Abstract
Background:
Breast cancer is one of the most common cancer in women and a proportion of patients experiences brain metastases with poor prognosis. The study aimed to construct a novel predictive clinical model to evaluate the overall survival (OS) of patients with postoperative brain metastasis of breast cancer (BCBM) and validate its effectiveness.
Methods:
From 2010 to 2020, a total of 310 female patients with BCBM were diagnosed in The Affiliated Cancer Hospital of Xinjiang Medical University, and they were randomly assigned to the training cohort and the validation cohort. Data of another 173 BCBM patients were collected from the Surveillance, Epidemiology, and End Results Program (SEER) database as an external validation cohort. In the training cohort, the least absolute shrinkage and selection operator (LASSO) Cox regression model was used to determine the fundamental clinical predictive indicators and the nomogram was constructed to predict OS. The model capability was assessed using receiver operating characteristic, C-index, and calibration curves. Kaplan–Meier survival analysis was performed to evaluate clinical effectiveness of the risk stratification system in the model. The accuracy and prediction capability of the model were verified using the validation and SEER cohorts.
Results:
LASSO Cox regression analysis revealed that lymph node metastasis, molecular subtype, tumor size, chemotherapy, radiotherapy, and lung metastasis were statistically significantly correlated with BCBM. The C-indexes of the survival nomogram in the training, validation, and SEER cohorts were 0.714, 0.710, and 0.670, respectively, which showed good prediction capability. The calibration curves demonstrated that the nomogram had great forecast precision, and a dynamic diagram was drawn to increase the maneuverability of the results. The Risk Stratification System showed that the OS of low-risk patients was considerably better than that of high-risk patients (P < 0.001).
Conclusion:
The nomogram prediction model constructed in this study has a good predictive value, which can effectively evaluate the survival rate of patients with postoperative BCBM.
Keywords: Breast cancer brain metastasis, Nomograms, Overall survival, Surveillance, Survival prediction model
Introduction
Breast cancer (BC) is the most common malignant tumor in women all over the world, and it is the most ubiquitous cause of cancer-related death in women. A total of 90% of BC patients eventually died of distant metastasis.[1] The most common metastatic organs include bone, regional lymph nodes, lungs, liver, and brain. Compared with other metastases, the prognosis of breast cancer patients with brain metastasis (BM) remains poor. Studies have shown that the difficulty in BM treatment is to overcome the complexity of the blood–brain barrier (BBB) for pharmacological treatments and the targets in the molecular routes related to the growth of brain tumor cells (TCs). Despite multimodal and systemic therapy for breast cancer brain metastasis (BCBM), including a combination of surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy, the prognosis of patients with BCBM is dismal, with high morbidity and mortality.[2] Inrecent years, many prognostic models were developed for caner types and widely used in clinical practice. These models may have good risk stratification ability, improving the accuracy of clinical decision. Therefore, identifying key clinical characteristics of patients with postoperative BCBM to construct a prognostic model is crucial to predict the overall survival (OS) of BCBM patients and determine the optimal treatment to prolong their life. Nomogram is an effective and a convenient predictive tool used to calculate the prognosis of many diseases by integrating diverse prognostic and determinant risk factors. It has been used as a prognostic devise in oncology and has showed promising utility in personalized medicine.
Prognostic models of recurrent BM in postoperative BC were rarely reported. Therefore, the current research aimed to construct a prognostic nomogram for BCBM patients based on the retrospective data from The Affiliated Cancer Hospital of Xinjiang Medical University and assess its accuracy and practicability using an independent external cohorts from the Surveillance, Epidemiology, and End Results Program (SEER) database.
Methods
The Research Ethics Committee of The Affiliated Cancer Hospital of Xinjiang Medical University has approved this study (No.K-2021015). The retrospectively collected data from this hospital have been consented by all patients, and for the anonymized data which were collected retrospectively from the SEER database, the need for informed consent was waived. The research was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection and selection standard
Clinicopathological data for this research were acquired from The Affiliated Cancer Hospital of Xinjiang Medical University from January 2010 to December 2020, including clinicopathological features, treatment, relevant serological and imaging data, and survival information of patients with postoperative BCBM. The tumor, regional lymph node, and metastasis (TNM) stage is classified based on the American Joint Committee on Cancer (AJCC) 2017 version of the guidelines.
Patients were included based on the following criteria: (1) breast cancer as the first primary tumor; (2) patients with breast cancer diagnosed pathologically after surgery; (3) patients with completed information on clinicopathological features, demographic information, and follow-up information; (4) postoperative breast cancer patients diagnosed as BCBM using pathology of brain tissue or brain computed tomography (CT) or magnetic resonance imaging (MRI). Two experienced radiologists read the CT and MRI images, and all the interpretation results were consistent.
Patients were excluded based on the following criteria: (1) male patients with breast cancer; (2) patients with suspected or confirmed distant metastasis before surgery; (3) patients with primary cancer of both breast and brain; (4) patients with bilateral primary breast cancer; and (5) patients with incomplete clinical data or interrupted treatment during follow-up.
