Abstract
Ixazomib (IXA) is an oral proteasome inhibitor (PI) used in combination with lenalidomide and dexamethasone (IXA-Rd) for patients with relapsed and/or refractory multiple myeloma (RRMM). The REMIX study is one of the largest prospective, real-world analysis of the effectiveness of IXA-Rd in the setting of RRMM. Conducted in France between August 2017 and October 2019, the REMIX study, a non-interventional prospective study, included 376 patients receiving IXA-Rd in second line or later and followed for at least 24 months. Primary endpoint was the median progression-free survival (mPFS). Median age was 71 years (Q1-Q3 65.0 – 77.5) with 18.4% of participants older than 80 years. IXA-Rd was initiated in L2, L3 and L4 + for 60.4%, 18.1% and 21.5%, respectively. mPFS was 19.1 months (95% CI [15.9, 21.5]) and overall response rate (ORR) was 73.1%. mPFS was 21.5, 21.9 and 5.8 months in patients receiving IXA-Rd as L2, L3, L4 + respectively. Among patients receiving IXA-Rd in L2 and L3, mPFS was similar for patients previously exposed to lenalidomide (19.5 months) than for those lenalidomide naive (not exposed, 22.6 months, p = 0.29). mPFS was 19.1 months in patients younger than 80 years and 17.4 months in those 80 years or older (p = 0.06) with similar ORR (72.4% and 76.8%) in both subgroups. Adverse events (AEs) were reported in 78.2% of patients including 40.7% of treatment-related AE. IXA discontinuation was due to toxicity in 21% of patients. To conclude, the results of the REMIX study are consistent with the results of Tourmaline-MM1 and confirm the benefit of IXA-Rd combination in real life. It shows the interest of IXA-Rd in an older and frailer population, with an acceptable effectiveness and tolerance.
Supplementary information
The online version contains supplementary material available at 10.1007/s00277-023-05278-3.
Keywords: Relapse and refractory multiple myeloma, Real world evidence, Ixazomib, Effectiveness, Safety, Elderly
Background
Among the many treatments available for patients with relapsed/refractory multiple myeloma (RRMM), new, promising therapies have recently emerged [1, 2]. These new therapies offer patients further therapeutic options to respond to inevitable relapses during disease evolution [3].
Ixazomib (IXA) is the first orally-administered in its class. It has been approved in Europe and in the USA combined with lenalidomide and dexamethasone (Rd) for treating RRMM after first-line treatment, based on the results of TOURMALINE-MM1 phase 3 clinical trial [4]. TOURMALINE-MM1 was conducted in a population of patients having received in median 1 prior line of treatment (1–3) and demonstrated a significant longer median progression-free survival (PFS) with ixazomib-Rd (IXA-Rd) than with placebo-Rd (20.6 versus 14.7 months; hazard ratio [HR] 0.74, P = 0.01) and a significant increased overall response rate (ORR) with limited additional toxicity and a maintained level of quality of life [5].
As with many novel chemotherapeutic agents, the choice to prescribe IXA is based on finding a balance between efficacy, toxicity, and patient characteristics including age, frailty, or cytogenetic abnormalities. At early relapse, treatment choice is majorly orientated by refractoriness to lenalidomide and / or bortezomib. In an elderly population, assessment of frailty and comorbidities is also highly weighting on treatment choice [6–9]. In the setting of a frail population, the availability of a fully oral combination can be of great interest. Additionally, it has been estimated that a substantial proportion of typical RRMM patients (approximately 40%), are excluded from clinical trials, which makes translating clinical development results to real-life practice uncertain [9–11]. Thus, defining the most appropriate treatment sequences for each patient, considering their characteristics and taking advantage of each therapy line requires more insight in real practice [8, 12, 13]. Real-world studies are needed to generalize results to real-life populations [14, 15].
The objective of the non-interventional REMIX study to evaluate IXA use in real life has been underway in France since IXA became available in 2017 to generate supplementary data derived from use in an unselected population of RRMM patients. The REMIX study is one of the largest prospective, studies to provide real-world evidence (RWE) of the IXA-Rd combination. The study has been designed to assess effectiveness and safety in the treated population as well as to refine the appropriate patient profile to receive the combination.
Material and methods
Study design
REMIX was a non-interventional, prospective, multicenter study conducted in France in patients with RRMM who received an oral formulation of IXA combined with lenalidomide and dexamethasone (IXA-Rd) in real-life conditions. The decision to treat with IXA-Rd was at the physician’s discretion. Patient management was performed according to standard of care at each site.
To be eligible, adult patients had to have received IXA-Rd after at least 1 prior line of chemotherapy according to the summary of product characteristics (SmPC) of each product and IXA had to be initiated concomitantly to Rd. If lenalidomide was started more than 6 weeks before IXA, it was considered non-concomitant and the patient excluded from the primary analyses. In France, IXA-Rd was available under compassionate access from August 2017 until October 2018 when IXA became commercially available. Patients were prospectively enrolled during the first four months after starting IXA-Rd. Patients were followed up for at least 24 months (max 49.5 months) or until the end of the study or death, whichever occurred first, according to the site's standard practices.
To ensure a representative patient population, sites participating in the nationwide compassionate access (n = 158) were invited to participate in the study. Among these sites, 64 accepted, which included both public and private hospitals located throughout France. Of these, 60 sites actively enrolled patients in the study.
Study endpoints
The primary endpoint measure was median progression-free survival (mPFS) and PFS rates assessed at 12, 18, 24, and 36 months. PFS was defined as the time interval from the date of first dose of IXA to the date of disease progression or death, whichever occurred first. Secondary endpoints included overall survival (OS) defined as the time interval from the date of first dose of IXA to the date of death, at 12, 18, 24, 36, 42 and 48 months, duration of response (DoR) defined as the time interval between the best response to treatment to progression or death, whichever occurred first among patients with at least a partial response (PR), and endpoints based on the response rates (RR): complete response (CR), very good partial response (VGPR), PR and the stable disease (SD) rates. Overall response rate (ORR) combined CR, VGPR and PR. Safety endpoints included incidences of adverse events (AEs), serious AE (SAEs), treatment-related AEs and SAE, and AE leading to treatment discontinuation.
Assessment and data collection
Data was collected every 3 months during the first two years, then every 6 months thereafter until study end, as per standard practice. An investigator assessed the therapeutic response including the refractory status to lenalidomide (Twenty-six (6.9%) patients were reported to be lenalidomide-refractory, although this was a non-inclusion criterion) and disease progression according to International Myeloma Working Group (IMWG) criteria [16] as in usual practice at each site (no central review). Safety data were collected for up to 30 days after the last treatment dose administration. Treatment discontinuation was based on the investigator judgment. Post-IXA-Rd data were collected for subsequent therapies description and survival status. Patient cytogenetic abnormalities, Eastern Cooperative Oncology Group performance status (ECOG-PS) and International Staging System (ISS) were not routinely conducted in all sites but were collected at IXA-Rd initiation when available. Comorbidities were estimated with the Charlson's Comorbidity Index and frailty was evaluated using the simplified frailty score based on age, Charlson’s Comorbidity Index and ECOG-PS [17].
