Highlights
-
•
We found a novel role for NPRA in gastric cancer, promoting fatty acid oxidation.
-
•
NPRA can be used as a therapeutic target for gastric cancer.
-
•
It was further verified that increased fatty acid metabolism could promote malignant behavior of gastric cancer cells.
-
•
The completion of this paper will greatly enrich the regulatory mechanism in the fatty acid oxidation process of gastric cancer, provide theoretical basis for the design of molecular targeted drugs targeting tumor energy metabolism pathways in the future, and also provide a new strategy for the clinical treatment of gastric cancer.
-
•
In recent decades, the Warburg theory has been revisited and a deeper understanding of the metabolism of tumor cells has been achieved. With the in-depth understanding of the changes of oncogenes and cellular energy metabolism, more potential targets for tumor treatment have been found. Among the many signaling pathways, the fatty acid oxidation pathway is receiving more and more attention.
Keywords: EMT, NPRA, PPARα, CPT1B, FAO
Abstract
Among cancers, gastric cancer (GC) ranks third globally in morbidity and mortality, particularly in East Asia. Natriuretic peptide receptor A (NPRA), a receptor for guanylate cyclase, plays important roles in regulating water and sodium balance. Recent studies have suggested that NPRA is involved in tumorigenesis, but its role in GC development remains unclear. Herein, we showed that the expression level of NPRA was positively correlated with gastric tumor size and clinical stage. Patients with high NPRA expression had a lower five-year survival rate than those with low expression, and NPRA was identified as an independent predictor of GC prognosis. NPRA knockdown suppressed GC cell proliferation, migration and invasion. NPRA overexpression enhanced cell malignant behavior. Immunohistochemistry of collected tumor samples showed that tumors with high NPRA expression had higher peroxisome proliferator-activated receptor α (PPARα) levels. In vivo and in vitro studies showed that NPRA promotes fatty acid oxidation and tumor cell metastasis. Co-IP showed that NPRA binds to PPARα and prevents PPARα degradation. PPARα upregulation under NPRA protection activates arnitine palmitoyl transferase 1B (CPT1B) to promote fatty acid oxidation. In this study, new mechanisms by which NPRA promotes the development of GC and new regulatory mechanisms of PPARα were identified.
Introduction
Among cancers, gastric cancer (GC) ranks third globally in morbidity and mortality rates, particularly in East Asia [1]. Although the average survival rate of patients with GC has improved significantly in recent years through advanced diagnosis and treatment techniques, the prognosis of patients with GC is still very poor when metastasis occurs [2]. Therefore, it is important to study more molecular mechanisms of GC metastasis and find some potential molecular biomarkers that can predict patient prognosis.
Metabolic abnormalities are considered to be an important mechanism of tumorigenesis and tumor progression. Fatty acids can be used as a main energy source to participate in various processes such as tumor proliferation, survival, invasion and angiogenesis [3]. The fatty acid oxidation (FAO) pathway can provide the required energy and biogenic macromolecules for rapidly proliferating tumor cells. Fatty acid oxidation is the primary pathway for the degradation of fatty acids and promotes NADPH and ATP production [4], NADPH can promote the reduction of GSSG to GSH, thereby reducing the level of reactive oxygen species (ROS) in cells. Abnormal lipid metabolism mediated by FAO plays an important role in tumor progression [5], [6], [7], [8].
NPRA acts as a receptor for guanylate cyclase and promotes the action of guanylate cyclase to produce the second messenger cGMP. Studies have shown that cGMP can promote the upregulation of the PPAR family of key proteins in fat metabolism and enhance the functions of PPARγ and PPARα [9]. As lipid-sensitive transcription factors, the PPAR family can provide a "rapid assessment" of the intracellular lipid metabolic environment. Compared with other family members, PPARα promotes fatty acid oxidation, especially in hungry mice [10,11]. Inhibition of PPARα can lead to decreased fatty acid oxidation, fatty acid accumulation and liver cirrhosis in mice and increase the risk of liver cancer [12,13].
The loading of fatty acyl groups onto carnitine is catalyzed by CPT1B, a crucial rate-limiting FAO enzyme [14], [15], [16], [17]. Recent research has demonstrated that CPT1B accelerates the growth of colorectal, prostate, and hepatocellular carcinomas [18,19]. CPT1B is upregulated in prostate cancer and is correlated with poor prognosis [20]. Patients with high-grade glioblastoma and ovarian cancer exhibit elevated CPT1B expression [21], [22], [23]. Known targets of fatty acid metabolism include CPT1B. A CPT1B enzyme inhibitor called etomoxir (ETO) can make human metastatic breast cancer, colon cancer, leukemia, and hepatocellular carcinoma more susceptible to chemotherapy [24], [25], [26], [27], [28], [29]. However, it is not yet known how CPT1B is regulated in GC, and learning about these mechanisms will help identify possible treatment targets.
NPRA is a guanylate cyclase-binding membrane receptor that mediates the role of atrial natriuretic peptide (ANP) in regulating water and sodium balance [30]. Our previous study showed that the expression level of NPRA was positively correlated with the size and clinical stage of GC. Inhibition of NPRA results in impaired proliferation and migration of GC cells in vitro and in vivo [31,32]. After NPRA interference, the mitochondrial function of tumor cells was impaired, and the intracellular ATP content decreased significantly. Although its physiological function is not entirely understood, we wondered whether NPRA might promote fatty acid oxidation to enhance GC formation. Since NPRA is a transmembrane polymer receptor protein that is recycled in cells, it may function physiologically by interacting with and controlling other proteins [33,34]. In the current work, we discovered by a series of studies that NPRA promotes the oxidation of fatty acids. We investigated the role of NPRA in GC and its regulation of the downstream signals PPARα and CPT1B. We found that tumors with high NPRA expression had higher PPARα expression than those with low NPRA expression. High NPRA expression predicted poorer survival in GC patients. In vivo and in vitro studies have shown that NPRA promotes fatty acid oxidation and tumor cell metastasis. Co-IP showed that NPRA binds to PPARα and prevents PPARα degradation. Increased PPARα expression under NPRA protection activates CPT1B to promote fatty acid oxidation. Thus NPRA is a potential target for the diagnosis and treatment of GC.
