Graphical Abstract
Graphical Abstract.
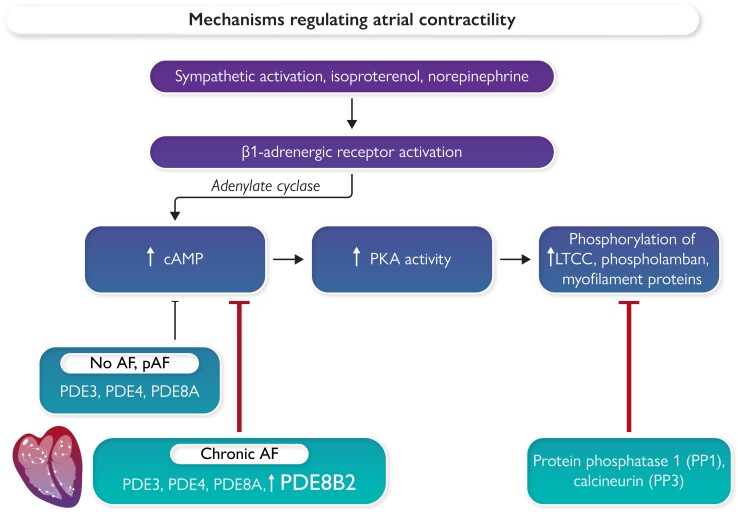
Atrial contractility is regulated by intracellular calcium levels; these reflect the balance of calcium influx, sequestration, and efflux. Beta-adrenergic activation with either norepinephrine (endogenous) or isoproterenol (pharmacological) increases the activity of adenylate cyclase, increasing cAMP levels and protein kinase A (PKA) activity. Degradation of cAMP is regulated by phosphodiesterases (PDEs). The phosphorylation state of relevant PKA targets, such as the L-type calcium channel (LTCC), the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), and myofilament proteins, is regulated by the balance of PKA activity and phosphatase activity. In patients with no history of atrial fibrillation (AF) or paroxysmal AF (pAF), basal phosphorylation of key LTCC subunits is higher than in atrial myocytes from patients with chronic (persistent) AF.14 Pavlidou et al.14 provide novel evidence that this is probably due to increased abundance and activity of phosphodiesterase isoform 8B, variant 2 (PDE8B2), in patients with chronic AF.
This editorial refers to ‘Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism’, by N.G. Pavlidou et al., https://doi.org/10.1093/eurheartj/ehad086.
Atrial fibrillation (AF), the most common arrhythmia, is associated with impaired contractile activity, and increased risk of left atrial thrombus formation and stroke. Contractile activity is dynamically regulated by intracellular calcium levels. Calcium influx into atrial myocytes is mediated by L-type calcium channels (LTCCs); lowering of cytosolic Ca2+ levels occurs as a result of re-uptake of Ca2+ into the sarcoplasmic reticulum by the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) and efflux of Ca2+ via the sodium calcium exchanger (NCX). Both Ca2+ influx and Ca2+–lowering processes are activated in response to phosphorylation of key targets by intracellular kinases, protein kinase A (PKA), and calcium-dependent calmodulin kinase II (CaMKII).1 Activation of these proteins is typically transient, as cyclic adenosine monophosphate (cAMP) is degraded by phosphodiesterases (PDEs),2 attenuating PKA activity. Dephosphorylation of the LTCCs, phospholamban (which inhibits SERCA2a), and other kinase targets is promoted by phosphatases that return phosphorylation to basal levels1 (see Graphical Abstract).
Dysregulation of intracellular Ca2+ cycling in the setting of AF underlies both impaired atrial contractility and excitability. More than 40 years ago, clinical electrophysiology studies demonstrated that the effective refractory period (ERP) was unable to adapt to changes in rate in patients in whom atrial arrhythmias (atrial flutter and AF) could be induced,3 and increased dispersion of refractoriness was evident.4 In experimental studies, Allessie and colleagues showed that rapid pacing caused a rapid shortening of the atrial ERP, and that the extent of ERP shortening was associated with the duration of induced AF episodes.5 Shortening of ERP facilitates re-entrant activity that can increase the vulnerability to and persistence of AF.6 Goette and colleagues were among the first to link rapid atrial pacing with Ca2+ overload, suggesting that Ca2+ overload was the proximal cause of pacing-induced electrical remodelling in AF,7 and that this remodelling was associated with changes in the structure and function of mitochondria in atrial cardiac myocytes. Atrial action potential morphology changes in response to rapid pacing or AF, and it was postulated that the abbreviation of action potential duration and loss of adaptation of atrial ERP to changes in rate might be due to either a reduction in LTCC current (ICa, L) or an increase in repolarizing potassium currents.7 As cytosolic Ca2+ overload can trigger Ca2+-mediated proteolysis (by calpains),8 apoptosis, and cell death, down-regulation of ICa, L may contribute to myocyte survival. At the same time, loss of ICa, L can simultaneously increase vulnerability to AF and impair atrial contractility.
