Abstract
Carob is botanically called as Ceratonia siliqua and belongs to the Legumes family. The fruit is derived from hermaphrodite trees and hard in shape. The carob contains high sugar contents in pulp, fat in seed and minerals like potassium, calcium, and phosphorus are present in pods. Polyphenols and antioxidants are abundant in leaves and pods. It can be used for enhancing human health due to its high nutritional profile. Carob gum is used in the pharmaceutical industry in the form of pomades, anti‐celiac ingredients, pills, and dental paste. The clinical carob can aid as an anti‐cancer, anti‐reflux, anti‐diabetic, anti‐diarrheal, anti‐hyperlipidemia, anti‐bacterial, anti‐microbial, and anti‐fungal. Nowadays, carob seeds are being used as an alternative to cocoa powder in food items whereas the leaves, pods, and seeds of carob are also historically used as food for animal feed. However, these parts of carob are available in markets with reasonable prices. Carob production, though with a rising contribution, contributes to the local economy. In this sense, we can incorporate knowledge on the chemical properties and the biological effect of carob fruits on human health. In this study, the supportive and health‐promoting impacts of carob are discussed along with the clinical testing obtained from natural constituents of carob. In addition, further studies can be performed to extract and separate polyphenols and antioxidant potential for the development of functional that play a valuable role in pharmaceutical and food sectors.
Keywords: carob, economic, food application, limitations, medicinal, nutrition, polyphenol
In this study, along with the clinical testing obtained from natural constituents of carob. In addition, further development of appropriate approaches for the study of extraction and separation of polyphenols should be performed to determine their antioxidant potential for pharmaceutical and food sectors.
1. INTRODUCTION
Ceratonia siliqua L. is the botanical name of carob. It belongs to the plant family and derives from the Greek word “Kera.” These fruits have keratomorphic shapes which are the Latin word siliqua that refers to the pods' hardness and shape (Papaefstathiou et al., 2018). The carob tree (C. siliqua L.) is a native tree in the region of Mediterranean, and there occupies great importance on account of economic and environmental conditions (Ali, Kousar, et al., 2022; Benković et al., 2016). Carob fruit has a tough‐to‐crack pod that is elongated, flat, arced or straight, and thick sutures. There are edges that are either rounded or somewhat pointed. Its size ranges from 10 to 30 cm. Its composition is on two crucial parts including pulp (90%) and seeds (10%) (Durazzo et al., 2014). Cosmetics, food, and pharmaceutical industries use it widely as raw material (Iqra et al., 2023; Rasool et al., 2023). The latest research by Food and Agriculture Organization exposes that carob fruit is worldly produced at the rate of 158.609 tons per year, derived from 66,874 hectares, where the contribution from Asia, Europe, and Africa is 11.3%, 75.55%, and 13%, respectively (Pernet & Ribi Forclaz, 2019).
Carob is a representative fruit of the areas with the climate of the Mediterranean. The carob tree belongs to the legumes family, and evergreen tree with sensitive temperature. In the past, carob was used for sweetening and treating different ailments. Carob is one of the oldest and most beneficial known plants on the earth (Goulas et al., 2016). The cultivation can be done in regions of less rainfall, and have a life of up to 150 years (El Hajaji et al., 2011). Carob has been taken as a cheap source of human and animal nutrition for years due to its different properties (Ramón‐Laca & Mabberley, 2004). Today, carobs are used as pharmaceuticals, cosmetics, and food (Kotrotsios et al., 2012). The whole year fresh green tree of carob has leaves with the thickly single‐layered upper epidermis. These green parts are composed of phenolic compounds in the leaves cells. The carob is a brown pod that is primarily used as a fruit. The carb surface with wrinkles later on ripening gives a leather smooth texture. There are two prime constituents of carbs including pulp and seeds. The pulp is the region in a pod of carob without seeds. However, the pericarp is its upper layer leathery in texture while the inner one is called the mesocarp. Biohydrogen was produced using solid carob waste from the food sector. Several reaction parameters, including pH, catalysis, nitrogen environment, and water supply, are explored for the process, and the yield is compared to glucose as a control substrate (Bahry et al., 2022). Water is used to remove the pods, and microorganisms fermented them. In place of ethylene glycol, a number of ionic liquids are investigated for extraction of dry ethanol. In comparison to nonthermal extraction, microwave‐assisted extraction of phenolics provided better yields (Sanchez‐Segado et al., 2019). Conditions for drying thin‐layer pulp were researched and improved. The quality and yields of drying carob juice is improved using spray drying. To attain the best sensory, antioxidant, and physiochemical qualities, a variety of roasting conditions are investigated. Pods are removed to determine their sugar content, and the extraction is planned using the mathematical Taguchi technique (Huma et al., 2018). In reality, pods are harvested four times in a row. It is discovered that dried ripe pod powder works well as a dye pollution absorber (methylene blue). By reacting with Ce(NO3)3 6H2O, an aqueous extract of leaves was utilized to create CeO2 nanoparticles (NPs) (Tagnamas et al., 2018). These NPs exhibited potent cytotoxic and antioxidant properties. To create ZnO‐NPs, Zn(CH3COO)2 was treated with an aqueous extract of leaves. Strong cytotoxic action is shown by these NPs against human breast cancer cells (Javadi et al., 2019).
Rats exposed to STZ‐nicotinamide‐induced hyperglycemia were protected by a methanolic extract of dry, unripe pods. Water and ethyl acetate are used to extract the carob honey (El‐Haskoury et al., 2019). Rats with diabetes induced by STZ had a hypoglycemic responses to both extracts. Unripe, dry pods were extracted using 70% aqueous methanol, and the extract was fractionated using dichloromethane and water. Both fractions had an active antidiarrheal and antiemetic in the rat model after being examined for their overall chemical makeup (El‐Haskoury et al., 2019).
Origin, harvesting timing, and cultivars are the main factors on which the chemical composition of carob pulp depends. The total contents of sugar (fructose, glucose, and sucrose) are high in the pulp. Moreover, 185v of cellulose and hemicellulose are also present. The high concentration of condensed tannins (16%–20% of dry weight) is also present in ripened pods of carob (Goulas, 2016).
2. NUTRITIONAL AND CHEMICAL COMPOSITION OF CARBS
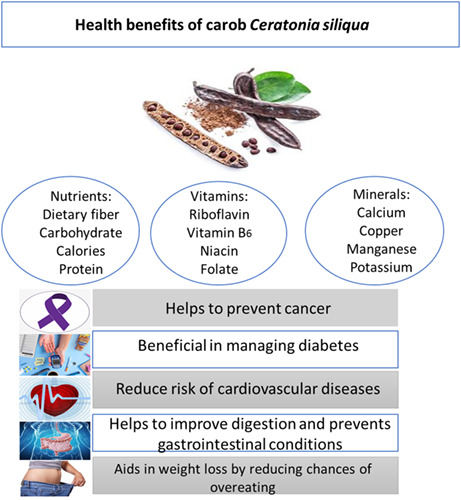
The carob fruit is complicated mixture of secondary metabolites. A variety of nutrients including fiber, sugar, and various polyphenols are present. Many minerals and amino acids are also abundant in carob. The nutritional makeup of carob and its health advantages are shown in Figure 1.
FIGURE 1.
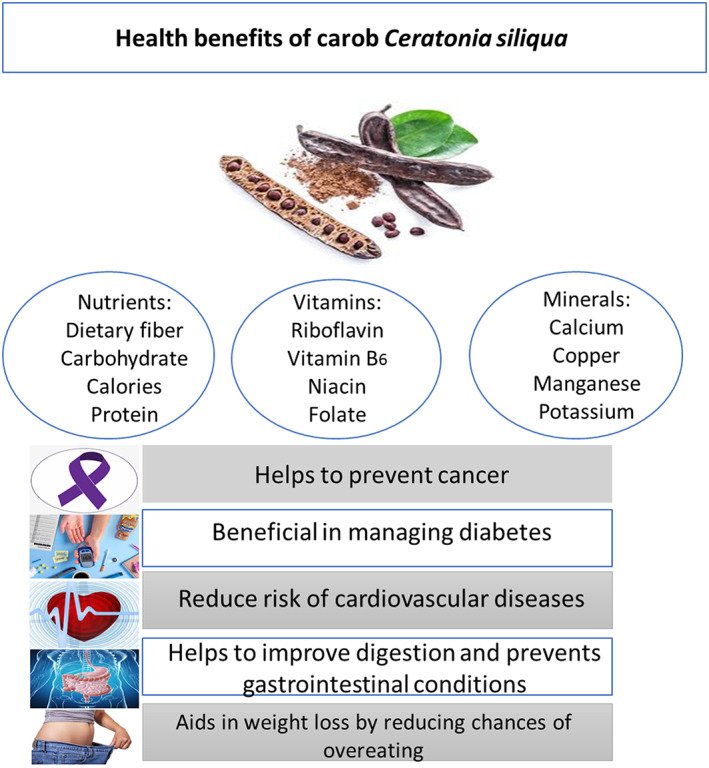
Nutritional composition of carob and health benefits.
