Abstract
Background
The effects of food on the pharmacokinetics and safety of metformin hydrochloride (MH) are unclear.
Objective
To discover the effects of food on the pharmacokinetics and safety of MH, and its influence factors.
Methods
English and Chinese databases, and grey (unpublished) literature were searched for eligible studies (registration No. CRD 42022321067 in PROSPERO network). The summary weighted mean difference for continuous variables, and the risk ratio for dichotomous variables was calculated for the main pharmacokinetic parameters. Heterogeneity among the included studies was analyzed using the I2 test. Subgroup analyses, meta-regression, sensitivity analysis, and publication bias test were conducted.
Results
Fourteen clinical trials were included, comprising 408 participants. The pooled AUC0→t, AUC0→∞, and Cmax were decreased by about 30.21% (I2 = 16.7%, p = 0.276), 28.00% (I2 = 73.6%, p < 0.001), and 40.38% (I2 = 92.8%, p < 0.001). Tmax was delayed by about 29.42% (I2 = 45.1%, p = 0.034). Subgroup analysis and meta-regression analysis revealed dosage of MH and gender composition as two significant sources of heterogeneity in AUC0→∞ and Cmax. Sensitivity analysis indicated that most of results were stable. The Egger’s regression test and the Begg test (p > 0.05) confirmed that there is no publication bias.
Conclusions
Pharmacokinetics parameters of MH were affected by food. High-fat, high-calorie diet lowered the extent and rate of absorption while slowing the absorption of metformin. These findings suggest that it is necessary to increase the dosage of MH in order to maintain the same treatment effect when administration of MH after a high fat, high calorie diet.
Keywords: Food, Effects, Metformin, Pharmacokinetics, Safety, Meta-analysis
Highlights
-
•
Pharmacokinetics parameters of MH were affected by food.
-
•
Diet lowers the extent & rate of absorption and slowing the absorption of metformin.
-
•
Dosage and gender impact the effects of food on AUC0→∞ and Cmax.
1. Introduction
Diabetes is a serious threat to human health [[1], [2], [3]]. Across the globe, 537 million adults (aged 20–79 years) are living with diabetes, which amounts to 1 in 10 individuals, and this number is predicted to rise to 643 million by 2030 and 783 million by 2045 [4]. Metformin, which acts as a double guanidine drug, is the first-line treatment for type 2 diabetes [[5], [6], [7], [8], [9], [10]]. The major glucose-lowering effects of metformin in patients with type 2 diabetes are mostly mediated through inhibition of hepatic gluconeogenesis and improving insulin sensitivity [11,12]. In addition, metformin acutely lowers blood glucose levels through the inhibition of intestinal glucose transport [13].
The pharmacokinetics of metformin, including Absorption, Distribution, Metabolism, and Excretion (ADME), are very significant in the treatment of diabetes by metformin. Metformin is absorbed preponderantly by the intestinum tenue, and gastrointestinal absorption is accomplished within about 6 h of swallowing [14]. The bioavailability of metformin tablets averaged 40–60% [14,15]. Metformin is rapidly distributed throughout the body following absorption [14]. The absence of liver metabolism, no conjugates or metabolites of metformin have been ascertained [14]. Metformin is excreted in the urine through the kidneys as a prototype drug, and the excretory half-life of oral metformin is 4.0–8.7 h [14,16].
The pharmacokinetics of metformin is mainly decided by membrane transporters, including the plasma membrane monoamine transporter (PMAT), the organic cation transporters (OCTs), the multidrug and toxin extrusion (MATE) transporters, and the critical protein kinase AMP activated protein kinase (AMPK) [16]. PMAT may play an important role in the uptake of metformin from the gastrointestinal tract, while OCTs induce intestinal absorption, hepatic uptake, and renal excretion of metformin [16]. MATEs are considered to conduce to the hepatic and renal excretion of the drug [16].
Metformin is administrated with meals, while insulin secretagogues and insulin preparation are administrated before meals [9]. The effects of food on pharmacokinetics and safety of metformin hydrochloride tablets (MH) are interesting and significant pharmacokinetic issues for the management of type 2 diabetes mellitus. MH is available in various dosage formulations. Glucophage and Glycoran are the innovative drugs of MH (850 mg) and MH (250 mg), respectively. The instruction manual of Glucophage states that food decreases the extent of absorption and slightly delays the absorption of metformin, as shown by an approximately 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the curve (AUC) of a plasma concentration versus time, and a 35-min prolongation of time to peak plasma concentration (Tmax) [17]. However, the effects of food on the pharmacokinetic parameters of Glycoran (innovative MH, 250 mg) [18], and most generic MH drugs (both 850 mg and 250 mg), are not quantified. Whether the effects of food on the pharmacokinetics and safety of MH are related to drug dosages and formulations, gender, age, body mass index, and race of participants or patients is an important unanswered question.
Recently, several clinical trials of pharmacokinetic evaluation of MH in healthy adults under both fasting and postprandial conditions have been reported [19,20]. This makes it possible to solve the problem. To our knowledge, no study has comprehensively summarized the evidence regarding the effects of food on the pharmacokinetics and safety of MH. Therefore, we reviewed existing evidence from clinical trials on the pharmacokinetics, bioavailability, drug consistency evaluation, and safety of innovative and generic MH under fasting and/or postprandial conditions in healthy adult participants, and performed a head-to-head meta-analysis. The purpose of this systematic review and meta-analysis is discovering the effects of food on the pharmacokinetics and safety of MH, and find the effects of dosage and brand of MH, gender, age, BMI, and race of participants on the pharmacokinetics of MH.
2. Material and methods
This systemic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [21,22] and was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42022321067). The protocol is listed in Supplementary, Appendix 1.
2.1. Information sources and search strategy
The published literature was identified by searching PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science with no language restriction from inception until March 5, 2022, for clinical trials investigating dedicated pharmacokinetics and safety of MH under fasting and/or postprandial conditions in healthy adult participants. Three Chinese databases (CNKI, WANFANG, and VIP database), were also systematically searched to identify any relevant study published between March 5, 2016 [23] and March 5, 2022. The search strategy included the key words: healthy adults AND metformin AND (fasting OR postprandial) AND (pharmacokinetic OR bioequivalence OR safety). In addition, relevant reviews, editorials, and the reference lists of the included articles were scanned for additional relevant studies. Grey (unpublished) literature was identified by searching the websites of clinical practice guideline collections, clinical trial registries, national and international medical specialty societies, and recent conference abstracts. Full-text articles of potentially relevant studies that were unavailable through the university library were requested from the authors (Supplementary, Appendix 2).
