Abstract
Background
Randomized trials have demonstrated that anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) can be safe and efficacious treatments for patients with ALK-positive advanced non-small-cell lung cancer (aNSCLC). However, their safety, tolerability, effectiveness, and patterns of use in real-world patients remain understudied.
Objective
We sought to assess the overall treatment pattern characteristics, safety, and effectiveness outcomes of real-world patients with ALK-positive aNSCLC receiving ALK TKIs.
Patients and Methods
This retrospective cohort study using electronic health record data included adult patients with ALK-positive aNSCLC receiving ALK TKIs between January 2012 and November 2021 at a large tertiary medical center, University of California, San Francisco (UCSF), with alectinib or crizotinib as the initial ALK TKI therapy. Our primary endpoints included the incidence of treatment changes (treatment dose adjustments, interruptions, and discontinuations) during the initial ALK TKI treatment, the count and type of subsequent treatments, rates of serious adverse events (sAEs), and major adverse events (mAEs) leading to any ALK TKI treatment changes. Secondary endpoints included the hazard ratios (HRs) for median mAE-free survival (mAEFS), real-world progression-free survival (rwPFS), and overall survival (OS) when comparing alectinib with crizotinib.
Results
The cohort consisted of 117 adult patients (70 alectinib and 47 crizotinib) with ALK-positive aNSCLC, with 24.8%, 17.9%, and 6.0% experiencing treatment dose adjustments, interruptions, and discontinuation, respectively. Of the 73 patients whose ALK TKI treatments were discontinued, 68 received subsequent treatments including newer generations of ALK TKIs, immune checkpoint inhibitors, and chemotherapies. The most common mAEs were rash (9.9%) and bradycardia (7.0%) for alectinib and liver toxicity (19.1%) for crizotinib. The most common sAEs were pericardial effusion (5.6%) and pleural effusion (5.6%) for alectinib and pulmonary embolism (6.4%) for crizotinib. Patients receiving alectinib versus crizotinib as their first ALK TKI treatment experienced significantly prolonged median rwPFS (29.3 versus 10.4 months) with an HR of 0.38 (95% CI 0.21–0.67), while prolonged median mAEFS (not reached versus 91.3 months) and OS (54.1 versus 45.8 months) were observed in patients receiving alectinib versus crizotinib but did not reach statistical significance. Yet, it is worth noting that there was a high degree of cross-over post-progression, which could significantly confound the overall survival measures.
Conclusions
We found that ALK TKIs were highly tolerable, and alectinib was associated with favorable survival outcomes with longer time to adverse events (AE) requiring medical interventions, disease progression, and death, in the context of real-world use. Proactive monitoring for adverse events such as rash, bradycardia, and hepatotoxicity may help further promote the safe and optimal use of ALK TKIs in the treatment of patients with aNSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-00973-7.
Key Points
| ALK TKIs in real-world patients were found highly tolerable with only 24.8%, 17.9%, and 6.0% of patients experiencing an AE requiring treatment dose adjustment, interruption, or discontinuation, respectively. |
| Our findings of longer survival outcomes of mAEFS, rwPFS, and OS highlighted the higher tolerability and effectiveness of alectinib compared with crizotinib. |
| Proactive monitoring for adverse events such as rash, bradycardia, and hepatotoxicity may help further promote the safe and optimal use of ALK TKIs in the treatment of patients with aNSCLC. |
Introduction
Lung cancer is the most prevalent cancer and the leading cause of cancer-related death worldwide with more than 2.20 million new cases and 1.79 million deaths per year [1]. Roughly 85% of all lung cancer can be classified as non-small-cell lung cancer (NSCLC), of which 4–6% harbor a rearrangement involving the anaplastic lymphoma kinase (ALK) gene [2–6]. ALK rearrangements are frequently detected by fluorescence in situ hybridization (FISH), next generation sequencing (NGS), or immunohistochemistry in real-world settings [7]. The most common type of fusion partner reported with ALK rearrangements is echinoderm microtubule-associated protein-like 4 (EML4) [8, 9]. While over 30 types of ALK variants have been identified to date, all are thought to remain sensitive to ALK tyrosine kinase inhibitors (TKIs) [10, 11]. ALK-rearranged (positive) NSCLC is often distinctively identified in light or never smokers and younger adults [8].
Randomized controlled trials have demonstrated that ALK TKIs are efficacious and safe [12–16]. Currently, there are five ALK inhibitors available for use—crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib—spanning three generations of ALK TKIs. Real-world studies focusing on ALK TKI use in the context of routine clinical care have thus far focused on effectiveness and identifying optimal treatment sequences of ALK TKIs or subsequent therapies, as patients eventually develop resistance [17–23]. Yet, detailed assessments and characterization of the potentially diverse treatment patterns surrounding the use of ALK TKIs have not been well-studied. In addition, studies investigating the reasons leading to diverse treatment patterns have been lacking. Understanding ALK TKI treatment patterns and the reasons underlying the changes in treatment patterns is important, as they can provide key insights into improving the use of ALK TKIs.
In this study, we sought to assess the real-world use and performance of ALK TKIs, including treatment patterns, safety, and effectiveness. Specifically, our primary outcomes focused on the characterization of the real-world ALK TKIs treatment patterns, including treatment changes (dose adjustments, interruptions, and discontinuation), subsequent treatments, and adverse events of the pooled population. Our secondary outcomes focused on assessing differences in median major-adverse-event-free survival (mAEFS), real-world progression-free survival (rwPFS), and overall survival (OS) outcomes in patients with ALK-positive advanced NSCLC (aNSCLC) receiving alectinib versus crizotinib.
Methods
Ethics
This retrospective study was approved by the University of California, San Francisco (UCSF) Institutional Review Board (UCSF IRB 21-3390).