2.1. Carbohydrates
Carob fruit is a vital source of sugar and has high nutritional value. Previous research revealed that the carbohydrates contents in the cultivated range about 40–55 g 100 g−1 dm (Turhan, 2014). Usually, the carob cultivar contains higher sugar content (Sigge et al. 2011). According to sugar composition, the carob, sucrose is vital source of CHO and its quantity is up to 52 g 100 g−1 dm (Diaz, 1997). Fructose and glucose concentrations may be as high as 1.8–12.5 g 100 g−1 dm and 1.8–10.2 g 100 g−1 dm respectively. Carob sugar is taken from the fruit and used to make natural carob syrup. The cutting‐edge method for its recovery to create carob syrup has been established (Livesey, 2003).
2.2. Proteins
There are several amino acids present in carob fruits such as sulfur‐containing amino acids (methionine and cysteine), acidic (aspartic acid and glutamic acid), hydroxylic (serine and tyrosine), aliphatic (alanine, glycine, isoleucine, leucine, proline and valine), basic (arginine, histidine, and lysine), and amidic (asparagine and glutamine) (Singh et al., 2007). Around 57% of the amino acid composition in pods is made up of basic and amidic amino groups. Carob fruits were examined as an excellent supply of amino acids in accordance with the World Health Organization's (WHO) requirements for protein. Especially, the concentration of required essential amino acids in carob fruit fairly crosses the WHO standards (Miś & Dziki, 2013).
2.3. Minerals
Calcium and potassium are both found in large quantities in carob fruits. Potassium concentrations may reach 970 mg 100 g−1 dry weight and 1120 mg 100 g−1 dry weight, whereas salt concentrations start at 300 mg 100 g−1 dry weight (Rizzo et al., 2004). As 1 L of milk contains 1200 mg of calcium whereas one cup of milk has the same amount of calcium as one piece of the carob fruit. Carob fruit contains little amounts of important bio components like magnesium and phosphorus. Microminerals including manganese, nickel, cobalt, zinc, barium, iron, and cobalt are also included in it. Iron is the micromineral with the greatest concentration. Compared to seeds, pods contain a lower concentration of bio components (Barak & Mudgil, 2014).
2.4. Fibers
By removing the water from carob pulp that makes up between 30% and 40% of the total carob pulp. The carob fibers are divided into two categories including soluble and insoluble fibers (Camero & Merino, 2004). The process of obtaining natural fibers of carob have also been licensed (Haber, 2002). Lignin, cellulose, and hemicellulose make up the insoluble portion of dietary fibers. However, the minimal quantity of polyphenols in carob spans the range of 70% of total fibers. The difference between carob fibers and other dietary fiber sources is due to the higher level of polyphenols. Carob fiber is a dietary fiber that is both insoluble and non‐fermentable (Nasar‐Abbas et al., 2016). The soluble fiber is less (maximum 10 g 100 g L−1 of that carob fiber) and consists of a simple category of carbohydrates. Ultimately, carob fibers have functions in the rheology of dough in bakery‐based products (Nawrocka et al., 2016).
3. PHYTOCHEMICAL COMPOSITION OF CARB
Polypchemical particularly dietary fibers (27%–50%), tannins (18%–20%), carbohydrates and minerals (iron, potassium, sodium, magnesium, zinc, and copper) are present in large quantity while proteins (3%–4%) and lipids (0.4%–0.8%) are present in fewer amounts in carob pods. The fruit is famous due to a high number of sugars which contains important constitutes including fructose (5%–7%), glucose (5%–6%), and sucrose (32%–38%). The large quantity of dietary fibers is also present in pods (Palafox‐Carlos et al., 2011). To identify the presence of polyphenolic compounds, the HPLC method of chromatography was utilized that exposed the existence of non‐hydrolyzed tannins. It is proanthocyanins made of flavan‐3‐ol groups and their galloyl esters, gallic acid, catechin, epicatechin gallate, epigallocatechin gallate, and quercetin glycosides (Ortega et al., 2009). Previous studies suggested that hydrolyzed tannins are also present in carob pods in the form of flavonoids (26%), phenolic acids (cinnamic, p‐coumaric, and gallic acids), tannins, hydroxytyrosol, flavone glycosides, and polyphenols (Papagiannopoulos et al., 2004). Electrolytes like sodium, zinc, manganese, potassium, iron, and copper are also found in large quantities in carob juice. The chemical composition of carob pods varies following the climate, specie, maturity stage, and various parts of the tree. Pyrogallol (48.02%–3.55%), catechin (19.10%–2.11%) and plus tannic acid (9.01%–1.40%) are found to be major components in mature pods (Toydemir et al., 2013). Nonetheless, the catechin (16.52%–2.34%), epicatechin (12.26%–1.04%), gallic acid (15.12%–2.31%), pyrogallol (26.45%–3.03%), and chlorogenic acid (15.01%–1.72%) are found in immature pods (Rtibi et al., 2015). Phenols in the form of tannins (13%–0.45%), kaempferol (77%–2.43%), polydatin (0.85%–0.22%), and catechin hydrate (4.30%–0.3%) are present in the leaves are identified. The chemical compositions of carob are shown in Figure 2.
FIGURE 2.
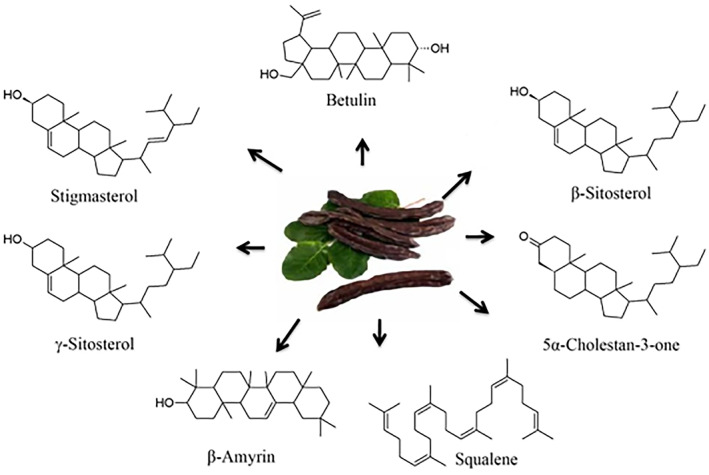
Chemical compositions of carob.
Similarly, it is also revealed that fibers are present richer in leaves than in pods. Carob extracts contain more reduced sugar than leaves (Rtibi et al., 2016).
4. BIOAVAILABILITY OF BIOACTIVE COMPOUNDS
The biological functions of phenols rely on their deportment in the digestive tract. A few polyphenolic compounds exist in medicinal plants. The sufficient amount of polyphenols is absorbed and transformed to enhance health. Bioactive compounds of carob are shown in Figure 3.
FIGURE 3.
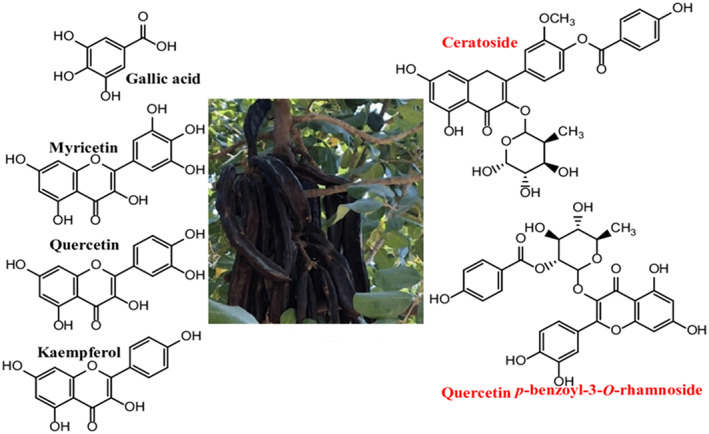
Bioactive compounds of carob.
Bio accessibility and their capability of crossing the mucosal barrier of the intestine are the typical factors that define the bioavailability of the constituents (Crozier et al., 2010). The extract of compounds from food matrices and their state of stability in the gastrointestinal condition is known as bioaccessibility (Ali, Manzoor, et al., 2022; Ali, Riaz, et al., 2022; Gawlik‐Dziki, 2012). This procedure of substances relies on different factors encompassing matric composition, initial concentration in the matrix, and the factors related to the host like the presence of enzymes that contributes to the mechanism of digestion and the physiochemical characteristics of the fluids of gastro‐intestine. Moreover, the polyphenols is combined with further ingredients of food like fibers, lipids, and sugars that improve or reduce the bioavailability by process of digestion (Tagliazucchi et al., 2010). The tannins and catechin bioavailability were examined outside the body of rats (small bowel parts). Despite are observed of both components by the intestine. However, catechin is passed through the gut slowly. The less molecular weight phenols like isoflavones and gallic acid superficially get assimilated via the tract (Tagliazucchi et al., 2012). Some bioactive compounds and their health benefits are demonstrated in Table 1.
