Abstract
Background:
STK11 is an important tumor suppressor gene reported to confer immunotherapy resistance in non-small cell lung cancers (NSCLC) especially in the presence of KRAS co-alterations.
Methods:
This study analyzed 4446 patients for whom next generation sequencing of tissue and/or circulating tumor DNA (ctDNA) had been performed.
Results:
Overall, 60 of 4446 tumors (1.35%) harbored STK11 alterations. STK11 alterations were associated with shorter median time-to-progression and overall survival (OS) across cancers from diagnosis: 6.4 months (5.1-7.9) versus 12 months (11.7-12.3) p=0.001); and 20.5 (17.4-23.5) versus 29.1 (26.9-31.3) (p=0.03), respectively (pan-cancer). Pan-cancers, the median PFS (95% CI) for first-line therapy (regardless of treatment type) for those with co-altered STK11 and KRAS (N = 27) (versus STK11-altered and KRAS wild-type (N = 33)), was significantly shorter (3 (1.3-4.7) versus 10 (4.9-15.7) months, p<0.0005, p multivariate, 0.06); median OS also was also shorter (p multivariate=0.02). In pan-cancer patients treated with checkpoint blockade, STK11 and KRAS co-altered versus STK11-altered/KRAS wild-type had a shorter median PFS and trend towards shorter OS (p=0.04 and 0.06 respectively). In contrast, in examining STK11-altered versus wild-type pan-cancer patients treated with checkpoint blockade immunotherapy, the two groups showed no difference in outcome (PFS (p=0.4); OS (p=0.7)); STK11-altered versus wild-type lung cancer patients also did not fare worse on immunotherapy.
Conclusions:
Across cancers, STK11 alterations correlated with a poor prognosis regardless of therapy. However, STK11 alterations alone did not associate with inferior immunotherapy outcome in the pan-cancer setting or in NSCLC. Pan-cancer patients with co-altered STK11/KRAS did worse, regardless of treatment type.
Keywords: STK11, Immunotherapy, non-small cell lung cancer, KRAS, cancer
INTRODUCTION
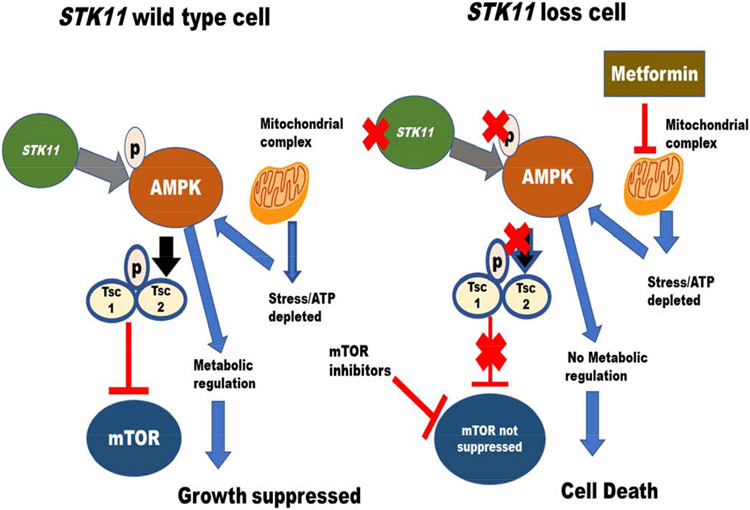
Germline mutations in STK11 (a serine threonine kinase), also known as liver kinase B1 (LKB1), were identified in 1998 as the cause for the rare autosomal dominant genetic disorder Peutz-Jeghers syndrome, which is characterized by hamartomatous polyps, pigmented macules in mouth and extremities, and a predisposition to early-onset colon, breast, stomach, ovarian and pancreatic cancers (1-3). STK11 has since been recognized as an important tumor suppressor gene with a wide variety of metabolic functions, some of which may be theoretically targetable (Supplemental Table 1). Tumor cells with inactivation or loss of STK11 will be particularly vulnerable to a state of energetic stress as they cannot activate adenosine monophosphate-activated protein (AMP) kinase (4). STK11 also negatively regulates mammalian target of rapamycin (mTOR) signaling through its substrate AMPK, and the loss of STK11 leads to the aberrant activation of mTOR in a variety of tissues, with a case study showing usefulness of the mTor inhibitor everolimus for therapy (5-6) (Figure 1). The intestinal polyps in Peutz-Jeghers syndrome have up-regulated mTORC1 signaling, supporting the idea that STK11 loss results in mTOR activation (7).
Figure 1.
STK11 Role in Cellular Processes – STK11 loss results in AMPK suppression. STK11 loss leads to inactivation of AMPK and cell death. Since AMPK suppresses mTOR activity, STK11 loss leads to mTOR activation
A variety of tumors have shown STK11 aberrations. For instance, STK11/KRAS-mutant lung cancers constitute a genetic subset of aggressive non-small cell lung cancer (NSCLC) with increased in vitro sensitivity to mitogen-activated protein kinases (MAPK) and mTOR signaling inhibition (8). Loss of heterozygosity of the STK11 locus in primary breast carcinomas is associated with metastases and low STK11 expression in these tumors portends a poor prognosis (9). The loss of STK11 in cervical cancer and melanoma correlates with widespread and high-grade metastasis (10-11).
STK11 anomalies have also been implicated in immune dysfunction associated with cancer. For instance, STK11-mutant lung cancers have suppression of the immune surveillance response (12). Immune inhibition may be due, at least in part, to neutrophil recruitment and suppression of T cell function through cytokines that promote inflammation (13). Further, STK11 co-mutations predict de novo clinical resistance to programmed cell death 1 or programmed cell death ligand 1 (PD-1/PD-L1) axis blockade in the presence KRAS alterations in lung cancer (14-15). Among lung cancers, STK11/LKB1 alterations were the only marker significantly associated with PD-L1 negativity in patients with high/intermediate tumor mutational burden (TMB) (16) (with both higher TMB and increased expression of PDL1 being associated with immunotherapy response) (17-18).
Herein, we examine the landscape of STK11 alterations in 4446 patients in the pan-cancer setting, as well as association with clinical variables and with treatment outcome.
METHODS
Patients
We reviewed the characteristics and clinical outcomes of 4446 patients with successfully analyzed ctDNA and/or tissue next generation sequencing (NGS) at University California Moores Cancer Center in order to delineate the STK11 mutational landscape and clinical correlates. These included 232 patients who received immunotherapy. Examples of drugs included in immunotherapy were anti-PD-1/PD-L1 agents, anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4), combination anti-CTLA4/anti-PD-1/PD-L1 regimens, and high dose interleukin-2 (IL-2). All investigations followed the guidelines of the UCSD Institutional Review Board for data collection (Profile Related Evidence Determining Individualized Cancer Therapy, NCT02478931) and any investigational therapies for which the patients consented.
Next Generation Sequencing (NGS)
For the purpose of analysis of tissue and blood NGS for alterations, only characterized alterations were assessed. Variants of unknown significance (VUS) were not assessed. If an alteration was found in tissue or in ctDNA, it was counted.
Tissue NGS:
Tissue NGS was performed by Foundation Medicine (FoundationOne™, Cambridge, Massachusetts, http://www.foundationone.com), which is a clinical grade Clinical Laboratory Improvement Amendments (CLIA) approved NGS test that interrogates up to 315 cancer-related genes plus introns from 28 genes often rearranged or altered in cancer to a typical median depth of coverage of greater than 500X (full list available at http://www.foundationone.com/learn.php#2). Pathologists at Foundation Medicine ensure 20% tumor infiltrate in samples for analysis.
This test can detect base substitutions, insertions, and deletions (indels), copy number alterations (CNAs) and rearrangements using a routine tissue sample (including core or fine needle biopsies) (18).
Circulating tumor DNA (ctDNA):
ctDNA analysis was performed by a CLIA-licensed and College of American Pathologist (CAP)-accredited clinical laboratory (Guardant Health, Inc., http://www.guardanthealth.com/) using NGS. Average sequencing depth of coverage was greater than 250×, with >100× at >99% of exons. Up to 73 genes were interrogated (19).
