Abstract
Aims
Oesophageal fistula represents a rare but dreadful complication of atrial fibrillation catheter ablation. Data on its incidence, management, and outcome are sparse.
Methods and results
This international multicentre registry investigates the characteristics of oesophageal fistulae after treatment of atrial fibrillation by catheter ablation. A total of 553 729 catheter ablation procedures (radiofrequency: 62.9%, cryoballoon: 36.2%, other modalities: 0.9%) were performed, at 214 centres in 35 countries. In 78 centres 138 patients [0.025%, radiofrequency: 0.038%, cryoballoon: 0.0015% (P < 0.0001)] were diagnosed with an oesophageal fistula. Peri-procedural data were available for 118 patients (85.5%). Following catheter ablation, the median time to symptoms and the median time to diagnosis were 18 (7.75, 25; range: 0–60) days and 21 (15, 29.5; range: 2–63) days, respectively. The median time from symptom onset to oesophageal fistula diagnosis was 3 (1, 9; range: 0–42) days. The most common initial symptom was fever (59.3%). The diagnosis was established by chest computed tomography in 80.2% of patients. Oesophageal surgery was performed in 47.4% and direct endoscopic treatment in 19.8% and conservative treatment in 32.8% of patients. The overall mortality was 65.8%. Mortality following surgical (51.9%) or endoscopic treatment (56.5%) was significantly lower as compared to conservative management (89.5%) [odds ratio 7.463 (2.414, 23.072) P < 0.001].
Conclusion
Oesophageal fistula after catheter ablation of atrial fibrillation is rare and occurs mostly with the use of radiofrequency energy rather than cryoenergy. Mortality without surgical or endoscopic intervention is exceedingly high.
Keywords: Atrial fibrillation, Catheter ablation, Radiofrequency energy, Oesophageal fistula
Structured Graphical Abstract
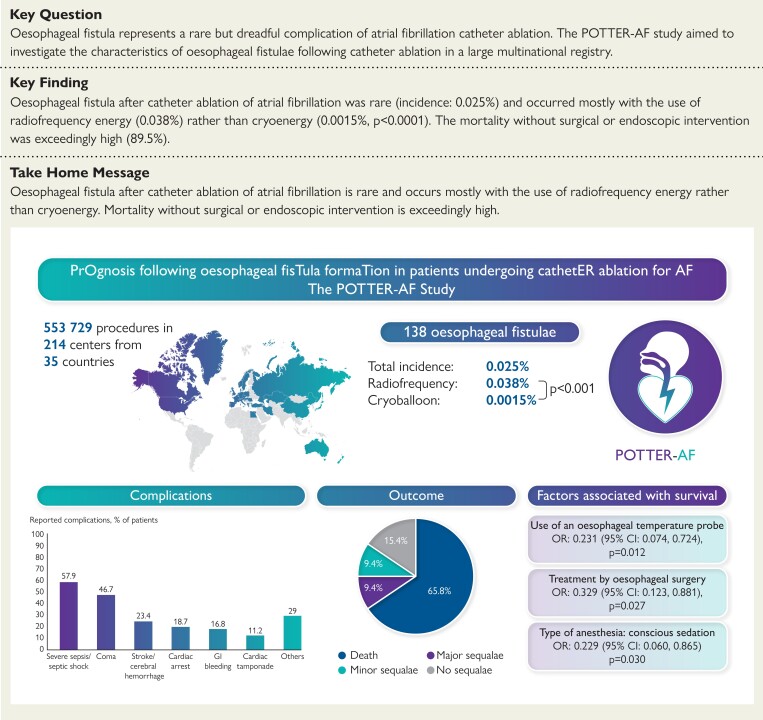
Structured Graphical Abstract.
Summary of the POTTER-AF study results. CI, confidence interval; OR, odds ratio.
See the editorial comment for this article ‘Putting fear into perspective: estimating the true incidence of oesophageal fistula formation post-atrial fibrillation ablation’, by M.W. Lim and J.M. Kalman, https://doi.org/10.1093/eurheartj/ehad309.
Introduction
Invasive treatment of atrial fibrillation (AF) by catheter ablation based pulmonary vein isolation (PVI) is being increasingly performed worldwide. Catheter ablation has shown high procedural and long-term follow-up success rates for treatment of paroxysmal and persistent AF.1 In general, the rate of severe peri- and post-procedural complications is low, and several technical improvements, novel technologies, and energy sources have increased the safety profile of this treatment strategy. However, oesophageal fistula (OF) is a devastating and potentially lethal complication of AF ablation procedures. Its incidence is known to be low and has been reported to range between 0.02% and 0.1% of cases, with a high mortality of 50% to 83%.2–5 Since OF is a rare complication, only limited information based on case reports, case series, and nationwide registries with a limited number of patients on its incidence, management, and outcome is available in the recent literature.2,3,6–12 The largest survey to date was conducted in 2015 and included 33 patients with OF after AF ablation.3 Meanwhile, the total number of AF ablation procedures has significantly increased. Additionally, AF ablation technologies have rapidly changed with increasing numbers of cryoballoon ablations, contact force guidance, and high-power short-duration based radiofrequency (RF) ablations.13–15 The aim of this worldwide study was to evaluate the incidence, management, and outcome of OF after catheter ablation procedures for AF or atrial tachycardia (AT) treatment.
Methods
Study design
The PrOgnosis following oesophageal fisTula formaTion in patients undergoing cathetER ablation for AF (POTTER-AF) study is designed as an international, multi-centre, anonymized registry study to evaluate the incidence, management and outcomes of post-procedural OF after catheter ablation of AF. The survey was conducted at the Department of Rhythmology at the Lübeck University Heart Center under the auspices of the Working Group of Cardiac Electrophysiology of the German Cardiac Society (AGEP, DGK). Experienced electrophysiological centres from all around the world were invited to participate. The registry was approved by the local ethical review board of the University of Lübeck, Germany (AZ 21–291). Each participating centre was responsible for its ethics approval by the local ethics committee. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All patient information was anonymized. The POTTER-AF study has been registered at clinicialtrials.gov (NCT05273645). Each participating centre provided data on the total number of patients treated with catheter ablation for AF or AT. Additionally, patients’ baseline characteristics, peri-procedural characteristics, and follow-up data were assessed for patients with OF according to a standardized and uniform online questionnaire survey (SurveyMonkey). Data acquired via SurveyMonkey were assessed for an individual patient level and used for further analysis. The inclusion criteria were patients with an OF (which included atrio-OF, oesophago-pericardial fistula, or oesophageal perforation) after catheter ablation for AF treatment. There were no exclusion criteria for this study. The primary endpoint was the occurrence of OF following catheter ablation for AF or AT treatment. The secondary endpoints were the diagnosis and management of OF as well as outcome and mortality.
Data management
Data were retrospectively and electronically collected. The analysis was performed using anonymized data only. The described data were retrospective data derived from the clinical routine of the participating centres, including routine follow-up visits. All the members of the research team were obliged to secrecy. All data were protected from unauthorized external access, as only members of the research team were permitted and enabled access to these data.
