Abstract
Aronia melanocarpa (Michx.) Ell. is a rich source of anthocyanins and proanthocyanidins with confirmed health benefits. Individual cyanidin glucosides (cyanidin 3‐galactoside, cyanidin 3‐arabinoside, cyanidin 3‐xyloside, and cyanidin 3‐glucoside) of anthocyanins (calculated by individual cyanin glycoside fractions was 419.9 mg/100 g FW) were isolated by Sephadex LH‐20 column and different parts of proanthocyanidins with a different mean degree of polymerization (mDP) were fractionated by the solubility differences in different solvents. The composition of different mDP of proanthocyanidins was as follows: monomers (1.51%), oligomer (mDP of 4.2 ± 0.9, 20.57%), CPP‐50 (mDP of 78.9 ± 4.1, 22.17%), CPP‐60 (mDP of 66.1 ± 1.2, 27.94%), CPP‐70 (mDP of 36.8 ± 3.9, 36.8%), CPP‐75 (mDP of 25.2 ± 1.3, 6.14%), CPP‐L (mDP of 10.2 ± 2.6, 6.95%), and there were recycling loss of 0.34%. Cyanidin 3‐glucoside showed the strongest inhibition effects on α‐amylase and lipase and cyanidin 3‐arabinoside showed the strongest inhibition effect on α‐glucosidase, while cyanidin 3‐xyloside has no inhibition effect on the α‐amylase, and cyanidin 3‐galactoside, cyanidin 3‐arabinoside, and cyanidin 3‐xyloside have no inhibition effects on lipase. The inhibition effect of proanthocyanidins with different mDP to the enzymes all showed high negative correlations between the mDP and IC50 (half‐maximal inhibitory concentration). This study suggests that A. melanocarpa (Michx.) Ell. can have beneficial effects due to inhibition of the digestion enzyme.
Keywords: anthocyanins, Aronia melanocarpa (Michx.) Ell., lipase, proanthocyanidins, α‐amylase, α‐glucosidase
The content, composition and enzyme inhibition activity of anthocyanin and proan‐thocyanidins of A. melanocarpa (Michx.) Ell. from China were similar to those in the United States and Europe. Anthocyanin and proanthocyanidins inhibited the activity of digestion enzyme, which is one of the important physiological functions of A. melanocarpa (Michx.) Ell.
1. INTRODUCTION
With the increase in people's income and living standard, consumers put forward higher requirements for the health, nutrition, safety, and function of food, resulting in the continuous growth of market demand for healthy food with biologically active compounds. It has received wide attention that berries with high content of bioactive substances such as different kinds of polyphenols (anthocyanins and proanthocyanidins), organic acid, and polysaccharides are associated with a lower risk of arteriosclerosis, anti‐diabetic effects, obesity (Gironés‐Vilaplana et al., 2014), etc.
Among the functional berries, greatly increased research interests have been put on A. melanocarpa (Michx.) Ell. with a high content of polyphenols (including anthocyanins, flavonols, flavanols, proanthocyanidins, phenolic acids, etc.) (Sidor et al., 2019) and possess one of the highest antioxidant activities among plant species (Denev et al., 2012). Anthocyanins are mainly composed of cyanidin 3‐glucoside, 3‐galactoside, 3‐xyloside, and 3‐arabinoside, which are the main source of black color (Veberic et al., 2015). Flavonols present in A. melanocarpa (Michx.) Ell. belong to a diverse group of compounds, which mainly consist of quercetin derivatives (quercetin‐3‐glucoside, 3‐galactoside, 3‐rutinoside, 3‐robinobioside, and 3‐vicianoside), isorhamnetin 3‐galactoside, 3‐glucoside, 3‐neohesperidoside, 3‐rutinoside, myricetin and kaempherol 3‐galactoside, and 3‐glucoside. Proanthocyanidin of A. melanocarpa (Michx.) Ell. is mainly composed of (−)‐epicatechin and trace amounts of (+)‐catechin with different mean degree of polymerization (mDP). A. melanocarpa (Michx.) Ell. also contain phenolic acids, among which dominating are chlorogenic and neochlorogenic acids (Sidor et al., 2019). These bioactive compounds contributing to the antioxidative potential and alleviation of the related diseases were confirmed in vitro, in vivo, and clinically. They were reported to have the effects of regulating the expression of genes critical for intestinal cholesterol flux in Caco‐2 cells, antiatherogenic, cardioprotective, gastroprotective, antioxidant, anti‐inflammation, anti‐aging, anti‐cancer, etc. (Bräunlich et al., 2013; Sosnowska et al., 2018). Thus, generous consumption of A. melanocarpa (Michx.) Ell. is recommended in dietary guidelines worldwide (Vázquez‐Espinosa et al., 2019). However, due to the bitterness or astringency taste, they are not usually consumed as fresh fruits, but processed as jams, jellies, fruit syrups, juice, energy drinks (Jurikova et al., 2017), or concentrated extracts (Jurgoński et al., 2008).
Extraction is the crucial step for isolation, identification, and use of phenolic compounds. However, there is no general extraction method, and different methods will be used according to different raw materials. Pretreatment may be a good choice to release the polyphenol compounds, such as fermentation, enzyme lysis, grinding, and freeze‐thawing. The most commonly used techniques for the isolation of phenolic compounds are solvent extraction (with or without assistance of microwave and ultrasound) and supercritical fluid extraction. This method has the advantages of being a simple process, low cost, and high purity. Different adsorbents were tested, such as Amberlite XAD7HP absorption and acidified ethanol elution (Galván D'Alessandro et al., 2013), Sep‐Pak C‐18 cartridges absorption, 1% acetic acid acidified water (Jing et al., 2008) or methanol (Sosnowska et al., 2018) elution for anthocyanin, and Sephadex LH‐20 for the purification of anthocyanins (Bräunlich et al., 2013) and proanthocyanidins (Fan et al., 2007) in order to remove the concomitant substances such as sugars, amino acids, and proteins from anthocyanin and/or proanthocyanidin‐containing extracts. For the fractionation of different mDP of proanthocyanidins, a rapid method based on liquid/liquid extraction and relative solubility of these compounds in different solvents (water, ethyl acetate, methanol, and chloroform) was established (Saucier et al., 2001).
The prevalence of diabetes and obesity is alarmingly increasing in the last few decades, leading to many serious public health concerns worldwide. α‐Glucosidase, α‐amylase, and lipase are the key enzymes that affect the digestion and absorption of major carbohydrates and lipids in diet (Türkan et al., 2020). Thus, inhibiting α‐glucosidase, α‐amylase, and pancreatic lipase would prevent the breakdown of carbohydrates and triglyceride and delay the absorption of glucose and fatty acids into the systemic circulation and adipocytes (Rajan et al., 2020). However, the conventional α‐glucosidase, α‐amylase, and lipase inhibitor available in clinics are all chemicals (side effects often occurred), and identifying safe clinical alternatives from plants to inhibit these enzymes have been considered a significant advancement (Rajan et al., 2020).
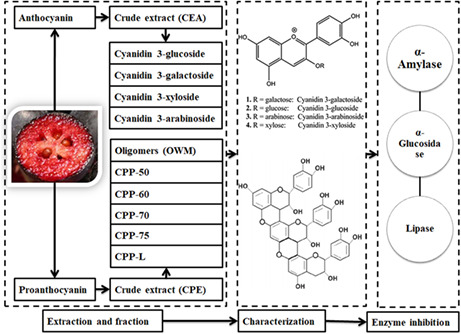
Reports indicated that phenolic in Aronia can inactivate α‐amylase, α‐glucosidase, and lipase through non‐specific binding to enzymes (Bräunlich et al., 2013). However, crude polyphenol was studied and the individual effects of pure chemicals have not been considered, which will be important for the studying of the mechanism of the inhibition. Therefore, the individual cyanidin glycosides of anthocyanins and proanthocyanidins with different mDP were fractionated and the enzyme inhibition effects were studied (Figure 1). This study will be useful to elucidate the inhibition effects of polyphenol from A. melanocarpa (Michx.) Ell. for obesity and diabetes and helpful for product development.
FIGURE 1.
Flow chart of extraction, fractionation, characterization, and enzyme inhibition.
