Abstract
Introduction
Hepatitis B virus disease is a global acute and chronic communicable disease. Mother-to-child transmission is the reason for high carrier rates. Unvaccinated newborns infected through mother-to-child transmission are at >95% risk of developing chronic hepatitis B virus disease. Vaccination is the most effective measure to reduce the global incidence of hepatitis B virus disease. Despite the World Health Organization's target to achieve 90% of the hepatitis B vaccine birth dose by 2030, little is known about the vaccination status of exposed newborns.
Objective
The present study aimed to determine the timing of the hepatitis B vaccine birth dose in exposed newborns in Southwest Ethiopia.
Methods
An institution-based cross-sectional study was employed on 422 systematically selected exposed newborns from April 2, 2022, to August 28, 2022. A pretested, interviewer-administered questionnaire was used for data collection. Data were entered into Epi data 3.1 and exported into SPSS version 23 software for analysis. Both bivariable and multivariable binary logistic regressions were performed. Variables with a p-value <.05 at a 95% confidence interval (CI) were considered statistically significant.
Results
The proportion of neonates who received their first dose of the hepatitis B vaccine on time was 57 (42.5%) (95% CI: 38.3–46.1%). A higher likelihood of vaccinating their exposed newborns on time was associated with formal education (adjusted odds ratio [AOR] = 3.01, 95% CI: 2.21–7.09), four or more ANC visits (AOR = 2.33, 95% CI: 2.05–6.21), and husband engagement (AOR = 4.31, 95% CI: 2.03–6.34).
Conclusion
The proportion of timely initiation of the hepatitis B vaccine birth dose in Southwest Ethiopia was low. Thus, strengthening health education on the hepatitis B vaccine, encouraging women to have at least four ANC visits, and encouraging male involvement help improve the timely administration of the hepatitis B vaccine.
Keywords: hepatitis B vaccine, timing of birth dose, exposed newborns, Southwest Ethiopia
Introduction
Hepatitis B virus (HBV) disease is a global acute and chronic communicable disease that causes major hepatic disease (WHO, 2017, 2022). Hepatitis B infection is caused by HBV that infects the liver and causes hepatocellular inflammation, cirrhosis, hepatocellular carcinoma, and death (CDC, 2020, 2023; WHO, 2015). Globally, more than 2 billion people have been infected with HBV, 240 million people are chronic carriers; and over 686,000 people die each year (WHO, 24 June 2022, 2021, December 2015). Mother-to-child transmission (MTCT) of HBV accounts for an estimated 21% of death worldwide, while regionally it ranges from 13% in the Eastern Mediterranean region to 26% in the Western Pacific region (Franco et al., 2012). Mother-to-child transmission is usually the reason for high chronic infection carrier rates (Machaira, Papaevangelou, Vouloumanou, Tansarli & Falagas, 2015; WHO, 27 July 2020). One of the distinctive features of HBV infection is that the risk of developing chronic infection varies greatly with the age at which the infection is acquired. Around 90% develop a chronic infection following perinatal infection (Lai, Ratziu, Yuen & Poynard, 2003; Orlando et al., 2015; WHO., 2015). Vaccination is the most effective measure to reduce the global incidence of HBV disease (CDC, 19 July 2021; Franco et al., 2012). Provision of the hepatitis B vaccine birth dose within 12–24 h can prevent up to 95% of HBV transmission (CDC, 19 July 2021; Reardon et al., 2019; Zhang et al., 2022). A single birth dose of the hepatitis B vaccination strategy is cost-effective (Biondi et al., 2023). In addition to the hepatitis B vaccine birth dose, administration of Tenofavir (TDF) for the mother at 28 weeks of gestation when HBV DNA is > 2 × 105 IU/mL reduces the risk of MTCT (Liu et al., 2022). Even though MTCT is a major contributor to the ongoing HBV epidemic, only 11 of 54 (20.3%) sub-Saharan African countries have introduced the birth dose of HBV vaccine into their regular immunization schedule which is not in Ethiopia (Boisson et al., 2022). Unvaccinated newborns infected through MTCT are at >95% risk of developing chronic hepatitis B virus disease (Joshi & Coffin, 2020; WHO, 24 June 2022). World Health Organization (WHO) target is to achieve 90% of hepatitis B vaccine birth dose by 2030 (Seaman et al., 2020; WHO, December 2015). Universal screening of all pregnant women, at-birth prophylaxis with specific anti-HBV immune globulin, and HBV vaccination for exposed newborns are effective in reducing the risk of vertical transmission. A good understanding of the current status of the hepatitis B vaccine birth dose also helps WHO 2030 target in improving exposed newborns’ vaccination status. Therefore, this study aims to assess the timing of the hepatitis B vaccine birth dose in exposed newborns and associated factors among exposed newborns at public health institutions in Southwest Ethiopia, 2022.
Literature Review
A study conducted in Central Europe, Eastern Europe, and Central Asia showed that the hepatitis B vaccine birth dose coverage was 63·5% in Southeast Asia and East Asia, 79.0% in Oceania, 33.8% in North Africa and the Middle East, and 10% in Sub-Saharan Africa (Seaman et al., 2020). According to the global report, the overall global coverage of the birth dose of the hepatitis B vaccine among exposed newborns that was given within 24 h after birth was 42% and only 10% in Africa (Zhao, Zhou & Zhou, 2020).