Statistical analysis
All categorical variables were reported as absolute and relative frequencies and were compared using Fisher’s exact test or the χ2 test. Continuous variables were tested for normal distribution using the Shapiro–Wilk test. They were reported as mean ± standard deviation (SD) in the case of normal distribution, otherwise as median and interquartile range (first quartile, third quartile). Continuous variables were compared using the non-paired Student’s t-test when normally distributed and the corresponding non-parametric test (Mann–Whitney U test) otherwise.
The association between different parameters and death was assessed using binary logistic regression and is reported as odds ratio (OR) and 95% confidence intervals (CIs).
Variables with a P-value <0.1 in the univariable model and considered clinically important for the outcome were included in a multivariable binary logistic regression model.
All parameters with perfect collinearity were excluded from the logistic regression analysis and were reported descriptively. The variables eligible for multivariable logistic regression are as follows: age, structural heart disease, left ventricular ejection fraction, coronary artery disease, congestive heart failure, duration of hospitalization, use of conscious sedation, use of thermal probe, anatomical PVI, and diagnostic method [computed tomography (CT), septic shock, coma, cardiac arrest, oesophageal surgery, direct oesophageal surgery without endoscopic treatment, conservative treatment]. All P-values are two sided. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 28.0 (IBM SPSS Statistics).
Results
Patients’ population
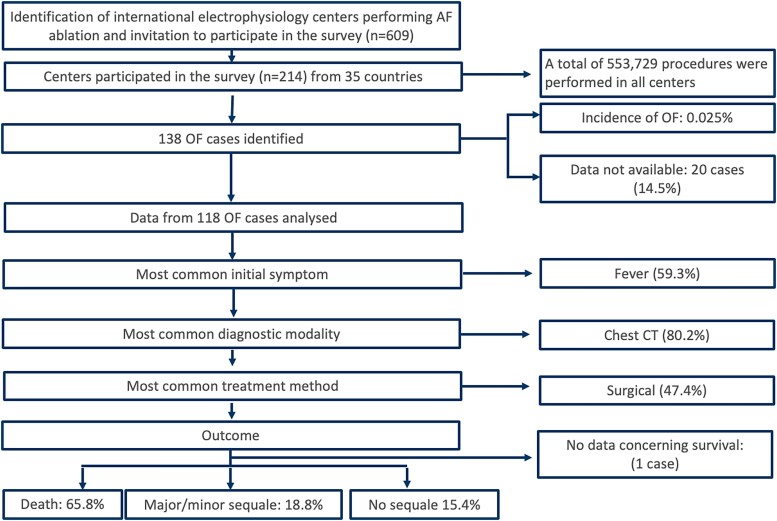
A total of 609 experienced electrophysiological centres around the world were invited to participate in this study (Figure 1). Data on overall conducted AF or AT catheter ablation procedures were obtained from 214 (35%) centres in 35 countries across 5 continents. The full list of centres included in this study is available in the supplementary data (see Supplementary data online, Table S1). A total of 553 729 patients underwent catheter ablation procedures for AF or AT treatment between 1996 and 2022. The mean percentage of energy source was RF in 62.9% ± 29.8%, cryoballoon in 36.2% ± 30%, laserballoon in 0.6% ± 3%, and others in 0.3% ± 1.3%. A total of 138 patients (0.025%) from 78 centres (21 countries) experienced post-procedural OF (Figure 2). The incidence of RF was 0.038%, while for cryoballoon it was 0.0015% (P < 0.0001). For other modalities, the incidence was 0.02%.
Figure 1.
Flowchart of the POTTER-AF study. AF, atrial fibrillation; OF, oesophageal fistula; CT, computed tomography.
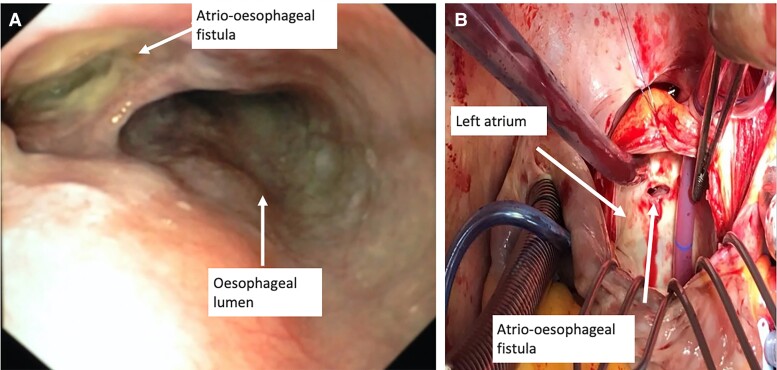
Figure 2.
(A) Endoscopic view of the oesophagus in a patient with an atrio-oesophageal fistula. (B) Intraoperative situ during oesophageal surgery in a patient with an atrio-oesophageal fistula.
Peri-procedural data, management, and outcomes were available for 118 patients (18 countries), while relevant data were not available for 20 patients (14.5%). The final diagnoses were atrio-OF in 113/118 (95.8%) patients, oesophago-pericardial fistula in 4/118 (3.4%) patients, and oesophageal perforation in 1 (0.8%) patient. The baseline characteristics are shown in Table 1. Forty-seven percent were female, with a mean age of 62.0 ± 11.4 years. A history of oesogastric pathology was reported in 8% of the patients, and 23% reported a pre-procedural proton pump inhibitor therapy.
Table 1.
Baseline characteristics
| Characteristics | All patients | Survivors | Non-survivors | P-value |
|---|---|---|---|---|
| Number of patients | 118 | 40 | 77 | |
| Age, years | 62.0 ± 11.4 | 58.8 ± 12.4 | 64 ± 10.5 | 0.045 |
| Female sex | 55 (47) | 20/40 (50) | 35/76 (46.1) | 0.700 |
| Body mass index, kg/m2 | 26.4 (23.6, 28.9) | 26.6 (24.6, 29.1) | 26.1 (23.1, 28.8) | 0.772 |
| Paroxysmal AF | 49 (42) | 19/40 (47.5) | 30/77 (39) | 0.432 |
| Persistent AF | 61 (52) | 19/40 (47.5) | 41/77 (53.2) | 0.566 |
| Long standing persistent AF | 8 (7) | 2/40 (5) | 6/77 (7.8) | 0.714 |
| Structural heart disease | 36 (31) | 8/40 (20) | 28/76 (36.8) | 0.090 |
| Coronary artery disease | 20/112 (18) | 2/38 (5.3) | 18/73 (24.7) | 0.017 |
| Congestive heart failure | 20/115 (17) | 3/39 (7.7) | 17/76 (22.4) | 0.068 |
| LA surface, cm² | 25.5 ± 15.4 | 25.32 ± 8.3 | 34.5 ± 20.2 | 0.0830 |
| LA volume, ml | 132.6 ± 57.9 | 199 ± 31.5 | 125.2 ± 29.6 | 0.0041 |
| LVEF, % | 60 (50, 65) | 60 (55, 65) | 60 (50, 60) | 0.038 |
| CHA2DS2-VASc score | 2.1 ± 1.4 | 1.9 ± 1.3 | 2.3 ± 1.4 | 0.192 |
| Arterial hypertension | 70/117 (60) | 23/40 (57.5) | 47/76 (61.8) | 0.692 |
| Type II diabetes mellitus | 17 (14) | 5/40 (12.5) | 12/76 (15.8) | 0.785 |
| Chronic kidney disease | 10/91 (11) | 1/29 (3.4) | 9/61 (14.8) | 0.158 |
| History of oesogastric pathology | 9/109 (8) | 1/38 (2.6) | 8/70 (11.4) | 0.156 |
| Pre-procedural PPI therapy | 25/110 (23) | 6/38 (15.8) | 19/71 (26.8) | 0.237 |
| OF after 1st ablation procedure | 105 (89) | 37/40 (92.5) | 67/77(87) | 0.538 |
| OF after >1st ablation procedure | 13 (11) | 3/40 (7.5) | 10/77 (13) | 0.538 |
Values are counts, n (%), mean ± SD, or median (first quartile, third quartile).