Data Source and Study Population
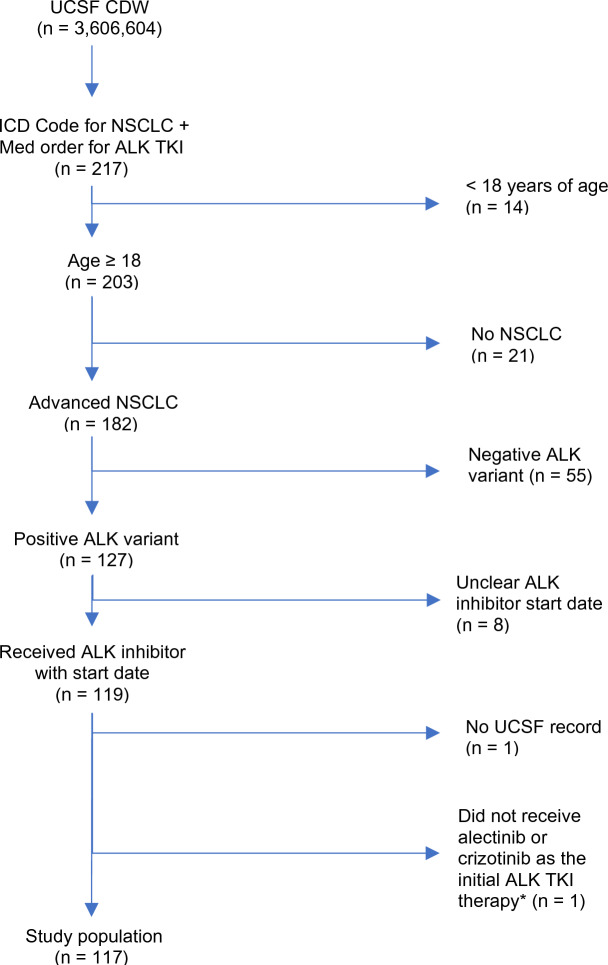
All patients included in the study and the associated patient-level data were identified and extracted by both queries of the de-identified clinical data warehouse and chart abstractions of electronic health records (EHR) at UCSF. Specifically, the inclusion criteria of this retrospective study were (1) being an adult patient with age > 18 years, (2) having documented ALK-positive aNSCLC status, (3) having received ALK TKI treatment and having been seen at UCSF between when EHR at UCSF became available, January 2012, and when the study was initiated, November 2021 (Fig. 1), and (4) having received alectinib or crizotinib as the initial ALK TKI therapy. Patients with cancer stage 3B or 4 were considered to have aNSCLC status. Through chart review, any adult patients without documented ALK-positive and aNSCLC status were excluded from this study. The receipt of ALK TKIs were initially identified by querying the ALK TKI prescription records in the UCSF clinical data warehouse, which were subsequently confirmed by chart review. Patients with no record of the start date (month and year) for their initial ALK TKI therapy or lack of any subsequent records in the UCSF EHR after the first ALK TKI exposure were excluded.
Fig. 1.
Patient selection. A total of 217 real-world patients were initially identified using electronic health records data available in UCSF’s de-identified clinical data warehouse. Following chart review, a total of 117 adult patients receiving ALK TKIs were confirmed as having ALK-positive advanced NSCLC. *One patient received ceritinib as the initial ALK TKI therapy
Study Variables and Endpoints
After identifying the study cohort, patient-level variables were extracted from UCSF’s EHR using both computational and manual chart review approaches. Patients’ baseline disease characteristics, ALK TKI treatment pattern characteristics, adverse events, and outcomes, including real-world disease progression, were manually abstracted by chart review. Baseline disease characteristics included the Eastern Cooperative Oncology Group (ECOG) performance status, insurance, ALK diagnosis test and sample types, ALK fusion partner, histology type, and presence and location of metastases. ALK TKI treatment pattern characteristics included variables such as initial start date and end date for the first ALK TKI treatment, any treatment changes, and the subsequent treatment after discontinuation. ALK TKI treatment changes included any treatment dose adjustments, interruptions, and discontinuation after the initiation of the first ALK TKI. Treatment dose adjustments were defined as any modification to frequency or dosage during the first ALK TKI therapy, whereas treatment interruption was defined as a pause in the first ALK TKI regimen with a subsequent restart of therapy. Treatment discontinuation was defined as the first discontinuation of an ALK TKI. The discontinuation could be due to several reasons summarized in this study including adverse events, disease progression, and death. Individual patients could contribute to multiple dose adjustments and/or treatment interruptions. Subsequent therapy was defined as any therapy immediately following the discontinuation of the first ALK TKI therapy. Adverse events (AEs) included both serious and major adverse events. Serious adverse events (sAEs) were defined as hospitalization-associated AEs, whereas major adverse events (mAEs) referred to AEs leading to treatment changes while receiving the first ALK TKI. The direct causal relations between mAEs and the resulting treatment changes were determined directly based on provider documentation. All AEs captured were standardized to the medical dictionary for regulatory activities (MedDRA) preferred term [24]. Other variables such as gender, race, Charlson Comorbidity Index scores, [25] areas of deprivation index (ADI), [26], and death dates were extracted and calculated computationally. Patient’s status of deceased or alive and the date of death were derived from both chart abstraction as well as queries of the EHR and clinical data warehouse at UCSF, which sources the death data from the California Electronic Death Registry System [27]. The last date of chart abstraction was 1 July 2022. Detailed definitions, sources, and extraction methods for all patient-level data included in this study have been summarized in eTable 1 [28].
The primary endpoints included incidence rates of sAEs, mAEs, and treatment changes during the initial ALK TKI and types of subsequent therapy. The secondary endpoints included the median survivals, major-adverse-event-free survival(mAEFS), real-world progression-free survival (rwPFS), [29, 30] and overall survival (OS) comparing alectinib with crizotinib. mAEFS was defined as the time from the start of first ALK TKI therapy to the first mAE. rwPFS was defined as the time between the initiation of the first ALK TKI to the first documentation of disease progression or death due to any cause, whereas OS was defined as the time between the initiation of the first ALK TKI to death due to any cause. If the event for mAEFS or rwPFS was not observed, the earliest date between the date of next treatment initiation, last date of clinical encounter, or last date of chart abstraction was the censoring date. For OS, the earliest of either the last date of clinical encounter or the last date of chart abstraction was the censoring date if patient was not deceased. Patients without any observable encounter with healthcare providers specializing in thoracic oncology for more than 6 months were deemed lost to follow-up, with the last observable encounter date with a thoracic oncology specialist as the censoring date.
