Abstract
Background
Most observational population-based studies identify respiratory syncytial virus (RSV) by nasal/nasopharyngeal swab reverse transcriptase real-time PCR (RT-PCR) only. We conducted a systematic review and meta-analyses to quantify specimen and diagnostic testing-based underascertainment of adult RSV infection.
Methods
EMBASE, PubMed, and Web of Science were searched (January 2000−December 2021) for studies including adults using/comparing >1 RSV testing approach. We quantified test performance and RSV detection increase associated with using multiple specimen types.
Results
Among 8066 references identified, 154 met inclusion. Compared to RT-PCR, other methods were less sensitive: rapid antigen detection test (RADT; pooled sensitivity, 64%), direct fluorescent antibody (DFA; 83%), and viral culture (86%). Compared to singleplex PCR, multiplex PCR's sensitivity was lower (93%). Compared to nasal/nasopharyngeal swab RT-PCR alone, adding another specimen type increased detection: sputum RT-PCR, 52%; 4-fold rise in paired serology, 44%; and oropharyngeal swab RT-PCR, 28%. Sensitivity was lower in estimates limited to only adults (for RADT, DFA, and viral culture), and detection rate increases were largely comparable.
Conclusions
RT-PCR, particularly singleplex testing, is the most sensitive RSV diagnostic test in adults. Adding additional specimen types to nasopharyngeal swab RT-PCR testing increased RSV detection. Synergistic effects of using ≥3 specimen types should be assessed, as this approach may improve the accuracy of adult RSV burden estimates.
Keywords: respiratory syncytial virus infections, adults, diagnosis, epidemiology, sensitivity and specificity
Most observational studies only identify RSV in adults by nasal/nasopharyngeal swab RT-PCR. Our review found that while RT-PCR is the most accurate, adding additional specimen types to nasopharyngeal swab RT-PCR testing increased RSV detection by up to 50%.
Respiratory syncytial virus (RSV) causes acute respiratory infection in persons of all ages. The Global Burden of Disease Study 2016 reported 25 million episodes of RSV infections and 77 000 associated deaths worldwide [1]. While mild in most healthy adults, RSV can result in severe illness and poor outcomes in persons who are frail, elderly, or have underlying health conditions [2].
Due to RSV's nonspecific clinical manifestations, laboratory testing of respiratory secretions is required to confirm infection [3]. In adults, low viral titers and shorter duration of viral shedding (compared to young children) limit RSV detection using current diagnostic modalities [2].
Historically, the gold standard diagnostic modality was viral culture but this has been largely replaced by more sensitive and less time-consuming methods [4]. Molecular techniques, such as nucleic acid amplification tests, which can detect very low viral titers, have improved test sensitivity in adults and produce results more quickly. Reverse transcriptase real-time polymerase chain reaction (RT-PCR) has become the reference diagnostic method for RSV detection due to its high diagnostic accuracy [3, 5].
A rise in serology titers (≥4-fold rise between acute/convalescence or pre-/postseason specimens) can identify recent RSV infection although some adults do not seroconvert [6, 7]. This method is largely limited to epidemiological studies because a retrospective diagnosis is less useful for clinical care [5]. Alternative diagnostic methods such as antigen detection assays (eg, direct fluorescent antibody [DFA] tests and rapid antigen detection tests [RADTs]) offer fast results and are easy to perform but have low sensitivity in adults [4, 5].
While RT-PCR increases RSV detection sensitivity compared to other testing modalities, the specimen used for diagnostic test can impact sensitivity of RSV detection. Specimen collection using nasopharyngeal swabs (NPS), although better tolerated than nasal wash or nasopharyngeal aspirate (NPA) in adults, yields lower viral titers and underestimates RSV infections [3, 5]. Studies combining sputum and paired serology with NP/nasal swab have further documented the underascertainment associated with single specimens, particularly NPS [7].
As a result of the diagnostic tests employed and the choice of specimen used, many RSV infections are likely missed. This in turn has implications for designing public health policies as these are heavily dependent on accurate determination of disease burden [3, 5]. Furthermore, underascertainment of RSV infections reduces statistical power and may lead to misclassification within vaccine efficacy trials, thus impeding vaccine development efforts [8].
We conducted a systematic literature review and meta-analysis to collate comparative literature on RSV diagnostic testing in adults and quantify the underascertainment associated with RSV diagnostic testing and sampling approaches. This quantification could allow adjustment of clinical burden estimates for diagnostic testing-related underascertainment and thus better estimate the true burden of disease.
METHODS
Protocol and Registration
This systematic review protocol adhered to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) for Diagnostic Test Accuracy (DTA) Studies [9] and is registered on the PROSPERO database (registration No. CRD42022313209).
Study Eligibility Criteria
Eligibility criteria were developed using the Population-Intervention-Comparator-Outcomes-Time Frame-Setting (PICOTS) framework (Supplementary Table 1). Briefly, primary studies were eligible if they reported on the diagnostic test performance or compared RSV detection rates using different specimens. We excluded secondary research (eg, reviews, meta-analyses), studies in children, and in vitro studies.
Information Sources and Search Strategy
Embase, MEDLINE (via PubMed), Web of Science, and nonindexed sources and RSV research networks (Supplementary Table 2) were searched for publications during 1 January 2000 to 27 December 2021. Additional relevant articles were sought by manual search of systematic reviews' bibliographies. Supplementary Table 3 presents detailed search strategies.
Study Selection
Retrieved articles were screened based on a priori eligibility criteria (Supplementary Table 1). First, titles and abstracts were screened to identify potentially relevant articles. Subsequently full-text screening was performed in duplicate by independent reviewers (L.M.M., B.M.) with a third reviewer resolving disagreements (C.O., S.M.).
Data Extraction
Predefined data items were extracted from each included article into a piloted structured extraction form on DistillerSR [10]. One reviewer extracted the data while a second reviewer cross-checked the collected data. Discrepancies were resolved by consensus or a third reviewer acted as an arbiter. Data items extracted for this review are summarized in the Supplementary Table 4.
