Abstract
To provide an overview of the current available data about FAPI PET in breast cancer patients, with a perspective point of view. A literature search for studies about FAPI PET in the last 5 years (from 2017 to January 2023) was carried out on MEDLINE databases, such as PubMed, EMBASE, Web of Science and Google Scholar using the following keywords: “PET” AND “FAPI” AND “Breast Cancer” AND “Fibroblast imaging”. The Critical Appraisal Skills Program (CASP) checklist for diagnostic test studies was used for testing the quality of selected papers. 13 articles were selected, including 172 patients affected by breast cancer who underwent FAPI-based PET images. CASP checklist was used in 5/13 papers, demonstrating a general low quality. Different types of FAPI-based tracers were used. No difference in terms of FAPI uptake was reported based on the histopathological characteristics, such as immunohistochemistry and grading of breast cancer. FAPI demonstrated more lesions and yielded much higher tumor-to-background ratios than 2-[18F]FDG. Preliminary experiences with FAPI PET in breast cancer showed some advantages than the current available 2-[18F]FDG, although prospective trials are needed to further evaluate its diagnostic utility in clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12149-023-01852-x.
Keywords: 68 Ga-FAPI, Positron emission tomography–computed tomography, Breast neo-plasms, Fluorodeoxyglucose F18
Introduction
Breast cancer is the most frequent cancer and the second major cause of tumor death in women, after lung cancer [1]. It is defined as an heterogenous cancer due to various factors, such as tumor type, histological grade, lymph node metastasis, estrogen receptor (ER), progesterone receptor (PR), and human epidermal factor receptors 2 (HER2). All these features affect the treatment approach and the prognosis. Breast cancer biology represents a key factor for guiding the appropriate therapy; therefore, the identification of specific targets, often expressed on cancer cells, can be used for diagnosis and therapy, thus improving therapeutic outcome. Several tumor entities, such as breast, colon, and pancreatic carcinomas, are characterized by a strong desmoplastic reaction [2]. The presence of cancer-associated fibroblasts and extra-cellular fibrosis is associated with the gross tumor mass. Conversely from the normal fibroblasts, the cancer-related fibroblasts express a specific protein, named fibroblast activation protein (FAP). FAP is a transmembrane glycoprotein consisting of an extra-cellular and intra-cellular domain and a transmembrane component [3]. It represents one of the crucial components of the extra-cellular matrix and modulates or remodels the tumor microenvironment. For its peculiarity, fibroblast activating protein inhibitor (FAPI) has gained an important role as therapeutic target in a variety of human malignancies. Consequently, also FAPI-based radiopharmaceutical agents were produced, firstly with the aim of exploiting FAPI as a diagnostic target, but after as a potential theragnostic agent [4]. In the last years, different type of radiopharmaceuticals has been tested, either labeled with [68 Ga]Ga, but also with [177Lu]Lu or [225Ac]Ac.
Molecular imaging in breast cancer has a long research history, including whole-body PET, PET/CT and PET/MR scanning, and also dedicated systems either with SPECT or PET [5]. However, the clinical application of 2-[18F]FDG PET/CT is currently limited to the evaluation of locally or metastatic breast cancer, due to its limited sensitivity in detecting small breast lesions, micrometastases and some tumors with specific biological features (i.e., lobular carcinoma or low-grade breast tumors) [6, 7]. Many authors have recently demonstrated the potential clinical utility of FAPI-based PET in patients affected by breast cancer, trying to solve the current imaging gap. Herein, we provide an overview of the current available data about FAPI PET in breast cancer patients, with a perspective point of view.
Materials and methods
Literature search
Two authors performed the literature search, study inclusion, and data extraction. A literature search for studies about FAPI PET in the last 5 years (from 2017 to January 2023) was carried out on MEDLINE databases, such as PubMed, EMBASE, Web of Science and Google Scholar using the following keywords: “PET” AND “FAPI” AND “Breast Cancer” AND “Fibroblast imaging”. No limits were applied to the search strategy. Congress materials, reviews, letters to editors, editorials and clinical cases were excluded. After the recovery of the PDF files, the references of the studies already selected were checked.
From each study, the following data were recovered: type of the study (prospective, retrospective, etc.), year and geographical origin, sample size, setting of disease, type of FAPI agent, and comparative data with other radiopharmaceuticals.
