Abstract
Background:
The recent mpox outbreak has disproportionately affected people with HIV (PWH) and resulted in the first widespread use of the novel antiviral tecovirimat. It is unknown whether there are differences in treatment outcomes for PWH compared to individuals without HIV.
Objective:
To compare the clinical presentation and treatment outcomes of PWH and HIV-negative persons with mpox virus (MPXV) treated with tecovirimat.
Design:
Retrospective cohort study of individuals treated with tecovirimat for confirmed mpox infection from June to August 2022.
Setting:
Two academic medical centers in New York City.
Participants:
196 persons treated with tecovirimat from June 20 to August 29, 2022. Of 154 testing positive for MPXV, 73 were PWH, and 4 had a CD4 count <200 cells/μL.
Measurements:
Patient demographic characteristics, clinical presentation, treatment outcomes, and safety data for tecovirimat.
Results:
Indications for tecovirimat treatment were similar between the PWH and HIV-negative groups. Four individuals experienced serious adverse events; none were attributed to tecovirimat. Three of these four participants had HIV and two had CD4 counts <200 cells/μL. 23% of all participants experienced non-severe side effects. Groups had similar rates of hospitalization, indications for treatment, and co-occurring infections, but PWH had fewer days from symptom onset to treatment (7.5 vs. 10). There was no difference in treatment outcomes including days to improvement or rate of persistent symptoms.
Limitation:
Patients with MPXV who were not treated with tecovirimat were not followed routinely and therefore lacked comparable outcome data, limiting evaluation of efficacy.
Conclusion:
In our cohort of patients treated with tecovirimat for severe mpox, HIV status did not appear to affect treatment outcomes.
Introduction
Mpox virus (MPXV) is a zoonotic orthopox DNA virus in the same genus as smallpox, originally described in 1958 among laboratory monkeys and first recognized to cause human infection during the 1970s in the Democratic Republic of Congo. Since discovery, there have been multiple documented outbreaks, primarily within countries of sub-Saharan Africa with a history of MPXV infections, with cases in other countries generally tied to travel or importation of infected animals (1). In May of 2022 a new outbreak emerged in Europe, which has since developed into a multi-national public health emergency.
The epidemiology of the current outbreak differs significantly from prior instances in that mpox infection is occurring almost exclusively among men who have sex with men, and has been associated with significant rates of coincident sexually transmitted infections (2-4). In addition to known skin-to-skin and respiratory transmission, MPXV has been identified in semen as well as fecal/rectal specimens, suggesting the potential for infection via exposure to bodily fluids (5, 6). This may occur in both symptomatic and asymptomatic individuals given MPXV has been found on rectal samples of asymptomatic men who have sex with men (7, 8).
Persons with HIV (PWH) are disproportionately affected, with between 35-47% of mpox cases occurring in individuals with concurrent HIV infection in large case series (4, 9-13), with the major caveat that ascertainment bias from conducting studies in dedicated HIV care settings may have played a role. At this time, it is unclear to what degree HIV acts as an independent risk factor for mpox acquisition, though emerging data suggest that uncontrolled HIV is a risk factor for developing severe disease(14). A study examining the 2017-2018 MPXV outbreak in Nigeria noted higher mortality rates in PWH, however, these individuals had a high prevalence of AIDS (15). This finding has not been reproduced in the recent outbreak, potentially due to high rates of HIV treatment adherence and viral suppression (3, 10). A recent CDC report noted higher rates of hospitalization among HIV-positive individuals infected with MPXV, but data regarding determinants of the decision for hospitalization was not reported (10).
For individuals presenting with severe mpox symptoms or elevated risk for progression to severe disease, the CDC has recommended offering treatment with tecovirimat under an NIAID-funded clinical trial, Study of Tecovirimat for Human Mpox Virus (STOMP) or an Expanded Access Investigational New Drug (EA-IND) protocol (16). This medication, originally developed and approved for treatment of smallpox, has been widely used in the current outbreak (17). Reports to date have noted that tecovirimat appears well-tolerated, with only mild side effects, and that treated individuals have high rates (>90%) of complete symptom resolution by the end of therapy (18-20).