AF, atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction; TIA, transitory ischaemic attack; PPI, proton pump inhibitors; OF, oesophageal fistula.
The highest amount of OF per centre was five in one centre. The maximum incidence of OF at the specified time was 0.4%, whereas the minimum incidence per study centre was 0.0066% (P < 0.01).
Peri-procedural characteristics
Procedural data are presented in Table 2. In 114/118 patients (96.6%), the catheter ablation energy source was RF; contact force measuring catheters were used in 46% of them. The median RF power when ablating at the posterior wall was 30 (interquartile range: 25, 30) W. Besides PVI, additional left atrial (LA) lines were deployed in 45.5% of RF patients. Ablation of a roof line was performed in 30.9%, a posterior line was performed in 24.5%, and ablation of complex fractionated atrial electrograms at the posterior wall was performed in 15.2% of patients.
Table 2.
Procedural data
| Characteristics | All patients | Survivors | Non-survivors | P-value |
|---|---|---|---|---|
| Usage of general anaesthesia | 56 (47.5) | 18/40 (45) | 38/77 (49.4) | 0.700 |
| Usage of deep analgosedation | 35 (29.7) | 12/40 (30) | 23/77 (29.9) | 1.000 |
| Procedure time, min | 147 (108, 180) | 135.5 (108.5, 177.5) | 150 (110.3, 191.3) | 0.124 |
| RF energy | 114 (96.6) | 39/40 (97.5) | 74/77 (96.1) | 1.000 |
| RF duration, min | 38.5 (23.8, 52.6) | 31 (22.5, 51) | 43.5 (27, 53.9) | 0.217 |
| RF contact force catheter | 52/113 (46.0) | 17/39 (43.6) | 35/73 (47.9) | 0.695 |
| RF power on LA posterior wall, Watts | 30 (25, 30) | 30 (25, 35) | 30 (25, 30) | 0.404 |
| Circumferential PVI | 102/109 (93.6) | 33/36 (91.7) | 68/72 (94.4) | 0.684 |
| Segmental ostial | 2/109 (1.8) | 0/36 (0) | 2/72 (2.8) | 0.551 |
| Anatomical ostial | 6/109 (5.5) | 4/36 (11.1) | 2/72 (2.8) | 0.094 |
| Additional LA line ablation | 50/110 (45.5%) | 20/37 (54.1) | 30/72 (41.6) | 0.231 |
| Roof line | 34/110 (30.9) | 10/37 (27) | 24/72 (33.3) | 0.663 |
| Posterior line | 27/110 (24.5) | 10/37 (27) | 17/72 (23.6) | 0.815 |
| Anterior lines | 8/110 (7.3) | 0/37 (0) | 8/72 (11.1) | 0.049 |
| Inferior lines | 2/110 (1.8) | 1/37 (2.7) | 1/72 (1.4) | 1.000 |
| Mitral isthmus line | 4/110 (3.6) | 1/37 (2.7) | 3/72 (4.2) | 1.000 |
| CFAE ablation at the LA posterior wall | 12/79 (15.2) | 2/25 (8) | 10/53 (18.9) | 0.319 |
| Cryoballoon | 3 (2.5) | 0/40 (0) | 3/77 (3.9) | 0.550 |
| 1st generation cryoballoon | 1/3 (33.3) | 0 | 1/3 (33.3) | |
| 2nd generation cryoballoon | 2/3 (66.6) | 0 | 2/3 (66.6) | |
| Range minimal temperature, C° | −69–−47 | −69–−47 | ||
| Laserballoon | 1 (0.8) | 1/40 (2.5) | 0/77 (0) | 0.342 |
| Maximal laser energy, Watts | 10 | 10 | ||
| Usage of oesophageal temperature probe | 29 (24.6) | 14/40 (35) | 15/77 (19.5) | 0.075 |
| Maximal temperature, C° | 40.2 ± 2.2 | 39.9 ± 2.6 | 40.7 ± 1.6 | 0.481 |
| Post-procedure prescription of PPI | 85/114 (74.6) | 29/39 (74.4) | 56/75 (74.7) | 1.000 |
Values are counts, n (%), mean ± SD, or median (first quartile, third quartile).
CFAE, complex fractionated atrial electrograms; LA, left atrium; PVI, pulmonary vein isolation; PPI, proton pump inhibitors; RF, radiofrequency.
In 3/118 patients (2.5%), a cryoballoon (n = 1: first generation, Arctic Front cryoballoon, Medtronic Inc. and n = 2: second generation Arctic Front Advanced cryoballoon, Medtronic Inc.) was used for PVI. The minimal reported temperature was −69°C during cryoballoon application to the RIPV. No oesophageal temperature probe was utilized in any of the cryoballoon OF cases. In 1/118 patients (0.8%), the laserballoon ablation (HeartLight, Cardiofocus) was used. The maximal laser energy at the posterior wall was reported to be 10 W, and the maximal oesophageal temperature as measured via an oesophageal temperature probe was reported to be 37.1°C. An oesophageal temperature probe was utilized in 24.6% of the total OF population, and data concerning the oesophageal temperature were available for 15.3% of patients. The mean maximum measured temperature was 40.2° ± 2.2°C. A total of 74.6% reported post-procedural proton pump inhibitor therapy.