Quality of the selected studies
Selected imaging studies were analyzed using a modified version of the Critical Appraisal Skills Program (CASP) (https://casp-uk.net/aboutus, accessed on 1st February 2023) checklist for diagnostic test studies. Critical appraisal was performed by 2 reviewers, and discrepancies, if any, were resolved by discussion among researchers.
Results
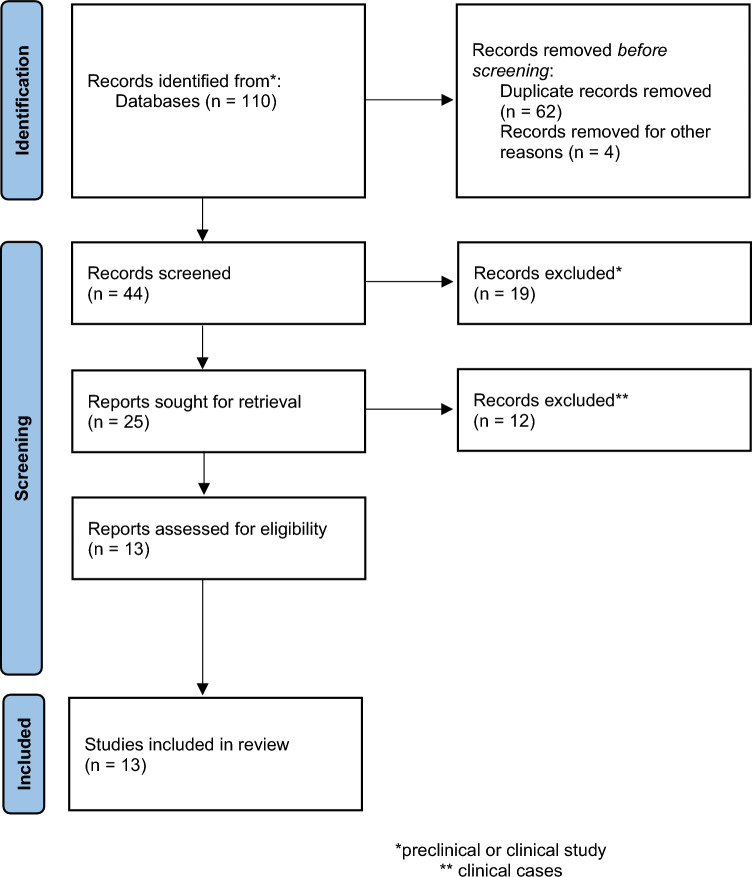
Based on the search criteria, 13 articles were selected (Fig. 1). In Table 1 are reported the main characteristics of the included reports. Totally, 172 patients affected by breast cancer underwent FAPI-based PET images. In most selected papers (n = 8; 62%, [8–15], FAPI-based PET/CT was used in other oncological diseases further than breast cancer. PET/MR was employed only in 2 studies [16, 17]. Different types of FAPI-based tracers were used; [Ga-]Ga-FAPI-04 was more often used [8–10, 12, 15, 18–20]. The setting of disease was not always available or not clearly stated, although restaging phase was more often a criterion of selection. The quality of papers was tested in only those considering patients affected by breast cancer (n = 5) [16–20]. Based on the CASP analysis, the quality of papers was generally low, because the standard of reference was often missed, too limited patient population were enrolled and only a descriptive analysis was made (Table 2 and Supplementary Table 1). However, the limited quality of the currently available papers represent an important challenges for the future clinical trials or studies.
Fig. 1.
PRISMA flow-chart of the identified and selected papers
Table 1.