TABLE 1.
Health benefits of bioactive compounds present in carob.
| Bioactive compounds | Health benefits | Source | Reference |
|---|---|---|---|
| Polyphenols, gallic acid, epigallocatechin, catechin, quercetin, myricetin, kaempferol and rutin | Antioxidant, anti‐inflammatory, antibacterial activities, effective against neurogenerative disorders and antitumoral activities | Carob pulp, carob fiber, carob pod and carob seed extract | Abulyazid et al. (2017); Rico et al. (2019); Rodríguez‐Solana et al. (2019) |
| D‐pinitol | Anticancer and antidiabetic effect | Carob pulp extract | Christou et al. (2019) |
| Tannins | Antidiarrheal effect | Carob bean juice | Sigge et al. (2011) |
| Cinnamic acid | Antioxidant activities and hepatoprotective effect | Carob fruit extract | Dhaouadi et al. (2014); Roseiro et al. (2013) |
| Galactomannan | Effective in gastrointestinal health | Carob pod endosperm | Xie et al. (2020) |
| Chlorogenic acid and epicatechin | Reduce intestinal glucose absorption and laxative activities | Carob seed | Abu Hafsa et al. (2017) |
| Flavonol glycosides | Increased lipid metabolism and reduce LDL level | Carob pulp extract | Gruendel et al. (2007) |
4.1. Carob polyphenols and their bioavailability
The carob fruits are high in phenolic acid that is compressed by gallotannins and flavonoids (Benchikh et al., 2014). Although polyphenols represent a broad range in in vitro biological properties, and their bioavailability reduces in vivo efficiency. However, they are the most abundant in the human diet and their behavior is not always parallel to the abundance due to poor absorption or rapid removal (Chahar et al., 2011). Polyphenols have a bioactivity of 10% (animal tests) with a range of around 2%–20%. It is extremely necessary to have a way of increasing their bioavailability because a broad sample of participants is needed in clinical trials to illustrate the efficacy and thus such tests are not affordable (Serafini et al., 2010). The first strategy should be to investigate the bioavailability as well as pharmacokinetic profile because these are the major factors causing its action. A lot of therapeutic potential phytochemicals have a high in vitro record but decreased in vivo due to their weak ADME (absorption, distribution, excretion, and metabolism) properties. The fundamental parameters to define a pharmacokinetic profile to an appropriate degree are T max, C max, t 1/2, and AUC. C max is the maximum reached the amount of medication after one dose and after the second dose; T max is the time taken to achieve the maximum concentration of a medication (C max); t 1/2 is the time for the medicine to achieve half the initial concentration; whereas AUC (the region below the curve) is referred to as the cumulative concentration of a drug over time (Lu et al., 2014). A more detailed evaluation of bioavailability in human and animal studies is needed for the low absorption as well as intensive metabolism of compounds with poor bioavailability (Cao et al., 2015). The number of research to quantify in vivo flavonoids was focused on LC–MS/MS platforms. Assessing their persistence in human plasma using novel pharmacokinetic techniques will provide the foundation on which scientists can build to resolve the impediment of poor bioavailability. In vivo studies, further work should be carried out on molecules with impressive outcomes as the distance between two situations is often impossible to overcome (Wong et al., 2014).
5. FOOD APPLICATIONS OF CAROB
Nowadays, carob seeds are being used as an alternative to cocoa powder in food items and scrutiny on comprehensive examination of chemical and sensory attributes of products (Srour et al., 2016). Currently, the replacement of cocoa in white milk chocolate with carob pod powder has been investigated. Protein contents were increased to a little extent by utilizing more carob powder instead of cocoa in white chocolate, but the fiber and sugar content upgrades highly. Some uses of carob are shown in Figure 4.
FIGURE 4.
The use of carob.
By increasing the concentration of carob powder in white chocolate, the calcium, potassium, magnesium, and sodium concentrations were investigated to high whereas iron and zinc concentrations was found to drop (Salem & Fahad 2012). The most important impact was the stepwise reduction of caffeine, which completely vanished at 100% concentration of powder carob. The aroma did not show any special difference by increasing carob flour quantity (Tsatsaragkou et al., 2012). The results demonstrated that nutritional status, sensory and functional properties are improved by using carob in milk chocolate. It has been understood as the best alternative to cocoa powder in the synthesis of products based on chocolate. In the manufacture of cakes that are free from gluten to banana and soy flour. The impact of carob flour was also studied by investigating the sensory and physicochemical characteristics of cakes of different formulations (Haber, 2003).
The calories, lipids, and carbohydrates are lowered while the concentration of dietary fibers increases by using carob powder in cakes. But the resilience, elasticity, and cohesiveness in cakes are lessened by the use of carob flour instead of cocoa powder. The cake with 75% replaced with carob powder that have no noticeable differences in sensory properties compared to cocoa‐based cakes (Rosa et al., 2015). The manufacture of cakes that are free of gluten have fewer calories and high in fiber and protein content. Its pleasing sensory attributes for celiac‐suffering patients. A substitute for chocolate milk carob relying on milk beverages by using carob powder is also manufactured (Iipumbu, 2008). The manufacture of different carob‐based beverages and powders is done by utilizing both unroasted and roasted pulp of carob of various varieties.
The roasted powder shows richness of antioxidant activity and phenolic contents and results. Moreover, the roasted powder enhanced the acceptability aspects of sensory properties carob coffee odor, flavor, caramel aroma, mocha odor, mouthfeel, viscosity, and afterward bitter taste (Moreira et al., 2017). It may be due to the association of pyrazines, pyridine, ketones, and aldehydes that are concerned with typical roasted taste and aroma (Afoakwa, 2016). Some functional food items are mentioned in Table 2.
TABLE 2.
Carob‐enriched functional foods and their impact on quality of product.
| Carob‐based food products | Positive impact | Reference |
|---|---|---|
| Bread fortified with carob pod flour | Bread quality improved, high antioxidant and phenolic content, and improve organoleptic attributes | Hoehnel et al. (2019) |
| Carob spread | Improved texture and color attributes, and good source of minerals | Aydın and Özdemir (2017) |
| Carob powder‐based milk beverages | Highest phenolic content, antioxidant activities, and improved color, taste texture, and overall acceptability | Srour et al. (2016) |
| Rice based snacks enriched with pea and carob fruit powder | Improved organoleptic and textural attributes, and high phenolic and antioxidants compounds | Arribas et al. (2019) |
| Carob powder‐based yogurt | Produce low‐fat yogurt, high fiber content, produce low lactose, and increased sweetness | Moreira et al. (2017) |
| Carob powder‐based gluten free cakes | Improved dietary fiber content, lower caloric content, rich in protein, decreased cohesiveness, and good sensory attributes | Rosa et al. (2015) |
| Carob flour‐based pasta | High phenolic contents, higher antioxidant capacities, higher glycemic index (GI), and improved sensory attributes | Arribas et al. (2020) |
| Carob syrup used as sugar replacer | Increased fermentation, reduce microbial activity, and higher yield of mannitol production | Nasar‐Abbas et al. (2016) |
| Edible coating of carob bean gum‐based products | Improve physical appearance, increased shelf life, minimize the losses of bioactive compounds, and reduce oxidation process | Rojas‐Argudo et al. (2009) |
| Carob bean gum‐based bakery products | Increase rheological properties, improve yield of bakery items, and increased water absorption capacity | Zhu et al. (2019) |
| Carob‐based ice cream | Used as stabilizer, decrease melting point, and increase viscosity | Bahramparvar and Tehrani (2011) |
6. BIOCHEMICAL APPLICATIONS OF CAROB
6.1. Antimicrobial and antifungal activities
Ceratonia siliqua methanol extract was tested for antimicrobial activities as contrasted to Plantago major methanol extract which was more effective for many of these bacteria. C. siliqua extract was more active in Enterococcus sp. Methanol and aqueous extracts were tested alone and in conjunction with many other antimicrobial agents (gentamicin, amikacin, ampicillin, and clindamycin) (Talibi et al., 2012). Extracts and antimicrobial agents combined were more successful than each independently. Antibacterial property against Pectobacterium atrosepticum was evaluated in potato soft red. The acetone extract was more active. It was found that the methanol extract of the leaves was active for Listeria monocytogenes. HPLC extract analysis provided seven antibacterial compounds and extract dry pods were screened for 14 forms of fungi and bacteria (Meziani et al., 2015).
Dried plant powder was absorbed into methanol, and their mixture was centrifuged, filtered, and screened for antifungal and antibacterial activity. The photosensitivity has also been investigated and proven to be adequate. It was found to be highly efficient against 11 of them in 1000 and 500 mg mL−1 amounts. Chloroform as well as hydroalcoholic (no indication of ratio) extracts of dry particles were processed and found to be active toward 15 fungal and bacterial organisms including three species of Caucasian albania (Aissani et al., 2012).
