Abstract
The aim of this research is to investigate lipid-lowering influence of dietary ginger (Zingier officinales Rocs) polysaccharides (GPS) on hyperlipidemia rats. Rat models with hyperlipidemia was established by high-fat food diet (HFD). Comparing to GP-negative model group, GPS attenuated several effects of HFD feeding, including the levels of blood lipid biochemistry, serum inflammatory markers (tumor necrosis factor TNF-a, interleukin IL-6), antioxidant capacity (superoxide dismutase SOD, glutathione peroxidase GSH-Px, total antioxidant capacity T-AOC, propylene dialdehyde MDA), uric acid and immune index. 16 S rDNA gene sequencing of fecal samples showed that GPS increased the growth of Akkermansia muciniphila and decreased the proportion of Firmicutes to Bacteroidetes; This changes in microbial community structure can help prevent diet-induced metabolic disease. These results suggest that GPs may act on the gut, changing the structure of the gut microbial community, thereby reducing intestinal and systemic inflammation, thus improved metabolic outcomes.
Keywords: Ginger polysaccharides (GPS), Blood lipids, Antioxidant capacity, Uric acid, Gut microbiota, Hyperlipidemia
1. Introduction
Hyperlipidemia is a kind of disease of lipid metabolism. The biomarkers of hyperlipidemia are high levels of total cholesterol (TC), low density lipoprotein (LDL), triacylglycerol (TG) and/or low level of high density lipoprotein (HDL) in the blood. It has now believes that hyperlipidemia is an important dangerous factors for coronary heart disease [1]. Epidemiological and research in vitro have suggested that hyperlipidemia/oxidized phospholipids may have adverse effects on the bones [[2], [3], [4]]. Hyperlipidemia-induced NALS (non-alcoholic liver steatosis) leading to remarkble cellular aggregation of hemostatic serpins in hepatic [5]. Increasing evidences have shown that hyperlipidemia can cause the body to be in an inflammatory state [6,7]. Inflammatory process plays a key role in the development of arteriosclerosis and occurrence of coronary heart disease (CHD) [8]. Hyperlipidemia, as well as hypertension, insulin resistance, and obesity, are a group of metabolic syndromes X (MSXs) that increase an interindividual risk of developing type 2 diabetes and cardiac vascular disease (CVD). Recent studies have also found that these metabolic changes are closely related to changes in the composition of the gut flora and rotator cuff tear [9,10].
The incidence and prevalence of hyperlipidemia are increasing all over the world, which has become an important public health problem in recent years. The study of blood lipid reduction drugs has also become a hot spot of research, especially in terms of herbal products and Traditional Chinese Medicine [[11], [12], [13], [14], [15], [16]]. Ginger (Zingiber officinale Roscoe, Zingiberacae) is the fresh root of perennial herb ginger, also known as “ginger root”, “spicy cloud”, or “hook fingers”. Ginger has been used as a traditional Chinese medicine for more than 2500 years. Ginger is widely believed to have the gastrointestinal benefits of promoting digestion. It is a common ingredient for food and beverages in many countries. As studies towards ginger proceed, a new beneficial aspect of ginger has been revealed to the public. In addition to the traditional efficacy of ginger, it may also have positive effects in terms of weight loss [17], anti-tumor [[18], [19], [20]], anti-inflammatory [21], and anti-oxidant [22] functionalities. Ginger contains a variety of different compounds. The major constituents are carbohydrates (60–70%), protein (9%), lipids (3–8%), crude fiber, terpenes and phenolic compound. With the development of separation technology, the efficacy of active components in ginger has drawn more and more attention in research. Researchers have recently verified polysaccharides in ginger as a type of antioxidant while few research focused on other bioactivities of ginger polysaccharides (Wang et al., 2018). Ginger polysaccharide can improve the contractility of toad gastrocnemius muscle by improving the contractility of toad gastrocnemius muscle. At the same time, ginger polysaccharide can significantly reduce the content of lactic acid and MDA in toad gastrocnemius muscle, indicating that ginger polysaccharide has anti-fatigue effect (Xia et al., 2014). Song et al. found that feeding ginger polysaccharide to cerebral ischemia-reperfusion injury model rats can significantly improve the behavior disorder of model rats, increase the activity of SOD in serum and brain tissue of model rats, and reduce the content of MDA. And reduce the NO content and water content in the brain tissue of model rats. It shows that ginger polysaccharide has protective effect on cerebral ischemia injury, and its possible mechanism is related to the scavenging of oxygen free radicals and anti-lipid peroxidation by ginger polysaccharide (Song et al., 2015). These research offered significant basics for further understanding the pharmacological effects of ginger, and whether the effect of ginger on metabolic disorders (such as hyperlipidemia and obesity) is related to changes in the structure of the intestinal microbiota.
To evaluate the effects on hyperlipidemia and/or efficacy on the metabolic syndrome (MSXs) of dietary GPs, this study used experimental hyperlipidemia rats as a model, and studied the effects of daily consumption of GPs on their lipid biochemistry, uric acid, immune and antioxidant function, and monitored the structure of their intestinal microbiota.
2. Materials and methods
2.1. Chemical reagents and apparatus
Reagents used in this study were ginger (Ginger provided by the Jiushan, Ganzhou (26.14° N,115.48° E). TG, TC, LDL, HDL, Cr (Creatinine), BUN (Blood urea nitrogen), UA (Uric acid), alkaline phosphatase (ALP), total bile acid (TBA) Content Testing Kitsupplied by Shanghai Cohua Biotechnology Co. Ltd., Blood SOD, GSH-Px, MDA, T-AOC, IL-6, TNF-α Content detection Kit supplied by Shanghai Herpen Biotechnology Co. Ltd. 4% Polyformaldehyde, HE dye, paraffin, and distilled water.
