Highlights
-
•
Gut microbiota depletion impairs systemic HBV-specific immune responses.
-
•
Gut microbiota depletion delays HBV antigen clearance.
-
•
PPs depletion dampens gut microbiota-boosted HBV-specific t cell response in liver.
-
•
The “Gut microbiota-PPs” axis manipulates HBV antigen clearance partially.
Keywords: Hepatitis B virus, Peyer's patch, Gut microbiota, Adaptive immune response, Cellular immunity
Abstract
Background
Gut microbiota is crucial for immune homeostasis and is associated with the prognosis of chronic hepatitis B infection. Peyer's patches (PPs), characterized by intestinal mucosa localization, are involved in the gut microbiota-mediated immune response. However, whether and how PPs orchestrate gut microbiota-modulated anti-hepatitis B virus (HBV) response remain elusive. This study aims to elucidate the role of PPs in gut microbiota-mediated anti-HBV adaptive immunity.
Methods
We investigated the effects of gut microbiota and PPs on adaptive immune responses by transcriptomic, phenotypic, and functional analyzes from an HBV mouse model with gut commensal microbiota and PP-depleting interventions.
Results
Depletion of gut microbiota impaired systemic adaptive immune responses, resulting in a delayed HBV antigen clearance. Differentially expressed genes analysis of PPs revealed that pathways related to adaptive immune responses were significantly downregulated in gut microbiota-deficient mice. Notably, the depletion of PPs could abolish gut microbiota-boosted intrahepatic HBV-specific T cell response, leading to a higher serum hepatitis B surface antigen level in mice.
Conclusion
PPs orchestrate gut microbiota-mediated intrahepatic anti-HBV cellular immunity, underlining the significance of remote manipulating the “gut microbiota-PPs” axis for achieving optimum anti-HBV response.
Graphical abstract

1. Introduction
Although the availability of preventive vaccines and first-line antiviral treatment regimens, hepatitis B virus (HBV) infection remains a substantial cause of morbidity and mortality (Nguyen et al., 2020). The host immune response, especially the adaptive immune arm, is indispensable for the control and clearance of HBV infection (Bertoletti et al., 2009). As such, an in-depth insight into the host's adaptive immunity is essential for functional cure-oriented treatment strategies.
Researchers have paid considerable attention to the local anti-HBV immunity of the periphery, spleen and liver in the past decades. In contrast, little is known regarding the gut tissue, which harbors a diverse microbiota involved in host homeostasis maintenance. The gut microbiota is a highly dynamic multispecies community, serving as a mighty regulator in maintaining the integrity of the mucosal barrier and shaping mucosal immunity as well as systemic immunity (Thursby and Juge, 2017; Sender et al., 2016). It should be pointed out that the gut microbiota must be kept in an interspecies balance, whereas any perturbation of the gut microbiota, termed dysbiosis, could lead the host susceptible to metabolic disorders, autoimmunity, infections, and cancer (Clemente et al., 2012).
Recently, several studies have reported the implications of gut microbiota in fighting against HBV infection. Wu et al. have clarified the necessity of the integrity and balance of gut microbiota for HBV clearance in a mouse model (Wu et al., 2019). Interestingly, the age-dependent clearing chance of HBV infection somewhat coincides with the timing of gut microbiota establishment (Ganem and Prince, 2004; Lozupone et al., 2012; Rodríguez et al., 2015), indicating the involvement of gut microbiota in the outcome of HBV infection. Nevertheless, the underlying mechanisms whereby the gut microbiota exerts anti-HBV effects remain elusive.
As the paramount structural components of the gut-associated lymphoid tissues responsible for mucosal immunity, peyer's patches (PPs) are the competent sentinels overlaid by follicle-associated epithelium rich in microfold cells which form pockets allowing direct interaction between antigens and immune cells, and the inside lymph are drained by mesenteric lymph nodes (MLNs) to distal sites (Mowat and Agace, 2014). Exposing to a tremendous diversity of external antigens, PPs are dedicated to adaptive immune priming with constant germinal center (GC) activity and providing eminent immune defense potency at the frontline (Mörbe et al., 2021). Of note, an elegant study on autoimmune arthritis confirmed the pivotal role of PPs on particular gut microbiota-mediated systemic adaptive immune responses by initiating the differentiation and egress of follicular helper T (Tfh) to distal tissues (Teng et al., 2016). These findings prompt us to dissect the possibility of manipulating the axis of “gut microbiota-PPs-anti-HBV systemic immunity” to achieve an optimum outcome in the context of HBV infection.
Here, we found that gut microbiota deficiency impaired anti-HBV adaptive immune responses in the PPs, spleen, and liver compartments, resulting in delayed HBV clearance. Moreover, the depletion of PPs dampened gut microbiota-boosted intrahepatic HBV-specific T cell response and serum hepatitis B surface antigen (HBsAg) decline. Collectively, our findings add detailed knowledge to the gut microbiota-mediated anti-HBV systemic immune response and emphasize “gut microbiota-PPs” axis-based remote manipulation for anti-HBV immunotherapeutic strategies.
2. Methods
2.1. Mice
C57BL/6 wild-type (WT) mice were purchased from Guangdong Medical Laboratory Animal Center (Foshan, PR China). Six- to eight-week-old age-matched male mice were used in the experiments. All animal experiments were approved by the Ethical Committee for Experimental Animal Care of the 458th Hospital of the Chinese People's Liberation Army (PLA) (Guangzhou, China) or Southern Medical University (Guangzhou, China). The studies reported in this paper are in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. HBV mouse model
We used the hydrodynamic injection (HDI) to establish the HBV mouse model as previously described (Huang et al., 2006). In short, six µg of pAAV/HBV1.2 contained a 1.2-fold full-length HBV A genome was injected via mouse tail vein within 5∼8 s in a volume of physiological saline solution equivalent to 8∼10% of the body weight.