Tumor mutational burden (TMB):
For tissue tumor mutational burden (TMB), the number of somatic mutations (non-driver, non-germline) detected on NGS (interrogating 1.2 megabase (mb) of the genome) was quantified and that value extrapolated to the whole exome using a validated algorithm. TMB was measured in mutations per (mb). TMB levels were divided into three groups: low (1–5 mutations/mb), intermediate (6–19 mutations/mb), and high (≥20 mutations/mb) (Foundation Medicine (FoundationOne™) (16).
Statistical Analysis and Outcomes
Patients’ baseline characteristics were evaluated using descriptive statistics. Multiple logistic regressions (multivariable analysis) were fit to analyze the association between STK11-mutations and other patients’ characteristics. For immunotherapy, overall survival (OS) was defined as the time from initiation of the first immunotherapy to patient death. Patients were excluded in the survival analysis if they were lost to follow up before their first restaging. Patients were censored at date of last follow up for PFS and OS if they had not progressed or died, respectively. For immunotherapy-treated patients, PFS was described as time to progression from date of first immunotherapy. For patients in general, time to progression refers to date of diagnosis to metastases, relapse, or progression.
Estimations for PFS, OS and time to progression were done using a Kaplan-Meier analysis, and were compared amongst subgroups by the log-rank test. The Cox Regression model was fit to assess the association between OS, PFS and multiple other patients’ characteristics (co-variables). Unless otherwise specified, only variables with P-values ≤ 0.25 were included in the multivariable models. All statistical analysis was performed with IBM SPSS version 25.0.
RESULTS
Data was curated for demographics, genomic co-alterations, outcome (regardless of treatment type) and correlation with outcome on immunotherapy (Tables 1 to 4, Figures 1 to 3).
Table 1.
Characteristics of 60 patients with STK11 alterations (VUS excluded) (N = 4,446 patients tested)
| Patient characteristics |
STK11 aberrant N = 60 |
STK11 wild type N = 4386 |
P value univariate |
|---|---|---|---|
| Women (N=2272/4446 or 51.1%) | 20/60 (33.3%) | 2252/4386 (51.3%) | 0.006 (Fisher’s Exact test) |
| Median age at diagnosis (range) (years) | 65 (30-87) | 63 (23-92) | 0.3 |
| Median (95% CI) number of tissue co-alterations (not including STK11 or VUS) tissue | 4 (3-5) | 4 (3-5) | 0.6 |
| Median (95% CI) number of ctDNA co-alterations (not including STK11 or VUS) | 4 (3-5) | 4 (3-5) | 0.7 |
| Median overall survival (95% CI) from diagnosis (months) (Kaplan Meier) | 20.5 (17.4-23.5) | 29.1 (26.9-31.3) | 0.03 |
| Median overall survival (95% CI) from diagnosis (months) (Kaplan Meier) in patients with NSCLC (N=384) | 20.5 months (14.7-26.1) N = 27 |
16.9 months (16.7-17.1) N = 357 |
0.1 |
| Median overall survival (95% CI) from diagnosis (months) (Kaplan Meier) in patients with cancers other than non-small cell lung cancer (N=4062) | 20 months (16.9-23.4) N= 33 |
34 months (31-37.1) N=4029 |
0.09 |
| **Median time to progression from date of diagnosis (months) in all patients (N= 4446) | 6.4 months (5.1-7.9) N=60 |
12 months (11.7-12.3) N=4386 |
0.001 |
| **Median time to progression from date of diagnosis (months) in patients with NSCLC (N= 384) | 6.2 months (4.8-7.6) N = 27 |
8.2 months (8-8.4) N = 357 |
0.6 |
| Median time to progression from date of diagnosis of KRAS-mutated all cancers (N=661) | 3.4 months (1.6-5.4) N=27 |
5.6 months (5.3-5.9) N=634 |
0.2 |
| Median time to progression from date of diagnosis of KRAS-mutated cancers other than NSCLC (N=587) | 3.3 months (3.1-3.6) N=11 |
5.5 months (5.2-5.8) N=576 |
0.07 |
| Median time to progression from date of diagnosis of KRAS-mutated NSCLC (N=74) | 3.4 months (0-7.7) N=16 |
6.8 months (6.2-7.4) N=58 |
0.5 |
| Median time to progression from date of diagnosis of KRAS-mutated cancers excluding patients on immunotherapy (N=627) | 3.3 months (3.1-3.4) N=21 |
5.6 months (5.3-5.9) N=606 |
0.4 |
| Median time to progression from date of diagnosis of KRAS-mutated cancers other than NSCLC excluding patients on immunotherapy (N=565) | 3.2 months (1.7-4.7) N=8 |
5.4months (5.2-5.6) N=557 |
0.08 |
| Median time to progression from date of diagnosis of KRAS-mutated NSCLC excluding patients on immunotherapy (N=62) | 3.4 months (0-6.9) N=13 |
6.8 months (6.3-7.3) N=49 |
0.9 |
| Median overall survival (95% CI) from diagnosis (months) (Kaplan Meier) in patients with KRAS mutated NSCLC (N=74) | 20.5 months (8-32.9) N=16 |
16.8 months (15.8-17.8) N=58 |
0.9 |
| Median overall survival (95% CI) from diagnosis (months) (Kaplan Meier) in patients with KRAS mutated cancers other than NSCLC (N=585) | 16.2 months (13-19.6) N=14 |
13 months (12.5-13.5) N= 571 |
0.9 |
| Median overall survival from date of diagnosis of KRAS-mutated cancers excluding patients on immunotherapy (N=627) | 16.2 months (10-22.4) N= 21 |
13.1 months (12.4-13.5) N=606 |
0.4 |
| Median overall survival from date of diagnosis of KRAS-mutated cancers other than NSCLC excluding patients on immunotherapy (N=565) | 13.1 months (0-27) N=8 |
12.7 months (12.4-13.1) N= 557 |
0.9 |
| Median overall survival from date of diagnosis of KRAS-mutated NSCLC excluding patients on immunotherapy (N=62) | 21.3 months (6-36.6) N=13 |
16.6 months (15.8-17.3) N=49 |
0.1 |
| Type of cancer | Cancers tested for STK11 (tissue and/or ctDNA NGS) (N) |
STK11 alterations N (% of cancers) |
STK11 wild type N (% of cancers) |
| All patients | 4446 | 60/4446 (1.4%) | 4386/4446 (98.6%) |
| Lung non-small cell cancers | 384 | 27/384 (7%) | 357/383 (93%) |
| Head and neck cancers | 92 | 4/92 (4.4%) | 88/92 (95.6%) |
| Anal Squamous cell cancer | 17 | 3/17 (17.6%) | 14/17 (82.4%) |
| Pancreatic adenocarcinoma | 160 | 1/160 (0.6%) | 159//160 (99.4%) |
| Breast cancer | 379 | 4/379 (1.1%) | 375//379 (98.9%) |
| Cholangiocarcinoma | 106 | 3/106 (2.8%) | 103/106 (97.2%) |
| Appendiceal cancer | 123 | 3/123 (2.4%) | 120/123 (97.6%) |
| Colorectal cancer | 421 | 2/421 (0.5%) | 419/421 (99.5%) |
| Carcinoma unknown primary | 287 | 3/287 (1.4%) | 284/287 (98.6%) |
| Other*** | 2477 | 10/2477 (0.4%) | 2467/2477 (99.6%) |
Median time to progression refers to time from diagnosis to metastases or progression and is used in Table 1.
Other consists of hematologic malignancies (N = 348); prostate cancer (N = 209, brain tumors (N=272), gastrointestinal cancers (N= 396), melanoma and other skin cancers (N=221), thyroid cancers (N= 198), ovarian cancer (N= 236), endometrial and cervical cancers (N=148 includes 23 cervical cancers), sarcomas (N= 249)
Abbreviations: CI = confidence interval; ctDNA = circulating tumor DNA; NGS = next generation sequencing; NSCLC = non-small cell lung cancer; VUS = variant of unknown significance
Table 4.