Apparatus used in this study were fully automatic biochemical instruments (Beckman AU480), Enzyme Marker (Thermo), Microscope (IX37 Biomicroscope), Electronic Balance (AUY120 Sajima Analysis Balance).
2.2. The extraction of ginger polysaccharide
Ginger was washed out of sediment, precipitated into pulp, then received freeze-drying by Nanchang Wanhua Biochemical Products Co., Ltd. and finally crushed into powder, sieved over No. 60mesh. GPs were extracted by hot-water extraction (HWE). HWE was carried out at 90 °C for 4 h in aqueous bath. The extract was then centrifuged at 4000 r/min for 20 min and the supernatant was concentrated to 1/4 vol under vacuum concentration. Then the crude GPs were obtained by a series of steps such as deproteinization with sevage reagents (Chloroform: pentanol or butanol, mixed at a ratio of 4:1), alcohol precipitation and vacuum drying at 40 °C (Yang et al., 2018).
Polysaccharide extraction rate (%) = (determined polysaccharide concentration × sugar liquid volume × Dilution multiple)/Ginger powder mass × 100%
2.3. Experiment animal
Fifty male S. D (Sprague Dawley Rat) rats, weighing 180–220 g were provided by the experimental Animal Science and Technology Research Center of Jiangxi University of Traditional Chinese Medicine (Nanchang, China). In this experiment, barrier housing facility was used, which met the National Standard “Environment and Housing Facilities of Laboratory Animal Requirements (GB 14925–2010) “. The rats were housed in acrylic cages lined with sawdust at a constant room temperature (23 ± 1 °C) and maintained in a 12 h: 12 h light/dark cycle. The nursing of the experimental animals and the operation of animal experiments were carried out in accordance with the provisions of Jiangxi University of Traditional Chinese Medicine committee. All animal experiments were conducted in accordance with the arrival guide and British animal (Scientific Procedures) Act1986 and the relevant guidelines. The whole experiment passed the review and supervision of the experimental animal Ethics Committee of Jiangxi University of TCM. The experimental animal ethics review number was JZSYDWLL20200801.
2.4. Induction of hyperlipidemia rats
In this study, high fat diet (HFD) was used to induce hyperlipidemia in the experimental rats (Table 1). High-fat food formula: protein26%, fat 35%, carbohydrate26% (Table 1). High fat feed and normal feed are provided by Wuhan Wanqian Jiaxing Biotechnology Co., Ltd.
Table 1.
High-fat food formula.
| Raw material name | gm | kcal |
|---|---|---|
| Casein | 200 | 800 |
| L-cystin | 3 | 12 |
| Corn Starch | 0 | 0 |
| LORD | 245 | 2205 |
| Maltodextrin | 125 | 500 |
| Sucrose | 68.8 | 275 |
| Cellulose | 50 | 0 |
| Soybean oi | 25 | 225 |
| Mineral mix | 10 | 0 |
| Vitamin mix | 10 | 40 |
| Calcium Carbonate | 5.5 | 0 |
| DiCalcium Phosphate | 13 | 0 |
| Potassium Bitartrat | 16.5 | 0 |
| Choline bitartrate | 2 | 0 |
| total | 773.85 | 4057 |
| gm% | Kcal% | |
| protein | 26% | 20% |
| fat | 35% | 60% |
| carbohydrate | 26% | 20% |
| Total kcal/kg | 5240 |
2.5. Animal treatment and group-specific treatments
50 SD rats were fed adaptively for 7 days, then randomly divided into 2 groups. 10 rats were treated as normal control group (one of them was disregarded in the final stage of experiment as no blood sample was obtained) receiving normal maintenance feed, 40 were used as experimental groups, receiving high-fat food diet (HFD). The levels of serum TC, TG and HDL-C of the 40 rats were determined after 2 weeks of HFD. Those with triglyceride levels above 1.9 entered the experimental administration. The selected rats (29 out of 40) were divided into 4 groups, namely the model group (M group, 8 rats), low dose (50 mg/kg) GPs groups (JL group, 7 rats), mid dose (100 mg/kg) GPs groups (JM group, 7 rats) and high dose (200 mg/kg) GPs groups (JH group,7 rats). The amount of intragastric administration in rat was 10 ml/kg. The control group (C group) was continually given maintenance feed, GPs group and model group received continuous high fat food.
2.6. Sample collection and serum analysis
After 8 week of intervention, the rats were sacrificed by CO2 asphyxiation. Blood from the femoral artery was collected into tubeand coagulated. Serum was collected by centrifugation at 5000 rpm for 10 min, and frozen at −80 °C for biochemical analysis. Serum samples obtained from rats were measured on the levels of blood lipid biochemistry, serum inflammatory markers, antioxidant capacity, uric acid and immune index. After the rats were sacrificed, theirs liver and abdominal fats (from Peritesticular) were removed prior to weighing the livers and calculation for liver index.
2.7. The tissue section observation
The liver and adipose tissue samples from the rats were fixed in a 4% paraformaldehyde solution for 72 h. After fixation, the samples were embedded in paraffin. Slices of the embedded tissue measuring 5 mm in thickness were then prepared. These sections were stained with hematoxylin and eosin (H&E). Finally, the cellular structure and lipid accumulation of the liver and adipocytes were observed under a light microscope at magnifications of 400 × and 200 × .