Materials and methods
The current study has been submitted to and approved by the Ethic Committee for Clinical Research of the First Affiliated Hospital of Wannan Medical College (IRB file No. 2020-10-07). All participants were given written informed consent. The experimental research on xenografted tumor model has been approved by the Ethic Committee for Clinical Research of the First Affiliated Hospital of Wannan Medical College (IRB file No. LLSC-2020-295) and reporting of these experiments complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. These studies were conducted in accordance with the Declaration of Helsinki (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/).
Human tissue samples
The para-cancerous tissues and gastric cancer specimens of 70 patients with gastric cancer were collected from the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Wannan Medical College (Wuhu, Anhui, PR China) from March 2021 to February 2022. All tissues were removed during surgery. The survey and experiments have obtained patients’ consent and been approved by the Ethic Committee for Clinical Research of the First Affiliated Hospital of Wannan Medical College. The study was conducted in accordance with the Declaration of Helsinki (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/).
Cell culture
MKN45 and HGC27, two human GC cell lines, were acquired from the Central Laboratory of Yijishan Hospital, Wannan Medical College. All cells were grown in RPMI 1640 (Gibco, USA) with 10% fetal bovine serum (FBS), and the cells were placed in a 37 °C, 5% CO2 incubator for culture.
Western blot analysis
Cells and tissues were collected with RIPA lysis buffer and detected as described above [35]. The antibodies were as follows: anti-NPRA (Santa Cruz sc-137041), anti-PPARα (Santa Cruz sc-398934), anti-CPT1B (Abcam ab134988), anti-CD44 (Abcam A19020), and anti-CD133 (Abcam A0818).
Immunoprecipitation (IP)
Total proteins from GC cells were extracted, and IP employing the designated primary antibody and protein A/G-agarose beads was carried out (Santa Cruz Biotechnology, USA).
RNA extraction and qRT‒PCR
Total RNA extraction and quantitative real-time PCR (qRT‒PCR) were performed as described previously [35]. The following primers were used in this study: NPRA forward, 5′- GGG ATA CAG TCA ACA CAG CCT CAA - 3′, NPRA reverse, 5′- CGAAGCTCCAGCTCGAAACC −3′; GAPDH forward, 5′- GAACGGGAAGCTCACTGG −3′, GAPDH reverse, 5′- GCCTGCTTCACCACCTTCT −3′. GAPDH was used as an endogenous control for mRNA expression.
Flow cytometry
Flow cytometry was utilized in conjunction with propidium iodide labeling to investigate the rate of apoptosis of GC cells. FlowJo was used to perform analysis of the data (FlowJo).
Lentivirus transfection and establishment of a stably transfected cell line and siRNA
The NPRA overexpression vector was constructed by JIMA (Shanghai, China) by inserting the NPRA sequence into the pcDNA3.1-3xFlag expression vector. SiRNA targeting PPARα was synthesized by JIMA (Shanghai, China). ShRNA sequences including shNPRA-1 and shNPRA-2 and a nonsense sequence (shCTL) packaged in lentivirus vectors were designed and synthesized by JIMA (Shanghai, China). Transfection was performed using Lipofectamine 3000 (Invitrogen, USA) following the manufacturer's instructions. All sequences are listed in Supplementary Table 2.
Cell counting kit-8 (CCK-8) assay
Cells were inoculated at 1 × 103 cells per well in a 96-well plate. Three wells for each condition and blank control wells without cells were set up. The plates were incubated at 37 °C and 5% CO2 for 0, 24, 48 and 72 h CCK-8 solution (100 μL) was added to each well, and the cell were cultured for 4 h. Finally, the absorption value at 450 nm was detected by a microplate reader, and the cell absorption value was equal to the absorption value of the sample well - the absorption value of the blank control well.
Invasion assay
Complete culture medium (containing 10% FBS) was first added to the lower part of the Transwell chamber, and 100 µL of cells was gently added to the upper chamber. The cells were incubated in a 5% CO2 incubator at 37 °C for 12 h, fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet for 15 min, and the cells on the membrane were gently removed with cotton swabs. Finally, the cells were observed under an inverted microscope.
Wound healing assay
The cancer cells were seeded into 6-well plates and cultured until the monolayer reached >90% confluency. On the second day, scratches were made on the cell layer perpendicular to the cell plane with a pipette tip. After the scratches were made, sterile PBS was used to wash the cells 3 times, and the nonadherent cells were washed away so that the scratches were clearly visible. Then, fresh serum-free medium was added, and the cells were placed in a 37 °C, 5% CO2 incubator for culture. At 0 h and 36 h, the width of the scratches was observed under a microscope and measured, and photographs were taken.
Xenograft nude mouse model
Female nude mice aged 4 weeks were purchased from Nanjing Institute of Biomedicine, Nanjing University (Nanjing, China). Gastric cancer cells were injected under the armpit at a concentration of 1 million cells/50 µl vehicle. Tumor formation was observed daily, and the tumor diameter and weight of the mice were measured every two days after tumor formation (approximately the third day).
Construction of GC organoids
Fresh human tissue was taken and crushed into 2–3 mm small tissue blocks using sterile instruments in cold PBS. Collagenase digestion was performed at 37 °C for 45 min; the sample was centrifuged, the supernatant was removed, and the pellet was washed with cold PBS. The gastric stem cells were placed into a centrifuge tube filtered through a 70-µm screen and resuspended in cold matrix glue premixed with various growth factors. A 50 µm cell-gel suspension was added into each well of a 24-well plate, and the gel was allowed to solidify in a 37 °C incubator for 20 min. Special medium for organoids was added, the medium was changed every 4 days, and photos were taken for observation every day.
ATP level detection
According to the guidelines provided by the manufacturer, the ATP Determination Kit manufactured by Beyotime (Shanghai, China) was utilized for analysis.