In America, the hepatitis B vaccine birth dose vaccination coverage ranged from 17% in St. Kitts and Nevis to 99% in Cuba (Álvarez et al., 2017). In the study conducted in the South-East Asia Region, the hepatitis B vaccine birth dose coverage status and progress of hepatitis B control through vaccination increased from 9% in 2011 to 34% in 2015 (Childs, Roesel & Tohme, 2018). According to a 2015 report of the study done in China, 95.14% of hepatitis B exposed newborns got their first dose of the hepatitis B vaccine within 24 h of birth (Li et al., 2012).
A multicenter study conducted in China on the Management Algorithm for Prevention of MTCT of the HBV showed that administration of TDF for the mother at 28 weeks of gestation when HBV DNA is > 2 × 105 IU/mL reduces risk of MTCT of the HBV (Liu et al., 2022). A study conducted in Greenland revealed that among the children born to hepatitis B positive women, 58% received a 3-dose hepatitis B vaccine series and 20% received no vaccinations (Børresen et al., 2012). A study conducted in the United Kingdom on the immunization of babies born to HBsAg-positive mothers revealed that 99.4% of exposed newborns received birth dose, and 9.8% has taken HBIG within 24 h (Keeble, Quested, Barker, Varadarajan & Shankar, 2015).
A study conducted in China on the Perinatal hepatitis B prevention program revealed that hepatitis B vaccine birth dose coverage (within 24 h) remained high (96.3–97.1%) during this period, and the coverage of HBIG increased from 85.0% (before July 2011) to 92.1% (after July 2011) (Zhang et al., 2014). According to the global, regional, and national burden of hepatitis B report by 2019, only 68 out of 194 countries had already achieved the 2030 target proposed by WHO (Lancet, 20 June 2022).
According to the vaccination registry report in Italy, 155 of the enrolled subjects 94% had received the hepatitis B vaccine birth dose (Zanella et al., 2020). A study done on newborns vaccination on the hepatitis B in China revealed that 97.34% of subjects received the hepatitis B vaccine first dose within 24 h after birth (Wu, Li & Zhou, 2016). A study conducted in Georgia on under vaccination of hepatitis B vaccine revealed that 14%–18.5% of exposed newborns received birth dose within 24 h of birth (Jiles, Daniels, Yusuf, McCauley & Chu, 2001).
A study conducted in USA showed that 63.9% hepatitis B exposed newborns received the hepatitis B vaccine birth dose and 64.8% received the HBIG timely within 12 h of birth (Schillie et al., 2015). Implementing a birth dose of the hepatitis B vaccine in five African countries revealed that 7% in the Gambia, 13% in Nigeria, and 74% in the Botswana of exposed newborns received the hepatitis B vaccine birth dose timely (Moturi et al., 2018). A study conducted in Nigeria on the prevention of MTCT of the hepatitis B infection and 9 months follow-up of the hepatitis B exposed infants showed that 327 exposed infants (89.6%) had received the hepatitis B vaccine within 24 h of delivery (Ndububa et al., 2022).
A study done in Ontario showed that a switch to a single birth dose of the hepatitis B vaccination strategy is cost-effective (Biondi et al., 2023). A study conducted at a tertiary hospital in North-Central Nigeria on the timing of receiving the hepatitis B vaccine birth dose showed that 133 exposed infants (33%) received a timely birth dose of hepatitis B vaccine birth dose (Bada et al., 2022). A study conducted in Arsi Zone showed that 20.7% of hepatitis B exposed newborns have received the hepatitis B vaccine birth dose, and 83 (54.6%) have received both the hepatitis B vaccine and HBIG (Bayu, Elias, Abdisa, Tune & Namo, 2020b).
Objectives of the Study
To assess the timing of hepatitis B vaccine birth dose in exposed newborns and associated factors at Gurage Zone public health institutions, SNNPR, Southwest Ethiopia, 2022. The results of this study will provide an important input for the health bureau of Southwest Ethiopia to determine possible interventions aimed at improving the hepatitis B vaccine birth dose and addressing child health problems.
Methodology
Study Design, Period, and Setting
An institution-based cross-sectional study design was employed from April 2, 2022, to August 28, 2022, in Gurage Zone public health institutions. Gurage Zone is located about 158 kilometers southwest of the capital city of Ethiopia, Addis Ababa, and 255 kilometers from Hawassa, the capital city of SNNPR. The study was conducted at four governmental hospitals situated in the Gurage zone. Wolkite University Specialized Referral Teaching Hospital was established in 2018 G.C. and is located 170 km away from the capital city of Ethiopia, Addis Ababa. It provides services for more than 1.2 million inhabitants living in the Gurage zone and surrounding districts. Attat Primary Hospital, which is 175 km from Addis Ababa, was established in 1969 and serves 800,000 people. Butajira General Hospital is situated 130 km South of the capital of Ethiopia, Addis Ababa, and 50 km West of Ziway town in the Rift Valley. The hospital provides clinical services for the town of Butajira and its surrounding communities, serving approximately 1,300,000 people, and Gunchire Primary Hospital has been in operation since 2015 G.C. and serves 270,000 people. The daily average patient flow is 250 patients.