2.2. Eligibility criteria and study selection
Two authors (Sun and Liu) independently screened the titles and abstracts to determine whether the articles were relevant to the meta-analysis based on the pre-defined inclusion criteria. The full texts of potentially eligible studies were then reviewed before the final selection. Any disagreement was resolved in consultation with the third author (Wang).
The inclusion criteria were as follows: (1) pharmacokinetics and safety clinical trials of MH under both fasting and postprandial conditions; (2) participants were healthy adults (age ≥18 years), not patient; (3) pharmacokinetic, bioavailability, or bioequivalence studies that include volunteers i.e., not patient. The exclusion criteria were as follows: (1) conference abstracts; (2) reviews; (3) case reports; (4) clinical trial register; (5) compound preparations, sustained release, or preparations other than general tablets; (6) clinical trials in patients instead of adult participants; (7) drugs other than metformin were used; (8) pre-clinical studies; (9) multiple dosages were given in succession.
2.3. Data extraction and quality assessment
Data were extracted from the included articles by two independent authors (Sun and Liu) using standardized data extraction sheets based on the defined inclusion criteria. Any disagreements were resolved in consultation with the third author (Wang), and disagreement was resolved by consensus. If available, the following information was extracted from each article: first author, year of publication, clinical trial site, the brand of MH, dosages, manufacturers, number of participants, main pharmacokinetics parameters (AUC0→t, AUC0→∞, Cmax, and Tmax), and safety outcomes (adverse reactions, severity classification, outcomes). The mean values and standard deviations of continuous data were extracted from the literature. If the mean and standard deviation were not available in an article, they were estimated by median and range [24]. If the description of relevant information in the article was unclear, the corresponding author or first author was contacted by phone, WeChat, or email.
Two authors (Yan and Chen) independently conducted quality assessment of all the included articles using the Cochrane risk of bias tool 2.0 in the following domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result, and overall bias [25]. Each domain was classified as low risk of bias, high risk of bias, or some concerns. Any disagreements were resolved in consultation with another author (Wang), and disagreement was resolved by consensus.
2.4. Statistical analysis
Effect size was calculated based on the weighted mean difference (WMD) for continuous variables (AUC0→t, AUC0→∞, Cmax, and Tmax) and the risk ratio (relative risk; RR) for dichotomous variables (the primary safety outcome, i.e., adverse reaction). The effect size of all combined results is represented by the 95% confidence interval (CI) with upper and lower limits. The pooled rates used a fixed-effects model or a random effects model. The fixed-effects model was used for pooled results with low heterogeneity (I2 ≤ 50%); otherwise, the random-effects model was used for analysis.
Heterogeneity among the included studies was analyzed using the I2 test [21,22] as follows: I2 = [(Q − df)/Q] × 100%, where Q is the χ2 heterogeneity statistic and df is the degrees of freedom. I2 values > 75% indicate high heterogeneity, whereas values between 50% and 75% indicate moderate heterogeneity. I2 values between 25% and 50% indicate low heterogeneity, and values below 25% indicate no heterogeneity.
To explore sources of heterogeneity, subgroup analyses, and meta-regression were conducted. We conducted subgroup analyses according to the following potential sources of heterogeneity: different dosages and formulations, as defined by generic and innovative drugs, mean of age and BMI of participants, race of participants. Univariate and multivariate meta-regression was performed to quantify the potential influence of participants (gender composition, mean of age, BMI, race) and drug (dosage and formulation) characteristics on parameters with moderate or high heterogeneity.
A sensitivity analysis was performed by omitting each study one by one. Potential publication bias was examined using the funnel plot and Egger linear regression [21,22].
Statistical significance and analysis software: Statistical significance was set at p < 0.05. The statistical software package Stata16 (Stata Corp., College Station, TX, USA) was used for the meta-analysis.
3. Results
3.1. Study selection and characteristics
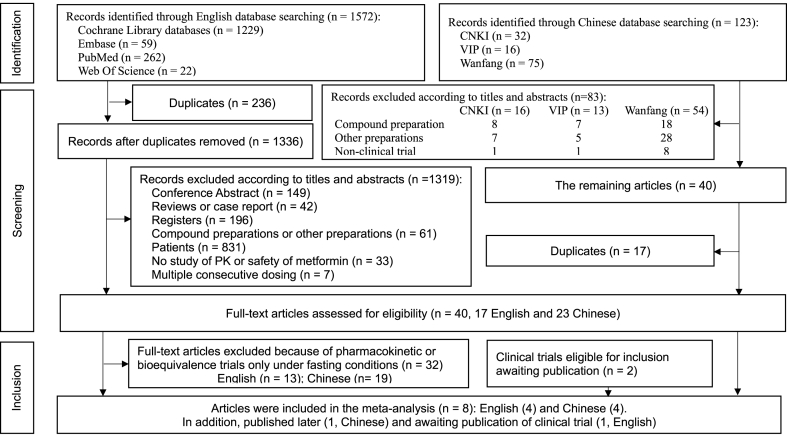
Overall, 1695 studies were retrieved from the electronic databases, including 1572 English and 123 Chinese studies (Fig. 1). After removing duplicates and conducting title and abstract review, we assessed 40 full-text articles for eligibility, including 17 English and 23 Chinese articles. Eight articles, including four English and four Chinese articles that collectively reported on 12 clinical trials (English: #3, #8, #9, #10; Chinese: #1, #2, #4, #7, in Supplementary, Appendix 3), met all the inclusion and exclusion criteria, and were thus included in our meta-analysis. In addition, two clinical trials of bioequivalence and safety evaluation of two low-dose MH (250 mg) treatments under fasting and postprandial conditions to be published (#5 and #6 in Supplementary, Appendix 3), also met the inclusion and exclusion criteria and were included in this meta-analysis.
Fig. 1.
Flow diagram of study selection.
The characteristics of the included trials have been summarized in Table 1. The included trials involved 408 healthy adult participants (24–36 participants in each clinical trial) comprising 284 men and 124 women, of which 180 participated in fasting trials, 180 participated in postprandial trials, and 48 participated in both fasting and postprandial trials. Two clinical trials were conducted in Canada and the United States, while the remaining clinical trials were performed in China. The trial drugs included innovative and generic MH at both 850 mg and 250 mg (Table 1).
Table 1.
