Abstract
Fibroblast growth factor 21 (FGF21), a member of the FGF subfamily, is produced primarily in the liver and adipose tissue. The main function of FGF21 is to regulate energy metabolism of carbohydrates and lipids in the body through endocrine and other means, making FGF21 have potential clinical value in the treatment of metabolic disorders. Although FGF21 and its receptors play a role in the regulation of bone homeostasis through a variety of signaling pathways, a large number of studies have reported that the abuse of FGF21 and its analogues and the abnormal expression of FGF21 in vivo may be associated with bone abnormalities. Due to limited research information on the effect of FGF21 on bone metabolism regulation, the role of FGF21 in the process of bone homeostasis regulation and the mechanism of its occurrence and development have not been fully clarified. Certainly, the various roles played by FGF21 in the regulation of bone homeostasis deserve increasing attention. In this review, we summarize the basic physiological knowledge of FGF21 and the effects of FGF21 on metabolic homeostasis of the skeletal system in animal and human studies. The information provided in this review may prove beneficial for the intervention of bone diseases.
Keywords: Fibroblast growth factor 21 (FGF21), Bone homeostasis, Bone metabolism
The human fibroblast growth factor (FGF) superfamily consists of 22 related proteins from FGF1 to FGF23 (FGF15 and FGF19 are mouse and human orthologs, respectively [1]), and is divided into seven sub-families based on genetic and functional similarities. Members of this superfamily have multiple biological functions [[2], [3], [4]]. They are cytokines with various functions in biological processes such as growth, development, and metabolism and are used for the treatment of diseases [[4], [5], [6]]. FGF21 (23 kDa, 209 amino acids) is an atypical member of the FGF19 subfamily and is mainly produced by the liver and adipose tissue [7,8]. In recent years, FGF21 has been shown to have excellent pharmacological effects in the treatment of metabolic disorders (diabetes and obesity), cardiovascular disorders, and other diseases, because of its ability to enhance insulin sensitivity and reduce body weight [[9], [10], [11], [12]]. This has made FGF21 have potential value in clinical application. However, there have been several safety concerns with FGF21 drug use, including growth inhibition, circadian rhythm disturbances, and bone loss [8,13]. Abnormal expression and signal transduction of FGF21 are associated with skeletal system abnormalities [[14], [15], [16]]. Therefore, as a candidate drug for the treatment of metabolic diseases, the related effects of FGF21 on the bone have attracted much attention. This review summarizes the basic knowledge of the physiology of FGF21 and the multiple effects it has on skeletal system homeostasis.
Physiology of FGF21
FGF21 has been studied extensively in rodents and primates. It belongs to the endocrine subfamily and is released into the peripheral blood circulation due to lack of a heparin-binding domain [13]. FGF21 is released almost exclusively by the liver into circulation under basal conditions or fasting [8,17]. Following its release into the systemic circulation, it can integrate metabolism in the liver, adipose tissue, skeletal muscle, pancreas, and other metabolic organs by controlling the expression of transcriptional factors that shape cellular phenotype and tissue metabolic functions [18,19]. FGF21 regulates the systemic energy metabolism, especially the homeostasis of glucose and lipid metabolism, in humans and animals by acting on central and peripheral organs and tissues [17,20,21]. It also acts as a stress hormone to excite sympathetic nerves and activate the hypothalamic-pituitary-adrenal axis to increase secretion of corticosteroids that regulate systemic energy metabolism [6,13,22,23]. However, due to difference in the positions of generation and action of FGF21, its function is still controversial [8,21,24].
FGF21 and other members of its family contain a highly conserved central core domain and a β-trefoil structure that are critical for their interaction with fibroblast growth factor receptors (FGFRs) [5]. FGFRs are a family of receptor tyrosine kinases comprising of four isoforms (FGFR1, FGFR2, FGFR3, and FGFR4) [5]. They are widely expressed in various tissues and organs, including liver, adipose tissue, cartilage tissue [25], skeletal muscle [26], colon, etc [[27], [28], [29]]. FGFR1 is mainly expressed in the testis, bone marrow, and small intestine. FGFR2-4 are mainly expressed in the pituitary gland, liver, and colon, respectively. Although FGFs typically bind to heparin for receptor activity, FGF21 has a weak heparin-binding capacity. Therefore, to exert its activity, it needs to bind to the receptor complex composed of FGFR and co-receptor β-klotho (KLB), a single-channel transmembrane protein [13,30]. KLB is expressed in limited tissues and cell types, mainly in the liver, pancreas, adipose tissue, pituitary gland, and hypothalamus [27,29,30]. In the presence of KLB, FGFR1 is the predominant isoform that mediates FGF21 activity [31]. FGF21 binds to the β-klotho-FGFR1c complex with the highest affinity [13], triggering autophosphorylation of FGFR1 and activation of the downstream MAPK (mitogen-activated protein kinase) cascade pathway and the PI3K/AKT (phosphatidylinositol 3-kinase / protein kinase B) signaling pathway to target cells to initiate multiple effects [5,21,32]. FGF21 can also signal through FGFR2 and FGFR3; however, their physiological role in FGF21 signaling is unclear. FGF21 does not activate downstream signaling through the β-klotho-FGFR4 complex [5,33]. Given that FGFR is widely expressed, and Klotho is an essential co-receptor for tissue-specific expression, restricted expression of Klotho determines the specificity and sensitivity of target tissues to FGF21. The liver, adipose tissue, pancreas, and central nervous system, which are the key target organs [5,[34], [35], [36], [37]], play an important role in the FGF21-regulated energy metabolism homeostasis.