Research Questions
What is the proportion of timing of hepatitis B vaccine birth dose to exposed newborns in public hospitals of the Gurage zone?
What are the factors associated with timing of hepatitis B vaccine birth dose to exposed newborns in public hospitals of the Gurage zone?
Study Population
The study population included all HBV-exposed newborns (newborns delivered to mothers who were already diagnosed HBV positive) delivered at Gurage Zone health institutions during the study period.
Study Unit
Exposed newborns were selected using a systematic sampling technique based on the chronological order of their admission with their mothers to the postnatal care ward.
Inclusion Criteria
All HBV-exposed newborns admitted with their mothers to the immediate postnatal care ward.
Exclusion Criteria
Those mothers of exposed newborns who were not willing to take part in the study after details of the study purpose were provided during data collection.
Study Variables
Dependent Variables
Timing of the hepatitis B vaccine birth dose (received within 24 h or not).
Independent Variables
Sociodemographic characteristics (age, residence, occupation, educational status, marital status, household size, income, religion, and ethnicity). Reproductive health variables (number of ANC visits, parity, and birth interval).
Operational Definitions
Exposed newborns: An infant that born from HBsAg positive mother (Chen, 2009).
Timely administration of hepatitis B birth dose: is the first dose of hepatitis B vaccine that is given as soon as possible after birth, ideally within 24 h (WHO, July 2017, July 2020; WHO, 2019). Yes/No.
Sample Size Determination
The actual sample size was determined for the present study by using a single population proportion formula with assumptions of (p = 50%, 95% confidence interval [CI], 5% margin of error, and 10% nonresponse rate, which yielded 422).
Sampling Techniques and Procedures
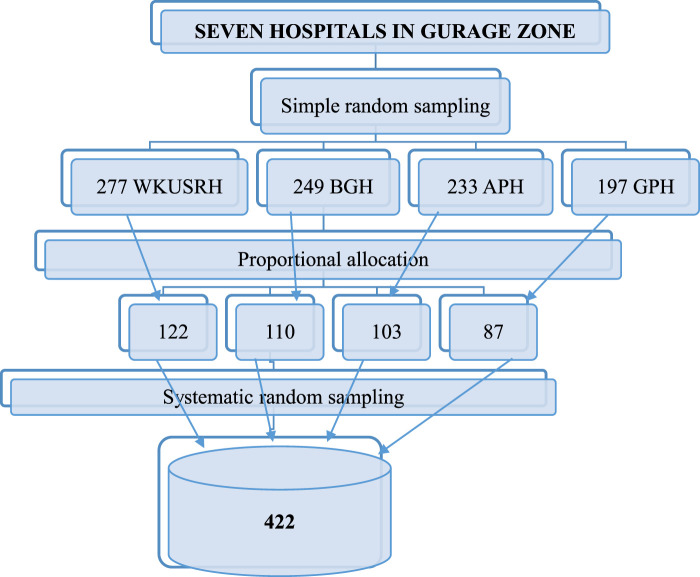
The Gurage zone has seven public hospitals: one specialized referral hospital, one general hospital, and five primary hospitals. First, from those hospitals, the researchers selected four hospitals by using a simple random sampling technique. The one-year total number of hepatitis B positive mothers in each hospital was identified (277 in Wolkite University Specialized Referral Hospital [WUSRH], 233 in Attat Primary Hospital [APH], 249 in Butajira General Hospital [BGH], and 197 in Gunchire Primary Hospital [GPH]). A total of 956 hepatitis B-positive mothers were identified in four selected hospitals. Then, the possible number of respondents in each hospital was allocated proportionally based on a one-year report of hospitals. Finally, systematic random sampling was used to select the study participants in each hospital as K = N/n = 956/422, where K = 2 (Figure 1).
Figure 1.
Schematic representation of sampling procedure for timing of hepatitis B vaccine birth dose in exposed newborns at public health institutions, Gurage Zone, SNNPR, Ethiopia, 2022.
Data Collection Tools
Data were collected using an interviewer-administered, pretested questionnaire containing questions related to sociodemographic characteristics, Obstetrics characteristics, and vaccination status of exposed newborns. The questionnaire was prepared in English after different literature reviews (Bayu et al., 2020b; Li et al., 2012; Organization, 2017, 2019; Poland & Jacobson, 2004; Zanella et al., 2020) and translated into Amharic, the local language. Three BSc midwives were recruited to collect data. The general content validity of the questionnaires was checked by relevant professionals against the conceptual framework of the study, and the reliability of the questionnaire was checked using Cronbach's alpha value, which was 0.75.
Data Quality Management
Training was given to the data collectors and supervisors before data collection. A 5% pretest was done so that the questionnaire was then assessed for its clarity, length, and completeness. The quality of the collected data was checked daily by the principal investigator and supervisors. Everyday, the collected data were reviewed and cross-checked for completeness and relevance by the supervisors and principal investigators.
