Summary
Background
The PISA-II trial showed that short-term anti-tumour necrosis factor (anti-TNF) therapy followed by surgical closure induces radiological healing of perianal fistulas in patients with Crohn's disease more frequently than anti-TNF therapy alone after 18 months. This study aimed to compare long-term outcomes of both treatment arms.
Methods
Follow-up data were collected from patients who participated in the PISA-II trial, an international patient preference randomised controlled trial. This multicentre trial was performed in nine hospitals in the Netherlands and one hospital in Italy. Patients with Crohn's disease above the age of 18 years with an active high perianal fistula and a single internal opening were asked to participate. Patients were allocated to anti-TNF therapy (intravenous infliximab, or subcutaneous adalimumab, at the discretion of the gastroenterologist) for one year, or surgical closure combined with 4-months anti-TNF therapy. Patients without a treatment preference were randomised (1:1) using random block randomisation (block sizes of six without stratification), and patients with a treatment preference were treated according to their preferred treatment arm. For the current follow-up study, data were collected until May 2022. Primary outcome was radiological healing on magnetic resonance imaging (MRI), including all participants with a MRI made less than 6 months ago at the time of data collection. Analysis was based on observed data.
Findings
Between September 14, 2013, and December 7, 2019, 94 patients were enrolled in the trial. Long-term follow-up data were available in 91 patients (36/38 (95%) anti-TNF + surgical closure, 55/56 (98%) anti-TNF). A total of 14/36 (39%) patients in the surgical closure arm were randomly assigned, which was not significantly different in the anti-TNF treatment arm (16/55 (29%) randomly assigned). Median follow-up was 5.7 years (interquartile range (IQR) 5–7). Radiological healing occurred significantly more often after anti-TNF + surgical closure (15/36 = 42% versus 10/55 = 18%; P = 0.014). Clinical closure was comparable (26/36 = 72% versus 34/55 = 62%; P = 0.18) in both groups. However, clinical closure in the surgical group was achieved with less re-interventions 4/26 (= 15%) versus 18/34 (= 53%), including (redo-)surgical closure procedures. Recurrences occurred in 0/25 (0%) patients with radiological healing versus 27/76 (36%) patients with clinical closure, sometime during follow-up. Anti-TNF trough levels were higher in patients with long-term clinical closure in both groups (P = 0.031 and P = 0.014). In 6/11 (55%) patients in the anti-TNF group with available trough levels, recurrences were diagnosed within three months of a drop under 3.5ug/ml. 36 patients stopped anti-TNF, after which 0/14 (0%) patients with radiological healing developed a recurrence and 9/22 (41%) with clinical closure. Self-rated (in)continence was comparable between groups, and 79% (60/76) of patients indicated comparable/improved continence after treatment. Decision-regret analysis showed that all (30/30) anti-TNF + surgical closure patients agreed or strongly agreed that surgery was the right decision versus 78% (36/46) in the anti-TNF arm. All surgical closure patients would go for the same treatment again, whereas this was 89% (41/46) in the anti-TNF arm.
Interpretation
This study confirmed that surgical closure should be considered in amenable patients with perianal fistulas and Crohn's disease as long-term outcomes were favourable, and that radiological healing should be the aim of treatment as recurrences only occurred in patients without radiological healing. In patients with complete MRI closure, anti-TNF could be safely stopped.
Funding
None.
Keywords: Perianal fistula, Crohn disease, Anti-TNF, Ligation of intersphincteric fistula tract (LIFT), Advancement flap
Research in context.
Evidence before this study
Current guidelines recommend anti-tumour necrosis factor (TNF) treatment and suggest considering surgical closure in patients with surgically amenable disease. However, the PISA-II trial showed that, at 18 months follow-up, short-term anti-TNF therapy combined with surgical closure induces radiological healing of perianal fistulas in patients with Crohn's disease more frequently than anti-TNF therapy alone.
Furthermore, current guidelines state that clinical assessment is sufficient to evaluate response to treatment of perianal fistulas in patients with Crohn's disease and only recommend additional magnetic resonance imaging (MRI) to evaluate improvement of fistula inflammation.
Added value of this study
Perianal fistulas in patients with Crohn's disease can have a great impact on patients' quality of life and are difficult to close permanently. To our knowledge, the PISA study was the first study to directly compare outcomes after short-term anti-TNF therapy combined with surgical closure to anti-TNF therapy. The current manuscript presents the long-term results with a median follow-up of 5.7 years. These data suggested that radiological healing occurred more often in the surgical closure arm, whereas clinical closure was comparable between the two treatment arms. However, this was after a re-intervention (including attempt of surgical closure) in more than half of the patients in the anti-TNF treatment arm. Long-term clinical closure was related to higher anti-TNF trough levels in both study arms. The study also showed that recurrences did not occur in patients with radiological healing during long-term follow-up, whereas recurrences were seen in more than one third of patients with clinical closure. The majority of recurrences after clinical closure in the anti-TNF group were directly related to a decrease in anti-TNF trough level.
Implications of all the available evidence
The current study indicates that long-term outcomes of short-term anti-TNF therapy combined with surgical closure are favourable compared to anti-TNF therapy alone, especially in terms of radiological healing. Radiological healing on MRI – defined as a completely fibrotic fistula tract – was found a reliable prognostic parameter for lasting clinical closure.
Introduction
Perianal fistulas in patients with Crohn's disease can be difficult to close, and achieving long-term closure is even more challenging. Over the years, multiple medical and surgical treatment approaches have been suggested in order to achieve closure, reduce perianal fistula symptoms such as pain, discharge, abscess formation and reduce the overall negative impact on quality of life. However, it is still unclear what the most optimal choice of treatment is for long-term closure of perianal fistulas in patients with Crohn's disease.