Despite increasing literature regarding mpox infection among PWH, including safety and clinical outcomes of tecovirimat treatment, no studies to date compare treatment outcomes among PWH to those of HIV negative individuals. Our study examines differences in demographics, clinical presentation, co-occurring infections, treatment safety, and clinical outcomes among patients treated with tecovirimat with and without HIV.
Methods
We conducted a retrospective cohort study of adults initiated on tecovirimat for MPXV under the CDC EA-IND protocol at New-York Presbyterian’s Weill Cornell Medical Center and New-York Presbyterian’s Columbia University Medical Center between June and August 2022.
Our study population included all individuals who initiated treatment with tecovirimat based on CDC eligibility guidelines. We excluded individuals without available confirmation of MPXV infection from all analyses except for safety outcomes. Confirmed MPXV infection was defined as a positive PCR from any of the following sources: 1) internal hospital lab testing, 2) send-out testing from commercial laboratories, 3) specimens sent to the CDC under the EA-IND protocol, 4) New York City Department of Health testing, 5) test results from outside facilities provided by the patient during the course of the study.
The CDC Central Institutional Review Board (11) approved the EA-IND protocol, and informed consent for tecovirimat treatment was carried out in accordance with their guidelines. The Columbia University IRB and the Biomedical Research Alliance of New York IRBs (the latter under a reliance agreement with the Weill Cornell Medicine IRB) approved this retrospective study, waiving the requirement for informed consent.
CDC treatment and reporting protocols changed over the duration of this case series. The original protocol required visits both during treatment, and after treatment completion, but an amended protocol dated July 20, 2022 required only one follow up either during or after treatment. Treatment initiation and follow up visits took place in-person in both the inpatient and outpatient settings, as well as via telehealth. Only individuals completing at least one follow up visit were included for analysis of safety outcomes, time until initial improvement, and presence of new lesions at 48 hours. Those completing a post-treatment follow up visit were included for analysis of the aforementioned outcomes plus additional clinical outcome measures, including resolution of pain at the end of treatment and any persistent symptoms.
Using the CDC EA-IND case report forms as well as other clinical documentation from the electronic health record, we collected demographic data including age, sex assigned at birth, gender identity, race/ethnicity, gender of sex partners, number of sex partners in the preceding month, HIV pre-exposure prophylaxis (PrEP) usage, HIV status (most recent viral load and whether CD4 count was currently <200 cells/μL), concomitant sexually transmitted infection (STI) diagnosis (both in prior two weeks before tecovirimat initiation and at initial study visit). Other data collected included indication for tecovirimat treatment, initial MPXV symptoms, presence of prodrome, anatomic distribution of skin lesions, total number of lesions, presence of bacterial superinfection, and need for specialty consultation. At follow up visits, data collected included days until first sign of improvement on therapy, whether new lesions appeared within 48 hours of treatment initiation, whether pain and other symptoms were resolved by end of treatment, and adverse events. All data were manually abstracted into a REDCap (21) database from the EHR by study authors (JM, KS) with quality monitoring by senior authors (JZ, MJG).
Statistical methods
We performed a descriptive analysis of the presentation and outcomes of mpox patients treated with tecovirimat. Quantitative variables were reported as medians with interquartile range, and categorical variables as percentages of the total cohort. Differences between the groups of individuals with HIV and those without were described standardized mean differences for demographic and outcome data. Instances in which the absolute value of the SMD is greater than 0.10 are commonly interpreted to represent significant differences between groups, although this cutoff is arbitrary. Prevalence ratios were calculated for setting, timing, and treatment indication, as well as clinical outcomes, and adjusted for age and race/ethnicity. Prevalence ratios and standardized mean difference were not calculated in instances where proportions were identical or categories contained zero individuals. Statistical analysis was performed using R software version 4.2.1 (22).