Statistical Analysis
The data were cleaned, entered into Epi Data 3.1, and exported to SPSS 23 for analysis. The data were cleaned by running Frequency, checking for missing values, and checking for the presence of outliers. We summarized participants’ characteristics using proportions of categorical variables and the mean with standard deviation (SD) based on the distribution of data for continuous variables. Normality was checked using the Shapiro–Wilk test. Factors associated with the outcome variable were analyzed using binary logistic regression. Bivariable analysis, a crude odds ratio with 95% CI, was used to see the association between each independent variable and the outcome variables. Variables with a p-value of ≤0.25 in the bivariable analysis were selected for the multivariable binary logistic regression model. Multi-collinearity was checked to see the linear correlation among the independent variables by using the variance inflation factor (VIF). The VIF of the data was <10, and no sign of multicollinearity was detected. A multivariable binary logistic regression analysis was computed to control the possible effects of confounders. The goodness of the fitness model was tested by the Hosmer–Lemeshow statistic, and its value was above 0.05. The strength of the association between dependent and independent variables was assessed using an adjusted odds ratio (AOR) with a 95% CI. A p-value was used to report the level of significance of each independent variable with respect to a dependent variable.
Results
Sociodemographic Characteristics of the Study Sample
All of the respondents responded to the questionnaire, making the response rate 100%. Nearly half 193 (45.7%) of the mothers of the exposed newborns were in the age group of 26–35 years, with a mean age of 28 years (28 ± 2.3 SD). More than two-thirds of the mothers of exposed newborns 292 (69.2%) were Gurage in ethnicity, more than half 214 (51.7%) were Orthodox religion followers, and more than half 240 (56.9%) were from rural areas. More than four-fifths of the mothers of exposed newborns 355 (84.1%) had formal education, and the average household size was 3.4 persons (3.4 ± 1.57) (Table 1).
Table 1.
Sociodemographic Characteristics of the Study Sample at Gurage Zone Public Health Institution, Southwest Ethiopia (N = 422).
| Variables | Frequency | Percentage (%) |
|---|---|---|
| Age | ||
| 15–25 | 132 | 31.3 |
| 26–35 | 193 | 45.7 |
| ≥36 | 97 | 22.9 |
| Residence | ||
| Urban | 182 | 43.1 |
| Rural | 240 | 56.9 |
| Marital status | ||
| Married | 417 | 98.8 |
| Unmarried | 5 | 1.2 |
| Ethnicity | ||
| Gurage | 292 | 69.2 |
| Amhara | 64 | 15.2 |
| Kembeta | 41 | 9.7 |
| Oromo | 25 | 5.9 |
| Religion | ||
| Orthodox | 214 | 50.7 |
| Muslim | 157 | 37.2 |
| Protestant | 51 | 12.1 |
| Educational status of women | ||
| No formal education | 67 | 15.9 |
| Formal education | 355 | 84.1 |
| Occupation of women | ||
| Housewife | 241 | 57.1 |
| Merchant | 127 | 30.1 |
| Government employee | 54 | 12.8 |
| Family monthly income(Birr) | ||
| <3000 | 93 | 22.0 |
| ≥3000 | 329 | 78.0 |
| Family Size | ||
| <4 | 193 | 45.7 |
| ≥4 | 229 | 54.3 |
Obstetric Characteristics of the Mothers
More than half 234 (55.5%) of the mothers of the exposed newborns had more than four children; two-thirds 281 (66.6%) had four or more ANC visits; and more than three-fourths 321 (76.1%) had a birth interval of more than two years (Table 2).
Table 2.
Obstetric Characteristics of the Mothers of the Exposed Newborns at Gurage Zone Public Health Institutions, Southwest Ethiopia (N = 422).
| Variables | Frequency | Percentage (%) |
|---|---|---|
| Number of alive children | ||
| <4 | 188 | 44.5 |
| ≥4 | 234 | 55.5 |
| Number of ANC Visits | ||
| Three or less times | 141 | 33.4 |
| Four and above | 281 | 66.6 |
| Birth Interval | ||
| <1 year | 101 | 23.9 |
| >2 years | 321 | 76.1 |
| Mode of delivery | ||
| SVD | 377 | 89.3 |
| Instrumental | 28 | 6.6 |
| Cesarean section | 17 | 4.0 |
| Birth weight | ||
| <2500gm | 47 | 11.1 |
| ≥2500gm | 375 | 88.9 |
Prevalence of Hepatitis B Birth Dose Vaccination Among Hepatitis B Exposed Newborns
Among the 422 neonates that were exposed to HBV, 134 (31.7%) received the birth dose of the hepatitis B vaccine, and 57 (42.5%) received the vaccination punctually within 24 h of birth. Only 19 (14.2%) exposed newborns received the hepatitis B Immunoglobulin G vaccine. Eighty-one (60.4%) of the fathers of the exposed newborns were took part in the immunization program (Table 3).
Table 3.