FGF21 and bone homeostasis
The bone is a special connective tissue. In the regulation of bone homeostasis, bone tissue undergoes constant transformation and remodeling to adapt to the changing environment. The gold standard for assessing human bone health is bone mineral density (BMD), which is co-mediated by osteoblasts and osteoclasts [38,39]. FGF21 has a strong pathophysiological connection to bone homeostasis regulation, and its expression has a spatio-temporal specificity and controls the process of bone development [[39], [40], [41]]. However, information on the relationship between FGF21 and bone homeostasis is limited, and the role of FGF21 in bone homeostasis has not been fully elucidated. Many diseases are related to bone loss and regeneration. Because the molecular mechanism and early treatment measures of bone-related diseases are not completely clear [14], the treatment costs are expensive, which brings much burden on individuals, families, and society. Therefore, it is necessary to explore the relevant knowledge on skeletal system homeostasis and seek effective treatment methods. In the following paragraphs, we summarize the possible effects of FGF21 in the homeostasis of the skeletal system, providing new insights for studying its relevance to bone-related diseases.
Effects of FGF21 on bone homeostasis in animal studies
Does FGF21 affect the bones of animals? If so, is the effect positive or negative? Some animal-based studies have reported that the indirect effect of FGF21 on the bone through increasing the activity of peroxisome proliferator-activated receptor-gamma (PPARγ) is considered to be a significant mechanism of bone resorption [8,42,43]. In Fgf21 transgenic (Fgf21-Tg ) mice, overexpressed circulating FGF21 stimulated lipogenesis of bone marrow precursors, inhibited osteoblast activity, and increased osteoclast activity by enhancing PPARγ activity in bone marrow mesenchymal stem cells (BMSCs). This resulted in decreased BMD, significant bone loss, and increased bone fragility due to decreased bone formation and increased bone resorption [43]. Research has also shown that elevated levels of FGF21 are associated with negative effects on bone metabolism. Conversely, increased markers of bone formation and decreased markers of bone resorption in Fgf21-knockout (Fgf21-KO) mice exhibited a high bone mass phenotype [43]. Inagaki, wang et al. [44,45] demonstrated that FGF21 treatment can regulate the growth hormone-insulin-like growth factor 1 axis (GH-IGF1). In transgenic mice with overexpressed FGF21, the expression of IGF1 in the liver was reduced to inhibit GH signaling, indirectly induce the secretion of insulin-like growth factor-binding protein 1 (IGFBP1) in the liver, and bind IGFBP1 to osteoclast precursor integrin β1, to enhance the receptor activator of nuclear factor κ-B ligand (RANKL)-stimulated extracellular regulated protein kinases (ERK) phosphorylation and nuclear factor of activated T cells 1 (NFATc1) activation, thereby promoting osteoclast differentiation and resulting in reduced BMD. Blockade of IGFBP1 abolished ovariectomy (OVX)-induced bone resorption and abrogated FGF21-mediated bone loss, while maintaining its insulin sensitivity [45]. Overexpression of FGF21 in mice resulted in decreased bone growth and decreased mouse body length [46]. In Kim et al.’s findings, treating obese non-human primates with the long-acting FGF21 analog PF-05231023 caused modest changes in bone resorption and formation markers without any weight loss [47]. Despite its low magnitude, this may suggest that FGF21 has an adverse effect on the bone. Another animal study also showed an inhibitory effect of FGF21 on bone formation, with serum FGF21 down-regulating bone formation during lactation in C57BL/6 mice and high circulating FGF21 negatively affecting BMD during lactation [48]. Compared to lactating control mice, Fgf21-deficient mice showed increased bone formation with no changes in bone resorption [48]. Li et al. [49] used Dystrophin/Utrophin (muscular dystrophy protein/dystrophin-related protein) double knockout (dKO) mouse model of duchenne muscular dystrophy (DMD) and found that in the DMD animal model, skeletal muscle-derived circulating FGF21 expression was significantly up-regulated. Furthermore, FGF21 significantly increased circulating levels of bone formation marker procollagen type I N-terminal propeptide (P1NP) and decreased levels of bone resorption marker carboxy-terminal cross-linked telopeptide of type 1 collagen (CTX1) in dKO mice. Following the injection of neutralizing anti-FGF21 antibody, progressive bone loss in weight-bearing (vertebrae, femur, and tibia) and non-weight-bearing bones (parietal bone) of dKO mice was significantly reduced, and bone mass was significantly increased in dystrophic mice, suggesting that myogenic FGF21 might be a negative regulator of bone homeostasis in DMD [49]. Additionally, FGFRs and KLB were significantly up-regulated in dKO mice, suggesting that bone tissue may be a direct target of FGF21. Therefore, FGF21 may directly affect osteoclast and adipocyte formation through the FGFRs-β-klotho axis. It may also promote RANKL-induced osteoclast formation in primary bone marrow macrophages (BMM), promote the adipogenic differentiation of BMSCs, and inhibit osteogenic differentiation [49]. In vivo bone morphometric analysis showed that neutralization of FGF21 primarily reduced the number of osteoclasts but had no significant effect on the number of osteoblasts. In vivo and in vitro studies of the effects of FGF21 indicated that FGF21 primarily affects osteoclast function but has no significant direct effects on osteoblast activity. They also found no statistically significant changes in circulating IGF1 and IGFBP1 levels in dKO mice compared to either WT mice or FGF21 after neutralization, suggesting that the negative effects of FGF21 on bone phenotype in DMD mice were not mediated by hepatic IGF1 and IGFBP1 expression and secretion [49]. Their findings were consistent with those of Wei et al. [43] but differed from those of Inagaki, wang et al. [44,45]. However, Charoenphandhu et al. [42] performed CT analysis of the tibia in high fat (HF) fed rats treated with FGF21, and showed that FGF21 specifically reduced the volume, thickness, and volumetric bone mineral density (vBMD) of the tibial trabecula, mineralized tissues, and osteoblasts. They also observed an increase in bone marrow adipocytes but no change in osteoclasts. In addition, there are bone-related factors in the process of vascular calcification (VC), and clinical evidence suggests that bone loss shares many risk factors, mechanisms, and etiologies with VC [50]. Therefore, in the study of the mechanism and effect of FGF21 on vitamin D3+nicotine (VDN)-induced VC in rats, Shi et al. [51] detected the expressions of bone marker proteins such as osteopontin (OPN), osteocalcin (OCN), and bone morphogenetic protein 2 (BMP2) using Western blot, and also found that FGF21 inhibited the expressions of OPN, OCN, and BMP2 at the protein level. Immunohistochemical staining further confirmed the inhibitory effect of FGF21 on the expression of BMP2. FGF21 inhibits calcification and osteogenic transformation of vascular smooth muscle cells (VSMCs) [51,52].
