Abstract
ALK-EML4 fusion-positive lung adenocarcinomas (LUADs) are effectively treated with ALK tyrosine kinase inhibitors, but most patients eventually develop resistance to these drugs owing to ALK-dependent or independent mechanisms. Endothelial to mesenchymal transformation with SCLC development is an ALK-independent mechanism of resistance that has not been previously reported with sequential ALK I1171T mutation while the patient is on treatment for the SCLC. Here, we report the first case of sequential SCLC transformation followed by ALK I1171T mutation in a patient with ALK-EML4 fusion-positive LUAD. After progression on multiple lines of therapy, we describe our experience of managing ALK-mutant LUAD and transformed SCLC with a novel combination of lorlatinib and temozolomide. We also briefly summarize cases of endothelial to mesenchymal transformation ALK-mutant LUAD from the literature.
Keywords: Case report, ALK-EML4, ALK-positive NSCLC, Small cell transformation, ALK I1171T mutation
Introduction
ALK-EML4 fusion is reported in around 3% to 7% of patients with non–small cell lung adenocarcinoma (LUAD).1 Second-generation ALK inhibitors such as alectinib now represent the standard first-line therapy for ALK mutant non–small cell LUAD with good responses. However, most patients will have a progression of disease owing to acquired resistance.2 This acquired resistance can be ALK-dependent, such as secondary ALK mutations or amplifications, or it can be related to ALK-independent (off-target) pathways, such as EGFR, KRAS, KIT, MET, and insulin-like growth factor 1 mutations. One ALK-independent resistant pathway is the histologic transformation of the tumor to SCLC.2 There is a paucity of data regarding combining the ALK inhibitor such as lorlatinib with SCLC therapy to prevent on-target escape of tumor cells. In this report, we describe the management of sequential histologic transformation of ALK-positive (ALK+) LUAD to small cell cancer and acquired ALK I1171T mutation in the non–small cell cancer clone with a combination of temozolomide and lorlatinib. There has been one previous report of acquired ALK G1202R mutation and SCLC transformation treated with a combination of carboplatin/etoposide and alectinib. However, that patient had rapid clinical deterioration and died.3 We also summarize the previously published literature regarding SCLC transformation in patients with ALK-EML4 fusion-positive LUAD.
Case Presentation
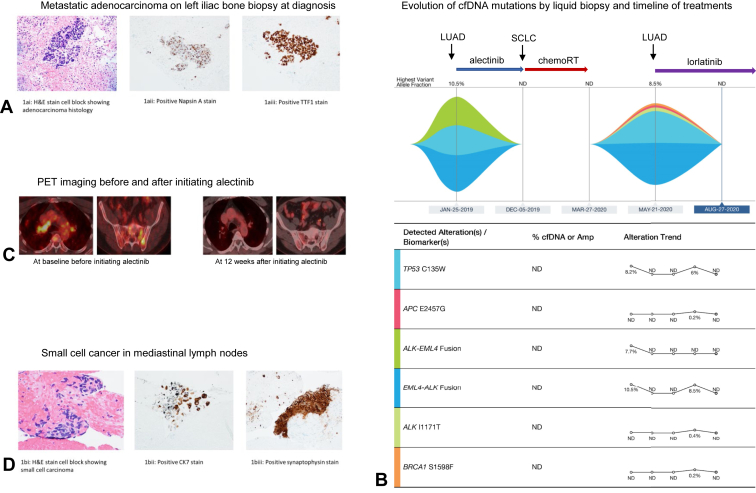
In January 2019, a 58-year-old nonsmoking man with no relevant past medical history was incidentally found to have a spiculated right upper lobe mass with diffuse sclerotic bone lesions on computed tomography (CT) scans. Endobronchial ultrasound (EBUS) guided fine needle aspiration of the lymph nodes at stations 4R and 10L revealed adenocarcinoma. The biopsy from the left iliac bone lesion confirmed metastatic adenocarcinoma of the lung (Fig. 1A). A liquid biopsy using Guardant360 was positive for EML4-ALK fusion with a variant allele frequency (VAF) of 10.5% and TP53 C135W mutation with VAF of 8.2% (Fig. 1B). Same mutations were found in the tissue biopsy by EBUS. No RB1 mutation was identified. The patient was started on alectinib 600 mg twice daily and received radiation therapy to the lumbar metastatic lesions. After 2 months, alectinib was put on hold owing to concerns of pneumonitis, which resolved with prednisone. In May 2019, repeat imaging studies revealed partial response to treatment per Response Evaluation Criteria in Solid Tumors criteria (Fig. 1C). Patient was restarted on alectinib at a lower dose and maintained at 450 mg twice daily.
