Background:
This meta-analysis aimed to investigate the diagnostic value of color ultrasound screening for fetal cardiovascular abnormalities during the second trimester in China.
Methods:
A literature search was conducted to evaluate the clinical value of color ultrasound screening for fetal cardiovascular abnormalities during the second trimester using English and Chinese databases. Numerical values for sensitivity and specificity were obtained from false-negative, false-positive, true-negative, and true-positive rates, presented alongside graphical representations with boxes marking the values and horizontal lines showing the confidence intervals. Summary receiver operating characteristic (SROC) curves were applied to assess the performance of the diagnostic tests. Data were processed using Review Manager 5.3.
Results:
Four studies (151 patients with fetal cardiovascular abnormalities and 3397 undiagnosed controls) met our evaluation criteria. The sensitivity was 0.91 and 0.96, respectively, and the specificity was 1.00. The Area Under the Curve (AUC) from the SROC curves was >90%; therefore, it was classified as excellent. Furthermore, there were 6 types of fetal cardiovascular abnormalities, and the pooled screening rate of atrioventricular septal defects was the highest.
Conclusion:
Our meta-analysis showed that the use of color ultrasound during the second trimester can be an excellent diagnostic tool for fetal cardiovascular abnormalities.
Keywords: cardiac abnormality, color doppler, meta-analysis, second trimester
1. Introduction
Congenital heart abnormalities are the most common structural abnormalities associated with fetal cardiovascular development.[1] An abnormal fetal cardiovascular function affects fetal health. The rate of congenital heart abnormalities is approximately 0.8% in fetuses, which reduces the population birth quality.[2] The embryo begins to form a cardiovascular system at approximately 3 weeks of pregnancy but is not able to circulate blood independently.[3] The internal structure of the fetal heart does not completely develop until 7 to 8 weeks of pregnancy.[4] The exposure of pregnant women to external adverse stimulation during fetal cardiovascular system development at 7 to 8 weeks of pregnancy may hinder its proper development. In severe cases, the fetus is delivered with congenital heart disease, which severely affects the fetal quality of life in the later years.[5,6]
The causes of fetal cardiovascular abnormalities include heredity factors, fetal factors, and the maternal environment.[7] Older women infected with viruses, or those who have congenital diseases, are more likely to give birth to newborns with fetal cardiovascular abnormalities.[7] Furthermore, abnormalities in fetal chromosomes and amniotic fluid, and developmental delay in utero can also lead to cardiovascular abnormalities.[8] After the birth of a newborn, its symptoms are not obvious and will delay detection time, resulting in increased difficulty in treatment.[8] Due to insufficient development of the fetal cardiovascular system in early pregnancy, there may be some deviation in the detection results of fetal heart disease by ultrasound examination. In the second trimester of pregnancy, fetal orientation and fetal heart development are relatively stable, so it may be the best time to explore fetal heart development.[9]
Color Doppler is the use of autocorrelation technology for Doppler signal processing, and the blood flow signal obtained by autocorrelation technology is color-coded and then superimposed on a 2-dimensional image in real time, leading to the formation of a color Doppler blood flow image.[10] It offers several advantages, including minimal trauma, no radiation exposure, quick and convenient operation, and widespread utilization in clinical practice.[11] It can be used for examination of the brain,[12] liver,[13] spleen,[14] stomach,[15] kidney,[16] breast[17] and uterus,[18] along with screening for vascular diseases, especially cardiovascular diseases.[19]
Color Doppler can be conveniently and accurately applied in the screening of fetal heart malformations during pregnancy, which can clearly diagnose and reduce the birth rate of newborns with congenital heart disease.[20] Color Doppler can be used to assess the intrauterine placenta, umbilical cord, and amniotic fluid status of pregnant women. Furthermore, it can clearly show the general structure of the fetus in utero and the 4 chambers of the heart. It aids in clear visualization of the cardiac vein, artery abnormality, ventricular outflow tract, blood vessels, and other conditions, leading to an improved detection rate.[20] This is of great significance for high-quality fertility.
Color Doppler has been widely used in prenatal fetal screening for cardiovascular abnormalities in China because of its convenience, low trauma, and lack of radiation. Several studies have evaluated the clinical compliance rate of color Doppler in screening for fetal cardiovascular abnormalities during pregnancy.[21–24] However, these studies were conducted with small sample sizes and were not representative of the general Chinese population. In addition, there have been few meta-analyses on the diagnostic accuracy of color Doppler in the diagnosis of fetal cardiovascular abnormalities during the second trimester in the Chinese population. Therefore, the purpose of this study was to use a meta-analysis to comprehensively evaluate these studies and the types of cardiovascular abnormalities in the Chinese population.
