Abstract
Curculigo latifolia is a plant in the Hypoxidaceae family commonly used in herbal medicine. The study objective was to evaluate the antioxidant and anti-elastase properties of C. latifolia extracts in vitro and silico as a candidate for antiaging active ingredients. This study identified secondary metabolites of the hexane (HE), ethyl acetate (EAE), and ethanol extracts (EE) from the root (R), stem (S), and leaf (L) organs by LC-ESI-MS and evaluated in vitro antioxidant and inhibitor elastase activity. An antioxidant evaluation was performed using ABTS, Beta Carotene Bleaching (BCB), and Ferric Reduction Antioxidant Power (FRAP). Evaluation of anti-elastase was carried out using elastase and followed by an in silico study of molecular docking using the target protein elastase (1B0F). Fifteen C. latifolia metabolites were identified in C. latifolia extracts, most of which were phenolic compounds. In antioxidant testing, REE, REAE, SEE, and SEAE extracts showed potent antioxidant activity based on the ABTS, BCB, and FRAP methods. In anti-elastase testing, it was found that SEE, REE, REAE, and RHE extracts gave powerful inhibition of elastase activity (in the ranges of 16.89 to 27.91 µg/mL). The in-silico study demonstrated the potential of the identified metabolites to bind to the target protein 1B0F involved in remodeling the skin aging process. This research concludes that the extracts from C. latifolia have the potential to serve as an active antiaging source.
Keywords: Anti-elastase, ABTS, Curculigo latifolia, LC-ESI-MS, Lipid peroxidation, Molecular docking, Reduction power
1. Introduction
Aging is a complex and continuous biological system involving changes in the cellular and molecular structure of the body. Intrinsic and extrinsic factors influence the maturation process of the skin. As the body ages, the intrinsic factor causes fine wrinkles and a thinned epidermis (Purohit et al., 2016, Zhang and Duan, 2018). Contrary to internal aging, extrinsic aging leads to deep wrinkles, weak skin structure, and hyperpigmentation, primarily due to prolonged sun exposure (Lago and Puzzi, 2019, Xia et al., 2015). As the skin ages, wrinkles appear, and the skin's elasticity is diminished, resulting in progressive dermal atrophy. Several factors contribute to the development of dermal atrophy, including a decrease in the extracellular matrix (ECM), which includes collagen and elastic fibers (Lukitaningsih et al., 2020).
Elastin is an ECM fibrous protein that plays a role in providing elasticity and repair to tissues. Collagen is a fundamental molecular unit in forming skin tissue produced from procollagen. In the dermis, dermal fibroblasts produce procollagen under the influence of transforming growth factor-β (TGF-β) and activator protein-1 (AP-1), where TGF-β regulates collagen production, and AP-1 regulates collagen breakdown. The presence of intrinsic and extrinsic effects, such as chronic UV radiation, promotes the breakdown and decreased protein synthesis due to the upregulation of matrix metalloproteinases (MMPs), including the decrease in elastase synthesis. This process causes tissue damage during photoaging and premature aging (Jadoon et al., 2015, Varani et al., 2000).
An increase in skin damage necessitates chemoprevention and therapy strategies. One promising strategy is by utilizing natural materials. In today's era, treatment based on natural materials is increasingly popular. Most people feel safe in its use due to the minimal side effects. A study by WHO stated that about 80% of the world population strongly believes in herbal medicine because of the therapeutic effects of natural ingredients. The increasing use of natural ingredients in medicine encourages investigation of chemical compounds that can provide pharmacological potential (Saleem et al., 2021, Zengin et al., 2018).
Curculigo latifolia is a plant from the Hypoxidaceae family widely distributed in China, Japan, Nepal, Malaya, India, Australia, and Africa. The original distribution of this plant comes from India, Myanmar, Thailand, Peninsular Malaysia, Singapore, Philippines and some parts in Indonesia such as Sumatra, Lingga Islands, Bangka Island, Borneo, Java, and Celebes (Wang et al., 2021). Scientifically, C. latifolia has been found to possess antioxidant, anti-diabetic, and antibacterial properties (Farzinebrahimi et al., 2016, Ooi et al., 2018, Umar et al., 2021). The bioactivities of C. latifolia are strongly supported by the content of secondary metabolites found in these plants. Previous reports showed that the roots of C. latifolia contain phenolic compounds like phloridzin, pomiferin, scandenin, and mundulone through the LC-MS analysis (Zabidi et al., 2019). As reported in a previously study, C. latifolia contains phenolic glycosides such as orchioside derivatives, curculigoside derivatives, and triterpenes (cycloartane) curculigosaponin derivatives determined based on the UHPLC-Q-Orbitrap HRMS. The leaves were reported to contain phenolic glycoside and curculigosaponin products (cycloartan triterpenes) (Umar et al., 2021).
Although C. latifolia has been reported to have various pharmacological effects, this study explored the antiaging effect through antioxidant activity and elastase inhibition. Some previous information stated that the leaf and root extracts of C. latifolia had antioxidant effects but were carried out with limited methods. This study explored the antioxidant activity using the ABTS radical reduction method, lipid peroxidation (BCB assay), and iron reduction (FRAP Assay). Antioxidant compounds in C. latifolia extract have a role in reducing the activity of skin-degrading enzymes, one of which is elastase. A study of the phytochemical content by LC-ESI-MS in the root, stem, and leaf extracts of C. latifolia was also conducted in order to enrich the data information and study its elastase inhibitory activity with an in silico approach through molecular docking.
2. Materials and methods
2.1. Plant collection and extraction
C. latifolia plant was obtained from the Research Institute for Spices and Medicinal Plants (BALITTRO), Bogor City, West Java, Indonesia (-6.576994315224358° N, 106.78637511741873° E). The specimen of C. latifolia was identified by Dr. Anang Setiawan Ahmad and stored at the Herbarium Bogoriense, Center for Biological Research, National Research and Innovation Agency (BRIN), Cibinong, West Java, with voucher code B-132/V/DI.05.07. Plant parts of C. latifolia were (the roots, stems, and leaves) cleaned and dried at 40 °C (72 h). Dry materials were powdered and extracted by maceration in stages with solvent variations starting from non-polar to polar solvents (n-hexane, ethyl acetate, and 70% ethanol, respectively). Solvents from each extract was evaporated to obtain n-hexane extract of root (HRE), stem (HSE), and leaf (HLE). Ethyl acetate extract of root (REAE), stem (SEAE), leaf (LEAE). Ethanol extracts of roots (REE), stems (SEE), and leaves (LEE). The extracts obtained were then subjected to further testing.
2.2. Phytochemical screening by LC-ESI-MS
The extracts of C. latifolia were analysed by LC-ESI-MS. LC-ESI-MS analysis was carried out using Waters Acquity UPLC I-Class and XEVO G2-XS QTof. Chromatographic separation was performed using an ACQUITY UPLC® BEH C18 (1.7 m, 2.1 x50 mm). The mobile phase used a mixture of A (H2O + 0.1% Formic Acid) and B: (ACN + 0.1% Formic Acid) with gradient concentration. The ionization was carried out in a positive ion mode in m/z range 100–1200 amu, with a capillary voltage of 2 kV and the source temperature of 120 °C, the desolvation temperature of 500 °C. The fragmentation amplitude was set to 40 eV, and MS2 data were acquired in positive ion mode (Hanafi et al., 2018).
2.3. Antioxidant analysis
The antioxidant activity of each extract was analyzed with different methods including ABTS (Nur et al., 2021b), lipid peroxidation by beta-carotene bleaching (Nur et al., 2021a), and FRAP assays (Dravie et al., 2020).
2.3.1. Radical ABTS reduction assay
A radical scavenging method based on ABTS was modified by Nur et al. (2021b). A mixture of ABTS (7 mM in 10 mL distillate water) and potassium persulfate (2.45 mM in 10 mL distillate water) was incubated for 12–16 h in the dark to form an ABTS radical solution. The solution mixture was then diluted with ethanol to 50 mL. A working solution of ABTS was prepared by mixing it with ethanol (1:10). 1 mL of ABTS working solution was diluted to 5 mL with ethanol and incubated for 30 min as a blank solution. The absorbance was measured with a UV–visible spectrophotometer (745 nm; Shimadzu UV-1900). The serial volumes of each C. latifolia extract were reacted with 1 mL of ABTS working solution, and the volumes were made up to 5 mL with ethanol. An absorbance measurement at 745 nm was performed after incubating the mixture for 30 min in the dark at room temperature. The positive control in this study used standard quercetin and ascorbic acid.
IC50 values were calculated by comparing the percentage of inhibition of ABTS radicals across different concentrations of sample solution with the formula:
% Inhibition = (Abs. blank – Abs. sample/Abs. blank) × 100%.
2.3.2. Lipid peroxidation inhibition by beta carotene bleaching (BCB) assay
Assays for BCB were performed in accordance with Nur et al. (2021a) with slight modifications. To create beta carotene emulsion (BCE), beta carotene powder (20 mg) was dissolved in chloroform (0.2 mL), followed by linoleic acid (0.2 mL) and tween 20 (2 mL). After mixing well, the remaining chloroform was evaporated. To form a transparent emulsion, the mixture was diluted with distilled water to 100 mL. A serial volume of each extract solution was prepared by mixing 1 mL of BCE with 5 mL of ethanol. After incubation at 50 °C for 20 and 120 min, the absorbance at 461 nm was measured by a spectrophotometer (Shimadzu UV-1900). A blank solution was prepared (1 mL of BCE in 5 mL of ethanol), and its absorption was measured in the same procedure. Quercetin and butyl hydroxytoluene (BHT) were used as positive controls.
The difference between the degradation of the sample and a blank solution was used to calculate antioxidant activity. The absorbance results were then processed to find the rate of degradation based on the formula (ln (a/b) × 1/t), where Ln is a corresponds to the natural logarithm, a is an absorbance value after 20 min of incubation, b is an absorbance value after 120 min, and t is the incubation time (120 min). IC50 values are determined by calculating the inhibition of lipid peroxidation at each concentration of sample solution using the formula:
2.3.3. FRAP (Ferric reducing antioxidant power) assay
This study involved the reduction of iron using the FRAP method (Dravie et al., 2020). FRAP reagent was made by reacting acetate buffer (pH 3.6), Tris Pyridyl Triazine (TPTZ, 1 mM), and FeCl3 (0.02 M) in a ratio of 10:1:1. Each extract solution (0.1% w/v) was mixed with 2 mL of FRAP reagent, and distilled water was added to make up the volume (5 mL). Incubation at room temperature for 30 min was followed by an absorbance measurement at 595 nm (Shimadzu UV-1900). The FRAP value for each extract was calculated using quercetin as the standard curve. The quercetin equivalent value per gram of extract (µMQEV/g extract) was used to estimate its antioxidant capability (Nur et al., 2019).
