Abstract
Background
Limited real-world data exist regarding the efficacy of palbociclib in combination with endocrine therapy in pre/perimenopausal women with metastatic breast cancer.
Objective
We aimed to compare real-world tumor responses among pre/perimenopausal women who initiated palbociclib plus an aromatase inhibitor (AI) or AI monotherapy as first-line treatment for hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer.
Methods
This retrospective observational cohort study (NCT05012644) used electronic health record data from The US Oncology Network. Tumor responses were determined based on treating clinicians’ assessments of radiologic evidence for changes in disease burden. Normalized inverse probability treatment weighting was used to balance baseline characteristics between treatment cohorts.
Results
Of 196 pre/perimenopausal women, 116 and 80 were in the palbociclib plus AI cohort and AI cohort, respectively. Real-world response rates (complete or partial response) were 52.1% and 46.2%, respectively (odds ratio, 1.27 [95% confidence interval 0.72‒2.24]). Among patients with one or more tumor assessments on treatment, real-world response rates were 60.0% in the palbociclib plus AI cohort (n = 103) and 49.9% in the AI cohort (n = 71; odds ratio, 1.51 [95% confidence interval 0.82‒2.77]).
Conclusions
This real-world analysis suggests that pre/perimenopausal patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer appear more likely to respond to palbociclib plus AI versus AI alone as first-line therapy, which may support the combination as a standard-of-care treatment for this patient population.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-00979-1.
Plain Language Summary
Palbociclib (Ibrance®) is a medicine for patients with metastatic breast cancer (MBC). Metastatic means that the cancer has spread to other places in the body. Patients take palbociclib with hormone therapy, such as an aromatase inhibitor (AI). Palbociclib plus an AI is a treatment for a type of MBC called HR+/HER2‒ MBC. HR+/HER2‒ stands for hormone receptor positive, human epidermal growth factor receptor 2 negative. Researchers wanted to observe responses to treatment in routine clinical practice among women with HR+/HER2– MBC who had not reached menopause. A response is if a tumor shrinks or disappears after treatment. This study used healthcare information reported in electronic medical records of patients seen by doctors in The US Oncology Network. This study included 196 women with HR+/HER2– MBC who had not reached menopause and had not received prior treatment for MBC. A total of 116 women received palbociclib plus an AI, and 80 women received an AI alone. Researchers used standard statistical approaches to balance baseline characteristics between the two treatment groups. These adjustments made the groups more similar so that researchers could compare treatment responses. Sixty percent of patients who took palbociclib plus an AI responded, compared with 50% of patients who took an AI alone. These results suggest that palbociclib plus an AI may benefit women with HR+/HER2‒ MBC who have not reached menopause.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-00979-1.
Key Points
| This real-world retrospective analysis evaluated the effect of first-line palbociclib plus an aromatase inhibitor (AI) versus AI alone on tumor responses in pre/perimenopausal women with hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. |
| The real-world response rates associated with palbociclib plus an AI were numerically higher than those associated with an AI alone. |
| These results suggest that pre/perimenopausal women with hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer appear more likely to respond to palbociclib plus an AI than an AI alone in the first-line setting. |
Introduction
Female breast cancer is the most prevalent cancer worldwide, comprising 2.26 million new cases (11.7% of all cancers) and accounting for nearly 700,000 deaths in 2020 [1]. Breast cancer will affect approximately 13% of US women in their lifetimes, with 287,850 estimated cases and 43,250 estimated deaths having occurred among US women in 2022 [2, 3]. Of approximately 4.1 million women with a history of breast cancer living in the USA in 2022, approximately 4% were living with metastatic disease, which has a 5-year survival rate of 29% [3]. Women < 50 years of age account for 17% of US breast cancer cases [3], with younger age being a recognized risk factor for more aggressive disease [4]. Most US breast cancer cases are hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2−) [3]. The incidence of HR+ cancers increased at an annual rate of 1.3%–5.7% among US women < 50 years of age between 2000 and 2015 [5].
Palbociclib is an orally administered CDK4/6 inhibitor that is approved by the US Food and Drug Administration (FDA) in combination with an AI as a first-line therapy for HR+/HER2− advanced breast cancer (ABC) or metastatic breast cancer (MBC) [6]. Of particular relevance to the findings presented herein, the FDA recently expanded the approved indication for palbociclib plus an AI to include pre/perimenopausal women in December 2022. The initial accelerated approval of palbociclib plus an AI was based on results from the phase I/II PALOMA-1 study [7] in which median progression-free survival (PFS) was significantly longer for the palbociclib plus letrozole arm compared with the letrozole-alone arm (20.2 vs 10.2 months; hazard ratio, 0.49 [95% confidence interval (CI) 0.32–0.75]; P < 0.001) in postmenopausal women with estrogen receptor-positive/HER2− ABC who did not receive systemic therapy for advanced disease (N = 165) [8]. These findings were subsequently supported by results from the confirmatory phase III PALOMA-2 study that demonstrated a statistically significant improvement in the primary endpoint of PFS for the palbociclib plus letrozole arm compared with the placebo plus letrozole arm (extended follow-up analysis: median PFS of 27.6 vs 14.5 months; hazard ratio, 0.56 [95% CI 0.46–0.69]; P < 0.0001) in a similar population (N = 666) [9–11]. Additionally, palbociclib in combination with fulvestrant is approved in the USA for patients with HR+/HER2− ABC or MBC that has progressed following endocrine therapy [6]. This is based on findings from the PALOMA-3 trial, which demonstrated a statistically significant improvement in median PFS for the palbociclib plus fulvestrant arm compared with the placebo plus fulvestrant arm (9.5 vs 4.6 months; hazard ratio, 0.46 [95% CI 0.36–0.59]; P < 0.0001) in this population (N = 521) [12–14]. A subgroup analysis of pre/perimenopausal patients (n = 108) in PALOMA-3 also demonstrated a clinical benefit, with longer median PFS (9.5 vs 5.6 months; hazard ratio, 0.50 [95% CI 0.29–0.87]) and a higher objective response rate (25.0% vs 11.1%; odds ratio (OR), 3.06 [95% CI 0.82–13.38]) for palbociclib plus fulvestrant (plus goserelin as a luteinizing hormone-releasing hormone [LHRH] agonist) compared with placebo plus fulvestrant (plus goserelin) [15].
Although pre/perimenopausal patients were excluded from the PALOMA-2 trial [9], there is evidence to suggest that palbociclib plus an AI is effective in that population. A subgroup analysis of PALOMA-2 supported improved PFS associated with palbociclib plus letrozole versus placebo plus letrozole in patients with a prior oophorectomy (n = 105; hazard ratio, 0.49 [95% CI 0.28–0.86]; Pfizer, data on file). Additionally, the phase II Young-PEARL study conducted in South Korea found that premenopausal women with HR+/HER2− ABC that progressed following tamoxifen treatment, and who could have had one or more previous lines of treatment for advanced disease (n = 178), had a median PFS of 20.1 months when treated with palbociclib plus exemestane plus leuprolide versus 14.4 months when treated with capecitabine (hazard ratio, 0.66 [95% CI 0.44–0.99]; P = 0.02) [16].
A recent real-world study in patients with HR+/HER2− MBC from the US Flatiron Health Analytic Database found that adjusted real-world PFS and overall survival (OS) were significantly longer in patients who received palbociclib plus letrozole (n = 839) compared with patients who received letrozole monotherapy (n = 698) [17]. In the small subgroup of patients aged 18–50 years (n = 104), used as a surrogate for pre/perimenopausal status, the real-world PFS in patients who received palbociclib plus letrozole was also longer compared with those who received letrozole monotherapy (hazard ratio, 0.82 [95% CI 0.48–1.39]). However, to date, the limited real-world data available for the pre/perimenopausal patient population have relied on age as a surrogate for menopausal status or have been from non-comparative single-arm studies. To supplement available clinical trial and limited existing comparative real-world data in a confirmed pre/perimenopausal patient population, this study aimed to compare real-world tumor responses among pre/perimenopausal women who initiated palbociclib plus AI or AI monotherapy as a first-line therapy for HR+/HER2− MBC. In this real-world study, tumor response was chosen as an endpoint versus real-world PFS to allow for a more objective comparison of treatment effectiveness given the small sample size and differences in treatment follow-up time periods.
Methods
Study Design and Data
This was a retrospective observational cohort study (NCT05012644) of patients with HR+/HER2− MBC who received care within The US Oncology Network (The Network). The Network includes > 1380 affiliated physicians operating in > 480 US sites of care that collectively treat > 1.2 million patients annually [18].
