Abstract
Background
Walking entails orchestration of the sensory, motor, balance, and coordination systems, and walking disability is a critical concern after stroke. How and to what extent these systems influence walking disability after stroke and recovery have not been comprehensively studied.
Methods
We retrospectively analyzed patients with stroke in the Post-acute care-Cerebrovascular Diseases (PAC-CVD) program. We compared the characteristics of patient groups stratified by their ability to complete the 5-m walk test across various time points of rehabilitation. We then used stepwise linear regression to examine the degree to which each stroke characteristic and functional ability could predict patient gait performance.
Results
Five hundred seventy-three patients were recruited, and their recovery of walking ability was defined by the timing of recovery in a 5-m walk test. The proportion of patients who could complete the 5-m walk test at admission, at 3 weeks of rehabilitation, at 6 weeks of rehabilitation, between 7 and 12 weeks of rehabilitation, and who could not complete the 5-m walk test after rehabilitation was 52.2%, 21.8%, 8.7%, 8.7%, and 8.6%, respectively. At postacute care discharge, patients who regained walking ability earlier had a higher chance of achieving higher levels of walking activity. Stepwise linear regression showed that Berg Balance Scale (BBS) (β: 0.011, p < .001), age (β: −0.005, p = .001), National Institutes of Health Stroke Scale (NIHSS) (6a + 6b; β: −0.042, p = .018), Mini-Nutritional assessment (MNA) (β: −0.007, p < .027), and Fugl–Meyer upper extremity assessment (FuglUE) (β: 0.002, p = .047) scores predicted patient's gait speed at discharge.
Conclusion
Balance, age, leg strength, nutritional status, and upper limb function before postacute care rehabilitation are predictors of walking performance after stroke.
Keywords: Postacute care, Stroke rehabilitation, Walking ability, Gait speed, Functional recovery
At a glance commentary
Scientific background on the subject
Patients who regained walking ability earlier had a higher chance of achieving higher levels of walking activity. Patient’s age and scores on Berg Balance Scale, the leg strength subscales of National Institutes of Health Stroke Scale, Mini-Nutritional assessment, and Fugl–Meyer upper extremity assessment predicted patient’s gait speed at discharge.
What this study adds to the field
The present study provided insights into the recovery of poststroke walking performance through high-intensity rehabilitation. We found that upper limb function is also a predictor of walking performance after stroke. Accurate prediction of patient ability to recover from walking disability can help tailor rehabilitation programs and discharge plans.
Walking disability is a major concern among patients undergoing poststroke rehabilitation because the inability to walk considerably compromises their functionality and quality of life [1]. Numerous factors, including ataxia [2,3], leg strength [4], sensory deficits [5,6], spasticity, and distal leg strength [7], and cognitive impairment [8], may contribute to walking disability. Because regaining walking ability is a major goal of poststroke rehabilitation [9], assessing how these factors contribute to walking disability is crucial for establishing a tailored rehabilitation strategy to facilitate recovery.
Hornby et al. [10] investigated the effects of training intensity on locomotion outcomes by pooling data from 3 randomized controlled trials. Among the 144 patients with subacute or chronic stroke, those who received high-intensity training and completed a high dose of training had the highest gains in locomotion. Hirano et al. [11] investigated the factors predicting independent walking at discharge from a rehabilitation program in 72 severely hemiplegic patients with stroke and developed a prediction formula based on age and knee extensor strength to body weight ratio with an accuracy of 91%. Smith et al. [12] analyzed the rehabilitation data of 41 patients and developed an algorithm to predict patients' ability to walk independently at 6 or 12 weeks after a stroke. The algorithm was based on the patients’ trunk control test and leg strength assessed 1 week after stroke and achieved an accuracy of 95%. Kollen et al. [13] investigated the factors that contribute to the improvement in walking ability by using sequential assessments for up to 1 year in 101 patients with stroke and found that standing balance control was a more accurate predictor of improvement in walking ability than leg strength or synergism. Park et al. [8] investigated the accuracy of poststroke cognitive function at 1 month in predicting walking ability at 6 months in 72 patients with stroke and found that executive function, memory, and visuospatial deficits were predictors of walking ability. In summary, training intensity, actual dose of practice, balance, leg strength, and cognitive function are predictors of poststroke walking ability.
The aforementioned studies, however, were confounded by predefined baseline characteristics (eg, patients were already able to walk at baseline [10] or had better insurance coverage for rehabilitation) [14], small sample size [8,[10], [11], [12], [13]], limited functional outcome assessment (eg, only cognition [8], motor, or balance domains) [8,[10], [11], [12], [13]], limited time points of assessments [8,10,11], or the use of only dichotomous outcomes [10,11]. Additional studies are therefore needed to address the recovery from walking disability in patients with stroke with various neurologic and functional impairments.
The Post-acute Care-Cerebrovascular Diseases (PAC-CVD) program [[15], [16], [17]] provides a unique opportunity to investigate this problem. Eligible patients enrolled in this program underwent high-intensity rehabilitation for 3 h/day on every weekday and 1 h/day on Saturday and comprehensive functional evaluation at regular time intervals up to 12 weeks. The rehabilitation program comprised physical therapy (PT), occupational therapy (OT), and speech and swallowing therapy (ST), and the functional assessments evaluated the performance in activities of daily living, quality of life, nutritional status, function of paretic limbs, gait speed and endurance, balance, and cognitive function.
We hypothesized that besides known factors such as age, balance, leg strength, and cognition, other factors can predict poststroke walking performance. In this study, we examined whether patients’ demographics, comorbidities, stroke lesion side and location, limb ataxia, aphasia, leg strength, stroke onset to post-acute care (PAC) rehabilitation interval, activities of daily living, upper limb synergism and function, nutritional status, somatosensation, balance, and cognitive function could predict the poststroke gait performance. Accurate prediction of patient ability to recover from walking disability after a stroke can help tailor rehabilitation programs and discharge plans.