For this review, we abstracted data on paired specimens (hence ≥ 2 specimen types from the same patient using the same diagnostic test) or paired testing (1 specimen type using ≥2 diagnostic methods). Overall RSV detection was defined as the number of specimens that tested positive for RSV by any testing approach. Consistent with PRISMA-DTA guidance, the index test was defined as the test under evaluation while the reference test was the comparator test [9].
Risk of Bias and Applicability
Study quality assessment was performed by one reviewer and verified by a second reviewer using Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria [11]. Disagreement between reviewers' assessments was resolved by consensus and, if required, a senior review team member resolved the conflict. To allow assessment of studies comparing RSV detection by different specimens, we included a domain to capture potential sources of bias due to specimen collection and flow. In Supplementary Table 5, we outline each domain component, highlighting how we made judgements concerning risk of bias.
Diagnostic Accuracy Measures and Synthesis of Results
Characteristics of studies and populations were analyzed and reported descriptively. We reported the overall detection rate (DR) and test-specific DR for each study. Subsequently, the DR ratio (DRR) was estimated as (positive by any method)/(positive by reference test). RT-PCR and NPS and nasal swabs were considered reference test and sampling methods, respectively. We chose NPS and nasal swab combined as the gold standard because they were the most commonly used tests for adult RSV detection in identified studies and because evidence from influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suggests that they may perform similarly [12, 13].
Data from 2-by-2 tables were used to measure diagnostic tests' sensitivity and specificity, calculate 95% confidence intervals (CIs), and were then presented graphically in a forest plot. For this review, sensitivity is defined as the proportion of RSV-positive specimens correctly identified, and specificity as the proportion of RSV-negative specimens correctly identified by a test against a reference. Diagnostic test performance summary is presented separately for RADTs, DFA, viral culture, and serology using any RT-PCR type, including singleplex, multiple, in-house, and commercial platforms, as a common reference and stratified by age groups. Age groups were adults only and combined results for adults and children where no stratification was reported. Meta-analyses of each diagnostic test's performance were performed where applicable using random-effects models. Additionally, we evaluated the performance of multiplex RT-PCR (targeting multiple respiratory pathogens) against singleplex RT-PCR (only RSV), and the concordance between multiplex RT-PCR platforms. All analyses were performed in R version 4.2.0, using the meta4diag and metafor package.
Sensitivity Analyses
Given the potentially higher sensitivity of diagnostic tests among children, a sensitivity analysis was conducted to explore whether results were affected by excluding studies involving both adults and children (mixed population) or not reporting the percentage of adults (unspecified population).
RESULTS
Study Selection
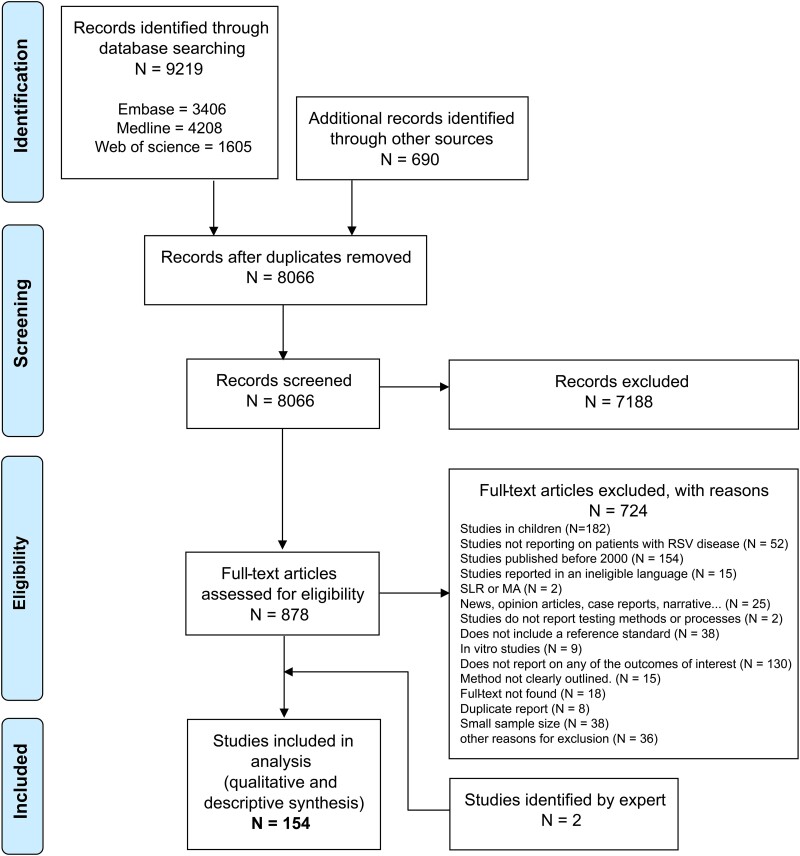
Our database search returned 9219 references and 690 references were identified from other sources (Supplementary Table 2), producing 8066 unique references. After screening, we included 154 articles in the review (Figure 1). Two additional articles were included based on expert recommendation (E.B.) [14, 15].
Figure 1.
PRISMA flow chart showing the study selection process. Abbreviations: MA, meta-analyses; SLR, Systematic literature reviews.
Study Characteristics
Supplementary Table 6 provides the complete list and detailed description of included studies. Most were conducted in North or South America (37%) or Europe (32%), and 25 studies included only adults (Table 1). Most studies had a cross-sectional design (137/154), while 8 were prospective surveillance studies, and 9 used other designs.
Table 1.