According to the same standards, clinicopathological materials of the external verification cohort were obtained from the SEER database from 2010 to 2016, which is an authoritative source for cancer statistics in the United States. The permission from the US National Cancer Institute (username number: 13013-Nov2019) were obtained before extracting the data.
The inclusion criteria of the 173 BCBM patients enrolled from SEER database were: (1) identified as breast cancer with International Classification of Diseases for Oncology-3 (ICD-O-3)/World Health Organization (WHO) 2008 histology codes (8500, 8520, 8522); (2) tumor anatomic site codes (C50.0–C50.9); (3) identified as brain metastasis with SEER Combined Mets at DX-brain (2010+); and (4) diagnosed between 2010 and 2016. The exclusion criteria were: (1) patients with bilateral primary breast cancer; and (2) missing or unknown clinical information.
The prognostic variables for the enrolled 173 patients were extracted from the SEER database including age at diagnosis, race, tumor location, tumor size, lymph node metastasis, molecular subtype, treatment strategy, vital status, and survival time.
Follow-up
The 310 patients enrolled from the hospital were followed up regularly by visiting physicians after the diagnosis of breast cancer, with an interval of once every 6 months within 5 years and once annually after 5 years, and terminated at the time of death. The deadline for follow-up information collection was December 31, 2020. The follow up information were collected by two methods: medical history review and telephone interview. The former included review of the patient's clinic and in-hospital data, while telephone interview was performed when the relevant information was not available in the medical records.
The following variables were included in the study: (1) basic information, such as age, marital status, menstruation, and nationality; (2) follow-up information, including recurrence or metastasis time, location of metastasis, the time of occurrence of the second and more primary malignant tumor, time and cause of death, and the time of the last follow-up.
Statistical modeling
Data of all the above qualified BCBM patients were processed. The descriptive statistics were used to describe the basic information, for example, the classified data were represented as n (%) and the continuous variable data were expressed as median with ranges or mean with standard deviation (SD). Classified indicators were compared using the chi-squared test, and the continuous variables were compared using student's t-test or Mann–Whitney U-test. The 310 patients were randomly assigned to a training cohort and a validation cohort in a 1:1 split ratio. R package "caret" (version 4.1.1, http://www.r-project.org), a type of random number generator that uses true random numbers as initial conditions and treats the generated numbers as objects, was used, a random seed was set, and the createDataPartition function was used to randomly split the data into training and validation sets in a 1:1 ratio.
The least absolute shrinkage and selection operator (LASSO) Cox regression model was used to identify independent risk factors of OS. Compared with conventional stepwise Cox regression analysis, LASSO Cox regression decreases the assessed variance and offers an interpretable final model that may be more precise. To offer clinicians a measurable tool to forecast the mortality of each patient, we constructed a nomogram according to the Cox analysis on the training cohort.
The capability of the predictive model was assessed in the training and validation groups. The C-index was used to assess the discriminant ability of the predictive model. The probability that the C-index evaluation model correctly ranked a sequence of events in a pair of randomly selected cases was assessed. The value of the C-index ranges from 0.5 to 1.0. A higher C-index indicates better predictive performance. The area under the curve (AUC) was another parameter used to measure the distinguishing force of the model. We drew a receiver operating characteristic curve (ROC) and calculated the AUC. The calibration curves were drawn to further evaluate the consistency between the predicted survival probability and the observed probability. Kaplan–Meier curves were calculated to compare the OS, the primary outcome of this research, between the two groups with high and low risks of BM. OS was defined as the duration from diagnosis to death (for any reason) or to the last follow-up. All statistical analyses were performed using R software. P <0.05 was considered statistically significant.
Results
Patient features
Based on the inclusion and exclusion criteria, a total of 310 postoperative BCBM patients (161 in the training cohort and 149 in the validation cohort) were selected from the medical record database of The Affiliated Cancer Hospital of Xinjiang Medical University during January 2010 to December 2020. Based on the same criteria, another 173 postoperative BCBM patients were enrolled from the SEER database during 2010 to 2016 for external verification [Supplementary Figure 1, http://links.lww.com/CM9/B542].
There was no statistically significant difference in demographic and clinical characteristics between the training cohort (n = 161) and the validation cohort (n = 149) [Supplementary Table 1, http://links.lww.com/CM9/B542]. In terms of tumor location, 42.9% (133/310) patients were in the left and 57.1% (177/310) were right. Patients mostly aged 35–56 years, accounting for 67.1% (208/310). In terms of tumor size, those with T1–T2 accounted for 70.6% (219/310), while those with T3–T4 accounted for 29.4% (91/310), and most patients presented T2 (159 [51.3%]). In terms of molecular subtypes of breast cancer, patients with human epidermal growth factor receptor-2 (HER2) enriched accounted for the largest proportion (103 [33.2%]). More patients received improved radical cure surgery (252 [81.3%]), chemotherapy (228 [73.5%]) and radiation therapy (208 [67.1%]). The mean survival time of the training and validation cohorts were 39.0 ± 26.3 months and 36.9 ± 22.8 months, respectively.