2. Materials and Methods
2.1. Study search
A systematic review was conducted according to the methods and recommendations of the PRISMA extension statement for reporting systematic reviews. The Cochrane Collaboration Systematic Reviews of Diagnostic Test Accuracy handbook was also consulted. We identified studies published in English between the years 2015 and 2022. English language (PubMed, Google Scholar, Cochrane Library, and Clinical Trials) and Chinese language databases (CNKI, Cqvip, WANFANG data, and Baidu scholar) were searched applying controlled vocabulary (MeSH keywords), as well as using the terms “cardiac abnormality,” “heart anomaly,” “heart disease,” “congenital,” “Color Doppler,” “second trimester,” “China,” and “Chinese,” or variants and combinations of these keywords. The reference lists of the included studies were reviewed to identify other potentially eligible studies.
2.2. Exclusion criteria
The selected publications were independently evaluated by 2 reviewers based on the established inclusion criteria. These publications were selected based on the information provided in the title and/or abstract as well as their full text, if available in English. Furthermore, these publications were studies on Color Doppler in fetal cardiovascular abnormalities in China. The samples used in these studies were human specimens. Additionally, the samples in these publications were diagnosed using 2-stage screening (a questionnaire for primary screening and formal diagnosis by physicians according to the conventional diagnostic criteria in China). The following studies were excluded from this systematic review and meta-analysis: non-research-based publications, such as reviews, press releases, newsletters, and forums and unclear diagnostic criteria or unconventional diagnostic tools.
2.3. Data extraction and quality assessment
The corresponding data were extracted from studies that met the inclusion criteria and contained the following information: first author, year of publication, country, and sample size (including the number of positive and negative groups). Indicators such as sensitivity and specificity and number of true positives, false positives, false negatives, and true negatives were recorded directly or calculated indirectly according to the original data of the enrolled studies. In addition, data on the disease types of the positive patients were extracted and collected. The quality of the selected publications was estimated following the criteria of the Grading of Recommendations Assessment, Development, and Evaluation method. The quality of the enrolled studies was assessed using the Cochrane Center Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2).
2.4. Statistical analysis
The meta-analysis was performed using Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Forest plots were used to summarize the estimates with 95% confidence intervals. The random-effects model was used to calculate the pooled sensitivity, specificity, positive predictive value, and negative predictive value. The summary receiver operating characteristic (SROC) curve was plotted and the area under the curve (AUC) of the SROC was then calculated. A rough guide for classifying the accuracy of a diagnostic test was based on the AUC. The criteria for AUC classification were 90% to 100% (excellent), 80% to 90% (good), 70% to 80% (fair), 60% to 70% (poor), and 50% to 60% (failure).[25]
For fetal cardiac anomaly studies, a random-effects model was used. The meta-analysis was performed using Review Manager 5.3. The screening rate was computed for each study to derive a pooled screening rate estimate using the inverse variance method, which involved calculating the weighted average using SEs. The variance of the screening rate of each estimate (known as effect size) was calculated as sqrt (pq/n), where p is the screening rate, q is 1-p, and n is the total sample size. Heterogeneity was anticipated in advance, and Cochrane Q test and Higgins I2 statistics were used. The I2 test was also used to assess the heterogeneity between studies (I2 = 75%–100%, P < .05). Forest plots were used to summarize the estimates with 95% confidence intervals.