Patient presentation
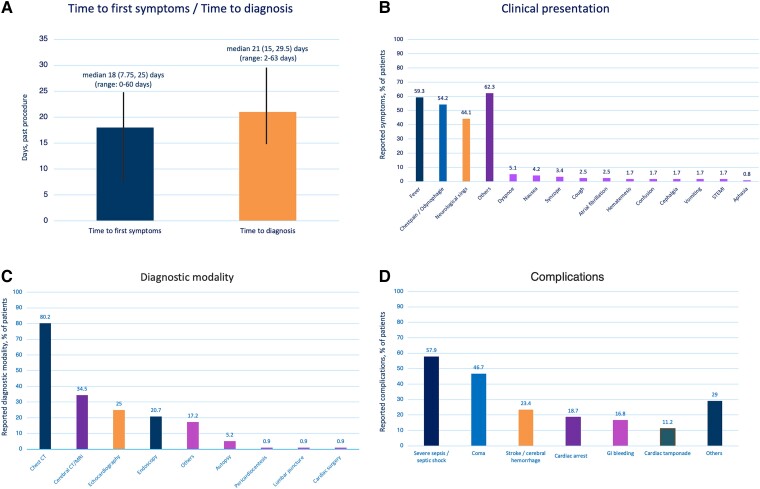
The findings on patients’ presentation, diagnostic modality, and complications are depicted in Figure 3. The median time between the procedure and earliest onset of symptoms was 18 (7.75, 25) days (range: 0–60 days), and the median time between the procedure and OF diagnosis was 21 (15, 29.5) days (range: 2–63 days). The median time from first symptom onset to OF diagnosis was 3 (1, 9) days (range: 0–42 days). One OF occurred on Day 2 (RF, non-contact force catheter, maximum of 25 W at posterior wall, PVI only). The first symptoms occurred already on Day 1 (fever, neurological symptoms, multi-organ dysfunction, septic shock, and death before interventional or surgical treatment). Two further OF were diagnosed on Day 4 and two OF on Day 5. All early diagnosed patients reported symptoms (fever and neurological symptoms).
Figure 3.
(A) Time to first symptoms and time to diagnosis. Overview of the clinical presentation (B), utilized diagnostic modalities (C), and complications (D) of all patients with oesophageal fistula (n = 118). Multiple symptoms were possible.
In patients treated with endoscopy alone, oesophageal surgery, or conservative treatment, the median time between the procedure and the earliest onset of symptoms was 10 (6, 15), 18 (11, 22.5) and 20.5 (10, 29) days, respectively (P = 0.03). The median times between the procedure and OF diagnosis were 18 (10, 25), 21 (15, 29) and 26.5 (19, 32) days, respectively (P = 0.03).
The primary initial symptoms were fever (n = 70, 59.3%), chest pain or odynophagia (n = 64, 54.2%), and neurological symptoms (stroke or seizures) (n = 52, 44.1%). Other symptoms were reported in 74 patients (62.3%: dyspnoea, nausea, syncope, cough, AF, haematemesis, confusion, vomiting, ST-elevation myocardial infarction, and aphasia). The symptoms occurred either in isolation or in association with one another. In one patient (0.8%), no symptoms were reported. Diagnosis was made by routine endoscopy on Day 10. In this case, a RF contact force measuring catheter with a maximum of 48 W at the posterior wall during deployment of a posterior line was utilized. After endoscopic treatment via clipping, no sequelae was reported.
The diagnosis was established by chest CT in 93 (80.2%) patients, with cerebral CT or cerebral magnetic resonance imaging in 40 (34.5%), with echocardiography in 29 (25%), with endoscopy in 24 (20.7%) patients, and other, in 20 (17.2%, autopsy, peri-cardiocentesis, lumbar puncture, cardiac surgery) of patients. Diagnosis was established using either an isolated modality or a combination of several modalities.
Clinical course, management, and outcome
During the clinical course, delayed complications were stroke or cerebral haemorrhages (25/107, 23.4%), severe sepsis or septic shock (62/107, 57.9%), coma (50/107, 46.7%), cardiac arrest (20/107, 18.7%), gastrointestinal bleeding (18/107, 16.8%), cardiac tamponade (12/107, 11.2%), or others in 31/107 (29%). A total of 5/107 (4.7%) patients reported no complications. In two of those patients, the OF was detected during routine endoscopic ultrasound assessment and was treated by endoscopic clipping and endoscopic stenting with no and minor sequelae, respectively. In one patient, a CT scan was performed due to confusion. After detection of an OF, the patient was treated with endoscopic stenting followed by oesophageal surgery with minor long-term sequelae. Gastric liquid pericardial effusion was detected in two patients; both treated with oesophageal surgery and did not report any sequelae.
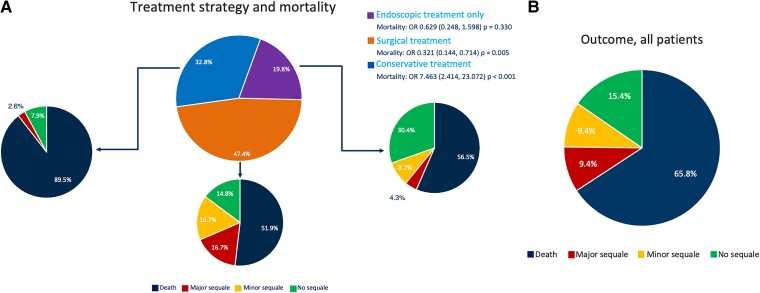
All patients were treated with intravenous antibiotic therapy. Among 116 patients diagnosed with OF, and treatment data available, n = 38 (32.8%) were treated conservatively, without endoscopic of surgical treatment attempts, and n = 34 (89.5%) of them died during follow-up. One patient (2.6%) had severe sequelae, and three (7.9%) reported no long-term sequelae.
A total of 31 (26.7%) patients were initially treated with endoscopic therapy [oesophageal stenting (n = 28), clipping (n = 2), or vacuum-assisted-closure therapy (n = 1)]. Due to their critical condition, 17/31 (54.8%) died or had severe sequalae (3/31, 9.7%). Minor sequelae were reported in 4/31 (12.9%) patients, while 7/31 (22.6%) had no sequalae. The oesophageal stent was removed in 4/31 (12.9%) patients after 30–75 days.
Isolated endoscopic therapy was performed in 23 (19.8%) patients (mortality: 13/23, 56.5%), whereas in eight patients the initial endoscopic therapy was switched to a surgical approach due to limited benefit (mortality: 4/8, 50%).
A total of 55/116 (47.4%) patients were treated using an oesophageal surgical approach. In one patient treated with oesophageal surgery, the data concerning mortality were not available. A total of 28/54 (51.9%) patients treated surgically died. A direct surgical approach without a previous endoscopic treatment attempt was conducted in 47/116 (40.5%) patients. In this group, 24/46 (52.2%) died. In terms of mortality, there were no significant differences between patients who underwent a direct surgical approach and those who underwent a direct endoscopic approach (P = 0.801). The overall mortality was 77/117 (65.8%), 11/117 (9.4%), and 11/117 (9.4%) experienced long-term major and minor sequalae, respectively. Only 18/117 (15.4%) reported no long-term sequalae. The mortality following surgical (51.9%) or endoscopic treatment (56.5%) was significantly lower than that following conservative management (89.5%) [OR 7.463 (2.414, 23.072) P < 0.001] (Figure 4). The median time to death was 28.5 days (19.3, 42).
Figure 4.