To date, only two randomised controlled trials (RCTs) were able to directly compare medical and surgical treatment of perianal fistulas in patients with Crohn's disease in the short and medium long-term. The first was the PISA-I study1 which compared re-intervention rates after chronic seton drainage, anti-tumour necrosis factor (anti-TNF) therapy and surgical closure after short-term anti-TNF treatment. Unfortunately, the study could not draw conclusions on closure rates due to early termination of the trial because of futility, with the highest re-intervention rates in the chronic seton drainage arm. Therefore, the PISA-II study2 was initiated, a patient preference RCT which focussed on radiological healing on magnetic resonance imaging (MRI), which is suggested as a superior assessment of deep healing compared to clinical evaluation.3 The PISA-II study showed more frequent induction of radiological healing after short-term anti-TNF treatment combined with surgical closure compared to anti-TNF therapy at 18 months follow-up.2 The study also showed that radiological healing was associated with no recurrences at the time.
Previous studies have suggested that recurrences can occur after cessation of anti-TNF therapy4 and that closure of Crohn's perianal fistulas may be achieved by anti-TNF dose intensification to increase serum drug levels.5 However, it is unknown whether a drop in anti-TNF trough level is associated with recurrences and whether radiological healing at 18 months follow-up is indeed associated with long-term clinical closure without recurrences during long-term follow-up after stopping anti-TNF. The aim of this study was therefore to compare long-term outcomes after short-term anti-TNF treatment combined with surgical closure to anti-TNF therapy alone.
Methods
Study design and participants
In this follow-up study, data were collected from patients who participated in the previously reported PISA-II trial.2 In short, the PISA-II trial was a multicentre, international patient preference RCT that compared short-term anti-TNF treatment combined with surgical closure to anti-TNF therapy as treatment of perianal fistulas in patients with Crohn's disease. A total of 94 patients were included between September 14, 2013 and December 7, 2019.
All patients with Crohn's disease above the age of 18 years with an active high perianal fistula, defined as a fistula located in the upper two-thirds of the striated anal muscles (i.e. located in the upper part of the external sphincter or located in the puborectal muscle), and a single internal opening were eligible for inclusion. Patients with a stoma, recto-vaginal fistula, active proctitis (defined as the presence of any active mucosal inflammation or ulcers >5 mm in diameter in the rectal mucosa), anorectal stenosis (defined as inability to introduce a proctoscope), submucosal and low, intersphincteric fistulas, multiple internal fistula openings, use of anti-TNF medication for more than 3 months before randomisation, contraindication or previous unsuccessful anti-TNF treatment for perianal fistula, and dementia or altered mental status that would prohibit the understanding and giving of informed consent were excluded. All included patients previously provided written informed consent.
Randomisation and masking
Patients without a preference for one of the treatment arms were randomised (1:1) and others were treated according to their preference. Central automated randomisation from the trial website of the Academic Medical Centre (ALEA Clinical, Abcoude, the Netherlands) was used for block randomisation with block sizes of six.2 It was not possible to mask treatment allocation from patients and medical personnel. The specialised radiologist scoring the primary outcome was masked for both treatment allocation and clinical outcome.
Procedures
The anti-TNF therapy arm consisted of 6 weeks of seton drainage and medication that consisted of either intravenous infliximab or subcutaneous adalimumab for 1 year, at the discretion of the gastroenterologist. Anti-TNF was initiated in combination with a tablet azathioprine 2.0–2.5 mg/kg once a day, or a tablet or suspension mercaptopurine 1.0–1.5 mg/kg. Surgical closure entailed the ligation of the intersphincteric fistula tract (LIFT) procedure or the advancement flap, at the discretion of the surgeon.2 Surgical closure procedures were performed after approximately 8–12 weeks seton drainage and after approximately 6–10 weeks anti-TNF therapy. Anti-TNF therapy was stopped at around 4 months, at the discretion of the treating gastroenterologist.
For the current study, long-term follow-up data were collected from the time of enrolment in the PISA-II trial until April 30th 2022. Data were collected by medical chart review, by telephone contact and, if needed, by e-mail. The PISA-II2 study received central approval from the Medical Ethical Committee at the Amsterdam University Medical Centers, location University of Amsterdam, and all other participating centres and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. For the current study, formal approval and individual patient informed consent was waived by the Medical Ethics Review Committee of the Amsterdam University Medical Centers. This long-term follow-up analysis was not prespecified in the original PISA-II study protocol.
Outcomes
The primary outcome of the PISA-II trial was radiological healing at MRI after 18 months follow-up.2 Radiological healing was defined as a completely hypointense, fibrotic fistula tract; absence of hyperintensity on T2-weighted sequences regardless of fat suppression and no enhancement of the tract after contrast administration on a fat suppressed T1-weighted sequence.6 In the current study, the primary outcome was long-term radiological healing at MRI according to the same definition and determined by a specialised radiologist (JS). Follow-up ended on April 30th 2022 for all included patients, however, a MRI was included as the long-term MRI if it was performed less than 6 months ago at the time of data collection (May 2022). In patients without a long-term MRI, with a clinically open fistula, the fistula was considered not radiologically healed. Secondary outcomes included long-term clinical closure, defined as closure of the external opening without discharge of pus or faeces on palpation which persisted until end of follow-up, and the effect of trough levels on clinical closure, re-interventions, defined as need for additional treatment besides the treatment(s) in the treatment arm in which the patient was enrolled, recurrences, defined as reopening of the external opening after clinical closure, and the influence of (suboptimal) anti-TNF serum trough levels on recurrence. Re-interventions were performed at the discretion of the treating physician and based on the clinical symptoms (with or without additional MRI). For both treatment groups the same criteria applied, both were at the discretion of the treating physician. Median trough levels were determined during maintenance anti-TNF therapy, and based on at least two consecutive measurements. In case of fistula recurrence, the specific trough level closest to date of recurrence was analysed and compared to the known median trough level before. A suboptimal anti-TNF trough level was defined as <7.1 ug/ml for infliximab and <6.8 ug/ml for adalimumab.7 Furthermore, patients were retrospectively asked about their pre- and post-treatment continence (improved, decreased or never had any incontinence problems), and asked to complete a decisional regret questionnaire. The validated Decision Regret Scale (DRS) was used at the end of follow-up to evaluate patients’ distress or remorse after anti-TNF induction combined with surgical closure or anti-TNF therapy.8 The DRS contains five questions and uses a Likert-type scale for the answers ranging from 1 (“strongly agree”) to 5 (“strongly disagree”). The final score could be converted to a score ranging from 0 (no regret) to 100 (highest regret).