2.8. Intestinal microbiota 16 S rDNA high-throughput sequencing analysis
To analyze the intestinal microbiota through high-throughput sequencing of 16 S rDNA, fresh fecal particles were collected directly from each rat near the anus. Three rats were randomly selected from each group, and their fecal samples were stored at −80 °C until DNA extraction and 16 S rRNA gene sequencing. The DP328 Fecal Genome Extraction Kit (Tiangen Biochemical Technology Co., Ltd) was used to extract total DNA following the manufacturer's instructions. Nanodrop 2000 was used to determine DNA concentration and purity. The V3–V4 regions of the 16 S rDNA gene were amplified using universal primers (338 F: 5ʹ-ACTCCTACGGGAGGCAGCAG-3ʹ and 806 R: 5ʹ-GGACTACCAGGGTATCTAAT-3ʹ), with a 12 bp barcode added in PCR reactions. The PCR reaction consisted of 25 μl 2x Premix Taq (Takara Biotechnology, Dalian Co. Ltd., China), 1 μl of each primer (10 M), and 3 μl of DNA (20 ng/μl) template in a total volume of 50 μl. The length and concentration of the PCR products were assessed by 1% agarose gel electrophoresis. The PCR products were then mixed in equimolar ratios using GeneTools Analysis Software (Version 4.03.05.0, SynGene). The PCR mixture was purified using the EZNA Gel Extraction Kit (Omega, USA). Next, sequencing libraries were prepared using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina® (New England Biolabs, USA) according to the manufacturer's recommendations, along with index codes. The library quality was evaluated using the Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina HiSeq 2500 platform, generating 250 bp paired-end reads. The high-quality clean reads were obtained by applying specific filtering conditions to the paired-end raw reads using Trimmomatic (V0.33). FLASH (V1.2.11) was used to merge the paired-end clean reads based on their overlapping regions. Raw Tags were generated by combining sequences that overlapped with at least 10 reads from the opposite end of the same DNA fragment, with a maximum allowable error ratio of 0.1. Mothur software (V1.35.1) was utilized to assign sequences to each sample based on their unique barcode and primer, resulting in effective Clean Tags. The usearch software (V10) was employed for sequence analysis. Sequences with ≥97% similarity were grouped into Operational Taxonomic Units (OTUs), which potentially represent species. The most frequently occurring sequence in each OTU was selected as a representative sequence and further annotated using the Ribosomal Database Project (RDP) Classifier v.2.2 trained on the Greengene_2013_5_99 database, with a cutoff confidence value of 0.6. To visualize the differences in OTU composition among the samples, Principal Component Analysis (PCA) was conducted to construct a 2-D graph, summarizing the main factors responsible for these differences. Samples located close together indicate high similarity. Alpha diversity indices, including observed species, chao, ace, shannon, and simpson, were used to analyze the complexity of species diversity within each sample. The first four values are directly proportional to the sample's complexity, while the simpson value has a negative correlation with the complexity.
2.9. Statistical analysis
All Biochemical data were expressed as mean ± standard error (SEM). Statview5.0.1 (SAS in statute Inc., USA) was used for statistical analysis. One-way analysis of variance was performed on data with homogeneity of variance. Dunnett's t-test was used to was compared each group with the model group. P < 0.05 was considered statistically significant. Principal Component Analysis was conducted with R package (v3.1.1).
3. Results
3.1. Effect of die GPs on serum lipid indicators in experimental hyperlipid rats
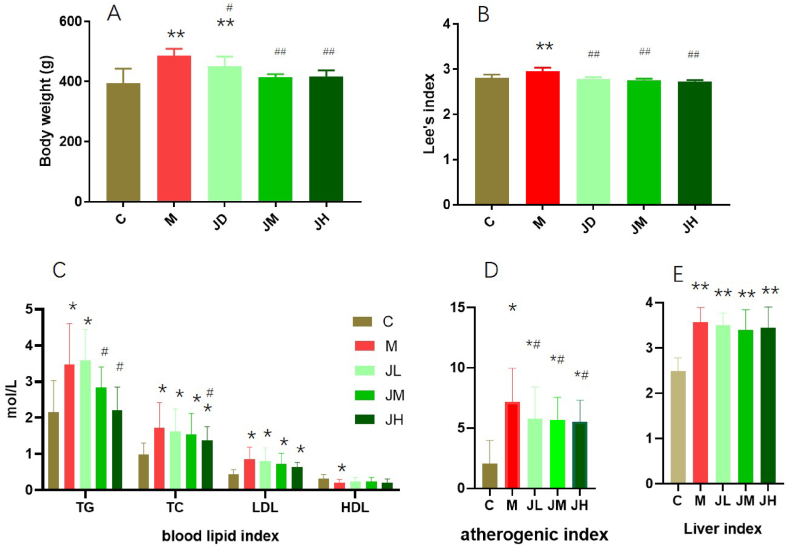
In this experiment, hot water method was used to extract ginger polysaccharides from ginger. The extraction rate of ginger polysaccharide was 6.5%.From Table 2, it can be seen that the TG content in the seurm of the model group was significantly higher than that of the control group, indicating that the experimental hyperlipidemia rat model was successful. Compared with the normal control group, the body weight and Lee's index of the model group increased significantly, and the difference was significant(P<0.01). After intragastric administration of Gps, the weight of Gps groups decreased significantly compared with the model group, and reached significant difference (Fig. 1A and B). Compared with the model group, three dose groups of Gps reduced the content of TG, TC, LDL-c and atherogenic index, liver index in the rat blood (Fig. 1C,D,E).
Table 2.
Blood triglyceride concentration after model establishment (‾X±SD).
| Experimental grouping | Triglyceride |
|---|---|
| C | 0.84 ± 0.25 |
| M | 2.88 ± 0.24* |
| JL | 2.86 ± 0.40* |
| JM | 2.89 ± 0.58* |
| JH | 2.93 ± 0.86* |
*indicates significant difference with C group (P < 0.05).
Fig. 1.
Effect of dietary GPs on blood lipid index in experimental hyper lipid rats (‾X±SD)
* indicates significant difference with C group (P < 0.05). ** indicates significant difference with C group (P < 0.01), # indicates significant difference with M group (P < 0.05). # # indicates significant difference with M group (P < 0.01). A: body weight, B: Lee's index, C:blood lipid index, D:atherogenic index, E:liver index.