Study data were captured from the electronic health record (EHR) from structured fields and a chart review of unstructured data of The Network, iKnowMed (iKM), with the Limited Access Death Master File used for supplemental mortality status. Previous studies have demonstrated the utility of the iKM EHR database for the evaluation of patient profiles, treatment patterns, and outcomes among patients with MBC [19, 20]. Structured EHR data were extracted using programmatic methods, and a systematic manual chart abstraction was used to capture additional information from unstructured fields. All study data were merged into a single data set and underwent a series of quality checks, which varied by the type and importance of variable.
First, 80 individual data elements identified by the team as having clinical and methodological relevance to the study were selected to undergo source data verification for 25% (n = 49) of eligible charts. These data elements included tumor assessments, treatment history, and vital status, among other select variables. During these checks, data quality specialists reviewed the source chart to confirm that the abstraction was comprehensive and accurate. Across all data elements checked, the overall success rate was 98.1%.
Analytic methods were also performed, consisting of over 90 separate programmatic checks of data elements the study team identified as being critical to the study and/or susceptible to systemic errors, including baseline, treatment, tumor assessment, and vital status data points. For these checks, values that were outlying, clinically or logically improbable, inconsistent with other data captured for the patient, or unexpectedly missing were flagged for resolution. When a potential data issue was identified through these analytic checks, source data verification was used to confirm what was documented in the underlying EHR data. If the data point was correctly captured in the study data set, it was documented; otherwise, it was corrected in the study data set. Following analytic checks of the data and source data verification if needed, data for key study variables were aggregated, and a board-certified oncologist reviewed the distribution of values to confirm clinical appropriateness of the data.
Additional checks were conducted for tumor assessment data, the primary endpoint of the study. Tumor assessment data were abstracted by certified tumor registrars and/or oncology care nurses with specialized chart review training specific to oncological abstraction methods. Then, all tumor assessment data underwent 100% source data verification by a data quality specialist, in addition to the quality checks described above.
Patients
Eligible patients were women who had a documented history of MBC and confirmed HR+/HER2− status, were ≥18 years of age and pre- or perimenopausal at initial MBC diagnosis, received palbociclib plus AI or AI monotherapy during 1 January, 2010–30 June, 2020 as a first-line treatment for MBC at a Network site(s) with full iKM EHR capabilities, and had EHR data available for research purposes. The 5-year period before the 2015 palbociclib approval was included to enable identification of additional patients in the AI monotherapy control cohort, considering the expected limited sample size owing to the epidemiology of the pre/perimenopausal patient population and increased use of CDK4/6 inhibitors in this patient population after FDA approval. If not explicitly documented by a physician, premenopausal status was determined based on a sequence of criteria, which, in addition to being < 60 years of age, included one or both of the following: (1) having menses (not amenorrheic) and/or (2) having no documented history of a prior bilateral oophorectomy and receiving an LHRH analog. Exclusion criteria were prior treatment with CDK4/6 inhibitors in the early breast cancer or MBC setting, documented history of another primary cancer within The Network, a first clinical visit > 120 days after the MBC diagnostic date (with chart review confirming no initial treatment outside The Network), or enrollment in any interventional clinical trial or receipt of treatment for another primary cancer during the study observation period (i.e., after initiation of palbociclib plus AI or AI monotherapy through the study data cut-off date of 31 December, 2020).
Outcomes
Real-world tumor responses were determined based on the treating clinician’s assessment of radiologic evidence for a change in the burden of disease over the course of treatment derived from a chart review [21]. Response assessments were captured as documented by treating clinicians in patients’ charts, and an independent review of radiology reports was not performed. As with real-world studies, assessments were not performed on a predefined schedule, and responses were not necessarily confirmed by a subsequent assessment. Each real-world response assessment was classified as a complete response (defined as the resolution of all evidence of disease), a partial response (defined as a partial reduction in the size of visible disease in some or all areas without increase in any area [any reduction described was included and did not need to meet any specific criteria, such as the Response Evaluation Criteria in Solid Tumors criteria]), stable disease (defined as disease that is stable [no progression or improvement] or as a mixed response [combination of improved and worsened disease]), progressive disease (defined as worsening of disease), or not evaluable (defined as no documentation of status of disease). A real-world best response was derived based on clinician-documented responses at different response assessment timepoints from treatment initiation until the first documentation of progressive disease during first-line therapy.
Statistical Analyses
The primary endpoint was a real-world response rate (rwRR), calculated by treatment cohort as the proportion of patients with a real-world best response of complete response or partial response during first-line therapy that occurred at least 30 days after treatment initiation without progressive disease at any prior assessment. Given the anticipated small sample size of this patient population, no formal hypothesis testing was planned; therefore, comparisons of rwRRs between treatment cohorts were descriptive only.