Materials and methods
Postacute care stroke rehabilitation program
This retrospective study analyzed the patients with acute stroke enrolled from March 2014 to December 2019 in the PAC-CVD program in Linkou Chang Gung Memorial Hospital. All patients received PAC provided by a multidisciplinary team. The details of this program have been described previously [15]. The enrollment criteria were as follows: (1) stroke onset time within 1 month, (2) stable hemodynamic parameters within 72 h, (3) no neurological deterioration within 72 h, and (4) sufficient cognitive function and ability to learn rehabilitation skills plus a modified Rankin Scale (mRS) score between 2 and 4 (between 3 and 4 since July 2017 due to the change in policy).
For each patient, our PAC program provides 3 h/day of rehabilitation on weekday and 1 h/day on Saturday for up to 12 weeks. Our PAC program provides no rehabilitation on Sunday and national holidays. For the 3 h/day of rehabilitation on weekday, the patient will have a combination of 1-h PT, 1-h OT, and 1-h ST if a patient needs ST. If a patient does not need ST, the patient will receive “2-h PT and 1-h OT” or “1-h PT and 2-h OT” according to the patient's rehabilitation needs. On Saturday, either 1 h of PT or OT is provided. The PT includes balance, gait, and robotic-assisted training; the OT includes posture training, transfers, activities of daily living, cognitive function, and constraint-induced movement therapy. Functional assessments were performed at admission, then every 3 weeks, and at the end of the program or at discharge. The closing regulation of the program was primarily based on the potential of further anticipated improvement and the necessity of in-hospital rehabilitation.
Data collection
Ethics
The study protocol conformed to the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital (IRB no 201900589B0A3).
Patient characteristics
The demographics (age and sex), stroke severity measured at the acute stage by using the National Institutes of Health Stroke Scale (NIHSS) [18], stroke lesion side (left, right, or bilateral) and location (supratentorial or infratentorial), functional assessments in the rehabilitation ward, stroke onset to PAC interval, and length of stays in rehabilitation wards were recorded.
National institutes of health stroke scale
The NIHSS is a 15-item scale that is used to record neurologic findings in acute stroke. It measures deficits affecting level of consciousness, visual field loss, extraocular movement, motor strength, ataxia, sensory loss, dysarthria, language, and neglect. The scores range from 0 (no deficit) to 42 (maximal deficits) [18].
Functional assessments
The functional assessments in this study included the mRS, Barthel Index (BI) [19], Berg Balance Scale (BBS) [20], Fugl–Meyer upper extremity assessment (FuglUE), modified Fugl–Meyer sensory assessment (FuglSEN) [21], Mini-Nutritional assessment (MNA) [22], Mini-Mental State Examination (MMSE) [23], and gait speed assessment by using the 5-m walk test [24].
Barthel index and modified Rankin scale
The BI assesses functional independence and mobility of a patient while performing 10 basic daily activities. The possible scores range from 0 (total dependency) to 100 (total independency). The mRS measures the global disability of a patient, with ordinal scores ranging from 0 (no symptom) to 6 (death).
Fugl–Meyer upper extremity assessment
The FuglUE assesses the synergism and function of the paretic upper limb, including the movement, coordination, and reflex action of the shoulder, elbow, forearm, wrist, and hand of the paretic upper limb, on a 3-point scale from 0 (cannot perform) to 2 (performs fully). The maximum score is 66.
Modified Fugl–Meyer sensory assessment
The modified FuglSEN assessment measures 4 sensory modalities, namely light touch, temperature, tactile localization, and joint position sensation, at various body parts on a 3-point scale from 0 (absent sensation) to 2 (normal). The maximum score is 44.
Mini-mental state examination
The MMSE is a widely used test of cognitive function and includes tests of orientation, attention, memory, language, and visuospatial skills. The maximum score is 30.
Berg balance scale
The BBS measures a patient's ability to maintain static and dynamic balance while performing 14 predetermined tasks. Task performance is ordinally scored from 0 to 4, with higher scores indicating better performance. The maximum score is 56.
Mini-nutritional assessment
The MNA [25] is composed of simple measurements and brief questions, including anthropometric measurements, global assessment (six questions related to lifestyle, medication, and mobility), dietary questionnaire (eight questions, related to number of meals, food and fluid intake, and autonomy of feeding), and subjective assessment (self-perception of health and nutrition). The maximum score is 30. The score distinguishes patients with 1. Adequate nutritional status, MNA ≥24; 2. Protein-calorie malnutrition, MNA <17; 3. At risk of malnutrition, MNA between 17 and 23.
Five-meter walk test (gait speed test)
A timed 5-m walk with nontimed acceleration and deceleration phases on a walkway was used to calculate gait speed. In this test, patients were asked to walk at a comfortable pace and were allowed to use a walking aid if needed.
Stratification of patients by walking ability
Patients were stratified by their ability to complete the 5-m walk test across different time points of rehabilitation: Group 1: at admission; Group 2: at 3 weeks; Group 3: at 6 weeks; Group 4: between 7 and 12 weeks; and Group 5: unable to complete the test at the end of PAC.
Stratification of walking activity by gait speed at PAC discharge
We adopted the criterion published in a recent study [9], which indicated that the walking activity of patients with stroke could be predicted by their comfortable gait speed, to stratify the level of walking activity of our patients at discharge. Cutoff gait speeds of 0.93 and 0.49 m/s were used.
Statistics
The differences in scores and ratios between walking categories were tested using the Kruskal–Wallis test or χ2 test. A stepwise linear regression model was applied to predict walking ability. Gait speed at discharge was the dependent variable in the linear regression model, and the independent variables included patient demographics (age and sex), comorbidities (diabetes mellitus, hypertension, dyslipidemia, coronary artery disease, atrial fibrillation, and previous stroke), stroke location (supratentorial or infratentorial), lesion side of stroke (left, right, or bilateral), NIHSS ataxia (NIHSS 7), lower limbs (NIHSS 6a+6b), and aphasia (NIHSS 1b+1c+9) subscores, stroke onset to PAC interval, performance in activities of daily living (mRS and BI), BBS, Fugl–Meyer assessments (FuglUE and FuglSEN), and MNA. We did not put MMSE into the final model since it was no longer performed since Jan 2018 and (the MMSE scores were missing in 35% of cases) and was not a predictor of gait speed when it was put into the regression model. Collinearity diagnostics monitored with a variance inflation factor <3 were considered acceptable. A p value of < 0.05 was considered significant.