Vaccination Status of the Hepatitis B Exposed Newborns That Born at Gurage Zone Public Health Institution, Southwest Ethiopia (N = 422).
| Variables | Frequency | Percentage | |
|---|---|---|---|
| Newborn vaccinated at birth (n = 422) | Yes | 134 | 31.7 |
| No | 288 | 68.3 | |
| Timing of vaccination (n = 134) | Within 24 hours | 57 | 42.5 |
| Within 48 hours | 77 | 57.5 | |
| HBIG received at birth (134) | Yes | 19 | 14.2 |
| No | 115 | 85.8 | |
| Timing of vaccination (n = 19) | Within 24 hours | 11 | 57.9 |
| Within 48 hours | 8 | 42.1 | |
| Husband involvement in vaccinating newborn (n = 134) | Yes | 81 | 60.4 |
| No | 53 | 39.6 | |
Factors Associated with Timing of the Hepatitis B Vaccine Birth Dose
Using a bivariable binary logistic regression model, it was discovered that the following factors were statistically significantly associated with the timing of the hepatitis B vaccine birth dose: residence, women's educational level, family size, birth interval, number of ANC visits, and husband involvement in newborn vaccination. The multivariable binary logistic regression analysis indicated that the husband's involvement, the women's educational level, and the number of ANC visits were statistically significant at the p-value 0.05 level. In this study, women with formal education had a 3.01 times higher likelihood of immunizing their exposed newborns on time than women without formal education (AOR = 3.01, 95% CI: 2.21–7.9); women who had four or more ANC visits were 2.33 times more likely to vaccinate their exposed newborns on time than women who had three or fewer visits (AOR = 2.33, 95% CI: 2.05–6.21); and husband participation in newborn vaccination increased the odds of giving the hepatitis B vaccine birth dose on time by 4.31 times compared to those who did not (AOR = 4.31, 95% CI: 2.03–6.34) (Table 4).
Table 4.
Bivariable and Multivariable Binary Logistic Regression Analyses for HBV Exposed Newborns at Public Health Institutions, Southwest Ethiopia (N = 422).
| Variables | Timely initiation of vaccine | COR (95% CI) | AOR (95% CI) | p value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Residence | |||||
| Rural | 20 | 220 | 1 | ||
| Urban | 37 | 145 | 2.81 (1.67–9.39) | 2.10 (0.94–6.41) | 0.103 |
| Marital status | |||||
| Married | 56 | 361 | 0.62 (0.32–2.27) | 0.45 (0.71–3.19) | 0.526 |
| Unmarried | 1 | 4 | 1 | ||
| Educational status of women | |||||
| No formal education | 4 | 63 | 1 | ||
| Formal education | 53 | 302 | 2.76 (2.34–7.01) | 3.01 (2.21–7.09) | 0.027 |
| Family income in birr | |||||
| <3000 | 16 | 77 | 1 | ||
| ≥3000 | 41 | 288 | 0.69 (0.54–4.31) | 0.76 (0.43–1.94) | 0.647 |
| Family size | |||||
| <4 | 37 | 156 | 2.47 (2.09–6.21) | 1.37 (0.97–2.71) | 0.385 |
| ≥4 | 20 | 209 | 1 | ||
| Number of ANC Visits | |||||
| Three or less | 10 | 131 | 1 | ||
| Four or more | 47 | 234 | 2.63 (1.98–5.20) | 2.33 (2.05–6.21) | 0.031 |
| Birth interval | |||||
| <1 year | 13 | 88 | |||
| ≥2 years | 44 | 277 | 1.08 (1.01–4.84) | 0.81 (0.23–2.03) | 0.427 |
| Husband involvement in newborn vaccination | |||||
| Yes | 38 | 43 | 1.58 (2.35–5.22) | 4.31 (2.03–6.34) | 0.016 |
| No | 19 | 34 | 1 | ||
p value less than 0.25 in bivariable analysis, and p value <0.05 in multivariable analysis indicates statistical significance.
Discussion
In the current study, 57 (42.5%) (95% CI: 38.3–46.1%) of the exposed neonates received their first dose of the hepatitis B vaccine within 24 h. This result is significantly lower than the outcome of a large-scale study conducted in Greenland, where 80% of the exposed babies to the HBV had received a birth dose immunization on schedule (Børresen et al., 2012). The result of this study was also lower than the studies conducted in China, which revealed 97.3% and 96.3–97.1% (Wu et al., 2016; Zhang et al., 2014); Italy 94% (Zanella et al., 2020); United Kingdom 99.4% (Keeble et al., 2015); South Tarawa 95% (Wang et al., 2017); Western Pacific Region 67% (Kabore et al., 2022); Botswana 74% (Moturi et al., 2018), Nigeria 89.6% (Ndububa et al., 2022); and Arsi Zone 52.6% (Bayu, Elias, Abdisa, Tune & Namo, 2020a). The sociodemographic and socioeconomic variations of the study participants are the cause of the differences between prior studies and the current study. The finding of the present study was higher than the study done in the African Region (Nigeria, Democratic Republic of the Congo, Ethiopia, Rwanda, Cabo Verde, Sao Tome, Principe, Algeria, Senegal, and Botswana), which found that 14% of exposed newborns received the birth dose of the hepatitis B vaccine on time during the COVID-19 pandemic outbreak (Kabore et al., 2022) and in North-Central Nigeria, 33% (Bada et al., 2022). This difference from the present study is due to differences in the study period and the sociodemographic characteristics of the study participants. The current study was also higher than the studies conducted in Gambia 7% and Nigeria 13% (Moturi et al., 2018). This wide gap is due to differences in the sociodemographic, and socioeconomic characteristics of the study participants.