Statistical analysis
Data were collected in an electronic database. All categorical data are presented as frequencies and percentages, continuous normally distributed data as mean and standard deviation (SD) and continuous non-normally distributed data as median and interquartile range (IQR). Chi-square or Fisher's exact test were used as appropriate to compare primary and secondary outcome parameters for the two treatment arms. Median anti-TNF trough levels were compared using the Mann–Whitney U test. Kaplan–Meier survival analysis was done for all included patients to estimate the time until reaching long-term clinical closure. Time-to-event is time until reaching long-term clinical closure or until the end of their follow-up. A two-sided P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (version 26.0).
Role of the funding source
There was no funding source for this study.
Results
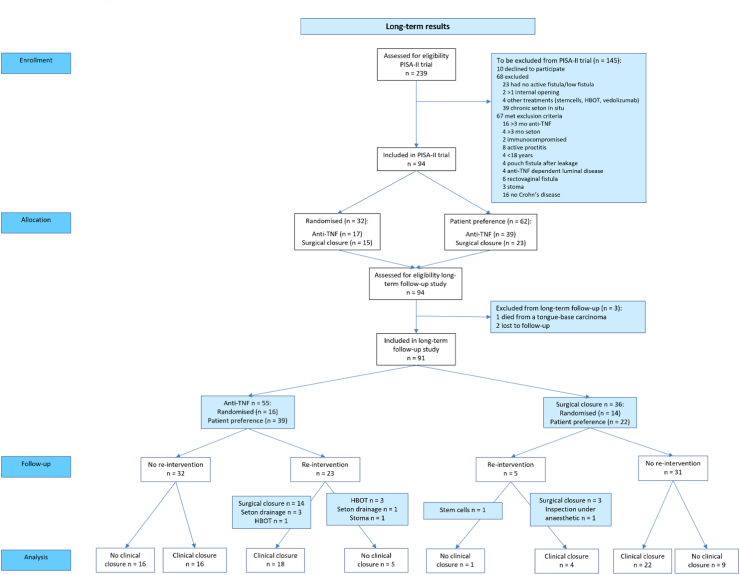
Long-term follow-up data were collected from 91 out of 94 patients (97%) included in the PISA-II trial, of whom 36/38 were allocated to the surgical closure arm and 55/56 to the anti-TNF treatment arm. Of the three excluded patients, one had died from a tongue-base carcinoma, not related to study treatment, and two patients from different hospitals were lost to follow-up and telephone contact as well as e-mail went unanswered. Of the 36 patients in the surgical closure arm, 14 were randomly assigned and 22 chose the treatment arm. Of the 55 patients in the anti-TNF treatment arm, 16 were randomly assigned and 39 chose the treatment arm. Patients were predominantly females (59%) and had a median age of 33 years (IQR 26–44). All baseline characteristics between the two treatment arms were comparable (Table 1). Median follow-up was 5.7 years (IQR 5–7).
Table 1.
Baseline characteristics included patients.
| Short-term anti-TNF treatment + surgical closure (n = 36) | Anti-TNF (n = 55) | |
|---|---|---|
| Follow-up, median years (IQR) | 6.1 (4.8–7.4) | 5.4 (4.3–6.8) |
| Age at inclusion, median years (IQR) | 32 (26–51) | 35 (26–46) |
| Female, n (%) | 21 (58) | 33 (60) |
| Male, n (%) | 15 (42) | 22 (40) |
| Active smoker at baseline, n (%) | 12 (33) | 16 (29) |
| BMI at baseline, median kg/m2(IQR) | 24 (21–27) | 23 (21–27) |
| Montreal disease location, n (%) | ||
| - L1: Ileal | 25 (69) | 33 (60) |
| - L2: Colonic | 6 (17) | 8 (15) |
| - L3: Ileocolonic | 5 (14) | 14 (25) |
| - L4: Isolated upper disease | 0 (0) | 0 (0) |
| Previous anti-TNF treatment at baselinea, n (%) | 15 (42) | 20 (36) |
| Crohn's disease duration at baseline, median years (IQR) | 3 (1–9) | 5 (1–14) |
| Number of external openings at baseline, median (IQR) | 1 (1–2) | 1 (1–2) |
IQR, interquartile range; BMI, Body Mass Index.
More than 6 months ago.
During long-term follow-up, a total of 25 patients reached radiological healing. This was significantly different between short-term anti-TNF therapy combined with surgical closure and anti-TNF therapy (15/36 = 42% versus 10/55 = 18%; P = 0.014). Long-term clinical closure occurred in 26/36 (72%) in the surgical closure arm and in 34/55 (62%) in the anti-TNF treatment arm (P = 0.18). In 18/34 (53%) patients in the anti-TNF treatment arm long-term clinical closure was reached after a re-intervention, compared to 4/26 (15%) in the surgical closure arm (P = 0.003). This re-intervention entailed a surgical closure procedure in 14/18 (78%) patient in the anti-TNF treatment arm (Fig. 1). In the anti-TNF treatment arm, 16/55 (29%) patients reached long-term clinical closure without additional treatment and 5/55 (9.1%) patients reached radiological healing without additional treatment.