(A) Overview of the different treatment strategies and outcome of patients. (B) Outcome of all patients with oesophageal fistula.
In order to better understand the differences between patients receiving conservative treatment and those receiving surgical/endoscopic treatment, the comparison between the two populations in terms of baseline characteristics, procedural data, complications, and survival was performed (see Supplementary data online, Table S4). It is important to note that the patients receiving conservative treatment had a longer time to initial symptoms [20.5 (10, 29.8) vs. 15 (6, 21.8) days; P = 0.037] and a longer time until OF diagnosis [26 (18, 33.5) vs. 19 (13, 28.5) days; P = 0.019], as compared to those receiving surgical/endoscopic treatment. Moreover, the patients receiving invasive treatment were more likely to be diagnosed by means of chest CT (86.8% vs. 65.8%; P = 0.013) and less likely to be diagnosed by other methods. In terms of complications, the patients receiving surgical/endoscopic treatment were less likely to have a diagnosis of stroke during the clinical course as compared to those treated conservatively (14.7% vs. 39.5%; P = 0.008).
Subgroup analysis of survivors vs. non-survivors
A detailed comparison of survivors and non-survivors is shown in Supplementary data online, Table S2. Concerning baseline characteristics, atrio-OF, older age, reduced left ventricular ejection fraction, and coronary artery disease showed significantly higher values in terms of mortality, while an oesophageal-pericardial fistula (n = 4 patients, 100% survival) showed a significantly lower mortality. An anterior line showed significantly higher mortality. The occurrence of septic shock, coma, cardiac arrest, and gastrointestinal bleeding was significantly higher in the non-survivors, while the rate of patients with no complications was significantly higher in the survivors. Patients with any interventional or surgical treatment, patients with oesophageal surgery as well as patients with direct oesophageal surgery without previous stenting or clipping showed a significantly lower mortality.
To identify mortality associated factors, a simple (univariable) binary logistics regression was performed (see Supplementary data online, Table S3). For identified factors with a P-value of <0.1 and considered of clinical importance for the prognosis, a multivariable binary logistic regression was conducted.
Although not significant, yet a trend towards a lower mortality was observed for the use of an oesophageal probe (OR: 0.449, P = 0.068) in the univariable logistic regression. After including this variable in the multivariable model, it was significantly associated with lower mortality (OR: 0.231, P = 0.012). Moreover, the use of conscious sedation and the treatment via oesophageal surgery were associated with better survival (Table 3).
Table 3.
Multivariable binary logistic regression
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| Age (years) | 1.039 | 0.995–1.084 | 0.081 |
| LVEF (%) | 0.992 | 0.942–1.044 | 0.748 |
| Coronary artery disease | 3.096 | 0.555–17.259 | 0.197 |
| Congestive heart failure | 2.625 | 0.555–12.411 | 0.223 |
| Conscious sedation | 0.229 | 0.060–0.865 | 0.030 |
| Use of thermal probe | 0.231 | 0.074–0.724 | 0.012 |
| Diagnostic: CT | 0.371 | 0.093–1.481 | 0.160 |
| Oesophageal surgery | 0.329 | 0.123–0.881 | 0.027 |
The continuous variables have the unit of measurement in brackets. All other variables are categorical variables.
CI, confidence interval; CT, computed tomography; OR, odds ratio; LVEF, left ventricular ejection fraction.
Discussion
OF represents a rare but dreadful complication of AF catheter ablation. The incidence varies between different studies; however, it may be underreported and the true incidence is unknown. This fearful complication is associated with a very high mortality rate.2–5 Due to the limited number of cases, data concerning the incidence, management, and outcome of OF are sparse. Therefore, this complication requires an international worldwide effort to allow for a better understanding of the factors contributing to its occurrence and the optimal management strategies. To address these issues, the POTTER-AF study was conducted.
The major findings were: (i) the incidence of OF after catheter ablation for AF/AT treatment was 0.025% with an incidence of 0.038% for RF and 0.0015% for Cryoballoon (P < 0.0001); (ii) the median time to symptoms [18 (7.75, 25) days], and the median time to diagnosis [21 (15, 29.5) days] occurred relatively late after the procedure, while the median time from first symptom onset to OF diagnosis was 3 (1, 9) days (range: 0–42 days); (iii) the most common initial symptom was fever (59.3%); (iv) the diagnosis was established using chest CT in 80.2%; (v) oesophageal surgery was performed in 47.4% and a direct endoscopic treatment was conducted in 19.8%, and conservative treatment was conducted in 32.8% of cases; (vi) the overall mortality was 65.8%, 18.8% experienced long term sequalae; (vii) mortality following surgical (51.9%) or endoscopic treatment (56.5%) was significantly lower as compared with conservative management (89.5%) (P < 0.001); and (viii) the multivariable binary logistic regression found the conscious sedation and the use of thermal probe, as well as the treatment by means of oesophageal surgery as significantly associated with a better prognosis in terms of survival (Structured Graphical Abstract).
This worldwide survey provides the largest dataset on OF today. It reports important data to allow a better understanding of the incidence, management, and outcomes of OF occurring after AF/AT ablation. With 0.025%, the incidence of OF is in line with recent literature of a nationwide survey from France (incidence of 0.026%), which was calculated from 33 OF in 129 286 AF/AT ablations procedures.3 Other surveys with limited patients number reported on incidences of between 0.016% and 0.15%.2,7,16
Impact of the energy source in oesophageal fistula formation
Although OF has been mainly reported for RF based catheter ablation procedures, the latest findings suggest that OF also may occur in cryoballoon and other balloon-based ablation procedures.10,17 The incidence of OF following cryoballoon based ablation was reported as <0.0001%, which maybe reflects the frequent use of oesophageal temperature probes during cryoballoon based procedures.10 The POTTER-AF study evaluated an OF in a total of 3 patients after cryoballoon based PVI which only reflects 2.5% of the analysed population. The incidence of RF (0.038%) was significantly higher than that of cryoballoon (0.0015%, P < 0.0001). This difference might partially be explained by additional ablation of LA lines in RF patients, yet most likely not completely. With a reported cryoballoon temperature of a minimum of −69°C, which is far below the suggested minimum temperature of −60°C for the Arctic Front Advanced cryoballoon, the combination of a temperature cut-off and the utilization of an oesophageal temperature probe might prevent OF in cryoballoon procedures. In 1/118 patients (0.8%), the laserballoon ablation was used. The maximal laser energy at the posterior wall was reported to be 10 W, which is in line with the recommendations.18
Since RF is still the most common energy source for cardiac ablation procedures, and the main proportion of patients included in the POTTER-AF study suffered OF after RF-based catheter ablation. The latest observations on pulsed field ablation based catheter ablation suggested that this novel non-thermal energy source might reduce oesophageal injuries and potentially OF due to its selectivity to cardiomyocytes.19
Prevention of oesophageal fistula
Utilization of oesophageal temperature probes is a common strategy to monitor the oesophageal temperature and potentially prevent oesophageal injuries. While for cryoballoon and laserballoon-based ablation, the use of an oesophageal temperature probe is a commonly accepted strategy. However, its usage during RF based ablation is less common, and one study showed that it could be a risk factor for the development of endoscopically detected esophageal lesions.20 However, in general, the use of a temperature probe seems to be a potential way to reduce the risk of oesophageal overheating and cooling and therefore a potential strategy to prevent oesophageal injuries. In the POTTER-AF study, an oesophageal temperature probe was utilized in 24.6% of POTTER-AF patients, and the mean maximal measured temperature was 40.2 ± 2.2°C.