Outcomes of 232 patients who received immunotherapy*
| Characteristics | Median PFS (95% CI) (months) univariate |
HR (95% CI) univariate |
P value univariate |
HR (95% CI)) multivariate** |
P value multivariate |
|---|---|---|---|---|---|
| Age ≥65 (n =91) versus < 65 years (N = 141) | 2.8 (2.1-3.6) versus 7.1 (3.2-11) | 2.9 (2.1-3.9) | <0.005 | 2.7 (1.9-4) | <0.005 |
| Women (N = 94) versus men (N = 138) | 3.8 (2.6-4.8) versus 5.7 (4.1-7.3) | 1.3 (1-1.8) | 0.07 | 1 (0.7-1.4) | 0.9 |
| STK11 mutated (N = 21) versus wild type (N = 211) | 3.2 (1-5.3) versus 4.7 (3.6-5.8) | 1.2 (0.8-2.0) | 0.4 | ||
| STK11 mutated NSCLC (N = 9) versus STK11 wild type lung cancer (N =49) | 5.5 (1.2-9.9) versus 3 (1.3-4.7) | 1.5 (1.1-2.2) | 0.3 | ||
| Lung cancer (N = 58) versus not (N = 174) | 3.6 (2.3-4.8) versus 5.1 (3.8-6.4) | 1.6 (1.1-2.2) | 0.005 | 1.5 (1.02-2.4) | 0.04 |
| Patients with >4 co-alterations versus (≤ 4 co-alterations *** | 4.7 (3.6-5.2) versus 5.1 (2.7-7.5) | 1.1 (0.9-1.5) | 0.8 | ||
| TMB high (N = 47) versus low-intermediate (N = 118) **** | 9.7 (1.5-17.9) versus 4.4 (3.6-5.2) | 0.6 (0.4-0.9) | 0.02 | 0.7 (0.4-.8) | 0.006 |
| STK11 and KRAS co-altered (N=6) versus STK11 wild-type and KRAS mutated (N=28) | 1.9 (0.2-3.9) versus 4.1 ((3.9-4.6) | 1.7 (0.9-2.4) | 0.02 | 1.9 (1-3.8) | 0.04 |
| STK11 and KRAS co-altered (N=6) versus STK11 altered and KRAS Wild type (N = 15) | 1.9 (0.1-3.9) versus 7.1 (0.1-15) | 2.9 (1-9) | 0.04 | 1.3 (0.6-3.1) | 0.5 |
| STK11 and KRAS co-altered cancers other than NSCLC (N=3) versus STK11 wild type and KRAS-mutated cancers other than NSCLC (N=17) | 1.9 (0.7-3.2) versus 5.5 (1.2-9.9) | 1.2 (0.4-3.2) | 0.7 | ||
| STK11 and KRAS co-altered NSCLC (N=3) versus STK11 wild type and KRAS mutated NSCLC (N=11) | 2.1 (0.9-3) versus 3 (1.9-4.2) | 1.6 (0.8-2.5) | 0.1 | 2.1 (1.9-3.4) | 0.3 |
| Characteristics | Median OS (95% CI) (months) univariate***** |
HR (95% CI) univariate |
P value univariate |
HR (95% CI)) multivariate |
P value multivariate |
| Age ≥65 (n =91) versus < 65 years (N = 141) | 7.5 (6-9.2) versus 29 (21.8-36.2) | 12.5 (7.7-20.4) | <0.005 | 12.5 (7-22) | <0.005 |
| Women (N = 94 versus men (N = 138) | 14.8 (10.9-18.8) versus 26.9(21-28.9) | 1.5 (1.02-2.2-) | 0.04 | 1.04 (0.7-1.7) | 0.9 |
| STK11 mutated (N = 21) versus wild type (N = 211) | 17.7 (10.2-25.2) versus 18 (11.2-21.5) versus | 1.2 (0.6-2.3) | 0.7 | ||
| STK11 mutated NSCLC (n = 9) versus STK11 wild type lung cancer (N = 49) | 11.4 (2.4-20.5) versus 12.8 (8.2-22.1) | 1.4 (1.2-2.2) | 0.04 | 1.3 (1.1-2.4) | 0.2 |
| Lung cancer (N = 58) versus not (N = 174) | 11.1 (1.7-20.5) versus 21 (15.3-24.1) | 1.7 (1.1-2.5) | 0.02 | 1.3 (0.7-2.2) | 0.3 |
| Patients with >4 co-alterations versus (≤ 4 co-alterations ** | 15.8 (12.9-23.4) versus 17.5(11.3-21.5) | 1.6 (0.5-3.6) | 0.6 | ||
| TMB high (N = 47) versus low-intermediate (N = 118) **** | 37.3 vs 15.8 | 0.7 (0.4-1.3) | 0.02 | 0.5 (0.3-0.9) | 0.01 |
| STK11 and KRAS co-altered (N=6) versus STK11 wild type and KRAS mutated (N=28) | 9.3 (2.4-16.3) versus 16.3 (10.8-21.8) | 1.2 (0.8-2) | 0.4 | ||
| STK11 and KRAS co-altered (N=6) versus STK11 altered and KRAS wild type (N = 15) | 9.3 (2.4-16.3) versus 17.2 (11.6-22.8) | 2.97 (1-9)) | 0.06 | 2 (0.2-3.6) | 0.5 |
| STK11 and KRAS co-altered cancers other than NSCLC (N=3) versus STK11 wild type and KRAS mutated cancers other than NSCLC (N=17) | 18.7 (2.3-23) versus 23.1 (1.9-8.4) | 1.9 (0.8-2.5) | 0.08 | 2.1 (0.7-3.3) | 0.3 |
| STK11 and KRAS co-altered NSCLC (N=3) versus STK11 wild type and KRAS mutated NSCLC (N=11) | 11.9 (1.6-17.3) versus 12.4 (10.7-37.2) | 1.3 (0.7-2.1) | 0.4 |
Examples of drugs included in immunotherapy were anti-PD-1/PD-L1 agents, anti-CTLA4, combination anti-CTLA4/anti-PD-1/PD-L1 regimens, and high dose interleukin-2 (IL-2).
Variables with p value <0.25 for association with PFS were included in the multivariate analysis. PFS is from first immunotherapy; the small numbers of patients in some rows precludes robust statistical analysis
Co-alterations were stratified at the median (≤ 4 versus >4) determined in Table 2.
TMB values were available for only 165 patients: TMB low to intermediate includes <20 mutations per megabase; TMB high, ≥ 20 mutations/mb.
OS is from initiation of the first immunotherapy to patient death.
Abbreviations: CI = confidence interval; HR = hazard ratio; PFS = progression-free survival; TMB = tumor mutational burden
Figure 3, Panels A and B.
PFS (panel A) and OS (Panel B) (from start of treatment) of patients on immunotherapy with STK11 mutations (N=21) versus those whose tumors are STK11 wild type (wt) (N=211).