3. Results
3.1. Description of studies
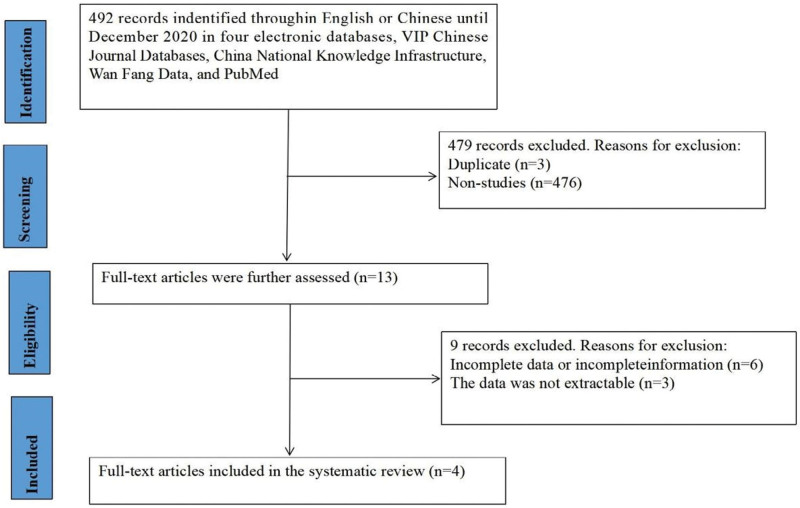
Based on the database search strategies, 492 Chinese or English articles were identified. Thirteen full-text articles were selected, and duplicate citations and studies irrelevant to the current meta-analysis were excluded. After excluding 9 articles with incomplete data, 4 met the inclusion criteria and were included in the systematic review (Fig. 1). All 4 articles were Chinese (Table 1). A total of 151 patients diagnosed with fetal cardiovascular abnormalities after delivery, and 3397 undiagnosed controls were included in the 3 studies. The basic characteristics of the included studies are presented in Table 1.
Figure 1.
Flow chart of the study selection process.
Table 1.
Characteristics of studies included in the present meta-analysis.
| Reference | Yr | Country | Patients/controls | Method | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Guohuai Zhang[11] | 2019 | China | 47/1433 | Color Doppler | 47 | 1 | 2 | 1433 | 96 (86,100) | 100 (100, 100) |
| Haijing Fan[12] | 2020 | China | 20/234 | Color Doppler | 20 | 1 | 1 | 234 | 95 (76,100) | 100 (98, 100) |
| Haohan Li[13] | 2015 | China | 74/2926 | Color Doppler | 74 | 8 | 4 | 2926 | 95 (87, 99) | 100 (99,100) |
| Jing Chen[14] | 2020 | China | 10/190 | Color Doppler | 10 | 0 | 1 | 190 | 91 (59, 100) | 100 (98, 100) |
FN = false negative, FP = false positive, TN = true negative, TP = numbers of true positive.
3.2. Publication bias
The QUADAS-2 tool was used to assess the quality of the included studies (Fig. 2). We evaluated the quality of the diagnostic studies based on the QUADAS-2 criteria in 6 key areas: patient selection, index testing, reference criteria, flow and timing, judgment bias, and applicability. Each area was assessed in terms of risk of bias, and the first 3 areas were assessed in terms of suitability. The outcome of the assessments could be “yes,” “no,” or “unclear” for each item. A “yes” implies a lower risk of bias, while a “no” or “unclear” outcome implies the opposite (Fig. 2).
Figure 2.
Quality of selected studies according to QUADAS-2 guidelines.
3.3. Analysis of diagnostic accuracy
The results of the diagnostic accuracy analysis showed that the sensitivity value was between 0.91 and 0.96, and the specificity value was 1.00 (Table 1 and Fig. 3). An SROC plot based on the results of this meta-analysis was constructed, and the pooled AUC of the SROC was found to be >90% (Fig. 4).
Figure 3.
Forest Plot of Color Doppler for the diagnosis of fetal cardiovascular abnormalities.
Figure 4.
SROC Plot for Color Doppler in the diagnosis of fetal cardiovascular abnormalities. SROC = summary receiver operating characteristic.
3.4. Analysis of types of fetal cardiac anomaly
As shown in Figure 5, there were 7 types of fetal cardiovascular abnormalities according to the type distribution: tricuspid atresia, tetralogy of Fallot, atrioventricular septal defect, single ventricle, ventricular septal defect, and single atrium. Screening rates were 16%, 11%, 36%, 16%, 13%, and 4%, respectively. The pooled screening rate of endocardial pad defects was the highest and was significantly different from that of the other types (P < .05).
Figure 5.
Analysis of types of fetal cardiac anomaly (A–F): tricuspid atresia, tetralogy of Fallot, atrioventricular septal defect, single ventricle, ventricular septal defect, and single atrium.