2.4. Elastase inhibition assay
Testing the elastase inhibitory activity of each extract of C. latifolia according to the modified assay protocol based on several studies conducted by Anggraini et al., 2020, Elgamal et al., 2021 dan Putri et al. (2019). Each extract of C. latifolia (10 mg) was weighed and dissolved in tris-buffer HCl up to 10 mL (1000 µg/mL). A concentration of 10–1000 µg/mL of the test solution was achieved by diluting the sample solution in series. As much as 20 µL of sample solution (10–1000 µg/mL) was inserted into 96 wells, added 120 µL of Triz-HCl buffer pH 8 to the well. The test solutions were added to 20 µL (0.22 U/mL) elastase (Sigma Aldrich SLBV 9311) and then incubated for 20 min at 25 °C. After incubation, the mixture was added of N-succinyl-(Ala-Ala-Ala) nitroanilide of 30 µL (Sigma Aldrich, S4760) to the well plate. After incubating at 25 °C for 50 min, the absorbance was measured at 405 nm with a 96-well Microplate Reader (Promega Glomax) (Anggraini et al., 2020).
2.5. In silico study by docking molecular
Molecular docking analysis of the fifteen compounds produced by the LC-ESI-MS analysis was carried out using the procedure described by Nursamsiar et al. (2020). In silico analysis of this enzyme's structure was conducted using the protein data bank (https://www.rcsb.org/structure/1B0F). Results of molecular docking were analysed regarding various evaluation parameters, i.e., the orientation of the ligand structure, hydrophobic interactions, hydrogen bonds formed, and the value of free energy from each ligand.
2.6. Data analysis
In vitro experiments were performed three times, with results reported as mean ± SD. To determine the significance level of the mean values, a one-way ANOVA was performed (Tukey HSD). The correlation between the antioxidant activity of each sample against elastase inhibition was analyzed through Pearson's correlation (Minitab 20 version). The in silico models were analyzed by Autodock Tools 4.2 program package.
3. Result
3.1. Extraction yields
The extraction process of plant organs of C. latifolia was carried out by maceration using solvents of different polarity to obtain their phytochemical profile. Based on the “like dissolved like” principle, n-hexane, ethyl acetate, and ethanol (70% v/v) were used to extract compounds that have low, semi, and high polarity from each plant organ, respectively (Chormey and Bakirdere, 2018, Zhuang et al., 2021). The ability of the solvents to extract compounds can be determined based on the yield percetages obtained. Results show that compared to n-hexane, ethyl acetate, and ethanol (70%v/v) gave the highest yield, obtaining yields of 48.32%, 46.75%, and 34.46%, from roots, stems, and leaves, respectively.
3.2. Phytochemical screening
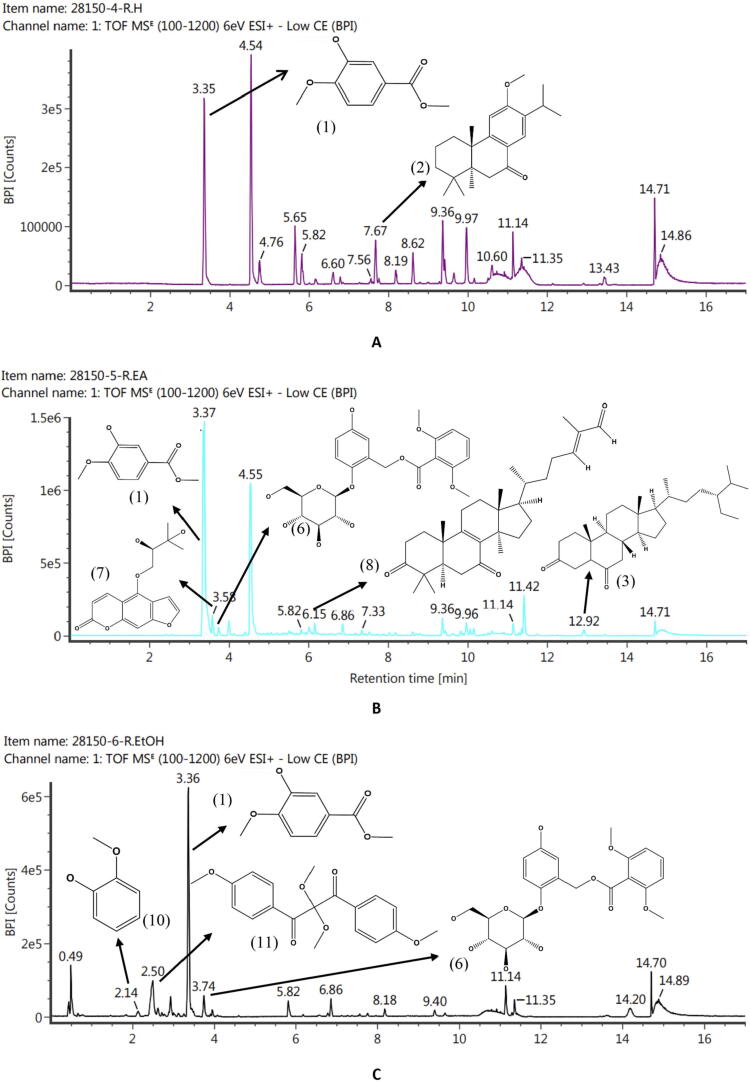
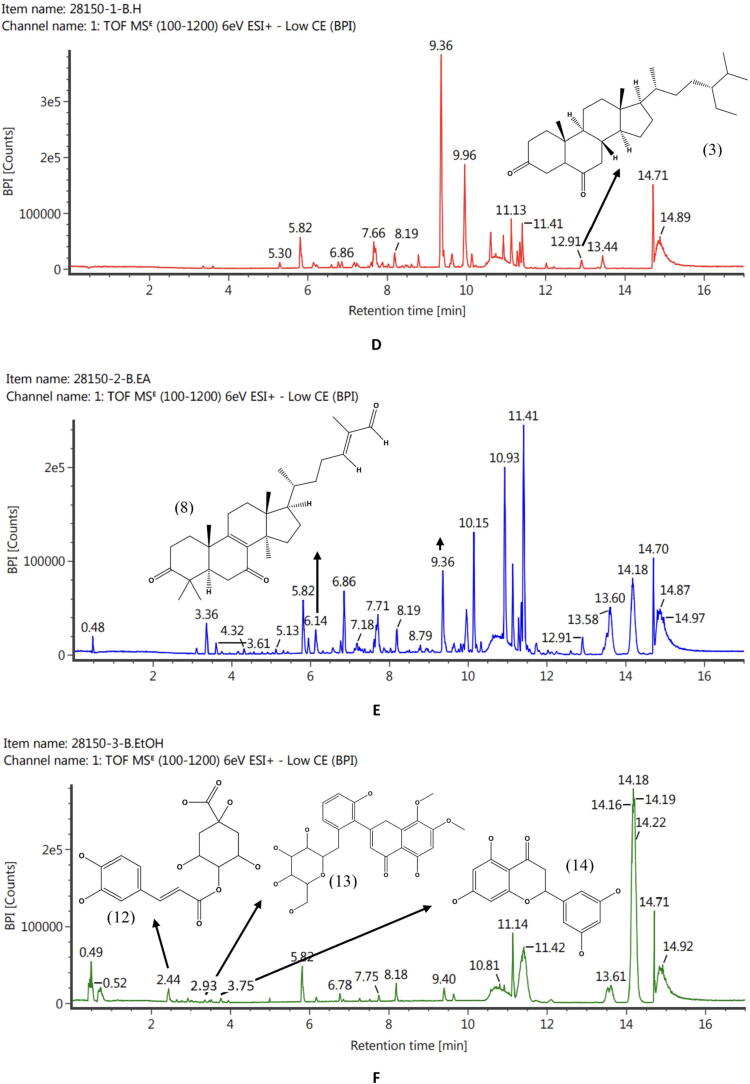
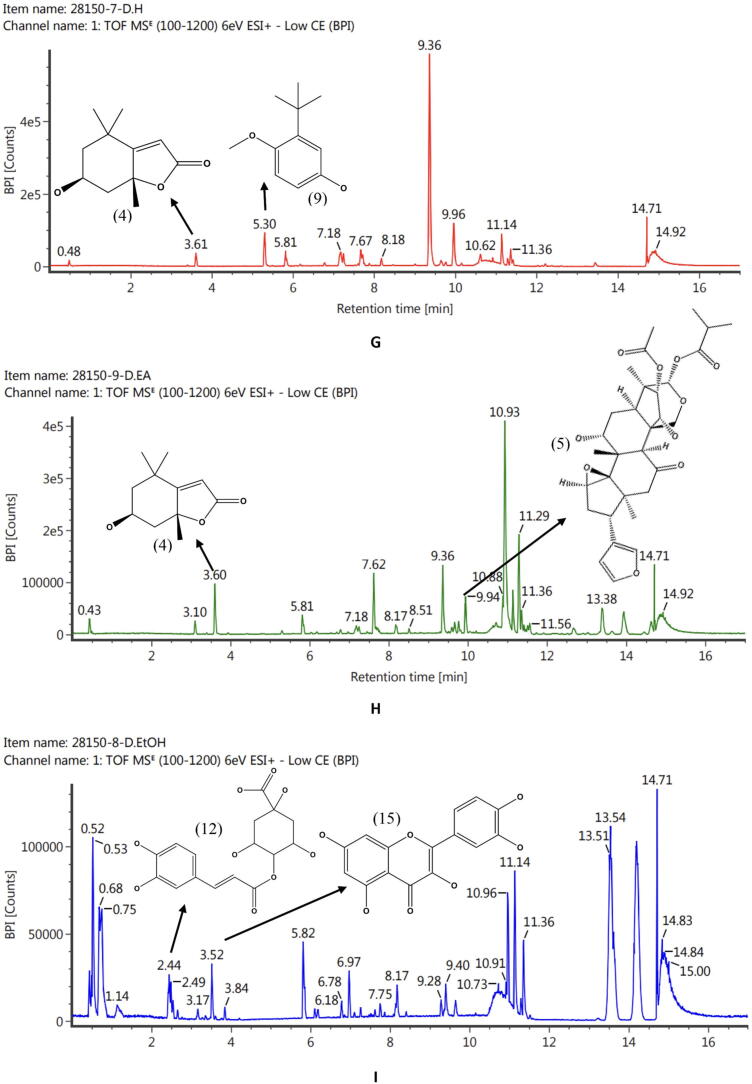
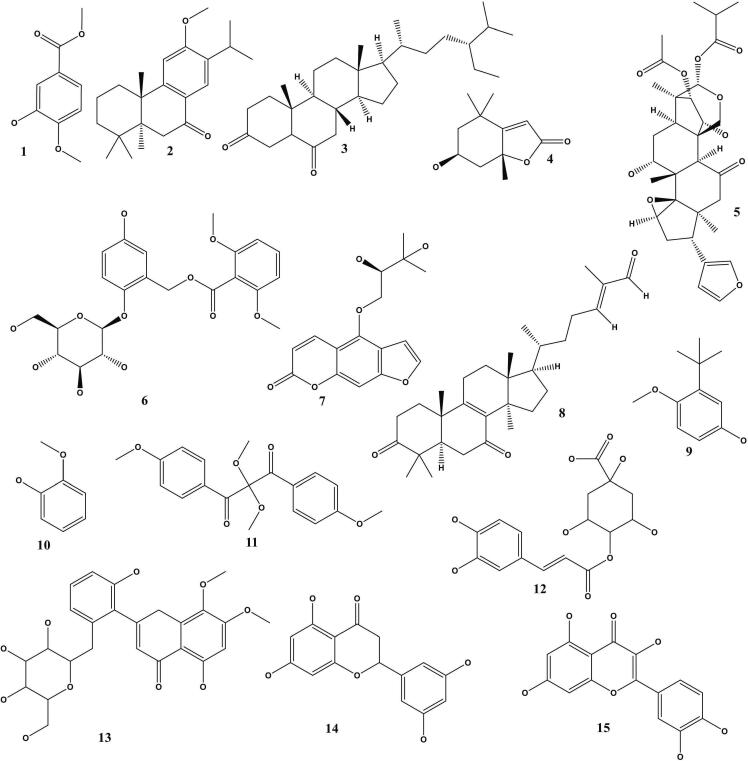
The phytochemicals of C. latifolia were analysed using LC-ESI-MS. Fig. 1, Fig. 2, Fig. 3 showed the LC-ESI-MS profiles of the root, stem, and leaf extracts. According to their mass accuracy and fragmentation pattern, as many as 15 compounds were identified. Ten of which were of phenolic, and four others were terpenoid/steroid derivates (Fig. 1). Information on compound names, retention times, and m/z fragmentation (positive molecule ion) can be seen in Table 1.