Using right atrial appendage tissue from patients undergoing cardiac surgery, Le Grand and colleagues studied the differences in cellular electrophysiology of atria and atrial myocytes enzymatically isolated from the atria of patients with dilated vs. normal size atria.9 Atrial tissues from patients with dilated atria had a shorter ERP, a loss of action potential plateau, and triangulation of action potentials, with an impaired ability to respond to changes in rate. In isolated right atrial myocytes studied with patch–clamp, peak ICa, L was reduced by ∼74%.9 In parallel studies, our lab evaluated changes in potassium currents10 and L-type calcium currents11 associated with AF in atrial myocytes enzymatically dissociated from atrial appendage tissues from cardiac surgery patients. Basal calcium current densities were reduced similarly (by 63%) in atrial myocytes isolated from patients with and without AF. This reduction would be expected to significantly shorten atrial action potential duration, and we and others12 showed that lower ICa, L was associated with impaired ability of the action potential to adapt to changes in rate. We also examined the impact of PKA activation using the beta-adrenergic agonist, isoproterenol. While ICa, L increased in response to isoproterenol in myocytes from both the AF and non-AF groups, it increased ICa, L density relatively more in atrial myocytes from patients with persistent AF than in those with no history of AF. Beta-adrenergic regulation of cardiac myocyte calcium channel activity is a critical regulator of both cardiac contractility and heart rate; transient activation is an important mechanism that can increase heart rate and contractility. In contrast, persistent elevation of catecholamines can cause heart failure due to calcium overload-induced modulation of proteolysis and impaired mitochondrial function. Our studies could not discern whether dysregulation of ICa, L in AF was due to transcriptional changes in subunits of the LTCC or to differences in phosphorylation of relevant calcium channel subunits or interacting proteins. Dephosphorylation of the LTCC and other PKA-targeted proteins is dependent in part on the activity of PDEs, several of which are present in atrial myocytes13 (Graphical Abstract). PDE activity targeting LTCCs attenuates calcium channel activity, resulting in less calcium influx and lower intracellular calcium levels, all contributing to reduced contractility and abbreviated action potential duration. Subcellular regional alterations in cAMP modulation of ICa, L in myocytes from patients with AF vs. control patients were recently reported, in which the authors identified differences in the regulation of calcium cycling via PDEs.2
In this issue of he European Heart Journal, Pavlidou and colleagues14 present an elegant multidisciplinary study in which they evaluated the abundance and activity of PDE8 isoforms A and B (PDE8A and PDE8B) with respect to their contribution to the down-regulation of ICa, L in atrial myocytes isolated from patients with AF. Their study analysed right atrial tissues from 165 well-phenotyped surgical patients. Among these, 67 had no history of AF, 38 had paroxysmal AF (pAF), and 60 had chronic (persistent) AF (cAF). The authors report that both PDE8A and PDE8B were up-regulated in the right atrium of patients with pAF relative to patients with no history of AF, and that PDE8A isoforms 1 and 2 (PDE8A1 and PDE8A2) were more abundant than the PDE8B isoforms 1 and 2 (PDE8B1 and PDE8B2) in patients with pAF. In contrast, they observed that PDE8B isoforms were more abundant than PDE8A in patients with cAF, and that PDE8B isoforms were the only PDE8 isoforms that were detectable in co-immunoprecipitation studies with the alpha-1C pore subunit of the LTCC. PDE8B mRNA and protein expression was up-regulated only in cAF, with PDE8B2 binding the LTCC more strongly than PDE8B1. Intriguingly, the authors report that, while PDE8A isoforms were found in both atrial and ventricular myocardium, PDE8B was expressed only in the atria. The authors further noted that lower LTCC alpha-subunit phosphorylation at Ser1928 in atria from cAF patients was associated with lower ICa, L density, and that exposure to isoproterenol increased cAMP, enhanced Ser1928 phosphorylation, and increased ICa, L in myocytes from patients with cAF, thus prolonging action potential duration at 50% repolarization. This was a careful study supported by high quality electrophysiology, immunohistochemistry, biochemical, and physiology studies.