Fig. 1.
Consort flow diagram towards long-term clinical closure. Fig. 1 shows the number of patients per treatment group that reached long-term clinical closure, and if a re-intervention was needed to achieve long-term clinical closure. Analysis after median 5.7 years follow-up. Abbreviations used: Anti-TNF, anti-tumour necrosis factor therapy; HBOT, hyperbaric oxygen therapy
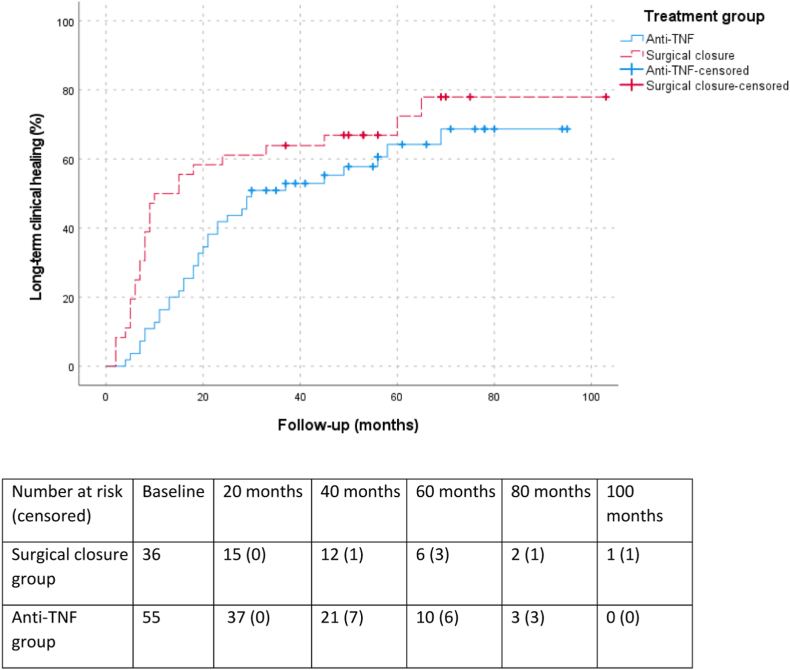
The Kaplan Meier of all included patients shows that patients in the surgical closure arm reached long-term clinical closure significantly earlier, after median 8 months (IQR 5–16), compared to patients in the anti-TNF treatment arm, after median 19 months (IQR 11–29) (P = 0.049) (Fig. 2). In the surgical closure arm, the interval between the surgical closure procedure and clinical closure is even shorter (median 4 months, IQR 1–13).
Fig. 2.
Kaplan Meier (one minus survival function) for time to clinical closure. Fig. 2 shows the Kaplan Meier curve (one minus survival function) of all included patients and the time-interval until reaching long-term clinical closure per treatment arm. In blue, the anti-TNF treatment arm is visualised and in red, the surgical closure treatment arm is shown. The X-axis shows the follow-up period in months, and the Y-axis shows the percentage of patients that reached long-term clinical closure at a certain time point.
Trough levels were available in 16/36 patients in the surgical closure under anti-TNF arm (median trough level 4.8, IQR 1.5–8.1) and in 32/55 patients in the anti-TNF arm (median trough level 6.6, IQR 2.4–10.7). In both groups, clinical closure was associated with a significantly higher trough level (surgical closure group 7.6 vs 3.0, P = 0.031 and anti-TNF group 8.6 vs 4.6, P = 0.014). A total 21/48 patients (44%) had a trough level >7.5. From these 21 patients 16 (76%) achieved clinical closure, whereas only 8/27 (30%) achieved clinical closure with a trough level <7.
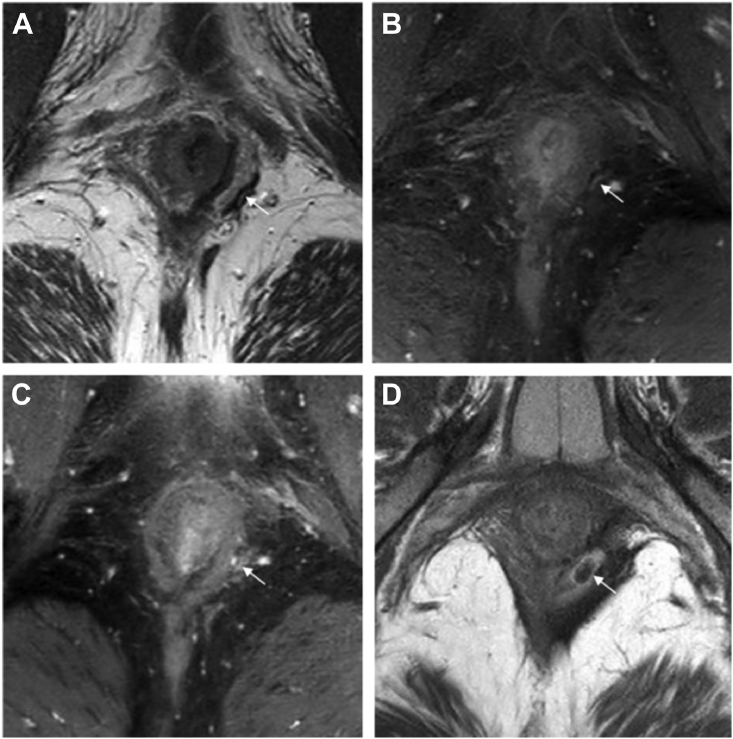
Recurrences occurred in 9/36 (22%) patients in the surgical closure arm compared to 19/55 (35%) in the anti-TNF treatment arm (P = 0.2 1). None of the 25 patients (0%) with radiological healing had a recurrence compared to 27 out of the in total 76 patients (36%) who reached clinical closure sometime during the median 5.7 years follow-up. Recurrences also occurred in patients with an almost completely fibrotic tract and only minimal activity on MRI (Fig. 3).