Clinical characteristics of included studies.
| Frist author; Publish time; (No.) | Drug (dosage), manufacturer, | Country of trial | Trial | Male (n) | Female (n) | Age (years) | Hight (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|
| Chen L. 2021 (#1) | (1) Generic metformin (250 mg), Penglai Nuokang Pharmaceutical Co., Ltd., China; (2) Glycoran® (250 mg). Nippon Shinyaku CO., LTD., Japan. | China | Fasting | 24 | 8 | 28.0 ± 7.89 | 167.98 ± 8.28 | 62.93 ± 7.22 | 22.31 ± 2.07 |
| Fed | 21 | 11 | 28.3 ± 8.56 | 167.44 ± 7.93 | 62.81 ± 6.94 | 22.42 ± 2.15 | |||
| Gao R. 2021 (#2) | (1) Generic metformin (250 mg), Harbin Zhenbao Pharmaceutical Co., Ltd. China; (2) Glycoran® (250 mg). Nippon Shinyaku CO., LTD., Japan. | China | Fasting | 14 | 10 | 33.83 ± 9.76 | 166.25 ± 6.86 | 67.82 ± 9.6 | 24.45 ± 2.26 |
| Fed | 16 | 8 | 35.88 ± 10.44 | 165.79 ± 9.13 | 64.73 ± 10.01 | 23.5 ± 2.43 | |||
| Huang X. 2020 (#3) | (1) Generic metformin (250 mg), Chongqing Kerui Pharmaceutical (Group) Co., LTD., China; (2) Glycoran® (250 mg). Nippon Shinyaku CO., LTD., Japan. | China | Fasting | 20 | 8 | 25.4 ± 6.4 | 164.57 ± 8.00 | 60.51 ± 7.41 | 22.3 ± 1.94 |
| Fed | 22 | 6 | 25.0 ± 4.7 | 165.21 ± 9.62 | 63.13 ± 9.84 | 23.02 ± 1.93 | |||
| Chen L. 2019 (#4) | (1) Generic metformin (250 mg), Shanxi Ante Bio-pharmaceutical Co., Ltd., China; (2) Glycoran® (250 mg). Nippon Shinyaku CO., LTD., Japan. | China | Fasting | 15 | 9 | 34.4 ± 8.0 | Not reported | Not reported | 23.46 ± 1.89 |
| Fed | 11 | 13 | 40.0 ± 8.1 | Not reported | Not reported | 24.05 ± 2.37 | |||
| Sun ML. To be published (#5, #6) | (1) Generic metformin (250 mg), Beijing Zhongxin Pharmaceutical Group Co., Ltd., China; (2) Glycoran® (250 mg), Nippon Shinyaku CO., LTD., Japan. | China | Fasting | 27 | 9 | 35.58 ± 10.55 | 166.97 ± 9.62 | 65.49 ± 9.79 | 23.42 ± 2.44 |
| Fed | 27 | 9 | 34.78 ± 9.44 | 166.55 ± 7.95 | 66.81 ± 9.43 | 24.06 ± 2.61 | |||
| Sun ML. 2021a, b (#7, #8) | (1) Generic metformin (850 mg), Guangdong Sinocorp pharmaceutical Co., Ltd. China; (2) Glucophage® (850 mg), the Merck UK corporate. | China | Fasting | 26 | 10 | 33.44 ± 8.62 | 166.38 ± 8.78 | 65.68 ± 10.38 | 23.65 ± 2.63 |
| Fed | 25 | 11 | 27.94 ± 6.35 | 165.94 ± 6.79 | 64.99 ± 9.62 | 23.55 ± 2.68 | |||
| Urban LE. 2018 (#9) | Glucophage® (850 mg), Bristol-Myers Squibb Co., USA. | Canada | Fasting + Fed | 12 | 12 | 47 ± 12 | 168 ± 8 | 84 ± 12 | 30 ± 3 |
| Sambol NC. 1996 (#10) | Metformin (850 mg), manufacturer was not reported. | USA | Fasting + Fed | 24 | 0 | 21–35 | NR | NR | NR |
Notes: BMI = body mass index. #, means the serial number of articles included in this study (Supplementary, Appendix 3).
3.2. Quality assessment
