Abstract
Background
The month of Ramadan is a holy month for Muslims. During this month, Muslims do not eat, drink, or smoke from sunrise to sunset. Patients with type 2 diabetes mellitus (T2DM) will also fast from dawn to dusk, creating a unique opportunity to study the effects of dietary changes during fasting period. One of the interesting results of Ramadan fasting is its effect on endothelial dysfunction, measured using Intercellular Adhesion Molecule-1 (ICAM-1) as a biological marker of endothelial function.
Aim
To determine the changes ICAM-1 levels in T2DM and non-DM patients during Ramadan fasting.
Methods
A retrospective cohort study was performed on 26 T2DM patients and 21 non-DM, age-matched patients (aged 19–60 years). Measurement of metabolic parameters (systolic and diastolic blood pressure, total calorie intake, and intensity of physical activity), anthropometry (body weight, body mass index (BMI) and abdominal circumference), total dietary intake, and laboratory analysis (blood glucose fasting, HbA1c, lipid profile, ICAM-1) were done at 4 weeks before (T0) and 14 days after Ramadan fasting (T1).
Result
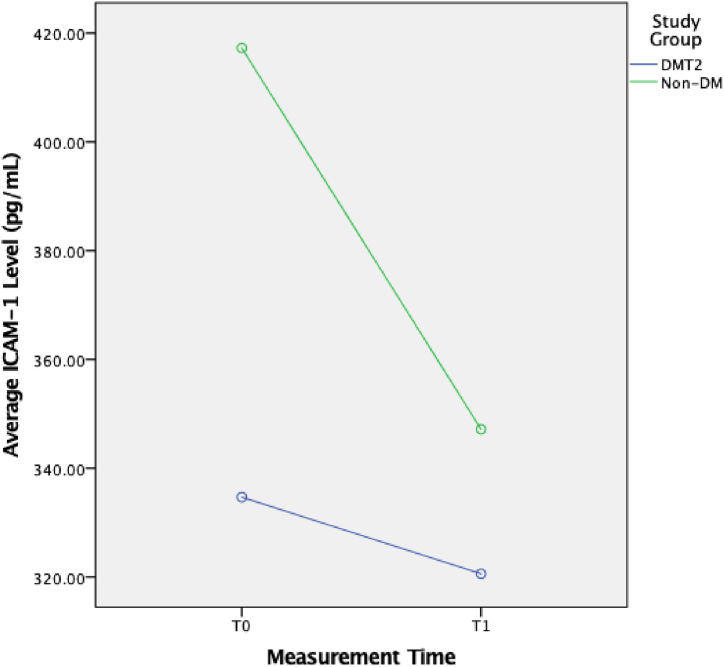
The median ICAM-1 level in T2DM patients at T0 was 340.9 (193–505) ng/mL and at T1 was 312.3 (158–581) ng/mL, while the ICAM-1 level in non-DM patients at T0 was 482 (305–653) and at T1 was 398.4 (202–526) ng/mL. There was no significant difference of ICAM-1 level between study groups at both T0 and T1 (p > 0.05). Both T2DM and non-DM patients had lower ICAM-1 level following Ramadan fasting. However, only non-DM patients had significantly lower post Ramadan ICAM-1 (p = 0.008)
Conclusion
There was a significant decrease in ICAM-1 level in both T2DM and non-DM patients after Ramadan fasting.
Keywords: Ramadan fasting, Diabetes mellitus, Intercellular adhesion Molecule-1 (ICAM-1)
1. Introduction
The prevalence of diabetes has increased worldwide in the last decade. Internationally, the burden of diabetes is also very large, consuming about 11.6% of global health expenditure in 2015 (673 million dollars) [1]. Diabetes mellitus type 2 (T2DM) is the most impactful independent risk factor for coronary heart disease, known to increase the risk of cardiovascular disease by two to three times [2]. Endothelial lining in diabetes mellitus patients is more prone to form atherosclerotic plaques than patients without diabetes mellitus, inducing endothelial dysfunction [2].