The normalized inverse probability treatment weighting (nIPTW) method (primary analysis) was used to balance baseline demographic and clinical characteristics and adjust for differences in observed potential confounders between the two treatment cohorts [22]. Inverse probability treatment weighting was normalized to stabilize weights and preserve the sample size of the original population. Propensity score matching (PSM) was performed as a sensitivity analysis using a 1:1 nearest neighbor matching with a caliper of 0.1 without replacement. A multivariate logistic regression model was used to generate a propensity score (PS) for each patient based on the prespecified baseline variables that were deemed associated with treatment assignment and/or the tumor response outcome (Tables 1 and 2) [23]. Missing information on the categorical variables of baseline characteristics was assigned to a new category of “unknown” and was always included in the model estimating the PS. Statistical analyses using PS methodology to balance baseline characteristics between treatment cohorts were conducted independently and blinded to tumor response outcome data, maintaining data integrity.
Table 1.
Demographic and clinical characteristics of patients with ≥1 tumor assessments before and after nIPTW
| Before nIPTW | After nIPTW | |||||
|---|---|---|---|---|---|---|
| Characteristica,b | Palbociclib + AI (n = 103)c |
AI monotherapy (n = 71)c |
Standardized mean differenced | Palbociclib + AI (n = 103) |
AI monotherapy (n = 71) |
Standardized mean differenced |
| Age, years | ||||||
| < 30 | 0 | 0 | 0 | 0 | ||
| 30−44 | 41 (39.8) | 29 (40.8) | 0.0212 | 44 (42.7) | 29 (40.8) | − 0.0496 |
| ≥ 45 | 62 (60.2) | 42 (59.2) | − 0.0212 | 59 (57.3) | 42 (59.2) | 0.0496 |
| Mean (SD) | 45.8 (6.4) | 46.1 (7.2) | 0.0428 | 45.9 (6.6) | 45.8 (6.8) | − 0.0038 |
| Median (min, max) | 46.0 (31.0, 58.0) | 46.0 (30.0, 60.0) | 46.0 (31.0, 58.0) | 46.0 (30.0, 60.0) | ||
| Race | ||||||
| Black | 12 (11.7) | 9 (12.7) | 0.0314 | 12 (11.7) | 9 (12.7) | 0.0527 |
| White | 68 (66.0) | 48 (67.6) | 0.0337 | 68 (66.0) | 46 (64.8) | − 0.0044 |
| Not reported/unknown | 23 (22.3) | 14 (19.7) | − 0.0641 | 23 (22.3) | 15 (21.1) | − 0.0370 |
| Weight, kg | ||||||
| n | 96 | 61 | 96 | 61 | ||
| Mean (SD) | 75.9 (17.7) | 74.6 (18.3) | − 0.0704 | 76.0 (17.3) | 74.0 (19.1) | − 0.1106 |
| Median (min, max) | 72.8 (47.8, 129.3) | 72.6 (44.7, 131.1) | 72.8 (47.8, 129.3) | 72.6 (44.7, 131.1) | ||
| BMI, kg/m2 | ||||||
| n | 96 | 61 | 96 | 61 | ||
| Mean (SD) | 28.2 (6.3) | 28.0 (6.5) | − 0.0326 | 28.1 (6.3) | 27.7 (6.8) | − 0.0611 |
| Median (min, max) | 27.3 (16.1, 46.0) | 26.2 (16.0, 51.2) | 27.3 (16.1, 46.0) | 26.2 (16.0, 51.2) | ||
| BMI ≥ 25 kg/m2 | ||||||
| Yes | 68 (66.0) | 37 (52.1) | − 0.2857 | 64 (62.1) | 40 (56.3) | − 0.1106 |
| No | 28 (27.2) | 24 (33.8) | 0.1441 | 30 (29.1) | 24 (33.8) | 0.0912 |
| Unknown | 7 (6.8) | 10 (14.1) | 0.2401 | 9 (8.7) | 7 (9.9) | 0.0409 |
| Tobacco use | ||||||
| No history of tobacco use | 69 (67.0) | 36 (50.7) | − 0.3356 | 71 (68.9) | 37 (52.1) | − 0.3350 |
| Current tobacco use | 7 (6.8) | 10 (14.1) | 0.2401 | 7 (6.8) | 9 (12.7) | 0.2103 |
| Prior tobacco use | 24 (23.3) | 11 (15.5) | − 0.1984 | 23 (22.3) | 11 (15.5) | − 0.1819 |
| Unknown | 3 (2.9) | 14 (19.7) | 0.5502 | 2 (1.9) | 14 (19.7) | 0.5673 |
| Family history of cancer | ||||||
| Yes | 69 (67.0) | 49 (69.0) | 0.0434 | 66 (64.1) | 49 (69.0) | 0.1071 |
| No | 34 (33.0) | 22 (31.0) | − 0.0434 | 37 (35.9) | 22 (31.0) | − 0.1071 |
| Hormone receptor status | ||||||
| ER+ only | 16 (15.5) | 12 (16.9) | 0.0371 | 17 (16.5) | 13 (18.3) | 0.0365 |
| PR+ only | 1 (1.0) | 0 | − 0.1400 | 1 (1.0) | 0 | − 0.1358 |
| ER+ and PR+ | 86 (83.5) | 59 (83.1) | − 0.0106 | 85 (82.5) | 58 (81.7) | − 0.0122 |
| Stage at diagnosis | ||||||
| Stage 0 or I | 21 (20.4) | 9 (12.7) | − 0.2087 | 18 (17.5) | 12 (16.9) | − 0.0223 |
| Stage II | 36 (35.0) | 25 (35.2) | 0.0054 | 35 (34.0) | 26 (36.6) | 0.0381 |
| Stage III | 17 (16.5) | 16 (22.5) | 0.1526 | 22 (21.4) | 14 (19.7) | − 0.0317 |
| Stage IV | 26 (25.2) | 18 (25.4) | 0.0025 | 24 (23.3) | 17 (23.9) | 0.0014 |
| Unknown | 3 (2.9) | 3 (4.2) | 0.0708 | 3 (2.9) | 2 (2.8) | 0.0137 |
| Disease histology | ||||||
| Ductal | 71 (68.9) | 55 (77.5) | 0.1935 | 72 (69.9) | 58 (81.7) | 0.2605 |
| Lobular | 12 (11.7) | 6 (8.5) | − 0.1066 | 13 (12.6) | 5 (7.0) | − 0.1686 |
| Mixed | 5 (4.9) | 5 (7.0) | 0.0926 | 4 (3.9) | 4 (5.6) | 0.0945 |
| Metaplastic | 0 | 0 | 0 | 0 | ||
| Mucinous | 1 (1.0) | 0 | − 0.1400 | 1 (1.0) | 0 | − 0.1150 |
| Other | 1 (1.0) | 0 | − 0.1400 | 1 (1.0) | 0 | − 0.1361 |
| Unknown | 13 (12.6) | 5 (7.0) | − 0.1882 | 12 (11.7) | 4 (5.6) | − 0.2283 |
| Prior neo/adjuvant chemotherapy | ||||||
| Yes | 43 (41.7) | 32 (45.1) | 0.0671 | 46 (44.7) | 29 (40.8) | − 0.0657 |
| No | 60 (58.3) | 39 (54.9) | − 0.0671 | 57 (55.3) | 42 (59.2) | 0.0657 |
| Prior neo/adjuvant endocrine therapy | ||||||
| Yes | 51 (49.5) | 43 (60.6) | 0.2235 | 50 (48.5) | 41 (57.7) | 0.1870 |
| No | 52 (50.5) | 28 (39.4) | − 0.2235 | 53 (51.5) | 30 (42.3) | − 0.1870 |
| ECOG PS score | ||||||
| 0 | 33 (32.0) | 24 (33.8) | 0.0375 | 33 (32.0) | 23 (32.4) | 0.0021 |
| 1 | 36 (35.0) | 22 (31.0) | − 0.0844 | 33 (32.0) | 22 (31.0) | − 0.0225 |
| ≥ 2 | 7 (6.8) | 7 (9.9) | 0.1110 | 10 (9.7) | 7 (9.9) | 0.0320 |
| Unknown | 27 (26.2) | 18 (25.4) | − 0.0197 | 27 (26.2) | 18 (25.4) | 0.0000 |
| CCI score | ||||||
| 0 | 84 (81.6) | 62 (87.3) | 0.1597 | 85 (82.5) | 63 (88.7) | 0.1997 |
| 1 | 12 (11.7) | 3 (4.2) | − 0.2773 | 12 (11.7) | 2 (2.8) | − 0.3393 |
| 2 | 4 (3.9) | 4 (5.6) | 0.0823 | 3 (2.9) | 4 (5.6) | 0.1377 |
| 3 | 3 (2.9) | 0 | − 0.2449 | 3 (2.9) | 0 | − 0.2424 |
| 4 | 0 | 1 (1.4) | 0.1690 | 0 | 1 (1.4) | 0.1479 |
| 6 | 0 | 1 (1.4) | 0.1690 | 0 | 1 (1.4) | 0.1269 |
| Metastatic disease site | ||||||
| Viscerale | 33 (32.0) | 23 (32.4) | 0.0076 | 32 (31.1) | 23 (32.4) | 0.0127 |
| Non-visceral | 40 (38.8) | 17 (23.9) | − 0.3251 | 32 (31.1) | 21 (29.6) | − 0.0308 |
| Bone onlyf | 30 (29.1) | 31 (43.7) | 0.3056 | 38 (36.9) | 27 (38.0) | 0.0172 |
| Number of metastatic sites | ||||||
| 1 | 28 (27.2) | 28 (39.4) | 0.2622 | 35 (34.0) | 26 (36.6) | 0.0513 |
| 2 | 39 (37.9) | 18 (25.4) | − 0.2716 | 32 (31.1) | 21 (29.6) | − 0.0238 |
| ≥ 3 | 36 (35.0) | 25 (35.2) | 0.0054 | 36 (35.0) | 24 (33.8) | − 0.0283 |
| Disease-free interval | ||||||
| < 12 months | 48 (46.6) | 29 (40.8) | − 0.1163 | 44 (42.7) | 30 (42.3) | − 0.0124 |
| ≥ 12 months | 13 (12.6) | 16 (22.5) | 0.2627 | 20 (19.4) | 13 (18.3) | − 0.0511 |
| De novo metastatic | 30 (29.1) | 19 (26.8) | − 0.0527 | 27 (26.2) | 18 (25.4) | − 0.0066 |
| Unknown | 12 (11.7) | 7 (9.9) | − 0.0578 | 11 (10.7) | 10 (14.1) | 0.0879 |
AI aromatase inhibitor, BMI body mass index, CCI Charlson Comorbidity Index, ECOG PS Eastern Cooperative Oncology Group performance status, ER estrogen receptor, max maximum, min minimum, nIPTW normalized inverse probability treatment weighting, PR progesterone receptor, SD standard deviation
aAll data are listed as n (%) unless otherwise indicated
bCharacteristics in bold were used for propensity scoring and subsequent nIPTW
cn values apply to each characteristic unless a different n value is provided
dStandardized mean differences < 0.1 were considered indicative of acceptable balance
eVisceral includes liver, lung, and pleura
fBone only includes bone, bone marrow, pelvis, ribs, skull, and spine
Table 2.