Hexane, ethyl‐acetate, chloroform, and methanol extracts from dry leaves were made and evaluated against citrus‐sour‐rot agent Geotrichum candidum for antifungal activity (Rahmoun et al., 2014). The hexane and chloroform were more inactive, whereas the methanol extract was more active than the ethyl acetate extract. It was militant against everybody. The total phenolic level was calculated to be 465.5 mg g−1 whereas gallic acid and the total flavonoid was found to be 24.6 mg g−1. The extract has been classified as antimicrobial properties and moderate antioxidant (DPPH). N‐hexane, ethyl acetate, and water have been extracted from dry leaves (Al‐fawwaz & Al‐Khaza'leh 2016).
6.2. Antidiabetic activities
The dry pod extract of ethanol/water has been studied for streptozotocin‐induced diabetes in rats. The blood glucose was found to be decrease (Hsouna et al., 2012). The combination of dried Roselle flowers (Hibiscus sabdariffa) and dry carob pods was extracted from water and administered to diabetic alloxane‐induced rats. The extract has been checked both with and without gamma rays combination of plant powder (Jamous et al., 2015).
In alloxane‐induced diabetic rats, an aqueous extract of the premature pod was evaluated for antidiabetic. This is more successful than an aqueous extract from mature pods. Powder from dry pods for phytosterols with n‐hexane was isolated (Mokhtari et al., 2011). The same party used the same extract for the study of the same disease except female rabbits are not pregnant. Fiber‐diluted aqueous extract of seed‐free dry pods and was prepared and tested to suppress the disease of the antidiabetic by inhibiting glucosidase. The appropriate activity has been found (Hamza & Al‐Seeni, 2015).
6.3. Antioxidant activities
Ethanolic leaves and pulp extracts (all sexes of the tree) have been screened for radical (DPPH) as well as antioxidant activities and extract leaves were observed to be more active. Dichloromethane, hexane, diethyl ether, methanol/water (8:2 v/v), and ethyl‐acetate have been extracted successively from the leaves. Extracts from the three tree varieties have been examined for antioxidant activity (DPPH) and total phenolic content (Rtibi et al., 2017).
Antioxidants, carotenoids, and polyphenols were evaluated from ethanol extract of dry pods. Eighty percent aqueous methanol extract was ready and measured on antioxidant activity (three methods). However, HPLC reverse stage analysis was performed for this purpose (Mounce & Al‐Saeed 2017). The pods (no seeds) were extracted using water, petroleum ether, methanol, ethanol, hexane, and acetone. Extracts were tested for total phenolic content, antioxidant activity (ABTS), and cerebral and myocardial lipid peroxidation in vitro and in vivo (rats). Polar extracts have been more active than non‐polar extracts.
Methanol extract from leaves was tested by various methods for antioxidant activity and was found to be very effective as compared to many other fruit plants. The total phenolic content and the antioxidant (DPPH and FRAP) ability of methane extract from dry leaves were identified. Compared to other plants studied, there were moderate activities in carob. Aquatic extract of dry carob pods was prepared and its total phenolic content and antioxidant activity were established. Both aqueous and methanol extracts have been found fairly high (plant component not indicated) and have been checked for antioxidant activity as well as total phenolic content (Macho‐Gonzalez et al., 2017).
6.4. Anti‐cancer effects
Colorectal cancer has become one of Western society's most common cancers (Johns & Houlston, 2001). Epidemiological and clinical studies have shown that a regulatory diet can suppress colorectal cancer. Researchers conclude that various phenolic compounds are highly promising associated substances (Nayak et al., 2015) for fruits, vegetables, rice, tea, and wine, which are very useful for the body of humans. The purpose is to minimize oxidative stress by chelating free radicals or redox activity. Some studies have also shown that the proliferation of different cancer cell line forms can be effectively inhibited (O'Keefe, 2016). Dietary fiber is another possible factor that can reduce the risk of colon cancer. High‐fat and high‐protein foods have a beneficial impact on colorectal cancer but have a detrimental relationship to high‐complex carbohydrates and high in fiber intake (Ali, Mughal, et al., 2021; Klenow et al., 2008). Earlier studies found that polyphenols and dietary fiber would reduce the risk of cancer, while carob fiber combines with those two nutrients (Klenow et al., 2009).
6.5. Anti‐reflux effects
Reflux in infants is normal. In 77% of infants under the age of 3 months, regurgitation was reported at least once a day. Carob bean gum is the most widely used milk thickener in European countries. Studies have shown that the number of carob bean gum regurgitation episodes decreases significantly and enhances other symptoms, such as crying, sleep disturbance, and gastroesophageal reflux (O'Keefe, 2016). Researchers investigated the effect on reflux as well as tolerance indicators in an adolescent gastroesophageal expression of carob bean gum thickened formulas. In the study, 56 qualified infants (1–6 months of age) were randomly assigned to either 0.33 g 100 mL−1 (Formula A) or 0.45 g 100 mL−1 (Formula B) of cold‐lösable gum bean or 0.45 g 100 mL−1 (Formula C) of hot‐lösble gum for 2 weeks. Results have shown that formula A (i.e., 0.33 g 100 mL−1) effectively decreased some pH‐monitoring levels of unpretentious gastroesophageal reflux, higher body weight, and well‐tolerated childhood. Thirty‐nine babies with three or more regurgitation episodes a day were studied in another study (Nayak et al., 2015). Besides, gastric emptying efficiency of different milk formulations was measured. The formulas are A, B, and C with 0.35 g of carbon beans per 100 mL−1 (HL‐350) and 0.45 g per 100 mL−1 (HL‐450) as well as (HL‐00), respectively. The results indicated that the HL‐350 or HL‐450 treatment of infants is greater than that of HL‐00 (reflux episodes). A comparison of two formulations revealed that HL‐450 had a slower growth rate of gastroesophageal reflux gastric emptying in children. In a further study, the authors proceeded to analyze the effect of the formula containing the bean gum (HL‐350 in younger infants), which can be used as a thickening formula of infant formula, significantly reducing the number of reflux episodes in babies and young children (Klenow et al., 2009).
6.6. Anti‐diabetic effects
The anti‐diabetic effects of carob‐containing herbal preparations and other natural products have been tested. The formulations were used as herbal remedies for people with diabetes who had a low glycemic index (Milek et al., 2015). The Carob gum diet has shown a drop in rat blood glucose levels. Dos Santos et al. further estimated in vitro the glycemic index of carob flour at 40.6. D‐pinitol may be present in carob products for anti‐diabetic properties, as it controls blood sugar levels in Type II diabetes mellitus patients by enhancing insulin sensitivity (Son et al., 2010). Carob syrup is viewed as a major source of D‐pinitol. Ten grams of it is adequate to decrease the sugar level in type II diabetes as compared to the normal dose (10 mg D‐pinitol/kg body weight). Tetik and Yüksel (2014) proposed that D‐pinitol could have strong measures with insulin, and therefore improve glycemic control by evaluating its effectiveness in diabetes animal models. The study also reported that D‐pinitol induced increased glucose absorption in the L6 line of muscles, indicating its presence in the glucose metabolic pathway of muscles rather than the increased development or action of insulin (Ali, Ain, et al., 2021; Coviello et al., 2015).
6.7. Anti‐diarrheal effects
The different percentages of carob are reduced the signs of diarrhea. The previous study has shown that 2% carob solution can prevent hemagglutination and adherence of Escherichia coli on specific epithelial cells of the intestines. The efficacy of the proposed fraction may be demonstrated by blocking the adherence of bacteria isolated from the highest small intestinal tract of children. The proportion consisted of 40% tannins or 21.2% polyphenols, and 26.4% dietary fiber. Tannins are not held responsible for anti‐diarrheal intervention for the first time. Liu et al. (2014) demonstrated by in vitro and in vivo models strategies that the tannin extraction of rhubarb downregulates the pathways PKA/p‐CREB and consequently influences water transport within the cells of aquaporins 2 and 3. Wursch (1991) also patented an anti‐diarrheal dietetic drug, which includes water‐insoluble tannins of at least 20% by weight by weight of particulate carob pods based on dry matter.