The results of the quality assessment are shown in Table 2. Some Concerns were given to the items of “bias due to deviations from intended interventions” because people delivering the interventions were aware of participants’ assigned intervention during the trial as per design of open-label trials in these included studies. As a result, overall bias was assessed as Some Concerns.
Table 2.
Quality assessment of the included studies using Cochrane risk of bias tool.
| Frist author; Publish time | Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | Over-all bias |
|---|---|---|---|---|---|---|
| Chen L. 2021 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Gao R. 2021 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Huang X. 2020 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Chen L. 2019 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Sun ML. 2023 and to be published | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Sun ML. 2021a, 2021b | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Urban LE. 2018 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
| Sambol NC. 1996 | fx1 | fx2 | fx1 | fx1 | fx1 | fx2 |
Notes: Low Risk of bias fx1; Some Concerns fx2.
3.3. Synthesis of main pharmacokinetic and safety parameters
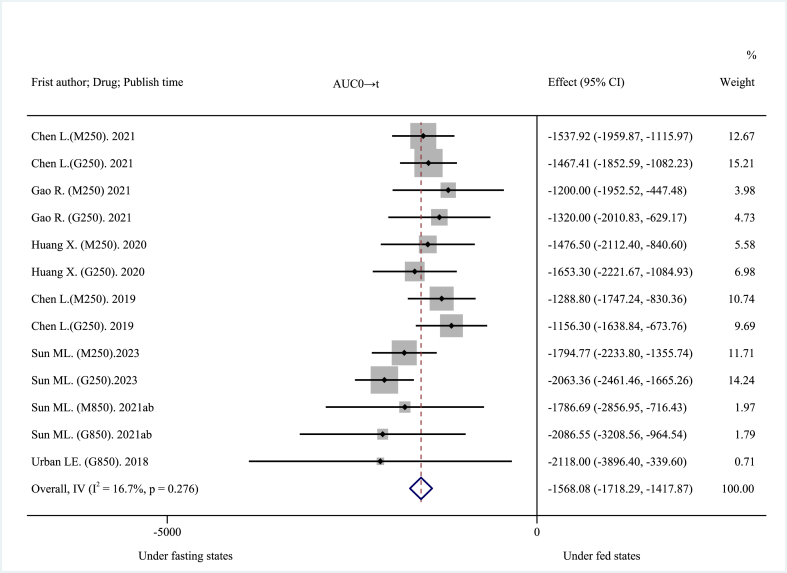
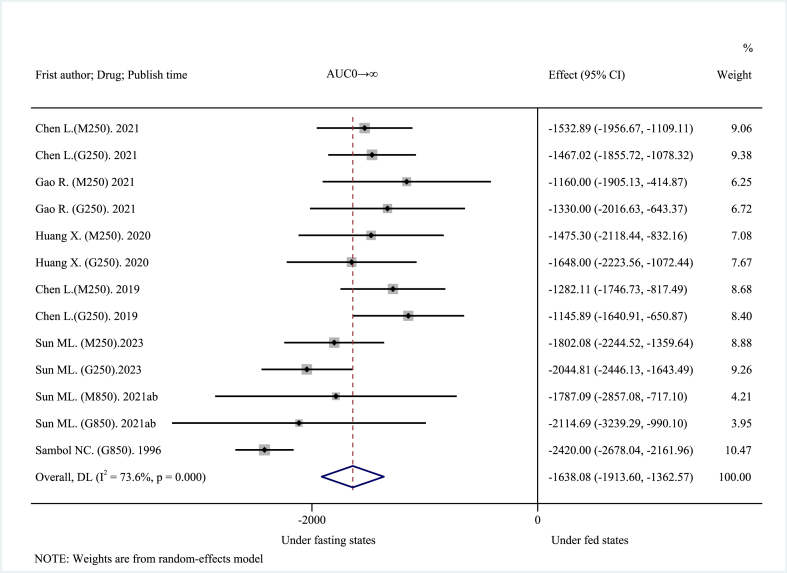
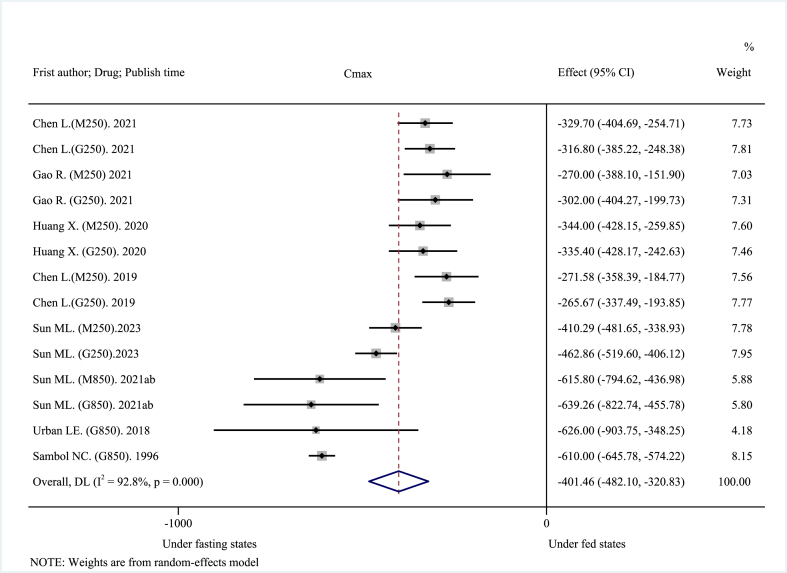
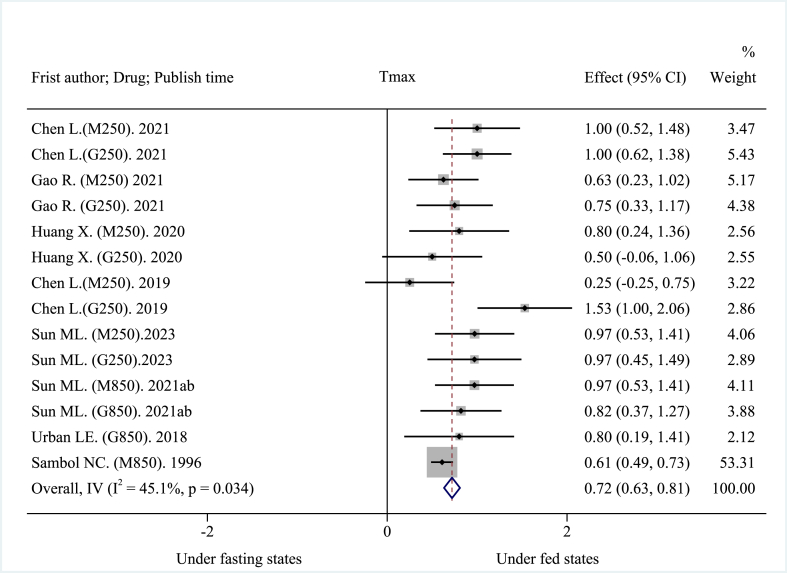
The 14 included clinical trials reported AUC0→t, AUC0→∞, Cmax, and Tmax as main pharmacokinetic outcomes. High-fat, high-calorie diet (about 800–1000 kcal: 150 kcal protein, 250 kcal carbohydrate, and 500–600 kcal fat), USA FDA-recommended standard meals [26] significantly reduced the mean AUC. The pooled AUC0→t was decreased by about 30.21% at −1568.08 ng h mL−1 (95% CI: −1718.29 to −1417.87 ng h mL−1; I2 = 16.7%, p = 0.276; z = −20.461, p < 0.001) (Fig. 2), and the pooled AUC0→∞was decreased by about 28.00% at −1638.08 ng h mL−1 (95% CI: −1913.60 to −1362.57 ng h mL−1; I2 = 73.6%, p < 0.001; z = −11.653, p < 0.001) (Fig. 3). These results suggest that a high-fat, high-calorie diet lowered the extent of absorption of MH. The mean Cmax was decreased by about 40.38% at −401.46 ng mL−1 (95% CI: −482.10 to −320.83 ng mL−1; I2 = 92.8%, p < 0.001; z = −9.758, p < 0.001) (Fig. 4). This result suggests that a high-fat, high-calorie diet reduced the rate of absorption of metformin. The pooled mean Tmax was delayed by about 29.42% at 0.72 h (95% CI: 0.63–0.81 h; I2 = 45.1%, p = 0.034; z = 15.894, p < 0.001) (Fig. 5). This result indicates that a high-fat, high-calorie meal slows the absorption of metformin.
Fig. 2.
Effects of high-fat, high-calorie meals on AUC0→t of metformin hydrochloride tablets.
Fig. 3.
Effects of high-fat, high-calorie meals on AUC0→∞ of metformin hydrochloride tablets.
Fig. 4.
Effects of high-fat, high-calorie meals on Cmax of metformin hydrochloride tablets.
Fig. 5.
Effects of high-fat, high-calorie meals on Tmax of metformin hydrochloride tablets.