In this study, women with formal education had a 3.01 times higher likelihood of immunizing their exposed newborns on time than women without formal education (AOR = 3.01, 95% CI: 2.21–7.9). This is due to the fact that women with formal education know the consequences of the disease on their newborns, so they vaccinate them timely. The study conducted in Kinshasa, Democratic Republic of the Congo strengthens the current study that intensified education initiatives in highly endemic areas to improve prevention of MTCT efforts (Thahir et al., 2022). Women who had four or more ANC visits were 2.33 times more likely to vaccinate their exposed newborns timely than women who had three or fewer visits (AOR = 2.33, 95% CI: 2.05–6.21). This is due to the fact that mothers who have more antenatal care visits may have more information about hepatitis, vaccines for newborns, and the consequences of the disease. This encourages the mothers to be ready ahead of time to buy a vaccine for their exposed newborns.
Husband participation in newborns vaccination increased the odds of giving the hepatitis B vaccine birth dose on time by 4.31 times compared to those who did not (AOR = 4.31, 95% CI: 2.03–6.34). This might be due to the fact that husbands empower the household economy and have the capability to buy vaccines for their exposed newborns. The women who keep a secret about their hepatitis sero-status from their husbands might be incapable of buying vaccines.
Strength and Limitations of the Study
The cross-sectional study design makes it impossible to measure cause-and-effect relationships, and selection bias could also be a weakness of this study.
Implications for Practice
The findings from this study have several implications for improving the timely administration of the hepatitis B vaccine birth dose in exposed newborns. This study revealed the factors that have a significant association with the timing of the hepatitis B vaccine birth dose in exposed newborns, and it provides important information for zonal health offices and their respective districts to improve the vaccination status of the hepatitis B vaccine birth dose in exposed newborns. It should also promote strong community-based behavioral change on the importance of timely administration of the hepatitis B vaccine birth dose. Health facilities should enhance linkage with health posts to increase ANC service utilization, and health extension workers should promote and give health education about the hepatitis B vaccine.
Conclusion and Recommendations
In Southwestern Ethiopia, timely delivery of the hepatitis B vaccine at birth to exposed newborns was not sufficient. Therefore, strengthening health education about hepatitis B and the hepatitis B vaccine, encouraging women to attend at least four antenatal care visits, and encouraging the participation of men in the vaccination process will improve the vaccination status of the exposed newborns. Currently, in Ethiopia, the government has not focused on vaccinating newborns exposed to hepatitis B. This vaccine is only available in private pharmacies and is not affordable for the vast majority of the population. So, the government has to get involved and provide vaccines to the exposed newborns at a reasonable cost.
Acknowledgments
The authors would like to thank Wolkite University for granting ethical approval. The authors also extend our gratitude to study participants and data collectors.
Footnotes
Authors’ Contributions: All authors equally participated.
ORCID iDs: Mebratu Demissie https://orcid.org/0000-0001-7566-4942
Haregwa Asnake Weldekidan https://orcid.org/0000-0003-4180-0779
Fikremariam Endeshew https://orcid.org/0000-0002-6698-6584
Seblework Abeje https://orcid.org/0000-0002-1096-5848
Data Availability: Data that support the findings are available from the corresponding author upon a reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval and Consent to Participants: The ethical clearance was obtained from the Institutional Review Board (IRB) of Wolkite University, College of Medicine and Health Sciences, with reference number CMHS/IRB's 0025/2022. A formal letter was written by the IRB to each hospital, and official permission was secured. Written informed consent was obtained from all mothers of exposed newborns before the study. The purpose and importance of the study were explained to women, and they were also informed about the possibility of refusing participation at any time during data collection. The confidentiality of the data was assured and maintained; a code number was assigned to the study participants without mentioning their names, and the information that was collected for the study was kept in a file and locked with a key.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Written informed consent was obtained from all subjects.
References
- Álvarez A. M. R., Pérez-Vilar S., Pacis-Tirso C., Contreras M., El Omeiri N., Ruiz-Matus C., Velandia-González M. (2017). Progress in vaccination towards hepatitis B control and elimination in the Region of the Americas. BMC Public Health, 17(1), 1–10. 10.1186/s12889-016-3954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada F. O., Stafford K. A., Osawe S., Wilson E., Sam-Agudu N. A., Chen H., Campbell J. D. (2022). Factors associated with receipt of a timely infant birth dose of hepatitis B vaccine at a tertiary hospital in north-central Nigeria. PLOS Global Public Health, 2(9), e0001052. 10.1371/journal.pgph.0001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayu H., Elias B., Abdisa S., Tune A., Namo H. (2020a). Post exposure prophylaxis coverage, vertical transmission and associated factors among hepatitis B exposed newborns delivered at Arsi zone health institutions, 2019. PLoS One, 15(10), e0238987. 10.1371/journal.pone.0238987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayu H., Elias B., Abdisa S., Tune A., Namo H. (2020b). Post exposure prophylaxis coverage, vertical transmission and associated factors among hepatitis B exposed newborns delivered at Arsi zone health institutions, 2019, p.5. PLoS One, 15(10). 10.1371/journal.pone.0238987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M. J., Estes C., Razavi-Shearer D., Sahdra K., Lipton N., Shah H., Feld J. J. (2023). Cost-effectiveness modelling of birth and infant dose vaccination against hepatitis B virus in Ontario from 2020 to 2050. Canadian Medical Association Open Access Journal, 11(1), E24–E32. 10.9778/cmajo.20210284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson A., Goel V., Yotebieng M., Parr J. B., Fried B., Thompson P. (2022). Implementation approaches for introducing and overcoming barriers to hepatitis B birth-dose vaccine in sub-Saharan Africa. Global Health: Science and Practice, 10(1), 8–9. 10.9745/GHSP-D-21-00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børresen M. L., Koch A., Biggar R. J., Ladefoged K., Melbye M., Wohlfahrt J., Krause T. G. (2012). Effectiveness of the targeted hepatitis B vaccination program in Greenland. American Journal of Public Health, 102(2), 277–284. 10.2105/AJPH.2011.300239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (19 July 2021). Global viral hepatitis report. https://www.cdc.gov/hepatitis/global/index.htm.