Results
A total of 573 patients (380 men and 193 women) with a mean age of 64.6 ± 12.6 years were included in this study. The clinical profiles of the patients are shown in Table 1.
Table 1.
Characteristics of patient groups stratified by walking ability.
| All | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | p value | |
|---|---|---|---|---|---|---|---|
| Patient number† | 573 | 299 (52.2) | 125 (21.8) | 50 (8.7) | 50 (8.7) | 49 (8.6) | |
| Age (year)‡ | 64.58 (12.6) | 62.3 (12.8) | 66.6 (11.9) | 66 (11.5) | 66.3 (13) | 68 (10.5) | <0.01 |
| Male | 380 (66.3) | 199 (66.6) | 82 (65.6) | 34 (68) | 32 (64) | 33 (67.3) | 1 |
| Female | 193 (33.7) | 100 (33.4) | 43 (34.4) | 16 (32) | 18 (36) | 16 (32.7) | 1 |
| Comorbidity | |||||||
| Previous stroke | 121 (21.1) | 54 (18.1) | 29 (23.2) | 8 (16) | 13 (26) | 17 (34.7) | 0.06 |
| Atrial fibrillation | 53 (9.2) | 26 (8.7) | 10 (8) | 8 (16) | 3 (6) | 6 (12.2) | 0.38 |
| CAD | 49 (8.6) | 25 (8.4) | 10 (8) | 5 (10) | 2 (4) | 7 (14.3) | 0.47 |
| Hypertension | 443 (52.9) | 222 (74.2) | 106 (84.8) | 37 (74) | 40 (80) | 38 (77.6) | 0.19 |
| Dyslipidemia | 270 (47.1) | 156 (52.2) | 48 (38.4) | 25 (50) | 18 (38) | 23 (46.9) | 0.05 |
| Diabetes mellitus | 219 (38.2) | 102 (34.1) | 53 (42.4) | 24 (48) | 21 (42) | 19 (38.8) | 0.25 |
| Stroke etiology | 0.34 | ||||||
| Hemorrhagic stroke | 84 (14.7) | 42 (14) | 16 (12.8) | 11 (22) | 10 (20) | 5 (10.2) | |
| Ischemic stroke | 489 (85.3) | 257 (86) | 109 (87.2) | 39 (78) | 40 (80) | 44 (89.8) | |
| Side of stroke | 0.54 | ||||||
| Left side | 261 (45.5) | 156 (52.2) | 57 (45.6) | 22 (44) | 27 (54) | 22 (44.9) | |
| Right side | 284 (49.6) | 132 (44.1) | 59 (47.2) | 24 (48) | 20 (40) | 26 (53.1) | |
| Bilateral | 28 (4.9) | 11 (3.7) | 9 (7.2) | 4 (8) | 3 (6) | 1 (2) | |
| Stroke location | 0.41 | ||||||
| Supratentorial | 416 (72.6) | 226 (75.6) | 88 (70.4) | 35 (70) | 37 (74) | 31 (63.3) | |
| Infratentorial | 156 (27.2) | 73 (24.4) | 37 (29.6) | 15 (30) | 13 (26) | 18 (36.7) | |
| Acute hospital | |||||||
| Initial NIHSS | 7.1 (4.4) | 6.4 (4.4) | 7.2 (4.2) | 8.1 (5) | 8.9 (4.1) | 8.3 (4.3) | <0.01 |
| Discharge NIHSS | 5.5 (3.1) | 4.5 (2.5) | 5.6 (2.9) | 6.4 (3.2) | 8 (4.1) | 7.2 (3.3) | <0.01 |
| Leg strengtha (NIHSS 6a+6b) | 1.2 (0.9) | 0.8 (0.6) | 1.4 (0.9) | 1.6 (1.1) | 1.8 (1.1) | 1.8 (1.1) | <0.01 |
| Ataxia (NIHSS 7)a | 0.5 (0.7) | 0.5 (0.7) | 0.5 (0.8) | 0.6 (0.8) | 0.3 (0.7) | 0.6 (0.8) | 0.37 |
| Aphasia (NIHSS 1b+1c+9) | 0.4 (1.1) | 0.4 (1.2) | 0.3 (1) | 0.4 (1.1) | 0.7 (1.5) | 0.3 (0.7) | 0.15 |
| Stroke onset to PAC interval (day) | 13.2 (5.3) | 12.7 (5.4) | 13.5 (5.3) | 14 (5.2) | 13.9 (4.8) | 14 (5.5) | 0.09 |
| Length of stays in PAC hospital (day) | 49 (8.6) | 46 (22.8) | 62.2 (21.4) | 64.2 (21.3) | 57.9 (24.7) | 46.9 (24) | <0.01 |
Data are expressed as †number (%) or ‡mean (SD). Abbreviations: CAD: coronary artery disease; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; PAC: postacute care.
At discharge from the acute hospital.
Of the included patients, 299 (52.2%) at admission (Group 1), 125 (21.8%) at 3 weeks (Group 2), 50 (8.7%) at 6 weeks (Group 3), and 50 (8.7%) during 7–12 weeks of rehabilitation (Group 4) were able to complete the gait speed test, and 49 (8.6%) patients were unable to complete the test after completing the PAC course (Group 5). The stroke onset to PAC interval was 13.2 ± 5.3 days and was not significantly different among groups (p = .09).