FAO rate detection
The Cell Mitochondrial Isolation Kit was used to separate the mitochondria of GC cells (Beyotime Biotechnology, Shanghai, China). The Fatty Acid-Oxidation Rate Colorimetric Assay Kit's instructions were followed to measure the oxidation rate (Genmed Scientifics Inc., USA).
Statistical analysis
Data from experiments are displayed as the mean ± standard deviation (SD). Means were computed based on at least three experiments that were conducted separately. Data analysis tools included SPSS software version 20.0 (IBM, USA) and GraphPad Prism 7.0 (GraphPad Software, USA). Analysis of variance (ANOVA), the χ2 test, and the two-tailed Student's t-test were used to determine group differences. Differences were considered significant when P values <0.05.
Results
The expression of NPRA was significantly increased in GC tissues vs normal tissues
Tissue samples were obtained from sixty-five cases of gastric cancer tissues were collected from the Department of Gastrointestinal Surgery, Yijishan Hospital of Wannan Medical College. NPRA in tissue samples was detected by immunohistochemistry with a monoclonal antibody against NPRA. The IRS method was used to analyze the immunohistochemical results. We assessed the association of NPRA expression levels with clinicopathological parameters in GC patients. The Pearson χ2 test showed that high NPRA expression was associated with tumor stage (TNM stage III + IV; P<0.001), large tumors (≥3.5 cm; P = 0.003) and PPARα expression (P = 0.044) (Supplementary Table 1). The expression level of NPRA was independent of age, histological differentiation and lymph node metastasis. These results suggest that NPRA overexpression may be involved in the aggressive phenotype of GC (Supplementary Table1).
NPRA protein expression was detected in sixty-five samples of human GC tissues and corresponding adjacent noncancerous gastric tissues by IHC. The results showed that the expression level of NPRA in cancer tissues was significantly higher than that in adjacent tissues. High NPRA expression was associated with increased PPARα expression (Fig. 1A). Nineteen fresh gastric cancer specimens were collected from gastric cancer patients who underwent surgery at Yijishan Hospital of Wannan Medical College (from 2021 to 2022). Qrt-PCR detected that the expression of NPRA mRNA in gastric cancer tissues was significantly higher than that in noncancerous tissues (P < 0.05) (Fig. 1E). We analyzed 19 gastric cancer tissues by qRT-PCR, and found that the expression level of PPARα in cancer tissues was significantly higher than that in adjacent cancer tissues, and was positively correlated with the expression level of NPRA in cancer tissues (Fig. S5A and B). We found that 5-year survival was poorer in patients with high expression of NPRA than in those with low expression. We conducted a survival analysis of the TCGA cohort with the help of GEPIA (http://gepia.cancer-pku.cn/). The results suggested that GC patients with high NPRA expression had worse overall survival (OS) (Fig. 1B and C). We performed organoid cultures of GC tissue from the patient, and NPRA expression was higher in patient#1 than in patient#2. The organoids of patient#1 were significantly larger than those of patient#2 (Fig. 1D). These data suggest that NPRA expression is increased in gastric cancer.
Fig. 1.
The expression of NPRA was significantly increased in GC tissues compared to control tissues and positively correlated with the expression of PPARα.
(A) IHC was used to detect the expression of NPRA and PPARα in GC tissues. (B) ROC time curves of NPRA. (C) Kaplan‒Meier overall survival (OS) curves were generated based on NPRA expression levels. (D) Organoids from two patients. (E) qRT‒PCR was used to analyze NPRA mRNA expression in GC tissues and corresponding adjacent noncancer tissues.
NPRA is required for GC cell proliferation, migration and FAO
To investigate whether NPRA was required for the invasive phenotypes of GC cells in vitro, we used two shRNAs, shNPRA-1 and shNPRA-2, to knock down NPRA expression in MKN45 and HGC27 cell lines. The Western blotting and qRT‒PCR results showed that compared with that in the control shRNA-transfected cells, NPRA protein expression and mRNA levels were dramatically decreased in NPRA-shRNA-1 and NPRA-shRNA-2 transfected cells (Figs. 2A, S1A). Colony formation experiments showed that silencing NPRA significantly reduced the proliferation of GC cells (Fig. 2B-C). Using the CCK-8 assay, we compared the proliferation curves between control shRNA cells and NPRA knockdown MKN45 and HGC27 cells. We observed that in both MKN45 and HGC27 cell lines, knocking down NPRA decreased cell proliferation (Fig. 2D). EdU experiments showed that downregulating NPRA significantly reduced EdU positivity in GC cells (Fig. 2E). TCGA detaset analysis of NPRA with Epithelial-Mesenchymal Transition (EMT) markers showed positive correlations between NPRA and EMT markers (Fig. 2G). Furthermore, we explored whether NPRA regulated the migration of GC cells. We scratched a line across the tumor cells and then observed the percentage of the wound area that had healed 36 h later. We found that knocking down NPRA expression significantly inhibited wound closure in MKN45 cells (Figs. 2H, I, S1B). To further validate this phenotype, we evaluated the effects of NPRA on GC cell migration by a Transwell migration assay. We plated tumor cells in the upper chamber without Matrigel and observed that the migration ability of tumor cells transfected with shNPRA was decreased compared with that of cells transfected with shCTL (Fig. 2J, K). GC cells were detected by flow cytometry, and the apoptosis rate of GC cells was significantly reduced in the NPRA interference group (Fig. 2L). Interference with NPRA expression significantly reduced the cellular ATP content in MKN45 and HGC27 cells (Fig. 2N). NPRA knockdown significantly suppressed FAO (Fig. 2O). Western blotting was conducted to determine the levels of the EMT related proteins E-Cadherin, N-Cadherin, Snail and Vimentin. β-Actin was used as a loading control. Furthermore, NPRA knockdown was companied by elevated expression of E-Cadherin and diminished expression of mesenchymal markers such as N-Cadherin, Snail and Vimentin in GC cells (Fig. 2M). These results indicated that NPRA is required for GC cell proliferation, migration and FAO.
Fig. 2.