2. MATERIALS AND METHODS
2.1. Chemicals and materials
All the chemicals used for analyses were of analytical grade and high‐performance liquid chromatography (HPLC) chemicals were of HPLC purity and were all purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Lipase from human porcine pancreas (EC 3.1.1.3), α‐amylase from human pancreas (EC 3.2.1.1), and α‐glucosidase from Bacillus stearothermophilus (EC 3.2.1.20) were obtained from Sigma‐Aldrich, Inc (Shanghai, China). Standards of cyanidin 3‐galactoside, cyanidin 3‐arabinoside, cyanidin 3‐xyloside, cyanidin 3‐glucoside, catechin, and epicatechin were obtained from Sigma‐Aldrich, Inc. (Shanghai, China). Amberlite XAD7HP, Sephadex LH‐20, and ENVI 18 DSK SPE were obtained from Sigma‐Aldrich, Inc (Shanghai, China).
Aronia melanocarpa (Michx.) Ell. were manually harvested at technological maturity by Liaoning Fukangyuan Black Chokeberry Technology Co., Ltd. (September 18, 2018) and was freeze stored until use.
2.2. Isolation of anthocyanins from A. melanocarpa (Michx.) Ell.
The pre‐grounded slurry of A. melanocarpa (Michx.) Ell. (4 kg) was subjected to n‐hexane (6:1, v/m) to remove lipophilic substances, after which was divided into two parts (part 1 for anthocyanins isolation and the other part for proanthocyanidins extraction) as pre‐treated slurry. Then, the slurry was extracted twice by ethanol solution (60% ethanol with 0.1% HCl, v/v) for 2 h at ambient temperature bubbled with nitrogen, and was centrifuged at 9090 g for 6 min and the supernatant was collected and combined. Then, the ethanol was removed from the supernatant and the retained solution was concentrated through rotatory evaporation under vacuum at 40°C to give the crude extract of anthocyanins (CEA). The CEA was subjected to a bed of Amberlite XAD‐7HP (5 × 50 cm column) until the eluate has no more anthocyanins detected by pH differential method (Jing et al., 2008), followed by elution with methanol (0.1% HCl) to give the anthocyanin‐enriched extract (AEE). The AEE was then purified by a Sephadex LH‐20 column (5 × 150 cm) by a step gradient of 15% and 30% methanol (0.1% HCl v/v), the fractionation steps follow Bräunlich et al. (2013), and was detected with HPLC at 520 and 280 nm for purity. Then, the individual of the cyanidin glycosides (cyanidin 3‐galactoside, cyanidin 3‐arabinoside, cyanidin 3‐xyloside, and cyanidin 3‐glucoside) were obtained.
2.3. Fractionation of proanthocyanidins from A. melanocarpa (Michx.) Ell.
2.3.1. Crude proanthocyanidins extract
The pre‐treated slurry of A. melanocarpa (Michx.) Ell. was extracted two times successively with 7 L of acetone:water (70:30, v/v) bubbled with nitrogen with mechanical agitation for 6 h. All the slurries were centrifuged at 8000 rpm for 6 min and the supernatants were combined and evaporated at 40°C using a vacuum rotary evaporator to remove acetone. And the aqueous solution was freeze‐dried to give the crude proanthocyanidins extract (CPE) powder.
2.3.2. Isolation of crude oligomer fraction (COF) and crude polymerized proanthocyanidins (CPP)from CPE
The CPE powder was dissolved in redistilled water to 5 g/L followed by ethyl acetate extraction two times with 1:1 (v/v) of the organic and aqueous phase. The ethyl acetate extract was evaporated at 40°C using a vacuum rotary evaporator to dry for crude oligomer fraction from CPE (COF). And the CPP was retained in the aqueous followed by freeze drying for the powder of CPP.
2.3.3. Removal of monomer from COF
The COF powder was dissolved to 150 mL by redistilled water. Then solid‐phase extraction (SPE) with column (ENVI 18, Supelco) was conducted by applying the dissolved COF on each column. The monomers were then removed by eluting diethyl ether until no monomers (epicatechin) were detected in the eluent by HPLC (Saito et al., 2006). Then, the remained oligomers were eluted with methanol until no more oligomers were detected by Folin–Ciocalteu method (Tan et al., 2017). Then, the methanol was evaporated, the residue was dissolved in minimum water, and freeze‐dried to obtain oligomers without monomers (OWM).
2.3.4. Fractionation of the polymer with different mDP from CPP
CPP powder was prepared by freeze drying the aqueous solution, followed by dissolving it with methanol. The same volume of chloroform (1:1, v/v) was added to the CPP–methanol solution. Then, the precipitate was harvested by filtration and recovered by methanol washing. Then, this methanol solution was evaporated and the residue was dissolved in water and freeze‐dried as a fraction of CPP fractionated by 50% chloroform (CPP‐50). The retained filtrate was repeatedly precipitated by adding more chloroform successively to the volume ratio of 1.5:1, 2.33:1, and 3:1 (v/v, chloroform:methanol), and the corresponding precipitate was harvested by filtration, washed in methanol, dissolved in water, and freeze‐dried to make the fraction of CPP‐60, CPP‐70, and CPP‐75. And the last filtrate was also evaporated, redissolved in water, and freeze‐dried to make CPP‐L fraction. All these samples were kept at −80°C until further studies (Saucier et al., 2001; Sosnowska et al., 2018).
2.4. Thiolysis of partially purified proanthocyanidins
Different kinds of partially purified A. melanocarpa (Michx.) Ell. proanthocyanidins (10 mg) were dissolved in 1.0 mL of 95% ethanol to prepare a 10 mg/mL proanthocyanidins solution. The thiolysis method was carried out according to Gao et al. (2018).
2.5. Determination of total phenolic contents
The total phenolic content was measured by the Folin–Ciocalteu method after adjustments (Vázquez‐Espinosa et al., 2019) with 60% ethanol (with 0.1% HCl) A. melanocarpa (Michx.) Ell. extracts. Gallic acid was used as a calibration standard, and results were expressed as gallic acid equivalents (GAE) per 100 g of fresh weight (FW) (mg of GAE/100 g FW).
2.6. Determination of total anthocyanins
Total anthocyanin contents of A. melanocarpa (Michx.) Ell. extracts were measured using the pH differential method (Lee et al., 2005) with 60% ethanol (with 0.1% HCl). Results are expressed as milligrams of cyanidin 3‐glucoside equivalents per 100 g of fresh weight (mg of cyanidin 3‐glucoside/100 g FW).
2.7. Determination of total proanthocyanidins
Proanthocyanidins were depolymerized into anthocyanidins by use of an n‐BuOH–HCl–ferric ammonium sulfate mixture and HPLC was used to determine the content as stated in Skupien and Oszmianski (2007). (−) Epicatechin was used as a calibration standard and results were expressed as mg (−)epicatechin equivalents (EE) per 100 g of FW (mg of EE/100 g FW).
2.8. HPLC and mass spectra determination of the cyanidin glycosides, proanthocyanidins, and thiolytic products
An Agilent 1260 Infinity HPLC (Agilent Technologies, Santa Clara, CA, USA) was used for the purity check of the cyanidin glycosides, (−)epicatechin, catechin, and thiolysis products by an Eclipse XDB‐C8 (4.6 × 150 mm, 5 μm) column (Agilent Technologies, Santa Clara, CA, USA) according to the method of Bräunlich et al. (2013) with a diode array detector (DAD). Anthocyanin standard stock solutions were prepared in methanol containing 0.1% formic acid and were used to identify the compounds. Wavelengths of (−)epicatechin at 280 nm and anthocyanin glycosides at 520 nm were determined. The mass spectra of thiolytic products were acquired in positive mode using electrospray ionization on an Agilent 6220 ESI‐TOF mass spectrometer. The mDP was calculated by the following formula (Ci et al., 2018):
| (1) |
2.9. Enzyme inhibition essays
2.9.1. α‐Amylase inhibition studies
α‐Amylase inhibitory activity was determined using the method from Tan et al. (2017), with a slight modification as Table 1 indicates. One hundred microliters of different concentrations of the fractionated compounds and 100 μL of the enzyme solution (5 mg/L) were mixed in a centrifuge tube at ambient temperature for 10 min. After adding 200 μL, 0.5% soluble starch with phosphate solution as a buffer (25 mmol/L, pH = 6.8), the tube was incubated at 37°C for 5 min. Then, 1 mL of DNS color reagent solution (96 mM 3,5‐dinitrosalicylic acid and 5.31 M sodium potassium tartrate in 2 M NaOH) was added into the tube. The tube was placed into a boiling water bath for 5 min to inactivate the enzyme. Then, the solution was diluted by adding 3 mL of distilled water. Then, 200 μL of the mixture was taken and added to a 96‐well plate and the absorption at 540 nm was determined. To eliminate the background absorbance produced by the fractionated compounds, an appropriate extract control without enzyme was included. α‐Amylase inhibitory activity was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (mg/mL) (Tan et al., 2017). The α‐amylase inhibitory activity was expressed as function 2 and the As, Ab, At, and Ac indicated the absorbance in different solutions as shown in Table 1. The inhibitory activity was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values.
| (2) |
TABLE 1.