More patients in the SEER cohort (97/173) were over 56 years old. In terms of molecular subtypes of breast cancer, luminal A accounted for the largest proportion (77 [44.5%]). More patients received chemotherapy (121 [69.9%]), and radiation therapy (129 [74.6%]). The mean survival time of the SEER cohort was 23.0 ± 20.9 months.
Univariate analysis of patients with BCBM
According to the univariate analysis, 11 variables were identified, including primary site, surgery method, lymph node metastasis, molecular subtype, HER2, Ki67 value, chemotherapy, radiotherapy, lung metastasis, vascular invasion, and nerve invasion (P <0.05) were associated with OS [Supplementary Table 2, http://links.lww.com/CM9/B542].
Construction and verification of the OS nomogram
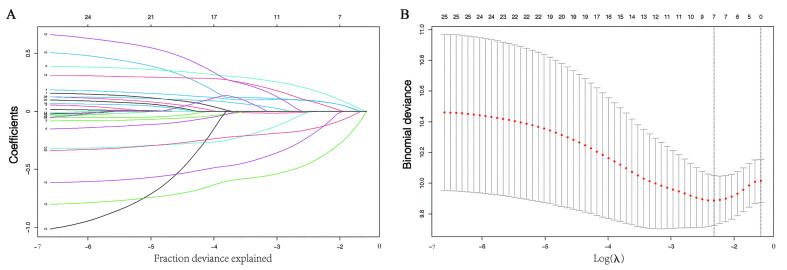
According to the outcomes of LASSO regression [Figure 1] and the univariate Cox analysis results, six characteristics (tumor size, lymph node metastasis status, molecular subtype, chemotherapy, radiotherapy, and lung metastasis) with non-zero coefficients were eventually incorporated into the development of the survival nomogram in the training cohort [Figure 2A].
Figure 1.
Selection of factors associated with OS using the LASSO Cox regression model. (A) The upper Abscissa is the number of non-zero coefficients in this model, and the ordinate is the coefficient value. LASSO coefficients of 25 candidate variables (age, laterality, different nationalities, tumor size, lymph node metastasis, neoadjuvant therapy, surgery method, grade, molecular subtype, ER, PR, HER2, Ki67, chemotherapy, target, endocrine, radiation, liver metastasis, lung metastasis, bone metastasis, vascular invasion, nerve invasion, and marital, menstrual, and vital status), including dummy variables in the training cohort. (B) The optimal penalization coefficient (λ) in the LASSO model was identified by 10-fold cross-validation and the minimum criterion in the training cohort. The left vertical dotted line represents the minimum error, and the right line represents the cross-validated error within one standard error of the minimum. The upper Abscissa indicates the number of independent variables that still exist in the model. ER: Estrogen receptor; HER2: Human epidermal growth factor receptor-2; LASSO: Least absolute shrinkage and selection operator; OS: Overall survival; PR: Progesterone receptor.
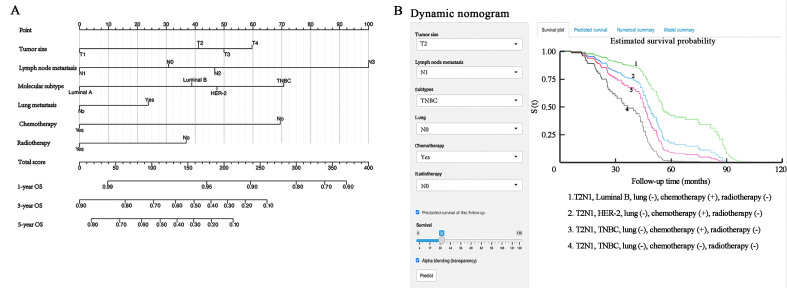
Figure 2.
The nomogram of predicting OS in patients with postoperative BCBM. (A) Survival nomogram for the prediction of 1-year, 3-year, and 5-year OS in BM patients. (B) Dynamic nomogram of predicting OS in patients with postoperative BCBM. BM: Brain metastasis; BCBM: Breast cancer brain metastasis; HER2: Human epidermal growth factor receptor 2; OS: Overall survival; TNBC: Triple negative breast cancer.
In addition to the ordinary nomogram, we also built a dynamic nomogram to facilitate the use for clinicians with an intuitive web-based interface [Figure 2B]. People can easily input values of the six predictors followed by a click of the "Predict" button, then the probability of survival and 95% confidence interval (CI) are exported on the right side of the interface.