In one study, diet-induced obesity (DIO) mice model was used to simulate human obesity and type 2 diabetes. In the study of Li et al. [53], while evaluating the bone metabolism effects of DIO mice treated with recombinant human fibroblast growth factor 21 (rhFGF21) and Fgf21-KO mice by using dual-energy X-ray absorptiometry (DXA) and μCT scanning, DIO mice treated with rhFGF21 showed the expected improvement in multiple metabolic parameters but did not change trabecular bone mass, bone formation and resorption biomarker expression, and bone marrow fat content. In addition, compared with WT mice, the Fgf21-KO did not exhibit a high bone mass profile, and there were no significant differences in bone mineral content (BMC) and BMD, and their responses to PPARα and PPARγ agonists were similar. Li et al. [53] inferred that PPARα and PPARγ have no direct role in the regulation of bone metabolism involving FGF21 under physiological conditions. Additionally, at the 75th American Diabetes Association Scientific Meeting, it was mentioned that FGF21 is not a regulator of bone homeostasis in DIO mice treated with rhFGF21. In a 12-weeks study of obese rhesus monkeys treated with FGF21, Andersen et al. [54] observed a decrease in IGF1 and an approximately 2-fold increase in CTX1 at the experimental dose of FGF21, with no increase in plasma cortisol and a decrease in BMD. Changes in body weight also increase bone turnover, altering blood levels of bone markers [55]. Therefore, Andersen et al. [54] inferred that FGF21 had no correlation with BMD. In a 14-weeks FGF21-treated obese OVX female Göttingen minipigs, Christoffersen et al. [18] observed significant improvements in glucose tolerance and insulin sensitivity; however no effect on lipids was observed. They also observed decreased plasma IGF1, peak GH, systemic BMD, and OCN levels, and a slight increase in CTX levels, but did not change in cortisol levels. They concluded that treatment with FGF21 reduced food intake in obese miniature pigs without changes in body composition or adverse effects on whole-body BMD and plasma cortisol [18]. In addition, Jimenez et al. used μCT to scan the skeletal structure of obese mice that were genetically modified with adeno-associated virus vector 8 (AAV8), encoded with optimized FGF21 sequence, and controlled by the synthetic liver-specific HAAT promoter; no significant differences in trabecular bone and cortical bone were found between the AAV8-HAAT -FGF21 mice and age-matched control mice with ineffective vector [56]. After 1 year of weekly subcutaneous injection of two different doses of Pegbelfermin (PGBF), a pegylated FGF21 analog, in adult male cynic monkeys, both doses (0.3, 0.75 mg/kg) exerted pharmacological effects and resulted in weight loss. Based on the evaluation of multiple clinically relevant anatomical sites, PGBF was considered to have no adverse effects on BMD and bone quality [57].
Paradoxically, Ishida et al. [50] found that FGF21 enhanced BMP2 dependent transcription and osteogenesis through the Smad signaling pathway in a high-glucose cultured C2C12 cell model. In the animal model study of oral bone defects by Yang et al. [58], it was reported that FGF21 treatment promoted bone formation, improve BMD, and significantly increase bone mass in rats with oral bone defect. They found that in BMSCs, FGF21 regulated vascular endothelial growth factor (VEGF) through hepatocyte growth factor (HGF)-mediated MAPK/pMAPK, (signal transducer and activator of transcription 3) STAT3/pSTAT3 pathways, promoted angiogenesis, and regulated Caspase-3 to inhibit cell apoptosis through HGF-mediated PI3K/AKT pathway. FGF21 also increased the expression of E-cadherin and fibronectin in BMSCs, promoted bone formation, and improved implant bone defect in experimental rats [58]. The destabilization of the medial meniscus (DMM) is an important model for studying post-traumatic osteoarthritis (OA). In a recent study, by constructing a DMM mouse model and in vitro cell culture, the researchers found that FGF21 administration alleviated oxidative stress-induced chondrocyte apoptosis and senescence in vitro by increasing autophagic flux [16]. Therefore, FGF21 has the potential to treat OA.