All patients provided their consent to participate to the study as per local regulations. Data were remotely monitored regularly during the study and each site was visited at least once by a monitoring Clinical Research Assistant. Monthly safety reconciliation with the sponsor safety database were performed and annual data reviews were organized.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki [18], the principles of the International Society for Pharmacoepidemiology guidelines for Good Pharmacoepidemiology Practice [19], and in compliance with the General Data Protection Regulation (GDPR) [20]. The protocol was approved by a French Ethical Committee on November 9th, 2017 (N° AU 1381). All patients provided their consent to participate to the study as per local regulations.
Sample size and statistical analysis
Analyses were performed in the eligible population.
Baseline patient characteristics, response, and safety data were summarized using descriptive statistics. Qualitative data are presented as numbers with the corresponding percentages. While quantitative data are presented as means with standard deviation (SD) and/or median with interquartile range (IQR). The numbers of patients with missing data are indicated. Missing data were not replaced.
Time-to-event analyses (PFS, OS, treatment duration, and DoR) were estimated using the Kaplan–Meier method, the 95% CI were estimated using the Greenwood formulae. Survival differences were compared in subgroups using the log rank test. Patients, still alive, with no disease progression at end of the study were censored at the date of their last disease assessment. Patients without response or progression assessments at the date of database lock were excluded from the analysis based on these data.
PFS, OS, and ORR were assessed overall and within subgroups: according to the lines of treatment (L2, L3, and L4 +), age groups (< 80 years old versus ≥ 80-years old), frailty (frail versus non frail), prior exposure to lenalidomide, time interval between last lenalidomide and IXA-Rd (≤ 12 months versus > 12 months), renal failure based on creatinine clearance at initiation (> 50 ml/min, 30–50 ml/min, ≤ 30 ml/min), autologous stem cell transplantation (ASCT), comorbidities (Charlson score), and cytogenetic abnormalities at baseline (standard risk (SR) versus high (HR) defined as del(17p) and/or t(4;14) and/or t(14;16)).
A total of 500 patients were expected to participate in the study. A sample size of 250 patients per subgroup would provide an accuracy of 6.2% in describing the study results.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
The REMIX study enrolled 376 patients who initiated IXA concurrently with Rd between August 2017 and October 2019 in 60 active participating sites: 197 during the compassionate access period and 179 thereafter. 32 patients were excluded from the analysis including 29 patients because lenalidomide was initiated more than 1 month before IXA, 2 patients because IXA-Rd was not initiated and 1 patient who did not complete its inclusion visit.
Patient demographics and disease characteristics are summarized in Table 1. At IXA-Rd treatment initiation, the median age was 71 years and 69 (18.4%) patients were 80 years or older. Among the 209 patients with available data, 18.2% patients had an ECOG performance status ≥ 2 including 4 patients (1.9%) with an ECOG = 3 (no patient with an ECOG = 4). Also, in the study population, 48.8% were frail and 62.8% had at least one comorbidity. Charlson score, lines of treatment, cytogenetic abnormalities, and the time intervals from diagnosis to IXA-Rd initiation were similar in the age groups except the frailty score (≥ 80 years: 96.7% and < 80 years: 35.9%).
Table 1.
Patient demographics and disease characteristics
| All patients (N = 376) |
|
|---|---|
| Median age at IXA-Rd start (in years) (IQR) | 71 (65.0–77.5) |
| ≥ 75, n (%) | 133 (35.4) |
| ≥ 80, n (%) | 69 (18.4) |
| Male Sex, n (%) | 185 (49.2) |
| Charlson index total score, n (%) | |
| 0 | 246 (65.4) |
| 1–2 | 100 (26.6) |
| 3–4 | 21 (5.6) |
| ≥ 5 | 9 (2.4) |
| ECOG at IXA-Rd start, n (%) | n = 209 |
| 0 | 69 (33.0) |
| 1 | 102 (48.8) |
| ≥ 2 | 38 (18.2) |
| Simplified frailty scale at IXA-Rd start, n (%) | n = 283 |
| Frail | 138 (48.8) |
| Non frail | 145 (51.2) |
| M Protein type,(n (%) | |
| IgG | 211 (56.1) |
| IgA | 81 (21.5) |
| None | 58 (15.4) |
| Other | 9 (2.4) |
| Data not available | 20 (5.3) |
| Light chain type, n (%) | |
| Kappa | 257 (68.4) |
| Lambda | 112 (29.8) |
| Data not available | 7 (1.9) |
| Cytogenetic features at IXA-Rd start, n (%) | |
| Standard-risk cytogenetic abnormalities | 167 (44.4) |
| High-risk cytogenetic abnormalities | 45 (12.0) |
| Data not available | 164 (43.6) |
| Median time since diagnosis (in years) | 4.0 |
| Line of treatment at IXA-Rd start, n (%) | |
| L2 | 227 (60.4) |
| L3 | 68 (18.1) |
| L4 + | 81 (21.5) |
| Creatinine clearance (ml/min) at IXA-Rd start, n (%) | n = 304 |
| > 50 | 238 (78.3) |
| 30–50 | 43 (14.1) |
| ≤ 30 | 23 (7.6) |
Prior therapy and pre-exposure to lenalidomide
Prior therapies before IXA-Rd start are described Table 2. Most patients, 227 (60.4%), had received only 1 previous line of therapy. IXA-Rd was prescribed second-line in 60.0% of patients, third-line in 18%, and fourth and further lines in 22%. Most patients, 344 (91.7%), were previously treated with bortezomib and 244 (65.1%) had prior immunomodulatory drug therapy, of whom 39.2% had been exposed to lenalidomide and 42.4% to thalidomide. 52 (14%) patients, had received daratumumab, which was only available on compassionate access in France during the study. About half of the patients, 167 (44.5%), had prior autologous stem cell transplantation (ASCT); 53.3% of patients in third or fourth lines of treatment or more versus 38.8% in second lines. Data about refractive status were available only for lenalidomide (n = 26 lena-refractory). Prior exposure to lenalidomide was present in 10.6% of patients in second line, 73.5% in third line, and 91.3% in fourth or further lines. Median prior lenalidomide duration was similar whatever the line (16.0–18.0 months) and median duration between the last lenalidomide dose and start of IXA-Rd therapy was 16 months with 59.5% patients having more than 12 months washout period before re-exposure.
Table 2.