A comparison of the walking ability groups revealed that group 1 was the youngest and group 5 was the oldest, with mean ages of 62.3 ± 12.8 and 69 ± 11.2 years, respectively (p < .01); groups 4 and 5 had a higher frequency of previous stroke (26% and 34.7%, respectively) than groups 1, 2, and 3 (18.1%, 23.2%, and 16%, respectively; p = .01). Groups 1 and 2 had milder stroke severity (mean NIHSS scores: 4.5 ± 2.5 and 5.6 ± 2.9, respectively) than groups 3, 4, and 5 (mean NIHSS scores: 6.4 ± 3.2, 8 ± 4.1, and 7.2 ± 3.3, respectively; p < .01). Groups 1 and 2 also had milder leg weakness [mean NIHSS (6a+6b): 0.8 ± 0.6 and 1.4 ± 0.9, respectively] than groups 3, 4, and 5 [mean NIHSS (6a+6b) scores: 1.6 ± 1.1, 1.8 ± 1.1, and 1.8 ± 1.1, respectively] (p < .01). The 5 groups did not differ in the proportions of hemorrhagic stroke (p = .34), left side stroke (p = .54), infratentorial stroke (p = .41), ataxia (p = .37) and aphasia subscores of NIHSS (p = .15). Groups 1 and 5 had shorter rehabilitation durations (46 ± 22.8 and 46.9 ± 24 days, respectively) than groups 2, 3, and 4 (62.2 ± 21.4, 64.2 ± 21.3, and 57.9 ± 24.7 days, respectively; p < .01).
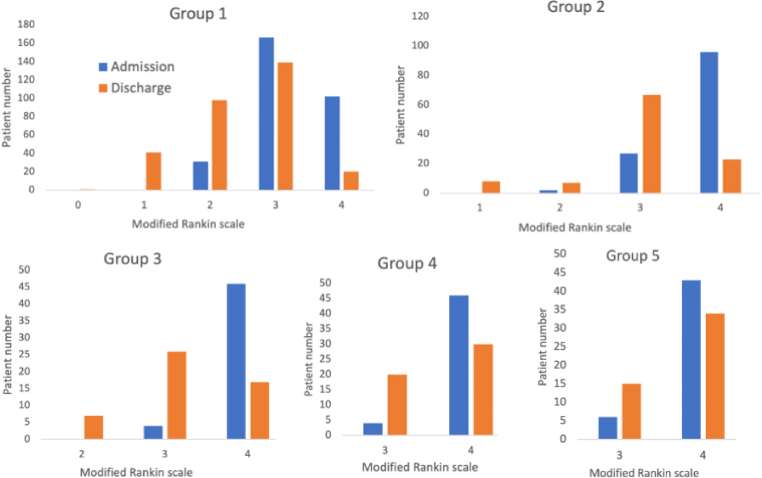
The mRS and other functional recovery profiles based on different walking categories are presented in Fig. 1 and Table 2. The mean BI and BBS scores at admission were the highest in group 1 and progressively decreased from group 1 to group 5 [mean BI scores were 56.9 ± 14.4, 43.4 ± 12.8, 39.3 ± 14.5, 36 ± 13.4, and 31.6 ± 14, respectively (p < .01); mean BBS scores were 42.2 ± 10, 21.2 ± 11.3, 12.4 ± 8.3, 11.3 ± 12.2, and 8.1 ± 8.5, respectively (p < .01)]. The mean mRS scores were significantly lower in group 1 (3.2 ± 0.6) than in the other groups (p < .01), indicating better performance in activities of daily living in group 1. The mean FuglUE scores progressively decreased from groups 1 to 4 (p < .01) and were similar between groups 4 and 5 (p = .48), indicating progressive worsening of synergism and function of the paretic upper limb from groups 1 to 4. The mean FuglSEN scores were higher in groups 1 and 2 than in groups 3, 4, and 5 (p < .01), indicating better somatosensation in groups 1 and 2 than in groups 3, 4, and 5. The mean MNA scores were higher in group 1 than in other groups (p < .01), indicating better nutritional status in group 1 than in the other groups. The mean MMSE scores were higher in groups 1, 2, 3 than in groups 4 and 5 (p < .01), indicating better cognitive function in groups 1, 2, 3 than in groups 4 and 5.
Fig. 1.
Distribution of (MRS) scores at admission and discharge in different patient groups.
Table 2.
Functional recovery profiles of walking categories.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | p value | |
|---|---|---|---|---|---|---|
| BI | ||||||
| At admission | 56.9 (14.4) | 43.4 (12.8) | 39.3 (14.5) | 36 (13.4) | 31.6 (14) | <0.01 |
| At discharge | 79.6 (14.2) | 70.8 (14.7) | 63.7 (14) | 52.7 (15.4) | 47 (14.8) | <0.01 |
| Δ BI | 22.7 14.7) | 27.3 (15.1) | 24.4 (15) | 16.8 (12.5) | 15.7 (10.9) | <0.01 |
| FuglUE | ||||||
| At admission | 50.4 (15) | 39.8 (19) | 30.2 (21.5) | 27.5 (21.1) | 27.7 (21.1) | <0.01 |
| At discharge | 58.5 (8.9) | 54.3 (12.7) | 46.6 (18.1) | 38 (10.5) | 33.1 (24.8) | <0.01 |
| Δ FuglUE | 8 (10.1) | 14.5 (13.5) | 16.5 (13.6) | 10.5 (11.2) | 5.4 (12.3) | <0.01 |
| FuglSEN | ||||||
| At admission | 37.8 (9.9) | 34.6 (12.4) | 28.1 (16.6) | 25.3 (16.7) | 26.5 (17.6) | <0.01 |
| At discharge | 40.6 (8.5) | 39.6 (9.1) | 34.6 (13.1) | 32.3 (14.5) | 29 (17.6) | <0.01 |
| Δ FuglSEN | 2.9 (6.6) | 5 (8) | 6.5 (9.2) | 7 (10.4) | 2.5 (9.8) | <0.01 |
| MMSEa | ||||||
| At admission | 23.7 (6.4) | 21.9 (6.3) | 24 (5) | 18.4 (9.6) | 18.6 (8.4) | <0.01 |
| At discharge | 27 (4.8) | 25.7 (5.8) | 28 (3.3) | 22.2 (9) | 21.9 (7.7) | <0.01 |
| Δ MMSE | 3.4 (3.8) | 4.1 (3.3) | 4.3 (3.7) | 4.1 (4.1) | 3.3 (4.4) | 0.21 |
| MNA | ||||||