Fig. 1.
LC-ESI-MS/MS profile accompanied by the structure of compounds identified from root extract. Fig. 1A (RHE) was predicted to contain methyl-3-hydroxy-4-methoxybenzoate (1) and sugiol (2). Fig. 1B (REAE) was predicted to contain the compounds methyl-3-hydroxy-4-methoxybenzoate (1), stigmastan-3,6-dione (3), curculigoside (6), aviprin (7), and lucialdehyde B (8). Fig. 1C (REE) was predicted to contain the compounds methyl-3-hydroxy-4-methoxybenzoate (1), curculigoside (6), guaiacol (10), and smilaxin (11).
Fig. 2.
LC-ESI-MS/MS profile accompanied by the structure of compounds identified from stem extract. Fig. 2D (SHE) was predicted to contain stigmastan-3,6-dione (3). Fig. 2E (SEAE) was predicted to contain lucialdehyde B (8), and Fig. 2F (SEE) was predicted to contain the compounds 5,2′,6′-trihydroxy-7,8-dimethoxy-flavone-2′-O-β-D-glucopyranoside (13), 5,7,3′,5′-tetrahydroxyflavanone (14), and quercetin (15).
Fig. 3.
LC-ESI-MS/MS profile accompanied by the structure of compounds identified from leaf extract. Fig. 3G (LHE) was predicted to contain digiprolactone (4) and 3-tert-butyl-4-methoxyphenol (5). Fig. 3H (LEAE) was predicted to contain digiprolactone (4) and azedarachin C (5). Fig. 3I was predicted contain 4-O-Caffeoylquinic acid-1 (12) and quercetin (15).
Table 1.
Phytochemical screening of C. latifolia extracts using an LC-ESI-MS.
| No | RT (min) | Observed MS (m/z) | Molecular ion | Compounds | Molecular Formula | Extracts | References |
|---|---|---|---|---|---|---|---|
| 1 | 3.36 | 183.06 | M + H, +Na | Methyl-3-hydroxy-4-methoxybenzoate | C9H10O4 | HRE, REAE, REE | (Seghiri et al., 2006) |
| 2 | 7.68 | 301.21 | M + H | Sugiol | C20H28O2 | HRE | (Chao et al., 2005, Simoneit et al., 2019) |
| 3 | 12.91 | 429.37 | M + H | Stigmastan-3,6-dione | C29H48O2 | HSE, REAE | (Lim et al., 2005) |
| 4 | 3.61 | 197.12 | M + H | Digiprolactone | C11H16O3 | HLE. LEAE | (Lestari et al., 2022) |
| 5 | 9.94 | 609.27 | M + H | Azedarachin C | C32H42O10 | LEAE | (Chao et al., 2005, Priyanto et al., 2023) |
| 6 | 3.75 | 489.14 | M + H, +Na | Curculigoside | C22H26O11 | REAE, REE | (Zhao et al., 2014) |
| 7 | 3.58 | 305.102 | M + H, +Na | Aviprin | C16H16O6 | REAE | (Ishihara et al., 2001) |
| 8 | 6.14 | 453.34 | M + H | Lucialdehyde B | C30H44O3 | SEAE, REAE | (Gao et al., 2010) |
| 9 | 5.30 | 181.12 | M + H | 3-tert-butyl-4-methoxyphenol | C11H16O2 | HLE | (Wiley and Sons., 2023) |
| 10 | 2.14 | 125.06 | M + H | Guaiacol | C7H8O2 | REE | (Dorfner et al., 2003) |
| 11 | 2.49 | 317.102 | M + H | Smilaxin | C17H16O6 | REE | (Woo et al., 1992) |
| 12 | 2.44 | 355.102 | M + H, +Na | 4-O-Caffeoylquinic acid-1 | C16H18O9 | SEE, LEE | (Li et al., 2016) |
| 13 | 2.93 | 492.13 | M + H, +Na | 5,2′,6′-Trihydroxy-7,8-dimethoxy-flavone-2′-O-β-D-glucopyranoside | C23H24O12 | SEE | (Nurul Islam et al., 2013) |
| 14 | 3.76 | 288.6 | M + H, +Na | 5,7,3′,5′-Tetrahydroxyflavanone | C15H12O6 | SEE | (Yang et al., 2016) |
| 15 | 3.52 | 303.05 | M + H | Quercetin | C15H10O7 | LEE | (Chen et al., 2015) |
3.3. ABTS radical reducing activity
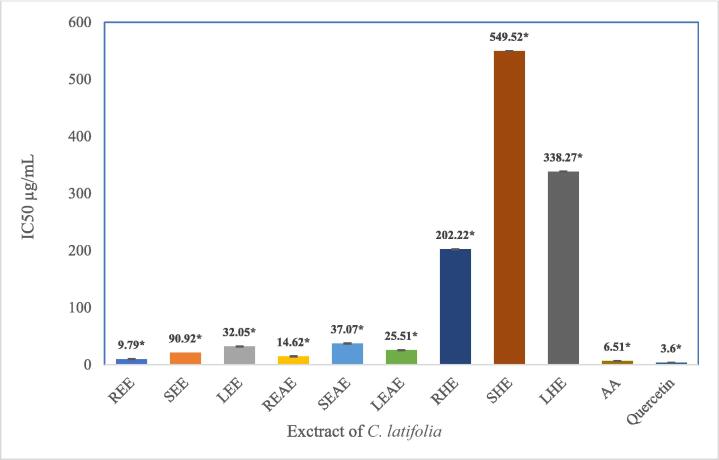
ABTS radical scavenging activity in the extract positively correlated with their concentrations. The radical scavenging activity of ABTS increases with increasing sample concentration. An inhibitor concentration of 50% (IC50) was used to measure the capacity of C. latifolia extract to lessen ABTS radicals. The test results in Fig. 4 show that the ethanol and ethyl acetate extracts in the roots, stems, and leaves of C. latifolia were highly active antioxidants with an IC50 value of 50 µg/mL, while the n-hexane extract in each organ had weak antioxidant activity (IC50 > 150 µg/mL). Among all C. latifolia, REE extract demonstrated the strongest antioxidant activity with an IC50 value of 9.79 µg/mL, significantly different from ascorbic acid (6.51 µg/mL) and three times weaker than the antioxidant power of quercetin (3.6 µg/mL) as a positive control.
Fig. 4.
Test the antioxidant activity of each C. latifolia extract by reducing ABTS radicals. This study used ascorbic acid (1–7.5 µg/mL) and quercetin (1–5 µg/mL) as positive controls. *indicates that the data are significantly different with a Tukey HSD of p ≤ 0.05 (n = 3).
3.4. BCB (Beta carotene bleaching) assay
Antioxidant activity testing the usage of the BCB technique was primarily based on the potential of antioxidant compounds from C. latifolia extract to prevent the rate of beta-carotene degradation because of the formation of peroxide radicals attributable to the oxidization of linoleic acid. In this study, the antioxidant capabilities of each extract of C. latifolia were expressed as IC50 (Table 2). The process of inhibiting the amount of beta-carotene degradation relies upon on increasing the concentration of the sample. The higher the concentration, the higher the inhibition amount of beta-carotene degradation. The test results for the ability of antioxidants to inhibit the beta-carotene degradation rate by lipid peroxidation showed that each C. latifolia extract had a significantly different antioxidant capacity among the samples (P < 0.05, n = 3). In Table 2, REAE and SEAE samples (IC50 < 20 µg/mL) showed high antioxidant properties compared to other extracts and quercetin as a positive control. However, compared with the BHT (IC50 = 0.55 µg/mL) as a positive control, the REAE and SEAE samples showed low antioxidant properties. BHT was used as a comparison because BHT is known to have specific antioxidant activity in preventing lipid peroxidation (Abdul Karim et al., 2014). At the same time, quercetin is a phenolic and flavonoid compound that is spread in natural ingredients, has potent antioxidant activity, and was used as a comparison to determine its action in preventing the oxidation process of beta carotene.
Table 2.