Silencing NPRA can suppress the proliferation of GC cell lines and inhibit their migration and FAO.(A) MKN45 and HGC27 cell lines were transfected with shNPRA-1 and shNPRA-2 and a blank control vector (shCTL), respectively. the expression of NPRA was analyzed by qRT‒PCR. (B) Colony formation experiments showed that silencing NPRA significantly reduced the proliferation of GC cells. (C) The statistical results of B. (D) A CCK-8 assay was applied to detect the viability of GC cells. (E) EdU was used to detect cell proliferation. (F) The statistical results of E. (G) TCGA detaset analysis shows the positive correlations between NPRA and EMT markers. (H) The wound healing assay indicated that NPRA downregulation inhibited MKN45 cell migration. (I) The statistical results of H. (J) The statistical results of K. (K) Transwell migration assays showed that knockdown of NPRA reduced the cell migration ability; scale bar: 200 μm. (L) Silencing NPRA significantly increased the apoptosis rate of GC cells in the cytometry experiment. (M) Western blot analyses of EMT marker proteins in NPRA knockdown GC cells. (N) ATP content detection. (O) NPRA knockdown significantly suppressed FAO.
Overexpression of NPRA enhanced GC cell proliferation, migration and FAO
To further validate NPRA functions, we repeated the experiments mentioned above by transfecting the NPRA plasmid to overexpress NPRA in MKN45 and HGC27 cells. Western blotting and qRT‒PCR results confirmed that NPRA expression was significantly upregulated in the NPRA overexpression plasmid transfection groups compared with the vector plasmid transfection groups in MKN45 and HGC27 cells (Fig. S2A, S2B). Colony formation experiments showed that NPRA significantly enhanced the proliferation of GC cells (Fig. 3A). Then, we investigated whether NPRA upregulation affected tumor cell proliferation. Using CCK-8 assays, we generated proliferation curves of tumor cells transfected with the vector plasmid and those transfected with the NPRA overexpression plasmid. The comparison results showed that both MKN45 and HGC27 cells overexpressing NPRA grew faster than control cells (Fig. 3B). We again observed that upregulation of NPRA dramatically increased the EdU positivity rate in MKN45 and HGC27 cell lines (Fig. 3C). Then, we assessed whether overexpressing NPRA could enhance tumor cell migration. Using a wound scratch assay, we observed that upregulation of NPRA dramatically promoted wound closure in both MKN45 and HGC27 cells compared with control cells (Figs. 3D, S2C). Moreover, using a transwell migration assay, we further confirmed that tumor cells overexpressing NPRA displayed an improved migration ability compared with control cells (Fig. 3E). Flow cytometry experiments showed that the apoptosis rate of GC cells was significantly reduced in the NPRA overexpression group (Fig. 3F). NPRA overexpression significantly increased the FAO rate and the ATP content (Fig. 3H-3I). Western blotting was used to determine the levels of E-Cadherin, N-Cadherin, Snail and Vimentin (Fig. 3G). In brief, these results suggested that NPRA overexpression enhances GC cell proliferation, migration and FAO in vitro.
Fig. 3.
Overexpression of NPRA enhances the proliferation, migration and FAO of GC cells. (A) Colony formation experiments showed that NPRA significantly enhanced the proliferation of GC cells. (B) CCK-8 was used to detect the viability of GC cells. (C) EdU was used to detect cell proliferation. (D) The wound healing assay indicated that NPRA upregulation enhanced MKN45 cell migration. (E) Transwell migration assays showed that increased NPRA expression enhanced GC cell migration ability. (F) Flow cytometry was used to detect cell apoptosis. (G) Western blotting was used to determine the levels of E-Cadherin, N-Cadherin, Snail and Vimentin. (H,I) NPRA overexpression significantly increased FAO and ATP values.
PPARα is required for GC cell proliferation, migration and FAO
Next, we investigated the mechanism by which NPRA regulates GC cell migration and FAO. Studies have shown that cGMP can promote the function of PPARγ and PPARα, which are key proteins in lipid metabolism. PPAR family menbers, as lipid-sensitive transcription factors, are responsible for the "rapid response" of the intracellular lipid metabolism environment evaluate, which can regulate key fatty acid oxidation enzymes. It is well known that NPRA acts as a guanylate cyclase receptor to facilitate the action of guanylate cyclase to produce the second messenger cGMP. Therefore we became interested in whether NPRA could directly affect PPARα. NPRA is a high-molecular-weight transmembrane protein, that is rapidly internalized, sequestrated, and redistributed into intracellular locations after binging the ligand. NPRA is thought to be a cellular protein that travels around different subcellular locations. This physiological characteristic indicates that NPRA may play a physiological role as a chaperone protein of other molecules in the cytoplasm. By reciprocal coimmunoprecipitation (Co-IP) experiments in the GC cell line MKN45, we found that NPRA interacts with PPARα and vice versa (Fig. 4A). We also confirmed their interaction by Co-IP in another GC cell line HGC27 (Fig. 4B). The western blotting and qRT‒PCR results confirmed that si-PPARα significantly reduced the expression of PPARαin both MKN45 and HGC27 cells (Fig. S3A, S3B). EdU assays and CCK-8 assays showed that PPARα increased the proliferation ability of GC cells (Fig. 4C–E). In addition, knockdown of PPARα significantly decreased the number of cell colonies and promoted apoptosis (Fig. 4F, G). Further transwell assays showed that PPARα promoted the migration of GC cells (Fig. 4H, I). Wound healing assays showed that PPARα promoted the migration of GC cells (Fig. 4J–L). ATP and FAO levels in GC cells were significantly decreased upon PPARα silencing (Fig. 4M, N).
Fig. 4.
PPARα is required for GC cell proliferation, migration and FAO. (A, B) Co-IP experiments in the GC cell lines MKN45 and HGC27showed that NPRA interacted with PPARα and vice versa. (C) CCK-8 assays showed that si-PPARα reduced the proliferation ability of GC cells. (D) EdU experiments showed that si-PPARα can reduce cell proliferation. (E) The statistical results of D. (F) Flow cytometry was used to detect cell apoptosis. (G) The statistical results of F. (H) Transwell assays showed that PPARα promoted the migration of GC cells. (I) The statistical results of H. (J, K) Wound healing assays showed that PPARα promoted the migration of GC cells. (L) The statistical results of J and K. (M, N) ATP and FAO levels in GC cells were significantly decreased by silencing PPARα.