Summary of Key Characteristics of Included Studies
| Characteristic | No. (%) (n = 154) |
|---|---|
| World Health Organization region | |
| Americas | 59 (37) |
| Europe | 53 (32) |
| Western Pacific | 38 (24) |
| Africa | 3 (1.9) |
| South-East Asia | 3 (1.9) |
| Study design | |
| Cross-sectional | 137 (89) |
| Surveillance | 8 (5.2) |
| Prospective cohort | 4 (2.6) |
| Case-control | 3 (1.9) |
| Retrospective cohort | 1 (0.6) |
| Randomized controlled trial | 1 (0.6) |
| Age group | |
| Adults and children | 80 (52) |
| Adults, 18 y and older | 25 (16) |
| Not reported, eg, based on stored samples | 49 (32) |
| Clinical setting | |
| Inpatient only, hospitalized and ICU | 29 (19) |
| Emergency department only | 7 (4.5) |
| Outpatient only, clinics and primary care centers | 8 (5.2) |
| Inpatient and emergency department, mixed | 3 (1.9) |
| Outpatient, inpatient, and emergency department, mixed | 8 (5.2) |
| Others, community-based and clinical trial sites | 4 (1.9) |
| Not reported | 95 (62) |
| Clinical presentation of the population studied | |
| LRTI onlya | 30 (19.5) |
| URTI only | 13 (8.4) |
| URTI or LRTI | 42 (27.3) |
| LRTI exacerbating preexisting conditions | 5 (3.2) |
| Otherb | 3 (1.9) |
| Not reported | 61 (39.6) |
| Specimen typec | |
| Nasopharyngeal swab | 102 (47.6) |
| Nasopharyngeal aspirate | 49 (31.8) |
| Bronchoalveolar lavage | 40 (18.7) |
| Oropharyngeal swab | 36 (16.8) |
| Othersd | 27 (12.6) |
| Sputum | 23 (10.7) |
| Nasal swab | 19 (12.3) |
| Nasal wash/aspirate | 12 (5.6) |
| Paired serology sample | 6 (2.8) |
| Not specified | 3 (1.4) |
| Saliva | 2 (0.6) |
| Diagnostic test | |
| Multiplex RT-PCR | 113 (68.5) |
| Singleplex RT-PCR | 26 (15.8) |
| Viral culture | 36 (21.8) |
| Direct fluorescent antibody test | 20 (12.1) |
| Rapid antigen detection tests | 18 (10.9) |
| Otherse | 10 (6.5) |
| Conventional PCR | 6 (3.6) |
| Serology | 6 (3.6) |
Abbreviations: ICU, intensive care unit; LRTI, lower respiratory tract infection; RT-PCR, reverse transcription-polymerase chain reaction; URTI, upper respiratory tract infection.
Includes pneumonia, acute bronchitis, bronchiolitis, and influenza-like illnesses.
Patients with respiratory illness or suspected respiratory illness with unclear category.
Eighty studies used a combination of respiratory samples for testing.
Unspecified upper respiratory swab, stored nucleic acid extract, unspecified respiratory secretions, pleural fluid, mouth wash, endotracheal tube specimen, and bronchial brushings.
Loop-mediated isothermal amplification (LAMP) = 4, nucleic acid sequence-based amplification (NASBA) = 3, reverse transcription strand invasion-based amplification (RT-SIBA) = 2 studies, Sanger sequencing = 1.
Overall, 77 studies recruited patients with lower respiratory tract infection or exacerbation of an underlying cardiac or pulmonary disease, while 55 studies included patients with upper respiratory tract infection. When reported (59 studies), study populations were most frequently hospitalized patients (66%).
The most common upper respiratory samples were NPS (102 studies), NPA (48 studies), and oropharyngeal swab (OPS, 36 studies). The most frequently used diagnostic approach was RT-PCR, with 68.5% (113 studies) using multiplex platforms and 15.8% (26 studies) using singleplex platforms. DFA testing and RADTs were reported in 20 studies and 18 studies, respectively, while 6 studies evaluated paired serological testing.
Risk of Bias and Applicability
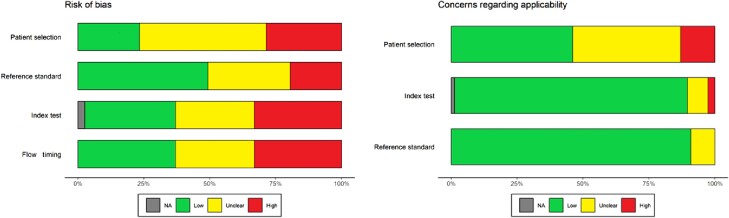
Overall, 99 of 154 (64.3%) included studies were graded as having a high risk of bias in ≥1 domain (46% for primary end point, single-sample RT-PCR vs combined testing approach). Quality assessment is presented in Figure 2 and Supplementary Table 7.
Figure 2.
Summary risk of bias assessment of included studies based on the QUADAS-2 tool. Abbreviation: NA, Not applicable.
In the patient selection domain, 74 studies (48.7%) had an unclear risk of bias due to lack of clear reporting of patient selection process and study setting (31% for primary end point) and 44 (29%) were graded as high risk of bias. In the index test domain, 51 studies (33.1%) had a high risk of bias due to either unblinded operators or lack of predefined threshold or internal control. While in the reference standard domain, 33 studies (21.4%) were assessed as high risk and 48 studies (31.1%) as unclear risk of bias. In the flow and timing domain, 51 studies (33%) were judged to be at high risk of bias. All 13 studies where our QUADAS-2 extension was used had low risk of bias due to sample flow.
For applicability assessment, 20 studies (13%) had patient selection domain risks, mostly studies without patient information or with inclusion of children without age-stratified results. In other domains, the number of studies with concerns were low.
Results of Individual Studies
The number of RSV infections detected overall, and by specific testing methods in each study, is presented in Supplementary Table 8.
RSV Detection by Single Sample RT-PCR Versus Combined Testing Approach
We identified 13 studies comparing RSV detection using RT-PCR based on NPS or nasal swab alone versus a combined testing approach [14–26]. The additional samples and testing approach included: paired serology testing (6 studies), RT-PCR on OPS (3 studies), sputum (3 studies), saliva (2 studies), and NPA (1 study) (Table 2).
Table 2.
