Abstract
Purpose
We evaluated the dosimetric effect of tumor changes in patients with fractionated brain stereotactic radiation therapy (SRT) on the tumor and normal brain using repeat verification magnetic resonance imaging (MRI) in the middle of the treatment period.
Methods and Materials
Fifteen large intracranial metastatic lesions with fractionated SRT were scanned employing standardized planning MRI (MRI-1). Repeat verification MRI (MRI-2) were performed during the middle of the irradiation period. Gross tumor volume (GTV) was defined as the volume of the contrast-enhancing lesion on T1-weighted MRI with gadolinium contrast agent. The doses to the tumor and normal brain were evaluated on the MRI-1 scan. Beam configuration and intensity on the initial volumetric modulated arc therapy plan were used to evaluate the dose to the tumor and the normal brain on MRI-2. We evaluated the effect of D98% (percent dose irradiating 98% of the volume) on the GTV using the plans on the MRI-1 and MRI-2 scans. For the normal brain, the V90%, V80%, and V50% (volume of the normal brain receiving >90%, 80%, and 50% of the prescribed dose, respectively) were investigated.
Results
Three (20% of the total) and 4 (26% of the total) tumors exhibited volume shrinkage or enlargement changes of >10%. Five (33% of the total) tumors exhibited volume shrinkage and enlargement changes of <10%. Three tumors (20% of the total) showed no volume changes. D98% of the GTV increased in patients with tumor shrinkage because of dose inhomogeneity and decreased in patients with tumor enlargement, with a coefficient of determination of 0.28. The V90%, V80%, and V50% increase with decreasing tumor volumes and were linearly related to the tumor volume difference with a coefficient of determination values of 0.97, 0.98, and 0.97, respectively.
Conclusions
Repeat verification MRI for brain fractionated SRT during the treatment period should be considered to reduce the magnitude of target underdosing or normal brain overdosing.
Introduction
Stereotactic radiosurgery and stereotactic radiation therapy (SRS/SRT) for benign and malignant brain tumors require a steep dose falloff around target volumes and tight margins to achieve local tumor control while sparing the dose to surrounding normal tissues.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Intrafractional and interfractional tumor changes in SRS and SRT could be a significant issue in terms of accuracy and precision of treatment delivery. Other studies have reported that intrafractional tumor changes during beam delivery are influenced by head immobilization.21, 22, 23 However, the potential for interfractional tumor changes during the treatment period, such as tumor size, shape, and geometry, must be considered to improve the accuracy of dose delivery. Edema, changes in tumor volume, shifting of fluids and muscle mass, and postoperative changes have been reported by several authors.11, 12, 13, 14, 15, 16, 17, 18, 19, 20
Fractionated SRT has been applied to large brain metastases to reduce the risk of radiation necrosis.1, 2, 3, 4, 5 The tumor volume and locational changes induced by the time between magnetic resonance imaging (MRI) acquisition and the start of radiosurgery may have a clinical effect on rapidly growing lesions.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 The possibility of tumor changes may increase as the number of fractions increases. Even slight tumor changes could potentially lead to treatment failure because of a steep dose falloff around target volumes and tight margins. The potential clinical effect of tumor changes on the dose distribution for both the gross tumor volume (GTV) and planning target volume (PTV) has been reported.18, 19, 20 Therefore, we also performed adaptive replanning for large brain metastases based on repeat MRI verification with a contrast agent in the middle of the treatment period. Some studies have focused on the dose to the target for interfractional target changes.18, 19, 20 To the best of our knowledge, the dose to the brain for interfractional target changes during fractionated brain SRT has not been reported.
This study aims to evaluate the dosimetric effect of the changes in tumor size, shape, and geometry on the doses to the targets and normal brain in patients with brain metastases undergoing fractionated SRT and demonstrate the usefulness of repeat verification MRI for adaptive radiation therapy in the middle of the treatment period.
Material and Methods
Patients
Thirteen patients (15 lesions) with large intracranial metastatic lesions treated with fractionated SRT at our institution between February 2018 and April 2022 were included in this study. Furthermore, 2 patients had 2 brain metastases each. Patients who received adjuvant radiation therapy, such as in the postoperative setting, were not included in this study. All patients had single or multiple brain metastases with a maximum diameter of >2 cm based on gadolinium (Gd) enhanced T1-weighted MRI. The prescribed dose and fractionation schedule were determined according to the size of the brain metastasis. The total dose was 35 Gy in 5 fractions for lesions between 2.1 and 3 cm, or 5.1 and 10 cc, and 40 Gy in 8 fractions for lesions between 3.1 and 4 cm, or 10.1 and 30 cc. Table 1 summarizes the patient characteristics.
Table 1.