- CDC (2020). Hepatitis B surveillance. https://www.cdc.gov/hepatitis/statistics/2020surveillance/hepatitis-b.htm.
- CDC (2023). Viral hepatitis B screening and testing recommendations. https://www.cdc.gov/mmwr/volumes/72/rr/rr7201a1.htm.
- Chen D.-S. (2009). Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. Journal of Hepatology, 50(4), 805–816. 10.1016/j.jhep.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Childs L., Roesel S., Tohme R. A. (2018). Status and progress of hepatitis B control through vaccination in the South-East Asia region, 1992–2015. Vaccine, 36(1), 6–14. 10.1016/j.vaccine.2017.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco E., Bagnato B., Marino M. G., Meleleo C., Serino L., Zaratti L. (2012). Hepatitis B: Epidemiology and prevention in developing countries. World (Oakland, Calif) , 4(3), 74–80. 10.4254/wjh.v4.i3.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiles R. B., Daniels D., Yusuf H. R., McCauley M. M., Chu S. Y. (2001). Undervaccination with hepatitis B vaccine: Missed opportunities or choice? American Journal of Preventive Medicine, 20(4 Suppl), 75–83. 10.1016/s0749-3797(01)00276-8 [DOI] [PubMed] [Google Scholar]
- Joshi S. S., Coffin C. S. (2020). Hepatitis B and pregnancy: Virologic and immunologic characteristics. Hepatology Communications, 4(2), 157–171. 10.1002/hep4.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabore H. J., Li X., Allison R. D., Avagyan T., Mihigo R., Takashima Y., Tohme R. A. (2022). Effects of decreased immunization coverage for hepatitis B virus caused by COVID-19 in World Health Organization Western Pacific and African Regions, 2020. Emerging Infectious Diseases, 28(Suppl 1), S217–S224. 10.3201/eid2813.212300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble S., Quested J., Barker D., Varadarajan A., Shankar A. G. (2015). Immunization of babies born to HBsAg positive mothers: An audit on the delivery and completeness of follow up in Norfolk and Suffolk, United Kingdom. Human Vaccines & Immunotherapeutics, 11(5), 1153–1156. 10.1080/21645515.2015.1019977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. L., Ratziu V., Yuen M.-F., Poynard T. (2003). Viral hepatitis B. The Lancet, 362(9401), 2089–2094. 10.1016/S0140-6736(03)15108-2 [DOI] [PubMed] [Google Scholar]
- Lancet T. (20 June 2022). Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterology and Hepatology, 7(1), 769–829. 10.1016/S2468-1253(22)00124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Wang Q., Zhang L., Su H., Zhang J., Wang T., Fan D. (2012). The risk factors of transmission after the implementation of the routine immunization among children exposed to HBV infected mothers in a developing area in northwest China. Vaccine, 30(49), 7118–7122. 10.1016/j.vaccine.2012.09.031 [DOI] [PubMed] [Google Scholar]
- Liu Z., Chen Z., Cui F., Ding Y., Gao Y., Han G., Liu Y. (2022). Management algorithm for prevention of mother-to-child transmission of hepatitis B virus (2022). Journal of Clinical and Translational Hepatology, 10(5), 1004–1010. 10.14218/JCTH.2022.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaira M., Papaevangelou V., Vouloumanou E. K., Tansarli G. S., Falagas M. E. (2015). Hepatitis B vaccine alone or with hepatitis B immunoglobulin in neonates of HBsAg1/HBeAg2 mothers: A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy, 70(2), 396–404. 10.1093/jac/dku404 [DOI] [PubMed] [Google Scholar]
- Moturi E., Tevi-Benissan C., Hagan J. E., Shendale S., Mayenga D., Murokora D., Mihigo R. (2018). Implementing a birth dose of hepatitis B vaccine in Africa: Findings from assessments in 5 countries. Journal of Immunological Sciences, 5(Suppl 1), 31–40. 10.29245/2578-3009/2018/si.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndububa D., Kuti O., Awowole I., Adekanle O., Ijarotimi O., Makinde O., Ijadunola M. (2022). Prospective cohort study of prevention of mother to child transmission of hepatitis B infection and 9 months follow-up of hepatitis B-exposed infants at Ile-Ife, Nigeria. BMJ Open, 12(11), e063482. 10.1136/bmjopen-2022-063482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W. H. (2017). Hepatitis B vaccines: WHO position paper-July 2017. Weekly Epidemiological Record, 92(27), 369–392. http://apps.who.int/.../WER9227.pdf28685564 [Google Scholar]
- Organization W. H. (2019). Hepatitis B vaccines: WHO position paper, July 2017—recommendations. Vaccine, 37(2), 223–225. 10.1016/j.vaccine.2017.07.046 [DOI] [PubMed] [Google Scholar]
- Orlando R., Foggia M., Maraolo A., Mascolo S., Palmiero G., Tambaro O., Tosone G. (2015). Prevention of hepatitis B virus infection: From the past to the future. European Journal of Clinical Microbiology and Infectious Diseases, 34(6), 1059–1070. 10.1007/s10096-015-2341-x [DOI] [PubMed] [Google Scholar]