4. Discussion
Color ultrasound is suitable for clinical applications because it poses little trauma, is convenient, and lacks radiation.[26] Color Doppler examination for pregnant women between 20 and 28 weeks of pregnancy is performed mainly to detect any cardiovascular abnormalities in the fetus. This information is then used to determine whether to induce labor surgically or to continue the pregnancy, which can help avoid fetal malformation to an extent and ensure the quality of the newborn.[27] It is important to screen for fetal heart malformation during pregnancy. If cardiovascular abnormalities are detected on time during the fetal period, the birth of such fetuses can be avoided.[28,29] Screening for fetal cardiovascular abnormalities using prenatal color ultrasound can provide a basis for pregnant women to continue or terminate pregnancy, thus helping to reduce the number of newborns with congenital heart diseases and improve the eugenic birth rate of the population.
Color ultrasound was developed based on B-ultrasound. It has multiple functions, multiple diagnostic methods, high accuracy, a wide examination range, and specific content. Studies have shown that the detection rate of congenital cardiovascular abnormalities using color ultrasound is higher than that of B-ultrasound.[20] Color ultrasound not only provides clear images intuitively but also monitors the growth process of the fetus and checks the amniotic fluid condition in the womb and placental umbilical cord.[30] In addition, color ultrasound can clearly show the structure of the fetal body, 4-chamber surface of the heart, vascular conditions, ventricular outflow tract, and arteriovenous abnormalities. This is beneficial for improving the diagnostic rate.[31] The results of this study showed that the pooled AUC of the SROC was >90%, indicating excellent diagnostic value. This result also supports the clinical application of color ultrasound.
The fetal cardiovascular system takes shape in the early stage; however, it cannot circulate blood effectively at this stage and is easily affected by fetal orientation.[32] After the second trimester, the heart structure is formed and is not affected by fetal orientation. Therefore, the second trimester is the best time to screen for fetal cardiovascular abnormalities. The second trimester was selected as the screening time for the 11 included studies. A systematic review showed that the sensitivity and specificity of color ultrasound in the diagnosis of fetal cardiovascular disorders in the first trimester were 55.80% and 99.98%, respectively, and suggested that the introduction of color Doppler in early ultrasound examination of fetal heart can improve the detection rate of f fetal cardiac pathology.[2] By meta-analysis of the present study, we found that the pooled AUC of SROC was >90% in the diagnosis, the sensitivity value was between 0.91 and 0.96, and the specificity value was 1.00. These results suggest that the diagnostic effect of color ultrasound in the second trimester may be more accurate.
Fetal cardiovascular abnormalities are mainly divided into 2 categories: nonstructural cardiovascular abnormalities, mainly caused by the complete development of the embryo, and structural cardiovascular abnormalities, mainly caused by embryonic development.[33] The results of this study showed that in the 4 included studies, the fetuses were all characterized by structural cardiovascular abnormalities, and the screening rate of atrioventricular septal defects was the highest among all types. The reason why this type of exposure has the highest rate in patients with fetal cardiovascular abnormalities requires further analysis.
The accurate evaluation of cardiac function is crucial for the early diagnosis of cardiovascular disease. Although this study found that color Doppler in the second trimester had an acceptably high accuracy in diagnosing fetal cardiovascular abnormalities. However, diagnostic techniques must be improved to improve diagnostic efficiency and accuracy. A video-based deep-learning algorithm, EchoNet-Dynamic, has outperformed human experts in segmenting the left ventricle, estimating ejection fraction, and evaluating diagnostic manifestations of cardiomyopathy.[34] This study reveals the importance of deep learning in automatically assessing cardiac function from echocardiographic videos. EchoNet-Dynamic can improve the precision and accuracy of existing methods, allowing earlier detection of subclinical cardiac dysfunction. Additionally, DPS-Net, a 2-dimensional echocardiogram based on deep learning, reportedly automatically measures the left ventricular ejection volume.[35] The combined evaluation of large-scale data sets shows that DPS-Net is highly adaptable in different echocardiographic systems. All of these have laid the foundation for the future development of imaging diagnosis of fetal cardiovascular abnormalities.
A limitation of this study is that in terms of diagnostic accuracy, only 4 studies met the inclusion criteria, and the diagnosis process was not described in detail after fetal induction or delivery, which may have affected the overall estimation. In addition, this meta-analysis included studies involving only the Chinese population; therefore, there may be some limitations to the generalization of the conclusions. Moreover, only papers published in Chinese were included, and papers in other languages were excluded, leading to inevitable errors. Nonetheless, our analysis shows that color ultrasound examination in the second trimester of pregnancy can be used as a clinical diagnostic tool for the diagnosis of fetal cardiovascular abnormalities. However, owing to the limitations of this study, high-quality, large sample sizes from multiple centers are still needed for further validation.