Patient characteristics
| MRI-1 |
MRI-2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. patient | No. tumor | Dose prescription | Primary tumor | Pathologic status | Time from MRI-1 to the first d of treatment (d) | Time from MRI-1 to MRI-2 (d) |
GTV (cc) | PTV (cc) | GTV (cc) | PTV (cc) | Replan | Reason | Timing of MRI-2 (before Xth treatment) |
| 1 | 1 | 7 Gy × 5 fr (80% isodose) | Nasal cavity | Small cell carcinoma | 4 | 6 | 4.2 | 6.4 | 5.2 | 7.6 | Yes | Enlargement | Third |
| 2 | 2 | 5 Gy × 8 fr (80% isodose) | Lung | Malignant neoplasms | 3 | 8 | 20.3 | 28.7 | 15.8 | 20.5 | Yes | Shrinkage | Fifth |
| 3 | 3 | 7 Gy × 5 fr (80% isodose) | Lung | Non-small cell carcinoma | 2 | 6 | 8.1 | 11 | 8.1 | 11 | no | Third | |
| 4 | 4 | 7 Gy × 5 fr (80% isodose) | Bladder | Urothelial caricinoma | 3 | 5 | 6.9 | 10 | 6.8 | 9.9 | Yes | Displacement | Fourth |
| 5 | 5 | 7 Gy × 5 fr (80% isodose) | Esophagus | Squamous cell carcinoma | 2 | 7 | 5.3 | 8.8 | 4 | 6.1 | Yes | Shrinkage | Fourth |
| 6 | 6 | 7 Gy × 5 fr (80% isodose) | Salivary gland | Adenocarcinoma | 2 | 7 | 9.8 | 13 | 10.4 | 13.7 | Yes | Enlargement | Fourth |
| 7 | 7 | 7 Gy × 5 fr (80% isodose) | Lung | Adenocarcinoma | 1 | 4 | 3.2 | 4.8 | 3.7 | 5.4 | Yes | Enlargement | Second |
| 8 | 8 | 5 Gy × 8 fr (80% isodose) | Lung | Small cell carcinoma | 2 | 7 | 14.8 | 18.8 | 14.8 | 18.8 | no | Fourth | |
| 9 | 5 Gy × 8 fr (80% isodose) | 2 | 7 | 23.8 | 30.4 | 23.8 | 30.4 | no | |||||
| 9 | 10 | 5 Gy × 8 fr (80% isodose) | Esophagus | Squamous cell carcinoma | 2 | 8 | 33 | 39.7 | 36.5 | 45.2 | Yes | Enlargement | Fourth |
| 10 | 11 | 5 Gy × 8 fr (80% isodose) | Breast | Invasive ductal carcinoma | 1 | 6 | 11.5 | 14.7 | 11 | 14.6 | Yes | Displacement | Fifth |
| 12 | 5 Gy × 8 fr (80% isodose) | 1 | 6 | 10.1 | 13 | 9.7 | 13 | Yes | Shrinkage | ||||
| 11 | 13 | 5 Gy × 8 fr (70% isodose) | Kidney | Renal cell carcinoma | 1 | 7 | 3.3 | 5 | 2.8 | 4.3 | Yes | Shrinkage | Fifth |
| 12 | 14 | 7 Gy × 5 fr (80% isodose) | Colon | Adenocarcinoma | 2 | 6 | 10.7 | 14.4 | 9.7 | 13.2 | Yes | Shrinkage | Third |
| 13 | 15 | 7 Gy × 5 fr (80% isodose) | Colon | Adenocarcinoma | 2 | 7 | 6.3 | 8.8 | 5.4 | 7.6 | Yes | Shrinkage | Fourth |
Abbreviations: fr = fraction; GTV = gross tumor volume; MRI-1 = standardized planning magnetic resonance imaging; MRI-2 = repeat verification magnetic resonance imaging; PTV = planning target volume.
MRI protocol
Standardized planning MRI (MRI-1) and repeat verification MRI (MRI-2) were performed on a 3.0 Tesla MRI system (Discovery MR750w; GE Healthcare, Milwaukee, WI). The axial slice thickness was 1.25 mm with noncontrast T1, T2, and FLAIR (fluid attenuated inversion recovery) sequences. Each case had a T1-weighted Gd contrast (0.2 mL/kg body weight) enhanced MRI with a voxel of 2.0 × 1.0 × 1.6 mm (field of view of 22 × 22 cm; matrix = 488 × 244; slice thickness of 1.6 mm).
Treatment procedure
Patients were immobilized in a thermoplastic mask (CIVCO Medical Solutions, Kalona, IA) and scanned using a computed tomography (CT) scan (Optima CT 580 W; GE Healthcare, Milwaukee, WI). The CT scan parameters were set as follows: the x-ray tube voltage was 120 kV, slice thickness was 1.25 mm, the field of view was 500 mm, and the mAs value was determined using an auto-exposure control function. The Eclipse treatment planning system (version 13.5, Varian Medical Systems, Palo Alto, CA) was used to combine MRI scans with CT scans and delineate the GTV and organs at risk (eg, normal brain, eyes, lens, optic chiasm, optic nerves, and brainstem). GTV was defined as the volume of the contrast-enhancing lesion on T1-weighted MRI with a Gd contrast agent. The clinical target volume was equal to that of the GTV. The PTV was created by adding an isotropic margin of 1 mm from the GTV in all directions. The isocenter was automatically placed at the center of the PTV. The dose was prescribed with a 70% to 80% isodose line covering the PTV. Plans were normalized such that PTV D95% or D98% (DX%: the percent dose irradiated X% of the volume) was equal to the prescribed dose. The maximum dose for the PTV ranged from 125% to 130%. Volumetric modulated arc therapy (VMAT) with beam energies of 6 MV (flattening filter-free mode) was delivered by TrueBeamSTx (Varian Medical Systems, Palo Alto, CA). For dose calculations, the Acuros XB algorithm was applied with a grid resolution of 1.0 mm. The ExacTrac system (BrainLAB AG, Heimstetten, Germany) was used for rigid image fusion with the skull anatomy using translational and rotational robotic couch shifts. The final patient translational and rotational positions were within tolerances of 0.5 mm and 0.5 degrees, respectively. Positioning verification was repeated until all shift values based on skull anatomy matched within tolerance with x-ray imaging.