Role of the Funding Source
The funding source had no role in the study design; collection, analysis, or interpretation of data; writing of the report; nor the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Results
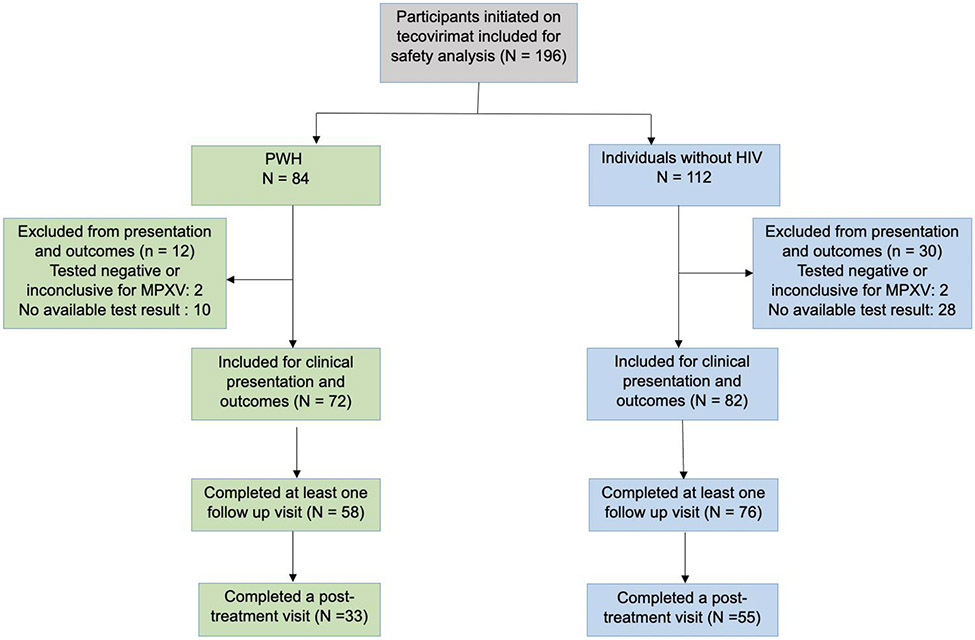
196 patients initiated treatment with tecovirimat. Of these 42 either tested negative for MPXV, or did not have available confirmation of a positive test. Of the 154 treated individuals with confirmed MPXV, 72 were PWH, and 82 were HIV-negative. 134 completed at least one follow up visit, and 88 completed a post-treatment follow-up.
There were a similar number of patients with and without HIV infection. All individuals were assigned male sex at birth, and only one patient reported female gender identity. Only two patients reported that their partners were exclusively women. PWH were older, and more likely to identify as Black or Hispanic. Of those with HIV, 14 had a viral load greater than 1000 copies/mL or CD4 count less than 200 cells/mm3. 70% of HIV-negative individuals reported taking HIV PrEP at the time of their initial visit.
Slightly more than half of individuals reported experiencing a prodrome, defined as fever, fatigue, malaise, lymphadenopathy, or other systemic symptoms prior to the development of skin lesions. While PWH were more likely to report skin lesions, fever or diarrhea on day one of illness, those without HIV were more likely to experience a prodrome and to develop additional symptoms including dysuria, odynophagia, dizziness, arthralgias, or lymphadenopathy (Supplemental Table 1). PWH were less likely to have exam findings including lymphadenopathy, pharyngitis, abdominal tenderness, penile discharge and anal discharge. Rates of co-incident STI were greater among individuals without HIV (38% vs 26%, SMD 0.26). The incidence of bacterial superinfection was overall high (33%), and slightly greater among patients without HIV (37% vs 31%, SMD −0.11) (Supplemental table 2).
The setting of treatment initiation was similar in both groups. Time from symptom onset to treatment initiation was shorter in PWH (7.5 vs 10 days) with a PR of an interval greater than 8 days of 0.56 (95% CI 0.38-0.80). This difference persisted when comparing PWH to HIV-negative individuals taking PrEP (7.5 days vs. 10 days, PR >8 days 0.65, 95% CI 0.45-0.84). Indications for treatment were similar between groups with the exception being indications related solely to HIV, and the most common being proctitis.