RSV Detection by Reference Testing and Additional testing
| Reference, Author (Year) | Country | Tested Population Description, All Adults Only Unless Otherwise Specified | Subjects With Paired Specimens, No. | Reference Specimen Type, All RT-PCR | RSV Detection Rate by Reference | RSV Detection Rate Using All Specimens | Detection Rate Ratio |
|---|---|---|---|---|---|---|---|
| Additional testing = paired serology | |||||||
| Luchsinger (2012) [16] | Chile | Hospitalized with CAP | 184 | Sputum, NP aspirate, or BAL | 10.8 | 19.6 | 1.80 |
| Falsey (2019) [14] | Europe/North America | ≥50 y with severe COPD/CHF with RS | 1111 | Nasal swab | 2.5 | 3.8 | 1.50 |
| Falsey (2002) [7] | USA | Healthy, CHF/COPD, inpatients, and nursing home residents | 1112 | NP swab | 7.8 | 10.5 | 1.34 |
| Feikin (2013) [18] | Kenya | Pediatric and adult outpatients with RS | 204 | NP swab | 8.3 | 11.3 | 1.36 |
| Korsten (2021) [15] | Belgium, The Netherlands, and England | Community-dwelling adults ≥60 y with RS (not medically attended and outpatients) | 1040 | NP swab | 3.5 | 5.7 | 1.64 |
| Zhang (2017) [19] | USA | Hospitalized 18 -64 y with CAP | 623 | NP swab | 2.2 | 2.9 | 1.29 |
| Zhang (2017) [19] | USA | Hospitalized ≥65 with CAP | 313 | NP swab | 3.2 | 4.8 | 1.50 |
| Additional testing = RT-PCR on oropharyngeal swabs | |||||||
| Ek (2019) [20] | Sweden | Attending emergency department with RS | 98 | NP swab | 6.1 | 6.1 | 1.00 |
| Kim (2011) [26] | Kenya | Pediatric and adult outpatients with RS | 2331 | NP swab | 10.8 | 14.1 | 1.30 |
| Lieberman (2009) [21] | Israel | Hospitalized with LRTI | 1000 | NP swab | 2.6 | 3.0 | 1.15 |
| Additional testing = RT-PCR on sputum | |||||||
| Branche (2014) [22] | USA | Hospitalized with RS | 965 | Nasal and throat swab | 5.1 | 6.5 | 1.29 |
| Falsey (2019) [14] | Europe/North America | ≥50 y with severe COPD/CHF with RS | 674 | Nasal swab | 2.4 | 4.7 | 2.00 |
| Jeong (2014) [23] | South Korea | Hospitalized and outpatient with RS | 154 | NP swab | 11.0 | 18.8 | 1.71 |
| Additional testing = RT-PCR on nasopharyngeal wash with or without oropharyngeal swabs | |||||||
| Lieberman (2009) [21] | Israel | Hospitalized with LRTI | 1000 | NP swab | 2.6 | 2.8 | 1.08 |
| Lieberman (2009) [21] | Israel | Hospitalized with LRTI | 1000 | NP swab | 2.6 | 3.1 | 1.19 |
| Additional testing = RT-PCR on saliva | |||||||
| To (2017) [25] | China | Hospitalized with RS | 159 | NP aspirate | 11.3 | 11.3 | 1.00 |
| To (2019) [24] | China | Hospitalized with RS | 212 | NP aspirate | 11.8 | 12.7 | 1.08 |
Abbreviations: CAP, community-acquired pneumonia; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; NP, nasopharyngeal; RS, respiratory symptoms; RSV, respiratory syncytial virus; RT-PCR, reverse transcription-polymerase chain reaction.
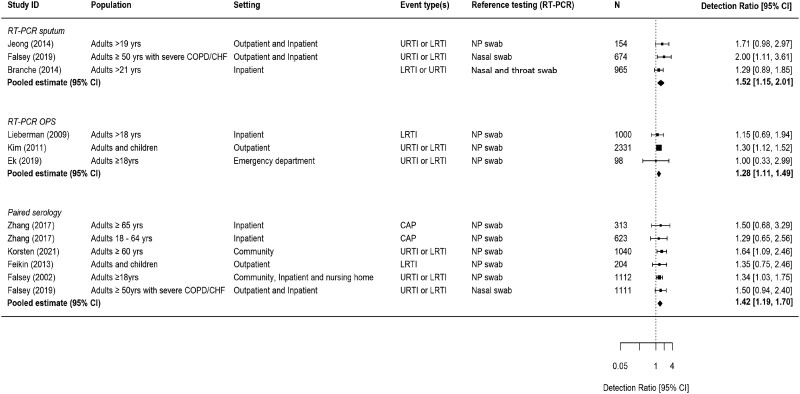
The addition of sputum RT-PCR to NP/nasal swab RT-PCR increased RSV detection by 52% (DRR, 1.52; 95% CI, 1.15–2.01), while adding RT-PCR of OPS increased detection by 28% (DRR, 1.28; 95% CI, 1.11–1.49) (Figure 3). Compared to RT-PCR on a NP/nasal swab sample only, the addition of paired serology testing increased detection by 42% (DRR, 1.42; 95% CI, 1.19–1.70).
Figure 3.
Increase in RSV detection rate due to the addition of another specimen testing to reference RT-PCR of NP swab or nasal swab. Detection ratio >1.0 indicates an increase in RSV detection associated with additional specimen testing. Abbreviations: CAP, community-acquired pneumonia; CHF, chronic heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; LRTI, lower respiratory tract infection; NP, nasopharyngeal; OPS, oropharyngeal swab; RT-PCR, reverse transcription polymerase chain reaction; URTI, upper respiratory tract infection.
Diagnostic Accuracy of Tests
We conducted meta-analyses of diagnostic test performance on index tests against a common reference of RT-PCR (Supplementary Table 9).