Verification MRI
MRI-2 was obtained to evaluate the changes in tumor size, shape, and geometry during the middle of the irradiation period using the same MRI protocol and Gd contrast agent as that used for MRI-1. In cases where there were 2 or more days off during the treatment period due to weekends or holidays, the verification MRI-2 was performed immediately after those days. Volume of interest for image registration was manually adjusted without including the oral area or mandible because the accuracy of automatic registration is dependent on the volume of interest. To delineate the target volume changes on MRI-2, the GTV contouring from the initial treatment plan on MRI-1 was copied and modified. The GTV on the MRI-2 scan was contoured by the same oncologist to avoid interobservation errors. Based on the previous report,18 the reasons for the replanning based on MRI-2 were classified as tumor shrinkage, tumor enlargement, and displacement. Tumor shrinkage was defined as a ≥1 mm shrinkage in the maximum tumor diameter and ≥10% shrinkage in the tumor volume. Tumor enlargement was defined as a ≥1 mm enlargement in the tumor diameter in each direction and ≥10% enlargement in the tumor volume. Displacement was defined as a ≥1 mm shift of center of tumor. The replanned treatment was delivered within 30 hours (the next day) of acquiring the MRI-2 scan. The doses to the target and normal brains were evaluated using the MRI-1 scan. Additionally, the beam configuration and intensity of the initial VMAT plan were used to evaluate the doses to the target and normal brain on MRI-2.
Data analysis
The tumor volumes obtained from the MRI-1 and MRI-2 scans were defined as V1 and V2, respectively. The relative interfractional tumor change rates were assessed between the GTV on MRI-1 and MRI-2 scans, which were calculated using the formula (V2 − V1) × 100 / V1. We evaluated the effect of D2%, D50%, and D98% on the replanned GTV and PTV using the initial SRT plan parameters (eg, beam configuration and intensity). For the normal brain, the V90%, V80%, and