The increasing rate of T2DM and obesity are substantial despite the efforts that have been made to reduce health costs [3]. Therefore, another unique approach is needed, one of which is to take advantage of the obligation to fast in some populations [4]. Intermittent fasting is a safe and cost-effective way to ameliorate health status [3]. Ramadan fasting is a form of intermittent fasting done by Muslims worldwide using the time-restricted feeding (TRF) method [3]. Existing studies support the notion that calorie restriction and intermittent fasting will improve the cerebrovascular, cardiovascular, and metabolic systems by decreasing insulin resistance and increasing insulin sensitivity. These processes could ameliorate the degree of endothelial dysfunction, especially in obese diabetic patients [3].
Although it is widely known as an important phenomenon in cardiovascular health, there has been no agreement on the ideal test for the evaluation of endothelial dysfunction [5]. Current measurements of circulating endothelial biomarkers is a potential alternative to be used in clinical trials of endothelial dysfunction [6]. Biomarker measurements are recommended as they are accurate, standardized, consistent, and suitable for most conditions, while also easily interpreted by clinicians [7]. One of the biomarkers in endothelial function is Intercellular Adhesion Molecule-1 (ICAM- 1). This biomarker are expressed by endothelial cells and leukocytes as a response to inflammatory cytokines increased levels of free fatty acids, oxidation by low-density lipoprotein (LDL), and the appearance of Advance Glycosylation End Products (AGEs) occurring in diabetes mellitus.
Serum ICAM-1 (sICAM-1) is positively correlated with leukocyte binding to the blood vessels, suggesting that sICAM-1 may be used as a reliable measure of inflammation and endothelial dysfunction in blood vessel walls [8,9]. Previous studies have shown that fasting regiments will effectively reduce inflammatory biomarkers, including Ramadan fasting [10,11]. Moreover, Ramadan fasting is known to improve endothelial function in patients with cardiovascular diseases [12]. However, there has been no studies assessing the change of ICAM-1 level in diabetes mellitus patients during Ramadan fasting. This study aims to assess changes of ICAM- 1 levels in from 4 weeks before Ramadan fasting to 14 days after Ramadan fasting, both in T2DM and non-DM adults.
2. Material and methods
2.1. Study subject
This is a retrospective cohort study involved 26 T2DM and 21 non-DM patients. Subjects was recruited using consecutive method. This research was conducted from March until May 2019 at the Metabolic Endocrine outpatient clinic of Cipto Mangunkusumo Hospital (RSCM) and the Metabolic Vascular and Aging Cluster (MVA). ICAM-1 analysis was conducted from June until July 2020 from participants’ blood samples that have been stored in The Indonesian Medical Education and Research Institute (IMERI)-Faculty of Medicine, Universitas Indonesia (FKUI).
The inclusion criteria for this study were type 2 diabetes mellitus patients aged 19–60 years old who fast during Ramadan for at least 14 days consecutively and age-matched non-diabetes mellitus patients who also fast during Ramadan for at least 14 days, consecutively. Patients with end-stage renal disease, liver cirrhosis, chronic gastrointestinal disease, cardiovascular disease, autoimmune disease, or having non-steroidal anti-inflammatory drugs, steroids, or antibiotics within 1 month were excluded from the study.
This study was approved by Ethical Committee of Faculty of Medicine Universitas Indonesia with ethical clearance number ND 673/UN2.FI/ETIK/PPM.February 00, 2019. The consent for study and publication was obtained from the participants of the study.
2.2. Ramadan fasting
Ramadan fasting is an annual holy period for Muslims which follows the Islamic calendar. Therefore, it is an annual event with irregular cycle. In Indonesia, there are only two seasons, namely dry and monsoon season. Being a tropical country, it is hot and humid throughout the year, creating a stable environment. The Ramadan fasting involves one month fasting from sunrise until sunset. During the fasting period, those who fast do not eat, drink, or smoke.
2.3. Examination and laboratory measurements
Research subjects who were willing to take part in the research underwent initial screening and collection of basic data including history taking, physical examination, and laboratory examinations. Waist circumference was measured using an ergonomic circumference measuring tape based on the WHO standard protocol. Body weight was measured in kilograms using Tanita MC780MA portable bio impedance analyzer (Tanita®, Japan), along with body composition such as fat percentage, lean mass, and total body water (TBW). The body mass index (BMI) was calculated by dividing the weight (kg) to the square of height (m) and categorized according to WHO Asia Pacific criteria.