Antioxidant activity of C. latifolia extract by beta carotene bleaching assay.
| Sample | Percentage of inhibition (%) in a concentration (µg/mL) of: |
IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 6.25 | 12.5 | 25 | 50 | 100 | 200 | ||
| REE | 4.11 ± 0.05 | 10.79 ± 1.08 | 28.13 ± 0.56 | 48.11 ± 1,96 | 73.03 ± 0.88 | – | 62.05*b |
| SEE | 6.80 ± 0.23 | 8.91 ± 0.4 | 17.76 ± 0.13 | 42.98 ± 0.53 | 74.91 ± 1.22 | – | 64.97*a |
| LEE | 22.34 ± 0.87 | 29.67 ± 1.35 | 39.38 ± 2.13 | 42.50 ± 0.98 | 59.43 ± 0.39 | – | 70.55*b |
| REAE | 38.44 ± 1.36 | 45.83 ± 0.97 | 59.93 ± 0.39 | 67.83 ± 1,3 | 82.15 ± 1.12 | – | 14.81*e |
| SEAE | 39.96 ± 1.33 | 48.23 ± 1.10 | 50.55 ± 0.64 | 67.29 ± 0.75 | 80.44 ± 0.88 | – | 19.42*d |
| LEAE | 8.66 ± 0.77 | 9.69 ± 0.89 | 37.77 ± 1.25 | 56.58 ± 5.19 | 82.16 ± 5.16 | – | 51.34* |
| RHE | – | 2.25 ± 0.14 | 9.96 ± 0.26 | 23.96 ± 0.61 | 44.65 ± 1.42 | 86.29 ± 4.60 | 115.1*h |
| SHE | – | 17.09 ± 1.12 | 18.63 ± 0.74 | 23.50 ± 0.40 | 47.23 ± 0.48 | 75.48 ± 1.27 | 118.7*g |
| LHE | – | 15.08 ± 0.92 | 19.53 ± 0.59 | 40.76 ± 0.87 | 63.17 ± 0.87 | 85.49 ± 0.61 | 94.92* |
| BHT* | IC50 (0.55)* | ||||||
| Quercetin* | IC50 (43.38)* | ||||||
Note: *Concentration series on positive control BHT (0.2 to 1 µg/mL) and quercetin (10 to 50 µg/mL). The percentage inhibition (%) of lipid peroxidation at each concentration is described as mean ± SD (n = 3). With a Tukey HSD of p ≤ 0.05, the data indicate a significant difference (*). abc..There was no extensive difference in data from the alphabetical order at p ≤ 0.05 (Tukey HSD).
3.5. Ferric reducing antioxidant capacity (FRAP) assay
To generate the standard calibration curve used in this study (Fig. 5). Quercetin is a phenolic-flavonoid compound that can represent the compound content of C. latifolia, which can reduce the Fe3+-TPTZ complex to Fe2+-TPTZ so that the results can be equivalent to its antioxidant capacity (Lim et al., 2007, Nur et al., 2019). Generally, each extract from C. latifolia can reduce iron complexes to Fe2+.
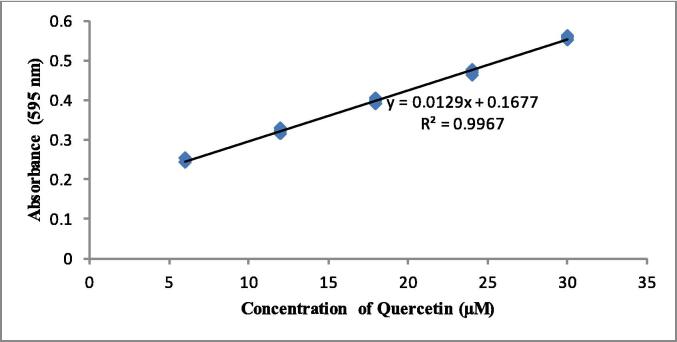
Fig. 5.
The quercetine calibration curve (6–30 µM), each data point were analyzed triplicates (n = 3).
The standard curve of quercetin (Fig. 5) obtained a linear regression value (r2) 0.9967 with 3 repetitions which showed good linearity so that it could be used to calculate the antioxidant power by reduction of Fe3+ to Fe2+ from C. latifolia extracts. Table 3 shows that the REAE extract had a significantly higher reducing ability (p ≤ 0.05) with a value of 833.33 ± 8.35 µMQEV/g among sample, followed by REE (462.12 ± 7.57), SEAE (422.98 ± 4.16), SEE (316.92 ± 9.53) and LEE (209.34 ± 7.04) extract. Meanwhile, LEAE, RHE, SHE, and LHE extracts provided a reduced power of < 100 µMQEV/g. The antioxidant capacity in reducing iron from REAE samples showed a reducing power close to that of ascorbic acid (997.26 ± 10.42 µMQEV/g extract) as a positive control.
Table 3.
Antioxidant activity in reducing iron using the FRAP method.
| Extract Samples | Reduction Power (µMQEV/g extract) (595 nm) |
|---|---|
| REE | 462.12 ± 7.57* |
| SEE | 316.92 ± 9.53* |
| LEE | 209.34 ± 7.04* |
| REAE | 833.33 ± 8.35* |
| SEAE | 422.98 ± 4.16* |
| LEAE | 88.38 ± 1.16* |
| RHE | 37.27 ± 2.48* |
| SHE | 18.61 ± 1.70*i |
| LHE | 14.57 ± 0.61*h |
| Ascorbic Acid | 997.26 ± 10.42* |
Note: Reduction power are represented as mean ± SD with three times replication (n = 3). *The data demonstrate a significant difference at the p ≤ 0.05 level (Tukey HSD). abc..Alphabetical differences showed that the data were not extensive different at the p ≤ 0.05 level (Tukey HSD).
3.6. In vitro anti-elastase assay
The in vitro anti-elastase test results (Table 4) showed that SEE, RHE, REE, and REAE extracts provide obtained powerful inhibitory action with IC50 values of 16.89, 19.22, 23.84, and 27.91 µg/mL, respectively. A similar action was also discovered for quercetin with an IC50 value of 4.88 µg/mL. The SEAE and LEE extracts showed strong activity with IC50 values > 50 µg/mL, while SHE extracts a moderate activity with IC50 values of 106.35 µg/mL. In contrast, LEAE and LHE extracts gave weak activity in inhibiting the action of elastase (IC50 > 150).
Table 4.
The anti-elastase activity of C. latifolia extract.
| Extract | Percentage of inhibition (µg/mL) in a concentration of: |
IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2.34 | 4.69 | 9.375 | 18.75 | 37.5 | 75 | 150 | ||
| REE | 31.76 ± 4.22 | 36.91 ± 0.59 | 45.14 ± 0.27 | 49.09 ± 0.73 | 52.87 ± 3.29 | 57.90 ± 2.04 | – | 23.84* |
| SEE | 36.14 ± 1.78 | 40.88 ± 1.05 | 44.12 ± 6.41 | 51.44 ± 1.62 | 56.21 ± 1.84 | 60.75 ± 3.77 | – | 16.89*g |
| LEE | 35.29 ± 0.78 | 37.91 ± 1.49 | 40.21 ± 3.25 | 44.35 ± 3.06 | 48.45 ± 4.79 | 51.32 ± 2.69 | – | 58.31*e |
| REAE | 26.12 ± 5.95 | 31.38 ± 4.06 | 39.85 ± 1.41 | 45.72 ± 1.53 | 52.04 ± 4.78 | 61.12 ± 1.59 | 66.06 ± 1.01 | 27.91* |
| SEAE | 24.66 ± 4.31 | 27.87 ± 3.28 | 33.10 ± 2.34 | 41.34 ± 3.00 | 45.84 ± 2.22 | 51.35 ± 0.86 | 59.12 ± 2.47 | 58.11*c |
| LEAE | 13.82 ± 4.21 | 17.49 ± 2.65 | 21.13 ± 2.93 | 26.95 ± 3.77 | 30.26 ± 3.27 | 36.13 ± 4.68 | 45.37 ± 2.03 | >150* |
| RHE | 31.37 ± 6.86 | 36.72 ± 3.33 | 46.93 ± 1.08 | 48.96 ± 1.79 | 54.39 ± 1.10 | 61.27 ± 0.59 | 68.79 ± 1.68 | 19.22*b |
| SHE | 14.91 ± 4.41 | 19.07 ± 3.72 | 23.91 ± 0.19 | 33.54 ± 3.63 | 40.36 ± 1.08 | 48.14 ± 1.69 | 52.60 ± 8.00 | 106.35* |
| LHE | 3.69 ± 1.91 | 7.70 ± 6.81 | 12.8 ± 7.19 | 17.4 ± 5.79 | 23.2 ± 1.64 | 27.1 ± 2.97 | 30.0 ± 1.35 | >150* |
| Quercetin | IC50 (4.88 µg/mL) | |||||||
Note: Concentration series on positive control quercetin (1.5 to 13.5 µg/mL). The percentage inhibition (%) of elastase at each concentration is expressed as mean ± SD (n = 3). *indicates the data is extensive different with p ≤ 0.05 level (Tukey HSD). abc..Alphabetical differences indicated that the data were not extensive different with a level of p ≤ 0.05 (Tukey HSD).
3.7. Pearson’s correlation between antioxidant and anti-elastase
The existence of a correlation between the antioxidant activity of each C. latifolia extract on elastase inhibition was performed through Pearson correlation statistical analysis. The correlation results of each antioxidant method on elastase inhibition can be seen in Table 5. The analysis results show that each extract's antioxidant activity correlates with elastase inhibition.
Table 5.
The Pearson’s correlation coefficient of BCB, ABTS, FRAP and anti-elastase activity.
| Correlation |
r-values |
Anti-elastase | ||
|---|---|---|---|---|
| BCB assay | ABTS assay | FRAP assay | ||
| BCB | 1 | |||
| ABTS | 0.796 | 1 | ||
| FRAP | −0.814 | −0.691 | 1 | |
| Anti-Elastase | 0.279 | 0.615 | −0.495 | 1 |
3.8. In silico anti-elastase assay
The Parameters in this docking study were amino acid residues, hydrogen bonds, and free bond energy (ΔG). Fig. 6 shows the compound (ligand) structure of C. latifolia obtained from the predicted LC-MS/MS for each extract. Primarily based at the visualization results, it is able to be seen that all compounds from C. latifolia extract were able to interact with the active site of elastase (Table 6). The data in Table 6 suggests that the compounds in the extract of C. latifolia have bond-free energies (ΔG) in the ranges of −4.18 to −8.27 kcal/mol concerning the active site of the elastase. Eight of these molecules form bonds with the amino acids Gly 193, Val 216, and Ser 195, key amino acids in elastase target proteins and capable of restate some of the reported interactions with native ligands (Fig. 7).
Fig. 6.
Chemical structure of the compound C. latifolia extract. Names of chemical compounds is according to the order in Table 1 and Table 6.
Table 6.