V50% (volumes of the normal brain receiving a dose of >90%, 80%, and 50% of the prescribed dose, respectively) were investigated. The relative doses calculated from the MRI-1 and MRI-2 scans were defined as D1 and D2, respectively. The dose difference was calculated using the formula (D2 − D1) × 100 / D1, because the prescribed dose was different for each lesion. The data were analyzed using Wilcoxon signed-rank tests with the statistical significance set at P < .05, using R version 3.5.2 (www.r-project.org).
Results
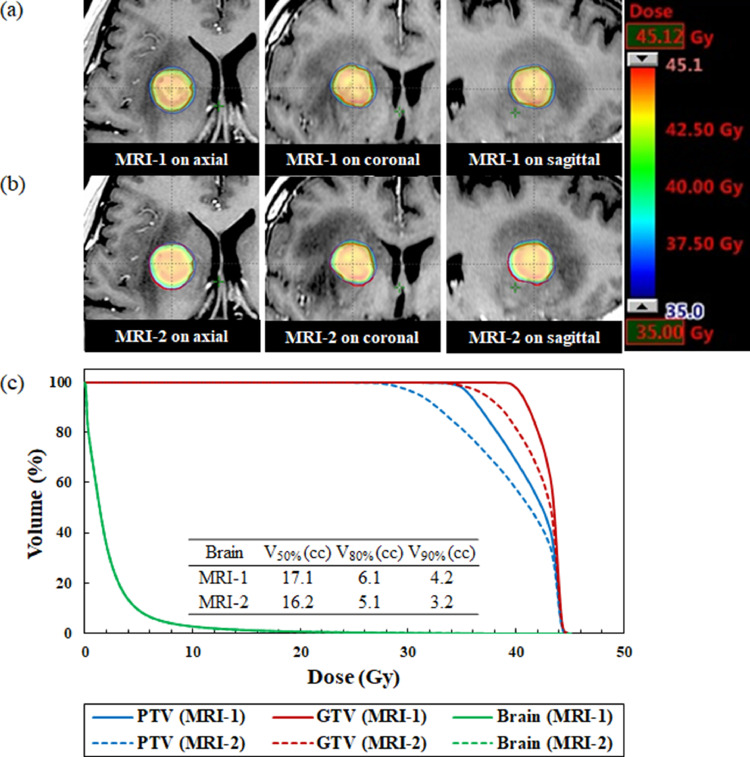
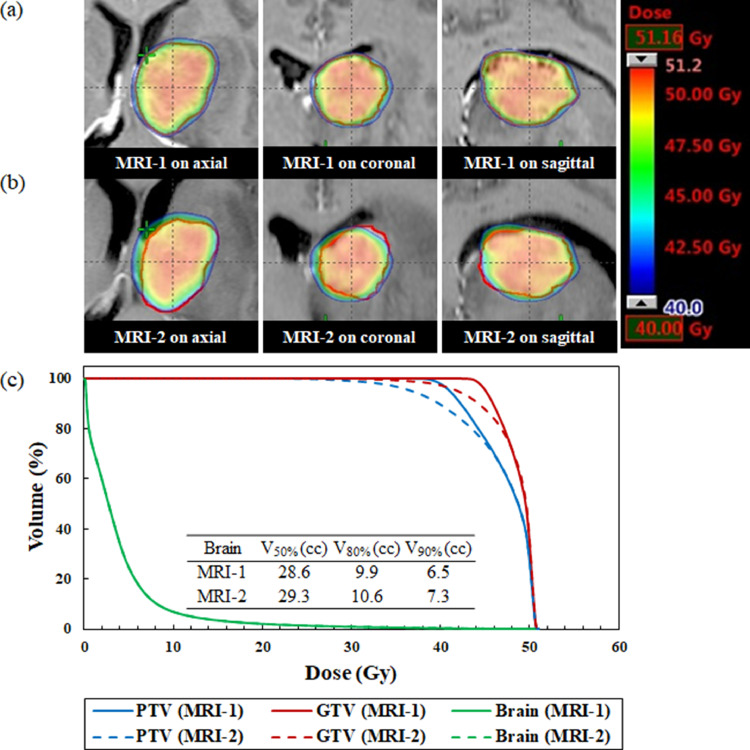
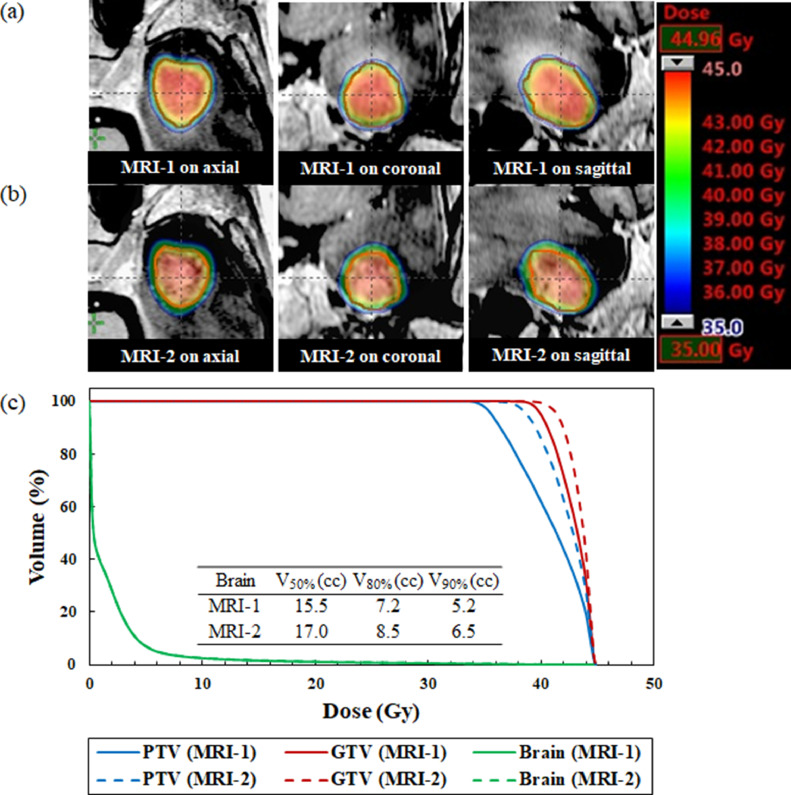
The median time between the MRI-1 scan and first day of treatment delivery was 2 days (range, 1-4 days). The median time between the MRI-1 and MRI-2 scans was 7 days (range, 4-8 days). Figures 1 to 3 illustrate an example of the dose distribution and dose volume histograms of the doses to the GTV, PTV, and normal brain on the MRI-1 and MRI-2 scans. Compared with the plan on the MRI-1 scan, the plan on the MRI-2 scan provided substantially lower doses to the GTV and PTV owing to tumor enlargement, as shown in Fig. 1. On the contrary, the MRI-2 plan provided slightly higher doses to the GTV and PTV because of tumor shrinkage, as shown in Fig. 2. Additionally, the dose administered to the normal brain appeared to considerably increase. A patient showed a significant dose reduction in the GTV because of tumor displacement, as shown in Fig. 3.
Fig. 1.
Dose distributions superimposed on the (a) standardized planning magnetic resonance imaging (MRI-1) and (b) repeat verification MRI (MRI-2) scans for a patient with enlarged brain tumor. (c) Comparison of dose volume histograms for the MRI-1 (solid line) and MRI-2 (dashed line) scans (No. 1). The gross tumor volume is expressed in red. The MRI-2 provided substantially lower doses to the GTV and PTV compared with the MRI-1 scan. The brain curve on MRI-1 is perfectly superimposed on MRI-2. Abbreviations: GTV = gross tumor volume; PTV = planning target volume.