Blood sampling for laboratory examination was taken at the clinical pathology installation and stored in the MVA cluster, IMERI-FKUI. Data were collected in two time frame, namely the pre-Ramadan period (four weeks before the start of Ramadan fasting (T0)) and the post-Ramadan period (14 days after the last day of Ramadan fasting (T1)). Blood samples were withdrawn after at least 10 h of fasting. A total of 5 ml of blood samples was collected through peripheral vein in vacutainer tubes containing Ethylenediaminetetraacetic acid (EDTA). Serum HbA1c level was measured using A1c EZ 2.0 Glycohemoglobin Analyzer [Biohermes®, USA] and the other blood parameters was measured using Abbott Architect c8000 device (Abbott®, USA) using standardized method. The ICAM-1 level was examined by Enzyme-Linked Immunosorbent Assay (ELISA) method using human ICAM-1 ELISA Kit (RnD system®, USA). Nutritional data was obtained using three days food record questionnaire that was confirmed by certified dietitian from the Southeast Asian Ministers of Education Organization Regional Center for Food and Nutrition (SEAMEO-REFCON). The nutritional components were analyzed using Nutrisurvey 2007 (Nutrisurvey®, Germany) consisting of total calorie intake, carbohydrates, fat, protein, and fiber.
2.4. Statistical analysis
Data processing was performed using Statistical Package for Social Sciences (SPSS) version 25 for Macintosh software (IBM®, USA). Data presentation was done in the form of proportions for categorical data, mean ± SB for numerical data with normal distribution, and median (minimum-maximum) for numerical data with abnormal distribution. Normality test was done using Kolmogorov-Smirnov test.
The changes of ICAM-1 level in T2DM and non-DM were analyzed using Wilcoxon non-parametric test. The difference between ICAM-1 in both groups was analyzed using Mann Whitney test. Multilevel linear model multivariate test was performed to assess the factors affecting the changes. Statistical significance was expressed as a p value < 0.05 with 95% confidence interval.
2.5. Result
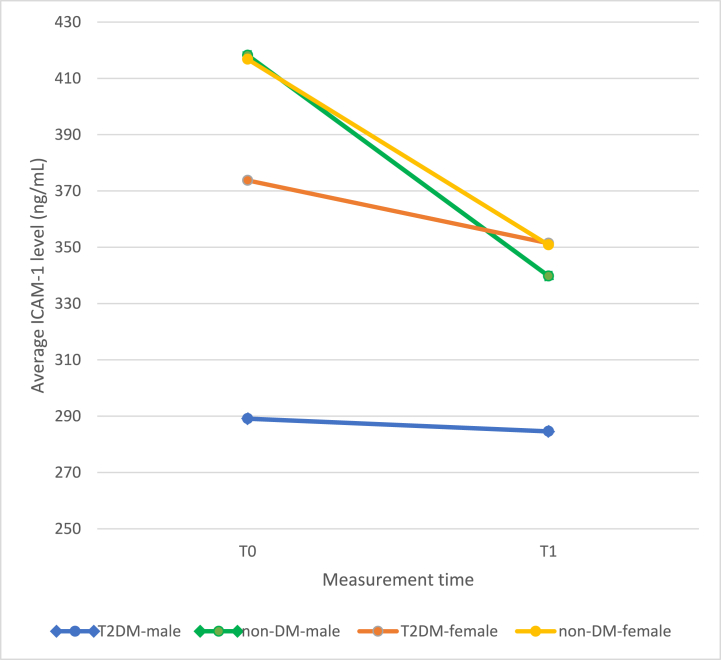
During the study, there were 26 T2DM subjects and 21 non-DM subjects recruited. The complete baseline characteristics of the subjects can be found in Table 1. There was no statistically significant difference in ICAM-1 level and ICAM-1 level change between the T2DM and non-DM groups (Table 2). However, there was a statistically significant change in ICAM-1 level in the non-DM group (p < 0.05). Although ICAM-1 change in the T2DM group was not statistically significant, it was clinically significant (Fig. 1, Fig. 2).
Table 1.