Patient characteristics
Demographics (Table 1):
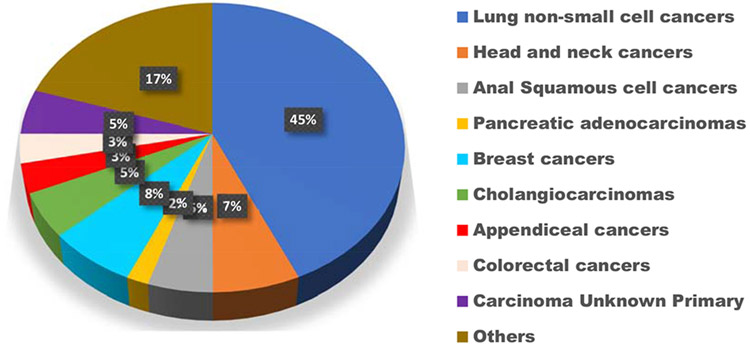
A total of 4446 patients had tissue or ctDNA NGS (2043 patients had tissue testing only; 1432 patients had ctDNA testing only; and 971 patients had both tissue and ctDNA testing). Of these, 60 patients’ tumors (1.35%) had STK11 alterations in either ctDNA, tissue or both (Supplemental Figure 1 (CONSORT diagram)). Median age at diagnosis of the STK11-altered patients was 65.5 versus 63 years in the STK11 wild type (p value, not significant). STK11 alterations were less common in women (33.3% of the 60 patients with STK11 alterations were women) versus 51.3% of patients with STK11 wild type (p = 0.006), though the number of woman versus men in the 4446 patient database was balanced (2272 (51.1%) versus 2174 (48.9%)). Of note, though the numbers are small, 3 of 17 patients with anal squamous cell cancer had STK11 alterations (18%); the percentage of patients with non-NSCLC and STK11 alterations was 7% (27 of 384 patients) (Table 1). Twenty-seven of the 60 STK11-alterred tumors (45%) were in patients with NSCLC and this formed the largest subgroup in the STK11-altered cohort (Figure 2, Panel A).
Figure 2, Panel A.
Distribution of cancer types in the STK11 cohort (N = 60 patients) (VUS excluded). The most common diagnosis was lung cancer, seen in 45% of patients (N = 27 patients)
Of the 60 patients with STK11 alterations, 27 had both tissue and blood ctDNA testing, and of these 27 patients, 9 (33%) had STK11 alterations in both tests, 11 (41%) had STK11 alterations in tissue DNA only and 7 (26%) had STK11 alterations in ctDNA only (Supplemental Figure 1).
Of the 4446 patients, 232 were treated with immunotherapy, mainly PD1/PDL1 checkpoint inhibitors; of these 232 patients, 21 patients (9.1%) had STK11 alterations (Table 2).
Table 2.
Characteristics of immunotherapy-treated patients (N = 232) with STK11-mutated tumors versus those with STK11 wild-type tumors
| Characteristic |
STK11-mutated (N = 21) * |
STK11 wild type (N = 211) * |
P value univariate |
|---|---|---|---|
| Median age (range) (years) | 64 (33-82) | 63 (24-92) | 0.7 (Fisher’s t test) |
| Percent women (N, %) (N = 94) | 6/21 (38%) | 88/211 (39.8%) | 0.4 (Fisher’s t test) |
| Median PFS (95% CI) from first immunotherapy (months) (all patients; N = 232) (Kaplan Meier) | 3.2 (1.02-5.3) | 4.7 (3.6-5.8) | 0.4 |
| Median overall survival ((95% CI) from first immunotherapy (months) (Kaplan Meier) | 18 (10.3-25.3) | 17.7 (10.2-25.2) | 0.7 |
| Frequency of lung cancer in STK11-mutated versus STK11-wild type immunotherapy-treated patients** | 9/21 (42.9%) | 49/211 (23.2%) | 0.06 (Fisher’s Exact test) |
| Median PFS****(95% CI) for TMB status high patients | 1.9 months (1.4-2.4) (N = 5 patients) |
13.6 months (4-23.3) (N = 42 patients) |
<0.005 |
| Median OS (95% CI) for TMB status high patients | 9.3 months (0-19.6) (N = 5 patients) |
37 months (N = 42 patients) |
0.3 |
| Median PFS (95% CI) for TMB status low/intermediate patients | 4.03 months (0-11) (N = 6 patients) |
4.4 months (0.5-5.2) (N = 112 patients) |
0.9 |
| Median OS (95% CI) for TMB status low/intermediate patients | 16.1months (12.1-23.8) (N = 6 patients) |
15.8 months (7.8-34) (N = 112 patients) |
0.5 |
| Median number (95% CI) of co-alterations (STK11 excluded; VUS excluded) (range) (tissue) | 4 (3-5) | 3 (2-4) | 0.3 |
| Median number (95% CI) of co-alterations (STK11 excluded; VUS excluded) (range) (ctDNA) | 4 (3-5) | 3 (2-4) | 0.4 |
|
Microsatellite status Microsatellite stable (N (%)) Microsatellite instability high (N (%)) |
8 patient results available 8/8 (100%) 0/8 (0%) |
117 patient results available 110/117 (94%) 7/117 (6%) |
0.7 (Fisher’s Exact test) |
Patients with either ctDNA and/or tissue positivity for a characterized STK11 mutation were considered to have an STK11-mutated tumor. A total of 21 patients with STK11 alterations and 211 patients wild type for STK11 had immunotherapy and available follow up data.
For immunotherapy treated STK-11 mutated tumors, 9 had lung cancer, 4 had gastrointestinal cancers, 3 had head and neck cancers and 2 had melanomas, 3 were other cancers; for immunotherapy treated STK-11 wild type tumors, 49 had lung cancer, 64 had melanomas, 28 had head and neck cancers, 13 had gastrointestinal cancers, 57 were other cancers.
PFS and OS was from first immunotherapy
TMB low to intermediate includes <20 mutations per megabase; TMB high, ≥ 20 mutations/mb
Abbreviations: ctDNA = circulating tumor DNA; CI = confidence interval; N = number; PFS = progression-free survival; TMB = tumor mutational burden; VUS = variants of unknown significance.
Genomic co-alterations
KRAS and TP53 were the genes most commonly co-altered with STK11 (Figure 2):
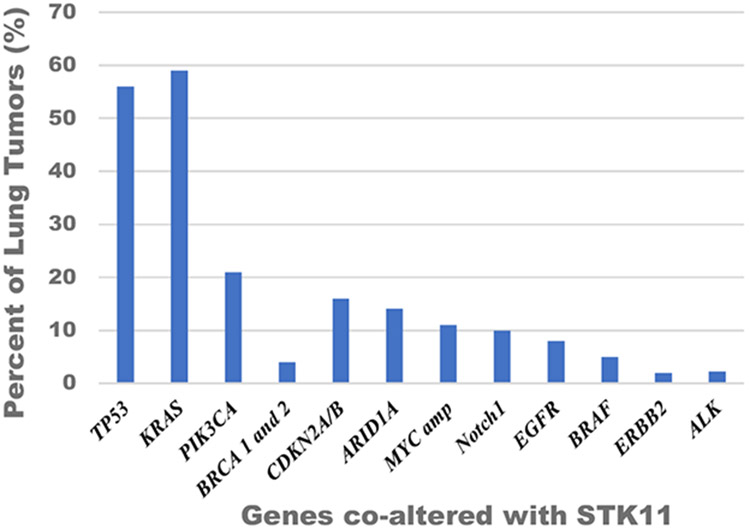
There was no difference in the median number of co-alterations in both groups (STK11-altered versus STK11 wild type) by tissue or circulating tumor DNA (median = 4 co-alteration in all groups (not including STK11 or VUSs) (Table 1). KRAS alterations (seen in 45% of STK11-altered cancers) and TP53 alterations (seen in 48% of STK11-altered tumors) (Figure 2, Panel B) were the most common co-altered genes. In lung cancer, 59% of the 27 STK11-altered tumors also had KRAS alterations and 56% had TP53 co-alterations (Figure 2, panel C).
Figure 2, Panel B.
Genes co-altered with STK11 across all tumors (%). VUS excluded, Total N = 60 patients. Percent of tumors with designated genes co-altered with STK11
Figure 2, Panel C.
Genes co-altered with STK11 across lung tumors (%). VUS excluded, Total N = 27 patients. Percent of lung tumors with designated genes co-altered with STK11
Prognosis and STK11 alterations
STK11 alterations were associated with shorter time to progression and survival across cancers regardless of therapy type (Table 1):
There was a significant difference in median time to progression (meaning metastases, relapse or progression regardless of treatment) from date of diagnosis in patients with STK11 alterations (N = 60) versus those with STK11 wild type (N = 4386) (median (95% confidence intervals (CI), 6.4 months (5.1-7.9) versus 12 months (11.7-12.3); log-rank p = 0.001). There was also a significant difference in median OS from date of diagnosis in patients with STK11 alterations (N = 60) versus those with STK11 wild type (N = 4386) (median (95% confidence intervals (CI)), 20.5 (17.4-23.5) versus 29.1 (26.9-31.3); log-rank p = 0.03).