Statistical Analyses
Rates of treatment changes, types of subsequent treatments, and rates of AEs (sAE and mAEs) were calculated to characterize real-world ALK TKI treatment patterns and AEs. Causal directed acyclic graphs (DAGs) [31] were constructed to develop the multivariate Cox regression models to estimate the median mAEFS, rwPFS, and OS and hazard ratios (HR) with 95% CI as the treatment effect of alectinib versus crizotinib on survival outcomes (Fig. 3). Key confounding variables that could affect ALK TKIs and/or outcomes were incorporated in the multivariate Cox proportional hazard models. The DAGs were constructed according to domain-specific knowledge. Specifically, the variables included were patients’ comorbidities, ECOG performance status, number of brain metastases, ADI scores, patients who were seen as a second opinion versus those who were not, gender, age, insurance (government versus private), and patients who received treatments prior to the first ALK TKI versus those who did not. Unmeasured variables such as health literacy and socioeconomic health status were controlled for by ADI as a proxy measure in these regression models. Missing ECOG scores were treated as a separate category (ECOG = 5). Patients receiving care at UCSF for secondary opinion, and ADI, were included to account for differing likelihood of detecting outcomes due to potential differences in adherence to treatments and follow-up visits, and health disparities.
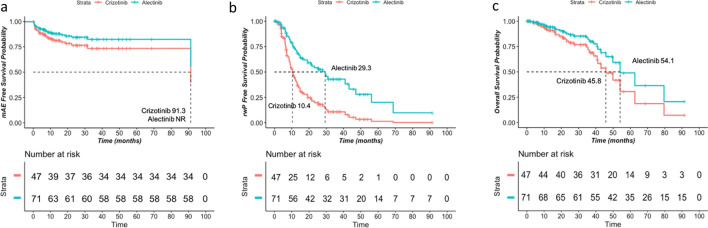
Fig. 3.
Adjusted survival probability estimates of mAEFS, rwPFS, and OS for alectinib and crizotinib using the Cox proportional hazard models. The adjusted survival probability estimates of a major-adverse-event-free survival, b real-world progression-free survival, and c overall survival for 117 assessable patients are shown. The adjusted survival probabilities shown were estimates of a typical patient with covariates corresponding to the most common category for categorical features and the mean for continuous features. The x-axis shows months since the initiation of the first ALK TKI treatment. Abbreviations: mAEFS: major-adverse-event-free survival, rwPFS: real-world progression-free survival, OS: overall survival
All analyses and data visualizations were conducted using Python 3.7.10 and R 3.6.1 with packages including survival and survminer [32, 33]. Simplified code and sample data for our computational pipeline is available at GitHub [34].
Results
Patient Baseline Characteristics
A total of 217 patients receiving ALK TKIs between January 2012 and November 2021 were identified by queries of the UCSF clinical data warehouse. Subsequently, 117 of these patients were confirmed to meet the criteria of ALK-positive aNSCLC and were treated with an ALK TKI through chart review (Fig. 1). Of the 99 patients who did not meet the inclusion criteria and were excluded from this analysis, 55 had no known, or did not have an, ALK mutation discernible from the medical records; 21 did not have NSCLC; and 14 were not older than 18 years. Of the 117 patients included in this study, 70 and 47 patients received alectinib and crizotinib, respectively, as the first ALK TKI treatment. Most patients were never smokers (7%), and the mean age was 55 years old. Of the patients, 65% had ECOG performance status scores of 0 or 1, and more than half (57.3%) of the patients received systemic therapies prior to initiation of ALK TKI. Additionally, nearly half (46.1%) of patients had brain metastases at baseline when beginning the first ALK TKI treatment.
ALK TKI Treatment Patterns and Adverse Events
Of the 117 patients, 70 (59.8%) and 47 (40.2%) received alectinib and crizotinib as their first ALK TKI, respectively (Table 1). The cohort experienced a wide range of disease trajectories while receiving ALK TKIs (Fig. 2A). Nineteen of 70 (27.1%) and 10 of 47 (21.3%) patients receiving alectinib and crizotinib required 19 and 10 dose adjustments, respectively (Table 2). The most common mAE leading to dose adjustments were bradycardia (20.1%) in patients receiving alectinib and liver toxicity (20.0%), bradycardia (20.0%), fatigue (20.0%), and diarrhea (20.0%) in patients receiving crizotinib (Table 3). ALK TKI therapy interruption duration ranged between 13 and 17 days in 16 (22.9%) and 5 (10.6%,) of patients receiving alectinib or crizotinib, respectively. The most common mAEs leading to treatment interruption were rash (25.0%) or liver toxicity (60.0%) in patients receiving alectinib or crizotinib, respectively (Table 3). Furthermore, financial toxicity contributed to two treatment dose adjustments and interruptions.
Table 1.