Prior therapy before IXA-Rd start
| All Patients (N = 376) |
Second Line (N = 227) |
Third Line (N = 68) |
≥ Fourth line (N = 81) |
|
|---|---|---|---|---|
| Prior proteasome inhibitor therapy, n (%) | 349 (93.1) | 210 (92.5) | 60 (88.2) | 79 (98.8) |
| bortezomib | 344 (91.7) | 207 (91.2) | 59 (86.8) | 78 (97.5) |
| carfilzomib | 28 (7.5) | 3 (1.3) | 6 (8.8) | 19 (23.8) |
| Prior immunomodulatory drug therapy, n (%) | 244 (65.1) | 105 (46.3) | 61 (89.7) | 78 (97.5) |
| lenalidomide | 147 (39.2) | 24 (10.6) | 50 (73.5) | 73 (91.3) |
| pomalidomide | 44 (11.7) | 1 (0.4) | 1 (1.5) | 42 (52.5) |
| thalidomide | 159 (42.4) | 84 (37.0) | 32 (47.1) | 43 (53.8) |
| Prior exposure to other therapy, n (%) | ||||
| melphalan | 170 (45.3) | 100 (44.1) | 26 (38.2) | 44 (55.0) |
| cyclophosphamide | 76 (20.3) | 27 (11.9) | 15 (22.1) | 34 (42.5) |
| daratumumab | 52 (13.9) | 10 (4.4) | 2 (2.9) | 40 (50.0) |
| bendamustine | 26 (6.9) | 2 (0.9) | 1 (1.5) | 23 (28.8) |
| vincristine | 24 (6.4) | 3 (1.3) | 5 (7.4) | 16 (20.0) |
| doxorubicin | 20 (5.3) | 1 (0.4) | 6 (8.8) | 13 (16.3) |
| panobinostat | 3 (0.8) | 0 (0.0) | 0 (0.0) | 3 (3.8) |
| At least one ASCT during previous therapy, n (%) | 167 (44.5) | 88 (38.8) | 37 (54.4) | 42 (52.5) |
| Last line before IXA-Rd exposure, n (%) | 94 (25.1) | 24 (10.6) | 46 (67.6) | 24 (30.0) |
| Median duration of exposure to lenalidomide (months) | 17.0 | 18.0 | 16.0 | 17.0 |
| Median duration between lenalidomide and IXA-Rd Start (months) | 16.0 | 20.0 | 9.0 | 19.0 |
| > 12 months, n (%) | 78 (59.5) | 16 (69.6) | 20 (47.6) | 42 (63.6) |
| > 24 months, n (%) | 42 (32.1) | 8 (34.8) | 10 (23.8) | 24 (36.4) |
| Refractory to lenalidomide, n (%) | 26 (6.9) | 2 (0.9) | 4 (5.9) | 20 (25.0) |
IXA-Rd initiation
Most patients (90.4%, n = 340) initiated IXA at the full dosage of 4 mg/day, while the remaining 36 patients were prescribed 3 mg/day or less. The starting daily dose of lenalidomide varied from 25 mg in 61.3% of patients (n = 228) to 20 mg in 4.0% (n = 15), 15 mg in 16.9% (n = 63) and 10 mg or less in 17.7% (n = 66) of them. Dexamethasone was associated with IXA-R at a daily dose of 40 mg or 20 mg in 52.7% (n = 195) and 43.0% of patients (n = 159), respectively.
Effectiveness
After a median follow-up of 28.7 (min: 0.4 – 49.5) months from patients’ enrolment to end of the study or death, whichever occurs first, 226 of 358 (63.1%) patients had progressed or died. At analysis, 1 patient was lost to follow-up, 17 patients had not been assessed for disease progression but were still alive and were not included in the PFS (n = 358) analysis.
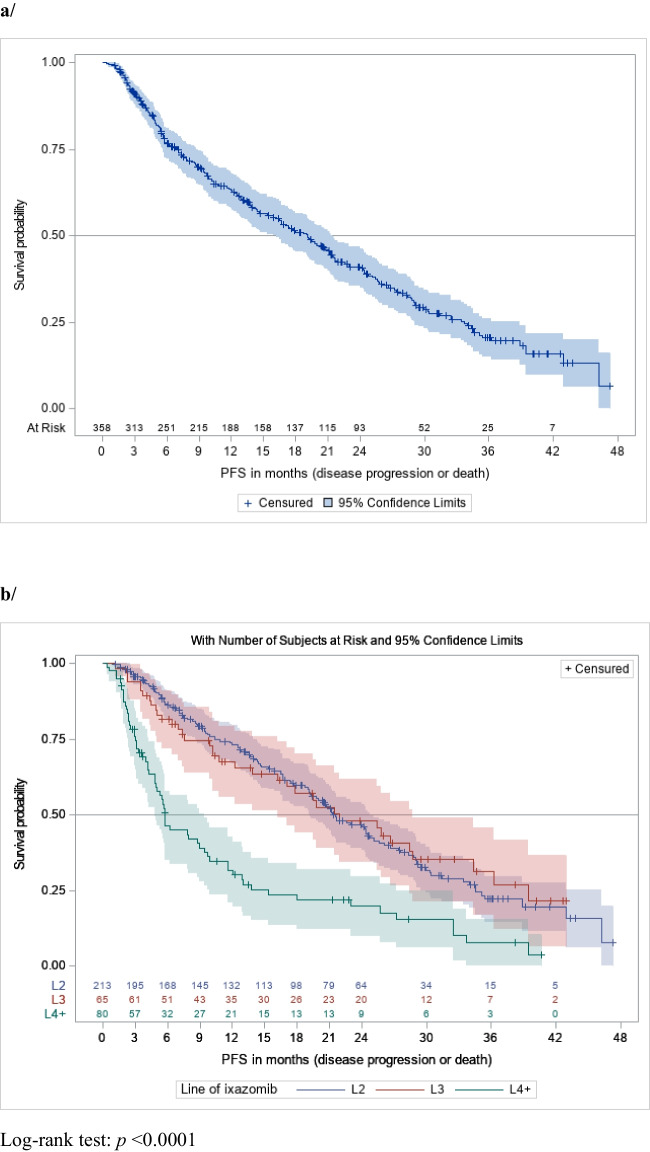
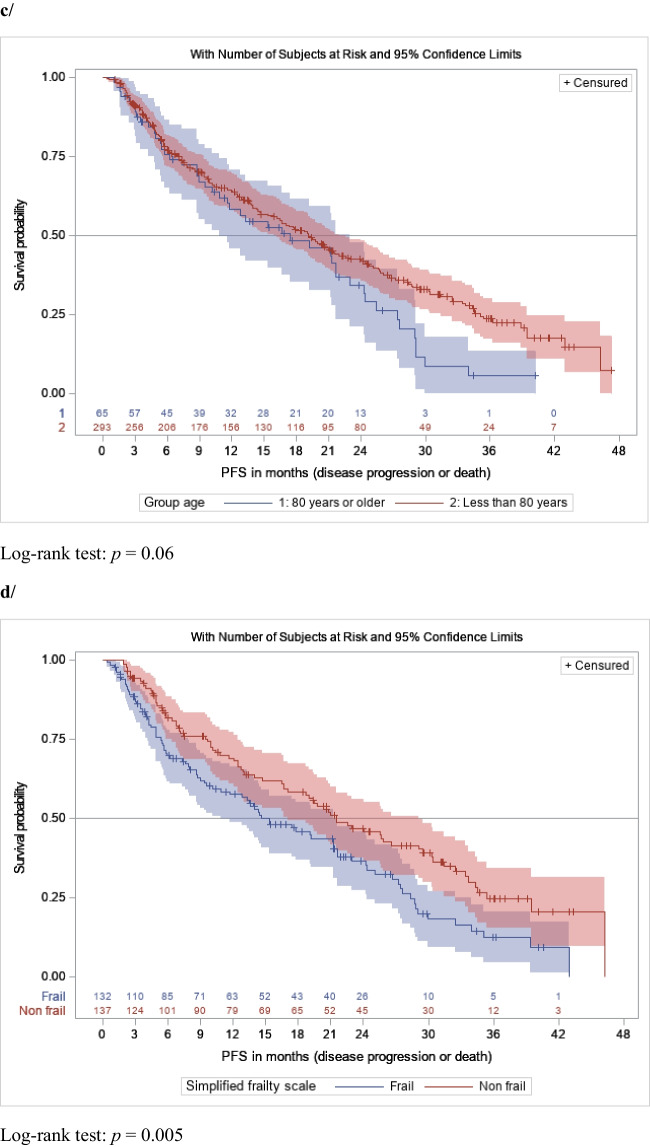
The Kaplan–Meier estimates of PFS are shown in Fig. 1. mPFS was 19.1 months (95% CI [15.9–21.5]) in the overall population (Fig. 1a). mPFS was 21.5 months (95% CI [19.2–24.8]) in patients receiving IXA-Rd as second-line treatment, 21.9 months (95% CI [16.2–28.7]) as third-line treatment, and 5.8 months (95% CI [4.8–9.4]) as fourth or further lines of treatment, respectively p < 0.01 (Fig. 1b). mPFS was 19.1 months (95% CI [15.9–21.9]) in patients younger than 80 years old and 17.4 months (95% CI [10.8–23.0]) in those 80 years old or older p = 0.06 (Fig. 1c). mPFS was significantly lower in frail patients versus non-frail (14.6 months (95% CI [10.8–21.3] versus 21.5 months (95%CI [17.0–29.1]), p < 0.01, Fig. 1d). mPFS was similar in patients with and without previous ASCT (19.8 months (95% CI [14.3, 24.8]) and 17.8 months (95% CI [14.4–21.5]) p = 0.30) or in comorbidities subgroups (with previous comorbidities: 19.5 months (95% CI [12.8–24.0]) and without comorbidities: 18.8 months (95% CI [15.3–21.9]; p = 0.67). Regarding cytogenetic abnormalities, mPFS was 21.2 months (95% CI [14.7–25.6]) in the standard risk group, 19.8 months (95% CI [16.4–29.0]) in the high-risk group and 15.4 months (95% CI [11.6–21.0]) in the group without the evaluation, p = 0.07).