6.8. Anti‐hyperlipidemia
Effects of high blood lipid or lipoprotein levels can cause atherosclerosis and later vascular and heart disease. The supplements that reduce the amount of lipids and cholesterol in the blood. Patents have been given for a cholesterol‐reducing preparation consisting of at least one dietary fiber picked from the carob‐fruit flesh group. In addition, the key ingredients for proprietary foodstuffs are carob fiber in combination with n‐3 fatty acids that benefit cardiovascular safety. The effects of carob constituents in hyperlipidemia have been extensively studied in vivo and in vitro models. LBG has been demonstrated to reduce cholesterol as well as lipid levels in rats. However, LBG had no significant impact on the cholesterol as well as triglycerol levels of diabetic rats in a more recent study (Yamamoto et al., 2000). The carob fruit findings are less controversial. Carob powder was applied to Sprague–Dawley rats together with a hyperlipidemic intake to dose‐dependent cholesterol as well as triglycerides. The findings also indicate that in carob powder ingested, the histopathological status of the heart, as well as the kidney of the animal while rats who adopted the hyperlipidemic diet had extreme disorders. Carob powder may be a possible choice for an overweight or obese diet (Hassanein et al., 2015). Valero‐Munoz et al. found that carob pod fibers have blocked physiological events contributing to atherosclerosis in rabbits with dyslipidemia. The function of SIRT1 and PGC‐1a, proteins that play a key role in the vascular as well as metabolic processes (Valero‐Munoz et al., 2014).
7. CAROB AND CLINICAL TRIALS
The clinical trials on carob is represented the use carob‐containing mixtures along with many other substances (Vandenplas et al., 2013). Results showed that only carbohydrates often include child hypercholesterolemia, regurgitation, and diarrhea. The majority of studies were performed on other diseases like cancer and diabetes. The outcomes of treatment for conditions such as hypercholesterolemia as well as diarrhea can be quickly discerned and control can be demonstrated over a shorter time period.
7.1. Infant regurgitation
The effectiveness of two ARFs for the therapy of infant regurgitation was evaluated and compared (Vandenplas et al., 2013). The ARF‐1 and ARF‐2 included LBG with a double‐blind cross‐over test in two groups of infants carried out for a month. For all classes and specific anthropometric criteria, the net number of babies was 115 with an average age of 9.1 weeks. ARF‐2 was systematically more successful than ARF‐1 and the total number of regurgitation episodes decreased from 8.25 to 2.32 in ARF‐1 as well as to 1.89 in ARF‐2. The same change was found for the mean volume of regurgitation with stronger action from ARF‐2.
Both ARF‐1 and ARF‐2 showed effectiveness in reducing infant volumes and the percentage of infant regurgitations that providing a broader spectrum of treatment options. Maiti et al. (2010) studied the results in infants with episodes of regurgitation of two formulae involving LBG at various concentrations. Thirty‐nine children with far more than three regurgitation episodes a day were investigated in this study. A comparison of the two milk‐based solutions in gastric emptying was measured at some times during feeding. The first solution contained LBG 0.35 g 100 mL portion 1 (HL‐350), whereas the second one contained 0.45 g 100 mL portion 1 (HL‐450). The best combination (HL‐00) was, on the other hand, clean of LBG. The impact assessment on regurgitation events was performed in 27 babies with episodes randomly allocated for 1 week with HL‐00 to obtain the formula. Results show that regurgitation seasons for infants who were fed HL‐350 or HL‐450, instead of HL‐00, were substantially lower.
Comparisons of the two formulas demonstrated that in infants with gastric esophageal reflux, HL‐450 showed a lower capacity of gastric emptying. Miyazawa et al. (2004) have examined the impact of both LBG‐based formulations at various rates in infants with episodes of regurgitation in earlier studies. Formula HL‐350 has shown promising promise by reducing episodes of regurgitation in 4‐month‐old children with reflux. For this study, researchers investigated the impact of HL‐350 for infants below 2 months. For this reason, a 2‐week study allocated 20 children with more than three regurgitation episodes a day. The babies were divided into two groups and the first was fed HL‐350 in the first week, and the second was controlled milk (HL‐00). The second party followed the opposite order. The number of regurgitation episodes in infants was substantially decreased during the week when they fed HL‐350 compared to the week of milk control. In addition, HL‐350 did not affect the delay in gastric acid secretion. The care of gastrointestinal bleeding in babies was performed successfully using LBG (Panghal et al., 2014).
8. ECONOMIC IMPACT
The harvesting of the carob tree seems to be great economic and environmental significance for the Island of Cyprus. The carob tree is regarded as the main crop for the long‐term survival of its high natural farms in terms of the climate. On the other hand, the high production of carob is well contributes to the local economy. In the last few decades carob has been recognized as the “black gold of Cyprus” due to financial value. However, it is the largest agricultural export. Carob production is currently substantially rising in Cyprus, because it is no longer viewed profitably. Carob fruit is mostly used to produce endosperm to the food industry in particular for the production of carob syrup. Similarly, the carob seed leaves are used for bioenergy and as animal feed as shown in Figure 5.
FIGURE 5.
Carob tree products.
The carob crops is able to deliver viability for production taking into account the health impacts of carob. Carob fibers and polyphenols are not used but pharmacological studies have related them to important health‐promoting properties. These compounds also become components in modern functional foods, dietary supplements, and plant‐based medications. Research is also required for the utilization of LBG as a drug carrier and D‐pinitol as an active compound for the treatment of diabetes. Ultimately, the use of such drug applications is utilized to achieve high‐value‐added goods from carob fruit (Goulas et al., 2016).
9. CONCLUSION
It is concluded that carob can be included in the regular diet of humans because it is nutrients dense plant source. In addition, it contains enough vegetative fat, protein amino acids, and minerals. However, carob can be used as a natural ingredient for producing fresh, nutritious foods that is depended on the manufacturing process. Carob is basically native to Cyprus that has a high biological value and needs to be protected. As a natural product, carob is not only beneficial to human health but also economically and environmentally important. The results of clinical trials showed that carbs can treat different diseases including hyperlipidemia, diabetes, colorectal cancer, and irritable bowel syndrome.
AUTHOR CONTRIBUTIONS
Ali ikram: Conceptualization (equal). khair‐ul‐Wajeeha Zafar: Data curation (equal). Muhammad Faizan Afzal: Software (equal). Afifa Aziz: Validation (equal). Izza Faiz ul Rasool: Visualization (equal). Ammar AL‐Farga: Investigation (equal).
FUNDING INFORMATION
The authors did not receive support from any organization for the submitted work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
There is no need for ethical approval as this was a review article.
ACKNOWLEDGMENTS
The authors are highly thankful to the Higher Education Commission of Pakistan and Government College University Faisalabad, Pakistan, for providing free full‐length papers.
Ikram, A. , Khalid, W. , Wajeeha Zafar, K.‐u.‐. , Ali, A. , Afzal, M. F. , Aziz, A. , Faiz ul Rasool, I. , Al‐Farga, A. , Aqlan, F. , & Koraqi, H. (2023). Nutritional, biochemical, and clinical applications of carob: A review. Food Science & Nutrition, 11, 3641–3654. 10.1002/fsn3.3367
Contributor Information
Waseem Khalid, Email: waseemkhalid@gcuf.edu.pk.
Faisal Aqlan, Email: aqlanfaisal@gmail.com.
DATA AVAILABILITY STATEMENT
Data used during the current study are available from the corresponding author.