Characteristics of the included studies
| Authors, referenes | Country | Type of study | Year of Pub | Study population | N pts | Setting of disease | Comparison with 2-[18F]FDG | Type of tracer (Injected dose) |
|---|---|---|---|---|---|---|---|---|
| Lindner et al. [8] | Germany | P | 2018 | Mixed | 2 | Restaging | No |
[68 Ga]Ga-FAPI-04 (80 nmol/GBq) |
| Kratochwil et al. [9] | Germany | R | 2019 | Mixed | 12 | Not clear | No |
[68 Ga]Ga-FAPI-04 (122–312 MBq) |
| Ballal et al. [11] | India | P | 2020 | Mixed | 19 | Staging and restaging | Yes |
[68 Ga]Ga- DOTA.SA.FAPi (mean: 174 MBq) |
| Chen et al. [10] | China | P | 2020 | Mixed | 4 | Staging and restaging | Yes |
[68 Ga]Ga-FAPI-04 (180–220 MBq) |
| Komek et al. [18] | Turkey | P | 2021 | Breast cancer | 20 | Staging and restaging | Yes |
[68 Ga]Ga-FAPI-04 (2 MBq/Kg) |
| Dendl et al. [12] | Germany | R | 2021 | Mixed | 14 | Not clear | Yes |
[68 Ga]Ga-FAPI-04 (52–325 MBq) |
| Elboga et al. [19] | Turkey | R | 2021 | Breast cancer | 48 | Staging and restaging | Yes |
[68 Ga]Ga-FAPI-04 (2 MBq/Kg) |
| Backhaus et al. [13] | Germany | R | 2022 | Mixed | 8 | Staging and Restaging | No |
[68 Ga]Ga-Onco-FAP (163.3 ± 50 MBq) |
| Backhaus et al. [16] | Germany | R | 2022 | Breast cancer | 19 | Staging and Restaging | No |
[68 Ga]Ga-FAPI-46 (149 ± 48 MBq) |
| Mona et al. [14] | US | P | 2022 | Mixed | 2 | Not clear | No |
[68 Ga]Ga-FAPI-46 (174–185 MBq) |
| Airo’ Farulla LS et al. [15] | Italy | R | 2022 | Mixed | 2 | Restaging | Yes |
[68 Ga]Ga-FAPI-04 (not available) |
| Eshet et al. [20] | Israel | P | 2022 | Breast cancer | 7 | Not clear | No |
[68 Ga]Ga-FAPI-04 (185 MBq) |
| Backhaus et al. [17] | Germany | R | 2022 | Breast cancer | 13 | Restaging (post-NAC) | No |
[68 Ga]Ga-FAPI-46 (99 ± 33 MBq) |
*Comparison [68 Ga]Ga-FAPI-46; NAC neoadjuvant chemotherapy; R = retrospective; P = prospective
Table 2.
CASP evaluation for 5 selected articles including breast cancer and FAPI PET
| Questions | [18] | [19] | [16] | [20] | [17] | ||
|---|---|---|---|---|---|---|---|
| Final agreement from 2 appraisers | |||||||
| 1 | Was there a clear question for the study to address? | Yes | Yes | Yes | Yes | Yes | |
| 2 | Was there a comparison with an appropriate reference standard? | No | No | Yes | No | Yes | |
| 3 | Did all patients get the diagnostic test and reference standard? | Can’t tell | Can’t tell | Yes | Can’t tell | Yes | |
| 4 | Could the results of the test have been influenced by the results of the reference standard? | Can’t tell | Can’t tell | No | Can’t tell | No | |
| 5 | Is the disease status of the tested population clearly described? | Yes | Yes | Yes | Yes | Yes | |
| 6 | Were the methods for performing the test described in sufficient detail? | Yes | Yes | Yes | Yes | Yes | |
| 7 | What are the results? | Sensitivity and specificity | Descriptive analysis | Descriptive analysis | Descriptive analysis | Descriptive analysis | |
| 8 | How sure are we about the results? Consequences and cost of alternatives performed? | Too preliminary | Too limited experience | Too limited experience | Too limited experience | Preliminary data | |
| 9 | Can the results be applied to your patients/the population of interest? | Yes | Yes | Yes | Yes | Yes | |
| 10 | Can the test be applied to your patient or population of interest? | Yes | Yes | Yes | Yes | Yes | |
| 11 | Were all outcomes important to the individual or population considered? | Yes | Yes | Yes | Yes | Yes | |
| 12 | What would be the impact of using this test on your patients/population? | The opportunity to have an alternative imaging | Interesting impact, further evaluations are need | Interesting impact, further evaluations are need | Interesting with an appropriate effect | Interesting impact, further evaluations are need | |
As illustrated in Table 1, different types of FAPI tracers have been tested across the studies, although in the those including only breast cancer, [68 Ga]Ga-FAPI-04 and [68 Ga]Ga-FAPI-46 were mostly used [16–20]. Indeed, Lindner et al. [6] found that diagnostic PET/CT scans performing after 10 min, 1 h and 3 h from the injection of [68 Ga]Ga-FAPI-04 in 2 patients with metastatic breast cancer, demonstrated a robust accumulation of tracer in the metastases, in contrast to the normal breast tissue where the tracer uptake was low. Moreover, due to its low retention, longer dwell times and no significant increase in background activity, [68 Ga]Ga-FAPI-04 is suitable also for the theragnostic purpose. In the study by Mona et al. [14], [68 Ga]Ga-FAPI-46 was tested also in 2 patients affected by breast cancer, showing a strong correlation with FAP expression in the cancer, thus rendering it a suitable radiopharmaceutical agent both for the diagnostic and therapeutic approach.