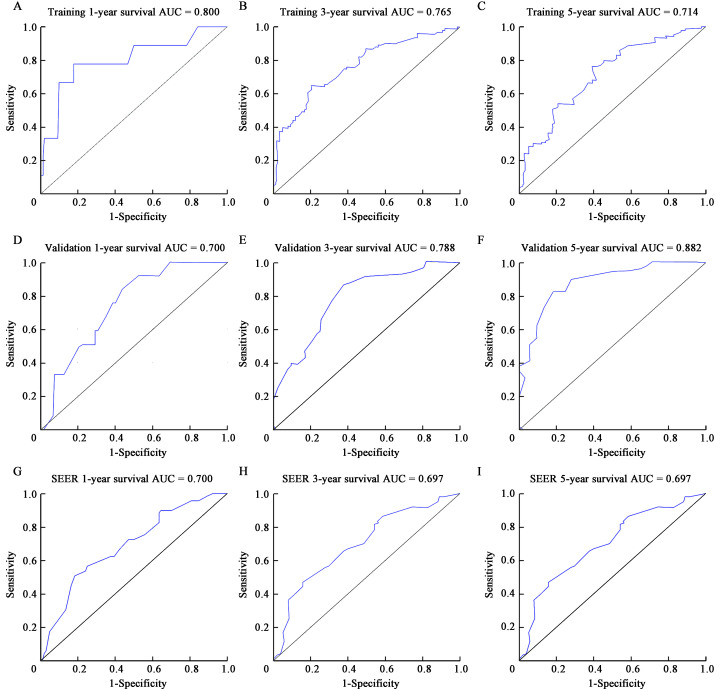
As shown in Figure 2, the survival nomogram intuitively predicted the 1-, 3- and 5-year OS rates of BCBM patients in the training cohort. The calibration curve of the survival nomogram is shown in Figures 3A–C. The curve was close to the ideal 45˚ line, which indicated that the survival nomogram was well calibrated in the training cohort. The C index of the training cohort was 0.714. Notably, ROC analysis showed that the survival nomogram correctly forecasted 1-year (AUC = 0.800), 3-year (AUC = 0.765), and 5-year (AUC = 0.714) survival rates in patients with BCBM [Figures 4A–C].
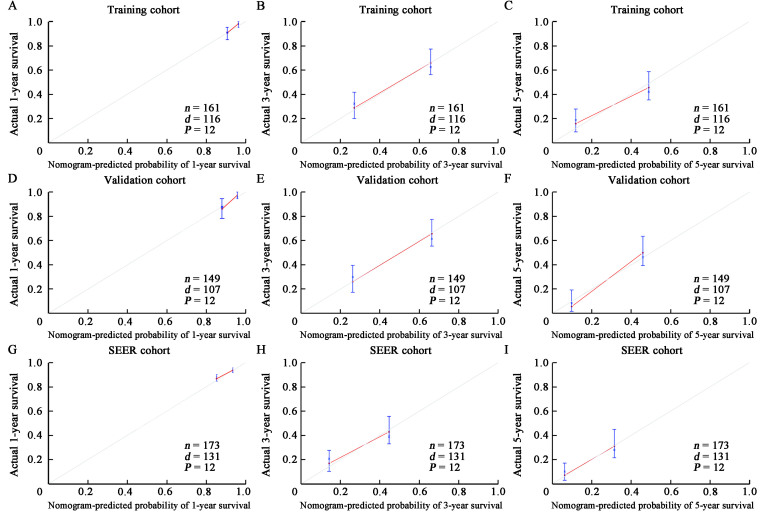
Figure 3.
The calibration curve of OS was predicted by the training, validation, and SEER groups of BCBM patients. 1-year, 3-year, and 5-year OS in the training cohort (A–C; C-index = 0.714), validation cohort (D–F; C-index = 0.710), and SEER cohort (G–I; C-index = 0.670). BCBM: Breast cancer brain metastasis; d: Number of deaths; n: Number of cases; OS: Overall survival; P: Sample size per calculation; SEER: Surveillance, Epidemiology, and End Results Program.
Figure 4.
Predictive performance of the survival nomogram of BCBM patients reflected by ROC curves. ROC curves for the 1-, 3- and 5-year OS in patients in the training cohort (A–C), validation cohort (D–F), and SEER cohort (G–I). AUC: Area under the curve; BCBM: Breast cancer brain metastasis; OS: Overall survival; ROC: Receiver operating characteristic; SEER: Surveillance, Epidemiology, and End Results Program.
To assess the calibration of the survival nomogram, we compared the predicted 1-, 3- and 5-year survival probabilities with the corresponding actual observations. As shown in Figures 3D–F, the calibration curve of the survival nomogram showed good agreement between the predicted probability and the actual results. The C index of the validation cohort was 0.710. Notably, The ROC curve also showed that the survival nomogram had good prediction for 1-, 3- and 5-year survival, with AUC values of 0.700, 0.788, and 0.882 [Figures 4D–F].
Analysis of the SEER database showed that the C index of the SEER validation cohort was 0.670 [Figures 3G–I]. The ROC curve analyses revealed that the survival nomogram in the SEER cohort had good predictive performance for 1-, 3-, and 5-year OS, with AUC values of 0.700, 0.697, and 0.697, respectively [Figures 4G–I]. Therefore, the internal and external verification of the model demonstrated that the nomogram had good prediction performance.