2.3. Gut commensal microbiota-depleted mouse model
Gut commensal microbiota was depleted using two well-established antibiotics (ABX) protocols, namely drinking and gavage (Rakoff-Nahoum et al., 2004; Gong et al., 2019). Briefly, for the drinking method, four ABX, neomycin sulfate (1 g/L), ampicillin (1 g/L), metronidazole (1 g/L), and vancomycin (0.5 g/L), were dissolved in sterile water, which was then supplied as drinking water for at least four weeks (Rakoff-Nahoum et al., 2004). For the gavage method, mice were gavaged with neomycin sulfate (200 mg/kg), vancomycin (100 mg/kg), metronidazole (200 mg/kg), and ampicillin (200 mg/kg) once a day for five consecutive days (Gong et al., 2019), and kept drinking ABX water until sacrifice.
2.4. PP-null mouse model
As described previously, pregnant WT breeders were intraperitoneally injected with 0.6 mg anti-interleukin (IL)-7Rα (BE0065, BioXCell) or control IgG (MF208-01, Mei5bio) on gestation day 14.5 to produce pups that were PP null (from anti-IL-7Rα-treated mothers) or PP sufficient (from IgG-treated mothers) (Teng et al., 2016).
2.5. Mononuclear cells and serum samples isolation
Lymphocytes from mice were isolated by standard density centrifugation as previously described (Li et al., 2020). Serum samples were immediately withdrawn and frozen at -20 °C until use.
2.6. Serological assays
The levels of mouse serum HBsAg and hepatitis B e antigen (HBeAg) were quantitatively determined by the Roche COBAS® 6000 analyzer (Roche Molecular Diagnostics). The concentrations of mouse serum antibodies to hepatitis B s antigen (anti-HBs), IL-21, and aspartate/alanine aminotransferase (AST/ALT) were measured by enzyme-linked immunosorbent assay kits following the manufacturer's instructions.
2.7. Cell surface and intracellular cytokine staining
Lymphocytes were stained with the Live/Dead staining (Thermo Fisher Scientific) to exclude dead cells and imcubated with mouse Fc Block (BD, Cat:553142) for 10 min at 4 °C to block the Fc receptors. Cells were subsequently stained with the indicated surface antibodies for 30 min at 4 °C. To detect the frequency of HBV-specific T cells, lymphocytes were stimulated with overlapping peptides spanning the whole HBV proteins (envelope, core, polymerase, and X; 2 µg/mL) in the presence of brefeldin A (1 µg/mL, BD Bioscience) for 6 h. For intracellular cytokine staining, cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) and stained with the corresponding antibodies (Supplementary Table 1). All samples were analyzed on a BD FACS Canto II or Aria III flow cytometer.
2.8. Enzyme-linked immunospot (ELISpot) assay
For detecting mouse total IgG, total IgA, and IgG-type anti-HBs, antibody to hepatitis B e antigen- or antibody to hepatitis B c antigen-secreting B cells, multi-Screen 96-well filtration plates (Millipore, Bedford, MA) were coated with anti-IgG (15 µg/mL), anti-IgA (15 µg/mL), recombined HBsAg (5 µg/mL), recombined HBeAg (2 µg/mL) or recombined hepatitis B core antigen (2 µg/mL) in phosphate-buffered saline at 4 °C. After overnight incubation, the plates were washed six times with phosphate-buffered saline and blocked with RPMI-1640 medium with 10% fetal bovine serum at room temperature for two h. Lymphocytes isolated from the spleen and PPs were resuspended for detection, and 200 μL of cell suspension was added into each well for 20 h incubation at 37 °C. Plates were then washed and incubated with biotin-labeled anti-IgG mAb or anti-IgA mAb (Mabtech, Sweden) and horseradish peroxidase-conjugated streptavidin (Mabtech, Nacka Strand, Sweden). Spots were counted by an Immuno-SpotS6 Ultra Analyzer (Cellular Technology, Inc., Santa Monica, CA).
2.9. Immunoflorence staining and confocal microscope
The mouse spleen tissue was fixed in 10% neutral-buffered formalin for two days. After dehydrating with graded ethanol, 4-μm sections of paraffin-embedded tissue were incubated with rabbit anti-mouse CD3 and biotinylated peptide nucleic acid overnight at 4 °C. Slides were washed in tris buffered saline and stained with secondary antibody (AF647 donkey anti-rabbit antibody, BV421 rat anti-mouse CD45R (B220), and streptavidin Dylight 549) for six h at 4 °C. Slides were then rewashed in tris-buffered saline and coverslipped with an anti-fade mounting medium (Leagene, China). Images were acquired on an LSM 980 confocal microscope (Zeiss, Germany) using ZEN software (V3.1).
2.10. RNA sequencing and data analysis
Total RNA from PPs was extracted using a Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol and subjected to library construction and deep sequencing on Illumina HiSeq™ 2500 by Gene Denovo Biotechnology Co., Ltd (Guangzhou, China). Gene expression levels were normalized based on the fragments per kilobase million reads. Fold changes were calculated for all possible comparisons, and a two-fold cutoff was used to select genes with expression changes. Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed for pathway analysis. Parallel identification of pathways significantly enriched or suppressed in PP lymphocytes after ABX usage was performed by Gene Set Enrichment Analysis (GSEA).