The biodistribution of FAPI in women can be affected by the hormonal status. The study from Dendl et al. [12] found that maximum standardized uptake value (SUVmax) was higher in premenopausal than post-menopausal patients in endometrium and breast tissue. Conversely, tracer uptake was similar between the two categories of patients in the ovaries. The uptake of FAPI in primary and metastatic breast cancer lesions has been reported in all the selected papers. In primary tumor, the SUVmax ranged between 2.6 and 17.0 [19]; similarly, high uptake values were reported in all metastatic sites (such as lymph nodes, lung, liver and bone). Interestingly, no difference in terms of FAPI uptake was reported based on the histopathological characteristics, such as immunohistochemistry and grading [12, 18, 19]. However, Elboga et al. [19] noticed that in HER2 patients, the FAPI uptake was higher as compared to the other luminal subtypes. Furthermore, a slightly higher mean uptake was observed in BRCA 1/2-positive patients than negative patients with regards to all lesions [12].
As illustrated in Table 1, 6 papers were focused on the comparison between 2-[18F]FDG and FAPI [10–12, 15, 18, 19]. Totally, data for 107 patients were now available. As emerged by the studies, FAPI demonstrated more lesions and yielded much higher tumor-to-background ratios than 2-[18F]FDG. As illustrated in Table 3, in all sites of disease, FAPI uptake was higher than 2-[18F]FDG also by a semiquantitative point of view, particularly when SUVmax was used. Furthermore, also the sensitivity for the breast cancer was higher in FAPI than 2-[18F]FDG being equal to 100% vs. 78.2%, respectively [18]; conversely, a slightly decrease in specificity was reported (95.8% vs. 100%, respectively for FAPI vs. 2-[18F]FDG; [18]). However, based on the study from Kömek et al. [18], FAPI PET was able to detect more lymph nodes, and sub centimetric lesions in hepatic tissue. Indeed, in their study, the authors reported that the median size of only FAPI positive lesions was 9 mm, therefore under the standard 10 mm. Interesting comparative data were reported by Elboga et al. [19], indeed the authors found that an early treatment response with 2-[18F]FDG PET/CT can display false-negative results, while FAPI can detect lesions even within the first month of post-chemotherapy period. Patients considered as responders or with a stable disease after chemotherapy at 2-[18F]FDG PET/CT, were later reclassified as progressive after FAPI PET imaging, thus approaching to a correct clinical management. The ability of FAPI PET to detect small lesions after chemotherapy was reported also by Backhaus et al. [17]. The authors found that FAPI PET/MR was able to classify responders vs. non-responders to neoadjuvant chemotherapy, otherwise incorrectly evaluated by MR alone. The same group confirmed that the combination of FAPI with PET/MR can significantly improve the detection of primary breast tumors and regional lymph node disease than MR alone.
Table 3.