Fig. 3.
Axial T1 and T2-weighted sequence of a patient with a recurrence after almost reaching radiological healing. Fig. 3 shows axial T1 and T2-weighted sequence of a patient that had a recurrent fistula after almost reaching radiological fistula healing. Figure A and B are of a fistula tract that was thought to be completely fibrotic. A is a T2-weighted sequences where a subtle linear hyperintense signal at the arrow in A is visible, which is also visible in fat-suppressed T1-weighted image B. Figure C and D are of the recurrent fistula tract. Figure C is a fat-suppressed T1-weighted sequence and figure D is a T2-weighted sequence. In figure C you can see the fistula tract at the same level as in B. Figure D is at a somewhat higher level where you can more clearly see the diameter of the recurrent fistula tract.
In 6/11 (55%) patients in the anti-TNF group with a clinical recurrence and available trough level, a recurrence was detected within three months of trough level decrease under 3.5. In the 25 patients with radiological healing, 14 patients stopped anti-TNF. None of them developed a recurrence. In the 76 patients with clinical closure sometime during follow-up, a recurrence was seen in 9/22 patients (41%) who stopped anti-TNF.
A total of 76/91 patients (84%) completed the self-rated incontinence questionnaire and the DRS: 30/36 (83%) in the surgical closure arm and 46/55 (84%) in the anti-TNF treatment arm. Overall, 21/76 (28%) had improved continence (less incontinence problems) after treatment, which was not significantly different between both treatment arms (11/30 (37%) versus 10/46 (22%); P = 0.16). In total, 60/76 (79%) of all patients indicated comparable/improved continence after treatment. In the surgical closure arm, 9/30 patients (30%) had more complaints of incontinence after treatment and in the anti-TNF treatment arm 7/46 patients (15%) had more incontinence problems. Of the 7 patients with increased incontinence in the anti-TNF treatment arm, 6 were after a surgical re-intervention (4 surgical closure procedures and 2 incision and (seton) drainage). There was no significant difference in incontinence between patients undergoing LIFT or advancement flap. Furthermore, patients with long-term clinical closure more often had an improved continence compared to those without long-term clinical closure (19/52 (37%) versus 2/24 (8%); P = 0.011).
Total DRS score was median 3 (IQR 0–25) in the surgical closure arm and median 10 (IQR 0–30) in the anti-TNF arm (P = 0.73). All patients (30/30) in the surgical closure arm agreed or strongly agreed that undergoing their treatment (surgery) was the right decision compared to 78% (36/46) in the anti-TNF arm (Table 2). Also, 100% (30/30) of the patients in the surgical closure arm would go for the same treatment if they had to do it over again, whereas this was 89% (41/46) for the patients in the anti-TNF group.
Table 2.
Decision regret scale.
| Strongly agree, n (%) | Agree, n (%) | Neither agree nor 3disagree, n (%) | Disagree, n (%) | Strongly disagree, n (%) | |
|---|---|---|---|---|---|
| It was the right decision | |||||
| Surgical closure (n = 30) | 20 (67) | 10 (34) | 0 (0) | 0 (0) | 0 (0) |
| Anti-TNF (n = 46) | 24 (52) | 12 (26) | 8 (17) | 2 (4) | 0 (0) |
| I regret the choice that was made | |||||
| Surgical closure (n = 30) | 0 (0) | 0 (0) | 1 (3) | 6 (21) | 23 (77) |
| Anti-TNF (n = 46) | 0 (0) | 1 (2) | 0 (0) | 12 (26) | 33 (72) |
| I would go for the same choice if I had to do it over again | |||||
| Surgical closure (n = 30) | 18 (60) | 8 (28) | 4 (14) | 0 (0) | 0 (0) |
| Anti-TNF (n = 46) | 24 (52) | 15 (33) | 2 (4) | 5 (11) | 0 (0) |
| The choice did me a lot of harm | |||||
| Surgical closure (n = 30) | 1 (3) | 3 (10) | 1 (3) | 7 (24) | 18 (60) |
| Anti-TNF (n = 46) | 0 (0) | 4 (9) | 3 (7) | 16 (35) | 23 (50) |
| The decision was a wise one | |||||
| Surgical closure (n = 30) | 22 (73) | 7 (24) | 1 (3) | 0 (0) | 0 (0) |
| Anti-TNF (n = 46) | 25 (54) | 18 (39) | 3 (7) | 0 (0) | 0 (0) |
Discussion
This long-term observational study of the PISA-II cohort showed that after a median follow-up of 5.7 years, 42% of patients in the surgical closure arm reached long-term radiological healing and 72% reached persistent clinical closure. In contrast, 18% of patients in the anti-TNF alone arm reached long-term radiological healing and 62% reached long-term clinical closure, which was after a surgical (re-)intervention in more than half of the patients. No recurrences occurred in any of the patients that reached radiological healing during follow-up, even in patients that stopped anti-TNF. These follow-up results confirm the notion that radiological healing is a valuable prognostic parameter for long-term fistula treatment success and should be the aim in fistula treatment. Furthermore, it can be used as a guide to stop biological therapy if given for perianal disease only. It furthermore encourages the use of surgical closure following anti-TNF induction as first-line treatment for high perianal fistulas with a single internal opening in patients with Crohn's disease.