2.11. Statistical analysis
All data are expressed as the mean ± SEM. SPSS Statistics 26.0 (Chicago, IL) and the GraphPad Prism 8 software were used for statistical analysis. Either unpaired two-tailed Student's t-test or Mann-Whitney test was used to compare variables between the two groups. All tests were two-sided; a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Depletion of gut microbiota disturbs systemic anti-HBV humoral immune response
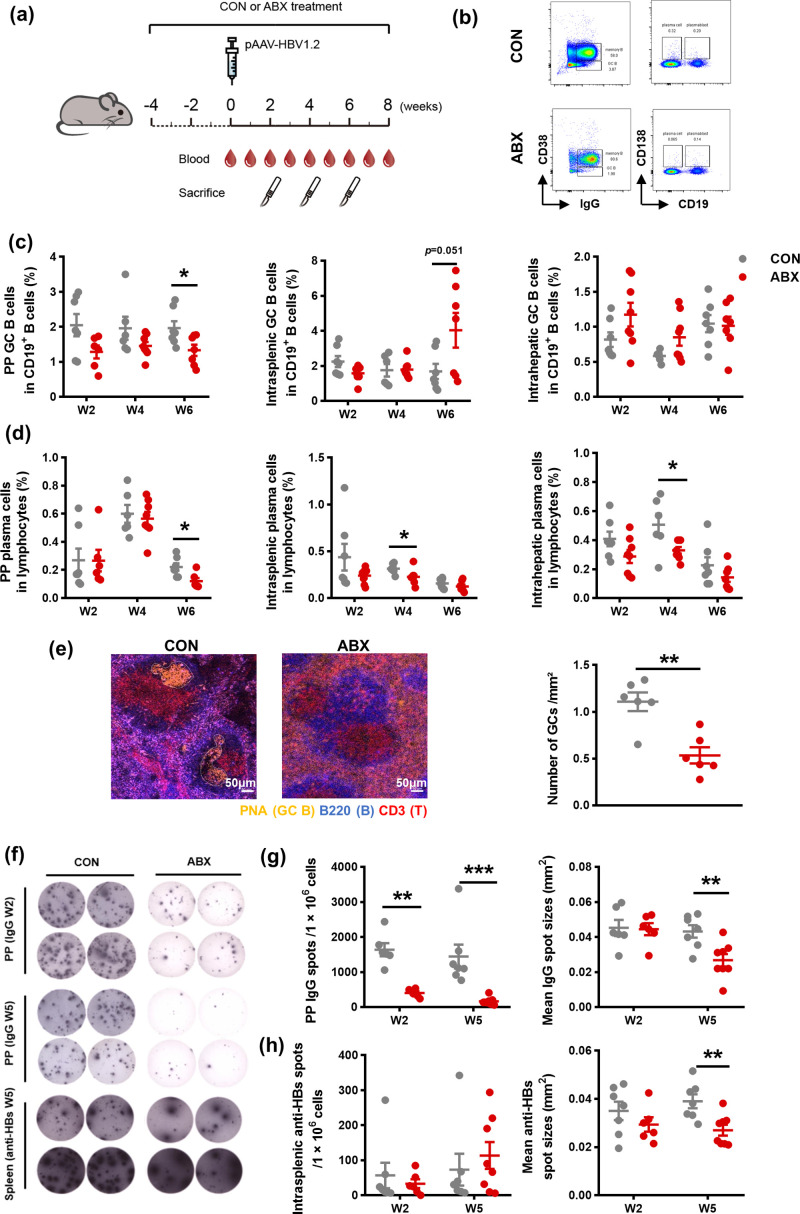
Given the appreciated role of humoral immunity on the functional cure in chronic hepatitis B (CHB) patients, we first dissected the impact of gut microbiota depletion on B cell response by treating WT mice with ABX drinking water four weeks before pAAV/HBV1.2 HDI until sacrifice (Fig. 1a). The absence of gut microbiota was confirmed by 16S analysis of fecal microbiota collected on the day before pAAV/HBV1.2 HDI (Supplementary Fig.1a). We focused on three distinct anatomic compartments, the PPs, spleen, and liver, which represented the gut location, the well-recognized secondary lymphoid tissue and the battlefield of anti-HBV immune reaction, respectively. Compared to the control counterparts, ABX treatment resulted in remarkable decreases in the frequencies of GC B cells, and plasma cells at the indicated time points, with the PP compartment most pronounced (Fig. 1b-d). We next sought morphological evidences for the ABX-induced impairment of humoral immunity. As shown in the immunofluorescent staining of spleen sections, GCs were under a disordered condition with severe atrophy in ABX-treated mice, in stark contrast to the decent formation of GC structure in control mice (Fig. 1e left). Further quantification analysis revealed the antibiotics-induced apparent reduction in the average number of GCs (Fig. 1e right and Supplementary Fig. 1b). In addition, we explored the antibody production attribute of B cells by using ELISpot assay. Since the mainstay of immunoglobulin isotype against HBV antigens are IgG antibodies (Hehle et al., 2020; Ismail et al., 2004), we mainly focused on dissecting IgG production herein. Consistently, we found that the ability of B cells to produce total IgG isotype declined significantly in PPs in ABX-treated mice, as supported by both spot counts and mean spot sizes detected at weeks two and five post HDI (Fig. 1f and g). Moreover, ABX-treated mice exhibited a significant shrinkage of mean spot sizes of intrasplenic anti-HBs (Fig. 1f and h; Supplementary Fig.1c and d). Besides, we also tested whether gut microbiota depletion changed IgA antibodies production, as IgA highly represents intestinal associated mucosa immunity modulated, and found that gut microbiota depletion also impeded IgA production in both PPs and spleen (Supplementary Fig. 1e and f). Taken together, our data unravel that the deficiency of gut microbiota extensively impedes systemic humoral immune response in the setting of HBV infection.
Fig 1.