Fig. 3.
Dose distributions superimposed on the (a) standardized planning magnetic resonance imaging (MRI-1) and (b) repeat verification MRI (MRI-2) scans for a patient with brain tumor displacement. (c) Comparison of dose volume histograms for the MRI-1 (solid line) and MRI-2 (dashed line) scans (No. 11). The gross tumor volume (GTV) is expressed in red. MRI-2 provided substantially lower doses to the gross tumor volume and planning target volume compared with the MRI-1 scan. The brain curve on MRI-1 is perfectly superimposed on MRI-2. Abbreviations: GTV = gross tumor volume; PTV = planning target volume.
Fig. 2.
Dose distributions superimposed on the (a) standardized planning magnetic resonance imaging (MRI-1) and (b) repeat verification MRI (MRI-2) scans for a patient with brain tumor shrinkage. (c) Comparison of dose volume histograms for the MRI-1 (solid line) and MRI-2 (dashed line) scans (No. 5). The gross tumor volume is expressed in red. The MRI-2 provided slightly higher doses to the gross tumor volume and planning target volume compared with the MRI-1 scan. The brain curve on MRI-1 is perfectly superimposed on MRI-2. Abbreviations: GTV = gross tumor volume; PTV = planning target volume.
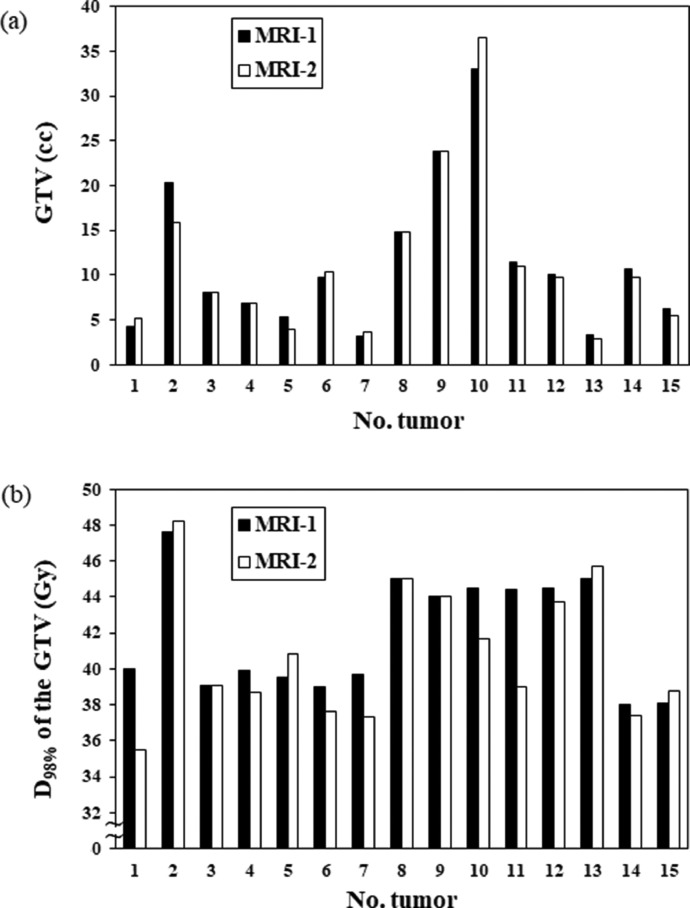
Figure 4 shows the volume and D98% of the tumor between the MRI-1 and MRI-2 scans. Table E1 shows the comparison of the doses to the GTV, PTV, and normal brain between the MRI-1 and MRI-2 scans, with the data shown as group medians with ranges. The median GTV changed from 9.8 cc (range, 3.2-33.0 cc) to 9.7 cc (range, 2.8-36.5 cc; P = .482). Three (20% of the total) and 4 (27% of the total) tumors exhibited volume shrinkage and enlargement changes of >10%. Five (33% of the total) tumors exhibited volume shrinkage and enlargement changes of <10%. Three tumors (20% of the total) showed no volume changes. Of the 15 large brain metastases, 12 (80% of the total) tumors required treatment plan modification. The dosimetric parameters of the GTV, PTV, and normal brain did not significantly differ between the MRI-1 and MRI-2 scans (P > .05).
Fig. 4.
Volume and D98% of the gross tumor volume between standardized planning magnetic resonance imaging and standardized planning magnetic resonance Abbreviations: GTV = gross tumor volume; MRI-1 = standardized planning magnetic resonance imaging; MRI-2 = repeat verification magnetic resonance imaging.
Compared with the plan on the MRI-1 scan, the plan on the MRI-2 scan showed that the D98% dose to the GTV tended to be lower, with an average of –3.1% (range, –12.2% to 3.3%). On the other hand, compared with the plan on the MRI-1 scan, the plan on the MRI-2 scan showed that the brain V90%, V80%, and V50% tended to be higher, with an average of 4.2% (range, –25.0% to 40.9%), 3.1% (range, –17.1% to 30.6%), and 1.5% (range, –3.4% to 13.1%), respectively. Table E2 shows the comparison of the irradiated volume of the brain between the MRI-1 and MRI-2 scans.