Overall, the incidence of adverse events was low. Of the four individuals experiencing serious adverse events during the study, all were deemed unlikely to be related to tecovirimat by the study investigators and treating clinicians. One patient had tecovirimat discontinued due to the appearance of a morbilliform drug rash ultimately attributed to trimethoprim-sulfamethoxazole. One patient experienced a GI bleed from an unclear source, another developed a bacterial superinfection of an anorectal ulcer, and one developed grade 4 transaminase elevation felt to be due to newly diagnosed acute HIV. In the case of the last patient, transaminase levels improved while tecovirimat was continued. Of these four patients, three were PWH and two had CD4 counts <200 cells/μL. Of the milder side effects, headache was the most common, followed by nausea and abdominal discomfort. The majority of individuals (148 of 197, 75%) reported no side effects. Two patients discontinued tecovirimat early due to mild side effects, one due to diarrhea and one due to palpitations.
Overall, treatment outcomes were similar between those with and without HIV. PWH started treatment 3 days sooner than HIV-negative patients, but both groups reported improvement with similar rapidity. The overwhelming majority of patients were pain-free at the end of the treatment period, with no difference between PWH and HIV-negative individuals. The majority of those reporting persistent symptoms at the end of the treatment period had incompletely healed skin lesions/ulcers at any body site. Rates of persistent symptoms did not vary by HIV status. Specialty consults were requested most often from dermatology, colorectal surgery, and urology, with no difference between groups.
Discussion
During the 2022 outbreak, MPXV infections have predominately affected MSM, with a disproportionate number of those infected also living with HIV. Tecovirimat, the agent most used for treatment, is being studied for efficacy against MPXV in randomized, controlled trials in humans (including the NIAID-funded STOMP trial), but results are not yet available (19). Anecdotal data supporting efficacy and safety has thus far relied exclusively on case series. To our knowledge, there are no published data comparing treatment outcomes between individuals with and without HIV infection. In this preliminary study, we report safety data for 197 individuals who received tecovirimat, and compared clinical presentation and treatment outcomes in 154 patients with confirmed MPXV treated with tecovirimat with and without HIV. We found no major differences in clinical presentation and treatment outcomes between the two groups.
Similar to other US data, patients in this report were almost exclusively men. PWH were slightly older (mean 39 vs 32 years), as in the largest US report (11). PWH were more likely than HIV-negative patients to identify as Black or Hispanic, which reflects both the disproportionate burden of HIV in these groups as well as the underlying demographics of patients receiving HIV care at Columbia University Medical Center and Weill Cornell Medical Center.
In this study PWH were more likely to report skin lesions, fever and diarrhea on day one of illness, while those without HIV were more likely to experience a prodrome and to develop additional symptoms or exam findings, including lymphadenopathy. This discrepancy in lymphadenopathy was also noted in a large US case series (10), but not in a similar German report (9). The significance of this difference is unclear, but it may contribute to diagnostic uncertainty in PWH between MPXV and herpesviruses such as HSV and VZV, which do not typically present with enlarged lymph nodes. Treatment eligibility criteria aside from HIV infection were not substantially different between groups, suggesting that severe mpox disease may present similarly between groups.
Prior reports suggest that PWH are hospitalized with MPX at higher rates than HIV negative individuals. There were no apparent differences in hospitalization rates in our cohort, but this may have been affected by the very low proportion of individuals in our cohort with CD4 count less than 200 cells/μL. Additional study comparing disease severity and therapeutic decision making between PWH with low CD4 and others with mpox is needed.
As in other reports, tecovirimat was well-tolerated in our case series, with no serious adverse events attributed to the medication. Rates of non-severe side effects in our series were similar to those reported from an initial series in California (18), but substantially higher than those noted by the CDC (20). This was likely due to our abstraction of all provider documentation rather than only the outcomes forms used by the CDC. Some symptoms noted as side effects (e.g. fatigue/malaise) are also symptoms of infection, and therefore attribution to medication alone is difficult. Regardless, we believe that our study adds substantially to the body of knowledge regarding patient experience with tecovirimat therapy, and can help inform patient counseling during treatment initiation.