| At admission | 17.2 (5.4) | 15.5 (5.2) | 15.9 (4.7) | 15.3 (5.2) | 16 (4.5) | <0.01 |
| At discharge | 19 (5.6) | 17.8 (5.6) | 17.9 (5.3) | 17.3 (5.2) | 17.3 (6.1) | 0.02 |
| Δ MNA | 1.8 (3.4) | 2.4 (2.9) | 2 (3.6) | 2 (3.2) | 1.2 (5.1) | 0.16 |
| BBS | ||||||
| At admission | 42.2 (10) | 21.2 (11.3) | 12.4 (8.3) | 11.3 (12.2) | 8.1 (8.5) | <0.01 |
| At discharge | 52.7 (4.6) | 48.2 (7.6) | 44.3 (9.6) | 37.9 (11.7) | 21.8 (12.8) | <0.01 |
| Δ BBS | 10.5 (0.5) | 27 (0.9) | 32.1 (1.2) | 27 (1.7) | 13 (1.5) | <0.01 |
| Comfortable gait speed (m/s) | ||||||
| At admission | 0.55 (0.27) | 0 | 0 | 0 | 0 | <0.01 |
| At 3 weeks | 0.70 (0.3) | 0.4 (0.24) | 0 | 0 | 0 | <0.01 |
| At 6 weeks | 0.77 (0.34) | 0.51 (0.27) | 0.3 (0.2) | 0 | 0 | <0.01 |
| At discharge | 0.87 (0.35) | 0.61 (0.32) | 0.48 (0.25) | 0.37 (0.24) | 0 | <0.01 |
Data are expressed as mean (SD). Abbreviations: BBS: Berg Balance Scale; BI: Barthel Index; FuglUE: Fugl–Meyer upper extremity assessment; FuglSEN: modified Fugl–Meyer sensory assessment; MMSE: Mini-Mental State Examination; MNA: Mini-Nutritional assessment.
The MMSE was no longer performed after Jan 1, 2018.
The mean mRS and BI showed greater improvement in groups 1, 2, and 3 than in groups 4 and 5 (both p < .01). The improvement in the mean FuglUE, FuglSEN, and BBS was significantly greater in groups 2, 3, 4 than in groups 1 and 5 (all p < .01).
Compared with group 4, group 3 exhibited greater improvements in BBS (p = .03) and FuglUE (p = .01). Compared with group 5, group 4 exhibited greater improvements in BBS (p < .01) and FuglSEN (p = .03) and a trend of greater improvements in FuglUE (p = .08); however, the improvements in BI were similar between groups 4 and 5 (p = .59).
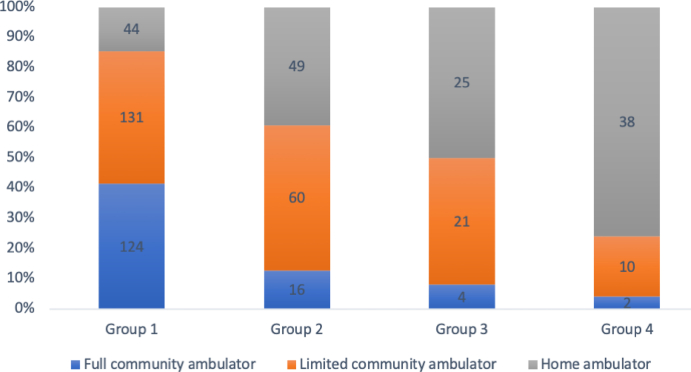
At the end of rehabilitation, the mean comfortable gait speeds in groups 1 to 4 were 0.86 ± 0.35, 0.61 ± 0.32, 0.48 ± 0.25, and 0.37 ± 0.24 m/s, respectively (p < .01). The number and proportion of patients at different levels of gait speed stratified by cutoffs of 0.49 and 0.93 m/s in each group are shown in Fig. 2. Group 1 had a significantly higher proportion of patients at the full community ambulators level (41.5%) than the other groups, and the proportions of home ambulators progressively increased from group 1 to 4 (14.7%, 39.2%, 50%, and 76%, respectively; p < .001).
Fig. 2.
Walking Activity of Patient Groups Stratified by Gait Speed at PAC Discharge. The patient numbers are shown in columns.
Linear regression model
A stepwise linear regression model was used to predict the patient's gait speed at discharge. The results showed that BBS, age, NIHSS (6a+6b), MNA, and FuglUE were predictors of gait speed (Table 3). The model explained 43% of the variance in gait speed (R2 = 0.43; adjusted R2 = 0.415). All the included variables had a variance inflation factor <3. The patient's sex, stroke side and location, stroke onset to PAC interval, the presence of aphasia or ataxia, comorbidities, and activities of daily living were not included in the final model.
Table 3.
Results of Stepwise Linear Regression Analysis of Factors Predicting Gait Speed at PAC discharge.
| Predictor | Unstandardized Coefficient | Coefficients standard error | Adjusted R Squarea | Standardized coefficient | 95% CI (lower and upper bound) | p value | |
|---|---|---|---|---|---|---|---|
| Constant | 0.8 | 0.131 | 0.543 | 1.058 | <0.001∗∗∗ | ||
| BBS | 0.011 | 0.001 | 0.371 | 0.505 | 0.009 | 0.014 | <0.001∗∗∗ |
| Age | −0.005 | 0.001 | 0.384 | −0.169 | −0.008 | −0.002 | 0.001∗∗ |
| NIHSS (6a+6b) | −0.042 | 0.018 | 0.400 | −0.124 | −0.077 | −0.007 | 0.018∗ |
| MNA | −0.007 | 0.003 | 0.408 | −0.107 | −0.013 | −0.001 | 0.027∗ |
| FuglUE | 0.002 | 0.001 | 0.415 | 0.111 | 0 | 0.004 | 0.047∗ |
Abbreviations: BBS: Berg Balance Scale; CI: confidence interval; FuglUE: Fugl–Meyer upper extremity assessment; MNA: mini-nutritional assessment; NIHSS: National Institutes of Health Stroke Scale.
