Abstract
Contrast agents is used in magnetic resonance imaging (MRI) to improve the visibility of the details of the organ structures. Gadolinium-based contrast agent (GBCA) has been used since 1988 in MRI for diagnostic and follow-up of patients, the gadolinium good properties make it an effective choice for enhance the signal in MRI by increase its intensity and shortening the relaxation time of the proton. Recently, many studies show a gadolinium deposition in different human organs due to release of free gadolinium various body organs or tissue, which led to increased concern about the use of gadolinium agents, in this study, the potential diseases that may affect the patient and side effects that appear on the patient and related to accumulation of gadolinium were clarified, the study focused on the organs such as brain and bones in which gadolinium deposition was found and the lesions associated with it, and the diseases associated with gadolinium retention includes Nephrogenic Systemic Fibrosis (NSF) and Gadolinium deposition disease (GDD). Some studies tended to improve the contrast agents by developing a new non-gadolinium agents or development of next-generation gadolinium agents. In this review article the latest knowledge about MRI contrast agent.
Keywords: MRI, Contrast medica, GBCA, GDD, NSF
1. Introduction
Magnetic resonance imaging (MRI) is non-invasive modality that uses radiofrequency to generate diagnostic images. MRI is characterized by its high image accuracy and considerable spatial resolution [1], [2], the anatomy and physiology of the patient can be accurately visualized using MRI [3]. There are many applications of MRI not only neuro-angiography [4] like Computed tomography and ultrasound [5], [6], [7]. MRI has become widely utilized in medical applications due to its excellent soft tissue contrast differentiation [8], and also it shared the safety procedure as ultrasonic modality [9], [6], [10], since the principle of MRI does not produce images using ionizing radiation [3].
MRI works on the basis of a magnetic field that primarily affects the distribution of hydrogen protons in water molecules and other odd elements throughout the body[11], [12], [13]. The basic physics in MRI dependent on radiofrequency (RF) electromagnetic signals [14], [15]. By relatively raising the static magnetic field intensity of magnets, the signal-to-noise ratio (SNR), spectrum dispersion, and susceptibility contrast can all be enhanced, but there are concerns with using a very strong magnetic field and the gradients that MRI magnets produce [16].
In order to improve the resolution and sensitivity of the MRI technique, the current trend in the MRI industry is toward strengthening the magnetic field, which necessitates the design of superconducting magnets. There is an increased likelihood that static magnetic fields will cause mechanical forces, which is dangerous [3]. Therefore, most MRI examinations use contrast agents to enhance image contrast and improve the image, different types of contrast agent are used, gadolinium-based contrast agent is the most common agent used in MRI due to its good properties, but several studies show gadolinium retention in human organs and tissues [17], [18]. In this study, gadolinium properties will be identified, advantages and disadvantages as a contrast agent, the different organs and tissues in which the gadolinium is likely to accumulate, the side effects and diseases caused by gadolinium accumulation, and the methods for measuring the amount of gadolinium in tissues.
2. Contrast agents in MRI
Since the manufacturer began using Gadolinium-based contrast agents, there have been more than 620 million contrast-enhanced MRI procedures. Today, there are roughly 30 million procedures annually in all countries around the world, and more than 8,000,000 liters of contrast agents are used [19]. According to earlier research, 40–50% of MRI examinations use contrast agents for their investigations, the contrast agent (CA) is used in the MRI technique to provide a strong signal in various tissues and so enhance the image contrast. A few contrast compounds are helpful in drug delivery targeting and early tumor identification [4], and contrast-enhanced MRI is significant for providing advanced pictures and disease management [20]. CA enhances image contrast by reducing relaxation durations due to its special characteristics, making pathological tissues appear in distinct signal from normal tissues [12] [1] [21].
The characteristics of MRI contrast agents should have strong stability, water solubility, non-toxicity and good circulation time for faster reach to the target, ability to be quickly released outside the body and the capability to reach a specific target [1], [22], [23]. Using affinity receptors on tumor target molecules, CAs are attached to targeting cells to achieve high selectivity of a specific target, where a high concentration contrast is used in cases where binding affinity is low [24], [25], [26]. A hydrophobic group like phenyl can be inserted to increase the target ability of the contrast agent, which was a tiny molecule with a relaxation efficiency of 3.5–5.5 mM/s, and was unable to track a specific target. Moreover, Porphyrins can also improve the target ability by binding them into ligands since they have a strong affinity for tumor tissue [1]. Based on relaxivity, chelate chemistry, viscosity, ionicity, stability and osmolality, CA differ from one another [24], [27], [28], which results in different types of CAs for clinical uses.
T1 and T2 contrast agents are the two different types of MRI contrast agents that are employed in clinical applications. Positive CAs are areas where T1-agents (T1, longitudinal relaxation) increase the signal intensities (SI) between tissues. Paramagnetic elements including gadolinium (Gd (III)) and iron (Fe (III)) are examples of T1 based contrast agents [29]. Negative CAs, also known T2-agents (T2, transverse relaxation), are large ferromagnetic iron oxide particles, compared to T2- CAs images. Comparing between two types, T1- CAs images exhibit a higher spatial resolution [12], [4].
To calculate the signal intensity (SI) between tissues, MR image's contrast are dependent on a number of factors, as illustrated in the following equation:
Where N is the proton spin density, T1 is the longitudinal relaxation time, T2 is the transverse relaxation time, TR is the repetition time, and TE is the echo time [30].
3. Gadolinium-based contrast agents (GBCAs)
In contrast enhanced magnetic resonance imaging (CE-MRI), the CA is intravenously administrated in patients, and then it diffuse in the blood [13], [3]. Gadolinium-based contrast agents (GBCAs) have been utilized in MRI in 1988 and have been administered more than 500 million times annually [31]. GBCAs are hydrophilic, anionic or neutral complex compounds that contain water coligand and octadentate chelator [32]. It is non-invasive and they are used for both diagnostic and follow-up purposes [33]. Comparing with CAs used in medical modalities, gadolinium contrast has a reduced risk of acute responses than iodine contrast, there is no substitute for MRI imaging that provides the same benefits as GBCAs due to gadolinium paramagnetism [34], [13].
The paramagnetic ions are necessary for the CAs in MRI to improve image contrast. This visual improvement is brought on by ions' unpaired electrons’ sensitivity to outside fields. The well-known ion utilized in MRIs is gadolinium (Gd3+), a paramagnetic ion that exhibits the greatest enhancement of the MRI image [13]. Gadolinium element is a naturally occurring heavy white silver rare earth metal with an atomic number of 64 and an ionic radius of 0.99 Å [35], [3], [36].
It is thought to be ferromagnetic in bulk [8] and also gadolinium ion characterized by strong paramagnetic properties above 68°F [37]. It is the most paramagnetic metal ion used in contrast agents due to its paramagnetic properties such as, and the presence of 7 unpaired electrons in the inner 4 f shell. Since it possesses the maximum number of unpaired electrons of any stable ions [20], [2], [12], it is considered an effective choice for enhancing the signal by increase its intensity and shortening the relaxation time of the proton [20], [25].
The ligand in metal complex which added into the GBCAs controlled the localization, behavior, and distribution in biological settings [21]. Free Gd+3 ion must be bonded with ligand since it is toxic [2]. Gadolinium requires a carrier molecule (chelating agent) to modify gadolinium ion distribution [38], and as a result, Gd+3 chelates with an organic ligand to reduce the release of toxic free gadolinium ion [39] and prevent gadolinium from connecting with tissues, making it safe to use in patients [13]. Gadolinium paramagnetism is unaffected when gadolinium links with a molecule or an anion in the majority of GBCAs that include one gadolinium element with chelate [40], reducing the toxicity of free Gd+3 ions while maintaining its properties [3]. However, un-chelated gadolinium can release when there are high concentrations of other cations. When free Gd+3 ion is released from an organic ligand, it can be associated with endogenous anions like CO3 2- and PO4 3-, which causes the formation of insoluble compounds that are free in the bloodstream and deposited in the target tissues [39].
The attachment of Gd+3 with chelate is dependent on thermodynamic stability, which refers to how much Gd+3 is released, and kinetic inertia, which it is the rate at which Gd+3 is released [13]. High kinetic stability means that gadolinium dissociation rate is slower than the elimination rate in patient body, and a small or negligible amount of free gadolinium is release during residence time in the body. The kinetic stability is the dissociation speed of gadolinium from its chelate. Free gadolinium is easily liberated from its chelates with high thermodynamic stability. In other words, thermodynamic stability is an equilibrium state between chelated and unchelated gadolinium. There are numerous methods for determining the thermodynamic stability constant (log Ktherm), including pH potentiometry, which records the value of pH changes, spectrophotometry, which records the value of absorbance intensity changes, and proton relaxometry. At lower pH levels, protons compete with gadolinium ions for binding to ligands. The conditional thermodynamic stability constant (log Kcond), which was calculated at 7.4 pH using a 1:1 ligand-to-metal ratio, takes this into consideration. Log Kcond is measured from log K and ligand protonation constants [21].