This is the first study to directly compare long-term outcomes after short-term anti-TNF therapy combined with surgical closure to anti-TNF therapy. Results of surgical closure were favourable, especially in terms of radiological healing. Previous studies showed comparable radiological healing rates after anti-TNF treatment ranging from 20% to 32% and higher radiological healing rates after surgical closure ranging from 48% to 56%.4,9, 10, 11 Acceptance of small residual abnormalities on MRI could explain these higher radiological healing rates.4,12 Our results showed that even a slight residue on MRI can result in a recurrence as indicated in Fig. 3, whereas no recurrences occurred in patients with radiological healing (i.e. deep healing).
Currently, the European Crohn's and Colitis Organisation guideline states that clinical assessment (a decrease in fistula drainage) is sufficient to evaluate response to treatment and only recommends an additional MRI to evaluate improvement of fistula inflammation.13 Also, the Selecting Therapeutic Targets in IBD program (STRIDE-II) does not define therapeutic targets for perianal fistulas to be used for a ‘treat-to-target’ strategy.14 However, in our study, recurrences occurred in 36% of patients that reached clinical closure sometime during follow-up and no recurrences occurred in patients with radiological healing. This emphasizes the importance of evaluation of fistula activity on MRI, especially when considering stopping anti-TNF. In literature it has already been suggested that MRI results best correlate with treatment response and can predict long-term outcome.3,15 Our study confirms that radiological healing on MRI – so a completely fibrotic fistula tract – is a reliable prognostic parameter for lasting clinical closure and should be a therapeutic target for perianal fistulas in patients with Crohn's disease.
Our results are in line with previous publications.16 In the anti-TNF treatment arm, more than half of the patients needed a re-intervention, and 78% of those underwent a surgical closure procedure before reaching long-term clinical closure. This could explain why patients in the surgical closure arm reached long-term clinical closure significantly earlier than patients in the anti-TNF treatment arm. Overall, we found that 29% of patients reached long-term clinical closure purely on anti-TNF treatment. This is comparable to the 35% maintained remission rates described in the systematic review of Lee et al.17 The slightly higher percentage could be explained by the shorter follow-up period of 26 and 54 weeks in the evaluated studies.18,19 Interestingly, in the current study, long-term clinical closure was associated with a higher median trough levels, and the majority of recurrences in the patient group with available trough levels were associated with a decrease to under 3.5. This could be another argument to aim for radiological healing, as although anti-TNF treatment can be continued for years, antibody formation, adverse events, development of an allergy, or a patient's wish to stop medication is seen quite frequently. The current data however suggest that stopping anti-TNF can only be advised when complete radiological healing has been achieved.
Patients treated with anti-TNF therapy only may benefit from higher trough levels.5,20 In our study we saw that in some patients timing of recurrences was associated to either a swift drop in anti-TNF trough level or a suboptimal anti-TNF trough level. Previous studies confirm this and showed that in patients treated with anti-TNF therapy without radiological healing, clinical recurrences can occur after cessation of therapy.4,10,21 We found that only patients without radiological healing had a recurrence. Current literature furthermore suggests that fistula closure is more likely with higher anti-TNF trough levels than needed for luminal disease.5,22, 23, 24 This could be due to higher production of local TNF in the fistula tract itself and the surrounding tissue.25 However, there is an ongoing discussion about what the most optimal therapeutic range for anti-TNF as treatment of perianal fistulas in patients with Crohn's disease is, for both infliximab and adalimumab.5,7,23,26,27 Obtaining clarity on this is especially important since higher trough levels possibly increase the chance of developing (serious) side effects, and a possible association has been found that higher anti-TNF concentrations may lead to higher overall disease burden and lower quality of life,28,29 although there are also studies that draw contrary conclusions.30, 31, 32
Control of pain and incontinence are among the main targets of treatment of perianal fistulas, and the latter is one of the most common reasons for patients to refrain from surgical procedures. Interestingly, the DRS showed us that 100% of the patients in the surgical closure arm agreed or strongly agreed that undergoing surgery was the right decision, including the nine patients (30%) with more incontinence problems after surgical closure, compared to 78% in the anti-TNF arm. This was in line with the Perianal Disease Activity Index (PDAI) after 18 months in the PISA-II trial, the reference standard for evaluating severity of perianal disease ranging from 0 to 20 points, with a median of 1 in the surgical closure arm and a median of 4 in the anti-TNF treatment arm (≤5 is indicative of non-severe disease activity).2 Our previous retrospective study also showed that almost half of the patients even had an improved continence after surgical closure and that in one third of the patients continence was clinically unaffected by the procedure.11 The DRS also showed that 13% of patients in the surgical closure arm, and 9% in the anti-TNF arm felt that their choice did them a lot of harm. Nevertheless, all patients in the surgical closure arm would furthermore go for the same treatment if they had to do it over again, whereas 11% of the patients in the anti-TNF group would not go for the same treatment. All this suggests that other factors might be more important than continence, such as recurrences or need for a re-intervention.
It is important that patients are properly counselled about their treatment options, especially since fistula treatment is all about quality of life. Counselling would optimally entail a consultation with both a surgeon and gastroenterologist. This multidisciplinary approach is gradually finding its way to standard practice and is increasingly recommended for patients with perianal Crohn's disease.13,33,34 Counselling patients for surgical closure should start early, since a significant proportion of patients in the anti-TNF treatment arm end op undergoing a surgical closure procedure during long-term follow-up anyway.
When interpreting current data, some limitations should be considered. The presented long-term results are for a specific type of perianal fistula (high, with one internal opening and without proctitis) and may therefore not be generalised to other perianal fistula types. It should also be realised that, after 18 months, no structured follow-up or treatment protocol was committed for the PISA-II patients. Therefore, data collected by medical chart review were in some patients incomplete, including data on smoking and on stopping, or continuing anti-TNF therapy in the surgical closure group, which might have contributed to the prevention of fistula recurrences. Incomplete data were complemented by data collected by telephone contact and, if needed, e-mail, which might have influenced the accuracy. Patients were also retrospectively asked about their pre-treatment continence, which could result in recall bias. However, a global change question was used to lower this risk.