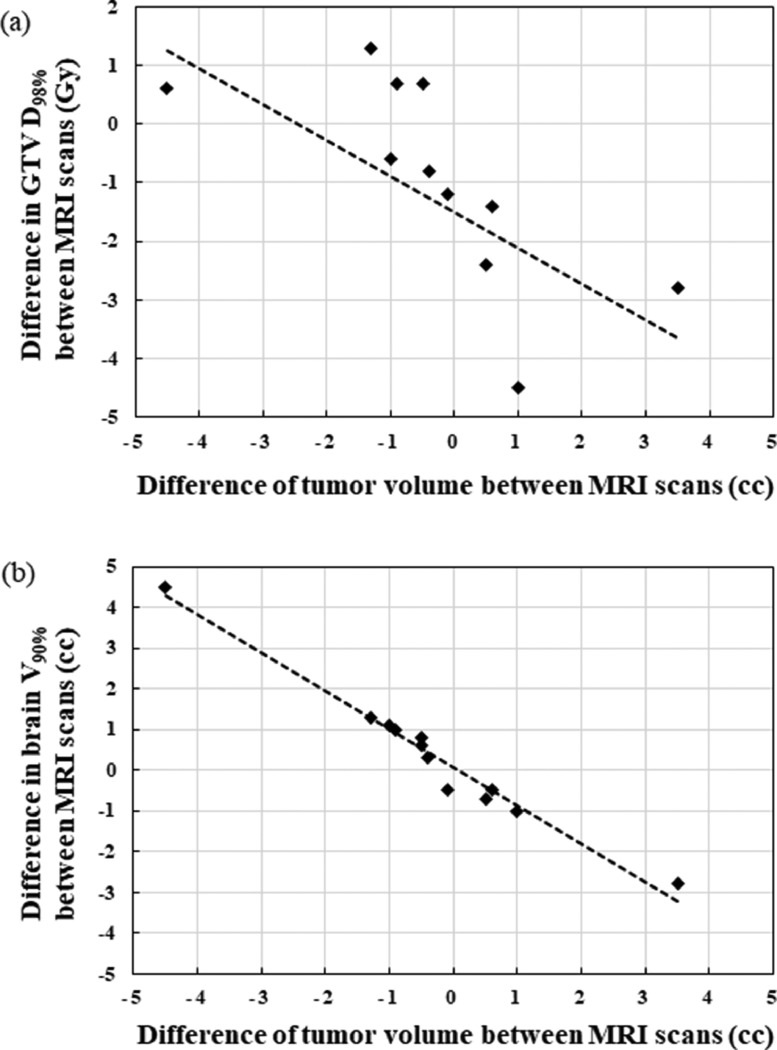
Figure 5 illustrates the plots of the absolute difference D98% dose to the GTV and brain V90% as a function of the absolute tumor volume difference between the MRI-1 and MRI-2 scans. Regarding the tumor dose, the D98% to the GTV increased in patients with tumor shrinkage because of dose inhomogeneity and decreased in patients with tumor enlargement, with a coefficient of determination (R2) of 0.28. The absolute volume difference between the tumor and the brain V90% between the MRI-1 and MRI-2 scans shows a considerable correlation. Brain V50% and V80% had the same trend as brain V90% (unshown). The V90%, V80%, and V50% increase with decreasing tumor volumes and were linearly related to the tumor volume difference with R2 of 0.97, 0.98, and 0.97, respectively.
Fig. 5.
Plots of the absolute difference (a) D98% of gross tumor volume and (b) brain V90% as a function of the absolute tumor volume difference between the standardized planning magnetic resonance imaging and repeat verification magnetic resonance imaging scans (n = 12). Brain V50% and V80% had the same trend as brain V90%. Abbreviations: GTV = gross tumor volume; MRI = magnetic resonance imaging.
Discussion
In this study, we observed that some patients with large brain tumors had significant target volumes and locational changes within a short period of time. The Wilcoxon signed-rank test showed no statistical difference between the 2 MRI scans on doses to the GTV, PTV, and normal brain because both enlargement and shrinkage tumor changes are possible. The largest tumor volume enlargement change observed was 23%, showing a significant dose reduction to the target. Insufficient doses to the GTV because of tumor enlargement during the treatment period could lead to poor tumor control.8 In contrast, sufficient doses to the GTV and overdoses to the normal brain caused due to tumor shrinkage could induce radiation necrosis more frequently. The location might be shifted by a change in the surrounding conditions (eg, edema) even if the tumor volume is almost unchanged between the MRI-1 and MRI-2 scans. In addition, GTV may be changed as a result of observer variation in delineation even in the short time interval between MRI-1 and MRI-2 scans. The dose distribution should be checked on the verification MRI to consider replanning rather than a target volume change. In this study, the treatment was started with a median time of 2 days after acquiring the treatment planning images because one of the crucial factors for treatment precision is the interval between MR imaging and treatment delivery. Seymour et al demonstrated the local freedom from progression would be worse in patients with an interval from MRI to treatment of more than 14 days.12 Salkeld et al found that changes in management required in 41% and 78% of patients with 7 days or delay longer than 7 days between standardized planning MRI and repeat verification MRI before treatment delivery.15 Therefore, we shorten the interval between MRI and treatment delivery as much as possible.