Accuracy of DFA
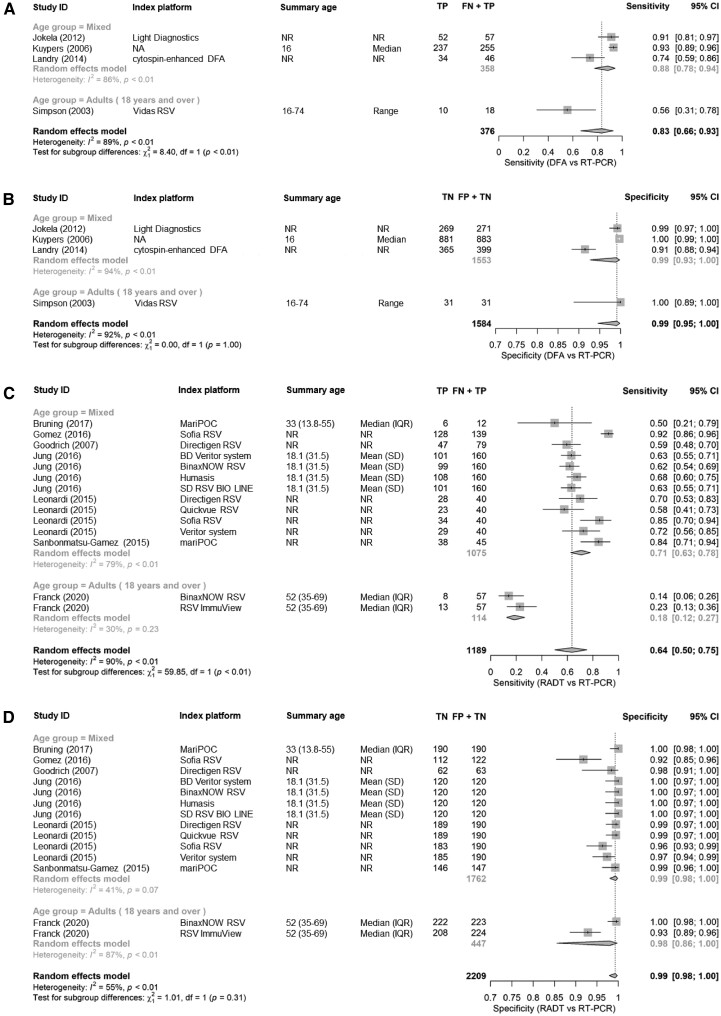
Five studies compared DFA to RT-PCR, of which 4 used a multiplex RT-PCR platform, and 1 study a simplex platform [27–31]. Pooled sensitivity versus multiplex RT-PCR was 83% (95% CI, 66%–93%) across all platforms, and the corresponding pooled specificity was 96.6% (95% CI, 96.2%–97.0%). The only sensitivity estimate limited to adults was the lowest reported at 56% (95% CI, 31%–78%) (Figure 4A and 4B).
Figure 4.
A, Sensitivity of DFA test using RT-PCR as reference test. B, Specificity of DFA test using RT-PCR as reference test. C, Sensitivity of RADT using RT-PCR as reference test. D, Specificity of RADT using RT-PCR as reference test. Abbreviations: CI, confidence interval; DFA, direct fluorescent antibody; FN, false negative; FP, false positive; IQR, interquartile range; NA, Not Applicable; NR, Not Reported; RADT, rapid antigen detection tests; RT-PCR, reverse transcription polymerase chain reaction; TN + FP, all reference negative; TN, true negative; TP, true positive; TP + FN, all reference positive.
Accuracy of RADT
Overall, RADTs had a pooled sensitivity of 64% (95% CI, 50%–75%) compared to a reference standard of RT-PCR based on 14 comparisons from 7 studies. The type of reference standard employed affected RADT sensitivity estimates [32–38]. Overall sensitivity was 77% with multiplex RT-PCR as reference test (n = 7 comparisons) and 50% with singleplex RT-PCR (n = 7). The studies limited to adults reported the lowest sensitivities: BinaxNOW (14%) and RSV ImmuView tests (23%) compared to singleplex RT-PCR [33]. Overall, RADT specificity was 99% (95% CI, 98%–100%) compared to RT-PCR (Figure 4C and 4D).
Accuracy of Viral Culture
We identified 3 studies that assessed viral culture versus a reference standard of RT-PCR, but 1 only included a single positive sample resulting in unstable results. The sensitivity ranged between 49% and 86%. Specificity was 100% in all 3 studies (Supplementary Table 9) [7, 39, 40].
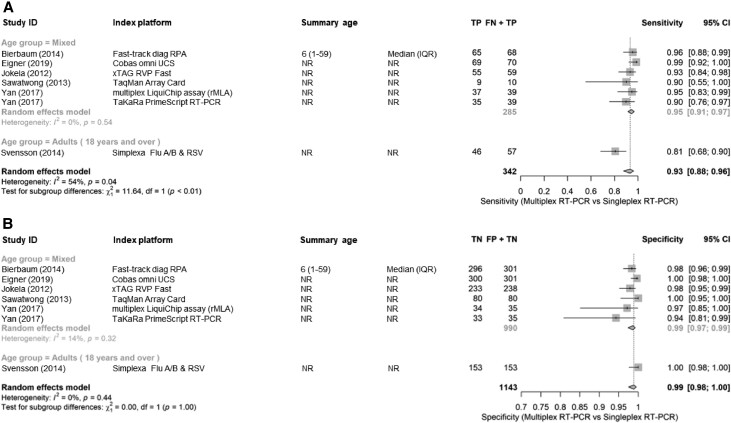
Accuracy of Multiplex Versus Singleplex and Agreement Between RT-PCR Platforms
Six studies evaluated the performance of a multiplex RT-PCR versus a gold standard singleplex platform [27, 41–45]. Pooled sensitivity was 92% (95% CI, 86%–96%) and specificity 99% (95% CI, 98%–100%) (Figure 5A and 5B). The single adults-only study had lowest sensitivity at 81%. Agreement between RT-PCR platforms was generally high, with a pooled κ value of 0.91 (Supplementary Figure 1).
Figure 5.
A, Sensitivity of multiplex RT-PCR using singleplex RT-PCR as reference test. B, Specificity of multiplex RT-PCR using singleplex RT-PCR as reference test. Abbreviations: CI, confidence interval; NR, Not Reported; FN, false negative; FP, false positive; IQR, interquartile range; RT-PCR, reverse transcription polymerase chain reaction; TN + FP, all reference negative; TN, true negative; TP, true positive; TP + FN, all reference positive.