NPRA promotes proliferation, migration and FAO through PPARα
Si-PPARα was transfected into NPRA-overexpressing cells, and proliferation (detected by CCK-8 assay), colony formation and EdU staining rates were decreased compared with those in control si-NC cells (Figs. 5A-D, S3C). The transfection of si-PPARα into NPRA-overexpressing cells significantly increased the apoptosis rate of GC cells according to the cytometry experiment (Fig. 5E and F). In addition, the application of siRNA to downregulate PPARα in NPRA-overexpressing cells significantly inhibited the migration (wound closure) and invasion of GC cells compared with that in the group with control siRNA treatment of NPRA-overexpressing cells (Fig. 5G-J). The transfection of si-PPARα into NPRA-overexpressing cells reduced the ATP content and FAO rate of GC cells (Fig. 5K and L).
Fig. 5.
NPRA promotes proliferation, migration and FAO through PPARα. (A, B, C) EdU experiments, CCK-8 assays and colony formation assays were used to detect cell proliferation. (D) The statistical results of B. (E) The transfection of si-PPARα into NPRA-overexpressing cells significantly increased the apoptosis rate of GC cells according to the cytometry experiment. (F) The statistical results of E. (G) The application of siRNA to downregulate PPARα in NPRA-overexpressing cells significantly inhibited the invasion of GC cells compared with that of NPRA-overexpressing control siRNA cells. (H) The statistical results of G. (I) The application of siRNA to downregulate PPARα in NPRA-overexpressing cells significantly decreased wound closure rate of GC cells compared with that of NPRA-overexpressing control siRNA cells. (J) The statistical results of I. (K, L) The transfection of si-PPARα into NPRA-overexpressing cells reduced the ATP content and FAO of GC cells.
NPRA regulates PPARα expression
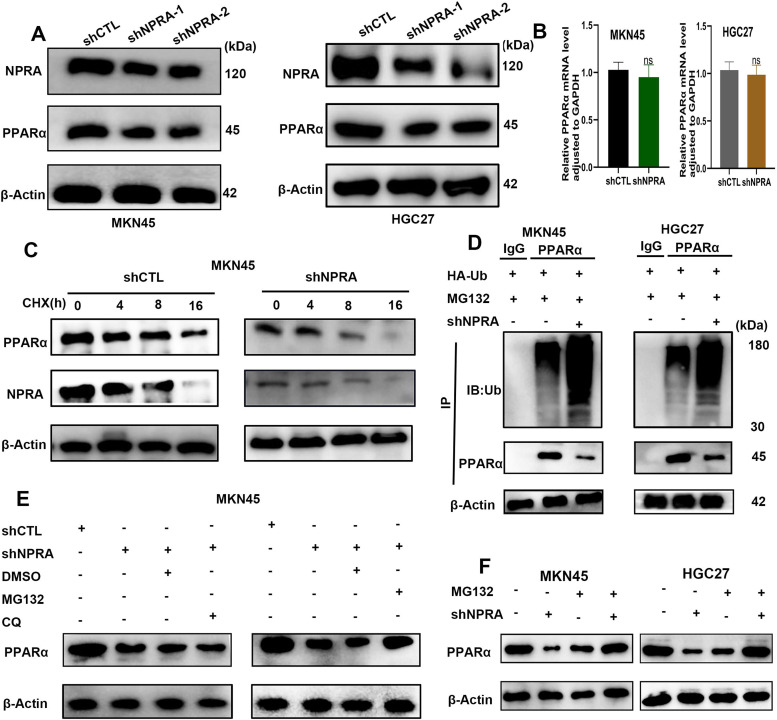
Western blot analysis showed that in both MKN45 and HGC27 cells, NPRA knockdown decreased PPARα expression compared with that in the control shRNA cells (Fig. 6A). Intriguingly, the level of PPARα mRNA was not significantly altered in NPRA-silenced cells compared with controls (Fig. 6B). Considering that NPRA alters PPARα protein levels but not mRNA levels, we speculated that NPRA may stabilize PPARα protein via the ubiquitination/degradation system. We then wondered whether NPRA affects the stability of PPARα. The protein stability of PPARα was assessed using cycloheximide (CHX, an inhibitor of protein synthesis), the experiment revealed that PPARα expression was decreased when NPRA was knocked down (Fig. 6C). The levels of ubiquitinated PPARα protein were increased in GC cells when NPRA was knocked in the presence of ubiquitin (Fig. 6D). The effects of MG132 and chloroquine (CQ) on NPRA knock down induced PPARα downregulation were determined by Western blotting, the proteasome inhibitor MG132 reversed the downregulation of PPARαprotein induced by NPRA knockdown, while the autophagy inhibitor chloroquine did not reverse the downregulation of PPARα protein induced by NPRA knockdown (Fig. 6E). As shown in Fig. 6F, the reduction in PPARα protein by shNPRA was restored by MG132.
Fig. 6.
NPRA regulates PPARα expression. (A) Western blot analysis showed that NPRA knockdown decreased PPARα expression. (B) The level of PPARα mRNA was not significantly altered in NPRA-silenced cells. (C) Western blot analysis of PPARα protein stability using CHX (an inhibitor of protein synthesis) when NPRA was knocked down. (D) The levels of ubiquitinated PPARα protein were increased in GC cells when NPRA was knocked in the presence of ubiquitin. (E) The proteasome inhibitor MG132 reversed the downregulation of PPARα protein induced by NPRA knockdown. (F) The reduction in PPARα protein by shNPRA was restored by MG132.