Other factors that influence GBCAs stability include ions concentration and the timing of interactions between gadolinium chelates and competitors. Gadolinium contrast agents are surrounded by competitors, such as endogenous cations that compete with free gadolinium ions for the ligand and endogenous anions, such as phosphate and carbonate, that compete for the free gadolinium ions [41].
The GBCAs stability also depends on the osmolality and viscosity. Since they are quickly excreted from the body and have low osmolality and low viscosity, GBCAs are generally thought to be safe and to have a high contrast efficiency. Low osmolality and viscosity are crucial for obtaining rapid infusion without causing an acute reaction [36].
Another factor controlled the contrast agents' effects is relaxivity. To reduce the amount of contrast agent required, contrast relaxivity should be increased. Relaxivity is the ability of contrast to increase the relaxation rate of surrounding water proton [39], and it is measurement of the changes in water proton relaxation rate with contrast agent concentration. GBCAs hydration state is associated with relaxivity of agents; the higher the relaxivity, the lower the thermodynamic stability. The hydration state is the number of organized water molecules in the inner sphere, and it contributes to contrast agent relaxivity. Increasing the hydration state is associated to an increase in relaxivity based on following equation:
Where the c represents concentration, q represents hydration state, r represents relaxivity, T1 m represents longitudinal water proton relaxation time, and τm represents water exchange lifetime.
Because trivalent gadolinium occupies 9 coordination number, one free accessible coordination site for water molecule in the inner sphere remains, enhancing the relaxation rate of the molecule and providing contrast. Furthermore, to improve contrast agent relaxivity, the contrast hydration state should be raised by limiting the number of coordination sites provided by the ligand. However, this reduces the contrast agent’s thermodynamic stability and allows gadolinium ion to reach endogenous anions, which raises toxicity concerns [21].
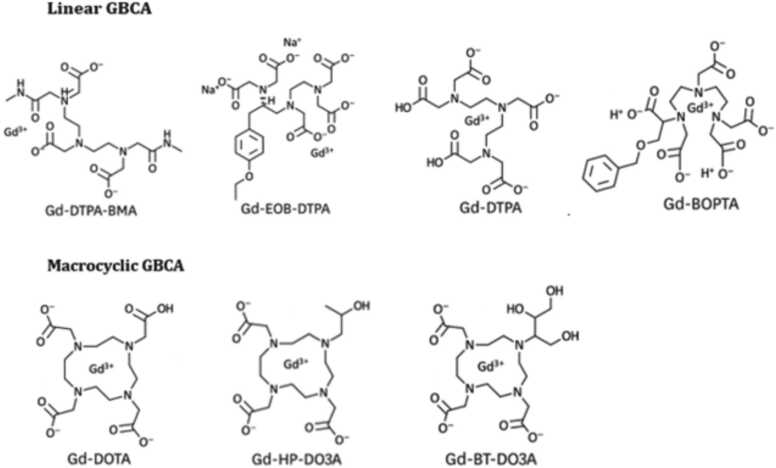
High-affinity bonds, which can be either linear or macrocyclic chelates, bind the gadolinium ion to their respective molecules [20], Since free gadolinium ions are toxic, the contrast agent’s ligand will determine whether contrast is classified as linear or macrocyclic [35]; liner agents have a chain-like structure that holds the Gd+3 while macrocyclic agents have a ring-like or cage-like structure [13]. Although linear has a greater potential to release gadolinium in the body because it is less strongly bound to gadolinium than macrocyclic [9], cage-like cyclic GBCAs are more stable than linear due to their higher kinetic inertness [42] and higher stability constant [2]. Fig. 1.
Fig. 1.
Types of linear and macrocyclic GBCAs.
Several polyaminocarboxylic acid ligands connected to Gd+ 3 to enhanced the ability of CA in MRI signal intensity including DOTA, DOTPA, DO3A, DTTA, DTPA and EDTA [12]. Examples of linear GBCA include gadopentetate dimeglumine (Gd-DTPA), gadoxetate disodium (Gd-EOB-DTPA), gadobenate dimeglumine (Gd-BOPTA), gadodiamide (Gd-DTPA-BMA), Gadoversetamide (Gd-DTPA-BMEA), and others. Gadobutrol (Gd-DO3A-butrol), gadoteridol (Gd-HP-DO3A), gadoterate meglumine (Gd-DOTA), and others are examples of macrocyclic GBCA [33] [43] [36]. Table 1 shows the structure, ionicity, stability, osmolality, viscosity, Log Ktherm, Log Kcond, and T1/2 of these different gadolinium contrast agents.
Table 1.
Demonstrates the structure, ionicity, stability, osmolality, viscosity, Log Ktherm, Log Kcond, and T1/2 of various gadolinium contrast agents.
| Gd-EOB-DTPA | Gd-DTPA-BMA | Gd-DTPA-BMEA | Gd-HP-DO3A | Gd-BOPTA | Gd-DO3A-butrol | Gd-DOTA | Gd-DTPA | |
|---|---|---|---|---|---|---|---|---|
| Structure | Linear | Linear | Linear | Macrocyclic | Linear | Macrocyclic | Macrocyclic | Linear |
| Ionicity | ionic | Nonionic | Nonionic | Nonionic | ionic | Nonionic | ionic | ionic |
| Stability | Intermediate | Low | Low | High | Intermediate | Intermediate | High | Intermediate |
| Osmolality | 688 | 789 | 110 | 630 | 1970 | 1603 | 1350 | 1960 |
| Viscosity | 12 | 1.4 | 2 | 1.3 | 5.3 | 5 | 2 | 2.9 |
| Log Ktherm | 23.5 | 16.9 | 16.6 | 23.8 | 22.6 | 21.8 | 25.6 | 22.1 |
| Log Kcond | 18.7 | 14.9 | 15 | 17.1 | 18.4 | 14.7 | 19.3 | 17.7 |
| T1/2 | < 5 s | < 5 s | < 5 s | 3.9 h | < 5 s | 43 h | 338 h | < 5 s |
The organic molecular chain warps around Gd+3 ion in linear, where it is elongated and flexible ligand [44], whereas in macrocyclic the ligand is preorganized into a rigid cage that chelates the ion. The presence of these types in ionic or non-ionic ligand affects the stability of the gadolinium-based contrast agent, where the ion has a strong binding with carboxyl groups in ionic ligand and a weakly binding with amide or alcohol functional groups in non-ionic ligand. Because negatively charged atoms bind more strongly to Gd+3, the ionic GBCAs are more stable than nonionic GBCAs [3] [13]; however, ionic GBCAs have higher osmolality [36]. The most stable and least toxic ionic-macrocyclic gadolinium-based contrast agents, such as gadoterate meglumine (Gd-DOTA), are recommended for clinical use, while nonionic linear chelates are the least stable [3] [13]. According to an in vitro study [45], kinetic stability varies depending on the agent type, with free gadolinium dissociating from its chelate in half-life of 35 s in pH of 1 in nonionic linear agent such as gadodiamide, while gadolinium in ionic macrocyclic agent such as gadoterate taking up to one month to dissociate in the same conditions [44].
GBCAs are categorized into extracellular agents, extracellular-intracellular agents, and intracellular agents based on their bio-distribution. For the extracellular compartment, the IV injection chelate is disseminated into the interstitial fluid compartments and intravascular. Alternatively, gadolinium chelate may circulate intracellularly, such as in the liver and kidney, via absorption mechanisms or passive diffusion, depending on the structure of the gadolinium chelate agents. Extracellular-intracellular agents are disseminated to extracellular and intracellular hepatocyte compartments; examples are gadobenate dimeglumine and gadoxetate disodium [41].
The half-life of GBCA is 90 min in healthy people and more than 30 h in patients with renal failure [46], 9.2 h in patients with severe chronic kidney disease [2], GBCAs can be used in different clinical applications such as imaging the central nervous system (CNS), vascular permeability detection, cardiac imaging, and cancer staging and detection [13]. it is also used to improve the contrast between the tumor and normal cells and to measure perfusion [8]. GBCAs are useful in pediatric patients' MRI scans for a variety of purposes, including the diagnosis and follow-up of central nervous system (CNS) tumors [34], the detection of small CSF flow disturbances, and the diagnosis of CNS tumors that are difficult to recognize without contrast enhancement [47].
Multiple sclerosis (MS) patients undergo regular MRI scans with GBCAs for follow-up. Patients with MS who received at least two doses of gadodiamide showed an increase in dentate nucleus T1 hyperintensity on unenhanced MRI; this increase was seen in linear GBCA [13], [35]. A study, which has been refuted by numerous other studies due to contradictions, found that patients with relapsing-remitting multiple sclerosis who were exposed to macrocyclic GBCA gadobutrol dosages experienced an increase in globus pallidus and dentate nucleus signal intensity [48]. This image can identify active inflammation during the inflammation process by detecting contrast lakes in the brain parenchyma in blood–brain barrier (BBB) disrupted areas [49]. The degree of grey matter (GM) atrophy seen on MRI in MS patients indicates a neurodegenerative change in the CNS, and the degree of atrophy is correlated with physical disability [50]. Increases in T1 signal intensity ratio were observed to be associated with cognitive impairment [51] in a follow-up study [52] of patients with multiple sclerosis.