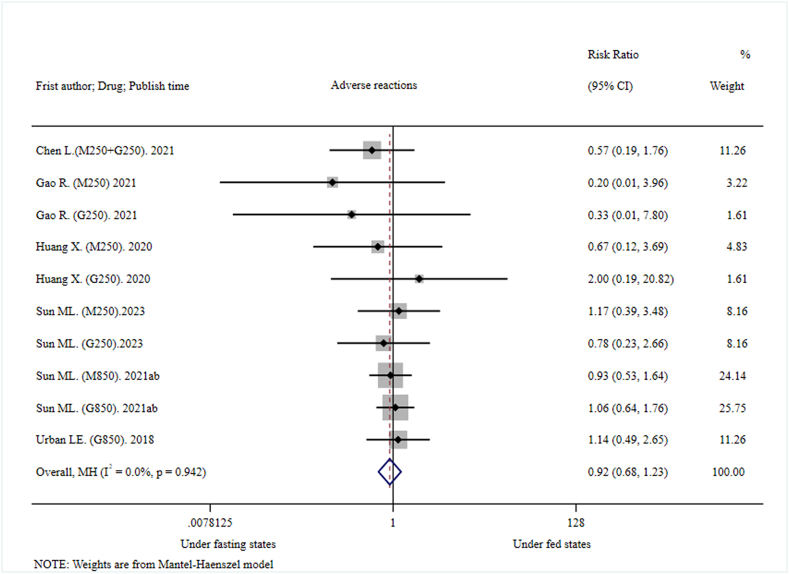
The pooled adverse reaction did not show significant differences under fasting conditions and postprandial conditions (RR = 0.92, 95%CI: 0.68–1.23, I2 = 0.0%; p = 0.942; z = −0.582, p = 0.561) (Fig. 6). There was only one Grade II adverse reaction reported in each of two fasting and three postprandial clinical trials, while the severity of other adverse reactions was Grade I. The pooled Grade II adverse reaction did not show significant difference under fasting conditions and postprandial conditions (RR = 2.35, 95% CI: 0.45–12.19, I2 = 0.0%; p = 0.928; z = 1.017, p = 0.309) (Supplementary, Appendix 4). In all included clinical trials, physical intervention (cold compress, physical cooling) and medication (upper respiratory tract infection) were performed on only two participants with grade II adverse reactions. All adverse reactions recovered except for two participants who were lost to follow-up. There were no unsolicited adverse events or serious adverse events in any of the included clinical trials. It could be safely concluded that MH is safe and well tolerated both under fasting and postprandial conditions. However, it was prudent to infer that there was no significant difference in adverse events between postprandial and fasting trials because of the small sample size.
Fig. 6.
Effects of high-fat, high-calorie meals on safety (adverse reaction) of metformin hydrochloride tablets.
3.4. Investigation of heterogeneity sources with subgroup analysis and meta-regression
Subgroup analysis revealed that different dosage of MH was source of AUC0→∞ heterogeneity (Supplementary, Appendix 5, 5.1–5.5, Figures A–E). The pooled AUC 0→∞ of the low-dose (250 mg) subgroup was decreased by about 30.34% at −1544.50 ng h mL−1 (95% CI: −1699.59 to −1389.42 ng h mL−1; I2 = 29.2%, p = 0.176; z = −19.519, p < 0.001), while the pooled AUC 0→∞ of the high-dose (850 mg) subgroup was decreased by about 23.41% at −2372.39 ng h mL−1 (95% CI: −2617.22 to −2127.56 ng h mL−1; I2 = 0.0%, p = 0.477; z = −18.992, p < 0.001) (Supplementary, Appendix 5, 5.1, Figure A). However, none of different doses or formulations of MH, average age, BMI, or race of participants was attributed to sources of Cmax heterogeneity. (Supplementary, Appendix 6, 6.1–5, Figure A–E).
In the multivariate meta-regression model, heterogeneity of AUC0→∞ was attributed to gender composition of the included studies [tau2 = 0, I2 res = 0.00%, adjusted R2 = 100.00%, Model F (3,9) = 13.67. Coef. = −2854.34 (95% CI: −5087.22 to −621.45), t = −2.89, p = 0.018, in random-effects model]; dosage of MH and gender composition contributed heterogeneity to Cmax [tau2 = 1308, I2 res = 39.60%, adjusted R2 = 91.10%, Model F (3,10) = 19.08. Dosage: Coef. = −0.46 (95% CI: −0.73 –0.19), t = −3.81, p = 0.003; Rate of male: Coef. = −405.80 (95% CI: −795.02 to −16.58), t = −2.32, p = 0.043 in random-effects model].
3.5. Sensitivity analysis
Sensitivity analysis of AUC0→t, AUC0→∞, Cmax, and safety (adverse reactions), using the leave-one-out method revealed that the results obtained when omitting each study were similar to those obtained when all studies were included (Supplementary, Appendix 7, 7.1–7.3 Figure A–C, and 7.5 Figure E). As such, sensitivity analysis indicated that the results were stable. Notably, sensitivity analysis revealed that the result of Tmax was unstable since the result obtained when omitting Sambol NC (M850, 1996) has a significant change from that obtained when all studies were included (Supplementary, Appendix 7, 7.4 Figure D).
3.6. Publication bias
Although the funnel plots look asymmetrical (Supplementary, Appendix 8), the Egger’s regression test (p = 0.896 for AUC0→t, p = 0.051 for AUC0→∞, p = 0.120 for Cmax, p = 0.059 for Tmax, and p = 0.134 for adverse reactions) and Beg’’s test (p = 0.855 for AUC0→t, p = 0.760 for AUC0→∞, p = 0.584 for Cmax, p = 0.913 for Tmax, and p = 0.107 for adverse reactions) reveal that there is no publication bias (Table 3).
Table 3.
Egger linear regression and Begg rank correlation.
| Item | Egger linear regression |
Begg rank correlation |
||||||
|---|---|---|---|---|---|---|---|---|
| Coef. | t | p | 95%CI | Adj. Kendall score (P-Q) | SD | z† | p† | |
| AUC0→t | −0.12 | −0.13 | 0.896 | −2.13–1.88 | −4 | 16.39 | 0.18 | 0.855 |
| AUC0→∞ | 2.84 | 2.19 | 0.051 | −0.02–5.70 | 6 | 16.39 | 0.31 | 0.760 |
| Cmax | 3.58 | 1.67 | 0.120 | −1.08–8.25 | −11 | 18.27 | 0.55 | 0.584 |
| Tmax | 1.26 | 2.08 | 0.059 | −0.06–2.58 | 3 | 18.27 | 0.11 | 0.913 |
| Adverse reactions | −0.58 | −1.67 | 0.134 | −1.38–0.22 | −19 | 11.18 | 1.61 | 0.107 |
Note: AUC = area under the curve, Cmax = peak plasma concentration, Tmax = time to peak plasma concentration. CI = confidence interval, SD = standard deviation of score; †, z and p values were continuity corrected.
4. Discussion
4.1. Summary of main findings
In this study, a high-fat, high-calorie diet decreased AUC0→t, AUC0→∞, and Cmax by approximately 30.21%, 28.00%, and 40.38%, respectively, and delayed Tmax by approximately 29.42%. These findings show that a high-fat, high-calorie meal decreased metformin absorption's extent and rate while also slowing it down. However, the I2 values for pooled AUC0→∞ and Cmax were 73.6% and 92.8%, respectively, which was quite heterogeneous. These findings suggest that it is necessary to increase the dosage of MH in order to maintain the same treatment effect when administration of MH after a high fat, high calorie diet.