In NSCLC, there was a non-significant difference in median time to progression (meaning metastases, relapse or progression regardless of treatment) from date of diagnosis in patients with STK11 alterations (N= 27) versus those with STK11 wild type (N = 357) (median (95% confidence intervals (CI)), 6.2 months (4.8-7.6) versus 8.2 months (8-8.4), p= 0.6) (Table 1); median OS was shorter in the STK11-mutated lung cancers, albeit it did not reach statistical significance (p = 0.1).
Prognostic effect of genes co-altered with STK11 (Table 3)
Table 3.
STK11 co-alterations and outcomes across all tumor types and therapies
|
STK11 alterations across all tumor types N=60, 1.7%) |
Median PFS from any first therapy* (95% CI) (months) univariate |
HR (95% CI) univariate |
P value univariate |
HR (95% CI)) Multivariate ** |
P value multivariate |
|---|---|---|---|---|---|
| STK11 and KRAS co-altered N=27/60 (45%) | 3 (1.3-4.7) | 3.1 (1.7-5.7) | <0.0005 | 2.3 (1-5.3) | 0.06 |
| STK11 altered with KRAS wild-type N= 33/60 (55%) (reference) | 10 (4.9-15.7) | ||||
| STK11 and TP53 co-altered N=29/60(48%) | 4.3 (0-10.8) | 1.3 (0.8-2.3) | 0.3 | ||
| STK11 altered with TP53 wild type=31/60 (52%) (reference) | 7.1 (2.3-11.9) | ||||
| STK11 and TP53 and KRAS co-altered N=15/60 (25%) | 1.9 (1.3-2.5) | 3.6 (1.9-6.7) | <0.0005 | 2 (0.9-4.4) | 0.08 |
| STK11 altered with either TP53 or KRAS or both wild type N=45/60 (75%) (reference) | 8.9 (5.1-12.7) | ||||
| STK11 altered with both KRAS and TP53 wild-type N=19/60 (32%) | 9.6 (5.1-14.2) | 0.6 (0.3-1) | 0.06 | 0.5 (0.2-1.4) | 0.9 |
| STK11 altered with KRAS or TP53 alteration N=41//60 (68%) (reference) | 4.6 (1.6-7.6) | ||||
| STK11 altered and KRAS co-altered lung cancers N=16/27 (59%) | 3 (1-5) | 1.7 (0.7-3.9) | 0.2 | 1.8 (0.5-6.1) | 0.3 |
| STK11 altered with KRAS wild type lung cancers N=11/27(41%) (reference) | 6.8 (3.5-10.2) | ||||
| STK11, KRAS, and TP53 co-altered lung cancers N=7/27 (26%) | 1.6 (1.1-2.1) | 2.8 (1.1-6.9) | 0.02 | 1 (0.2-5.7) | 0.9 |
| STK11 altered with either KRAS or TP53 or both wild type lung cancers N=20/27 (74%) (reference) | 5.9 (3.7-8.1) | ||||
| STK11 alteration only lung cancer N=6/27 (22%) | 7.1 (0.3-13.9) | 0.4 (0.1-1.2) | 0.09 | 0.997 (.2-5.7) | 0.9 |
| STK11 altered with KRAS, or TP53 alteration lung cancer N=21/27 (78%) (reference) | 4 (1.6-6.4) | ||||
| STK11 and TP53 co-altered lung cancers N=15/27 (56%) | 1.9 (1.2-2.6) | 2.3 (1.0-25.2) | 0.04 | 2.4 (0.6-9.3) | 0.2 |
| STK11 altered with TP53 wild type lung cancers N=12/27 (44%) (reference) | 6.5 (3.3-9.7) | ||||
| Median OS from any first therapy (95% CI) (months) univariate |
HR (95% CI) univariate |
P value univariate |
HR (95% CI)) multivariate |
P value multivariate |
|
| STK11 and KRAS co-altered N=27/60 (45%) | 16.2 (12.5-20) | 2.8 (1.5-5.4) | 0.001 | 2.9 (1.2-7.2) | 0.02 |
| STK11 altered with KRAS wild-type N= 33/60 (55%) (reference) | 26.3 (0-77.3) | ||||
| STK11 and TP53 co-altered N=29/60 (48%) | 16.2 (15.2-17.2) | 1.6 (0.9-3.1) | 0.1 | 1.7 (0.8-3.9) | 0.2 |
| STK11 altered with TP53 wild type, N=31/60 (52%) (reference) | 21.2 (13.8-28.6) | ||||
| STK11 and TP53 and KRAS co-altered N=15/60 (25%) | 11.9 (3.8-20) | 2.8 (1.5-5.5) | 0.001 | 1.2 (0.3-4.2) | 0.8 |
| STK11 altered with either TP53 or KRAS or both wild type N=45/60 (75%) (reference) | 21.2 (12.3-39) | ||||
| STK11 altered without KRAS or TP53 alteration N=19//60 (32%) | 25.1 (11-19.4) | 0.4 (0.2-0.9) | 0.02 | 0.85 (0.2-3.1) | 0.8 |
| STK11 altered with KRAS or TP53 alteration N=41//60 (68%) (reference) | 18.1 (16.2-23.1) | ||||
| STK11 altered and KRAS co-altered lung cancers N=16/27 (59%) | 18 (4.4-31.7)) | 0.5 (0.2-1.3) | 0.1 | 0.4 (0.1-1.7) | 0.2 |
| STK11 altered with KRAS wild type lung cancers N=11/27(41%) (reference) | 17.8 (11.6-32.2) | ||||
| STK11, KRAS, and TP53 co-altered lung cancers 7/27(26%) | 9.2 (5.9-12.5) | 2.2 (0.8-5.9) | 0.1 | 1.6 (0.2-13) | 0.7 |
| STK11 altered with either KRAS or TP53 or both wild type lung cancers 20/27(74%) (reference) | 20.5 (15.1-26) | ||||
| STK11 altered and TP53 co-altered lung cancers 15/27 (56%) | 11.9 (1.7-22.1) | 1.8 (0.7-4.6) | 0.2 | 2.6 (.4-15.7) | 0.3 |
| STK11 altered with TP53 wild type lung cancers 12/27 (44%) (reference) | 21.2 (16.8-25.6) |
Median PFS and OS is from any first therapy started after diagnosis. STK11 and KRAS co-altered patients 6/27 (22%) received immunotherapy; 18/27 (67%), cytotoxic and antiangiogenic therapy, 3/27 (11%), supportive care.
STK11 and KRAS co-altered lung cancers (16/27), 3/16(19%) received immunotherapy; 10/16(63%), cytotoxic therapy; and 3/16 (19%), supportive care.
Of the STK11-altered KRAS wild type cancers, 6/33 (18%) received immunotherapy; 21/33(64%), cytotoxic therapy; 6 /33 (18%) received other therapies.
Variables with p value <0.25 for association with PFS and OS were included in the multivariate analysis
Across cancers, patients with STK11 and KRAS co-alterations had worse outcome after first therapy (regardless of treatment type) as compared to those with STK11 alterations/KRAS wild type:
Across cancers, the median PFS (95% CI) for the first-line therapy (regardless of type of treatment) for those with co-altered STK11 and KRAS (N = 27) (versus STK11-altered and KRAS wild type (N = 33)), was significantly lower (3 (1.3-4.7) versus 10 (4.9-15.7) months, log-rank p univariate <0.0005, Cox p multivariate, 0.06) (Table 3). The median OS from the first-line therapy (regardless of treatment type) also was lower across cancers in STK11 and KRAS co-altered versus only STK11-altered; Cox p multivariate=0.02) (Table 3).