Clinical presentation and diagnostics
Although the pathophysiology of OF development is not completely understood, there is agreement that ablation energy from any source delivered to the posterior LA wall leads to thermal damage to the oesophagus. Since lesion formation requires time to progress, the first symptoms typically occur within 60 days after the procedure.21 The symptoms are diverse and not specific, consisting primarily of fever and neurological abnormalities, sometimes mimicking a cerebral vascular accident.22 The findings of POTTER-AF are in line with these observations since most patients reported fever (59.3%), chest pain/odynophagia (54.2%), and neurological signs (44.1%). With a median of 18 (7.75, 25) days (range 0–60), the observed time to symptoms was shorter than previously reported. Additionally, the time to diagnosis [median 21 (15, 29.5) days, range: 2–63 days] and time from first symptom onset to OF diagnosis [3 (1, 9) days (range: 0–42 days)] were relatively short but showed a relatively wide range. This observation might reflect the possibility that some patients presented at hospitals where they had not received the catheter ablation procedure which might lead to a longer time to diagnosis compared to the patients who presented at experienced electrophysiological centres who are potentially more aware of OF. In order to plan rapid treatment of OF, it is essential to detect this complication early. In more than 80% of patients, a chest CT was the most common diagnostic method, which has been previously recommended by other authors. In the CT scan, signs such as oesophageal opacification and air detected inside the left atrium are highly suggested to be associated with OF. It is important to state that endoscopy is discouraged that air should not be injected into the oesophagus due to the potential development of air embolism.
Treatment strategies and outcome
Since most patients reported fever and developed sepsis and/or septic shock, all patients were treated with antibiotics. The overall mortality was 65.8%, with significantly reduced mortality in patients undergoing surgical repair (51.9%) compared with endoscopic treatment only (56.5%) and conservative management (89.5%) [OR 7.463 (2.414, 23.072) P < 0.001], compared with conservative treatment).
Although these observed improvements in mortality are highly significant, the reason for the different treatment strategies shows a selection bias, since patients who were not able to undergo any oesophageal surgery or endoscopic treatment and received conservative treatment due to critical illness had the worst outcome. In fact, the time to the earliest onset of symptoms and the time to OF diagnosis were the shortest in patients who received endoscopic treatment only followed by oesophageal surgery and conservative treatment. Patients with an early OF detection received an early treatment via endoscopy or oesophageal surgery with a lower mortality while patients with late detection more often received a conservative treatment with a higher mortality. This observation again underlines the importance of early diagnosis, detection, and treatment of OF. The findings of the multivariable binary logistic regression analysis detected an intervention of OF patients via an oesophageal surgery as a factor that was associated with a lower mortality. Coronary artery disease, coma, and cardiac arrest were identified as the factors associated with a higher mortality. The use of an oesophageal thermal probe and the use of conscious sedation were also associated with better survival.
These observations are in line with a large meta-analysis conducted in 2017 with 120 reported OF cases from a total of 85 studies. The overall mortality was reported to be 55%, with significantly reduced mortality in patients undergoing surgical repair (33%) compared to endoscopic treatment (65%) and conservative management (97%).21
Limitations
The POTTER-AF study has several limitations. First, the findings were based on a retrospective analysis. Nevertheless, the data were obtained from a large number of centres across the globe represent the largest database on OF up to date. Second, since only data from patients with peri-procedural OF were collected, we were unable to assess predictors of its occurrence. Third, not all data were available and some patients were lost to follow-up. Fourth, because OF typically occurs relatively late after the procedure, the incidence may be underreported, and the true incidence remains unknown. Fifth, there were no data on how the temperature measured by the oesophageal temperature probe was utilized in these patients, and no cut-off values were available. Sixth, no accurate data were available reporting on the specific ablation design for the participating centres. Seventh, no subgroup analysis on the incidence of the use of contact force catheters, ablation index, and lesion size index was available. Eighth, the absence of data from a significant proportion of invited centres strongly limits the applicability of the present results to a general population and may result in underestimation of the true incidence of OF. Finally, our findings concerning different treatment strategies and outcomes show the above-mentioned selection bias concerning critical illness and operability of the patients.
Conclusions
In this large worldwide registry, the incidence of OF was very low; in general, the incidence was lower for cryoballoon- compared to RF-based procedures. The observed time to symptoms was shorter than previously reported. Additionally, the time to diagnosis and the time from first symptom onset to OF diagnosis were relatively short but showed a relatively wide range. The overall prognosis was poor. Surgical or endoscopic intervention is mandatory for improving patient survival.
Supplementary Material
Acknowledgements
We thank all the local investigators and assistant personnel for their great effort. Furthermore, we thank all the POTTER-AF collaborators listed in the Supplementary data online, Table S2.
Contributor Information
Roland Richard Tilz, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Vanessa Schmidt, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Helmut Pürerfellner, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Philippe Maury, Department of Cardiology, University Hospital Rangueil, Toulouse, France.
K R J ulian Chun, MVZ CCB am Agaplesion Markus Krankenhaus, Frankfurt a.M., Germany.
Martin Martinek, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Christian Sohns, Kliniken für Elektrophysiologie/Rhythmologie, Herz- und Diabeteszentrum NRW, Universitätsklinik der Ruhr-Universität Bochum, Bad Oeynhausen, Germany.
Boris Schmidt, MVZ CCB am Agaplesion Markus Krankenhaus, Frankfurt a.M., Germany.
Franck Mandel, Department of Cardiology, University Hospital Rangueil, Toulouse, France.
Estelle Gandjbakhch, Sorbonne Université, APHP, Pitié Salpêtrière University Hospital, Cardiology Institute, Paris, France.
Mikael Laredo, APHP, Pitié Salpêtrière University Hospital, Cardiology Institute, Paris, France.
Melanie Anuscha Gunawardene, Klinik für Kardiologie und Internistische Intensivmedizin, Asklepios Klinik St. Georg, Hamburg, Germany.
Stephan Willems, Klinik für Kardiologie und Internistische Intensivmedizin, Asklepios Klinik St. Georg, Hamburg, Germany.
Thomas Beiert, Heart Center Bonn, Department of Internal Medicine II, University Hospital Bonn, Bonn, Germany.
Martin Borlich, Heart Center, Segeberger Kliniken (Academic Teaching Hospital of the Universities of Kiel, Lübeck and Hamburg), Bad Segeberg, Schleswig-Holstein, Germany.