Roles of gut microbiota in the systemic anti-HBV humoral immune response. a Schematic representation of ABX treatment and pAAV/HBV1.2 hydrodynamic injection via the tail veins of WT mice. b Representative flow plots of B cell subsets. Mononuclear cells from PPs, spleen and liver were stained with anti-mouse CD19-APC, IgM-PerCP-Cy5.5, IgG-PE, CD38-FITC, and CD138-PE-Cy7. GC B cell (CD19+IgM−IgG+CD38−) population was calculated as a percentage of CD19+ cells, and plasma cell (CD19−CD138+) population was calculated as a percentage of live lymphocytes. c-d The frequencies of the PP, intrasplenic, and intrahepatic GC B cells (c) and plasma cells (d) were detected at the indicated time points in CON (n = 6–8) or ABX (n = 6–8) mice. e Representative immunofluorescent staining of GCs in sections from spleen samples in CON and ABX mice at week five post HDI. Scale bars: 50 µm (left). The number of GCs per μm² was compared between CON (n = 6) and ABX (n = 6) mice (right). f Representative ELISpot images showing results of total IgG isotype antibodies produced by B cells in PPs and anti-HBs produced by B cells in the spleen at the indicated time points. g The number and mean spot sizes of IgG spots of PPs B lymphocytes were measured at the indicated time points (n = 6–8/group). h The number and mean spot sizes of anti-HBs spots of intrasplenic B lymphocytes were measured at the indicated time points (n = 6–8/group). Data are combined from at least two independent experiments, with each experiment containing at least 3 mice per group. *p < 0.05, ** p < 0.01, *** p < 0.001. ABX, antibiotics; anti-HBs, hepatitis B surface antibodies; CHB, chronic viral hepatitis B; CON, control; ELISpot, Enzyme-linked immunospot; GC, germinal center; HBV, hepatitis B virus; PP, peyer's patch; WT, wild type.
3.2. Depletion of gut microbiota impairs systemic anti-HBV cellular immunity
As the dominant executor of antiviral response, the detection of the cellular immune arm is considered a leading indicator reflecting the host's anti-HBV immunity. Therefore, we next investigated whether gut microbiota orchestrated the dynamics of frequency as well as the function of anti-HBV T cell response. As expected, compared with control mice, the proportion of PP Tfh cells was contracted, and corresponding downtrends were observed in the spleen and liver compartments at week two post HDI in the ABX-treated group (Fig. 2a and b). Accordingly, gut microbiota depletion resulted in a substantial decrease of serum IL-21 at week two post HDI (Fig. 2c). These collectively suggested that the aforementioned impaired B cell response may be attributed to the compromised helper function of Tfh cells, which were the major source of IL-21. Besides, the percentages of PP and intrahepatic effector CD4+ T cells were also substantially reduced at week two post HDI upon gut microbiota depletion (Fig. 2a and d). We then determined the HBV-specific T cell response by stimulating with overlapping HBV peptide pools in vitro. Intriguingly, we found that the depletion of gut microbiota led to the downregulation of intrasplenic HBV-specific IL-21-CD4+ T cells. However, no significant statistical difference was found. At the same time, barely HBV-specific IL-21-producing CD4+ T cells in the PPs were detected (Fig. 2e). Similarly, ABX treatment engendered the decrease of HBV-specific interferon (IFN)-γ-producing CD8+ T cells in the spleen and liver (Fig. 2f). In brief, our data demonstrate the indispensable role of gut microbiota on effective systemic anti-HBV cellular immunity.
Fig 2.
Roles of gut microbiota in the systemic anti-HBV cellular immune response. a Representative flow plots of T cell subsets. Mononuclear cells from PPs, spleen and liver were stained with anti-mouse CD4-BB700, CD8-BV510, CD44-APC, CD62L-PE-Cy7, and CXCR5-BV421. Tfh cell (CD4+CXCR5+) and Teff cell (CD4+CD44hiCD62L−) population were calculated as percentages of CD4+ cells. b The frequencies of PP, intrasplenic and intrahepatic Tfh cells were detected at week two post HDI in CON (n = 7) or ABX (n = 8) mice. c The serum levels of IL-21 were detected in CON or ABX mice (n = 6/group). d The frequencies of PP (left), intrasplenic (middle), and intrahepatic (right) Teff cells were detected at the indicated time points in CON (n = 6–7) or ABX (n = 6–8) mice. For HBV-specific T cell detection, mononuclear cells from PPs, spleen and liver were stained with anti-mouse CD4-BB700, CD8-BV510, CXCR5-BV421, IFN-γ-APC, and IL-21-PE. e Representative flow plots of IL-21-CD4+ T cells with or without stimulated by overlapping HBV peptide pools in vitro in CON or ABX mice (left). The frequencies of PP, intrasplenic, and intrahepatic HBV-specific IL21-CD4+ T cells were detected at week five post HDI in CON (n = 7) or ABX (n = 7) mice (right). f Representative flow plots of IFN-γ-CD8+ T cells with or without stimulated by overlapping HBV peptide pools in vitro in CON or ABX mice (left). The frequencies of PP, intrasplenic, and intrahepatic HBV-specific IFN-γ-CD8+ T cells were detected at week five post HDI in CON (n = 7) or ABX (n = 7) mice (right). Data are combined from at least two independent experiments, with each experiment containing at least 3 mice per group. *p < 0.05, *** p < 0.001. ABX, antibiotics; CON, control; HBV, hepatitis B virus; HDI, hydrodynamic injection; IFN, interferon; IL, Interleukin; PP, peyer's patch; Teff, effector T; Tfh, follicular helper T.
3.3. Depletion of gut microbiota results in delayed HBV antigen clearance
The above results prompted us to explore whether the depletion of gut microbiota that modulated anti-HBV adaptive immunity hampered the clearance of HBV. We detected the dynamics of serum HBV antigens and found that antibiotics resulted in dramatically higher levels of serum HBsAg and HBeAg (Fig. 3a and b). Moreover, impaired production of serum anti-HBs was observed in ABX-treated mice (Fig. 3c). It was not until week seven post HDI that harbored detectable serum anti-HBs in the ABX-treated mice, and the levels were significantly lower than those in the control group. However, serum ALT and AST levels exhibited no significant difference between both groups (Fig. 3d), suggesting that the non-cytolytic mechanism might implicate the gut microbiota-regulated HBV clearance.