Figure 1.
(A) H&E stain and IHC stain on left iliac bone biopsy at diagnosis in January 2019. (B) Evolution of mutations by cfDNA analysis and timeline of biopsies and treatments from January 2019 to August 2020. (C) PET images at baseline before initiating alectinib in January 2019 and at 12 weeks after initiating alectinib, exhibiting treatment response. (D) H&E stain and IHC stain on mediastinal lymph node biopsy at disease progression in December 2019. Amp, amplification; cfDNA, cell-free DNA; H&E, hematoxylin and eosin; IHC, immunohistochemistry; LUAD, lung adenocarcinoma; PET, positron emission tomography; RT, radiation therapy.
In December 2019 (11 mo after diagnosis), a restaging positron emission tomography (PET) scan revealed a new right perihilar 1.5 cm lymph node. EBUS fine needle aspiration of the lymph node at station 11R revealed histologic transformation with the presence of small cell carcinoma (Fig. 1D). Foundation CDx confirmed ALK-EML4 fusion and TP53 C135W in the tumor cells, similar to what was seen from Guardant 360 liquid biopsy at diagnosis. Alectinib was stopped and the patient was started on treatment for SCLC with cisplatin plus etoposide for four cycles concurrently with radiation therapy for limited-stage SCLC. Subsequent imaging after completion of concurrent chemotherapy radiation therapy revealed stable disease. In March 2020, Guardant 360 liquid biopsy did not exhibit any circulating cell-free DNA (Fig. 1B).
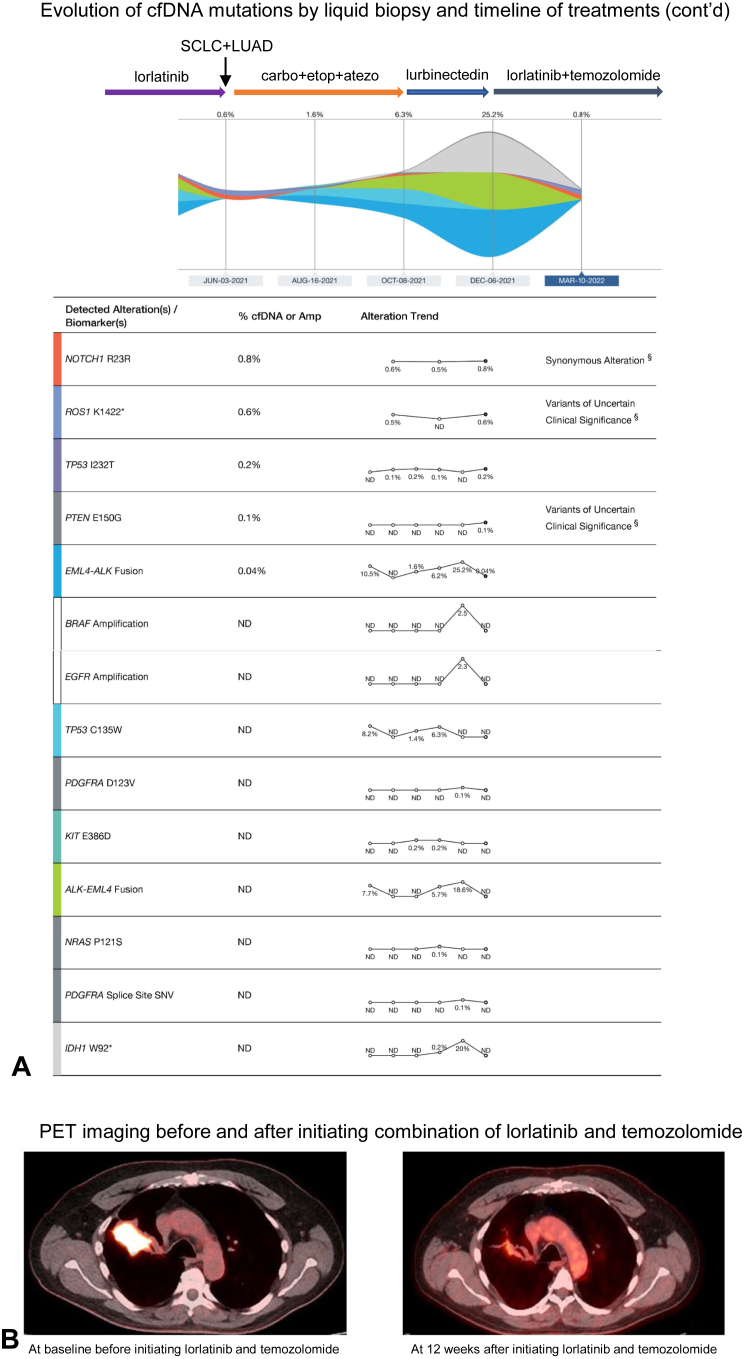
In May 2020, PET/CT scan discovered a new left adrenal gland hypermetabolic nodule with biopsy revealing recurrent metastatic NSCLC. A subsequent liquid biopsy revealed EML4-ALK fusion with VAF of 8.5% and a new ALK I1171T mutation was also noted (Fig. 1B). Patient was started on another ALK inhibitor, lorlatinib. His disease remained stable on serial imaging studies over the next year until June 2021 when a PET/CT scan revealed an enlarging right upper paratracheal lymph node and a new lesion in L3 vertebral body. Biopsies of the right upper paratracheal lymph node revealed recurrent SCLC and the new L3 vertebral body lesion confirmed metastatic adenocarcinoma of lung origin, respectively. Lorlatinib was discontinued and the patient was started on carboplatin, etoposide, and atezolizumab. However, after four cycles of treatment, restaging PET revealed the progression of the disease. The patient was subsequently started on lurbinectedin in October 2021; however, restaging scans after two cycles of therapy