- Poland G. A., Jacobson R. M. (2004). Prevention of hepatitis B with the hepatitis B vaccine. New England Journal of Medicine, 351(27), 2832–2838. 10.1056/NEJMcp041507 [DOI] [PubMed] [Google Scholar]
- Reardon J. M., O'Connor S. M., Njau J. D., Lam E. K., Staton C. A., Cookson S. T. (2019). Cost-effectiveness of birth-dose hepatitis B vaccination among refugee populations in the African region: A series of case studies. Conflict and Health; 13(5), 1–10. 10.1186/s13031-019-0188-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillie S., Walker T., Veselsky S., Crowley S., Dusek C., Lazaroff J., Fenlon N. (2015). Outcomes of infants born to women infected with hepatitis B. Pediatrics, 135(5), e1141–e1147. 10.1542/peds.2014-3213 [DOI] [PubMed] [Google Scholar]
- Seaman C. P., Morgan C., Howell J., Xiao Y., Spearman C. W., Sonderup M., Scott N. (2020). Use of controlled temperature chain and compact prefilled auto-disable devices to reach 2030 hepatitis B birth dose vaccination targets in LMICs: A modelling and cost-optimisation study. 10.1016/S2214-109X(20)30231-X [DOI]
- Thahir S., Tulenko S. E., Ngimbi P., Ntambua S., Matondo J., Mwandagalirwa K., Parr J. B. (2022). Low knowledge about hepatitis B prevention among pregnant women in Kinshasa, democratic Republic of Congo. PLOS Global Public Health, 2(9), e0000450. 10.1371/journal.pgph.0000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zhang G., Zheng H., Miao N., Shen L., Wang F., Zhang X. (2017). Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China. Vaccine, 35(33), 4229–4235. 10.1016/j.vaccine.2017.06.019 [DOI] [PubMed] [Google Scholar]
- WHO (24 June 2022). Hepatitis B key facts. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- WHO (27 July 2020). Preventing mother-to-child transmission of the hepatitis B virus. https://www.who.int/publications-detail-redirect/978-92-4-000270-8.
- WHO (December 2015). Preventing Perinatal Hepatitis B Virus Transmission: A Guide for Introducing and Strengthening Hepatitis B Birth Dose Vaccination. https://apps.who.int/iris/handle/10665/208278.
- WHO (July 2017). WHO Report Hepatitis B vaccines: WHO position paper Recommendations. https://pubmed.ncbi.nlm.nih.gov/28743487/.
- WHO (July 2020). Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. https://www.who.int/publications-detail-redirect/978-92-4-000270-8. [PubMed]
- WHO (2015). Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. https://www.who.int/publications-detail-redirect/9789241549059.
- WHO (2017). GLOBAL HEPATITIS REPORT. 83. https://apps.who.int/iris/handle/10665/255016.
- WHO (2019). Hepatitis B vaccines: WHO position paper, July 2017–recommendations. Vaccine, 37(2), 223–225. https://pubmed.ncbi.nlm.nih.gov/28743487/ . 10.1016/j.vaccine.2017.07.046 [DOI] [PubMed] [Google Scholar]
- WHO (2021). Hepatitis B facts and figures. https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/facts-and-figures/.
- WHO (2022). Global report of hepatitis B virus. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- Wu J. N., Li D. J., Zhou Y. (2016). Association between timely initiation of hepatitis B vaccine and completion of the hepatitis B vaccine and national immunization program vaccine series. International Journal of Infectious Diseases, 51(1), 62–65. 10.1016/j.ijid.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Zanella B., Bechini A., Boccalini S., Sartor G., Tiscione E., Bonanni P. (2020). Hepatitis B seroprevalence in the pediatric and adolescent population of Florence (Italy): An update 27 years after the implementation of universal vaccination. Vaccines, 8(2), 156. 10.3390/vaccines8020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ko S., Lv J., Ji F., Yan B., Xu F., Xu A. (2014). Perinatal hepatitis B prevention program in Shandong Province, China: Evaluation and progress. Human Vaccines & Immunotherapeutics, 10(9), 2755–2760. 10.4161/hv.29648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xu C., Rui Y., Chen J., Chen T., Dai Y., Zhou Y.-H. (2022). Efficacy of the hepatitis B vaccine alone in the prevention of hepatitis B perinatal transmission in infants born to hepatitis B e antigen-negative carrier mothers. Journal of Virus Eradication, 8(2), 100076. 10.1016/j.jve.2022.100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhou X., Zhou Y. H. (2020). Hepatitis B vaccine development and implementation. Human Vaccines & Immunotherapeutics, 16(7), 1533–1544. 10.1080/21645515.2020.1732166 [DOI] [PMC free article] [PubMed] [Google Scholar]