Baseline characteristics of the subjects.
| Variable | T2DM |
Non-DM |
p |
|---|---|---|---|
| n = 26 | n = 21 | ||
| Age | 54,4 ± 7,4 | 51,7 ± 7,1 | 0.210 |
| Male sex | 12 (46.1%) | 7 (33.3%) | 0.373 |
| Body Weight (kg) | 65.18 ± 10.9 | 63.65 ± 9.04 | 0.398 |
| Body Mass Index (kg/m2) | 0.670 | ||
| Underweight | 0 | 1 (4.8%) | |
| Normoweight | 6 (23.1%) | 6 (28.6%) | |
| Overweight | 4 (15.4%) | 3 (14.3%) | |
| Obese | 16 (61.5%) | 11 (52.4%) | |
| Systolic blood pressure (mmHg) | 127 (100–160) | 105 (90–150) | 0.053 |
| Diastolic blood pressure (mmHg) | 80 (65–100) | 70 (60–80) | 0.131 |
| Waist circumference (cm) | 90.2 ± 10,5 | 83,6 ± 9.7 | 0.068 |
| Physical Activity (METs) | 1.97 ± 0,4 | 2.01 ± 0,4 | 0.642 |
| Laboratory results | |||
| Fasting Blood Glucose (g/dL) | 121.5 (81–297) | 88 (79–116) | < 0.001 |
| Hb1Ac (%) | 8.3 (5.6–14.2) | 5.45 (4.1–6) | < 0.001 |
| Total cholesterol (mg/dL) | 186.7 (1.93–467) | 116.4 (61–285) | 0.638 |
| Dietary intake before Ramadan | |||
| Total nutrient (kcal) | 1.255 (818–2205) | 1.248 (876–1672) | 0.748 |
| Carbohydrate (gram) | 176.7 (104–235) | 175.2 (102–233) | 0.416 |
| Fat (gram) | 49.85 (19.5–107.8) | 38.17 (31.0–56.8) | 0.293 |
| Protein (gram) | 45.3 (21.8–95.7) | 42.3 (32.5–63.3) | 0.207 |
| Dietary intake after Ramadan | |||
| Total nutrient (kcal) | 1.035 (393–2.048) | 989 (583.6) | 0.257 |
| Carbohydrate (gram) | 147.5 (77.6–266.8) | 135.9 (76.5–170) | 0.346 |
| Fat (gram) | 43.3 (7.19–85.36) | 33.2 (20.1–60.6) | 0.134 |
| Protein (gram) | 35.8 (5.54–85) | 34.78 (16.7–42.1) | 0.095 |
*Numerical data with normal distribution are presented with means and standard deviations. Numeric data with abnormal distribution are presented with median and minimum-maximum values. Categorical data are displayed as absolute amounts and percentages.
Table 2.
Changes in ICAM-1 Level in T2DM Patients vs Non-DM.
| Endothelial Biomarker | T2DM [1] (N = 26) | P(1)a | Non-DM [2] (N = 26) | P(2)a | P(1,2)b |
|---|---|---|---|---|---|
| Pre-Ramadan ICAM-1 (ng/mL) | 340.9 (193–505)a | 0.228ac | 482 (305–653)b | 0.008bd | 0.106ab |
| Post-Ramadan ICAM-1 (ng/mL) | 312.3 (158–581)c | 398.4 (202–526)d | 0.363cd | ||
| ΔICAM-1 (ng/mL) | −44.7 (-215–366)e | −52.6 (-279–42.8)f | 0.422ef |
Wilcoxon test.
Kruskal Wallis test.
Fig. 1.
Changes of ICAM-1 Level in T2DM and non-DM Groups.
Fig. 2.
Changes of ICAM-1 level according to sex and T2DM group.
There was no statistically significant difference during the subgroup analysis between sex. Moreover, there was no correlation between ICAM-1 change to A1c and initial body weight.
3. Discussion
The results of this study in the T2DM group found no significant difference in the decrease in ICAM-1 level before and after Ramadan fasting in T2DM patients. However, the change of ICAM-1 level was significant in non-DM patients. This phenomenon may be explained by examining the comparison between pre- and post- Ramadan fasting dietary intake between study groups. There was only a minimal changes on the dietary intake on T2DM group, while the changes were more prominent in the non-DM group. Although there was no statistically significant difference in dietary intake between groups, there was a significant decrease in the consumption of fat and protein, while there was less difference in carbohydrate consumption. However, we did not record the dietary intake during the Ramadan fasting period, thus limiting the significance of our analysis.