Mean ± standard deviation or median (range) of the semiquantitative data from 2-[18F]FDG and FAPI in primary and in metastatic site of disease
| Authors, references | Primary | Lymph node | Distant | |||
|---|---|---|---|---|---|---|
| FAPI | 2-[18F]FDG | FAPI | 2-[18F]FDG | FAPI | 2-[18F]FDG | |
| Ballal et al. [11] |
6.5 ± 3.3* 4.9 ± 2.5** |
6.2 ± 1.6* 4.7 ± 2.3** |
– – |
– – |
– – |
– – |
| Komek et al. [18] | 17.4 (10.4–22.8)Ϯ | 5.8 (1.3–15.5)Ϯ | 16.7 (3.1–23.5)Ϯ | 5.1 (1–12)Ϯ |
9.2 (5.5–19)Ϯ (LiM) 6.1 (1.3–16.6)Ϯ (LM) 6 (3.7–15.1)Ϯ (BM) |
6.1 (3.5–14)Ϯ (LiM) 2.6 (1.2–10.1)Ϯ (LM) 4.4 (2.5–8.1)Ϯ (BM) |
| Elboga et al. [19] | 10 (2.6–17.00)Ϯ | 3.1 (1.1–13.2)Ϯ | 12.7 (4.1–36.6)Ϯ | 3.9 (1.7–20.6)Ϯ |
22.3 (20–23)Ϯ (LM) 17.9 (7.7–28)Ϯ LiM 14.9 (9.9–25)Ϯ BM |
7.1 (2.2–14)Ϯ (LM) 3.5 (3.1–3.8)Ϯ LiM 3.2 (1.4–5.2)Ϯ BM |
*SULpeak, **SULavg; ϮSUVmax; LM lung metastases; LiV liver metastases; BM bone metastases
Discussion
Until to date, 172 patients affected by breast cancer were studied with radiolabeled FAPI PET/CT. Therefore, limited data are available for drawing final comments, particularly in an oncological disease with a high incidence and prevalence in females. However, many concepts can be extrapolated from the 13 available papers that evaluated the role of different FAPI agents, also in comparison with 2-[18F]FDG. Indeed, currently the glucose-based agent, 2-[18F]FDG, is still considered the most common PET agent in breast cancer imaging, but it is linked by many clinical issues that are currently unsolved. First, 2-[18F]FDG uptake is low in some histopathological types, i.e., luminal A (positive estrogen receptor and a low proliferation index), lobular cancer, and in HER2-positive disease, thus significantly affected its diagnostic performance in these settings. Recently, [18F]Fluoroestradiol ([18F]FES) has been introduced in the clinical practice for overpassing some limitations of 2-[18F]FDG in patients with lobular cancer and luminal A subtypes, but its availability is still limited at few centers. Second, 2-[18F]FDG cannot be used to distinguish between malignant and benign disease because of a low target/background ratio in small lesions and to the partial volume effect [25, 26], although the current availability of dedicated breast scanners. Third, current International guidelines suggested to use [18F]FDG as on optional imaging in stage II or stage III breast cancer, when conventional imaging is negative or inconclusive. The limited employment of this imaging technique in the initial staging of disease, also in case of locally advanced breast cancer, is relative to its limited sensitivity and specificity, although the rate of metastases in this setting can arrive to 40% [27]. Fourth, 2-[18F]FDG is not indicated for the evaluation of response to therapy, either in adjuvant and in neoadjuvant setting, depending by its limited ability to identify small residual cancer tissue. Finally, there is not a specific radiopharmaceutical agent that can be used either in diagnostic or in therapeutic field for breast cancer. Therefore, on the basis of the abovementioned limitations, some additional agents for PET imaging, and not only, are strongly required.
The expression of FAP on activated fibroblasts in tumor stroma was quantified in 1990, when also a high correlation was reported in breast cancer [21]. The decision for the best peptides for breast cancer in a large amount of available FAPI agents depends on a lot of considerations: (1) the ability to detect the cancer lesions, (2) a high tumor-to-background ratio, (3) the capacity of distinguish between benign and malignant breast lesions and (4) the opportunity to use it either for diagnostic or therapeutic purpose. Based on this assumption, in the present systematic review, emerged that [68 Ga]Ga-FAPI-04 showed a high SUVs in many studies and when compared to the others, such as [68 Ga]Ga-DOTA-SA-FAPI [9]. However, a proof of principle work on [68 Ga]Ga-DOTA-SA-FAPI PET/CT-guided radioligand therapy with 177Lu-DOTA-SA-FAPI exists, thus opening the way for its theragnostic application in breast cancer [22].