3.2. Effect of dietary GPs on biochemical index and liver index of experimental hyperlipid rats (X ± S)
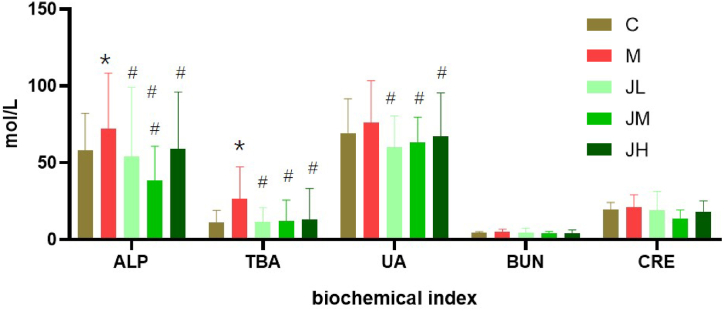
The GPs consumption can lower the levels of creatinine, urea nitrogen and alkaline phosphatase and liver index with noticeable differences (p < 0.05) (Fig. 2).
Fig. 2.
Effect of dietary GPs on biochemical index in experimental hyper lipid rats (‾X±SD)
* indicates significant difference with C group (P < 0.05). # indicates noticeable difference with M group (P < 0.05).
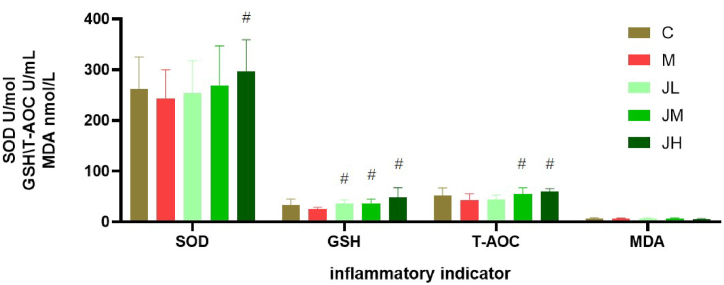
3.3. Effect of dietary GPs on antioxidant function of experimental hyperlipid rats
From Fig. 3, compared to model group, GPs fed group had noticeable increase in SOD, GSH-Px, T-AOC but GPs consumption did not affect MDA (propylene dialdehyde) (P > 0.05).
Fig. 3.
Impact of diet GPs on serum antioxidant indicator in experimental hyper lipid rats (‾X±SD)
* indicates significant difference with C group (P < 0.05). # indicates significant difference with Model rat group (P < 0.05).
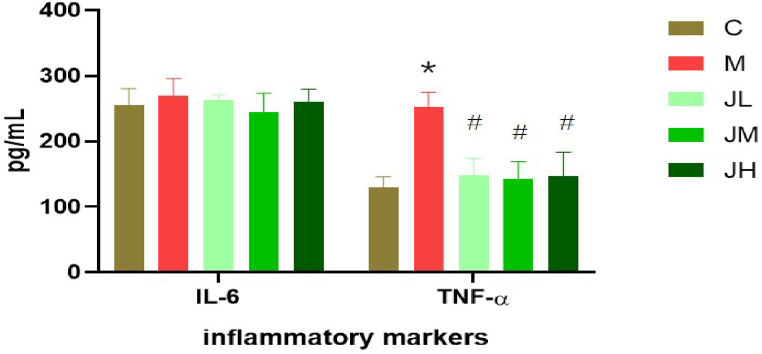
3.4. Effect of dietary GPs on serum inflammatory markers in experimental hyperlipid rats
As shown in Fig. 4, The GPs consumption was able to bring down these values, with differences in TNF-α level significant (p < 0.05), whereas in IL-6, insignificant (P > 0.05).
Fig. 4.
Effect of dietary GPs on serum inflammatory markers in experimental hyper lipid rats (‾X±SD)
* indicates significant difference with C group (P < 0.05). # indicates significant difference with Model rat group (P < 0.05).
3.5. The pathological observation of liver and adipose tissue slice in experimental hyperlipidemia rats with dietary GPs
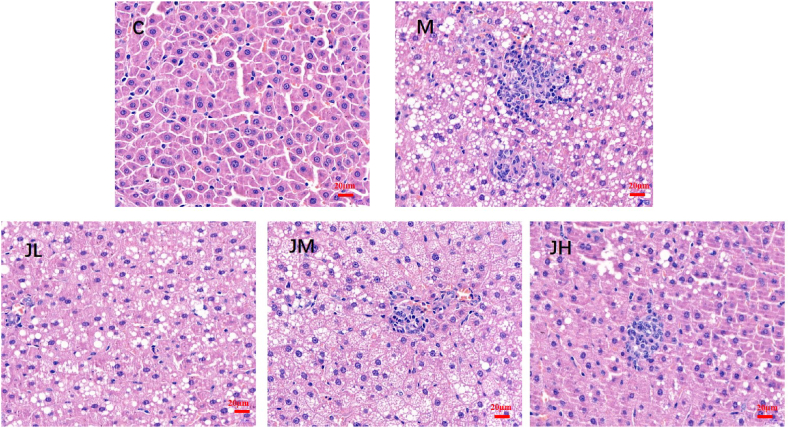
Under the microscope, the hepatocytes in the control group were arranged in a normal rope shape, with normal histological structure, no inflammatory infiltration, and no vacuolar degeneration. The liver tissue cells in the model group were disorderly arranged, with vascular fat droplets and inflammatory infiltration. Compared with the model group, the liver tissue cells in the low dose GPs group were disorderly arranged, lipid droplets were reduced, and the degree of inflammatory infiltration and vacuolar variability were reduced. In the medium dose group, the liver tissue cells of GPs were disorderly arranged, but the degree of vacuolar degeneration was further reduced and inflammatory infiltration was reduced. The liver tissue lipid droplets in the high dose group of GPs also further decreased, the degree of vacuolar degeneration decreased to the lowest among the three dose groups, and there was no inflammatory infiltration, as shown in Fig. 5.