∗p < .05, ∗∗p < .01, ∗∗∗p < .001.
Adjusted for age, sex, diabetes mellitus, hypertension, dyslipidemia, coronary artery disease, atrial fibrillation, previous stroke, FuglSEN, mRS, BI, and NIHSS aphasia and ataxia subscores.
Discussion
The present study demonstrated the effectiveness of PAC in facilitating walking ability recovery. Initially, 47.8% of patients admitted to the PAC ward were unable to complete the 5-m walk test, but the proportion decreased to 8.6% at discharge from PAC. Most importantly, the gait speed at PAC discharge could be predicted by balance, age, leg strength, nutritional status, and upper limb function at PAC admission. However, our study did not reveal the value of limb ataxia or somatosensation in accurately predicting post-PAC walking performance. One of the possible reasons is that previous studies enrolled patients with highly specific deficits. For instance, Kim et al. investigated the influence of ataxia on walking ability after stroke, and all the recruited patients with ataxia had limb muscle strength ≥4 on the Medical Resource Council Scale and NIHSS ≤5 [26]. Lynch et al. investigated the effect of sensory retraining on gait speed, and all the recruited patients had significant sensory impairment and were already able to walk 10 m at baseline [27].
Gait speed is a key indicator of general gait performance and can be used to discriminate among levels of walking disability. Cutoffs of gait speeds used to discriminate among home, limited community, and full community ambulators were 0.4 and 0.8 m/s [28]. The cutoffs were recently updated to 0.49 and 0.93 m/s in a study that measured poststroke walking activity in real-world settings [9]. Using the updated criteria, we found a significant differences in walking activity at PAC discharge across walking categories. Patients who regained walking ability earlier had a higher chance of achieving higher levels of walking activity, suggesting the importance of optimizing training strategies to achieve early ambulation during PAC.
Besides age, balance, leg strength, cognitive function, and training intensity, the present study demonstrated the value of upper limb function as a predictor of walking performance after stroke. Because walking relies on the coordinated movements of 4 limbs, it is not unexpected that upper extremity function was also a predictor of walking performance, although the coefficient for this factor was relatively small compared with those of other predictors in the regression model. Studies on arm swing during human walking have revealed that the arms not only act as passive mass dampers, powered by the movement of the lower body [29], but also actively participate in the walking action by moving backwards to counteract the torque around the body axis that is generated by striding legs [30]. Apart from the functions of maintaining balance and center of mass movement control, the upper limbs are also necessary for gait recovery because they are used to handle supports, such as walking aids [29].
Malnutrition is prevalent among stroke patients, and a recent study showed that malnutrition was associated with poor functional outcome [31]. Surprisingly, better nutritional state as measured by the MNA is predictive of slower gait speed in our regression model. One of the possible reasons is that the MNA includes anthropometry scoring, such as body mass index and extremities’ circumference, which may be higher in patients with obesity, a factor negatively affect the gait speed [32]. Further studies are needed to validate our finding.
The BBS has been used to predict walking activity and risk of incidental falls [33,34] in patients with stroke. In chronic stages of stroke, the optimal cutoff for predicting home versus community ambulators was 48 [9]. Patients with stroke and a lower BBS score had a lower walking speed, smaller stride length, longer stance time, longer asymmetrical stance time [35], and more variation in walking velocity and walking direction, suggesting a less efficient walking ability [36].
Cognition is commonly considered a predictor of motor outcome in stroke [37], and impaired cognition was reported to be a predictor of impaired walking ability [8]. Notably, the MMSE does not specifically address executive function and visuospatial deficits, which, along with memory, were shown to be predictors of walking ability by Park et al. [8] The negative effect of cognitive function on the prediction of walking ability may be because the cognitive domains assessed by the MMSE are limited. Some patients had mRS scores ≤3, indicating the ability to walk without support, but they were unable to complete the 5-m walk test. One of the possible reasons for this inability is poor cognitive function, which may have impaired their ability to comprehend or cooperate in performing the commands. Other possible reasons include poor exercise tolerance or unwillingness to participate.
The use of a patient cohort from the PAC-CVD program has advantages. First, the rehabilitation data in the program could be integrated with the acute-stage data recorded in the Stroke Registry of the Chang Gung Healthcare System [38,39]; thus, the rehabilitation outcomes could be analyzed in combination with detailed clinical information (a total of 130 variables in the combined dataset). By analyzing the combined dataset, the recovery trajectory of neurologic and functional impairments in patients with stroke from acute to recovery stages could be appreciated. Moreover, the temporal functional assessments from the acute to rehabilitation stages could allow comparisons and correlations between various functionalities. Second, because the PAC-CVD was implemented under National Health Insurance, which covers >99% of Taiwan's population, all eligible patients were enrolled in the program, reducing patient selection bias [17]. Finally, the execution of the rehabilitation program under the same multidisciplinary rehabilitation team in a single institution reduced the heterogeneity of care and ensured that the rehabilitation program was tailored to each patient.