Demographic and clinical characteristics of the overall analysis set before and after nIPTW
| Before nIPTW | After nIPTW | |||||
|---|---|---|---|---|---|---|
| Characteristica,b | Palbociclib + AI (n = 116)c |
AI monotherapy (n = 80)c | Standardized mean differenced | Palbociclib + AI (n = 116) |
AI monotherapy (n = 80) | Standardized mean differenced |
| Age, years | ||||||
| < 30 | 2 (1.7) | 0 | – 0.1873 | 6 (5.2) | 0 | – 0.3195 |
| 30–44 | 45 (38.8) | 29 (36.3) | – 0.0525 | 46 (39.7) | 30 (37.5) | – 0.0399 |
| ≥ 45 | 69 (59.5) | 51 (63.8) | 0.0878 | 64 (55.2) | 50 (62.5) | 0.1385 |
| Mean (SD) | 45.5 (7.0) | 46.8 (7.2) | 0.1876 | 45.0 (7.9) | 46.3 (6.8) | 0.1714 |
| Median (min, max) | 46.0 (26.0, 58.0) | 47.5 (30.0, 60.0) | 46.0 (26.0, 58.0) | 46.0 (30.0, 60.0) | ||
| Race | ||||||
| Black | 14 (12.1) | 9 (11.3) | − 0.0255 | 13 (11.2) | 10 (12.5) | 0.0561 |
| White | 76 (65.5) | 55 (68.8) | 0.0689 | 74 (63.8) | 51 (63.8) | 0.0009 |
| Not reported/unknown | 26 (22.4) | 16 (20.0) | − 0.0591 | 29 (25.0) | 18 (22.5) | − 0.0443 |
| Weight, kg | ||||||
| n | 108 | 67 | 108 | 67 | ||
| Mean (SD) | 75.5 (18.3) | 74.3 (18.2) | – 0.0651 | 75.6 (17.7) | 73.5 (19.3) | – 0.1163 |
| Median (min, max) | 72.4 (44.8, 129.3) | 72.2 (44.7, 131.1) | 72.7 (44.8, 129.3) | 72.2 (44.7, 131.1) | ||
| BMI, kg/m2 | ||||||
| n | 108 | 67 | 108 | 67 | ||
| Mean (SD) | 28.1 (6.5) | 27.9 (6.4) | – 0.0215 | 28.0 (6.3) | 27.6 (6.8) | – 0.0595 |
| Median (min, max) | 27.2 (16.1, 46.0) | 26.2 (16.0, 51.2) | 27.1 (16.1, 46.0) | 26.2 (16.0, 51.2) | ||
| BMI ≥ 25 kg/m2 | ||||||
| Yes | 75 (64.7) | 41 (51.3) | − 0.2741 | 69 (59.5) | 44 (55.0) | − 0.0880 |
| No | 33 (28.4) | 26 (32.5) | 0.0881 | 34 (29.3) | 27 (33.8) | 0.1026 |
| Unknown | 8 (6.9) | 13 (16.3) | 0.2956 | 14 (12.1) | 9 (11.3) | − 0.0125 |
| Tobacco use | ||||||
| No history of tobacco | 76 (65.5) | 39 (48.8) | – 0.3438 | 79 (68.1) | 41 (51.3) | – 0.3487 |
| Current tobacco use | 8 (6.9) | 11 (13.8) | 0.2267 | 7 (6.0) | 10 (12.5) | 0.2032 |
| Prior tobacco use | 28 (24.1) | 14 (17.5) | – 0.1640 | 26 (22.4) | 14 (17.5) | – 0.1413 |
| Unknown | 4 (3.4) | 16 (20.0) | 0.5324 | 3 (2.6) | 15 (18.8) | 0.5487 |
| Family history of cancer | ||||||
| Yes | 80 (69.0) | 54 (67.5) | – 0.0315 | 78 (67.2) | 54 (67.5) | – 0.0103 |
| No | 36 (31.0) | 26 (32.5) | 0.0315 | 38 (32.8) | 26 (32.5) | 0.0103 |
| Hormone receptor status | ||||||
| ER+ only | 19 (16.4) | 12 (15.0) | – 0.0379 | 20 (17.2) | 13 (16.3) | – 0.0176 |
| PR+ only | 1 (0.9) | 0 | – 0.1319 | 1 (0.9) | 0 | – 0.1231 |
| ER+ and PR+ | 96 (82.8) | 68 (85.0) | 0.0610 | 95 (81.9) | 67 (83.8) | 0.0375 |
| Stage at diagnosis | ||||||
| Stage I | 22 (19.0) | 9 (11.3) | − 0.2167 | 18 (15.5) | 11 (13.8) | − 0.0376 |
| Stage II | 38 (32.8) | 26 (32.5) | − 0.0055 | 38 (32.8) | 28 (35.0) | 0.0635 |
| Stage III | 21 (18.1) | 21 (26.3) | 0.1970 | 29 (25.0) | 18 (22.5) | − 0.0447 |
| Stage IV | 30 (25.9) | 20 (25.0) | − 0.0198 | 27 (23.3) | 19 (23.8) | 0.0013 |
| Unknown | 5 (4.3) | 4 (5.0) | 0.0327 | 5 (4.3) | 3 (3.8) | 0.0096 |
| Disease histology | ||||||
| Ductal | 79 (68.1) | 62 (77.5) | 0.2124 | 82 (70.7) | 65 (81.3) | 0.2640 |
| Lobular | 14 (12.1) | 8 (10.0) | – 0.0661 | 15 (12.9) | 6 (7.5) | – 0.1579 |
| Mixed | 5 (4.3) | 5 (6.3) | 0.0868 | 4 (3.4) | 4 (5.0) | 0.0850 |
| Metaplastic | 1 (0.9) | 0 | – 0.1319 | 1 (0.9) | 0 | – 0.1380 |
| Mucinous | 1 (0.9) | 0 | – 0.1319 | 1 (0.9) | 0 | – 0.1087 |
| Other | 1 (0.9) | 0 | – 0.1319 | 1 (0.9) | 0 | – 0.1271 |
| Unknown | 15 (12.9) | 5 (6.3) | – 0.2284 | 13 (11.2) | 4 (5.0) | – 0.2081 |
| Prior neo/adjuvant chemotherapy | ||||||
| Yes | 50 (43.1) | 36 (45.0) | 0.0382 | 53 (45.7) | 33 (41.3) | − 0.0909 |
| No | 66 (56.9) | 44 (55.0) | − 0.0382 | 63 (54.3) | 47 (58.8) | 0.0909 |
| Prior neo/adjuvant endocrine therapy | ||||||
| Yes | 55 (47.4) | 47 (58.8) | 0.2286 | 52 (44.8) | 45 (56.3) | 0.2342 |
| No | 61 (52.6) | 33 (41.3) | – 0.2286 | 64 (55.2) | 35 (43.8) | – 0.2342 |
| ECOG PS | ||||||
| 0 | 34 (29.3) | 27 (33.8) | 0.0957 | 35 (30.2) | 25 (31.3) | 0.0106 |
| 1 | 42 (36.2) | 24 (30.0) | − 0.1322 | 38 (32.8) | 25 (31.3) | − 0.0207 |
| ≥ 2 | 8 (6.9) | 9 (11.3) | 0.1520 | 10 (8.6) | 8 (10.0) | 0.0562 |
| Unknown | 32 (27.6) | 20 (25.0) | − 0.0588 | 33 (28.4) | 22 (27.5) | − 0.0264 |
| CCI score | ||||||
| 0 | 97 (83.6) | 67 (83.8) | 0.0035 | 98 (84.5) | 69 (86.3) | 0.0484 |
| 1 | 12 (10.3) | 4 (5.0) | – 0.2018 | 12 (10.3) | 3 (3.8) | – 0.2519 |
| 2 | 4 (3.4) | 7 (8.8) | 0.2229 | 3 (2.6) | 7 (8.8) | 0.2519 |
| 3 | 3 (2.6) | 0 | – 0.2304 | 3 (2.6) | 0 | – 0.2345 |
| 4 | 0 | 1 (1.3) | 0.1591 | 0 | 1 (1.3) | 0.1386 |
| 6 | 0 | 1 (1.3) | 0.1591 | 0 | 1 (1.3) | 0.1199 |
| Metastatic disease site | ||||||
| Viscerale | 43 (37.1) | 29 (36.3) | − 0.0170 | 43 (37.1) | 28 (35.0) | − 0.0332 |
| Non-visceral | 42 (36.2) | 17 (21.3) | − 0.3352 | 33 (28.4) | 23 (28.8) | − 0.0093 |
| Bone onlyf | 31 (26.7) | 34 (42.5) | 0.3363 | 39 (33.6) | 29 (36.3) | 0.0424 |
| Number of metastatic sites | ||||||
| 1 | 31 (26.7) | 30 (37.5) | 0.2323 | 36 (31.0) | 27 (33.8) | 0.0668 |
| 2 | 42 (36.2) | 20 (25.0) | − 0.2450 | 34 (29.3) | 24 (30.0) | − 0.0007 |
| ≥ 3 | 43 (37.1) | 30 (37.5) | 0.0089 | 46 (39.7) | 29 (36.3) | − 0.0639 |
| Disease-free interval | ||||||
| < 12 months | 54 (46.6) | 32 (40.0) | − 0.1325 | 48 (41.4) | 33 (41.3) | − 0.0028 |
| ≥ 12 months | 14 (12.1) | 18 (22.5) | 0.2785 | 24 (20.7) | 14 (17.5) | − 0.0818 |
| De novo metastatic | 35 (30.2) | 22 (27.5) | − 0.0590 | 31 (26.7) | 21 (26.3) | 0.0003 |
| Unknown | 13 (11.2) | 8 (10.0) | − 0.0392 | 13 (11.2) | 11 (13.8) | 0.1012 |
AI aromatase inhibitor, BMI body mass index, CCI Charlson Comorbidity Index, ECOG PS Eastern Cooperative Oncology Group performance status, ER estrogen receptor, max maximum, min minimum, nIPTW normalized inverse probability treatment weighting, PR progesterone receptor, SD standard deviation
aAll data are listed as n (%) unless otherwise indicated
bCharacteristics in bold were used for propensity scoring and subsequent nIPTW
cn values apply to each characteristic unless a different n value is provided
dStandardized mean differences <0.1 were considered indicative of acceptable balance
eVisceral includes liver, lung, and pleura
fBone only includes bone, bone marrow, pelvis, ribs, skull, and spine