Some studies have reported that target volume and locational changes during fractionated SRT results in insufficient doses to the target may be delivered.18, 19, 20 Uto et al analyzed 23 patients for a median time of 6 days from SRT initiation to the midtreatment MRI scan and observed tumor shrinkage and enlargement.20 Among this sample, a decrease in the minimum dose (D98%) to the GTV in 20% of the patients was observed by recalculating the treatment plan. Hessen et al reported that an analysis of 18 tumors for a median time of 8.5 days from planning to repeated MRI scans showed that the PTV dose coverage decreased up to –34.8% (median, 3.2%), and target volume changes affected the minimum dose in the PTV.19 Their dosimetric analysis focused on the dose delivered to the GTV or PTV. To the best of our knowledge, this is the first study to examine changes in dose to normal brain caused by interfractional target changes during fractionated SRT. Our results indicated that adaptive radiation therapy for fractionated brain SRT is required for both increased and decreased tumor volume because the irradiated brain volume could increase with decreasing tumor volumes, as shown in Fig. 5. Milano et al performed a literature review and found that 3 or 5 fraction fSRT for brain metastases, with normal brain tissue V20 Gy or V24 Gy <20 cm3 are associated with <10% risk of radiation necrosis.5 In addition, increased normal brain volume receiving more than 12 Gy (V12 Gy), where the linear-quadratic model with alpha-beta ratio of 2 is used to convert doses to single-fraction equivalent dose, was associated with increased toxicity risks.
It is important that physicians try to reduce unnecessary irradiation of the normal brain because the risk of radiation necrosis is related to the irradiated dose and volume. Certain studies have reported that multifraction SRS has superior local control and low risk of radiation necrosis than single-fraction SRS.1, 2, 3, 4, 5 Their clinical results imply the elimination of replanning during the treatment period, despite our findings. Whether replanning using repeated MRI in the middle of the treatment period resulted in better local control and reduction in the risk of radiation necrosis remains controversial. In addition, tumor volume and location changes during the period from the planning image acquisition to the treatment completion stage remain unpredictable. Further investigations are required to elucidate these phenomena.
Adaptive radiation therapy is an attractive strategy in which the treatment plan for fractionated brain SRT can be adapted for the changing tumor rather than assuming that it is identical to the MRI for treatment planning. Treatment plans must be adapted to brain tumor changes to deliver an accurate dose to a tumor with a tight PTV margin. The definition of PTV margin does not compensate for tumor biologic responses that might influence the tumor volume, position, or shape. Large brain tumor cases had a cystic component, and tumor enlargement or shrinkage were factors that increased or decreased the cystic component. The correlation between the shift in the center of PTV and the change in edema volume was reported by Hassen et al.13 Larger margins used to compensate for uncertainties in treatment volume result in increased risk to normal tissue.6 Therefore, the PTV margin for fractionated brain SRT should be considered based on the registration of the repeated MRI, the treatment preparation schedule. Replanning based on tumor visualization MRI scans during the treatment period may be an unrealistic approach in clinical practice. Although repeat MRI for modifications during the treatment period may be unnecessary in SRT for large brain metastases with pathologies other than adenocarcinoma and in the absence of pre-RT steroid administration,18 we perform repeat verification MRI for large brain tumors at least once during the treatment period. In the presence of clinically usable MRI devices, adaptive planning allows a significant improvement in target volume coverage and normal brain sparing, which may lead to local tumor control. GTV D98% is a strong and reproducible predictive factor for the local control of brain SRT.8 Therefore, covering a sufficient dose to the GTV in cases of tumor changes using adaptive radiation therapy is clinically important. The criteria of replanning for brain SRT in our institution was defined as changes in 10% or 1 mm of tumor volume and location. However, these criteria may be affected by several factors, such as PTV margin, preparation time, and quality of the plan. (eg, inhomogeneity within PTV, or dose fall-off outside PTV).
Our study has some limitations. First, the sample size was relatively small. Second, we did not assess the relationship between the predictive factors and tumor changes. The predictive factors of tumor changes that require modification are the pathologic status, timing of steroid administration, and changes in target volume before treatment.18,19 Finally, we did not assess local tumor control or radiation necrosis. Further clinical outcome studies will be reported for fractionated SRT with brain tumors using MRI scans in the middle of the treatment period.
Conclusion
Our study indicates the usefulness of repeat verification MRI for adaptive radiation therapy in the middle of the treatment period owing to changes in tumor size, shape, and geometry in patients with brain metastases. Some patients with large brain tumors experienced volume changes during fractionated SRT treatment. Repeated MRI should be considered to evaluate the dose to the target and normal brain, which improves tumor local control and reduces brain necrosis, to reduce the magnitude of underdosing to the target or overdosing to the normal brain during the treatment period.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This study was supported by JSPS KAKENHI grant number 21K15846.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101264.