The value of bond-free energy and amino acid residues that bind to the elastase (1B0F).
| No. | Compounds | Bond-free energy (kcal/mol) | H-bond/van der waals Interaction* |
|---|---|---|---|
| Native ligand | (1-{3-methyl-2-[4-(morpholine-4-carbonyl)-benzoyl-amino]-butyryl} pyrrolidine-2-carboxylic acid-(3,3,4,4,4-pentafluoro-1-isopropyl-2-oxo-butyl)-amide | −7.69 |
Gly 193, Val 216 Ser 195 |
| 1. | Methyl-3-hydroxy-4-methoxybenzoat | −4.71 |
Gly 193, Ser 195 Val 216 |
| 2. | Sugiol | −6.50 |
Gly 193, Ser 195 Ser 214 |
| 3. | Stigmastan-3,6-dione | −8.27 | Arg 217, Gly 218 |
| 4. | Diprolactone | −5.67 | Val 216 |
| 5. | Azedarachin C | −5.30 |
Gly 193, Ser 195 Phe A41 |
| 6. | Curculigoside | −4.90 | Gly 193, Ser 195, Val 216, Phe215 |
| 7. | Aviprin | −6.46 |
Gly 193, Ser 195, Val 216 |
| 8. | Lucialdehyde B | −6.81 | Ser 195, Arg 217 |
| 9. | 3-Tert-butyl-4-methoxyphenol | −5.76 |
Gly 193, Ser 195 Val 216 |
| 10. | Guaiacol | −4.18 |
Gly 193, Ser 195 Asp 194 |
| 11. | Smilaxin | −5.26 |
Ser 195 Val 216 Phe 192 |
| 12. | 4-O-caffeoylquinic acid-1 | −5.27 | Ser 195, Val 216 (phi-aril), Gly 193 |
| 13. | 5,2,6-Trihydroxy-7,8 dimethoxy-flavone-2-O-β-D-gluc | −4.27 |
Gly 193, Ser 195, Val 216, Asp 194 Ser 214 |
| 14. | 5,7,3,5-Tetrahydroxyflavanone | −6.34 | Ser 195, Val 216 |
| 15. | Quercetin | −6.06 |
Gly 193, Ser 195 Val 216 |
Note: *no bold letter in the column indicates as fol other amino acid residues that interact with hydrogen/van der waals on the C. latifolia compounds and are not the same as co-crystals.
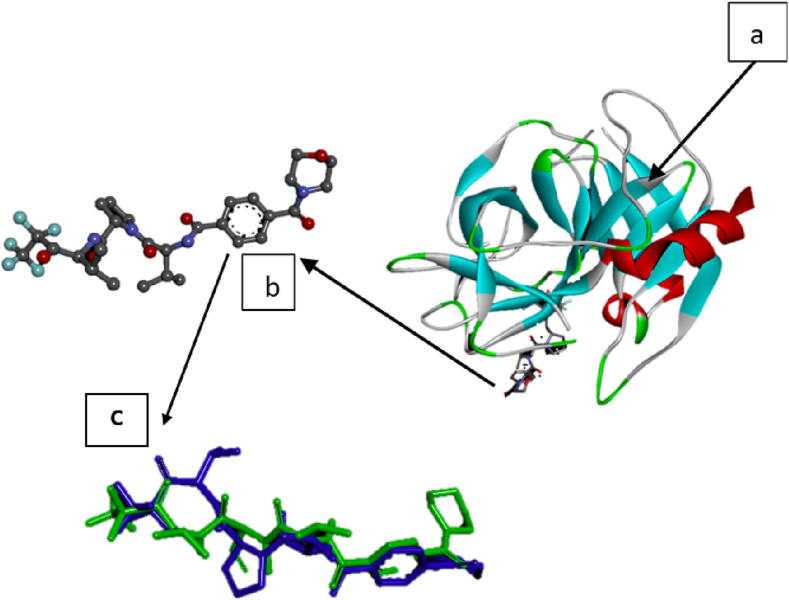
Fig. 7.
Elastase Receptor (1B0F) Structure (a), the native ligand (1-{3-methyl-2-[4-(morpholine-4-carbonyl)-benzoyl-amino]-butyryl} pyrrolidine-2-carboxylic acid-(3,3,4,4,4-pentafluoro-1-isopropyl-2-oxo-butyl)-amide (b) and re-docking native ligand (co-crystal) result in elastase pocket for validate the method with RMSD value of 1.678 Å (c).
4. Discussion
The current study focused on screening the antiaging activity of extracts from several parts of the C. latifolia plant based on antioxidant activity and in vitro and molecular docking approaches to anti-elastase activity. Three solvents (n-hexane, ethyl acetate, ethanol 70% v/v) were used to extract the phytochemical compounds in the dried plant parts of C. latifolia. The percentage yields value of C. latifolia extracts differs based on the polarity of the solvent used. This study considered that the use of ethanol as a solvent was effective in extracting components from each extract. The polarity index of ethanol is 5.2, while its dielectric constant is 24.55, which allow it to extract compounds with high polarity. In this study, ethanol (70% v/v) was used, indicating a water content of 30% v/v so that the polarity of the solvent was increasing (Abarca-Vargas et al., 2016, Hikmawanti et al., 2021). The use of ethanol solvents has been reported to attract components of the carbohydrate group, phenolic flavonoids, alkaloids, steroids, and terpenoids to allow a large percentage of yield value compared to the use of other solvents such as ethyl acetate and n-hexane, which have low polarity (Lapornik et al., 2005, Zabidi et al., 2019).
Based totally on the results of the LC-ESI-MS analysis, the majority of the compounds were obtained from the ethanol extract of each organ of C. latifolia (Table 1). In the root (Fig. 1) of C. latifolia, curculigoside is dominant. Comparable results were also found in (Ooi et al., 2016, Umar et al., 2021), which identified curculigoside compounds in the ethanolic extract of C. latifolia roots and leaves. However, the compounds methyl-3-hydroxy-4-methoxybenzoate found in HRE, REAE, and REE, sugiol (HRE), aviprin (REAE), guaiacol (lignin), and smilaxin found in REE were reported in the first time in the roots of the C. latifolia and stigmastan-3,6-dione and lucyaldehyde B in the roots and stem of C. latifolia (Fig. 2). Some of these compounds have pharmacological effects, including curculigoside, a phenolic compound, has been widely reported to have an antioxidant effect (Ooi et al., 2016, Wang et al., 2012, Zhang et al., 2019) and act as an anti-aging agent in inhibiting MMP-1 (Lee et al., 2009). Methyl-3-hydroxy-4-methoxybenzoate (Wang et al., 2016), aviprin (Zahri et al., 2012), guaiacol (Azadfar et al., 2015), and sugiol (Bajpai et al., 2014) also been reported to have an antioxidant effect. Phenolic derivatives in the stem of C. latifolia were also found in the form of 4-O-caffeoylquinic acid-1, 5,2′,6′-trihydroxy-7,8-dimethoxy-flavone-2′-O-β-D-glucopyranoside, and 5,7,3′,5′- tetrahydroxyflavanone and they are confirmed that compounds have the anti-radical capacity (Ganzon et al., 2018, Sun et al., 2007, Wang et al., 2018). In addition to the stem, 4-O-caffeoylquinic acid-1 (phenolic) compounds were found in the C. latifolia (LEE) leaf. The leaf of C. latifolia (Fig. 3) also contains quercetin (flavonoid) compounds (LEE), which have very potent antioxidant bioactivity (Chen et al., 2015) and terpenoid derivatives such as digiprolactone (HLE, LEAE) and azedarachin C (LEAE). The profile of chemical compounds produced from each extract of C. latifolia plant organs strongly supports the development of C. latifolia in delivering natural products that have pharmacological effects and are beneficial in medicine.
The phenolic content in C. latifolia extract acts as an antioxidant in inhibiting the activity of free radicals. In this study, antioxidant activity was quantified using different methods to confirm further the compounds' role in C. latifolia as antioxidants. In the ABTS method, antioxidant activity is measured by the potential of antioxidant compounds to contribute proton radicals to free radical compounds (Ahmad Wani and Tirumale, 2018, Morales and Paredes, 2014, Nur et al., 2021b). In the BCB method, Antioxidant potential compounds from the extract C. latifolia work by preventing beta carotene decomposition when linoleic acid is oxidized to hydroperoxide radicals at 50 °C. Hydroperoxide radicals formed attack the double bonds in beta carotene resulting in oxidation, which causes the loss of chromophore groups that give the color orange of beta carotene (Amiri, 2014, Bogacz-Radomska and Harasym, 2018). Both ABTS and BCB methods work in reducing radical reactions through the proton coupled electron transfer mechanism. The strength of antioxidant activity in the ABTS and BCB methods was determined based on the IC50 value. An extract with a smaller IC50 value has greater antioxidant activity. The IC50 values that are in the range of < 50 µg/mL, 50–100 µg/mL, >100–150 µg/mL, and > 150 µg/mL indicate very strong, strong, moderate, and weak activity, respectively (Nur et al., 2021a, 2021b).
The ABTS method found that the ethanol and ethyl acetate extracts from the parts of the roots, stems, and leaves of C. latifolia tended to have powerful activity (Fig. 4). This result is different from the results described by Ooi et al. (2018) which stated that the methanol extract from C. latifolia had an IC50 value of 431 ± 36.9 in ABTS assay with a weak category. At the same time, the extract on the stems and leaves of this plant has not been reported.
A BCB study indicated that the ethyl acetate extract of C. latifolia plant's roots, stems, and leaves were the most effective antioxidants. This was followed by ethanol extract and n-hexane extract, respectively (Table 2). These compounds can penetrate the lipid phase of the BCE and make it simpler to counteract hydroperoxide radicals from the linoleic acid oxidation process (Nur et al., 2021a). The phenolic compounds are methyl-3-hydroxy-4-methoxybenzoate, curculigoside, quercetin, 4-O-caffeoylquinic acid-1, 5,2′,6′-trihydroxy-7,8-dimethoxy-flavone-2′-O-β-D-glucopyranoside, and 5,7,3′,5′- tetrahydroxy-flavanone are thought to work in inhibiting lipid peroxidation in the initiation phase and also in the propagation phase (Marco, 1968, Nickavar and Esbati, 2012). Studies on the anti-radical capacity of C. latifolia plant extracts in inhibiting the rate of beta-carotene degradation have not been reported. Therefore, this study provides information on the antioxidant bioactivity profile of C. latifolia extract in inhibiting lipid peroxidation by the beta-carotene bleaching method, which is relevant to peroxidation that occurs biologically (Aminjafari et al., 2016, Koleva et al., 2002).