The primary analysis of an rwRR using the nIPTW method was performed in: (1) all patients (overall analysis set), where patients without tumor assessments on treatment were considered non-responders in the estimation of an rwRR, and (2) patients with at least one documented tumor assessment during first-line therapy. The rwRR adjusted by nIPTW was estimated for each treatment cohort with a corresponding two-sided 95% CI using the Wilson score method. The ORs and 95% CIs for the adjusted rwRR were estimated using the Cochran-Mantel-Haenszel method. All analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
A total of 196 pre/perimenopausal women were included in the study, with 116 patients receiving palbociclib plus an AI and 80 patients receiving AI monotherapy as a first-line therapy. In total, 103 patients in the palbociclib plus AI cohort and 71 patients in the AI monotherapy cohort had one or more tumor assessments on treatment during first-line therapy. Of the total study population, 81.9% and 63.8% of the patients in the palbociclib plus AI cohort and AI monotherapy cohort, respectively, had documentation of receiving an LHRH agonist in their medical record during first-line treatment (documentation for LHRH agonists initiated outside The Network was not accessible). Demographic and clinical characteristics for both cohorts are provided in Tables 1 and 2. In the unadjusted population, median (range) ages among patients with one or more tumor assessments were similar in both groups (46.0 [31.0–58.0] and 46.0 [30.0–60.0] years in the palbociclib plus AI and AI monotherapy cohorts, respectively); approximately two-thirds of participants in both groups were White. In terms of clinical characteristics, approximately one-quarter had stage IV disease at diagnosis (25.2% and 25.4%, respectively), and approximately two-thirds had Eastern Cooperative Oncology Group performance status scores of 0 or 1 (67.0% and 64.8%). In the palbociclib plus AI and AI monotherapy cohorts, respectively, 41.7% and 45.1% of patients received prior neo/adjuvant chemotherapy and 49.5% and 60.6% received prior neo/adjuvant endocrine therapy. Demographic and clinical characteristics were generally similar in the overall analysis set.
In the overall analysis set, letrozole was the most common AI taken by patients receiving palbociclib plus an AI (93/116; 80.2%). Of patients receiving AI monotherapy, similar percentages received letrozole (37/80; 46.3%) or anastrozole (38/80; 47.5%). Median (range) treatment duration was 12.1 (0.3–65.8) and 11.0 (0.5–115.7) months in the palbociclib plus AI and AI monotherapy cohorts, respectively.
As of the data cut-off date (31 December, 2020), 64 patients (55.2%) in the palbociclib plus AI cohort and 67 patients (83.8%) in the AI monotherapy cohort discontinued first-line treatment; 40.5% and 65.0% discontinued because of disease progression, respectively, and 8.6% and 5.0% because of toxicity; 44.0% and 16.3% were ongoing with the first-line treatment.
Normalized inverse probability treatment weighting was able to effectively balance the eight measured confounders, with the exception of a body mass index ≥ 25 kg/m2 in patients with one or more tumor assessments and a body mass index < 25 kg/m2 and unknown disease-free interval in the overall analysis cohort having standardized mean differences slightly higher than the 0.1 cut-off value, which was considered indicative of acceptable balance (Tables 1 and 2). In the sensitivity analysis, after PSM, there were 51 patients with one or more tumor assessments in each treatment cohort and 62 patients in each cohort in the overall analysis set (Electronic Supplementary Material, Online Resources 1 and 2). Although the eight measured confounders were generally balanced between the matched cohorts, applicability of this strategy was limited owing to the overall small sample size and the larger proportion of patients in the palbociclib plus AI cohort.
Real-World Tumor Responses
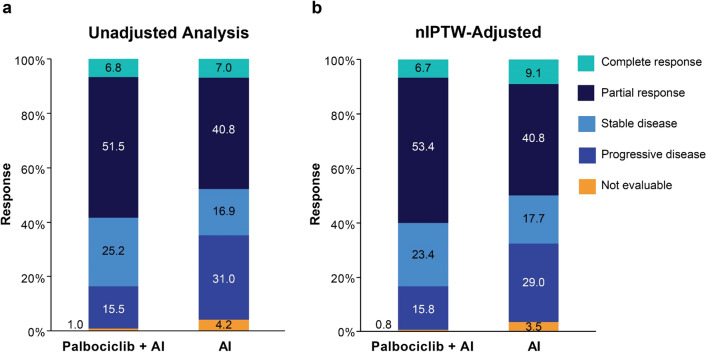
In patients with one or more tumor assessments on treatment, the unadjusted rwRRs were 58.3% for palbociclib plus an AI (n = 103) and 47.9% for AI monotherapy (n = 71; Fig. 1 and Table 3) with an associated OR of 1.52 (95% CI 0.79–2.92). The rwRRs adjusted by nIPTW were 60.0% and 49.9%, respectively (OR, 1.51 [95% CI 0.82–2.77]). In the sensitivity analysis of the matched patients receiving palbociclib plus AI (n = 51) and AI monotherapy (n = 51), rwRRs were 56.9% and 45.1%, respectively (OR, 1.61 [95% CI 0.68–3.78]; Electronic Supplementary Material, Online Resources 3 and 4).
Fig. 1.
Real-world tumor responses among patients with one or more tumor assessments. Percentage of patients with a tumor response in the unadjusted analysis (a) and the analysis adjusted by nIPTW (b). AI aromatase inhibitor, nIPTW normalized inverse probability treatment weighting
Table 3.
Real-world unadjusted and nIPTW-adjusted response rates
| Patients with ≥ 1 tumor assessments | Overall analysis set | |||
|---|---|---|---|---|
| Palbociclib + AI | AI monotherapy | Palbociclib + AI | AI monotherapy | |
| Tumor response unadjusted | ||||
| n | 103 | 71 | 116 | 80 |
| rwRR, CR + PR, n (%) | 60 (58.3) | 34 (47.9) | 60 (51.7) | 34 (42.5) |
| 95% CIa | 48.6, 67.3 | 36.7, 59.3 | 42.7, 60.6 | 32.3, 53.4 |
| Comparison vs AI monotherapy | ||||
| Odds ratiob | 1.518 | – | 1.449 | – |
| 95% CIb | 0.790, 2.918 | – | 0.785, 2.681 | – |
| Two-sided P-valueb | 0.2327 | – | 0.2605 | – |
| Tumor response adjusted by nIPTW | ||||
| n | 103 | 71 | 116 | 80 |
| rwRR, CR + PR, n (%) | 62 (60.0) | 35 (49.9) | 60 (52.1) | 37 (46.2) |
| 95% CIa | 50.4, 69.0 | 38.6, 61.2 | 43.0, 60.9 | 35.7, 57.0 |
| Comparison vs AI monotherapy | ||||
| Odds ratioc | 1.508 | – | 1.266 | – |
| 95% CIc | 0.819, 2.774 | – | 0.715, 2.241 | – |
| Two-sided P-valuec | 0.1874 | – | 0.4186 | – |
AI aromatase inhibitor, CI confidence interval, CMH Cochran-Mantel-Haenszel, CR complete response, nIPTW normalized inverse probability treatment weighting, PR partial response, rwRR real-world response rate
aBased on the Wilson score method
bBased on the CMH method
cBased on the CMH method using weighted rwRR
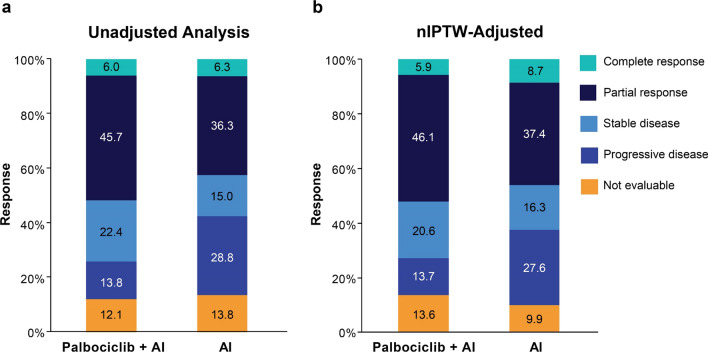
Among all patients, the unadjusted rwRRs were 51.7% for palbociclib plus AI (n = 116) and 42.5% for AI monotherapy (n = 80; Fig. 2 and Table 3); the associated OR comparing palbociclib plus AI to AI monotherapy was 1.45 (95% CI 0.79–2.68). The rwRRs adjusted by nIPTW were 52.1% and 46.2%, respectively (OR, 1.27 [95% CI 0.72–2.24]). A sensitivity analysis using PSM yielded the rwRRs of 48.4% for patients in the palbociclib plus AI cohort (n = 62) and 43.5% for those in the AI monotherapy cohort (n = 62; OR 1.22 [95% CI 0.56–2.62]; Electronic Supplementary Material, Online Resources 3 and 4).