The mixture solution for α‐amylase inhibitory activity determination.
| Treatments | Fractionated compounds | Starch solution | α‐Amylase | DNS solution | A540nm |
|---|---|---|---|---|---|
| Samples | √ | √ | √ | √ | As |
| Enzyme blank | √ | √ | X | √ | Ab |
| Sample blank | B | √ | √ | √ | At |
| Blank | B | √ | X | √ | Ac |
Note: √ Indicates that included in the reaction mixture; X indicates that not included in the reaction mixture; and B indicates only buffer.
2.9.2. α‐Glucosidase inhibition studies
α‐Glucosidase inhibitory activity was determined according to Gong et al. (2020) and Tan et al. (2017) with modifications (Table 2). Ten μL of each fractionated compound with appropriate concentration were mixed with 20 μL of 2.4 mM 4‐nitrophenyl‐β‐D‐glucuronide (pNPG) solution (dissolved in 0.1 M, pH 6.8 phosphate buffer), and 10 μL of 3 U/mL enzyme solution was added into a 96‐well plate to start the reaction at 37°C for 10 min. Then, 40 μL of Na2CO3 (1 mol/L) was added to the reaction mixture to terminate the reaction followed by determining the absorbance at 405 nm by a microtiter plate reader. The percentage of inhibition was calculated using Equation 3. α‐Glucosidase inhibitory activity was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (Gong et al., 2020; Tan et al., 2017).
| (3) |
TABLE 2.
The mixture solution for α‐ glucosidase inhibitory activity determination.
| Treatments | Fractionated compounds | pNPG solution | α‐Glucosidase | A540nm |
|---|---|---|---|---|
| Samples | √ | √ | √ | As |
| Enzyme blank | √ | √ | X | Ab |
| Sample blank | B | √ | √ | At |
| Blank | B | √ | X | Ac |
Note: √ Indicates that included in the reaction mixture; X indicates that not included in the reaction mixture; and B indicates only buffer.
2.9.3. Lipase inhibition assay
The lipase inhibitory activity was determined according to the method described by Tan et al. (2017) with modifications (Table 3). Three hundred and fifty μL phosphate buffer (0.05 M, pH 7.6), 150 μL of porcine lipase enzyme solution (50 mg/mL), and 50 μL of fractionated compounds were added in a centrifuge tube (1.5 mL size) and incubated at 37°C for exactly 10 min. Then, 450 μL pNP laurate substrate was added to the reaction mixture and blended, followed by incubation at 37°C for 120 min without light. The reaction mixture was centrifuged for 10 min at 8000 g and the supernatant of 200 μL was taken into a 96‐well plate to determine the absorbance at 405 nm. The percentage inhibition was calculated using Equation 4. Lipase inhibitory activity was measured at five different concentrations, and a logarithmic regression curve was established to calculate IC50 values (Tan et al., 2017).
| (4) |
TABLE 3.
The mixture solution with different compositions for lipase inhibitory activity determination.
| Treatments | Fractionated compounds | pNPP solution | Lipase | A405nm |
|---|---|---|---|---|
| Samples | √ | √ | √ | As |
| Enzyme blank | √ | √ | X | Ab |
| Sample blank | B | √ | √ | At |
| Blank | B | √ | X | Ac |
Note: √ Indicates that included in the reaction mixture; X indicates that not included in the reaction mixture; and B indicates only buffer.
2.10. Statistics
All measurements were performed in triplicate and the results were presented as mean ± standard deviation (SD). The data and figures were processed with Origin 9.0 and Adobe Photoshop CC 2018. And SPSS 26.0 was used to evaluate whether the data of mDP and IC50 of different enzymes conform to normal distribution.
3. RESULTS AND DISCUSSION
3.1. Characterization of the A. melanocarpa (Michx.) Ell. Fruits
The contents of total polyphenol, proanthocyanidins, and anthocyanin of A. melanocarpa (Michx.) Ell. are indicated in Table 4 and were also compared with several studies. It indicates that A. melanocarpa (Michx.) Ell. also is a good source of phenol, anthocyanin, and proanthocyanidins planted in China. However, different sources of the Aronia fruits showed different contents of these compounds, which probably depend on variety, cultivation conditions, and harvest date (Kokotkiewicz et al., 2010). Proanthocyanidins accounted for 42.20%, while anthocyanins contributed only 17.28% of the total phenolic content for A. melanocarpa (Michx.) Ell.
TABLE 4.
Content of total polyphenol, proanthocyanidins, and anthocyanin of A. melanocarpa (Michx.) Ell.
| Total polyphenol (mg of GAE/100 g FW) | Total proanthocyanidins (mg of EE/100 g FW) | Total anthocyanin (mg of cyanidin 3‐glucoside/100 g FW) | References | |
|---|---|---|---|---|
| A. melanocarpa (Michx.) Ell. | 3414.1 ± 12.2 | 1440.7 ± 15.1 | 590.0 ± 13.6 | This study |
| A. prunifolia | 2996.0 ± 172 | 4790.0 | 497.0 ± 20 | Wangensteen et al. (2014) |
| A. melanocarpa | 2556.0 | – | 429.0 | Zheng and Wang (2003) |
| A. melanocarpa | 2010.0 | 663.7 | 1480.0 | Wu et al. (2004) |
3.2. Extraction, fractionation, and characterization of the phenolic compounds
3.2.1. Extraction, fractionation, and characterization of anthocyanins
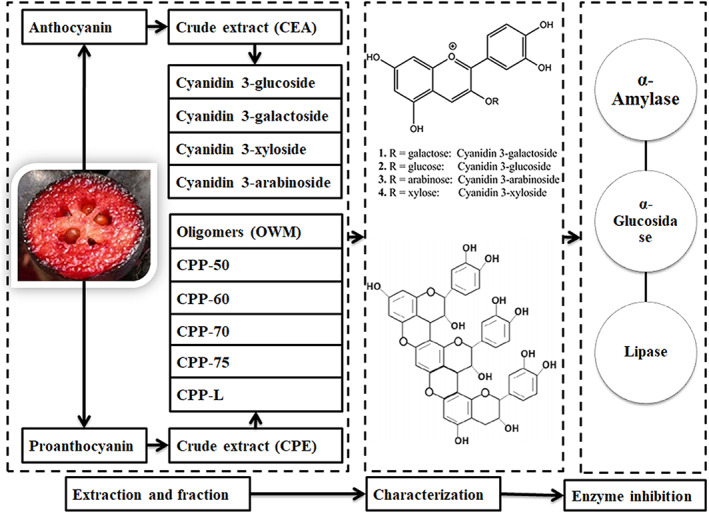
Two‐thousand‐gram A. melanocarpa (Michx.) Ell. was used to extract and purify the crude anthocyanins and its cyanidin glycosides. The results are shown in Table 5 and Figure 2. For crude extract of anthocyanins (CEA), there were not only anthocyanins but also water‐soluble compounds such as protein, sugar, and vitamin. Thus, more compounds were obtained at this stage. Followed by the absorbance of anthocyanins using Amberlite XAD7HP, most of the water‐soluble compounds have been washed off and methanol was used to get the high‐purity anthocyanin complex. There were mainly four cyanidin glycosides found in A. melanocarpa (Michx.) Ell. such as cyanidin 3‐galactoside, cyanidin 3‐arabinoside, cyanidin 3‐xyloside, and cyanidin 3‐glucoside, which were purified and showed the same migration time compared with the standard individual anthocyanins (Figure 2). Their chromatographic spectrum was in agreement with previous studies (Bräunlich et al., 2013). Unlike other berries, A. melanocarpa (Michx.) Ell. anthocyanin is very simple, consisting almost exclusively of the cyanidin glycoside as shown in our results in Table 5. However, Wu et al. (2004) found that there were minor amounts of pelargonidin‐3‐arabinoside and traces of pelargonidin‐3‐galactoside in A. melanocarpa (Michx.) Ell. (Wu et al., 2004).
TABLE 5.