Patient baseline characteristics
| Overall | |
|---|---|
| Study cohort—N | 117 |
| ALK inhibitor—count (%) | |
| Alectinib (2nd generation) | 70 (59.8) |
| Crizotinib (1st generation) | 47 (40.2) |
| Gender—count (%) | |
| Female | 65 (55.6) |
| Male | 52 (44.4) |
| Smoking status—count (%) | |
| Former | 33 (28.2) |
| Never | 84 (71.8) |
| Insurance type—count (%) | |
| Government | 50 (42.7) |
| Private | 63 (53.8) |
| Unknown or none | 4 (3.4) |
| Age (in years)—mean (std) | 55.6 (12.9) |
| Race—count (%) | |
| Asian | 36 (30.8) |
| Black or African American | 3 (2.6) |
| Declined/other/unknown | 21 (17.9) |
| Native Hawaiian or Other Pacific Islander | 4 (3.4) |
| White or Caucasian | 53 (45.3) |
| Comorbidity Index score—mean (std) | 6.5 (5.5) |
| Areas of Deprivation Index (ADI)—count (%) | |
| 1–3 | 78 (66.7) |
| 4–6 | 20 (17.1) |
| 7–10 | 19 (16.2) |
| Performance status (ECOG)—count (%) | |
| 0 | 18 (15.4) |
| 1 | 59 (50.4) |
| 2 | 7 (6.0) |
| 3 | 8 (6.8) |
| 4 | 1 (0.9) |
| Unknown | 24 (20.5) |
| ALK diagnostic test—count (%) | |
| FISH | 49 (41.9) |
| NGS | 47 (40.2) |
| FISH and NGS | 15 (12.8) |
| ALK test sample—count (%) | |
| Biopsy | 93 (79.5) |
| Blood | 10 (8.5) |
| Fluid | 3 (2.6) |
| Blood and biopsy | 6 (5.1) |
| ALK fusion partner—count (%) | |
| EML4 | 57 (96.6) |
| Intron 19 | 2 (3.4) |
| Histology type—count (%) | |
| Adenocarcinoma | 111 (94.9) |
| Squamous cell carcinoma | 2 (1.7) |
| Other | 4 (3.4) |
| De novo status—count (%) | 101 (86.3) |
| NSCLC stage—count (%) | |
| III | 11 (9.4) |
| IV | 106 (90.6) |
| Metastasis—count (%) | |
| Brain | 54 (46.1) |
| Not brain | 58 (49.6) |
| No metastasis | 5 (4.3) |
| Prior therapies | |
| Yes | 67 (57.3) |
| No | 50 (42.7) |
std standard deviation
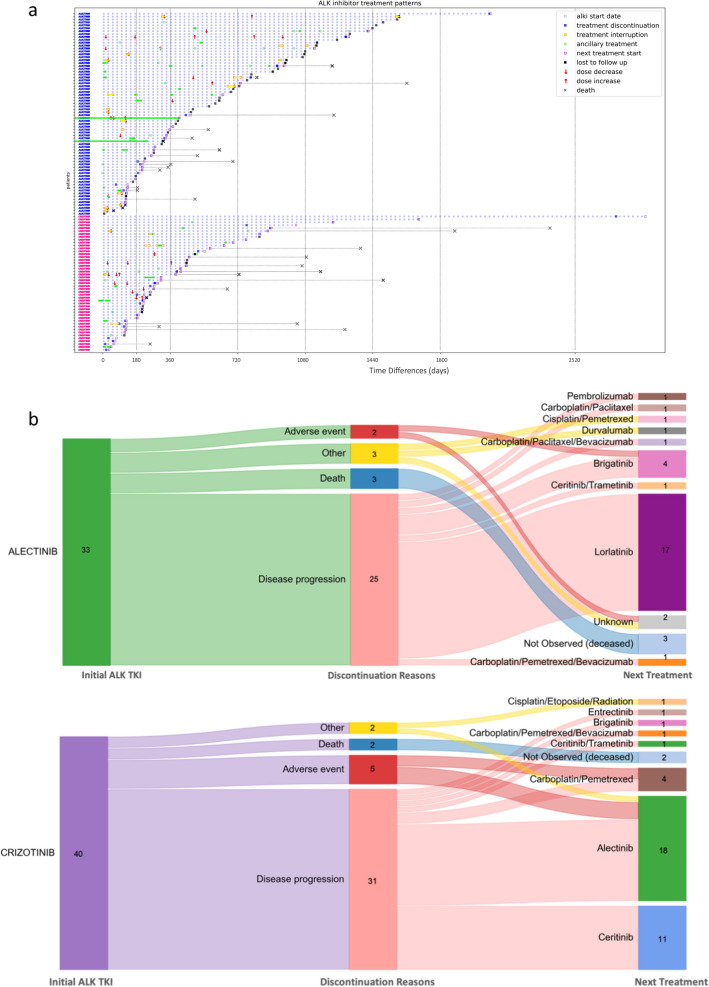
Fig. 2.
ALK TKI treatment trajectories. a ALK TKI treatment patterns in 117 real-world patients. Treatment pattern is characterized with the first ALK TKI, time between initiation of the first ALK TKI and dose increase or decrease, therapy interruptions, and discontinuation, death, or lost to follow-up. Next treatment initiation as well as ancillary treatments were also annotated. The y-axis was labeled with the first ALK TKI treatment each individual patient received, with blue indicating alectinib and pink indicating crizotinib. b A Sankey plot to present quantitatively the sequential drug choices and rationales for discontinuing the first ALK TKI
Table 2.
Characterizations of dose adjustment, regimen interruption, and treatment discontinuations
| Alectinib | Crizotinib | |
|---|---|---|
| Patient (count—n) | 70 | 47 |
| Dose adjustment—unique patient count (%) | 19 (27.1) | 10 (21.3) |
| Time to first dose adjustment—mean (std) | 206.6 (230.4) | 108.8 (89.6) |
| Number of dose adjustment incidences—count | 30 | 17 |
| Adverse events—count (%) | 23 (76.7) | 13 (76.5) |
| Financial toxicity—count (%) | 1 (4.3) | |
| Disease progression—count (%) | 2 (8.7) | 1 (5.9) |
| Death—count (%) | ||
| Other—count (%) | ||
| Resume to prior doses—count (%) | 4 (17.4) | 3 (17.6) |
| Regimen interruption—unique patient count (%) | 16 (22.9) | 5 (10.6) |
| Time to first regiment interruption—mean (std) | 187.8 (335.3) | 113.6 (115.5) |
| First regimen interruption duration—mean (std) | 13.1 (11.2) | 17.4 (13.4) |
| Number of regimen interruption incidences | 21 | 6 |
| Adverse events—count (%) | 18 (85.7) | 5 (83.3) |
| Financial toxicity—count (%) | 1 (4.8) | |
| Disease progression—count (%) | ||
| Death—count (%) | ||
| Other—count (%) | 2 (9.5) | 1 (16.7) |
| Treatment discontinuation—unique patient count (%) | 33 (47.1) | 40 (85.1) |
| Time to discontinuation—mean (std) | 439.5 (460.3) | 453.0 (513.7) |
| Number of treatment discontinuation incidences | 33 | 40 |
| Adverse events—count (%) | 2 (6.1) | 5 (12.5) |
| Financial toxicity—count (%) | ||
| Disease progression—count (%) | 25 (75.8) | 31 (77.5) |
| Death—count (%) | 3 (9.1) | 2 (5.0) |
| Other—count (%) | 3 (9.1) | 2 (5.0) |
Table 3.