Subgroup analysis revealed that different dosage of MH was source of AUC0→∞ heterogeneity. As per the results of meta-regression analyses, gender composition of the included studies was another important source of heterogeneity in AUC0→∞. The meta-regression analyses identified heterogeneity in Cmax was attributed to dosage of MH and gender composition (accounting for 91.10% of heterogeneity). The findings demonstrated that the impact of a high-fat, high-calorie diet on AUC0→∞ and Cmax was more pronounced in the low-dose subgroup than in the high-dose subgroup (AUC0→∞: 30.34% vs. 23.41%; and Cmax: 42.24% vs. 38.25%). However, there was no statistically significant difference when stratified by formulation. Heterogeneity in the primary metformin pharmacokinetic parameters cannot be attributed to age, gender, BMI, and race of participants. The findings demonstrated that there is no significant correlation between the impact of a high-fat, high-calorie diet on the primary metformin pharmacokinetic parameters and gender composition, average age, BMI, race of participants, in the included studies.
In addition, the pooled adverse reaction did not show significant statistical difference under fasting and postprandial conditions (RR = 0.92, 95%CI: 0.68–1.23). It might be attributed to the better safety and tolerance of MH both under fasting and postprandial conditions. In conclusion, the issue of the small sample size should be taken into consideration with caution.
4.2. Comparison with the existing literature
Effects of diet on the pharmacokinetics and safety of MH have been rarely reported. In 2016, National Medical Products Administration (NMPA) required that all generic drugs should be evaluated and compared for consistency in quality and efficacy with the innovative drug, and bioequivalence should be proven under both fasting and postprandial conditions [27,28]. This new regulation promotes research into the effects of diet on the pharmacokinetics and safety of MH.
The instruction manual of Glucophage mentioned that pharmacokinetics parameters of the drug (850 mg) were affected by food, including a 40% lower Cmax, a 25% lower AUC, and a 35-min prolongation of Tmax [17]. Sambol et al. reported that compared with the fasting state, the Tmax was delayed by 37 min, the Cmax was 39% lower, and the AUC was 24% lower when MH (850 mg) was administered after a high-fat, high-calorie breakfast in 24 healthy participants [29]. In the present study, the pooled mean Cmax and AUC0→∞ of the high-dose (850 mg) subgroup were decreased by approximately 38.25% and 23.41%, respectively, and the pooled mean Tmax was delayed by approximately 0.65 h (95% CI: 0.54–0.76 h) (our calculation results). The results of this study were generally consistent with the instruction manual of Glucophage and the study by Sambol and his colleagues.
Huang et al. reported that when comparing the main pharmacokinetics parameters in both fed and fasting states, Tmax of MH (0.25 g) produced by Chongqing Kerui Pharmaceutical (Group) Co., Ltd. And Glycoran (0.25 g) manufactured by Nippon Shinyaku Co., Ltd. in Japan was delayed by approximately 1 h and 0.5 h, respectively, the mean Cmax was decreased by about 41%, and the mean AUC of the two formulations was significantly reduced by about 28% and 31%, respectively [19]. In the present study, the pooled mean Tmax of the low-dose (250 mg) subgroup was delayed by about 0.84 h (95% CI: 0.69–0.99 h)(our calculation results), and the pooled mean Cmax, AUC0→t, and AUC0→∞ were decreased by approximately 42.24%, 30.78%, and 30.34%, respectively, under postprandial conditions compared with those observed under the fasting conditions. The results of this study were consistent with those of Huang et al., which suggests that a high-fat, high-calorie diet may lower the extent and rate of absorption of low-dose of MH (250 mg). Pharmacokinetic parameters of the drug were affected by food.
The Instruction manual mentioned that Glucophage should be taken with meals in order to reduce its side effects [17]. However, in the present study, the pooled adverse reaction did not show a significant difference under fasting conditions and postprandial conditions (RR = 0.92, 95%CI: 0.68–1.23). In our opinion, this observation may be related to the higher safety and well tolerance of MH both under fasting and postprandial conditions. MH does not generally cause hypoglycemia in healthy participants since it increases endogenous glucose production in non-diabetic individuals [[30], [31]]. The main side effects of MH are gastrointestinal reactions [30]. However, caution is needed regarding this conclusion due to the small sample size of the study.
4.3. Strength and limitations
Although there have been many bioequivalence trials of MH [[32], [33], [34], [35], [36], [37], [38], [39]], few studies have addressed the effects of food on MH metabolism and safety [19,29]. The NMPA requires that the consistency evaluation of generic drugs must demonstrate bioequivalence and safety in both fasting and postprandial conditions before approval, which makes it easier to study the effects of diet on the pharmacokinetics and safety of MH [,27,28]. The strength of this study is that it is the first systematic review and meta-analysis of the effects of high-fat, high-calorie meals on the pharmacokinetics and safety of MH. The effects of diet on the pharmacokinetics and safety of MH were confirmed by scientific methods and rigorous logical reasoning.
The instructions for MH call for simultaneous use with food, and thus the issue of the effects of food on pharmacokinetics (ADME) and the safety of MH is particularly important for patients with type 2 diabetes and endocrinologists. This study suggest that it is necessary to increase the dosage of MH in order to maintain the same treatment effect when administration of MH after a high fat, high calorie diet. The results of this study have guiding significance for MH in patients with type 2 diabetes.
There were several limitations in this study, which include the following: (1) Currently, there is not enough of randomized, two-period, two cross-over, single-dose clinical trials under fasting and postprandial conditions in the same healthy adult participants. We had to conduct systematic reviews and meta-analyses using contemporaneous clinical trials of pharmacokinetic, bioavailability, or bioequivalence and safety under fasting and postprandial conditions in the participants without significant statistical differences of demographic characteristics at the baseline. (2) The sample size included in this study is small. (3) Although FDA-approved dosages for MH are 500 mg, 850 mg and 1000 mg, the formulations of 500 mg and 1000 mg were not included in this study due to the lack of pharmacokinetic and safety data under both fasting and postprandial conditions [35]. Hopefully, more clinical data will fill in the gaps. (4) In addition to high-fat and high-calorie meals, there are also low-protein, low-fat, and low-carbohydrate diets [40]. As FDA defined a high-fat, high-calorie diet as the standard diet in phase I clinical trials [26], there is currently a lack of the effects of low-protein, low-fat, and low carbohydrate diet on the pharmacokinetics and safety of MH in healthy volunteers. (5) Due to language limitations, this study only included studies written in English and Chinese. Most of the clinical trials we included were conducted in China. (6) Sensitivity analysis revealed that the result of Tmax was unstable. Study of #10 differs from other studies in that only male participants, 4-period crossover trial (similar to study of # 9), low Tmax variability, and other potential factors may be responsible for the instability of Tmax.