In cardiac magnetic resonance (CMR), GBCA diffuse into the extracellular space and accumulate in the interstitial fluid of healthy and affected myocardium due to their slower washout and increased distribution in sick myocardium that enhance retention of the GBCAs. In the late gadolinium enhancement (LGE) technique used in CMR, Gadolinium retention in expanded extracellular space can be seen in fibrosis and myocardial disarray, allowing characterization of myocardial diseases based on assessment of myocardial scar distribution [39].
4. Effects of contrast agents on the MR Image
Contrast agent used during application to increase the specificity, sensitivity and visibility of images by altering the intrinsic properties of tissues [39]; it contains various pharmaceuticals [9]. Using contrast in MRI massively enhances the signal intensity by binding to serum proteins, and highlights contrast [44] [35], where the area where the gadolinium contrast accumulate is bright on T1-weighted images [50]. In T1- and T2-weighted images, the relaxation time of the water proton is affected, due to high magnetic moment that Gd produces, the GBCA shortens the T1 and T2 relaxation times, improving contrast and increased signal in an MRI scan [13], the nuclear spin relaxation that results from the magnetic dipole–dipole interaction between Gd+3 unpaired f-electrons and water molecules is what causes the longitudinal relaxation time T1 to become shorten and the T1-weighted MRI images to become much brighter and have better contrast. CAs also promotes the contrast of soft tissues, which is useful for identifying pathologies such as fibrosis, inflammatory processes, marrow disorders, malignant conditions and perfusion disorders [53].
Gd+3 complex MRI probes utilize the inner-sphere water molecules changes (q), rotational times (τR) and the mean lifetime (τM) of water molecule bounded to Gd+3 [12], The relaxation efficiency of T1 contrast agent is affected by (τR) and (τM) of the water molecule [54]. The signal intensity is influenced by the longitudinal relaxation time (T1), transverse relaxation time (T2), and Spin density (ρ) [55]. The agent's longitudinal relaxivity (r1) and transverse relaxivity (r2) values determine its capacity to reduce the T1- and T2- relaxation times [12].
Age-related changes in signal intensity have recently been given some thought as a potential source of gadolinium-dependent brain hyperintense. The T2 in gray matter nuclei and basal ganglia is shortened as a result of increased mineralization brought on by aging processes that change the distribution of paramagnetic elements, create weight matter hyper intensities, and cause quantitative neurological changes that affect MRI metrics [42].
Recently, gadolinium- based contrast agents were incorporated into nanoparticles; the relaxation rate is influenced by the nanoparticle size [1]. Based on pHs distinctions across of tissues, stimulus-responsive nanoparticles, such as pH responsiveness, are crucial for tumor targeting. The internalization of contrast agents is negatively impacted by negative charge contrast agents, which have a single surface charge, extended metabolic times, and small molecule size. Positive charges are it is easily removed by blood vessels, accelerate the active internalization of contrast agents by tumor cells, and increase the accumulation of contrast agents in tumors by promoting the permeability and retention (EPR) effect. As a result, the pH-responsive capability and the use of contrast agents provide better MR imaging of tumors [4]. According to several studies, contrast agents utilized in nanoparticles form provide agents with great efficiency and sensitivity. The size of the nanoparticles is around between 3 and 350 nm. Nanoparticles can be coated with substances like diethyleneglycol (DEG) to lessen their toxicity [30].
5. Gadolinium deposition toxicity
Concerns about gadolinium-based contrast agent and its considerable side effects have arisen due to increased usage of MRI in diagnostic and follow-up procedures for some patients who need to receive GBCA repeatedly [3]. Despite being widely used, it has a number of shortcomings, including a short circulation time and the lack of a target specificity [1], the non-tissue specificity, high metabolic rate, low longitudinal relative axiality (r1) and toxicity of GBCAs are some of the issues that impact their use in clinical settings [4]. Although GBCAs have a safety profile and don't aggravate renal insufficiency [2], uncommon cases such as episodes of neurotoxic and severe hypersensitivity reactions have been known to occur [31].
Mild reactions are likely to occur after clinical GBCAs administration and include nausea, vomiting, warmth, pain at the injection site, paraesthesia, coldness, headache, and dizziness. They are rare reactions, with incidences between 0.07% and 2.4% [34], and they may be brought on by the contrast agent directly damaging cells with direct toxicity due to an increase in osmolality which is the concentration of solutes in a fluid (number of particles / weight) [56]; however, allergic-like reaction rates only range from 0.004% to 0.7% [34], and the incidence of severe reactions is only about 0.001% [31]. Acute adverse events in cardiac magnetic resonance (CMR) images have been evaluated in European Cardiovascular Magnetic Resonance (EuroCMR), with adverse events ranging from 0.17% of 17,767 patients to 0.12% of 37,788 patients [57]. Very few patients with gadolinium-induced acute pancreatitis developed symptoms right away; in one case, symptoms took up to 3 h to manifest because the patient had previously been exposed to gadolinium, while in another case, symptoms took 4 h to manifest because it was the patient's first expose to gadolinium [38]. Patients who received high amounts of gadolinium unintentionally experienced symptoms such as hypertension, global aphasia, stupor, rigidity, confusion, seizures, and vomiting [47].
For patients with normal kidney function, about 90% of GBCAs are excreted in the urine within 24 h of administration [9]; 98% of GBCAs are eliminated through kidneys without any changes or biotransformation [53]; later studies also demonstrate the persistence of gadolinium in the body even months or years after GBCAs administration. Gadolinium has recently been found in glioma patients who underwent CE-MRI.
Gadolinium retention and agent stability are slightly correlated, with linear agents being less stable than macrocyclic compounds with less gadolinium retention. Studies show that linear GBCAs have a greater retention than macrocyclic GBCAs [31], and that the brain's T1 hyperintensity is connected to linear GBCA [33]. Non-ionic linear chelates' free gadolinium ions accumulate in body organs and have negative effects. Ionic macrocyclic chelates are not entirely safe, which is why the US Food and Drug Administration only allows the use of gadolinium-based contrast agents when absolutely necessary [3]. According to the Canadian Association of Radiology (CAR), gadoversetamide and gadodiamide, which are lower stability linear nonionic GBCAs, and gadopentetate dimeglumine, which is an ionic linear agent, are all classified as high-risk GBCAs, and using these agents in patients with stages 4 or 5 of CKD is completely forbidden. Non-ionic macrocyclic gadobutrol, non-ionic macrocyclic gadoteridol, and ionic macrocyclic gadoterate meglumine are classified as low risk. Ionic linear gadobenate dimeglumine and ionic linear gadoxetate disodium are rated as medium risk [58]. According to ACR guidelines, advanced CKD patients can use gadoterate meglumine, gadobenate dimeglumine, gadobutrol, and gadoteridol without risk if they underwent dialysis after exposure to these agents. Patients at risk should use these medications with caution [59].
Free gadolinium may release and bind to metals in extracellular or blood, and transmetalation reactions may occur [9], where the transmetalation or dechelation could occur in less stable agents, this is related to thermodynamic stability and kinetic stability, where the macrocyclic agents have higher stability than liner agents, the gadolinium ion may have deposited as a salt following transmetalation, such as gadolinium phosphate, gadolinium hydroxide and gadolinium carbonate, or it may attach to macromolecules like metalloenzymes, peptides and proteins [60] [61]. Calcium is significant factor that contributes to human body processes like heart muscle contraction and nerve transmission, and due to the proximity of the gadolinium radius to the calcium (Ca2+) radius and the gadolinium's ion charge (+3), free gadolinium remains in calcium site for a long time and interfere with biological processes, act as a blocker for calcium channels, and impair physiological processes [62] [61]. The presence of free gadolinium in salt, which is soluble in water, may be due to calcium channel blocking, known as acute effects, or it may be due to profibrotic and proinflammatory effects, known as chronic effects.
Chelated iron has a higher thermodynamic stability than chelated gadolinium, which suggests that it may be involved in the phenomena of gadolinium transmetalation. Because iron and chelate ligands form a stable complex, areas with high iron concentration also exhibit greater levels of gadolinium accumulation. Free gadolinium that is released from gadolinium chelates has a high affinity for carbonate ions and phosphate, and it may also attach to proteins. Therefore, excessive iron would prefer the gadolinium separate from its chelate [63].