Testing of antioxidant activity on C. latifolia extract was also performed the use of the FRAP technique. In the FRAP assay, the compounds in the extract redox react with iron complexes as a substrate. In principle, the FRAP test measures the ability of C. latifolia compounds to oxidize Fe3+ to Fe2+ under acidic conditions (Aminjafari et al., 2016, Rabeta and Nur Faraniza, 2013). In FRAP method, the antioxidant strenght of each extract was determined that the root organ of C. latifolia plants tended to have extreme activity in reducing Fe3+ to Fe2+ complexes (Table 3). Based totally on the results of the phytochemical screening, the majority of compounds identified in the root extracts were phenolic compounds (Table 1). Hydroxyl groups in phenolic aromatics can chelate iron and reduce it by the redox reaction (Simamora et al., 2020, Spiegel et al., 2020). Testing of antioxidant activity using the FRAP assay on C. latifolia extract has not been widely informed. This test describes biological events in the body through the Fenton reaction, which aims to inhibit the conversion process of hydrogen peroxide produced in the metabolism into hydroxyl radicals, which negatively affect the body (Caillet et al., 2007, Kulisic et al., 2004, Nur et al., 2021b). The same thing for n-hexane extract showed a lower effect than other extracts among the ABTS, BCB, and FRAP methods. This result may be influenced by the lack of phenolic in the n-hexane extract.
The three antioxidant quantification methods that have been carried out tend to provide similar activity between samples with a Pearson correlation coeffiicient (r) of 0.691 to 0.814 (Table 5). Antioxidant activity in the BCB method against ABTS and FRAP is strongly correlated with r-values of 0.796 and −0.814, which means that the BCB/ABTS method has similar activity up to 79.6% (strong correlation) and BCB/FRAP reaches 81.4% (strong correlation). The moderate correlation occurs in the ABTS/FRAP method with an r-value of 0.691, which means that the similarity of activities reaches 69.1%.
This study also tested the anti-elastase activity of C. latifolia extract. Inhibition of elastase activity is one of the parameters to determine the ability of a compound to inhibit skin aging. The inhibition of elastase by the inhibitor can be observed visually through the yellow color fading that occurs due to the inhibition of the interaction between elastase and N-succinyl-(Ala-Ala-Ala) nitroanilide as a substrate by compounds from the extract of C. latifolia (Desmiaty et al., 2021). Extracts from the roots of C. latifolia (HRE, REAE, and REE) confirmed the most efective inhibition of elastase with an IC50 value < 50 µg/mL (Table 4). Although the IC50 value was significantly different (p ≤ 0.05) from quercetin as a positive control, the HRE, REAE, and REE extracts had the same potency, which was in the powerful category (IC50 value < 50 µg/mL). The results of identification carried out by LC-ESI-MS (Table 1) showed that extracts from the root of C. latifolia were generally a phenolic group of compounds. The previous studies from Szewczyk et al. (2020) and Pientaweeratch et al. (2016) confirmed that phenolic compounds can inhibit skin-degrading enzymes, through hydrogen binding from the elastase's active site and chelating ions from metalloenzymes.
The relationship study between anti-elastase and antioxidant activity (Table 5) showed that ABTS/FRAP was moderately correlated with anti-elastase activity, which reached 61.5% (r-value of 0.615) and 49.5% (r-value of 0.495), respectively. Unlike the case with anti-elastase/BCB, it shows a weak correlation with an r-value of 0.279, which means that anti-elastase activity is only influenced by 27.9% of the antioxidant capacity by the BCB test. However, the effect of the antioxidant action of each extract of C. latifolia on anti-elastase activity still needs to be further studied to confirm the relationship between the two mechanisms.
In the present study, the mechanism of interaction and correlation of the phenolic compound of C. latifolia to its bioactivity on elastase was described in silico. In silico anti-elastase activity of compounds (Fig. 6) from C. latifolia extract was carried out by molecular docking to predict the interactions between ligands (compounds in C. latifolia) against skin-degrading enzymes’ target proteins (1B0F) (Cregge et al., 1998). The molecular structure of the target protein elastase, native ligan (co-crystal), and overlay of co-crystal re-docking result can be seen in Fig. 7 (7a-c). Re-docking of the native ligand into the target protein pocket was carried out to validate the docking method so that the Root Means Standard Deviation (RMSD) value was obtained. RMSD value, which is<2 Å, indicates that the molecular docking method parameters provide results closer to the experimental results (Fig. 7c). The validation results show that the RMSD value of 1.678 Å indicating that the molecular docking method parameters used meet the requirements. Smaller RMSD indicates a closer alignment of the crystallographic ligand position with the re-bonded ligand position (Kontoyianni et al., 2004, Nursamsiar et al., 2020).
The compounds that showed the best interactions were compounds 2, 7, 14, 15, 9, and 12, with bond-free energy values of −6.60, −6.46, −6.34, −6.06, −5.76, and −5.27 kcal/mol, respectively (Table 6). It was found that the six compounds interact similarly with co-crystals and have free energy values that are close to those for the co-crystals binding to the target protein (Fig. 8). However, compounds 3 and 8 have bond-free energies close to the bond-free energies of co-crystals, but the binding pattern to key amino acids is different from that of co-crystals. The more negative the bond free energy (ΔG) value indicates good stability between the ligand and the target protein (receptor) so that the bond formed are more powerful (Elgamal et al., 2021).
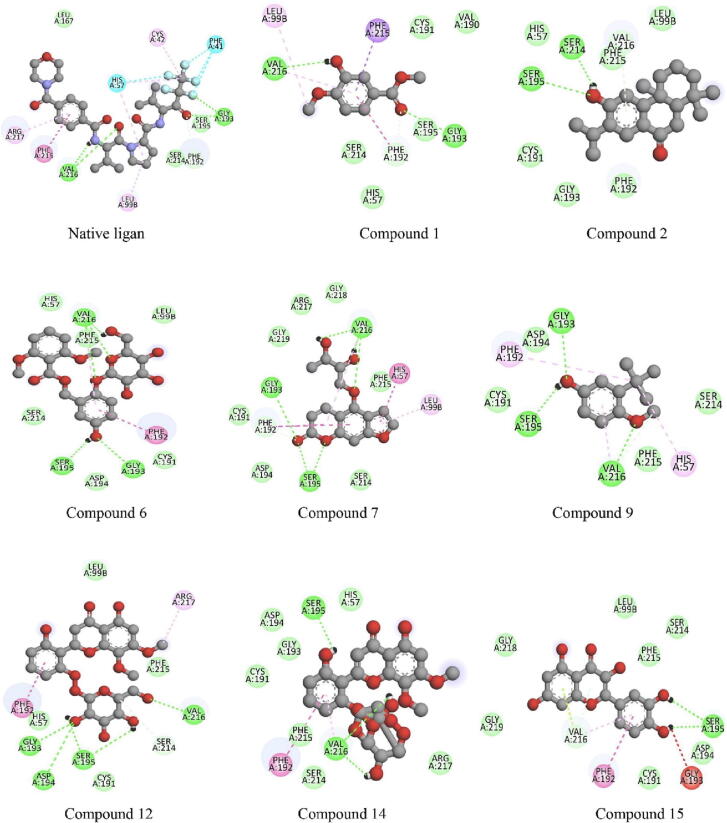
Fig. 8.
2D interactions of co-crystals 1B0F (native ligands) and compounds 1 (methyl-3-hydroxy-4-methoxybenzoat), 2 (sugiol), 6 (curculigoside), 7 (aviprin), 9 (3-tert-butyl-4-methoxyphenol), 12 (4-O-caffeoylquinic acid-1), 14 (5,7,3,5-tetrahydroxyflavanone), and 15 (quercetin) with amino acid residues of elastase (1B0F). Numbering of a chemical compound according to the order in Table 5.
Based on Fig. 8, the of the compound that best contributes to its interaction with the receptor is the hydrogen bonding of the hydroxyl group (O–H). Furthermore, the nucleophilic water molecule interacts with the hydroxyl group attached to the aromatics. Essentially, this prevents the substrate from functioning by interfering with the hydrolysis reaction. Hydrogen bonding is an interaction that can stabilize ligand binding to receptors. Another interaction that occurs in the C. latifolia compound with the amino acid residue of the elastase is the van der Waals interaction which can also increase the conformational stability (Williams et al., 2012). The four best compounds from C. latifolia are phenolic derivatives with a planar-shaped aromatic ring that allows for Van der Waals and π-interactions. Electron-rich aromatic systems and electron-deficient anions can form π-anion interactions. The π-anion interaction plays a vital role in protein stability and ligand binding, and this interaction is energetically beneficial (Elgamal et al., 2021, Nursamsiar et al., 2020).
An overview of the in silico studies indicate that most of the phenolic group compounds identified in the extracts are well accommodated at the binding site of the target enzyme with a relatively large free binding energy averaging −6 kcal/mol. Findings the in silico molecular docking supported the in vitro results that provide anti-elastase effects, especially in extracts of the roots and stems of the C. latifolia plant.
5. Conclusion
Examination of the phytochemicals of the plant organ extract of C. latifolia identified 15 secondary metabolites, and the majority were phenolic compounds. Each extract of C. latifolia showed antioxidant and anti-elastase activities with moderate to powerful ability. Ethanol and ethyl acetate extracts of the root and stem (REE, SEE, REAE, and SEAE) gave the best antioxidant activity based on in the three methods applied. REE, SEE, REAE, and RHE extracts also provided very strong bioactivity against elastase inhibition. The anti-elastase effect of C. latifolia extracts is associated with its action as an antioxidant with a moderate correlation. The in vitro anti-elastase activity was correlated with the in silico activity against the elastase target protein (1B0F). The compounds of sugiol, aviprin, 4-O-caffeoylquinic acid-1, quercetin, and 5,7,3,5-tetrahydroxyflavanone gave the best interaction on the target protein with the most negative binding free energy of < -6 kcal/mol. It has been suggested that the presence of identified phenolic compounds in C. latifolia extract contributes to its ability to act as a powerful antioxidant and anti-elastase in vitro and in silico studies. Through both in vitro and silico investigations, C. latifolia can be considered as an antiaging candidate.
CRediT authorship contribution statement
Syamsu Nur: Conceptualization, Investigation. Heri Setiawan: Validation. Muhammad Hanafi: Methodology, Investigation. Berna Elya: Conceptualization, Methodology.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Berna Elya reports financial support, article publishing charges, equipment, drugs, or supplies, and statistical analysis were provided by the Indonesian Ministry of Higher Education and Culture. Muhammad Hanafi reports a relationship with the National Research and Innovation Agency Republic of Indonesia that includes: consulting or advisory.