Leon Iden, Heart Center, Segeberger Kliniken (Academic Teaching Hospital of the Universities of Kiel, Lübeck and Hamburg), Bad Segeberg, Schleswig-Holstein, Germany.
Anna Füting, Department of Electrophysiology, Alfred Krupp Hospital, Essen, Germany; Department Of Medicine, Witten/Herdecke University, Witten, Germany.
Raphael Spittler, Department of Cardiology II/Electrophysiology, Center for Cardiology, University Hospital Mainz, Mainz, Germany.
Thomas Gaspar, Department of Internal and Cardiovascular Medicine, Herzzentrum Dresden, University Clinic, Technische Universität Dresden, Dresden, Germany.
Sergio Richter, Department of Internal and Cardiovascular Medicine, Herzzentrum Dresden, University Clinic, Technische Universität Dresden, Dresden, Germany.
Anja Schade, Department of Interventional Electrophysiology, Helios Hospital Erfurt, Erfurt, Germany.
Malte Kuniss, Department of Cardiology Kerckhoff Heart Center, Bad Nauheim, Germany.
Thomas Neumann, Department of Cardiology Kerckhoff Heart Center, Bad Nauheim, Germany.
Alexander Francke, Helios Klinikum Pirna, Klinik für Innere Medizin II, Pirna, Germany.
Carsten Wunderlich, Helios Klinikum Pirna, Klinik für Innere Medizin II, Pirna, Germany.
Dong-In Shin, Department of Cardiology, Heart Centre Niederrhein, Helios Clinic Krefeld, Krefeld, Germany; Faculty of Health, School of Medicine, University Witten/Herdecke, Witten, Germany.
Dirk Grosse Meininghaus, Department of Cardiology, Carl-Thiem-Klinikum gGmbH Cottbus, Cottbus, Germany.
Mike Foresti, Kliniken Maria Hilf GmbH, Mönchengladbach, Germany.
Marc Bonsels, Kliniken Maria Hilf GmbH, Mönchengladbach, Germany.
David Reek, Department of Cardiology, University Hospital Augsburg, Augsburg, Germany.
Uwe Wiegand, Sana-Klinikum Remscheid GmbH, Akademisches Lehrkrankenhaus der Universität zu Köln, Remscheid, Germany.
Alexander Bauer, Diak-Klinikum Schwäbisch Hall und Klinikum Crailsheim, Schwäbisch Hall, Germany.
Andreas Metzner, Universitäres Herz- und Gefäßzentrum, Klinik für Kardiologie, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany.
Lars Eckardt, Department of Cardiology II (Electrophysiology), University Hospital Münster, Germany.
Sorin Ștefan Popescu, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Olaf Krahnefeld, Sana Kliniken Lübeck, Lübeck, Germany.
Christian Sticherling, Deaprtment of Cardiology, University Hospital Basel, Münster, Switzerland.
Michael Kühne, Deaprtment of Cardiology, University Hospital Basel, Münster, Switzerland.
Dinh Quang Nguyen, St. Vinzenz-Hospital Köln, Köln, Germany.
Laurent Roten, Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Basel, Switzerland.
Ardan M Saguner, Department of Cardiology, University Heart Center, University Hospital Zurich, Zurich, Switzerland.
Dominik Linz, Department of Cardiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands.
Pepijn van der Voort, Catharina Hospital, Eindhoven, The Netherlands.
Bart A Mulder, Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Johan Vijgen, Heart Center Hasselt, Jessa Hospital, Hasselt, Belgium.
Alexandre Almorad, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Charles Guenancia, Cardiology Department, Dijon University Hospital, Dijon, France.
Laurent Fauchier, Service de Cardiologie, Centre Hospitalier Universitaire Trousseau, Tours, France.
Serge Boveda, Cardiology—Heart Rhythm Management Department, Clinique Pasteur, Toulouse, France; Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Y De Greef, Department of Cardiology, ZNA Heart Centre, Antwerp, Belgium; Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Antoine Da Costa, Division of Cardiology, Jean Monnet University, Saint-Etienne, Créteil, France.
Pierre Jais, CHU Bordeaux, Univ. Bordeaux, IHU LIRYC ANR-10-IAHU-04, France.
Nicolas Derval, CHU Bordeaux, Univ. Bordeaux, IHU LIRYC ANR-10-IAHU-04, France.
Antoine Milhem, La Rochelle Hospital, France.
Laurence Jesel, University Hospital Strasbourg, France.
Rodrigue Garcia, University Hospital Poitier, France.
Hervé Poty, Clinique Tonkin, Lyon, France.
Ziad Khoueiry, Clinique Saint Pierre, Perpignan, France.
Julien Seitz, Hospital St. Joseph, Marseille, France.
Julien Laborderie, Bayonne Hospital, France.
Alexis Mechulan, Hospital Clairval, Marseille, France.
Francois Brigadeau, University Hospital Lille, France.
Alexandre Zhao, Clinique Ambroise Parée, Paris, France.
Yannick Saludas, Clinique Pôle Santé République, Clermont Ferrand, France.
Olivier Piot, Centre Cardiologie du Nord, Saint Denis, France.
Nikhil Ahluwalia, Barts Heart Centre, Barts Health NHS Trust, London, UK; Wiliam Harvey Heart Centre, Queen Mary University of London, UK.
Claire Martin, Royal Papworth Hospital, Cambridge, UK.
Jian Chen, Department of Heart Disease, Haukeland University Hospital, University of Bergen, Bergen, Norway.
Bor Antolic, Department of Cardiology, University Medical Center Ljubljana, Slovenia.
Georgios Leventopoulos, University of Patras, Greece.
Emin Evren Özcan, Heart Rhythm Management Center, Dokuz Eylul University, Izmir, Turkey.
Hikmet Yorgun, Department of Cardiology, Hacettepe University, Ankara, Turkey.
Serkan Cay, Department of Cardiology, Division of Arrhythmia and Electrophysiology, University of Health Sciences, Yuksek Ihtisas Cardiovascular Building, Ankara City Hospital, Ankara, Turkey.
Kivanc Yalin, Cerrahpasa Faculty of Medicine, Department of Cardiology, Istanbul University-Cerrahpasa, Istanbul, Turkey.
Maichel Sobhy Botros, Department of critical care medicine, Faculty of medicine, Cairo University, Cairo, Egypt.
Ahmed Taher Mahmoud, Department of critical care medicine, Faculty of medicine, Cairo University, Cairo, Egypt.
Ewa Jędrzejczyk-Patej, Department of Cardiology, Congenital Heart Diseases and Electrotherapy, Silesian Centre for Heart Diseases, Zabrze, Poland.
Osamu Inaba, Department of Cardiology, Japanese Red Cross Saitama Hospital, Japan.
Ken Okumura, Division of Cardiology, Saiseikai Kumamoto Hospital, Kumamoto, Japan.
Koichiro Ejima, Department of Cardiology, Tokyo Women’s Medical University, Shinjuku-ku, Tokyo, Japan.
Houman Khakpour, UCLA Cardiac Arrhythmia Center, Los Angeles, USA.