5. Conclusions and implications
Pharmacokinetics parameters of MH were affected by food. High-fat, high-calorie diet lowered the extent and rate of absorption, while slowing the absorption of metformin. The dosage and gender impact the effects of high-fat, high-calorie diet on AUC0→∞ and Cmax. These findings suggest that it is necessary to increase the dosage of MH in order to maintain the same treatment effect when administration of MH after a high fat, high calorie diet.
Food had little effect on the safety of MH, which may be related to the fact that the drug was well tolerated and safe under both fasting and postprandial conditions. However, caution is needed regarding this conclusion due to the small sample size of the study.
Author contribution statement
Ming-Li Sun, Fang Liu: Conceived and designed the study; Contributed materials; Wrote the paper. Ping Yan: Conceived and designed the study; Analyzed and interpreted the data, analysis tools or data; Wrote the paper. Wei Chen: Analyzed and interpreted the data; analysis tools or data; Wrote the paper. Xing-He Wang: Contributed materials, Analyzed and interpreted the data; Wrote the paper. Ming-Li Sun, Fang Liu, Ping Yan, Wei Chen, Xing-He Wang: Performed the experiments.
Data availability statement
Data included in article/supp. material/referenced in article.
Ethics declarations
This research is exempt from ethics approval because the work is carried out on published documents.
Funding
This work was supported by the Open Research Funding of Central Laboratory, Beijing Shijitan Hospital Affiliated to Capital Medical University [Grant No. 2020-KF28], Phase 0 Platform Construction Project Funded by Beijing Municipal Commission of Science and Technology [Grant No. 2018LQYJ], and National Key Research and Development Program of China (Grant No. 2020YFC2005403). The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the Article.
Data sharing statement
The supplementary data can be found in the Supplemental Materials section of the online article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank deputy chief pharmacist Rong Gao, the first author of the study listed in supplementary, appendix 3, #2, for providing more information about the adverse drug reactions in the study. We also appreciate the assistance and support provided by Dr. Xiao-Yun Liu in the statistical process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17906.
Contributor Information
Ming-Li Sun, Email: sunmingli@bjsjth.cn.
Wei Chen, Email: hanwa63@126.com.
Xing-He Wang, Email: wangxh@bjsjth.cn.
List of Abbreviations
- AMPK
AMP activated protein kinase
- AUC
area under the curve
- ADME
drug Absorption, Distribution, Metabolism, and Excretion
- BMI
body mass index
- CENTRAL
Cochrane Central Register of Controlled Trials Library
- CI
confidence interval
- Cmax
peak plasma concentration
- CNKI
China National Knowledge Infrastructure
- MATE
the multidrug and toxin extrusion
- MH
metformin hydrochloride
- NMPA
National Medical Products Administration
- OCTs
the organic cation transporters
- PK
pharmacokinetics
- PMAT
the plasma membrane monoamine transporter
- PRISMA
the Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROSPERO
the International Prospective Register of Systematic Reviews
- RR
risk ratio = relative risk
- SMD
standardized mean difference
- Tmax
time to peak plasma concentration
- WMD
the weighted mean difference
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Amanat S., Ghahri S., Dianatinasab A., Fararouei M., Dianatinasab M. Exercise and type 2 diabetes. Adv. Exp. Med. Biol. 2020;1228:91–105. doi: 10.1007/978-981-15-1792-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Wang H., Tang C., Gao Z., Huang Y., Zhang B., Wei J., Zhao L., Tong X. Potential role of natural plant medicine cyclocarya paliurus in the treatment of type 2 diabetes mellitus. J. Diabetes Res. 2021;2021 doi: 10.1155/2021/1655336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Peng W., Zhao Z., Zhang M., Shi Z., Song Z., Zhang X., Li C., Huang Z., Sun X., Wang L., Zhou M., Wu J., Wang Y. Prevalence and treatment of diabetes in China, 2013-2018. JAMA. 2021;326:2498–2506. doi: 10.1001/jama.2021.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDF . International Diabetes Federation; Brussels: 2022. Atlas 10th Edition [Internet]. C1950-2022.https://diabetesatlas.org/atlas/tenth-edition/ [accessed Dec 6, 2021]. [cited Apr 07, 2022]. Available from: [Google Scholar]
- 5.Moran G.M., Bakhai C., Song S.H., Agwu J.C., Guideline Committee Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Professional Practice Committee 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Professional Practice Committee Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144–S174. doi: 10.2337/dc22-S010. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y.F. Continuous improvement of patient-centered type 2 diabetes management strategy:a brief introduction and comment of Standards of Medical Care in Diabetes-2022. Zhonghua Nei Ke Za Zhi. 2022;61:363–366. doi: 10.3760/cma.j.cn112138-20220123-00075. Chinese. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital) Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition) Zhonghua Nei Ke Za Zhi. 2022;61:12–50. doi: 10.3760/cma.j.cn112138-20211027-00751. Chinese. [DOI] [PubMed] [Google Scholar]
- 10.Flory J., Lipska K. Metformin in 2019. JAMA. 2019;321:1926–1927. doi: 10.2337/dc22-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foretz M., Guigas B., Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 12.LaMoia T.E., Shulman G.I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horakova O., Kroupova P., Bardova K., Buresova J., Janovska P., Kopecky J., Rossmeisl M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019 Apr 16;9(1):6156. doi: 10.1038/s41598-019-42531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheen A.J. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 15.Pentikäinen P.J., Neuvonen P.J., Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur. J. Clin. Pharmacol. 1979;16:195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Zhou J., Xi M., Jia Y., Wong Y., Zhao J., Ding L., Zhang J., Wen A. Pharmacogenetic variation and metformin response. Curr. Drug Metabol. 2013;14:1070–1082. doi: 10.2174/1389200214666131211153933. [DOI] [PubMed] [Google Scholar]
