To the Editor: Multiple myeloma (MM) is a plasma cell disorder characterized by heterogeneous features.[1] Accurate risk stratification could predict diverse prognoses of patients with myeloma and attain risk-adapted therapy to extend their lifespan. Recently, the European Myeloma Network (EMN) conducted a large retrospective analysis involving more than 7000 patients with myeloma and developed a new risk model defined as the Second Revision of International Staging System (R2-ISS), with excellent risk distribution among patients enrolled in clinical trials.[2] The R2-ISS stratifications were based on weighted risk scores of different prognostic factors: ISS II 1.0 point, ISS III 1.5 points, del(17p) 1.0 point, elevated lactate dehydrogenase (LDH) 1.0 point, t(4;14) 1.0 point, and 1q21+ 0.5 points. In terms of cumulative total scores, patients were further distributed into four risk groups: R2-ISS I (0 points), II (0.5–1.0 points), III (1.5–2.5 points), and IV (3.0–5.0 points). However, the evidence about the applicability and predictive performance of the R2-ISS in the real world was limited. Thus, this study aimed to determine whether R2-ISS was valid for patients with MM undergoing real-world treatment patterns in China.
This retrospective study included patients with newly-diagnosed multiple myeloma (NDMM) who were diagnosed and treated at the Institute of Hematology and Blood Diseases Hospital in China between January 2013 and December 2019. All patients received either a proteasome inhibitor (PI) or an immunomodulatory drug (IMiD)-based treatment. This study was approved by the Ethics Committee of Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences (No. IIT2020023-EC-1) in compliance with the Declaration of Helsinki. The medical records of our institution were reviewed, and clinical resources were collected from our database. Cytogenetics abnormalities were detected by fluorescence in situ hybridization (FISH) panels (del (17p), t (11;14), t (14;16), t (4;14), t (14;20), and chromosome 1q21) on CD138+ enriched cells (≥95% purity). The threshold levels were set at 20% for the numerical aberrations and 10% for the immunoglobulin heavy (IgH) locus translocations.
Overall survival (OS) was defined as the time from the date of the initial therapy to the time of death or last follow-up. Progression-free survival (PFS) was defined as the time from diagnosis to the time of the first documented disease progression, death, or last follow-up. Survival curves were plotted using the Kaplan–Meier method and compared using the two-sided log-rank test. A P-value <0.05 was considered statistically significant in all analyses. Statistical analyses were performed using the SPSS software (version 26.0; IBM, Armonk, NY, USA) or R version 4.1.2 (http://www.r-project.org).
In total, 505 NDMM patients eligible for R2-ISS stratification were included in this study. The median age was 58 (range: 29–83) years, and 97 patients (19.2%) were aged >65 years. Regarding ISS staging, patients were stratified as follows: ISS I, 91 (18.0%); ISS II, 183 (36.2%); and ISS III, 231 (45.7%). As first-line treatment, 322 (63.8%) of patients received PI-based therapy and 132 (26.1%) received both PIs and IMiDs simultaneously. After induction treatment, 163 (32.3%) patients underwent autologous stem cell transplantation (ASCT). With a median follow-up of 38.3 months, the median PFS and OS were 41.6 months and 71.5 months, respectively. The baseline features and treatment are shown in Supplementary Table 1, http://links.lww.com/CM9/B592.
Compared to the EMN training set[2] and Myeloma and Related Diseases Registry (MRDR) set[3], our patients exhibited a significantly higher proportion of ISS stage III (45.7% [231/505] vs. 24.8% [551/2226] vs. 29.6% [381/1289]; P <0.001) and 1q21+ (47.0% [233/496] vs. 36.8% [820/2226] vs. 13.3% [171/1289]; P <0.001). Regarding first-line treatment, 66.7% (1485/2226) of patients in the EMN training set received PIs + IMiDs-based therapy, evidently higher than our cohort (26.1% [132/505]; P <0.001). Similarly, patients in EMN and MRDR cohorts received a higher proportion of ASCT than our cohort (83.3% [1855/2226] vs. 55.2% [552/1000] vs. 32.3% [163/505]; P <0.001)[Supplemental Table 2, http://links.lww.com/CM9/B592]. Of note, there were some discrepancies in the clinical features and therapeutic options for patients between different regions.