Although 2-[18F]FDG PET/CT is commonly used in recurrence, as previously mentioned, it is not generally recommended in initial staging of breast cancer [20]. Only aggressive invasive ductal breast cancer, such as triple negative and grade III tumors showed a moderate-high 2-[18F]FDG uptake, while a controversial uptake has been reported in HER2-positive tumors [23, 24]. FAPI uptake is not correlated with histopathological, molecular feature and tumor grade, being equally increased in all types of breast cancer. This behavior can solve the limitation of 2-[18F]FDG, with a strong clinical importance, either in patients with lobular cancer or in those with HER2-positive, luminal A and luminal B disease. Currently, preliminary data by Eshet et al. [20] reported the ability of FAPI PET in detecting more lesions than CT in seven women with lobular cancer, in many distant organs, such as orbits, posterior mediastinum, internal mammary, retroperitoneum and pelvis. Due to the limited available data about the correlation among histology, molecular subtypes and FAPI uptake, prospective studies are mandatory. Although, the independence of FAPI uptake from the biological characteristics of breast cancer can be an advantage in terms of lesions’ detection, FAPI PET cannot predict the aggressiveness of the lesion and therefore it cannot be considered a prognostic parameter. Probably, the combination of FAPI and 2-[18F]FDG would be suggested to cover both the information (either diagnostic or prognostic) in breast cancer patients. In this way, no current data about the correlation between prognosis and FAPI are available.
Some studies have demonstrated that an early treatment response with 2-[18F]FDG PET/CT can display false-negative results [28, 29]. To monitor the response to therapy, mainly in neoadjuvant setting is an important clinical issue for 2 main reasons: (1) the opportunity to early test the chemosensitivity of the tumor and (2) to early change the therapeutic scheme in case of treatment failure. To date, MR is the imaging of choice for these endpoints, but some studies have demonstrated its limited sensitivity and specificity also when compared to 2-[18F]FDG PET/CT [30]. The study by Elboga et al. [19] tried to overpass this limitation, using FAPI. The opportunity to recognize the presence of residual active disease, within one month from the start of chemotherapy, can be useful for planning the therapeutic management. Therefore, FAPI-based PET can help in this way and further evaluation should be performed, either in neoadjuvant, adjuvant or metastatic setting.
The advantages from FAPI in comparison to 2-[18F]FDG are relative also to the radiation burden and some practical issues. First, based on Table 1, the amount of injected radiolabeled FAPI was about 200 MBq (5.4 mCi), therefore with an effective whole-body dose of 1.56 ± 0.26 mSv for a PET scan. When including a low-dose CT scan (3.7 mSv), the dose is approximately 5.3 mSv in total [31]. Conversely, in the current clinical setting, the radiation burden of FDG in oncological setting range between 5 and 15 mSv for a PET/CT scan [32]. Furthermore, the opportunity to use digital or whole-body PET scanner can further reduce the radiation exposure in this setting of disease, that often affects young women.
Second, FAPI uptake is independent from the fasting and resting time; moreover the images can be obtained soon after 10 min from the tracer’s injection, because of its fast clearance and lower-off target accumulation. However, also some disadvantages have been reported from the use of FAPI in breast cancer as compared to 2-[18F]FDG. The first one is the influence on FAPI uptake due to the changes in hormone status, thus altering the interpretation of the images in premenopausal women [3, 12]. The differences in pre- and post-menopausal patients are relative to a major uptake in the health breast tissue that can influence the tumor-to-background ratio, but larger studies in these two categories of patients are required for better understanding the hormonal effects of the FAPI uptake. The second disadvantage of FAPI is the detection of more false-positive findings than 2-[18F]FDG due to various fibrotic processes, such as myelofibrosis, granulomatous disease, liver cirrhosis [19] and inflammatory processes (i.e., tuberculosis, [33]). These false-positive findings can reduce the specificity and the positive predictive value of FAPI PET, mainly soon after surgery, radiation therapy and during follow-up.
As largely known, MRI is better than CT in the evaluation of primary breast cancer, due to its high contrast resolution and also for the ability to differentiate between malignant and benign lesions in contrast-enhanced sequences. However, FAPI can be complementary to MRI, overpassing its limited specificity. Indeed, in the study by Backhaus et al. [16], no FAPI uptake was reported in patients with papilloma, ductal carcinoma in situ and in BIRADS-2 lesions. In another study by the same group [17], FAPI PET/MR was able to correctly identify all patients with a complete pathological response after neoadjuvant chemotherapy, otherwise missed by MRI alone. Although these encouraging preliminary results, additional data are needed, also considering the limited number of hybrid PET/MR scanner worldwide. Alternatively, combined PET and MR images would be considering in the future studies for improving the differential diagnosis in indeterminate breast lesions.
The present review form the literature has some limitations. First, the limited number of included papers. Second, several types of FAPI tracers were reported, but currently no specific indications on how can be better use in each disease has been extensively demonstrated. Third, the diagnostic performance of FAPI has not been reported in all papers, but only in the study by Kömek et al. [18]. Finally, some papers [13, 16, 17] were made by the same group of researchers, thus with a potential overlap of the patient population.