Fig. 2.
Real-world tumor responses among all patients. Percentage of patients with a tumor response in the unadjusted analysis (a) and the analysis adjusted by nIPTW (b). AI aromatase inhibitor, nIPTW normalized inverse probability treatment weighting
An additional sensitivity analysis was conducted to assess the robustness of the primary analysis, removing eight patients from the AI monotherapy cohort who were found to be postmenopausal at treatment initiation after “unblinding” to the outcome data. Exclusion of these patients did not change the study outcomes (patients with one or more tumor assessments, nIPTW-adjusted OR of 1.62 [95% CI 0.86–3.03]; overall analysis set, nIPTW-adjusted OR of 1.35 [95% CI 0.75–2.43]).
Discussion
Evaluation of tumor responses is an important indicator of treatment effectiveness and may allow for objective treatment comparisons in real-world settings without the confounders that may affect time-related endpoints [24]. In real-world studies, such evaluations may provide evidence regarding the effectiveness of a given therapy to supplement clinical trial findings [25]. Electronic health record data from The Network, a large community-based database, were used to provide a descriptive comparison of real-world tumor responses among pre/perimenopausal patients who received palbociclib plus AI or AI monotherapy as a first-line treatment for HR+/HER2− MBC. Results indicated that the percentage of patients receiving palbociclib plus an AI with complete or partial responses was higher than that for patients receiving AI monotherapy, with nIPTW-adjusted rwRRs among patients with one or more tumor assessments of 60.0% and 49.9%, respectively (OR, 1.51 [95% CI 0.82–2.77]).
Real-world data are important for generating evidence regarding drug safety and efficacy under conditions of actual clinical practice and in patients who do not meet inclusion/exclusion criteria for clinical trials [25]. In recognition of the value provided by real-world evidence, the FDA has issued guidance establishing expectations for the quality of real-world data and a priori finalization of statistical analyses before conducting the final outcomes analyses for regulatory purposes, which can provide guidance for all real-world studies being conducted [26, 27]. Analysis of data for patients with MBC has shown that clinically meaningful information can be derived from the assessment of real-world PFS and real-world tumor responses based on EHR data abstraction when proper quality controls and analytic methods are incorporated based on a concordance shown between randomized clinical trials and real-world data. The concordance of real-world and clinical trial tumor responses in the MBC setting is supported by a study observing similar response rates between patients in the placebo plus letrozole arm of the PALOMA-2 study and patients from the US Flatiron Health Analytic Database who received first-line letrozole [21]. The study closely followed new FDA guidance surrounding real-world evidence, including seeking study design feedback, extensive quality control measures, clear documentation of data handling, and maintenance of data integrity [28]. Tumor response outcome data were not transferred to investigators for analysis until the statistical analysis plan using PS methodology to balance baseline characteristics was finalized. Estimation of a PS, generation of nIPTW, and feasibility assessment of the balancing process were therefore not influenced by the outcome data. Good practice and guidelines have underscored the importance of using real-world data subject to rigorous standards to maintain credibility [29, 30].
The current findings support clinical trial evidence regarding treatment with a CDK4/6 inhibitor in combination with an AI for pre/perimenopausal women with HR+/HER2− MBC. Notably, the FDA recently (13 December, 2022) expanded the palbociclib label to include pre/perimenopausal women in the indication of palbociclib plus an AI as an initial endocrine-based therapy for patients with HR+/HER2− ABC or MBC [6]. In PALOMA-2, which was limited to postmenopausal women (including those considered postmenopausal because of a prior bilateral oophorectomy), the confirmed objective response rate among patients receiving palbociclib plus letrozole was 42.1% compared with 34.7% in the placebo plus letrozole group; when limited to patients with measurable disease according to Response Evaluation Criteria in Solid Tumors, rates were 55.3% and 44.4%, respectively [9]. Subgroup analyses from PALOMA-2 compared outcomes between the palbociclib plus letrozole cohort and the placebo plus letrozole cohort in the intent-to-treat population (n = 444 vs n = 222, respectively), patients without a prior oophorectomy (n = 379 vs n = 182), and patients with a prior oophorectomy (n = 65 vs n = 40; Pfizer, data on file). In this analysis, median PFS was consistently prolonged in the palbociclib plus letrozole cohort versus the placebo plus letrozole cohort in the intent-to-treat population (24.8 vs 14.5 months, respectively; hazard ratio, 0.58 [95% CI 0.46–0.72]), patients without a prior oophorectomy (24.9 vs 15.9 months; hazard ratio, 0.60 [95% CI 0.47–0.77]), and patients with a prior oophorectomy (23.9 vs 13.8 months; hazard ratio, 0.49 [95% CI 0.28–0.86]). The objective response rate (including confirmed and unconfirmed responses) was higher in the palbociclib plus letrozole cohort compared with the placebo plus letrozole cohort in the intent-to-treat population (46.4% vs 38.3%, respectively) and in patients without a prior oophorectomy (42.7% vs 33.5%), whereas patients with a prior oophorectomy demonstrated similar response rates between the palbociclib and letrozole cohort (38.5%) and the placebo and letrozole cohort (40.0%). The subgroup of patients with a prior oophorectomy was small in this analysis, limiting interpretation. Similar CDK4/6 inhibitor class effects were observed in the large, multicenter, MONALEESA-7 phase III trial, which evaluated ribociclib or placebo in combination with goserelin and endocrine therapy (AI or tamoxifen) in pre/perimenopausal women with HR+/HER2− ABC or MBC [31, 32]. The ribociclib group (n = 335) compared with the placebo group (n = 337) had a significantly greater overall tumor response rate (41% versus 30%; P < 0.001), and median PFS (23.8 vs 13.0 months; hazard ratio, 0.55 [95% CI 0.44–0.69]; P < 0.0001) [31]. In the protocol-specified final OS analysis, median OS was not estimable in the ribociclib group and 40.9 months in the placebo group (hazard ratio, 0.71 [95% CI 0.54–0.95]; P = 0.00973) [32]. Premenopausal women evaluated in the Young-PEARL study showed a significantly longer median PFS in the palbociclib arm than in the capecitabine arm (20.1 vs 14.4 months; hazard ratio, 0.66 [95% CI 0.44–0.99]; P = 0.02); however, tumor response rates among the two arms were similar (37% vs 34%) in this population, in which approximately 50% of patients had received prior therapy for MBC [16]. The FDA pooled analysis of four phase III trials of patients with HR+/HER2− ABC or MBC receiving an AI in combination with a CDK4/6 inhibitor (mostly in the first line) or placebo demonstrated that median PFS associated with a CDK4/6 inhibitor plus an AI was significantly longer compared with an AI plus placebo in female patients ≤ 40 years of age (n = 193; 19.8 months vs 11.2 months; hazard ratio, 0.50 [95% CI 0.34–0.74]) [33], with age used as a surrogate for pre/perimenopausal status. Taken together, this evidence across clinical trials is supportive of a clinical benefit of a CDK4/6 inhibitor plus an AI in pre/perimenopausal women with HR+/HER2− MBC who have achieved ovarian suppression via surgical or chemical means, such as an oophorectomy or the use of an LHRH agonist.