Finally, individuals in this series had similar treatment outcomes regardless of HIV status. As in other reports, almost all patients experienced complete resolution of pain by the end of treatment. Skin lesions developing more than 48 hours after starting tecovirimat and persistent skin lesions after completing therapy were similarly uncommon among PWH and HIV-negative patients. Among those with persistent symptoms, all were noted to be in the process of healing at the time of treatment completion.
Individuals with HIV started tecovirimat sooner than those without HIV. Notably, this difference persisted when comparing PWH to HIV-negative individuals taking PrEP, suggesting that the difference was not driven by unequal access to care. It is unclear whether these shorter times to treatment initiation may have masked differences in outcomes. Those who started treatment earlier in the course of disease may have experienced faster symptom resolution—extrapolating from what is known about antiviral treatment of other acute infections—but those who started tecovirimat later could also have had relatively shorter times to full symptom resolution if their symptoms had already begun to improve. Additional studies to demonstrate the effect of tecovirimat on disease progression and to more closely track the timeline of symptom resolution are needed.
There are several limitations to our study. First, there was no control group of patients infected with MPXV who did not take tecovirimat. Those individuals diagnosed with MPXV who did not receive treatment were not followed routinely for clinical outcomes, which limited our ability to draw conclusions about therapeutic efficacy. In addition, some of the patients in this study were lost to follow up before completing their on-treatment or post-treatment follow-up visit, which reduced our safety and clinical outcomes data. In particular, data on time to resolution of rash missing for all but 7 individuals. The comparisons between PWH and individuals without HIV may have also been affected by selection bias, since merely having HIV could have been interpreted as a treatment indication in the CDC expanded access program. However, only 3 patients with HIV received treatment in the absence of another indication. Last, the majority of PWH in our study had well-controlled disease, with viral loads <1000 copies/mL and CD4 > 200 cells/mm3, so our insight into the effect of advanced HIV on clinical presentation and outcomes of MPXV was limited.
While it remains unclear how the incidence and demographic features of the current mpox outbreak will develop going forward, the current scenario requires better understanding of both disease and treatment in those individuals who bear the greatest burden of disease to date – primarily MSM and PWH. Tecovirimat is a promising treatment whose efficacy will hopefully be borne out in future rigorous studies.
Supplementary Material
Figure 1. Study flow diagram.
PWH: people with HIV, MPXV: mpox virus
Table 1.
Demographic characteristics of patients with confirmed Mpox Virus Infection treated with tecovirimat
| Overall (N = 154) n, % |
PWH (N= 72) n, % |
HIV Negative (N= 82) n, % |
Unadjusted Standardized Mean Difference |
|
|---|---|---|---|---|
| Age (median, Q1, Q3) | 35 (30, 42) | 39 (33, 46) | 32 (29, 36) | 0.86 |
| Sex at Birth | ||||