Appendix. Supplementary materials
Table S1. Dosimetric comparisons between the MRI-1 and MRI-2 scans (n = 15).
Table S2. Brain V90%, V80%, V50% on the MRI-1 and MRI-2 scans (n = 15).
References
- 1.Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011;81:483–489. doi: 10.1016/j.ijrobp.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Lehrer EJ, Peterson JL, Zaorsky NG, et al. Single versus multifraction stereotactic radiosurgery for large brain metastases: An international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys. 2019;103:618–630. doi: 10.1016/j.ijrobp.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–1148. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Chon H, Yoon K, Lee D, Kwon DH, Cho YH. Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neurooncol. 2019;145:49–56. doi: 10.1007/s11060-019-03265-1. [DOI] [PubMed] [Google Scholar]
- 5.Milano MT, Grimm J, Niemierko A, et al. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. 2021;110:68–86. doi: 10.1016/j.ijrobp.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Loganadane G, Dhermain F, Louvel G, et al. Brain radiation necrosis: Current management with a focus on non-small cell lung cancer patients. Front Oncol. 2018;8:336. doi: 10.3389/fonc.2018.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupic G, Brun L, Molnar I, et al. Significant correlation between gross tumor volume (GTV) D98% and local control in multifraction stereotactic radiotherapy (MF-SRT) for unresected brain metastases. Radiother Oncol. 2021;154:260–268. doi: 10.1016/j.radonc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Lucia F, Key S, Dissaux G, et al. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother Oncol. 2019;130:132–138. doi: 10.1016/j.radonc.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 10.Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Ohtakara K, Hoshi H. Target volume geometric change and/or deviation from the cranium during fractionated stereotactic radiotherapy for brain metastases: potential pitfalls in image guidance based on bony anatomy alignment. J Med Imaging Radiat Oncol. 2014;58:729–736. doi: 10.1111/1754-9485.12194. [DOI] [PubMed] [Google Scholar]
- 12.Seymour ZA, Fogh SE, Westcott SK, et al. Interval from imaging to treatment delivery in the radiation surgery age: How long is too long? Int J Radiat Oncol Biol Phys. 2015;93:126–132. doi: 10.1016/j.ijrobp.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Hessen ED, van Buuren LD, Nijkamp JA, et al. Significant tumor shift in patients treated with stereotactic radiosurgery for brain metastasis. Clin Transl Radiat Oncol. 2017;2:23–28. doi: 10.1016/j.ctro.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia MA, Anwar M, Yu Y, et al. Brain metastasis growth on preradiosurgical magnetic resonance imaging. Pract Radiat Oncol. 2018;8:e369–e376. doi: 10.1016/j.prro.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Salkeld AL, Hau EKC, Nahar N, et al. Changes in brain metastasis during radiosurgical planning. Int J Radiat Oncol Biol Phys. 2018;102:727–733. doi: 10.1016/j.ijrobp.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Bronnimann C, Huchet A, Benech-Faure J, et al. Interval between planning and frameless stereotactic radiosurgery for brain metastases: Are our margins still accurate? Neurooncol Pract. 2020;7:211–217. doi: 10.1093/nop/npz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutuk T, Tolakanahalli R, Williams A, et al. Impact of MRI timing on tumor volume and anatomic displacement for brain metastases undergoing stereotactic radiosurgery. Neurooncol Pract. 2021;8:674–683. doi: 10.1093/nop/npab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubo K, Kenjo M, Doi Y, et al. MRI appearance change during stereotactic radiotherapy for large brain metastases and importance of treatment plan modification during treatment period. Jpn J Radiol. 2019;37:850–859. doi: 10.1007/s11604-019-00886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessen E, Nijkamp J, Damen P, et al. Predicting and implications of target volume changes of brain metastases during fractionated stereotactic radiosurgery. Radiother Oncol. 2020;142:175–179. doi: 10.1016/j.radonc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Uto M, Ogura K, Katagiri T, Takehana K, Mizowaki T. Interfractional target changes in brain metastases during 13-fraction stereotactic radiotherapy. Radiat Oncol. 2021;16:140. doi: 10.1186/s13014-021-01869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keeling V, Hossain S, Jin H, et al. Quantitative evaluation of patient setup uncertainty of stereotactic radiotherapy with the frameless 6D ExacTrac system using statistical modeling. J Appl Clin Med Phys. 2016;17:111–127. doi: 10.1120/jacmp.v17i3.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infusino E, Trodella L, Ramella S, et al. Estimation of patient setup uncertainty using BrainLAB Exatrac X-Ray 6D system in image-guided radiotherapy. J Appl Clin Med Phys. 2015;16:5102. doi: 10.1120/jacmp.v16i2.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guckenberger M, Baier K, Guenther I, et al. Reliability of the bony anatomy in image-guided stereotactic radiotherapy of brain metastases. Int J Radiat Oncol Biol Phys. 2007;69:294–301. doi: 10.1016/j.ijrobp.2007.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dosimetric comparisons between the MRI-1 and MRI-2 scans (n = 15).
Table S2. Brain V90%, V80%, V50% on the MRI-1 and MRI-2 scans (n = 15).