Fig. 1.
PFS distributions with 95% confidence intervals in a/ the overall population; b/ patients receiving IXA-Rd as second-line therapy or as third line therapy and beyond; c/ patients less than 80 years-old and of 80 years-old and more; d/ frail and non-frail patients
Best response rates are detailed in Table 3. The investigator-assessed ORR was 73.1% with IXA-Rd. The best response was CR in 14.5% of patients, VGPR in 30.5%, PR in 28.1% and SD in 10.6% of patients with available response assessment (n = 331). ORR was similar in the age groups: 72.4% in those < 80 years old and 76.8% in those ≥ 80 years old. ORR was increased when IXA-Rd was taken second or third line (80.3% and 70%, respectively) and decreased when taken in fourth line or further (54.4%). In the study population, median DoR was estimated at 10.9 months (95% CI [8.7–14.8]).
Table 3.
Best response and overall survival
| All Patients (N = 376) |
Second Line (N = 227) |
Third Line (N = 68) |
≥ Fourth line (N = 81) |
< 80 YO (N = 307) |
≥ 80 YO (N = 69) |
|
|---|---|---|---|---|---|---|
| Best response, n (%) | N = 331 | N = 203 | N = 60 | N = 68 | N = 275 | N = 56 |
| Complete Response (CR) | 48 (14.5) | 32 (15.8) | 10 (16.7) | 6 (8.8) | 39 (14.2) | 9 (16.1) |
| Very Good Partial Response (VGPR) | 101 (30.5) | 75 (36.9) | 15 (25.0) | 11 (16.2) | 90 (32.7) | 11 (19.6) |
| Partial Response (PR) | 93 (28.1) | 56 (27.6) | 17 (28.3) | 20 (29.4) | 70 (25.5) | 23 (41.1) |
| Stable Disease (SD) | 35 (10.6) | 18 (8.9) | 6 (10.0) | 11 (16.2) | 27 (9.8) | 8 (14.3) |
| Progressive Disease (PD) | 54 (16.3) | 22 (10.8) | 12 (20.0) | 20 (29.4) | 49 (17.8) | 5 (8.9) |
| Duration of best response (months) | N = 242 | N = 163 | N = 42 | N = 37 | N = 91 | N = 23 |
| Median (95% CI) | 10.9 [8.7; 14.8] | 10.9 [8.5; 15.9] | 14.7 [6.9; 23.6] | 9.1 [3.5; 19.5] | 10.8 [7.8; 13.7] | 16.1 [5.1; 21.2] |
| IQR | [3.5; 20.0] | [3.9; 19.6] | [5.0; 23.9] | [2.4; 19.9] | [3.5; 19.6] | [3.3; 22.1] |
| Overall survival, survival rate % [CI] | N = 375 | N = 226 | N = 68 | N = 81 | N = 306 | N = 69 |
| Median (95% CI) | NE | NE | NE | 18.5 [11.0; 33.7] | NE | 31.6 [23;.] |
| 12 months | 82.2 [78.3; 86.1] | 89.2 [85.1; 93.3] | 86.6 [78.4; 94.7] | 59.2 [48.5; 69.9] | 83.8 [79.7; 88.0] | 75.0 [64.7; 85.3] |
| 24 months | 71.6 [67.0; 76.3] | 79.3 [73.9; 84.7] | 80.3 [70.7; 89.9] | 43.0 [31.9; 54.0] | 74.0 [69.0; 79.0] | 60.9 [49.1; 72.7] |
| 36 months | 58.3 [52.6; 63.9] | 63.4 [56.1; 70.8] | 68.9 [57.1; 80.7] | 35.3 [23.9; 46.7] | 61.7 [55.6; 67.8] | 41.4 [26.6; 56.2] |
| 42 months | 55.4 [49.4; 61.5] | 62.3 [54.8; 69.8] | 60.9 [46.1; 75.7] | 31.8 [19.6; 43.9] | 58.5 [51.9; 65] | 41.4 [26.6; 56.2] |
| 48 months | 52.4 [44.2; 60.5] | 57.5 [46.1; 68.9] | 60.9 [46.1; 75.7] | . % [.;.] | 54.8 [45.5; 64.1] | 41.4 [26.6; 56.2] |
NE: not estimated
At the time of the present analysis the median OS had not been yet been reached (Online Resource 1). The estimated OS rate was 82.2% (78.3; 86.1) at 12 months, 71.6% (67.0; 76.3) at 24 months, 58.3% (52.6; 63.9) at 36 months, 55.4% (49.4; 61.5) at 42 months and 52.4% (44.2; 60.5) at 48 months. In the subgroup of patients treated in the fourth line or further the median OS was 18.5 months (95% CI [11.0, 33.7]). In patients older than 80 years the median OS was 31.6 months (95% CI [23.0, not reached]).