Fig. 5.
Liver histopathological sections of rats after feeding GPs. Note:C, control group M, high fat model group. JL, low dose ginger group. JM, medium dose ginger group,JH, high dose ginger group. (HE staining x 400).
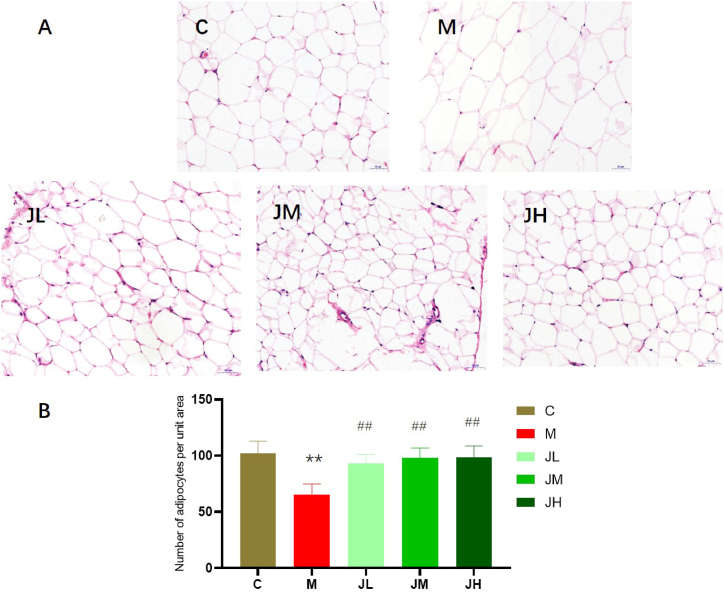
Compared with the periepididymal fat of rats in the control group, the adipocytes in the model group significantly increased, and the number of adipocytes per unit area decreased. After intragastric administration of ginger polysaccharide, the number of adipocytes per unit area increased (Fig. 6A and B).
Fig. 6.
Adipose histopathological sections of rats after feeding GPs (HE staining x 200) (6A). 6B the number of adipocytes per unit area.
3.6. The OTU PCA analysis in the feces of experimental hyperlipidemia rats
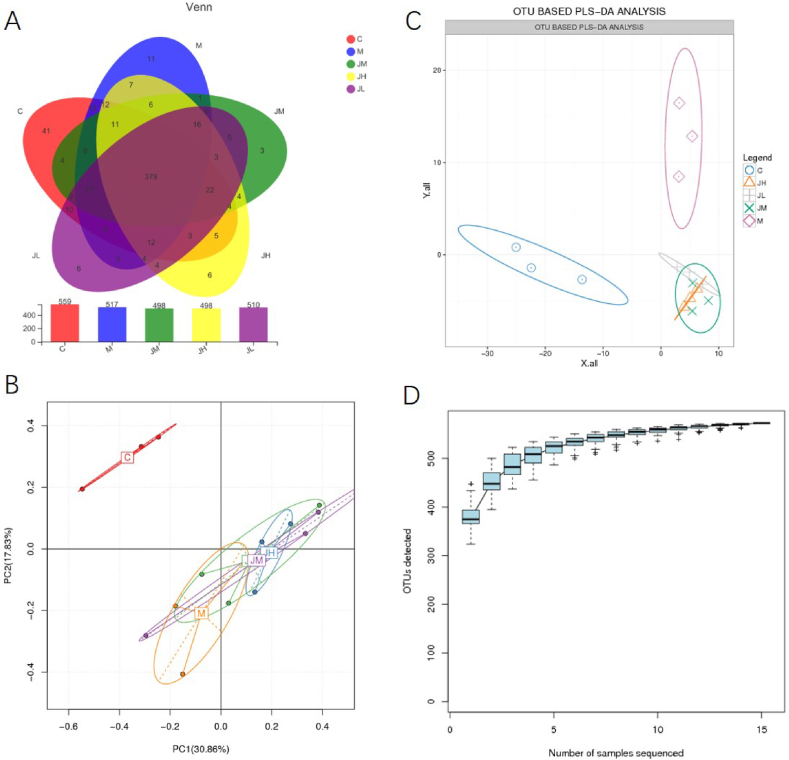
We performed 16 S rRNA sequencing to describe the composition of the gut microbiota and used a Venn diagram to show shared the number of shared and unique OTUs. There were 379 co-ownership OTUs, 41 specially owned OTUs is ascribed to the matched group, 11 unique OTUs to the HFD group and 6,3,6 specially owned OTUs to the L, M and H dose GPs groups respectively (Fig. 7A). Based on OTU abundance information, the relative amount of each OTU in each sample would be numerical, and the OTU was analyzed by PCA of using the relative abundance value. As shown in Fig. 7B and C, control Group (C) was the farthest from M, JL, JM, and JH groups, indicating the significant difference of intestinal microbiota abundance between group C and the other four groups. At the same time, the distance between the three samples collected in each group is relatively short, which indicates that the differences in intestinal microbiota abundance among the three samples in the same group were small. Species Accumulation (SA) analysis Used SA plots to show an increase in OTUs detected after each sample was added. The curve obtained using all OTU data is shown in the diagram (Fig. 7D).
Fig. 7.
Effect of HFD and Gps on the distribution of common/specific OTUs in rats (7A). PCA based on OTU abundance (Description).
Fig. 7B The numbers in brackets represents contributions of principal components to the differences between samples. Fig. 7C OTU based PLS-DA analysis. Fig. 7D In the analysis of species numbers detected.