NPRA promoted CPT1B expression and tumorigenesis in mice
Western blot analysis showed that in both MKN45 and HGC27 cells, NPRA knockdown decreased PPARα and CPT1B expression compared with that in the control shRNA cells (Fig. 7A). Similarly, Western blotting showed that in both MKN45 and HGC27 cells, PPARα knockdown decreased PPARα and CPT1B expression (Fig. 7B). To validate whether NPRA regulates CPT1B expression by activating PPARα. SiRNA was used to downregulate PPARα in NPRA-overexpressing MKN45 and HGC27 cells, and we found that PPARα and CPT1B expression was obviously decreased compared with that in siRNA control cells (Fig. 7C). To further validate our observations in vivo, we established a MKN45 subcutaneous tumor model in mice. We assessed whether NPRA depletion could inhibit tumor growth. We injected cells transfected with control shRNA and NPRA shRNA into mice subcutaneously and monitored tumor growth. MKN45 control shRNA cells formed larger tumors than MKN45 NPRA shRNA cells within 35 days. Tumor growth curves over 35 days showed that NPRA downregulation slowed MKN45 tumor growth (Fig. 7D). Consistently, the weight of tumors derived from MKN45 NPRA knockdown cells was significantly less than that of tumors derived from MKN45 shRNA control cells on the 35th day after tumor cell injection (Fig. 7E and F). A schematic diagram of the role of NPRA in GC is shown in (Fig. 7G).
Fig. 7.
NPRA promoted CPT1B expression and tumorigenesis in mice. (A) NPRA knockdown decreased PPARα and CPT1B expression. (B) PPARα knockdown decreased CPT1B expression. (C) By applying si-PPARα to NPRA-overexpressing cells, we found that PPARα and CPT1B expression obviously decreased. (D, E, F) NPRA knockdown MKN45 cells showed decreased tumor formation ability compared with control cells, as reflected by tumor size (D&E) and weight (F). (G) Schematic diagram of the role of NPRA in GC. In conclusion, NPRA activates CPT1B by binding PPARα, which promotes the interaction between NPRA and PPARα proteins in GC, stabilizes the PPARα protein, and promotes the downstream pathway and FAO.
Discussion
Gastric cancer is a serious threat to human health, especially in China. The early diagnosis rate of GC is still low, and the overall survival rate of GC patients is unsatisfactory [1]. To improve the diagnosis and treatment effect of GC, it is urgent to study the mechanism of the occurrence and development of GC and find diagnostic markers and intervention measures for GC. In previous studies, after NPRA interference, the mitochondrial function of tumor cells was impaired, and the intracellular ATP content decreased significantly, suggesting that NPRA may be involved in the metabolism and energy production process of GC cells [36]. The traditional idea is that tumor cells can only produce energy through glycolysis, which is not particularly related to mitochondrial respiration. Therefore, most studies on cancer are limited to a few signaling pathways. In recent decades, the Warburg theory has been revisited and a deeper understanding of the metabolism of tumor cells has been achieved. With an in-depth understanding of the changes in oncogenes and cellular energy metabolism, more potential targets for tumor treatment have been found. Among the many signaling pathways, the fatty acid oxidation pathway is receiving increasing attention. In our study, high NPRA expression was associated with advanced TNM stage and large tumor size. In addition, our data showed that high NPRA expression predicts poor 5-year survival in gastric cancer patients. NPRA is an independent predictor of OS in gastric cancer patients. Our data demonstrate the importance of NPRA in GC. FAs are important energy sources produced by FA oxidation (FAO) and have been shown to be essential for the growth and survival of cancer cells. It has recently been reported that FAO can support the self-renewal and drug resistance of breast cancer stem cells. Inhibition of FAO inhibits tumor stemness and reduces tumor growth [7,8].
It has been reported that some tumor cells have stem cell characteristics, and the enhancement of tumor stem cell features is an important factor in rapid tumor cell proliferation, drug resistance, recurrence and metastasis and other malignant biological behaviors [37,38]. Enhancement of stem cell properties in lung, ovarian and GCs has been reported to promote tumor progression [39], [40], [41]. Many factors promote the maintenance or acquisition of tumor stem cell characteristics, and among these factors, local tumor environmental factors are particularly important. To preserve their stemness, cancer cells are more prone to settle in an environment rich in energy-related substances with low acidity and low oxidative stress than in an environment with other conditions, cell metabolism, as a regulator of the above environmental conditions, has gradually been assessed for its role in regulating tumor stem cells [42,43]. FAO can maintain tumor cell stemness and promote tumor progression by regulating the tumor environment. In recent years, many studies have shown that fatty acid metabolism can promote the characteristics of tumor stem cells [44]. It has been reported that inhibition of FAO in breast cancer cells can increase the sensitivity of chemotherapy [45]. It has been reported that FAO can return the ROS content in tumor cells to normal levels and maintain the stem cell characteristics of tumor cells [46]. Moreover, it has been reported that fatty acid oxidation can maintain the characteristics of stem cells by promoting autophagy, eliminating excessive deposition of "long-lived proteins" and aging damaged organelles in cells [47]. Therefore, FAO plays an important role in maintaining the stemness of tumor cells. Epidemiological investigation has shown that with the increase in the lipid food proportion in Chinese people's diet, the incidence of GC also increases [48]. It was also found that fatty acid oxidative metabolites in the tumor area of GC patients increased [49].
Studies conducted in the past have demonstrated that NPRA can stimulate the growth of GC cells by lowering the levels of ROS in GC cells [50]. After it has bound ligands, the high-molecular-weight transmembrane protein known as NPRA quickly internalizes, isomerizes, and redistributes itself to different locations inside the cell [51]. Because of this, NPRA is regarded as a cellular protein because it can be upregulated in a variety of subcellular locations. Because of this quality, it may exert a physiological function in the cytoplasm by acting as a chaperone protein for other molecules. Physiological roles can be determined according to the physiological properties of an organism. The results of this investigation demonstrated that NPRA is capable of binding to and stabilizing PPARα, providing evidence that NPRA can boost FAO. According to the findings of our study, reducing the level of NPRA expression reduces PPARα protein expression, and the results of our Co-IP assays verified the combination of PPARα and NPRA. Since NPRA is a membrane protein, it has the potential to serve as a therapeutic target for drugs.