Effects of FGF21 on bone homeostasis in human studies
Although some studies on humans have shown the effects of FGF21 and its analogs on bone health, only few clinical studies have been reported and the results have been controversial. Talukdar et al. [20] studied overweight subjects with type 2 diabetes using a long-acting FGF21 analog, PF-05231023, and found that PF-05231023 treatment resulted in significant body weight loss, improved plasma lipoprotein spectrum, and increased adiponectin levels. In addition, circulating levels of IGF1 and various markers of bone formation, such as OCN, P1NP, and bone-specific alkaline phosphatase (BAP), were significantly decreased in a dose-dependent way, while the bone resorptive marker CTX1 was increased [20]. Similar to the observations in the previous study in which Kim et al. [47] treated obese animals with PF-05231023 caused small changes in bone biomarkers in the absence of body weight loss, all indicated bone loss. In the previous section, it was stated that Inagaki et al. demonstrated that FGF21 could regulate the GH-IGF1 axis in transgenic mice, while Talukdar et al. were the first to unveil the regulation of IGF1 signaling pathway and bone turnover pathway in humans [20,[43], [44], [45]]. Previously, a negative correlation between serum IGFBP1 concentrations and BMD has been reported in older men between the ages of 40 and 79 years [59]. However, Lee et al. [12] demonstrated in healthy elderly populations that the FGF21-regulatory axis is associated with age-related declines in BDM in humans, but IGFBP1 was not directly involved in healthy adults, and whether the FGF21-IGFBP 1-RANKL pathway mediates human bone metabolism remains unclear. Although IGFBP1 concentrations were not associated with either BMD or FGF21 concentrations, FGF21 concentrations were inversely associated with local human bone mineral density and were not sex specific [12]. An experimental study by Hao et al. [39] in Chinese Han adults statistically supported the physiological findings of the adverse effect of FGF21 on bone metabolism in Ward's triangle region, showing that plasma FGF21 increases with a concomitant decrease in BMD, and mutations in the FGF21 gene are concomitantly associated with increased BMD and decreased FGF21 expression levels. It was observed that FGF21 levels were inversely correlated with BMD of the femoral neck and hip, while no sex-specific effects were observed. In addition, elevated serum FGF21 was also associated with bone loss in the knee [60]. Fazeli et al. [61] used high-resolution peripheral quantitative computed tomography (HRPQCT) to show that serum FGF21 levels were associated with worsening radial trabecular microstructure and decreased radial bone strength in women with anorexia nerves. Although the FGF21 and BMD changes in Fazeli's study were not statistically significant, the results of their study supported the inference that there is an unfavorable relationship between circulating FGF21 levels and bone mass. Gallego-Escuredo et al. [62] found abnormally increased in both bone resorption and formation in HIV-1 infected patients, but a significant negative correlation was observed between high FGF21 levels and decreased BMD and BMC. Confirming the results of previous studies published by Brown and McComsey on low BMD and BMC in AIDS patients [63,64], serum osteoprotegerin (OPG) and CTX1 levels were significantly positively correlated with FGF21 levels, with bone formation lagging bone resorption, suggesting that FGF21 was directly related to bone loss in HIV-1 infected patients [62]. Wu et al. evaluated the relationship between BMD and serum FGF21 levels in 95 patients with chronic renal disease who were receiving hemodialysis (HD) using DXA, and found a negative correlation between serum FGF21 levels and BMD [11], similar to Zhu et al.’s assessment of the correlation between CT attenuation values and serum FGF21 in 339 patients undergoing HD [65]. Lui et al. [66] evaluated the BMD and trabecular bone score (TBS) of Chinese postmenopausal women with impaired glucose tolerance (IGT) prediabetes. In their study, although there may be a lack of significant correlation between serum FGF21 levels and BMD due to individual patient influence, FGF21 was significantly and independently negatively correlated with TBS, suggesting that FGF21 has a direct adverse effect on bone. A randomized controlled trial evaluating the safety and potential efficacy of LLF580, an FGF21 analog for lowering triglycerides, observed that LLF580 lowered blood lipid markers over 12 weeks, with no effect on fasting glucose or (Hemoglobin A1c) HbA1c. Although LLF580 decreased biomarkers of bone formation (BAP, P1NP, OCN), there were no differences in markers of bone resorption (CTX1, N-telopeptides of type I collagen [NTX1]). The researchers believed that LLF580 was relatively safe for treating patients with obesity or fatty liver, except for mild to moderate gastrointestinal adverse reactions [15].
Conversely, in a study of 115 healthy postmenopausal women with an average age of 60.2 ± 7.2 years, Choi et al. [67] found that serum FGF21 levels were negatively correlated with BMD, but the difference was not statistically significant. After adjusting for age and body mass index, serum FGF21 levels were not significantly associated with BMD, OCN, and C-peptide levels, indicating a minimal role for FGF21 in bone metabolism [67].
Furthermore, Lee et al. [68] reported an independent positive correlation between plasma FGF21 levels and total BMD of female subjects and spine BMD after adjusting for age, race, and body composition. Interestingly, no association was found between circulating FGF21 concentrations and BMD in young men [68]. In a cross-sectional study, Hu et al. [4]analyzed the associations among circulating FGF21 levels, BMD, and bone turnover biochemical markers (BTM) in a large sample of healthy Chinese Han postmenopausal women, verified that serum FGF21 level was positively correlated with age [[69], [70], [71]], and serum FGF21 levels had a significant positive correlation with BMD of lumbar spine in healthy postmenopausal women, but no significant correlation with BTM. At the same time, they pointed out that BMD in postmenopausal women in this study was generally lower than that in normal individuals, and that the conclusion reached could not directly reflect the relationship between FGF21 and BMD in normal people [4]. Therefore, due to inconsistency in the results of studies on the effects of FGF21 on human bone health, there is a need for more comprehensive studies on more participants to further verify the potential effects of FGF21 on human bones.