Extracts and fractions of anthocyanins from A. melanocarpa (Michx.) Ell.
| Samples | Yields (mg) | Contents (mg/100 g FW) |
|---|---|---|
| Crude extract of anthocyanins (CEA) | 67543.0 ± 505 | 3377.2 |
| Anthocyanin‐enriched extract (AEE) | 9442.3 ± 30 | 472.1 |
| Cyanidin 3‐galactoside | 5705.0 ± 23 | 285.3 |
| Cyanidin 3‐arabinoside | 2091.5 ± 11 | 104.6 |
| Cyanidin 3‐xyloside | 507.1 ± 5 | 25.4 |
| Cyanidin 3‐glucoside | 92.5 ± 2 | 4.6 |
| Total anthocyanins content calculated by individual cyanin glycoside fractions | – | 419.9 |
FIGURE 2.
HPLC profile of different individual anthocyanins. Profile a–d are the solutions fractionated by a Sephadex LH‐20 column. (a) for cyanidin 3‐xyloside, (b) for cyanidin 3‐arabinoside, (c) for cyanidin 3‐glucoside, and (d) for cyanidin 3‐galactoside, (e) is the standard individual anthocyanins as the reference compounds to identify the different fractions, I for cyanidin 3‐galactoside, II for cyanidin 3‐glucoside, III for cyanidin 3‐arabinoside, and IV for cyanidin 3‐xyloside.
Cyanidin 3‐galactoside was the highest content individual anthocyanin with 60.42% content in AEE, followed by cyanidin 3‐arabinoside with 22.15% content, cyanidin 3‐xyloside with 5.37% content, and cyanidin 3‐glucoside with 0.98% content. Also, there still was an 11.08% loss of AEE which was ascribed to the impurity substances in AEE, and elute and recycle loss during the fractionation process. Cyanidin 3‐galactoside and cyanidin 3‐arabinoside are the predominant representatives of 82.57%, although this was lower than Oszmiański and Wojdylo (2005) reported with a cumulative content of >90% in the berries (Oszmiański & Wojdylo, 2005).
The total anthocyanins content calculated by individual cyanin glycoside fractions was 419.9 mg/100 g FW, which was lower than that determined by pH differentiation method as shown in Table 5. This error may have come from the recycle loss of the fractionation method, and impurities were calculated as anthocyanins during pH differentiation determination.
3.3. Extraction, fractionation, and characterization of proanthocyanidins
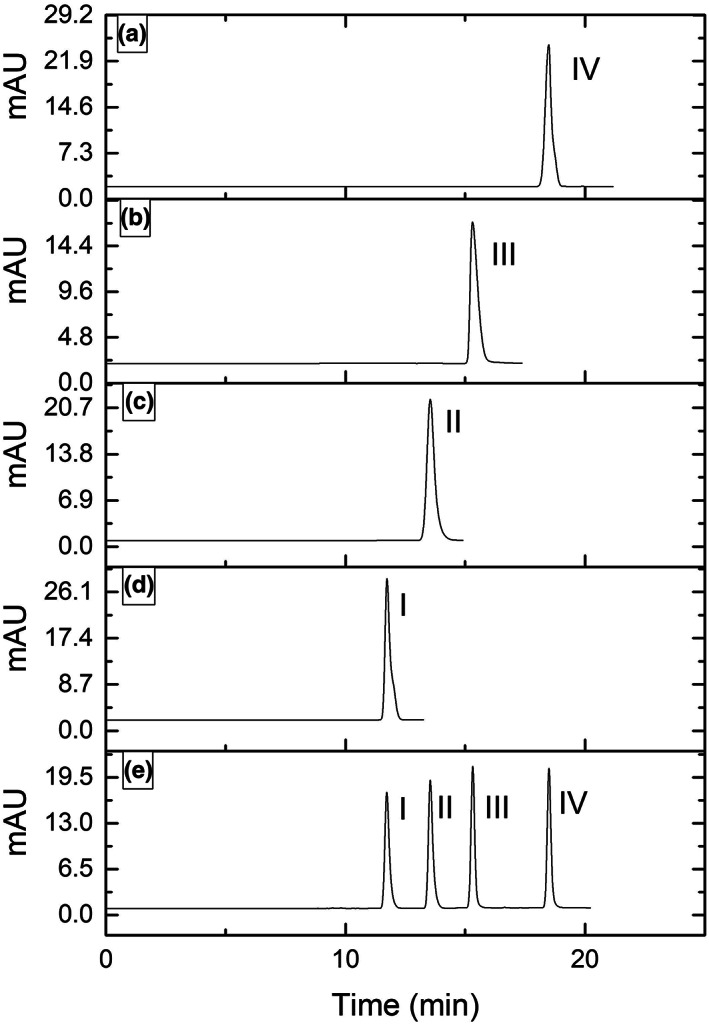
Two thousand gram of A. melanocarpa (Michx.) Ell. was used to fractionate the proanthocyanidins and found that polymeric proanthocyanidins constitute the major class of phenolics in A. melanocarpa (Michx.) Ell. Proanthocyanidins of A. melanocarpa (Michx.) Ell. are mainly composed of (−) epicatechin with trace amounts of catechin (Figure 3 and Table 6). The chain extension units and chain‐terminating units in proanthocyanidins were predominant as (−) epicatechin trace catechin (Figure 3), and the small peak 1 may be catechin. The mDP value has been calculated according to the thiolysis of proanthocyanidins as indicated in Table 7.
FIGURE 3.
HPLC analysis of thiolysis solution of PP‐50 detected at 280 nm.
TABLE 6.
MS spectrum of thiolysis solution of PP‐50.
| Peak number | RT (min) | [M‐H]−(m/z) | Compounds |
|---|---|---|---|
| 1 | 13.30 | 289 | Catechin |
| 2 | 14.91 | 289 | (−) epicatechin |
| 3 | 22.30 | 413 | heterocyclic ring cleavage products of epicatechin |
| 4 | 24.60 | 412 | Epicatechin benzyl thioether |
| 5 | 27.71 | 535 | Heterocyclic ring cleavage products of epicatechin benzyl thioether |
| 6 | 29.60 | 535 | Heterocyclic ring cleavage products of epicatechin benzyl thioether |
| 7 | 37.11 | 123 | Benzyl mercaptan |
TABLE 7.
Extracts and fractions of proanthocyanidins with different IC50 from A. melanocarpa (Michx.) Ell.
| Yield (mg) | Content (mg/100 g FW) | mDP | IC50 values (μg/mL) | |||
|---|---|---|---|---|---|---|
| α‐Amylase (Worsztynowicz et al., 2014) | α‐Glucosidase (Bräunlich et al., 2013) | Lipase | ||||
| Crude Proanthocyanidins extract (CPE) | 29678.6 ± 30.5 | 1483.9 ± 1.5 | 49.3 ± 3.8 | 1.16 ± 0.12 | 0.81 ± 0.15 | 1.79 ± 0.06 |
| Crude oligomer fraction from CPE (COF) | 6618.5 ± 36.6 | 330.9 ± 1.8 | 1.8 ± 0.5 | – | – | – |
| Monomers | 446.1 ± 26.0 | 22.3 ± 1.3 | – | – | – | – |
| Oligomers without monomers (OWM) | 6072.0 ± 22.1 | 303.6 ± 3.1 | 4.2 ± 0.9 | 4.32 ± 0.21 | 3.99 ± 0.42 | 5.03 ± 0.55 |
| Crude polymerized proanthocyanidins (CPP) | 22906.4 ± 28.9 | 1145.3 ± 1.4 | 56.9 ± 2.5 | 1.01 ± 0.06 | 0.75 ± 0.03 | 1.61 ± 0.02 |
| CPP‐50 | 6546.8 ± 24.5 | 327.3 ± 1.2 | 78.9 ± 4.1 | 0.23 ± 0.01 | 0.15 ± 0.06 | 0.31 ± 0.13 |
| CPP‐60 | 8248.0 ± 21.6 | 412.4 ± 1.1 | 66.1 ± 1.2 | 0.83 ± 0.05 | 0.72 ± 0.02 | 1.57 ± 0.06 |
| CPP‐70 | 3956.0 ± 30.1 | 197.8 ± 1.5 | 36.8 ± 3.9 | 1.39 ± 0.02 | 0.98 ± 0.03 | 2.05 ± 0.20 |
| CPP‐75 | 1814.2 ± 27.3 | 90.7 ± 1.4 | 25.2 ± 1.3 | 2.46 ± 0.05 | 1.41 ± 0.04 | 3.03 ± 0.11 |
| CPP‐L | 2052.1 ± 24.4 | 102.6 ± 1.2 | 10.2 ± 2.6 | 3.01 ± 0.01 | 2.56 ± 0.05 | 4.56 ± 0.31 |
Abbreviation: – not determined.