Adverse events contributing to regimen adjustment, interruption, or discontinuation
| Alectinib | Crizotinib | |||||||
|---|---|---|---|---|---|---|---|---|
| sAE | Dose adjustment | Regimen interruption | Treatment discontinuation | sAE | Dose adjustment | Regimen interruption | Treatment discontinuation | |
| N | 23 | 19 | 16 | 2 | 9 | 10 | 5 | 5 |
| Rash | 1 (4.3) | 3 (15.8) | 4 (25.0) | 1 (11.1) | 1 (10.0) | 1 (20.0) | ||
| Liver toxicity | 2 (8.7) | 2 (10.5) | 1 (6.2) | 1 (50.0) | 1 (11.1) | 2 (20.0) | 3 (60.0) | 4 (80.0) |
| Bradycardia | 4 (20.1) | 1 (6.2) | 2 (20.0) | 1 (20.0) | ||||
| Pneumonia | 2 (8.7) | 1 (5.3) | 2 (12.5) | 2 (22.2) | 1 (20.0) | |||
| Fatigue | 3 (15.8) | 1 (6.2) | 2 (20.0) | 1 (20.0) | ||||
| Pulmonary embolism | 2 (8.7) | 3 (33.3) | 1 (10.0) | |||||
| Nausea | 1 (4.3) | 1 (50.0) | 1 (11.1) | 1 (10.0) | 1 (20.0) | |||
| Blood bilirubin increased | 1 (4.3) | 2 (10.5) | 2 (12.5) | |||||
| Fever | 1 (5.3) | 2 (12.5) | 1 (11.1) | 1 (20.0) | ||||
| Pericardial effusion | 4 (17.4) | 1 (11.1) | ||||||
| Pleural effusion | 4 (17.4) | 1 (20.0) | ||||||
| Diarrhea | 1 (4.3) | 1 (6.2) | 2 (20.0) | |||||
| Constipation | 1 (4.3) | 2 (10.5) | 2 (12.5) | |||||
| Vomiting | 1 (4.3) | 1 (50.0) | 1 (11.1) | 2 (20.0) | ||||
| Pyelonephritis | 2 (8.7) | 1 (5.3) | 1 (6.2) | |||||
| Hematoma NOS | 2 (8.7) | 1 (6.2) | 1 (11.1) | |||||
| Sepsis | 2 (8.7) | 1 (6.2) | ||||||
| Diabetic ketoacidosis | 1 (4.3) | 1 (5.3) | 1 (6.2) | |||||
| Edema peripheral | 1 (5.3) | 1 (10.0) | 1 (20.0) | |||||
| Hyponatremia | 2 (8.7) | 1 (5.3) | ||||||
| Swelling face | 1 (5.3) | 1 (6.2) | 1 (20.0) | |||||
| Muscle weakness NOS | 1 (5.3) | 1 (6.2) | ||||||
| Deep vein thrombosis | 1 (4.3) | 1 (11.1) | ||||||
| Face edema | 1 (5.3) | 1 (6.2) | ||||||
| Anemia | 2 (10.5) | |||||||
| Mouth ulceration | 1 (5.3) | 1 (6.2) | ||||||
| Dysphagia | 1 (11.1) | 1 (10.0) | ||||||
| Ileus paralytic | 1 (4.3) | 1 (6.2) | ||||||
| Renal abscess NOS | 1 (5.3) | 1 (6.2) | ||||||
| Pain | 1 (4.3) | 1 (11.1) | ||||||
| Shingles | 1 (4.3) | 1 (6.2) | ||||||
| Cardiac failure | 1 (4.3) | 1 (11.1) | ||||||
| Gastrointestinal toxicity | 1 (4.3) | 1 (10.0) | ||||||
| Pancytopenia | 1 (11.1) | 1 (20.0) | ||||||
| Urinary tract infection | 1 (4.3) | 1 (5.3) | ||||||
| CK increased | 1 (5.3) | 1 (6.2) | ||||||
| Drug toxicity | 1 (5.3) | |||||||
| Neutrophil count decreased | 1 (20.0) | |||||||
| Serum ferritin increased | 1 (20.0) | |||||||
| Drug intolerance | 1 (5.3) | |||||||
| Acute generalized exanthematous pustulosis | 1 (6.2) | |||||||
| Chills | 1 (6.2) | |||||||
| Dizziness | 1 (5.3) | |||||||
| Muscle spasms | 1 (6.2) | |||||||
| Pulmonary edema | 1 (20.0) | |||||||
| Chylous ascites | 1 (10.0) | |||||||
| Q-T interval prolonged | 1 (20.0) | |||||||
| Alopecia | 1 (11.1) | |||||||
| Leukopenia | 1 (5.3) | |||||||
| Decreased appetite | 1 (10.0) | |||||||
| Insomnia | 1 (10.0) | |||||||
| Bacteremia | 1 (4.3) | |||||||
| Acute kidney injury | 1 (4.3) | 1 (6.2) | ||||||
| Acute myocardial infarction | 1 (11.1) | |||||||
| Encephalopathy | 1 (4.3) | |||||||
| Libido decreased | 1 (20.0) | |||||||
CK creatine kinase, NOS not otherwise specified
A diverse set of subsequent treatments were received by patients whose initial ALK TKI treatments (33 alectinib and 40 crizotinib) were discontinued, shown in Fig. 2b. The first ALK TKI treatments were discontinued in 75.8% and 77.5% of patients receiving alectinib and crizotinib, respectively, due to disease progression. Of the 25 patients receiving alectinib as their first ALK TKI treatment, 22 (88.0%) were treated with other ALK TKIs, including brigatinib (18.2%), lorlatinib (77.3%), and the combination of ceritinib and trametinib (4.5%), or immune checkpoint inhibitors, pembrolizumab (4.5%) or durvalumab (4.5%), as the subsequent treatment. Of the 31 patients receiving crizotinib as their first ALK TKI treatment, 25 (80.6%) were treated with later generations of ALK TKIs, including brigatinib (4.0%), alectinib (56.0%), ceritinib (44.0%), and the combination of ceritinib and trametinib (4.0%), upon disease progression. Only 2 (6.0%) and 5 (12.5%) patients experienced treatment discontinuation due to adverse events for patients receiving alectinib and crizotinib as their first ALK TKI treatment, respectively (Table 3). Overall, 24.8%, 17.9%, and 6.0% of patients experienced an mAE requiring treatment dose adjustment, interruption, or discontinuation, respectively. The most common mAEs were rash (10.0%) and bradycardia (7.1%) in patients receiving alectinib and liver toxicity (19.1%) for patients receiving crizotinib. The most common sAEs were pericardial effusion (5.7%) and pleural effusion (5.7%) for alectinib and pulmonary embolism (6.4%) for crizotinib (Table 3).