Risk hierarchical system
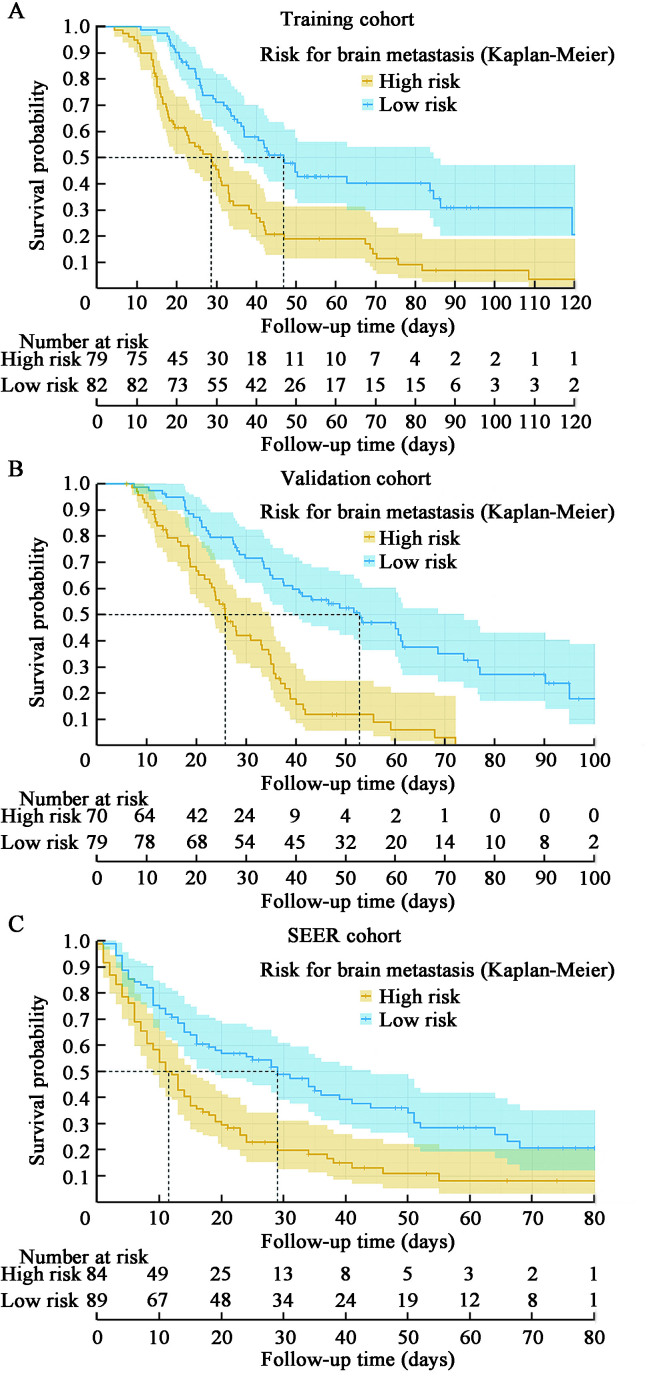
The above analyses demonstrated the good predictive effect of the survival nomogram. We calculated the prediction score based on the six variables in the nomogram. A median cutoff value was used to separate the patients in the training cohort into a low-risk group (risk score ≤0.6485) and a high-risk group (risk score >0.6485). The median survival time for low-risk and high-risk patients in the training cohort was 48 months and 29 months, respectively [Figure 5A]. Kaplan–Meier analysis indicated that the OS of low-risk patients (n = 82) was significantly better than the high-risk patients (n = 79) (P <0.001).
Figure 5.
Kaplan–Meier curve to test the stratification system of BCBM in the training cohort (A), validation set (B), and SEER set (C). BCBM: Breast cancer brain metastasis; SEER: Surveillance, Epidemiology, and End Results Program.
The median survival time of low-risk and high-risk patients in the validation cohort was 52 months and 26 months, respectively [Figure 5B]. Kaplan–Meier analysis demonstrated that the OS of low-risk patients (n = 79) was significantly better than the high-risk patients (n = 70) (P<0.001). The median survival times of low-risk and high-risk patients in the SEER cohort were 29 months and 12 months, respectively [Figure 5C]. The OS of low-risk patients was still better than the high-risk patients (P<0.001). All these results prove that the brain metastasis risk stratification system has good predictive value.
Discussion
The present study was a population-based single-center retrospective study aimed at establishing a robust prognostic model with risk stratification to forecast the survival probability of patients with postoperative BCBM. This model could be a reference for future clinical treatment.
Previous studies mostly reported the prognostic factors of BCBM survival. Based on LASSO Cox regression, the present study identified six variables (tumor size, lymph node metastasis status, molecular subtype, chemotherapy, radiotherapy, and lung metastasis). Some of the variables we reported are consistent with those of previous studies. In addition, estrogen receptor (ER), progesterone receptor (PR), age, number of brain metastases, the time interval from the first diagnosis of cancer to BM, primary tumor size, extracranial metastasis, primary tumor control, radiation dose, and isolated metastasis were also reported by previous studies.[3]
Several similarities and differences between the hospital cohort and SEER validation cohort were observed in this study. The patients' age range, tumor size, lymph node metastases, distant metastases, and molecular types of hospital cohort were statistically significantly different from those of the SEER verification cohort. The present study found that the proportion of patients receiving radiotherapy and chemotherapy was essentially the same in three cohorts. The survival rate of postoperative BCBM patients in the SEER verification cohort was not significantly different from that in the hospital cohorts, but the survival time was slightly shorter. Therefore, there may be differences in the survival rates of postoperative BM after breast cancer in the hospital population and the SEER database population, and further research is needed. Patients in the SEER verification cohort tended to have more poorly differentiated tumors than those in the hospital cohorts. The difference was partially due to race, geographical patterns, and dietary variations. Other factors, such as drinking, being overweight, and using hormones, were associated with the risk of breast cancer.[4] The differences between the population in our study and the SEER database may reflect the complex interactions between race, geography, environment, socioeconomic status, and genetic inequality.