Then we evaluated the effect of 1q21+ and the number of variations on survival. The median PFS and OS of patients carrying the 1q21+ mutation were 33.3 months and 62.6 months, respectively, significantly shorter than those without 1q21 abnormality (PFS: 50.8 months, OS: not reached [NR]; P <0.001). Furthermore, patients with 1q21 gain achieved a similar PFS to the 1q21 amplification group (33.3 vs. 30.7 months, respectively; P = 0.707). A comparable OS was also observed in both groups (56.5 vs. 62.6 months, respectively; P = 0.574) [Supplementary Figure 1, http://links.lww.com/CM9/B592]. Our results provide a complement to the reliability and integrity of the role of 1q21+ in the R2-ISS.
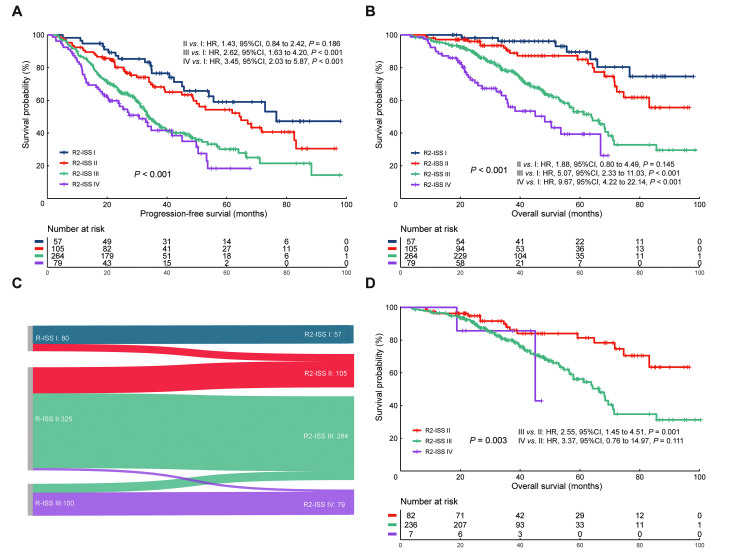
Based on the calculation of risk scores in R2-ISS, 57 (11.3%), 105 (20.8%), 264 (52.3%), and 79 (15.6%) patients were attributed as R2-ISS I, II, III, and IV, respectively. The median OS was NR, NR, 63.8, 48.2 months, and the median PFS times were 76.6, 64.3, 33.8, and 30.7 months in the R2-ISS I, II, III, and IV groups, respectively [Figures 1A,B]. Compared to these patients in R2-ISS I, there were significantly higher risks of death of patients in R2-ISS III (hazard ratio [HR]: 5.07, 95% confidence interval [95% CI]: 2.33–11.03; P <0.001) and of R2-ISS IV group (HR: 9.67, 95% CI: 4.22–22.14; P <0.001). However, the difference in risk of death was not statistically significant for R2-ISS I vs. II (HR: 1.88, 95% CI: 0.80–4.49; P = 0.145). Similar results could be found in the analyses of PFS. These patients classified as R2-ISS III/IV exhibited a higher risk of progression or relapse than R2-ISS I (III vs. I: HR: 2.62, 95% CI: 1.63–4.20, P <0.001; IV vs. I: HR: 3.45, 95% CI: 2.03–5.87, P <0.001). Nevertheless, the hazard ratio of progression or relapse was comparable between R2-ISS I and II (HR: 1.43, 95% CI: 0.84–2.42; P = 0.186) [Figures 1A,B].
Figure 1.
The performance of R2-ISS risk stratification. (A,B) PFS and OS according to R2-ISS. (C) The redistribution of patients with MM from R-ISS to R2-ISS. (D) OS of patients with R-ISS II redistributed by R2-ISS. CI: Confidence interval; HR: Hazard ratio;MM: Multiple myeloma; OS: Overall survival; PFS: Progression-free survival; R2-ISS: Second Revision of the International Staging System; R-ISS: Revision of the International Staging System.
We also explored the discriminating ability of the R2-ISS in specific subgroups, including different age groups, diverse induction treatments, and ASCT options. The R2-ISS accurately depicted the OS rates in patients from either the PI-based, IMiD-based, or PI + IMiD-based groups. The same applied to patients not receiving ASCT and patients belonging to either the younger (≤65 years) or older (>65 years) groups [Supplementary Figure 2, http://links.lww.com/CM9/B592]. Surprisingly, the OS rate of patients receiving ASCT could not be well distinguished by the R2-ISS model (P = 0.230) [Supplementary Figure 2E, http://links.lww.com/CM9/B592].