Time to the First mAE, Real-world Disease Progression, and Death
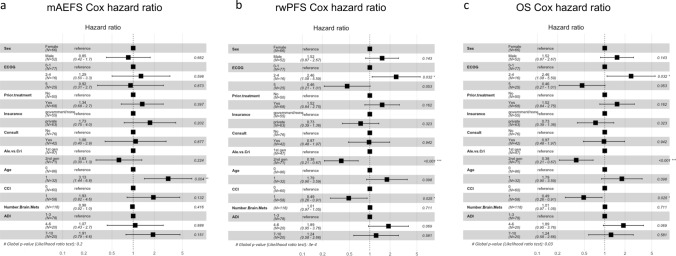
Patients receiving alectinib versus crizotinib as their first ALK TKI treatment experienced significantly prolonged median rwPFS (29.3 versus 10.4 months) with an HR of 0.38 (95% CI 0.21–0.67, p < 0.001; Figs. 3 and 4), as well as longer median mAEFS (not reached versus 91.3 months) with an HR of 0.63 (95% CI 0.30–1.33, p = 0.224). The OS of patients receiving alectinib as first ALK TKI was numerically larger than that of patients receiving crizotinib (54.1 versus 45.8 months), having an HR of 0.60 (95% CI 0.23–1.56, p = 0.295; Figs. 3 and 4). The high degree of cross-over post-progression is worth noting, as it could significantly confound the overall survival measures.
Fig. 4.
Forest plots of Cox proportional hazard models for mAEFS, rwPFS, and OS. Effects of alectinib versus crizotinib as the first ALK TKI treatment in a major-adverse-event-free survival, b real-world progression-free survival, and c overall survivals were estimated using multivariable Cox proportional hazard regression models. Abbreviations: mAEFS: major-adverse-event-free survival, rwPFS: real-world progression-free survival, OS: overall survival
We have conducted a sub-analysis focusing on patients who received ALK TKIs without prior therapies, and a sub-analysis focusing on patients with baseline brain metastases. The results for these two comparisons have been included in the supplemental material (eFigs. 2 and 3).
Discussion
This retrospective study sought to characterize real-world treatment patterns and safety, including sAEs and mAEs leading to any treatment changes in ALK-positive aNSCLC patients receiving their initial ALK TKI therapy. Overall, 34.2% (40 of 117) of real-world patients experienced an mAE that led to any treatment change, including treatment dose adjustment, interruption, and discontinuation, during the first ALK TKI treatment. When comparing rates in real-world patients to results reported in the ALEX randomized clinical trial numerically, we found slightly higher rates of dose adjustments (alectinib, 27.1% versus 20.4%; crizotinib, 21.3% versus 19.9%), but lower rates of interruption (alectinib, 22.9% versus 26.3%; crizotinib, 10.6% versus 26.5%), and much lower rates of treatment discontinuation (alectinib, 2.9% versus 14.5%; crizotinib, 10.6% versus 14.6%) due to adverse events [13, 35]. These differences, particularly the higher rates of dose adjustments due to adverse events, could be attributed to the fact that our real-world patient cohort has a larger proportion of patients with higher ECOG performance status scores, brain metastases at baseline, and receipt of systemic treatments prior to the first ALK TKI treatments. However, it is worth noting that lower rates of treatment interruptions and discontinuation due to adverse events were observed in our real-world patient cohort. It is likely due to differences between treatment protocols in the randomized clinical trials versus real-world clinical practices. Randomized clinical trials impose strict protocols to ensure treatment adherence and monitoring, which could lead to higher rates of adverse events reported. Contrarily, clinical decisions and patients’ adherence to treatment regimen may depend on the individual treating physicians and patients in real-world settings, which could contribute to the lower rates observed.
To profile the AEs that were clinically significant and required medical attention, we focused on the mAEs that led to treatment changes during the first ALK TKI treatment. The direct causal relations between mAEs and the resulting treatment changes were determined directly based on provider documentation. We note that our definition of mAEs differ from the definition of AEs used in trials, where AEs are captured at pre-specified timepoints and irrespective of clinical importance. Conversely, we would argue that our definition is more clinically relevant, as the mAEs were the events that triggered interventions and resulted in a change in dose, treatment interruption, or treatment discontinuation in real-world patients. While we noticed similar composition of AEs (bradycardia, rash, and liver toxicity) as reported in other studies [12, 13, 36], the rates found in our study cohort were relatively low in general. Although, this could indicate the favorable safety profile of ALK TKIs, the stringent AE definitions we used could also play a key contributing factor to the lower AE rates. However, the low rates of mAEs reported in this study highlighted the high tolerability of ALK TKIs, as the initial treatment regimen in real-world patients. The high tolerability of ALK TKIs was also evident in the long mAEFS estimated [not reached (alectinib) versus 91.3 months (crizotinib)].
We reported long survival outcomes, mAEFS (not reached versus 91.3 months), rwPFS (29.3 versus 10.4 months), and OS (54.1 versus 45.8 months) for alectinib versus crizotinib (Fig. 3). However, significant treatment effects of alectinib were only observed in rwPFS with an HR of 0.38 (95% CI 0.21–0.67, p < 0.001) (Fig. 4). This HR estimate was comparable to the ALEX randomized clinical trial as well as other real-world studies [13, 35, 37–39]. We developed causal DAGs to improve the estimation of treatment effects and minimize bias, a common limitation to retrospective research. This approach identified many important confounding covariates that are not commonly found in other real-world studies. For example, social determinants of health (ADI) may contribute to receiving necessary clinical care; also, whether or not patients were seen as consults at UCSF could attribute to the varying visit schedule, intervention and monitoring of toxicities, or assessment of disease progression. Under this framework, we believe that our multivariate Cox proportional analysis adjusting for the key covariates may result in higher confidence in HR estimates with less potential residual bias. It is worth noting that, while we found longer OS (54.1 versus 45.8 months) for alectinib versus crizotinib with an HR of 0.60 (95% CI 0.23–1.56, p = 0.295), the benefit of alectinib in OS did not reach statistical significance as reported in other real-world studies or the ALEX randomized clinical trial [35, 40]. This is possibly due to more than one-third (38.3%) of patients receiving crizotinib as the first ALK TKI treatment being treated with alectinib as the subsequent treatment immediately after discontinuation. As a result, the benefit of alectinib may have been partially reflected in the crizotinib cohort when trying to compare between alectinib and crizotinib.