The crude proanthocyanidins extract (CPE) was fractionated into six parts with different mDP. The mDP of the CPE was 49.3 ± 3.8, almost as reported by Skupien and Oszmianski (2007). After fractionation of the CPE, the proanthocyanidins were nicely fractionated with different mDP from 4.2 to 78.9, which will be ready for the studying of the enzyme inhibition with different mDP of proanthocyanidins as indicated in Table 7. The sum of OWM and CPP contents were the total proanthocyanidins, which was a little bit high than Table 4 indicated which was caused by different determination methods.
The mDP fractions of A. melanocarpa (Michx.) Ell. ranged from 1.8 ± 0.5 of COF to 78.9 ± 4.1 of CPP‐50 and that of the whole A. melanocarpa (Michx.) Ell. was 49.3 ± 3.8. The mDP of proanthocyanidins from A. melanocarpa (Michx.) Ell. was 12–70 in most of the reports and also has reported an exceptionally high mDP > 100 (Skupien & Oszmianski, 2007), differences may be caused by the different processing, storage, cultivation, determination methods, etc. Labarbe et al. (1999) also found that the proanthocyanidins from the seed and skin of grape also can be fractionated with different mDP, ranging increasingly from 4.7 to 17.4 in seed (8.1 for total extract) and from 9.3 to 73.8 in skin (34.9 for total extract). The monomer content of proanthocyanidins was the highest compared with several reports with content of 5.89 mg ECE/100 g FW (Dudonné et al., 2015), 5.17 mg/100 g FW (Wu et al., 2004), 0.01–0.02 μg CE/g DM (Taheri et al., 2013).
The composition of different mDP of proanthocyanidins was as follows: monomers (1.51%), oligomer (mDP of 4.2 ± 0.9, 20.57%), CPP‐50 (mDP of 78.9 ± 4.1, 22.17%), CPP‐60 (mDP of 66.1 ± 1.2, 27.94%), CPP‐70 (mDP of 36.8 ± 3.9, 36.8%), CPP‐75 (mDP of 25.2 ± 1.3, 6.14%), CPP‐L (mDP of 10.2 ± 2.6, 6.95%), and there were recycling loss of 0.34%. Wu et al. (2004) reported that the proanthocyanidins composition in Aronia is as follows: monomers (0.78%), dimers (1.88%), trimers (1.55%), 4–6‐mers (6.07%), 7–10‐mers (7.96%), and >10‐mers (81.72%) (Wu et al., 2004). This confirmed the conclusion that there was much more tannin in A. melanocarpa (Michx.) Ell., although there were 22.08% of monomer and oligomer higher than Wu et al. (2004) studies and 77.58% of mDP > 10 proanthocyanidins which are lower than that of other studies (Wu et al., 2004).
Proanthocyanidins' tendency to link with proteins is responsible for the astringency of A. melanocarpa (Michx.) Ell. (Tomar et al., 2022). Also, their pharmaceutical effects are interesting because they act beneficially on the circulatory system and are efficient free radical scavengers (Rigaud et al., 1993). Several studies found that proanthocyanidins with high mDP are more potent antioxidants than simple phenolics, and the increasing mDP may enhance the antioxidant power and lipase inhibition of proanthocyanidins (condensed tannins) (Sosnowska et al., 2016), which will influence its organoleptic or pharmacological properties. However, the high mDP feature should limit their absorption through the gut barrier; oligomers larger than trimers are unlikely to be absorbed in the small intestine in their native forms (Denev et al., 2012).
These studies established a rapid method to fractionate different mDP proanthocyanidins by different solvents only, which avoid the poor resolution and irreversible adsorption during chromatographic separations of the highly polymerized fraction (Labarbe et al., 1999).
3.4. Inhibition of the enzyme
The effects of crude extractions of anthocyanins and proanthocyanidins, the individual cyanin glycoside and proanthocyanidins with different mDP on the α‐amylase, α‐glucosidase and lipase are studied to identify the fractions of anthocyanins and proanthocyanidins that are responsible for the anti‐diabetes and obesity functions in A. melanocarpa (Michx.) Ell., The results were showed in Figure 4 and Table 7.
FIGURE 4.
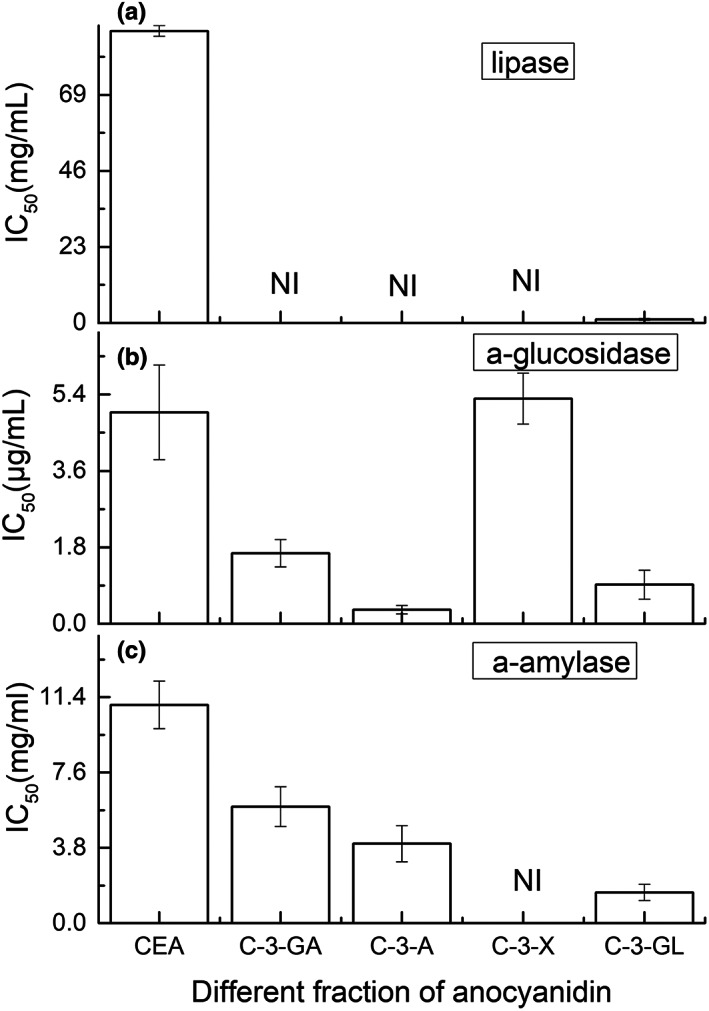
IC50 of enzyme inhibition effects by different individual cyanidin glycosides. NI indicates no inhibition.
Among the individual cyanidin glycoside and crude anthocyanins extracts, cyanidin 3‐glucoside showed the strongest inhibition effects on α‐amylase and lipase and cyanidin 3‐arabinoside showed the strongest inhibition effect on α‐glucosidase, while cyanidin 3‐xyloside has no effect for the inhibition of α‐amylase. And cyanidin 3‐galactoside, cyanidin 3‐arabinoside, and cyanidin 3‐xyloside have no inhibition effects for the lipase. Cyanidin 3‐arabinoside showed the strongest inhibition effect on the three enzymes of the anthocyanins studied. These results showed the same tendency as indicated in Table 8. Considering the data obtained from our investigations (Figure 4) and the reports in Table 8, cyanidin glycosides may be one of the reasons for the diabetes treatment effect of A. melanocarpa (Michx.) Ell. (Chrubasik et al., 2010), and studies suggested that intake of cyanidin and glycosides‐enriched plant foods together with acarbose may lead to the development of a novel combined therapy in type 2 diabetic patients (Akkarachiyasit et al., 2010).
TABLE 8.