Moreover, the limited decrease of ICAM-1 level in T2DM might be explained by other variables. First, the duration of T2DM in patients varied widely. The early stages of T2DM start with abnormalities in the form of insulin resistance. However, the later stage of T2DM is the complicated failure of pancreatic beta cells, making insulin deficiency more prominent [13]. Second, although the total calorie intake did decrease significantly, the carbohydrate consumption did not change significantly. This phenomenon occurred because of the tendency of consuming sweet and carbohydrate-heavy food [1] Third, the total cholesterol level in T2DM subjects was already high at baseline, while fasting did not cause a significant decrease. This would further worsen the outcome, as the oxidation of LDL plays a role in increasing ICAM-1 expression [8,14].
Moreover, there were more obese subjects with higher waist circumference in the T2DM group. In previous study by Rochlani et al. (2017), BMI and waist circumference were positively correlated with ICAM-1 level due insulin resistance, of which the deposit of visceral fat is more affected than subcutaneous fat [15] Insulin increases glucose uptake in muscles and liver, thereby inhibiting lipolysis and gluconeogenesis. Insulin resistance in fat tissue also inhibits insulin-mediated lipolysis [15].
Another issue surrounding the accuracy of this study was the timing of laboratory tests. Subsequent circadian changes during fasting such as sleep deprivation were associated with decreased glucose tolerance and increased insulin resistance. Moreover, the blood tests were carried out in the afternoon when the cortisol level was higher. Furthermore, during the fasting month of Ramadan, the variability of blood sugar sharply changes due to depletion and repletion of blood sugar. It is thought that endothelial damage due to blood sugar levels is not only associated with constant high blood sugar, but also the fluctuation of blood sugar levels [4]. There was also the issue of patient compliance during Ramadan fasting which was thought to affect changes in ICAM-1 level in T2DM patients during fasting.
The ICAM-1 level observed in this study was also higher in the non-DM group. This phenomenon might be explained by the presence of insulin resistance, hypertension, and obesity [16]. Although the differences between groups are statistically insignificant, overweight and obese subjects are more prevalent in the T2DM group. Moreover, the systolic blood pressure was higher in T2DM group. At the beginning of the examination, ICAM-1 levels in DM patients were lower than in non-DM patients. With the pathophysiological assumptions that occur in T2DM patients, ICAM-1 levels in DM patients when compared to non-DM patients should be higher. This was in accordance with the HOORN study which resulted in a higher ICAM-1 level in T2DM patients compared to non-DM patients [17,18]. However, the lower level of ICAM-1 level could be caused by the use of oral antidiabetic drugs such as the biguanide and thiazolidinediones. Metformin improves endothelial function by increasing insulin sensitivity [19], while thiazolidinediones also increase insulin-mediated glucose uptake via activation of PPARs [20]. In addition, it also has an effect on adipose tissue suppression by suppressing TNF, suppressing lipolysis which in turn suppresses fatty acid production and increases adiponectin [20]. Other drugs that can affect ICAM-1 level are ACE-I (Angiotensin Converting Enzyme-Inhibitor), ARB (Angiotensin Receptor Blocker) and statin drugs. Angiotensin has a pro-oxidative effect on blood vessels which in turn will reduce NO bioavailability, while statins are known to reduce LDL levels and improve insulin resistance, which will ultimately improve endothelial function [2]. Besides treatment, the rate of obese and overweight subjects in the non-DM group was relatively high, possibly affecting the ICAM-1 level.
The limitation of this study was the absence of dietary intake record during Ramadan fasting and the measurement of subjects’ compliance to the fasting regiment. However, this study proved that there was an improvement in endothelial function, both in T2DM and non-DM patients, after Ramadan fasting.
4. Conclusion
There was a significant decrease in ICAM-1 level in both T2DM and non-DM patients after Ramadan fasting. This phenomenon shows that Ramadan fasting has a beneficial effect of reducing the inflammation markers for both T2DM and non-DM population.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by a grant from the University of Indonesia (Grant No: NKB-1523/UN2.RST/HKP.February 05, 2020). We would like to thank all those who contributed to this study. We also would like to thank Cluster Metabolic and Vascular Aging, Indonesian Medical Education and Research Institute (IMERI) team for their support in this research.