Acknowledgments
Acknowledgments
It is with the utmost gratitude that the authors acknowledge the centers, scientific, and technical assist that was provided by the Advanced laboratories, the National Research and Innovation Institute Serpong, and Badan Riset dan Inovasi Nasional (BRIN). The author would like to thank the Indonesian Ministry of Education, Culture, Research Technology andthe degree by research (BRIN) program for supporting this research as well.
Funding
A Doctoral Dissertation Grant was provided by the Indonesian Ministry of Education, Culture, Research, and Technology under contract numbers 021/E5/PG.02.00.PL/2023 and NKB-865/UN2.RST/HKP.05.00/2023.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103716.
Contributor Information
Syamsu Nur, Email: syamsu.nur11@ui.ac.id.
Heri Setiawan, Email: heri.setiawan@farmasi.ui.ac.id.
Muhammad Hanafi, Email: muha002@brin.go.id.
Berna Elya, Email: berna.elya@farmasi.ui.ac.id.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abarca-Vargas R., Peña Malacara C.F., Petricevich V.L. Characterization of chemical compounds with antioxidant and cytotoxic activities in bougainvillea x buttiana holttum and standl, (Var. rose) extracts. Antioxidants. 2016 doi: 10.3390/antiox5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Karim A., Azlan A., Ismail A., Hashim P., Abd Gani S.S., Zainudin B.H., Abdullah N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014 doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Wani, N., Tirumale, S., 2018. Evaluation of antioxidant properties of different extracts of Chaetomium cupreum SS02. Bull. Fac. Pharmacy, Cairo Univ. https://doi.org/10.1016/j.bfopcu.2018.08.001.
- Aminjafari A., Miroliaei M., Angelova V.T., Emamzadeh R., Djukic M.M., Djuric A., Saso L. Antioxidant activity and protective role on protein glycation of synthetic aminocoumarins. Electron. J. Biotechnol. 2016 doi: 10.1016/j.ejbt.2016.08.004. [DOI] [Google Scholar]
- Amiri H. Chemical composition and antioxidant activity of essential oil and methanolic extracts of ferula microcolea (Boiss.) Boiss (Apiaceae) Int. J. Food Prop. 2014 doi: 10.1080/10942912.2012.665403. [DOI] [Google Scholar]
- Anggraini, N.B., Elya, B., Iskandarsyah, 2020. Anti-elastase, antioxidant, total phenolic and total flavonoid content of macassar kernels (Rhus javanica L) from pananjung pangandaran nature tourism park- Indonesia. J. Nat. Remedies. https://doi.org/10.18311/jnr/2020/24240
- Azadfar M., Gao A.H., Chen S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015 doi: 10.1016/j.ijbiomac.2014.12.049. [DOI] [PubMed] [Google Scholar]
- Bajpai, V.K., Sharma, A., Kang, S.C., Baek, K.H., 2014. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac. J. Trop. Med. https://doi.org/10.1016/S1995-7645(13)60183-2. [DOI] [PubMed]
- Bogacz-Radomska L., Harasym J. β-Carotene-properties and production methods. Food Qual. Saf. 2018 doi: 10.1093/fqsafe/fyy004. [DOI] [Google Scholar]
- Caillet S., Yu H., Lessard S., Lamoureux G., Ajdukovic D., Lacroix M. Fenton reaction applied for screening natural antioxidants. Food Chem. 2007 doi: 10.1016/j.foodchem.2005.10.009. [DOI] [Google Scholar]
- Chao K.P., Hua K.F., Hsu H.Y., Su Y.C., Chang S.T. Anti-inflammatory activity of sugiol, a diterpene isolated from Calocedrus formosana bark. Planta Med. 2005 doi: 10.1055/s-2005-864094. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yu H., Wu H., Pan Y., Wang K., Jin Y., Zhang C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen typhae for transformation rule exploration. Molecules. 2015 doi: 10.3390/molecules201018352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chormey, D.S., Bakirdere, S., 2018. Principles and Recent Advancements in Microextraction Techniques, in: Comprehensive Analytical Chemistry. https://doi.org/10.1016/bs.coac.2018.03.011.
- Cregge R.J., Durham S.L., Farr R.A., Gallion S.L., Hare C.M., Hoffman R.V., Janusz M.J., Kim H.O., Koehl J.R., Mehdi S., Metz W.A., Peet N.P., Pelton J.T., Schreuder H.A., Sunder S., Tardif C. Inhibition of human neutrophil elastase. 4. Design, synthesis, X-ray crystallographic analysis, and structure-activity relationships for a series of P2-modified, orally active peptidyl pentafluoroethyl ketones. J. Med. Chem. 1998 doi: 10.1021/jm970812e. [DOI] [PubMed] [Google Scholar]
- Desmiaty, Y., Hanafi, M., Saputri, F.C., Elya, B., Rifai, E.A., Syahdi, R.R., 2021. Two triterpenoids from Rubus fraxinifolius leaves and their tyrosinase and elastase inhibitory activities. Sci. Rep. https://doi.org/10.1038/s41598-021-99970-x. [DOI] [PMC free article] [PubMed]
- Dorfner R., Ferge T., Kettrup A., Zimmermann R., Yeretzian C. Real-time monitoring of 4-vinylguaiacol, guaiacol, and phenol during coffee roasting by resonant laser ionization time-of-flight mass spectrometry. J. Agric. Food Chem. 2003 doi: 10.1021/jf0341767. [DOI] [PubMed] [Google Scholar]
- Dravie, E.E., Kortei, N.K., Essuman, E.K., Tettey, C.O., Boakye, A.A., Hunkpe, G., 2020. Antioxidant, phytochemical and physicochemical properties of sesame seed (Sesamum indicum L). Sci. African. https://doi.org/10.1016/j.sciaf.2020.e00349.
- Elgamal A.M., El Raey M.A., Gaara A., Abdelfattah M.A.O., Sobeh M. Phytochemical profiling and anti-aging activities of Euphorbia retusa extract: In silico and in vitro studies. Arab. J. Chem. 2021 doi: 10.1016/j.arabjc.2021.103159. [DOI] [Google Scholar]
- Farzinebrahimi, R., Mat Taha, R., Rashid, K.A., Ali Ahmed, B., Danaee, M., Rozali, S.E., 2016. Preliminary Screening of Antioxidant and Antibacterial Activities and Establishment of an Efficient Callus Induction in Curculigo latifolia Dryand (Lemba). Evidence-based Complement. Altern. Med. https://doi.org/10.1155/2016/6429652. [DOI] [PMC free article] [PubMed]
- Ganzon J.G., Chen L.G., Wang C.C. 4-O-Caffeoylquinic acid as an antioxidant marker for mulberry leaves rich in phenolic compounds. J. Food Drug Anal. 2018 doi: 10.1016/j.jfda.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.-J., Min B.-S., Ahn E.-M., Nakamura N., Lee H.-K., Hattori M. ChemInform abstract: New Triterpene Aldehydes, Lucialdehydes A-C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. ChemInform. 2010 doi: 10.1002/chin.200248182. [DOI] [PubMed] [Google Scholar]
- Hanafi, H., Irawan, C., Rochaeni, H., Sulistiawaty, L., Roziafanto, A.N., Supriyono, 2018. Phytochemical screening, LC-MS studies and antidiabetic potential of methanol extracts of seed shells of Archidendron bubalinum (Jack) I.C. Nielson (Julang Jaling) from Lampung, Indonesia. Pharmacogn. J. https://doi.org/10.5530/pj.2018.6s.15.
- Hikmawanti, N.P.E., Fatmawati, S., Asri, A.W., 2021. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts, in: IOP Conference Series: Earth and Environmental Science. https://doi.org/10.1088/1755-1315/755/1/012060.
- Ishihara K., Fukutake M., Asano T., Mizuhara Y., Wakui Y., Yanagisawa T., Kamei H., Ohmori S., Kitada M. Simultaneous determination of byak-angelicin and oxypeucedanin hydrate in rat plasma by column-switching high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Biomed. Sci. Appl. 2001 doi: 10.1016/S0378-4347(00)00569-7. [DOI] [PubMed] [Google Scholar]
- Jadoon, S., Karim, S., Asad, M.H.H. Bin, Akram, M.R., Kalsoom Khan, A., Malik, A., Chen, C., Murtaza, G., 2015. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2015/709628. [DOI] [PMC free article] [PubMed]
- Koleva I.I., Van Beek T.A., Linssen J.P.H., De Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002 doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Kontoyianni M., McClellan L.M., Sokol G.S. Evaluation of docking performance: Comparative data on docking algorithms. J. Med. Chem. 2004 doi: 10.1021/jm0302997. [DOI] [PubMed] [Google Scholar]
- Kulisic T., Radonic A., Katalinic V., Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004 doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- Lago J.C., Puzzi M.B. The effect of aging in primary human dermal fibroblasts. PLoS One. 2019 doi: 10.1371/journal.pone.0219165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapornik B., Prošek M., Wondra A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005 doi: 10.1016/j.jfoodeng.2004.10.036. [DOI] [Google Scholar]
- Lee S.Y., Kim M.R., Choi H.S., Moon H.I., Chung J.H., Lee D.G., Woo E.R. The effect of curculigoside on the expression of matrix metalloproteinase-1 in cultured human skin fibroblasts. Arch. Pharm. Res. 2009 doi: 10.1007/s12272-009-2013-4. [DOI] [PubMed] [Google Scholar]
- Lestari O.A., Palupi N.S., Setiyono A., Kusnandar F., Yuliana N.D. In vitro antioxidant potential and phytochemical profiling oMelastoma malabathricum leaf water extract. Food Sci. Technol. 2022 doi: 10.1590/fst.92021. [DOI] [Google Scholar]
- Li Y., Tang W., Chen J., Jia R., Ma L., Wang S., Wang J., Shen X., Chu Z., Zhu C., Ding X. Development of marker-free transgenic potato tubers enriched in Caffeoylquinic acids and flavonols. J. Agric. Food Chem. 2016 doi: 10.1021/acs.jafc.6b00270. [DOI] [PubMed] [Google Scholar]
- Lim Y.Y., Lim T.T., Tee J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007 doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- Lim J.C., Park J.H., Budesinsky M., Kasal A., Han Y.H., Koo B.S., Lee S.I., Lee D.U. Antimutagenic constituents from the thorns of gleditsia sinensis. Chem. Pharm. Bull. 2005 doi: 10.1248/cpb.53.561. [DOI] [PubMed] [Google Scholar]
- Lukitaningsih E., Nur S., Qonithah F., Zulbayu A., Kuswahyuning R., Rumiyati R. In vitro anti-wrinkle and tyrosinase inhibitory activities of grapefruit peel and strawberry extracts. Maj. Obat Tradis. 2020;25:182–189. doi: 10.22146/mot.59194. [DOI] [Google Scholar]
- Marco G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968 doi: 10.1007/BF02668958. [DOI] [Google Scholar]
- Morales G., Paredes A. Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complement. Altern. Med. 2014 doi: 10.1186/1472-6882-14-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickavar, B., Esbati, N., 2012. Evaluation of the Antioxidant Capacity and Phenolic Content of Three Thymus Species. JAMS J. Acupunct. Meridian Stud. https://doi.org/10.1016/j.jams.2012.03.003. [DOI] [PubMed]
- Nur, S., Mubarak, F., Jannah, C., Winarni, D.A., Rahman, D.A., Hamdayani, L.A., Sami, F.J., 2019. Total phenolic and flavonoid compounds, antioxidant and toxicity profile of extract and fractions of paku atai tuber (Angiopteris ferox Copel). Food Res. https://doi.org/10.26656/fr.2017.3(6).135.