KRAS, TP53 and STK11 all co-altered were associated with worse outcome on any treatment (Table 3):
Overall, 25% of the total STK11-altered tumors (15/60) had co-alterations in both KRAS and TP53. In univariate analysis, across all tumor types with all three alterations, the median PFS was significantly shorter than in those patients with STK11 alterations and either KRAS or TP53 or both wild-type (1.9 versus 8.9 months, log-rank p<0.005); this held true for lung cancers as well (p = 0.02). OS was also shorter for those with all three co-alterations in univariate, but not in multivariate analysis (log-rank univariate p=0.001, Cox multivariate p=0.8). perhaps due to confounders and/or small numbers of patients.
Across cancers, patients with TP53 and STK11 co-alterations trended towards worse PFS but not OS as compared to those with STK11 alterations/TP53 wild type (Table 3):
Across all patients, the median PFS for the first-line therapy (regardless of type of treatment) for those with co-altered STK11 and TP53 (N = 29) (versus STK11-altered and TP53 wild type (N = 31)) trended lower (4.3 versus 7.1 months, p not significant). This was also true in the NSCLC subset (median PFS was 1.9 versus 6.5 months, univariate p=0.04) (p multivariate, 0.1). The OS however showed no difference for all patients (p=0.2) or for the lung cancer patients (p multivariate = 0.3).
STK11 alterations and immunotherapy treatment
Demographics of patients treated with immunotherapy (Table 4):
Overall, 232 patients treated with various immunotherapy agents were included in the analysis (Supplemental Figure 1). Of these, 21 patients had STK11 alterations. There were no significant differences in the percentage of women or median age (Table 2). There was also no significant difference in TMB status, microsatellite instability (MSI) status, and median number of co-alterations by tissue or ctDNA sequencing between the groups. The percentage of patients with a diagnosis of NSCLC was higher in the patients on immunotherapy with STK11 alterations (9/21 or 42.9%) than those with STK11 wild type (49/211 or 23.2%, p = 0.06), probably because NSCLC was the largest subgroup of patients with STK11 alterations (Table 1 and Figure 2).
Pan-cancer and Immunotherapy
Across cancers, TMB-high status, age less than 65 and being male correlated with better outcomes after immunotherapy (Table 4):
In multivariate analysis, TMB-high status versus TMB-low/intermediate correlated with better outcomes (median PFS = 4.4 versus 9.7 months; TMB- low/intermediate (N = 118) versus TMB-high (N = 51), p multivariate =0.006; median OS = 15.8 versus 37.3 months (multivariate p = 0.01 ). Age less than 65 was associated with significantly longer PFS and OS (p<0.005). In univariate analysis, men also did better than women for both PFS and OS (P<0.05).
Across cancers, patients with STK11-altered tumors did not do worse on immunotherapy than those with STK11-wild type cancers (Table 4):
There was no difference in PFS (p=0.4) or OS (p=0.7) in patients bearing STK11-altered (N = 21) versus wild-type tumors (N = 211) (Tables 2 and 4 and Figure 3).
Across cancers, in patients with STK11 mutations, those with KRAS co-alterations had worse outcome after immunotherapy (Table 4):
Though the numbers of patients are small, STK11 and KRAS co-altered (N=6) versus STK11-altered and KRAS wild-type (N = 15) had a median PFS after immunotherapy of 1.9 versus 7.1 months (p=0.04); median OS was 9.3 versus 17.2 months (p=0.06). (Multivariate analysis was not significant, but the numbers of patients are small).
Across cancers, patients with high TMB and STK11 alterations (versus high TMB and STK11 wild-type) had worse PFS after immunotherapy (Table 2):
Median PFS was 1.9 versus 13.6 months (p <0.005) after immunotherapy in patients with high TMB and STK11 alterations (N = 5) versus those with high TMB and STK11 wild type (N=46); median OS was numerically shorter in the former group (9.3 versus 37 months), but this difference was statistically insignificant (p =0.3) possibly due to the small number of patients. In TMB-low/intermediate patients, median PFS (p=0.9) and OS (p=0.5) did not differ in patients with STK11 alterations versus STK11 wild type. The numbers of patients in the STK11-altered group was small in each calculation.
NSCLC and Immunotherapy
Patients with STK11-altered NSCLCs did not do worse on immunotherapy than those with STK11-wild type cancers (Table 4):
The median PFS from first immunotherapy for STK11-mutated NSCLC (n = 9) versus STK11 wild-type lung cancer (N=49) was not significantly different (median 5.5 versus 3 months; p=0.3): the median OS on immunotherapy for STK11-mutated NSCLC (n = 9) was shorter as compared to STK11 wild-type lung cancer (N = 49) (11.4 versus 12.8 (months) but this difference was insignificant in multivariate analysis (p = 0.04 univariate; p=0.2, multivariate) (Table 4). The numbers were too small to compare STK11-altered/KRAS-altered NSCLC versus STK11-altered/KRAS wild-type NSCLC.
DISCUSSION
To our knowledge, this is the first study examining the clinical correlates of STK11 alterations in a pan-cancer setting. We used both tissue and circulating tumor DNA (ctDNA). The study was prompted by previous work that had identified STK11/LKB1 alterations as the most prevalent genomic driver of primary resistance to PD-1 axis checkpoint inhibitors in KRAS-mutant lung adenocarcinoma (15), perhaps because these tumors expressed lower levels of immune markers, including PD-L1 (14-15). STK11 mutations were also associated with lack of benefit when pembrolizumab was given with chemotherapy in lung cancer (26) and with primary resistance to PD-1/PD-L1 axis blockade in PD-L1-positive non-squamous NSCLC (15,26). Recently, STK11 alterations have been associated with poor prognosis in cervical cancers (27). Genomic factors have been previously implicated in immunotherapy outcome at multiple levels, such as in hyper-progression after immunotherapy (28) and in predicting responsiveness as well (29), making a better understanding of the specific role of STK11 important.
We found that 60 of 4446 (1.35%) of our patients had STK11 alterations in their cancers. There was no difference in the median number of characterized co-alterations in STK11-altered versus STK11 wild-type cancers (median = 4 characterized co-alterations in all groups (not including STK11 or VUS)). The genes most commonly altered with STK11 were KRAS and TP53; KRAS alterations were present in 45% of STK11-altered cancers and TP53 alterations were present in 48% of STK11-altered tumors.
Of the 60 patients with STK11 alterations, 45% (N = 27) had a diagnosis of NSCLC, making it the largest sub-group. The incidence of STK11 alterations in NSCLC in our study was 7%, which is comparable to the data from a study by Facchinetti et.al. (30), wherein STK11 mutations were found in 25 of 302 patients with NSCLC, an 8% incidence, and were co-altered with KRAS in 52% of the patients, again similar to our STK11-altered NSCLC in whom 59% had KRAS alterations (Table 3). In our NSCLC cohort, 56% had TP53 co-altered with STK11 (Table 3).
A recent report (26) presented the results in 49 patients with NSCLC treated with a common chemo-immunotherapy regimen--pemetrexed/carboplatin/pembrolizumab. It was demonstrated that the disease control rate (stable disease ≥6 months or partial remissions) and PFS differed significantly between STK11-altered and STK11 wild-type tumors (response rate, 31.3% vs 72.7%, p=0.01; median PFS 4.4 months vs 11.0 months, P=0.04) (26). However, in our study, STK11 alterations were associated with a poor prognosis, including shorter time to progression (regardless of treatment) (p = 0.001) and shorter OS from diagnosis in all patients (p = 0.03), as well as a shorter time to progression (metastases, relapse or progression) from diagnosis (p = 0.001) and trend towards shorter OS in NSCLC (p = 0.1) as compared to STK11 wild-type patients (Table 1), regardless of therapy. Similarly, in a recent report employing sequencing of 82 genes in 225 NSCLCs in Swedish patients that had surgically resected cancers, STK11 alterations even without KRAS co-alterations were an independent poor prognostic factor for survival (31). Moreover, a recent analysis of 2276 patients with lung adenocarcinoma showed that mutations in STK11 (39% also had KRAS mutations) were associated with poor outcomes, regardless of class of therapeutic agents, and were not specifically associated with poor outcomes only in those on immune checkpoint inhibitors (32). Taken together with our current observations, it appears that STK11 is associated with a poor prognosis across cancers as well as in NSCLC, regardless of the treatment regimen.