AJCC 8th Edition Cancer Staging indicates that molecular subtype is a considerable prognostic factor for BC,[5] and that breast cancer metastatic characteristics vary among the molecular subtypes. Breast cancer can be divided into four molecular subtypes based on histological biomarkers. Gerratana et al[6] demonstrated that the risk of postoperative recurrence of the four subtypes from high to low was HER2-positive breast cancer (HER2+), triple-negative breast cancer (TNBC), luminal B, and luminal A. Additionally, Kennecke et al[7] suggested that the incidence of BM was higher in TNBC (25–27%) and HER2+ (11–20%), and the incidence of BM was much lower in luminal A and luminal B (8–15% and 11%, respectively). In the present study, the incidence of HER2 in hospital cohorts was higher than that of luminal type, which was consistent with the findings of many studies.[6,7,8,9] It may be related to the fact that HER-2-positive immunotargeted therapy significantly increased the incidence of recurrence and metastasis and survival rate in this type of patients.
In addition, lymph node metastasis in patients with postoperative BCBM are associated with the survival rate. Rack et al[10] reported that the initial tumor size of the primary focus had little correlation with prognosis, but lymph node metastasis had a considerable impact on prognosis. Rapiti et al[11] also reported that the degree of lymph node metastasis and tumor size were related to prognosis. Considering the significance of tumor size in some studies and real clinical practice, tumor size was included in LASSO regression in this study, which confirmed that tumor size is of significance to the prognosis and survival of patients with postoperative brain metastasis of breast cancer, which is basically consistent with the above conclusions.
Biological evidence is available to elucidate the link between lung metastasis and subsequent BM. Stromal cell-derived factor-1α (SDF-1α) is the only chemokine ligand-receptor CXCR4. SDF-1α is expressed in the brain and lungs,[15] which causes BC cells to extravasate into the lungs and tends to form brain metastases. Slimane et al[13] included patients with metastatic breast carcinoma (MBC) and determined that lung metastasis was an independent prognostic covariate of BM in a multivariate analysis. Sezgin et al[14] demonstrated that lung metastasis, the first site of recurrence, was highly correlated with the occurrence of BCBM. Therefore, it can be deduced that lung metastasis is a significant risk factor for postoperative BCBM (hazard ratio [HR] 0.56, 95% confidence interval [CI] 0.39–0.81, P = 0.002).
The BBB is a physical and chemical barrier between the blood vessels and the brain that maintains the dynamic balance of the brain by restricting the entry of potentially toxic substances into the central nervous system. Under general physiological conditions, the BBB represents an obstacle for circulating tumor cells entering the brain, but it plays a protective role against immune cell and toxic agents once metastatic cells have colonized the cerebral compartment.[16] In addition, current studies show that brain endothelial cells provide a cellular barrier and actively support the growth and invasion of tumor cells.[17] However, the extent of BBB protection in the brains of cancer patients from peripheral effects is unclear. The BBB also restricts the entry of chemotherapeutic drugs, making it difficult for chemotherapeutic drugs to reach effective concentrations in brain tissue. As a result, the therapeutic effect of most chemotherapeutic drugs on brain tumors is significantly weakened. Related studies have shown that the effective rate of chemotherapeutic drugs in patients with brain metastasis of breast cancer is 4–38%.[18] Consequently, chemotherapeutic drug effects on brain metastases after breast cancer surgery remain controversial, and no standard chemotherapy regimen is available for brain metastases. Therefore, systemic chemotherapy is the only auxiliary treatment to improve symptoms in later stages. However, the combination of targeted drugs with higher BBB permeability with the addition of surgery and radiotherapy, may achieve better control of intracranial lesions, improve the quality of life of patients, prolong survival time, and reduce toxicity. This hypothesis is consistent with national and international research.[19]
Breast tumor diagnosis shows that BC cells take a long time to colonise the brain due to the impermeable BBB. In malignant intracranial tumors, the BBB is ultimately transformed into a blood-tumor barrier with poor permeability. Because of this characteristic, the drugs used to treat BM are only targeted at any molecule that plays a role in brain transmission across BBB or TCs. Monoclonal antibodies with actions against HER2, such as trastuzumab, pertuzumab, and trastuzumab emtansine, are considered to be too large to cross an intact BBB for an effective chemoprevention. The BBB may be destroyed after radiotherapy; however, continued use of trastuzumab after bone marrow formation may provide survival benefits. Numerous studies have indicated that high-dose trastuzumab can treat HER2+ BM.[20] Continuous treatment with trastuzumab in patients with BM is advantageous, but it is unclear whether this advantage is due to the drug's effect on the brain or powerful systematic control.[21] Contrastingly, some studies showed that HER2+ patients treated with trastuzumab have a higher incidence of BM. These data suggest that while trastuzumab is excellent at controlling extracranial relapse, the monoclonal antibody appears to have limited values in preventing CNS recurrence.