Sensitivity Analyses
To obtain adult-specific results for the DRRs (section “RSV Detection by Single Sample RT-PCR Versus Combined Testing Approach”), we excluded those studies that involved both adults and children [18, 26]. Compared to the primary analysis, the exclusion of Feikin et al 2013 [18] from the analysis for added paired serology produced similar findings (DRRsubanalysis = 1.43 vs DRRfull-analysis = 1.42), while exclusion of Kim et al 2011 [26] resulted in a lower DRR for RT-PCR on OPS (DRRsubanalysis = 1.12 vs DRRfull-analysis = 1.28) (Supplementary Figure 2). We included only 2 studies on adults ≥60 years old, hence we could not conduct a subanalysis in this group.
To investigate study quality's influence on our results, we excluded studies with high risk of bias and mixed adult and children study population from the primary outcome analysis and produced similar results for serology and sputum. For OPS, only 1 study was retained, which showed no increase in RSV detection by (DRR, 1.00; 95% CI, .33–2.99; Supplementary Figure 3A and 3B).
Investigation of Heterogeneity and Subgroup Analysis
We investigated heterogeneity by conducting a stratified analysis of test performance by adult and mixed populations (Figure 4A–4D). While the number of studies in only adults precluded meta-analyses, the overall reported sensitivities of RADT and DFA were higher in mixed adult and pediatric populations than in adults only.
DISCUSSION
To our knowledge, this is the first systematic review that quantifies the underascertainment of RSV infection associated with diagnostic testing and specimen collection approach. RT-PCR remains the most sensitive testing approach, and NPSs are the most used specimen type for this testing. Despite RT-PCR's high sensitivity, adding specimen types such as paired serology and sputum to NP/nasal swab RT-PCR increased RSV detection by 50% to 66% on average, respectively. The use of singleplex rather than multiplex PCR was associated with an additional 7% increase in ascertainment. Furthermore, sensitivity estimates limited to only adults were consistently the lowest reported across several comparisons. Because most RSV incidence studies in adults use only RT-PCR of NPS to detect RSV infection and many use a multiplex RT-PCR platform, our results support a substantial underascertainment of RSV burden in adults, particularly in the hospital setting.
All sample types were found to increase RSV detection when added to NP/nasal swab. Multiple reasons likely account for this increased RSV detection. At the time of presentation to a health care provider or to a hospital, the infection may be predominately focused in the lower rather than the upper respiratory tract, and thus sputum may be more likely to be positive than NP/nasal swab. Nasal viral shedding in adults begins 1–2 days after symptom onset and starts declining by days 5–6, thus because persons are hospitalized on average 5 to 6 days after illness onset, nasal shedding may be limited at the time of testing [46]. Over half of the populations from included studies were hospitalized, where material captured with NP/nasal swabs can be limited by nasal dryness from nasal oxygen use as well as potentially diuretic administration and dry indoor air due to air handling systems. Sputum is from within the lung and OPS from within the mouth/throat, which would likely be less impacted by such factors [46, 47]. Paired serology can detect recent RSV infections missed by direct viral detection in respiratory samples and may have important epidemiological application. Because of past exposure to RSV, adults can have a rapid and marked increase in humoral immune response allowing for RSV diagnosis based on a 4-fold rise in immunoglobulin G (IgG) antibody levels between the initial acute specimen and convalescent specimen (4–6 weeks later). However, not all with confirmed RSV-infection by PCR or viral culture seroconvert and false-negative serology can also occur due to already elevated IgG level in the acute specimen [46], especially if collected at hospital admission after several days of illness [7, 46].
Because of the limited number of studies reporting on exclusively adult populations, we included estimates from mixed-age populations. We conducted a sensitivity analysis excluding these for the primary end point, but not for other comparisons because the number of adult-only articles were too small. To address this, we presented results by age group (adults vs mixed/unspecified results) and included age in our figures. The lowest sensitivity estimates across multiple comparisons (ie, DFA, RADT, and singleplex vs multiplex) were those including adults only. Adults are known to have lower viral loads in their respiratory secretions than children [2], likely explaining these lower results. On this basis, the inclusion of mixed populations likely resulted in our estimates of RSV underascertainment being conservative.
We have collated a body of literature that documents that testing single NPS or nasal swab with RT-PCR for RSV diagnosis results in systematic underestimation of the frequency of RSV infection. While 2 prior reviews have highlighted the poor performance and variability in the performance of RADTs when compared with RT-PCR [4, 42], no reviews have collated information on the additional case yield associated with adding additional specimen types, making our systematic literature review the first to undertake this. Our review further highlights the limited level of agreement between point-of-care molecular tests and conventional RT-PCR (pooled estimated 70% for adult studies only; Supplementary Figure 1). However, a recent study published after this study's search period found 99.3% overall agreement between Xpert Xpress Flu/RS and an in-house RT-PCR in RSV detection [48].
This has important implications for burden of disease estimates, because nearly all RSV incidence studies among adults depend exclusively on RT-PCR NPS for RSV diagnosis [6]. Recently, a published meta-analysis of US incidence studies included an adjustment factor of 1.5-fold for underascertainment based on diagnostic testing, which increased the pooled prospective incidence estimate of RSV-related hospitalizations among persons 65 years and older from 189 to 282 per 100 000 [49]. Our results suggest this adjustment is conservative because all our collated comparisons were pairwise and did not take into account the additional cases that could result from adding more than 2 specimen types. A prospective study is underway in North America to collect a wide range of specimens for acute respiratory events to describe this overall increase in sensitivity. While there is some overlap among the additional positives identified by sputum, saliva, and paired serology testing compared to NPS alone, each specimen type contributes some additional unique positives [50].