There is a lot of evidence confirming the deposition of gadolinium in cells or tissues such as bone, kidney, brain, skin [9] and lymph nodes [46], but the specific form of these depositions is still unknown [62], and there is currently no evidence of tissue damage related to gadolinium accumulation [64]. Although gadolinium has been found in the skin of patients with renal insufficiency, the deposition of gadolinium in tissues occurs even in those with normal renal function [58].
Retention of gadolinium can activate dendritic cells, which then produce transforming growth factor beta 1 and start the fibrosis process. Phagocytosis of free gadolinium by macrophages can also start the fibrosis process [46]. The cortical neurons can be destroyed by free gadolinium through the oxidative stress pathway [63]. Subpial space, which located near the cortical veins, is enhanced a short time after GBCAs are injected. Additionally, contrast agents begin to leak into the nearby subarachnoid space 4 h after GBCA injection [65]. The releasing of free gadolinium may enhance chemokines releasing and CD34 + fibrocytes subsequent attraction, which cause fibrosis. Also, stimulate the expression and cytokines releasing caused fibrosis [38].
Studies reveal that the use of contrast-enhanced MRI has genotoxic consequences, it has been discovered that the gadolinium contrast agents and GdCl3 increase reactive oxygen species on the RAW264.7 cell line. However, several research have disputed this [9]. There are no known risks associated with utilizing GBCAs for atrophy measurement [50].
6. Gadolinium deposition in brain
Repeated intravenous administration of gadolinium contrast increased signal intensity and caused hyperintensities in the globus pallidus and deep cerebellar nucleus on unenhanced T1-weighted images, according to studies [31]; other research suggests that this increase is related to exposure to gadodiamide and gadobenate dimeglumine [53]; this increase was therefore considered to be indirect evidence of gadolinium retention [49]. Another study shows that repeated gadobutrol or gadoterate meglumine injection is associated with increased T1 signal intensity [33]. Additionally, few studies reported that this increased in signal intensity of dentate nucleus and globus pallidus associated with linear gadopentetate dimeglumine and not with macrocyclic gadoterate meglumine, while others show that macrocyclic gadobutrol do not affect their signal intensity. In some studies, gadobenate dimeglumine administration was not associated with an increase in the T1 signal intensity for dentate nucleus and globus pallidus. Therefore, it is unclear at this time whether the agent structure is associated with brain gadolinium deposition [53]. Following the release of gadolinium ions from the chelate, the toxicity manifests [49]. Additionally, although gadolinium is deposited in the brain’s basal ganglia, it is unclear if routine examinations with gadolinium contrast have an impact on mental or physical abilities [35].
When there is a BBB disorder, such as stroke, cancer, or multiple sclerosis, the brain experiences GBCA enhancement. The brain's neuronal interstitium and capillary endothelium with no BBB turbulence were discovered to have gadolinium accumulation [13]. Gadolinium is believed to penetrate the blood-cerebrospinal fluid barrier to enter the brain even in the absence of BBB problems. The exact mechanism for this penetration is unknown [66]. Molecules may enter the brain as a result of BBB disorders that restrict the entry of cells and pathogens.
Other cerebral areas also contained gadolinium, although only in smaller amounts. The marketing licenses were suspended by EMA in November 2017 as a precaution [31]. Pediatric patients who had received at least three doses of gadodiamide were similarly found to have increased concentrations of gadolinium in their pons and dentate nuclei [13]. According to a study [67], gadolinium accumulates in neuronal tissue in patients with normal renal function patients after they have been exposed to the gadodiamide agent at least four times. Studies also show that patients who have previously been exposed to GBCAs have higher concentrations of gadolinium in their cerebellar white matter and frontal lobe cortex and white matter [53]. Low doses of GBCAs do not alter physical examinations or electroencephalography, according to several safety trials [47]. Additionally, there is evidence that gadolinium exists in the brain tissue's interstices, where it can be taken by glia and neurons, but it is still unclear whether GBCAs have any toxic effects on the neurons’ cellular function [68].
7. Gadolinium deposition in bones
Studies show that gadolinium retention in bones is higher than other tissues [13]. One study found that cortical bone had a high level of gadolinium compared to brain tissues [53], and that the concentration of gadolinium in bones is 13–23 times higher than that in the globus pallidus and dentate nucleus. Gadolinium can remain in the bones for more than 8 years after GBCA injection. It can also replace the calcium in bone matrix and act as a gadolinium reservoir. When comparing the two types of agents, linear agents accumulate gadolinium 4.4 times more than macrocyclic agents. Gadolinium is retained in the bones and tissues following exposure to linear agents, according to a study [69], conducted on people without kidney disease and based only on symptoms [13]. According to certain studies, patients who had hip replacement surgery had gadolinium retained in their femoral bones, where it might stay for up to 8 years after the surgery [37].
8. Hypersensitivity reactions
The two types of adverse reactions to contrast agents are toxic reactions and hypersensitivity reactions (HRs). the toxic reactions are dose-dependent and based on the chemical properties of contrast agents, whereas HRs are dose-independent and unpredictable [70], and they have generally been evaluated as low risk for the administration of GBCAs [13]. It is known as "immediate reactions" when patients develop HRs within one hour of exposure to GBCAs.
In general, these reactions were observed in 0.7–3% of patients who had injections of various non-ionic contrast agents. Patients with allergies and asthma are more likely to develop HRs, and other risk factors for HRs include repeated exposure to GBCAs, being a woman, and systemic mastocytosis. While the serve immediate HRs range from 0.003% to 0.008% [70], the immediate HRs rate of GBCA administration is between 0.07% and 0.3%, with 9.2 administrations per 10,000 exhibiting the HRs condition. skin manifestations are present in 75–100% of HR cases [56]; erythema and urticaria with or without angioedema are the most prevalent clinical manifestations of GBCA hypersensitivity and are present in 50–90% of HR cases.
HRs may be related to mast cell activation and non-immunological processes that cause anaphylactoid reactions [43], anaphylactic shock, which is classified as an immediate HR and has a 0.01% incidence rate, as well as conjunctivitis, gastrointestinal symptoms, rhinitis, anaphylaxis, and bronchospasm [56]. Some symptoms are more severe, such as vomiting, nausea, hypotension, and dyspnea [71] [70]. In severe cases, acute coronary syndrome may occur [70]. Rarely, delayed hypersensitivity reactions occur [43]. These reactions are referred to as "non-immediate reactions", and symptoms can appear from 1 h to 10 days after contrast agent injection. DHRs include fixed drug eruptions, maculopapular eruptions, vasculitis, severe cutaneous adverse reactions (SCAR) and delayed urticaria [56].
9. Nephrogenic systemic fibrosis (NSF)
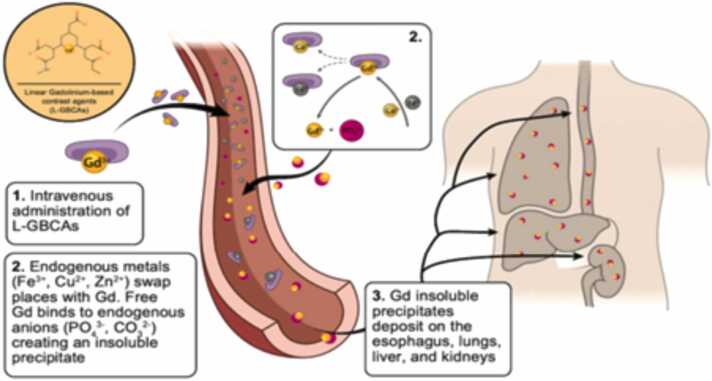
In patients with severe renal impairment associated with nephrogenic systemic fibrosis (NSF) disease, linear gadolinium-based contrast agents were first used in 2006; since then, macrocyclic contrast agents have been used [20]. Nephrogenic Systemic Fibrosis (NSF), a rare disease that was first discovered in 1997 [46] and has only recently been detected with high dosages or after recurrent exposure to GBCA on MRI [1], is a condition that affects patients with severe chronic kidney disease (CKD) and GBCA exposure [13]. NSF is life threatening fibrosing disorder that resembles scleroderma and causes fibrosis of the subcutaneous tissues in the skin and has signs of erythema and skin edema. Following the skin, the muscle is the organ that is most commonly affected by NSF [13]. NSF is a progressive disease that can result in significant impairment, joint spasms, muscle weakness, pain and severe disabilities [46]. Fig. 2.
Fig. 2.
Following the administration of linear gadolinium-based contrast agents (L-GBCAs), gadolinium-based contrast undergoes transmetalation and deposits.