Continuous treatment with trastuzumab in patients with BM is advantageous, but it is not clear whether this advantage is due to the effect of the drug in the brain or powerful systematic control.[21] Lapatinib, a small molecule kinase inhibitor of epidermal growth factor receptor (EGFR) and HER2, is thought to be able to cross the BBB.[22] A clinical study showed that lapatinib limits the ability of drugs to penetrate the complete BBB.[25] In another study, the lapatinib response rate as a single drug was 6% but increased to 66% with the addition of capecitabine.[23] The potential chemopreventive activity of lapatinib was suggested by the results of a phase III randomized trial, in which the effects of lapatinib plus capecitabine versus capecitabine alone were compared in patients with advanced breast cancer who had progressed on trastuzumab: fewer patients with CNS involvement at first progression were in the lapatinib-containing arm (2% vs. 6%).[24] These studies show that lapatinib may effectively prevent BM, but no concrete evidence supports this claim.
The novel HER2-targeted tyrosine kinase inhibitors have potential activity in the bone marrow. Preclinical data have shown that it may penetrate the complete BBB and overcome drug resistance to trastuzumab or lapatinib.[26] CDK4/6 inhibitors, especially abexilide, show good central nervous system permeability in preclinical models and reach the treatment level in BM. The incidence and survival rate of BM depend primarily on the breast cancer subtype, which indicates the need for personalised BCBM treatment.[16] The incidence of BM in HER2 was significantly higher than luminal type in our study, and the incidence of TNBC was lower than that of the other three subtypes.
Postoperative TNBC BM may destroy the BBB. In contrast, the postoperative BM of HER2+ BC tends to preserve the BBB.[27] Many national and international studies have shown the complexity of the BBB in BM and the characteristics of the molecular pathways related to brain TCs growth and high-risk groups. Fortunately, HER2-positive and triple-negative treatments have apparent preventive effects and improve survival.[8] However, there were few patients having a life expectancy of >3 years. How existing clinical treatment methods play a role in patients with BM is unclear, and these methods have several limitations. There is still no global consensus on a sustainable, safe, and effective treatment approach. Many trials are underway, and it is hoped that more valuable results will be found to optimize treatment and improve the survival rate and quality of life of patients with postoperative BCBM.
This study showed that molecular subtypes, chemotherapy, radiotherapy, lung metastasis, tumor size, and lymph node metastasis are risk factors for prognosis and survival. Domestic and foreign studies have shown that age, Ki67, histological grade, hormone receptor-negative status, and lymph node metastasis are essential factors in the prognosis of postoperative BM in BC.[12] Future experiments are hoped to confirm the results more accurately. The present study quantified all the above-influencing factors, and a relatively good evaluation system was constructed. The verification results showed that the C-indexes of the survival nomogram in the training, validation, and SEER cohorts were 0.714, 0.710, and 0.667, respectively, indicating potential prediction performance. The calibration curve of the predicted survival probability was consistent with the actual survival probability, and a dynamic diagram was constructed to increase the operability of the results. The results of the risk stratification showed that the OS of low-risk patients was significantly higher than that of high-risk patients (P <0.001).
Although there were some differences between the training and verification cohorts of The Affiliated Cancer Hospital of Xinjiang Medical University and the external verification of the SEER database, the line diagram of the hospital showed acceptable consistency in the external verification cohort. The diagram enables clinicians to identify patients with high risks of low survival rates and therefore to make better clinical decisions and provide follow-up monitoring for patients with BM after BC surgery.
However, our study had some limitations. First, because of the nature of retrospective research and the inclusion of only surgical resection of breast cancer patients with BM, the selection bias might exist and a lack of standardized sample treatment is inevitable. Second, the data of this study were obtained from a single center, the sample size was small, and the follow-up time only up to five years. These factors limited the representativeness of our study patients and external utility of our study findings, and the future studies with randomized, multi-center, large-sample size, and longer follow-up design are strongly encouraged.
In summary, based on The Affiliated Cancer Hospital of Xinjiang Medical University and SEER database, we determined the prognostic factors of patients with postoperative BM from BC. Based on these factors, we constructed and verified a diagram to forecast the survival rate of patients with postoperative BM in BC. The nomogram will also help clinicians personalize the prediction of patient survival and provide suggestions for improved therapy.
Acknowledgements
The authors thank the Record Room of The Affiliated Cancer Hospital of Xinjiang Medical University (Urumqi, China) and SEER public database for providing medical records.