REFERENCES
- Abu Hafsa, S. H. , Ibrahim, S. A. , & Hassan, A. A. (2017). Carob pods (Ceratonia siliqua L.) improve growth performance, antioxidant status and caecal characteristics in growing rabbits. Journal of Animal Physiology and Animal Nutrition, 101(6), 1307–1315. [DOI] [PubMed] [Google Scholar]
- Abulyazid, I. , Abd Elhalim, S. A. , Sharada, H. M. , Aboulthana, W. M. , & Abd Elhalim, S. T. (2017). Hepatoprotective effect of carob pods extract (Ceratonia siliqua L.) against cyclophosphamide induced alterations in rats. International Journal of Current Pharmaceutical Review and Research, 8(2), 149–162. [Google Scholar]
- Afoakwa, E. O. (Ed.). (2016). World cocoa production, processing and chocolate consumption pattern. In Chocolate science and technology (pp. 17–48). Wiley. 10.1002/9781118913758.ch2 [DOI] [Google Scholar]
- Aissani, N. , Coroneo, V. , Fattouch, S. , & Caboni, P. (2012). Inhibitory effect of carob (Ceratonia siliqua) leaves methanolic extract on Listeria monocytogenes. Journal of Agricultural and Food Chemistry, 60(40), 9954–9958. [DOI] [PubMed] [Google Scholar]
- Al‐fawwaz, A. T. , & Al‐Khaza'leh, K. A. (2016). Antibacterial and antifungal effect of some natural extracts and their potential use as photosensitizers. European Scientific Journal, 12, 147. 10.19044/esj.2016.v12n6p147 [DOI] [Google Scholar]
- Ali, A. , Ain, Q. , Saeed, A. , Khalid, W. , Ahmed, M. , & Bostani, A. (2021). Bio‐molecular characteristics of whey proteins with relation to inflammation. In Qureshi M. S. (Ed.), New advances in the dairy industry. Intechopen. 10.5772/intechopen.99220 [DOI] [Google Scholar]
- Ali, A. , Kousar, S. , Khalid, W. , Maqbool, Z. , Aziz, A. , Arshad, M. S. , & Manzoor, M. F. (2022). Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Frontiers in Nutrition, 3015, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A. , Manzoor, M. F. , Ahmad, N. , Aadil, R. M. , Qin, H. , Siddique, R. , & Aizhong, L. (2022). The burden of cancer, government strategic policies, and challenges in Pakistan: A comprehensive review. Frontiers in Nutrition, 1553, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A. , Mughal, H. , Ahmad, N. , Babar, Q. , Saeed, A. , Khalid, W. , & Liu, A. (2021). Novel therapeutic drug strategies to tackle immune‐oncological challenges faced by cancer patients during COVID‐19. Expert Review of Anticancer Therapy, 21(12), 1371–1383. [DOI] [PubMed] [Google Scholar]
- Ali, A. , Riaz, S. , Sameen, A. , Naumovski, N. , Iqbal, M. W. , Rehman, A. , & Manzoor, M. F. (2022). The disposition of bioactive compounds from fruit waste, their extraction, and analysis using novel technologies: A review. Processes, 10(10), 2014. [Google Scholar]
- Arribas, C. , Cabellos, B. , Cuadrado, C. , Guillamón, E. , & Pedrosa, M. M. (2019). The effect of extrusion on the bioactive compounds and antioxidant capacity of novel gluten‐free expanded products based on carob fruit, pea and rice blends. Innovative Food Science & Emerging Technologies, 52, 100–107. [Google Scholar]
- Arribas, C. , Cabellos, B. , Cuadrado, C. , Guillamón, E. , & Pedrosa, M. M. (2020). Cooking effect on the bioactive compounds, texture, and color properties of cold‐extruded rice/bean‐based pasta supplemented with whole carob fruit. Food, 9(4), 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydın, S. , & Özdemir, Y. (2017). Development and characterization of carob flour based functional spread for increasing use as nutritious snack for children. Journal of Food Quality, 2017, 1–7. [Google Scholar]
- Bahramparvar, M. , & Tehrani, M. M. (2011). Application and functions of stabilizers in ice cream. Food Reviews International, 27(4), 389–407. [Google Scholar]
- Bahry, H. , Abdallah, R. , Chezeau, B. , Pons, A. , Taha, S. , & Vial, C. (2022). Biohydrogen production from carob waste of the Lebanese industry by dark fermentation. Biofuels, 13(2), 219–229. [Google Scholar]
- Barak, S. , & Mudgil, D. (2014). Locust bean gum: Processing, properties and food applications—A review. International Journal of Biological Macromolecules, 66, 74–80. [DOI] [PubMed] [Google Scholar]
- Benchikh, Y. , Louaileche, H. , George, B. , & Merlin, A. (2014). Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Industrial Crops and Products, 60, 298–303. [Google Scholar]
- Benković, M. , Srečec, S. , Bauman, I. , Ježek, D. , Karlović, S. , Kremer, D. , Karlović, K. , & Erhatić, R. (2016). Assessment of drying characteristics and texture in relation with micromorphological traits of carob (Ceratonia silliqua L.) pods and seeds. Food Technology and Biotechnology, 54(4), 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camero, B. M. , & Merino, C. S. (2004). Carob Bean (Ceratonia siliqua L.) and Its Products. (U.S. Patent No. 6,699,511). U.S. Patent and Trademark Office.
- Cao, S. , Ni, B. , Feng, L. , Yin, X. , Dou, H. , Fu, J. , Lin, L. , & Ni, J. (2015). Simultaneous determination of typhaneoside and isorhamnetin‐3‐O‐neohesperidoside in rats after oral administration of pollen Typhae extract by UPLC–MS/MS. Journal of Chromatographic Science, 53(6), 866–871. [DOI] [PubMed] [Google Scholar]
- Chahar, M. K. , Sharma, N. , Dobhal, M. P. , & Joshi, Y. C. (2011). Flavonoids: A versatile source of anticancer drugs. Pharmacognosy Reviews, 5(9), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou, C. , Poulli, E. , Yiannopoulos, S. , & Agapiou, A. (2019). GC–MS analysis of D‐pinitol in carob: Syrup and fruit (flesh and seed). Journal of Chromatography B, 1116, 60–64. [DOI] [PubMed] [Google Scholar]
- Coviello, T. , Trotta, A. M. , Marianecci, C. , Carafa, M. , Di Marzio, L. , Rinaldi, F. , & Matricardi, P. (2015). Gel‐embedded niosomes: Preparation, characterization and release studies of a new system for topical drug delivery. Colloids and Surfaces B: Biointerfaces, 125, 291–299. [DOI] [PubMed] [Google Scholar]
- Crozier, A. , Del Rio, D. , & Clifford, M. N. (2010). Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine, 31(6), 446–467. [DOI] [PubMed] [Google Scholar]
- Dhaouadi, K. , Belkhir, M. , Akinocho, I. , Raboudi, F. , Pamies, D. , Barrajón, E. , Estevan, C. , & Fattouch, S. (2014). Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT‐Food Science and Technology, 57(1), 1–8. [Google Scholar]
- Diaz, C. S. (1997). Antioxidants and atherosclerotic heart disease. (U.S. Patent No. 5,624,500). U.S. Patent and Trademark Office.
- Durazzo, A. , Turfani, V. , Narducci, V. , Azzini, E. , Maiani, G. , & Carcea, M. (2014). Nutritional characterisation and bioactive components of commercial carobs flours. Food Chemistry, 153, 109–113. [DOI] [PubMed] [Google Scholar]
- El Hajaji, H. , Lachkar, N. , Alaoui, K. , Cherrah, Y. , Farah, A. , Ennabili, A. , & Lachkar, M. (2011). Antioxidant activity, phytochemical screening, and total phenolic content of extracts from three genders of carob tree barks growing in Morocco. Arabian Journal of Chemistry, 4(3), 321–324. [Google Scholar]
- El‐Haskoury, R. , Al‐Waili, N. , El‐Hilaly, J. , Al‐Waili, W. , & Lyoussi, B. (2019). Antioxidant, hypoglycemic, and hepatoprotective effect of aqueous and ethyl acetate extract of carob honey in streptozotocin‐induced diabetic rats. Veterinary World, 12(12), 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik‐Dziki, U. (2012). Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. Journal of Functional Foods, 4(4), 872–882. [Google Scholar]
- Goulas, V. , Stylos, E. , Chatziathanasiadou, M. V. , Mavromoustakos, T. , & Tzakos, A. G. (2016). Functional components of carob fruit: Linking the chemical and biological space. International Journal of Molecular Sciences, 17(11), 1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruendel, S. , Otto, B. , Garcia, A. L. , Wagner, K. , Mueller, C. , Weickert, M. O. , Heldwein, W. , & Koebnick, C. (2007). Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. British Journal of Nutrition, 98(1), 101–105. [DOI] [PubMed] [Google Scholar]
- Haber, B. (2002). Carob fiber benefits and applications. Cereal Foods World, 47(8), 365. [Google Scholar]
- Haber, B. D. (2003). Carob product based antiinflammatory or chemopreventative agent . Europe Patent EP1, 36, A3.
- Hamza, R. G. , & Al‐Seeni, M. (2015). Effect of using gamma‐irradiated mixture extract of carob and roselle in diabetic rats. International Journal of Pharma and Bio Sciences, 6(1), B951–B960. [Google Scholar]
- Hassanein, K. , Youssef, M. K. E. , Ali, H. M. , & El‐Manfaloty, M. M. (2015). The influence of carob powder on lipid profile and histopathology of some organs in rats. Comparative Clinical Pathology, 24(6), 1509–1513. [Google Scholar]
- Hoehnel, A. , Axel, C. , Bez, J. , Arendt, E. K. , & Zannini, E. (2019). Comparative analysis of plant‐based high‐protein ingredients and their impact on quality of high‐protein bread. Journal of Cereal Science, 89, 102816. [Google Scholar]
- Hsouna, A. B. , Alayed, A. S. , & Abdallah, E. M. (2012). Evaluation of antimicrobial activities of crude methanolic extract of pods of Ceratonia siliqua L. against some pathogens and spoilage bacteria. African Journal of Microbiology Research, 6(14), 3480–3484. [Google Scholar]
- Huma, Z. E. , Jayasena, V. , Nasar‐Abbas, S. M. , Imran, M. , & Khan, M. K. (2018). Process optimization of polyphenol extraction from carob (Ceratonia siliqua) kibbles using microwave‐assisted technique. Journal of Food Processing and Preservation, 42(2), e13450. [Google Scholar]
- Iipumbu, L. (2008). Compositional analysis of locally cultivated carob (Ceratonia siliqua) cultivars and development of nutritional food products for a range of market sectors . [Doctoral dissertation, Stellenbosch University].