Additional real-world studies support improved survival and/or response rates for patients with HR+/HER2− MBC treated with palbociclib plus AI versus AI monotherapy. A study of pre/perimenopausal women with HR+/HER2− MBC retrospectively analyzed data abstracted from medical records by US oncologists from a nationwide physician network [34]. Patients receiving a CDK4/6 inhibitor regimen (CDK4/6 inhibitor monotherapy, n = 3; CDK4/6 inhibitor plus endocrine therapy plus chemotherapy, n = 11; and CDK4/6 inhibitor plus endocrine therapy, n = 169) had unadjusted complete response, partial response, and stable disease rates of 24.0%, 66.7%, and 7.7%, respectively. The recent study using the US Flatiron Health Analytic Database found that the adjusted real-world PFS was 20.0 months among patients with HR+/HER2− MBC, regardless of menopausal status, who received palbociclib plus letrozole (n = 839) compared with 11.9 months for letrozole monotherapy (n = 698; hazard ratio, 0.58 [95% CI 0.49–0.69]; P < 0.0001); adjusted OS was also significantly longer among patients who received palbociclib plus letrozole (not reached vs 43.1 months; hazard ratio, 0.66 [95% CI 0.53–0.82]; P < 0.001) [17]. In the small subgroup of patients aged 18–50 years (n = 104; age used as a surrogate for pre/perimenopausal status), patients who received palbociclib plus letrozole also had longer real-world PFS (hazard ratio, 0.82 [95% CI 0.48–1.39]), as well as longer OS (hazard ratio, 0.71 [95% CI 0.27–1.89]) than those who received letrozole monotherapy. Another analysis from the US Flatiron Health Analytic Database supported these findings and demonstrated a superior, adjusted, real-world best tumor response (i.e., complete response or partial response) among women (aged ≥ 18 years) who received palbociclib plus letrozole versus letrozole monotherapy (58.6% vs 39.1%; P < 0.0001; n = 215 per group) [35]. Although not comparative, an analysis conducted in the large non-interventional, prospective, multicenter, real-world POLARIS study found that pre/perimenopausal women with HR+/HER2− ABC or MBC treated with first-line palbociclib plus AI/fulvestrant/other (n = 110) had a rwRR of 31.8% and a median PFS of 20.3 months (Pfizer, data on file).
The efficacy of palbociclib plus fulvestrant is well established in patients with HR+/HER2− MBC whose disease had progressed following endocrine therapy, including in premenopausal women receiving goserelin to suppress estrogen activity [6, 15, 36]. In PALOMA-3, a study in which patients had to have progressed on at least one line of endocrine therapy either in the adjuvant or advanced setting, the objective response rate observed in premenopausal women was 25.0% for patients treated with palbociclib plus fulvestrant plus goserelin versus 11.1% for patients treated with placebo plus fulvestrant plus goserelin, and the median PFS was 9.5 versus 5.6 months, respectively (hazard ratio, 0.50 [95% CI 0.29–0.87]) [36]. A later data cut found that median PFS in this subpopulation of premenopausal women was 11.3 and 5.6 months, respectively [15]. Considering that palbociclib acts downstream of the estrogen receptor, which is effectively inhibited by both fulvestrant and AI, the efficacy of palbociclib plus fulvestrant in premenopausal women can be extrapolated to further support the use of palbociclib plus AI in premenopausal women [37], in addition to the clinical and real-world evidence discussed above.
In this study, pre/perimenopausal women were identified who initiated palbociclib plus an AI as first-line treatment for HR+/HER2− MBC, with evidence of rwRR consistent with randomized clinical trials and other real-world evidence. In the USA, National Comprehensive Cancer Network Guidelines® recommend use of a CDK4/6 inhibitor (palbociclib, ribociclib, or abemaciclib) in combination with an AI or fulvestrant as a first-line therapy for women with recurrent unresectable or stage IV HR+/HER2− disease who are postmenopausal or premenopausal undergoing ovarian ablation or suppression [38]. While ribociclib in combination with an AI has a category 1 recommendation as a first-line therapy in this patient population, no head-to-head studies comparing agents are available and there are some differences between the phase III study patient populations and palbociclib remains a preferred first-line treatment. In the European Union, palbociclib plus an AI is approved for treatment of HR+/HER2− ABC or MBC in pre/perimenopausal women in combination with an LHRH agonist based on extrapolation of efficacy data from PALOMA-2 and PALOMA-3, as well as a pharmacologic justification for use of palbociclib as an add-on to AI plus an LHRH agonist based on the downstream activity of palbociclib relative to that of the estrogen receptor, which is effectively inhibited by AI or fulvestrant (plus an LHRH agonist) [37, 39]. Findings from this study support the use of palbociclib combination therapy in pre/perimenopausal women with HR+/HER2− MBC.
Study strengths include the approach taken to ensure data quality and integrity, including the use of a standardized EHR, a chart review process to verify key data elements, 100% data source verification of tumor assessment given it was the primary endpoint, implementation of an analytic quality control process, and the approach of performing a PS-based balancing analysis before accessing tumor response outcome data. Because of the non-randomized nature of the study, statistical approaches were used to balance observed potential confounders and were able to create more homogeneous and comparable treatment cohorts, mimicking a randomized study. The Network also reflects a diverse and broad set of patients across the USA in a community network setting.
Limitations include the reliance of tumor response assessments on the treating clinician’s assessment in routine practice (rather than a standardized objective method such as Response Evaluation Criteria in Solid Tumors on a prespecified tumor assessment schedule), the lack of a blinded independent radiology review in routine clinical practice and the vulnerability of the iKM database to potential measurement errors, misclassifications, and missing or unavailable data, which is a challenge for real-world datasets. For example, documentation of the use of LHRH agonists in premenopausal women was lacking in this study. Only 81.9% of women in the palbociclib plus AI cohort and 63.8% in the AI cohort had a documented history of receiving LHRH agonists, even though its use in pre/perimenopausal women is essential when receiving an AI. This discrepancy is likely due to poor documentation in the EHR and women receiving LHRH agonists in other health networks. Nevertheless, women were designated as premenopausal based on other explicit EHR documentation and not only on their use of LHRH agonists. Quality control assessments and checks were performed to minimize this risk. However, as demonstrated by a retrospective identification of eight postmenopausal patients after the final data transfer, this strategy was not completely fail-safe. The varying time periods in which cohorts had initiated treatment are a limitation; palbociclib initiation started in 2015 when it was approved, whereas the monotherapy cohort could date back to 2010. Given the limited number of control patients available in the contemporaneous time period, obtaining historical control patients was warranted. Given the difference that would arise in a follow-up, a tumor response endpoint allowed for a more objective comparison of treatment effectiveness as a marker of treatment activity and avoided confounders that may affect a time-to-event endpoint [24], such as PFS or OS. Interestingly, obtaining historical control patients may have led to the similar duration of treatment observed between the two treatment arms, as patients treated with AI monotherapy would have had an earlier index date starting from 2010 than patients who would have started treatment with palbociclib only after its FDA approval in 2015. The non-randomized nature of the study may have enabled selection biases influencing the choice of treatment for individual patients that may not be fully addressed by the PS-based methodology. However, the use of nIPTW was demonstrably successful in balancing select baseline demographic and clinical factors while retaining all patients in the original unweighted population, although this does not eliminate the risk of unmeasured confounders. In addition, the PSM sensitivity analysis was limited given the small sample size after matching, and the larger proportion of patients in the palbociclib plus AI cohort. Finally, the generalizability of study findings may be limited to other US community oncology practices; however, the iKM EHR database covers more than 500 sites of care across the USA.
Conclusions
This retrospective observational study of pre/perimenopausal women with HR+/HER2− MBC demonstrated that rwRRs associated with palbociclib plus an AI used as a first-line therapy were numerically higher than those associated with AI monotherapy. A limited sample size could have impacted the ability to detect a significant difference; however, no formal hypothesis testing was performed. These real-world findings may supplement existing data suggesting a therapeutic benefit of palbociclib plus an AI in this population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Editorial/medical writing support was provided by Judith Kandel, PhD, of ICON plc (Blue Bell, PA, USA) and Diana Avery, PhD, of Oxford PharmaGenesis Inc. (Newtown, PA, USA), and was funded by Pfizer Inc.
Declarations
Funding
This study was sponsored by Pfizer Inc.