Effectiveness and pre-exposure to lenalidomide
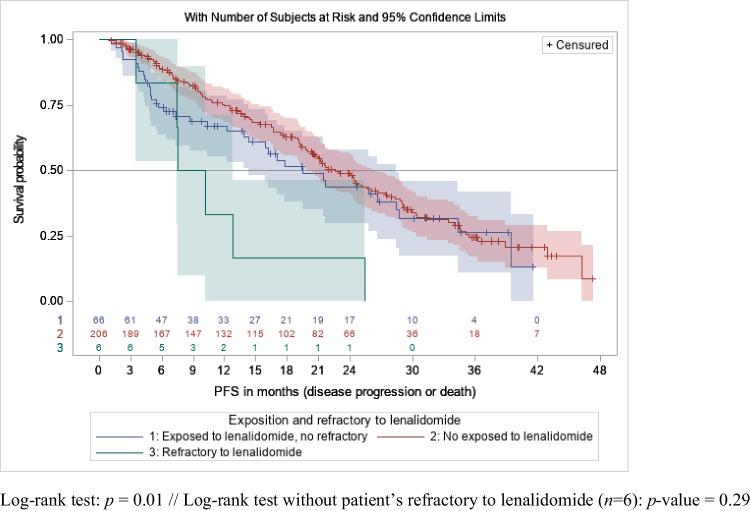
When focusing on second and third lines (n = 272), mPFS was similar in patients previously treated with lenalidomide (mPFS of 19.5 months (95% CI [14.3–28.4]) and in patients not exposed to lenalidomide (mPFS of 22.6 months, 95% CI [20.0–26.7]), p = 0.29 (Fig. 2) without any differences in patients’ characteristics in both groups. Those results were similar when the analysis focused on patients of second line (not enough patients to estimate results for third line).
Fig. 2.
PFS distributions with 95% confidence intervals in patients receiving IXA-Rd in L2 and L3 according to prior exposure to lenalidomide
In pre-exposed patients (n = 64), when time between last lenalidomide dose and IXA-Rd start was ≤ 12 months, mPFS was 7.4 months (95% CI [4.9–17.8]). It was 25.8 months (95% CI [15.9-not reached]) when this washout period exceeded 12 months (p = 0.0043, Online Resource 2).
Safety
A dose reduction was observed in 99 patients (26.4%) for IXA and in 129 patients (34.4%) for lenalidomide during treatment. Treatment temporary suspensions were reported in 83 patients (22.1%) with IXA and in 80 patients (21.3%) with lenalidomide. At analysis, the median duration of treatment with IXA was 12.4 months. At final analysis, 278 (74.1%) had permanently discontinued IXA and 215 (57.3%) lenalidomide. IXA discontinuation was due to toxicity in 21% (79/376) of patients and progression in 34.6% (130/376) of patients. Respectively, 69.6% and 75.2% of patients ≥ 80 years and < 80 years discontinued IXA of whom 21.7% (15/69) and 20.9% (64/306) due to adverse events.
AEs were reported in 294 patients (78.2%) treated with IXA-Rd including 54.3% of patients with SAE and 40.7% with treatment-related AE. The most frequently treatment-related AEs (> 10 patients) were diarrhea (13.9%), thrombocytopenia (12.6%), nausea (8.5%), asthenia (7.1%), anemia (4.4%), neutropenia (4.4%), vomiting (4.1%), peripheral neuropathy (4.1%) and unspecified cytopenia (3.4%). The incidence of AE, AE related to treatment, SAE, and SAE related to treatment are 77.2%, 41.7%, 54.1% and 16.0% in patients < 80 and 82.6%, 36.2%, 55.1%, 11.6% in patients ≥ 80-year-old. Overall, most frequent SAE included thrombocytopenia (12.2% of patients with at least one AE), plasma cell myeloma (9.5%), death (7.8%), neutropenia (5.8%), general physical health deterioration (5.4%), diarrhea (4.4%) and anemia (4.4%) as presented in Table 4.
Table 4.
Adverse events reported during the study in patients treated with IXA-Rd
| Patients (N = 376) | ||
|---|---|---|
| Patients with at least one AE (N = 294) |
Patients with at least one treatment-related AE (N = 153) |
|
| Patients with: | ||
| any AE, n (%) | 294 (78.2) | 153 (40.7) |
| any serious AE, n (%) | 204 (54.3) | 57 (15.2) |
| any AE leading to death, n (%)* | 67 (17.8) | 1 (0.3) |
| Most frequent AE (n patients, %): | ||
| Diarrhea | 77 (26.2) | 41 (13.9) |
| Thrombocytopenia | 68 (23.1) | 37 (12.6) |
| Asthenia | 44 (15.0) | 21 (7.1) |
| Neutropenia | 35 (11.9) | 13 (4.4) |
| Nausea | 34 (11.6) | 25 (8.5) |
| Anemia | 33 (11.2) | 13 (4.4) |
| Plasma cell myeloma | 29 (9.9) | 1 (0.3) |
| Death | 23 (7.8) | 0 (0.0) |
| Neuropathy peripheral | 21 (7.1) | 12 (4.1) |
| Vomiting | 19 (6.5) | 12 (4.1) |
| General physical health deterioration | 17 (5.8) | 1 (0.3) |
| Cytopenia | 16 (5.4) | 10 (3.4) |
| Constipation | 15 (5.1) | 9 (3.1) |
| Fatigue | 14 (4.8) | 8 (2.7) |
| Oedema peripheral | 14 (4.8) | 1 (0.3) |
| Pancytopenia | 14 (4.8) | 8 (2.7) |
| Back pain | 14 (4.8) | 2 (0.7) |
| Muscle spasms | 13 (4.4) | 3 (1.0) |
| Paresthesia | 11 (3.7) | 0 (0.0) |
| Acute kidney injury | 11 (3.7) | 1 (0.3) |
| Bronchitis | 10 (3.4) | 4 (1.4) |
| Insomnia | 10 (3.4) | 2 (0.7) |
*There were 21 patients with AE leading to death while being treated with IXA-Rd
Subsequent therapy
After IXA-RD treatment discontinuation, in the 177 patients with available data, subsequent therapies mostly comprised pomalidomide (n = 99, 55.9%), daratumumab (n = 91, 51.4%), carfilzomib (n = 63, 35.6%), bortezomib (n = 60, 33.9%) and cyclophosphamide (n = 53, 29.9%).