References
- 1.Hassanein M., Al-Arouj M., Hamdy O., Bebakar W.M.W., Jabbar A., Al-Madani A., et al. Diabetes and Ramadan: practical guidelines. Diabetes Res. Clin. Pract. 2017;126:303–316. doi: 10.1016/j.diabres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Tousoulis D., Kampoli A.-M., Stefanadis C. Diabetes mellitus and vascular endothelial dysfunction: current perspectives. Curr. Vasc. Pharmacol. 2012;10(1):19–32. doi: 10.2174/157016112798829797. [DOI] [PubMed] [Google Scholar]
- 3.Mo'ez Al-Islam E.F., Jahrami H.A., Obaideen A.A., Madkour M.I. Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: systematic review and meta-analysis. Journal of Nutrition & Intermediary Metabolism. 2019;15:18–26. [Google Scholar]
- 4.Golbidi S., Daiber A., Korac B., Li H., Essop M.F., Laher I. Health benefits of fasting and caloric restriction. Curr. Diabetes Rep. 2017;17(12):1–11. doi: 10.1007/s11892-017-0951-7. [DOI] [PubMed] [Google Scholar]
- 5.Verma S., Buchanan M.R., Anderson T.J. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 6.Abebe W., Mozaffari M. Endothelial dysfunction in diabetes: potential application of circulating markers as advanced diagnostic and prognostic tools. EPMA J. 2010;1(1):32–45. doi: 10.1007/s13167-010-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storch A.S., Mattos JDd, Alves R., Galdino IdS., Rocha H.N.M. Methods of endothelial function assessment: description and applications. International Journal of Cardiovascular Sciences. 2017;30:262–273. [Google Scholar]
- 8.Van Buul J.D., Van Rijssel J., Van Alphen F.P., van Stalborch A.-M., Mul E.P., Hordijk P.L. ICAM-1 clustering on endothelial cells recruits VCAM-1. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meigs J.B., Hu F.B., Rifai N., Manson J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Yang Q., Liao Q., Li M., Zhang P., Santos H.O., et al. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. 2020:79–80. doi: 10.1016/j.nut.2020.110974. [DOI] [PubMed] [Google Scholar]
- 11.Askari V.R., Alavinezhad A., Boskabady M.H. The impact of “Ramadan fasting period” on total and differential white blood cells, haematological indices, inflammatory biomarker, respiratory symptoms and pulmonary function tests of healthy and asthmatic patients. Allergol. Immunopathol. 2016;44(4):359–367. doi: 10.1016/j.aller.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Yousefi B., Faghfoori Z., Samadi N., Karami H., Ahmadi Y., Badalzadeh R., et al. The effects of Ramadan fasting on endothelial function in patients with cardiovascular diseases. Eur. J. Clin. Nutr. 2014;68(7):835–839. doi: 10.1038/ejcn.2014.61. [DOI] [PubMed] [Google Scholar]
- 13.Almulhem M., Susarla R., Alabdulaali L., Khunti K., Karamat M.A., Rasiah T., et al. The effect of Ramadan fasting on cardiovascular events and risk factors in patients with type 2 diabetes: a systematic review. Diabetes Res. Clin. Pract. 2020;159 doi: 10.1016/j.diabres.2019.107918. [DOI] [PubMed] [Google Scholar]
- 14.Goncharov N.V., Nadeev A.D., Jenkins R.O., Avdonin P.V. Markers and biomarkers of endothelium: when something is rotten in the state. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/9759735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochlani Y., Pothineni N.V., Kovelamudi S., Mehta J.L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Therapeutic advances in cardiovascular disease. 2017;11(8):215–225. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding E., Song Y., Manson J., Rifai N., Buring J., Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 17.de Jager J., Dekker J.M., Kooy A., Kostense P.J., Nijpels G., Heine R.J., et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler. Thromb. Vasc. Biol. 2006;26(5):1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4. [DOI] [PubMed] [Google Scholar]
- 18.Wu J., Liang Z., Zhou J., Zhong C., Jiang W., Zhang Y., et al. Association of biomarkers of inflammation and endothelial dysfunction with fasting and postload glucose metabolism: a population-based prospective cohort study among inner Mongolians in China. Can. J. Diabetes. 2016;40(6):509–514. doi: 10.1016/j.jcjd.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Nizami H.L., Banerjee S.K. Mechanisms of Vascular Defects in Diabetes Mellitus. Springer; 2017. Mechanisms of action of drugs for treating endothelial dysfunction in diabetes mellitus; pp. 483–514. [Google Scholar]
- 20.Hadi H.A., Suwaidi J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007;3(6):853–876. [PMC free article] [PubMed] [Google Scholar]