The primary strengths of this review were a comprehensive systematic literature review that included 3 global databases, inclusion of other potential sources of relevant studies, and broad inclusion criteria. The inclusion of many large studies and multiple comparisons allowed for a broader scope of diagnostic tests comparisons. Furthermore, these results come from a variety of treatment settings (inpatients, outpatients, nonmedically attended events from community, nursing home residents), populations (older and younger adults, persons with underlying congestive heart failure, chronic obstructive pulmonary disease, as well as healthy adults), event types (any acute respiratory symptoms, all lower respiratory tract infection, and limited to pneumonia), and geographic regions (Asia, Africa, North and South America, and Europe) supporting the generalizability of these results.
However, there are limitations in this study. First, about one-third (46 of 154) of included studies had fewer than 200 participants and hence small overall numbers of RSV detected. Second, about two-thirds of articles had at least 1 high or unclear risk of bias. This was most often observed in studies comparing diagnostic test performance and less often in studies comparing detection rates using combined specimens. Overall, 49 studies had an unclear risk of bias, due to inadequate available information in at least 1 domain. This highlights the gaps in reporting of DTA studies and calls for wider adoption of DTA reporting guidelines. Lastly, as mentioned above, many of the studies included in the analysis of diagnostic test performance provided sparse demographic data or did not provide disaggregated information. This lack of detailed demographic and clinical information precluded the investigation of heterogeneity by subgroup analysis, including one for older adults, likely to benefit most from future vaccines.
CONCLUSIONS
Accurate diagnostic methodologies are critical for determining RSV disease burden, public health impact of preventative interventions (including vaccines), and generating accurate economic models. With multiple RSV vaccine candidates currently under development, the estimation of population-level effects will rely heavily on an accurate diagnosis of infected persons. Our review indicates that, while RT-PCR using NPS is the most sensitive currently available diagnostic methodology, the addition of other testing approaches—including collection of different specimens and potentially use of serology—substantially boosts RSV detection and these results should be considered when estimating disease burden and the subsequent economic value of RSV immunization of adults. Additional research on synergistic effects of using multiple specimens could further inform this issue and lead to more accurate disease burden estimates and economic models, for example by adding a multiplier to adjust published RSV lower respiratory tract infection incidence estimates.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Chukwuemeka Onwuchekwa, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Laura Mora Moreo, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Sonia Menon, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Belen Machado, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Daniel Curcio, Pfizer Vaccine, Ireland.
Warren Kalina, Pfizer Vaccine, Ireland.
Jessica E Atwell, Pfizer Vaccine, Ireland.
Bradford D Gessner, Pfizer Vaccine, Ireland.
Mariana Siapka, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium; Impact Epilysis, Thessaloniki, Greece.
Neha Agarwal, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Michelle Rubbrecht, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Harish Nair, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom.
Mark Rozenbaum, Pfizer Inc, Capelle aan den Ijssel, The Netherlands.
Zuleika Aponte-Torres, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Hilde Vroling, P95 Epidemiology and Pharmacovigilance, Leuven, Belgium.
Elizabeth Begier, Pfizer Vaccine, Ireland.
Notes
Financial support. This work was funded by Pfizer Inc, USA. Funding to pay the Open Access publication charges for this article was provided by Pfizer Inc, USA.
References
- 1. GBD 2016 Lower Respiratory Infections Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi T, Denouel A, Tietjen AK, et al. . Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2019; 222:S577–83. [DOI] [PubMed] [Google Scholar]
- 3. Chartrand C, Tremblay N, Renaud C, Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J Clin Microbiol 2015; 53:3738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henrickson KJ, Hall CB. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J 2007; 26:S36–40. [DOI] [PubMed] [Google Scholar]
- 5. Popow-Kraupp T, Aberle JH. Diagnosis of respiratory syncytial virus infection. Open Microbiol J 2011; 5:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically-attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis 2022; 9:ofac300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002; 40:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine 2018; 36:3199–207. [DOI] [PubMed] [Google Scholar]
- 9. Salameh JP, Bossuyt PM, McGrath TA, et al. . Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ 2020; 370:m2632. [DOI] [PubMed] [Google Scholar]
- 10. DistillerSR . Version 2.35. Evidence partners.https://www.evidencepartners.com.
- 11. Whiting PF, Rutjes AW, Westwood ME, et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–36. [DOI] [PubMed] [Google Scholar]
- 12. Irving SA, Vandermause MF, Shay DK, Belongia EA. Comparison of nasal and nasopharyngeal swabs for influenza detection in adults. Clin Med Res 2012; 10:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsujimoto Y, Terada J, Kimura M, et al. . Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect Dis (Lond) 2021; 53:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falsey AR, Walsh EE, Esser MT, Shoemaker K, Yu L, Griffin MP. Respiratory syncytial virus-associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J Med Virol 2019; 91:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korsten K, Adriaenssens N, Coenen S, et al. . Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J 2021; 57:2002688. [DOI] [PubMed] [Google Scholar]
- 16. Luchsinger V, Piedra PA, Ruiz M, et al. . Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis 2012; 54:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falsey AR, Formica MA, Walsha EE. Yield of sputum for viral detection by reverse transcriptase PCR in adults hospitalized with respiratory illness. J Clin Microbiol 2012; 50:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feikin DR, Njenga MK, Bigogo G, et al. . Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol 2013; 20:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Sakthivel SK, Bramley A, et al. . Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017; 55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ek P, Böttiger B, Dahlman D, Hansen KB, Nyman M, Nilsson AC. A combination of naso- and oropharyngeal swabs improves the diagnostic yield of respiratory viruses in adult emergency department patients. Infect Dis 2019; 51:241–8. [DOI] [PubMed] [Google Scholar]