Fig 3.
Roles of gut microbiota in HBV antigen clearance. The serum levels of HBsAg (a), HBeAg (b), anti-HBs (c), ALT (d, left), and AST (d, right) were detected in CON (n = 6) or ABX (n = 6) mice. Data are combined from at least two independent experiments, with each experiment containing at least 3 mice per group. *p < 0.05, ** p < 0.01. ABX, antibiotics; ALT, alanine aminotransaminase; anti-HBs, antibody to hepatitis B surface antigen; AST, aspartate aminotransferase; CON, control; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
3.4. Adaptive immunity-related pathways were downregulated in PPs after the depletion of gut microbiota
Our above findings revealed the dampened anti-HBV adaptive immunity of PPs in the absence of gut microbiota. To gain a comprehensive insight into the role of gut microbiota in manipulating PPs in fighting against HBV, we performed transcriptomic profile analysis of PPs under gut microbiota depletion or not in the HBV mouse model. A total of 180 differential expressed genes (DEGs) (fold change > two and p < 0.05) were detected, of which 123 transcripts were significantly downregulated and 57 transcripts were markedly upregulated in PPs of the ABX group as compared to the control group (Fig. 4a). Notably, most of these downregulated DEGs were related to the immunoglobulin-related gene family and defensin-α-related gene family, which were reported to be associated with positive regulation of immune response and antiviral activity (Holly et al., 2017) (Fig. 4b). Furthermore, both GO analysis and KEGG pathway enrichment analyses consistently revealed significant enrichment in the humoral immune response process, such as B cell receptor signaling pathway, immunoglobulin-mediated immune response, and B cell-mediated immunity in PP tissues compared to antibiotics and control treatment groups (Fig. 4c and d). Correspondingly, GSEA was also performed to conceal useful information from minor effect genes in traditional enrichment analysis by GO and KEGG. As expected, in addition to the negative enrichment of mucosal immune responses, B cell receptor signaling pathways were substantially downregulated in the ABX group (Fig. 4e and f). In particular, cellular immune responses, including T cell activation involved in immune response, Th1, Th2, and Th17 cell differentiation, and T cell receptor signaling pathway together with other pathways relevant to response to exogenous microorganisms were uniformly negative enriched in ABX-treated group compared to the control group (Fig. 4e and f). Overall, our transcriptomic analysis of PPs corroborates the extensive and profound modulation of gut microbiota on the adaptive immunity of PPs in the setting of HBV infection.
Fig 4.
Transcriptomic profile analysis of PPs with gut microbiota depletion or not in HBV mouse model (n = 3/group). a The heatmap of the DEGs of PPs from CON and ABX groups. b The radar plot of 18 selected DEGs related to immune response and defense against bacteria. c The top 20 significantly enriched biological processes from DEGs by GO analysis. GO terms were labeled by name, and red arrows highlighted interesting terms. d The top 20 significantly enriched pathways from DEGs by KEGG analysis. KEGG terms were marked by name, and red arrows highlighted interesting pathways. e Representative GSEA-GO results showing that immune-related biological processes were significantly downregulated in the ABX group. f Representative GSEA-KEGG results showing that immune-related pathways were significantly downregulated after ABX usage. ABX, antibiotics; CON, control; DEGs, differentially expressed genes; GO, Gene Ontology; GSEA, Gene Set Enrichment Analysis; HBV, hepatitis B virus; KEGG, Kyoto Encyclopedia of Genes and Genomes; PP, peyer's patch.
3.5. PPs are partially required for gut microbiota-mediated HBV-specific immune response
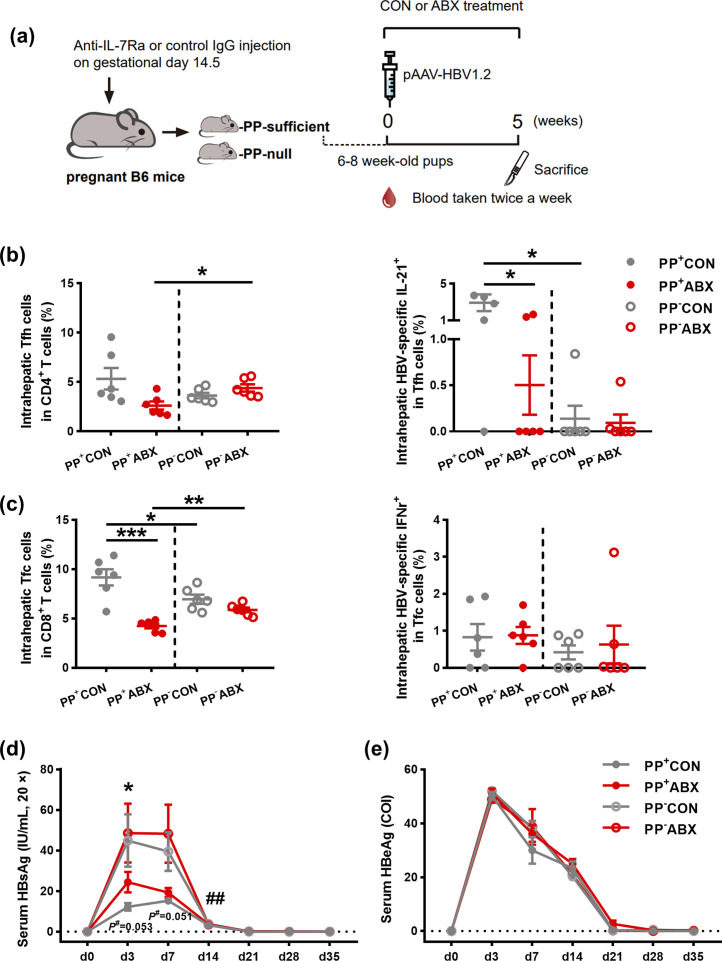