3.7. Alpha variety analyze of variety
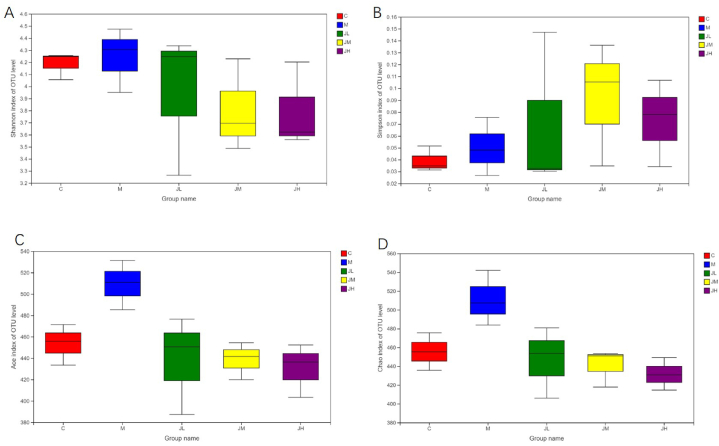
Alpha diversity is referred to the variety in a specified area or ecosystem. Common used measurement include chao, ace, shannon, simpson. Chao and ace indices are generally used to reflect community abundance, and shannon and simpson indices reflect microbial diversity. Community richness and diversity changed after HFD and ginger polysaccharide feeding, but the difference was not significant (Fig. 8).
Fig. 8.
Alpha diversity index statistics of five groups of fecal samples.
The boxplot was used to visually display the differences in the alpha diversity between groups. The five elements from bottom to top are the minimal value, the first quartile, the median, the third quartile and the maximum. A: shannon index,B:Simpson index,C:Ace index, D:Chao index.
3.8. Influence of GPs on the floristic composition and abundance of gut microbiota in hyperlipidemia rats
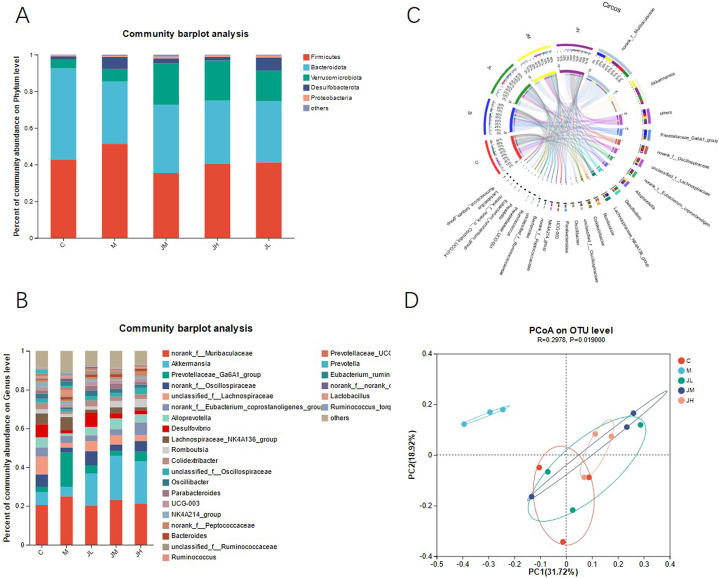
Total amountfive phyla were detect from in the intestinal feces of five groups of rats, including Firmicutes, bacteroideta, verrucomicrobiota, desulfobactereta, and Proteobacteria, accounting for more than 98% of the total number of bacteria in the fecal samples of the five groups of rats (Fig. 9A). Compared with the control group, the fat-rich diet decreased the abundance of Firmicutes and verrucomicrobiota, while increased the abundance of bacteroideta. After eating low, medium, and high doses of ginger polysaccharide, the abundance of bacteroideta decreased. The abundance of Firmicutes did not increase significantly, but that of verrucomicrobiota was increased, with a marked difference comparing to the HDF M group (P < 0.05) (Table 7).
Fig. 9.
Effect of HFD and Gps on abundance of gut bacterial microbiota of rats. A: Effects of HFD and GPs on abundance of intestinal flora at phylum classification level of rats
B: Effects of HFD and GPs on abundance of intestinal flora at genus classification level of rats.
C: The relationship between specimens and species. The visual circle chart diagram reflects the distribution proportion of predominant species in each (or group) sample and the distribution ratio of dominant species in different specimens (groups).
D: Principal component analysis (PCA) was used to construct two-dimensional map to summarize factors causing this difference. If the two samples are closely, the similarity is high.
Table 7.
Effects of HFD and GPs on abundance of intestinal flora at phylum classification level of rats(‾X ± SD).
| Phylum | C | M | JL | JM | JH |
|---|---|---|---|---|---|
| Bacteroidetes | 36.01 ± 12.67 | 50.27 ± 8.08* | 35.81 ± 6.58 | 38.33 ± 10.81 | 34.80 ± 2.75 |
| Firmicutes | 55.46 ± 10.44 | 43.12 ± 4.88* | 44.50 ± 11.77# | 35.76 ± 9.95# | 41.43 ± 8.45# |
| Proteobacteria | 1.15 ± 0.59 | 1.16 ± 0.62 | 1.88 ± 0.21 | 2.23 ± 0.31 | 1.06 ± 0.46 |
| Tenericutes | 0.02 ± 0.01 | 0.28 ± 0.15 | 0.05 ± 0.03 | 0.06 ± 0.04 | 0.05 ± 0.04 |
| Verrucomicrobia | 7.23 ± 4.52 | 4.91 ± 5.02* | 19.06 ± 17.57# | 23.20 ± 20.22# | 22.25 ± 10.99# |
* indicate significant difference with C group (P < 0.05). # indicate marked difference with M group (P < 0.05).