Conclusions
In conclusion, preliminary experiences with FAPI PET in breast cancer are interesting for a lot of reasons: (1) the ability to detect more lesions than 2-[18F]FDG, (2) the independence of FAPI uptake from the molecular and histopathological features and (3) the opportunity to detect small lesions after chemotherapy, thus guiding to a further appropriate therapy. Future studies are warranted, in large population, to confirm these assumptions.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. None.
Data availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lacey JV, Devesa SS, Brinton LA. Recent trends in breast cancer incidence and mortality. Environ Mol Mutagen. 2002;39:82–88. doi: 10.1002/em.10062. [DOI] [PubMed] [Google Scholar]
- 2.Siveke JT. Fibroblast-activating protein: targeting the roots of the tumor microenvironment. J Nucl Med. 2018;59:1412–1414. doi: 10.2967/jnumed.118.214361. [DOI] [PubMed] [Google Scholar]
- 3.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Prot Clin Appl. 2014;8:454–463. doi: 10.1002/prca.201300095. [DOI] [PubMed] [Google Scholar]
- 4.Jokar N, Velikyan I, Ahmadzadehfar H, Rekabpour SJ, Jafari E, Ting HH, et al. Theranostic approach in breast cancer: a treasured tailor for future oncology. Clin Nucl Med. 2021;46:e410–e420. doi: 10.1097/RLU.0000000000003678. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan D, Berg WA. Dedicated breast gamma camera imaging and breast PET: current status and future directions. PET Clin. 2018;13:363–381. doi: 10.1016/j.cpet.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evangelista L, Cervino AR, Michieletto S, Saibene T, Ghiotto C, Guarneri V, et al. Diagnostic and prognostic impact of fluo-rine-18-fluorodeoxyglucose PET/CT in preoperative and postoperative setting of breast cancer patients. Nucl Med Commun. 2017;38:537–545. doi: 10.1097/MNM.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista L, Baretta Z, Vinante L, Bezzon E, De Carolis V, Cervino AR, et al. Comparison of 18F-FDG positron emission tomography/computed tomography and computed tomography in patients with already-treated breast cancer: diagnostic and prognostic implications. Q J Nucl Med Mol Imaging. 2012;56:375–384. [PubMed] [Google Scholar]
- 8.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–1422. doi: 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 9.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–1832. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 11.Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur J Nucl Med Mol Imaging. 2021;48:1915–1931. doi: 10.1007/s00259-020-05132-y. [DOI] [PubMed] [Google Scholar]
- 12.Dendl K, Koerber SA, Finck R, Mokoala KMG, Staudinger F, Schillings L, et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur J Nucl Med Mol Imaging. 2021;48:4089–4100. doi: 10.1007/s00259-021-05378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backhaus P, Gierse F, Burg MC, Büther F, Asmus I, Dorten P, et al. Translational imaging of the fibroblast activation protein (FAP) using the new ligand [68Ga]Ga-OncoFAP-DOTAGA. Eur J Nucl Med Mol Imaging. 2022;49:1822–1832. doi: 10.1007/s00259-021-05653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mona CE, Benz MR, Hikmat F, Grogan TR, Lueckerath K, Razmaria A, et al. Correlation of 68Ga-FAPi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: interim analysis of a prospective translational exploratory study. J Nucl Med. 2022;63:1021–1026. doi: 10.2967/jnumed.121.262426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Airò Farulla LS, Demirci E, Castellucci P, Alan-Selçuk N, Fortunati E, Gilardi L, et al. Radiolabeled FAP inhibitors as new pantumoral radiopharmaceuticals for PET imaging: a pictorial essay. Clin Transl Imaging. 2023;11:95–106. doi: 10.1007/s40336-022-00506-8. [DOI] [Google Scholar]
- 16.Backhaus P, Burg MC, Roll W, Büther F, Breyholz H-J, Weigel S, et al. Simultaneous FAPI PET/MRI targeting the fibro-blast-activation protein for breast cancer. Radiology. 2022;302:39–47. doi: 10.1148/radiol.2021204677. [DOI] [PubMed] [Google Scholar]