The above results demonstrated that gut microbiota homeostasis tightly controlled the anti-HBV adaptive immunity of PPs. We next sought to determine whether PPs participated in gut microbiota-orchestrated anti-HBV response in the spleen and liver. We obtained the PP-null offspring by administering anti-IL-7Rα mAb into WT pregnant mice, which was required for the embryonic formation of PPs (Yoshida et al., 1999). We confirmed the successful depletion of PPs by visually checking their existence (Fig. 5a and Supplementary Fig. 2a). Additionally, the general constitutions of immune subsets in mesenteric lymph nodes and spleen were comparable between PP-sufficient and PP-null group (Supplementary Fig. 2b-e), indicating the unaffected remaining secondary lymphoid tissues and exclusive abolition of PPs in our PP-deficient mouse model. Subsequently, we analyzed the responses of both HBV-specific cellular and humoral immunity. Intriguingly, gut microbiota-induced expansion of intrahepatic HBV-specific IL-21-Tfh cells and Tfc cells, which was associated with the control of viral infection in B cell follicles, was abolished in the setting of PP deficiency, whereas neither the frequency of intrahepatic Tfh cells nor HBV-specific IFN-γ-Tfc cells exhibited differentiated changes under PP depletion or not (Fig. 5b and c). In contrast, such effects did not present in the spleen compartment (Supplementary Fig. 3a and b). Unlike the distinct impact of PP depletion on HBV-specific cellular immune response, the anti-HBV-specific humoral immunity scarcely showed any difference in the effects of gut microbiota-meditated antiviral response in the spleen (Supplementary Fig. 3c and d). We next tested whether anti-HBV effects induced by gut microbiota were impaired under PP abrogation. As shown in Fig. 5d and e, PP depletion significantly resulted in the disappearance of gut microbiota-induced HBV clearance as compared PP-null control group with the PP-null ABX group, and higher levels of serum HBsAg at days 3, 7, and 14 post HDI were observed as compared PP-null control group with PP-sufficient control group, in contrast to the unaffected serum levels of HBeAg among groups. These findings, taken together, suggest that although the impact of gut microbiota on HBV-related immune response is not entirely dependent on PPs, PP depletion partially impeded cellular immune response in the liver and HBV clearance.
Fig 5.
Roles of PPs in gut microbiota-mediated HBV-specific immune response. a Schematic representation of the establishment of PP-depleted mice, and the subsequent treatment. Mononuclear cells from liver were stained with anti-mouse CD4-BB700, CD8-BV510, CXCR5-BV421, IFN-γ-APC, and IL-21-PE. Tfh cell (CD4+CXCR5+) and Tfc cell (CD8+CXCR5+) population were calculated as percentages of CD4+ or CD8+ cells. b The frequencies of intrahepatic Tfh cells (left) and HBV-specific IL-21-Tfh cells (right) were detected at week five post HDI in indicated mice groups. c The frequencies of intrahepatic Tfc cells (left) and HBV-specific IFN-γ-Tfc cells (right) were detected at week five post HDI in indicated mice groups. d-e The dynamic serum levels of HBsAg (d) and HBeAg (e) were detected in indicated mice groups (n = 6/group). *, PP+CON vs. PP+ABX; #, PP+CON vs. PP−CON. Data are combined from at least two independent experiments, with each experiment containing at least 3 mice per group. *p < 0.05, ** p < 0.01, *** p < 0.001. ABX, antibiotics; CON, control; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HDI, hydrodynamic injection; IFN interferon; IL, interleukin; PP, peyer's patch; Tfc, follicular cytotoxicity T; Tfh, follicular helper T.
4. Discussion
Emerging evidence has been gained regarding the orchestration of gut microbiota to both local mucosal and systemic immune responses. Notably, researchers have proposed the leading role of gut microbiota in age-related HBV immune clearance and regulating intrahepatic anti-HBV response (Wu et al., 2019; Chou et al., 2015), providing a basis for the benefits of gut microbiota-based anti-HBV immunotherapy strategies. The results herein demonstrate that gut microbiota contributes to systemic anti-HBV adaptive immune responses, facilitating the clearance of HBV, amid which PPs play an indispensable role in gut microbiota-modulated intrahepatic HBV-specific cellular immunity. Our findings highlight the therapeutic potential of remote manipulating the “gut microbiota-PPs” axis for an optimum anti-HBV response.