revealed progressive supraclavicular and mediastinal lymphadenopathy with worsening left adrenal and bone metastatic disease. A 2-mm focus of enhancement was also seen in the periventricular white matter suggesting central nervous system metastasis. The patient was started on temozolomide in December 2021. Given the progression of both the SCLC and LUAD tumor clones with high ALK fusion VAF (Fig. 2A), it was decided to add lorlatinib to temozolomide. Interval CT and PET scans in March 2022 revealed a substantial response to therapy with a decrease in the size of lobulated central RUL mass (Fig. 2B), resolution of right clavicular, mediastinal lymphadenopathy, and remarkably smaller left adrenal, and left paraaortic nodal metastasis. Brain MRI revealed resolution of punctate periventricular white matter enhancement. The patient’s disease remained in good control without remarkable adverse effects from the combination therapy for 6 months. Unfortunately, the patient recently had cancer progression when the patient is currently enrolled in a clinical trial.
Figure 2.
(A) Continued evolution of mutations by cfDNA analysis and timeline of further biopsies and treatments from August 2020 to March 2022. (B) PET images at a new baseline before initiating a combination of lorlatinib and temozolomide in December 2021 and at 12 weeks after exhibiting treatment response. Amp, amplification; cfDNA, cell-free DNA; LUAD, lung adenocarcinoma; PET, positron emission tomography.
Discussion
This is the first case report to describe the sequential off-target epithelial-to-mesenchymal transition (EMT) followed by on-target ALK I1171T mutation after ALK-targeted therapy with alectinib. In the literature, SCLC transformation has been reported in a total of eight patients after progression on crizotinib or alectinib. In Table 1,3, 4, 5, 6, 7, 8, 9, 10, 11, 12 we summarize reported cases of SCLC transformation in ALK+ NSCLC. In our patient, the rebiopsied SCLC revealed retained ALK rearrangement confirming true transformation. The tumor and blood next-generation sequencing confirmed the presence of p53 mutation with the presence of single-hit RB1 with a low expression suggesting epigenetic silencing. As previously described by Ou et al.,3 the presence of either RB1 or p53 mutation at the time of diagnosis may indicate a higher risk of EMT. We believe it was a subsequent transformation rather than coexisting upfront because the initial biopsy did not exhibit a neuroendocrine component or RB1 mutation.
Table 1.