The detailed mechanism by which NPRA promotes GC development needs to be further studied. Although we found that NPRA mediated activation of PPARα may promote gastric cancer progression, the binding sites of NPRA to PPARα needs to be further investigated. The results of our study may provide valuable information for GC prognosis prediction and the development of future therapies. This study can be used as a basis for studies on FAO inhibition via NPRA in GC.
Conclusion
NPRA is upregulated in gastric cancer and is correlated with poor prognosis, which binds to PPARα, and promotes PPARα expression. NPRA and PPARα synergistically promote the expression of CPT1B, and promote FAO. NPRA provides energy and metabolic substances for tumor cells. NPRA is involved in the adaptation of GC stem cells to the metabolic and environmental requirements, the maintains the stem cell characteristics of GC cells, and promotes the malignant biological behavior of GC cells.
Disclosure statement
No potential conflict of interest was reported by the author(s).
CRediT authorship contribution statement
Tingting Cao: Conceptualization, Writing – original draft. Song Wang: Conceptualization, Supervision, Writing – review & editing. Long Qian: Conceptualization, Supervision, Writing – review & editing. Chengwei Wu: Writing – review & editing. Tao Huang: Writing – review & editing. Ye Wang: Writing – review & editing. Qian Li: Writing – review & editing. Jiawei Wang: Writing – review & editing. Yabin Xia: Conceptualization, Supervision, Writing – review & editing. Li Xu: Writing – review & editing. Luman Wang: Conceptualization, Writing – original draft. Xiaoxu Huang: Conceptualization, Writing – original draft.
Declaration of Competing Interest
The authors have no conflict of interest.
Acknowledgments
The authors thank the patients, their families, and the investigators and staff involved in the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101734.
Appendix. Supplementary materials
Fig. S1. (A) The western blotting results showed that compared with control shRNA-transfected cells, NPRA protein expression was dramatically decreased in both NPRA-shRNA-1 and NPRA-shRNA-2 transfected cells. (B) The wound healing assay indicated that NPRA downregulation inhibited HGC27 cell migration.
Fig. S2. (A, B) The western blotting and qRT‒PCR results confirmed that NPRA was significantly upregulated in cell with NPRA overexpression plasmid transfection compared with cells with vector plasmid transfection in both MKN45 and HGC27 cells. (C) The wound healing assay indicated that NPRA upregulation enhanced HGC27 cell migration.
Fig. S3. (A, B) The western blotting and qRT‒PCR results confirmed that PPARα expression was significantly decreased in cells transfected with si-PPARαcompared with cells transfected with NC in both MKN45 and HGC27 cells. (C) EdU experiments were used to detect cell proliferation in HGC27 cells.
Fig. S4. (A, B) In gastric cancer cell lines MKN45 and HGC27, NPRA increased the expression of protein CD44 and protein CD133, indicated that NPRA can promote the stemness of gastric cancer cells.
Fig. S5. (A) qRT-pcr showed that the expression of PPARα in cancer tissues was significantly higher than that in adjacent tissues. (B) In gastric cancer tissue samples, NPRA expression was positively correlated with PPARα.
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Li M., et al. Fatty acid oxidation: driver of lymph node metastasis. Cancer Cell Int. 2021;21(1):339. doi: 10.1186/s12935-021-02057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.K., et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363(6427):644–649. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld M.G., Lunyak V.V., Glass C.K. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20(11):1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 7.Houten S.M., Wanders R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 2010;33(5):469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X., et al. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer. 2017;16(1):76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashmore T., et al. Nitrate enhances skeletal muscle fatty acid oxidation via a nitric oxide-cGMP-PPAR-mediated mechanism. BMC Biol. 2015;13:110. doi: 10.1186/s12915-015-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., et al. Activation of PPARalpha by oral clofibrate increases renal fatty acid oxidation in developing pigs. Int. J. Mol. Sci. 2017;18(12) doi: 10.3390/ijms18122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakhia R., et al. PPARalpha agonist fenofibrate enhances fatty acid beta-oxidation and attenuates polycystic kidney and liver disease in mice. Am. J. Physiol. Renal. Physiol. 2018;314(1):F122–F131. doi: 10.1152/ajprenal.00352.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin M.P., Sathyanarayan A., Mashek D.G. Acyl-CoA Thioesterase 1 (ACOT1) regulates PPARalpha to couple fatty acid flux with oxidative capacity during fasting. Diabetes. 2017;66(8):2112–2123. doi: 10.2337/db16-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Q., et al. PPARalpha-Deficient ob/ob obese mice become more obese and manifest severe hepatic steatosis due to decreased fatty acid oxidation. Am. J. Pathol. 2015;185(5):1396–1408. doi: 10.1016/j.ajpath.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louet J.F., et al. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB) J. Biol. Chem. 2002;277(41):37991–38000. doi: 10.1074/jbc.M205087200. [DOI] [PubMed] [Google Scholar]
- 15.Vega R.B., Huss J.M., Kelly D.P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 2000;20(5):1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S., et al. Peroxisomal proliferator activated receptor gamma coactivator (PGC-1alpha) stimulates carnitine palmitoyltransferase I (CPT-Ialpha) through the first intron. Biochim. Biophys. Acta. 2004;1679(2):164–173. doi: 10.1016/j.bbaexp.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., et al. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha) J. Biol. Chem. 2004;279(52):53963–53971. doi: 10.1074/jbc.M406028200. [DOI] [PubMed] [Google Scholar]
- 18.Bonnefont J.P., et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol. Asp. Med. 2004;25(5–6):495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Khomenko, V.L., et al., Use of visken in hypertension associated with ischemic heart disease and nutritional obesity. Sov. Med., 1990(3): p. 64–7. [PubMed]
- 20.Abudurexiti M., et al. Targeting CPT1B as a potential therapeutic strategy in castration-resistant and enzalutamide-resistant prostate cancer. Prostate. 2020;80(12):950–961. doi: 10.1002/pros.24027. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.N., et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37(46):6025–6040. doi: 10.1038/s41388-018-0384-z. [DOI] [PubMed] [Google Scholar]