| Male | 154 (100%) | 72 (100%) | 82 (100%) | - |
| Gender Identity | ||||
| Male | 120 (99%) | 63 (98%) | 57 (100%) | −0.18 |
| Gender(s) of Sex Partners | ||||
| Men | 136 (94%) | 66 (100%) | 70 (90%) | 0.48 |
| Women | 2 (1.4%) | 0 (0%) | 2 (2.6%) | −0.23 |
| Men and Women | 6 (4.2%) | 0 (0%) | 6 (7.7%) | −0.41 |
| Race/Ethnicity | ||||
| Non-Hispanic white | 50 (33%) | 17 (24%) | 33 (41%) | −0.36 |
| Non-Hispanic Black | 40 (26%) | 26 (37%) | 14 (17%) | 0.46 |
| Hispanic/Latino | 37 (25%) | 21 (30%) | 16 (20%) | 0.24 |
| Other | 24 (16%) | 6 (8.6%) | 18 (22%) | −0.39 |
| Uncontrolled HIV (CD4 < 200 or VL > 1000) | 14 (9.1%) | 14 (19%) | NA | - |
| CD4 <200 | 4 (2.6%) | 4 (5.6%) | NA | - |
| Taking PrEP | 57 (37%) | 0 (0%) | 57 (70%) | - |
PrEP: HIV pre-exposure prophylaxis, VL: viral load, NA: Not applicable
Table 2:
Setting, timing, and treatment indication of patients with confirmed mpox virus infection treated with tecovirimat
| Overall (N = 154) n, % |
PWH (N = 72) n, % |
HIV Negative (N = 82) n, % |
Unadjusted Prevalence Ratio 95% CI |
Adjusted Prevalence Ratio∥ 95% CI |
|
|---|---|---|---|---|---|
| Setting | |||||
| Outpatient | 138 (90%) | 61 (85%) | 77 (94%) | - | - |
| Inpatient | 16 (10%) | 11 (15%) | 5 (6.1%) | 1.56 [0.98, 2.16] | 1.4 [0.91, 1.90] |
| Days from symptom onset to treatment initiation (median, Q1, Q3) | 8.0 (6.0, 12.0) | 7.5 (5.0, 11.0) | 10.0 (7.0, 12.0) | - | - |
| >8 days from symptom onset to treatment | 75 (49%) | 25 (35%) | 50 (61%) | 0.56 [0.38-0.80] | 0.59 [0.40,0.80] |
| Treatment Indication | |||||
| Proctitis or rectal lesions | 80 (52%) | 34 (47%) | 46 (56%) | 0.83 [0.59, 1.16] | 1.01 [0.69, 1.33] |
| Other anatomically sensitive or confluent lesions * | 36 (23%) | 22 (31%) | 14 (17%) | 0.76 [0.46, 1.27] | 1.05 [0.09, 2.00] |
| Lesions involving urethra | 22 (14%) | 7 (9.7%) | 15 (18%) | 0.65 [0.30, 1.11] | 0.86 [0.41, 1.30] |
| Uncontrolled HIV (CD4<200 OR VL not suppressed) | 8 (5.2%) | 8 (11%) | 0 (0%) | - | - |
| Hospitalized for mpox | 2 (1.3%) | 2 (2.8%) | 0 (0%) | - | - |
| Significant active exfoliative dermatologic conditions | 1 (0.6%) | 0 (0%) | 1 (1.2%) | - | - |
| Other high-risk comorbidity† | 1 (0.6%) | 1 (1.4%) | 0 (0%) | - | - |
| HIV positive (not uncontrolled) § | 3 (2.1%) | 3 (4.3%) | 0 (0%) | - | - |
Includes ocular lesions, facial lesions, and genital lesions not involving the urethra
Includes autoimmune conditions with immunosuppressive component and history of solid organ transplant
Centers for Disease Control eligibility guidelines initially listed HIV infection without specifying advanced disease as a potential indication for treatment for mpox.
Adjusted for age and race/ethnicity
Table 3.
Adverse events reported by patients with or without confirmed mpox virus infection who initiated tecovirimat
| Overall (N = 196) n, % |
|
|---|---|
| Serious Adverse Event | 4 (2.0%) |
| No reported side effect | 148 (75%) |
| Headache | 12 (6.1%) |
| Nausea | 10 (5.1%) |
| Abdominal discomfort | 8 (4.1%) |
| Diarrhea | 5 (2.5%) |
| Fatigue or drowsiness | 5 (2.5%) |
| Anorexia | 4 (2.0%) |
| Chest discomfort or palpitations | 4 (2.0%) |
| Dizziness | 3 (1.5%) |
| Rash | 2 (1.0%) |
| Paresthesia | 2 (1.0%) |
| Other ** | 14 (7.1%) |
Other side effects included diaphoresis, flushing, insomnia, pruritis, low back pain, thirst, and “brain fog”
Table 4.