IC50 values of individual cyanidin glycosides in different references.
| Individual cyanidin glycoside/mDP | IC50 values | ||
|---|---|---|---|
| α‐Amylase | α‐Glucosidase | Lipase | |
| Cyanidin 3‐galactoside |
>1.00 mM (Akkarachiyasit et al., 2010) 5.16 ± 0.06 mg/mL (Worsztynowicz et al., 2014) |
224.5 ± 22.45 μg/mL for sucrose (Adisakwattana et al., 2009) 1.54 ± 0.1 μg/mL (Bräunlich et al., 2013) |
nf (Worsztynowicz et al., 2014) |
| Cyanidin 3‐arabinoside | 3.98 ± 0.10 mg/mL (Worsztynowicz et al., 2014) | 0.37 ± 0.08 μg/mL (Bräunlich et al., 2013) | nf (Worsztynowicz et al., 2014) |
| Cyanidin 3‐xyloside | nf (Worsztynowicz et al., 2014) | 5.5 ± 1.6 μg/mL (Bräunlich et al., 2013) | nf (Worsztynowicz et al., 2014) |
| Cyanidin 3‐glucoside |
134.7 ± 4.49 μg/mL (Akkarachiyasit et al., 2010) 1.74 ± 0.04 mg/mL (Worsztynowicz et al., 2014) |
435.53 ± 22.45 μg/mL for sucrose (Akkarachiyasit et al., 2010); 0.87 ± 0.2 μg/mL (Bräunlich et al., 2013) |
84.54 ± 2.94 μg/mL (Vijayaraj et al., 2019) 1.17 ± 0.04 mg/mL (Worsztynowicz et al., 2014) |
| Proanthocyanidins with different mDP (Data shown as mDP‐IC50) |
3.3‐(0.075 ± 0.003) mg/mL (Fu et al., 2015) (11.8 ± 0.1)‐1.7 μg/mL (Kato et al., 2017) 32.6–2.9 μg/mL (Kato, 2019) 9.0–4.2 μg/mL (Kato, 2019) (4–8)‐38 μg/mL (Kato, 2019) (7.54 ± 0.22)–(1022.84 ± 31.50) μg/mL (Li et al., 2015) 11.69 ± (0.23–541.26) ± 20.30 μg/mL (Li et al., 2015) |
(3.2–14.0)–(1–0.015) μg/mL (Hsu et al., 2018) (7.54 ± 0.22)‐(4.08 ± 0.56) μg/mL (Li et al., 2015) (11.69 ± 0.23)‐(3.12 ± 0.79) μg/mL (Li et al., 2015) (7.3 ± 0.1)‐(0.037 ± 0.001) mg/mL (Wang et al., 2019) |
3.8‐(3.88 ± 0.35) μg/mL (Ci et al., 2018) 9.6‐(1.84 ± 0.46) μg/mL (Ci et al., 2018) |
| One more study for mDP and IC50 with figure is Zhou et al. (2018) | |||
Abbreviation: nf, not found.
This was the first study to investigate all the individual cyanidins and glycoside interactions on the enzyme inhibition effects from A. melanocarpa (Michx.) Ell. It indicated that the structural difference between glycoside at the 3‐O‐position of cyanidin was an important factor for modulating the inhibition of the three enzymes. The glycosides (glucose and galactose, arabinose, and xylose) of the individual anthocyanins have the same formulas but different structural formulas with different positions of the hydroxyl (‐OH) group on C‐4 for cyanidin 3‐galactoside and cyanidin 3‐glucoside and c‐3 for cyanidin 3‐arabinoside and cyanidin 3‐xyloside as indicated in Figure 5 with arrows, which suggests that the structural difference in the sugar at the 3‐O‐position may be an important factor for modulating the inhibition of the enzyme studied (Akkarachiyasit et al., 2010). Also, the B‐ring increases the hydrophobic characteristics of the compounds, thus enhancing higher affinity toward the enzyme (Vijayaraj et al., 2019). However, it is still difficult to tell the reasons why these other three anthocyanins have no inhibition effects on lipase, and more studies such as the interaction between the enzyme and anthocyanins molecules are needed.
FIGURE 5.
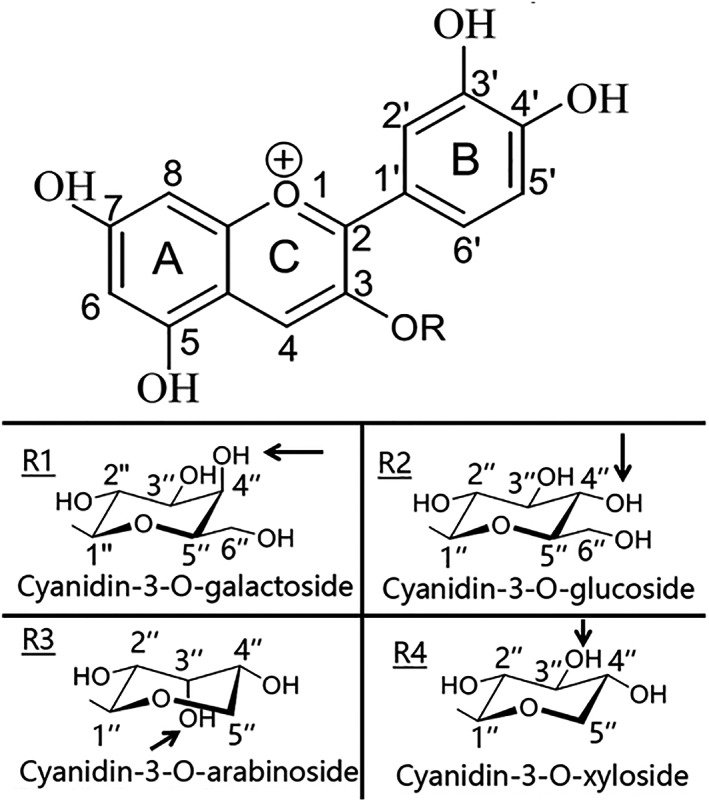
Different molecule structures of cyanidin glycosides.
Table 8 also indicated the inhibition effects of the proanthocyanidins with different mDP, which are normally distributed. According to the results, inhibition of α‐glucosidase is more specific than inhibition of lipase and α‐amylase. And PP‐50 showed the strongest inhibition effects on the three enzymes. However, the effects of the three enzymes showed high negative correlations between the mDP and IC50 (Figure 6). Thus, it indicated that the enzyme inhibition effect of proanthocyanidins from A. melanocarpa (Michx.) Ell. was mDP correlated and the presence of epicatechin as the extension unit may play an important role (Kato et al., 2017). The main inhibitory mechanism of proanthocyanidins on the enzyme studied may be due to the insertion of proanthocyanidins into the pocket of the enzyme altering the catalytic configuration of the active site in a manner, thus reducing substrate‐binding affinity (Wei et al., 2017). All the proanthocyanidins fractions with different mDP showed strongest inhibition effects than anthocyanins individuals on α‐amylase. And CPP‐50 showed stronger α‐glucosidase and lipase inhibition than that of all the anthocyanins. This may be ascribed to the most effective protein‐precipitating effect of large molecules of proanthocyanidins (Hofmann et al., 2006), which is of particular importance, as bioavailability is not needed for proanthocyanidins of any size to exert activity by inhibiting the digestion of lipids or carbohydrates in the gastrointestinal lumen (Neilson et al., 2016).
FIGURE 6.
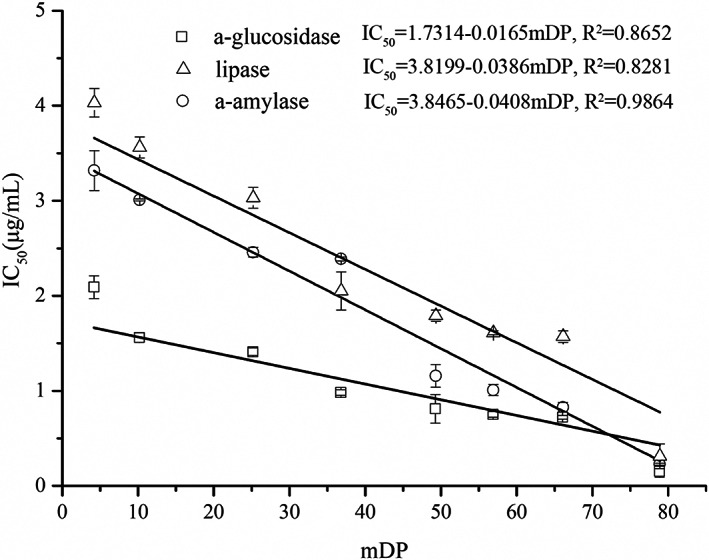
Correlation of the mDP and IC50 of proanthocyanidins.
4. CONCLUSIONS
A. melanocarpa (Michx.) Ell. has been transplanted to China for about 12 years, and it has only been allowed to be sold as food in China for not more than 4 years. Therefore, the functional components and functions of the fruit planted in China still need further study. The results showed that the content, composition, and enzyme inhibition activity of anthocyanin and proanthocyanidins of A. melanocarpa (Michx.) Ell. from China were similar to those in the United States and Europe. Anthocyanin and proanthocyanidins inhibited the activity of the digestion enzyme, which is one of the important physiological functions of A. melanocarpa (Michx.) Ell. However, the interaction and mechanism between anthocyanin and proanthocyanidins and enzymes need to be further studied for the development of functional foods.