Multiple tissues, including the skin, muscles, lungs, esophagus, and kidneys are affected by the multisystemic fibrotic disease NSF [39]. Patients with CKD are at higher risk for developing NSF due to their longer gadolinium half-lives and higher transmetalation reaction risk. However, pediatric patients, including fetuses and neonates with immature kidney functioning, are more likely to acquire NSF [37]. Due to the increased probability that linear chelates may release free gadolinium ions, NSF is linked to exposure to linear GBCA [31]. In NSF, the injection of GBCAs causes chelates to become unstable and enhances the release of free gadolinium, which is then able to bind with endogenous anions and dissociate through transmetalation by zinc and copper metal ions [2] [39]. This insoluble precipitate then enters the interstitial tissue of the esophagus, liver, lung and, kidneys [39]. Additionally, it is thought that CKD patients' delayed clearance of GBCAs is what causes the separation of gadolinium from its chelating agent [58]. As the incidence of NSF has dramatically dropped, some institutions have adopted restrictive policies on the use of GBCAs, and as a result, more stable GBCAs have been used [2], where contrast agents are avoided for patients at risk [59] and some institutions have not documented any new cases since [58]. According to studies conducted on CKD patients to assess the risk of NSF, the risk of contrast agents gadobenate dimeglumine, Gadobutrol, and Gadoteridol is extremely low. As a result, these contrast agents can be employed in patients with severe CKD [72]. Gd-DTPA, Gd-DTPA-BMA, and Gd-DTPA-BMEA are categorized as high risk to NSF, whereas Gd-BOPTA and Gd-EOB-DTPA are moderate risk of NSF [36]. According to studies, the pathogenesis of NSF may be associated to the induction of fibronectin expression in fibroblasts [38].
10. Nephropathy
There is not much research on the relationship between GBCAs administration and renal toxicity. Proximal tubular vacuolation can be brought on by high volume contrast agents and hypertonic solutions, and its incidence is related to gadolinium contrast storage. Gadolinium can remain in the kidney for a long period of time due to renal dysfunction, which increases the amount of gadolinium deposited in the patient’s body and decreases the clearance of GBCAs from the body with regard to nephrotoxicity. The Biliary is engaged in combined intracellular–extracellular excretion in patients with renal function disorders [13] [53].
The third most frequent cause of acute renal failure for hospitalized patients is contrast-induced nephropathy (CIN), an acute disease that affects the kidney after the administration of contrast agents. There is no known treatment for CIN, so preventative precautions must be performed. The occurrence rate of CIN relies on a number of variables, including the quantity and type of contrast utilized. It has been determined gadolinium-based contrast agents are more toxic to kidneys than iodine contrast at the same X-ray attenuation dose [19]. There is a case report [73] of a patient who underwent two consecutive MRI scans in 2006 due to clinical needs. Two days later, the patient developed acute renal failure, and kidney biopsies revealed acute tubular necrosis [38].
11. Gadolinium deposition disease (GDD)
Gadolinium deposition disease is a new pathology that was proposed in 2016, and a study described self-reported symptoms from patients with normal renal function as a symptom of new GDD. A patient must exhibit at least three of the following five symptoms in order to be diagnosed with GDD [74]: (1) bone pain,(2) peripheral leg and arm pain, (3) central torso pain, (4) headache and clouded mentation and (5) peripheral leg and arm thickening and discoloration [75]. Studies indicate that the symptoms of GDD are likely connected to a hereditary impairment of heavy metal metabolism. Chelation therapy is the recommended course of action for GDD, although it is not recommended because GDD is currently only a proposal [74].
The terms "GDD" and "other GDD-equivalent terms" are all refer to symptoms that are not associated with disorders affecting kidney function or with early-onset adverse events, such as physiologic reactions and acute allergic-like, that occur less than 24 h after the administration of GBCA, or late-onset adverse events, such as NSF, that occur more than 24 h after the administration of GBCA [31].
12. Exposure to GBCAs during pregnancy and lactation
Diagnostic imaging during pregnancy and lactation poses specific difficulties due to the sensitivity of both the developing fetus and the breast tissue to radiation. As a result, imaging protocols in these situations heavily rely on ultrasound and MRI techniques to minimize the potential risks associated with ionizing radiation exposure [76].
Because of its propensity to cross the placenta and enter the fetal circulation, where it may deposit in the developing fetus [77,78], the use of gadolinium-based contrast agents in pregnant women is contentious. However, it is unclear what the long-term implications will be. Therefore, a pregnant woman or someone who may become pregnant should only be exposed to GBCAs in extremely required cases. When there are very strong signs of MRI enhancement, the most stable GBCSs should be administered to a pregnant woman in the least dose possible [49]. Because the organs of the fetus are physiologically immature, they may be more susceptible to gadolinium toxicity issues. A study [79] on fetal exposure to linear or macrocyclic gadolinium contrast agents made the assumption that there would be a higher risk of inflammatory skin conditions, infiltrative or rheumatologic skin conditions for newborns, as well as a higher risk of neonatal deaths and stillbirths. There are beliefs that gadolinium enters the mother through the placenta and is then eliminated through the mother's urine [80,77].
As was previously mentioned, gadolinium is deposited in many bodily parts, and in the event that remodeling processes are activated during pregnancy, this gadolinium may be expelled in the circulation together with other components. The concentration of gadolinium in cord blood thus indicates the rate of gadolinium release, and the fetal is exposed to gadolinium by endogenous release of gadolinium ion [78].
The amount of intravenous contrast agent that is excreted into breast milk is less than 1% of the dose administered to a nursing mother. Therefore, the effective dose received by an infant through ingestion of breast milk containing the contrast agent is at least 10,000 times lower than the dose they would typically receive through an intravenous contrast-enhanced MRI for a neonatal indication. Currently, there is a lack of specific safety data regarding the risks associated with the ingestion of gadolinium-based contrast agents (GBCA) by breast-feeding infants. Despite conducting a comprehensive literature search, no case reports have been found that attribute adverse events in infants to the consumption of breast milk containing GBCA.
It is generally recommended to avoid breastfeeding for 24 h after the administration of contrast medium, especially if high-risk agents are used. This precaution is taken to minimize potential risks to the infant, as some contrast agents may pass into breast milk in small amounts. Consulting with a healthcare provider is important to determine the specific recommendations based on the type of contrast medium used and individual circumstances [76].
13. Using the GBCAs in MRI-linac
The use of MRI technology has grown to be a significant component of radiotherapy plans. MRI-linac treatment, which uses gadolinium-based contrast agents, is now localized in the treatment room to provide direct images with better anatomical vision. Gadolinium is inherently radioactive since its half-life of removal from the body is 1.6 h, and the interval between the start of imaging and the completion of dose delivery is roughly 30 min. When administering a dose, the cinematic MRI may be utilized to verify target and stop the delivery of the target shifts. Due to possible breaking or modifications due to the fact that X-rays carry a higher energy than the bond dissociation energy for chemical compounds, this results in gadolinium retention in the body parts, which has the above-mentioned negative health effects [8].
14. Improve the contrast agents
There is a need to find a gadolinium substitute after estimating the associated with the release of free gadolinium and its deposition in various body organs or tissues. Super-magnetic iron oxide nanoparticles coated with carboxymethyldextran were used as a contrast agent, and this substance is known as ferumoxytol. Its properties are different from those of gadolinium; the ferumoxytol agent is large and does not cross blood vessels to tumor rapidly due to compromised BBB. the lesion is enhanced one day after injection, whereas gadolinium agents enhance the lesion after minutes. The ferumoxytol half-life is approximately 14 h; this long time allows to demonstrate tumor vasculature and calculate the relative volume of cerebral blood for potentially malignant tumors. Manganese (Mn2+) is a good substitute for gadolinium since it exhibits strong paramagnetism and possess long electronic T1. Manganese is also a significant element in the body, endogenous mechanisms can remove it [32].
The development of next-generation gadolinium agents with enhanced relaxivity has the potential to decrease dosage while improving lesion characterization, diagnosis, lesion enhancement and clinical efficacy. Tetrameric gadolinium-based newly designed contrast agent (gadoquatrane) shows high relaxation and high stability [20]. Gadopiclenol, a newly developed agent created by [81], has a nonionic macrocyclic GBCA structure, one gadolinium ion, and was designed to produce T1 relaxivity that is two to three times greater than that of current gadolinium factors while maintaining the physical qualities. The new agent was tested on animals to see if there may be cerebral deposition, and it was discovered that after 5 months of exposure to gadopiclenol, the amount of gadolinium deposited with cerebellum is comparable to that of macrocyclic gadobutrol [82]. The new type of contrast agent uses DO3A, to which a phenyl group has been added. Therefore, the Gd-DOTA without the phenyl group modification has a shorter relaxation period than contrast agent changed with it [1].
15. Gadolinium measurement
Studies estimated that the presence of gadolinium was found in all samples taken from patients exposed to linear GBCA, not macrocyclic GBCA, but some studies said that gadolinium retention occurs after exposure to either linear or macrocyclic GBCA administration [13]. These results were obtained using the gadolinium measurement techniques, a studies use a technique called inductively coupled plasma mass spectrometry (ICP-MS). Gadolinium deposition effects are localized, quantified, and evaluated using transmission electron microscopy and light microscopy [53]. Only the amount of gadolinium, not its chemical form, is determined by the ICP-MS, but when combined with Laser Ablation system (LA-ICP-MS), it is possible to assess the distribution of gadolinium in tissues [83].