Although therapeutic drug monitoring nowadays is becoming more prevalent, this was still quite unusual at the start of the study back in 2013. As anti-TNF trough levels were not part of the initial predefined outcomes, they were irregularly measured and available in a minority of patients. There may therefore be selection bias as to who received testing. However, the available data on trough levels are in line with previous literature.
In conclusion, this long-term follow-up study shows favourable long-term outcomes of short-term anti-TNF therapy combined with surgical closure compared to anti-TNF therapy alone as treatment of perianal fistulas with a single internal opening in patients with Crohn's disease. We found comparable clinical closure rates, however, after re-interventions in more than half of the patients in the anti-TNF treatment arm. We also found higher radiological healing rates after surgical closure with no recurrences even after stopping anti-TNF, which supports the finding that radiological healing – so a completely fibrotic fistula tract - should be the aim of treatment.
These data therefore support that patients with a perianal fistula and Crohn's disease amenable for surgical closure should be offered this in combination with short-term anti-TNF therapy in order to increase the chance of achieving deep radiological healing.
Contributors
GRD, CYP, KBG, WAB and CJB initiated this study and designed the trial. MGWD contributed with the statistics. All other authors contributed to the study conception and data collection. The underlying data was accessed, verified and interpreted by EMMP and CJB. The first draft of the manuscript was written by EMMP and CJB. All other authors critically reviewed previous versions of the manuscript and approved the final manuscript for submission.
The underlying data was accessed, verified and interpreted by EMMP and CJB, and these authors had final responsibility for the decision to submit for publication.
Data sharing statement
Bonafide, qualified, scientific and medical researchers may request access to deidentified study data by contacting the corresponding author. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data access.
Declaration of interests
EMMP, MAJB, KLR, KATGMW, JS, MGWD, and AS have nothing to declare.
GRH has received grants from Bristol Meiers Squibb, Pfizer and Takeda, has served as a consultant and/or received speaker's fees from AbbVie, Alimentiv, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion Healthcare, Cytoki Pharma, Eli Lilly, Ferring, Galapagos, Glaxo SmithKline, Gossamer Bio, Immunic, InDex Pharmaceuticals, Janssen, Johnson & Johnson, Kaleido, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Takeda, Tillotts, Ventyx Biosciences. He furthermore received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer, Boehringer Ingelheim, Takeda and Abbvie, and participated on a Data Safety Monitoring Board or Advisory Board of Astrazeneca, Abbvie and Seres Health.
CYP has received grants from Perspectum and Gilead; consulting fees from Shire, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Tillotts.
KBG has received grants from Pfizer, Galapagos, Celltrion, consultancy fees from.
AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, ImmunicTherapeutics, Janssen Pharmaceuticals, Novartis, Pfizer Inc., Samsung Bioepis and Takeda, and speaker's honoraria from Celltrion, Ferring, Janssen Pharmaceuticals, Novartis, Pfizer Inc, Samsung Bioepis, Takeda and Tillotts.
SD has received consulting fees from Abbvie, Alimentiv, Allergan, Amgen, AstraZaneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial and Vifor. He furthermore received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer and Takeda.
WAB has received grants from Braun and Vifor, and has served as speaker for Takeda, Johnson and Johnson and Braun.
CJB has an unrestricted grant from Boehringer Ingelheim and Roche. She has also received consultancy fees and/or speaker's honoraria from AbbVie, MSD, Tillotts, Janssen and Takeda.
Acknowledgments
This paper was presented during the annual meeting of the European Crohn and Colitis Organisation (February 2022).
References
- 1.Wasmann K.A., de Groof E.J., Stellingwerf M.E., et al. Treatment of perianal fistulas in crohn's disease, seton versus anti-TNF versus surgical closure following anti-TNF [PISA]: a randomised controlled trial. Journal of Crohn's & colitis. 2020;14(8):1049–1056. doi: 10.1093/ecco-jcc/jjaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meima-van Praag E.M., van Rijn K.L., Wasmann K.A.T.G.M., et al. Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): a patient preference randomised trial. Lancet Gastroenterol Hepatol. 2022;7(7):617–626. doi: 10.1016/S2468-1253(22)00088-7. [DOI] [PubMed] [Google Scholar]
- 3.Maaser C., Sturm A., Vavricka S.R., et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. Journal of Crohn's & colitis. 2019;13(2):144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 4.Tozer P., Ng S.C., Siddiqui M.R., et al. Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn's perianal fistulas. Inflamm Bowel Dis. 2012;18(10):1825–1834. doi: 10.1002/ibd.21940. [DOI] [PubMed] [Google Scholar]
- 5.Strik A.S., Löwenberg M., Buskens C.J., et al. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn's disease patients. Scand J Gastroenterol. 2019;54(4):453–458. doi: 10.1080/00365521.2019.1600014. [DOI] [PubMed] [Google Scholar]
- 6.van Rijn K.L., Meima-van Praag E.M., Bossuyt P.M., et al. Fibrosis and MAGNIFI-CD activity index at magnetic resonance imaging to predict treatment outcome in perianal fistulizing crohn's disease patients. Journal of Crohn's & colitis. 2022;16(5):708–716. doi: 10.1093/ecco-jcc/jjab168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plevris N., Jenkinson P.W., Arnott I.D., Jones G.R., Lees C.W. Higher anti-tumor necrosis factor levels are associated with perianal fistula healing and fistula closure in Crohn's disease. Eur J Gastroenterol Hepatol. 2020;32(1):32–37. doi: 10.1097/MEG.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 8.Brehaut J.C., O'Connor A.M., Wood T.J., et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 9.Jayne D.G., Scholefield J., Tolan D., et al. Anal fistula plug versus surgeon's preference for surgery for trans-sphincteric anal fistula: the FIAT RCT. Health Technol Assess. 2019;23(21):1–76. doi: 10.3310/hta23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng S.C., Plamondon S., Gupta A., et al. Prospective evaluation of anti-tumor necrosis factor therapy guided by magnetic resonance imaging for Crohn's perineal fistulas. Am J Gastroenterol. 2009;104(12):2973–2986. doi: 10.1038/ajg.2009.509. [DOI] [PubMed] [Google Scholar]