AUTHOR CONTRIBUTIONS
Limei Chen: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Wuxi Chen: Data curation (equal); methodology (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Demao Li: Formal analysis (equal); funding acquisition (equal); investigation (equal); software (equal); writing – review and editing (equal). Xiumin Liu: Conceptualization (equal); funding acquisition (equal); project administration (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
This research was funded by the National Key R&D Program of the Ministry of Science and Technology of China (2018YFA0902200).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
ACKNOWLEDGMENTS
Many thanks to Mr. Shifu Chen and Liaoning Fukangyuan Black Chokeberry Technology Co., Ltd. for their support with Aronia melanocarpa (Michx.) Ell.
Chen, L. , Chen, W. , Li, D. , & Liu, X. (2023). Anthocyanin and proanthocyanidin from Aronia melanocarpa (Michx.) Ell.: Purification, fractionation, and enzyme inhibition. Food Science & Nutrition, 11, 3911–3922. 10.1002/fsn3.3377
Limei Chen and Wuxi Chen contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adisakwattana, S. , Charoenlertkul, P. , & Yibchok‐anun, S. (2009). α‐Glucosidase inhibitory activity of cyanidin‐3‐galactoside and synergistic effect with acarbose. Journal of Enzyme Inhibition and Medicinal Chemistry, 24(1), 65–69. 10.1080/14756360801906947 [DOI] [PubMed] [Google Scholar]
- Akkarachiyasit, S. , Charoenlertkul, P. , Yibchok‐anun, S. , & Adisakwattana, S. (2010). Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α‐glucosidase and pancreatic α‐amylase. International Journal of Molecular Sciences, 11(9), 3387–3396. 10.3390/ijms11093387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräunlich, M. , Slimestad, R. , Wangensteen, H. , Brede, C. , Malterud, K. E. , & Barsett, H. (2013). Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients, 5(3), 663–678. 10.3390/nu5030663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrubasik, C. , Li, G. , & Chrubasik, S. (2010). The clinical effectiveness of chokeberry: A systematic review. Phytotherapy Research, 24(8), 1107–1114. 10.1002/ptr.3226 [DOI] [PubMed] [Google Scholar]
- Ci, Z. , Jiang, C. , Feng, S. , Wu, S. , Cui, Y. , Sasaki, Y. , & Kojima, M. (2018). Anti‐obesity effect of proanthocyanidins from the coat of scarlet runner beans on high‐fat diet‐fed mice. Journal of Food Nutrition Research, 6(2), 103–109. 10.12691/jfnr-6-2-6 [DOI] [Google Scholar]
- Denev, P. N. , Kratchanov, C. G. , Ciz, M. , Lojek, A. , & Kratchanova, M. G. (2012). Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: in vitro and in vivo evidences and possible mechanisms of action: A review. Comprehensive Reviews in Food Science and Food Safety, 11(5), 471–489. 10.1111/j.1541-4337.2012.00198.x [DOI] [Google Scholar]
- Dudonné, S. , Dubé, P. , Anhê, F. F. , Pilon, G. , Marette, A. , Lemire, M. , Harris, C. , Dewailly, E. , & Desjardins, Y. (2015). Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. Journal of Food Composition and Analysis, 44, 214–224. 10.1016/j.jfca.2015.09.003 [DOI] [Google Scholar]
- Fan, J. , Ding, X. , & Gu, W. (2007). Radical‐scavenging proanthocyanidins from sea buckthorn seed. Food Chemistry, 102(1), 168–177. 10.1016/j.foodchem.2006.05.049 [DOI] [Google Scholar]
- Fu, C. , Yang, X. , Lai, S. , Liu, C. , Huang, S. , & Yang, H. (2015). Structure, antioxidant and α‐amylase inhibitory activities of longan pericarp proanthocyanidins. Journal of Functional Foods, 14, 23–32. 10.1016/j.jff.2015.01.041 [DOI] [Google Scholar]
- Galván D'Alessandro, L. , Vauchel, P. , Przybylski, R. , Chataigné, G. , Nikov, I. , & Dimitrov, K. (2013). Integrated process extraction–adsorption for selective recovery of antioxidant phenolics from Aronia melanocarpa berries. Separation and Purification Technology, 120, 92–101. 10.1016/j.seppur.2013.09.027 [DOI] [Google Scholar]
- Gao, C. , Cunningham, D. G. , Liu, H. , Khoo, C. , & Gu, L. (2018). Development of a thiolysis HPLC method for the analysis of procyanidins in cranberry products. Journal of Agricultural and Food Chemistry, 66(9), 2159–2167. 10.1021/acs.jafc.7b04625 [DOI] [PubMed] [Google Scholar]
- Gironés‐Vilaplana, A. , Baenas, N. , Villaño, D. , Speisky, H. , García‐Viguera, C. , & Moreno, D. A. (2014). Evaluation of Latin‐American fruits rich in phytochemicals with biological effects. Journal of Functional Foods, 7, 599–608. 10.1016/j.jff.2013.12.025 [DOI] [Google Scholar]
- Gong, T. , Yang, X. , Bai, F. , Li, D. , Zhao, T. , Zhang, J. , Sun, L. , & Guo, Y. (2020). Young apple polyphenols as natural α‐glucosidase inhibitors: In vitro and in silico studies. Bioorganic Chemistry, 96, 103625. 10.1016/j.bioorg.2020.103625 [DOI] [PubMed] [Google Scholar]
- Hofmann, T. , Glabasnia, A. , Schwarz, B. , Wisman, K. N. , Gangwer, K. A. , & Hagerman, A. E. (2006). Protein binding and astringent taste of a polymeric procyanidin, 1,2,3,4,6‐Penta‐O‐galloyl‐β‐d‐glucopyranose, castalagin, and grandinin. Journal of Agricultural and Food Chemistry, 54(25), 9503–9509. 10.1021/jf062272c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C. Y. , Lin, G. M. , Lin, H. Y. , & Chang, S. T. (2018). Characteristics of proanthocyanidins in leaves of Chamaecyparis obtusa var. formosana as strong α‐glucosidase inhibitors. Journal of the Science of Food and Agriculture, 98(10), 3806–3814. 10.1002/jsfa.8894 [DOI] [PubMed] [Google Scholar]
- Jing, P. , Bomser, J. A. , Schwartz, S. J. , He, J. , Magnuson, B. A. , & Giusti, M. M. (2008). Structure−function relationships of anthocyanins from various anthocyanin‐rich extracts on the inhibition of colon cancer cell growth. Journal of Agricultural and Food Chemistry, 56(20), 9391–9398. 10.1021/jf8005917 [DOI] [PubMed] [Google Scholar]
- Jurgoński, A. , Juśkiewicz, J. , & Zduńczyk, Z. (2008). Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods for Human Nutrition, 63(4), 176–182. 10.1007/s11130-008-0087-7 [DOI] [PubMed] [Google Scholar]
- Jurikova, T. , Mlcek, J. , Skrovankova, S. , Sumczynski, D. , Sochor, J. , Hlavacova, I. , Snopek, L. , & Orsavova, J. (2017). Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules, 22(6), 944. 10.3390/molecules22060944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, E. (2019). Bioactive compounds in plant materials for the prevention of diabetesand obesity. Bioscience, Biotechnology, and Biochemistry, 83(6), 975–985. 10.1080/09168451.2019.1580560 [DOI] [PubMed] [Google Scholar]
- Kato, E. , Kushibiki, N. , Inagaki, Y. , Kurokawa, M. , & Kawabata, J. (2017). Astilbe thunbergii reduces postprandial hyperglycemia in a type 2 diabetes rat model via pancreatic alpha‐amylase inhibition by highly condensed procyanidins. Bioscience, Biotechnology, and Biochemistry, 81(9), 1699–1705. 10.1080/09168451.2017.1353403 [DOI] [PubMed] [Google Scholar]
- Kokotkiewicz, A. , Jaremicz, Z. , & Luczkiewicz, M. (2010). Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. Journal of Medicinal Food, 13(2), 255–269. 10.1089/jmf.2009.0062 [DOI] [PubMed] [Google Scholar]
- Labarbe, B. , Cheynier, V. , Brossaud, F. , Souquet, J.‐M. , & Moutounet, M. (1999). Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. Journal of Agricultural and Food Chemistry, 47(7), 2719–2723. 10.1021/jf990029q [DOI] [PubMed] [Google Scholar]