Energy dispersive X-ray spectroscopy and scanning electron microscopy have been used to measure the level of gadolinium in skin in patients with nephrogenic systemic fibrosis (NSF) disease, which is linked to the administration of GBCAs. However, when ICP-MS and spectroscopy have been used to measure the level of gadolinium in patients who have a history of chronic kidney disease and have previously been exposed to GBCAs, it was found that there is no skin gadolinium retention [13]. Rare earth elements (RREs) are determined in materials using ICP-MS, however GBCAs analysis has several drawbacks. For example, separation techniques should be used in low REE contents to separate the RRE from main elements, but in this case, gadolinium is both the RRE and the main element to be analyzed, making it impossible to do so. Additionally, when gadolinium oxides and hydroxides of gadolinium are analyzed by ICP-MS, they produce isobar [84].
In one study [85], hydrophilic interaction liquid chromatography (HILIC) with ICP-MS was used to assess the presence of gadolinium in NSF skin biopsies. In a separate study, the presence of gadolinium was discovered in samples taken from patients with brain tumor, where the gadolinium deposition is recognized by scanning electron microscopy/energy dispersive ray spectroscopy (SEM/EDS). Imaging technique, such as synchrotron X-ray fluorescence (SXRF), extended X-ray absorption fine structure (EXAFS) spectroscopy, element specific transmission electron microscopy (TEM), secondary ion mass spectrometry (SIMS), and SEM/EDX, are used to observe the small gadolinium deposition and coprecipitation with calcium and phosphorus.Because the hair has concentrated heavy metals, it can be easily obtained using a non-invasive approach in studies that use it to measure gadolinium levels using ICP-MS [62].
16. conclusion
Gadolinium-based contrast agents are commonly used in medical imaging, particularly in MRI, to enhance image contrast and improve diagnostic accuracy. These contrast agents contain paramagnetic ions that help create clearer images. However, it is important to ensure the safe administration of GBCAs. To prevent the toxicity of free gadolinium ions, they are bound to organic ligands, forming stable Gd+3 chelates. This reduces the release of free gadolinium ions and prevents their association with endogenous anions such as CO3-2 and PO4-3, which can lead to the formation of insoluble compounds in the bloodstream and deposition in tissues. GBCAs are generally considered safe and do not exacerbate renal insufficiency. However, mild adverse reactions can occur following their administration, including nausea, vomiting, pain at the injection site, headache, and dizziness. There is evidence confirming the deposition of gadolinium in various tissues such as bone, kidney, brain, skin, and lymph nodes. The specific form of these depositions is still unknown, and currently, there is no evidence of tissue damage directly associated with gadolinium accumulation. Nevertheless, studies have reported certain disorders related to gadolinium retention, including hypersensitivity reactions, nephrogenic systemic fibrosis, and gadolinium deposition disease. These conditions are rare and usually occur in individuals with pre-existing risk factors or compromised renal function. It's important for healthcare providers to consider the benefits and risks of using GBCAs and to closely monitor patients, particularly those at higher risk, to ensure their safety during diagnostic procedures. Please note that the information provided is a general overview and should not be considered as medical advice. It's always recommended to consult with a healthcare professional for specific concerns or questions regarding the use of contrast agents.
Consent for publication
The participants signed consent regarding publishing their data (and/or photographs).
Ethics approval and consent to participate
This study was not dealing with patient or animals.
Funding
There is no source of funding.
Authors' contributions
The study was designed by Muntaser S.Ahmad. Material preparation and data collection were performed by Nebal Iyad and Osama Makhamrah. The first draft of the manuscript was written by Sanaa G. Alkhatib, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
CRediT authorship contribution statement
S.Ahmad Muntaser: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Sanaa G. Alkhatib sanaa: Writing – review & editing. Makhamrah Osama: Data curation, Investigation. Iyad Nebal: Conceptualization, Writing – original draft.
Declaration of Competing Interest
Nebal Iyad, Sanaa G. Alkhatib, Osama Makhamrah, and Muntaser S. Ahmad declare that they have no conflict of interest.
Contributor Information
Muntaser S.Ahmad, Email: wmuntaser@gmail.com.
Mohammad Hjouj, Email: mhjouj@staff.alquds.edu.
Data Availability
The data that support the findings of this study are openly available. It can be used by other Authors.
References
- 1.Kang Y., Zhao Y. Preparation of magnetic resonance contrast agent gadolinium-containing organic nanoparticles and their electrochemical behavior investigation. Int. J. Electrochem. Sci. 2022;vol. 17:1–10. doi: 10.20964/2022.07.62. [DOI] [Google Scholar]
- 2.Cheong B.Y.C., Wilson J.M., Preventza O.A., Muthupillai R. Gadolinium-based contrast agents: updates and answers to typical questions regarding gadolinium use. Tex. Hear. Inst. J. 2022;vol. 49(3):7–9. doi: 10.14503/THIJ-21-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanana P., et al. The effect of magnetic field gradient and gadolinium-based mri contrast agent dotarem on mouse macrophages. Cells. 2022;vol. 11(5):757. doi: 10.3390/cells11050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Z., et al. Sericin-based gadolinium nanoparticles as synergistically enhancing contrast agents for pH-responsive and tumor targeting magnetic resonance imaging. Mater. Des. 2021;vol. 203 doi: 10.1016/j.matdes.2021.109600. [DOI] [Google Scholar]
- 5.Al-Tell A. Justification of urgent brain CT examinations at medium size hospital, Jerusalem. Atlas J. Biol. 2019:655–660. doi: 10.5147/ajb.v0i0.213. [DOI] [Google Scholar]
- 6.Oglat A., et al. Chemical items used for preparing tissue-mimicking material of wall-less flow phantom for doppler ultrasound imaging. J. Med. Ultrasound. 2018;vol. 26(3):123. doi: 10.4103/JMU.JMU_13_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammar A.O., et al. Characterization and construction of a robust and elastic wall-less flow phantom for high pressure flow rate using doppler ultrasound applications. Nat. Eng. Sci. 2018;vol. 3(3):359–377. doi: 10.28978/nesciences.468972. [DOI] [Google Scholar]
- 8.Mahmood F., Nielsen U.G., Jørgensen C.B., Brink C., Thomsen H.S., Hansen R.H. Safety of gadolinium based contrast agents in magnetic resonance imaging-guided radiotherapy – an investigation of chelate stability using relaxometry. Phys. Imaging Radiat. Oncol. 2022;vol. 21:96–100. doi: 10.1016/j.phro.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobanoglu H. Assessment of genetic damage induced by gadolinium-based radiocontrast agents. J. Trace Elem. Med. Biol. 2022;vol. 70(October 2021) doi: 10.1016/j.jtemb.2021.126914. [DOI] [PubMed] [Google Scholar]
- 10.Oglat A.A., Alshipli M., Sayah M.A., Ahmad M.S. Artifacts in diagnostic ultrasonography. J. Vasc. Ultrasound. 2020;vol. 44(4):212–219. doi: 10.1177/1544316720923937. [DOI] [Google Scholar]
- 11.Ahmad M.S., Arab A. Ability of MRI diagnostic value to detect the evidence of physiotherapy outcome measurements in dealing with calf muscles tearing. J. Med. Clin. Res. Rev. 2022;vol. 6(10):6–11. doi: 10.33425/2639-944X.1292. [DOI] [Google Scholar]
- 12.Meng Q., Wu M., Shang Z., Zhang Z., Zhang R. Responsive gadolinium(III) complex-based small molecule magnetic resonance imaging probes: Design, mechanism and application. Coord. Chem. Rev. 2022;vol. 457 doi: 10.1016/j.ccr.2021.214398. [DOI] [Google Scholar]
- 13.Davies J., Siebenhandl-Wolff P., Tranquart F., Jones P., Evans P. Gadolinium: pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022;vol. 96(2):403–429. doi: 10.1007/s00204-021-03189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad M.S., et al. Agarose and wax tissue-mimicking phantom for dynamic magnetic resonance imaging of the liver. J. Med. - Clin. Res. Rev. 2021;vol. 5(11) doi: 10.33425/2639-944X.1250. [DOI] [Google Scholar]
- 15.Ban C.C., et al. Modern heavyweight concrete shielding: Principles, industrial applications and future challenges; review. J. Build. Eng. 2021;vol. 39(December 2020) doi: 10.1016/j.jobe.2021.102290. [DOI] [Google Scholar]
- 16.Ahmad M.S., et al. A recent short review in non-invasive magnetic resonance imaging on assessment of HCC Stages: MRI findings and pathological diagnosis. J. Gastroenterol. Hepatol. Res. 2020;vol. 9(2):3113–3123. doi: 10.17554/j.issn.2224-3992.2020.09.881. [DOI] [Google Scholar]
- 17.Ahmad M.S., et al. Current status regarding tumour progression, surveillance, diagnosis, staging, and treatment Of HCC a literature review. J. Gastroenterol. Hepatol. Res. 2019;vol. 8(2):2841–2852. [Google Scholar]
- 18.Makhamrah O., Ahmad M.S., Doufish D., Mohammad H. Internal auditory canal (IAC) and cerebellopontine angle (CPA): comparison between T2-weighted SPACE and 3D-CISS sequences at 1.5T. Radiat. Phys. Chem. 2023;vol. 206(December 2022) doi: 10.1016/j.radphyschem.2023.110797. [DOI] [Google Scholar]
- 19.Zheng H., Wang G., Cao Q., Ren W., Xu L., Bu S. A risk prediction model for contrast-induced nephropathy associated with gadolinium-based contrast agents. Ren. Fail. . 2022;vol. 44(1):741–747. doi: 10.1080/0886022X.2022.2069579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohrke J., et al. Preclinical profile of gadoquatrane. Invest. Radiol. 2022;vol. 57(10):629–638. doi: 10.1097/RLI.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clough T.J., Jiang L., Wong K., Long N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019:1–14. doi: 10.1038/s41467-019-09342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O. Makhamrah, M.S. Ahmad, and M. Hjouj, Evaluation of Liver Phantom for Testing of the Detectability Multimodal for Hepatocellular Carcinoma, in Proceedings of the 2019 2nd International Conference on Digital Medicine and Image Processing, Nov. 2019, pp. 17–21. doi: 10.1145/3379299.3379307.