- 11.van Praag E.M., Stellingwerf M.E., van der Bilt J.D.W., Bemelman W.A., Gecse K.B., Buskens C.J. Ligation of the intersphincteric fistula tract and endorectal advancement flap for high perianal fistulas in crohn's disease: a retrospective cohort study. Journal of Crohn's & colitis. 2020;14(6):757–763. doi: 10.1093/ecco-jcc/jjz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panés J., García-Olmo D., Van Assche G., et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet (London, England) 2016;388(10051):1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 13.Gionchetti P., Dignass A., Danese S., et al. European evidence-based consensus on the diagnosis and management of crohn's disease 2016: Part 2: surgical management and special situations. Journal of Crohn's & colitis. 2017;11(2):135–149. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 14.Turner D., Ricciuto A., Lewis A., et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 15.van Rijn K.L., Lansdorp C.A., Tielbeek J.A.W., et al. Evaluation of the modified Van Assche index for assessing response to anti-TNF therapy with MRI in perianal fistulizing Crohn's disease. Clin Imag. 2020;59(2):179–187. doi: 10.1016/j.clinimag.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Yassin N.A., Askari A., Warusavitarne J., et al. Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn's disease. Aliment Pharmacol Therapeutics. 2014;40(7):741–749. doi: 10.1111/apt.12906. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.J., Parker C.E., Taylor S.R., et al. Efficacy of medical therapies for fistulizing crohn's disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(12):1879–1892. doi: 10.1016/j.cgh.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber S., Lawrance I.C., Thomsen O., Hanauer S.B., Bloomfield R., Sandborn W.J. Randomised clinical trial: certolizumab pegol for fistulas in Crohn's disease - subgroup results from a placebo-controlled study. Aliment Pharmacol Therapeutics. 2011;33(2):185–193. doi: 10.1111/j.1365-2036.2010.04509.x. [DOI] [PubMed] [Google Scholar]
- 19.Sands B.E., Anderson F.H., Bernstein C.N., et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 20.Gu B., Venkatesh K., Williams A.J., et al. Higher infliximab and adalimumab trough levels are associated with fistula healing in patients with fistulising perianal Crohn's disease. World J Gastroenterol. 2022;28(23):2597–2608. doi: 10.3748/wjg.v28.i23.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Assche G., Vanbeckevoort D., Bielen D., et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol. 2003;98(2):332–339. doi: 10.1111/j.1572-0241.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 22.Ungar B., Levy I., Yavne Y., et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14(4):550–557.e2. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Yarur A.J., Kanagala V., Stein D.J., et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn's disease. Aliment Pharmacol Therapeutics. 2017;45(7):933–940. doi: 10.1111/apt.13970. [DOI] [PubMed] [Google Scholar]
- 24.Davidov Y., Ungar B., Bar-Yoseph H., et al. Association of induction infliximab levels with clinical response in perianal crohn's disease. Journal of Crohn's & colitis. 2017;11(5):549–555. doi: 10.1093/ecco-jcc/jjw182. [DOI] [PubMed] [Google Scholar]
- 25.Scharl M., Weber A., Fürst A., et al. Potential role for SNAIL family transcription factors in the etiology of Crohn's disease-associated fistulae. Inflamm Bowel Dis. 2011;17(9):1907–1916. doi: 10.1002/ibd.21555. [DOI] [PubMed] [Google Scholar]
- 26.El-Matary W., Walters T.D., Huynh H.Q., et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal crohn's disease in children. Inflamm Bowel Dis. 2019;25(1):150–155. doi: 10.1093/ibd/izy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Gregorio M., Lee T., Krishnaprasad K., et al. Higher anti-tumor necrosis factor-α levels correlate with improved radiologic outcomes in crohn's perianal fistulas. Clin Gastroenterol Hepatol. 2022;20(6):1306–1314. doi: 10.1016/j.cgh.2021.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Hendler S.A., Cohen B.L., Colombel J.F., Sands B.E., Mayer L., Agarwal S. High-dose infliximab therapy in Crohn's disease: clinical experience, safety, and efficacy. J Crohn's Colitis. 2015;9(3):266–275. doi: 10.1093/ecco-jcc/jju026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandse J.F., Vos L.M., Jansen J., et al. Serum concentration of anti-TNF antibodies, adverse effects and quality of life in patients with inflammatory bowel disease in remission on maintenance treatment. Journal of Crohn's & colitis. 2015;9(11):973–981. doi: 10.1093/ecco-jcc/jjv116. [DOI] [PubMed] [Google Scholar]
- 30.Bonovas S., Fiorino G., Allocca M., et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14(10):1385–1397.e10. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Moćko P., Kawalec P., Pilc A. Safety profile of biologic drugs in the treatment of inflammatory bowel diseases: a systematic review and network meta-analysis of randomized controlled trials. Clin Drug Invest. 2017;37(1):25–37. doi: 10.1007/s40261-016-0459-y. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein G.R., Feagan B.G., Cohen R.D., et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107(9):1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz D.A., Ghazi L.J., Regueiro M., et al. Guidelines for the multidisciplinary management of Crohn's perianal fistulas: summary statement. Inflamm Bowel Dis. 2015;21(4):723–730. doi: 10.1097/MIB.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 34.Lamb C.A., Kennedy N.A., Raine T., et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]