Characteristics of Clinical Cases of SCLC Transformation as Resistance Mechanism to ALK Inhibitors
| Authors | Date of Publication | Sex | Age (y) | Histologic Diagnosis | Most Immediate Prior TKI | Duration of TKI Before Transformation | ALK Translocation in SCLC | Treatment After Transformation |
|---|---|---|---|---|---|---|---|---|
| Fujita et al.4 | 2016 | F | 67 | Adenocarcinoma | Crizotinib Alectinib |
7 mo 3 mo |
Present | Irinotecan and alectinib |
| Caumont et al.5 | 2016 | F | 63 | Adenocarcinoma | Crizotinib | 6 mo | Present | Alectinib: partial response |
| Cha et al.6 | 2016 | M | 72 | Adenocarcinoma | Crizotinib | 2 mo | Present | Unknown |
| Takegawa et al.7 | 2016 | F | 43 | Adenocarcinoma | Crizotinib Alectinib |
6 mo 13 mo |
Present | none (patient deceased) |
| Miyamoto et al.8 | 2016 | F | 56 | Adenocarcinoma | Crizotinib Alectinib |
2 y 8 mo |
Unknown | Cisplatin irinotecan |
| Levacq et al.9 | 2016 | F | 53 | Adenocarcinoma | Crizotinib Ceritinib |
7 mo 7 mo |
Present with atypical pattern | Cisplatin etoposide: mixed evolution |
| Ou et al.3 | 2017 | M | 35 | Adenocarcinoma | Ceritinib Alectinib |
8 mo 2 mo |
Present | Carboplatin etoposide |
| Zhu et al.10 | 2017 | M | 49 | Adenocarcinoma | Crizotinib | 17 mo | Present | Etoposide |
| Oya et al.11 | 2017 | M | 62 | Adenocarcinoma | Alectinib | 24 mo | Present | Cisplatin etoposide |
| Balla et al.12 | 2018 | F | 73 | Adenocarcinoma | None | 24 mo | Present | Cisplatin etoposide |
F, female; M, male; TKI, tyrosine kinase inhibitor.
After control of SCLC, a repeat liquid biopsy did not detect the presence of any circulating tumor DNA. However, shortly thereafter, restaging PET revealed a new adrenal lesion with a biopsy confirming metastatic LUAD. Liquid biopsy at that time revealed the development of the ALK I1171T-activating mutation, which has been reported as an on-target resistance mechanism to crizotinib and alectinib. It is a missense alteration that occurs in the cytoplasmic kinase domain of the ALK protein resulting in increased phosphorylation of ALK.13 In eight patients with ALK I1171T mutation who were treated with subsequent ALK inhibitors (brigatinib, lorlatinib, ceritinib), the objective response rate was 75%, the median duration of response was 4.2 months, and the median progression-free survival was 5.5 months.14 Because of the sensitivity of this mutation to lorlatinib, our patient responded well with radiologic response to treatment and clearance of ALK I1171T-resistant cells on liquid biopsy. However, a year later, the patient had progression of the SCLC clone requiring treatment with systemic chemotherapy with immunotherapy, and lorlatinib therapy had to be put on hold. The patient then had biopsy-proven progression of both the NSCLC and SCLC clones. Given the aggressive nature of patients with SCLC, he was treated with SCLC systemic therapy regimens, carboplatin, etoposide, and atezolizumab, and then lurbinectedin after progression without any response or disease control. As a last-ditch effort, the patient was started on temozolomide, especially given the concern for central nervous system metastatic disease. Guided by liquid biopsy (Fig. 2A), we decided to add lorlatinib to control the progressive LUAD. The patient has not only been able to tolerate this combination therapy without any adverse effects, but his 3-month interval scans also revealed a near-complete response on imaging. This case highlights the dilemma of needing to continue the TKI with the SCLC regimen to prevent the on-target escape of tumor cells, underscoring the need for studies looking at the safety of combining TKIs with SCLC regimens. Our case can serve as a possible proof of concept of combining these therapies to induce durable responses. Larger studies are needed to vigorously study the safety and efficacy of this combination.
Conclusion
In summary, our case highlights the importance of repeating biopsy to rule out EMT in patients with ALK+ LUAD as this can completely change the treatment course. We also described the safety and efficacy of combining lorlatinib with temozolomide to control LUAD and EMT-acquired SCLC, respectively, providing proof of concept for future studies.
Credit Authorship Contribution Statement
Umair Majeed: Conceptualization, Data curation, Investigation, Writing-original draft, Writing-review and editing.
Shenduo Li: Conceptualization, Data curation, Investigation, Writing- original draft, Writing-review and editing.
Karan Seegobin: Writing-review and editing.
Aziza Nassar: Writing-review and editing.
Rami Manochakian: Writing-review and editing.
Yujie Zhao: Writing-review and editing.
Yanyan Lou: Conceptualization, Project administration, Supervision, Writing-review and editing.
Acknowledgments
The authors thank the patient for providing informed consent. This study was approved by our institutional review board.
Footnotes
Drs. Majeed and Li contributed equally to this work.