- 23.Ahmad M.S., et al. Hepatocellular carcinoma liver dynamic phantom for MRI. MR Imaging Intern. Audit. Canal Inn. Ear 3T Comp. 3D Driven Equilib. 3D Balanc. Fast F. Echo Seq. 2021;vol. 188 doi: 10.1016/j.radphyschem.2021.109632. [DOI] [Google Scholar]
- 24.Jakobsen J.Å., et al. Patterns of use, effectiveness and safety of gadolinium contrast agents: a European prospective cross-sectional multicentre observational study. BMC Med. Imaging. 2021;vol. 21(1):74. doi: 10.1186/s12880-021-00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil R., et al. Single- and multi-arm gadolinium MRI contrast agents for targeted imaging of glioblastoma. Int. J. Nanomed. 2020;vol. Volume 15:3057–3070. doi: 10.2147/IJN.S238265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A.M. Iordache et al., The incidence of skin lesions in contrast media- induced chemical hypersensitivity, pp. 1113–1124, 2019, doi: 10.3892/etm.2018.7056. [DOI] [PMC free article] [PubMed]
- 27.Ahmad M.S., et al. Dynamic hepatocellular carcinoma model within a liver phantom for multimodality imaging. Eur. J. Radiol. Open. 2020;vol. 7 doi: 10.1016/j.ejro.2020.100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad M., et al. Chemical characteristics, motivation and strategies in choice of materials used as liver phantom: a literature review. J. Med. Ultrasound. 2020;vol. 28(1):115–117. doi: 10.4103/JMU.JMU_4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad M.S., Makhamrah O., Hjouj M. Multimodal imaging of hepatocellular carcinoma using dynamic liver phantom. intechopen, no. Tour. 2021:13. doi: 10.1016/j.radphyschem.2021.109632. [DOI] [Google Scholar]
- 30.Azizian G., et al. Synthesis route and three different core-shell impacts on magnetic characterization of gadolinium oxide-based nanoparticles as new contrast agents for molecular magnetic resonance imaging. Nanoscale Res. Lett. 2012;vol. 7:1–10. doi: 10.1186/1556-276X-7-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahid I., Joseph A., Lancelot E. Use of real-life safety data from international pharmacovigilance databases to assess the importance of symptoms associated with gadolinium exposure. Invest. Radiol. 2022;vol. 57(10):664–673. doi: 10.1097/RLI.0000000000000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.E.M. Gale and P. Caravan, Gadolinium-Free Contrast Agents for Magnetic Resonance Imaging of the Central Nervous System, pp. 395–397, 2018, doi: 10.1021/acschemneuro.8b00044. [DOI] [PMC free article] [PubMed]
- 33.Bi Q., et al. Gadolinium deposition in the brain is related to various contrast agents: a matched case–control study. Clin. Radiol. 2022;vol. 77(4):299–306. doi: 10.1016/j.crad.2021.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Hojreh A., Peyrl A., Bundalo A., Szepfalusi Z. Subsequent MRI of pediatric patients after an adverse reaction to Gadolinium-based contrast agents. PLoS One. 2020;vol. 15(4) doi: 10.1371/journal.pone.0230781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.V.A. Athavale, Athavale VA, Application of Artificial Intelligence in Magnetic Resonance Imaging to reduce Gadolinium accumulation in patients with multiple sclerosis: A Review Application of artificial intelligence in magnetic resonance imaging to reduce gadolinium acc, vol. 2, no. 1, pp. 478–490, 2022, doi: 10.52152/spr/2022.157.
- 36.gadolinium deposition after administration of gadolinium ‑ based contrast agents Kanda T., Oba H., Toyoda K., Kitajima K. Brain gadolinium deposition after administration of gadolinium ‑ based contrast agents. Jpn. J. Radiol. 2016;vol. 34(1) doi: 10.1007/s11604-015-0503-5. [DOI] [PubMed] [Google Scholar]
- 37.Uosef A., Villagran M., Kubiak J.Z., Wosik J., Ghobrial R.M., Kloc M. Side effects of gadolinium MRI contrast agents. Pediatr. i Med. Rodz. 2020;vol. 16(1):49–52. doi: 10.15557/PiMR.2020.0008. [DOI] [Google Scholar]
- 38.Rogosnitzky M., Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;vol. 29(3):365–376. doi: 10.1007/s10534-016-9931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo-Bernal S., et al. Nephrogenic systemic fibrosis in patients with chronic kidney disease after the use of gadolinium-based contrast agents: a review for the cardiovascular imager. Diagnostics. 2022;vol. 12(8):1816. doi: 10.3390/diagnostics12081816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A. Balachandran, P. Kala, S. Ashwathappa, M. Varunya, R. Kollu, and H.V. Ramprakash, O riginal R esearch A rticle Gadolinium-based Contrast Agents: Evaluation of Effect On Renal Function Parameters, vol. 3, no. 1, pp. 166–169, 2018.