- 17.Instruction manual of Glucophage . Bristol-Myers Squibb Company; New York: 2022. Last Reviewed on RxList: 5/20/2020 [Internet]. C1995-2022.https://www.rxlist.com/lucophage-drug.htm#indications [accessed May 20, 2020]. [cited 2022.04.07]. Available from: [Google Scholar]
- 18.Instruction Manual of Metformin Hydrochloride Tablets (Glycoran Tablets 250 Mg) [Internet]. C1911-2022. Nippon Shinyaku CO. LTD.; Kyoto: 2022. https://zy.yaozh.com/data/pdf/00052403.pdf [accessed Oct 2020]. [cited 2022.04.07]. Available from: [Google Scholar]
- 19.Huang X.M., Wang G.Z., He B.B., Gao T., Long P., Zhang B.K. Bioequivalence and pharmacokinetic evaluation of two metformin hydrochloride tablets under fasting and fed conditions in healthy Chinese volunteers. Clin Pharmacol Drug Dev. 2020;9:910–917. doi: 10.1002/cpdd.849. [DOI] [PubMed] [Google Scholar]
- 20.Urban L.E., Audet D., Ron E.S., Sannino A., Zohar Y., Demitri C., Panteca E., Surano I., Heshmati H.M. Effect of a nonsystemic, orally administered hydrogel, GS100, on metformin pharmacokinetics. Can. J. Physiol. Pharmacol. 2018;96:1127–1131. doi: 10.1139/cjpp-2018-0123. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Green S. The Cochrane Collaboration; Oxford: 2022. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Internet]. C1993-2022.https://handbook-5-1.cochrane.org/ [accessed 2011]. [cited Apr 7, 2022]. Available from: [Google Scholar]
- 23.NMPA . 2016. Opinions of the General Office of the State Council of the People’s Republic of China on Consistency Evaluation of Quality and Efficacy of Generic Drugs (No. 8 of 2016)https://www.nmpa.gov.cn/zhuanti/ypqxgg/ggzhcfg/20160305170501942.html accessed 15 March 2016. [Google Scholar]
- 24.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in andomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Assessing the Effects of Food on Drugs in INDs and NDAs — Clinical Pharmacology Considerations Guidance for Industry [Internet]. C1906-2022. Food and Drug Administration Center; Virginia: 2022. https://www.fda.gov/media/121313/download [accessed Feb 2019]. [cited Apr 07, 2022]. Available from: [Google Scholar]
- 27.NMPA . National Medical Products Administration; Beijing: 2022. Opinions of the General Office of the State Council on the Consistency Evaluation of Quality and Efficacy of Generic Drugs (No. 8 of 2016) [Internet]. C1978-2022.https://www.nmpa.gov.cn/xxgk/fgwj/qita/20160305170501219.html [accessed Mar 5, 2018]. [cited Apr 07, 2022]. Available from: [Google Scholar]
- 28.NMPA . National Medical Products Administration; Beijing: 2022. Circular of NMPA on Issuing Statistical Guidelines for Bioequivalence Studies (No. 103 of 2018) [Internet]. C1978-2022.https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20181029173101911.html [accessed Oct 29, 2018] [cited Apr 07, 2022]. Available from: [Google Scholar]
- 29.Sambol N.C., Brookes L.G., Chiang J., Goodman A.M., Lin E.T., Liu C.Y., Benet L.Z. Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br. J. Clin. Pharmacol. 1996;42:510–512. doi: 10.1111/j.1365-2125.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 30.Domagała-Rodacka R., Cibor D., Szczeklik K., Rodacki T., Mach T., Owczarek D. Gastrointestinal tract as a side-effect target of medications. Przegl. Lek. 2016;73:652–658. PMID: 29688675. [PubMed] [Google Scholar]
- 31.Gormsen L.C., Søndergaard E., Christensen N.L., Brøsen K., Jessen N., Nielsen S. Metformin increases endogenous glucose production in non-diabetic individuals and individuals with recent-onset type 2 diabetes. Diabetologia. 2019;62:1251–1256. doi: 10.1007/s00125-019-4872-7. [DOI] [PubMed] [Google Scholar]
- 32.Al Hawari S., AlGaai E., Yusuf A., Abdelgaleel A., Hammami M.M. Bioequivalence study of two metformin formulations. Arzneimittelforschung. 2007;57:192–195. doi: 10.1055/s-0031-1296605. [DOI] [PubMed] [Google Scholar]
- 33.Atanasova I., Bozhinova K., Todorova D., Terziivanov D. Pharmacokinetics and comparative bioavailability of two metformin formulations after single-dose administration in healthy subjects. Clin. Drug Invest. 2003;23:743–749. doi: 10.2165/00044011-200323110-00007. [DOI] [PubMed] [Google Scholar]
- 34.Holguín G., Cuesta F., Archbold R., Restrepo M., Parra S., Peña L., Montoya B., Ríos J.C., Toro V.E., Ruiz A. Bioavalaibility and pharmacokinetic comparison of two formulations of metformin 850 mg tablets in healthy Colombian volunteers. Colomb. Méd. 2011;1:81–87. [Google Scholar]
- 35.Najib N., Idkaidek N., Beshtawi M., Bader M., Admour I., Alam S.M., Zaman Q., Dham R. Bioequivalence evaluation of two brands of metformin 500 mg tablets (Dialon & Glucophage)—in healthy human volunteers. Biopharm. Drug Dispos. 2002;2:301–306. doi: 10.1002/bdd.326. [DOI] [PubMed] [Google Scholar]
- 36.Noh Y.H., Lim H.S., Jung J.A., Jin S.J., Kim M.J., Kim Y.H., Park H.J., Bae K.S. A single-dose, crossover study comparing the pharmacokinetics and pharmacodynamics of 2 formulations of metformin in healthy volunteers. Int. J. Clin. Pharm. Ther. 2012;50:605–613. doi: 10.5414/CP201715. [DOI] [PubMed] [Google Scholar]
- 37.Valizadeh H., Nayyeri-Maleki P., Ghanbarzadeh S., Sheikhloo A., Servat H., Nemati M., Zakeri-Milani P. Pharmacokinetics and bioequivalence of two brands of metformin 500 mg tablets in Iranian healthy volunteers. Int J Pharm Investig. 2014;1:61–68. [Google Scholar]
- 38.Yuen K.H., Wong J.W., Billa N., Julianto T., Toh W.T. Bioequivalence of a generic metformin tablet preparation. Int. J. Clin. Pharm. Ther. 1999;37:319–322. PMID: 10442505. [PubMed] [Google Scholar]
- 39.Garza-Ocañas L., González-Canudas J., Tamez-dela O.E., Badillo-Castañeda C., Gómez-Meza M.V., Romero-Antonio Y., Molina-Pérez A., Amador-Hernández A.G. Comparative bioavailability of metformin hydrochloride oral solution versus metformin hydrochloride tablets in fasting Mexican healthy volunteers. Adv. Ther. 2019;36:407–415. doi: 10.1007/s12325-018-0853-3. [DOI] [PubMed] [Google Scholar]
- 40.Saffar F., Aiache J.M., Andre P. Influence of food on the disposition of the antidiabetic drug metformin in diabetic patients at steady-state. Methods Find Exp. Clin. Pharmacol. 1995;17:483–487. PMID: 8577211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.