5. Conclusions
The use of color ultrasound by pregnant women during the second trimester is a good diagnostic tool for fetal cardiovascular abnormalities. In addition, this diagnostic method can be used for early diagnosis of pregnant women in advance, which contributes to high quality fertility.
Author contributions
Conceptualization: Wenxiu Li.
Data curation: Bingye Shi, Zhe Han.
Methodology: Bingye Shi, Zhe Han.
Software: Bingye Shi, Zhe Han.
Validation: Wei Zhang, Wenxiu Li.
Writing – original draft: Wenxiu Li.
Writing – review & editing: Wei Zhang, Wenxiu Li.
Abbreviations:
- AUC
- area under the curve
- SROC
- summary receiver operating characteristic
The authors have no conflicts of interest to disclose.
Ethics approval was not required because this review contains only published literature.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was supported by the Natural Science Foundation of Beijing (7202040).
BS and ZH contributed equally to this work.
How to cite this article: Shi B, Han Z, Zhang W, Li W. The clinical value of color ultrasound screening for fetal cardiovascular abnormalities during the second trimester: A systematic review and meta-analysis. Medicine 2023;102:28(e34211).
Contributor Information
Bingye Shi, Email: 2435231348@qq.com.
Zhe Han, Email: hdfyhanzhe@163.com.
Wei Zhang, Email: 690285303@qq.com.
References
- [1].Williams K, Carson J, Lo C. Genetics of congenital heart disease. Biomolecules. 2019;9:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karim JN, Bradburn E, Roberts N, et al. First-trimester ultrasound detection of fetal heart anomalies: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2022;59:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Perak AM, Lancki N, Kuang A, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo J, Chaemsaithong P, Huang J, et al. Comparison of uterine artery Doppler measurements at 6 weeks of pregnancy after IVF between pregnancies that resulted in miscarriage and ongoing pregnancies. Int J Gynaecol Obstet. 2021;152:249–55. [DOI] [PubMed] [Google Scholar]
- [5].Scott M, Neal AE. Congenital heart disease. Prim Care. 2021;48:351–66. [DOI] [PubMed] [Google Scholar]
- [6].Linglart L, Gelb BD. Congenital heart defects in noonan syndrome: diagnosis, management, and treatment. Am J Med Genet C Semin Med Genet. 2020;184:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moore-Morris T, van Vliet PP, Andelfinger G, et al. Role of epigenetics in cardiac development and congenital diseases. Physiol Rev. 2018;98:2453–75. [DOI] [PubMed] [Google Scholar]
- [8].Yoshizato T, Kozuma Y, Horinouchi T, et al. Diagnosis of fetal abnormalities during the first trimester. Kurume Med J. 2021;66:85–92. [DOI] [PubMed] [Google Scholar]
- [9].Volpe P, De Robertis V, Campobasso G, et al. Diagnosis of congenital heart disease by early and second-trimester fetal echocardiography. J Ultrasound Med. 2012;31:563–8. [DOI] [PubMed] [Google Scholar]
- [10].Paladini D, Bottelli L, Lops G, et al. Color Doppler visualization of fetal coronary arteries is enhanced by high resolution and Radiantflow. Ultrasound Obstet Gynecol. 2022;60:432–4. [DOI] [PubMed] [Google Scholar]
- [11].Cannella R, Giambelluca D, Pellegrinelli A, et al. Color doppler ultrasound in portal hypertension: a closer look at left gastric vein hemodynamics. J Ultrasound Med. 2021;40:7–14. [DOI] [PubMed] [Google Scholar]
- [12].Pedicelli A, Bartocci M, Lozupone E, et al. The role of cervical color Doppler ultrasound in the diagnosis of brain death. Neuroradiology. 2019;61:137–45. [DOI] [PubMed] [Google Scholar]
- [13].Jia P, Liu R, Liu Y, et al. Comparison of the value of color doppler ultrasound and multislice spiral CT in the differential diagnosis of benign and malignant nodules in the liver. Contrast Media Mol Imaging. 2022;2022:5251966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giunta M, La Mura V, Conti CB, et al. The role of spleen and liver elastography and color-doppler ultrasound in the assessment of transjugular intrahepatic portosystemic shunt function. Ultrasound Med Biol. 2020;46:1641–50. [DOI] [PubMed] [Google Scholar]