Outcomes from initial and post-treatment follow up visits
| Outcomes from initial follow-up visit |
Overall (N = 134) n, % |
PWH (N = 58) n, % |
HIV Negative (N = 76) n, % |
Unadjusted Prevalence Ratio [95% CI] |
Adjusted Prevalence Ratio* 95% CI |
|---|---|---|---|---|---|
| Days until first improvement (median, Q1, Q3) | 2.00 (1.00,3.75) | 2.00 (1.00,3.00) | 2.00 (2.00,4.00) | - | - |
| New lesions after 48 hours | 22 (18%) | 8 (15%) | 14 (20%) | 0.80 [0.32-1.27] | 0.94 [0.53, 1.35] |
| Required additional specialty consult | 18 (15%) | 9 (18%) | 9 (13%) | 1.25 [0.60, 1.90] | 1.1 [0.49, 1.70] |
| Outcomes from post- treatment follow-up visit |
Overall (N = 88) n, % |
PWH (N = 33) n, % |
HIV Negative (N = 55) n, % |
Unadjusted PR [95% CI] |
Adjusted Prevalence Ratio* 95% CI |
| Pain resolved at end of treatment | 73 (90%) | 27 (96%) | 46 (87%) | 2.96 [0.46, 18.96] | 1.1 [0.49, 1.70] |
| Any persistent symptom at end of treatment | 26 (32%) | 7 (24%) | 19 (36%) | 0.69 [0.19, 1.18] | 0.73 [0.28, 1.18] |
| Skin lesions | 13 (15%) | 5 (15%) | 8 (15%) | 1.03 [0.26, 1.80] | 1.0 [0.43, 1.58] |
| Rectal pain | 7 (8.0%) | 1 (3.0%) | 6 (11%) | 0.36 [0.06, 2.26] | 0.54 [0.13, 2.29] |
| Fatigue/malaise | 3 (3.4%) | 0 (0%) | 3 (5.5%) | - | - |
| Anal bleeding or discharge | 2 (2.3%) | 0 (0%) | 2 (3.6%) | - | - |
| Anal fissure | 2 (2.9%) | 0 (0%) | 2 (4.3%) | - | - |
| Dysuria | 1 (1.1%) | 0 (0%) | 1 (1.8%) | - | - |
| Other | 3 (3.4%) | 1 (3.0%) | 2 (3.6%) | 0.89 [0.17, 4.49] | 1.16 [0.36, 3.62] |
Adjusted for age and race/ethnicity
Acknowledgements:
The authors thank Susan Ball, Christopher Brown, Tanya Ellman, Grant Ellsworth, Daniel Finn, Caroline Greene, David Helfgott, Jonathan Jacobs, Carrie Johnston, Shashi Kapadia, Yesha Malik, Kristen Marks, Samuel Merrick, Kohta Saito, Joshua Rosenblatt, Lawrence Siegel, Matthew Simon, Harjot Singh, Ole Vielemeyer, Carlos Vaamonde, Mary Vogler, Cecilia Yoon, Lars Westblade, Michelle Chang, Caroline Carnevale, Hannah Catan, Clare DeLaurentis, Peter Gordon, Shauna Gunaratne, Nuwan Gunawardhana, Ian Horton, Susan Olender, Edward Perez, Lawrence Purpura, Orlando Rosario, Mascha Elskamp, and Ariana Pazmino for contributing data and facilitating treatment implementation, Daniela Quigee and Delivette Castor for contributing statistical expertise, and Roy Gulick and Magda Sobieszczyk for their support and helpful discussions.
Primary Funding Source:
National Institutes of Health.