Funding
The study was supported by National Natural Science Foundation of China (No. 82060520), and Tianshan Cedar Talent Training Project of Science and Technology Department of Xinjiang Uygur Autonomous Region (No. 2020XS14).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Nie Y, Ying BC, Lu ZN, Sun TH, Sun G. Predicting survival and prognosis of postoperative breast cancer brain metastasis: a population-based retrospective analysis. Chin Med J 2023; 136:1699–1707. doi: 10.1097/CM9.0000000000002674
References
- 1.Luo C Li N Lu B Cai J Lu M Zhang Y, et al. Global and regional trends in incidence and mortality of female breast cancer and associated factors at national level in 2000 to 2019. Chin Med J 2021;135: 42–51. doi: 10.1097/CM9.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achrol AS Rennert RC Anders C Soffietti R Ahluwalia MS Nayak L, et al. Brain metastases. Nat Rev Dis Primers 2019;5: 5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Y Cao H Zhang Y Pan Z Dong S Wang G, et al. Nomogram-predicted survival of breast cancer brain metastasis: A SEER-based population study. World Neurosurg 2019;128: e823–e834. doi: 10.1016/j.wneu.2019.04.262. [DOI] [PubMed] [Google Scholar]
- 4.Nindrea RD, Aryandono T, Lazuardi L. Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia: A meta-analysis. Asian Pac J Cancer Prev 2017;18: 3201–3206. doi: 10.22034/APJCP.2017.18.12.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkes A Warneke CL Clifton K Al-Awadhi A Oke O Pestana RC, et al. Prognostic factors in patients with metastatic breast cancer with bone-only metastases. Oncologist 2018;23: 1282–1288. doi: 10.1634/theoncologist.2018-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerratana L Fanotto V Bonotto M Bolzonello S Minisini AM Fasola G, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 2015;32: 125–133. doi: 10.1007/s10585-015-9697-2. [DOI] [PubMed] [Google Scholar]
- 7.Kennecke H Yerushalmi R Woods R Cheang MC Voduc D Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28: 3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 8.Niikura N Hayashi N Masuda N Takashima S Nakamura R Watanabe K, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: A multicenter retrospective analysis. Breast Cancer Res Treat 2014;147: 103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 9.Voduc KD Nielsen TO Perou CM Harrell JC Fan C Kennecke H, et al. αB-crystallin expression in breast cancer is associated with brain metastasis. NPJ Breast Cancer 2015;21: 15014. doi: 10.1038/npjbcancer.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rack B Janni W Gerber B Strobl B Schindlbeck C Klanner E, et al. Patients with recurrent breast cancer: Does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat 2003;82: 83–92. doi: 10.1023/B:BREA.0000003955.73738.9e. [DOI] [PubMed] [Google Scholar]
- 11.Rapiti E Verkooijen HM Vlastos G Fioretta G Neyroud-Caspar I Sappino AP, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol 2006;24: 2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 12.Rogoz B, Houzé de l′Aulnoit A, Duhamel A, Houzé de l′Aulnoit D. Thirty-year trends of survival and time-varying effects of prognostic factors in patients with metastatic breast cancer–A single institution experience. Clin Breast Cancer 2018;18: 246–253. doi: 10.1016/j.clbc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Slimane K Andre F Delaloge S Dunant A Perez A Grenier J, et al. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol 2004;15: 1640–1644. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]
- 14.Sezgin C, Gokmen E, Esassolak M, Ozdemir N, Goker E. Risk factors for central nervous system metastasis in patients with metastatic breast cancer. Med Oncol 2007;24: 155–161. doi: 10.1007/BF02698034. [DOI] [PubMed] [Google Scholar]
- 15.Arya M, Ahmed H, Silhi N, Williamson M, Patel HR. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol 2007;28: 123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- 16.Witzel I, Oliveira-Ferrer L, Pantel K, Müller V, Wikman H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res 2016;18: 8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kienast Y von Baumgarten L Fuhrmann M Klinkert WE Goldbrunner R Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 2010;16: 116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi G, Di Stefano AL, Farina P, Zagonel V, Tabouret E. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: An overview of the literature. Cancer Treat Rev 2014;40: 951–959. doi: 10.1016/j.ctrv.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012;118: 2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 20.Bartsch R Rottenfusser A Wenzel C Dieckmann K Pluschnig U Altorjai G, et al. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol 2007;85: 311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- 21.Witzel I Kantelhardt EJ Milde-Langosch K Ihnen M Zeitz J Harbeck N, et al. Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie 2011;34: 304–308. doi: 10.1159/000328679. [DOI] [PubMed] [Google Scholar]
- 22.Li L Zhang D Liu B Lv D Zhai J Guan X, et al. Antibody-drug conjugates in HER2-positive breast cancer. Chin Med J 2021;135: 261–267. doi: 10.1097/CM9.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachelot T Romieu G Campone M Diéras V Cropet C Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol 2013;14: 64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland S Ashley S Miles D Chan S Wardley A Davidson N, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases – The UK experience. Br J Cancer 2010;102: 995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krop IE Lin NU Blackwell K Guardino E Huober J Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann Oncol 2015;26: 113–119. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bose R Kavuri SM Searleman AC Shen W Shen D Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3: 224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yonemori K Tsuta K Ono M Shimizu C Hirakawa A Hasegawa T, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010;116: 302–308. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.