This study has some limitations. First, because all the patients were selected based on the criteria established by the PAC-CVD program and treated in a single acute care and PAC hospital, the results may not be generalizable to all patients with stroke. Nevertheless, the patients qualified for the PAC-CVD program are likely those who benefit most from rehabilitation; therefore, our findings are particularly applicable to patients in need of intensive rehabilitation. Second, the NIHSS ataxia and leg strength subscales do not reflect truncal ataxia or distal leg strength [18]. Using scales that specifically measure ataxia, leg strength or function, may better reflect their impairments and reveal their association with walking disability. Moreover, the ataxia NIHSS subscore may not be sufficiently comprehensive (score range, 3) to grade limb ataxia, and patients who score 0 (indicates no deficit) [26,40] may have limb weakness that may have rendered them unable to perform the coordination tests instead of having no limb ataxia [3]. Third, although all the patients received high-intensity rehabilitation, the training strategy and dosage of training may differ to tailor each patient's deficits, leading to heterogeneity of treatments, and we have no detailed recording of rehabilitation frequency and training strategies of our patients. Finally, the differences in rehabilitation durations between walking categories may account for the differences in functional improvements. However, because the closing regulation of the program was primarily based on the potential of further anticipated improvement and the necessity of in-hospital rehabilitation, we think our model may be reliable in a real-world setting.
Further researches should add more information that are candidates to predict the recovery of walking ability. Candidate variables would include leg function, which can be measured by Fugl-Meyer lower extremity assessment [21], limbs spasticity, which can be measured by modified Ashworth Scale or modified Tardieu scale [41], and severity of ataxia, which can be measured by Scale for Assessment and Rating of Ataxia [26]. In addition, the details of PT/OT/ST training programs should be considered in future researches for poststroke gait recovery.
Conclusions
Our study provided insights into the recovery of poststroke walking performance through high-intensity rehabilitation. After PAC stroke rehabilitation, although most patients regained their ability to walk, a substantial portion of them still had limited walking ability at home or in the community. Balance, age, leg strength, nutritional status, and upper limb function are important predictors of poststroke walking performance. Additional studies are warranted to reveal the intricate relationships between factors that contribute to walking disability to help tailor individualized rehabilitation strategies and discharge plans.
Sources of funding
This study was supported by Chang Gung Medical Foundation Grants, CMRPG3K0231-2 and BMRPB67.
Conflicts of interest
None.
Acknowledgement
This manuscript was edited by Wallace Academic Editing.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Chih-Kuang Chen, Email: leonard@cgmh.org.tw.
Yu-Cheng Pei, Email: yspeii@gmail.com.
References
- 1.Schmid A., Duncan P.W., Studenski S., Lai S.M., Richards L., Perera S., et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 2.Bultmann U., Pierscianek D., Gizewski E.R., Schoch B., Fritsche N., Timmann D., et al. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture. 2014;39:563–569. doi: 10.1016/j.gaitpost.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Choi S.W., Han N., Jung S.H., Kim H.D., Eom M.J., Bae H.W. Evaluation of ataxia in mild ischemic stroke patients using the scale for the assessment and rating of ataxia (SARA) Ann Rehabil Med. 2018;42:375–383. doi: 10.5535/arm.2018.42.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentiplay B.F., Adair B., Bower K.J., Williams G., Tole G., Clark R.A. Associations between lower limb strength and gait velocity following stroke: a systematic review. Brain Inj. 2015;29:409–422. doi: 10.3109/02699052.2014.995231. [DOI] [PubMed] [Google Scholar]
- 5.Chia F.S., Kuys S., Low Choy N. Sensory retraining of the leg after stroke: systematic review and meta-analysis. Clin Rehabil. 2019;33:964–979. doi: 10.1177/0269215519836461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolognini N., Russo C., Edwards D.J. The sensory side of post-stroke motor rehabilitation. Restor Neurol Neurosci. 2016;34:571–586. doi: 10.3233/RNN-150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esquenazi A., Moon D., Wikoff A., Sale P. Hemiparetic gait and changes in functional performance due to OnabotulinumtoxinA injection to lower limb muscles. Toxicon. 2015;107:109–113. doi: 10.1016/j.toxicon.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Park J., Lee S.U., Jung S.H. Prediction of post-stroke functional mobility from the initial assessment of cognitive function. NeuroRehabilitation. 2017;41:169–177. doi: 10.3233/NRE-171469. [DOI] [PubMed] [Google Scholar]
- 9.Fulk G.D., He Y., Boyne P., Dunning K. Predicting home and community walking activity poststroke. Stroke. 2017;48:406–411. doi: 10.1161/STROKEAHA.116.015309. [DOI] [PubMed] [Google Scholar]
- 10.Hornby T.G., Henderson C.E., Holleran C.L., Lovell L., Roth E.J., Jang J.H. Stepwise regression and latent profile Analyses of locomotor outcomes poststroke. Stroke. 2020;51:3074–3082. doi: 10.1161/STROKEAHA.120.031065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano Y., Hayashi T., Nitta O., Takahashi H., Nishio D., Minakawa T., et al. Prediction of independent walking ability for severely hemiplegic stroke patients at discharge from a rehabilitation hospital. J Stroke Cerebrovasc Dis. 2016;25:1878–1881. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Smith M.C., Barber P.A., Stinear C.M. The TWIST algorithm predicts time to walking independently after stroke. Neurorehabil Neural Repair. 2017;31:955–964. doi: 10.1177/1545968317736820. [DOI] [PubMed] [Google Scholar]
- 13.Kollen B., Van De Port I., Lindeman E., Twisk J., Kwakkel G. Predicting improvement in gait after stroke a longitudinal prospective study. Stroke. 2005;36:2676–2680. doi: 10.1161/01.STR.0000190839.29234.50. [DOI] [PubMed] [Google Scholar]
