652–64. [DOI] [PubMed] [Google Scholar]
- 20.Bertoletti A, Boni C. HBV antigens quantity: duration and effect on functional cure. Gut. 2022;71:2149–51. [DOI] [PubMed] [Google Scholar]
- 21.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–14. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld MJ, Chiu SM, Park JY, Brakenhoff SM, Kaewdech A, Seto WK, Tanaka Y, et al. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J Hepatol. 2022;76:1042–50. [DOI] [PubMed] [Google Scholar]
- 23.Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64:S71–S83. [DOI] [PubMed] [Google Scholar]
- 24.Bertoletti A, Tan AT. HBV as a target for CAR or TCR-T cell therapy. Curr Opin Immunol. 2020;66:35–41. [DOI] [PubMed] [Google Scholar]
- 25.Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021;15:1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014;35:61–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Yang L, Liu S, Zhang M, Jin Z. Interleukin-35 Suppresses interleukin-9-secreting CD4(+) T cell activity in patients with hepatitis B-related hepatocellular carcinoma. Front Immunol. 2021;12:645835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinker F, Zimmer CL, Honer Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584–93. [DOI] [PubMed] [Google Scholar]
- 29.Fumagalli V, Di Lucia P, Venzin V, Bono EB, Jordan R, Frey CR, et al. Serum HBsAg clearance has minimal impact on CD8+ T cell responses in mouse models of HBV infection. J Exp Med. 2020;217:e20200298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–973. e969. [DOI] [PubMed] [Google Scholar]
- 31.Tang L, Chen C, Gao X, Zhang W, Yan X, Zhou Y, et al. Interleukin 21 reinvigorates the antiviral activity of hepatitis B virus (HBV)-specific CD8+ T cells in chronic HBV infection. J Infect Dis. 2019;219:750–9. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Ma S, Tang L, Li Y, Wang W, Huang X, et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology. 2013;58:1277–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