Mechanisms of FGF21 in bone metabolism
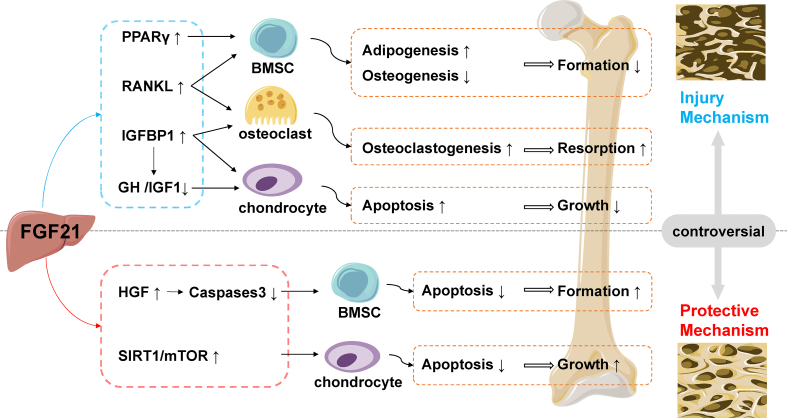
The molecular mechanism of FGF21 in bone homeostasis has not been fully elucidated, and there is still controversy among various experimental data (Fig. 1). Further studies on the molecular mechanism of FGF21 in bone homeostasis need to be conducted. Some studies reported that FGF21 stimulated adipogenesis of BMSCs, inhibited osteoblast activity, and increased osteoclast activity by activating PPARγ [43,49]. However, a study has reported that FGF21 has no direct effect on the PPARα and PPARγ pathways in the regulation of bone homeostasis [53].
Fig. 1.
Injury and protective mechanisms of FGF21 on the bone. FGF21 stimulates BMSCs adipogenesis by activating PPARγ, and decreased osteogenic differentiation leads to reduced bone formation. FGF21 increases osteoclast formation by inducing IGFBP1 or RANKL signaling, resulting in increased bone resorption. FGF21 inhibits the proliferation and differentiation of chondrocytes by inactivating the GH/IGF1 growth axis, and promotes apoptosis leading to attenuated bone growth. FGF21 regulates Caspase3 through HGF, protects BMSCs from oxidative stress-inducedapoptosis, and inhibits chondrocyte apoptosis by activating the SIRT1/mTOR signaling pathway, further promoting bone growth and protecting bones. The figure contains modified templates from Servier Medical Art, licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). PPARγ: peroxisome proliferator-activated receptor-gamma; IGFBP1: insulin-like growth factor binding protein 1; RANKL: receptor activator of nuclear factor κ-B ligand; IGF1/GH: growth hormone-insulin-like growth factor 1 axis; HGF: hepatocyte growth factor; SIRT1/mTOR: silent Information Regulator 1/mammalian target of rapamycin; BMSC: bone marrow mesenchymal stem cell.
The expression of FGFRs and β-klotho has been significantly upregulated in the bones of dystrophic mice, suggesting that bone may serve as a direct target tissue for FGF21 [49,72,73]. FGF21 may directly promote RANKL-induced osteoclastogenesis in BMM through the FGFRs-β-klotho complex, as well as promote bone marrow adipogenesis, while inhibiting the osteogenic differentiation of BMSCs [49]. FGF21 directly binds to the FGFR1-β-klotho axis in growth plate chondrocytes and activates ERK1/2 cascade signaling, thereby inhibiting cell proliferation and differentiation [72,73]. However, the expression of FGFRs and β-klotho in bone tissue has not been fully determined.
Studies have shown that FGF21 is also closely related to the GH-IGF1 growth axis, and downregulation of IGF1-GH and GH-STAT5 by FGF21 inhibited bone growth in mice [44,74]. IGFBP1 has been identified as a novel bone resorption factor secreted by the liver, and the growth axis plays an important role in protein metabolism and bone homeostasis [44,75,76]. FGF21 inhibits GH signaling by decreasing hepatic IGF1 expression and indirectly induces hepatic IGFBP1 secretion. IGFBP1 binds to the precursor osteoclast integrin β1 receptor and enhances ERK phosphorylation and NFATc1 activation stimulated by RANKL, thereby promoting osteoclast formation and leading to bone loss [41,44,45]. Fgf21-deficient mice significantly expressed osteocalcin, suggesting that the regulation of FGF21-IGFBP 1 not only affects the generation and activation of osteoclasts, but also affects the production of osteocalcin in osteoblasts [75]. In addition, FGF21 can also inhibit proliferation and differentiation of growth plate chondrocytes by directly antagonizing GH [73], thus affecting bone homeostasis. However, Li and Lee et al. suggested that the regulation of bone homeostasis by FGF21 was unrelated to hepatic IGF1 and IGFBP1 [12,49,53].
FGF21 activated Smad-BMP2 signaling to promote osteogenesis in the C2C12 cell line [50]. FGF21 treatment in DMM mice upregulated SIRT1/mTOR and induced enhanced autophagic flux, which in turn inhibited chondrocyte apoptosis [16]. FGF21 promotes bone neovascularization by upregulating hepatocyte growth factor (HGF), up-regulating MAPK/pMAPK and down-regulating STAT3/pSTAT3, and up-regulating PI3K/AKT to inhibit Caspase-3 and BMSCs apoptosis, thereby improving bone defects [58].