- Nur, S., Aisyah, A.N., Lukitaningsih, E., Rumiyati, Juhardi, R.I., Andirah, R., Hajar, A.S., 2021a. Evaluation of antioxidant and cytotoxic effect against cancer cells line of Angiopteris ferox Copel tuber and its compounds by LC-MS analysis. J. Appl. Pharm. Sci. https://doi.org/10.7324/JAPS.2021.110808.
- Nur, S., Angelina, A.A., Aswad, M., Yulianty, R., Burhan, A., Nursamsiar, 2021b. In vitro anti-aging activity of Muntingia calabura L. fruit extract and its fractions. J. Pharm. Pharmacogn. Res.
- Nursamsiar, Siregar, M., Awaluddin, A., Nurnahari, N., Nur, S., Febrina, E., Asnawi, A., 2020. Molecular docking and molecular dynamic simulation of the aglycone of curculigoside a and its derivatives as alpha glucosidase inhibitors. Rasayan J. Chem. https://doi.org/10.31788/RJC.2020.1315577
- Nurul Islam M., Downey F., Ng C. Comprehensive profiling of flavonoids in Scutellaria incana L. using LC-Q-TOF-MS. Acta Chromatogr. 2013 doi: 10.1556/AChrom.25.2013.3.11. [DOI] [Google Scholar]
- Ooi D.J., Chan K.W., Sarega N., Alitheen N.B., Ithnin H., Ismail M. Bioprospecting the curculigoside-cinnamic acid-rich fraction from Molineria latifolia rhizome as a potential antioxidant therapeutic agent. Molecules. 2016 doi: 10.3390/molecules21060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi D.J., Azmi N.H., Imam M.U., Alitheen N.B., Ismail M. Curculigoside and polyphenol-rich ethyl acetate fraction of Molineria latifolia rhizome improved glucose uptake via potential mTOR/AKT activated GLUT4 translocation. J. Food Drug Anal. 2018 doi: 10.1016/j.jfda.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pientaweeratch S., Panapisal V., Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: an in vitro comparative study for anti-aging applications. Pharm. Biol. 2016 doi: 10.3109/13880209.2015.1133658. [DOI] [PubMed] [Google Scholar]
- Priyanto, J.A., Prastya, M.E., Primahana, G., Randy, A., Utami, D.T., 2023. Paederia foetida Linn Leaves-Derived Extract Showed Antioxidant and Cytotoxic Properties Against Breast Carcinoma Cell. HAYATI J. Biosci. https://doi.org/10.4308/hjb.30.2.271-280.
- Purohit T., He T., Qin Z., Li T., Fisher G.J., Yan Y., Voorhees J.J., Quan T. Smad3-dependent regulation of type I collagen in human dermal fibroblasts: Impact on human skin connective tissue aging. J. Dermatol. Sci. 2016 doi: 10.1016/j.jdermsci.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Putri I.R., Handayani R., Elya B. Anti-elastase activity of Rumput Teki (Cyperus rotundus L.). rhizome extract. Pharmacogn. J. 2019 doi: 10.5530/pj.2019.11.119. [DOI] [Google Scholar]
- Rabeta, M.S., Nur Faraniza, R., 2013. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of garcinia atrovirdis and cynometra cauliflora. Int. Food Res. J.
- Saleem, H., Zengin, G., Sarfraz, M., Alafnan, A., Locatelli, M., Tartaglia, A., Ahmad, I., Mahomoodally, M.F., Khurshid, U., Ahemad, N., 2021. Phytochemical composition and in -vitro pharmacological evaluation of Emex australis Steinh: A natural source of enzyme inhibitors. South African J. Bot. https://doi.org/10.1016/j.sajb.2021.02.023.
- Seghiri R., Mekkiou R., Boumaza O., Benayache S., Bermijo J., Benayache F. Phenolic compounds from Centaurea africana. Chem. Nat. Compd. 2006 doi: 10.1007/s10600-006-0228-x. [DOI] [Google Scholar]
- Simamora, A., Santoso, A.W., Timotius, K.H., Rahayu, I., 2020. Antioxidant Activity, Enzyme Inhibition Potentials, and Phytochemical Profiling of Premna serratifolia L. Leaf Extracts. Int. J. Food Sci. https://doi.org/10.1155/2020/3436940. [DOI] [PMC free article] [PubMed]
- Simoneit B.R.T., Otto A., Oros D.R., Kusumoto N. Terpenoids of the swamp cypress subfamily (Taxodioideae), Cupressaceae, an overview by GC-MS. Molecules. 2019;24:1–18. doi: 10.3390/molecules24173036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Kapusta K., Kołodziejczyk W., Saloni J., Zbikowska B., Hill G.A., Sroka Z. Antioxidant activity of selected phenolic acids-ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules. 2020 doi: 10.3390/molecules25133088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.M., Yang J.S., Zhang H. Two new flavanone glycosides of Jasminum lanceolarium and their anti-oxidant activities. Chem. Pharm. Bull. 2007 doi: 10.1248/cpb.55.474. [DOI] [PubMed] [Google Scholar]
- Szewczyk K., Miazga-Karska M., Pietrzak W., Komsta Ł., Krzemińska B., Grzywa-Celińska A. Phenolic composition and skin-related properties of the aerial parts extract of different hemerocallis cultivars. Antioxidants. 2020 doi: 10.3390/antiox9080690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar A.H., Ratnadewi D., Rafi M., Sulistyaningsih Y.C. Untargeted metabolomics analysis using ftir and uhplc-q-orbitrap hrms of two curculigo species and evaluation of their antioxidant and α-glucosidase inhibitory activities. Metabolites. 2021 doi: 10.3390/metabo11010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Warner R.L., Gharaee-Kermani M., Phan S.H., Kang S., Chung J.H., Wang Z.Q., Datta S.C., Fisher G.J., Voorhees J.J. Vitamin A antagonizes decreased cell growth and elevated collagen- degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Invest. Dermatol. 2000 doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Zhao L., Xu J., Nie Y., Guo Y., Tong Y., Qin L., Zhang Q. Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts. Acta Biochim. Biophys. Sin. (Shanghai). 2012 doi: 10.1093/abbs/gms014. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H., Su X., Yang H., Sun W. Flavonoids and Phenolic Compounds of Petrosimonia sibirica. Chem. Nat. Compd. 2016 doi: 10.1007/s10600-016-1679-3. [DOI] [Google Scholar]
- Wang Y., Li J., Li N. Phytochemistry and pharmacological activity of plants of genus Curculigo: An updated review since 2013. Molecules. 2021 doi: 10.3390/molecules26113396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of scutellaria baicalensis. Pharm. Biol. 2018 doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, J., Sons, Inc. SpectraBase; https://spectrabase.com/ad?sid=5tD8wBuqn2&r=https://spectrabase.com/spectrum/5tD8wBuqn2 (accessed 6/11/2023).
- Williams L.K., Li C., Withers S.G., Brayer G.D. Order and disorder: Differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J. Med. Chem. 2012 doi: 10.1021/jm301273u. [DOI] [PubMed] [Google Scholar]
- Woo M.H., Do J.C., Son K.H. Five new spirostanol glycosides from the subterranean parts of smilax sieboldii. J. Nat. Prod. 1992 doi: 10.1021/np50086a015. [DOI] [Google Scholar]
- Xia W., Quan T., Hammerberg C., Voorhees J.J., Fisher G.J. A mouse model of skin aging: Fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase-1. J. Dermatol. Sci. 2015 doi: 10.1016/j.jdermsci.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Yang Y., Sun X., Liu J., Kang L., Chen S., Ma B., Guo B. Quantitative and qualitative analysis of flavonoids and phenolic acids in snow chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules. 2016 doi: 10.3390/molecules21101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabidi N.A., Ishak N.A., Hamid M., Efliza Ashari S. Subcritical water extraction of antioxidants from Curculigo latifolia root. J. Chem. 2019 doi: 10.1155/2019/8047191. [DOI] [Google Scholar]
- Zahri S., Razavi S.M., Moatamed Z. Antioxidant activity and cytotoxic effect of aviprin and aviprin-3″-O-D-glucopyranoside on LNCaP and HeLa cell lines. Nat. Prod. Res. 2012 doi: 10.1080/14786419.2010.529442. [DOI] [PubMed] [Google Scholar]
- Zengin G., Zheleva-Dimitrova D., Gevrenova R., Nedialkov P., Mocan A., Ćirić A., Glamočlija J., Soković M., Aktumsek A., Mahomoodally M.F. Identification of phenolic components via LC–MS analysis and biological activities of two Centaurea species: C. drabifolia subsp. drabifolia and C. lycopifolia. J. Pharm. Biomed. Anal. 2018 doi: 10.1016/j.jpba.2017.11.045. [DOI] [PubMed] [Google Scholar]
- Zhang S., Duan E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018 doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Quanlong, Zhao, L., Shen, Y., He, Y., Cheng, G., Yin, M., Zhang, Qiaoyan, Qin, L., 2019. Curculigoside Protects against Excess-Iron-Induced Bone Loss by Attenuating Akt-FoxO1-Dependent Oxidative Damage to Mice and Osteoblastic MC3T3-E1 Cells. Oxid. Med. Cell. Longev. https://doi.org/10.1155/2019/9281481. [DOI] [PMC free article] [PubMed]
- Zhao G., Yuan F., Zhu J. An LC-MS/MS method for determination of curculigoside with anti-osteoporotic activity in rat plasma and application to a pharmacokinetic study. Biomed. Chromatogr. 2014 doi: 10.1002/bmc.3025. [DOI] [PubMed] [Google Scholar]
- Zhuang, B., Ramanauskaite, G., Koa, Z.Y., Wang, Z.G., 2021. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. https://doi.org/10.1126/sciadv.abe7275. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.