At the genus level, it involves the changes of 47 bacterial communities. Among them, muribaculaceae, akkermansia, prevotellaceae, oscilillospiraceae and lachnospiraceae are the main dominant flora (Fig. 9B and C). Compared with the blank control group, the rich of muribaculaceae in the intestine of HFD M group was significantly higher than that of the C group (P < 0.05), while its abundance in akkermansia, lachnospiraceae and oscilillospira decreased from the control level (P < 0.05). After eating various doses of ginger polysaccharide, the levels of muribaculaceae and prevotellaceae in the intestine of rats almost fell to the normal level, the abundance of akkermansia increased significantly, even beyond the level of the C group, and the abundance levels of lachnospiraceae and oscilillospira increased (Table 8).
Table 8.
Effects of HFD and GPs on abundance of intestinal flora at genus classification level of rats (%).
| genus | C | M | JL | JM | JH |
|---|---|---|---|---|---|
| muribaculaceae | 20.46 | 24.93 | 20.17 | 23.22 | 21.11 |
| akkermansia | 6.68 | 4.85 | 16.65 | 22.61 | 21.86 |
| Prevotellaceae | 2.78 | 17.81 | 4.11 | 2.52 | 5.02 |
| oscilillospiraceae | 6.34 | 2.48 | 7.34 | 3.32 | 5.49 |
| lachnospiraceae | 9.17 | 2.51 | 5.02 | 4.78 | 3.08 |
4. Discussion
HFD is a method of making experimental hyperlipidemia models. By contrast with the model group, dietary GPs attenuated several effects of HFD feeding, including the serum lipid, blood inflammatory markers (such as TNF-a, IL-6), antioxidant capacity (SOD, GSH-Px, T-AOC), uric acid and immune index in our study. Previous research has shown that GPs can lower blood lipids and improve antioxidant capacity; Ahmed et al. (2000) and his fellows reported that administration of Zingiber officinales Rosc (ginger 1%, w/w) reduced markedly malathion-derivant lipid peroxidation and oxidant stress in these rats. The results of this experiment showed that high-fat diet also led to the decrease of SOD and GSH-Px, and the increase of T-AOC activity. After feeding with ginger polysaccharide, SOD, GSH-Px and T-AOC were close to normal levels, while MDA content was not significantly affected. The results showed that dietary GPs could increase the antioxidant ability of hyperlipidmia rats in some degree.
The high-calorie diet in modern society not only leads to hyperlipidemia, but also increases the accumulation of fat. The increase in fat leads to an increase in the secretion of fatty factors, which is the main factor of the body's chronic inflammatory response. Dietary ginger can significantly reduce IL-6 and TNF-a levels of experimental hyperlipidemia rats. Among them, the level of decrease in TNF-alpha achieved significant differences. Based on the results of our study, combined with the pathological results, blood lipid indicators, antioxidant indicators, and decreased levels of inflammatory cells, one can conclude that dietary GPs improved the inflammatory responses of the HFD model.
Hyperlipidemia often leads to abnormal metabolism. The ALP and TBA in the HFD M group are higher than those in the C group. After GPs was fed, the values have decreased and approached the normal control level. Because serum ALP and TBA are sensitive diagnostic indicators for parenchymal liver damage and digestive system diseases, once liver cells have lesions or intestinal-hepatic circulation disorders, it can cause total bile acid to increase. The decrease of ALP and TBA content in this experiment shows that dietary GPs can reduce the content of ALP and TBA. The degree of liver damage on experimental hyperlipidemia rats was induced by HFD. Uric acid is a product of purine metabolism. Under normal circumstances, the rate of uric acid production and excretion is relatively constant. The change in uric acid content in body fluids can reflect the body's metabolic and immune status. Due to the intake of high-fat and high-purine foods, Hyperlipidemia and the extend of obesity may also increase abnormally. Abnormal uric acid metabolism can lead to hyperuricemia and eventually induce ventilation. In this experiment, we found that in terms of the blood biochemical index of uric acid in rats, the JL group and the JM group had lower levels than the C group, indicating GPs might reduce the uric acid content in the blood of hyperlipidemia rats.
Gut microbiota enhances the host's ability to obtain nutrition and energy from food. Under normal physiological conditions, the metabolic activity of the intestinal flora helps to extract calories from the intake of dietary substances and store these calories in the host adipose tissue for future use. Bacterial lipopolysaccharide in intestinal microbiota can cause low-level inflammatory response, which makes it easy to associate inflammation with HFD induced metabolic syndrome. In HFD induced hyperlipidemia rats, the dominant gut bacteria were also Firmicutes and Bacteroides. Compared to the C group, the number of opportunistic pathogen such as gram-negative Bacteroides in the hyperlipidemia model group increased significantly, and akkermansia, oscillospiraceae and lachnospiraceae, which are negatively related to obesity, on the contrary, decreased significantly. Through the analysis of gut microbiota level, we found that Akkermansia decreased markedly in the HFD M group, but increased after given GPs. As a symbiotic genus of the Verrucomicrobia phylum, Akkermansia contains only one member: Akkermansia muciniphila. A. muciniphila degrades mucin and is a rich member of the human intestinal microbiota [23]. Colonization of A. muciniphila has been reported to have a protective effect on diet-induced obesity [24], ability to promote mucosal wound healing, and can increase antitumor responses during anti-PD-1 immunotherapy. Akkermansia muciniphila inversely correlates with the onset of inflammation; it can adjust adipose tissue metabolism and metabolic disorder during obesity in mice [25]. We also know that oscillospiraceae can produce short chain fatty acids (SCFAs) such as butyrate, which is beneficial for improving the metabolism of hyperlipidemia. The abundance of Bacteroides had no significant effect in the intestinal tract of rats after low, medium and high dose intake of GPs. Interestingly, the number of akk bacteria of verruca increased significantly, suggesting that GPs can positively adjust the gut micro inflammation caused by HFD diet by increasing the abundance of intestinal oscillospiraceae, lachnospiraceae, especially uncreased akkkermansia flora. This ginger polysaccharide can improve the metabolism of HFD by increasing akk bacteria, which needs to be further verified later.