This study is the first to document a significant and specific role for increased PDE8B2 as a mediator of reduced atrial calcium current density in cAF, and the authors have documented an intriguing ‘switch’ that is associated with the transition from paroxysmal to chronic (persistent) AF. The authors suggest that drugs or interventions that selectively inhibit PDE8B2 may have therapeutic benefit for treating patients with persistent AF.14 The incidence of stroke and heart failure is higher in patients with persistent AF than for those with paroxysmal AF. Unbiased genome-wide analyses of atrial appendage mRNA abundance document dramatically different gene expression patterns associated with the switch from paroxysmal to persistent (chronic) AF.15 The causes of this switch and its downstream consequences are of interest for future studies. While PDE8B2 expression is relatively atrial specific, PDE8B1 is more highly expressed in brain, vascular, and thyroid tissues than in the atria, so caution and transcript specificity may be warranted when targeting PDE8B.
Dysregulation of calcium cycling is a critical aspect of atrial arrhythmogenesis. Mechanism-based therapies that improve calcium homeostasis may offer hope for slowing the progression of AF, and lowering the risk of stroke and AF-related mortality. We congratulate Pavlidou et al. for providing new insights into the mechanisms of AF progression.
Contributor Information
Julie H Rennison, Department of Cardiovascular & Metabolic Sciences, Cleveland Clinic, M/S ND-50, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
David R Van Wagoner, Department of Cardiovascular & Metabolic Sciences, Cleveland Clinic, M/S ND-50, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Funding
Funding was provided by the American Heart Association (18SFRN34170442) and the National Institutes of Health (1P01HL158502-01A1).
References
- 1. Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: mechanistic insights and therapeutic opportunities. Eur Heart J 2012;33:1870–1877. 10.1093/eurheartj/ehs079 [DOI] [PubMed] [Google Scholar]
- 2. Reinhardt F, Beneke K, Pavlidou NG, Conradi L, Reichenspurner H, Hove-Madsen L, et al. Abnormal calcium handling in atrial fibrillation is linked to changes in cyclic AMP dependent signaling. Cells 2021;10:3042. 10.3390/cells10113042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int J Cardiol 1982;2:179–197. 10.1016/0167-5273(82)90032-8 [DOI] [PubMed] [Google Scholar]
- 4. Boutjdir M, Le Heuzey JY, Lavergne T, Chauvaud S, Guize L, Carpentier A, et al. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol 1986;9:1095–1100. 10.1111/j.1540-8159.1986.tb06676.x [DOI] [PubMed] [Google Scholar]
- 5. Allessie MA, Wijffels MC, Kirchhof CJ. Experimental models of arrhythmias: toys or truth? Eur Heart J 1994;15:2–8. 10.1093/eurheartj/15.suppl_a.2 [DOI] [PubMed] [Google Scholar]
- 6. Lammers WJ, Kirchhof C, Bonke FI, Allessie MA. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am J Physiol 1992;262:H47–H55. 10.1152/ajpheart.1992.262.1.H47 [DOI] [PubMed] [Google Scholar]
- 7. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation 1996;94:2968–2974. 10.1161/01.CIR.94.11.2968 [DOI] [PubMed] [Google Scholar]
- 8. Brundel BJ, Ausma J, van Gelder IC, Van der Want JJ, van Gilst WH, Crijns HJ, et al. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc Res 2002;54:380–389. 10.1016/S0008-6363(02)00289-4 [DOI] [PubMed] [Google Scholar]
- 9. Le Grand BL, Hatem S, Deroubaix E, Couetil JP, Coraboeuf E. Depressed transient outward and calcium currents in dilated human atria. Cardiovasc Res 1994;28:548–556. 10.1093/cvr/28.4.548 [DOI] [PubMed] [Google Scholar]
- 10. Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 1997;80:772–781. 10.1161/01.res.80.6.772 [DOI] [PubMed] [Google Scholar]
- 11. Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 1999;85:428–436. 10.1161/01.res.85.5.428 [DOI] [PubMed] [Google Scholar]
- 12. Yue L, Feng J, Gaspo R, Li G-R, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 1997;81:512–525. 10.1161/01.RES.81.4.512 [DOI] [PubMed] [Google Scholar]
- 13. Rivet-Bastide M, Vandecasteele G, Hatem S, Verde I, Bénardeau A, Mercadier JJ, et al. cGMP-stimulated cyclic nucleotide phosphodiesterase regulates the basal calcium current in human atrial myocytes. J Clin Invest 1997;99:2710–2718. 10.1172/JCI119460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pavlidou NG, Dobrev S, Beneke K, Reinhardt F, Pecha S, Jacquet E, et al. Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism. Eur Heart J 2023;44:2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshmukh A, Barnard J, Sun H, Newton D, Castel L, Pettersson G, et al. Left atrial transcriptional changes associated with atrial fibrillation susceptibility and persistence. Circ Arrhythm Electrophysiol 2015;8:32–41. 10.1161/CIRCEP.114.001632 [DOI] [PMC free article] [PubMed] [Google Scholar]