Conflicts of Interest/Competing Interests
Angela DeMichele reports institutional research support from Calithera, Genentech, Novartis, Pfizer, and Inivata; research writing support from Pfizer; and that her spouse serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to cancer. Nicholas Robert, Kathleen M. Aguilar, and Yunfei Wang are employees of and hold stock/stock options in Ontada, a brand within McKesson, and have performed contracted research for Pfizer. Connie Chen, Sindy Kim, Zhe Zhang, Dongrui Ray Lu, Benjamin Li, Michael Gaffney, and Lynn McRoy are employees of and hold stock/stock options in Pfizer. Sebastian Schneeweiss reports employment with Brigham and Women’s Hospital; participating in investigator-initiated grants to his institution from Boehringer Ingelheim, Takeda, and UCB Pharma on topics unrelated to this study; consulting fees and equity from Aetion; and an advisory role for Temedica GmbH. Jeremy A. Rassen has an ownership interest in and is an employee of Aetion.
Ethics Approval
The study was submitted to the US Oncology Institutional Review Board, but a full evaluation was deemed not required as it was a secondary data collection study.
Consent to Participate
This study was granted an exception and waiver of informed consent from the US Oncology Institutional Review Board, and a deidentified data set was used for analysis.
Consent for Publication
Not applicable.
Availability of Data and Material
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Code Availability
Not applicable.
Authors’ Contributions
Study conceptualization: AD, NR, CC, KMA, SS, JAR, and LM Methodology (design or development): AD, NR, CC, SK, ZZ, DRL, KMA, BL, SS, JAR, MG, and LM Data analysis: CC, SK, ZZ, DRL, BL, MG, and LM Validation (verification of results/data): YW Investigation (conducting research/experiments): CC, ZZ, DRL, BL, MG, and LM Resources (provision of study materials, samples, analysis tools): NR, CC, ZZ, DRL, KMA, BL, MG, and LM Data curation (data management/organization): NR, KMA, and YW Supervision (oversight for research planning and execution): NR, CC, ZZ, DRL, KMA, BL, MG, and LM Writing (original draft preparation): AD, CC, SK, SS, JAR, and LM Writing (review and editing): all authors.
Contributor Information
Angela DeMichele, Email: angela.demichele@uphs.upenn.edu.
Nicholas Robert, Email: nicholas.robertmd@mckesson.com.
Connie Chen, Email: connie.chen@pfizer.com.
Sindy Kim, Email: sindy.kim@pfizer.com.
Zhe Zhang, Email: zhe.zhang@pfizer.com.
Dongrui Ray Lu, Email: dongrui.lu@pfizer.com.
Kathleen M. Aguilar, Email: kat.aguilar@mckesson.com
Yunfei Wang, Email: yunfei.wang@mckesson.com.
Benjamin Li, Email: benjamin.li@pfizer.com.
Sebastian Schneeweiss, Email: schneeweiss@aetion.com.
Jeremy A. Rassen, Email: jeremy.rassen@aetion.com
Michael Gaffney, Email: michael.gaffney@pfizer.com.
Lynn McRoy, Email: lynn.mcroy@pfizer.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 4.Shah AN, Metzger O, Bartlett CH, Liu Y, Huang X, Cristofanilli M. Hormone receptor-positive/human epidermal growth receptor 2-negative metastatic breast cancer in young women: emerging data in the era of molecularly targeted agents. Oncologist. 2020;25(6):e900–e908. doi: 10.1634/theoncologist.2019-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas A, Rhoads A, Pinkerton E, Schroeder MC, Conway KM, Hundley WG, et al. Incidence and survival among young women with stage I-III breast cancer: SEER 2000–2015. JNCI Cancer Spectr. 2019;3(3):pkz040. doi: 10.1093/jncics/pkz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Palbociclib (IBRANCE): highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212436s003lbl.pdf. Accessed 2 Mar 2023.
- 7.Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res. 2015;21(21):4760–4766. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 10.Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Palbociclib (IBRANCE). https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance. Accessed 7 Nov 2022.
- 12.Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 13.Walker AJ, Wedam S, Amiri-Kordestani L, Bloomquist E, Tang S, Sridhara R, et al. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res. 2016;22(20):4968–4972. doi: 10.1158/1078-0432.CCR-16-0493. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 15.Rugo HS, Cristofanilli M, Loibl S, Harbeck N, DeMichele A, Iwata H, et al. Prognostic factors for overall survival in patients with hormone receptor-positive advanced breast cancer: analyses from PALOMA-3. Oncologist. 2021;26(8):e1339–e1346. doi: 10.1002/onco.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YH, Kim TY, Kim GM, Kang SY, Park IH, Kim JH, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20(12):1750–1759. doi: 10.1016/S1470-2045(19)30565-0. [DOI] [PubMed] [Google Scholar]
- 17.DeMichele A, Cristofanilli M, Brufsky A, Liu X, Mardekian J, McRoy L, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021;23(1):37. doi: 10.1186/s13058-021-01409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The US Oncology Network. Empowering local cancer care. https://usoncology.com/our-company/. Accessed 4 Mar 2022.
- 19.Patt DA, Jiao X, Fonseca E, Clark J, Fox PS, Horblyuk R, et al. Real-world use of first-line chemotherapy in post-menopausal patients with HR-positive HER2-negative metastatic breast cancer (mBC) in a US community oncology network. J Clin Oncol. 2016;34(15_Suppl):561. doi: 10.1200/JCO.2016.34.15_suppl.561. [DOI] [Google Scholar]
- 20.Robert NJ, Goertz HP, Chopra P, Jiao X, Yoo B, Patt D, et al. HER2-positive metastatic breast cancer patients receiving pertuzumab in a community oncology practice setting: treatment patterns and outcomes. Drugs Real World Outcomes. 2017;4(1):1–7. doi: 10.1007/s40801-016-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Bartlett C, Mardekian J, Cotter MJ, Huang X, Zhang Z, Parrinello CM, et al. Concordance of real-world versus conventional progression-free survival from a phase 3 trial of endocrine therapy as first-line treatment for metastatic breast cancer. PLoS ONE. 2020;15(4):e0227256. doi: 10.1371/journal.pone.0227256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50(Suppl 1):1S–10S. doi: 10.2967/jnumed.108.057174. [DOI] [PubMed] [Google Scholar]
- 25.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence: what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration . Real-world data: assessing electronic health records and medical claims data to support regulatory decision-making for drug and biological products. Silver Spring: US Food and Drug Administration; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration . Considerations for the use of real-world data and real-world evidence to support regulatory decision-making for drug and biological products. Silver Spring: US Food and Drug Administration; 2021. [Google Scholar]
- 28.US Food and Drug Administration . Framework for FDA’s real-world evidence program. Silver Spring: US Food and Drug Administration; 2018. [Google Scholar]
- 29.Concato J, Corrigan-Curay J. Real-world evidence: where are we now? N Engl J Med. 2022;386(18):1680–1682. doi: 10.1056/NEJMp2200089. [DOI] [PubMed] [Google Scholar]
- 30.Dodwell D, Shakir R. Assessing new drugs in advanced cancer: beyond randomised evidence. Clin Oncol (R Coll Radiol) 2021;33(4):e201–e202. doi: 10.1016/j.clon.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 32.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 33.Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250–260. doi: 10.1016/S1470-2045(19)30804-6. [DOI] [PubMed] [Google Scholar]
- 34.Dalal AA, Goldschmidt D, Romdhani H, Guerin A, Wang H, Costa O, et al. Treatment duration and response among pre/perimenopausal women with HR+/HER2- metastatic breast cancer: a chart review study in the US. In: Poster Presented at Miami Breast Cancer Conference 36th Annual Meeting; 7-10 March 2019; Miami (FL).
- 35.Brufsky A, Liu X, Li B, McRoy L, Layman RM. Real-world tumor response of palbociclib plus letrozole versus letrozole for metastatic breast cancer in US clinical practice. Target Oncol. 2021;16(5):601–611. doi: 10.1007/s11523-021-00826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loibl S, Turner NC, Ro J, Cristofanilli M, Iwata H, Im SA, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22(9):1028–1038. doi: 10.1634/theoncologist.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Medicines Agency. Assessment report: IBRANCE. Available from: https://www.ema.europa.eu/en/documents/assessment-report/ibrance-epar-public-assessment-report_en.pdf. Accessed 10 Mar 2022.
- 38.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed April 4, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 39.European Medicines Agency. Summary of product characteristics: IBRANCE. Available from: https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf. Accessed 22 Mar 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.