Conclusion
In summary, the clinical validation of FGF21 as a biomarker of metabolic and bone-related diseases and as a therapeutic intervention target is still needed. Currently, most of the literature has shown that FGF21 has adverse effects on the bone. However, the information on the relationship between FGF21 and bone homeostasis is still limited, and its effect on the bone as well as its mechanism of action are still uncertain. In animal and human studies, different conclusions have been drawn about the relationship between FGF21 levels and bone metabolism regulation. The discrepancy may be due to the differences in FGF21 expression and regulation between animals and humans, such as the inherent differences among different species, the differences in response to FGF21 among different species, and the significant differences and wide variability within the species themselves. Since 2005, when the metabolic activity of FGF21 was first determined by Kharitonenkov et al. [77] in a cell-based high-throughput screening study, many in vitro and in vivo experiments have been conducted to better understand the physiological and pharmacological effects of FGF21 in biological systems. FGF21 is well known for its outstanding role in the treatment of diabetes and obesity-related metabolic disorders; however, it also has many adverse effects on the body. This calls to mind that FGF21-based therapy has potentially deleterious side effects on the skeletal system and further studies are needed to guide clinical application of FGF21 medications and drug development.
Funding
Financial support from Science and Technology Department of Sichuan Province (grant no. 2020YFH0114).
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Owen B.M., Mangelsdorf D.J., Kliewer S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metabol. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H., Sherrier M., Li H. Skeletal muscle and bone - emerging targets of fibroblast growth factor-21. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.625287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Li J., Zhang D., Zhou X., Xie J. Role of the fibroblast growth factor 19 in the skeletal system. Life Sci. 2021;265 doi: 10.1016/j.lfs.2020.118804. [DOI] [PubMed] [Google Scholar]
- 4.Hu W., He J., Fu W., Wang C., Yue H., Gu J., et al. Fibroblast growth factor 21 is associated with bone mineral density, but not with bone turnover markers and fractures in Chinese postmenopausal women. J Clin Densitom. 2019;22:179–184. doi: 10.1016/j.jocd.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Geng L., Lam K.S.L., Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 6.Salminen A., Kaarniranta K., Kauppinen A. Integrated stress response stimulates FGF21 expression: systemic enhancer of longevity. Cell Signal. 2017;40:10–21. doi: 10.1016/j.cellsig.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Fasshauer M., Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Staiger H., Keuper M., Berti L., Hrabe de Angelis M., Haring H.U. Fibroblast growth factor 21-metabolic role in mice and men. Endocr Rev. 2017;38:468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 9.Dong J.Q., Rossulek M., Somayaji V.R., Baltrukonis D., Liang Y., Hudson K., et al. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br J Clin Pharmacol. 2015;80:1051–1063. doi: 10.1111/bcp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y., et al. FGF21 regulates sweet and alcohol preference. Cell Metabol. 2016;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y.T., Hsu B.G., Wang C.H., Lin Y.L., Lai Y.H., Kuo C.H. Lower serum fibroblast growth factor 21 levels are associated with normal lumbar spine bone mineral density in hemodialysis patients. Int J Environ Res Publ Health. 2020;17:1938. doi: 10.3390/ijerph17061938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.Y., Fam K.D., Chia K.L., Yap M.M.C., Goh J., Yeo K.P., et al. Age-related bone loss is associated with FGF21 but not IGFBP1 in healthy adults. Exp Physiol. 2020;105:622–631. doi: 10.1113/EP088351. [DOI] [PubMed] [Google Scholar]
- 13.Kuro O.M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Liu S., Li J., Yi Z. The role of the fibroblast growth factor family in bone-related diseases. Chem Biol Drug Des. 2019;94:1740–1749. doi: 10.1111/cbdd.13588. [DOI] [PubMed] [Google Scholar]
- 15.Rader D.J., Maratos-Flier E., Nguyen A., Hom D., Ferriere M., Li Y., et al. LLF580, an FGF21 analog, reduces triglycerides and hepatic fat in obese adults with modest hypertriglyceridemia. J Clin Endocrinol Metab. 2022;107:e57–e70. doi: 10.1210/clinem/dgab624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H., Jia C., Wu D., Jin H., Lin Z., Pan J., et al. Fibroblast growth factor 21 (FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021;12:865. doi: 10.1038/s41419-021-04157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maratos-Flier E. Fatty liver and FGF21 physiology. Exp Cell Res. 2017;360:2–5. doi: 10.1016/j.yexcr.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Christoffersen B., Straarup E.M., Lykkegaard K., Fels J.J., Sass-Orum K., Zhang X., et al. FGF21 decreases food intake and body weight in obese Gottingen minipigs. Diabetes Obes Metabol. 2019;21:592–600. doi: 10.1111/dom.13560. [DOI] [PubMed] [Google Scholar]
- 19.Tillman E.J., Rolph T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metabol. 2016;23:427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Platek T., Polus A., Goralska J., Razny U., Dziewonska A., Micek A., et al. Epigenetic regulation of processes related to high level of fibroblast growth factor 21 in obese subjects. Genes. 2021;12:307. doi: 10.3390/genes12020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookout A.L., de Groot M.H., Owen B.M., Lee S., Gautron L., Lawrence H.L., et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K.H., Lee M.S. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab J. 2014;38:245–251. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M., Cao H., Hou Y., Sun G., Li D., Wang W. Liver plays a major role in FGF-21 mediated glucose homeostasis. Cell Physiol Biochem. 2018;45:1423–1433. doi: 10.1159/000487568. [DOI] [PubMed] [Google Scholar]