- 22.Schlaepfer I.R., et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol. Cancer Ther. 2014;13(10):2361–2371. doi: 10.1158/1535-7163.MCT-14-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu A., et al. Diet-induced hepatic steatosis activates Ras to promote hepatocarcinogenesis via CPT1alpha. Cancer Lett. 2019;442:40–52. doi: 10.1016/j.canlet.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Winocour P.H., et al. The prevalence of hyperlipidaemia and related clinical features in insulin-dependent diabetes mellitus. Q. J. Med. 1989;70(263):265–276. [PubMed] [Google Scholar]
- 25.Camarda R., et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016;22(4):427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J.H., et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14(9):2154–2165. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samudio I., et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 2010;120(1):142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C.L., et al. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23(1):206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernlund E., et al. Potentiation of chemotherapeutic drugs by energy metabolism inhibitors 2-deoxyglucose and etomoxir. Int. J. Cancer. 2008;123(2):476–483. doi: 10.1002/ijc.23525. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins M.R., Redondo J., Brown L.A. The natriuretic-peptide family. Lancet. 1997;349(9061):1307–1310. doi: 10.1016/S0140-6736(96)07424-7. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Q., et al. ANP promotes proliferation and inhibits apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex and improves ovary functions of PCOS rats. Cell Death Dis. 2017;8(10):e3145. doi: 10.1038/cddis.2017.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakao Y., et al. Natriuretic peptide receptor A is related to the expression of vascular endothelial growth factors A and C, and is associated with the invasion potential of tongue squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2017;46(10):1237–1242. doi: 10.1016/j.ijom.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Misono K.S., et al. Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J. 2011;278(11):1818–1829. doi: 10.1111/j.1742-4658.2011.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey K.N. Endocytosis and trafficking of natriuretic peptide receptor-a: potential role of short sequence motifs. Membranes. 2015;5(3):253–287. doi: 10.3390/membranes5030253. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X., et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol. Cancer. 2019;18(1):71. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., et al. Natriuretic peptide receptor A inhibition suppresses gastric cancer development through reactive oxygen species-mediated G2/M cell cycle arrest and cell death. Free Radic. Biol. Med. 2016;99:593–607. doi: 10.1016/j.freeradbiomed.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y., et al. Nuclear EGFR-PKM2 axis induces cancer stem cell-like characteristics in irradiation-resistant cells. Cancer Lett. 2018;422:81–93. doi: 10.1016/j.canlet.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Liu L., et al. Chemotherapy induces breast cancer stemness in association with dysregulated monocytosis. Clin. Cancer Res. 2018;24(10):2370–2382. doi: 10.1158/1078-0432.CCR-17-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu T., et al. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett. 2018;423:36–46. doi: 10.1016/j.canlet.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Liu W., et al. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/beta-catenin signaling pathway. Cell Death. Dis. 2018;9(3):343. doi: 10.1038/s41419-018-0363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y., et al. Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-beta/catenin signaling pathway. Stem Cell Res. Ther. 2017;8(1):98. doi: 10.1186/s13287-017-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munro M.J., et al. Cancer stem cells in colorectal cancer: a review. J. Clin. Pathol. 2018;71(2):110–116. doi: 10.1136/jclinpath-2017-204739. [DOI] [PubMed] [Google Scholar]
- 43.Cacheux W., et al. Mutational analysis of anal cancers demonstrates frequent PIK3CA mutations associated with poor outcome after salvage abdominoperineal resection. Br. J. Cancer. 2016;114(12):1387–1394. doi: 10.1038/bjc.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beyaz S., et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531(7592):53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T., et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27(1):136–150. doi: 10.1016/j.cmet.2017.11.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju H.Q., et al. Redox regulation of stem-like cells though the CD44v-xCT axis in colorectal cancer: mechanisms and therapeutic implications. Theranostics. 2016;6(8):1160–1175. doi: 10.7150/thno.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito K., et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science. 2016;354(6316):1156–1160. doi: 10.1126/science.aaf5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan S., et al. Clinical epidemiology of gastric cancer in Hehuang valley of China: a 10-year epidemiological study of gastric cancer. World J. Gastroenterol. 2014;20(30):10486–10494. doi: 10.3748/wjg.v20.i30.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song H., et al. Tissue metabolomic fingerprinting reveals metabolic disorders associated with human gastric cancer morbidity. Oncol. Rep. 2011;26(2):431–438. doi: 10.3892/or.2011.1302. [DOI] [PubMed] [Google Scholar]
- 50.Jin Y.X., et al. Performance of nitrogen and phosphorus removal from municipal wastewater of different C/N ratios using intelligent controlled systems sequencing batch biofilm reactor (SBBR) Huan Jing Ke Xue. 2011;32(3):729–735. [PubMed] [Google Scholar]
- 51.Pandey K.N., et al. Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J. Biol. Chem. 2002;277(7):4618–4627. doi: 10.1074/jbc.M106436200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) The western blotting results showed that compared with control shRNA-transfected cells, NPRA protein expression was dramatically decreased in both NPRA-shRNA-1 and NPRA-shRNA-2 transfected cells. (B) The wound healing assay indicated that NPRA downregulation inhibited HGC27 cell migration.
Fig. S2. (A, B) The western blotting and qRT‒PCR results confirmed that NPRA was significantly upregulated in cell with NPRA overexpression plasmid transfection compared with cells with vector plasmid transfection in both MKN45 and HGC27 cells. (C) The wound healing assay indicated that NPRA upregulation enhanced HGC27 cell migration.
Fig. S3. (A, B) The western blotting and qRT‒PCR results confirmed that PPARα expression was significantly decreased in cells transfected with si-PPARαcompared with cells transfected with NC in both MKN45 and HGC27 cells. (C) EdU experiments were used to detect cell proliferation in HGC27 cells.
Fig. S4. (A, B) In gastric cancer cell lines MKN45 and HGC27, NPRA increased the expression of protein CD44 and protein CD133, indicated that NPRA can promote the stemness of gastric cancer cells.
Fig. S5. (A) qRT-pcr showed that the expression of PPARα in cancer tissues was significantly higher than that in adjacent tissues. (B) In gastric cancer tissue samples, NPRA expression was positively correlated with PPARα.