Disclosures: Dr. Lou reports serving as an advisory board member for AstraZeneca, Novocure, and Janssen Pharmaceuticals; serving as a consultant for AstraZeneca; receiving an honorarium from Clarion health care; research funding support from Merck, MacroGenics, Tolero Pharmaceuticals, AstraZeneca, Vaccinex, Blueprint Medicines, Harpoon Therapeutics, Sun Pharma Advanced Research, Bristol-Myers Squibb, Kyowa Pharmaceuticals, Tesaro, and Bayer HealthCare. Dr. Manochakian reports serving on advisory boards for AstraZeneca, Guardant Health, Novocure, Takeda, and Janssen Pharmaceuticals; and consulting for AstraZeneca. Dr. Zhao reports receiving research funding support from Zai Lab, PDS Biotechnology, Transgen, Mereo BioPharma, Mirati Therapeutics, Inc. Incyte Pfizer Inc. Alpine Immune Science Merck & Co., Inc., and Elucida Oncology. The remaining authors declare no conflict of interest.
Cite this article as: Majeed U, Li S, Seegobin K, et al. First report of management of sequential small cell transformation and ALK I1171T mutation as resistance mechanisms in a patient with ALK-EML4 fused non–small cell lung adenocarcinoma with a novel combination of temozolomide and lorlatinib: a case report. JTO Clin Res Rep. 2023;4:100536.
References
- 1.Shaw A.T., Yeap B.Y., Mino-Kenudson M., et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y., Deng C., Qiu Z., Cao C., Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.713530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ou S.H.I., Lee T.K., Young L., et al. Dual occurrence of ALK G1202R solvent front mutation and small cell lung cancer transformation as resistance mechanisms to second generation ALK inhibitors without prior exposure to crizotinib. Pitfall of solely relying on liquid re-biopsy? Lung Cancer. 2017;106:110–114. doi: 10.1016/j.lungcan.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Fujita S., Masago K., Katakami N., Yatabe Y. Transformation to SCLC after treatment with the ALK inhibitor alectinib. J Thorac Oncol. 2016;11:e67–e72. doi: 10.1016/j.jtho.2015.12.105. [DOI] [PubMed] [Google Scholar]
- 5.Caumont C., Veillon R., Gros A., Laharanne E., Bégueret H., Merlio J.P. Neuroendocrine phenotype as an acquired resistance mechanism in ALK-rearranged lung adenocarcinoma. Lung Cancer. 2016;92:15–18. doi: 10.1016/j.lungcan.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Cha Y.J., Cho B.C., Kim H.R., Lee H.J., Shim H.S. A case of ALK-rearranged adenocarcinoma with small cell carcinoma-like transformation and resistance to crizotinib. J Thorac Oncol. 2016;11:e55–e58. doi: 10.1016/j.jtho.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 7.Takegawa N., Hayashi H., Iizuka N., et al. Transformation of ALK rearrangement-positive adenocarcinoma to small-cell lung cancer in association with acquired resistance to alectinib. Ann Oncol. 2016;27:953–955. doi: 10.1093/annonc/mdw032. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S., Ikushima S., Ono R., et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol. 2016;46:170–173. doi: 10.1093/jjco/hyv173. [DOI] [PubMed] [Google Scholar]
- 9.Levacq D., D’Haene N., de Wind R., Remmelink M., Berghmans T. Histological transformation of ALK rearranged adenocarcinoma into small cell lung cancer: a new mechanism of resistance to ALK inhibitors. Lung Cancer. 2016;102:38–41. doi: 10.1016/j.lungcan.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y.C., Liao X.H., Wang W.X., et al. Patients harboring ALK rearrangement adenocarcinoma after acquired resistance to crizotinib and transformation to small-cell lung cancer: a case report. Onco Targets Ther. 2017;10:3187–3192. doi: 10.2147/OTT.S139718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oya Y., Yoshida T., Uemura T., Murakami Y., Inaba Y., Hida T. Serum ProGRP and NSE levels predicting small cell lung cancer transformation in a patient with ALK rearrangement-positive non-small cell lung cancer: a case report. Oncol Lett. 2018;16:4219–4222. doi: 10.3892/ol.2018.9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balla A., Khan F., Hampel K.J., Aisner D.L., Sidiropoulos N. Small-cell transformation of ALK-rearranged non-small-cell adenocarcinoma of the lung. Mol Case Stud. 2018;4 doi: 10.1101/mcs.a002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama R., Friboulet L., Koike S., et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20:5686. doi: 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw A.T., Solomon B.J., Besse B., et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non–small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]