Discussion
The REMIX study is the largest prospective, real-world study to evaluate orally administered combination IXA-Rd in patients with RRMM and confirms the efficacy and safety of the IXA-Rd triplet oral regimen with a mPFS of 19.1 months in RRMM patients with in median 1 prior line of treatment and a proportion of 39% prior exposure to lenalidomide.
The REMIX study confirms that IXA-Rd is safe and effective in elderly patients. The study included a high proportion of patients older than 75 years (35%) and 80 years (18%), and the median age (71 years) was higher than in any other real-world study [21–25], as presented in Table 5. The efficacy in terms of mPFS and ORR remains meaningful in these elderly patients, particularly in those aged 80 years and over (mPFS of 17.4 months, ORR of 76.8%); the mPFS was similar in younger patients (p = 0.06). The median OS was reached in the elderly subgroup, however, as expected, younger patients had not yet reached the median overall survival. This is important because a well-tolerated, oral triplet regimen is particularly advantageous for older patients compared to other available treatment options, which are more intensive and require hospital administration. Patients older than 80 years are generally excluded from clinical trials and there are few data regarding their outcomes in the literature. However, these patients are crucial, as they represent a quarter of the patients seen in routine clinical practice. To our knowledge, this is the first study to publish this insight into older RRMM patients and the demonstrated benefits of the IXA-Rd triplet mean this could be an alternative treatment option for a population that clinicians deal with daily [6, 8].
Table 5.
The REMIX study and other IXA-Rd papers
| Reference | Name of the study | Design | Country | Number of patients | Patient characteristics | Effectiveness | Safety | Subgroups of interest |
|---|---|---|---|---|---|---|---|---|
| Moreau P et al. [4] / Richardson et al. [26] | TOURMALINE-MM1 | Clinical trial | 26 countries | 722 patients (360 in IXA-Rd group) |
Median age: 66 years, % > 65: 53% L2 (62%), L3 (27%), L4 (11%) Previously exposed to R: 12% |
ORR: 78% ≥ VGPR: 48% mPFS: 20.6 months mPFS > 75 years: 18.5 mPFS ISS III: 18.4 months mPFS high-risk cytogenetics: 21.4 months mOS: 53.6 months |
Discontinuation due to toxicity: 17% | Age, ISS stage, cytogenetic risk, number of prior therapies, prior exposition to IP and IMID, refractory to last therapy, relapsed or refractory |
| Macro M et al. 2023 (present study) | REMIX study | Real-world prospective study | France | 376 patients across 60 sites |
Median age: 71 years, %80 + : 18% L2 (60%), L3 (18%), L4 + (22%) Previously exposed to R: 39.2% |
ORR: 73% ≥ VGPR: 45% mPFS: 19.1 months L2 and L3: 22 months vs L4 + : 6 months ≥ 80y: 17 months vs 19 months in < 80y frail: 15 months vs 22 months in non-frail mOS not reached |
Discontinuation due to toxicity: 21% | Age, frailty, line of treatment, renal failure, comorbidities, previous ASCT, prior exposure to R |
| Varga G et al. [21] | - | Real-world retrospective study | Hungary | 77 patients treated at 7 centers |
Median age: 66 years L2 (27%), L3 (35%), L4 (39%) |
ORR: 67% ≥ VGPR: 23% mPFS: 11.4 months mPFS not reached in L2, was 10 months in L3 and 8.8 months in L4 No difference according ISS and cytogenetic profile |
No permanent drug interruptions due to AEs |
ISS classification Cytogenetic profile |
| Cohen YC et al. [22] | - | Real-world retrospective study | Israel | 78 patients across 7 sites |
Median age: 68 years L2 (64%), L3 (19%), L4 + (17%) Previously exposed to R: 26% |
ORR: 88% ≥ VGPR: 45% mPFS: 24 months mPFS not reached vs 20.2 months for age ≤ 65 vs > 65, respectively mOS not reached |
Discontinuation due to toxicity: 14% |
Age (≤ 65) No effect on PFS was found for gender, BSA, ixazomib line number, diagnosis paraprotein and involved light chain, cytogenetic risk, ISS, presence of CRAB or EMD levels above ULN, prior drug exposure (IMiDs, PIs), and prior ASCT |
| Terpos E et al. [23] | - | Real-world retrospective study | Greece, the UK, and the Czech Republic | 155 patients who received IXA via early access programs |
Median age: 68 years L2 (51%), L3 (28%), L4 + (21%) Previously exposed to R: 17% |
ORR: 74% (76.5% in L2, 71.2% in L3 +) ≥ VGPR: 35% mPFS: 27.6 months L2: 27.6 months L3 + : 19.9 months prior exposure to R: mPFS 4.8 months (n = 26) no prior exposure to R: 27.6 (n = 129) |
Discontinuation due to adverse events/toxicity: 9% |
Gender Prior ASCT Length of ixazomib exposure Prior IMID exposure |
| Minarik J et al. [24] | - | Real-world prospective study | The Czech Republic | 127 patients |
Median age: 66 years L2 (58%), L3 (24%), L4 + (19%) Previously exposed to R: 17% (6% refractory) |
ORR: 73% ≥ VGPR: 33.3% mPFS: 17.5 months L2: 32.8 months L3: 23.1 months L4: 9.7 months L5 + : 5 months > 75 years: 11.1 months mOS: 36.6 months |
Discontinuation due to toxicity: 3.1% |
Age, ISS stage, ASCT, cytogenetics, maximal treatment response, and pretreatment |
| Hajek R et al. [25] | - | Real-world study | 13 countries (INSIGHT MM and the Czech RMG) | 263 patients |
Median age: 68 years with 15% > 75 years L2 (44%), L3 (35%), L4 + (21%) Previously exposed to R: 27% (7% refractory) |
ORR: 73% ≥ VGPR: 37% mPFS: 21 months L2: 26 months L3: 24 months L4: 14 months |
Discontinuation due to AEs: 32% | Line of treatment |
Frail patients included in the REMIX study also benefited from IXA-Rd treatment. Although mPFS was shorter in this subgroup than non-frail patients (14.6 months versus 21.5 months, p < 0.01), this result is nevertheless positive considering the acceptable tolerance profile, which is difficult to compare as frail patients are often excluded from trials. This means IXA-Rd could also be an interesting alternative for frail patients.
Overall, the efficacy of IXA-Rd in a real-world situation (mPFS of 19.1 months, ORR of 73%) was similar to the controlled, registration study TOURMALINE-MM1 (mPFS of 20.6 months, ORR of 78%) [4]. Yet, the REMIX population was older (median age, 71 years) compared with TOURMALINE-MM1 (median age 66 years), and included more patients with advanced disease (L4 +) 21.5% compared with 11% in TOURMALINE-MM1 and an ECOG > 1 (18.2%) compared with 5% in TOURMALINE-MM1. Importantly, more patients in the REMIX study had been previously exposed to lenalidomide (39.2%) than in TOURMALINE-MM1 (12%) or bortezomib (REMIX 91.7%; TOURMALINE MM1 69%). Also, some REMIX patients had previously received carfilzomib (7.5%), pomalidomide (11.7%) or daratumumab (13.9%) unlike TOURMALINE MM1. Thus, patients were more extensively poly-treated than in TOURMALINE-MM1, which is recognized to have a negative impact on effectiveness. Moreover, 49.8% of the REMIX patients were frail and two third had comorbidities in contrast to the TOURMALINE-MM1 in which frail patients were excluded. The majority of real-world studies conducted are retrospective with small study sample. The mPFS varied from 11.4 to 27.6 months. Patients are younger than in REMIX study and less previously exposed to lenalidomide (maximum: 27%).