- 25.Biosse Duplan M., Dambroise E., Estibals V., Veziers J., Guicheux J., Legeai-Mallet L. An Fgfr3-activating mutation in immature murine osteoblasts affects the appendicular and craniofacial skeleton. Disease model mech. 2021;14:048272. doi: 10.1242/dmm.048272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benoit B., Meugnier E., Castelli M., Chanon S., Vieille-Marchiset A., Durand C., et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. 2017;23:990–996. doi: 10.1038/nm.4363. [DOI] [PubMed] [Google Scholar]
- 27.Wang D., Eraslan B., Wieland T., Hallström B., Hopf T., Zolg D.P., et al. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol Syst Biol. 2019;15 doi: 10.15252/msb.20188503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 29.Markan K.R., Potthoff M.J. Metabolic fibroblast growth factors (FGFs): mediators of energy homeostasis. Semin Cell Dev Biol. 2016;53:85–93. doi: 10.1016/j.semcdb.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng L., Liao B., Jin L., Yu J., Zhao X., Zhao Y., et al. β-Klotho promotes glycolysis and glucose-stimulated insulin secretion via GP130. Nature metabolism. 2022;4:608–626. doi: 10.1038/s42255-022-00572-2. [DOI] [PubMed] [Google Scholar]
- 31.Adams A.C., Yang C., Coskun T., Cheng C.C., Gimeno R.E., Luo Y., et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metabol. 2012;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minard A.Y., Tan S.X., Yang P., Fazakerley D.J., Domanova W., Parker B.L., et al. mTORC1 is a major regulatory node in the FGF21 signaling network in adipocytes. Cell Rep. 2016;17:29–36. doi: 10.1016/j.celrep.2016.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V., et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anuwatmatee S., Tang S., Wu B.J., Rye K.A., Ong K.L. Fibroblast growth factor 21 in chronic kidney disease. Clin Chim Acta. 2019;489:196–202. doi: 10.1016/j.cca.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Lewis J.E., Ebling F.J.P., Samms R.J., Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metabol. 2019;30:491–504. doi: 10.1016/j.tem.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Salminen A., Kauppinen A., Kaarniranta K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. J Mol Med (Berl) 2017;95:123–131. doi: 10.1007/s00109-016-1477-1. [DOI] [PubMed] [Google Scholar]
- 37.Yan H., Xia M., Chang X., Xu Q., Bian H., Zeng M., et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca H., Moreira-Goncalves D., Coriolano H.J., Duarte J.A. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44:37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 39.Hao R.H., Gao J.L., Li M., Huang W., Zhu D.L., Thynn H.N., et al. Association between fibroblast growth factor 21 and bone mineral density in adults. Endocrine. 2018;59:296–303. doi: 10.1007/s12020-017-1507-y. [DOI] [PubMed] [Google Scholar]
- 40.Wan Y. Bone marrow mesenchymal stem cells: fat on and blast off by FGF21. Int J Biochem Cell Biol. 2013;45:546–549. doi: 10.1016/j.biocel.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L., Song H.Y., Gao L.L., Yang L.Y., Mu S., Fu Q. MicroRNA-100-5p inhibits osteoclastogenesis and bone resorption by regulating fibroblast growth factor 21. Int J Mol Med. 2019;43:727–738. doi: 10.3892/ijmm.2018.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charoenphandhu N., Suntornsaratoon P., Krishnamra N., Sa-Nguanmoo P., Tanajak P., Wang X., et al. Fibroblast growth factor-21 restores insulin sensitivity but induces aberrant bone microstructure in obese insulin-resistant rats. J Bone Miner Metabol. 2017;35:142–149. doi: 10.1007/s00774-016-0745-z. [DOI] [PubMed] [Google Scholar]
- 43.Wei W., Dutchak P.A., Wang X., Ding X., Wang X., Bookout A.L., et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci USA. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inagaki T., Lin V.Y., Goetz R., Mohammadi M., Mangelsdorf D.J., Kliewer S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metabol. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Wei W., Krzeszinski J.Y., Wang Y., Wan Y. A liver-bone endocrine relay by IGFBP1 promotes osteoclastogenesis and mediates FGF21-induced bone resorption. Cell Metabol. 2015;22:811–824. doi: 10.1016/j.cmet.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 47.Kim A.M., Somayaji V.R., Dong J.Q., Rolph T.P., Weng Y., Chabot J.R., et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metabol. 2017;19:1762–1772. doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- 48.Bornstein S., Brown S.A., Le P.T., Wang X., DeMambro V., Horowitz M.C., et al. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014;155:3516–3526. doi: 10.1210/en.2014-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Sun H., Qian B., Feng W., Carney D., Miller J., et al. Increased expression of FGF-21 negatively affects bone homeostasis in dystrophin/utrophin double knockout mice. J Bone Miner Res. 2020;35:738–752. doi: 10.1002/jbmr.3932. [DOI] [PubMed] [Google Scholar]
- 50.Ishida K., Haudenschild D.R. Interactions between FGF21 and BMP-2 in osteogenesis. Biochem Biophys Res Commun. 2013;432:677–682. doi: 10.1016/j.bbrc.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y., Lu W., Hou Y., Fu K., Gan F., Liu J. Fibroblast growth factor 21 ameliorates vascular calcification by inhibiting osteogenic transition in vitamin D3 plus nicotine-treated rats. Biochem Biophys Res Commun. 2018;495:2448–2455. doi: 10.1016/j.bbrc.2017.10.115. [DOI] [PubMed] [Google Scholar]