Limitations
This retrospective study is subject to selection bias, as all study cohort and covariates were retrospectively identified and abstracted using the EHR data at UCSF. In addition, the study cohort included all adult patients with ALK-positive aNSCLC who received ALK TKIs rather than an intent-to-treat cohort. Causal DAGs were applied to minimize potential biases in estimating treatment effects on survival outcomes (eFig. 1).
To estimate effects of alectinib versus crizotinib on survival outcomes (mAEFS, rwPFS, and OS), several key covariates were identified based on the causal DAGs constructed. While we intended to construct causal DAGs that depict the intricate correlations between key players affecting decisions of ALK TKI treatment exposure and outcomes, we acknowledge that the construction could be incomplete. Potential confounding covariates may be missed and not incorporated. While we believe that the key covariates were identified, future studies with larger sample sizes are needed to further validate our findings. Additionally, our estimations could be biased due to informative censoring where decisions of ALK TKI treatment discontinuations may be influenced by prior AE experiences. Furthermore, while competing events (death could occur prior to sAE) could contribute to potential bias, we did not account for competing risks due to the small number of events. Our AEs were captured based on clinical notes documenting the occurrence of AEs, which could result in potential recording bias, as the treating physicians could miss or not report the AEs mentioned by patients.
Lastly, our study represents real-world experiences from a single tertiary treatment center with a relatively small sample size. As a result, the generalizability of our findings may be limited. However, the study method, specifically, the computational code for the analysis, as well as code to construct causal DAGs, are being shared publicly on GitHub [34] for other researchers to adopt and study real-world ALK TKI treatment patterns and outcomes. Furthermore, the trends identified are worth exploring in future studies with larger cohort size from a more diverse patient population.
Conclusion
We found that ALK TKIs in real-world patients were highly tolerable, with only 24.8%, 17.9%, and 6.0% of patients experiencing an AE requiring treatment dose adjustment, interruption, or discontinuation, respectively. We found that rash, bradycardia, and liver toxicity were the most common AEs leading to modifying or discontinuing ALK TKI treatment. Our findings with longer survival outcomes of mAEFS, rwPFS, and OS highlighted the high tolerability and effectiveness of alectinib compared with crizotinib in real-world patient populations. The detailed characterization of AEs may provide insights into improving clinical care and management for patients receiving ALK TKI treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the staff of the Bakar Computational Health Sciences Institute, UCSF Information Commons, and the Center for Data-driven Insights and Innovation within University of California Health. We thank the UCSF Clinical and Translational Science Institute (UL1TR001872) for their help on retrieving clinical notes at UCSF for chart review and validation. MW was supported by the US National Institutes of Health T32 training Grant (5T32GM007175-43). Partial grant support was provided by Genentech Inc. The content reflects the views of the authors and should not be construed to represent the views or policies of the National Institutes of Health (NIH) or Genentech.
Declarations
Funding
Research reported in this publication was partially supported by Genentech Inc (CW266073), the UCSF Bakar Computational Health Sciences Institute, the UCSF Division of Gastroenterology and Hepatology, and the National Center for Advancing Translational Sciences (UL1TR001872), and the US National Institutes of Health T32 (5T32GM007175‐43).
Conflicts of Interest
V.A.R. receives research support from Janssen, Genentech, Blueprint Medicines, Mitsubishi Tanabe, Takeda, Merck, and Alnylam Inc. L.B. owns stocks in Epic sciences and has served consulting or advisory roles for Genentech/Roche, Regeneron, Merk, Johnson and Johnson, Daichi, neuvogen, Bayer, Sanofi, ORCIC, Novocure, Mirati, Turning Point Therapeutics, Abbvie, InterVenn Biosciences, and Elevation Oncology. J.A.S., C.W.L., and S.O. are employed by Genentech and own stock in F. Hoffmann-La Roche. A.J.B is a co-founder and consultant to Personalis and NuMedii; is a consultant to Mango Tree Corporation, and in the recent past, Samsung, 10x Genomics, Helix, Pathway Genomics, and Verinata (Illumina); has served on paid advisory panels or boards for Geisinger Health, Regenstrief Institute, Gerson Lehman Group, AlphaSights, Covance, Novartis, Genentech, and Merck, and Roche; is a shareholder in Personalis and NuMedii; is a minor shareholder in Apple, Meta (Facebook), Alphabet (Google), Microsoft, Amazon, Snap, 10x Genomics, Illumina, Regeneron, Sanofi, Pfizer, Royalty Pharma, Moderna, Sutro, Doximity, BioNtech, Invitae, Pacific Biosciences, Editas Medicine, Nuna Health, Assay Depot, and Vet24seven, and several other non-health related companies and mutual funds; and has received honoraria and travel reimbursement for invited talks from Johnson and Johnson, Roche, Genentech, Pfizer, Merck, Lilly, Takeda, Varian, Mars, Siemens, Optum, Abbott, Celgene, AstraZeneca, AbbVie, Westat, and many academic institutions, medical or disease-specific foundations and associations, and health systems. A.J.B receives royalty payments through Stanford University, for several patents and other disclosures licensed to NuMedii and Personalis. Atul Butte’s research has been funded by the NIH, Peraton (as the prime on an NIH contract), Genentech, Johnson and Johnson, the Food and Drug Administration (FDA), Robert Wood Johnson Foundation, Leon Lowenstein Foundation, Intervalien Foundation, Priscilla Chan and Mark Zuckerberg, the Barbara and Gerson Bakar Foundation, and in the recent past, the March of Dimes, Juvenile Diabetes Research Foundation, California Governor’s Office of Planning and Research, California Institute for Regenerative Medicine, L’Oreal, and Progenity. None of these organizations have had any influence on this manuscript. M.W., S.S., and D.D. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
This retrospective study was approved by the University of California, San Francisco (UCSF) Institutional Review Board (UCSF IRB 21-3390).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and analyzed during the current study are not publicly available due to privacy concerns of health records but may be available from the corresponding author on reasonable request.
Code Availability
The analysis codes for this study have been shared on GitHub [34].