- 17.Backhaus P, Burg MC, Asmus I, Pixberg M, Büther F, Breyholz H-J, et al. Initial results of FAPI-PET/MRI to assess response to neoadjuvant chemotherapy in breast cancer. J Nucl Med. 2023;64:717–723. doi: 10.2967/jnumed.122.264871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kömek H, Can C, Güzel Y, Oruç Z, Gündoğan C, Yildirim ÖA, et al. 68Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: a comparative pilot study with the 18F-FDG PET/CT. Ann Nucl Med. 2021;35:744–752. doi: 10.1007/s12149-021-01616-5. [DOI] [PubMed] [Google Scholar]
- 19.Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Uzun E, et al. Superiority of 68Ga-FAPI PET/CT scan in detecting additional lesions compared to 18FDG PET/CT scan in breast cancer. Ann Nucl Med. 2021;35:1321–1331. doi: 10.1007/s12149-021-01672-x. [DOI] [PubMed] [Google Scholar]
- 20.Eshet Y, Tau N, Apter S, Nissan N, Levanon K, Bernstein-Molho R, et al. The role of 68Ga-FAPI PET/CT in detection of meta-static lobular breast cancer. Clin Nucl Med. 2023 doi: 10.1097/RLU.0000000000004540. [DOI] [PubMed] [Google Scholar]
- 21.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballal S, Yadav MP, Kramer V, Moon ES, Roesch F, Tripathi M, et al. A theranostic approach of [68Ga]Ga-DOTA SA FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021;48:942–944. doi: 10.1007/s00259-020-04990-w. [DOI] [PubMed] [Google Scholar]
- 23.Groheux D, Hindie E. Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging. 2021;9:221–231. doi: 10.1007/s40336-021-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarikaya I. Breast cancer and PET imaging. Nucl Med Rev Cent East Eur. 2021;24:16–26. doi: 10.5603/NMR.2021.0004. [DOI] [PubMed] [Google Scholar]
- 25.Spadafora M, Pace L, Evangelista L, Mansi L, Del Prete F, Saladini G, et al. Risk-related 18F-FDG PET/CT and new diagnostic strategies in patients with solitary pulmonary nodule: the ITALIAN multicenter trial. Eur J Nucl Med Mol Imaging. 2018;45:1908–1914. doi: 10.1007/s00259-018-4043-y. [DOI] [PubMed] [Google Scholar]
- 26.Redondo-Cerezo E, Martínez-Cara JG, Jiménez-Rosales R, Valverde-López F, Caballero-Mateos A, Jérvez-Puente P, et al. Endoscopic ultrasound in gastric cancer staging before and after neoadjuvant chemotherapy. a comparison with PET-CT in a clinical series. United Eur Gastroenterol J. 2017;5:641–647. doi: 10.1177/2050640616684697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan ME, Houssami N. Evaluation of the evidence on staging imaging for detection of asymptomatic distant metastases in newly diagnosed breast cancer. Breast. 2012;21(112–23):28. doi: 10.1016/j.breast.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of 18F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol. 2017;27:4786–4796. doi: 10.1007/s00330-017-4831-y. [DOI] [PubMed] [Google Scholar]
- 29.Keam B, Im SA, Koh Y, Han SW, Oh DY, Cho N, et al. Early metabolic response using FDG PET/CT and molecular phenotypes of breast cancer treated with neoadjuvant chemotherapy. BMC Cancer. 2011;11:452. doi: 10.1186/1471-2407-11-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Yao L, Jin P, Hu L, Li X, Guo T, Yang K. MRI and PET/CT for evaluation of the pathological response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast. 2018;40:106–115. doi: 10.1016/j.breast.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2020;61:1171–1177. doi: 10.2967/jnumed.119.236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sah BR, Ghafoor S, Burger IA, Ter Voert EEGW, Sekine T, Delso G, et al. Feasibility of 18F-FDG dose reductions in breast cancer PET/MRI. J Nucl Med. 2018;59:1817–1822. doi: 10.2967/jnumed.118.209007. [DOI] [PubMed] [Google Scholar]
- 33.Gu B, Luo Z, He X, Wang J, Song S. 68Ga-FAPI and 18F-FDG PET/CT Images in a patient with extrapulmonary tuberculosis mimicking malignant tumor. Clin Nucl Med. 2020;45:865–867. doi: 10.1097/RLU.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.