- Iqra, Sughra, K. , Ali, A. , Afzal, F. , Yousaf, M. J. , Khalid, W. , Faizul Rasul, H. , Aziz, Z. , Aqlan, F. M. , Al‐Farga, A. , & Arshad, A. (2023). Wheat‐based gluten and its association with pathogenesis of celiac disease: A review. International Journal of Food Properties, 26(1), 511–525. [Google Scholar]
- Jamous, R. , Zaitoun, S. , Husein, A. , Qasem, I. , & Ali‐Shtayeh, M. (2015). Screening for biological activities of medicinal plants used in traditional Arabic Palestinian herbal medicine. European Journal of Medicinal Plants, 9(1), 1–13. 10.9734/EJMP/2015/17429 [DOI] [Google Scholar]
- Javadi, F. , Yazdi, M. E. T. , Baghani, M. , & Es‐haghi, A. (2019). Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Materials Research Express, 6(6), 065408. [Google Scholar]
- Johns, L. E. , & Houlston, R. S. (2001). A systematic review and meta‐analysis of familial colorectal cancer risk. The American Journal of Gastroenterology, 96(10), 2992–3003. [DOI] [PubMed] [Google Scholar]
- Klenow, S. , Glei, M. , Haber, B. , Owen, R. , & Pool‐Zobel, B. L. (2008). Carob fibre compounds modulate parameters of cell growth differently in human HT29 colon adenocarcinoma cells than in LT97 colon adenoma cells. Food and Chemical Toxicology, 46(4), 1389–1397. [DOI] [PubMed] [Google Scholar]
- Klenow, S. , Jahns, F. , Pool‐Zobel, B. L. , & Glei, M. (2009). Does an extract of carob (Ceratonia siliqua L.) have chemopreventive potential related to oxidative stress and drug metabolism in human colon cells? Journal of Agricultural and Food Chemistry, 57(7), 2999–3004. [DOI] [PubMed] [Google Scholar]
- Kotrotsios, N. , Christaki, E. , Bonos, E. , & Florou‐Paneri, P. (2012). Dietary carob pods on growth performance and meat quality of fattening pigs. Asian‐Australasian Journal of Animal Sciences, 25(6), 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , Zheng, Y. , Xu, W. , Wang, H. , & Lin, N. (2014). Rhubarb tannins extract inhibits the expression of aquaporins 2 and 3 in magnesium sulphate‐induced diarrhoea model. BioMed Research International, 2014, 619465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey, G. (2003). Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutrition Research Reviews, 16(2), 163–191. [DOI] [PubMed] [Google Scholar]
- Lu, K. , Yang, X. , Shen, J. , Robinson, B. , Huang, H. , Liu, D. , Bolan, N. , Pei, J. , & Wang, H. (2014). Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agriculture, Ecosystems & Environment, 191, 124–132. [Google Scholar]
- Macho‐González, A. , Garcimartín, A. , López‐Oliva, M. E. , Bertocco, G. , Naes, F. , Bastida, S. , Sánchez‐Muniz, F. J. , & Benedi, J. (2017). Fiber purified extracts of carob fruit decrease carbohydrate absorption. Food and Function, 8(6), 2258–2265. 10.1039/c7fo00166e [DOI] [PubMed] [Google Scholar]
- Maiti, S. , Dey, P. , Banik, A. , Sa, B. , Ray, S. , & Kaity, S. (2010). Tailoring of locust bean gum and development of hydrogel beads for controlled oral delivery of glipizide. Drug Delivery, 17(5), 288–300. [DOI] [PubMed] [Google Scholar]
- Meziani, S. , Oomah, B. D. , Zaidi, F. , Simon‐Levert, A. , Bertrand, C. , & Zaidi‐Yahiaoui, R. (2015). Antibacterial activity of carob (Ceratonia siliqua L.) extracts against phytopathogenic bacteria Pectobacterium atrosepticum . Microbial Pathogenesis, 78, 95–102. [DOI] [PubMed] [Google Scholar]
- Milek, A. , Butler, E. A. , & Bodenmann, G. (2015). The interplay of couple's shared time, women's intimacy, and intradyadic stress. Journal of Family Psychology, 29(6), 831. [DOI] [PubMed] [Google Scholar]
- Miś, A. , & Dziki, D. (2013). Extensograph curve profile model used for characterising the impact of dietary fibre on wheat dough. Journal of Cereal Science, 57(3), 471–479. [Google Scholar]
- Miyazawa, R. , Tomomasa, T. , Kaneko, H. , & Morikawa, A. (2004). Effect of locust bean gum in anti‐regurgitant milk on the regurgitation in uncomplicated gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition, 38(5), 479–483. [DOI] [PubMed] [Google Scholar]
- Mokhtari, M. , Sharifi, S. , & Shahamir, T. M. (2011). The effect of hydro alcoholic seeds extract of Ceratonia siliqua on the blood glucose and lipids concentration in diabetic male rats. In 2011 International Conference on Life Science and Technology, IPCBEE (Vol. 3). Lorestan University of Medical Sciences.
- Moreira, T. C. , da Silva, A. T. , Fagundes, C. , Ferreira, S. M. R. , Cândido, L. M. B. , Passos, M. , & Krüger, C. C. H. (2017). Elaboration of yogurt with reduced level of lactose added of carob (Ceratonia siliqua L.). LWT‐Food Science and Technology, 76, 326–329. [Google Scholar]
- Mounce, F. S. , & Al‐Saeed, M. H. (2017). Study the effect of phytoesterol of Ceratoina siliqua fruit and insulin on hematological and biochemical parameters in diabetic pregnant female rabbits induced by alloxan. Basrah Journal of Veterinary Research, 16(1), 219–242. [Google Scholar]
- Nasar‐Abbas, S. M. , E‐Huma, Z. , Vu, T. H. , Khan, M. K. , Esbenshade, H. , & Jayasena, V. (2016). Carob kibble: A bioactive‐rich food ingredient. Comprehensive Reviews in Food Science and Food Safety, 15(1), 63–72. [DOI] [PubMed] [Google Scholar]
- Nawrocka, A. , Miś, A. , & Szymańska‐Chargot, M. (2016). Characteristics of relationships between structure of gluten proteins and dough rheology–influence of dietary fibres studied by FT‐Raman spectroscopy. Food Biophysics, 11(1), 81–90. [Google Scholar]
- Nayak, B. , Liu, R. H. , & Tang, J. (2015). Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Critical Reviews in Food Science and Nutrition, 55(7), 887–918. [DOI] [PubMed] [Google Scholar]
- O'keefe, S. J. (2016). Diet, microorganisms and their metabolites, and colon cancer. Nature reviews Gastroenterology & Hepatology, 13(12), 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, N. , Macia, A. , Romero, M. P. , Trullols, E. , Morello, J. R. , Angles, N. , & Motilva, M. J. (2009). Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 57(16), 7239–7244. [DOI] [PubMed] [Google Scholar]
- Palafox‐Carlos, H. , Ayala‐Zavala, J. F. , & González‐Aguilar, G. A. (2011). The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. Journal of Food Science, 76(1), R6–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panghal, D. , Nagpal, M. , Thakur, G. S. , & Arora, S. (2014). Dissolution improvement of atorvastatin calcium using modified locust bean gum by the solid dispersion technique. Scientia Pharmaceutica, 82(1), 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaefstathiou, E. , Agapiou, A. , Giannopoulos, S. , & Kokkinofta, R. (2018). Nutritional characterization of carobs and traditional carob products. Food Science & Nutrition, 6(8), 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiannopoulos, M. , Wollseifen, H. R. , Mellenthin, A. , Haber, B. , & Galensa, R. (2004). Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC‐UV‐ESI/MSn. Journal of Agricultural and Food Chemistry, 52(12), 3784–3791. [DOI] [PubMed] [Google Scholar]
- Pernet, C. A. , & Ribi Forclaz, A. (2019). Revisiting the Food and Agriculture Organization (FAO): international histories of agriculture, nutrition, and development. The International History Review, 41(2), 345–350. [Google Scholar]
- Rahmoun, N. M. , Ziane, H. , & Boucherit‐Otmani, Z. (2014). Antibacterial and antifungal screening of four medicinal plants. Journal of Coastal Life Medicine, 2(12), 975–979. [Google Scholar]
- Ramón‐Laca, L. , & Mabberley, D. J. (2004). The ecological status of the carob‐tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Botanical Journal of the Linnean Society, 144(4), 431–436. [Google Scholar]