The mPFS of patients in the REMIX study treated with IXA-Rd in L2 (21.5 months) and in L3 (21.9 months) were longer than that of patients in L4 and above (5.8 months), p < 0.01. This is in line with TOURMALINE-MM1 and most real-world studies [21–25]. Similarly, the best treatment response (CR and VGPR) occurred more frequently in L2 and L3 than in subsequent lines. As with TOURMALINE-MM1, efficacy remains high in second relapse (L3) and is equivalent to first relapse (L2). In practice, these results are not surprising, and it is now recognized that treatment benefits are reduced at advanced stages of the disease [23, 25, 27].
In the REMIX study, a large proportion of patients were pre-exposed to lenalidomide before initiating IXA-Rd. This was especially true for L3 (73.5%) and L4 + (91.3%) patients but rarer for L2 patients (10.6%). It is noteworthy that in this study, pre-exposure to lenalidomide in non-refractory second- and third-line patients is not associated with reduced IXA-Rd efficacy, with mPFS remaining equivalent in both groups (19.5 months versus 22.6 months, p = 0.29). For this population, re-using lenalidomide may be of benefit to those whom are sensitive to lenalidomide. Few data are available on lenalidomide pre-exposure, as few patients in TOURMALINE-MM1 had been pre-exposed to lenalidomide, preventing this association from being studied [4, 28]. Results from other real-world studies suggest the benefit may be limited in lenalidomide-pre-treated patients [23]. However, as lenalidomide exposure increases with advanced disease, it is difficult to discern between the real impact of lenalidomide pre-exposure and late disease relapse. Further analysis of REMIX data suggests that a washout period of approximately 12 months between prior lenalidomide exposure and IXA-Rd initiation may improve IXA-Rd efficacy. However, these results should be interpreted with caution, as the washout period duration may be related to other factors such as response to the previous line which are not considered in this analysis.
In addition to lenalidomide pre-exposure, L4 patients had also been exposed to multiple immunomodulatory drugs (IMiD), proteasome inhibitors (PI) and anti-CD38s. Specifically, half of these patients had received pomalidomide or daratumumab and nearly a quarter had received carfilzomib. Conversely, very few L2 or L3 patients had received these immunomodulatory treatments. The multiple exposure of L4 + patients to various agents may reflect the RRMM resistance to treatment and is expected to be associated with the lower IXA-Rd efficacy in this subgroup.
Dose reductions or treatment interruptions for IXA-Rd are reported for approximately one quarter of patients, similarly with IXA or lenalidomide. Treatment discontinuation related to AEs was noted in 24.5% of patients, in this frailer and older population, which is slightly higher than TOURMALINE-MM1 (17%). As in other studies, the most frequently reported AEs were digestive or hematological, with no new signals identified [23–25, 29]. Unlike most other real-world studies, the REMIX study is prospective and is therefore likely to be more comprehensive and accurate in reporting AEs during follow-up than in retrospective studies.
The limitations of the REMIX study are those inherent to real-world observational studies, notably relating to treatment response or progression assessments, which are assessed by the investigator. Frailty score calculation was based on ECOG-PS if patients were ≤ 80 years, which is less collected in routine clinical practice than in clinical trials. Due to missing data on ECOG-PS, the simplified frailty score was only available for 283 patients (75.0%) The prospective patient recruitment at the start of treatment does not predict the response to treatment and limits the impact of this bias on the efficacy assessment. The representativeness of the patients recruited is still questionable even though the centers were encouraged to propose the study to all their eligible patients. Lastly, the study started when IXA became available in a compassionate program in France. A total of 500 patients were expected to participate in the study with a sample size of 250 patients per subgroup. Even if the total of 500 patients was not reached, an accuracy of 5% was sufficient to estimate the survival analyses and the proportion of patients was similar in each subgroup (N = 197 during the compassionate access period before the treatment was marketed and reimbursed and N = 179 thereafter), is in line with what was intended. Sensitivity analyses conducted to identify potential selection bias related to the compassionate program showed that those patients recruited during the compassionate access period were similar to those recruited afterwards, although those patients recruited during compassionate access were slightly younger. This can be explained contextually and historically by the treatments available to clinicians during the compassionate program.
In conclusion, the results from the REMIX study are consistent with the TOURMALINE-MM1 results and confirms the benefit of the all-oral IXA-Rd triplet in real life, particularly in early relapse. It reveals that pre-exposure to lenalidomide in non-refractory second and third-line patients is not associated with reduced efficacy and suggests beneficial re-use in early relapses. While many treatments are available to the clinician to manage RRMM patients, REMIX demonstrates the value of the oral IXA-Rd in an elderly population (> 80 years) in which efficacy and acceptable tolerance (they do not experience higher rates of AE or treatment discontinuation) are maintained.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors sincerely thank the patients who make the study possible, the medical writer, the clinical study teams, the investigators, and Kappa Santé for coordinating the study and analysis the results.
Abbreviations
- AEs
Adverse Events
- ASCT
Autologous Stem Cell Transplantation
- CR
Complete Response
- DoR
Duration of Response
- ECOG
Eastern Cooperative Oncology Group performance status
- HR
High Risk
- ISS
International Staging System
- IMIDs
Immunomodulatory drugs
- IQR
Interquartile range
- IXA
Ixazomib
- IXA-Rd
Ixazomib-Rd
- Rd
Lenalidomide and dexamethasone
- mPFS
Median Progression Free Survival
- ORR
Overall Response Rate
- OS
Overall Survival
- PR
Partial Response
- PI
Proteasome inhibitors
- RWE
Real-world evidence
- RRMM
Relapsed/Refractory Multiple Myeloma
- RR
Response Rate
- SAEs
Serious AE
- SD
Stable Disease
- SR
Standard Risk
- VGPR
Very Good Partial Response
Authors’ contribution
ACR, LB, AMS, KL, LCF, HZ, FH, VR, CH, FM, LK, as investigators, have included and followed patients.