The highly dynamic gut microbial community mutually interacts with the host immunity, playing a prominent role in keeping individuals in a steady state. Nevertheless, the gut microbiota is not always innocuous and might become the culprit of a particular disease once the homeostasis is broken. Remarkably, HBV infection hampers the gut microbiota development and dynamically changes the specific gut bacteria ratio in the HBV mouse model, and vice versa (Guo et al., 2021; Zhu et al., 2019). The disruption of intestinal homeostasis damages gut barrier function and suppresses the antiviral cellular immune response in the liver. Here, we focus on the latter issue by exploring the influence of gut microbiota on anti-HBV adaptive immunity and attempting to address the involved pathway by using an HBV mouse model mimicking chronic HBV infection in humans. Consistently, our results showed that the depletion of gut microbiota impaired anti-HBV adaptive immunity in PPs, the spleen, and the liver to a different extent, among which the PP compartment was the most susceptible site. Notably, albeit the depletion of gut microbiota, a paradoxical expansion on the intrasplenic GC B cells at week six post HDI was found (Fig. 1c, p = 0.051). Onging fight against HBV by activating GC immune response in the ABX group with lagged viral elimination, the compensative boosting of GC reaction resulted from decreased plasma cells and disturbed transformation from GC B cells into plasma cells might account for such phenomenon. Besides, a decent research has revealed that the effect of vancomycin in certain contexts may support a more robust adaptive immune response in the spleen (Mandal et al., 2021). Nevertheless, the inner mechanism regarding how antibiotics usage modulates germinal centers remains unknown and needs further research for certain. Although previous studies have identified the necessity of gut microbiota in generating efficient anti-HBV response as mentioned above, our findings further confirmed these existent researches by simultaneously scrutinizing both HBV-specific humoral and cellular immunity and unveiling the unappreciated roles of PPs intensively engaging in the intrahepatic T cell activity. Of note, as a secondary lymphoid tissue, PPs are characterized by the uncovering of capsules typically existing in other lymph nodes, constantly encountering intestinal antigens with persistent GC reaction, the nexus between host and gut commensal (Fenton et al., 2020; Cornes, 1965). It is conceivable that in such a highly functioning and finely regulated tissue, once the symbiosis is broken, the immunological equilibrium in PPs is readily tipped when a virus invades, resulting in infection onset and even chronicity. Unsurprisingly, the anti-HBV immune response in PPs is most profoundly affected under dysbiosis. However, limited by the insufficient amount of isolated mouse PPs, we failed to detect HBV-specific B cell response in PP by ELISpot assay, which belongs to another essential adaptive immunity arm accounting for achieving a functional cure. Further improvement of the PP isolation technique and exploiting alternative sensitive indicators, such as the recently reported fluorochrome-labeled HBV-specific B cell quantitation (Burton et al., 2018; Le Bert et al., 2020), will facilitate our understanding of the extent of PPs manipulated by gut microbiota.
In a model of autoimmune arthritis, PPs were confirmed to be essential for gut microbiota-triggered and -exacerbated systemic arthritis by the egress of differentiated PP Tfh cells into systemic sites, thereby boosting the generation of auto-antibody responses in systemic lymphoid tissues (Teng et al., 2016). In line with this finding, we herein report that the devoid of PPs substantially reduces intrahepatic HBV-specific IL-21-Tfh cells and Tfc cells. In addition, our published data revealed that the antiviral efficacy of intrahepatic Tfc cells was superior to the circulating counterparts in chronic HBV infection (Li et al., 2020). Hence, it is plausible to conceive a pathway whereby PP Tfc cells migrate into the liver serving as executors fighting against HBV. Nevertheless, we cannot exclude the possibility that, in addition to the mechanism of immune subset migration, the circulation of PP-derived soluble mediators might also account for intrahepatic immunity. Specifically, PP dendritic cells imprint gut-homing specificity on T cells with the marker of α4β7 integrin, which allows the general tracking of PP T cells in systemic location (Mora et al., 2003). As such, whether the reduction of intrahepatic T cell subsets is attributed to the impaired egress of PP subsets imprinted with α4β7 integrin or the downregulation of in situ intrahepatic proliferation and differentiation of effector T cells warrants further investigation.
Notably, we found systemic anti-HBV humoral immunity was more vulnerable to the anti-HBV cellular immunity upon depletion of gut microbiota. In contrast, the absence of PPs resulted in more severe gut microbiota-mediated intrahepatic cellular immunity damage. We infer that gut microbiota-induced intrahepatic anti-HBV immunity was PP-dependent. In contrast, gut microbiota-derived components or metabolic products released to the periphery might account for the systemic anti-HBV activity in the other compartments. Detailed molecules and metabolites analyses of microbiota are essential to address our assumption.
Numerous studies have focused on identifying new therapeutic strategies, such as more potent antiviral drugs and immune interventions aimed at the functional cure in CHB patients have been performed (Fanning et al., 2019), among which clinical trials based on fecal microbiota transplantation (FMT) have been launched in several medical centers recently (Chauhan et al., 2021; Ren et al., 2017). Correspondingly, our findings provide the concept of adapting gut microbiota to bolster systemic anti-HBV response. FMT could be designed as a novel strategy for combination therapy of CHB infection. Alternatively, given that gut microbiota could also be shaped by personal diet, manipulating the gut microbiota through diet seems to be another promising and more acceptable option (Valdes et al., 2018). Conversely, the evaluation and dynamic monitoring of HBV serum indicators during antibiotics treatment in CHB patients should be carefully considered in case of the aggravation of HBV infection or hepatic flares.
Admittedly, some fundamental questions remain to be unresolved. In particular, we mainly focused on how gut microbiota changes anti-HBV immune response, though it's certain that the interaction is bidirectional, and how HBV infection works on the intestinal microecology needs further research. Besides, we tested whether HBV infection affected the basic constitution of B cell and T cell subtypes in both PPs and the spleen in mice and found that HBV infection barely disturbed the constitution of B cell and T cell subtypes especially in PPs (data not shown). Moreover, given the multispecies community of gut microbiota, whether and which specific commensal species account for the systemic anti-HBV response remains to be determined. Additionally, we herein only assess the adaptive immune arm, and further exploration of the involvement of innate immunity will help to gain comprehensive insights into the gut microbiota-mediated anti-HBV response. Likewise, how other lymphoid tissues like MLNs served as important intestinal immune compartments which drain lymph from PPs deserves further study. Besides, underlying mechanisms regarding the effective genes and molecular pathways in PPs should be undertaken, which could be hinted by exploiting the RNA sequencing datasets. Lastly, it should be noted that only small group of mice experiments were performed, and discrepancies exist between humans and mice, including gut microbiota composition, the distribution of PPs as well as the HBV model (Nguyen et al., 2015; Lorenz and Newberry, 2004; Hwang and Park, 2018). However, human samples are unavailable in our study since the current invasive process of human PP collection. Advances in human PP sampling and the subsequent exploration will favor the interpretation and translation of our findings into clinical practice.
5. Conclusions
Our data reveals that gut microbiota modulates systemic anti-HBV adaptive immune responses and exploits PPs to orchestrate intrahepatic anti-HBV cellular immunity remotely. Therapeutic strategies targeting the “gut microbiota-PPs” axis might be prospective combination interventions for treating CHB patients.