Importantly, across cancers in our study, patients with STK11-altered tumors did not do worse on immunotherapy than those with STK11-wild type cancers (Table 4). Our data does not clarify whether or not patients with NSCLC and STK11 alterations do worse on immunotherapy than STK11 wild-type patients because the number of individuals in each subgroup is small.
Skoulidis et al. previously reported a significantly poorer PFS and OS in KRAS-mutated NSCLC with co-alterations in STK11 treated with anti-PDL1 therapy compared to KRAS-mutated, STK11 wild-type NSCLC (14-15). In our study, as mentioned above, STK11 alterations alone did not predict worse immunotherapy outcome, but STK11 alterations together with KRAS alterations (versus KRAS alterations alone), and STK11 alterations in TMB-high (versus TMB-high alone) predicted worse immunotherapy outcome. On the other hand, across cancers, patients with KRAS and STK11 co-alterations also had worse outcome as compared to those with STK11 alterations/KRAS wild type, regardless of therapy. Furthermore, in the literature, STK11-deficient KRAS-mutated tumors have been shown to have a more invasive and metastatic phenotype with marked decrease in survival in NSCLC as compared to STK11 wild-type KRAS mutated NSCLC (33). Therefore, it is conceivable that the poorer outcome in STK11/KRAS altered tumors versus STK11 wild type/KRAS altered tumors reflects the poor prognosis of these cancers regardless of therapy, rather than being immunotherapy specific.
In an analysis of resected lung adenocarcinomas, STK11-mutated tumors show a decrease in intra-tumoral lymphocytes CD4+, CD8+, and CD3+, suggesting that loss of STK11 function suppresses the body’s immune system response (34,35). In a recent analysis of 75 patients with lung cancer, there were zero responses to checkpoint inhibitors in all seven patients with STK11 alterations (36). Similarly, an analysis of response to anti-PDL1/PD1 therapy in KRAS-mutated NSCLC showed resistance in the STK11 co-altered, KRAS-mutated cancers as compared to STK11 wild-type, KRAS-mutated NSCLC (15). In the SU2C (Stand up to cancer) cohort of patients with lung adenocarcinoma recently reported by Skoulidis et. al., STK11 alterations in the KRAS-mutated lung adenocarcinoma cases were the only marker associated with PDL-1 being negative even in the TMB intermediate/high group (15), the latter being a marker for immunotherapy response (16,37-38). There is data to suggest that tumors that overexpress PDL-1 detectable by immunohistochemistry respond better to anti-PDL1 therapies, though lack of expression may not be exclusionary to any benefit (39). In our study, across cancers, TMB-high status was associated with better outcomes after immunotherapy. However, as mentioned, across cancers, our patients with STK11-altered tumors did not do worse on immunotherapy than those with STK11-wild type cancers. On the other hand, pan-cancer_patients with high TMB and STK11 alterations had worse PFS after immunotherapy than those with high TMB and STK11 wild type. These data suggest that STK11 alterations may predict poorer immunotherapy outcome in specific situations, such as those patients with high TMB.
We found that 25% of the patients with STK11 alterations across all cancers had both KRAS and TP53 alterations concomitantly. This subgroup with all three genes co-altered had a significantly shorter PFS and OS from any first therapy than if the STK11 alteration was by itself or with only KRAS or TP53 co-altered (p <0.005 PFS, p= 0.001 OS Table 3); this held true across cancers and in NSCLC. In contrast to our findings, a recent publication (40) found that those with STK11 and KRAS and TP53 mutations had a significantly longer PFS and OS from any first therapy than the STK11 and KRAS co-altered or only STK11-altered group. Further studies would be needed to reconcile the differences in these findings for patients with STK11, KRAS, and TP53 co-alterations.
Our study has limitations, primarily due to the small number of patients with STK11 alterations (despite a large sample size); only 60 patients out of 4446 had a deleterious STK11 alteration. Moreover, only 21 out of the 232 patients (9%) on immunotherapy had STK11 alterations. Further, analysis of outcome after therapy was retrospective. Finally, other limitations of the study included that the number of patients with additional alterations that may be of interest in diseases such as lung cancer (e.g., KEAP1) was small, precluding analysis. Future studies may also want to evaluate disease-specific risk factors, such as tobacco use, ethnicity and so forth.
CONCLUSIONS
In summary, to our knowledge, this is the first study to examine the clinical implications of STK11 alterations across cancer types. In the pan-cancer setting, STK11 alterations portended a poor prognosis, regardless of the therapy. KRAS and TP53 were the most common genes co-altered with STK11.
In our patients, when treated with checkpoint inhibitor immunotherapy, the presence of STK11 across cancers by itself did not correlate with a worse outcome; however, if KRAS was co-altered or TMB was high, the presence of an STK11 alteration correlated with a shorter PFS. On the other hand, when STK11 and KRAS were co-altered, outcome was worse than in those with STK11 wild-type/KRAS-altered tumors, regardless of type of therapy, suggesting that these co-alterations are prognostic factors in addition to or rather than predictive factors for therapy outcome.
Taken together, these data suggest that STK11 alterations may have prognostic effects, regardless of therapy, but do not predict a poor outcome, in of themselves, after immunotherapy in the pan-cancer setting. Similarly, concurrent STK11 and KRAS alterations also are associated with poor outcome in the pan-cancer setting, regardless of treatment. Future studies may need to consider the potentially confounding poor prognostic implications of STK11 alterations when trying to determine the role of this gene in understanding resistance to immunotherapy.
Supplementary Material
STK11 alterations had shorter median time-to-progression and overall survival (OS)
Pan-cancer co-altered STK11/KRAS did worse, regardless of treatment type.
STK11 alterations alone did not associate with inferior immunotherapy outcome
STK11 and KRAS and Tp53 mutations had a significantly shorter PFS and OS
Funding
Funded in part by National Cancer Institute grant P30 CA023100 and the Joan and Irwin Jacobs Fund philanthropic fund.
List of abbreviations
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide
- AMP
Adenosine monophosphate
- AMPK
Adenosine monophosphate-activated protein kinase
- ATP
Adenosine tri-phosphate
- Chk1
Checkpoint kinase 1
- ctDNA
Circulating tumor DNA
- CLIA
Clinical Laboratory Improvement Amendments
- CAP
College of American Pathologist
- CI
Confidence intervals
- CNAs
Copy number alteration
- CTLA4
Cytotoxic T-Lymphocyte Associated Protein 4
- EGFR
Epidermal growth factor receptor
- FAK
Focal adhesion kinase
- HR
hazard ratio
- Indels
Insertions and deletions
- IL-2
Interleukin-2
- LKB1
Liver kinase B1
- Mtorc
Mammalian target of rapamycin complex
- Mtor
Mammalian target of rapamycin
- mb
Megabase
- MSI
Micro-satellite instability
- MAPK
Mitogen-activated protein kinase
- MEF
Mouse embryonic fibroblasts
- MDM2
Murine double minute 2
- N
number
- NGS
Next Generation Sequencing
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PD-L1
Programmed cell death ligand 1
- PD-1
Programmed cell death 1
- PFS
Progression free survival
- Src
Steroid receptor coactivator
- S2UC
Stand up to cancer
- STK11
serine threonine kinase
- TMB
Tumor mutational burden
- VUS
Variants of unknown significance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
All investigations followed the guidelines of the UCSD Institutional Review Board for data collection (Profile Related Evidence Determining Individualized Cancer Therapy, NCT02478931) and any investigational therapies for which the patients consented.
Availability of data and material
All data generated or analyzed during this study are included in this published article. Raw data is available on reasonable request.