- 41.RGadolinium-based contrast agent accumulation and toxicity: An updateamalho J., Semelka R.C., Ramalho M., Nunes R.H., AlObaidy M., Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. Am. J. Neuroradiol. 2016;vol. 37(7):1192–1198. doi: 10.3174/ajnr.A4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green C., Jost G., Frenzel T., Boyken J., Schwenke C., Pietsch H. The Effect of Gadolinium-Based Contrast Agents on Longitudinal Changes of Magnetic Resonance Imaging Signal Intensities and Relaxation Times in the Aging Rat Brain. Invest. Radiol. 2022;vol. 57(7):453–462. doi: 10.1097/RLI.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grueber H.P., Helbling A., Joerg L. Skin test results and cross-reactivity patterns in IgE- And T-cell-mediated allergy to gadolinium-based contrast agents. Allergy, Asthma Immunol. Res. 2021;vol. 13(6):933–938. doi: 10.4168/AAIR.2021.13.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Layne K.A., Dargan P.I., Archer J.R.H., Wood D.M. Gadolinium deposition and the potential for toxicological sequelae – a literature review of issues surrounding gadolinium‐based contrast agents. Br. J. Clin. Pharmacol. 2018;vol. 84(11):2522–2534. doi: 10.1111/bcp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morcos S.K. Extracellular gadolinium contrast agents: differences in stability. Eur. J. Radiol. 2008;vol. 66(2):175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Lange S., et al. Nephrogenic systemic fibrosis as a complication after gadolinium-containing contrast agents: a rapid review. Int. J. Environ. Res. Public Health. 2021;vol. 18(6):3000. doi: 10.3390/ijerph18063000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel M., Atyani A., Salameh J.-P., McInnes M., Chakraborty S. Safety of intrathecal administration of gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology. . 2020;vol. 297(1):75–83. doi: 10.1148/radiol.2020191373. [DOI] [PubMed] [Google Scholar]
- 48.Moser F.G., et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: comparison between gadobutrol and linear gadolinium-based contrast agents. Am. J. Neuroradiol. 2018;vol. 39(3):421–426. doi: 10.3174/ajnr.A5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao X., et al. Gadolinium retention in the brain of mother and pup mouse: effect of pregnancy and repeated administration of <scp>gadolinium‐based</scp> contrast agents. J. Magn. Reson. Imaging. 2022;vol. 56(3):835–845. doi: 10.1002/jmri.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lie I.A., et al. The effect of gadolinium-based contrast-agents on automated brain atrophy measurements by FreeSurfer in patients with multiple sclerosis. Eur. Radiol. 2022;vol. 32(5):3576–3587. doi: 10.1007/s00330-021-08405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forslin Y., Martola J., Bergendal Å., Fredrikson S., Wiberg M.K., Granberg T. Gadolinium retention in the brain: An MRI relaxometry study of linear and macrocyclic gadolinium-based contrast agents in multiple sclerosis. Am. J. Neuroradiol. 2019;vol. 40(8):1265–1273. doi: 10.3174/ajnr.A6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.X.T. Granberg, Retention of Gadolinium-Based Contrast Agents in Multiple Sclerosis: Retrospective Analysis of an 18-Year Longitudinal Study, 2017. [DOI] [PMC free article] [PubMed]
- 53.Ranga A., Agarwal Y., Garg K. Gadolinium based contrast agents in current practice: risks of accumulation and toxicity in patients with normal renal function. Indian J. Radiol. Imaging. 2017;vol. 27(02):141–147. doi: 10.4103/0971-3026.209212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallo E., Diaferia C., Di Gregorio E., Morelli G., Gianolio E., Accardo A. Peptide-based soft hydrogels modified with gadolinium complexes as MRI contrast agents. Pharmaceuticals. 2020;vol. 13(2):19. doi: 10.3390/ph13020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fatima A., Ahmad M.W., Al Saidi A.K.A., Choudhury A., Chang Y., Lee G.H. Recent advances in gadolinium based contrast agents for bioimaging applications. Nanomaterials. 2021;vol. 11(9):2449. doi: 10.3390/nano11092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bara M.T.G., et al. Hypersensitivity to gadolinium-based contrast media. 2022;vol. 3(March) doi: 10.3389/falgy.2022.813927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uhlig J., et al. Acute adverse events in cardiac MR imaging with gadolinium-based contrast agents: results from the European Society of Cardiovascular Radiology ( ESCR) MRCT Registry in 72. 839 Patients Am. Coll. Radiol. 2019:3686–3695. doi: 10.1007/s00330-019-06171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soloff E.V., Wang C.L. Safety of gadolinium-based contrast agents in patients with stage 4 and 5 chronic kidney disease: a radiologist’s perspective. Kidney360. 2020;vol. 1(2):123–126. doi: 10.34067/KID.0000502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.S. Maripuri and K.L. Johansen, Risk of Gadolinium-Based Contrast Agents in Chronic Kidney Disease — Is Zero Good Enough ?, pp. 1–2, 2019, doi: 10.1002/jmri.25625. [DOI] [PubMed]
- 60.Runge V.M. Dechelation (Transmetalation): consequences and safety concerns with the linear gadolinium-based contrast agents, in view of recent health care rulings by the EMA (Europe), FDA (United States), and PMDA (Japan) Invest. Radiol. 2018;vol. 53(10):571–578. doi: 10.1097/RLI.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 61.Dekkers I.A., Roos R., van der Molen A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European medicines agency. Eur. Radiol. 2018;vol. 28(4):1579–1584. doi: 10.1007/s00330-017-5065-8. [DOI] [PubMed] [Google Scholar]
- 62.Among O.T. HHS Public Access. 2021;vol. 55(10):636–642. doi: 10.1097/RLI.0000000000000681.Human. [DOI] [Google Scholar]
- 63.Kartamihardja A.A.P., Ariyani W., Hanaoka H., Taketomi-Takahashi A., Koibuchi N., Tsushima Y. The role of ferrous ion in the effect of the gadolinium-based contrast agents (GBCA) on the purkinje cells arborization: an in vitro study. Diagnostics. 2021;vol. 11(12):2310. doi: 10.3390/diagnostics11122310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah R., D’Arco F., Soares B., Cooper J., Brierley J. Use of gadolinium contrast agents in paediatric population: donald rumsfeld meets hippocrates! Br. J. Radiol. 2019;vol. 92(1094) doi: 10.1259/bjr.20180746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naganawa S., et al. Relationship between time-dependent signal changes in parasagittal perivenous cysts and leakage of gadolinium-based contrast agents into the subarachnoid space. Magn. Reson. Med. Sci. 2021;vol. 20(4):378–384. doi: 10.2463/mrms.mp.2020-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.T. Frenzel, C. Apte, G. Jost, L. Schöckel, J. Lohrke, and H. Pietsch, Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents Comparative Study in Rats, vol. 52, no. 7, pp. 18–20, 2017, doi: 10.1097/RLI.0000000000000352. [DOI] [PMC free article] [PubMed]
- 67.R.J. Mcdonald et al., Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging1, vol. 000, no. 0, pp. 1–11, 2015. [DOI] [PubMed]
- 68.J.K. Richter, H. Von Tengg-kobligk, J.T. Heverhagen, and V.M. Runge, Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent, vol. 54, no. 8, pp. 453–463, 2019, doi: 10.1097/RLI.0000000000000567. [DOI] [PubMed]
- 69.Burke L.M.B., Ramalho M., AlObaidy M., Chang E., Jay M., Semelka R.C. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn. Reson. Imaging. 2016;vol. 34(8):1078–1080. doi: 10.1016/j.mri.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Nucera E., et al. Contrast medium hypersensitivity: a large Italian study with long-term follow-up. Biomedicines. 2022;vol. 10(4):759. doi: 10.3390/biomedicines10040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nucera E., et al. Gadolinium-based contrast agents hypersensitivity: a case series. J. Asthma Allergy. 2021;vol. Volume 14:241–244. doi: 10.2147/JAA.S295748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudnick M.R., Wahba I.M., Leonberg-Yoo A.K. Use of gadolinium-based contrast agents in patients with severe renal impairment. absence of risk versus caution: a nephrologist’s perspective. Kidney360. 2020;vol. 1(6):433–435. doi: 10.34067/KID.0003022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A.R.B. Study, Are Gadolinium-Based Contrast Media Nephrotoxic ?”.
- 74.Harvey H.B., Gowda V., Cheng G. Gadolinium deposition disease: a new risk management threat. J. Am. Coll. Radiol. 2019;vol. 17(4):546–550. doi: 10.1016/j.jacr.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Semelka R.C., et al. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn. Reson. Imaging. 2016;vol. 34(10):1383–1390. doi: 10.1016/j.mri.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Fraum T.J., Ludwig D.R., Bashir M.R., Fowler K.J. Gadolinium-based contrast agents: a comprehensive risk assessment. J. Magn. Reson. Imaging. 2017;vol. 46(2):338–353. doi: 10.1002/jmri.25625. [DOI] [PubMed] [Google Scholar]
- 77.Bird S.T., et al. First-trimester exposure to gadolinium-based contrast agents: a utilization study of 4.6 Million U.S. Pregnancies. Radiology. 2019;vol. 293(1):193–200. doi: 10.1148/radiol.2019190563. [DOI] [PubMed] [Google Scholar]
- 78.R. Amin, T. Darrah, H. Wang, and S. Amin, Editor’s Highlight: In Utero Exposure to Gadolinium and Adverse Neonatal Outcomes in Premature Infants, Toxicol. Sci., vol. 156, no. 2, pp. 520–526, Apr. 2017, doi: 10.1093/toxsci/kfx013.
- 79.Ray J.G., Vermeulen M.J., Bharatha A., Montanera W.J., Park A.L. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;vol. 316(9):952. doi: 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- 80.Kanda T. The new restrictions on the use of linear gadolinium-based contrast agents in Japan. Magn. Reson. Med. Sci. 2019;vol. 18(1):1–3. doi: 10.2463/mrms.e.2017-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robert P., et al. Contrast-to-dose relationship of gadopiclenol, an MRI macrocyclic gadolinium-based contrast agent, compared with gadoterate, gadobenate, and gadobutrol in a rat brain tumor model. Radiology. 2020;vol. 294(1):117–126. doi: 10.1148/radiol.2019182953. [DOI] [PubMed] [Google Scholar]
- 82.Tweedle M.F. Next-Generation MRI contrast agents: still including gadolinium. Radiology. 2020;vol. 294(1):127–128. doi: 10.1148/radiol.2019192113. [DOI] [PubMed] [Google Scholar]
- 83.M. Le Fur, P. Caravan, and M.G. Hospital, The biological fate of gadolinium-based MRI contrast agents: a call to action for bioinorganic chemists, vol. 11, no. 2, pp. 240–254, 2020, doi: 10.1039/c8mt00302e.The. [DOI] [PMC free article] [PubMed]
- 84.Ben Salem D., Barrat J.A. Determination of rare earth elements in gadolinium-based contrast agents by ICP-MS. Talanta. 2021;vol. 221(September 2020) doi: 10.1016/j.talanta.2020.121589. [DOI] [PubMed] [Google Scholar]
- 85.Birka M., Roscher J., Holtkamp M., Sperling M., Karst U. Investigating the stability of gadolinium based contrast agents towards UV radiation. Water Res. 2016;vol. 91:244–250. doi: 10.1016/j.watres.2016.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available. It can be used by other Authors.