Author Contributions
V.A.R. designed the study and oversaw research development; S.S. and M.W. performed data extractions; V.A.R. and L.B. ensured data quality; M.W. developed statistical methods and analyzed the data; L.B., V.A.R., A.J.B., D.D., J.A.S., C.W.L., and S.O. provided domain knowledge expertise; M.W. and V.R wrote the initial draft of the manuscript; M.W., S.S., L.B., A.J.B., D.D., J.A.S., C.W.L., S.O., and V.A.R. reviewed, edited, and approved the manuscript.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clinic. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. The Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 6.Gainor JF, Varghese AM, Ou S-HI, Kabraji S, Awad MM, Katayama R, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendrell JA, Taviaux S, Béganton B, Godreuil S, Audran P, Grand D, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep. 2017;7:12510. doi: 10.1038/s41598-017-12679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon B, Varella-Garcia M, Camidge DR. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol. 2009;4:1450–1454. doi: 10.1097/JTO.0b013e3181c4dedb. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–1780. doi: 10.1016/j.ejca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018;16:1724–1736. doi: 10.1158/1541-7786.MCR-18-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36(12):1199–1206. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 13.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 14.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 15.Camidge DR, Kim HR, Ahn M-J, Yang JC-H, Han J-Y, Lee J-S, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Eng J Med. 2018;379:2027–39. [DOI] [PubMed]
- 16.Kim D-W, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britschgi C, Addeo A, Rechsteiner M, Delaloye R, Früh M, Metro G, et al. Real-world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer patients. Front Oncol. 2020;10:1299. doi: 10.3389/fonc.2020.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahanzeb M, Lin HM, Pan X, Yin Y, Wu Y, Nordstrom B, et al. Real-world treatment patterns and progression-free survival associated with Anaplastic Lymphoma Kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small cell lung cancer. Oncologist. 2020;25:867–877. doi: 10.1634/theoncologist.2020-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskovitz M, Dudnik E, Shamai S, Rotenberg Y, Popovich-Hadari N, Wollner M, et al. ALK Inhibitors or Chemotherapy for Third Line in ALK-positive NSCLC? Real-world Data. Oncologist. 2022;27:e76–e84. doi: 10.1093/oncolo/oyab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterhouse DM, Espirito JL, Chioda MD, Baidoo B, Mardekian J, Robert NJ, et al. Retrospective observational study of ALK-inhibitor therapy sequencing and outcomes in patients with ALK-positive non-small cell lung cancer. Drugs Real World Outcomes. 2020;7:261–269. doi: 10.1007/s40801-020-00207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goto Y, Yamamoto N, Masters ET, Kikkawa H, Mardekian J, Wiltshire R, et al. Treatment sequencing in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer in Japan: a real-world observational study. Adv Ther. 2020;37:3311–3323. doi: 10.1007/s12325-020-01392-0. [DOI] [PubMed] [Google Scholar]
- 22.Ganti AK, Lin C-W, Yang E, Wong WB, Ogale S. Real-world adherence and persistence with anaplastic lymphoma kinase inhibitors in non–small cell lung cancer. JMCP Acad Managed Care Pharmacy. 2022;28:305–314. doi: 10.18553/jmcp.2021.21310. [DOI] [PubMed] [Google Scholar]
- 23.Koopman B, Groen HJM, Schuuring E, Hiltermann TJN, Timens W, den Dunnen WFA, et al. Actionability of on-target ALK resistance mutations in patients with non-small cell lung cancer: local experience and review of the literature. Clin Lung Cancer. 2022;23:e104–e115. doi: 10.1016/j.cllc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20:109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12:188–197. [PMC free article] [PubMed] [Google Scholar]
- 26.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Data-driven Insights and Innovation Annual Report 2020. Available from: https://www.ucop.edu/uc-health/_files/cdi2-2019-2020-annual-report.pdf
- 28.Slatter S, Wang M, Lin C-W, Sussell J, Ogale S, Datta D, et al. Methods for clinical chart abstraction: real-world ALK inhibitor treatment patterns and reasons for discontinuation study 2022. Available from: https://www.protocols.io/view/methods-for-clinical-chart-abstraction-real-world-cf3btqin
- 29.Griffith SD, Miksad RA, Calkins G, You P, Lipitz NG, Bourla AB, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;1–13. [DOI] [PMC free article] [PubMed]
- 30.Griffith SD, Tucker M, Bowser B, Calkins G, Chang C, Guardino E, et al. Generating real-world tumor burden endpoints from electronic health record data: comparison of RECIST, radiology-anchored, and clinician-anchored approaches for abstracting real-world progression in non-small cell lung cancer. Adv Ther. 2019;36:2122–2136. doi: 10.1007/s12325-019-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 32.Kassambara A, Kosinski M, Biecek P, Fabian S. Survminer: drawing survival curves using “ggplot2”. 2021. Available from: https://CRAN.R-project.org/package=survminer.
- 33.Therneau TM, until 2009) TL (original S->R port and R maintainer, Elizabeth A, Cynthia C. survival: Survival Analysis. 2022. Available from: https://CRAN.R-project.org/package=survival.
- 34.GitHub. ALK TKI cox analyses. https://git.ucsf.edu/Michelle-Wang2/ALKTKI_coxanalyses.
- 35.Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim D-W, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. [DOI] [PubMed] [Google Scholar]
- 36.Cirne F, Zhou S, Kappel C, El-Kadi A, Barron CC, Ellis PM, et al. ALK inhibitor-induced bradycardia: a systematic-review and meta-analysis. Lung Cancer. 2021;161:9–17. doi: 10.1016/j.lungcan.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Shen S, Hu P, Geng D, Zheng R, Li X. Alectinib versus crizotinib in ALK-positive advanced non-small cell lung cancer and comparison of next-generation TKIs after crizotinib failure: Real-world evidence. Cancer Med. 2022;11:4491–4500. doi: 10.1002/cam4.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang H, Jin L, Zhang Z, Jiang Z, Malik Z. Comparison of clinical efficacy of alectinib versus crizotinib in ALK-positive non-small cell lung cancer: a meta-analysis. Front Oncol. 2021;11:646526. doi: 10.3389/fonc.2021.646526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou C, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7:437–446. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 40.Tilkema-Tiebosch M, Damhuis R, Vijftigschild S, Wekken AJVD. 1131P Overall survival after treatment with first-line crizotinib or alectinib in patients with stage IV NSCLC and ALK rearrangement: a real-world nationwide cohort study from the Netherlands. Ann Oncol. 2022;33:S1068. doi: 10.1016/j.annonc.2022.07.1255. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.