- 14.Medford-Davis L.N., Fonarow G.C., Bhatt D.L., Xu H., Smith E.E., Suter R., et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines-stroke. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu C.L., Chen Y.P., Chen C.C.P., Chen C.K., Chang H.N., Chang C.H., et al. Functional recovery patterns of hemorrhagic and ischemic stroke patients under post-acute care rehabilitation program. Neuropsychiatr Dis Treat. 2020;16:1975–1985. doi: 10.2147/NDT.S253700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh C.Y., Tsao W.C., Lin R.T., Chao A.C. Three years of the nationwide post-acute stroke care program in Taiwan. J Chin Med Assoc. 2018;81:87–88. doi: 10.1016/j.jcma.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang C.Y., Chen Y.R., Hong J.P., Chan C.C., Chang L.C., Shi H.Y. Rehabilitative post-acute care for stroke patients delivered by per-diem payment system in different hospitalization paths: a Taiwan pilot study. Int J Qual Health Care. 2017;29:779–784. doi: 10.1093/intqhc/mzx102. [DOI] [PubMed] [Google Scholar]
- 18.Lyden P., Lu M., Jackson C., Marler J., Kothari R., Brott T., et al. Underlying structure of the National Institutes of Health stroke scale: results of a factor analysis. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 19.Lees K.R., Bath P.M., Schellinger P.D., Kerr D.M., Fulton R., Hacke W., et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke. 2012;43:1163–1170. doi: 10.1161/STROKEAHA.111.641423. [DOI] [PubMed] [Google Scholar]
- 20.Blum L., Korner-Bitensky N. Usefulness of the Berg balance scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan K.J., Tilson J.K., Cen S.Y., Rose D.K., Hershberg J., Correa A., et al. Fugl-meyer assessment of sensorimotor function after stroke. Stroke. 2011;42:427–432. doi: 10.1161/STROKEAHA.110.592766. [DOI] [PubMed] [Google Scholar]
- 22.Foley N.C., Salter K.L., Robertson J., Teasell R.W., Woodbury M.G. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke. 2009;40:e66–e74. doi: 10.1161/STROKEAHA.108.518910. [DOI] [PubMed] [Google Scholar]
- 23.Bour A., Rasquin S., Boreas A., Limburg M., Verhey F. How predictive is the MMSE for cognitive performance after stroke? J Neurol. 2010;257:630–637. doi: 10.1007/s00415-009-5387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson C.M., Kostsuca S.R., Boura J.A. Utilization of a 5-meter walk test in evaluating self-selected gait speed during preoperative screening of patients scheduled for cardiac surgery. Cardiopulm Phys Ther J. 2013;24:36–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Vellas B., Guigoz Y., Garry P.J., Nourhashemi F., Bennahum D., Lauque S., et al. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim B.R., Lim J.H., Lee S.A., Park S., Koh S.E., Lee I.S., et al. Usefulness of the scale for the assessment and rating of ataxia (SARA) in ataxic stroke patients. Ann Rehabil Med. 2011;35:772–780. doi: 10.5535/arm.2011.35.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch E.A., Hillier S.L., Stiller K., Campanella R.R., Fisher P.H. Sensory retraining of the lower limb after acute stroke: a randomized controlled pilot trial. Arch Phys Med Rehabil. 2007;88:1101–1107. doi: 10.1016/j.apmr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Middleton A., Fritz S.L., Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephenson J.L., Lamontagne A., De Serres S.J. The coordination of upper and lower limb movements during gait in healthy and stroke individuals. Gait Posture. 2009;29:11–16. doi: 10.1016/j.gaitpost.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Kuhtz-Buschbeck J.P., Jing B. Activity of upper limb muscles during human walking. J Electromyogr Kinesiol. 2012;22:199–206. doi: 10.1016/j.jelekin.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Aliasghari F., Izadi A., Khalili M., Farhoudi M., Ahmadiyan S., Deljavan R. Impact of premorbid malnutrition and dysphagia on ischemic stroke outcome in elderly patients: a community-based study. J Am Coll Nutr. 2019;38:318–326. doi: 10.1080/07315724.2018.1510348. [DOI] [PubMed] [Google Scholar]
- 32.Mendes J., Borges N., Santos A., Padrão P., Moreira P., Afonso C., et al. Nutritional status and gait speed in a nationwide population-based sample of older adults. Sci Rep. 2018;8:4227. doi: 10.1038/s41598-018-22584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda N., Kato J., Shimada T. Predicting the probability for fall incidence in stroke patients using the Berg Balance Scale. J Int Med Res. 2009;37:697–704. doi: 10.1177/147323000903700313. [DOI] [PubMed] [Google Scholar]
- 34.Tilson J.K., Wu S.S., Cen S.Y., Feng Q., Rose D.R., Behrman A.L., et al. Characterizing and identifying risk for falls in the LEAPS study: a randomized clinical trial of interventions to improve walking poststroke. Stroke. 2012;43:446–452. doi: 10.1161/STROKEAHA.111.636258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Meulen F.B., Weenk D., Buurke J.H., Van Beijnum B.J., Veltink P.H. Ambulatory assessment of walking balance after stroke using instrumented shoes. J Neuroeng Rehabil. 2016;13:48. doi: 10.1186/s12984-016-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Meulen F.B., Weenk D., Van Asseldonk E.H., Schepers H.M., Veltink P.H., Buurke J.H. Analysis of balance during functional walking in stroke survivors. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Özdemir F., Birtane M., Tabatabaei R., Ekuklu G., Kokino S. Cognitive evaluation and functional outcome after stroke. Am J Phys Med Rehabil. 2001;80:410–415. doi: 10.1097/00002060-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Liu C.H., Wei Y.C., Lin J.R., Chang C.H., Chang T.Y., Huang K.L., et al. Initial blood pressure is associated with stroke severity and is predictive of admission cost and one-year outcome in different stroke subtypes: a SRICHS registry study. BMC Neurol. 2016;16:27. doi: 10.1186/s12883-016-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo L.L.L., Chien S.C., Lin J.R., Liow C.W., Lee J.D., Peng T.I., et al. Derivation and validation of a scoring system for intravenous tissue plasminogen activator use in asian patients. J Stroke Cerebrovasc Dis. 2017;26:1695–1703. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 40.Kwong P.W.H., Ng S.S.M. Cutoff score of the lower-extremity motor subscale of Fugl-Meyer assessment in chronic stroke survivors: a cross-sectional study. Arch Phys Med Rehabil. 2019;100:1782–1787. doi: 10.1016/j.apmr.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Francisco G.E., McGuire J.R. Poststroke spasticity management. Stroke. 2012;43:3132–3136. doi: 10.1161/STROKEAHA.111.639831. [DOI] [PubMed] [Google Scholar]