- Lee, J. , Durst, R. , & Wrolstad, R. (2005). AOAC official method 2005.02: Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method. In Horwitz W. (Ed.), Official methods of analysis of AOAC international (18th ed.). AOAC. [PubMed] [Google Scholar]
- Li, Q. , Chen, J. , Li, T. , Liu, C. , Zhai, Y. , McClements, D. J. , & Liu, J. (2015). Separation and characterization of polyphenolics from underutilized byproducts of fruit production (Choerospondias axillaris peels): Inhibitory activity of proanthocyanidins against glycolysis enzymes. Food & Function, 6(12), 3693–3701. 10.1039/C5FO00939A [DOI] [PubMed] [Google Scholar]
- Neilson, A. P. , O'Keefe, S. F. , & Bolling, B. W. (2016). High‐molecular‐weight proanthocyanidins in foods: Overcoming analytical challenges in pursuit of novel dietary bioactive components. Annual Review of Food Science Technology, 7, 43–64. 10.1146/annurev-food-022814-015604 [DOI] [PubMed] [Google Scholar]
- Oszmiański, J. , & Wojdylo, A. (2005). Aronia melanocarpa phenolics and their antioxidant activity. European Food Research and Technology, 221(6), 809–813. 10.1007/s00217-005-0002-5 [DOI] [Google Scholar]
- Rajan, L. , Palaniswamy, D. , & Mohankumar, S. K. (2020). Targeting obesity with plant‐derived pancreatic lipase inhibitors: A comprehensive review. Pharmacological Research, 104681, 1–34. 10.1016/j.phrs.2020.104681 [DOI] [PubMed] [Google Scholar]
- Rigaud, J. , Escribano‐Bailon, M. , Prieur, C. , Souquet, J.‐M. , & Cheynier, V. (1993). Normal‐phase high‐performance liquid chromatographic separation of procyanidins from cacao beans and grape seeds. Journal of Chromatography A, 654(2), 255–260. 10.1016/0021-9673(93)83368-3 [DOI] [Google Scholar]
- Saito, S. T. , Welzel, A. , Suyenaga, E. S. , & Bueno, F. (2006). A method for fast determination of epigallocatechin gallate (EGCG), epicatechin (EC), catechin (C) and caffeine (CAF) in green tea using HPLC. Food Science and Technology, 26, 394–400. 10.1590/S0101-20612006000200023 [DOI] [Google Scholar]
- Saucier, C. , Mirabel, M. , Daviaud, F. , Longieras, A. , & Glories, Y. (2001). Rapid fractionation of grape seed proanthocyanidins. Journal of Agricultural and Food Chemistry, 49(12), 5732–5735. 10.1021/jf010784f [DOI] [PubMed] [Google Scholar]
- Sidor, A. , Drożdżyńska, A. , & Gramza‐Michałowska, A. (2019). Black chokeberry (Aronia melanocarpa) and its products as potential health‐promoting factors – an overview. Trends in Food Science & Technology, 89, 45–60. 10.1016/j.tifs.2019.05.006 [DOI] [Google Scholar]
- Skupien, K. , & Oszmianski, J. (2007). The effect of mineral fertilization on nutritive value and biological activity of chokeberry fruit. Agricultural and Food Science, 16(1), 46–55. 10.2137/145960607781635822 [DOI] [Google Scholar]
- Sosnowska, D. , Podsędek, A. , Kucharska, A. Z. , Redzynia, M. , Opęchowska, M. , & Koziołkiewicz, M. (2016). Comparison of in vitro anti‐lipase and antioxidant activities, and composition of commercial chokeberry juices. European Food Research and Technology, 242(4), 505–515. 10.1007/s00217-015-2561-4 [DOI] [Google Scholar]
- Sosnowska, D. , Podsędek, A. , Redzynia, M. , & Kucharska, A. Z. (2018). Inhibitory effect of black chokeberry fruit polyphenols on pancreatic lipase – Searching for most active inhibitors. Journal of Functional Foods, 49, 196–204. 10.1016/j.jff.2018.08.029 [DOI] [Google Scholar]
- Taheri, R. , Connolly, B. A. , Brand, M. H. , & Bolling, B. W. (2013). Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. Journal of Agricultural and Food Chemistry, 61(36), 8581–8588. 10.1021/jf402449q [DOI] [PubMed] [Google Scholar]
- Tan, Y. , Chang, S. K. C. , & Zhang, Y. (2017). Comparison of α‐amylase, α‐glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry, 214, 259–268. 10.1016/j.foodchem.2016.06.100 [DOI] [PubMed] [Google Scholar]
- Tomar, M. , Bhardwaj, R. , Verma, R. , Singh, S. P. , Dahuja, A. , Krishnan, V. , Kansal, R. , Yadav, V. K. , Praveen, S. , & Sachdev, A. (2022). Interactome of millet‐based food matrices: A review. Food Chemistry, 385, 132636. 10.1016/j.foodchem.2022.132636 [DOI] [PubMed] [Google Scholar]
- Türkan, F. , Atalar, M. N. , Aras, A. , Gülçin, İ. , & Bursal, E. (2020). ICP‐MS and HPLC analyses, enzyme inhibition and antioxidant potential of Achillea schischkinii Sosn. Bioorganic Chemistry, 94, 103333. 10.1016/j.bioorg.2019.103333 [DOI] [PubMed] [Google Scholar]
- Vázquez‐Espinosa, M. , González‐de‐Peredo, A. V. , Espada‐Bellido, E. , Ferreiro‐González, M. , Toledo‐Domínguez, J. J. , Carrera, C. , Palma, M. , & Barbero, G. F. (2019). Ultrasound‐assisted extraction of two types of antioxidant compounds (TPC and TA) from black chokeberry (Aronia melanocarpa L.): Optimization of the individual and simultaneous extraction methods. Agronomy, 9(8), 456. 10.3390/agronomy9080456 [DOI] [Google Scholar]
- Veberic, R. , Slatnar, A. , Bizjak, J. , Stampar, F. , & Mikulic‐Petkovsek, M. (2015). Anthocyanin composition of different wild and cultivated berry species. LWT – Food Science and Technology, 60(1), 509–517. 10.1016/j.lwt.2014.08.033 [DOI] [Google Scholar]
- Vijayaraj, P. , Nakagawa, H. , & Yamaki, K. (2019). Cyanidin and cyanidin‐3‐glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. Journal of Food Biochemistry, 43(3), e12774. 10.1111/jfbc.12774 [DOI] [PubMed] [Google Scholar]
- Wang, M. , Jiang, J. , Tian, J. , Chen, S. , Ye, X. , Hu, Y. , & Chen, J. (2019). Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α‐glucosidase. Journal of Functional Foods, 56, 286–294. 10.1016/j.jff.2019.03.026 [DOI] [Google Scholar]
- Wangensteen, H. , Bräunlich, M. , Nikolic, V. , Malterud, K. E. , Slimestad, R. , & Barsett, H. (2014). Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. Journal of Functional Foods, 7, 746–752. 10.1016/j.jff.2014.02.006 [DOI] [Google Scholar]
- Wei, M. , Chai, W.‐M. , Yang, Q. , Wang, R. , & Peng, Y. (2017). Novel insights into the inhibitory effect and mechanism of proanthocyanidins from Pyracantha fortuneana fruit on α‐glucosidase. Journal of Food Science, 82(10), 2260–2268. 10.1111/1750-3841.13816 [DOI] [PubMed] [Google Scholar]
- Worsztynowicz, P. , Napierała, M. , Białas, W. , Grajek, W. , & Olkowicz, M. (2014). Pancreatic α‐amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochemistry, 49(9), 1457–1463. 10.1016/j.procbio.2014.06.002 [DOI] [Google Scholar]
- Wu, X. , Gu, L. , Prior, R. L. , & McKay, S. (2004). Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. Journal of Agricultural and Food Chemistry, 52(26), 7846–7856. 10.1021/jf0486850 [DOI] [PubMed] [Google Scholar]
- Zheng, W. , & Wang, S. Y. (2003). Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. Journal of Agricultural and Food Chemistry, 51(2), 502–509. 10.1021/jf020728u [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Zhang, L. , Li, W. , Zhang, S. , Luo, L. , Wang, J. , & Sun, B. (2018). In vitro evaluation of the anti‐digestion and antioxidant effects of grape seed procyanidins according to their degrees of polymerization. Journal of Functional Foods, 49, 85–95. 10.1016/j.jff.2018.08.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