Noel Boyle, UCLA Cardiac Arrhythmia Center, Los Angeles, USA.
John N Catanzaro, University of Florida Health, Jacksonville, FL, USA.
Vivek Reddy, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Sanghamitra Mohanty, St. David’s Medical Center, Texas Cardiac Arrhythmia Institute, Austin, TX, USA.
Andrea Natale, St. David’s Medical Center, Texas Cardiac Arrhythmia Institute, Austin, TX, USA; International Electrophysiology, Scripps Clinic, San Diego, CA, USA; Metro Health Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Hermann Blessberger, Department of Cardiology, Kepler University Hospital, Linz, Austria.
Bing Yang, Department of Cardiology, Shanghai East Hospital, Tongji University, Shanghai, China.
Irene Stevens, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Philipp Sommer, Kliniken für Elektrophysiologie/Rhythmologie, Herz- und Diabeteszentrum NRW, Universitätsklinik der Ruhr-Universität Bochum, Bad Oeynhausen, Germany.
Christian Veltmann, Heart Center Bremen, Electrophysiology Bremen, Bremen, Germany.
Daniel Steven, Department for Electrophysiology, Heart Center University Cologne, Cologne, Germany.
Julia Vogler, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Karl-Heinz Kuck, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
José Luis Merino, La Paz University Hospital, Universidad Autónoma de Madrid, Idipaz, Madrid, Spain.
Ahmad Keelani, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Christian-H Heeger, Department of Rhythmology, University Heart Center Lübeck, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Supplementary data
Supplementary data is available at European Heart Journal online.
Pre-registered clinical trial number
The pre-registered clinical trial number is ClinicalTrials.gov Identifier: NCT05273645.
Ethical approval
The registry was approved by the local ethical review board of the University of Lübeck, Germany (AZ 21–291). Each participating centre was responsible for its ethics approval by the local ethics committee. The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. All patient information was anonymized.
Data availability
Non-digital data supporting this study are curated at the Study Center of the Department of Rhythmology, University Hospital Schleswig-Holstein, German.
Funding
All authors declare no funding for this contribution.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2. Ghia KK, Chugh A, Good E, Pelosi F, Jongnarangsin K, Bogun F, et al. . A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2008;24:33–36. 10.1007/s10840-008-9307-1 [DOI] [PubMed] [Google Scholar]
- 3. Gandjbakhch E, Mandel F, Dagher Y, Hidden-Lucet F, Rollin A, Maury P. Incidence, epidemiology, diagnosis and prognosis of atrio-oesophageal fistula following percutaneous catheter ablation: a French nationwide survey. Europace 2021;23:557–564. 10.1093/europace/euaa278 [DOI] [PubMed] [Google Scholar]
- 4. Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J, et al. . Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2009;3:32–38. 10.1161/CIRCEP.109.859116 [DOI] [PubMed] [Google Scholar]
- 5. Chavez P, Messerli FH, Dominguez AC, Aziz EF, Sichrovsky T, Garcia D, et al. . Atrioesophageal fistula following ablation procedures for atrial fibrillation: systematic review of case reports. Open Heart 2015;2:e000257. 10.1136/openhrt-2015-000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nair KKM, Shurrab M, Skanes A, Danon A, Birnie D, Morillo C, et al. . The prevalence and risk factors for atrioesophageal fistula after percutaneous radiofrequency catheter ablation for atrial fibrillation: the Canadian experience. J Interv Card Electrophysiol 2013;39:139–144. 10.1007/s10840-013-9853-z [DOI] [PubMed] [Google Scholar]
- 7. Barbhaiya CR, Kumar S, Guo Y, Zhong J, John RM, Tedrow UB, et al. . Global survey of esophageal injury in atrial fibrillation ablation: characteristics and outcomes of esophageal perforation and fistula. JACC Clin Electrophysiol 2016;2:143–150. 10.1016/j.jacep.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 8. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–1255. 10.1161/CIRCULATIONAHA.117.025827 [DOI] [PubMed] [Google Scholar]
- 9. Mohanty S. Outcomes of atrio-esophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol 2014;25:E6. 10.1111/jce.12494 [DOI] [PubMed] [Google Scholar]
- 10. John RM, Kapur S, Ellenbogen KA, Koneru JN. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm 2017;14:184–189. 10.1016/j.hrthm.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 11. Tilz RR, Chun KRJ, Metzner A, Buchard A, Wissner E, Koektuerk B, et al. . Unexpected high incidence of esophageal injury following pulmonary vein isolation using robotic navigation. J Cardiovasc Electrophysiol 2010;21:853–858. 10.1111/j.1540-8167.2010.01742.x [DOI] [PubMed] [Google Scholar]
- 12. Steinbeck G, Sinner MF, Lutz M, Müller-Nurasyid M, Kääb S, Reinecke H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: a nationwide in-hospital analysis of administrative data for Germany in 2014. Eur Heart J 2018;39:4020–4029. 10.1093/eurheartj/ehy452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–2245. 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- 14. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak R, et al. . EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–1235. 10.1093/europace/euv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heeger CH, Sano M, Popescu SȘ, Subin B, Feher M, Phan HL, et al. . Very high-power short-duration ablation for pulmonary vein isolation utilizing a very-close protocol—the FAST AND FURIOUS PVI study. Europace 2023;25:880–888. 10.1093/europace/euac243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YG, Shim J, Kim D, Choi J, Park S, Pak H, et al. . Characteristics of atrial fibrillation patients suffering atrioesophageal fistula after radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2018;29:1343–1351. 10.1111/jce.13671 [DOI] [PubMed] [Google Scholar]
- 17. Sarairah SY, Woodbury B, Methachittiphan N, Tregoning DM, Sridhar AR, Akoum N. Esophageal thermal injury following cryoballoon ablation for atrial fibrillation. JACC Clin Electrophysiol 2020;6:262–268. 10.1016/j.jacep.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 18. Schmidt B, Metzner A, Chun KR, Leftheriotis D, Yoshiga Y, Fuernkranz A, et al. . Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol 2010;3:481–488. 10.1161/CIRCEP.110.954149 [DOI] [PubMed] [Google Scholar]
- 19. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner A, et al. . Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–1266. 10.1093/europace/euac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller P, Dietrich J-W, Halbfass P, Abouarab A, Fochler F, Szöllösi A, et al. . Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm 2015;12:1464–1469. 10.1016/j.hrthm.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 21. Han HC, Ha FJ, Sanders P, Spencer R, Teh AW, O’Donnell D, et al. . Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol 2017;10:e005579. 10.1161/CIRCEP.117.005579 [DOI] [PubMed] [Google Scholar]
- 22. Pappone C, Vicedomini G, Santinelli V. Atrio-esophageal fistula after AF ablation: pathophysiology, prevention &treatment. J Atr Fibrillation 2013;6:860. 10.4022/jafib.860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Non-digital data supporting this study are curated at the Study Center of the Department of Rhythmology, University Hospital Schleswig-Holstein, German.