Data availability statement
Data are available upon reasonable request. All raw transcriptome data have been deposited in GSA (CRA accession number: PRJCA011926, https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA011926).
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC2303600), the National Natural Science Foundation of China (81971933 and 82022007), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S131), and the Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University (2020J003).
CRediT authorship contribution statement
Yifan Li: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. Shihong Zhong: Investigation, Formal analysis, Writing – original draft. Zihan Jin: Investigation. Guofu Ye: Investigation. Tianling Zhang: Investigation. Zhipeng Liu: Investigation. Zhenguo Liu: Investigation. Zhaofeng Zeng: Investigation. Qiong Li: Investigation. Yuhao Wang: Investigation. Yanda Zhao: Investigation. Libo Tang: Writing – original draft, Supervision. Huaihong Chen: Supervision, Writing – review & editing. Yongyin Li: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Professor Pei-Jer Chen for generously providing pAAV/HBV1.2 plasmids.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199129.
Contributor Information
Libo Tang, Email: tanglibosmu@foxmail.com.
Huaihong Chen, Email: chh19760306@163.com.
Yongyin Li, Email: yongyinli@foxmail.com.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Bertoletti A., Tan A.T., Gehring A.J. HBV-specific adaptive immunity. Viruses. 2009;1 doi: 10.3390/v1020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A.R., Pallett L.J., McCoy L.E., et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Investig. 2018;128:4588–4603. doi: 10.1172/JCI121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A., Kumar R., Sharma S., et al. Fecal microbiota transplantation in hepatitis B e antigen-positive chronic hepatitis b patients: a pilot study. Dig. Dis. Sci. 2021;66:873–880. doi: 10.1007/s10620-020-06246-x. [DOI] [PubMed] [Google Scholar]
- Chou H.H., Chien W.H., Wu L.L., et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2015;112:2175–2180. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornes J.S. Number, size, and distribution of Peyer's patches in the human small intestine: part I the development of Peyer's patches. Gut. 1965;6:225–229. doi: 10.1136/gut.6.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning G.C., Zoulim F., Hou J., Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 2019;18:827–844. doi: 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- Fenton T.M., Jørgensen P.B., Niss K., et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity. 2020;52 doi: 10.1016/j.immuni.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Prince A.M. Hepatitis B virus infection–natural history and clinical consequences. N. Engl. J. Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- Gong S., Yan Z., Liu Z., et al. Intestinal microbiota mediates the susceptibility to polymicrobial sepsis-induced liver injury by granisetron generation in mice. Hepatology. 2019;69:1751–1767. doi: 10.1002/hep.30361. [DOI] [PubMed] [Google Scholar]
- Guo W., Zhou X., Li X., et al. Depletion of gut microbiota impairs gut barrier function and antiviral immune defense in the liver. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.636803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehle V., Beretta M., Bourgine M., et al. Potent human broadly neutralizing antibodies to hepatitis B virus from natural controllers. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly M.K., Diaz K., Smith J.G. Defensins in Viral Infection and Pathogenesis. Annu. Rev. Virol. 2017;4:369–391. doi: 10.1146/annurev-virology-101416-041734. [DOI] [PubMed] [Google Scholar]
- Huang L.R., Wu H.L., Chen P.J., Chen D.S. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.R., Park S.G. Mouse models for hepatitis B virus research. Lab. Anim. Res. 2018;34:85–91. doi: 10.5625/lar.2018.34.3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N., Fish G.E., Smith M.B. Laboratory evaluation of a fully automated chemiluminescence immunoassay for rapid detection of HBsAg, antibodies to HBsAg, and antibodies to hepatitis C virus. J. Clin. Microbiol. 2004;42:610–617. doi: 10.1128/JCM.42.2.610-617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Salimzadeh L., Gill U.S., et al. Comparative characterization of B cells specific for HBV nucleocapsid and envelope proteins in patients with chronic hepatitis B. J. Hepatol. 2020;72:34–44. doi: 10.1016/j.jhep.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Li Y., Tang L., Guo L., et al. CXCL13-mediated recruitment of intrahepatic CXCR5CD8 T cells favors viral control in chronic HBV infection. J. Hepatol. 2020;72:420–430. doi: 10.1016/j.jhep.2019.09.031. [DOI] [PubMed] [Google Scholar]
- Lorenz R.G., Newberry R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann. N. Y. Acad. Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal R.K., Denny J.E., Namazzi R., et al. Dynamic modulation of spleen germinal center reactions by gut bacteria during Plasmodium infection. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J.R., Bono M.R., Manjunath N., et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mörbe U.M., Jørgensen P.B., Fenton T.M., et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14:793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Nguyen M.H., Wong G., Gane E., Kao J.H., Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.L.A., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8 doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ren Y.D., Ye Z.S., Yang L.Z., et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765–1768. doi: 10.1002/hep.29008. [DOI] [PubMed] [Google Scholar]
- Rodríguez J.M., Murphy K., Stanton C., et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Teng F., Klinger C.N., Felix K.M., et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer's patch T follicular helper cells. Immunity. 2016;44:875–888. doi: 10.1016/j.immuni.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Li F., Chen Y., et al. CD4 T cells play a critical role in microbiota-maintained anti-HBV immunity in a mouse model. Front. Immunol. 2019;10:927. doi: 10.3389/fimmu.2019.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Honda K., Shinkura R., et al. IL-7 receptor alpha+ CD3(-) cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- Zhu Q., Xia P., Zhou X., et al. Hepatitis B virus infection alters gut microbiota composition in mice. Front. Cell Infect. Microbiol. 2019;9:377. doi: 10.3389/fcimb.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All raw transcriptome data have been deposited in GSA (CRA accession number: PRJCA011926, https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA011926).
Data will be made available on request.