Competing interests
Dr. Kurzrock has the following disclosure information: Stock and Other Equity Interests (IDbyDNA, CureMatch, Inc., and Soluventis); Consulting or Advisory Role (Gaido, LOXO, X-Biotech, Actuate Therapeutics, Roche, NeoMed, Soluventis, Pfizer, and Merck); Speaker’s fee (Roche); Research Funding (Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, DeBiopharm, Boerhringer Ingelheim, and OmniSeq [All institutional]); Board Member (CureMatch, Inc., and CureMetrix, Inc.). .).
Dr. Goodman consults for Seattle Genetics and EUSA Pharma. Dr. Barkauskas has no disclosures to report. Nithya Krishnamurthy has no disclosures to report.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998. Jan; 391(6663):184. [DOI] [PubMed] [Google Scholar]
- 2.Su GH, Hruban RH, Bansal RK, Bova GS, Tang DJ, Shekher MC, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999. Jun 1; 154(6):1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turpin A, Cattan S, Leclerc J, Wacrenier A, Manouvrier-Hanu S, Buisine MP, et al. Hereditary predisposition to cancers of the digestive tract, breast, gynecological and gonadal: Focus on the Peutz-Jeghers. Bulletin du Cancer. 2014. Sep; 101(9):813–22. [DOI] [PubMed] [Google Scholar]
- 4.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell metabolism. 2013. Oct 1; 18(4):556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahoney CL, Choudhury B, Davies H, Edkins S, Greenman C, Van Haaften G, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br J Cancer. 2009. Jan; 100(2):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parachoniak CA, Rankin A, Gaffney B, Hartmaier R, Spritz D, Erlich RL, Miller VA, Morosini D, Stephens P, Ross JS, Keech J. Exceptional durable response to everolimus in a patient with biphenotypic breast cancer harboring an STK11 variant. Molecular Case Studies. 2017. Sep 1;3(5): a000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, et al. MTOR and HIF-1α-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proceedings of the National Academy of Sciences. 2009. Jul 7; 106(27): 11137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz–Jeghers syndrome. Oncogene. 2007. Dec; 26(57):7825. [DOI] [PubMed] [Google Scholar]
- 9.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clinical Cancer Research. 2002. Jul 1; 8(7):2085–90. [PubMed] [Google Scholar]
- 10.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PloS one. 2009. Apr 2; 4(4): e5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Monahan KB, Pfefferle AD, Shimamura T, Sorrentino J, Chan KT, et al. LKB1/STK11 inactivation leads to expansion of a prometastatic tumor subpopulation in melanoma. Cancer Cell. 2012. Jun 12; 21(6):751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schabath MB, Welsh EA, Fulp WJ, Chen L, Teer JK, Thompson ZJ, et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation, and immune surveillance in lung adenocarcinoma. Oncogene. 2016. Jun; 35(24):3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016. Mar 1; 76(5):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discovery. 2015. Aug 1; 5(8):860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoulidis F, Carter BW, Zhang J, Wistuba II, Papadimitrakopoulou V, Heymach J. Association of STK11/LKB1 mutations with primary resistance to PD-1/PD-L1 axis blockade in PD-L1 positive non-squamous NSCLC. Journal of Clinical Oncology 2017. 35:15_suppl, 9016–9016. [Google Scholar]
- 16.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Molecular cancer therapeutics. 2017. Nov 1; 16(11):2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA oncology. 2018. Sep 1; 4(9): 1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature Biotechnology. 2013. Nov; 31(11):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PloS one. 2015. Oct 16; 10(10): e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Molecules and Cells. 2013. Oct 1; 36(4):279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. Journal of Biological Chemistry. 2005. Nov 25; 280(47):39582–93. [DOI] [PubMed] [Google Scholar]
- 22.Andrade-Vieira R, Xu Z, Colp P, Marignani PA. Loss of LKB1 expression reduces the latency of ErbB2-mediated mammary gland tumorigenesis, promoting changes in metabolic pathways. PloS one. 2013. Feb 22;8(2):e56567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Li Y, Wang X, Liu F, Gao P, Quinn MM, et al. Gemcitabine and Chk1 inhibitor AZD7762 synergistically suppress the growth of Lkb1-deficient lung adenocarcinoma. Cancer Res. 2017. Sep 15; 77(18):5068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline ER, Shupe J, Gilbert-Ross M, Zhou W, Marcus AI. LKB1 represses focal adhesion kinase (FAK) signaling via a FAK-LKB1 complex to regulate FAK site maturation and directional persistence. Journal of Biological Chemistry. 2013. Jun 14; 288(24):17663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang KJ, Constanzo JD, Venkateswaran N, Melegari M, Ilcheva M, Morales JC, et al. Focal adhesion kinase regulates the DNA damage response and its inhibition radiosensitizes mutant KRAS lung cancer. Clinical Cancer Research. 2016. Dec 1; 22(23):5851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skoulidis F, Elamin Y, Lam V, Zhang J, Lewis J, Rinsurongkawong W, et al.MA19. 10 Impact of STK11/LKB1 Genomic Alterations on Clinical Outcomes with Chemo-Immunotherapy in Non-Squamous NSCLC. Journal of Thoracic Oncology. 2018. Oct 1; 13(10): Supplement 424–5. [Google Scholar]
- 27.Hirose S, Murakami N, Takahashi K, Kuno I, Takayanagi D, Asami Y, et al. Genomic alterations in STK11 can predict clinical outcomes in cervical cancer patients. Gynecologic Oncology. 2020. Jan 1;156(1):203–10. [DOI] [PubMed] [Google Scholar]
- 28.Adashek JJ, Kato S, Ferrara R, Russo GL, Kurzrock R. Hyperprogression and immune checkpoint inhibitors: hype or progress? The oncologist. 2020. Feb;25(2):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz J, Swanton C, Kurzrock R. Molecular profiling and the reclassification of cancer: divide and conquer. American Society of Clinical Oncology Educational Book. 2013;33(1):127–34. [DOI] [PubMed] [Google Scholar]
- 30.Facchinetti F, Bluthgen MV, Tergemina-Clain G, Faivre L, Pignon JP, Planchard D, et al. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer. 2017. Oct 1; 112:62–8. [DOI] [PubMed] [Google Scholar]
- 31.La Fleur L, Falk-Sörqvist E, Smeds P, Berglund A, Sundström M, Mattsson JS, et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Cancer. 2019. Apr 1; 130:50–8. [DOI] [PubMed] [Google Scholar]
- 32.Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open. 2020. Apr 1;5(2): e000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcus AI, Zhou W. LKB1 regulated pathways in lung cancer invasion and metastasis. Journal of Thoracic Oncology. 2010. Dec 1; 5(12):1883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calles A, Sholl LM, Rodig SJ, Pelton AK, Hornick JL, Butaney M, et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS-mutant lung adenocarcinoma. Clinical Cancer Research. 2015. Jun 15; 21(12):2851–60. [DOI] [PubMed] [Google Scholar]
- 35.Skoulidis F, Elamin Y, Papadimitrakopoulou V, Tong P, Wang J, Lewis J, et al. MA04.07 impact of major co-mutations on the immune contexture and response of KRAS-mutant lung adenocarcinoma to immunotherapy. Journal of Thoracic Oncology. 2017. Jan 1; 12(1): Supplement 361–2. [Google Scholar]
- 36.Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018. May 14;33(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman AM, Sokol ES, Frampton GM, Lippman SM, Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer immunology research. 2019. Oct 1;7(10):1570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman AM, Kato S, Chattopadhyay R, Okamura R, Saunders IM, Montesion M et al. , Phenotypic and genomic determinants of immunotherapy response associated with squamousness. Cancer immunology research. 2019. Jun 1;7(6):866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Molecular cancer therapeutics. 2015. Apr 1; 14(4):847–56. [DOI] [PubMed] [Google Scholar]
- 40.Bange E, Marmarelis ME, Hwang WT, Yang YX, Thompson JC, Rosenbaum J, et al. Impact of KRAS and TP53 co-mutations on outcomes after first-line systemic therapy among patients with STK11-mutated advanced non-small-cell lung Cancer. JCO Precision Oncology. 2019. May10; 3:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.