- 52.Liu X., Cao F., Liu S., Mi Y., Liu J. BMP2/Smad signaling pathway is involved in the inhibition function of fibroblast growth factor 21 on vascular calcification. Biochem Biophys Res Commun. 2018;503:930–937. doi: 10.1016/j.bbrc.2018.06.098. [DOI] [PubMed] [Google Scholar]
- 53.Li X., Stanislaus S., Asuncion F., Niu Q.T., Chinookoswong N., Villasenor K., et al. FGF21 is not a major mediator for bone homeostasis or metabolic actions of PPARalpha and PPARgamma agonists. J Bone Miner Res. 2017;32:834–845. doi: 10.1002/jbmr.2936. [DOI] [PubMed] [Google Scholar]
- 54.Andersen B., Straarup E.M., Heppner K.M., Takahashi D.L., Raffaele V., Dissen G.A., et al. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int J Obes. 2018;42:1151–1160. doi: 10.1038/s41366-018-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinton P.S., Rector R.S., Linden M.A., Warner S.O., Dellsperger K.C., Chockalingam A., et al. Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur J Clin Nutr. 2012;66:606–612. doi: 10.1038/ejcn.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jimenez V., Jambrina C., Casana E., Sacristan V., Munoz S., Darriba S., et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol Med. 2018;10:e8791. doi: 10.15252/emmm.201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson K.E., Guillot M., Graziano M.J., Mangipudy R.S., Chadwick K.D. Pegbelfermin, a PEGylated FGF21 analogue, has pharmacology without bone toxicity after 1-year dosing in skeletally-mature monkeys. Toxicol Appl Pharmacol. 2021;428 doi: 10.1016/j.taap.2021.115673. [DOI] [PubMed] [Google Scholar]
- 58.Yang S., Guo Y., Zhang W., Zhang J., Zhang Y., Xu P. Effect of FGF-21 on implant bone defects through hepatocyte growth factor (HGF)-mediated PI3K/AKT signaling pathway. Biomed Pharmacother. 2019;109:1259–1267. doi: 10.1016/j.biopha.2018.10.150. [DOI] [PubMed] [Google Scholar]
- 59.Pye S.R., Almusalam B., Boonen S., Vanderschueren D., Borghs H., Gielen E., et al. Influence of insulin-like growth factor binding protein (IGFBP)-1 and IGFBP-3 on bone health: results from the European Male Ageing Study. Calcif Tissue Int. 2011;88:503–510. doi: 10.1007/s00223-011-9484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z.C., Xiao J., Wang G., Li M.Q., Hu K.Z., Ma T., et al. Fibroblast growth factor-21 concentration in serum and synovial fluid is associated with radiographic bone loss of knee osteoarthritis. Scand J Clin Lab Invest. 2015;75:121–125. doi: 10.3109/00365513.2014.992942. [DOI] [PubMed] [Google Scholar]
- 61.Fazeli P.K., Faje A.T., Cross E.J., Lee H., Rosen C.J., Bouxsein M.L., et al. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. 2015;77:6–11. doi: 10.1016/j.bone.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallego-Escuredo J.M., Lamarca M.K., Villarroya J., Domingo J.C., Mateo M.G., Gutierrez M.D.M., et al. High FGF21 levels are associated with altered bone homeostasis in HIV-1-infected patients. Metabolism. 2017;71:163–170. doi: 10.1016/j.metabol.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 64.McComsey G.A., Tebas P., Shane E., Yin M.T., Overton E.T., Huang J.S., et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L., Li M., Zha Q., Yang M., Yu J., Pan M., et al. Fibroblast growth factor 21 (FGF21) is a sensitive marker of osteoporosis in haemodialysis patients: a cross-sectional observational study. BMC Nephrol. 2021;22:183. doi: 10.1186/s12882-021-02393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lui D.T.W., Lee C.H., Chau V.W.K., Fong C.H.Y., Yeung K.M.Y., Lam J.K.Y., et al. Potential role of fibroblast growth factor 21 in the deterioration of bone quality in impaired glucose tolerance. J Endocrinol Invest. 2021;44:523–530. doi: 10.1007/s40618-020-01337-y. [DOI] [PubMed] [Google Scholar]
- 67.Choi H.S., Lee H.A., Kim S.W., Cho E.H. Association between serum fibroblast growth factor 21 levels and bone mineral density in postmenopausal women. Endocrinol Metab (Seoul) 2018;33:273–277. doi: 10.3803/EnM.2018.33.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee P., Linderman J., Smith S., Brychta R.J., Perron R., Idelson C., et al. Fibroblast growth factor 21 (FGF21) and bone: is there a relationship in humans? Osteoporos Int. 2013;24:3053–3057. doi: 10.1007/s00198-013-2464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taniguchi H., Tanisawa K., Sun X., Higuchi M. Acute endurance exercise lowers serum fibroblast growth factor 21 levels in Japanese men. Clin Endocrinol. 2016;85:861–867. doi: 10.1111/cen.13162. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F., et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 71.Hanks L.J., Gutierrez O.M., Bamman M.M., Ashraf A., McCormick K.L., Casazza K. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J Clin Transl Endocrinol. 2015;2:77–82. doi: 10.1016/j.jcte.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubicky R.A., Wu S., Kharitonenkov A., De Luca F. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology. 2012;153:2287–2295. doi: 10.1210/en.2011-1909. [DOI] [PubMed] [Google Scholar]
- 73.Wu S., Levenson A., Kharitonenkov A., De Luca F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol Chem. 2012;287:26060–26067. doi: 10.1074/jbc.M112.343707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keipert S., Ost M., Johann K., Imber F., Jastroch M., van Schothorst E.M., et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab. 2014;306:E469–E482. doi: 10.1152/ajpendo.00330.2013. [DOI] [PubMed] [Google Scholar]
- 75.Chung C., Insogna K.L. The liver throws the skeleton a bone (resorption factor) Hepatology. 2016;64:977–979. doi: 10.1002/hep.28688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milman S., Huffman D.M., Barzilai N. The somatotropic Axis in human aging: framework for the current state of knowledge and future research. Cell Metabol. 2016;23:980–989. doi: 10.1016/j.cmet.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J., et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]