5. Conclusion
Unhealthy dietary patterns, especially high fat diet intake, can lead to the development of various metabolic disorders, including obesity, hyperlipidemia, and other metabolic disorders. HFD can accelerate de novo fat synthesis and increase LDL levels. Gut microbiota is closely related to metabolic disorders induced by HFD, and dietary intervention of intestinal microbiota is an effective treatment strategy for these metabolic disorders. Polysaccharides derived from plants are sources of polymeric carbohydrate macromolecules and fermentable dietary fiber, and exhibit excellent biological activity in the prevention and treatment of HFD induced metabolic diseases. Many reports suggest that natural polysaccharides are one of the most effective modulators of intestinal microbiota composition. Polysaccharides are also one of the main functional and active ingredients in ginger, and have broad development value and application prospects in the fields of medicine, health, food, condiments, and cosmetics. We have found that Oral administration of GPs in the industry can change the internal gut microbial community structure and reduce metabolic abnormalities caused by HDF diet. This study has a good reference value for the role of ginger polysaccharides in regulating intestinal flora and further developing and utilizing ginger polysaccharides.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
This research was supported by Double-First Class discipline construction project: JXSYLXK-ZHYAO115, and Health Commission of Jiangxi Province (No. 20204766). Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (No. CXTD-22004), Jiangxi Provincial Scientific Research Infrastructure Platform Project (20212bcd46005).
We thank Zhongrui Wu,Suyun Xiao,Weiyue Deng, and Xiaoqin Tan for the Part of the work on animal husbandry and data collection of this study.
Contributor Information
Tao Hong, Email: 372729181@qq.com.
Li Liu, Email: 13870803370@163.com.
Zhi-yong Liu, Email: liuzhiyong0791@163.com.
References
- 1.Assmann G., Schulte H. The Prospective Cardiovascular Münster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am. Heart J. 1989;116(6 Pt 2):1713–1724. doi: 10.1016/0002-8703(88)90220-7. [DOI] [PubMed] [Google Scholar]
- 2.Huang M.S., et al. Hyperlipidemia impairs osteoanabolic effects of PTH. J. Bone Miner. Res. 2010;23(10):1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirih F., et al. 2012. Adverse Effects of Hyperlipidemia on Bone Regeneration and Strength. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S., et al. Hyperlipidemia compromises homing efficiency of systemically transplanted BMSCs and inhibits bone regeneration. Int. J. Clin. Exp. Pathol. 2014;7(4):1580–1587. [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez-Espinosa D., et al. Intracellular retention of hepatic serpins caused by severe hyperlipidemia. Liver Int. 2006;26(6):708–715. doi: 10.1111/j.1478-3231.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- 6.Tietge U.J. Hyperlipidemia and cardiovascular disease: inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014;25(1):94. doi: 10.1097/MOL.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 7.Clarke M.C., et al. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Curr. Opin. Lipidol. 2010;106(2):363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 8.Madjid M., Willerson J.T. Inflammatory markers in coronary heart disease. Br. Med. Bull. 2011;4(2):124–131. doi: 10.1093/bmb/ldr043. [DOI] [PubMed] [Google Scholar]
- 9.Vijay-Kumar M. 2010. Altered Microbiota and Metabolic Syndrome in Tlr5 Deficient Mice. [Google Scholar]
- 10.Garcia G.H., et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J. Shoulder Elbow Surg. 2017;26(12):2086–2090. doi: 10.1016/j.jse.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Ahmida M.H., Abuzogaya M.H. The effects of oral administration of green tea and ginger extracts on serum and hepatic lipid content in rats fed a hyperlipidemic diet. J. Appl. Sci. Res. October. 2009;5(10):56–64. [Google Scholar]
- 12.Bagri, P., et al., Antidiabetic effect of Punica granatum flowers: effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol.. 47(1): p. 0-54. [DOI] [PubMed]
- 13.Liu J., et al. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials. Chin. Med. 2006;1(1):4. doi: 10.1186/1749-8546-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross, Maxine S. Red yeast rice: the efficacy of monascus purpureus yeast for treatment of hyperlipidemia a modifiable risk factor of cardiovascular disease. Holist. Nurs. Pract. 2017;31(1):52–58. doi: 10.1097/HNP.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 15.Zhen-Nan Y.E., et al. Effect of cyclocarya paliurus polysaccharide on hyperlipidemia and anti-lipid peroxidation in hyperlipidemic rats. Modern Food Sci. Technol. 2014;30(4):1–5+20. [Google Scholar]
- 16.Yang W., et al. Nuclc Acids Research; 2018. SymMap: an Integrative Database of Traditional Chinese Medicine Enhanced by Symptom Mapping; p. D1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maharlouei N., et al. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018;59(11):1–14. doi: 10.1080/10408398.2018.1427044. [DOI] [PubMed] [Google Scholar]
- 18.Surh Y.J., et al. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger. J. Environ. Pathol. Toxicol. & Oncol. Official Organ of the Int. Soc. for Environ. Toxicol. Cancer. 1999;18(2):131–139. [PubMed] [Google Scholar]
- 19.Jung, P.Y., et al., [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med. J.. 47(5): p. 688-. [DOI] [PMC free article] [PubMed]
- 20.Murakami A., et al. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int. J. Cancer. 2004;110(4):481–490. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]
- 21.Justo O.R., et al. Evaluation of in vitro anti-inflammatory effects of crude ginger and rosemary extracts obtained through supercritical CO2 extraction on macrophage and tumor cell line: the influence of vehicle type. BMC Compl. Alternative Med. 2015;15:390. doi: 10.1186/s12906-015-0896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B.S., et al. Separation of 6-gingerol from ginger (zingiber officinale Roscoe) and antioxidative activity. KSBB Journal. 2006;21(6) [Google Scholar]
- 23.Ansaldo E., et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–1184. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everard A., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeberger M., et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5 doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]