Furthermore, we depicted the redistribution of patients from the Revision of ISS (R-ISS) to the R2-ISS stratification in Figure 1C. In light of the excessive proportion (64.4% [325/505]), these patients classified as R-ISS II still have heterogeneity in their prognoses. Our results showed that ISS II patients could be divided as follows: 82 patients (25.2%) to R2-ISS II, 236 (72.6%) to R2-ISS III, and 7 (2.2%) to R2-ISS IV. These newly classified patients showed a statistically significant difference in the OS rate (P = 0.003) [Figure 1D]. These results demonstrated that the R2-ISS classification could provide better risk stratification than the R-ISS and mitigate the heterogeneity of the survival rates among R-ISS II patients.
In this study, we showed that the new R2-ISS scoring model is a reliable and reproducible method for adequately predicting the prognosis of patients with MM. Compared with the R-ISS, the risk stratification of R2-ISS appeared more discriminatory, displaying four proportional groups with diverse prognoses. The prediction of survival outcomes in patients classified through the R2-ISS is refined. This improvement can be attributed to the redistribution by R2-ISS for the heterogeneous R-ISS II group.
It should be noted that the proportion of R2-ISS III/IV in our cohort was evidently higher than that in the EMN training set (67.9% [343/505] vs. 50.0% [1112/2226])[2] and MRDR cohort (67.9% [343/505] vs. 48.3% [623/1289])[3], which may be attributed to the higher prevalence of 1q21+ (47.0% [233/496]) in our cohort. However, the proportions of R2-ISS stratification are roughly similar when only considering our patients and those in Chen's cohorts[4] [Supplementary Table 2, http://links.lww.com/CM9/B592]. These results highlighted the inherent variations of patients in different regions, especially for the prevalence of 1q21 gain, which may question whether the current weighted score of 1q21+ in R2-ISS, mainly derived from the European MM population, was somewhat overestimated. Thus, several multicenter studies, including patients among diverse regions, should be conducted to further assess the most appropriate weighted score of 1q21+ in the future.
In subgroup analyses, the R2-ISS model adequately predicted the OS rates in most patients except those receiving ASCT, but the survival curve depicting the OS in ASCT group showed a trend consistent with other subgroups. It could be explained that the unsatisfactory risk stratification may have been influenced by the relatively short follow-up time (38.3 months). In addition, these results of subgroup analyses need to be interpreted with caution, considering the small number of patients in such subgroups.
This study should be viewed considering its limitations. One limitation of our study is that it was conducted in a single center. Moreover, fewer than 50% of patients with MM in our study had received ASCT, thus limiting the extrapolation of our results to other regions. However, our cohort potentially represents the current therapeutic patterns of myeloma patients in a developing country. Lastly, we did not perform further analysis on the accurate weighted value of 1q21+ in the R2-ISS since we were limited by the insufficient number of cases.
In conclusion, the new R2-ISS risk scoring shows better prognostic accuracy than the R-ISS model. It retains its effective risk distribution ability for the Chinese NDMM population in the real world.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81920108006, 82270218) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Nos. 2021-I2M-1-041, 2022-I2M-1-022).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Yan WQ, Fan HS, Xu JY, Liu JH, Li LN, Du CX, Deng SH, Sui WW, Xu Y, Zou DH, Qiu LG, An G. Prognostic value of the Second Revision of the International Staging System (R2-ISS) in a realworld cohort of patients with newly-diagnosed multiple myeloma. Chin Med J 2023;136:1744–1746. doi: 10.1097/CM9.0000000000002735
References
- 1.van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. The Lancet 2021;397: 410–427. doi: 10.1016/s0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- 2.D'Agostino M Cairns DA Lahuerta JJ Wester R Bertsch U Waage A, et al. . Second revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: a European Myeloma Network (EMN) report within the HARMONY project. J Clin Oncol 2022;23: Jco2102614. doi: 10.1200/jco.21.02614. [DOI] [PubMed] [Google Scholar]
- 3.Tan JLC Wellard C Moore EM Mollee P Rajagopal R Quach H, et al. . The Second Revision of the International Staging System (R2-ISS) stratifies progression-free and overall survival in multiple myeloma: real world data results in an Australian and New Zealand population. Br J Haematol 2023;200: e17–e21. doi: 10.1111/bjh.18536. [DOI] [PubMed] [Google Scholar]
- 4.Chen H Zhou N Hu X Wang D Wei W Peng R, et al. . The applicability of the Second Revision of the International Staging System for patients with multiple myeloma receiving immunomodulatory drugs or proteasome inhibitor-based regimens as induction treatment: a real-world analysis. Hematol Oncol 2023;41: 139–146. doi: 10.1002/hon.3090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.