5T32AI100852-10 (JM), NIAID K23AI150378 (JZ), UL1TR002384 (KS, MJG), UM1AI69419 (KS, MJG), 5UM1AI069470-17 (JM, BG, JZ)
References
- 1.Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infect Dis. 2022;9(7):ofac310. Epub 2022/07/28. doi: 10.1093/ofid/ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spicknall IH PE, Clay PA, et al. Modeling the Impact of Sexual Networks in the Transmission of Monkeypox virus Among Gay, Bisexual, and Other Men Who Have Sex With Men — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1131–5. doi: 10.15585/mmwr.mm7135e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679–91. Epub 2022/07/23. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 4.Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. Epub 2022/07/29. doi: 10.1136/bmj-2022-072410. https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiro-Mestres A, Fuertes I, Camprubi-Ferrer D, Marcos MA, Vilella A, Navarro M, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28). Epub 2022/07/16. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palich R, Burrel S, Monsel G, Nouchi A, Bleibtreu A, Seang S, et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. The Lancet Infectious Diseases. 2022. doi: 10.1016/s1473-3099(22)00586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferre VM, Bachelard A, Zaidi M, Armand-Lefevre L, Descamps D, Charpentier C, et al. Detection of Monkeypox Virus in Anorectal Swabs From Asymptomatic Men Who Have Sex With Men in a Sexually Transmitted Infection Screening Program in Paris, France. Ann Intern Med. 2022;175(10):1491–2. Epub 20220816. doi: 10.7326/M22-2183. [DOI] [PubMed] [Google Scholar]
- 8.De Baetselier I, Van Dijck C, Kenyon C, Coppens J, Michiels J, de Block T, et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med. 2022;28(11):2288–92. Epub 20220812. doi: 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann C, Jessen H, Wyen C, Grunwald S, Noe S, Teichmann J, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: A large outbreak cohort in Germany. HIV Med. 2022. Epub 2022/09/06. doi: 10.1111/hiv.13378. [DOI] [PubMed] [Google Scholar]
- 10.Curran KG EK, Russell OO, et al. HIV and Sexually Transmitted Infections Among Persons with Monkeypox — Eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1141–7. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughan AM, Cenciarelli O, Colombe S, Alves de Sousa L, Fischer N, Gossner CM, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill. 2022;27(36). Epub 2022/09/10. doi: 10.2807/1560-7917.ES.2022.27.36.2200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catala A, Clavo-Escribano P, Riera-Monroig J, Martin-Ezquerra G, Fernandez-Gonzalez P, Revelles-Penas L, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022. Epub 2022/08/03. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 13.Philpott D HC, Alroy KA, et al. Epidemiologic and Clinical Characteristics of Monkeypox Cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018–22. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severe Manifestations of Monkeypox among People who are Immunocompromised Due to HIV or Other Conditions: Centers for Disease Control and Prevention Center for Preparedness and Response 2022. [updated September 29October 4, 2022]. Available from: https://emergency.cdc.gov/han/2022/han00475.asp. [Google Scholar]
- 15.Yinka-Ogunleye A, Aruna O, Dalhat M, Ogoina D, McCollum A, Disu Y, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. The Lancet Infectious Diseases. 2019;19(8):872–9. doi: 10.1016/s1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Shea J F T, Morris SB, Weiser J, Petersen B, Brooks JT. Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection — United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1023–8. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M. RJ Editorial: herpes zoster ophthalmicus and AIDS. Br J Ophthalmol. 1987;71:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai AN, Thompson GR, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate Use of Tecovirimat for the Treatment of Monkeypox Infection. JAMA. 2022. Epub 2022/08/23. doi: 10.1001/jama.2022.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matias WR, Koshy JM, Nagami EH, Kovac V, Moeng LR, Shenoy ES, et al. Tecovirimat for the Treatment of Human Monkeypox: An Initial Series From Massachusetts, United States. Open Forum Infect Dis. 2022;9(8):ofac377. Epub 2022/08/12. doi: 10.1093/ofid/ofac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Laughlin K TF, Elmor R, et al. Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol — United States, May–August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1190–5. doi: 10.15585/mmwr.mm7137e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P, Talyor R, Minor BL et al. . The REDCap consortium: Building an international community of software partners. Journal of Biomedical Informatics. 2019. doi: doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Core TR. R: A language and environment for statistical computing. . R Foundation for Statistical Computing, Vienna, Austria; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.