- [15].Jang HS, Lee JS, Lim GY, et al. Correlation of color Doppler sonographic findings with pH measurements in gastroesophageal reflux in children. J Clin Ultrasound. 2001;29:212–7. [DOI] [PubMed] [Google Scholar]
- [16].Petrucci I, Clementi A, Sessa C, et al. Ultrasound and color Doppler applications in chronic kidney disease. J Nephrol. 2018;31:863–79. [DOI] [PubMed] [Google Scholar]
- [17].Moustafa AF, Cary TW, Sultan LR, et al. Color doppler ultrasound improves machine learning diagnosis of breast cancer. Diagnostics (Basel). 2020;10:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Keller CA, Antil N, Jeffrey RB, et al. Color doppler imaging of vascular abnormalities of the uterus. Ultrasound Q. 2022;38:72–82. [DOI] [PubMed] [Google Scholar]
- [19].Rice MJ, McDonald RW, Sahn DJ. Contributions of color Doppler to the evaluation of cardiovascular abnormalities in the fetus. Semin Ultrasound CT MR. 1993;14:277–85. [DOI] [PubMed] [Google Scholar]
- [20].Takita H, Matsuoka R, Tokunaka M, et al. Usefulness of color Doppler mode for fetal cardiac ultrasound screening in the second trimester: a study at a single perinatal center. J Matern Fetal Neonatal Med. 2020;33:3695–8. [DOI] [PubMed] [Google Scholar]
- [21].Guohuai Z. Clinical study on screening fetal cardiovascular abnormalities by color ultrasound during pregnancy. Electron J Clin Med Lit. 2019;47:163. [Google Scholar]
- [22].Haijing F. Electronic journal of clinical medical literature. Contemp Med. 2020;31:153–4. [Google Scholar]
- [23].Haohan L. Analysis of the clinical effect of pregnant women in pregnancy ultrasound screening for fetal cardiovascular abnormality. W Med Inf Digest. 2015;8:42–3. [Google Scholar]
- [24].Jing C. Clinical study on screening fetal cardiovascular abnormalities by color ultrasound during pregnancy. Ima Res Med Appl. 2020;10:221–2. [Google Scholar]
- [25].Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318:2199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Q, Qi J, Xiao S. Application of color ultrasound in the diagnosis of gynecological endocrine disorders and study of ultrasonic image characteristics. Contrast Media Mol Imaging. 2022;2022:4773179. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [27].Wei Z, Mu M, Li M, et al. Color Doppler ultrasound detection of hemodynamic changes in pregnant women with GDM and analysis of their influence on pregnancy outcomes. Am J Transl Res. 2021;13:3330–6. [PMC free article] [PubMed] [Google Scholar]
- [28].Allan LD, Apfel HD, Printz BF. Outcome after prenatal diagnosis of the hypoplastic left heart syndrome. Heart. 1998;79:371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holland BJ, Myers JA, Woods CR, Jr. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol. 2015;45:631–8. [DOI] [PubMed] [Google Scholar]
- [30].Yoo SJ, Min JY, Lee YH. Normal pericardial fluid in the fetus: color and spectral Doppler analysis. Ultrasound Obstet Gynecol. 2001;18:248–52. [DOI] [PubMed] [Google Scholar]
- [31].Zhao BW, Yang Y, Pan M, et al. Color Doppler twinkling artifact in fetuses with echogenic intracardiac foci: echocardiographic observation and clinical significance. Ultrasound Obstet Gynecol. 2010;35:548–51. [DOI] [PubMed] [Google Scholar]
- [32].Baschat AA. Examination of the fetal cardiovascular system. Semin Fetal Neonatal Med. 2011;16:2–12. [DOI] [PubMed] [Google Scholar]
- [33].Dorfman AL, Geva T, Samyn MM, et al. SCMR expert consensus statement for cardiovascular magnetic resonance of acquired and non-structural pediatric heart disease. J Cardiovasc Magn Reson. 2022;24:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ouyang D, He B, Ghorbani A, et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Fan Y, Li S, et al. Deep learning-based automated left ventricular ejection fraction assessment using 2-D echocardiography. Am J Physiol Heart Circ Physiol. 2021;321:H390–9. [DOI] [PubMed] [Google Scholar]