- Rasool, I. F. U. , Aziz, A. , Khalid, W. , Koraqi, H. , Siddiqui, S. A. , Al‐Farga, A. , & Ali, A. (2023). Industrial application and health prospective of fig (Ficus carica) by‐products. Molecules, 28(3), 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico, D. , Martín‐Diana, A. B. , Martínez‐Villaluenga, C. , Aguirre, L. , Silván, J. M. , Dueñas, M. , De Luis, D. A. , & Lasa, A. (2019). In vitro approach for evaluation of carob by‐products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon, 5(1), e01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, V. , Tomaselli, F. , Gentile, A. , La Malfa, S. , & Maccarone, E. (2004). Rheological properties and sugar composition of locust bean gum from different carob varieties (Ceratonia siliqua L.). Journal of Agricultural and Food Chemistry, 52(26), 7925–7930. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Solana, R. , Coelho, N. , Santos‐Rufo, A. , Gonçalves, S. , Pérez‐Santín, E. , & Romano, A. (2019). The influence of in vitro gastrointestinal digestion on the chemical composition and antioxidant and enzyme inhibitory capacities of carob liqueurs obtained with different elaboration techniques. Antioxidants, 8(11), 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas‐Argudo, C. , Del Río, M. A. , & Pérez‐Gago, M. B. (2009). Development and optimization of locust bean gum (LBG)‐based edible coatings for postharvest storage of ‘Fortune’ mandarins. Postharvest Biology and Technology, 52(2), 227–234. [Google Scholar]
- Rosa, C. S. , Tessele, K. , Prestes, R. C. , Silveira, M. , & Franco, F. (2015). Effect of substituting of cocoa powder for carob flour in cakes made with soy and banana flours. International Food Research Journal, 22(5), 2111–2118. [Google Scholar]
- Roseiro, L. B. , Duarte, L. C. , Oliveira, D. L. , Roque, R. , Bernardo‐Gil, M. G. , Martins, A. I. , Sepúlveda, C. , Almeida, J. , Meireles, M. , Gírio, F. M. , & Rauter, A. P. (2013). Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Industrial Crops and Products, 47, 132–138. [Google Scholar]
- Rtibi, K. , Jabri, M. A. , Selmi, S. , Souli, A. , Sebai, H. , El‐Benna, J. , Amri, M. , & Marzouki, L. (2015). Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS‐scavenging activity. RSC Advances, 5(102), 84207–84215. [Google Scholar]
- Rtibi, K. , Selmi, S. , Grami, D. , Amri, M. , Eto, B. , El‐Benna, J. , Sebai, H. , & Marzouki, L. (2017). Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomedicine & Pharmacotherapy, 93, 522–528. 10.1002/jsfa.8091 [DOI] [PubMed] [Google Scholar]
- Rtibi, K. , Selmi, S. , Jabri, M. A. , Mamadou, G. , Limas‐Nzouzi, N. , Sebai, H. , El‐Benna, J. , Marzouki, L. , Eto, B. , & Amri, M. (2016). Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Advances, 6(50), 44345–44353. [Google Scholar]
- Salem, E. M. , & Fahad, A. O. (2012). Substituting of cacao by carob pod powder in milk chocolate manufacturing. Australian Journal of Basic and Applied Sciences, 6(3), 572–578. [Google Scholar]
- Sanchez‐Segado, S. , Salar‐García, M. J. , Ortiz‐Martínez, V. M. , de los Ríos, A. P. , Hernández‐Fernández, F. J. , & Lozano‐Blanco, L. J. (2019). Evaluation of ionic liquids as in situ extraction agents during the alcoholic fermentation of carob pod extracts. Fermentation, 5(4), 90. [Google Scholar]
- Serafini, M. , Peluso, I. , & Raguzzini, A. (2010). Flavonoids as anti‐inflammatory agents. Proceedings of the Nutrition Society, 69(3), 273–278. [DOI] [PubMed] [Google Scholar]
- Sigge, G. O. , Lipumbu, L. , & Britz, T. J. (2011). Proximate composition of carob cultivars growing in South Africa. South African Journal of Plant and Soil, 28(1), 17–22. [Google Scholar]
- Singh, G. , Arora, S. , Sharma, G. S. , Sindhu, J. S. , Kansal, V. K. , & Sangwan, R. B. (2007). Heat stability and calcium bioavailability of calcium‐fortified milk. LWT‐Food Science and Technology, 40(4), 625–631. [Google Scholar]
- Son, D. W. , Lee, J. W. , Lee, P. J. , & Bae, K. H. (2010). Glycemic index of Insu 100® herbal preparation containing Korean red ginseng, carob, mulberry, and banaba. Journal of Ginseng Research, 34(2), 89–92. [Google Scholar]
- Srour, N. , Daroub, H. , Toufeili, I. , & Olabi, A. (2016). Developing a carob‐based milk beverage using different varieties of carob pods and two roasting treatments and assessing their effect on quality characteristics. Journal of the Science of Food and Agriculture, 96(9), 3047–3057. 10.1002/jsfa.7476 [DOI] [PubMed] [Google Scholar]
- Tagliazucchi, D. , Helal, A. , Verzelloni, E. , & Conte, A. (2012). The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. Journal of Agricultural and Food Chemistry, 60(44), 11056–11064. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi, D. , Verzelloni, E. , Bertolini, D. , & Conte, A. (2010). In vitro bio‐accessibility and antioxidant activity of grape polyphenols. Food Chemistry, 120(2), 599–606. [Google Scholar]
- Tagnamas, Z. , Bahammou, Y. , Kouhila, M. , Lamharrar, A. , & Idlimam, A. (2018). Thin layer solar drying of Moroccan carob pulp (Ceratonia siliqua L.). In IOP conference series: Earth and environmental science (161, 1, 012007). IOP Publishing. [Google Scholar]
- Talibi, I. , Askarne, L. , Boubaker, H. , Boudyach, E. H. , Msanda, F. , Saadi, B. , & Aoumar, A. A. B. (2012). Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Protection, 35, 41–46. 10.1111/j.1472-765X.2012.03273.x [DOI] [Google Scholar]
- Tetik, N. , & Yüksel, E. (2014). Ultrasound‐assisted extraction of d‐pinitol from carob pods using response surface methodology. Ultrasonics Sonochemistry, 21(2), 860–865. [DOI] [PubMed] [Google Scholar]
- Toydemir, G. , Capanoglu, E. , Kamiloglu, S. , Boyacioglu, D. , De Vos, R. C. , Hall, R. D. , & Beekwilder, J. (2013). Changes in sour cherry (Prunus cerasus L.) antioxidants during nectar processing and in vitro gastrointestinal digestion. Journal of Functional Foods, 5(3), 1402–1413. [Google Scholar]
- Tsatsaragkou, K. , Yiannopoulos, S. , Kontogiorgi, A. , Poulli, E. , Krokida, M. , & Mandala, I. (2012). Mathematical approach of structural and textural properties of gluten free bread enriched with carob flour. Journal of Cereal Science, 56(3), 603–609. 10.1016/j.jcs.2012.07.007 [DOI] [Google Scholar]
- Turhan, I. (2014). Relationship between sugar profile and D‐pinitol content of pods of wild and cultivated types of carob bean (Ceratonia siliqua L.). International Journal of Food Properties, 17(2), 363–370. [Google Scholar]
- Valero‐Muñoz, M. , Martín‐Fernández, B. , Ballesteros, S. , Lahera, V. , & de las Heras, N. (2014). Carob pod insoluble fiber exerts anti‐atherosclerotic effects in rabbits through sirtuin‐1 and peroxisome proliferator‐activated receptor‐γ coactivator‐1α. The Journal of Nutrition, 144(9), 1378–1384. [DOI] [PubMed] [Google Scholar]
- Vandenplas, Y. , Leluyer, B. , Cazaubiel, M. , Housez, B. , & Bocquet, A. (2013). Double‐blind comparative trial with 2 antiregurgitation formulae. Journal of Pediatric Gastroenterology and Nutrition, 57(3), 389–393. [DOI] [PubMed] [Google Scholar]
- Wong, K. C. , Law, M. C. , Wong, M. S. , & Chan, T. H. (2014). Development of a UPLC–MS/MS bioanalytical method for the pharmacokinetic study of (−)‐epiafzelechin, a flavan‐3‐ol with osteoprotective activity, in C57BL/6J mice. Journal of Chromatography B, 967, 162–167. [DOI] [PubMed] [Google Scholar]
- Wursch, P. (1991). The physiological and nutritional importance of dietary fibre. (U.S. Patent No. 5,043,160). U.S. Patent and Trademark Office. [DOI] [PubMed]
- Xie, J. , Wang, Z. , Cui, H. , Nie, H. , Zhang, T. , Gao, X. , & Qiao, Y. (2020). Effects of enzymatic hydrolysate of locust bean gum on digestibility, intestinal morphology and microflora of broilers. Journal of Animal Physiology and Animal Nutrition, 104(1), 230–236. [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y. , Sogawa, I. , Nishina, A. , Saeki, S. , Ichikawa, N. , & Iibata, S. (2000). Improved hypolipidemic effects of xanthan gum‐galactomannan mixtures in rats. Bioscience, Biotechnology, and Biochemistry, 64(10), 2165–2171. [DOI] [PubMed] [Google Scholar]
- Zhu, B. J. , Zayed, M. Z. , Zhu, H. X. , Zhao, J. , & Li, S. P. (2019). Functional polysaccharides of carob fruit: A review. Chinese Medicine, 14(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used during the current study are available from the corresponding author.