- 21. Lieberman D, Lieberman D, Shimoni A, Keren-Naus A, Steinberg R, Shemer-Avni Y. Identification of respiratory viruses in adults: nasopharyngeal versus oropharyngeal sampling. J Clin Microbiol 2009; 47:3439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Branche AR, Walsh EE, Formica MA, Falsey AR. Detection of respiratory viruses in sputum from adults by use of automated Multiplex PCR. J Clin Microbiol 2014; 52:3590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeong JH, Kim KH, Jeong SH, Park JW, Lee SM, Seo YH. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J Med Virol 2014; 86:2122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. To KKW, Yip CCY, Lai CYW, et al. . Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect 2019; 25:372–8. [DOI] [PubMed] [Google Scholar]
- 25. To KK, Lu L, Yip CC, et al. . Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect 2017; 6:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim C, Ahmed JA, Eidex RB, et al. . Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One 2011; 6:e21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jokela P, Piiparinen H, Mannonen L, Auvinen E, Lappalainen M. Performance of the Luminex xTAG respiratory viral panel fast in a clinical laboratory setting. J Virol Methods 2012; 182:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuypers J, Wright N, Ferrenberg J, et al. . Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44:2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Landry ML, Ferguson D. Comparison of Simplexa flu A/B & RSV PCR with cytospin- immunofluorescence and laboratory-developed TaqMan PCR in predominantly adult hospitalized patients. J Clin Microbiol 2014; 52:3057–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rovida F, Percivalle E, Zavattoni M, et al. . Monoclonal antibodies versus reverse transcription-PCR for detection of respiratory viruses in a patient population with respiratory tract infections admitted to hospital. J Med Virol 2005; 75:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simpson JL, Moric I, Wark PAB, Johnston SL, Gibson PG. Use of induced sputum for the diagnosis of influenza and infections in asthma: a comparison of diagnostic techniques. J Clin Virol 2003; 26:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruning AHL, de Kruijf WB, van Weert HCPM, et al. . Diagnostic performance and clinical feasibility of a point-of-care test for respiratory viral infections in primary health care. Fam Pract 2017; 34:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franck KT, Schneider UV, Ma CMG, Knudsen D, Lisby G. Evaluation of Immuview RSV antigen test (SSI Siagnostica) and BinaxNOW RSV card (Alere) for rapid detection of respiratory syncytial virus in retrospectively and prospectively collected respiratory samples. J Med Virol 2020; 92:2992–8. [DOI] [PubMed] [Google Scholar]
- 34. Gomez S, Prieto C, Folgueira L. A prospective study to assess the diagnostic performance of the Sofia® immunoassay for influenza and RSV detection. J Clin Virol 2016; 77:1–4. [DOI] [PubMed] [Google Scholar]
- 35. Goodrich JS, Miller MB. Comparison of Cepheid's analyte-specific reagents with BD Directigen for detection of respiratory syncytial virus. J Clin Microbiol 2007; 45:604–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jung BK, Choi SH, Lee JH, Lee J, Lim CS. Performance evaluation of four rapid antigen tests for the detection of respiratory syncytial virus. J Med Virol 2016; 88:1720–4. [DOI] [PubMed] [Google Scholar]
- 37. Leonardi GP, Wilson AM, Dauz M, Zuretti AR. Evaluation of respiratory syncytial virus (RSV) direct antigen detection assays for use in point-of-care testing. J Virol Methods 2015; 213:131–4. [DOI] [PubMed] [Google Scholar]
- 38. Sanbonmatsu-Gámez S, Pérez-Ruiz M, Lara-Oya A, Pedrosa-Corral I, Riazzo-Damas C, Navarro-Marí JM. Analytical performance of the automated multianalyte point-of-care mariPOC® for the detection of respiratory viruses. Diagn Microbiol Infect Dis 2015; 83:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liolios L, Jenney A, Spelman D, Kotsimbos T, Catton M, Wesselingh S. Comparison of a multiplex reverse transcription-PCR-enzyme hybridization assay with conventional viral culture and immunofluorescence techniques for the detection of seven viral respiratory pathogens. J Clin Microbiol 2001; 39:2779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li PQ, Yang ZF, Chen JX, et al. . Simultaneous detection of different respiratory virus by a multiplex reverse transcription polymerase chain reaction combined with flow-through reverse dot blotting assay. Diagn Microbiol Infect Dis 2008; 62:44–51. [DOI] [PubMed] [Google Scholar]
- 41. Bierbaum S, Forster J, Berner R, et al. . Detection of respiratory viruses using a multiplex real-time PCR assay in Germany, 2009/10. Arch Virol 2014; 159:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svensson MJ, Lind I, Wirgart BZ, Östlund MR, Albert J. Performance of the Simplexa™ flu A/B & RSV direct kit on respiratory samples collected in saline solution. Scand J Infect Dis 2014; 46:825–31. [DOI] [PubMed] [Google Scholar]
- 43. Yan Y, Luo JY, Chen Y, et al. . A multiplex liquid-chip assay based on Luminex xMAP technology for simultaneous detection of six common respiratory viruses. Oncotarget 2017; 8:96913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eigner U, Reucher S, Hefner N, et al. . Clinical evaluation of multiplex RT-PCR assays for the detection of influenza A/B and respiratory syncytial virus using a high throughput system. J Virol Methods 2019; 269:49–54. [DOI] [PubMed] [Google Scholar]
- 45. Sawatwong P, Chittaganpitch M, Paveenkittiporn W, Baggett HC, Olsen SJ, Whistler T. Evaluation of a molecular diagnostic platform for simultaneous detection of multiple respiratory pathogens in Thailand. Am J Trop Med Hyg 2013; 89:124. [Google Scholar]
- 46. Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis 2010; 50:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zuurbier RP, Korsten K, Verheij TJM, et al. . Performance assessment of a rapid molecular respiratory syncytial virus point-of-care test: a prospective community study in older adults. J Infect Dis 2022; 226:S63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Norton Infectious Disease Institute . Norton Infectious Diseases Institute has been selected by Pfizer as the only site in United States to study how to effectively detect respiratory syncytial virus (RSV) in adults.https://nortonhealthcare.com/news/study-looks-at-how-to-detect-rsv-in-adults/. Accessed 4 July 2021.
- 50. Ramirez JA, Carrico R, Wilde AM, et al. Adding sputum and saliva to nasopharyngeal swab samples for PCR detection of respiratory syncytial virus in adults hospitalized with acute respiratory illness may double case detection . ID week 2022. Washington, DC: Infectious Diseases Society of America, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.