JB has participated in the review and interpretation of the analyses and the exploitation of the results. LV, CC, MM, and XL as members of the scientific committee, have participated in the design of the protocol, the review and interpretation of the analyses, and the exploitation of the results. All the authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The study, including medical writing funding, was supported by Takeda France.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was performed in line with the ethical principles of the Declaration of Helsinki, the principles of the International Society for Pharmacoepidemiology guidelines for Good Pharmacoepidemiology Practice, and in compliance with the General Data Protection Regulation (GDPR). Approval was granted by the French Ethics Committee of Clermont-Ferrand (CPP SUD-EST VI) on November 9th, 2017 (N° AU 1381).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
MM has received Honoraria from Janssen, Sanofi, GSK and Takeda, travel support from Janssen and Takeda and research funding from Janssen and Takeda. CH has received honoraria from Janssen, BMS, GSK, Takeda, Sanofi and Amgen. LV has been a member on an entity’s board of directors or advisory committee for BMS, Janssen and Takeda, received honoraria from Janssen and Sanofi and funding for meeting participation for BMS, Janssen, Takeda, Sanofi and Pfizer. KL has received honoraria from AbbVie, AstraZeneca, Beigene, Iqone, Janssen, Novartis and Takeda. LK has received travel support from Janssen. JB is a Takeda employee. CC has been consultant for Amgen, AstraZeneca and Roche and has received honoraria and research funding for Amgen, AstraZeneca, BMS, GSK, Merck, Novartis, Pfizer, Roche and Takeda. XL is a consultant and has received honoraria from Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron and Iteos. The other authors declare they have no financial interests.
Footnotes
M. Macro and C. Hulin are first co-authors.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson KC. Progress and paradigms in multiple myeloma. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:5419–5427. doi: 10.1158/1078-0432.CCR-16-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudreault J-S, Touzeau C, Moreau P. Triplet combinations in relapsed/refractory myeloma: update on recent phase 3 trials. Expert Rev Hematol. 2017;10:207–215. doi: 10.1080/17474086.2017.1285694. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol Off J Eur Soc Med Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 5.Leleu X, Masszi T, Bahlis NJ, et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am J Hematol. 2018 doi: 10.1002/ajh.25134. [DOI] [PubMed] [Google Scholar]
- 6.Willan J, Eyre TA, Sharpley F, Watson C, King AJ, Ramasamy K. Multiple myeloma in the very elderly patient: challenges and solutions. Clin Interv Aging. 2016;11:423–435. doi: 10.2147/CIA.S89465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweegman S, Engelhardt M, Larocca A, EHA SWG on ‘Aging and Hematology’ Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29:315–321. doi: 10.1097/CCO.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 8.Fraz MA, Warraich FH, Warraich SU, et al. Special considerations for the treatment of multiple myeloma according to advanced age, comorbidities, frailty and organ dysfunction. Crit Rev Oncol Hematol. 2019;137:18–26. doi: 10.1016/j.critrevonc.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17:575–583.e2. doi: 10.1016/j.clml.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Hungria VTM, Lee HC, Abonour R, et al. Real-world (RW) multiple myeloma (MM) patients (Pts) remain under-represented in clinical trials based on standard laboratory parameters and baseline characteristics: analysis of over 3,000 Pts from the Insight MM Global, Prospective, Observational Study. Blood. 2019;134:1887. doi: 10.1182/blood-2019-125749. [DOI] [Google Scholar]
- 11.Chari A, Romanus D, Palumbo A, Blazer M, Farrelly E, Raju A, Huang H, Richardson P. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark RCTs in relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019 doi: 10.1016/j.clml.2019.09.625. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hari P, Romanus D, Luptakova K, Blazer M, Yong C, Raju A, Farrelly E, Labotka R, Morrison VA. The impact of age and comorbidities on practice patterns and outcomes in patients with relapsed/refractory multiple myeloma in the era of novel therapies. J Geriatr Oncol. 2018;9:138–144. doi: 10.1016/j.jgo.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Davies F, Rifkin R, Costello C, et al. Real-world comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in the US. Ann Hematol. 2021;100:2325–2337. doi: 10.1007/s00277-021-04534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chari A, Richardson PG, Romanus D, et al. Real-world outcomes and factors impacting treatment choice in relapsed and/or refractory multiple myeloma (RRMM): a comparison of VRd, KRd, and IRd. Expert Rev Hematol. 2020;13:421–433. doi: 10.1080/17474086.2020.1729734. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 17.Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34:224–233. doi: 10.1038/s41375-019-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Epstein M, International Society of Pharmacoepidemiology Guidelines for good pharmacoepidemiology practices (GPP) Pharmacoepidemiol Drug Saf. 2005;14:589–595. doi: 10.1002/pds.1082. [DOI] [PubMed] [Google Scholar]
- 20.Official Journal of the European Communities (1995) Directive 95/46/EC of the European parliament and of the council of 24 october 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data. 281
- 21.Varga G, Nagy Z, Demeter J, et al. Real world efficacy and safety results of ixazomib lenalidomide and dexamethasone combination in relapsed/refractory multiple myeloma: data collected from the Hungarian ixazomib named patient program. Pathol Oncol Res POR. 2019;25:1615–1620. doi: 10.1007/s12253-019-00607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen YC, Magen H, Lavi N, et al. Ixazomib-based regimens for relapsed/refractory multiple myeloma: are real-world data compatible with clinical trial outcomes? A multi-site Israeli registry study. Ann Hematol. 2020;99:1273–1281. doi: 10.1007/s00277-020-03985-9. [DOI] [PubMed] [Google Scholar]
- 23.Terpos E, Ramasamy K, Maouche N, et al. Real-world effectiveness and safety of ixazomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Ann Hematol. 2020;99:1049–1061. doi: 10.1007/s00277-020-03981-z. [DOI] [PubMed] [Google Scholar]
- 24.Minarik J, Pika T, Radocha J, et al. Survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (Rd) in relapsed and refractory multiple myeloma patients in routine clinical practice. BMC Cancer. 2021;21:73. doi: 10.1186/s12885-020-07732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hájek R, Minařík J, Straub J, et al. Ixazomib-lenalidomide-dexamethasone in routine clinical practice: effectiveness in relapsed/refractory multiple myeloma. Future Oncol Lond Engl. 2021;17:2499–2512. doi: 10.2217/fon-2020-1225. [DOI] [PubMed] [Google Scholar]
- 26.Richardson PG, Kumar SK, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, Stoppa AM, Gimsing P, Garderet L, Touzeau C, Buadi FK, Laubach JP, Cavo M, Darif M, Labotka R, Berg D, Moreau P (2021) Final overall survival analysis of the tourmaline-mm1 phase iii trial of ixazomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol 39(22):2430–2442. 10.1200/JCO.21.00972 [DOI] [PubMed]
- 27.Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–2275. doi: 10.1038/s41375-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos M-V, Masszi T, Grzasko N, et al. Impact of prior therapy on the efficacy and safety of oral ixazomib-lenalidomide-dexamethasone vs. placebo-lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma in TOURMALINE-MM1. Haematologica. 2017;102:1767–1775. doi: 10.3324/haematol.2017.170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Moreau P, Hari P, et al. Management of adverse events associated with ixazomib plus lenalidomide/dexamethasone in relapsed/refractory multiple myeloma. Br J Haematol. 2017;178:571–582. doi: 10.1111/bjh.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.